Abstract
The ability to penetrate tissue is an important virulence factor for pathogenic spirochetes. Previous studies have recognized the role of motility in allowing pathogenic spirochetes to invade tissues and migrate to sites favorable for bacterial proliferation. However, the nature of the movements, whether they are random or controlled by chemotaxis systems, has yet to be established. In this study, we addressed the role of motility and chemotaxis in tissue penetration by the periodontal disease-associated oral spirochete Treponema denticola using an oral epithelial cell line-based experimental approach. Wild-type T. denticola ATCC 35405 was found to penetrate the tissue layers effectively, whereas a nonmotile mutant was unable to overcome the tissue barrier. Interestingly, the chemotaxis mutants also showed impaired tissue penetration. A cheA mutant that is motile but lacks the central kinase of the chemotaxis pathway showed only about 2 to 3% of the wild-type penetration rate. The two known chemoreceptors of T. denticola, DmcA and DmcB, also appear to be involved in the invasion process. The dmc mutants were actively motile but exhibited reduced tissue penetration of about 30 and 10% of the wild-type behavior, respectively. These data suggest that not only motility but also chemotaxis is involved in the tissue penetration by T. denticola.
Motility is now widely recognized as a virulence factor for many pathogenic organisms (39). Movement of motile organisms is usually guided by a sophisticated chemotaxis system (29, 56). Motility and chemotaxis are known to allow bacteria efficient nutrient acquisition, avoidance of toxic substances, or translocation to optimal colonization sites. In a variety of pathogenic bacteria, including the human gastric and gastrointestinal pathogens Helicobacter pylori (5) and Campylobacter jejuni (57, 62), the cholera agent Vibrio cholerae (8–10), the fish pathogen Vibrio anguillarum (36–38) and the plant root pathogen Agrobacterium tumefaciens (13), chemotaxis appears to be an important factor for successful colonization of their respective hosts. Interestingly, a strong chemotactic response towards substances that are present at their site of infection has been demonstrated for many gastrointestinal pathogens, including H. pylori, V. cholerae, and the spirochete Brachyspira hyodysenteriae, each of which is highly attracted by mucin. It has been discussed that this feature enables H. pylori to direct itself against the gastric flow toward the epithelium (5). Fully motile but nonchemotactic mutant strains were shown to be avirulent even though they appeared to be able to persist in the stomach for an extended period of time.
Pathogenesis-associated spirochetes are motile bacteria that can be found in the most advanced regions of infected tissue (34). Previous studies have shown that the motility of spirochetes is a key virulence factor, since spirochete motility mutants fail to infect their host (18, 48). While it is evident that pathogenic spirochetes do move within the tissues of their respective hosts, it is still unclear whether these cellular movements are random or directed by chemotaxis systems. Genome sequence analyses of Borrelia burgdorferi, Treponema pallidum, and Treponema denticola revealed that these spirochetes not only have complete flagellum-based motility systems but also possess the genes necessary for chemotaxis that could direct the flagellar movement (6, 7, 11, 14, 22a, 54; see also data available at www.tigr.org). Earlier experiments in our laboratory as well as those of other groups have also indicated that pathogenic spirochetes do indeed perform chemotaxis towards substances present at their site of infection (15, 19, 23, 30, 33, 52, 63). Based on these findings, we have hypothesized that chemotaxis may play a role in guiding motility of pathogenic spirochetes during penetration and further invasion of the host tissues (28). In this study, we addressed this question by analyzing the tissue penetration ability of chemotaxis mutants of the oral spirochete T. denticola.
T. denticola is frequently isolated from inflamed sites of the periodontal pocket and is thought to be implicated in periodontal disease (27, 43, 49, 53, 61). Periodontal disease appears to be a very complex mixed infection involving virulence factors such as adhesion to the tissue, immune suppression, and tissue invasion and destruction (1). Several of these virulence factors have been characterized in T. denticola, including tissue-destroying enzymes that exhibit proteolytic, collagenolytic, or fibrinolytic activities (31, 46, 47, 60) and immune suppression (50, 51). Furthermore, the bacterium has been shown to be able to penetrate endothelial tissue layers (42).
In this study we addressed the role of motility and especially chemotaxis in tissue penetration of T. denticola. Several gene inactivation mutants in some of the motility- and chemotaxis-related genes have already been constructed by other investigators and in our laboratory (17, 24–26; R. Lux, J.-H. Sim, J. P. Tsai, and W. Shi, unpublished data). We also developed an oral epithelial cell line-based tissue penetration assay, since the oral epithelium constitutes the first barrier that T. denticola must overcome to initiate its tissue invasion. By analyzing the tissue penetration ability of a set of defined motility and chemotaxis mutants, we obtained the first experimental evidence that not only cellular motility but also chemotaxis is important for T. denticola to penetrate tissue layers.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Strains used in this study and their relevant genotypes are listed in Table 1. T. denticola ATCC 35405 and its mutant derivatives HL51, HL0501, and HL503 were kind gifts from Howard Kuramitsu (State University of New York, Buffalo). T. phagedenis biotype Reiter was a gift from Ulf B. Göbel (Humboldt University, Berlin, Germany), Treponema pallidum subsp. pallidum Nichols was maintained in one of our laboratories (J.N.M.) by rabbit testicular passage. The cheA mutant (RL101) was constructed through insertion of an erm cassette in the middle of the cheA gene according to the methods described by Li et al. (25). Detailed information about the cheA mutant will be published elsewhere (Lux et al., unpublished). All treponemes except T. pallidum were grown in TYGVS medium (35) at 35°C under anaerobic conditions (85% N2, 10% H2, 5% CO2). On the day of the experiment, T. pallidum was extracted from the orchitis of an infected rabbit with heat-inactivated (56°C for 30 min) fresh normal rabbit serum that was diluted 50% with phosphate-buffered saline (PBS) (pH 7.2) (2, 32).
