Abstract
In this study, we evaluated the ability of DNA vaccines encoding the bacterioferritin (BFR) or P39 proteins of Brucella spp. to induce cellular and humoral immune responses and to protect BALB/c mice against a challenge with B. abortus 544. We constructed eukaryotic expression vectors called pCIBFR and pCIP39, encoding BFR or P39 antigens, respectively, and we verified that these proteins were produced after transfection of COS-7 cells. PCIBFR or pCIP39 was injected intramuscularly three times, at 3-week intervals. pCIP39 induced higher antibody responses than did the DNA vector encoding BFR. Both vectors elicited a T-cell-proliferative response and also induced a strong gamma interferon production upon restimulation with either the specific antigens or Brucella extract. In this report, we also demonstrat that animals immunized with these plasmids elicited a strong and long-lived memory immune response which persisted at least 3 months after the third vaccination. Furthermore, pCIBFR and pCIP39 induced a typical T-helper 1-dominated immune response in mice, as determined by cytokine or immunoglobulin G isotype analysis. The pCIP39 delivered by intramuscular injection (but not the pCIBFR or control vectors) induced a moderate protection in BALB/c mice challenged with B. abortus 544 compared to that observed in positive control mice vaccinated with S19.
The first report of the protective efficacy of a nucleic acid-based vaccine in an animal model was published by J. B. Ulmer et al. (57). Since then, several reports have shown that DNA vaccination engenders long-lived humoral and cellular immune responses in vivo against virus, bacterium, or parasite but could also be of potential interest for the treatment of autoimmunity, cancer, and allergy in a variety of animal models (10, 20, 22, 33, 54).
DNA vaccine provides prolonged antigen expression, leading to amplification of immune response and induces memory responses against infectious agents (23, 30). Moreover, endogenous expression of antigen from DNA introduced into host cells leads to processed peptides presented with the major histocompatility complex class I, able to induce cytotoxic T-lymphocyte (9, 58). Finally, specific nucleotidic sequences present in the plasmid play a critical role in the immunogenicity of these vaccines by acting as adjuvant (39, 47). This type of vaccine is capable of eliciting the strong cell-mediated immunity that is required for control of infection by many intracellular agents (24, 35, 44). This kind of immune response is of paramount importance against Brucella spp. (19, 41, 50).
Brucella are gram-negative facultative intracellular bacteria that cause brucellosis in animals and humans. Their ability to survive and replicate within host phagocytic and nonphagocytic cells seems to be responsible for the duration of the disease, which may remain active for years (8, 45). The protection against this infection requires a long-lived cellular immune response, depending on the processing of the bacteria by macrophages (3, 4). Survival of the vaccine-strain should be crucial for the development of a protective cellular immune response against B. abortus in cattle. Understanding the mechanisms by which long-lived cellular immune responses are generated following vaccination will be important for the rational design of vaccines against brucellosis.
The vaccination against bovine brucellosis with live attenuated B. abortus strain S19 is used in most of the world because it has been useful for the control and eradication of this disease (18). However, S19 presents several drawbacks: it is difficult to differentiate between vaccinated and naturally infected animals, because S19 elicit antibodies against smooth lipopolysaccharide, and S19 can also cause abortion in pregnant cattle and is still fully virulent for humans (7, 50). In order to avoid these drawbacks alternative vaccine approaches are needed.
Our laboratory has previously described bacterioferritin (BFR) (13) and P39 (a putative periplasmic binding protein) (11) as T-cell immunodominant Brucella antigens (12) that elicit both a strong delayed-type hypersensitivity reaction in guinea pigs sensitized with brucellin and in vitro proliferation or gamma interferon (IFN-γ) production by peripheral blood mononuclear cells from infected cattle. The potential of these antigens to induce a Th1-oriented immune response makes them attractive candidates for DNA vaccination. We have recently demonstrated that these recombinant antigens (BFR and P39) adjuvanted with CpG oligodeoxynucleotide (ODN) induced a Th1-type immune response, but only the recombinant P39 plus CpG ODN induced a significant level of protection against B. abortus 544 challenge (2).
The present study shows that intramuscular (i.m.) immunization of mice with pCIBFR or pCIP39 (expression vectors for BFR and P39, respectively) generates a strong and long-lived specific T-cell response, whereas only pCIP39 induces a strong humoral response. However, the Th1 immune response induced by pCIBFR does not protect mice against B. abortus 544 following intraperitoneal (i.p.) challenge, whereas pCIP39 induced a moderate level of protection compared to the S19 vaccine.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. abortus 544 (virulent strain) was obtained from J.-M. Verger (Institut National de la Recherche Agronomique, Pathologie Infectieuse et Immunologie, Nouzilly, France), and B. abortus S19 was obtained from J. Godfroid. Brucella cells were grown on 2YT agar. For vaccination or challenge, the colonies were suspended in a sterile phosphate-buffered saline (PBS), bacterial cells were washed twice, and the number of bacteria was measured by determining the CFU on 2YT (10 g of yeast extract, 10 g of tryptone, and 5 g of NaCl per liter) agar plates.
Plasmid preparation.
The coding sequences of the Brucella p39 gene (flanked by XbaI/EcoRI sites) and the Brucella bfr gene (flanked by XbaI/XhaI sites) were ligated into the multiple-cloning site of the mammalian expression vector pCI (Promega, Madison, Wis.), giving pCIP39 and pCIBFR, respectively. Escherichia coli DH5α cells (Stratagene, La Jolla, Calif.) were transformed with the plasmids and grown in Luria-Bertani broth containing ampicillin (100 μg/ml). Plasmid DNA for in vitro transfection or mouse immunization, was extracted from a 16-h culture and purified using the Endo-Free Plasmid Giga kit (Qiagen, Chatsworth, Calif.). The concentration and purity of the plasmid was determined by measuring the optical density ratio A260/A280. Plasmid DNA was adjusted to a final concentration of 1 mg/ml in PBS and stored at −80°C.
Polyclonal antibodies against BFR.
