Abstract
Background
There is limited evidence exploring the maternal and neonatal complications of gestational diabetes mellitus (GDM) following singleton or twin pregnancies. Further, there have been no reviews completed examining the possible risk factors associated with GDM in singleton compared to twin pregnancies. This study assesses the impact of GDM in singleton and twin pregnancies on maternal and neonatal outcomes.
Methods
From 1954 to December 2021, a thorough literature search was conducted in the EMBASE, Cochrane, MEDLINE, ScienceDirect, and Google Scholar databases and search engines. The risk of bias was calculated using the Newcastle Ottawa (NO) scale. A random-effects model was applied and interpreted as pooled odds ratio (OR) with 95% confidence intervals (CI).
Results
Eight studies satisfied the inclusion criteria, with the quality of most studies being good to satisfactory. The risk of caesarean section (pooled OR = 0.32; 95%CI: 0.22 to 0.46), small-for-gestational age (SGA) neonates (pooled OR = 0.40; 95%CI: 0.19 to 0.84), preterm delivery (pooled OR = 0.07; 95%CI: 0.06 to 0.09), respiratory morbidity (pooled OR = 0.26; 95%CI: 0.19 to 0.37), neonatal hyperbilirubinemia (pooled OR = 0.19; 95%CI: 0.10 to 0.40), and NICU admission (pooled OR = 0.18; 95%CI: 0.14 to 0.25) was significantly lower in singleton pregnancies with GDM than in twin pregnancies with GDM.
Conclusion
Maternal outcomes like caesarean section and neonatal outcomes like SGA neonates, preterm delivery, respiratory morbidity, hyperbilirubinemia, and NICU admission were significantly greater in twin pregnancies with GDM. It is important for clinicians and policymakers to focus intervention strategies on twin pregnancies with GDM.
Introduction
Gestational diabetes mellitus (GDM), which is defined as glucose intolerance that develops and is first recognized during pregnancy, is one of the most common complications in pregnant women. The burden of GDM has been increasing globally, over the past decade [1]. There has been a concurrent rise in the rate of twin pregnancy [2–4]. The risk factors associated with GDM and twin pregnancy are shared, such as high body mass index (BMI) and advancing maternal age [5]. GDM increases the risk of preterm delivery and a twin pregnancy with GDM is associated with a higher incidence of adverse pregnancy outcomes, such as gestational hypertension, pre-eclampsia, premature rupture of membranes, and adverse neonatal outcomes, such as small for gestational age (SGA) offspring, low birth injuries, hypoglycaemia, hypothermia, respiratory morbidity and neonatal intensive care unit (NICU) admission [6–9]. Of note, a singleton pregnancy with GDM has its own set of complications such as respiratory morbidity, macrosomia, hypoglycaemia and shoulder dystocia [10, 11]. However, the limited literature examining the link between GDM in singleton and twin pregnancies and maternal and neonatal complications is conflicting [12–14]. The available evidence is limited by small sample sizes or lack of a control group of singleton pregnancies, thereby removing the possibility of assessing the impact of plurality on the clinical outcomes due to GDM [12–14]. To date, there are no studies that attempted to summarize data on the difference in outcomes between singleton and twin pregnancies in patients with GDM. Thus, the aim of the current review and meta-analysis was to summarize data from studies that examine the difference in maternal and neonatal outcomes between singleton and twin pregnancies in women with GDM.
Methods
Eligibility criteria
Study design
Observational studies that meet the criteria for inclusion (cross-sectional, cohort, case-control, etc.) were included. Conference abstracts, case studies, case reports, and grey literature were excluded whereas full-text papers were included. This study was registered in PROSPERO (CRD42022301059).
Study participants
Studies containing women diagnosed with GDM were included, while studies with pregnant women with pre-existing diabetes were excluded.
Exposure
Studies evaluating the difference in maternal and neonatal outcomes in singleton and twin pregnancies complicated by GDM.
Outcome
Maternal outcomes. Gestational hypertension, pre-eclampsia, caesarean section.
Neonatal outcomes. Preterm delivery, small for gestational age (SGA) neonates, low birth weight, hypoglycaemia, hyperbilirubinemia or jaundice, respiratory morbidity, and NICU admission.
Search strategy
EMBASE, Cochrane Library, MEDLINE, and search engines like Google Scholar and ScienceDirect were used in the literature search (S1 Appendix). The search was conducted using free-text phrases and medical subject headings (MeSH). The time period (January 1954 to December 2021), language (English only), and design filters were all used during the final search, which was carried out using appropriate Boolean operators ("AND" & "OR") and observational study filter. The relevant articles’ references were checked for any new publications.