TABLE 1.
Strains used in the study
| Strain | Relevant genotype or description | Reference |
|---|---|---|
| T. denticola | ||
| ATCC 35405 | Serovar A | 3 |
| HL51 | ATCC 35405 flgE::ermF ermAM | 25 |
| HL0501 | ATCC 35405 dmcA::ermF ermAM | 17 |
| HL503 | ATCC 35405 dmcB::ermF ermAM | 24 |
| RL101 | ATCC 35405 cheA::ermF ermAM | Lux et al., unpublished |
| T. pallidum | Subsp. pallidum, Nichols strain | |
| T. phagedenis ATCC 51274 | Biotype Reiter |
Gingival keratinocyte cell line and tissue layer growth conditions.
The human gingival keratinocyte cell line HOK-16B was used in this study. HOK-16B was immortalized by transfection with cloned type 16 human papillomavirus (40). HOK-16B was maintained in keratinocyte basic medium (KBM) (Clonetics, San Diego, Calif.) supplemented with bovine pituitary extract (0.03 mg/ml), human epidermal growth factor (0.1 ng/ml), insulin (5.0 μg/ml), hydrocortisone (0.5 μg/ml), and antibiotics (gentamicin at 50 μg/ml and amphotericin B at 50 ng/ml) in a humidified atmosphere with 5% CO2 at 37°C. Cells were detached from plastic tissue culture flasks (Cellstar, Greiner Labortechnik, Frickenhausen, Germany) by trypsinization (2.5 mg of trypsin-EDTA per ml; GIBCO, Grand Island, N.Y.) and washed three times in prewarmed medium. Cells (106/cm2) were seeded onto a 3-μm-pore-size polycarbonate filter of a 24-well plate of the Transwell two-chamber tissue culture system (Costar, Cambridge, Mass.). The supplemented KBM was carefully exchanged on a daily basis until the tissue developed tight junctions. Integrity of the tissue was assessed using an Ohmmeter (World Precision Instruments, Sarasota, Fla.). Tissues typically reached their peak resistance of up to 35Ω at day 3 or 4 after seeding. A resistance of >10 Ω was considered to indicate a tight-junctioned tissue layer (12).
Tissue penetration assay.
Tight-junctioned HOK-16B tissue layers were incubated in supplemented KBM without antibiotics overnight. TYGVS-grown T. denticola derivatives and Treponema phagedenis were examined for motility, diluted 1:2 in unsupplemented KBM, and incubated anaerobically overnight. The tissues were transferred into KBM, and tissue resistance was measured directly before and after the experiment. The spirochetes were harvested by low-speed centrifugation (1,000 × g for 6 min) and resuspended in prereduced KBM to a density of 5 × 108 cells/ml. A 200-μl portion of this suspension was added to the upper well of the Transwell two-chamber system. The tissues were coincubated with the bacteria for 8 h under anaerobic conditions as used for growth of T. denticola. A filter insert without tissue served as a control. In this control experiment, T. pallidum was maintained in 50% normal rabbit serum–PBS (pH 7.2) as described above, because a considerable loss of motility of this spirochete was observed when no tissue was present. The bacteria were collected from the lower well and counted using a Petroff-Hausser bacterial counting chamber (Hausser Scientific Partnership, Horsham, Pa.). Penetration rates were calculated as the percentage of the total motile bacteria that penetrated through the system in the control experiment without tissue. In this control experiment, typically 50 to 80% of the cells that were initially added to the upper well migrated through the filter support of the system without a tissue barrier.
Tissue embedding.
The filter of the Transwell system that the tissue layer was grown on was transferred from the culture medium into PBS containing 2% glutaraldehyde for cross-linking. The sample was further prefixed with 1% OsO4, dehydrated with ethanol, and embedded in Epon. Sections 1 to 2 μm thick were mounted on slides and stained with toluidine blue.
Microscopy.
Dark-field microscopy (Leitz, Wetzlar, Germany) at a ×1,000 magnification was used to examine the motility of the spirochetes and to count bacteria in the counting chamber at a ×200 magnification. Tissue sections were observed using phase-contrast microscopy at a ×400 magnification, and tissue layers were observed at a ×320 magnification with an inverted microscope (Leitz). Pictures were taken with a digital camera (SPOT; Diagnostic Instruments Inc., Sterling Heights, Mich.).
RESULTS
Development of an oral epithelial cell line-based in vitro tissue penetration assay under anaerobic conditions.
In vitro tissue penetration experiments have become a tool to assess invasiveness of pathogenic spirochetes. Strains that fail to penetrate tissue layers in vitro usually prove to be impaired in virulence as well (48). In vitro tissue penetration experiments for spirochetes are typically performed aerobically using endothelial tissue layers (4, 42, 58, 59) in the growth medium for the cell line used or multilayered intact tissue preparation of mouse abdominal cell walls (44, 45). Since the oral epithelium is the first barrier that T. denticola must overcome to initiate its tissue penetration, we decided to adapt this assay to an oral epithelial cell line to analyze the role of motility and chemotaxis for this oral spirochete.
Various human oral epithelial cell lines (such as SCC9, CAL27, and HOK-16B) were tested, and the immortalized human gingival keratinocyte cell line HOK-16B (40) was chosen for the in vitro tissue penetration assay. This cell line is able to form tight-junctioned tissue layers with a resistance of >10Ω. A thin section of a typical tissue layer at 14 to 17Ω is shown in Fig. 1. HOK-16B is normally grown aerobically in supplemented KBM medium. Unfortunately, under these growth conditions, the percentage of motile T. denticola cells dropped dramatically (Fig. 2). Good bacterial viability and motility, however, are crucial for the analysis of tissue penetration experiments. Therefore, we performed a series of experiments to search for conditions that ensured epithelial cell integrity as well as motility of T. denticola.
