Abstract
To study the utility of in vitro-polarized intestinal cell monolayers for modeling Vibrio cholerae-host cell interactions, we added live V. cholerae bacteria to the apical surfaces of polarized T84 cell monolayers and monitored changes in electrical properties. We found that both classical and El Tor strains produce cholera toxin after addition to the monolayer, but induction is most likely due to medium components rather than bacterium-cell interactions. We also found that the RTX toxin is produced by El Tor strains. This toxin caused a loss of the barrier function of the paracellular tight junction that was measured as a decrease in transepithelial resistance. This decrease occurred when bacteria were added to either the apical or basolateral surfaces, indicating that the RTX toxin receptor is expressed on both surfaces. These results are discussed with regard to the applicability of the polarized T84 cell monolayers as an in vitro model of host-pathogen interactions.
Vibrio cholerae is a devastating bacterial pathogen capable of causing pandemic diarrheal disease. The major virulence factor responsible for the severe diarrhea of cholera disease is cholera toxin (CT). CT is an A-B subunit toxin composed of five CtxB subunits that facilitate binding of the toxin to the GM1-ganglioside receptor. After toxin binding, the catalytic moiety CtxA is translocated into the target cell. Within the cell, CtxA ADP-ribosylates Gα protein, leading to constitutive production of the second messenger cyclic 3′,5′-AMP (cAMP) by adenylate cyclase. This up-regulation of cAMP signals the opening of chloride ion channels and a subsequent net loss of salt and water from the intestines. Hence, the cholera victim perishes from dehydration (16, 30).
Production of CT is regulated by a two-component regulatory system composed of a sensor kinase, ToxR, and a response regulator, ToxT. A third component of the regulator system is the modulator ToxS (2, 31). Of keen interest are the environmental stimuli that lead to induction of the toxR regulon. In vitro, classical strains are induced in Luria broth (LB) and grown at 30°C, 66 mM NaCl, and pH 6.5 (31), while El Tor strains are induced in the specialized medium AKI (13).
In vivo, the cue for synthesis of CT appears to be quite distinct. CT synthesis is apparently initiated after the bacteria adhere to the epithelium, suggesting that contact with intestinal cells signals the bacterium to produce CT (18).
The intestine is a complex environment in which many host factors and signal transduction events could contribute to the stimulation of CT production and many structures could be involved in adherence of the bacterium to the host cell. We would like to develop an in vitro model to mimic the in vivo observations to study these processes in a controlled environment.
Recent efforts in our lab have utilized polarized T84 intestinal epithelial cells to study V. cholerae host-pathogen interactions. T84 cells are a human colonic cell line that can be grown in a Transwell to form a model intestinal epithelial monolayer (22). This model has been used to study other pathogens, including pathogenic Escherichia coli, Helicobacter pylori, Neisseria gonorrhoeae, and Salmonella enterica serovar Typhimurium (9, 11, 12, 23).
Unfortunately, the development of in vitro systems for the study of V. cholerae has been hampered by the large number of proteases and toxins exported by V. cholerae that affect tissue culture cells. In a recent study, we measured the electrical response of a T84 monolayer to the addition of culture supernatant preparations from various V. cholerae vaccine strains (26). We found that the secreted metalloprotease hemagglutinin/protease (HA/protease) in culture supernatant fluids of El Tor and O139 strains causes a loss of transepithelial resistance (TER) across a T84 intestinal monolayer (26), consistent with earlier studies using polarized MDCK cells (33). V. cholerae strains with a deletion in the gene for HA/protease did not export proteins that affect the integrity of the T84 monolayer and thus had little effect on the electrical properties of the monolayer (26).
However, the potential exists that toxins expressed not in broth media but in response to culture conditions or the cells themselves will have a deleterious impact on the polarized monolayer when viable bacteria are added to the cells. One toxin that may have a detrimental effect on the monolayer is the newly discovered RTX toxin (21). This toxin is produced by El Tor and O139 strains, but not by classical strains (21). We have shown that expression of this toxin is essential for depolymerization of cellular actin by an unknown mechanism involving covalent linkage of actin monomers (6). Other toxins that cause actin depolymerization have been shown to cause a breakdown of paracellular tight junctions that maintain the stability of the monolayer, indicating that the RTX toxin might have a similar effect on T84 cells (10).
In this study we sought to adapt the T84 system for the in vitro analysis of V. cholerae-intestinal cell interactions. In the course of our efforts, we discovered that both classical and El Tor strains elicit dramatic electrical changes in the monolayer due to the expression of toxin genes. We find that both classical and El Tor strains express CT in response to culture conditions. This production of CT by V. cholerae can be measured directly in the T84 cells by an increase in short-circuit current (Isc) and a concurrent decrease in TER as Cl− channels are opened. A different electrical response predominated when El Tor strains were added to T84 cell monolayers. RTX toxin expression could be detected as a decrease in the TER that resulted from loosening of the paracellular tight junctions of the monolayer.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Bacterial strains used in this study are listed in Table 1. ΔhapA mutations were introduced into the El Tor strains using the sacB-based counterselectable plasmid pCVDHapSal1 as previously described (3, 5). ΔrtxCABD mutations were introduced by first amplifying the DNA flanking the naturally occurring 7.9-kb deletion in O395 (21) and then cloning the fragment into the sacB vector pWM91 (27). The mutation was transferred into El Tor strains by recombination followed by sacB-based counterselection as previously described (5).
