Abstract
Eosinophilic gastrointestinal diseases are delayed-type chronic allergic disorders that show gastrointestinal eosinophil dense infiltration, with an exaggerated Th2-type immune reaction considered to be an important mechanism. These diseases can be roughly divided into two types: eosinophilic esophagitis, mainly found in young and middle-aged men, and eosinophilic gastroenteritis, which is found in both genders equally. A diagnosis of eosinophilic esophagitis is suspected when characteristic endoscopic findings, including longitudinal furrows and rings, are noted. However, characteristic endoscopic abnormalities are rarely found in cases with eosinophilic gastroenteritis, so multiple biopsy sampling from the apparently normal gastrointestinal mucosal surface is important for making an accurate diagnosis. The administration of systemic glucocorticoid is the standard treatment for eosinophilic gastroenteritis, while acid inhibitors and topical glucocorticoid swallowing therapy are effective for eosinophilic esophagitis. Anti-cytokine therapies for eosinophilic gastrointestinal diseases are currently under development.
Keywords: proton pump inhibitor, endoscopy, allergy, elimination diet
Introduction
Allergic diseases caused by an exaggerated or inappropriate immune response to exogenous antigens are increasing in many countries including Japan. The prevalence of three major types of allergic diseases including bronchial asthma, allergic rhinitis, and atopic dermatitis has been steadily rising, and they are now commonly encountered. Food allergies are also increasing in prevalence and regarded as one of the most important classifications of allergic disease, as anaphylactic shock can result in some cases. In addition to acute food allergy caused by an immunoglobulin E (IgE)-mediated mechanism, delayed-type food allergy has been proposed as a new disease entity (1).
Eosinophilic gastrointestinal diseases are considered to be a pathologic condition caused by delayed-type food allergy and characterized by the dense infiltration of eosinophils in the esophago-gastro-intestinal tract. Recently, they have been established as a disease entity, although information concerning their diagnosis and treatment has not yet been widely distributed. In addition, as with other allergic diseases, the prevalence of eosinophilic gastrointestinal diseases is rapidly increasing in Japan as well as in Western countries (2,3).
We herein review the pathogenesis, classification, epidemiology, diagnosis, and treatment of eosinophilic gastrointestinal diseases.
Pathogenesis
Although the pathogenesis and pathophysiology of eosinophilic gastrointestinal diseases are not completely understood, an exaggerated Th2-type immune response to exogenous antigens, especially food and airborne antigens, is considered the main etiological mechanism (4). After starting the oral administration of allergens for allergic rhinitis, up to 5% of treated patients reportedly develop an eosinophilic gastrointestinal disease (5,6). However, up to 70% of affected patients can be treated by an elimination diet for the exclusion of several different types of allergens (7,8).
Epidemiological investigations including twin and family studies have suggested that a greater contribution of environmental factors than genetic factors to the development of eosinophilic esophagitis, the most prevalent type of eosinophilic gastrointestinal disease (9-11), which provides a reasonable explanation for the recently observed rapid increase in cases with these diseases. Regarding environmental factors, proton pump inhibitors and antibiotics administered during the neonatal period and for Caesarian delivery are reported to be risk factors for the development of pediatric eosinophilic esophagitis (12-14). Those agents change the gut microbiome and suppress the adequate digestion of food antigens; furthermore, they may induce allergic reactions to ingested foods or airborne antigens (15,16).
Various research attempts, including a genome-wide association study, have identified several risk-related genes. Among the reported genes, thymic stromal lymphopoietin and calpain 14 are potentially important, as their variations may influence the occurrence of eosinophilic gastrointestinal diseases by altering the Th2-type immune response and epithelial permeability to allergens (17-19).
A microarray analysis of gut mucosal biopsy specimens showed an increased expression of Th2-type cytokines, including interleukin (IL)-5, IL-13, and eotain3, and decreased expression of epithelial adhesion molecules, such as desmoglein (20). Furthermore, the expression of TGF-β and periostin, cytokines related to increased remodeling and fibrosis of the gut wall, has also been reported to be increased (21).
Based on these findings, eosinophilic esophageal diseases are speculated to occur in cases with increased levels of Th2-type immune reaction diathesis. When immunogenic food or airborne allergens penetrate the epithelial barrier without appropriate digestion and degradation in the gut, they can initiate Th2-type immune reactions in gastrointestinal tissues (Fig. 1).
Figure 1.
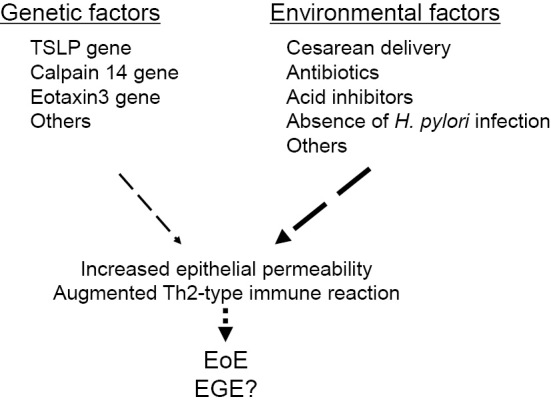
Influence of genetic and environmental factors on the development of eosinophilic gastrointestinal diseases.
Classification
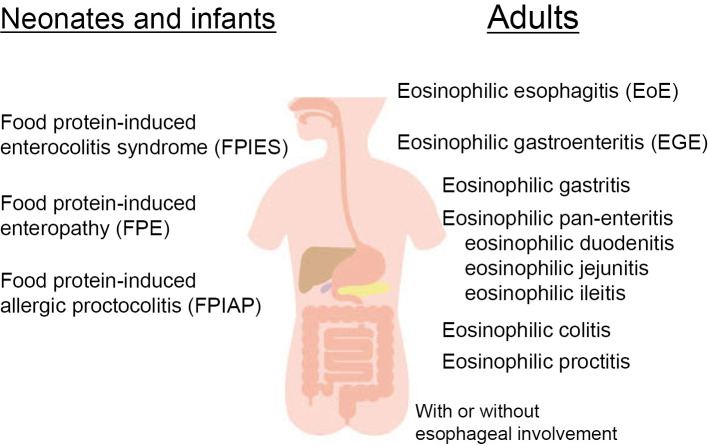
Eosinophilic esophageal diseases are classified based on the involved segment and wall layer of the gastrointestinal tract. When dense eosinophilic infiltration is limited to the esophageal epithelial layer, the disease is classified as eosinophilic esophagitis, which is the most common eosinophilic gastrointestinal disease in Japan as well as Western countries (3). When pathologically dense infiltration and activation of eosinophils is identified in other segments of the gastrointestinal tract, the disease is classified as eosinophilic gastroenteritis, irrespective of esophageal eosinophil infiltration. Eosinophilic gastroenteritis may then be further classified as eosinophilic gastritis, eosinophilic duodenitis, eosinophilic ileitis, or eosinophilic colitis depending on the segment involved (Fig. 2).