Study selection process
The titles and abstracts of the papers that had been identified were examined by two separate researchers (FT & AF). If a study met the inclusion requirements, the full text of the study was retrieved.
The same group of investigators (FT & AF) then conducted a screening of the retrieved full texts and evaluated them in light of the inclusion criteria. Studies that met the criteria were included, and the reasons for excluding the others were noted.
Any discrepancies were discussed with the second investigator in order to be rectified (AF). The study was reported in accordance with the "Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 checklist" [15].
Data extraction process
Data extracted for the review consisted of the following information: Authors, the title of the study, the year it was published, the study period, the study design, the setting, the country or region, the total sample size, the details of the outcome assessment, the average age, and the primary and secondary outcomes in each group. The first author (FT) entered the data, while the second author (AF) double-checked the accuracy of entry.
Risk of bias assessment
The Newcastle Ottawa (NO) scale for observational studies was used to assess the likelihood of bias independently by two investigators (FT and AF). The domains it covers are Selection (four stars), Comparability (two stars), and Outcome (two stars). The final score can vary from 0 to 8 stars; studies with a score of 7–8 stars are considered to be of "good," "satisfactory," or "unsatisfactory" quality, respectively [16].
Statistical analysis
STATA version 14.2 was used for the statistical analysis (StataCorp, CollegeStation, TX, USA). The data were presented as a pooled odds ratio (OR) because the primary outcomes were binary, and the number of events and sample size within each group were entered. The methodological heterogeneity was taken into account using a random effects model with inverse-variance.
The chi-square test and the I2 statistic were used to assess heterogeneity [17]. The pooled estimate and study-specific estimate were graphically represented using a forest plot, and the robustness of the pooled estimate was assessed using a sensitivity analysis. Due to the final inclusion of less than 10 studies, a meta-regression or assessment of publication bias could not be done (8 studies).
Results
Study selection
In total, 1,167 records were identified through literature search. Of them, 73 pertinent study full texts were acquired. By looking through the references of the obtained full texts during primary screening, two additional publications were found. The final decision was made to include 8 studies with 41,548 participants after the final screening against eligibility criteria (Fig 1) [12–14, 18–22].
Fig 1. PRISMA flowchart.
Characteristics of the included studies
Included studies were all retrospective in nature. Most were conducted in Canada (3 studies) followed by Spain (2 studies). The sample sizes varied from 184 to 18,942. The average age of women with a singleton pregnancy complicated with GDM ranged from 32 to 35.5 years and women with a twin pregnancy and GDM from 31.9 to 35.8 years. Seven of the studies reported on the SGA outcome, 6 studies each on pre-eclampsia and caesarean section outcomes, 5 studies on preterm birth, and 4 studies each on respiratory morbidity, hypoglycaemia, hyperbilirubinemia, and NICU admission, and 3 studies on gestational hypertension (Table 1). The majority (5 out of 6) of the studies were of good to satisfactory quality (Table 2).
Table 1. Characteristics of the included studies (N = 8).
| Author and year | Country | Study design | Sample size in singleton GDM | Sample size in twin GDM | Study participants | Mean age |
|---|---|---|---|---|---|---|
| Ashwal 2021 | Canada | Retrospective cohort | 1893 | 180 | All women with a singleton or twin pregnancy (with outcome reported separately for GDM patients with singleton or twin pregnancy) who were followed up at a single tertiary referral center (Sunnybrook Health Sciences Center, Toronto, Ontario, Canada) between January 1, 2011, and April 31, 2020 | S = 34.6 T = 35.1 |
| Guillén-Sacoto 2017 | Spain | Retrospective observational | 122 | 62 | GDM pregnancies, women were followed throughout their pregnancy, then underwent postpartum examination between January 1991 and December 2015. | S = 35.5 T = 35.8 |
| Guillén-Sacoto 2018 | Spain | Retrospective observational | 240 | 120 | Pregnant women with GDM: 120 pregnancies twins and 240 singleton pregnancies as controls | S = 35.1 T = 35.3 |
| Hiersch 2019 | Canada | Retrospective cohort | 16731 | 649 | All twin and singleton live births in Ontario, Canada, 2012–2016 with pregnancy outcomes compared between women with vs without gestational diabetes mellitus, analysed separately for twin and singleton births | S = 33.1 T = 34 |
| Lai 2015 | Canada | Retrospective analysis | 18137 | 805 | Singleton and twin GDM pregnancies resulting in live/still births in Alberta | NR |
| Sheehan 2019 | Australia | Retrospective cohort | 1370 | 39 | All women who delivered at the Royal Women’s Hospital, Melbourne, between January 2016 and December 2017 with outcome reported separately between GDM singleton and GDM twin pregnancy | NR |
| Weiner 2018 | Israel | Retrospective analysis | 228 | 114 | All deliveries of singleton and twin pregnancies complicated by GDM at a single university-affiliated hospital between 1/2008-10/2016 | S = 32.3 T = 31.9 |
| Xue 2019 | China | Retrospective analysis | 572 | 286 | Twin and singleton pregnancies complicated by GDM, which delivered in Peking University First Hospital from January 1st, 2012—December 31st, 2017 | S = 32 T = 33 |
S-Singleton pregnancy; T-Twin pregnancy; NR-Not reported
Table 2. Quality assessment of the included studies (N = 8).
