Abstract
Human milk is composed of complex microbial and non-microbial components that shape the infant gut microbiome. Although several maternal and infant factors have been associated with human milk microbiota, no study has investigated this in an Australian population. Therefore, we aimed to investigate associations between human milk bacterial composition of Australian women and maternal factors (body mass index (BMI), mode of delivery, breast pump use, allergy, parity) and infant factors (sex, mode of feeding, pacifier use, and introduction of solids). Full-length 16S rRNA gene sequencing was used to characterise milk bacterial DNA profiles. Milk from mothers with a normal BMI had a higher relative abundance of Streptococcus australis than that of underweight mothers, while milk from overweight mothers had a higher relative abundance of Streptococcus salivarius compared with underweight and obese mothers. Mothers who delivered vaginally had a higher relative abundance of Streptococcus mitis in their milk compared to those who delivered via emergency caesarean section. Milk of mothers who used a breast pump had a higher relative abundance of Staphylococcus epidermidis and Streptococcus parasanguinis. Milk of mothers whose infants used a pacifier had a higher relative abundance of S. australis and Streptococcus gwangjuense. Maternal BMI, mode of delivery, breast pump use, and infant pacifier use are associated with the bacterial composition of human milk in an Australian cohort. The data from this pilot study suggests that both mother and infant can contribute to the human milk microbiome.
Introduction
Human milk (HM) is made up of nutritive components, immune factors, and microbial communities [1–3]. It contributes to seeding of the infant gut [4–8] and oral cavity [9] microbiomes. Bifidobacterium breve, B. adolescentis, B. dentium, B. infantis, B. longum, B. bifidum, B. angulatum, Staphylococcus epidermidis, and Veillonella parvula have been reported among the shared bacterial species between HM and the infant gut microbiome [4, 6, 10, 11], while S. epidermidis, S. auricularis, Streptococcus parasanguinis/gordonii, S. mitis/oralis, and S. salivarius have been reported to be shared between HM and the infant oral microbiome [9]. Infant gut bacterial communities are important for immune development [12, 13] and may modify the risk of developing early-life [14, 15] as well as later-life diseases [16, 17]. Thus, HM microbial communities likely have important implications for infant health.
The composition of the HM microbiota varies between individuals [18]. Metataxonomic and metagenomic studies have revealed that Staphylococcus spp. and Streptococcus spp. are consistently present and highly abundant in HM [19–25], whereas the presence and abundance of other bacterial species are variable between individuals and populations [26]. Early-life maternal and infant factors have been associated with the bacterial composition of HM. Maternal factors such as mode of delivery [27–35], body mass index (BMI) [27, 30, 35–38], and breast pump use [34, 39] have been reported to be associated with HM bacterial profiles. Infant factors such as gestational age at delivery [28], sex [34, 38], and feeding method [34, 39–42] have also been reported to be associated with HM bacterial profiles.
While previous studies have sought to identify associations between maternal and infant factors and the HM microbiome in various populations, to date, no study has assessed these in an Australian setting. This is important, as the HM microbiome [26, 30, 32, 35, 43, 44], and the human microbiome [45–47] more generally, have been shown to vary between geographically distinct populations (due to a combination of genetic, local environment, and dietary factors). Being an isolated island nation, it is important to characterise the HM microbiota and describe any associated maternal and infant factors. This may aid in identifing potential avenues to alter HM microbial profiles in a manner that supports infant health.
In this pilot study, we analysed the milk microbiota of Western Australian women. We sought to determine the influence of maternal and infant factors on HM bacterial profiles in this population. Further, we have improved upon previous studies in this field by utilising full-length 16S rRNA gene sequencing, allowing deeper taxonomic resolution.
Materials and methods
Study population
Twenty nine predominantly breastfeeding women (those using HM as the main source of infant nourishment), with healthy infants aged 3–83 weeks and no nipple infection or nipple pain were recruited for this study. All mothers provided written informed consent prior to participation. Ethical approval was obtained from The University of Western Australia’s Human Research Ethics Committee (RA/4/1/2369).
Maternal and infant demographic data collection
Data regarding maternal BMI, mode of delivery, maternal allergies, parity, infant sex, and pacifier use were collected via an online questionnaire. Mothers who self-reported having an allergic skin reaction or allergy to any food, medication, or animal were classified as having an allergy. Participants were assigned into one of the following BMI categories: underweight if less than 18.5, normal if between 18.5–24.9, overweight if between 25.0–29.9, Obesity class I if between 30.0–34.9, Obesity class II if between 35.0–39.9, and Obesity class III if above 40. Data regarding infant formula and solid intake, nipple pain, and breast pump use were collected at the time of sample collection.
Sample collection
Milk samples were collected aseptically using a Symphony electric breast pump (Medela AG, Baar, Switzerland) with a sterile pump kit (microwave steam sterilised). To reduce contamination from the skin, participants cleaned the nipple and areola of the expressing breast with chlorhexidine wipes followed by rinsing with sterile saline and drying with sterile gauze. Up to 10 mL of post-milk ejection milk was collected directly into a sterile 50mL tube. Milk samples were kept on ice and immediately transported to the laboratory where they were aliquoted and stored at -80°C until DNA extraction.
DNA extraction
Milk samples (1 mL) were centrifuged at 40,000 x g for 5 min at 4°C and the supernatant and fat were removed. DNA was extracted from the cell pellet using the MagAttract Microbial DNA Kit (QIAGEN) on the Kingfisher Flex platform according to the manufacturer’s instructions. Two negative extraction controls were processed alongside the samples. The negative extraction controls consisted of 1 ml of sterile DNA-free water (Integrated DNA Technologies, Queenstown, Singapore) were placed at the centre of the 96-well extraction plate.
16S rRNA gene amplification and PacBio HiFi sequencing
PCR amplification and PacBio High-Fidelity (HiFi) sequencing was performed as previously described [48]. Briefly, the full-length 16S rRNA gene was amplified using the PacBio uni-tagged primers 27F (5’-gcagtcgaacatgtagctgactcaggtcacAGRGTTYGATYMTGGCTCAG-3’) and 1492R (5’-tggatcacttgtgcaagcatcacatcgtagRGYTACCTTGTTACGACTT-3’). Asymmetric barcoding was performed using the uni-tagged PacBio forward barcodes 1F-4F and reverse barcodes 16R-30R. Barcoded amplicons were pooled in equimolar concentrations and gel purified using a QIAGEN Gel Extraction Kit. Samples were sequenced using PacBio single molecule HiFi sequencing on a single SMRT cell at the Ramaciotti Centre for Genomics (NSW, Australia).
Raw sequence analysis
Raw data were processed at the Ramaciotti Centre for Genomics using PacBio SMRTLink analysis software v6.0 to generate demultiplexed.fastq files. Demultiplexed HiFi reads were filtered, aligned, clustered, and taxonomically assigned using Mothur v.1.44.1 [49] against the SILVA reference database (v138) [50] as previously described [48]. Rarefaction was performed based on the smallest library size (1007 reads).