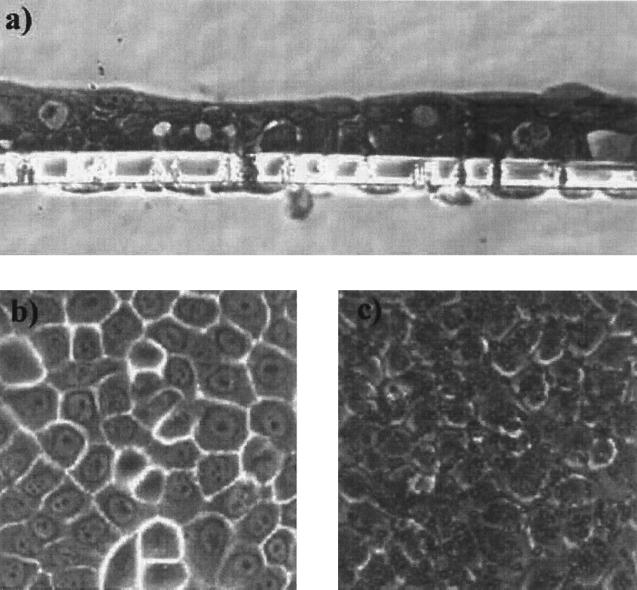
FIG. 1.
Tissue layers formed by HOK-16B cell lines on the membrane support of the Transwell two-chamber system after 4 days of incubation in supplemented KBM in a 5% CO2 atmosphere. (a) Side view of the tissue layers through thin section; (b) top view. (c) The circular spots as seen in the top view are the 3-μm holes in the membrane support. Pictures were taken through 40× (a) and 32× (b and c) objective lenses. Similar cellular structures were observed for the tissue layers maintained in KBM under anaerobic conditions (data not shown).
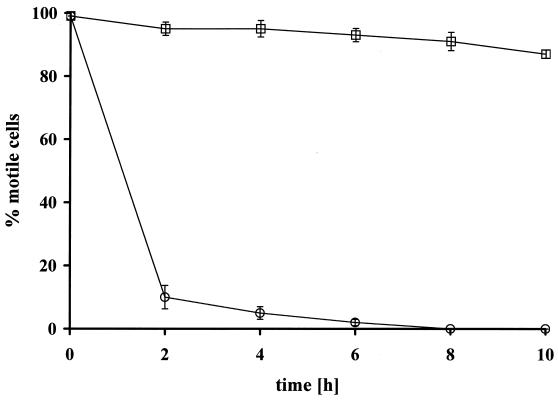
FIG. 2.
Motility of wild-type T. denticola ATCC 35405 in KBM for epithelial cell lines in a 5% CO2 atmosphere at 35°C (○) and under anaerobic conditions (85% N2, 10% H2, 5% CO2) at 35°C (□). Values for each condition were obtained in triplicate in two independent experiments. More than 600 cells were examined for cellular motility in each experiment. All mutant derivatives except the nonmotile strain HL53 showed similar results (data not shown).
All T. denticola derivatives that were used in this study remained motile in unsupplemented KBM for more than 48 h (Fig. 2) if incubated anaerobically. Anaerobic conditions, however, interrupt the electron transfer chain of eukaryotic cells due to lack of the end acceptor O2, resulting in loss of energy and tissue integrity. Eukaryotic cells that lack a complete electron transfer chain can be rescued in the presence of pyruvate and uridine (21). We found that the pyruvate concentration (55 mg/liter) in the unsupplemented growth medium KBM was sufficient to keep HOK-16B cells healthy under anaerobic conditions. Therefore, tissues of HOK-16B cells were first grown aerobically in a 5% CO2 atmosphere until they exhibited a tissue resistance of >10Ω, and then they were transferred to an anaerobic chamber containing 85% N2, 10% H2, and 5% CO2. The presence of pyruvate was sufficient to maintain tight junctions for more than 12 h. In the additional presence of uridine (110 mg/liter), the tissue cells were healthy for 2 to 3 days (data not shown). Based on these findings, we decided to use anaerobic coincubation of T. denticola ATCC 35405 and its various mutant derivatives with tight-junctioned tissue layers of HOK-16B in unsupplemented KBM as the experimental medium. Under these conditions, both the bacterial strains and the eukaryotic tissue appeared to remain viable for the duration of the experiment (8 to 10 h).
Tissue penetration by wild-type T. denticola.
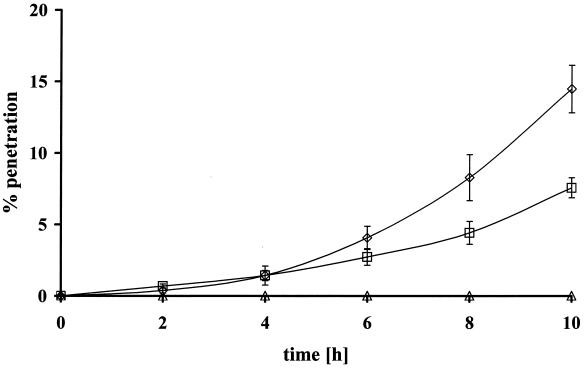
Using the assay developed above, we tested the tissue penetration ability of wild-type T. denticola. The pathogenic spirochete T. pallidum subsp. pallidum Nichols was used as a positive control, and T. phagedenis biotype Reiter, a spirochete that is not pathogenic to humans, was used as a negative control. Experiments with the microaerophilic T. pallidum were performed under both aerobic and anaerobic conditions. No significant difference in penetration rates was observed. As shown in Fig. 3, T. pallidum actively penetrated epithelial cell layers (about 14% of the cells pervaded the tissue in 10 h), whereas T. phagedenis was unable to overcome the tissue barrier. Wild-type T. denticola ATCC 35405 was also found to be able to penetrate oral epithelial tissue layers but only at about half the rate observed for T. pallidum (about 8% in 10 h) (Fig. 3). It is interesting that both T. denticola and T. pallidum exhibited an exponential increase of penetration efficiency over time, unlike the linear or hyperbolic increase that was described for their respective penetration of endothelial tissue layers (12, 42, 59).