Figure 2.
Classification of eosinophilic gastrointestinal diseases.
Eosinophil infiltration can occur in any layer of the gastrointestinal tract, including mucosal, muscle, and sub-serosal layers, so eosinophilic gastrointestinal diseases are classified based on the involved layer, such as mucosal, muscle layer, and sub-serosal types (22). However, the mechanisms by which pathological eosinophil infiltration occurs in various segments and layers of the gastrointestinal tract have not been elucidated.
Epidemiology
Epidemiological data for eosinophilic esophagitis and eosinophilic gastroenteritis differ in several aspects (Table 1). In Western countries, the incidence and prevalence of eosinophilic esophagitis are reported to be approximately 10 and 50, respectively, per 100,000 individuals in the general population (23). Its prevalence in Japan is considered to be increasing rapidly (2,24,25), and several reports that analyzed cases examined with endoscopy have suggested the rate of prevalence to be as high as noted in Western studies (24,25). However, to our knowledge, the prevalence of eosinophilic esophagitis in the general Japanese population has not yet been investigated. Regarding eosinophilic gastroenteritis, its prevalence in Western countries is lower than that of eosinophilic esophagitis and reported to range from 2 to 8 per 100,000 in the general population (26,27). In Japan as well, the prevalence of eosinophilic esophagitis has recently become higher than that of eosinophilic gastroenteritis due to an increasing trend in the diagnosis (3,28).
Table 1.
Diagnosis of Eosinophilic Esophagitis (EoE) and Eosinophilic Gastroenteritis (EGE).
| EoE | EGE | |
|---|---|---|
| Age | 30-50 years | All ages |
| Male/female ratio | 4:1 | 1:1 |
| Symptoms | Dysphagia | Abdominal pain |
| Heartburn | Nausea/vomiting | |
| Diarrhea | ||
| Laboratory tests | Eosinophilia 30% | Eosinophilia 80% |
| Elevated TARC | ||
| Endoscopy | Characteristics | Non-specific |
| longitudinal furrows | normal >60% | |
| white plaque | edema | |
| rings | redness | |
| others | erosion/ulcer | |
| CT | Rare | Frequent |
| thickened esophageal wall | segmental thickening of gut ascites | |
| Histology | Eosinophils >15/HPF Basal zone hyperplasia Dilated intracellular space Papilla elongation |
No consensus regarding eosinophil density Intraepithelial eosinophils Villous atrophy |
| Surface layering of eosinophils Eosinophilic microabscess Mast cell infiltration |
Crypt hyperplasia Eosinophilic abscess Mast cell infiltration |
|
| Others | Others |
Regarding affected ages and gender, eosinophilic esophagitis is found most frequently in middle-age (30-50 years) men with a man-to-woman ratio of 4-5 to 1. In contrast, eosinophilic gastroenteritis is found similarly in all age groups, and the gender ratio is reported to be roughly equal (26,28). These different epidemiological characteristics suggest a similar but unique pathogenesis for these two types of eosinophilic gastrointestinal disease.
The Diagnosis
The diagnosis of eosinophilic gastrointestinal diseases is based on a precise collection of the medical history, appropriate physical examinations, laboratory tests of peripheral blood, a gastrointestinal endoscopic study, and the results of a histopathological examination of biopsy specimens (Table 1). Notably, taking the medical history for subjective symptoms and the histopathological detection of dense eosinophil infiltration in the gut wall are considered to be especially important (29).
Medical history collection
Half of patients with eosinophilic gastrointestinal diseases are affected by at least one atopic disease, such as bronchial asthma, allergic rhinitis, atopic dermatitis, and IgE-mediated food allergy. Therefore, taking the history with a focus on atopic diseases is important (2,3,28).
Adults with eosinophilic esophagitis mainly complain of dysphagia and heartburn, while pediatric cases are often affected by non-specific symptoms, such as abdominal pain. Patients with eosinophilic gastroenteritis will note epigastralgia, nausea, and/or vomiting if they have gastro-duodenal lesions, whereas those with eosinophilic gastroenteritis have ileal and/or colonic lesions and will be affected by diarrhea and lower abdominal pain. Noting the presence of these bothersome symptoms is considered necessary for an accurate diagnosis of eosinophilic gastrointestinal diseases (29).
A physical examination
Although the role of physical examination findings in the diagnosis of eosinophilic gastrointestinal diseases is not substantial, skin lesions suggesting atopic dermatitis and signs suggesting intestinal inflammation may be detected.
Laboratory tests
Peripheral blood eosinophilia can be found in approximately one-third of cases of eosinophilic esophagitis, although the grade of eosinophilia is very mild and easily overlooked (Table 1). In contrast to eosinophilic esophagitis, 80% of patients with eosinophilic gastroenteritis show peripheral eosinophilia, and its severity grade is higher than eosinophilic esophagitis (28). In addition to peripheral eosinophilia, the plasma IgE concentration is elevated in 70% of patients with eosinophilic gastrointestinal diseases. Furthermore, higher concentrations of an antigen-specific IgE against various foods and airborne antigens, including pollen, are frequently observed in patients with these diseases (28,30). Thus, eosinophilia, as well as specific and non-specific findings indicating an increased IgE concentration in plasma are suggestive of atopic diathesis in eosinophilic gastrointestinal disease cases.
The prevalence of Helicobacter pylori infection has been shown to be lower in patients with eosinophilic esophagitis as well as those with eosinophilic gastroenteritis than in control populations (31,32). An elevated Th1 immune reaction, observed in cases with H. pylori infection, may inhibit the Th2 immune response, which is necessary for the development of eosinophilic gastrointestinal diseases.