Statistical analyses
Alpha diversity was measured using richness (number of different OTUs) and Shannon diversity. Differences in alpha diversity measures between maternal and infant characteristics were assessed using Wilcoxon Rank Sum tests for categorical characteristics and Kruskal Wallis tests for continuous characteristics. A continuity correction was included in the Wilcoxon Rank Sum tests to account for ties in richness. Differences in beta diversity between the categorical and continuous maternal and infant characteristics were assessed through univariate PERMANOVAs on Bray-Curtis distances. These analyses were performed using the R environment for statistical computing [51]. To analyse whether any OTUs were differentially abundant based on maternal or infant factors, the metastats tool [52] was used in Mothur v.1.44.1 [49]. The relative abundance of OTUs was compared across the groups by conducting a two-sample t-test. OTUs of interest were mapped taxonomically using BLAST [53, 54] with a sequence identity score of > 97.8% being considered a good match. Significant results were reported only for OTUs with an average relative abundance of ≥ 1% across all samples that were present in > 1 sample. For all tests, p-values < 0.05 were considered significant. Bonferroni correction was applied to the significance level.
Results
Characteristics of the 29 mothers and their infants are included in Table 1. 13 OTUs made up ≥1% relative abundance in these HM samples. The five most abundant OTUs mapped to Staphylococcus epidermidis (mean relative abundance: 27.3%), Streptococcus parasanguinis (14.0%), Streptococcus mitis (9.5%), Streptococcus gwangjuense (6.2%), and Haemophilus parainfluenzae (4.9%) (Fig 1a). At the genus level, Streptococcus spp. (52.3%) and Staphylococcus spp. (34.7%) dominated the profiles (Fig 1b).
Table 1. Participant characteristics (n = 29).
| Variables | Mean (range) or n (%) |
|---|---|
| Maternal age (years) | 32.8 (24–40) |
| Maternal ethnicity:a | |
| Caucasian | 26 (89.6%) |
| Aboriginal Australian | 1 (3.4%) |
| Maternal BMIb | 25.1 (16.9–38.3) |
| Obesity class: | |
| Normal | 13 (44.8%) |
| Overweight | 9 (31%) |
| Obesity class II | 3 (10.3%) |
| Underweight | 3 (10.3%) |
| Primiparous | 15 (51.7%) |
| Maternal allergy | 11 (37.9%) |
| Previous mastitisc | 4 (13.7%) |
| Breast pump use | 24 (82.7%) |
| Mode of delivery:b | |
| Vaginal | 19 (65.5%) |
| Emergency caesarean section | 5 (17.2%) |
| Elective caesarean section | 4 (13.7%) |
| Current maternal antibiotic intakea | 1 (3.4%) |
| Infant age (weeks) | 23.3 (3.4–83.3) |
| Gestational age at delivery (weeks) | 39.2 (35–41) |
| Male infant | 16 (55.1%) |
| Mode of feeding: | |
| Exclusive breastfeeding | 15 (51.7%) |
| Human milk and formula | 4 (13.7) |
| Human milk and solids | 10 (34.4%) |
| Pacifier use | 15 (51.7%) |
a missing variable value for two participants
b missing variable value for one participant
c none of the mothers presented with symptoms of mastitis at the time of milk sample collection
Fig 1. The relative abundance of bacterial OTUs and genera in HM samples.
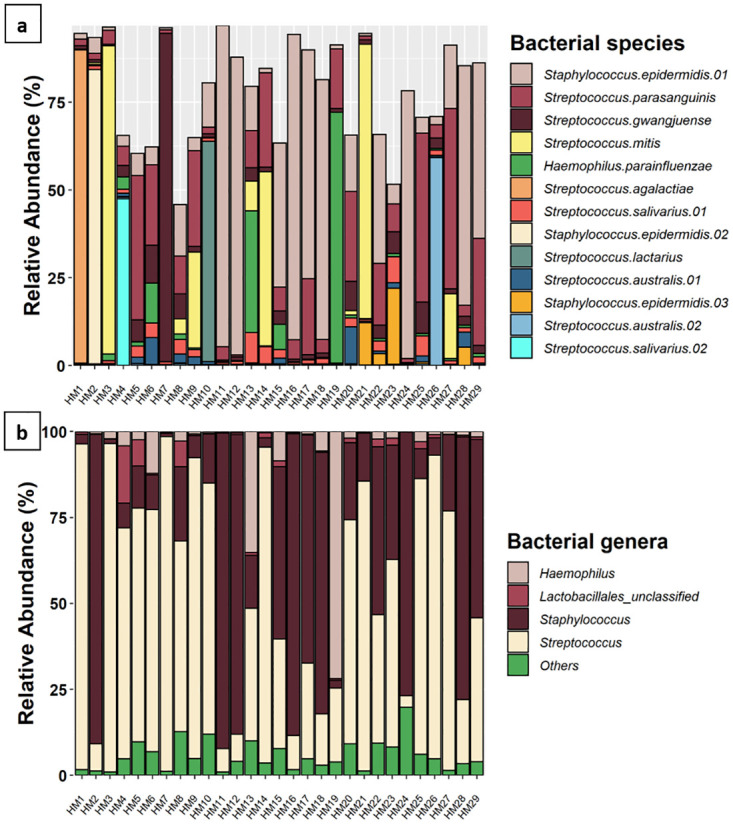
(a) The relative abundance of OTUs which made up ≥1% overall relative abundance. Species assigned to each OTU are noted in the legend. Where multiple OTUs mapped to the same species, they are numbered. (b) The relative abundance of bacterial genera which made up ≥ 1% relative abundance across all samples. Genera which accounted for < 1% relative abundance are grouped together as “others”.
HM bacterial profiles are associated with maternal and infant factors
Differences in HM bacterial composition were observed relative to maternal BMI, mode of delivery, breast pump use, and pacifier use. However, lactation stage, maternal allergy, parity, infant sex, mode of feeding, and solid food intake showed no significant relationship with the HM bacterial profiles.
Maternal BMI
Milk of overweight mothers (n = 9) showed a significantly higher relative abundance of an OTU which mapped to Streptococcus salivarius compared to underweight mothers (n = 3) (mean relative abundance: 3.45% vs 1.09%, P = 0.03) and obese mothers (n = 3) (3.45% vs 1.19%, P = 0.04) (Fig 2a). Additionally, milk from normal weight mothers (n = 13) showed a significantly higher relative abundance of Streptococcus australis compared with milk from underweight mothers (1.34% vs 0.33%, P = 0.02) (Fig 2b). No differences in richness, beta diversity, or Shannon diversity were detected based on maternal BMI.
Fig 2. The relative abundance of Streptococcus spp. is associated with maternal BMI.