FIG. 3.
Time course of tissue penetration for different treponemata over 10 h. Diamonds, T. pallidum (n = 4); squares, T. denticola (n = 5); triangles, T. phagedenis (n = 4). The tissue resistance was about 12 to 13Ω.
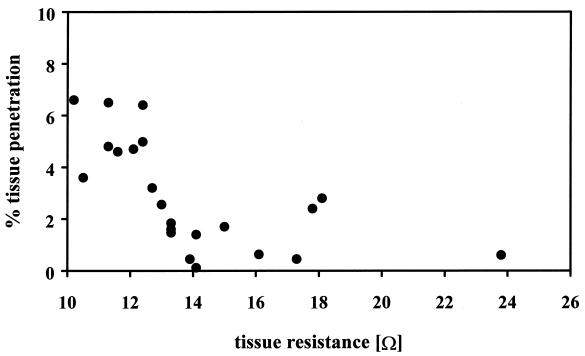
We found that tissue penetration rates were also dependent on the tissue resistance (Fig. 4). The onset of detectable penetration was delayed with increased tissue resistance, but the penetration kinetics remained exponential (data not shown). It turned out, however, that the increased resistance was apparently due to the tissue growing into a multilayer rather than forming increased numbers of tight junctions. Tissues that consisted mainly of a monolayer had resistances of 10 to 13Ω. To rule out experimental ambiguities due to formation of cellular multilayers, tissue penetration rates of different strains were compared only for tissues that exhibited a resistance corresponding to predominantly monolayer or double-layer formation.
FIG. 4.
Correlation of penetration rate and tissue resistance. Penetration rates were determined after 8 h of coincubation of the tissues with wild-type T. denticola. Tissue resistance was measured before and after the experiment.
Tissue penetration by T. denticola mutant strains defective in chemotaxis or motility.
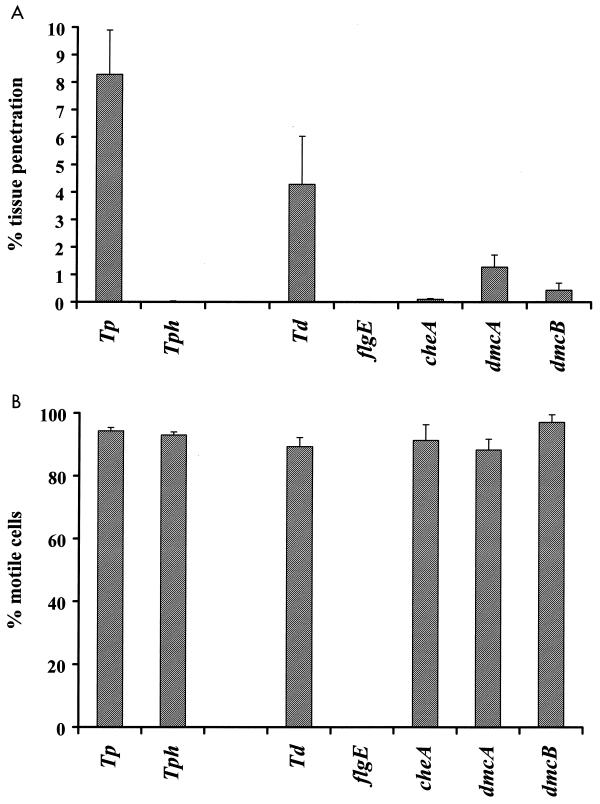
Several defined motility and chemotaxis mutants have been previously constructed in other laboratories and in our laboratory, including strains that are nonmotile (flgE) or are defective in the central kinase of the general chemotaxis pathway (cheA) or one of the chemoreceptors (dmcA or dmcB). All these mutants were derived from wild-type T. denticola ATCC 35405. We examined these motility and chemotaxis mutants for their ability to penetrate oral epithelial cell layers. As expected, the nonmotile flgE mutant HL51 was unable to penetrate the epithelial tissue layer, confirming that motility is crucial for this feature (Fig. 5). Interestingly, the chemotaxis mutants (cheA, dmcA, and dmcB) showed significantly reduced penetration rates compared to the wild-type cells (Fig. 5A), even though they were fully motile throughout the experiment (Fig. 5B). The cheA mutant strain RL101 penetrated only at about 2 to 3% of the rates observed for the wild type. Both chemoreceptor mutants, the dmcA (HL0501) and dmcB (HL503) strains, also showed significantly decreased penetration rates, about 30 and 10% of the wild-type behavior, respectively. These data show that not only cellular motility but also chemotaxis is important for T. denticola to penetrate tissue layers.
FIG. 5.
Tissue penetration rates (A) and motility (B) of wild-type T. denticola (Td), T. pallidum (Tp), and T. phagedenis (Tph) and various T. denticola motility and chemotaxis mutant strains. Values were determined after 8 h of coincubation of the bacteria with the tissue. Tissues used for these experiments exhibited resistances between 11 and 14Ω. More than 600 cells of each strain were examined for cellular motility.