| S.N. | Author and year | Representativeness | Sample size justification | Non-response | Ascertainment of exposure | Control for confounding | Assessment of outcome | Statistical tests | Overall Quality |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Ashwal 2021 | * | 0 star | 0 star | * | ** | * | * | Satisfactory |
| 2. | Guillén-Sacoto 2017 | * | * | * | * | * | * | * | Good |
| 3. | Guillén-Sacoto 2018 | * | 0 star | * | * | * | * | * | Good |
| 4. | Hiersch 2019 | * | * | 0 star | * | * | * | * | Good |
| 5. | Lai 2015 | 0 star | 0 star | 0 star | * | ** | * | * | Satisfactory |
| 6. | Sheehan 2019 | * | 0 star | 0 star | * | ** | * | * | Satisfactory |
| 7. | Weiner 2018 | * | * | * | * | * | * | * | Good |
| 8. | Xue 2019 | * | 0 star | * | * | ** | * | * | Good |
Maternal outcomes
Gestational hypertension
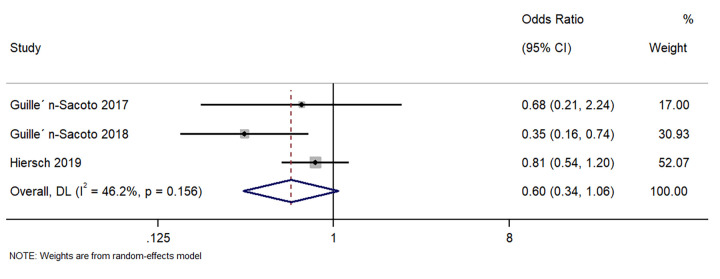
Three studies described the difference in gestational hypertension between singleton GDM and twin GDM pregnancies. The pooled OR was 0.60 (95%CI: 0.34 to 1.06; I2 = 46.2%), indicating a similar incidence of gestational hypertension in women with singleton GDM and twin GDM pregnancies(p = 0.08) (Fig 2).
Fig 2. Forest plot showing the difference in gestational hypertension between women with a singleton pregnancy or twin pregnancy and GDM.
NOTE: Weights are from random-effects model.
Pre-eclampsia
Six studies described the difference in pre-eclampsia between singleton GDM and twin GDM pregnancies. The pooled OR was 0.70 (95%CI: 0.25 to 2.02; I2 = 92.6%), suggesting no difference in the rate of pre-eclampsia between singleton GDM and twin GDM pregnancies (p = 0.51) (Fig 3).
Fig 3. Forest plot showing the difference in pre-eclampsia between women with a singleton pregnancy or twin pregnancy and GDM.
NOTE: Weights are from random-effects model.
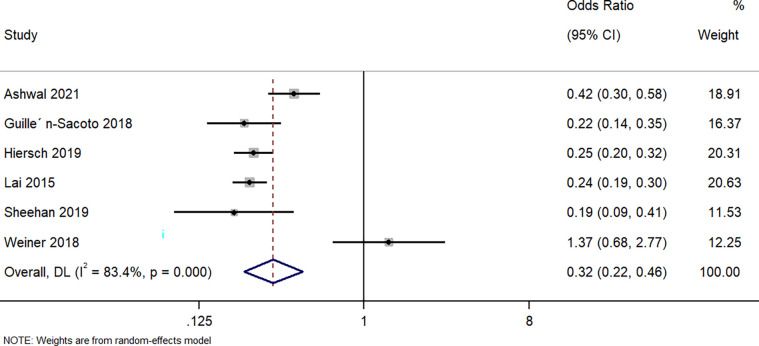
Caesarean section
Six studies described the difference in the caesarean section between singleton GDM and twin GDM pregnancies. The pooled OR was 0.32 (95%CI: 0.22 to 0.46; I2 = 83.4%), indicating that a singleton pregnancy complicated with GDM correlates with a markedly (68%) lower risk of the caesarean section when compared to a twin GDM pregnancy (p<0.001) (Fig 4).