TABLE 1.
V. cholerae strains used in this study
| Strain | Genotypea | Toxin production
|
Reference | ||
|---|---|---|---|---|---|
| CT | RTX | HAPb | |||
| Classical | |||||
| O395 | Wild type, Stpr | + | − | − | 24 |
| O395N1 | O395 ΔctxA | − | − | − | 25 |
| O395NT | O395 ΔctxAB | − | − | − | 25 |
| KKV62 | O395 ΔtoxR | − | − | − | 29 |
| KKV163 | O395 ΔtoxT | − | − | − | 29 |
| E1 Tor | |||||
| N16961 | Inaba wild type, Stpr | + | + | + | 5 |
| E7946 | Ogawa wild type, Stpr | + | + | + | 24 |
| Bah1 | E7946 Δcore | − | + | + | 32 |
| Bah1P | E7946 Δcore ΔhapA | − | + | − | 6 |
| Bah2P | E7946 ΔattRS ΔhapA | − | − | − | 6 |
| P27459 | Inaba wild type | + | + | + | 24 |
| Bang1P | P27459 Δcore ΔhapA | − | + | − | This study |
| Bang2P | P27459 ΔattRS ΔhapA | − | − | − | This study |
| P4 | P27459 ΔctxAB | − | + | + | 8 |
| KFV82 | P27459 ΔhapA ΔrtxCABD | + | − | − | This study |
| KFV105 | P27459 ΔctxAB ΔhapA ΔrtxCABD | − | − | − | This study |
Δcore mutations have deletion of orfU, ace, cep, zot, and ctxAB from the CTXΦ core region (32). ΔattRS refers to deletion of the entire CTXΦ prophage and flanking DNA, including the 3′ end of the rtxA gene (21, 32) ΔrtxCABD is identical to the naturally occurring deletion of classical strain O395 (21).
HA/protease.
LB, AKI (20), and T84 medium (low-glucose Dulbecco's modified Eagle's medium mixed 1:1 with Ham's F-12 nutrient mixture and glutamine and supplemented with 0.014 M sodium carbonate and 0.015 M HEPES) were used to grow bacteria as appropriate.
Addition of bacteria to polarized T84 epithelial cells.
T84 cells (passages 75 to 90) were cultured in collagen-treated commercially available 0.33-cm2 Transwell inserts (Costar Laboratories, Cambridge, Mass.) as previously described in T84 medium containing 6% newborn calf serum (20). Experiments were performed 10 to 12 days after plating, when resistances consistently reached >1,000 Ω cm2. Cells were rinsed in Hanks' balanced salt solution containing CaCl2 and were transferred into T84 medium without serum or antibiotics. Transwells were kept without CO2 at 37°C on a plate warmer or in an incubator for the course of the experiments. Isc was measured at 10- to 15-min intervals using a dual voltage clamp device and 25-μA current pulses, and the resistances were calculated according to Ohm's law as previously described (20). V. cholerae cultures were grown overnight at 30°C in LB, washed twice in phosphate-buffered saline (PBS), and diluted to 109 CFU per ml. After at least three baseline electrical readings, 10 μl of PBS-washed bacteria was pipetted into the apical chamber of the Transwell. In all experiments, 10 μl of PBS was used as a negative control. Experiments were terminated when the TER of PBS control monolayers decreased by over 20% or after 300 min, when acidification of the medium by bacterial growth compromised the integrity of the monolayers.
Dextran flux studies.
The electrical properties of infected monolayers were monitored until the TER of V. cholerae-infected cells dropped to <500 Ω · cm2. Fluorescein dextran (200 ng, 3,000 Da; Molecular Probes) was then added to the apical chamber. Paracellular transfer of dextran was measured by sampling 50 μl from the basolateral chamber at 15-min intervals for a total of 90 min. Fresh medium (50 μl) was added to the basolateral chamber to restore a 1-ml volume. The amount (in picograms) of dextran in the 50-μl sample was determined with a Millipore (Bedford, Mass.) Cytofluor 2300 fluorescent plate reader (excitation, 485 nm; emission, 530 nm) using fluorescein dextran to establish a standard curve, and values were then adjusted for volume difference due to sampling. The rate of flux (picograms per minute) was determined from the slope of the linear curve of six time points. Values were normalized to picograms per hour per square centimeter.
Measurement of CT concentration.
Growth in LB and AKI was carried out as previously described for in vitro induction of CT (7, 13). For growth in T84 medium, 50 μl of washed bacterial culture was added to 1 ml of T84 medium without serum in a 24-well dish. The plate was incubated at 37°C for 4 h to mimic the T84 cell culture conditions. Concentration of CT in the T84 culture supernatant fluid was measured by the GM1-ganglioside enzyme-linked immunosorbent assay as previously described (7).
RESULTS
Effect of O395 and N16961 on polarized T84 intestinal epithelial monolayers.