DISCUSSION
The ability of spirochetes to invade tissue is an important virulence factor. It has been shown previously that B. burgdorferi, Leptospira interrogans, T. pallidum, and also T. denticola can cross barriers of endothelial cell layers whereas nonpathogenic species of the same genera are unable to do so (4, 42, 58, 59). T. pallidum and some oral spirochetes are apparently also able to invade the complex tissue of mouse abdominal wall preparations (44, 45) but interestingly only from the epithelial and not from the connective tissue side (44). Loss of motility was correlated with drastically reduced pathogenesis (48). As expected, the T. denticola derivative lacking flagella was completely unable to penetrate the tissue, supporting the idea that active bacterial movement and not passive translocation via endocytosis by the eukaryotic cells or other cellular processes is required for tissue invasion. This is in agreement with previous studies on other pathogenic spirochetes and a variety of other pathogenic bacteria that demonstrated the requirement of motility for virulence.
Although the role of chemotaxis in the tissue invasion process of spirochetes has been considered (28), experiments addressing this issue have not yet been conducted. Chemotaxis has been described to be a virulence factor for some pathogenic bacteria, such as H. pylori, C. jejuni, V. cholerae, and others (5, 8–10, 57, 62), whereas it appears to be only indirectly involved in the pathogenesis of Salmonella enterica serovar Typhimurium (16, 22). In this study, we provide evidence, for the first time, that chemotaxis is implicated in tissue penetration by T. denticola, since chemotaxis mutants were impaired in tissue penetration despite being motile. At present, however, we do not know the exact nature of the relationship between chemotaxis and tissue penetration. Chemotaxis could serve as a means to direct the bacterium into and through the tissue. Chemotaxis mutants of V. cholerae were found to migrate into the mucus-filled intervillous spaces of rabbit intestines at a much lower rate than their wild-type parent strain (10). It is also conceivable that chemotaxis plays an indirect role by maintaining the motility pattern of reversals and flexing at a certain frequency. Alternatively, chemotaxis mutations may affect the expression of other virulence factors related to tissue penetration.
For the oral bacterium T. denticola, the epithelium of the gingival tissue constitutes a natural site of entry into its host. In this study, we established an in vitro tissue penetration assay for an immortalized human keratinocyte cell line. Tissue layers of this cell line maintained integrity even under anaerobic conditions for more than 48 h. Wild-type T. denticola was able to penetrate this tissue layer at rates that are comparable to those described for penetration of endothelial cell layers (42) (Fig. 3). T. pallidum, which we used as a positive penetration control, showed only about half of the penetration that was observed by other investigators for endothelial monolayers (12, 59). As expected, the nonpathogenic T. phagedenis was unable to overcome the epithelial barrier. Interestingly, the time course for penetration seems to be exponential rather than linear or following a saturation curve as found for penetration of endothelial tissues (12, 42, 59). This finding implies that during penetration certain events increase the likeliness that other spirochetes penetrate the tissue. These events could involve tissue destruction that facilitates entry into the tissue. This possible tissue destruction, however, has to be very limited, because a significant loss of tissue resistance that would correlate with massive tissue damage was never observed during the experiments. This exponential increase in tissue penetration could be explained if chemotaxis-guided targeting of damaged tissue is involved in the penetration process. Molecules that are released by injured or diseased tissue cells could attract the bacteria towards these weakened spots, thus facilitating penetration.
The hypothesis that chemoattraction could play a role in tissue penetration is supported by the result that a mutant strain lacking CheA, the central kinase in chemotactic signal transduction, is severely impaired in tissue penetration. It exhibits only 2 to 3% of the wild-type penetration rates. This mutant is fully motile but unable to respond to a mix of nutrients (Lux et al., unpublished). The lack of a chemotaxis response would disable the mutant to detect “weak spots” within the tissue, and each bacterium would randomly try to migrate into the cell layer rather than taking advantage of existing “passages.” The motility pattern of this mutant, however, also appears to have a greatly decreased reversal frequency compared to that of the wild type (data not shown). This leaves open the possibility that a certain frequency of reversal might be necessary for efficient migration through a tissue. A more detailed analysis of the influence of motility patterns on the tissue penetration ability of T. denticola is in progress.
Two other mutant strains, HL0501 and HL503, that were tested are lacking the DmcA and DmcB chemoreceptors, respectively. Both mutants have reduced penetration rates but are not as severely impaired as the cheA mutant. DmcB appears to have a greater influence on tissue penetration than DmcA. It was shown previously, however, that loss of DmcB has a dominant effect on DmcA methylation (24). This additive effect could explain why the DmcB mutant strain has a significant lower penetration rate than the DmcA mutant strain. These chemoreceptors were previously shown to be involved in migration towards nutrients (17, 24). A BLAST search of the unfinished genome sequence of T. denticola ATCC 35405 (www.tigr.org) revealed the existence of nine more open reading frames that contain the highly conserved domain described for methyl-accepting chemotaxis proteins (55) and therefore possibly encode chemoreceptors. Therefore, it appears unlikely that inactivation of one or two chemoreceptors would completely abolish the response to attractants. The possible presence of more than two chemoreceptors in T. denticola could explain why tissue penetration of HL0501 (dmcA) and HL503 (dmcB) is reduced but to a lesser extent than in the generally nonchemotactic cheA mutant RL101. The dmcA mutant HL0501 exhibits a motility pattern that is similar to that of wild type. In contrast, HL503, which lacks dmcB, appears to have an elevated reversal frequency. As both RL101 and HL0501, which exhibit the greatest decrease in tissue penetration rates, also differ in their motility pattern from the wild type, we cannot rule out the possibility that this altered motility pattern also affects tissue penetration.
