Abstract
AIM:
The “2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease” provides recommendations to guide clinicians in the diagnosis, genetic evaluation and family screening, medical therapy, endovascular and surgical treatment, and long-term surveillance of patients with aortic disease across its multiple clinical presentation subsets (ie, asymptomatic, stable symptomatic, and acute aortic syndromes).
METHODS:
A comprehensive literature search was conducted from January 2021 to April 2021, encompassing studies, reviews, and other evidence conducted on human subjects that were published in English from PubMed, EMBASE, the Cochrane Library, CINHL Complete, and other selected databases relevant to this guideline. Additional relevant studies, published through June 2022 during the guideline writing process, were also considered by the writing committee, where appropriate.
STRUCTURE:
Recommendations from previously published AHA/ACC guidelines on thoracic aortic disease, peripheral artery disease, and bicuspid aortic valve disease have been updated with new evidence to guide clinicians. In addition, new recommendations addressing comprehensive care for patients with aortic disease have been developed. There is added emphasis on the role of shared decision making, especially in the management of patients with aortic disease both before and during pregnancy. The is also an increased emphasis on the importance of institutional interventional volume and multidisciplinary aortic team expertise in the care of patients with aortic disease.
TOP 10 TAKE-HOME MESSAGES FOR THE DIAGNOSIS AND MANAGEMENT OF AORTIC DISEASE
1. Because outcomes for patients with aortic disease are enhanced at programs with higher volumes, experienced practitioners, and extensive management capabilities, Multidisciplinary Aortic Team care is considered in determining the appropriate timing of intervention.
2. Shared decision-making involving the patient and a multidisciplinary team is highly encouraged to determine the optimal medical, endovascular, and open surgical therapies. In patients with aortic disease who are contemplating pregnancy or who are pregnant, shared decision-making is especially important when considering the cardiovascular risks of pregnancy, the diameter thresholds for prophylactic aortic surgery, and the mode of delivery.
3. Computed tomography, magnetic resonance imaging, and echocardiographic imaging of patients with aortic disease should follow recommended approaches for image acquisition, measurement and reporting of relevant aortic dimensions, and the frequency of surveillance before and after intervention.
4. At centers with Multidisciplinary Aortic Teams and experienced surgeons, the threshold for surgical intervention for sporadic aortic root and ascending aortic aneurysms has been lowered from 5.5 cm to 5.0 cm in selected patients, and even lower in specific scenarios among patients with heritable thoracic aortic aneurysms.
5. In patients who are significantly smaller or taller than average, surgical thresholds may incorporate indexing of the aortic root or ascending aortic diameter to either patient body surface area or height, or aortic cross-sectional area to patient height.
6. Rapid aortic root growth or ascending aortic aneurysm growth, an indication for intervention, is defined as ≥0.5 cm in 1 year or ≥0.3 cm per year in 2 consecutive years for those with sporadic aneurysms and ≥0.3 cm in 1 year for those with heritable thoracic aortic disease or bicuspid aortic valve.
7. In patients undergoing aortic root replacement surgery, valve-sparing aortic root replacement is reasonable if the valve is suitable for repair and when performed by experienced surgeons in a Multidisciplinary Aortic Team.
8. Patients with acute type A aortic dissection, if clinically stable, should be considered for transfer to a high-volume aortic center to improve survival. The operative repair of type A aortic dissection should entail at least an open distal anastomosis rather than just a simple supracoronary interposition graft.
9. There is an increasing role for thoracic endovascular aortic repair in the management of uncomplicated type B aortic dissection. Clinical trials of repair of thoracoabdominal aortic aneurysms with endografts are reporting results that suggest endovascular repair is an option for patients with suitable anatomy.
10. In patients with aneurysms of the aortic root or ascending aorta, or those with aortic dissection, screening of first-degree relatives with aortic imaging is recommended.
PREAMBLE
Since 1980, the American College of Cardiology (ACC) and American Heart Association (AHA) have translated scientific evidence into clinical practice guidelines with recommendations to improve cardiovascular health. These guidelines, which are based on systematic methods to evaluate and classify evidence, provide a foundation for the delivery of quality cardiovascular care. The ACC and AHA sponsor the development and publication of clinical practice guidelines without commercial support, and members volunteer their time to the writing and review efforts. Guidelines are official policy of the ACC and AHA. For some guidelines, the ACC and AHA collaborate with other organizations.
Intended Use
Clinical practice guidelines provide recommendations applicable to patients with or at risk of developing cardiovascular disease. The focus is on medical practice in the United States, but these guidelines are relevant to patients throughout the world. Although guidelines may be used to inform regulatory or payer decisions, the intent is to improve quality of care and align with patients’ interests. Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances and should not replace clinical judgment.
Clinical Implementation
Management, in accordance with guideline recommendations, is effective only when followed by both practitioners and patients. Adherence to recommendations can be enhanced by shared decision-making between clinicians and patients, with patient engagement in selecting interventions on the basis of individual values, preferences, and associated conditions and comorbidities.
Methodology and Modernization
The AHA/ACC Joint Committee on Clinical Practice Guidelines (Joint Committee) continuously reviews, updates, and modifies guideline methodology on the basis of published standards from organizations, including the Institute of Medicine,1,2 and on the basis of internal reevaluation. Similarly, presentation and delivery of guidelines are reevaluated and modified in response to evolving technologies and other factors to optimally facilitate dissemination of information to health care professionals at the point of care.
Numerous modifications to the guidelines have been implemented to make them shorter and enhance “user friendliness.” Guidelines are written and presented in a modular, “knowledge chunk” format, in which each chunk includes a table of recommendations, a brief synopsis, recommendation-specific supportive text and, when appropriate, flow diagrams or additional tables. Hyperlinked references are provided for each modular knowledge chunk to facilitate quick access and review.
In recognition of the importance of cost–value considerations, in certain guidelines, when appropriate and feasible, an analysis of value for a drug, device, or intervention may be performed in accordance with the ACC/AHA methodology.3
To ensure that guideline recommendations remain current, new data will be reviewed on an ongoing basis by the writing committee and staff. Going forward, targeted sections/knowledge chunks will be revised dynamically after publication and timely peer review of potentially practice-changing science. The previous designations of “full revision” and “focused update” will be phased out. For additional information and policies on guideline development, readers may consult the ACC/AHA guideline methodology manual4 and other methodology articles.5–7
Selection of Writing Committee Members
The Joint Committee strives to ensure that the guideline writing committee contains requisite content expertise and is representative of the broader cardiovascular community by selection of experts across a spectrum of backgrounds, representing different geographic regions, sexes, races, ethnicities, intellectual perspectives/biases, and clinical practice settings. Organizations and professional societies with related interests and expertise are invited to participate as partners or collaborators.
Relationships With Industry and Other Entities
The ACC and AHA have rigorous policies and methods to ensure that documents are developed without bias or improper influence. The complete policy on relationships with industry and other entities (RWI) can be found online. Appendix 1 of the guideline lists writing committee members’ relevant RWI. For the purposes of full transparency, their comprehensive disclosure information is available in a Supplemental Appendix. Comprehensive disclosure information for the Joint Committee is also available online.
Evidence Review and Evidence Review Committees
In developing recommendations, the writing committee uses evidence-based methodologies that are based on all available data.4,5 Literature searches focus on randomized controlled trials (RCTs) but also include registries, nonrandomized comparative and descriptive studies, case series, cohort studies, systematic reviews, and expert opinion. Only key references are cited.
An independent evidence review committee is commissioned when there are ≥1 question(s) deemed of utmost clinical importance and merit formal systematic review to determine which patients are most likely to benefit from a drug, device, or treatment strategy, and to what degree. Criteria for commissioning an evidence review committee and formal systematic review include absence of a current authoritative systematic review, feasibility of defining the benefit and risk in a time frame consistent with the writing of a guideline, relevance to a substantial number of patients, and likelihood that the findings can be translated into actionable recommendations. Evidence review committee members may include methodologists, epidemiologists, clinicians, and biostatisticians. Recommendations developed by the writing committee on the basis of the systematic review are marked “SR”.
Guideline-Directed Medical Therapy
The term guideline-directed medical therapy encompasses clinical evaluation, diagnostic testing, and both pharmacological and procedural treatments. For these and all recommended drug treatment regimens, the reader should confirm dosage with product insert material and evaluate for contraindications and interactions. Recommendations are limited to drugs, devices, and treatments approved for clinical use in the United States.
Keywords: AHA Scientific Statements, abdominal aortic aneurysm, aortic dissection, aortitis, aortopathy, bicuspid aortic valve, blunt traumatic aortic injury, cardiac surgery, guidelines, endovascular aortic repair, heritable thoracic aortic disease, intramural hematoma, malperfusion syndrome, Marfan syndrome, Loeys-Dietz syndrome, penetrating atherosclerotic ulcer, thoracic aortic aneurysm, thoracoabdominal aortic aneurysm, thoracic endovascular aortic repair, vascular surgery
1. INTRODUCTION
1.1. Methodology and Evidence Review
The recommendations listed in this guideline are, whenever possible, evidence based. An initial extensive evidence review, which included literature derived from research involving human subjects, published in English, and indexed in MEDLINE (through PubMed), EMBASE, the Cochrane Library, the Agency for Healthcare Research and Quality, and other selected databases relevant to this guideline, was conducted from February 2021 to April 2021. Search terms included both key words and index terms (eg, MeSH, Emtree); search terms included but were not limited to the following: aortic occlusion; aortic aneurysm; aortic aneurysm, thoracic; aortic aneurysm, abdominal; surveillance after endovascular aneurysm repair; diagnostic imaging; monitoring; surveillance; imaging; aorta; aortic; computed tomography; ultrasound; magnetic resonance imaging; arterial occlusive diseases; aortic diseases; aortic atherosclerosis; atherosclerosis; clinical trial; observational study; randomized controlled trial; review; atherosclerotic aortic disease; plaque, atherosclerotic; aorta; aortitis; infectious; autoimmune; aortic rupture; penetrating aortic ulcers; comparative studies; nonexperimental studies; type A aortic dissection; type A; type B; aneurysm, dissecting; aorta and echocardiography. The final evidence tables are included in the Online Data Supplement and summarize the evidence used by the writing committee to formulate recommendations. References selected and published in the present document are representative and not all-inclusive.
1.2. Organization of the Writing Committee
The writing committee consisted of clinicians, cardiologists, internists, interventionalists, surgeons, radiologists, anesthesiologists, a nurse practitioner, and a lay/patient representative. The writing committee included representatives from the ACC, AHA, American Association for Thoracic Surgery, American College of Radiology, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons (STS), and Society for Vascular Surgery (SVS). Appendix 1 of the present document lists writing committee members’ relevant RWI. For the purposes of full transparency, the writing committee members’ comprehensive disclosure information is available in a Supplemental Appendix.