Polarized T84 cells monolayers maintained at 37°C in T84 medium without serum were stable, with resistance values of >1,000 Ω · cm2 for about 5 h. For mock-treated control cells, a change in Isc was not generally observed and a 20% decrease in resistance was measured after 4 to 5 h (data not shown). The use of T84 medium without serum or antibiotics in place of HBSS in these experiments permitted growth of the bacteria, so that any observed effects would be due to bacterial interactions or de novo export of proteins.
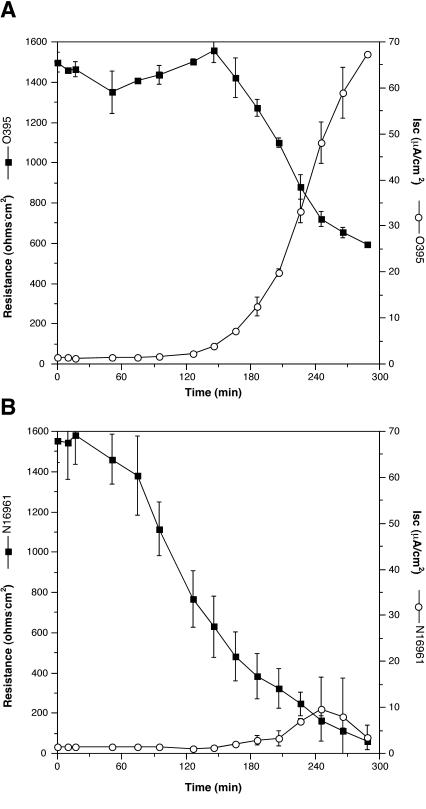
As an initial experiment, prototypical wild-type V. cholerae strains of the El Tor biotype, N16961, and the classical biotype, O395, were used. Both strains elicited responses from the cells, but the responses were different (Fig. 1).
FIG. 1.
Responses of T84 polarized monolayers to the addition of V. cholerae differ between classical and El Tor strains. PBS-washed classical strain O395 (A) and El Tor strain N16961 (B) were added to the apical chamber after the first three readings were recorded. Values are means and standard deviations of triplicates in a single experiment.
Classical strain O395 elicited a strong increase in the Isc (Fig. 1A). The measurable Isc peaked at 65 μA/cm2 just prior to the termination of the experiment. An inversely proportional loss of TER was also measured (Fig. 1A). The increase in Isc occurred about 100 min after the addition of the bacteria, suggesting that synthesis of a protein was essential for elicitation of this T84 cellular response.
The El Tor strain N16961 elicited a dramatically different response. Only a slight increase in the Isc was observed late in the experiment (Fig. 1B). However, after only 60 min, a decrease in TER was observed that was markedly greater than the decrease observed in the monolayers infected with the classical strain (Fig. 1).
Thus, our initial experiment shows that both classical and El Tor strains elicit a decrease in the TER but only the classical strain induces an increase in the Isc. The differences in electrical properties and the 40-min discrepancy in the time to initiation of the change implicates two distinct factors.
Increase in Isc is due to expression of CT by O395.
Purified CT has been shown to cause an increase in Isc with a concurrent decrease in TER when added to the apical surfaces of T84 cells (20). These responses are due to the opening of membrane ion-conducting channels following up-regulation of cAMP production by CT. The Cl− ion-secretory response following channel opening is measured as an increase in current (Isc), while the simultaneous decrease in resistance corresponds to current passing through the open channels of the apical and basolateral membranes.
To see if we were detecting CT production by the classical strain O395 after addition to cells, we examined the mutant O395N1, a derivative of O395 bearing a deletion in the gene ctxA, which encodes the catalytic subunit of CT (Table 1). O395N1 did not elicit the increase in Isc, indicating that the catalytic action of CT causes the increase in Isc when O395 is incubated with T84 cells (Table 2). This observed increase was not due to inefficient removal of CT after overnight growth in LB, because addition of chloramphenicol to block de novo protein synthesis inhibited the stimulation of Isc by 81% (Table 2). In addition, deletion of either toxR or toxT from O395 decreased the Isc 84 and 90%, respectively, indicating that induction of CT occurs through the stimulation of the ToxR-ToxT two-component regulatory system (Table 2). In all cases where the Isc was inhibited, the concurrent drop in TER was also inhibited (Table 2), consistent with the observed changes in Isc being caused by opening of ion channels due to production of CT by the classical V. cholerae strain.
TABLE 2.
Changes in Isc and TER elicited by classical V. cholerae strains is due to de novo synthesis of CT
| Strain or treatment | Relevant genotype | Isc (μA/cm2)a | TER (% of initial) |
|---|---|---|---|
| Saline | 1.4 ± 0.1 | 99 ± 15 | |
| O395 | Classical wild type | 59 ± 6 | 44 ± 2 |
| O395N1 | ctxA | 2.0 ± 0.4 | 146 ± 22 |
| KKV62 | toxR | 9.6 ± 2.0 | 110 ± 12 |
| KKV163 | toxT | 5.9 ± 1.6 | 137 ± 17 |
| O395 + 5 μg of chloramphenicol/ml | No bacterial protein synthesis | 11 ± 1.6 | 93 ± 9 |
| 0.12 nM purified CT | 33 ± 4.5 | 48 ± 8 |
Isc and TER for bacterial mutants were measured at 266 min (206 min after addition of washed cultures) in the experiment shown in Fig. 1A. Isc and TER for addition of purified CT were measured at 195 min after addition in a separate experiment. Data are means and standard deviations of duplicates from a single experiment.