We are currently in the process of constructing other chemotaxis mutants that allow potential determination of the impact of reversal frequency on tissue penetration. A cheRB double mutant of Escherichia coli that exhibits a wild type-like swimming behavior but is greatly imparied in chemotactic signaling has been described (20, 41). Single inactivation of either gene locks the bacterium in an extremely tumbly (cheB) or smooth (cheR) swimming pattern (41). Mutations in these genes might result in similar phenotypes in T. denticola and be useful in elucidating the importance of swimming pattern versus chemoattraction in tissue penetration.
ACKNOWLEDGMENTS
We thank Jee-Hyun Sim and Birgitta Sjostrand for technical support, Xiaoyang Wu for providing freshly prepared suspensions of T. pallidum, Howard Kuramitsu and UlfB. Göbel for providing strains, Sharon Hunt-Gerardo for editing, and all lab members for their great support.
This work was supported by NIH grants DE12532 and GM54666 to Wenyuan Shi.
REFERENCES
- 1.Carranza F A J, Newman M G. Clinical periodontology. 8th ed. Philadelphia, Pa: W. B. Saunders Company; 1996. [Google Scholar]
- 2.Champion C I, Miller J N, Borenstein L A, Lovett M A, Blanco D R. Immunization with Treponema pallidum endoflagella alters the course of experimental rabbit syphilis. Infect Immun. 1990;58:3158–3161. doi: 10.1128/iai.58.9.3158-3161.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan E C, Siboo R, Keng T, Psarra N, Hurley R, Cheng S L, Iugovaz I. Treponema denticola (ex Brumpt 1925) sp. nov., nom. rev., and identification of new spirochete isolates from periodontal pockets. Int J Syst Bacteriol. 1993;43:196–203. doi: 10.1099/00207713-43-2-196. [DOI] [PubMed] [Google Scholar]
- 4.Comstock L E, Thomas D D. Penetration of endothelial cell monolayers by Borrelia burgdorferi. Infect Immun. 1989;57:1626–1628. doi: 10.1128/iai.57.5.1626-1628.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foynes S, Dorrell N, Ward S J, Stabler R A, McColm A A, Rycroft A N, Wren B W. Helicobacter pylori possesses two CheY response regulators and a histidine kinase sensor, CheA, which are essential for chemotaxis and colonization of the gastric mucosa. Infect Immun. 2000;68:2016–2023. doi: 10.1128/iai.68.4.2016-2023.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Venter J C, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 7.Fraser C M, Norris S J, Weinstock G M, White O, Sutton G G, Dodson R, Gwinn M, Hickey E K, Clayton R, Ketchum K A, Sodergren E, Hardham J M, McLeod M P, Salzberg S, Peterson J, Khalak H, Richardson D, Howell J K, Chidambaram M, Utterback T, McDonald L, Artiach P, Bowman C, Cotton M D, Venter J C, et al. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281:375–388. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- 8.Freter R, Allweiss B, O'Brien P C, Halstead S A, Macsai M S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vitro studies. Infect Immun. 1981;34:241–249. doi: 10.1128/iai.34.1.241-249.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freter R, O'Brien P C. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: fitness and virulence of nonchemotactic Vibrio cholerae mutants in infant mice. Infect Immun. 1981;34:222–233. doi: 10.1128/iai.34.1.222-233.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freter R, O'Brien P C, Macsai M S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect Immun. 1981;34:234–240. doi: 10.1128/iai.34.1.234-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene S R, Stamm L V. Molecular characterization of a chemotaxis operon in the oral spirochete, Treponema denticola. Gene. 1999;232:59–68. doi: 10.1016/s0378-1119(99)00115-8. [DOI] [PubMed] [Google Scholar]
- 12.Haake D A, Lovett M A. Interjunctional invasion of endothelial cell monolayers. In: Clark V L, Bavoil P M, editors. Bacterial pathogenesis. San Diego, Calif: Academic Press; 1997. pp. 711–727. [Google Scholar]
- 13.Hawes M C, Smith L Y. Requirement for chemotaxis in pathogenicity of Agrobacterium tumefaciens on roots of soil-grown pea plants. J Bacteriol. 1989;171:5668–5671. doi: 10.1128/jb.171.10.5668-5671.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinzerling H F, Olivares M, Burne R A. Genetic and transcriptional analysis of flgB flagellar operon constituents in the oral spirochete Treponema denticola and their heterologous expression in enteric bacteria. Infect Immun. 1997;65:2041–2051. doi: 10.1128/iai.65.6.2041-2051.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiramune T, Shiraiwa C, Kikuchi N, Yanagawa R. A basic study of chemotaxis of leptospiras. Zentbl Vetmed. 1990;37:749–752. doi: 10.1111/j.1439-0450.1990.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 16.Jones B D, Lee C A, Falkow S. Invasion by Salmonella typhimurium is affected by the direction of flagellar rotation. Infect Immun. 1992;60:2475–2480. doi: 10.1128/iai.60.6.2475-2480.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kataoka M, Li H, Arakawa S, Kuramitsu H. Characterization of a methyl-accepting chemotaxis protein gene, dmcA, from the oral spirochete Treponema denticola. Infect Immun. 1997;65:4011–4016. doi: 10.1128/iai.65.10.4011-4016.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy M J, Rosey E L, Yancey R J., Jr Characterization of flaA− and flaB− mutants of Serpulina hyodysenteriae: both flagellin subunits, FlaA and FlaB, are necessary for full motility and intestinal colonization. FEMS Microbiol Lett. 1997;153:119–128. doi: 10.1111/j.1574-6968.1997.tb10472.x. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy M J, Yancey R J., Jr Motility and chemotaxis in Serpulina hyodysenteriae. Vet Microbiol. 1996;49:21–30. doi: 10.1016/0378-1135(95)00174-3. [DOI] [PubMed] [Google Scholar]