Fig 4. Forest plot showing the difference in caesarean section between women with a singleton pregnancy or twin pregnancy and GDM.
NOTE: Weights are from random-effects model.
Neonatal outcomes
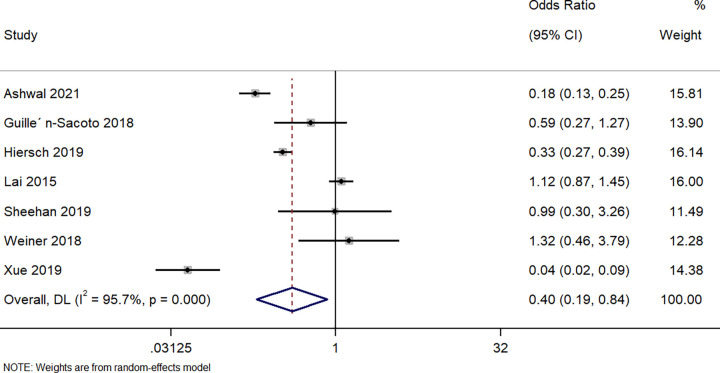
Small for gestational age neonates
Seven studies described the difference in delivering an SGA neonate between singleton GDM and twin GDM pregnancies. The pooled OR was 0.40 (95%CI: 0.19 to 0.84; I2 = 95.7%), indicating that the women with a singleton pregnancy and GDM had a significantly (60%, p = 0.01) lower risk of delivering small for gestational age offspring than women with a twin pregnancy and GDM (Fig 5).
Fig 5. Forest plot showing the difference in small for gestational age neonates between women with a singleton pregnancy or twin pregnancy and GDM.
NOTE: Weights are from random-effects model.
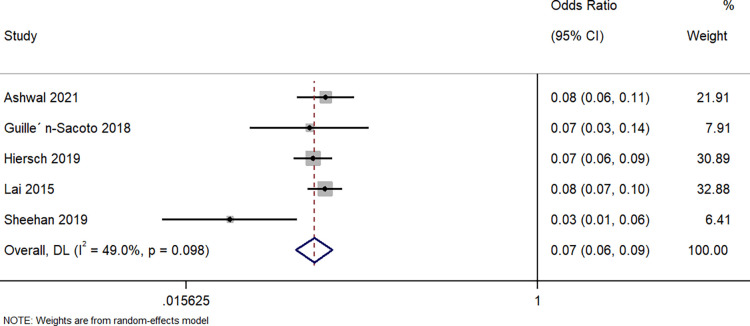
Preterm delivery
Five studies described the difference in having a preterm delivery between singleton GDM and twin GDM pregnancies. The pooled OR was 0.07 (95%CI: 0.06 to 0.09; I2 = 49%), indicating that the women with a singleton pregnancy and GDM had a 93% lower risk of preterm delivery when compared to women with a twin pregnancy and GDM (p<0.001) (Fig 6).
Fig 6. Forest plot showing the difference in preterm delivery between women with a singleton pregnancy or twin pregnancy and GDM.
NOTE: Weights are from random-effects model.
Respiratory morbidity
Four studies described the difference in respiratory morbidity in neonates born to GDM women with a singleton pregnancy and those born to women with a twin pregnancy and GDM. The pooled OR was 0.26 (95%CI: 0.19 to 0.37; I2 = 28.3%), indicating that the neonates born to women with a singleton pregnancy and GDM had a significantly lower (74%) risk of having respiratory morbidity when compared to neonates born to women with a twin pregnancy and GDM (p<0.001) (Fig 7).
Fig 7. Forest plot showing the difference in respiratory morbidity in neonates between women with a singleton pregnancy or twin pregnancy and GDM.
NOTE: Weights are from random-effects model.