CT production is induced in T84 medium.
These results could indicate that we were detecting synthesis of CT stimulated by contact with the target cell or by a component of the T84 medium. O395 and O395NT, a mutant of O395 with both ctxA and ctxB deleted (Table 1), were grown statically in 1 ml of T84 medium at 37°C for 4 h to mimic incubation temperature and aeration of the tissue culture system. The secretion of CT into the culture medium was measured by the GM1-ganglioside enzyme-linked immunosorbent assay that detects the presence of the CtxB subunit of CT in supernatant fluids. After 4 h of static incubation, the concentration of CT in the T84 medium without serum reached 15.7 nM. This amount is about 10-fold lower than the concentration of CT when O395 is grown overnight in LB under toxin-inducing conditions (Table 3). However, addition of only 0.12 nM CT is sufficient for an Isc response that reaches a maximum at 33 μA/cm2 (Table 2). Thus, the amount of toxin produced in T84 medium alone is sufficient to cause the Isc response observed when bacteria are added to the cells. These results suggest that a component of the medium, rather than cell contact, causes induction of CT in classical strains at 37°C.
TABLE 3.
Concentration of CT in culture media after growth in inducing conditions or in T84 medium
| Straina | Growth conditions | [CT] (nM)b |
|---|---|---|
| O395 | LB, 30°C, overnight, rolling | 205 |
| O395NT | LB, 30°C, overnight, rolling | 0 |
| O395 | T84 medium, 37°C, 4 h, static | 15.7 |
| O395NT | T84 medium, 37°C, 4 h, static | 0 |
| P27459 | AKI, 37°C, overnight, shaking | 11 |
| P4 | AKI, 37°C, overnight, shaking | 0 |
| P27459 | T84 medium, 37°C, 4 h, static | 0.060 |
| P4 | T84 medium, 37°C, 4 h, static | 0 |
O395NT and P4 are derivatives of O395 and P27459, respectively, with deletions of ctxA and ctxB genes.
CT was measured by the GM1-ganglioside enzyme-linked immunosorbent assay with commercially available CT (Sigma) used for a standard curve.
The loss of resistance in El Tor strains is not due to Zot, Ace, CT, or HA/protease.
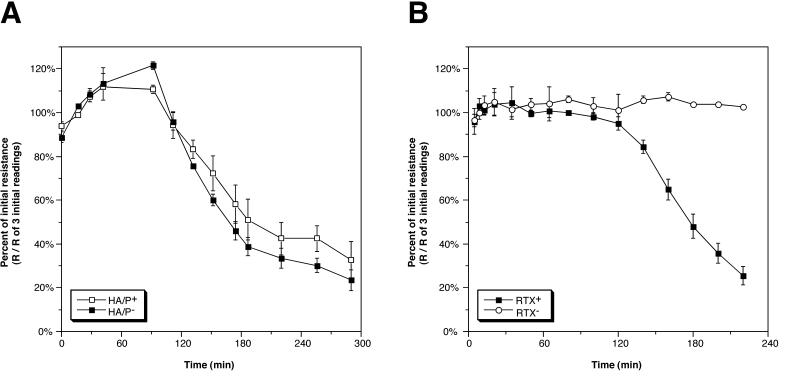
To assess the cause of the loss of resistance when the El Tor strain was added to cells, we first examined Bah1, an El Tor strain with a core deletion removing genes for putative accessory toxins Zot and Ace and the genes for CT (Table 1). The wild-type parent of Bah1, E7946, behaved similarly to N16961, eliciting a drop in TER without a corresponding increase in Isc (data not shown). Strain Bah1 elicited a drop in resistance similar to that observed for its wild-type parent, E7946, indicating that loss of the toxins Zot, Ace, and CT was not responsible for this observed drop in resistance (Fig. 2A and data not shown).
FIG. 2.
Changes in TER after addition of V. cholerae El Tor bacteria are due to RTX toxin, not HA/protease. (A) V. cholerae Bah1 (HA/protease+ [HA/P+]) is compared to Bah1P (HA/P−). (B) Bah1P (RTX+) is compared to Bah2P (RTX−). Values were derived by dividing the recorded resistance (R) value by the mean of three initial resistance values recorded before addition of bacteria and are means and standard deviations of triplicates in a single experiment.
HA/protease in V. cholerae culture supernatants causes a loss of TER across polarized T84 cell monolayers (26) by cleavage of the tight junction protein occludin (34). To see if the loss of resistance we observed when V. cholerae was added to T84 cells was due to production of HA/protease, we tested Bah1P, a derivative of Bah1 with a deletion in the gene that encodes HA/protease. This strain also showed a loss in resistance identical to that observed for Bah1, indicating that the drop is not due to production of HA/protease (Fig. 2A). This result is not surprising, since hapA, the gene for HA/protease, is under stationary-phase control via an autoinduction system (14) and would not be expected to be expressed in the time frame of these experiments.