Purified BFR (0.1 mg) was mixed with 1 ml of Freund's adjuvant (complete for the initial injection; incomplete for subsequent intramuscular injections) and injected into a rabbit that was previously bled to collect preimmune serum. Three inoculations were performed at 3-week intervals. Antisera were collected 10 days after the last injection; antisera used in this work were used at working dilutions of 1:500.
Antigen expression in COS-7 cell line.
Monkey kidney COS-7 epithelial cells were grown at 37°C in 5% CO2 in six-well plates (Falcon) containing Dulbecco's modified Eagle's medium (DMEM) (Gibco, BRL) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, penicillin (100 U/ml), and gentamicin (50 μg/ml), and subconfluence monolayers were washed once with serum-free DMEM; afterwards, 500 μl of DMEM (supplemented as above, but without FBS) was added. Then, 100 μl of transfection mixture (100 μl of serum-free DMEM containing 6 μg of Fugene6 [Boehringer, Mannheim, Germany]) and 1 μg of plasmid DNA were held at room temperature (RT) for 5 min and added to the cells, which were then incubated at 37°C in 5% CO2 overnight. Expression of BFR and P39 proteins was detected by immunoblotting.
SDS-PAGE and immunoblotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting were performed as previously described (56, 61). After 24 h of transfection COS-7 cells were lysed by freeze-thawing cycles. Cell lysates were centrifuged at 12,000 × g for 5 min and then analyzed by immunoblotting, by using anti-BFR or anti-P39 antibodies (37).
Mice DNA vaccination and challenge.
Specific-pathogen-free 4-week-old BALB/c female mice were purchased, from Iffa-Credo, Brussels, Belgium. Six-week-old mice randomly allocated in three groups of 16 mice, received i.m. injections in the tibialis anterior muscles with 100 μg of pCIBFR, pCIP39, or pCI as a negative control in 50 μl sterile saline (PBS), by using a 1-ml insulin syring with a 28-gauge needle. Three vaccinations at 3-week intervals were performed. The immune response (four mice per group) was analyzed 3 and 12 weeks after the last DNA vaccination. Positive control mice (n = 8) received an intraperitoneal (i.p.) injection with 5 × 104 CFU of B. abortus vaccine strain S19 in 100 μl of sterile PBS 4 weeks before the challenge.
For the challenge, mice received an i.p. injection with approximately 5 × 104 CFU of B. abortus 544 in 100 μl of sterile PBS, 4 weeks after the last vaccination.
Quantitation of bacteria in the spleen.
At 4 and 8 weeks postchallenge, mice (n = 4) were killed by cervical dislocation, and their spleens were removed, homogenized in 2 ml of sterile PBS, serially 10-fold diluted, and plated in triplicate. B. abortus 544 colonies were counted after 3 days of incubation at 37°C with 10% CO2.
In the positive control group vaccinated with strain S19, dilutions were spared on 2YT agar alone incubated with or without CO2 and on 2YT agar plus 0.1% erythritol to distinguish between B. abortus 544 and S19 strains (46).
Isotype-specific Ig ELISAs.
Expression and purification of the recombinant P39 and recombinant BFR were described previously (2, 37). Briefly the respective genes were cloned into a pET-15b expression vector (Novagen, Madison, Wis.), and the resulting plasmid was introduced in E. coli BL21 (DE3). After induction with IPTG (isopropyl-β-d-thiogalactopyronoside) (Promega) the His-tagged proteins were purified from the pellet lysate on a Ni-affinity chromatography column (Pharmacia Biotech).
The wells of polystyrene plates (MaxiSorp; Nunc A/S, Roskilde, Denmark) were coated overnight at 4°C with recombinant protein BFR, P39 (50 μl per well) at a final concentration of 1 μg/ml in PBS (pH 7.4) or with the B. abortus 544 or B. melitensis 16M bacterial lysate at a concentration 3 μg/ml. After three wash cycles with PBS, the plates were saturated for 2 h at RT with 150 μl of blocking buffer (PBS with 2.5% casein). The wells were washed with PBS containing 0.1% Tween 20. Fifty microliters of serially twofold-diluted individual serum samples, starting at a 1/100 dilution in buffer (PBS with 1.25% casein, 50 mM EDTA, and 0.05% Tween 20), was added to the plates and incubated for 1 h at RT. The serum from nonimmunized mice was used as a negative control. After five washing cycles, plates were incubated with a 1,000-fold dilution of goat biotinylated anti-mouse immunoglobulin G1 (IgG1) or IgG2a (Amersham) for 1 h at RT. After the plates were washed, 1,000-fold-diluted streptavidin-horseradish peroxidase (Amersham) was added for IgG isotype detection for 1 h at RT. The excess reagent was removed by five washing cycles. Finally TMB (3,3′,5,5′-tetramethyl benzidine) in citrate-phosphate buffer (0.05 M Na2HPO4, 0.025 M citric acid, pH 5.0) and 2 mM H2O2 were added to monitor the peroxidase activity. The reaction was stopped after 20 min by addition of 2 M H2SO4. The optical density was measured at 450 and 630 nm on a Bio Kinetics Reader EL-340 (Biotek Instruments, Winooski, Vt.). The end point titer was defined as the highest dilution of serum giving an optical reading of two times the reading of the negative control.
Lymphocyte proliferation assay.