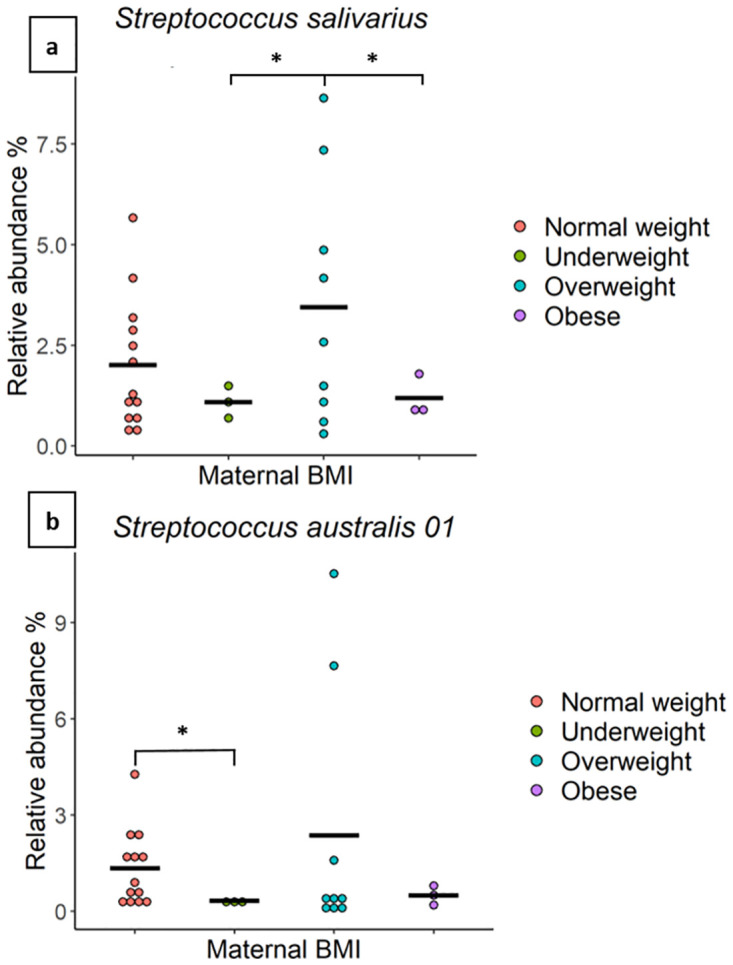
The relative abundance of two OTUs, mapping to (a) Streptococcus salivarius and (b) Streptococcus australis in milk from mothers of different BMI classes (normal weight n = 13, overweight n = 9, obese n = 3, and underweight n = 3). The average value for each group is indicated with a line. * indicate significant results.
Mode of delivery
Milk from mothers who delivered vaginally (n = 19) had a significantly higher relative abundance of an OTU which mapped to S. mitis (14.01% vs 1.95%, P = 0.05) (Fig 3a) than that of mothers who delivered via emergency caesarean section (CS) (n = 5). Although no significant differences were detected in Shannon or beta diversities based on mode of delivery, HM from mothers who delivered via CS (n = 10) showed a trend toward a higher bacterial richness compared to those who delivered vaginally (n = 19), however, this did not reach statistical significance (P = 0.07) (Fig 3b).
Fig 3. The relative abundance of Streptococcus mitis and bacterial richness are associated with the mode of delivery.
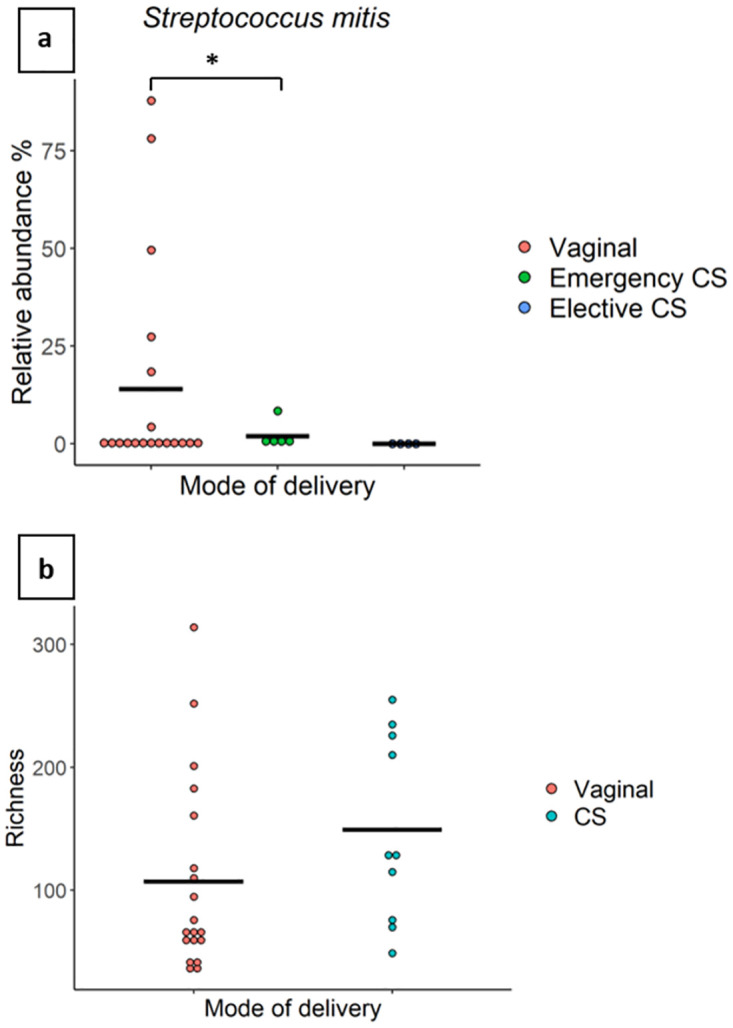
(a) The relative abundance of Streptococcus mitis in milk from mothers who delivered vaginally (n = 19), via emergency caesarean section (n = 5), or via elective caesarean section (n = 4). The average value for each group is indicated with a line. (b) Richness of milk samples from mothers who delivered via caesarean section (n = 10) or vaginally (n = 19). * indicate significant results.
Breast pump use
Milk of mothers who used a breast pump (n = 24) had a higher relative abundance of OTUs which mapped to S. epidermidis (30.95% vs 9.51%, P = 0.000002), S. parasanguinis (15.52% vs 6.42%, P = 0.000002), S. mitis (11.50% vs 0%, P = 0.000002), S. salivarius (2.47% vs 0.97%, P = 0.000002), and S. australis (1.59% vs 0.69%, P = 0.000002) compared to milk of mothers who did not use a breast pump (n = 5) (Fig 4). Richness, beta diversity, and Shannon diversity did not differ according to breast pump use.
Fig 4. The relative abundance of Streptococcus spp. and Staphylococcus sp. is associated with breast pump use.