1.3. Document Review and Approval
The Joint Committee appointed a peer review committee to review the document. The peer review committee was comprised of individuals nominated by ACC, AHA, and the collaborating organizations. Reviewers’ RWI information was distributed to the writing committee and is published in this document (Appendix 2).
This document was approved for publication by the governing bodies of the ACC and the AHA and was endorsed by the American Association for Thoracic Surgery, American College of Radiology, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, Society for Vascular Medicine, and Society for Vascular Surgery.
1.4. Scope of the Guideline
In developing the “2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease” (2022 aortic disease guideline), the writing committee reviewed previously published guidelines. Table 1 contains a list of these publications deemed pertinent to this writing effort and is intended for use as a resource, thus obviating the need to repeat existing guideline recommendations.
Table 1.
Associated Guidelines
| Title | Organization | Publication Year (Reference) |
|---|---|---|
| Guidelines | ||
| Thoracic endovascular aortic repair for descending thoracic aortic aneurysms | SVS | 20211 |
| Valvular heart disease | ACC/AHA | 20202 |
| Large vessel vasculitis | EULAR | 20203 |
| Blood cholesterol | AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA | 20194 |
| Congenital heart disease | AHA/ACC | 20195 |
| Abdominal aortic aneurysm | SVS | 20186 |
| High blood pressure | ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA | 20187 |
| Lower extremity peripheral artery disease | AHA/ACC | 20178 |
| Descending thoracic aorta diseases | ESVS | 20179 |
| Bicuspid aortic valves statement of clarification | ACC/AHA | 201610 |
| Vascular graft infections, mycotic aneurysms, and endovascular infections | AHA | 201611 |
| Endovascular repair of traumatic thoracic aortic injury | SVS | 201112 |
| Thoracic aortic disease | ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM | 201013 |
| Coronary and other atherosclerotic vascular disease | AHA/ACC | 200614 |
| Acute type A aortic dissection | AATS | 202115 |
| Type B aortic dissection | STS | 202216 |
AACVPR indicates American Association of Cardiovascular and Pulmonary Rehabilitation; AAPA, American Academy of Physician Assistants; AATS, American Association for Thoracic Surgery; ABC, Association of Black Cardiologists; ACC, American College of Cardiology; ACCF, American College of Cardiology Foundation; ACPM, American College of Preventive Medicine; ACR, American College of Radiology; ADA, American Diabetes Association; AGS, American Geriatrics Society; AHA, American Heart Association; APhA, American Pharmacists Association; ASA, American Society of Anesthesiologists; ASH, American Society of Hematology; ASPC, American Society for Preventive Cardiology; ESVS, European Society for Vascular Surgery; EULAR, European League Against Rheumatism; NLA, National Lipid Association; NMA, National Medical Association; PCNA, Preventive Cardiovascular Nurses Association; SCA, Society of Cardiovascular Anesthesiologists; SCAI, Society for Cardiovascular Angiography and Interventions; SIR, Society of Interventional Radiology; STS, Society of Thoracic Surgeons; SVM, Society for Vascular Medicine; and SVS, Society for Vascular Surgery.
1.5. Class of Recommendations and Level of Evidence
The Class of Recommendation (COR) indicates the strength of recommendation, encompassing the estimated magnitude and certainty of benefit in proportion to risk. The Level of Evidence (LOE) rates the quality of scientific evidence supporting the intervention on the basis of the type, quantity, and consistency of data from clinical trials and other sources (Table 2).1
Table 2.
Applying American College of Cardiology/American Heart Association Class of Recommendation and Level of Evidence to Clinical Strategies, Interventions, Treatments, or Diagnostic Testing in Patient Care* (Updated May 2019)
| CLASS (STRENGTH) OF RECOMMENDATION | |
|
| |
| CLASS 1 (STRONG) | Benefit >>> Risk |
|
| |
| Suggested phrases for writing recommendations: | |
| • Is recommended | |
| • Is indicated/useful/effective/beneficial | |
| • Should be performed/administered/other | |
| • Comparative-Effectiveness Phrases†: | |
| – Treatment/strategy A is recommended/indicated in preference to treatment B | |
| – Treatment A should be chosen over treatment B | |
|
| |
| CLASS 2a (MODERATE) | Benefit >> Risk |
|
| |
| Suggested phrases for writing recommendations: | |
| • Is reasonable | |
| • Can be useful/effective/beneficial | |
| • Comparative-Effectiveness Phrases†: | |
| – Treatment/strategy A is probably recommended/indicated in preference to treatment B | |
| – It is reasonable to choose treatment A over treatment B | |
|
| |
| CLASS 2b (WEAK) | Benefit ≥ Risk |
|
| |
| Suggested phrases for writing recommendations: | |
| • May/might be reasonable | |
| • May/might be considered | |
| • Usefulness/effectiveness is unknown/unclear/uncertain or not well-established | |
|
| |
| CLASS 3: No Benefit (MODERATE) (Generally, LOE A or B use only) | Benefit = Risk |
|
| |
| Suggested phrases for writing recommendations: | |
| • Is not recommended | |
| • Is not indicated/useful/effective/beneficial | |
| • Should not be performed/administered/other | |
|
| |
| Class 3: Harm (STRONG) | Risk > Benefit |
|
| |
| Suggested phrases for writing recommendations: | |
| • Potentially harmful | |
| • Causes harm | |
| • Associated with excess morbidity/mortality | |
| • Should not be performed/administered/other | |
|
| |
| LEVEL (QUALITY) OF EVIDENCE ‡ | |
|
| |
| LEVEL A | |
|
| |
| • High-quality evidence‡ from more than 1 RCT | |
| • Meta-analyses of high-quality RCTs | |
| • One or more RCTs corroborated by high-quality registry studies | |
|
| |
| LEVEL B-R | (Randomized) |
|
| |
| • Moderate-quality evidence‡ from 1 or more RCTs | |
| • Meta-analyses of moderate-quality RCTs | |
|
| |
| LEVEL B-NR | (Nonrandomized) |
|
| |
| • Moderate-quality evidence‡ from 1 or more well-designed, well-executed nonrandomized studies, observational studies, or registry studies | |
| • Meta-analyses of such studies | |
|
| |
| LEVEL C-LD | (Limited Data) |
|
| |
| • Randomized or nonrandomized observational or registry studies with limitations of design or execution | |
| • Meta-analyses of such studies | |
| • Physiological or mechanistic studies in human subjects | |
|
| |
| LEVEL C-EO | (Expert Opinion) |
|
| |
| • Consensus of expert opinion based on clinical experience | |
COR and LOE are determined independently (any COR may be paired with any LOE).
A recommendation with LOE C does not imply that the recommendation is weak. Many important clinical questions addressed in guidelines do not lend themselves to clinical trials. Although RCTs are unavailable, there may be a very clear clinical consensus that a particular test or therapy is useful or effective.
The outcome or result of the intervention should be specified (an improved clinical outcome or increased diagnostic accuracy or incremental prognostic information).
For comparative-effectiveness recommendations (COR 1 and 2a; LOE A and B only), studies that support the use of comparator verbs should involve direct comparisons of the treatments or strategies being evaluated.
The method of assessing quality is evolving, including the application of standardized, widely-used, and preferably validated evidence grading tools; and for systematic reviews, the incorporation of an Evidence Review Committee.
COR indicates Class of Recommendation; EO, expert opinion; LD, limited data; LOE, Level of Evidence; NR, nonrandomized; R, randomized; and RCT, randomized controlled trial.
2. NORMAL ANATOMY, ABNORMAL ANATOMY, AND DEFINITIONS
2.1. Normal Aortic Anatomy
The aorta is the largest artery in the body and can be divided into 5 main anatomic segments (Figure 1): the root or sinus segment, which extends from the aortic valve annulus to the sinotubular junction; the ascending thoracic aorta, which extends from the sinotubular junction to the innominate artery; the aortic arch, which extends from the innominate to the left subclavian artery; the descending thoracic aorta, which extends from the left subclavian artery to the diaphragm; and the abdominal aorta, which extends from the diaphragm to the level of the aortic bifurcation.
Figure 1.
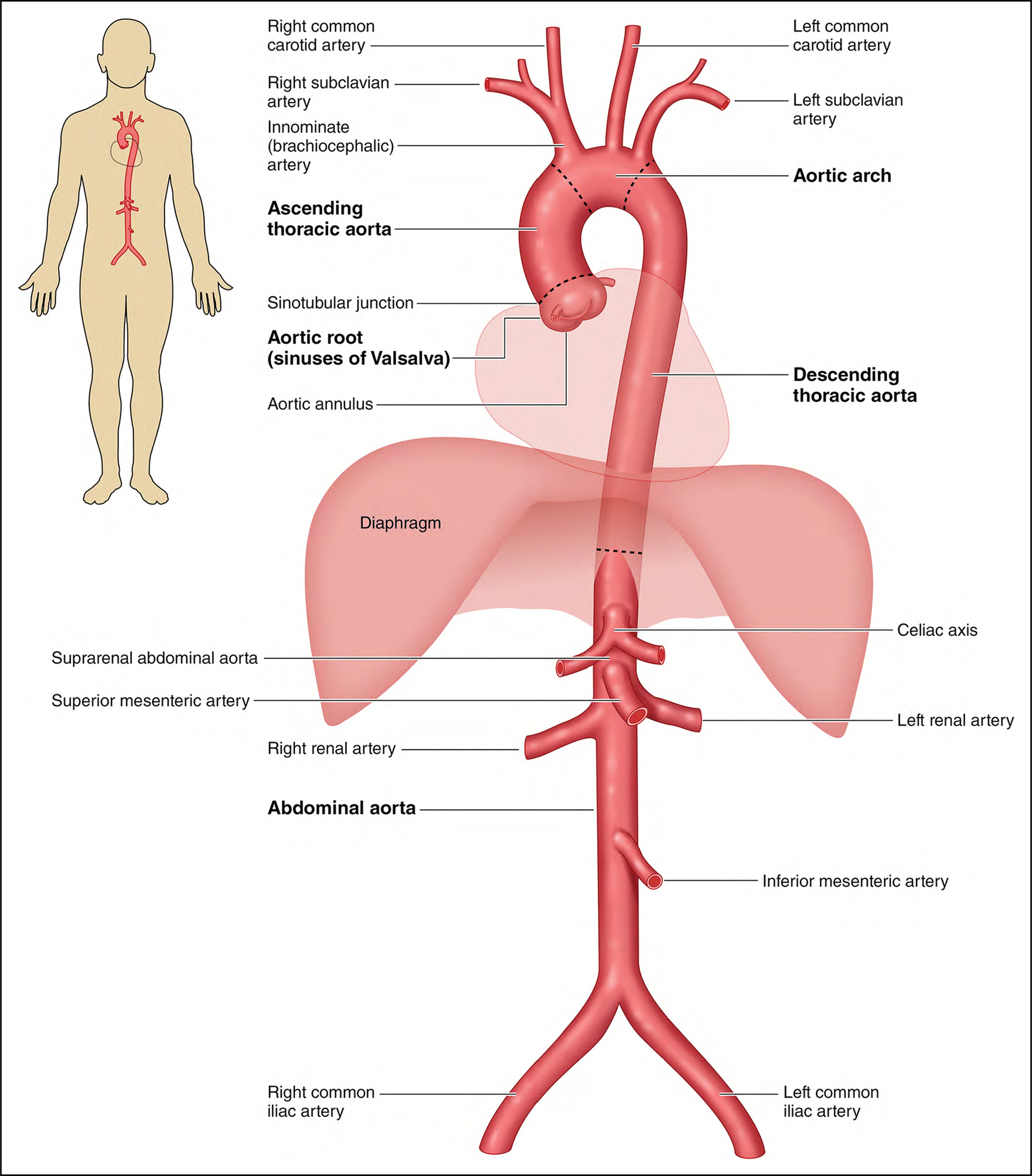
The Anatomy of the Aorta and Its Main Branches.