Proliferation is determined by measuring the level of incorporation of [3H]thymidine into the DNA of actively dividing cells. The spleens were removed and homogenized with RPMI 1640 medium (Gibco, BRL) containing 10% FBS, the cells were centrifuged for 10 min at 1,200 × g, and the cell-containing pellets were washed two times with RPMI 1640 supplemented with 5% FBS. Erythrocytes in spleen cell preparations were lysed with Gey's solution, by incubating the mixture for 10 min at 4°C. The lymphocytes were washed two times in RPMI 1640 containing 5% FBS by centrifugation. Splenocytes resuspended in complete medium (RPMI 1640 supplemented with 10% FBS, 2 mM l-glutamine, penicillin-streptomycin [100 U/ml], gentamicin [50 μg/ml], and 5 × 10−5 M 2-mercaptoethanol) were cultured at 2 × 105 cells per well in 96-well flat-bottom microwell plates. Cultures in quadruplicate were stimulated with 1 μg of BFR or P39 or 3 μg of B. abortus 544 or B. melitensis 16M. Concanavalin A (ConA) (Sigma, St. Louis, Mo.) at a concentration of 0.5 μg per well and RPMI were used as positive and unstimulated controls, respectively. After 72 h at 37°C in a humidified 5% CO2 incubator, cells were pulsed with 0.5 μCi of [3H]thymidine/well (ICN) for 18 h. The cells were harvested onto glass filter strips (Skatron Inc., Sterling, Va.). Tritiated-thymidine incorporation was counted by liquid scintillation spectroscopy with a Betaplate counter (WALLAC Oy, Turku, Finland). The mean number of counts per minute and standard error of the mean for each quadruplicate set of cells were determined.
Cytokine quantitation.
Levels of IFN-γ and interleukin-5 (IL-5) in murine splenocyte culture supernatants were measured after 96 h of incubation with antigen or mitogen as described for the lymphocyte proliferation assay. IFN-γ and IL-5 were assayed according to the manufacturer's instructions using specific enzyme-linked immunosorbent assay (ELISA) kits (Pharmingen, San Diego, Calif.).
Statistical analysis.
The P value was calculated by using the Student t test, and a P value of <0.05 was considered to be significant.
RESULTS
Construction of pCIBFR and pCIP39 expression vectors.
To evaluate the relative role of the BFR and P39 proteins in inducing an immune response and protective immunity against brucellosis, the bfr and p39 coding sequences were inserted into eukaryotic expression vector, pCI, to produce plasmids pCIBFR and pCIP39, respectively.
To examine protein expression from these plasmids in eukaryotic cells, COS-7 cells were transiently transfected with plasmid pCIBFR or pCIP39 or with the parental plasmid (pCI), serving as a negative control, and we analyzed BFR and P39 proteins expression by immunoblotting analysis of whole-cell lysates (Fig. 1).
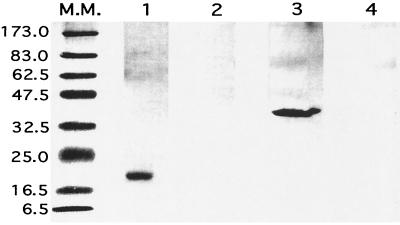
FIG. 1.
In vitro expression of BFR and P39 was tested by transient transfection of COS-7 cells. The cells were transfected with either pCI (lanes 2 and 4), pCIBFR (lane 1), or pCIP39 (lane 3). After 24 h, the COS-7 cells were washed and lysed. The proteins were separated by SDS-PAGE, and the antigens were then detected by immunoblotting, using rabbit anti-BFR polyserum (lanes 1 and 2), or anti-P39 monoclonal antibody (lanes 3 and 4). Molecular mass standards (lane M.M.) are indicated in kilodaltons.
Figure 1 shows that pCIBFR and pCIP39 DNA-transfected cells express the respective BFR and P39 proteins, with an apparent molecular mass of 20 kDa for BFR and 39 kDa for P39 (lanes 1 and 3), while these proteins were not detected in control pCI-transfected COS-7 cells (lanes 2 and 4).
The humoral response to BFR or P39 induced by DNA vaccination.
Sera collected 3 and 12 weeks after the last vaccination were assayed for the presence of BFR- or P39-specific antibodies by indirect ELISA using the relevant purified recombinant proteins, and Brucella whole-cell extracts as antigens. As shown in Table 1, 3 weeks after the last immunization, mice injected with pCIBFR have weak antibody IgG2a titers to BFR protein and Brucella extracts while no IgG1 could be detected. The pCIP39 immunizations induced antibody IgG2a responses to P39 and Brucella extracts that were 1.5 to 2 logs higher than these induced by pCIBFR injections. This antigen also induced IgG1 titers which were slightly lower than the IgG2a titers. Antibodies against P39 could already be detected 1 week after the first pCIP39 DNA injection (data not shown). Immunization with pCI or pCIBFR did not induce any production of anti-P39 antibodies. Humoral response measured 12 weeks postvaccination indicates that pCIP39 DNA vaccine leads to the generation of long-lived IgG1 and IgG2a responses (Table 2). By contrast, at 12 weeks postvaccination, no more antibody against BFR could be demonstrated.
TABLE 1.
IgG responses after 3 weeks in mice vaccinated with various plasmidsa
| Antigen | Log10 antibody titer in mice vaccinated with:
|
|||||
|---|---|---|---|---|---|---|
| pCl
|
pCIBFR
|
pCIP39
|
||||
| IgG1 | IgG2a | IgG1 | IgG2a | IgG1 | IgG2a | |
| BFR | 2.3 ± 0.06 | |||||
| P39 | 4.7 ± 0.12 | 5.0 ± 0.1 | ||||
| 16M | 2.0 ± 0.02 | 3.2 ± 0.05 | 3.5 ± 0.09 | |||
| 544 | 2.0 ± 0.04 | 3.2 ± 0.06 | 3.5 ± 0.04 | |||
Sera from four mice per group, collected 3 weeks after the last vaccination, were assayed individually by ELISA.
TABLE 3.
Protection against B. abortus 544 provided to BALB/c mice by vaccination with pCIBFR and pCIp39 compared to live strain B. abortus B19a or pCI alone
| Treatment group (n = 4) | Vaccine | Log10 brucellae in spleen (mean ± SD) at wk postchallenge
|
Log units of protection at wk
|
||
|---|---|---|---|---|---|
| 4 | 8 | 4 | 8 | ||
| 1 | —b | 4.95 ± 0.04 | 5.13 ± 0.23 | ||
| 2 | B19 | 2.95 ± 0.18 | 2.05 ± 0.28 | 2c | 3.08c |
| 3 | pCI | 4.96 ± 0.10 | 5.02 ± 0.10 | ||
| 4 | pCIBFR | 4.98 ± 0.61 | 5.0 ± 0.28 | ||
| 5 | pCIP39 | 4.80 ± 0.15 | 4.40 ± 0.15 | 0.15 | 0.73c |
Group 2 was vaccinated i.p. with 5 × 104 CFU of strain B19.