To determine possible peripheral blood biomarkers for the diagnosis as well as grading of disease activity, the concentrations of various cytokines were measured and compared with those in healthy individuals (33,34). Although eotaxin3, IL-5, IL-13, and thymic stromal lymphopoietin (TSLP) showed elevated peripheral blood concentrations in patients with eosinophilic gastrointestinal diseases, marked overlap was seen with values in the healthy subjects in those studies, indicating their limited utility as biomarkers. Nevertheless, TSLP and IL-33 are being further investigated as potential biomarkers of infantile eosinophilic gastroenteritis (35). At present, a diagnosis based on blood biomarkers is considered to be difficult.
When ascitic fluid is present, a large number of eosinophils in ascites may be found, a sign of sub-serosal type of eosinophilic gastroenteritis. Eosinophil-associated protein concentrations measured in esophageal mucosal surface fluid collected by a swallowed string can be used to diagnose eosinophilic esophagitis (36). In addition, the examination of stool samples to determine the concentrations of eosinophil granular proteins, including eosinophil-derived neurotoxin, can also be utilized, as those are diagnostic markers of esophageal gastrointestinal diseases (37). The nitric oxide concentration in exhaled breath has also been reported to be a potential biomarker for eosinophilic esophagitis, although research data concerning this point remain inadequate (38,39).
Although each of these laboratory tests can be useful for the diagnosis and grading of eosinophilic gastrointestinal disease activity, their value remains limited, and further studies are necessary.
Radiology
Plain chest and abdominal X-ray are not sensitive enough to detect abnormalities in cases with eosinophilic gastrointestinal diseases. In contrast, computed tomography (CT) is useful, and its diagnostic yield is high, especially for determining eosinophilic gastroenteritis. In cases with eosinophilic esophagitis, esophageal wall thickening may be detected by CT. However, in eosinophilic gastroenteritis cases, segmental gut wall thickening and collection of ascites, signs indicating the sub-serosal type, are frequently observed as abnormal findings.
Endoscopy
The diagnostic value of endoscopic examination findings varies among the different types of eosinophilic gastrointestinal diseases (Table 1).
• For the diagnosis of eosinophilic esophagitis
These findings have been shown to be useful and provide important clues for the diagnosis of eosinophilic esophagitis. Longitudinal furrows, frequently found on the lower esophageal mucosal surface between longitudinal esophageal folds, are a characteristic finding noted in 90% of cases (40-42). Furthermore, white plaque and localized esophageal constriction, termed rings, are also frequently observed. These three findings of longitudinal furrows, white plaque, and rings are considered important endoscopic findings for the detection of eosinophilic esophagitis (2).
In addition to those major findings, a crepe paper-like appearance suggesting epithelial fragility (43), pull sign suggesting subepithelial fibrosis (44), and Ankylosaurus back sign suggesting a favorable response to proton pump inhibitor administration (45) have been reported. A crepe paper-like appearance is noted when a wide portion of epithelial tissue can be removed as a sheet during biopsy sample collection from the mucosal surface, while the pull sign refers to the sensation of resistance when pulling the forceps to obtain a tissue sample. Evidence of longitudinally arrayed whitish epithelial thickening resembling the nodules found on the back of a dinosaur is referred to as Ankylosaurus back sign.
To confirm the histopathological diagnosis of eosinophilic esophagitis, at least four biopsy specimens are reportedly necessary for an accurate diagnosis with minimal possibility of a false-negative result (46). Biopsy specimens obtained from the lower segment of the esophagus tend to have denser eosinophil infiltration than those obtained from the upper segment (25). In addition, specimens obtained from lesions shown by endoscopy as longitudinal furrows or white plaque also tend to have denser eosinophil infiltration than specimens obtained from apparently normal mucosa (42). Therefore, biopsy specimens should be obtained from different sites, mainly in the lower esophagus as well as from lesions identifiable by endoscopy, to ensure the most accurate pathological diagnosis.
• For the diagnosis of eosinophilic gastroenteritis
In contrast to eosinophilic esophagitis, no endoscopic abnormalities are detected in 60-70% of patients with eosinophilic gastroenteritis, regardless of the gastrointestinal tract segment (47,48). Histological results often show dense eosinophil infiltration in these endoscopically normal-appearing segments. Endoscopic abnormalities found in cases with eosinophilic gastroenteritis include mucosal edema, redness, erosion, ulcers, and nodularity (Table 1) (48). However, such abnormalities are generally non-specific, and these endoscopic findings are associated with many different types of gastrointestinal disease.
The frequent absence of an endoscopic abnormality and non-specific findings make the endoscopic diagnosis of eosinophilic gastroenteritis difficult and distinct from eosinophilic esophagitis. As a result, multiple biopsy samples obtained from different gastrointestinal mucosa sites are necessary for the diagnosis of eosinophilic gastroenteritis, irrespective of the presence of specific endoscopic findings. Unfortunately, whether or not samples obtained from lesions identifiable by endoscopy show greater levels of eosinophil infiltration has not been clarified. It is important to note that samples taken from apparently normal mucosa frequently show eosinophil infiltration in pathological results.
Histopathology
To obtain appropriate histological examination results, the biopsy samples must be properly processed. Sections taken perpendicular to the surface of mucosa are necessary for counting the number of infiltrated eosinophils in different layers of the mucosa. Physical pressure applied to a sample just after obtaining it may facilitate eosinophil and/or mast cell degranulation, hampering identification. Various histopathological abnormalities in addition to increased eosinophil infiltration in cases with eosinophilic esophagitis as well as those with eosinophilic gastroenteritis have also been reported (49,50).
• For the diagnosis of eosinophilic esophagitis
For the diagnosis of eosinophilic esophagitis, eosinophil infiltration >15 eosinophils/high-power field (HPF) (×400) in the esophageal epithelial layer in at least 1 microscopic field is necessary (51), although just one field with dense eosinophil infiltration is considered adequate for a diagnosis. In addition to dense eosinophil infiltration in the epithelial layer, mast cell infiltration, basal cell zone hyperplasia, and dilated intercellular space in the epithelial layer have also been reported in affected patients. Epithelial papilla elongation and subepithelial dense fibrosis can be observed when a denser mucosal layer is obtained from the esophagus (52). In these patients, eosinophil infiltration is mainly found in the epithelial layer, whereas no eosinophil infiltration is found in normal healthy individuals. Rarely, dense eosinophil infiltration is found only in a muscle layer with impaired esophageal motility and can be classified as eosinophilic esophageal myositis. This type of esophageal eosinophil infiltration is considered as a muscle layer type of eosinophilic esophagitis (53).