The aortic wall is composed of 3 layers (Figure 2): a thin inner intima, a thicker central media, and a thin outer adventitia. The intima consists of a layer of endothelial cells within a matrix of connective tissue. The media consists of smooth muscle cells, elastic fibers, collagen proteins, and polysaccharides sandwiched in >50 layers known as elastic lamellae. The media provides strength and distensibility to the aorta, features that are critical to circulatory function. The adventitia is composed of connective tissue, fibroblasts, nerves, and the vasa vasorum, which perfuse the outer aortic wall and a substantial portion of the media.
Figure 2. A Simplified Diagram Depicting the Key Histologic Components of the Aortic Wall.
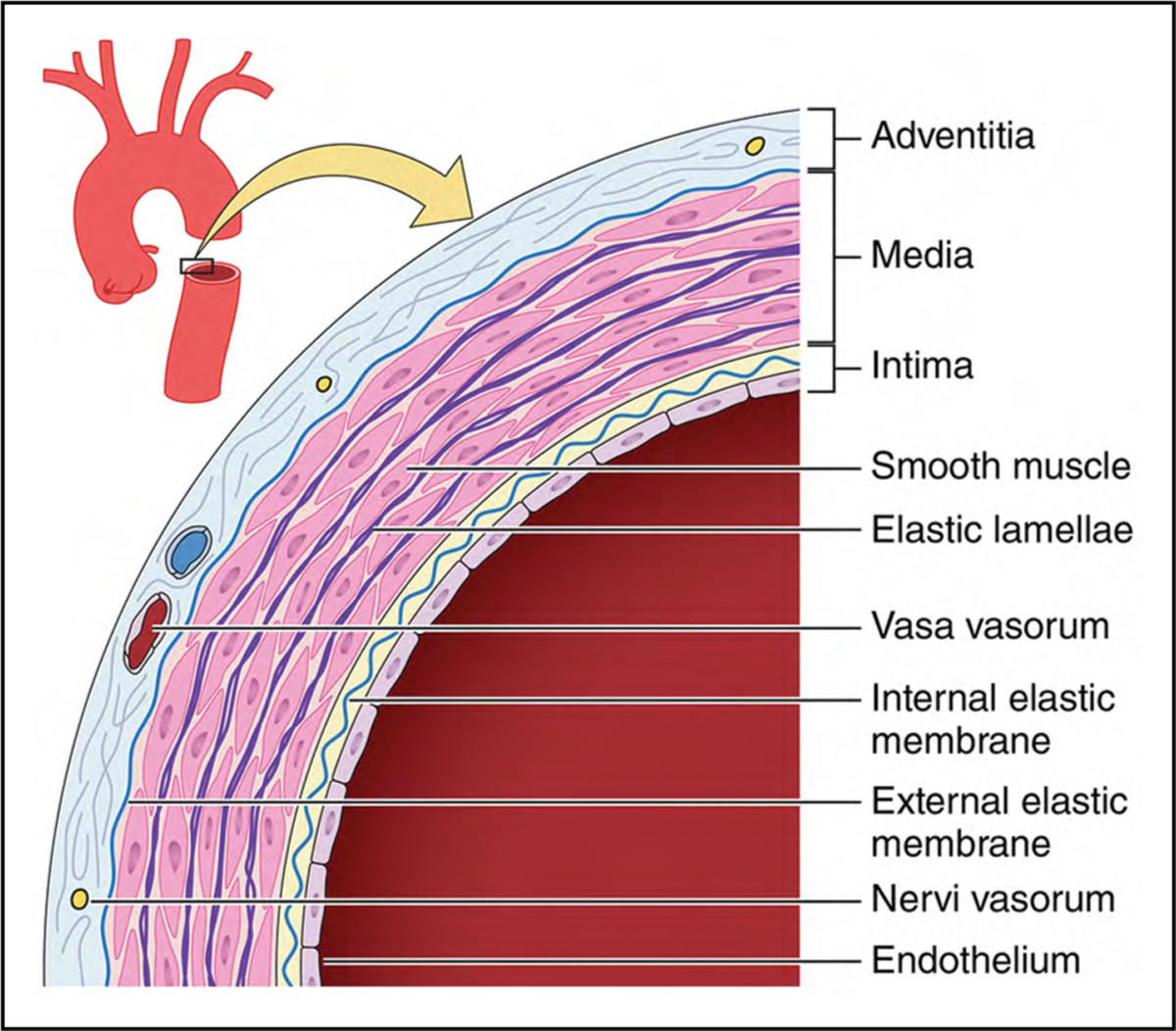
The medial layer in human aortas contains >50 alternating layers of elastin and smooth muscle cells (whereas only 5 are shown in this simplified illustration). Adapted (cropped) from “Illustration of tunics of the arteries vs veins” by Malgosia Wilk-Blaszczak, used under CC-BY 4.0. “Illustration of tunics of the arteries vs veins” is adapted (cropped) from figure 20.3 in BC OpenStax Anatomy and Physiology used under CC-BY 4.0.
2.2. Aortic Landing Zones
In addition to the standard anatomic descriptors of the aortic anatomy, there is a more technical classification of aortic anatomy that is used to plan, guide, and report aortic interventions, especially endovascular stent-grafting. Because the clinical success of thoracic aortic endovascular procedures is influenced by the proximal sealing zone, in this system the thoracic and abdominal aorta are divided into 11 landing zones, as detailed in Figure 3.
Figure 3. Classification of Aortic Anatomic Segments by 11 Landing Zones.
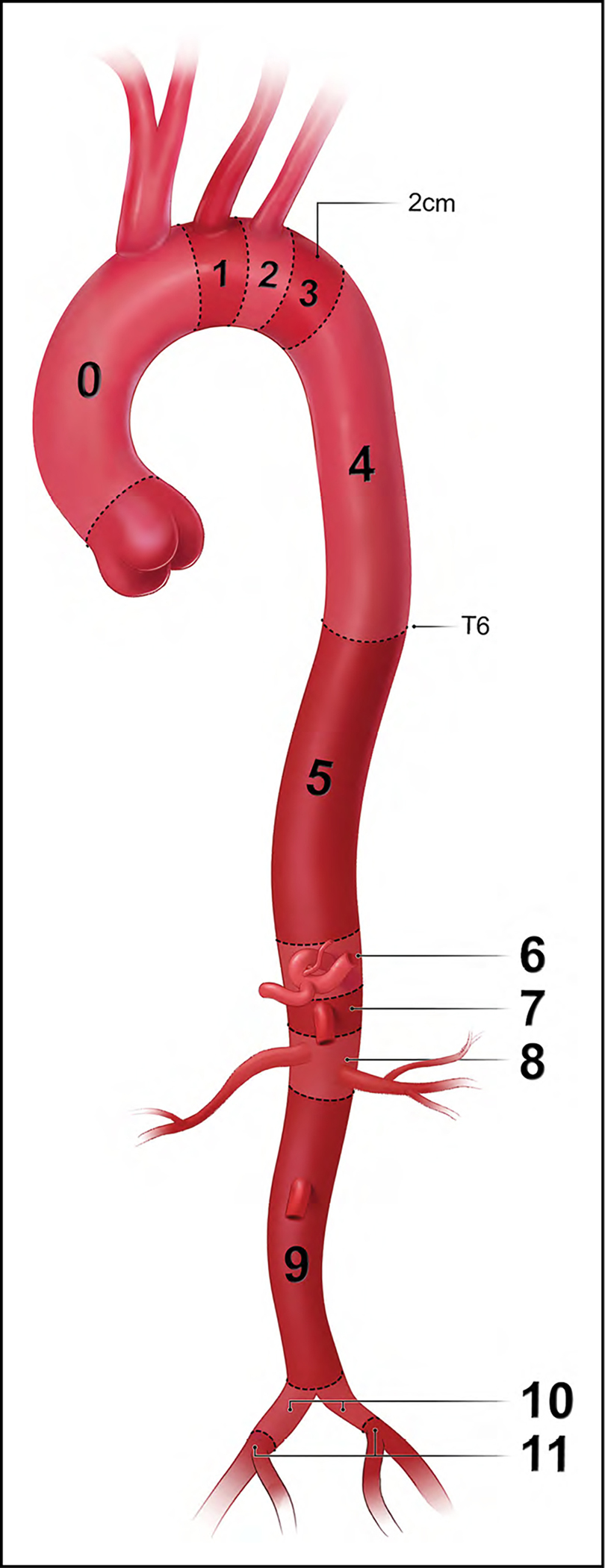
Zone 0 (involves the ascending to distal end of the origin of the innominate artery); Zone 1 (involves the origin of the left common carotid; between the innominate and the left carotid); Zone 2 (involves the origin of the left subclavian; between the left carotid and the left subclavian); Zone 3 (involves the proximal descending thoracic aorta down to the T4 vertebral body; the first 2 cm distal to the left subclavian); Zone 4 (the end of zone 3 to the mid-descending aorta – T6); Zone 5 (the mid-descending aorta to the celiac); Zone 6 (involves the origin of the celiac; the celiac to the superior mesenteric); Zone 7 (involves the origin of the superior mesenteric artery; the superior mesenteric to the renals); Zone 8 (involves the origin of the renal arteries; the renal to the infrarenal abdominal aorta); Zone 9 (the infrarenal abdominal aorta to the level of aortic bifurcation ); Zone 10 (the common iliac); Zone 11 (involves the origin of the external iliac arteries). From Czerny et al.1 Copyright 2019, with permission from Elsevier, Inc., Now Medical Studios, and Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery.
Note that Roselli et al2 have proposed a modification of zone 0, dividing it into 3 subsegments, in which 0A extends from the annulus to the distal margin of the highest coronary, 0B extends above the coronary to the distal margin of the right pulmonary artery, and 0C extends from the right pulmonary artery to the distal end of the origin of the innominate artery.