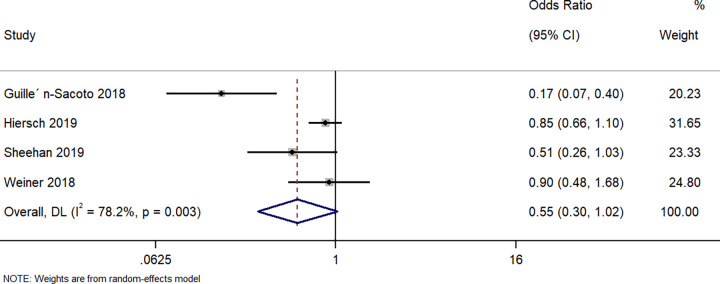
Hypoglycaemia
Four studies described the difference in hypoglycaemia in neonates between singleton GDM and twin GDM pregnancies. The pooled OR was 0.55 (95%CI: 0.30 to 1.02; I2 = 78.2%), indicating that there was no difference in the rate of neonatal hypoglycaemia between both groups (p = 0.06) (Fig 8).
Fig 8. Forest plot showing the difference in neonatal hypoglycaemia between women with a singleton pregnancy or twin pregnancy and GDM.
NOTE: Weights are from random-effects model.
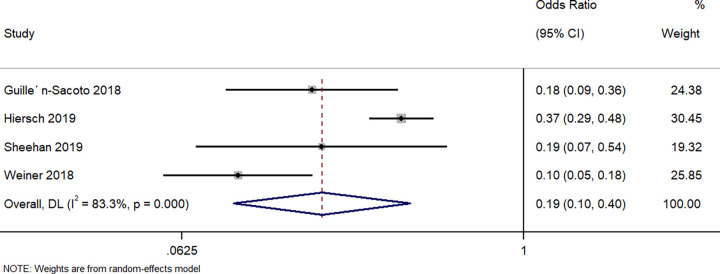
Hyperbilirubinemia
Four studies described the difference in neonates having hyperbilirubinemia between the neonates born to singleton GDM and those born to twin GDM pregnancies. The pooled OR was 0.19 (95%CI: 0.10 to 0.40; I2 = 83.3%), indicating that the neonates born to women with a singleton pregnancy and GDM had a significantly lower (81%) risk of having hyperbilirubinemia when compared to neonates born to women with a twin pregnancy and GDM (p<0.001) (Fig 9).
Fig 9. Forest plot showing the difference in neonatal hyperbilirubinemia between women with a singleton pregnancy or twin pregnancy and GDM.
NOTE: Weights are from random-effects model.
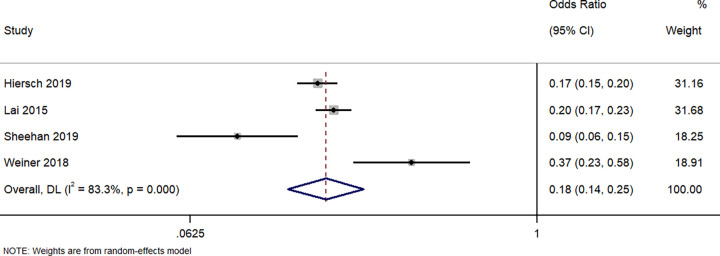
NICU admission
Four studies have described the difference in neonates requiring NICU admission between the neonates born to singleton GDM and those born to twin GDM pregnancies. The pooled OR was 0.18 (95%CI: 0.14 to 0.25; I2 = 83.3%), indicating that the neonates born to women with a singleton pregnancy and GDM have a significantly lower (82%) risk of requiring NICU admission when compared to neonates born to women with a twin pregnancy and GDM (p<0.001) (Fig 10).
Fig 10. Forest plot showing the difference in NICU admission between women with a singleton pregnancy or twin pregnancy and GDM.
NOTE: Weights are from random-effects model.
Additional analysis
The sensitivity analysis did not find any appreciable variation in the effect size (in terms of magnitude and direction). This suggests that there was no overall single-study effect on the estimate of any outcome. Due to the small number of papers, a funnel plot for publication bias and meta-regression for examining heterogeneity cannot be performed.
Discussion
This systematic review and meta-analysis aimed to examine the risk of various maternal and neonatal outcomes in women with a singleton pregnancy and a twin pregnancy that are complicated by GDM. In total, 8 studies fulfilled the eligibility criteria. Most of these studies were conducted in Western countries, like Canada and Spain. While all the studies followed the retrospective design, most of them were of good to a satisfactory quality.