The gene expression pattern in biopsy specimens functions as a more sensitive and specific diagnostic marker of eosinophilic esophagitis than histopathological observation. An eosinophilic esophagitis diagnostic panel was shown able to determine the expression of more than 90 different messenger RNAs in esophageal mucosa and was used to suggest a diagnosis. This messenger RNA panel has also been reported to be more sensitive than the histological measurement of eosinophil infiltration and requires only a single biopsy specimen for an accurate diagnosis (54-56).
• For the diagnosis of eosinophilic gastroenteritis
Eosinophilic gastroenteritis can be associated with increased eosinophil infiltration in the lamina propria, intraepithelial eosinophils, eosinophils in Peyer's patches, degranulation of eosinophils, extracellular deposition of eosinophil granular proteins, villous atrophy, crypt hyperplasia, eosinophil abscess, and mast cell infiltration (49). The number of eosinophils present in the lamina propria of different segments of the gastrointestinal tract in normal healthy individuals has been reported by several investigators (57,58). In the esophageal epithelial layer of healthy subjects, no eosinophil infiltration is shown, whereas in the stomach, duodenum, distal ileum, ascending colon, descending colon, and rectum, eosinophil infiltration up to 10, 20, 30, 30, 20, and 10 per HPF field, respectively, can be found (Fig. 3). The density of eosinophil infiltration is greatest in the distal ileum and ascending colon and then becomes lower in the more oral and anal segments of the gastrointestinal tract. Therefore, the infiltration of eosinophils should be evaluated in consideration of the segment from which the biopsy specimens were taken.
Figure 3.
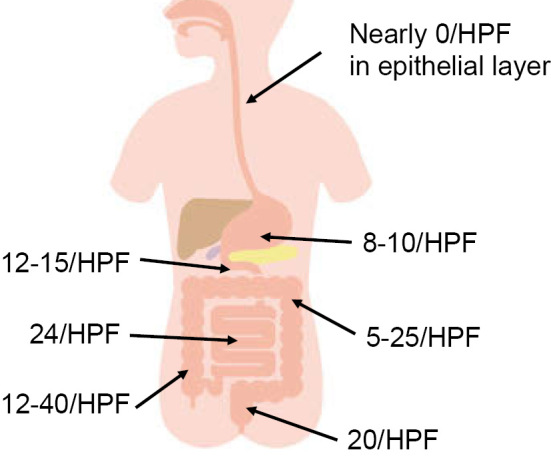
Maximal eosinophil infiltration in different segments of the gastrointestinal tract in a normal healthy individual.
A consensus has not yet been reached regarding the optimum cut-off points for the pathological density of eosinophil infiltration in each segment of the gastrointestinal tract, so other histopathological findings, including intraepithelial eosinophils, should be considered for the histological diagnosis of eosinophilic gastroenteritis different from that of eosinophilic esophagitis.
Treatment
The therapeutic responses to treatments differ between eosinophilic esophagitis and eosinophilic gastroenteritis (Table 2). For eosinophilic esophagitis, the response rate to remission induction therapy is usually good, and maintenance therapy is also successful in the majority of cases (59). In contrast, difficulties with remission and maintenance treatment are often encountered with eosinophilic gastroenteritis (60).
Table 2.
Treatment of Eosinophilic Esophagitis (EoE) and Eosinophilic Gastroenteritis (EGE).
| EoE | EGE | |
|---|---|---|
| Drugs | PPI | Systemic glucocorticoid |
| Vonoprazan | Topical glucocorticoid | |
| Topical glucocorticoid fluticasone budesonide |
Anti-allergic drugs montelukast histamine H1 receptor antagonist |
|
| Systemic glucocorticoid | ||
| Dietary therapy | Six-food elimination diet | Elimination diet |
| Four-food elimination diet | Elemental diet | |
| Two-food elimination diet | ||
| Targeted elimination diet | ||
| Elemental diet | ||
| Other therapy | Balloon dilatation | Surgery for perforation or obstruction |
Treatment of eosinophilic esophagitis
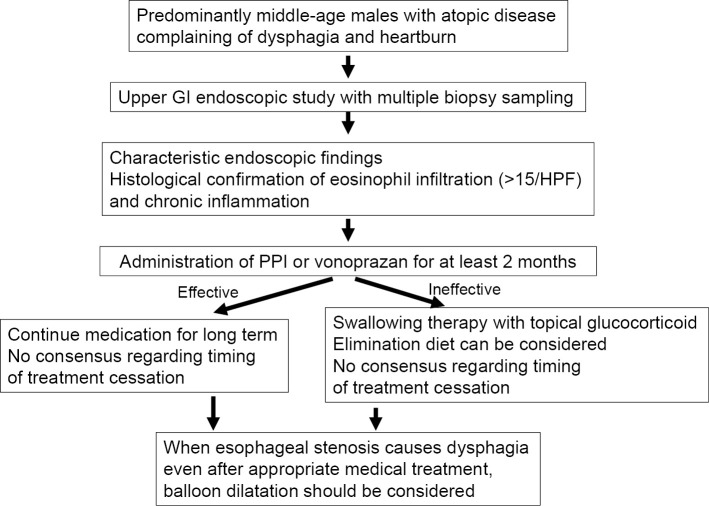
The first-line treatment for eosinophilic esophagitis is the administration of a gastric acid inhibitor, such as a high-dose proton pump inhibitor, or vonoprazan, a potassium competitive acid blocker (Fig. 4). Therapy with a proton pump inhibitor for 2 months induces remission in over 50% of patients with eosinophilic esophagitis (61,62), while vonoprazan treatment is also reported to be more or as effective as that with proton pump inhibitors (63,64). Cases in which esophageal eosinophil infiltration can be treated by the administration of a proton pump inhibitor are termed proton pump inhibitor-responsive esophageal eosinophilia, and in the past, these cases were separated from eosinophilic esophagitis. However, several recent studies have indicated that the clinical, endoscopic, and histopathological characteristics of these two diseases are quite similar, and their gene expression patterns nearly the same (41,65). Therefore, esophageal eosinophil infiltration irrespective of responsiveness to acid inhibitors is diagnosed as eosinophilic esophagitis when some symptoms potentially originating from the esophageal abnormalities are noted by the patient (61). The mechanism by which acid inhibitors cause remission of eosinophilic esophagitis has not been clarified. However, an acid inhibitor-related decrease in esophageal acid exposure and resulting decrease in esophageal mucosal permeability caused by physiological or pathological esophageal acid reflux must be considered (66,67).