2.3. Definitions of Dilation and Aneurysm of the Aortic Root and Ascending Thoracic Aorta
The conventional definition of an arterial aneurysm is any artery that is dilated to at least 1.5 times its expected normal diameter.3 This definition applies well to the abdominal and descending thoracic aorta. However, it has long been recognized that this definition fails when it comes to defining aneurysms of the aortic root and ascending thoracic aorta. For example, a man in his 40s would be expected to have an average aortic root diameter of 3.5 cm; applying the standard definition of ≥1.5 times reference diameter, his aortic root would have to reach 5.25 cm before it would be considered an aneurysm, whereas most experts would consider his aorta to be an aneurysm well below that diameter. Indeed, if this patient had Marfan syndrome or a familial thoracic aortic aneurysm, aortic repair would be recommended at a diameter of ≤5.0 cm, a size that would not even be large enough to be termed an “aneurysm.”
The most important consideration in deciding the diameter thresholds at which to call the root and ascending aorta dilated or aneurysmal is based on the natural history of such abnormal aortas. Borger et al4 studied 201 patients with bicuspid aortic valve (BAV) undergoing aortic valve replacement (AVR) (those undergoing concomitant replacement of the ascending aorta were excluded) and followed them for 10 to 15 years; they found that those with baseline aortic diameters of 4.5 cm to 4.9 cm had a significantly increased risk of aneurysm, dissection, or sudden death (P<0.001) compared with those with diameters <4.5 cm (Figure 4).
Figure 4. Freedom From Ascending Aortic Complications for Patients With Bicuspid Aortic Valve Disease.
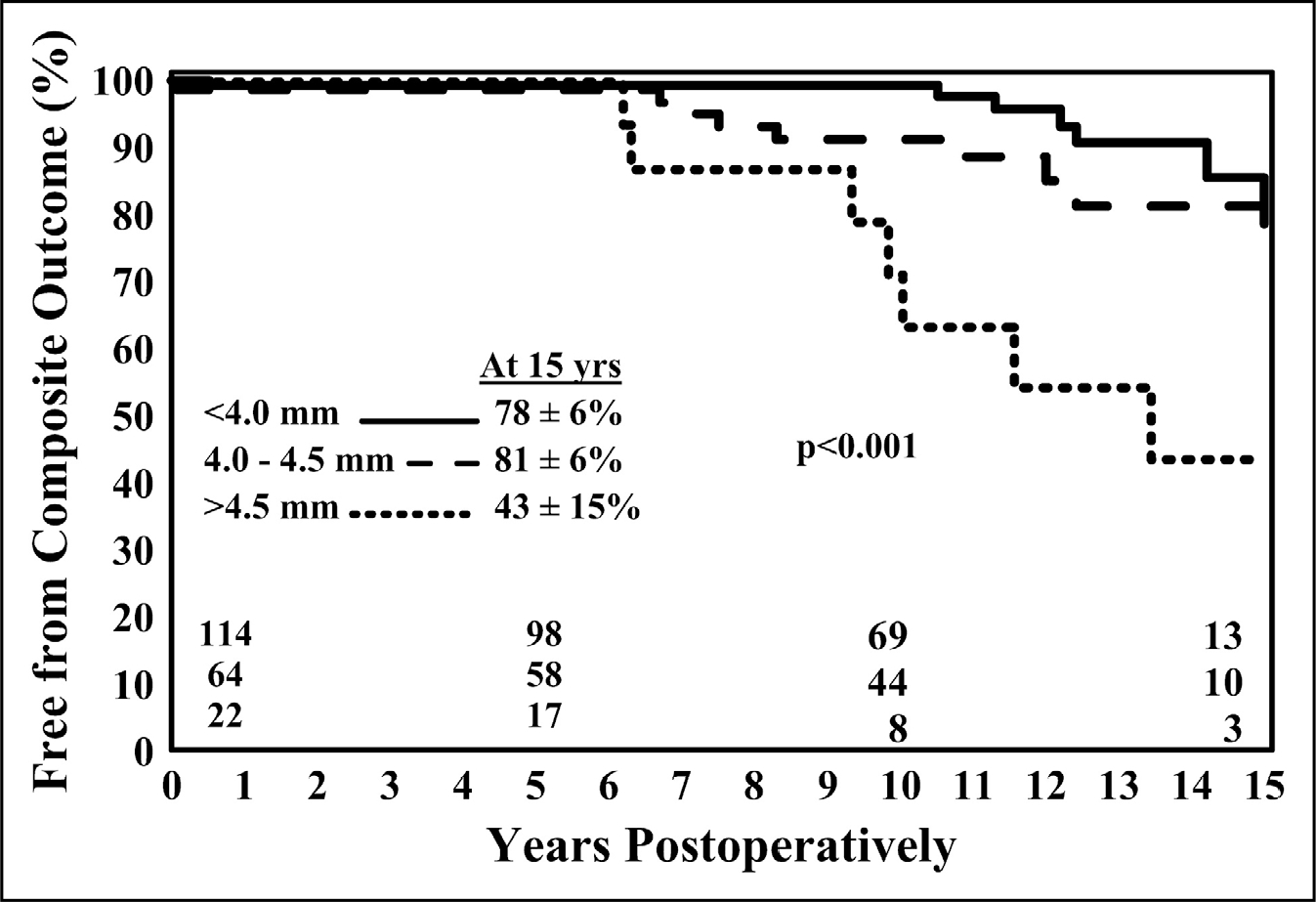
Patients with moderate dilation of the ascending aorta (4.5 cm–4.9 cm) had a significantly increased risk of future aortic complications (aneurysm, dissection, or sudden death). Reprinted from Borger et al.4 Copyright 2004, with permission from Elsevier, Inc. and the American Association for Thoracic Surgery.
To evaluate the risk of type A aortic dissection at various diameters below the traditional 5.5 cm threshold for prophylactic aortic repair, Paruchuri et al5 plotted a distribution curve of ascending aortic size in a community sample from the MESA (Multi-Ethnic Study of Atherosclerosis) database. They then analyzed the number of dissections (numerator) at each aortic diameter and the population at risk at each aortic diameter (denominator). They found that, relative to a control aortic diameter of ≤3.4 cm, a diameter of 4.0 cm to 4.4 cm conferred an 89-fold increased risk of dissection, and a diameter of ≥4.5 cm conferred a 6000-fold increased risk (Figure 5), albeit these are only relative risk estimates and do not inform absolute risk. It follows that the increase in risk at 4.0 cm to 4.4 cm justifies defining an aorta of this size “dilated,” and the abrupt increase in risk at a diameter of ≥4.5 cm justifies defining an aorta of this size as an “aneurysm.” Using this approach, of the subjects in the MESA database, only 2.6% would be considered to have a dilated aorta and only 0.2% to have an aneurysm.
Figure 5. Relative Risk of Aortic Dissection by Size Range.
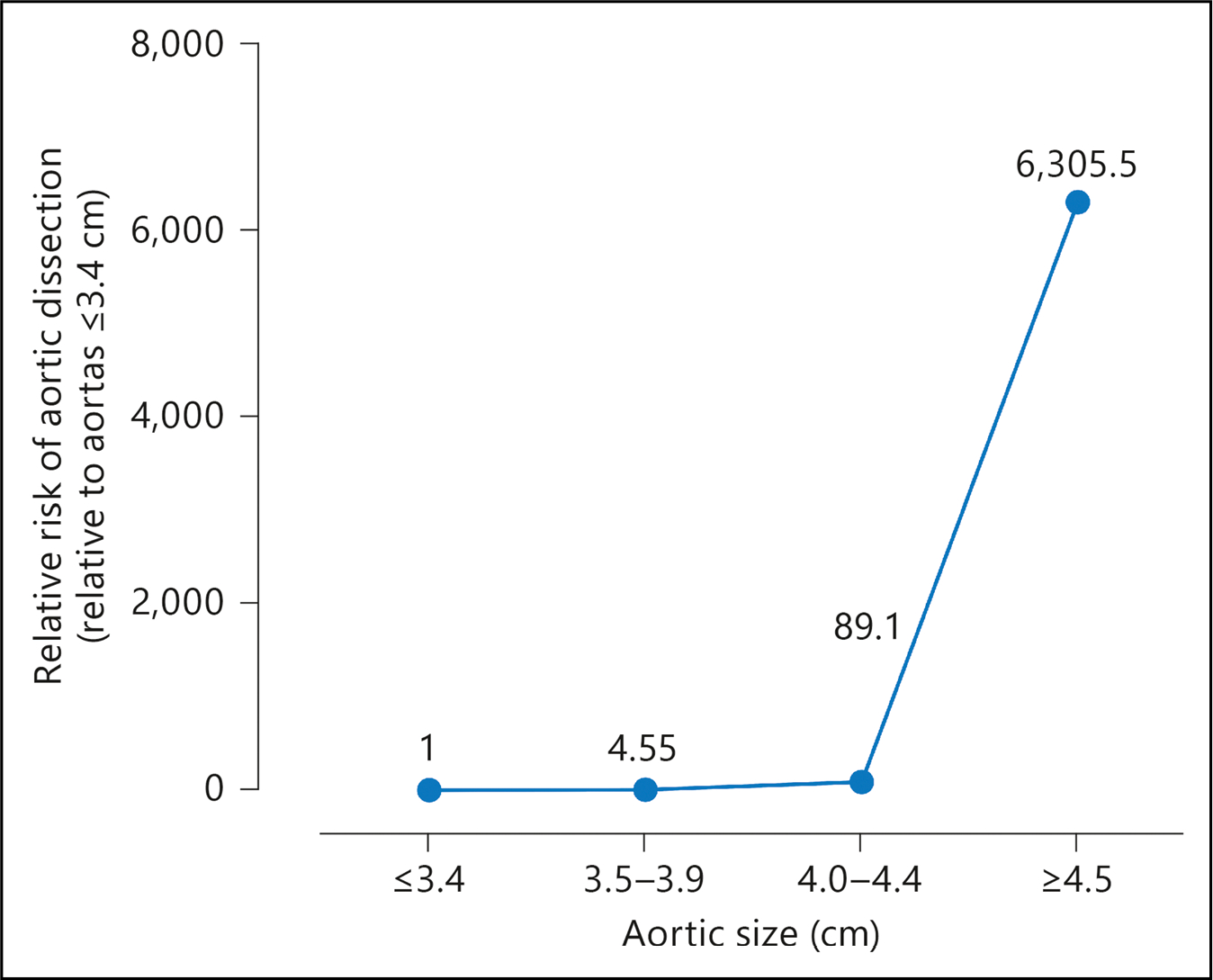
The relative risk of aortic dissection begins to increase appreciably at a diameter of 4.0 cm to 4.4 cm and then increases dramatically at a diameter of ≥4.5 cm. Reprinted from Paruchuri et al.5 Copyright 2005, with permission from Karger Publishers, Basel, Switzerland.