The results presented here highlight the divergent effect of GDM on almost all neonatal outcomes (except neonatal hypoglycaemia), especially the risk of caesarean section. Sensitivity analysis did not reveal any significant single-study effect on the magnitude or direction of the association.
While there were no previous attempts to compare maternal and neonatal outcomes in GDM women with singleton and twin pregnancies, we explored the potential impact of such association using the previous literature. Our results show that women with a singleton pregnancy and GDM had a significantly lower risk of having a caesarean section than women with a twin pregnancy and GDM. These results are surprising given that the growth potential during the third trimester in a twin pregnancy is lower, leading to a reduced possibility of developing macrosomia [23]. However, these results could be due to the inherent indications for caesarean delivery associated with GDM or twin pregnancies, such as a discrepancy in fetal growth, higher risk of placenta previa, malpresentation, and patient and doctor preference [13].
Our study also shows that GDM women with a singleton pregnancy have a significantly lower risk of having a preterm delivery and SGA babies compared to women with a twin pregnancy and GDM. It can be argued that GDM-induced accelerated growth in twins should not be concerning, given that it is unlikely to cause neonatal complications like birth trauma or shoulder dystocia. GDM-induced growth in twins could actually be beneficial, given the slower rate of growth in twins during the third trimester, thus raising the risk of growth restriction [24–27]. These factors are most likely why the impact of GDM in excess foetal growth is counteracted. However, accelerated growth within the foetus may be associated with similar foetal programming as singleton pregnancies with GDM, ultimately leading to long-term metabolic complications including diabetes, obesity, and cardiovascular disease [28–32]. Hence, longitudinal studies conducted over a longer period among the twin GDM infants are necessary to answer this question.
Our results suggest that women with a singleton pregnancy and GDM had a significantly lower risk of delivering neonates with hyperbilirubinemia or respiratory morbidity than women with a twin pregnancy. The mechanism behind such association was unclear and further longitudinal studies are required to identify the possible reason(s) for the association with adverse metabolic neonatal outcomes and twin GDM. We also found a significantly lower NICU admission rate in women with a singleton pregnancy and GDM. We may speculate that GDM women with twin pregnancies have a significantly higher overall risk of adverse neonatal outcomes leading to admission to NICU for closer monitoring and observation till their recovery. An alternative hypothesis could be that twin pregnancies are associated with an overall high risk which may be further increased by maternal GDM. Hence, the neonates might be admitted to the NICU irrespective of the development of complications for close monitoring [9].
The main strength of this review was the accurate methodology and comprehensive literature search. In addition, this review adds to the limited evidence available on the association between singleton & twin pregnancies with GDM and adverse maternal and neonatal outcomes. We found that the majority of the included studies were of better quality and had little to no outcome heterogeneity. This raises the evidence’s credibility and the findings’ external validity (generalizability). Sensitivity analysis revealed no discernible alteration in the magnitude or direction of study estimate.
However, there are some limitations to this study. For some of the outcomes, significant between-study variability was found. It is difficult to explore such significant heterogeneity by meta-regression because there are so few papers. Due to similar factors, we were unable to evaluate the probability of publication bias with any of the results. Since almost all of the investigations were retrospective, it was difficult to determine any causal relationships. The determination of the proper effect size and the development of evidence-based recommendations for the hospital context require further longitudinal research.
Despite the listed limitations, this meta-analysis has specific implications for the clinical practice. Women with twin pregnancy and GDM should be classified as a specific target group for providing focussed care during their antenatal period. These patients require more stringent follow-ups and high level of clinical care throughout their antenatal, natal and postnatal period. The neonates born out of these women also requires intensive targeted care throughout their neonatal period and intermittent follow-ups till they pass the infancy period.
Conclusions
The results presented here highlight how maternal outcomes like caesarean section and neonatal outcomes like SGA neonates, preterm delivery, respiratory morbidity, hyperbilirubinemia, and NICU admission are significantly greater in women with twin pregnancies and GDM. While several outcomes were similar between the two groups, a thorough understanding of the intervention strategies for women with twin pregnancies who are diagnosed with GDM is of great importance. Further, it is paramount that clinicians are aware of the significantly increased risk in GDM women with a twin pregnancy.
