Figure 1. Reconstitution of α-syn secretion regulated by palmitoylated DNAJC5 in HEK293T cells.
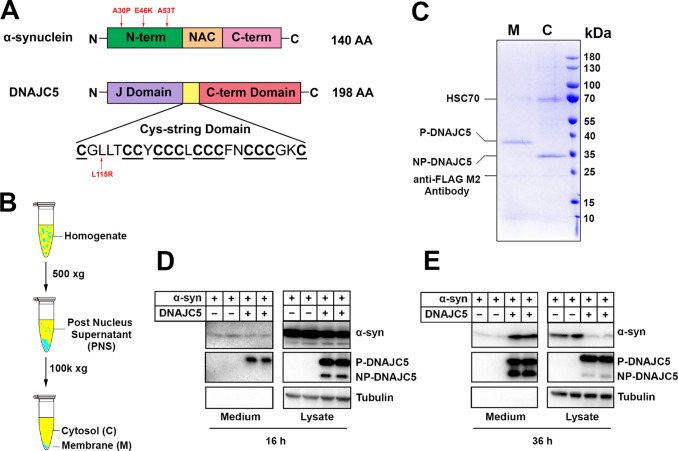
(A) Schematic diagrams of α-syn and DNAJC5. Domains are highlighted in different colors. Red arrows indicate known disease-causing mutations on each protein. (B) Membrane and cytosol fractionation scheme. Briefly, homogenized HEK293T cells were centrifuged at low speed to prepare a post-nuclear supernatant (PNS). High-speed centrifugation was then performed to separate the sedimentable membrane (M) from cytosol (C). (C) Partition of palmitoylated DNAJC5 (P-DNAJC5) and non-palmitoylated DNAJC5 (NP-DNAJC5) between the membrane (M) and cytosol (C) fractions. DNAJC5 was immunoprecipitated from cytosol and membrane with anti-FLAG resin and evaluated by Coomassie-blue stained SDS-PAGE. (D) α-syn secretion 16 h after transfection. The secretion of P-DNAJC5 in the medium was detected. (E) α-syn secretion 36 h after transfection. NP-DNAJC5 was also secreted in the medium together with α-syn.