This definition of a dilated ascending aorta being ≥4.0 cm is consistent with what was proposed in the 2014 European Society of Cardiology guidelines on the diagnosis and treatment of aortic diseases, in which aortic “dilation” was similarly defined as an aorta diameter of >4.0 cm.6
Finally, in the clinical setting, the term “dilation” is preferred to “ectasia” to describe mild aortic enlargement. Historically, there has been a lack of uniformity in the use of “ectasia” in image interpretation. Many radiologists use “ectatic” rather than “dilated” to describe a mildly enlarged aorta, whereas others use “ectatic” to describe an abnormal aortic shape, such as a “tortuous” aorta.7 Even more problematic is the fact that some imaging groups use the term “ectasia” to describe larger aortas, such as those 4.5 cm to 5.4 cm in diameter,8 which overlaps with what most experts would consider to be an aneurysm. Lastly, in imaging of the coronary arteries, “ectasia” is typically used to describe diffuse (rather than focal) coronary artery dilation,9 which may lead to some clinical uncertainty when “ectasia” is applied to the aorta.
2.3.1. Normalizing Aortic Root and Ascending Aortic Diameters for Body Size
As with the aortic diameter thresholds for surgery presented in this guideline, it recognized that the 4.0 cm and 4.5 cm diameter thresholds discussed previously are intended for those whose height, body surface area (BSA), or both is within 1 to 2 standard deviations of the mean. For male and female patients who are significantly shorter or taller than average, these diameters need to be adjusted downward or upward, accordingly. Several methods to normalize aortic diameter are currently used in clinical practice and clinical research.
The Z-Score
The z-score is routinely used to assess aortic dilation in the pediatric population, as changes in a child’s age and body size make it especially challenging to define normal aortic size and to distinguish normal from pathologic aortic growth. Nomograms have been established correlating BSA and aortic root diameter to generate the z-score. One limitation of the reliance on BSA is that there are multiple formulae to calculate BSA that yield different results for the same patient. A second limitation is that multiple z-score calculators exist, each performing differently.10 Finally, most of the literature on the natural history of acute aortic syndromes (AAS) is based on aortic diameters, whereas reports of outcome based on z-scores are limited, so the z-score is not typically used to report the degree of aortic dilation in adults.
The Aortic Size Index and Aortic Height Index
Most often, in the clinical care of adult patients, aortic diameters are normalized using a ratio of aortic diameter to BSA or aortic diameter to the patient’s height. In 2006, Davies et al11 showed that aortic size index (ASI), which is defined as aortic diameter (cm)/BSA(m2), is a better predictor of adverse aortic events than diameter alone, and that a simple nomogram could be used to stratify those with aortic aneurysms into low-, medium-, and high-risk groups. However, it is unclear whether the weight of an adult has a significant impact on the expected normal aortic diameter, and one would not expect a patient’s aorta to grow or shrink with significant fluctuations in weight. Zafar et al12 therefore examined whether aortic height index (AHI), which is defined as aortic diameter (cm)/patient height (m), might perform better than the ASI, and they reported that the AHI performed at least as well as the ASI12 and had the advantage of being simpler to calculate.
The Cross-Sectional Area to Height Ratio
Another approach to normalizing aortic size to height was proposed by Svensson et al in 200213 in which they calculated a ratio of the cross-sectional area of the aorta (cm) to the patient’s height (m). The initial studies used a cross-sectional area to height ratio of >10 cm2/m as a threshold for intervention because of a significant increase in risk of adverse events; notably, in more contemporary reports, this group has shown the simpler cross-sectional area to height ratio of ≥10 cm2/m (rather than >10 cm2/m) as the threshold predictive of increased risk.14,15
2.4. Definitions and Classification of Acute Aortic Syndrome (AAS)
AAS are life-threatening conditions in which there is a breach in the integrity of the aortic wall. The most common AAS are aortic dissection, intramural hematoma (IMH), and penetrating atherosclerotic ulcer (PAU), all of which can lead to rupture (Figure 6).
Figure 6. Acute Aortic Syndromes.
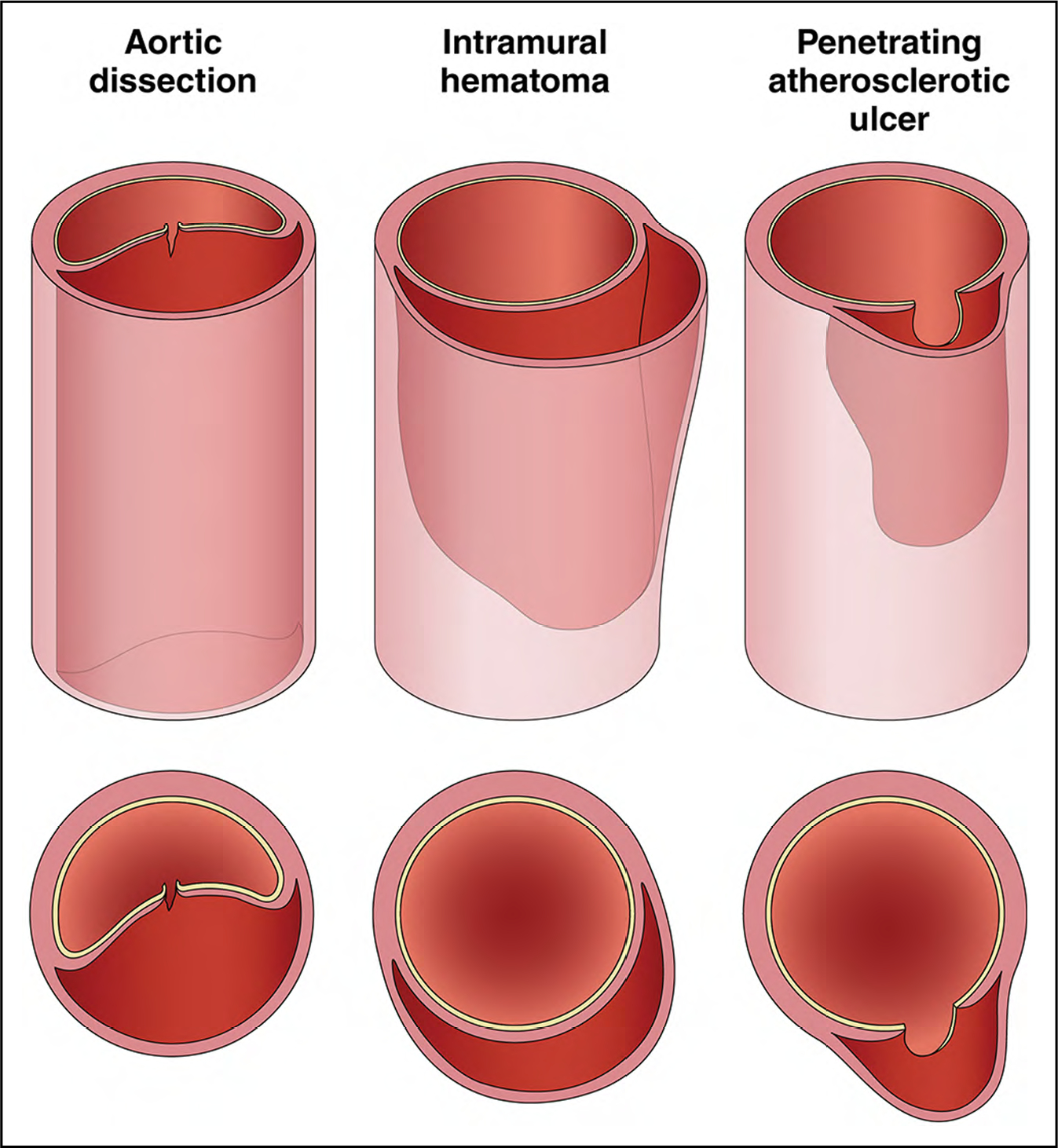
In aortic dissection, a tear in the aortic intima allows blood to penetrate the aortic media, pushing the dissection flap into the middle of the aorta, separating the true from the false lumen. In intramural hematoma, blood leaks into the aortic media at low pressure, forming a thrombus that pushes the outer wall of the aorta outward, leaving a relatively normal appearing aortic lumen. A penetrating atherosclerotic ulcer allows blood to enter the aortic media, but atherosclerotic scarring of the aorta typically confines the blood collection, often resulting in a localized dissection or pseudoaneurysm. Adapted with permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Clough et al,1 Copyright 2015.
2.4.1. Aortic Dissection
Aortic dissection is the most common of the AAS. Aortic dissection occurs when there is an intimal tear that allows the blood to pass through the tear and into the aortic media, splitting the intima in 2 longitudinally, creating a dissection flap that divides the true lumen from a newly formed false lumen (Figure 6). The dissection flap can propagate in an antegrade or retrograde fashion and lead to a number of life-threatening complications, including acute aortic regurgitation (AR), myocardial ischemia, cardiac tamponade, acute stroke, or malperfusion syndromes. The blood surging in the false lumen may rupture back through the intima into the true lumen, creating a reentry tear. If the blood in the false lumen instead tears through the outer media and adventitia, aortic rupture will result. The incidence of aortic dissection is estimated to be 5 to 30 cases per million people per year, with men more commonly affected. Most dissections occur in those between the ages of 50 to 70 years, although patients with Marfan syndrome, BAV, Loeys-Dietz syndrome, and vascular Ehlers-Danlos syndrome, present at younger ages.
2.4.1.1. Definition
Aortic dissection has traditionally been defined as “acute” during the first 2 weeks after symptom onset and “chronic” when beyond the second week. Investigators from the International Registry of Acute Aortic Dissection (IRAD) proposed that aortic dissection be divided into 4 temporal types: hyperacute (<24 h), acute (2–7 d), subacute (8–30 d), and chronic (>30 d).2 The most contemporary temporal classification system, proposed by the SVS and STS, similarly divides aortic dissection into 4 temporal types, as shown in Table 3, to improve prognostication and guide decision making about the timing and types of potential intervention.
Table 3.
Classification of Aortic Dissection Chronicity Based on the 2020 SVS/STS Reporting Standards
| Chronicity | Time From Onset of Symptoms |
|---|---|
| Hyperacute | <24 h |
| Acute | 1–14 d |
| Subacute | 15–90 d |
| Chronic | >90 d |
Adapted with permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Clough RE, et al.1 Copyright 2015.
STS indicates Society of Thoracic Surgeons; and SVS, Society for Vascular Surgery.
Acute aortic dissection of the ascending aorta is highly lethal in symptomatic patients left untreated, with an early mortality of 1% to 2% per hour after symptom onset.3 The mortality rate is increased among patients who present with or develop complications of cardiac tamponade (with or without cardiogenic shock), acute myocardial ischemia or infarction, stroke, or organ malperfusion.3 Patients with uncomplicated acute type B aortic dissection have a 30-day mortality rate of 10%. However, when patients with acute type B aortic dissection develop complications, such as malperfusion or rupture, the mortality rate increases to 20% by day 2 and to 25% by day 30.3
2.4.1.2. Classification
There are 2 commonly used anatomic classification systems for aortic dissection (Figure 7): the DeBakey system and the Stanford system.
