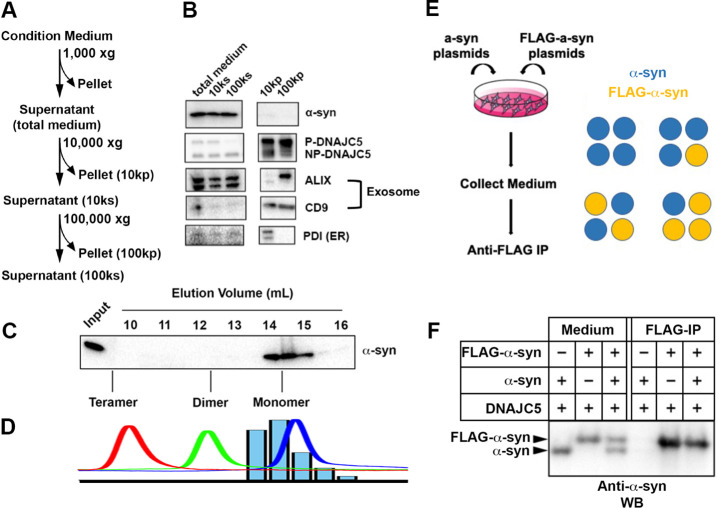
Figure 2. Characterization of secreted α-syn.
(A) Medium fractionation scheme. (B) Secreted α-syn was soluble. Differential centrifugation was performed with conditioned medium from HEK293T cells transfected with DNAJC5 and α-syn. Alix and CD9, exosome markers. PDI, an endoplasmic reticulum (ER) marker, was used as exosome-negative control. (C) Gel filtration fractionation of medium. Conditioned medium was concentrated and subjected to gel filtration fractionation. Fractions were evaluated by anti-α-syn immunoblot. (D) Chromatograms of tandem α-syn monomer (blue curve), dimer (green curve), and tetramer (red curve) were overlaid. In comparison, the relative intensity of secreted α-syn in each fraction was plotted as blue bars. (E) Schematic diagram of co-immunoprecipitation (co-IP) of secreted α-syn and FLAG-α-syn. Shown here is possible interaction between α-syn (blue circle) and FLAG-α-syn (yellow circle) in a representative tetrameric conformation. (F) Anti-FLAG immunoprecipitation (FLAG-IP) of media from cells transfected with indicated plasmids. Both the medium input and FLAG-IP samples were evaluated with anti-α-syn immunoblot (anti-α-syn WB).