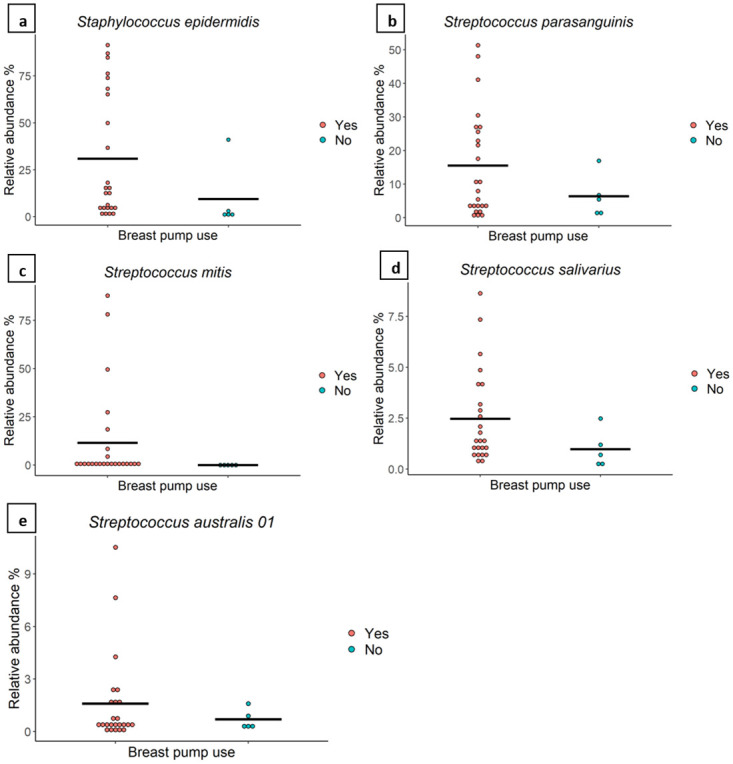
The relative abundance of various bacterial taxa in milk from mothers who did (n = 24) or did not (n = 5) use a breast pump. The average value for each group is indicated with a line.
Pacifier use
Milk of mothers whose infants used a pacifier (n = 15) had a significantly higher relative abundance of OTUs which mapped to S. australis (4.19% vs 0.09%, P = 0.003) and S. gwangjuense (10.10% vs 1.92%, P = 0.02) compared to that of mothers whose infants had never used a pacifier (n = 14) (Fig 5). Richness, beta diversity, and Shannon diversity did not differ by pacifier use.
Fig 5. The relative abundance of Streptococcus spp. is associated with pacifier use.
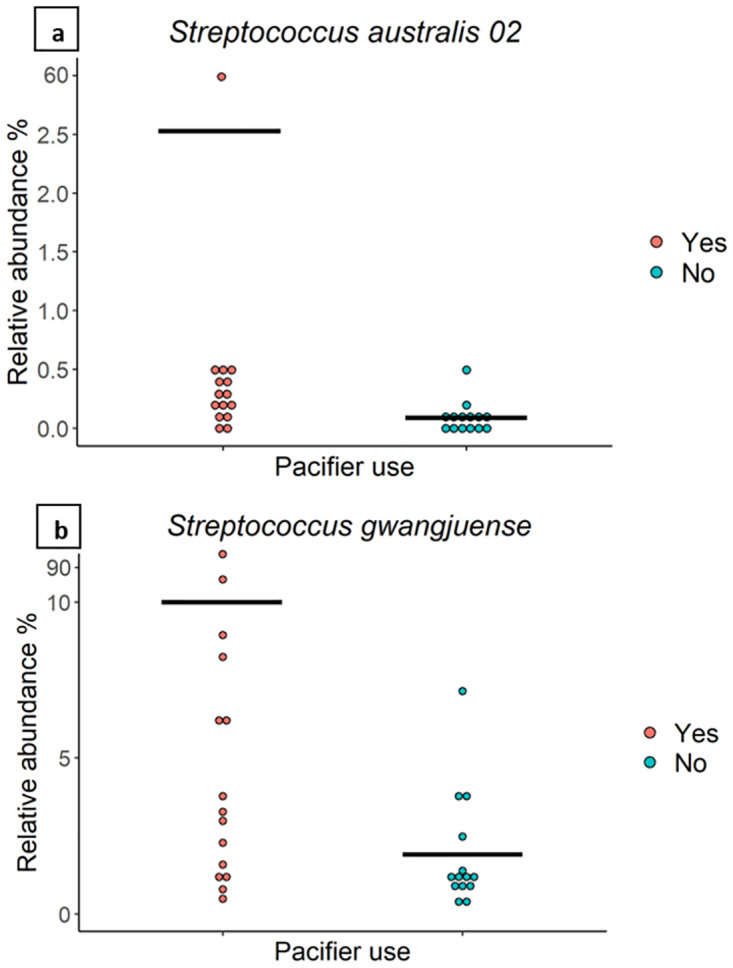
The relative abundance of bacterial taxa in milk from mothers whose infants did (n = 15) or did not (n = 14) use a pacifier. The average value for each group is indicated with a line.
Lactation stage
No significant differences in the relative abundance of the most abundant bacterial species (relative abundance of ≥ 1%) were detected based on samples collected < 6 months postpartum (n = 19) and those collected > 6 months postpartum (n = 10). Richness (113.5 ± 82.1, 137.8 ± 75.5, P = 0.2), beta diversity (PERMANOVA, P = 0.5) (S1 Fig), and Shannon diversity (1.7 ± 1.1, 2.3 ± 0.9, P = 0.1) did not differ significantly by lactation stage.
Discussion
In this pilot study, we identified maternal BMI, mode of delivery, breast pump use, and pacifier use as factors that were associated with HM bacterial composition. While similar studies have been performed elsewhere in the world [27–31, 33, 34, 36–39, 55], this is the first study to assess such associations in an Australian population. This is important given that the HM microbiome has been shown to vary geographically (due to a combination of genetic, local environment, and dietary factors) [3, 26, 30]. Further, by using full-length 16S rRNA gene sequencing, we have been able to resolve these associations to the species level, whereas previous such studies have been limited to the genus or family level [27, 34, 42].
Several studies have reported an association between maternal BMI and the HM microbiome [27, 30, 35–38]. In the present cohort, milk from overweight mothers was associated with a higher abundance of S. salivarius compared to milk from obese mothers (3.45% vs 1.19%) and underweight mothers (3.45% vs 1.09%). Additionally, we showed that milk from normal weight mothers had a higher abundance of S. australis compared with milk from underweight mothers (1.34% vs 0.33%) (Fig 2). These findings may be reflective of BMI-related differences in the human microbiome [56–61] or differences in maternal diet [38, 62–65]. However, with only three overweight and three underweight mothers in this study, the results should be interpreted with caution. High maternal BMI has been associated with a higher abundance of Staphylococcus spp. [35], Akkermansia spp. [27, 36], and Granulicatella spp. [38], and a decreased abundance of Bifidobacterium spp. [27, 36], Lactobacillus spp., and Streptococcus spp. [35] in HM. In addition, milk from mothers with a high BMI has been reported to have a lower bacterial diversity and a higher total bacterial count [27]. We could not identify such changes in the present study, potentially due to population and methodologic differences. Nevertheless, these preliminary results suggest that maternal BMI is associated with HM bacterial composition in an Australian cohort.