Figure 7. Classification of Acute Aortic Dissection.
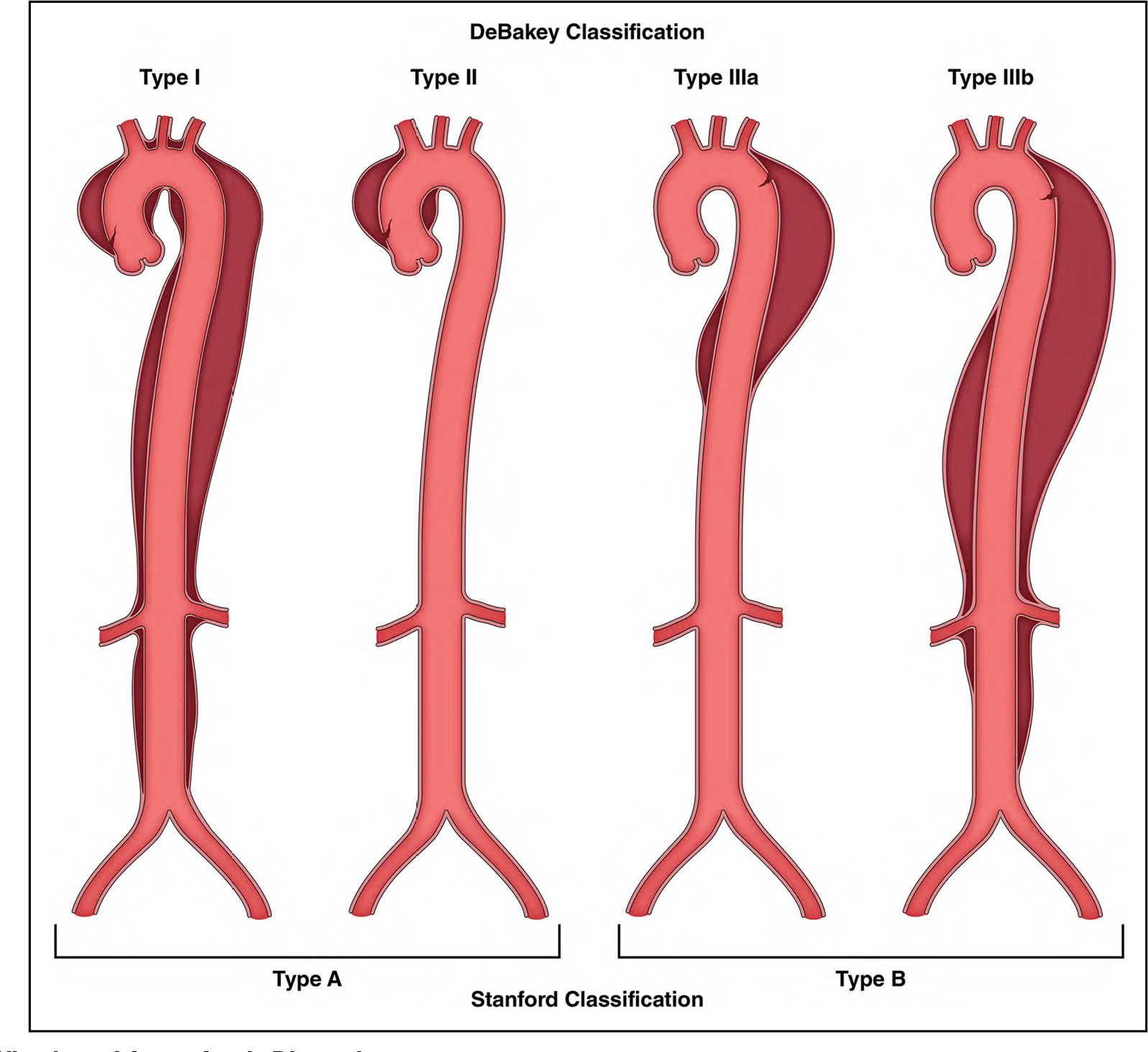
The DeBakey and Stanford classification systems are used most commonly. The DeBakey system offers greater anatomic detail, whereas the Stanford system is simpler, essentially distinguishing those dissections that involve the ascending thoracic aorta from those that do not.
The DeBakey system categorizes dissections into types I, II, and III, based on the origin of the intimal tear and the extent of the dissection:
Type I: Dissection tear originates in the ascending aorta and propagates distally to include the aortic arch and typically the descending aorta
Type II: Dissection tear is confined only to the ascending aorta
- Type III: Dissection tear originates in the descending thoracic aorta and propagates most often distally
- Type IIIa: Dissection tear is confined only to the descending thoracic aorta
- Type IIIb: Dissection tear originates in the descending thoracic aorta and extends below the diaphragm
- Type A: All dissections involving the ascending aorta, irrespective of the site of the intimal tear
- Type B: All dissections that do not involve the ascending aorta (including dissections that involve the aortic arch but spare the ascending aorta)
Figure 8. Anatomic Reporting of Aortic Dissection Based on the 2020 SVS/STS Reporting Standards.
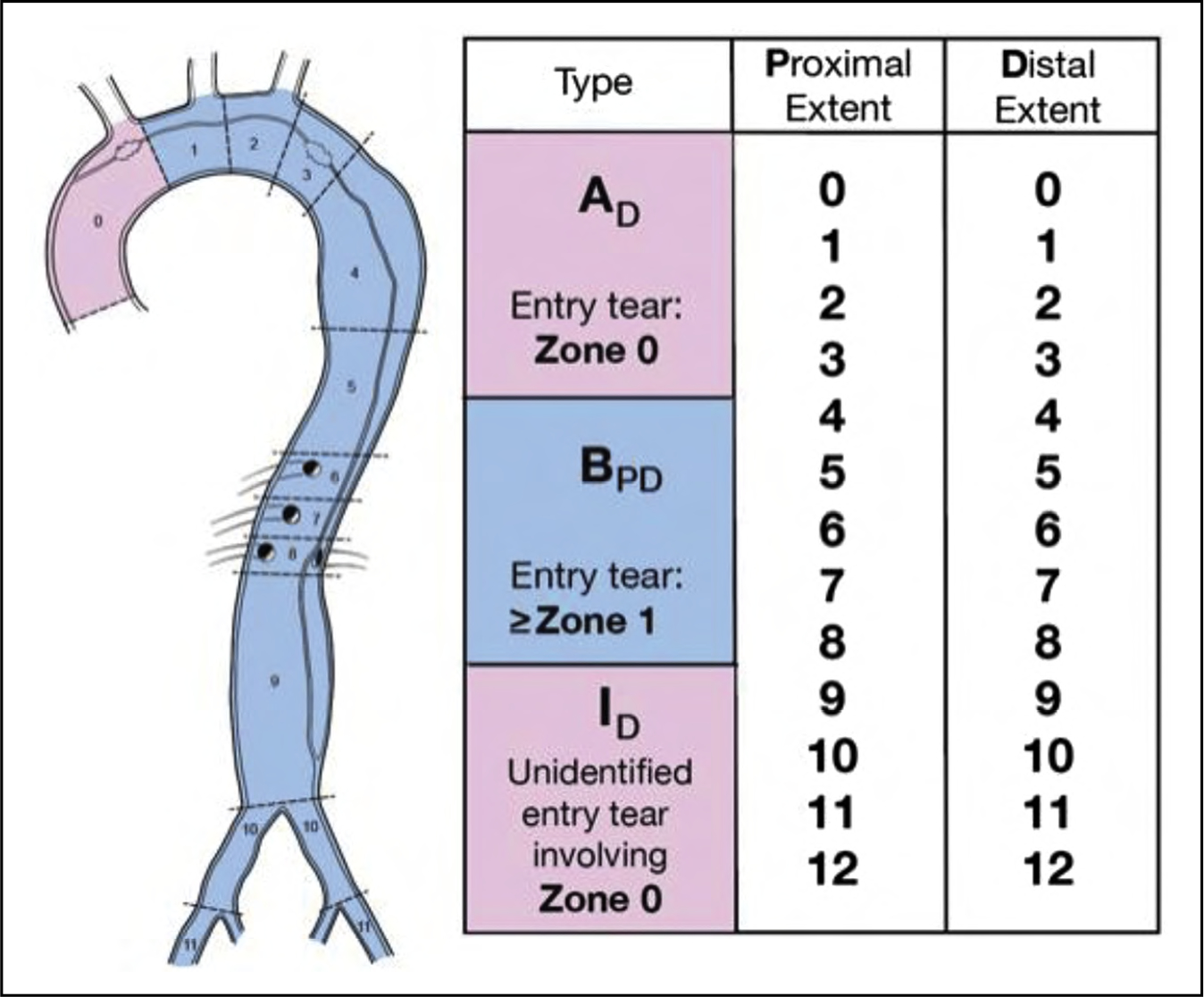
STS indicates Society of Thoracic Surgeons; and SVS, Society for Vascular Surgery. Reprinted from Lombardi et al.5 Copyright 2020, with permission from Elsevier, Inc., the Society of Thoracic Surgeons, and the Society for Vascular Surgery.
AD indicates type A is used for any dissection with an entry tear in zone 0 and extends distally the zone denoted by the subscript D (eg, A9); BPD, type B is used for any dissection with an entry tear in zone 1 or beyond; the proximal and distal extents of the dissection are denoted by subscripts P and D, respectively (eg, B39). ID, when a dissection begins in zone 0 but the location of the entry tear has not been identified, it will be considered “Indeterminate”; it will be designated with an I and its distal extent denoted by the subscript D (eg, I9).
2.4.1.3. Malperfusion
Malperfusion syndrome occurs when there is end-organ ischemia related to inadequate perfusion of the aortic branch vessels. The relationship of the true and false lumens in an aortic dissection has a critical role in maintaining stable perfusion of end-organs. Initially, the true lumen collapses because of the loss of transmural pressure across the dissection flap and the subsequent elastic recoil of the medial smooth muscle. Simultaneously, the false lumen expands immediately because of reduced elastic recoil, depth of the dissection plane within the media, and percentage of the wall circumference involved. Any of the aortic branches are at risk for malperfusion as the false lumen expands and compresses the true lumen and can occur in multiple vascular beds simultaneously as the dissection propagates distally. Dynamic obstruction occurs when the septum of the dissected intima prolapses across into the ostia of a branch, usually during systole, thereby not allowing adequate flow to perfuse the vessel (Figure 9). The ostia itself remains anatomically undamaged. When the dissection tear extends into the vessel proper and creates a stenosis or thrombosis in the artery, static obstruction occurs (Figure 9).