Loss of resistance is due to the V. cholerae RTX toxin.
We have recently shown that the RTX toxin of V. cholerae causes depolymerization of actin in a variety of tissue culture cell lines (6). Other toxins that target actin have been shown to cause a loss of TER in T84 cell monolayers without a proportional increase in Isc (10). To test if RTX toxin is causing the loss of TER, Bah2P, which bears a deletion in the 3′ end of rtxA in addition to the core deletion, was tested on T84 cells. This strain did not elicit a decrease of TER (Fig. 2B). Indeed, this strain frequently causes about a 10 to 20% increase in TER over the course of the assay.
The drop in TER was not observed in the presence of the bacterial protein synthesis inhibitor chloramphenicol, indicating that de novo synthesis of the RTX toxin by V. cholerae is required. Addition of the eukaryotic protein synthesis inhibitor cycloheximide (50 μg/ml) did not affect the drop in resistance, indicating that protein synthesis by the host cell is not required for RTX activity (data not shown).
Thus, the loss of TER in T84 cell monolayers when a V. cholerae El Tor strain is added to the apical surface is due to de novo synthesis of RTX toxin.
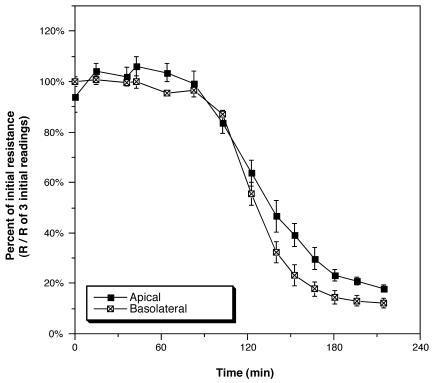
The receptor for RTX toxin is both apically and basolaterally exposed.
The sensitivity of T84 cells to some toxins, including anthrax toxin, is surface dependent. In the case of anthrax toxin, the appropriate receptor is present only on the basolateral surface (1). To examine the polarity localization of the RTX receptor, Bah1P was added to either the apical or the basolateral chamber in parallel samples (Fig. 3). Addition to the basolateral side did not change the pattern of loss of TER relative to addition to the apical surface. In addition, no new toxicities were detected when the RTX− strain Bah2P was added to the basolateral chamber (data not shown). Thus, the receptor for the RTX toxin is present on both surfaces of polarized intestinal cells.
FIG. 3.
RTX toxin is active when bacteria are added to either the apical or basolateral chamber. PBS-washed Bah1P (10 μl) was added to the apical chamber or Bah1P (50 μl) was added to the basolateral chamber. Both additions correspond to a 1:20 dilution in the total volume of the chamber. Values were derived by dividing the recorded resistance (R) value by the mean of three initial resistance values recorded before addition of bacteria and are means and standard deviations of triplicates in a single experiment.
The RTX toxin affects tight junctions.
Loss of TER by a T84 cell monolayer can occur for a variety of reasons, including opening of membrane channels or pores, breakdown of the tight junction between cells, and cell death. Loss of TER due to channel opening is generally accompanied by an increase in Isc. Since stimulation of Isc is not observed in the presence of the RTX toxin, and RTX toxin does not cause cell death (6), we investigated changes in the permeability of the paracellular tight junctions.
Fluorescein dextran is not membrane permeative, and thus, its transit across a polarized epithelial layer from the apical to the basolateral chamber must be paracellular. The flux rate of a 3,000-Da fluorescein dextran molecule across the T84 monolayer after incubation of cells with rtxA+ strain Bah1P (1,170 ± 211 pg/h/cm2) was 6.6-fold greater than the flux rates for the rtxA− strain Bah2P and uninoculated cells (175 ± 29 and 176 ± 60 pg/h/cm2, respectively). Thus, T84 cells incubated with V. cholerae undergo a breakdown of the barrier function of the paracellular tight junction.
CT induction in El Tor strains.
El Tor strains produce CT in vitro only under specialized AKI growth conditions (13). Since CT was produced by O395 cultured statically in T84 medium, we asked if an El Tor strain would also stimulate CT under these conditions. Such an observation might be expected, since El Tor strains are known to produce CT when grown without shaking in AKI (13). For this experiments we used the El Tor Inaba strain P27459 as the parent. This strain produced 11 nM CT when grown in AKI but only 60 pM when grown statically in T84 medium, representing nearly a 200-fold decrease (Table 3).