Figure 4.
Flowchart of the diagnosis and treatment of eosinophilic esophagitis.
When proton pump inhibitor or vonoprazan administration is not adequately effective, second-line treatment is necessary. Two possible options are peroral administration of a topical glucocorticoid and an elimination diet. Oral administration of a topical glucocorticoid, such as fluticasone or budesonide, and slow swallowing of a glucocorticoid twice a day results in remission in over 70% of examined cases (68,69). However, continued maintenance treatment is necessary for long-term disease control, and the interruption of administration is followed by immediate exacerbation (70,71). In contrast, an elimination diet may be effective for remission induction and long-term control. The effective elimination of allergic foods based on peripheral blood allergen-specific IgE concentrations or skin prick or patch tests is reportedly difficult (62,72). Therefore, the empirical elimination of the six most frequently observed allergic foods is recommended. A six-food elimination diet excludes dairy products, soy, eggs, wheat, nuts, and seafood. Approximately 70% of patients with eosinophilic esophagitis treated with a 6-food empirical elimination diet are reported to enter a state of remission (73). Once the elimination diet has been shown to be effective, it is then possible to detect the responsible food by returning each food into the diet one by one.
Treatment of eosinophilic gastroenteritis
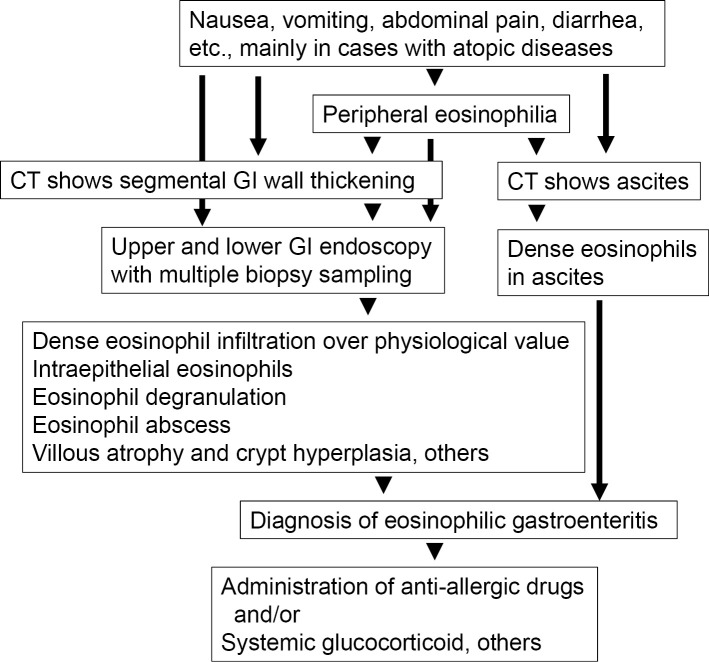
Anti-allergic drugs including histamine H1 receptor and leukotriene receptor antagonists, as well as mast cell stabilizers have been used to treat eosinophilic gastroenteritis, although evidence supporting their effectiveness is lacking (Fig. 5) (74). Based on limited study data currently available, montelukast may be effective (75,76). Systemic glucocorticoid administration has traditionally been used for remission induction therapy, with favorable results shown. According to previous reports, approximately 40% of patients with eosinophilic gastroenteritis show a complete response to glucocorticoid therapy, and no recurrence is observed after stopping administration (60). Another 40% of patients show a good response to remission induction therapy with a glucocorticoid, although their disease activity returns after stopping administration, while the remaining 20% show resistance to such therapy, and their disease activity cannot be controlled. For these cases, various immune-modulating therapies have been reported to be effective, although additional evidence is needed.
Figure 5.
Flowchart of the diagnosis and treatment of eosinophilic gastroenteritis.
Therapy under development
For the treatment of refractory cases with eosinophilic esophagitis or eosinophilic gastroenteritis, new molecular-targeted therapies focusing on Th2 immune reaction are under development (77). Placebo-controlled randomized studies have presented promising results demonstrating the therapeutic efficacy of anti-α4βintegrin (78), anti-IL-5 (79), anti-IL-13 (80), anti-IL-4/13 receptor (81), and anti-siglec-8 (82) antibodies. Clinical research investigations concerning these new anti-cytokine therapies are currently in progress in Japan.