—, injected with PBS.
P < 0.05 (significant) compared with value for control mice.
TABLE 2.
IgG responses after 12 weeks in mice vaccinated with various plasmidsa
| Antigen | Log10 antibody titer in mice vaccinated with:
|
|||||
|---|---|---|---|---|---|---|
| pCI
|
pCIBFR
|
pCIP39
|
||||
| IgG1 | IgG2a | IgG1 | IgG2a | IgG1 | IgG2a | |
| BFR | ||||||
| P39 | 4.4 ± 0.12 | 4.4 ± 0.08 | ||||
| 16M | 3.8 ± 0.1 | 3.8 ± 0.09 | ||||
| 544 | 3.8 ± 0.07 | 3.8 ± 0.02 | ||||
Sera from four mice per group, collected 12 weeks after the last vaccination, were assayed individually by ELISA.
T-cell-proliferative response by splenocytes from DNA-vaccinated mice.
To further investigate the cellular immune response induced by the plasmid vectors we analyzed the proliferative T-cell response.
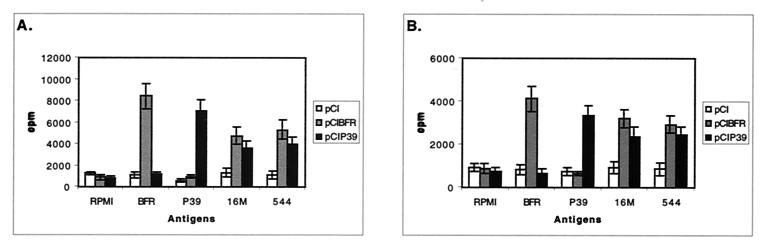
As shown in Fig. 2, both BFR and P39 DNA vaccination resulted in specific T-cell proliferation in response to either the related recombinant antigen or to Brucella extracts. This specific induced proliferative response was also found at 12 weeks postvaccination (Fig. 2B), although to a lesser extent. In contrast, immunization with pCI appeared to have no effect on the level of T-cell proliferative response (Fig. 2). The ConA mitogen was able to induce T-cell proliferation in all cases (data no shown).
FIG. 2.
Lymphocyte proliferation assay. BALB/c mice were immunized with pCI, pCIBFR, or pCIP39. T-cell-proliferative responses were measured at week 3 (A) and 12 (B) after the last immunization. Splenocytes from each mouse were prepared and stimulated in vitro with purified recombinant BFR or P39 (1 μg/ml) and Brucella extracts (3 μg/ml) as the antigen. After 72 h of culture, [3H]thymidine was added; 18 h later, cells were harvested and the counts per minute were determined. Each sample was assayed in quadruplicate. Data represent the mean ± standard deviation (error bars) from the four mice.
IFN-γ and IL-5.
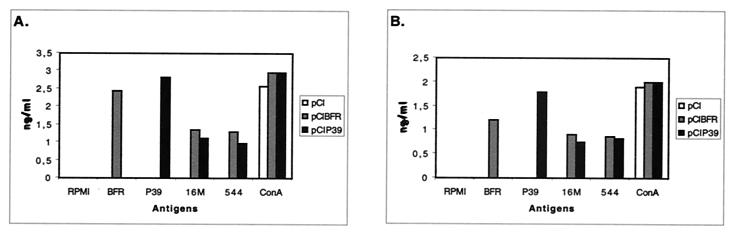
Cytokine production profiles from splenocytes of four vaccinated mice per group, 3 and 12 weeks after the last vaccination, were examined after restimulation with different antigens. As shown in Fig. 3, splenic lymphocytes from pCIP39-vaccinated mice produced up to 2.6 ng of IFN-γ per ml 3 weeks postvaccination or up to 1.7 ng per ml 12 weeks postvaccination upon restimulation with P39. Lower, while significant, IFN-γ levels were obtained upon stimulation with Brucella extract. By contrast, little if any IL-5 was measured (data not shown). Spleen cells from animals immunized with pCIBFR also secreted large amounts of IFN-γ upon restimulation with BFR, 2.4 ng per ml at 3 weeks and 1 ng per ml at 12 weeks (Fig. 3A). We could not detect IL-5 secretion from cells obtained from this group (data not shown). Mice vaccinated with pCI produced neither IFN-γ or IL-5 upon restimulation with the antigens. The RPMI did not induce IFN-γ or IL-5 production, whereas ConA induced a large quantity of IFN-γ (Fig. 3) and a minimal amount of IL-5 (data no shown). Taken together, these results indicate that there was a significant Th1 biased response after the immunization with pCIBFR or pCIP39.
FIG. 3.
Quantitative ELISA analysis of IFN-γ secretion by murine lymphocytes isolated after DNA immunization (pCI, pCIBFR, or pCIP39) and subsequent stimulation in culture using different antigens. Splenocytes from the immunized mice (four mice/group) were harvested at week 3 (A) and 12 (B) after the last immunization. Assays were performed on supernatants of 2 × 106 lymphocytes per ml. Lymphocytes were restimulated with the indicated antigens. At 96 h after restimulation, supernatants were analyzed for IFN-γ levels. Samples were tested in duplicate.
Protection against B. abortus 544 challenge in DNA-immunized mice.
To determine whether the immunization with the candidate DNA vaccines induced protection against B. abortus 544 infection, mice immunized three times with either pCIBFR or pCIP39 or pCI were infected 4 weeks later with B. abortus 544. Four and eight weeks after challenge, mice were killed and CFU in the spleens were quantitated. The mice which were immunized by pCIP39 showed only a slight but significant level of protection at 8 weeks after challenge (Table 3). No significant difference in the number of the bacteria isolated from the spleens of the nonvaccinated and pCIBFR- or pCI-vaccinated animals was observed (Table 3). In contrast, at a vaccination-to-challenge interval of 4 weeks, mice vaccinated with strain S19 demonstrated a significant protection, which increased between 4 (2 log10) and 8 weeks postinfection (3.08 log10).