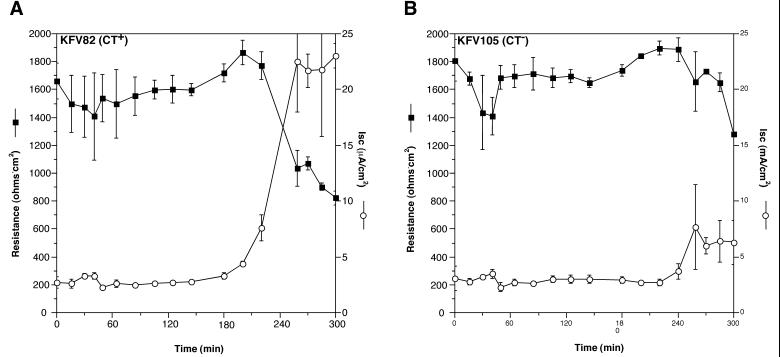
In order to measure CT production on T84 cells, appropriate El Tor genetic backgrounds that cannot produce RTX or HA/protease were constructed. Strain KFV82 is a derivative of P27459 with deletions in hapA and a deletion in the rtx locus that eliminates RTX toxin (Table 1). This strain elicited an increase in Isc to 27 μA/cm2 late in the experiment (Fig. 4A). This change in Isc is about threefold greater than the increase the Isc observed for the isogenic ctxAB deletion mutant KFV105 (Fig. 4B). Thus, the stimulation of Isc by KFV82 is due primarily to CT, and the magnitude of the increase is consistent with the amount of CT produced in T84 medium alone.
FIG. 4.
CT expression in El Tor V. cholerae. Both KFV82 (A) and KFV105 (B) have deletions in the rtx locus and in hapA eliminating factors that disrupt integrity of the paracellular tight junctions. The effect of CT expression on TER and Isc in a CT-positive strain (A) is shown in contrast to results with a ΔctxAB isogenic mutant (B). All values are means and standard deviations from triplicates in a single experiment.
DISCUSSION
In vitro study of host-bacterium interactions requires assembly of a controlled system in which all potential factors can be monitored and manipulated. Such systems have proven both illuminating and problematic for the study of V. cholerae pathogenesis. This study was initiated in hopes of developing an in vitro system for the study of V. cholerae-host communication and adherence. It has been proposed that some V. cholerae genes, including the ctx genes, are induced following colonization of the intestinal epithelium in vivo (18), and we hoped to mimic that interaction in a polarized cell line. To our surprise, V. cholerae does elicit a number of cellular responses upon coincubation with T84 cells, although they were quite unexpected.
The first important observation of this study is the production of CT in vitro within the 4-h time course of these experiments. Induction of CT production was most obvious for the classical strain (Fig. 1A) but was also detectable in the El Tor strain once other toxins were eliminated by mutagenesis (Fig. 4). However, this induction could not be ascribed to contact with epithelial cells, as might be suggested by in vivo experimentation, because in vitro culture conditions also induce toxin production (Table 3). The induction of the CT genes in T84 medium was unexpected, since the conditions used (37°C, 100 mM NaCl, and pH 7.4) are generally considered nonpermissive for induction of the ToxR regulon (7).
The question of what factor in the T84 medium or what environmental growth conditions affected stimulation of CT expression now arises. One possibility is that the ToxR regulon is regulated by a chemical component of the tissue culture medium. Several bacterial membrane signal transduction proteins have been shown to directly respond to small molecules. Some examples include the binding of aspartate by the chemotaxis methyl-accepting protein Tar to stimulate methylation of the receptor (28), the binding of the homoserine lactone of Vibrio harveyi by LuxN to initiate a phosphorelay essential for induction of the luminescence genes (4), and the binding of acetosyringone by the VirA protein of Agrobacterium tumefaciens to initiate transcription of genes necessary for T-DNA transfer (19). In each of these cases, association of the regulator with the small molecule leads to enhanced production of the corresponding genetic regulon. However, a small molecule has never been found to play a role in V. cholerae gene induction, although only complex media such as LB and AKI have been utilized in the past. If such a small molecule is essential for ToxR stimulation, it should be unveiled by careful dissection of the chemically defined T84 medium, since addition of serum was found to not be essential for CT induction.
Another possible inducer of CT production under the T84 test conditions is the microaerophilic environment presented by the Transwell. Several Salmonella intestinal pathogens have virulence genes controlled by such conditions, including the invasion genes of S. enterica serovars Choleraesuis and Typhimurium (17). As in the Salmonella studies, the induction of CT under controlled oxygen concentration could be demonstrated if decreased aeration is an important contributing factor.
Regardless of the mechanism, it is both intriguing and confounding that CT is induced under these culture conditions. Induction in a defined medium has never been observed, and yet the induction within the medium alone complicates use of the T84 system for study of host contact-induced CT production. Defining the precise environmental cue that V. cholerae responds to under these conditions will be necessary before such a system can be adequately developed.
The second important observation of this study is the loss of the integrity of the T84 cell monolayer dependent on production of the RTX toxin by V. cholerae El Tor strains. This loss of TER was not due to channel opening, as indicated for the CT response, but rather due to loosening of the paracellular tight junctions, as indicated by an increase in passive transcellular diffusion of a membrane-impermeative solute (see Results). At this time our evidence linking the RTX toxin to changes in the intestinal cell tight junctions is limited to a comparison of mutants with and without the rtxA gene. Further data to demonstrate the role of the RTX toxin in this activity would require purification of the toxin. However, the enormous size of the toxin (an estimated 484,000 Da) has proven to be a significant technical hurdle. Yet our results are similar to those obtained with the Rho-modifying toxin Clostridium difficile toxin A, indicating that the actin-depolymerizing RTX toxin affects polarized T84 cells in a similar manner even though the mechanisms of actin depolymerization in these two toxins are distinct (6, 10, 15).