Mode of delivery has been repeatedly associated with the composition of the HM microbiome [27–35]. We found a higher abundance of the typical oral taxa S. mitis [66] (14.01% vs 1.95%) in milk from mothers who delivered vaginally compared to those who delivered via emergency CS (Fig 3a). The frequency of breastfeeding may play a role in the increase of oral bacteria in HM of women who delivered vaginally, as CS delivery is associated with more breastfeeding difficulties [67]. Unfortunately, we do not have a complete data set on breastfeeding difficulties in this cohort. Moreover, in Australia, intrapartum antibiotic prophylaxis is administered during CS. Antibiotics can induce changes to the maternal gut microbiome [68, 69], which may affect HM bacteria via the enteromammary route [4, 5, 10, 11]. Antibiotics can also have a direct effect on the HM microbiome [33]. Hermansson et al. provided evidence that exposure to intrapartum antibiotics is associated with alterations to the HM microbiome, regardless of delivery mode [33]. Therefore, the observed associations between HM bacterial composition and CS delivery may be, at least in part, driven by intrapartum antibiotic administration.
Milk from mothers who habitually used a breast pump (on a daily or almost daily basis) was associated with a significantly higher relative abundance of typical oral taxa including S. parasanguinis (15.52% vs 6.42%) [70], S. salivarius (2.47% vs 0.97%) [71], S. mitis (11.50% vs 0%) [66], and S. australis (1.59% vs 0.69%), as well as the typical skin taxon S. epidermidis (30.95% vs 9.51%) compared to the milk of mothers who did not use a breast pump (Fig 4). It is unclear why these taxa were at a higher relative abundance in milk from mothers who used a breast pump; however, the high abundance of S. epidermidis, may be related to the disturbance of skin bacteria during the application of vacuum during pumping, allowing easier entry through the nipple to the mammary gland. Analysis of samples collected from nipples and areola before sterilisation might provide some evidenc. More research is needed to confirm whether bacteria enter the mammary gland through the nipple or whether milk inoculation occurs as milk is expressed. In addition, cleaning practices of breast pump parts between each pumping session could have contributed to the observed differences. Steam decontamination of the breast pump kit has been reported to significantly decrease milk contamination with Enterobacteriaceae and Candida spp. compared with milk samples collected with breast pump accessories that were only washed and not decontaminated [72]. Further research should be performed to investigate whether the observed differences in milk associated with use of a breast pump originate from pump cleaning practices. We are not the first to report that breast pump use is associated with alterations to the HM microbiome. Fehr et al. reported that HM of mothers who sometimes used a breast pump had a significantly lower relative abundance of Veillonella dispar, Haemophilus parainfluenzae, Streptococcus spp., and Bifidobacterium spp. compared with those who only directly breastfed their infants [39]. In contrast to our results, Moosavi et al. reported that direct breastfeeding was associated with a higher abundance of Actinobacteria and Veillonellaceae, while the use of a breast pump at least once during the last two weeks was associated with the presence of potential opportunistic pathogens such as Stenotrophomonas spp. and Pseudomonadaceae [34].
Infant pacifier use was associated with a significantly higher relative abundance of S. australis (4.19% vs 0.09%), a species first isolated from the oral cavities of Australian children in 2001 [66], and S. gwangjuense (10.10% vs 1.92%), a species first isolated from human pericoronitis, an inflammation of the periodontal tissue surrounding unerupted teeth [73] (Fig 5). To our knowledge, this is the first cohort in which S. gwangjuense has been identified in HM. Sharing of bacterial taxa between the infant oral cavity and HM has been repeatedly demonstrated in previous studies [9, 10, 41]. Retrograde flow occurs during breastfeeding, with HM from the infant’s mouth flowing back through the milk ducts to the alveoli during the second half of milk ejection as oxytocin is degraded and intraductal pressure reduces [74].
Pacifier use has been shown to be associated with differences in infant oral microbiome composition. Two studies have reported a significant positive association between pacifier use and the count of microbes such as lactobacilli and Candida spp. in the infant oral microbiome [75, 76]. Other studies reported a significantly higher prevalence of Candida spp. colonisation in the oral microbiota of infants who use a pacifier compared with those who did not [77, 78]. Pacifier use may influence oral sugar clearance in a manner similar to removable dentures, which have been implicated in less effective clearance [79]. This would prolong low pH conditions, making the oral cavity favourable to aciduric microorganisms [80, 81]. In this manner, pacifier use may alter the composition of the oral microbiota and thereby influence microbes contributing to the HM microbiome through retrograde flow. Thus, this study provides preliminary evidence that pacifier use is associated with the HM bacterial profile; however, larger studies are required to replicate these findings with the addition of information on frequency of pacifier use and pacifier cleaning practices.
We did not identify significant associations between maternal allergy, parity, infant sex, mode of feeding, and introduction of solids and the bacterial composition of HM. For parity and infant sex, this is supported by previous findings from Williams et al. [38] and Urbaniak et al. [24], respectively. In contrast, one large cohort study reported an association between maternal atopy, parity, and infant sex and HM bacterial composition [34]. Multiparous mothers and those with atopy had a higher Actinobacteria richness in their milk, while mothers with male infants had a decreased bacterial richness [34]. However, milk samples were not collected aseptically, which may have contributed to the observed differences in results. In a different study, mothers with male infants had an increased relative abundance of Streptococcus spp. and a lower relative abundance of Staphylococcus spp. in their milk [38]. Only one study has investigated the association between exclusive breastfeeding and the HM microbiome, and this was in an area of high HIV prevalence. Milk from mothers who fed their infants exclusively showed an increased relative abundance of Streptococcus parasanguis than those who used mixed feeding methods [40]. These contradictory results could be attributed to the use of short amplicon sequencing, geographically different populations (genetic, local environment, and dietary factors), sample size, and use of non-sterile sample collection techniques by these studies.
A number of limitations in this pilot study need to be acknowledged. The major limitation of this study is the small sample size, which limits the generalisability of these results and their relevance to infant health. Further study in larger cohorts may strengthen the conclusions drawn from this pilot study. Future studies with larger sample sizes will also be able to employ multivariable analyses. Milk samples were collected from participants over a large range of lactation stages (mean: 23.4 weeks ± 18.9 weeks, range 3.4–83.3 weeks, median: 17.3 weeks) among the 29 milk samples. The study is also limited by the lack of information on maternal breast pump cleaning practices, intrapartum antibiotic administration, frequency of breastfeeding, and breastfeeding difficulties.
Conclusions
The current study demonstrates that maternal BMI, mode of delivery, breast pump use, and pacifier use are significantly associated with the bacterial composition of HM in a small cohort of exclusively breastfeeding Western Australian women. The association of these factors with HM bacterial profiles highlights the importance of both mother and infant as contributors to the HM microbiome; however, these conclusions remain statistically insignificant. Therefore, further research is needed to investigate and validate these relationships in a larger cohort and determine if these relationships are related to infant health and development.
Supporting information
(TIF)
Data Availability
All FASTQ files are available from the NCBI SRA database (PRJNA817009).