- 20.Khan S, Castellano F, Spudich J L, McCray J A, Goody R S, Reid G P, Trentham D R. Excitatory signaling in bacterial probed by caged chemoeffectors. Biophys J. 1993;65:2368–2382. doi: 10.1016/S0006-3495(93)81317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King M P, Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989;246:500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- 22.Lee C A, Jones B D, Falkow S. Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc Natl Acad Sci USA. 1992;89:1847–1851. doi: 10.1073/pnas.89.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.Li C, Motaleb A, Sal M, Goldstein S T, Charon N W. Spirochete periplasmic flagella and motility. J Mol Microbiol Biotechnol. 2000;2:345–354. [PubMed] [Google Scholar]
- 23.Li C Y, Tsai J P, Han Y W, Yang Z, Wolinsky L E, Kuramitsu H, Shi W. Chemotaxis and the cheA mutant of Treponema denticola. J Dent Res. 1998;77:228. [Google Scholar]
- 24.Li H, Arakawa S, Deng Q D, Kuramitsu H. Characterization of a novel methyl-accepting chemotaxis gene, dmcB, from the oral spirochete Treponema denticola. Infect Immun. 1999;67:694–699. doi: 10.1128/iai.67.2.694-699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Ruby J, Charon N, Kuramitsu H. Gene inactivation in the oral spirochete Treponema denticola: construction of an flgE mutant. J Bacteriol. 1996;178:3664–3667. doi: 10.1128/jb.178.12.3664-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Limberger R J, Slivienski L L, Izard J, Samsonoff W A. Insertional inactivation of Treponema denticola tap1 results in a nonmotile mutant with elongated flagellar hooks. J Bacteriol. 1999;181:3743–3750. doi: 10.1128/jb.181.12.3743-3750.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loesche W J. Bacterial mediators in periodontal disease. Clin Infect Dis. 1993;16:S203–S210. doi: 10.1093/clinids/16.supplement_4.s203. [DOI] [PubMed] [Google Scholar]
- 28.Lux R, Moter A, Shi W. Chemotaxis in pathogenic spirochetes: directed movement toward targeting tissues? J Mol Microbiol Biotechnol. 2000;2:355–364. [PubMed] [Google Scholar]
- 29.Manson M D, Armitage J P, Hoch J A, Macnab R M. Bacterial locomotion and signal transduction. J Bacteriol. 1998;180:1009–1022. doi: 10.1128/jb.180.5.1009-1022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayo J A, Blake A, Donze D. Chemotaxis by Treponema denticola. J Dent Res. 1990;69:382. [Google Scholar]
- 31.Mikx F H, de Jong M H. Keratinolytic activity of cutaneous and oral bacteria. Infect Immun. 1987;55:621–625. doi: 10.1128/iai.55.3.621-625.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller J N. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by gamma-irradiation. J Immunol. 1973;110:1206–1215. [PubMed] [Google Scholar]
- 33.Milner J A, Sellwood R. Chemotactic response to mucin by Serpulina hyodysenteriae and other porcine spirochetes: potential role in intestinal colonization. Infect Immun. 1994;62:4095–4099. doi: 10.1128/iai.62.9.4095-4099.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moter A, Leist G, Rudolph R, Schrank K, Choi B K, Wagner M, Gobel U B. Fluorescence in situ hybridization shows spatial distribution of as yet uncultured treponemes in biopsies from digital dermatitis lesions. Microbiology. 1998;144:2459–2467. doi: 10.1099/00221287-144-9-2459. [DOI] [PubMed] [Google Scholar]
- 35.Ohta K, Makinen K K, Loesche W J. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun. 1986;53:213–220. doi: 10.1128/iai.53.1.213-220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ormonde P, Horstedt P, O'Toole R, Milton D L. Role of motility in adherence to and invasion of a fish cell line by Vibrio anguillarum. J Bacteriol. 2000;182:2326–2328. doi: 10.1128/jb.182.8.2326-2328.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Toole R, Lundberg S, Fredriksson S A, Jansson A, Nilsson B, Wolf-Watz H. The chemotactic response of Vibrio anguillarum to fish intestinal mucus is mediated by a combination of multiple mucus components. J Bacteriol. 1999;181:4308–4317. doi: 10.1128/jb.181.14.4308-4317.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Toole R, Milton D L, Wolf-Watz H. Chemotactic motility is required for invasion of the host by the fish pathogen Vibrio anguillarum. Mol Microbiol. 1996;19:625–637. doi: 10.1046/j.1365-2958.1996.412927.x. [DOI] [PubMed] [Google Scholar]
- 39.Ottemann K M, Miller J F. Roles for motility in bacterial-host interactions. Mol Microbiol. 1997;24:1109–1117. doi: 10.1046/j.1365-2958.1997.4281787.x. [DOI] [PubMed] [Google Scholar]
- 40.Park N H, Min B M, Li S L, Huang M Z, Cherick H M, Doniger J. Immortalization of normal human oral keratinocytes with type 16 human papillomavirus. Carcinogenesis. 1991;12:1627–1631. doi: 10.1093/carcin/12.9.1627. [DOI] [PubMed] [Google Scholar]