The effect of the RTX toxin was surprising, since we had previously observed that V. cholerae culture supernatant fluids devoid of HA/protease elicited little change in the TER of a T84 polarized cell monolayer (26). Since the RTX toxin is secreted (6), it should have been active and detectable in the supernatant fluids used in the previous study. The difference in the conclusions of these two studies likely lies in the growth phase-dependent expression of both RTX and HA/protease. RTX activities have only been detected in log-phase culture supernatants and have never been detected in the culture supernatant fluids of stationary-phase cultures similar to those used in the prior investigation. However, the system used in this study depended on detection of toxins produced during logarithmic growth in tissue culture medium. Conversely, HA/protease is regulated by autoinduction and is not produced until stationary phase (14), and thus, it would not be produced in the system used here. Hence, the experimental conditions necessary to unveil the role of both of these toxins depend on growth conditions. These data indicate that other toxins may also affect the integrity of the tight junctions but they simply are not produced under any test conditions applied to date.
As indicated above, this study was designed in part to identify a genetic background and a set of growth conditions that would be amenable to the study of V. cholerae interactions with polarized intestinal cells. We have successfully demonstrated that V. cholerae can be genetically manipulated to produce strains that do not compromise intestinal monolayers. For the classical strains, O395N1, a ctxA deletion mutant of O395, was electrically neutral in the experiments, demonstrating little change in both Isc and TER. Similarly, the combination of El Tor mutants leading to loss of HA/protease, RTX, and CT expression showed only slight effects on TER and Isc late in the experiment. These genetically modified variants of V. cholerae can now be used for further study of host-cell interactions without further concerns of monolayer disruption.
ACKNOWLEDGMENTS
We thank M. Ferguson-Maltzman for her technical assistance and the Lencer lab members for their helpful suggestions. S. Colgan is thanked for his assistance on the flux studies.
This work was supported by NIH grants AI-18045 to J.J.M and DK-48106 to W.I.L. K.J.F. was supported by NRSA postdoctoral fellowship AI-10385.
REFERENCES
- 1.Beauregard K E, Wimer-Mackin S, Collier R J, Lencer W I. Anthrax toxin entry into polarized epithelial cells. Infect Immun. 1999;67:3026–3030. doi: 10.1128/iai.67.6.3026-3030.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotter P A, DiRita V J. Bacterial virulence gene regulation: an evolutionary perspective. Annu Rev Microbiol. 2000;54:519–565. doi: 10.1146/annurev.micro.54.1.519. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein R A, Boesman-Finkelstein M, Chang Y, Häse C C. Vibrio cholerae hemagglutinin/protease, colonial variation, virulence, and detachment. Infect Immun. 1992;60:472–478. doi: 10.1128/iai.60.2.472-478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freeman J A, Lilley B N, Bassler B L. A genetic analysis of the functions of LuxN: a two-component hybrid sensor kinase that regulates quorum sensing in Vibrio harveyi. Mol Microbiol. 2000;35:139–149. doi: 10.1046/j.1365-2958.2000.01684.x. [DOI] [PubMed] [Google Scholar]
- 5.Fullner K J, Mekalanos J J. Genetic characterization of a new type IV pilus gene cluster found in both classical and El Tor biotypes of Vibrio cholerae. Infect Immun. 1999;67:1393–1404. doi: 10.1128/iai.67.3.1393-1404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fullner K J, Mekalanos J J. In vivo covalent crosslinking of actin by the RTX toxin of Vibrio cholerae. EMBO J. 2000;19:5315–5323. doi: 10.1093/emboj/19.20.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardel C L, Mekalanos J J. Regulation of cholera toxin by temperature, pH, and osmolarity. Methods Enzymol. 1994;235:517–526. doi: 10.1016/0076-6879(94)35167-8. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg I, Mekalanos J J. Cloning of the Vibrio cholerae recA gene and construction of a Vibrio cholerae recA mutant. J Bacteriol. 1986;165:715–722. doi: 10.1128/jb.165.3.715-722.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hecht G, Koutsouris A. Enteropathogenic E. coli attenuates secretagogue-induced net intestinal ion transport but not Cl− secretion. Am J Physiol. 1999;276:G781–G788. doi: 10.1152/ajpgi.1999.276.3.G781. [DOI] [PubMed] [Google Scholar]
- 10.Hecht G, Pothoulakis C, LaMont J T, Madara J L. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Investig. 1988;82:1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofman V, Ricci V, Galmiche A, Brest P, Auberger P, Rossi B, Boquet P, Hofman P. Effect of Helicobacter pylori on polymorphonuclear leukocyte migration across polarized T84 epithelial cell monolayers: role of vacuolating toxin VacA and cag pathogenicity island. Infect Immun. 2000;68:5225–5233. doi: 10.1128/iai.68.9.5225-5233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hopper S, Wilbur J S, Vasquez B L, Larson J, Clary S, Mehr I J, Seifert H S, So M. Isolation of Neisseria gonorrhoeae mutants that show enhanced trafficking across polarized T84 epithelial monolayers. Infect Immun. 2000;68:896–905. doi: 10.1128/iai.68.2.896-905.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwanaga M, Yamamoto K, Higa N, Ichinose Y, Nakasone N, Tanabe M. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol Immun. 1986;30:1075–1083. doi: 10.1111/j.1348-0421.1986.tb03037.x. [DOI] [PubMed] [Google Scholar]