Funding Statement
ASS, ASC, LFS, and DTG are supported by an unrestricted research grant from Medela AG, administered by The University of Western Australia. MSP is supported by a National Health and Medical Research Council Project Grant (APP1144040). Umm Al-Qura University, Saudi Arabia, provides a PhD scholarship for ASS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Andreas N, Kampmann B, Le-Doare K. Human breast milk: A review on its composition and bioactivity. Early human development. 2015;91:629–35. doi: 10.1016/j.earlhumdev.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 2.Aakko J, Kumar H, Rautava S, Wise A, Autran C, Bode L, et al. Human milk oligosaccharide categories define the microbiota composition in human colostrum. Beneficial microbes. 2017;8:563–7. doi: 10.3920/BM2016.0185 [DOI] [PubMed] [Google Scholar]
- 3.Ojo-Okunola A, Nicol M, du Toit E. Human breast milk bacteriome in health and disease. Nutrients. 2018;10:1643. doi: 10.3390/nu10111643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jost T, Lacroix C, Braegger C, Rochat F, Chassard C. Vertical mother–neonate transfer of maternal gut bacteria via breastfeeding. Environmental microbiology. 2014;16:2891–904. doi: 10.1111/1462-2920.12238 [DOI] [PubMed] [Google Scholar]
- 5.Milani C, Mancabelli L, Lugli GA, Duranti S, Turroni F, Ferrario C, et al. Exploring vertical transmission of bifidobacteria from mother to child. Applied and Environmental Microbiology. 2015;81:7078–87. doi: 10.1128/AEM.02037-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asnicar F, Manara S, Zolfo M, Truong DT, Scholz M, Armanini F, et al. Studying vertical microbiome transmission from mothers to infants by strain-level metagenomic profiling. MSystems. 2017;2:e00164–16. doi: 10.1128/mSystems.00164-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duranti S, Lugli G, Mancabelli L, Armanini F, Turroni F, James K, et al. Maternal inheritance of bifidobacterial communities and bifidophages in infants through vertical transmission. Microbiome. 2017;5:66–13. doi: 10.1186/s40168-017-0282-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pannaraj PS, Li F, Cerini C, Bender JM, Yang S, Rollie A, et al. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA pediatrics. 2017;171:647–54. doi: 10.1001/jamapediatrics.2017.0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz L, Bacigalupe R, García-Carral C, Boix-Amoros A, Argüello H, Silva CB, et al. Microbiota of human precolostrum and its potential role as a source of bacteria to the infant mouth. Scientific reports. 2019;9:8435. doi: 10.1038/s41598-019-42514-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kordy K, Gaufin T, Mwangi M, Li F, Cerini C, Lee DJ, et al. Contributions to human breast milk microbiome and enteromammary transfer of Bifidobacterium breve. PLoS One. 2020;15:e0219633. doi: 10.1371/journal.pone.0219633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duranti S, Lugli GA, Mancabelli L, Armanini F, Turroni F, James K, et al. Maternal inheritance of bifidobacterial communities and bifidophages in infants through vertical transmission. Microbiome. 2017;5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grönlund M, Arvilommi H, Kero P, Lehtonen O, Isolauri E. Importance of intestinal colonisation in the maturation of humoral immunity in early infancy: a prospective follow up study of healthy infants aged 0–6 months. Archives of Disease in Childhood-Fetal and Neonatal Edition. 2000;83:F186–F92. doi: 10.1136/fn.83.3.f186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chichlowski M, Guillaume De Lartigue J, Raybould HE, Mills DA. Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. Journal of pediatric gastroenterology and nutrition. 2012;55:321. doi: 10.1097/MPG.0b013e31824fb899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giongo A, Gano KA, Crabb DB, Mukherjee N, Novelo LL, Casella G, et al. Toward defining the autoimmune microbiome for type 1 diabetes. The ISME journal. 2011;5:82–91. doi: 10.1038/ismej.2010.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arrieta M-C, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Science translational medicine. 2015;7:307ra152–307ra152. doi: 10.1126/scitranslmed.aab2271 [DOI] [PubMed] [Google Scholar]
- 16.Kalliomäki M, Carmen Collado M, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. The American journal of clinical nutrition. 2008;87:534–8. doi: 10.1093/ajcn/87.3.534 [DOI] [PubMed] [Google Scholar]
- 17.Stinson L. Establishment of the early-life microbiome: a DOHaD perspective. Journal of developmental origins of health and disease. 2019;11:1–10. doi: 10.1017/S2040174419000588 [DOI] [PubMed] [Google Scholar]
- 18.Martín R, Heilig HG, Zoetendal EG, Jiménez E, Fernández L, Smidt H, et al. Cultivation-independent assessment of the bacterial diversity of breast milk among healthy women. Research in microbiology. 2007;158:31–7. doi: 10.1016/j.resmic.2006.11.004 [DOI] [PubMed] [Google Scholar]
- 19.Hunt KM, Foster JA, Forney LJ, Schütte UM, Beck DL, Abdo Z, et al. Characterization of the diversity and temporal stability of bacterial communities in human milk. PloS one. 2011;6:e21313. doi: 10.1371/journal.pone.0021313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jost T, Lacroix C, Braegger C, Chassard C. Assessment of bacterial diversity in breast milk using culture-dependent and culture-independent approaches. British Journal of Nutrition. 2013;110:1253–62. doi: 10.1017/S0007114513000597 [DOI] [PubMed] [Google Scholar]
- 21.Ward T, Hosid S, Ioshikhes I, Altosaar I. Human milk metagenome: a functional capacity analysis. BMC microbiology. 2013;13:116. doi: 10.1186/1471-2180-13-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiménez E, de Andrés J, Manrique M, Pareja-Tobes P, Tobes R, Martínez-Blanch JF, et al. Metagenomic analysis of milk of healthy and mastitis-suffering women. Journal of Human Lactation. 2015;31:406–15. doi: 10.1177/0890334415585078 [DOI] [PubMed] [Google Scholar]
- 23.Sakwinska O, Moine D, Delley M, Combremont S, Rezzonico E, Descombes P, et al. Microbiota in breast milk of Chinese lactating mothers. PLoS One. 2016;11. doi: 10.1371/journal.pone.0160856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urbaniak C, Angelini M, Gloor GB, Reid G. Human milk microbiota profiles in relation to birthing method, gestation and infant gender. Microbiome. 2016;4:1–9. doi: 10.1186/s40168-015-0145-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy K, Curley D, O’Callaghan T, O’Shea C-A, Dempsey E, O’Toole P, et al. The composition of human milk and infant faecal microbiota over the first three months of life: a pilot study. Scientific reports. 2017;7:40597. doi: 10.1038/srep40597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lackey KA, Williams JE, Meehan CL, Zachek JA, Benda ED, Price WJ, et al. What’s normal? microbiomes in human milk and infant feces are related to each other but vary geographically: the INSPIRE study. Frontiers in Nutrition. 2019;6:45. doi: 10.3389/fnut.2019.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabrera-Rubio R, Collado C, Laitinen K, Salminen S, Isolauri E, Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. The American Journal of Clinical Nutrition. 2012;96:544–51. doi: 10.3945/ajcn.112.037382 [DOI] [PubMed] [Google Scholar]