Supporting information
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Martin B, Sacks DA. The global burden of hyperglycemia in pregnancy—Trends from studies in the last decade. Diabetes Res Clin Pract. 2018;145: 17–19. doi: 10.1016/j.diabres.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 2.Alkaabi J, Almazrouei R, Zoubeidi T, Alkaabi FM, Alkendi FR, Almiri AE, et al. Burden, associated risk factors and adverse outcomes of gestational diabetes mellitus in twin pregnancies in Al Ain, UAE. BMC Pregnancy and Childbirth. 2020;20: 612. doi: 10.1186/s12884-020-03289-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz DB, Daoud Y, Zazula P, Goyert G, Bronsteen R, Wright D, et al. Gestational diabetes mellitus: metabolic and blood glucose parameters in singleton versus twin pregnancies. Am J Obstet Gynecol. 1999;181: 912–914. doi: 10.1016/s0002-9378(99)70324-8 [DOI] [PubMed] [Google Scholar]
- 4.Roach VJ, Lau TK, Wilson D, Rogers MS. The incidence of gestational diabetes in multiple pregnancy. Aust N Z J Obstet Gynaecol. 1998;38: 56–57. doi: 10.1111/j.1479-828x.1998.tb02958.x [DOI] [PubMed] [Google Scholar]
- 5.Ram M, Berger H, Lipworth H, Geary M, McDonald SD, Murray-Davis B, et al. The relationship between maternal body mass index and pregnancy outcomes in twin compared with singleton pregnancies. Int J Obes (Lond). 2020;44: 33–44. doi: 10.1038/s41366-019-0362-8 [DOI] [PubMed] [Google Scholar]
- 6.HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358: 1991–2002. doi: 10.1056/NEJMoa0707943 [DOI] [PubMed] [Google Scholar]
- 7.Ghai V, Vidyasagar D. Morbidity and mortality factors in twins. An epidemiologic approach. Clin Perinatol. 1988;15: 123–140. [PubMed] [Google Scholar]
- 8.Büscher U, Horstkamp B, Wessel J, Chen FC, Dudenhausen JW. Frequency and significance of preterm delivery in twin pregnancies. Int J Gynaecol Obstet. 2000;69: 1–7. doi: 10.1016/s0020-7292(99)00223-4 [DOI] [PubMed] [Google Scholar]
- 9.McGrath RT, Hocking SL, Scott ES, Seeho SK, Fulcher GR, Glastras SJ. Outcomes of twin pregnancies complicated by gestational diabetes: a meta-analysis of observational studies. J Perinatol. 2017;37: 360–368. doi: 10.1038/jp.2016.254 [DOI] [PubMed] [Google Scholar]
- 10.Kevat DAS, Sinha AK, McLean AG. Lower treatment targets for gestational diabetes: is lower really better? Med J Aust. 2014;201: 204–207. doi: 10.5694/mja14.00099 [DOI] [PubMed] [Google Scholar]
- 11.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of Treatment of Gestational Diabetes Mellitus on Pregnancy Outcomes. New England Journal of Medicine. 2005;352: 2477–2486. doi: 10.1056/NEJMoa042973 [DOI] [PubMed] [Google Scholar]
- 12.Ashwal E, Berger H, Hiersch L, Yoon EW, Zaltz A, Shah B, et al. Gestational diabetes and fetal growth in twin compared with singleton pregnancies. Am J Obstet Gynecol. 2021; S0002-9378(21)00454–3. doi: 10.1016/j.ajog.2021.04.225 [DOI] [PubMed] [Google Scholar]
- 13.Sheehan ACM, Umstad MP, Cole S, Cade TJ. Does Gestational Diabetes Cause Additional Risk in Twin Pregnancy? Twin Res Hum Genet. 2019;22: 62–69. doi: 10.1017/thg.2018.72 [DOI] [PubMed] [Google Scholar]
- 14.Weiner E, Barber E, Feldstein O, Schreiber L, Dekalo A, Mizrachi Y, et al. The placental component and neonatal outcome in singleton vs. twin pregnancies complicated by gestational diabetes mellitus. Placenta. 2018;63: 39–44. doi: 10.1016/j.placenta.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 15.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372: n160. doi: 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells G, Shea B, O’Connell D, Robertson J, Peterson J, Welch V, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta- Analysis.: 21. [Google Scholar]
- 17.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. [cited 22 Aug 2021]. Available: https://handbook-5-1.cochrane.org/ [Google Scholar]