DISCUSSION
Vaccination continues to be the most successful procedure for preventing losses in animals due to infectious diseases. The development of new-generation vaccine systems to prevent brucellosis is important to avoid the disadvantages of the currently used live vaccines. These new vaccines will be designed to generate immune responses mimicking those found in animals during natural infection. It is well established that protection against infection by an intracellular pathogen, including Brucella, requires the generation of potent cell-mediated immunity linked to the induction of a Th1-type immune response over time (19, 41). IFN-γ, which is a key Th1 cytokine, plays a prominent function in up-regulation of macrophage anti-Brucella activity and, with tumor necrosis factor alpha, is considered crucial for protection against Brucella spp. (5, 6, 28, 29, 52, 62).
The achievement of this goal for a new brucellosis vaccine implies both the identification of adequate delivery or adjuvant system and the analyses of major immunodominant antigens. Many antigens of Brucella have been tested with or without a variety of adjuvants. Among these antigens, Cu-Zn superoxide dismutase, Yajc, L7-L12, and GroELs could induce a humoral or/and cellular immune response in mice, but only L7-L12 and certain epitopes of the Cu-Zn superoxide dismutase could induce some level of protection (38, 42, 53, 60). We recently described that the addition of CpG ODN adjuvants to the recombinant P39 protein can induce a strong anti-Brucella Th1 response that is able to significantly reduce the splenic bacterial load after a challenge with B. abortus 544 (2). With the P39-CpG ODN vaccine the level of protection was high (2.48 log10) 4 weeks after the challenge and similar to the protection conferred by the S19 vaccine control. Nevertheless, at 8 weeks postinfection while the S19 induced protection remained high, the protective activity of the P39 subunitary vaccine vanished (1.21 log10). In order to achieve a long-term protection with this promising P39 antigen, we investigated the naked DNA vaccine approach.
DNA vaccines, because of prolonged in situ antigen production, can elicit long-lasting humoral and cellular immune responses (21, 25, 36, 57). DNA vaccines also produce antigen in a highly immunogenic form because of the processing via major histocompatibility complex class I and II (14, 16, 48, 51). Furthermore, the plasmidic vector contains a built-in adjuvant effect (51). The CpG motifs in the bla gene stimulate the innate immune system to create a cytokine milieu (e.g., IL-12, IFN-α, IFN-γ, IL-2, and tumor necrosis factor beta [1, 15, 59]) that favors the generation of Th1 response against the antigen encoded by the plasmid (26, 32).
In this study, we constructed eukaryotic expression vectors for BFR and P39 Brucella proteins, and we showed that the BFR and P39 proteins were expressed intracellularly in COS-7 cells transfected with pCIBFR and pCIP39 plasmids, respectively. We evaluated the capacity of these constructs to elicit immune responses and protective immunity in BALB/c mice.
Three weeks after the last vaccination we found a weak titer of specific IgG2a in mice immunized i.m. with plasmid pCIBFR (Table 1). In contrast, pCIP39 induced high titers of anti-P39 antibodies (IgG1 and IgG2a), also reacting against Brucella extracts that were still present up to 12 weeks after the last vaccination (Table 2). The IgG1/IgG2a ratio was, approximately, equal to 1. Since the isotype profile of antibody response is a reflection of the T-helper-cell types (27, 40), these results suggest that i.m. pCIP39 or pCIBFR DNA vaccination induced Th1 responses. Mice vaccinated i.m. with pCI did not produce detectable antibodies against BFR or P39 antigens (Tables 1 and 2). Those data paralleled the results obtained with the adjuvanted recombinant proteins (2), the BFR antigen being less able to induce a strong antibody response than P39. This is also in agreement with the demonstrated potency of recombinant P39 protein to serve as serological diagnostic antigen in ELISA (38).
The induction of T-cell immune responses after DNA immunization was then evaluated by measuring T-cell-proliferative and cytokine responses after in vitro stimulation of splenic cells with purified recombinant BFR or P39 proteins or Brucella total extracts. Both BFR and P39 induced a high T-cell-proliferative response (Fig. 2) and high levels of IFN-γ (Fig. 3) but no IL-5. While we do not look for IL-4 synthesis, the existence of IgG1 antibodies specific for P39 indicates that IL-4 is also somehow involved in this response. Furthermore, this DNA immunization induced memory T cells since we still found a good immune response 12 weeks postvaccination. Even a single dose of DNA immunization (pCIBFR and pCIP39) was able to elicit a detectable immune response (data not shown). Once against these results are in agreement with those obtained with the CpG adjuvanted recombinant proteins, which were both able to induce a strong Th1 type immune response.
On the basis of these data we started to analyze the protective efficacy of the pCIBFR or pCIP39 DNA vaccines against a B. abortus challenge.
Whatever the time postchallenge considered, neither the pCIBFR vaccine nor pCI vector alone was able to confer a significant protection level (Table 3). Those results were reminiscent of the results gained with recombinant BFR, which was unable to protect, while inducing a strong specific Th1 immune response (2).
The level of protection conferred by the pCIP39 vaccine was low becoming only significant (0.73 logs) at 8 weeks postchallenge, while still remaining less efficient than the S19 vaccine. We also found that coinjection of both expression vectors did not enhance the immune response or the level of protection (data non shown).
It was previously reported that a DNA vaccine encoding the L7-L12 ribosomal protein induced an appropriate immune response, and conferred, in a B. abortus 2308 challenge experiment, a significant level of protection of 0.53 and 1.26 log units at 20 and 30 days after the last immunization, respectively (34). On the other hand the recombinant L7-L12 adjuvanted with monophosphoryl lipid A induced 1.13 and 1.21 log units of protection at 14 and 28 days after the last immunization, respectively (42). These results demonstrated that these two vaccine preparations induced approximately the same level of protection 4 weeks postinfection. Surprisingly, in our case, the pCIP39 vaccine was less efficacious than the recombinant P39 protein adjuvanted with CpG ODN. Considering the overall similarity (as far as we can see) of the immune response induced by the two different vaccinal preparations, the discrepancy between the level of protection achieved is striking.