- 28.Khodayar-Pardo P, Mira-Pascual L, Collado M, Martinez-Costa C. Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. Journal of Perinatology. 2014;34:599–605. doi: 10.1038/jp.2014.47 [DOI] [PubMed] [Google Scholar]
- 29.Cabrera-Rubio R, Mira-Pascual L, Mira A, Collado M. Impact of mode of delivery on the milk microbiota composition of healthy women. Journal of Developmental Origins of Health and Disease. 2016;7:54–60. doi: 10.1017/S2040174415001397 [DOI] [PubMed] [Google Scholar]
- 30.Kumar H, du Toit E, Kulkarni A, Aakko J, Linderborg KM, Zhang Y, et al. Distinct patterns in human milk microbiota and fatty acid profiles across specific geographic locations. Frontiers in Microbiology. 2016;7:1619. doi: 10.3389/fmicb.2016.01619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toscano M, De Grandi R, Peroni DG, Grossi E, Facchin V, Comberiati P, et al. Impact of delivery mode on the colostrum microbiota composition. BMC Microbiology. 2017;17:205. doi: 10.1186/s12866-017-1109-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S-W, Watanabe K, Hsu C-C, Chao S-H, Yang Z-H, Lin Y-J, et al. Bacterial composition and diversity in breast milk samples from mothers living in Taiwan and mainland China. Frontiers in microbiology. 2017;8:965. doi: 10.3389/fmicb.2017.00965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hermansson H, Kumar H, Collado MC, Salminen S, Isolauri E, Rautava S. Breast milk microbiota is shaped by mode of delivery and intrapartum antibiotic exposure. Frontiers in nutrition. 2019;6:4. doi: 10.3389/fnut.2019.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moossavi S, Sepehri S, Robertson B, Bode L, Goruk S, Field C, et al. Composition and variation of the human milk microbiota are influenced by maternal and early-life factors. Cell Host & Microbe. 2019;25:324–35. e4. [DOI] [PubMed] [Google Scholar]
- 35.Ding M, Qi C, Yang Z, Jiang S, Bi Y, Lai J, et al. Geographical location specific composition of cultured microbiota and Lactobacillus occurrence in human breast milk in China. Food & function. 2019;10:554–64. doi: 10.1039/c8fo02182a [DOI] [PubMed] [Google Scholar]
- 36.Collado MC, Laitinen K, Salminen S, Isolauri E. Maternal weight and excessive weight gain during pregnancy modify the immunomodulatory potential of breast milk. Pediatric Research. 2012;72:77. doi: 10.1038/pr.2012.42 [DOI] [PubMed] [Google Scholar]
- 37.Lundgren S, Madan J, Karagas M, Morrison H, Hoen A, Christensen B. Microbial communities in human milk relate to measures of maternal weight. Frontiers in Microbiology. 2019;10:2886. doi: 10.3389/fmicb.2019.02886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams J, Carrothers J, Lackey K, Beatty N, York M, Brooker S, et al. Human milk microbial community structure is relatively stable and related to variations in macronutrient and micronutrient intakes in healthy lactating women. The Journal of Nutrition. 2017;147:1739–48. doi: 10.3945/jn.117.248864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fehr K, Moossavi S, Sbihi H, Boutin RC, Bode L, Robertson B, et al. Breastmilk feeding practices are associated with the co-occurrence of bacteria in mothers’ milk and the infant gut: the CHILD cohort study. Cell Host & Microbe. 2020;28:285–97. e4. [DOI] [PubMed] [Google Scholar]
- 40.González R, Maldonado A, Martín V, Mandomando I, Fumadó V, Metzner KJ, et al. Breast milk and gut microbiota in African mothers and infants from an area of high HIV prevalence. PloS one. 2013. doi: 10.1371/journal.pone.0080299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biagi E, Aceti A, Quercia S, Beghetti I, Rampelli S, Turroni S, et al. Microbial community dynamics in mother’s milk and infant’s mouth and gut in moderately preterm infants. Frontiers in microbiology. 2018;9:2512. doi: 10.3389/fmicb.2018.02512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez Leyva L, Gonzalez E, Li C, Ajeeb T, Solomons NW, Agellon LB, et al. Human Milk Microbiota in an Indigenous Population Is Associated with Maternal Factors, Stage of Lactation, and Breastfeeding Practices. Current developments in nutrition. 2021;5:nzab013. doi: 10.1093/cdn/nzab013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hospodsky D, Pickering AJ, Julian TR, Miller D, Gorthala S, Boehm AB, et al. Hand bacterial communities vary across two different human populations. Microbiology. 2014;160:1144–52. doi: 10.1099/mic.0.075390-0 [DOI] [PubMed] [Google Scholar]
- 44.Drago L, Toscano M, De Grandi R, Grossi E, Padovani EM, Peroni DG. Microbiota network and mathematic microbe mutualism in colostrum and mature milk collected in two different geographic areas: Italy versus Burundi. The ISME Journal. 2017;11:875–84. doi: 10.1038/ismej.2016.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yatsunenko T, Rey F, Manary M, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7. doi: 10.1038/nature11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Quinque D, Horz H-P, Li M, Rzhetskaya M, Raff JA, et al. Comparative analysis of the human saliva microbiome from different climate zones: Alaska, Germany, and Africa. BMC microbiology. 2014;14:1–13. doi: 10.1186/s12866-014-0316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mobeen F, Sharma V, Tulika P. Enterotype variations of the healthy human gut microbiome in different geographical regions. Bioinformation. 2018;14:560. doi: 10.6026/97320630014560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheema AS, Lai CT, Dymock M, Rae A, Geddes DT, Payne MS, et al. Impact of expression mode and timing of sample collection, relative to milk ejection, on human milk bacterial DNA profiles. Journal of Applied Microbiology. 2021;131:988–95. doi: 10.1111/jam.14998 [DOI] [PubMed] [Google Scholar]
- 49.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and environmental microbiology. 2009;75:7537–41. doi: 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glöckner FO, Yilmaz P, Quast C, Gerken J, Beccati A, Ciuprina A, et al. 25 years of serving the community with ribosomal RNA gene reference databases and tools. Journal of biotechnology. 2017;261:169–76. doi: 10.1016/j.jbiotec.2017.06.1198 [DOI] [PubMed] [Google Scholar]
- 51.R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. 2020.