Summary
The eosinophilic gastrointestinal diseases of eosinophilic esophagitis and eosinophilic gastroenteritis are chronic delayed-type allergic diseases caused mainly by food and airborne antigens. Their prevalence is increasing in Japan as well as in Western countries, along with several other allergic diseases. For the diagnosis, the presence of gastrointestinal symptoms and identification of gastrointestinal eosinophil infiltration in histopathological results are necessary. The first-line treatment for eosinophilic esophagitis is the administration of a proton pump inhibitor or potassium-competitive acid inhibitor. When such efforts are not adequately effective, topical glucocorticoid administration or an elimination diet is usually selected as a second-line treatment option. For the treatment of eosinophilic gastroenteritis, glucocorticoid administration is the most widely used. Anti-Th2 cytokine therapies are currently under development for refractory cases of eosinophilic esophagitis or eosinophilic gastroenteritis.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Cianferoni A. Eosinophilic esophagitis and other eosinophilic disorders of the gastrointestinal tract. Pediatr Allergy Immunol Suppl 24: 25-27, 2020. [DOI] [PubMed] [Google Scholar]
- 2. Kinoshita Y, Ishimura N, Oshima N, Ishihara S. Systematic review: eosinophilic esophagitis in Asian countries. World J Gastroenterol 21: 8433-8440, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yamamoto M, Nagashima S, Yamada Y, et al. Comparison of non-esophageal eosinophilic gastrointestinal disorders with eosinophilic esophagitis: a nationwide survey. J Allergy Clin Immunol Pract 3339-3349, 2021. [DOI] [PubMed] [Google Scholar]
- 4. Kinoshita Y, Oouchi S, Fujisawa T. Eosinophilic gastrointestinal diseases - Pathogenesis, diagnosis, and treatment. Allergol Int 68: 420-429, 2019. [DOI] [PubMed] [Google Scholar]
- 5. Kawashima K, Ishihara S, Masuhara M, et al. Development of eosinophilic esophagitis following sublingual immunotherapy with cedar pollen extract: a case report. Allergol Int 67: 515-517, 2018. [DOI] [PubMed] [Google Scholar]
- 6. Petroni D, Spergel JM. Eosinophilic esophagitis and symptoms possibly related to eosinophilic esophagitis in oral immunotherapy. Ann Allergy Asthma Immunol 120: 237-240, 2018. [DOI] [PubMed] [Google Scholar]
- 7. Wolf WA, Jerath MR, Sperry SL, Shaheen NJ, Dellon ES. Dietary elimination therapy is an effective option for adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol 12: 1272-1279, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okimoto E, Ishimura N, Okada M, et al. Successful food-elimination diet in an adult with eosinophilic gastroenteritis. ACG Case Rep J 5: e38, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alexander ES, Martin LJ, Collins MH, et al. Twin and family studies reveal strong environmental and weaker genetic cues explaining heritability of eosinophilic esophagitis. J Allergy Clin Immunol 134: 1084-1092, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Allen-Brady K, Firszt R, Fang JC, Wong J, Smith KR, Peterson KA. Population-based familial aggregation of eosinophilic esophagitis suggests a genetic contribution. J Allergy Clin Immunol 140: 1138-1143, 2017. [DOI] [PubMed] [Google Scholar]
- 11. Peterson K, Clayton F, Qeadan F, et al. Esophageal eosinophilia is common among relatives of eosinophilic esophagitis patients. Clin Gastroenterol Hepatol S1542-3565(20)31557-3, 2020. [DOI] [PubMed] [Google Scholar]
- 12. Jensen ET, Dellon ES. Environmental factors and eosinophilic esophagitis. J Allergy Clin Immunol 142: 32-40, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jensen ET, Kuhl JT, Martin LJ, Langefeld CD, Dellon ES, Rothenberg ME. Early-life environmental exposures interact with genetic susceptibility variants in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol 141: 632-637, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jensen ET, Kuhl JT, Martin LJ, Rothenberg ME, Dellon ES. Prenatal, intrapartum, and postnatal factors are associated with pediatric eosinophilic esophagitis. J Allergy Clin Immunol 141: 214-222, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Untersmayr E, Bakos N, Schöll I, et al. Anti-ulcer drugs promote IgE formation toward dietary antigens in adult patients. FASEB J 19: 656-658, 2005. [DOI] [PubMed] [Google Scholar]
- 16. Pali-Schöll I, Jensen-Jarolim E. Anti-acid medication as a risk factor for food allergy. Allergy 66: 469-477, 2011. [DOI] [PubMed] [Google Scholar]
- 17. Rothenberg ME, Spergel JM, Sherrill JD, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet 42: 289-291, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kottyan LC, Davis BP, Sherrill JD, et al. Genome-wide association analysis of eosinophilic esophagitis provides insight into the tissue specificity of this allergic disease. Nat Genet 46: 895-900, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sleiman PM, Wang ML, Cianferoni A, et al. GWAS identifies four novel eosinophilic esophagitis loci. Nat Commun 5: 5593, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shoda T, Morita H, Nomura I, et al. Comparison of gene expression profiles in eosinophilic esophagitis (EoE) between Japan and Western countries. Allergol Int 64: 260-265, 2015. [DOI] [PubMed] [Google Scholar]
- 21. Wen T, Stucke EM, Grotjan TM, et al. Molecular diagnosis of eosinophilic esophagitis by gene expression profiling. Gastroenterology 145: 1289-1299, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut 31: 54-58, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dellon ES, Hirano I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology 154: 319-332, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fujishiro H, Amano Y, Kushiyama Y, Ishihara S, Kinoshita Y. Eosinophilic esophagitis investigated by upper gastrointestinal endoscopy in Japanese patients. J Gastroenterol 46: 1142-1144, 2011. [DOI] [PubMed] [Google Scholar]
- 25. Adachi K, Mishiro T, Tanaka S, Kinoshita Y. Suitable biopsy site for detection of esophageal eosinophilia in eosinophilic esophagitis suspected cases. Dig Endosc 28: 139-144, 2016. [DOI] [PubMed] [Google Scholar]