Reasonable explanations for the low protection conferred by pCIP39 may be searched for at the level of the antigen (quantity, localization, etc.), at the level of the adjuvantation or may be caused by subtle differences between immune response elicited by the two vaccinal preparations.
The production of P39 detected in transiently transfected COS-7 cells and the immune response induced in vaccinated mice argue for an in vivo expression. Nevertheless, the amount of antigen produced in vivo after DNA immunization is usually in the picogram or the nanogram range (23, 43). So the total level of P39 protein production in vivo, while believed to be sustained, could still be less than the amount of recombinant protein administered (three administrations of 20 μg) in the previous experiment, although the systemic immune parameters we measured were almost undistinguishable from the parameters measured when the mice were immunized with recombinant proteins. So, notwithstanding the quantity of antigen, the naked DNA immunization, compared to the adjuvanted recombinant protein, could also evoke an immune response subtly different in quality or localization, leading to the observed difference in the protective efficacy.
Alternatively, we demonstrated previously that neither the recombinant protein nor the CpG ODN given alone was able to induce protection and that the combination of both was required. So, another explanation could be that the adjuvant effect of the CpG in the naked DNA vaccine is less potent than the CpG ODN used with the recombinant protein, probably because it is less abundant.
In spite of the relative failure of the pCIP39 vaccine as used in this study, we remain confident of the potential of this antigen, and the testing of new formulations of the pCIP39 plasmid (e.g., adding CpG ODN as adjuvant [32], combining the DNA plasmid with a final protein boost [55], or using a live delivery vector [18, 50]) offers the opportunity to increase the potency of this candidate vaccine. This is currently under investigation in our laboratory. In addition, it would also be interesting to use several antigens as DNA-encoded vaccines (e.g., encoding L7-L12 and P39) in order to increase the level of protection against the Brucella challenge.
ACKNOWLEDGMENTS
We thank G. Houbeau and C. Evrard for their help with the mouse model.
Ayman Al-Mariri holds a bursary from the Atomic Energy Commission of Syria (AECS). This work was performed with the help of the Commission of European Communities (CEE), contact QLK2-CT-1999-00014.
REFERENCES
- 1.Akbari O. DNA Vaccination: transfection and activation of dendritic cells as key events for immunity. J Exp Med. 1999;189:169–177. doi: 10.1084/jem.189.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Mariri A, Tibor A, Mertens P, De Bolle X, Michel P, Godefroid J, Walravens K, Letesson J-J. Protection of BALB/c mice against Brucella abortus 544 challenge by vaccination with bacterioferritin or P39 recombinant proteins with CpG oligodeoxynucleotides as adjuvant. Infect Immun. 2001;69:4816–4822. doi: 10.1128/IAI.69.8.4816-4822.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araya L N, Elzer P H, Rowe G E, Enright F M, Winter A J. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J Immunol. 1989;143:3330–3337. [PubMed] [Google Scholar]
- 4.Baldwin C L, Winter A J. Macrophages and Brucella. Immunol Ser. 1994;60:363–380. [PubMed] [Google Scholar]
- 5.Caron E, Gross A, Liautard J P, Dornand J. Brucella species release a specific, protease-sensitive, inhibitor of TNF-alpha expression, active on human macrophage-like cells. J Immunol. 1996;156:2885–2893. [PubMed] [Google Scholar]
- 6.Caron E, Peyrard T, Kohler S, Cabane S, Liautard J P, Dornand J. Live Brucella spp. fail to induce tumor necrosis factor alpha excretion upon infection of U937-derived phagocytes. Infect Immun. 1994;62:5267–5274. doi: 10.1128/iai.62.12.5267-5274.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheville N F, Stevens M G, Jensen A E, Tatum F M, Halling S M. Immune responses and protection against infection and abortion in cattle experimentally vaccinated with mutant strains of Brucella abortus. Am J Vet Res. 1993;54:1591–1597. [PubMed] [Google Scholar]
- 8.Corbel M J. Recent advances in brucellosis. J Med Microbiol. 1997;46:101–103. doi: 10.1099/00222615-46-2-101. [DOI] [PubMed] [Google Scholar]
- 9.Corr M, Lee D J, Carson D A, Tighe H. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J Exp Med. 1996;184:1555–1560. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denis O, Tanghe A, Palfliet K, Jurion F, van den Berg T P, Vanonckelen A, Ooms J, Saman E, Ulmer J B, Content J, Huygen K. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect Immun. 1998;66:1527–1533. doi: 10.1128/iai.66.4.1527-1533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denoël P A, Vo T K, Tibor A, Weynants V E, Trunde J M, Dubray G, Limet J N, Letesson J J. Characterization, occurrence, and molecular cloning of a 39-kilodalton Brucella abortus cytoplasmic protein immunodominant in cattle. Infect Immun. 1997;65:495–502. doi: 10.1128/iai.65.2.495-502.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denoël P A, Vo T K, Weynants V E, Tibor A, Gilson D, Zygmunt M S, Limet J N, Letesson J J. Identification of the major T-cell antigens present in the Brucella melitensis B115 protein preparation, Brucellergene OCB. J Med Microbiol. 1997;46:801–806. doi: 10.1099/00222615-46-9-801. [DOI] [PubMed] [Google Scholar]