Figure 9. Mechanisms of Dynamic and Static Obstruction in Aortic Dissection.
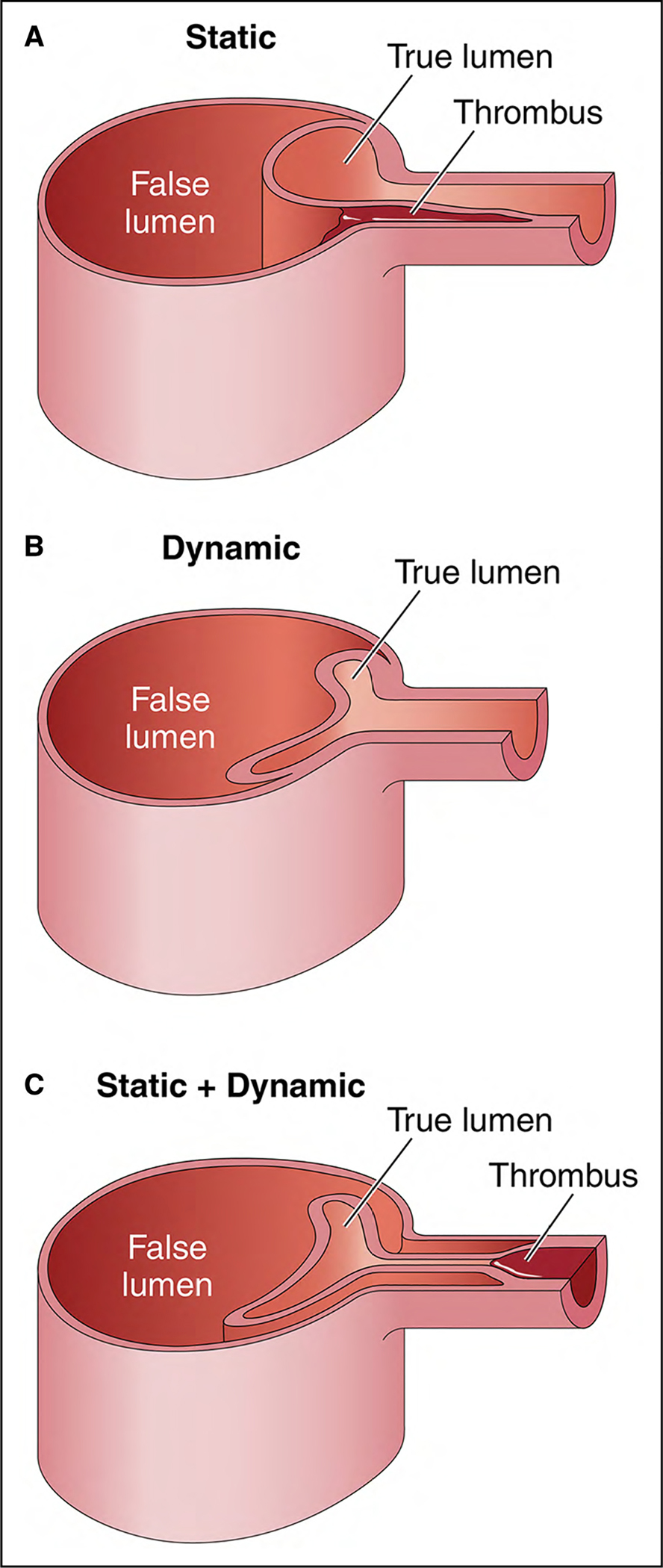
(A) Static obstruction occurs when the dissection flap extends from the aortic lumen into the ostium of the affected branch vessel, leading to localized thrombosis of the branch false lumen that narrows or colludes the branch true lumen and, consequently, impairs distal branch perfusion. (B) Dynamic obstruction occurs when the false lumen becomes persistently pressurized and compresses the true lumen, in turn pushing the dissection flap up against the ostium of the affected branch vessel, significantly reducing or occluding its flow. (C) Sometimes, a branch vessel can suffer from both static and dynamic obstruction at the same time. Adapted with permission from Grewal et al.6 Copyright 2021, Elsevier, Inc.
2.4.2. Intramural Hematoma
IMH describes the presence of blood within the medial layer of the aortic wall in the absence of an overt intimal tear or patent false lumen. The blood may arise from either rupture of the vasa vasorum causing bleeding within the media7 or small intimal tears that are not visualized on standard imaging examinations.8 IMH is diagnosed by computed tomographic angiography (CTA), magnetic resonance imaging (MRI), and echocardiography by the presence of a circular or crescent-shaped thickening of the aortic wall of >5 mm in the absence of detectable blood flow9 (Figure 6). Of patients presenting with suspected AAS, studies suggest that 5% to 25% have IMH, a proportion that approaches 30% to 40% in the Asian literature.8–11
The natural history of IMH is variable. Fewer than 10% of IMH cases resolve spontaneously, whereas 16% to 47% progress to aortic dissection if the intimal layer ruptures and creates an entry tear.7,12
2.4.3. Penetrating Atherosclerotic Ulcer
A PAU begins with an ulceration of an atherosclerotic plaque, which leads to a focal disruption in the aortic intima that allows blood to penetrate into the medial layer and is often associated with an IMH of variable size.10 PAUs most often appear in the middle or distal descending thoracic aorta, less frequently in the aortic arch and abdominal aorta, and rarely in the ascending aorta.8,10 PAUs can vary in size, and often multiple PAUs are present.10 The true incidence is unknown but is estimated to account for 2% to 7% of all cases of AAS.10 Typically, patients with PAU are older (>70 years of age) than those with classic aortic dissection and present more often with extensive and diffuse atherosclerotic disease involving both the aorta and coronary arteries.10 Additional common comorbidities include hypertension, tobacco use, chronic obstructive pulmonary disease, and renal insufficiency. PAU can occur in younger patients but often in the setting of a connective tissue disorder, and men are more commonly affected than women.8The natural history of PAU is not well defined, as they can remain stable, enlarge, or progress to either IMH, dissection, pseudoaneurysm, or aortic rupture.8 The risk of rupture has been reported to be up to 40%.13 The optimal management strategy must be individualized, considering the clinical presentation, the imaging features of the PAU, and the patient’s comorbidities.
2.5. Classification of Thoracoabdominal Aortic Aneurysm (TAAA)
When descending thoracic aortic aneurysms (TAA) extend into the abdominal aorta, they are referred to as thoracoabdominal aortic aneurysms (TAAA). The Crawford classification of TAAA, later modified by Safi et al1 (Figure 10), not only describes the extent of an aneurysm but also may predict the morbidity and mortality associated with aneurysm repair.2
Figure 10. Classification of Thoracoabdominal Aortic Aneurysms.
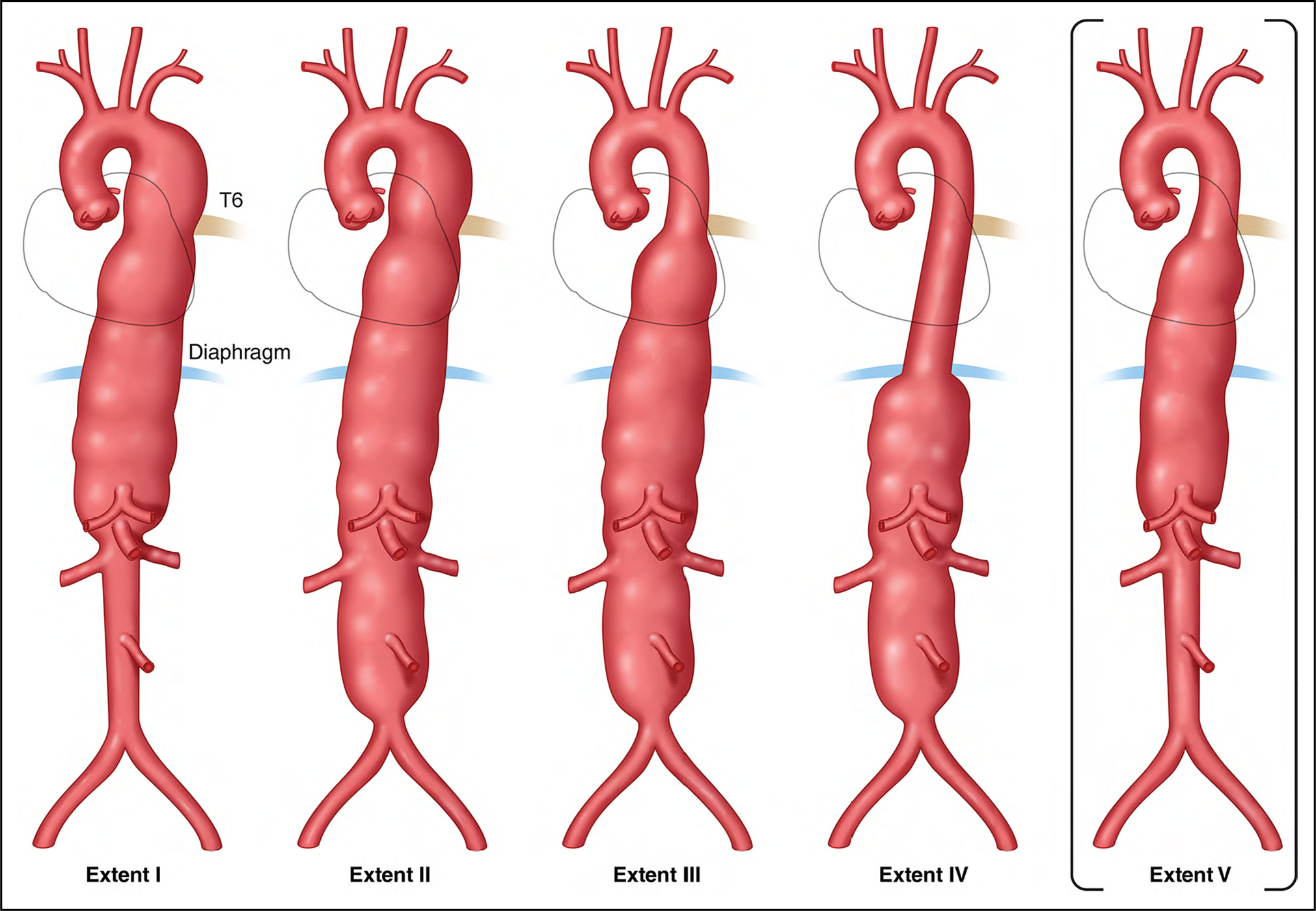
The classification of thoracoabdominal aortic aneurysms according to extent of aortic involvement as originally proposed by Crawford is as follows3: Extent I, below the left subclavian to above the celiac axis or opposite the superior mesenteric and above the renal arteries; Extent II, below the left subclavian and including the infrarenal abdominal aorta to the level of the aortic bifurcation; Extent III, below T6 intercostal space, tapering to just above the infrarenal abdominal aorta to the iliac bifurcation; and Extent IV, below T12, tapering to above the iliac bifurcation. Safi et al1 proposed expanding the classification with the addition of Extent V, below T6, tapering to just above the renal arteries.