- 26. Jensen ET, Martin CF, Kappelman MD, Dellon ES. Prevalence of eosinophilic gastritis, gastroenteritis, and colitis: estimates from a national administrative database. J Pediatr Gastroenterol Nutr 62: 36-42, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mansoor E, Saleh MA, Cooper GS. Prevalence of eosinophilic gastroenteritis and colitis in a population-based study, from 2012 to 2017. Clin Gastroenterol Hepatol 15: 1733-1741, 2017. [DOI] [PubMed] [Google Scholar]
- 28. Kinoshita Y, Furuta K, Ishimaura N, et al. Clinical characteristics of Japanese patients with eosinophilic esophagitis and eosinophilic gastroenteritis. J Gastroenterol 48: 333-339, 2013. [DOI] [PubMed] [Google Scholar]
- 29. Lucendo AJ, Molina-Infante J, Arias Á, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J 5: 335-358, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ishimura N, Furuta K, Sato S, Ishihara S, Kinoshita Y. Limited role of allergy testing in patients with eosinophilic gastrointestinal disorders. J Gastroenterol Hepatol 28: 1306-1313, 2013. [DOI] [PubMed] [Google Scholar]
- 31. Furuta K, Adachi K, Aimi M, et al. Case-control study of association of eosinophilic gastrointestinal disorders with Helicobacter pylori infection in Japan. J Clin Biochem Nutr 53: 60-62, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang L, Duan L, Ding S, et al. Eosinophilic gastroenteritis: clinical manifestations and morphological characteristics, a retrospective study of 42 patients. Scand J Gastroenterol 46: 1074-1080, 2011. [DOI] [PubMed] [Google Scholar]
- 33. Kinoshita Y, Furuta K, Ishimura N, Ishihara S. Elevated plasma cytokines in Japanese patients with eosinophilic esophagitis and gastroenteritis. Digestion 86: 238-243, 2012. [DOI] [PubMed] [Google Scholar]
- 34. Ishihara S, Shoda T, Ishimura N, et al. Serum biomarkers for the diagnosis of eosinophilic esophagitis and eosinophilic gastroenteritis. Intern Med 56: 2819-2825, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shoda T, Matsuda A, Arai K, et al. Sera of patients with infantile eosinophilic gastroenteritis showed a specific increase in both thymic stromal lymphopoietin and IL-33 levels. J Allergy Clin Immunol 138: 299-303, 2016. [DOI] [PubMed] [Google Scholar]
- 36. Ackerman SJ, Kagalwalla AF, Hirano I, et al. One-hour esophageal string test: a nonendoscopic minimally invasive test that accurately detects disease activity in eosinophilic esophagitis. Am J Gastroenterol 114: 1614-1625, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Subbarao G, Rosenman MB, Ohnuki L, et al. Exploring potential noninvasive biomarkers in eosinophilic esophagitis in children. J Pediatr Gastroenterol Nutr 53: 651-658, 2011. [DOI] [PubMed] [Google Scholar]
- 38. Lanz MJ, Guerrero RA, Gonzalez-Vallina R. Measurement of exhaletd nitric oxide in the evaluation for eosinophilic esophagitis in children. Ann Allergy Asthma Immunol 109: 81-82, 2012. [DOI] [PubMed] [Google Scholar]
- 39. Johnson K, Iyer V, Katzka D, et al. Poor relationship between fractionated exhaled nitric oxide and disease activity in eosinophilic esophagitis. Dysphagia 34: 138-144, 2019. [DOI] [PubMed] [Google Scholar]
- 40. Shimura S, Ishimura N, Tanimura T, et al. Reliability of symptoms and endoscopic findings for diagnosis of esophageal eosinophilia in a Japanese population. Digestion 90: 49-57, 2014. [DOI] [PubMed] [Google Scholar]
- 41. Jiao D, Ishimura N, Maruyama R, et al. Similarities and differences among eosinophilic esophagitis, proton-pump inhibitor-responsive esophageal eosinophilia, and reflux esophagitis: comparisons of clinical, endoscopic, and histopathological findings in Japanese patients. J Gastroenterol 52: 203-210, 2017. [DOI] [PubMed] [Google Scholar]
- 42. Okimoto E, Ishimura N, Okada M, et al. Specific locations of linear furrows in patients with esophageal eosinophilia. Dig Endosc 29: 49-56, 2017. [DOI] [PubMed] [Google Scholar]
- 43. Matsuura H, Muro S, Yamauchi K. Eosinophilic esophagitis: crepe paper-like appearance. Am J Med 131: e99-e100, 2018. [DOI] [PubMed] [Google Scholar]
- 44. Dellon ES, Gebhart JH, Higgins LL, Hathorn KE, Woosley JT, Shaheen NJ. The esophageal biopsy “pull” sign: a highly specific and treatment-responsive endoscopic finding in eosinophilic esophagitis (with video). Gastrointest Endosc 83: 92-100, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ishimura N, Sumi S, Okada M, et al. Ankylosaurus back sign: novel endoscopic finding in esophageal eosinophilia patients indicating proton pump inhibitor response. Endosc Int Open 6: E165-E172, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nielsen JA, Lager DJ, Lewin M, Rendon G, Roberts CA. The optimal number of biopsy fragments to establish a morphologic diagnosis of eosinophilic esophagitis. Am J Gastroenterol 109: 515-520, 2014. [DOI] [PubMed] [Google Scholar]
- 47. Hui CK, Hui NK. A prospective study on the prevalence, extent of disease and outcome of eosinophilic gastroenteritis in patients presenting with lower abdominal symptoms. Gut Liver 12: 288-296, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pesek RD, Reed CC, Collins MH. Association between endoscopic and histologic findings in a multicenter retrospective cohort of patients with non-esophageal eosinophilic gastrointestinal disorders. Dig Dis Sci 65: 2024-2035, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hurrell JM, Genta RM, Melton SD. Histopathologic diagnosis of eosinophilic conditions in the gastrointestinal tract. Adv Anat Pathol 18: 335-348, 2011. [DOI] [PubMed] [Google Scholar]
- 50. Genevay M, Rubbia-Brandt L, Rougemont AL. Do eosinophil numbers differentiate eosinophilic esophagitis from gastroesophageal reflux disease? Arch Pathol Lab Med 134: 815-825, 2010. [DOI] [PubMed] [Google Scholar]
- 51. Dellon ES. Eosinophilic esophagitis: diagnostic tests and criteria. Curr Opin Gastroenterol 28: 382-388, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee S, de Boer WB, Naran A, et al. More than just counting eosinophils: proximal oesophageal involvement and subepithelial sclerosis are major diagnostic criteria for eosinophilic oesophagitis. J Clin Pathol 63: 644-647, 2010. [DOI] [PubMed] [Google Scholar]
- 53. Spechler SJ, Konda V, Souza R. Can eosinophilic esophagitis cause achalasia and other esophageal motility disorders? Am J Gastroenterol 113: 1594-1599, 2018. [DOI] [PubMed] [Google Scholar]