- 13.Denoël P A, Zygmunt M S, Weynants V, Tibor A, Lichtfouse B, Briffeuil P, Limet J N, Letesson J J. Cloning and sequencing of the bacterioferritin gene of Brucella melitensis 16M strain. FEBS Lett. 1995;361:238–342. doi: 10.1016/0014-5793(95)00189-g. [DOI] [PubMed] [Google Scholar]
- 14.Dertzbaugh M T. Genetically engineered vaccines: an overview. Plasmid. 1998;39:100–113. doi: 10.1006/plas.1997.1329. [DOI] [PubMed] [Google Scholar]
- 15.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 16.Dupuis M, Denis-Mize K, Woo C, Goldbeck C, Selby M J, Chen M, Otten G R, Ulmer J B, Donnelly J J, Ott G, McDonald D M. Distribution of DNA vaccines determines their immunogenicity after intramuscular injection in mice. J Immunol. 2000;165:2850–2858. doi: 10.4049/jimmunol.165.5.2850. [DOI] [PubMed] [Google Scholar]
- 17.Ellis R W. New technologies for making vaccines. Vaccine. 1999;17:1596–1604. doi: 10.1016/s0264-410x(98)00416-2. [DOI] [PubMed] [Google Scholar]
- 18.Fensterbank R, Plommet M. Vaccination against bovine brucellosis with a low dose of strain 19 administered by the conjunctival route. IV. Comparison between two methods of vaccination. Ann Rech Vet. 1979;10:131–139. [PubMed] [Google Scholar]
- 19.Golding B, Scott D E, Scharf O, Huang L, Zaitseva M, Lapham C, Eller N, Golding H. Immunity and protection against Brucella abortus. Microbes Infect. 2001;3:43–48. doi: 10.1016/s1286-4579(00)01350-2. [DOI] [PubMed] [Google Scholar]
- 20.Goodman J S, Van Uden J H, Kobayashi H, Broide D, Raz E. DNA immunotherapeutics: new potential treatment modalities for allergic disease. Int Arch Allergy Immunol. 1998;116:177–187. doi: 10.1159/000023943. [DOI] [PubMed] [Google Scholar]
- 21.Gregoriadis G. Genetic vaccines: strategies for optimization. Pharm Res. 1998;15:661–670. doi: 10.1023/a:1011950415325. [DOI] [PubMed] [Google Scholar]
- 22.Gupta P K, Saini M, Gupta L K, Rao V D, Bandyopadhyay S K, Butchaiah G, Garg G K, Garg S K. Induction of immune responses in cattle with a DNA vaccine encoding glycoprotein C of bovine herpesvirus-1. Vet Microbiol. 2001;78:293–305. doi: 10.1016/s0378-1135(00)00304-7. [DOI] [PubMed] [Google Scholar]
- 23.Gurunathan S, Klinman D M, Seder R A. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 24.Gurunathan S, Wu C Y, Freidag B L, Seder R A. DNA vaccines: a key for inducing long-term cellular immunity. Curr Opin Immunol. 2000;12:442–447. doi: 10.1016/s0952-7915(00)00118-7. [DOI] [PubMed] [Google Scholar]
- 25.Hassett D E, Whitton J L. DNA immunization. Trends Microbiol. 1996;4:307–312. doi: 10.1016/0966-842x(96)10048-2. [DOI] [PubMed] [Google Scholar]
- 26.Jakob T, Walker P S, Krieg A M, von Stebut E, Udey M C, Vogel J C. Bacterial DNA and CpG-containing oligodeoxynucleotides activate cutaneous dendritic cells and induce IL-12 production: implications for the augmentation of Th1 responses. Int Arch Allergy Immunol. 1999;118:457–461. doi: 10.1159/000024163. [DOI] [PubMed] [Google Scholar]
- 27.Janeway C A, Carding S, Jones B, Murray J, Portoles P, Rasmussen R, Rojo J, Saizawa K, West J, Bottomly K. CD4+ T cells: specificity and function. Immunol Rev. 1988;101:39–80. doi: 10.1111/j.1600-065x.1988.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 28.Jiang X, Baldwin C L. Effects of cytokines on intracellular growth of Brucella abortus. Infect Immun. 1993;61:124–134. doi: 10.1128/iai.61.1.124-134.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones S M, Winter A J. Survival of virulent and attenuated strains of Brucella abortus in normal and gamma interferon-activated murine peritoneal macrophages. Infect Immun. 1992;60:3011–3014. doi: 10.1128/iai.60.7.3011-3014.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klinman D M, Sechler J M, Conover J, Gu M, Rosenberg A S. Contribution of cells at the site of DNA vaccination to the generation of antigen-specific immunity and memory. J Immunol. 1998;160:2388–2392. [PubMed] [Google Scholar]
- 31.Klinman D M, Verthelyi D, Takeshita F, Ishii K J. Immune recognition of foreign DNA: a cure for bioterrorism? Immunity. 1999;11:123–129. doi: 10.1016/s1074-7613(00)80087-4. [DOI] [PubMed] [Google Scholar]
- 32.Krieg A M. The role of CpG motifs in innate immunity. Curr Opin Immunol. 2000;12:35–43. doi: 10.1016/s0952-7915(99)00048-5. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan B R. Current status of DNA vaccines in veterinary medicine. Adv Drug Deliv Rev. 2000;43:3–11. doi: 10.1016/s0169-409x(00)00073-9. [DOI] [PubMed] [Google Scholar]
- 34.Kurar E, Splitter G A. Nucleic acid vaccination of Brucella abortus ribosomal L7/L12 gene elicits immune response. Vaccine. 1997;15:1851–1857. doi: 10.1016/s0264-410x(97)00140-0. [DOI] [PubMed] [Google Scholar]
- 35.Lai W C, Bennett M. DNA vaccines. Crit Rev Immunol. 1998;18:449–484. doi: 10.1615/critrevimmunol.v18.i5.30. [DOI] [PubMed] [Google Scholar]
- 36.Lee D J, Corr M, Carson D A. Control of immune responses by gene immunization. Ann Med. 1998;30:460–468. doi: 10.3109/07853899809002487. [DOI] [PubMed] [Google Scholar]
- 37.Letesson J J, Tibor A, van Eynde G, Wansard V, Weynants V, Denoël P, Saman E. Humoral immune responses of Brucella-infected cattle, sheep, and goats to eight purified recombinant Brucella proteins in an indirect enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 1997;4:556–564. doi: 10.1128/cdli.4.5.556-564.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin J, Adams L G, Ficht T A. Immunological response to the Brucella abortus GroEL homolog. Infect Immun. 1996;64:4396–4400. doi: 10.1128/iai.64.10.4396-4400.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mor G. Plasmid DNA: a new era in vaccinology. Biochem Pharmacol. 1998;55:1151–1153. doi: 10.1016/s0006-2952(97)00529-7. [DOI] [PubMed] [Google Scholar]