- 52.White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS computational biology. 2009;5:e1000352. doi: 10.1371/journal.pcbi.1000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of molecular biology. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 54.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic acids research. 1997;25:3389–402. doi: 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davé V, Street K, Francis S, Bradman A, Riley L, Eskenazi B, et al. Bacterial microbiome of breast milk and child saliva from low-income Mexican-American women and children. Pediatric Research. 2016;79:846. doi: 10.1038/pr.2016.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dominianni C, Sinha R, Goedert JJ, Pei Z, Yang L, Hayes RB, et al. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PloS one. 2015;10. doi: 10.1371/journal.pone.0124599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yun Y, Kim H-N, Kim SE, Heo SG, Chang Y, Ryu S, et al. Comparative analysis of gut microbiota associated with body mass index in a large Korean cohort. BMC microbiology. 2017;17:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao X, Zhang M, Xue J, Huang J, Zhuang R, Zhou X, et al. Body Mass Index Differences in the Gut Microbiota Are Gender Specific. Frontiers in Microbiology. 2018;9. doi: 10.3389/fmicb.2018.01250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Y, Cai Q, Zheng W, Steinwandel M, Blot WJ, Shu X-O, et al. Oral microbiome and obesity in a large study of low-income and African-American populations. Journal of oral microbiology. 2019;11:1650597. doi: 10.1080/20002297.2019.1650597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vongsa R, Hoffman D, Shepard K, Koenig D. Comparative study of vulva and abdominal skin microbiota of healthy females with high and average BMI. BMC microbiology. 2019;19:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alqaderi H, Ramakodi MP, Nizam R, Jacob S, Devarajan S, Eaaswarkhanth M, et al. Salivary Microbiome Diversity in Kuwaiti Adolescents with Varied Body Mass Index—A Pilot Study. Microorganisms. 2021;9:1222. doi: 10.3390/microorganisms9061222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Padilha M, Danneskiold-Samsøe N, Brejnrod A, Hoffmann C, Cabral V, Iaucci J, et al. The human milk microbiota is modulated by maternal diet. Microorganisms. 2019;7:502. doi: 10.3390/microorganisms7110502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Babakobi M, Reshef L, Gihaz S, Belgorodsky B, Fishman A, Bujanover Y, et al. Effect of Maternal Diet and Milk Lipid Composition on the Infant Gut and Maternal Milk Microbiomes. Nutrients. 2020;12:2539. doi: 10.3390/nu12092539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cortes-Macías E, Selma-Royo M, García-Mantrana I, Calatayud M, González S, Martínez-Costa C, et al. Maternal Diet Shapes the Breast Milk Microbiota Composition and Diversity: Impact of Mode of Delivery and Antibiotic Exposure. The Journal of Nutrition. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.LeMay-Nedjelski L, Asbury M, Butcher J, Ley S, Hanley A, Kiss A, et al. Maternal Diet and Infant Feeding Practices Are Associated with Variation in the Human Milk Microbiota at 3 Months Postpartum in a Cohort of Women with High Rates of Gestational Glucose Intolerance. The Journal of Nutrition. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holgerson PL, Esberg A, Sjödin A, West CE, Johansson I. A longitudinal study of the development of the saliva microbiome in infants 2 days to 5 years compared to the microbiome in adolescents. Scientific Reports. 2020;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang F, Cheng J, Yan S, Wu H, Bai T. Early feeding behaviors and breastfeeding outcomes after cesarean section. Breastfeeding Medicine. 2019;14:325–33. doi: 10.1089/bfm.2018.0150 [DOI] [PubMed] [Google Scholar]
- 68.Rogers MA, Aronoff DM. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clinical Microbiology and Infection. 2016;22:178. e1–.e9. doi: 10.1016/j.cmi.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Antibiotics Francino M. and the human gut microbiome: dysbioses and accumulation of resistances. Frontiers in microbiology. 2016;6:1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Q, Wu G, Chen H, Li H, Li S, Zhang C, et al. Quantification of Human Oral and Fecal Streptococcus parasanguinis by Use of Quantitative Real-Time PCR Targeting the groEL Gene. Frontiers in microbiology. 2019;10:2910. doi: 10.3389/fmicb.2019.02910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burton J, Chilcott C, Moore C, Speiser G, Tagg J. A preliminary study of the effect of probiotic Streptococcus salivarius K12 on oral malodour parameters. Journal of applied microbiology. 2006;100:754–64. doi: 10.1111/j.1365-2672.2006.02837.x [DOI] [PubMed] [Google Scholar]
- 72.Flores-Antón B, Martín-Cornejo J, Morante-Santana M, García-Lara N, Sierra-Colomina G, De la Cruz-Bértolo J, et al. Comparison of two methods for cleaning breast pump milk collection kits in human milk banks. Journal of Hospital Infection. 2019;103:217–22. doi: 10.1016/j.jhin.2019.07.007 [DOI] [PubMed] [Google Scholar]
- 73.Park S-N, Lim YK, Shin JH, Chang Y-H, Shin Y, Paek J, et al. Streptococcus gwangjuense sp. nov., isolated from human pericoronitis. Current microbiology. 2019;76:799–803. doi: 10.1007/s00284-019-01687-8 [DOI] [PubMed] [Google Scholar]
- 74.Ramsay D, Kent J, Owens R, Hartmann P. Ultrasound imaging of milk ejection in the breast of lactating women. Pediatrics. 2004;113:361–7. doi: 10.1542/peds.113.2.361 [DOI] [PubMed] [Google Scholar]
- 75.Ollila P, Niemelä M, Uhari M, Larmas M. Risk factors for colonization of salivary lactobacilli and Candida in children. Acta Odontologica Scandinavica. 1997;55:9–13. [DOI] [PubMed] [Google Scholar]
- 76.Ersin NK, Eronat N, Cogulu D, Uzel A, Aksit S. Association of maternal-child characteristics as a factor in early childhood caries and salivary bacterial counts. Journal of dentistry for children. 2006;73:105–11. [PubMed] [Google Scholar]
- 77.Darwazeh A, Al‐Bashir A. Oral candidal flora in healthy infants. Journal of oral pathology & medicine. 1995;24:361–4. doi: 10.1111/j.1600-0714.1995.tb01200.x [DOI] [PubMed] [Google Scholar]
- 78.Mattos-Graner RO, de Moraes AB, Rontani R, Birman EG. Relation of oral yeast infection in Brazilian infants and use of a pacifier. ASDC journal of dentistry for children. 2001;68:33–6, 10. [PubMed] [Google Scholar]
- 79.Hase J, Birkhed D. Oral Sugar Clearance in Elderly People With Prosthodontic Reconstructions. Scandinavian journal of dental research. 1991;99:333–9. doi: 10.1111/j.1600-0722.1991.tb01037.x [DOI] [PubMed] [Google Scholar]
- 80.Arendorf T, Addy M. Candidal carriage and plaque distribution before, during and after removable orthodontic appliance therapy. Journal of clinical periodontology. 1985;12:360–8. doi: 10.1111/j.1600-051x.1985.tb00926.x [DOI] [PubMed] [Google Scholar]
- 81.Bradshaw D, McKee A, Marsh P. Effects of carbohydrate pulses and pH on population shifts within oral microbial communities in vitro. Journal of dental research. 1989;68:1298–302. doi: 10.1177/00220345890680090101 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Data Availability Statement
All FASTQ files are available from the NCBI SRA database (PRJNA817009).