- 18.Guillén-Sacoto MA, Barquiel B, Hillman N, Burgos MA, Herranz L. Metabolic syndrome and impaired glucose metabolism during early postpartum after twin pregnancies complicated by gestational diabetes mellitus: Is the risk comparable to singleton pregnancies? Diabetes Metab. 2019;45: 390–393. doi: 10.1016/j.diabet.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 19.Guillén-Sacoto MA, Barquiel B, Hillman N, Burgos MÁ, Herranz L. Gestational diabetes mellitus: glycemic control during pregnancy and neonatal outcomes of twin and singleton pregnancies. Endocrinol Diabetes Nutr (Engl Ed). 2018;65: 319–327. doi: 10.1016/j.endinu.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 20.Hiersch L, Berger H, Okby R, Ray JG, Geary M, McDonald SD, et al. Gestational diabetes mellitus is associated with adverse outcomes in twin pregnancies. Am J Obstet Gynecol. 2019;220: 102.e1-102.e8. doi: 10.1016/j.ajog.2018.10.027 [DOI] [PubMed] [Google Scholar]
- 21.Lai FY, Johnson JA, Dover D, Kaul P. Outcomes of singleton and twin pregnancies complicated by pre-existing diabetes and gestational diabetes: A population-based study in Alberta, Canada, 2005–11. J Diabetes. 2016;8: 45–55. doi: 10.1111/1753-0407.12255 [DOI] [PubMed] [Google Scholar]
- 22.Xue CY, Su RN, Yang HX. [Analysis of the maternal glucolipid metabolism in twin pregnancies complicated by gestational diabetes mellitus]. Zhonghua Fu Chan Ke Za Zhi. 2019;54: 741–746. doi: 10.3760/cma.j.issn.0529-567x.2019.11.005 [DOI] [PubMed] [Google Scholar]
- 23.Sankilampi U, Hannila M-L, Saari A, Gissler M, Dunkel L. New population-based references for birth weight, length, and head circumference in singletons and twins from 23 to 43 gestation weeks. Ann Med. 2013;45: 446–454. doi: 10.3109/07853890.2013.803739 [DOI] [PubMed] [Google Scholar]
- 24.Grantz KL, Grewal J, Albert PS, Wapner R, D’Alton ME, Sciscione A, et al. Dichorionic twin trajectories: the NICHD Fetal Growth Studies. Am J Obstet Gynecol. 2016;215: 221.e1-221.e16. doi: 10.1016/j.ajog.2016.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendez-Figueroa H, Truong VTT, Pedroza C, Chauhan SP. Growth among Twins: Use of Singleton versus Twin-Specific Growth Nomograms. Am J Perinatol. 2018;35: 184–191. doi: 10.1055/s-0037-1606381 [DOI] [PubMed] [Google Scholar]
- 26.Muhlhausler BS, Hancock SN, Bloomfield FH, Harding R. Are twins growth restricted? Pediatr Res. 2011;70: 117–122. doi: 10.1203/PDR.0b013e31821f6cfd [DOI] [PubMed] [Google Scholar]
- 27.Fox NS, Rebarber A, Klauser CK, Roman AS, Saltzman DH. Intrauterine growth restriction in twin pregnancies: incidence and associated risk factors. Am J Perinatol. 2011;28: 267–272. doi: 10.1055/s-0030-1270116 [DOI] [PubMed] [Google Scholar]
- 28.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115: e290–296. doi: 10.1542/peds.2004-1808 [DOI] [PubMed] [Google Scholar]
- 29.McLean M, Chipps D, Cheung NW. Mother to child transmission of diabetes mellitus: does gestational diabetes program Type 2 diabetes in the next generation? Diabet Med. 2006;23: 1213–1215. doi: 10.1111/j.1464-5491.2006.01979.x [DOI] [PubMed] [Google Scholar]
- 30.Tam WH, Ma RCW, Yang X, Ko GTC, Tong PCY, Cockram CS, et al. Glucose intolerance and cardiometabolic risk in children exposed to maternal gestational diabetes mellitus in utero. Pediatrics. 2008;122: 1229–1234. doi: 10.1542/peds.2008-0158 [DOI] [PubMed] [Google Scholar]
- 31.Blondeau B, Joly B, Perret C, Prince S, Bruneval P, Lelièvre-Pégorier M, et al. Exposure in utero to maternal diabetes leads to glucose intolerance and high blood pressure with no major effects on lipid metabolism. Diabetes Metab. 2011;37: 245–251. doi: 10.1016/j.diabet.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 32.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab. 2009;94: 2464–2470. doi: 10.1210/jc.2009-0305 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.