- 40.Mosmann T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 41.Oliveira S C, Splitter G A. CD8+ type 1 CD44hi CD45RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur J Immunol. 1995;25:2551–2557. doi: 10.1002/eji.1830250922. [DOI] [PubMed] [Google Scholar]
- 42.Oliveira S C, Splitter G A. Immunization of mice with recombinant L7/L12 ribosomal protein confers protection against Brucella abortus infection. Vaccine. 1996;14:959–962. doi: 10.1016/0264-410x(96)00018-7. [DOI] [PubMed] [Google Scholar]
- 43.Prud'homme G J, Lawson B R, Chang Y, Theofilopoulos A N. Immunotherapeutic gene transfer into muscle. Trends Immunol. 2001;22:149–155. doi: 10.1016/s1471-4906(00)01822-6. [DOI] [PubMed] [Google Scholar]
- 44.Ramshaw I A, Ramsay A J. The prime-boost strategy: exciting prospects for improved vaccination. Immunol Today. 2000;21:163–165. doi: 10.1016/s0167-5699(00)01612-1. [DOI] [PubMed] [Google Scholar]
- 45.Sangari F J, Aguero J. Molecular basis of Brucella pathogenicity: an update. Microbiologia. 1996;12:207–218. [PubMed] [Google Scholar]
- 46.Sangari F J, Garcia-Lobo J M, Aguero J. The Brucella abortus vaccine strain S19 carries a deletion in the erythritol catabolic genes. FEMS Microbiol Lett. 1994;121:337–342. doi: 10.1111/j.1574-6968.1994.tb07123.x. [DOI] [PubMed] [Google Scholar]
- 47.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen M D, Silverman G J, Lotz M, Carson D A, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 48.Scheerlinck J Y. Genetic adjuvants for DNA vaccines. Vaccine. 2001;19:2647–2656. doi: 10.1016/s0264-410x(00)00495-3. [DOI] [PubMed] [Google Scholar]
- 49.Shata M T, Stevceva L, Agwale S, Lewis G K, Hone D M. Recent advances with recombinant bacterial vaccine vectors. Mol Med Today. 2000;6:66–71. doi: 10.1016/s1357-4310(99)01633-0. [DOI] [PubMed] [Google Scholar]
- 50.Smith L D, Ficht T A. Pathogenesis of Brucella. Crit Rev Microbiol. 1990;17:209–230. doi: 10.3109/10408419009105726. [DOI] [PubMed] [Google Scholar]
- 51.Stan A C, Casares S, Brumeanu T D, Klinman D M, Bona C A. CpG motifs of DNA vaccines induce the expression of chemokines and MHC class II molecules on myocytes. Eur J Immunol. 2001;31:301–310. doi: 10.1002/1521-4141(200101)31:1<301::AID-IMMU301>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 52.Stevens M G, Pugh G W, Tabatabai L B. Effects of gamma interferon and indomethacin in preventing Brucella abortus infections in mice. Infect Immun. 1992;60:4407–4409. doi: 10.1128/iai.60.10.4407-4409.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tabatabai L B, Pugh G W., Jr Modulation of immune responses in Balb/c mice vaccinated with Brucella abortus Cu-Zn superoxide dismutase synthetic peptide vaccine. Vaccine. 1994;12:919–924. doi: 10.1016/0264-410x(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 54.Tanghe A, Lefevre P, Denis O, D'Souza S, Braibant M, Lozes E, Singh M, Montgomery D, Content J, Huygen K. Immunogenicity and protective efficacy of tuberculosis DNA vaccines encoding putative phosphate transport receptors. J Immunol. 1999;162:1113–1119. [PubMed] [Google Scholar]
- 55.Tanghe A, D'Souza S, Rosseels V, Denis O, Ottenhoff T H M, Dalemans W, Wheeler C, Huygen K. Improved immunogenicity and protective efficacy of Tuberculosis DNA vaccine encoding Ag85 by protein boosting. Infect Immun. 2001;69:3041–3047. doi: 10.1128/IAI.69.5.3041-3047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tibor A, Weynants V, Denoël P, Lichtfouse B, De Bolle X, Saman E, Limet J N, Letesson J J. Molecular cloning, nucleotide sequence, and occurrence of a 16.5-kilodalton outer membrane protein of Brucella abortus with similarity to Pal lipoproteins. Infect Immun. 1994;62:3633–3639. doi: 10.1128/iai.62.9.3633-3639.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ulmer J B, Donnelly J J, Liu M A. DNA vaccines Promising: A new approach to inducing protective immunity. ASM News. 1996;62:476–479. [Google Scholar]
- 58.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 59.Ulmer J B, Sadoff J C, Liu M A. DNA vaccines. Curr Opin Immunol. 1996;8:531–536. doi: 10.1016/s0952-7915(96)80042-2. [DOI] [PubMed] [Google Scholar]
- 60.Vemulapalli R, Duncan A J, Boyle S M, Sriranganathan N, Toth T E, Schurig G G. Cloning and sequencing of yajC and secD homologs of Brucella abortus and demonstration of immune responses to YajC in mice vaccinated with B. abortus RB51. Infect Immun. 1998;66:5684–5691. doi: 10.1128/iai.66.12.5684-5691.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weynants V, Gilson D, Cloeckaert A, Denoël P A, Tibor A, Thiange P, Limet J N, Letesson J J. Characterization of a monoclonal antibody specific for Brucella smooth lipopolysaccharide and development of a competitive enzyme-linked immunosorbent assay to improve the serological diagnosis of brucellosis. Clin Diagn Lab Immunol. 1996;3:309–314. doi: 10.1128/cdli.3.3.309-314.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhan Y, Cheers C. Endogenous gamma interferon mediates resistance to Brucella abortus infection. Infect Immun. 1993;61:4899–4901. doi: 10.1128/iai.61.11.4899-4901.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]