- 41.Parkinson J S, Houts S E. Isolation and behavior of Escherichia coli deletion mutants lacking chemotaxis functions. J Bacteriol. 1982;151:106–113. doi: 10.1128/jb.151.1.106-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters S R, Valdez M, Riviere G, Thomas D D. Adherence to and penetration through endothelial cells by oral treponemes. Oral Microbiol Immunol. 1999;14:379–383. doi: 10.1034/j.1399-302x.1999.140609.x. [DOI] [PubMed] [Google Scholar]
- 43.Riviere G R, Elliot K S, Adams D F, Simonson L G, Forgas L B, Nilius A M, Lukehart S A. Relative proportions of pathogen-related oral spirochetes (PROS) and Treponema denticola in supragingival and subgingival plaque from patients with periodontitis. J Periodontol. 1992;63:131–136. doi: 10.1902/jop.1992.63.2.131. [DOI] [PubMed] [Google Scholar]
- 44.Riviere G R, Thomas D D, Cobb C M. In vitro model of Treponema pallidum invasiveness. Infect Immun. 1989;57:2267–2271. doi: 10.1128/iai.57.8.2267-2271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riviere G R, Weisz K S, Adams D F, Thomas D D. Pathogen-related oral spirochetes from dental plaque are invasive. Infect Immun. 1991;59:3377–3380. doi: 10.1128/iai.59.10.3377-3380.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosen G, Naor R, Kutner S, Sela M N. Characterization of fibrinolytic activities of Treponema denticola. Infect Immun. 1994;62:1749–1754. doi: 10.1128/iai.62.5.1749-1754.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosen G, Naor R, Rahamim E, Yishai R, Sela M N. Proteases of Treponema denticola outer sheath and extracellular vesicles. Infect Immun. 1995;63:3973–3979. doi: 10.1128/iai.63.10.3973-3979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sadziene A, Thomas D D, Bundoc V G, Holt S C, Barbour A G. A flagella-less mutant of Borrelia burgdorferi. Structural, molecular, and in vitro functional characterization. J Clin Invest. 1991;88:82–92. doi: 10.1172/JCI115308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlegel-Bregenzer B, Persson R E, Lukehart S, Braham P, Oswald T, Persson G R. Clinical and microbiological findings in elderly subjects with gingivitis or periodontitis. J Clin Periodontol. 1998;25:897–907. doi: 10.1111/j.1600-051x.1998.tb02388.x. [DOI] [PubMed] [Google Scholar]
- 50.Sela M N, Bolotin A, Naor R, Weinberg A, Rosen G. Lipoproteins of Treponema denticola: their effect on human polymorphonuclear neutrophils. J Periodontal Res. 1997;32:455–466. doi: 10.1111/j.1600-0765.1997.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 51.Shenker B J, Listgarten M A, Taichman N S. Suppression of human lymphocyte responses by oral spirochetes: a monocyte-dependent phenomenon. J Immunol. 1984;132:2039–2045. [PubMed] [Google Scholar]
- 52.Shi W, Yang Z, Geng Y, Wolinsky L E, Lovett M A. Chemotaxis in Borrelia burgdorferi. J Bacteriol. 1998;180:231–235. doi: 10.1128/jb.180.2.231-235.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Socransky S S, Haffajee A D, Cugini M A, Smith C, Kent R L., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 54.Stamm L V, Bergen H L. Molecular characterization of a flagellar (fla) operon in the oral spirochete Treponema denticola ATCC 35405. FEMS Microbiol, Lett. 1999;179:31–36. doi: 10.1111/j.1574-6968.1999.tb08703.x. [DOI] [PubMed] [Google Scholar]
- 55.Stock J B, Lukat G S, Stock A M. Bacterial chemotaxis and the molecular logic of intracellular signal transduction networks. Annu Rev Biophys Biophys Chem. 1991;20:109–136. doi: 10.1146/annurev.bb.20.060191.000545. [DOI] [PubMed] [Google Scholar]
- 56.Stock J B, Surette M G. Chemotaxis. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: ASM Press; 1996. pp. 1103–1129. [Google Scholar]
- 57.Takata T, Fujimoto S, Amako K. Isolation of nonchemotactic mutants of Campylobacter jejuni and their colonization of the mouse intestinal tract. Infect Immun. 1992;60:3596–3600. doi: 10.1128/iai.60.9.3596-3600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas D D, Higbie L M. In vitro association of leptospires with host cells. Infect Immun. 1990;58:581–585. doi: 10.1128/iai.58.3.581-585.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas D D, Navab M, Haake D A, Fogelman A M, Miller J N, Lovett M A. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc Natl Acad Sci USA. 1988;85:3608–3612. doi: 10.1073/pnas.85.10.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uitto V J, Grenier D, Chan E C, McBride B C. Isolation of a chymotrypsinlike enzyme from Treponema denticola. Infect Immun. 1988;56:2717–2722. doi: 10.1128/iai.56.10.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ximenez-Fyvie L A, Haffajee A D, Socransky S S. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. J Clin Periodontol. 2000;27:648–657. doi: 10.1034/j.1600-051x.2000.027009648.x. [DOI] [PubMed] [Google Scholar]
- 62.Yao R, Burr D H, Guerry P. CheY-mediated modulation of Campylobacter jejuni virulence. Mol Microbiol. 1997;23:1021–1031. doi: 10.1046/j.1365-2958.1997.2861650.x. [DOI] [PubMed] [Google Scholar]
- 63.Yuri K, Takamoto Y, Okada M, Hiramune T, Kikuchi N, Yanagawa R. Chemotaxis of leptospires to hemoglobin in relation to virulence. Infect Immun. 1993;61:2270–2272. doi: 10.1128/iai.61.5.2270-2272.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]