- 14.Jobling M G, Holmes R K. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol Microbiol. 1997;26:1023–1034. doi: 10.1046/j.1365-2958.1997.6402011.x. [DOI] [PubMed] [Google Scholar]
- 15.Just I, Wilm M, Selzer J, Rex G, von Eichel-Steiber C, Mann M, Aktories K. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J Biol Chem. 1995;270:13932–13936. doi: 10.1074/jbc.270.23.13932. [DOI] [PubMed] [Google Scholar]
- 16.Kaper J B, Morris J G J, Levine M M. Cholera. Clin Microbiol Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee C A, Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci USA. 1990;87:4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S H, Hava D L, Waldor M K, Camilli A. Regulation and temporal expression patterns of Vibrio cholerae virulence genes during infection. Cell. 1999;99:625–634. doi: 10.1016/s0092-8674(00)81551-2. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y W, Jin S, Sim W S, Nester E W. Genetic evidence for direct sensing of phenolic compounds by the VirA protein of Agrobacterium tumefaciens. Proc Natl Acad Sci USA. 1995;92:12245–12249. doi: 10.1073/pnas.92.26.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lencer W I, Delp C, Neutra M R, Madara J L. Mechanism of cholera toxin action on a polarized human intestinal epithelial line: role of vesicular traffic. J Cell Biol. 1992;117:1197–1209. doi: 10.1083/jcb.117.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin W, Fullner K J, Clayton R, Sexton J A, Rogers M B, Calia K E, Calderwood S B, Fraser C, Mekalanos J J. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc Natl Acad Sci USA. 1999;96:1071–1076. doi: 10.1073/pnas.96.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madara J L, Stafford J, Dharmsathaphorn K, Carlson S. Structural analysis of a human intestinal epithelial cell line. Gastroenterology. 1987;92:1133–1145. doi: 10.1016/s0016-5085(87)91069-9. [DOI] [PubMed] [Google Scholar]
- 23.McCormick B A, Parkos C A, Colgan S P, Carnes D K, Madara J L. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol. 1998;160:455–466. [PubMed] [Google Scholar]
- 24.Mekalanos J J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983;35:252–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- 25.Mekalanos J J, Swartz D J, Pearson G D N, Harford N, Groyne F, de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983;306:551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- 26.Mel S F, Fullner K J, Wimer-Mackin S, Lencer W I, Mekalanos J J. Association of protease activity in Vibrio cholerae vaccine strains with decreases in transcellular epithelial resistance of polarized T84 intestinal cells. Infect Immun. 2000;68:6487–6492. doi: 10.1128/iai.68.11.6487-6492.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metcalf W M, Jiang W, Daniels L L, Kim S-K, Haldimann A, Wanner B L. Conditionally replicative and conjugative plasmids carrying lacZα for cloning, mutagenesis, and allele replacement in bacteria. Plasmid. 1996;35:1–13. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 28.Mowbray S L, Koshland D E. Mutations in the aspartate receptor of Escherichia coli which affects aspartate binding. J Biol Chem. 1990;265:15638–15643. [PubMed] [Google Scholar]
- 29.Provenzano D, Schuhmacher D A, Barker J L, Klose K E. The virulence regulatory protein ToxR mediates enhanced bile resistance in Vibrio cholerae and other pathogenic Vibrio species. Infect Immun. 2000;68:1491–1497. doi: 10.1128/iai.68.3.1491-1497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sears C L, Kaper J B. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol Rev. 1996;60:167–215. doi: 10.1128/mr.60.1.167-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skorupski K, Taylor R K. Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol Microbiol. 1997;25:1003–1009. doi: 10.1046/j.1365-2958.1997.5481909.x. [DOI] [PubMed] [Google Scholar]
- 32.Taylor D N, Killeen K P, Hack D C, Kenner J R, Coster T S, Beattie D T, Ezzell J, Hyman T, Trofa A, Sjogren M H, Friedlander A, Mekalanos J J, Sadoff J C. Development of a live, oral, attenuated vaccine against El Tor cholera. J Infect Dis. 1994;170:1518–1523. doi: 10.1093/infdis/170.6.1518. [DOI] [PubMed] [Google Scholar]
- 33.Wu Z, Milton D, Nybom P, Sjö A, Magnusson K-E. Vibrio cholerae hemagglutinin/protease (HA/protease) causes morphological changes in cultured epithelial cells and perturbs their paracellular barrier function. Microb Pathog. 1996;21:111–123. doi: 10.1006/mpat.1996.0047. [DOI] [PubMed] [Google Scholar]
- 34.Wu Z, Nybom P, Magnusson K-E. Distinct effects of the Vibrio cholerae haemagglutinin/protease on the structure and localization of the tight junction-associated proteins occludin and ZO-1. Cell Microbiol. 2000;2:11–18. doi: 10.1046/j.1462-5822.2000.00025.x. [DOI] [PubMed] [Google Scholar]