- 54. Wen T, Stucke EM, Grotjan TM, et al. Molecular diagnosis of eosinophilic esophagitis by gene expression profiling. Gastroenterology 145: 1289-1299, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dellon ES, Yellore V, Andreatta M, Stover J. A single biopsy is valid for genetic diagnosis of eosinophilic esophagitis regardless of tissue preservation or location in the esophagus. J Gastrointestin Liver Dis 24: 151-157, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dellon ES, Veerappan R, Selitsky SR, et al. A gene expression panel is accurate for diagnosis and monitoring treatment of eosinophilic esophagitis in adults. Clin Transl Gastroenterol 8: e74, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Matsushita T, Maruyama M, Ishikawa N, et al. The number and distribution of eosinophils in the adult human gastrointestinal tract: a study and comparison of racial and environmental factors. Am J Surg Pathol 39: 521-527, 2015. [DOI] [PubMed] [Google Scholar]
- 58. DeBrosse CW, Case JW, Putnam PE, Collins MH, Rothenberg ME. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr Dev Pathol 9: 210-218, 2006. [DOI] [PubMed] [Google Scholar]
- 59. Laserna-Mendieta EJ, Casabona S, Guagnozzi D, et al. Efficacy of proton pump inhibitor therapy for eosinophilic oesophagitis in 630 patients: results from the EoE connect registry. Aliment Pharmacol Ther 52: 798-807, 2020. [DOI] [PubMed] [Google Scholar]
- 60. Pineton de Chambrun G, Gonzalez F, Canva JY, et al. Natural history of eosinophilic gastroenteritis. Clin Gastroenterol Hepatol 9: 950-956, 2011. [DOI] [PubMed] [Google Scholar]
- 61. Dellon ES, Liacouras CA, Molina-Infante J, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology 155: 1022-1033, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hirano I, Chan ES, Rank MA, et al. ; AGA Institute Clinical Guidelines Committee; Joint Task Force on Allergy-Immunology Practice Parameters. AGA institute and the joint task force on allergy-immunology practice parameters clinical guidelines for the management of eosinophilic esophagitis. Gastroenterology 158: 1776-1786, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ishimura N, Ishihara S, Kinoshita Y. Sustained acid suppression by potassium-competitive acid blocker (P-CAB) may be an attractive treatment candidate for patients with eosinophilic esophagitis. Am J Gastroenterol 111: 1203-1204, 2016. [DOI] [PubMed] [Google Scholar]
- 64. Kuzumoto T, Tanaka F, Sawada A, et al. Vonoprazan shows efficacy similar to that of proton pump inhibitors with respect to symptomatic, endoscopic, and histological responses in patients with eosinophilic esophagitis. Esophagus 18: 372-379, 2021. [DOI] [PubMed] [Google Scholar]
- 65. Shoda T, Matsuda A, Nomura I, et al. Eosinophilic esophagitis versus proton pump inhibitor-responsive esophageal eosinophilia: Transcriptome analysis. J Allergy Clin Immunol 139: 2010-2013, 2017. [DOI] [PubMed] [Google Scholar]
- 66. van Rhijn BD, Weijenborg PW, Verheij J, et al. Proton pump inhibitors partially restore mucosal integrity in patients with proton pump inhibitor-responsive esophageal eosinophilia but not eosinophilic esophagitis. Clin Gastroenterol Hepatol 12: 1815-1823, 2014. [DOI] [PubMed] [Google Scholar]
- 67. Frazzoni M, Penagini R, Frazzoni L, et al. Role of reflux in the pathogenesis of eosinophilic esophagitis: comprehensive appraisal with off- and on PPI impedance-pH monitoring. Am J Gastroenterol 114: 1606-1613, 2019. [DOI] [PubMed] [Google Scholar]
- 68. Lucendo AJ, Miehlke S, Schlag C, et al. Efficacy of budesonide orodispersible tablets as induction therapy for eosinophilic esophagitis in a randomized placebo-controlled trial. Gastroenterology 157: 74-86, 2019. [DOI] [PubMed] [Google Scholar]
- 69. Andreae DA, Hanna MG, Magid MS, et al. Swallowed fluticasone propionate is an effective long-term maintenance therapy for children with eosinophilic esophagitis. Am J Gastroenterol 111: 1187-1189, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Straumann A, Conus S, Degen L, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 9: 400-409, 2011. [DOI] [PubMed] [Google Scholar]
- 71. Dellon ES, Woosley JT, Arrington A, et al. Rapid recurrence of eosinophilic esophagitis activity after successful treatment in the observation phase of a randomized, double-blind, double-dummy trial. Clin Gastroenterol Hepatol 18: 1483-1492, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immuno 128: 3-20, 2011. [DOI] [PubMed] [Google Scholar]
- 73. Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 142: 1451-1459, 2012. [DOI] [PubMed] [Google Scholar]
- 74. Sunkara T, Rawla P, Yarlagadda KS, Gaduputi V. Eosinophilic gastroenteritis: diagnosis and clinical perspectives. Clin Exp Gastroenterol 12: 239-253, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schwartz DA, Pardi DS, Murray JA. Use of montelukast as steroid-sparing agent for recurrent eosinophilic gastroenteritis. Dig Dis Sci 46: 1787-1790, 2001. [DOI] [PubMed] [Google Scholar]
- 76. De Maeyer N, Kochuyt AM, Van Moerkercke W, Hiele M. Montelukast as a treatment modality for eosinophilic gastroenteritis. Acta Gastroenterol Belg 74: 570-575, 2011. [PubMed] [Google Scholar]
- 77. Greuter T, Hirano I, Dellon ES. Emerging therapies for eosinophilic esophagitis. J Allergy Clin Immunol 145: 38-45, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Grandinetti T, Biedermann L, Bussmann C, Alex Straumann A, Hruz P. Eosinophilic gastroenteritis: clinical manifestation, natural course, and evaluation of treatment with corticosteroids and vedolizumab. Dig Dis Sci 64: 2231-2241, 2019. [DOI] [PubMed] [Google Scholar]
- 79. Markowitz JE, Jobe L, Miller M, Frost C, Laney Z, Eke R. Safety and efficacy of reslizumab for children and adolescents with eosinophilic esophagitis treated for 9 years. J Pediatr Gastroenterol Nutr 66: 893-897, 2018. [DOI] [PubMed] [Google Scholar]
- 80. Hirano I, Collins MH, Assouline-Dayan Y, et al. RPC4046, a monoclonal antibody against IL13, reduces histologic and endoscopic activity in patients with eosinophilic esophagitis. Gastroenterology 156: 592-603, 2019. [DOI] [PubMed] [Google Scholar]
- 81. Sastre J, Dávila I. Dupilumab: a new paradigm for the treatment of allergic diseases. J Investig Allergol Clin Immunol 28: 139-150, 2018. [DOI] [PubMed] [Google Scholar]
- 82. Dellon ES, Peterson KA, Murray JA, et al. Anti-Siglec-8 antibody for eosinophilic gastritis and duodenitis. N Engl J Med 383: 1624-1634, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]