2.6. Classification of Endoleaks
Endovascular stent-grafting is widely used in the repair of aortic aneurysms. One of the limitations of endografting is the occurrence of endoleaks, either early or late following the procedure. There are 5 types of endoleaks, as detailed in Figure 11. An endoleak results in the persistence of blood flow outside the graft and within the aneurysm sac, preventing its complete thrombosis. Consequently, patients with endografts require lifelong surveillance imaging to monitor for the appearance of endoleaks.1
Figure 11. Classification of Endoleak Types.
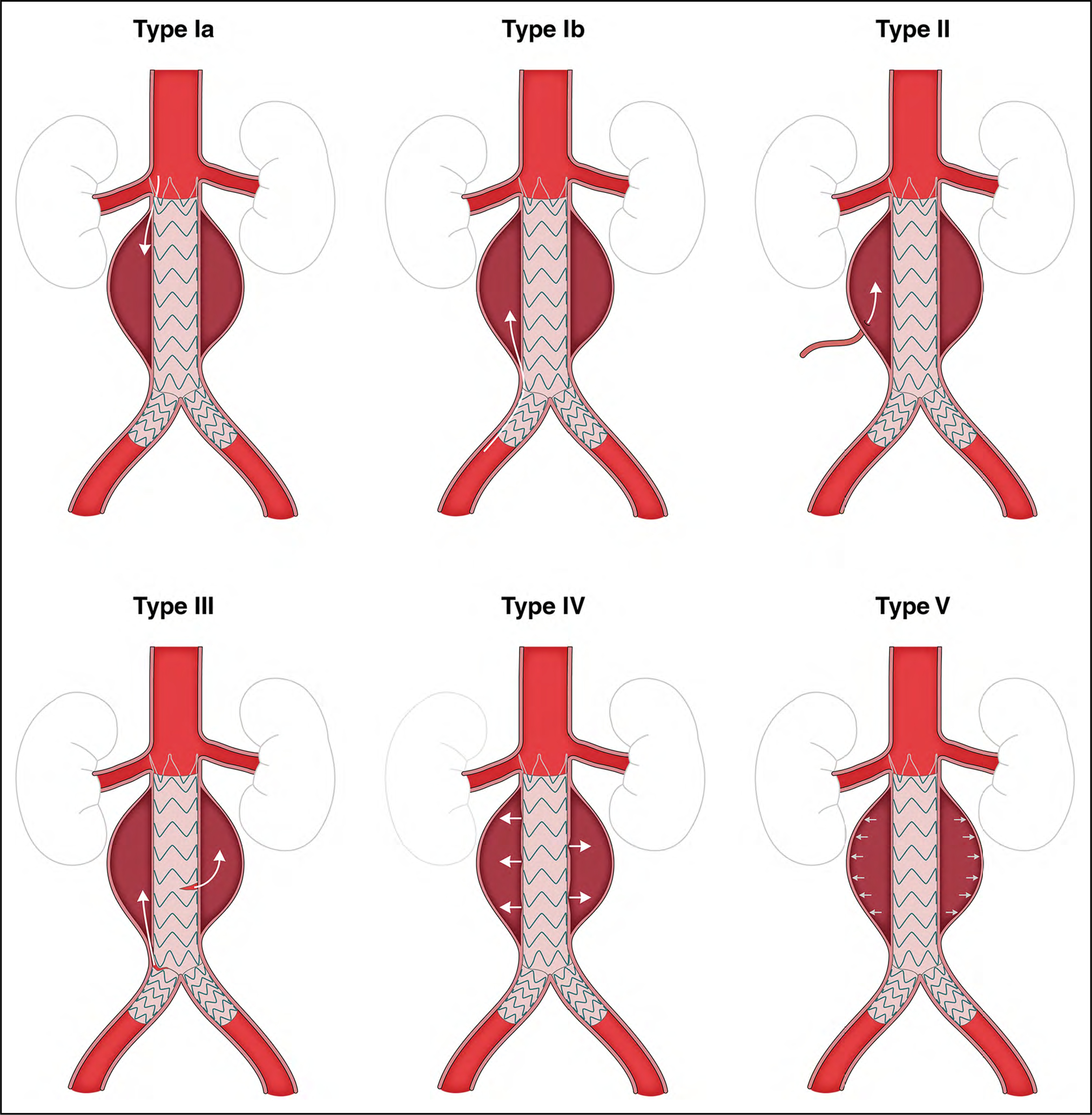
Endoleaks are classified by 5 types: Type Ia, proximal attachment site endoleak; Type Ib, distal attachment site endoleak; Type II, backfilling of the aneurysm sac through branch vessels of the aorta; Type III, graft defect or component misalignment; Type IV, leakage through the graft wall attributable to endograft porosity; and Type V, caused by “endotension,” possibly resulting from aortic pressure transmitted through the graft/thrombus to the aneurysm sac. Adapted from Rokosh et al.2 Copyright 2021, with permission from Elsevier, Inc., and the Society for Vascular Surgery.
3. IMAGING AND MEASUREMENTS
3.1. Aortic Imaging Techniques to Determine Presence and Progression of Aortic Disease
Recommendations for Aortic Imaging Techniques to Determine Presence and Progression of Aortic Disease
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with known or suspected aortic disease, aortic diameters should be measured at reproducible anatomic landmarks perpendicular to axis of blood flow, and these measurement methods should be reported in a clear and consistent manner. In cases of asymmetric or oval contour, the longest diameter and its perpendicular diameter should be reported.3,4 |
| 1 | C-LD | 2. In patients with known or suspected aortic disease, episodic and cumulative ionizing radiation doses should be kept as low as feasible while maintaining diagnostic image quality.5–7 |
| 1 | C-EO | 3. In patients with known or suspected aortic disease, when performing CT or MR imaging, it is recommended that the root and ascending aortic diameters be measured from inner-edge to inner-edge, using an electrocardiographic-synchronized technique. If there are aortic wall abnormalities, such as atherosclerosis or discrete wall thickening (more common in the distal aorta), the outer-edge to outer-edge diameter should be reported (Table 4). |
| 1 | C-EO | 4. In patients with known or suspected aortic disease, the aortic root diameter should be recorded as maximum sinus to sinus measurement. In the setting of known asymmetry, multiple measurements should be reported, and both short- and long-axis images of the root should be obtained to avoid underestimation of the diameter. |
| 2a | C-LD | 5. In patients with known or suspected aortic disease, it is reasonable that a dilated root or ascending aorta be indexed to patient height or BSA in the report, to aid in clinical risk assessment.8–11 |
| 2a | C-EO | 6. In patients with known or suspected aortic disease, when performing echocardiography, it is reasonable to measure the aorta from leading-edge to leading-edge, perpendicular to the axis of blood flow. Using inner-edge to inner-edge measurements may also be considered, particularly on short-axis imaging. |
| 2b | C-EO |
Table 4.
Essential Elements of CT and MRI Aortic Imaging Reports
| 1. Maximum aortic diameter at each level of dilation, perpendicular to the axis of blood flow. In cases of asymmetric or oval contour, the longest diameter and its perpendicular diameter should be reported. Standard measurement levels may be included, even when normal. |
| 2. Wall changes suggestive of atherosclerosis, diffuse thickening (eg, aortitis), or mural thrombus. |
| 3. Evidence of luminal stenosis/occlusion, including location, severity, and length. |
| 4. Findings suggestive of acute aortic syndrome (eg, communicating dissection, intramural hematoma, penetrating atherosclerotic ulcer, focal intimal tear), including proximal/distal extension (Figure 7), suspected entry tear site (if visible), and complications (eg, active contrast extravasation, rupture, contained rupture, rupture including periaortic hemorrhage, pericardial and pleural fluid, mediastinal stranding). |
| 5. Extension of aortic disease process (acute or chronic) into branch vessels, findings suggestive of end-organ injury, and suspected malperfusion. |
| 6. Direct comparison with previous examinations should be detailed to identify pertinent changes. |
| 7. Presence and extent of repair (eg, interposition graft, endovascular stent graft), as well as any evidence of complication. |
| 8. Impression regarding disease classification (eg, acute aortic syndrome, aneurysm/pseudoaneurysm, luminal stenosis, atherosclerotic aortic disease). |
| 9. Relevant details regarding method of image acquisition (eg, use of electrocardiographic-gating and phase of acquisition) and measurement (eg, axial versus double oblique, inner-edge versus outer-edge) should be included. |
CT indicates computed tomography; and MRI, magnetic resonance imaging.
Synopsis
Optimized depiction of aortic anatomy and pathology requires dedicated aortic imaging protocols. Computed tomography (CT), magnetic resonance imaging (MRI), transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE), and abdominal aortic ultrasound all have important roles in these evaluations (Table 5). Selection of an imaging modality may be based on patient-specific factors, including hemodynamic stability, contrast allergy, renal function, and patient tolerance (eg, given relatively longer examination times and the confined space associated with MRI, occasionally requiring sedation). The institutional availability of an imaging modality or an expert imaging physician may also direct modality selection. The ubiquity of CT scanners, combined with rapid acquisition of intuitive, high-resolution 3-dimensional (3D) imaging data sets, has led to the wide adoption of this modality for the assessment of suspected aortic pathology and for periprocedural vascular evaluation, in most cases supplanting diagnostic catheter angiography.12
Table 5.
Diagnostic Performance of Aortic Imaging Modalities
| Parameter | CT | MRI | TTE | TEE | US |
|---|---|---|---|---|---|
| Availability | +++ | ++ | +++ | ++ | +++ |
| Portability | - | - | +++ | +++ | +++ |
| Speed of acquisition | +++ | + | ++ | ++ | ++ |
| Spatial resolution | +++ | ++ | ++ | +++ | ++ |
| Temporal resolution | + | ++ | +++ | +++ | +++ |
| Three-dimensional data set | +++ | ++ | + | + | + |
| Arch branch vessel evaluation | +++ | +++ | ++ | + | NA |
| Evaluation of valve and ventricular function | + | ++ | +++ | +++ | NA |
CT indicates computed tomography; MRI, magnetic resonance imaging; NA, not applicable; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography; US, abdominal aortic ultrasound; +++ excellent results; ++ good results; + fair results; and -, not available.
Recommendation-Specific Supportive Text
Measurements should be obtained perpendicular to the long axis of the aorta at specified segmental locations (Figure 12), with measurements also taken at the locations of any abnormalities. If a 3D data set has been acquired, dedicated multiplanar reformats orthogonal to aortic flow axis should be created at each level of measurement. This approach provides structured, repeatable measurement reporting on serial imaging and avoids oblique imaging that may overestimate the aortic diameter at levels of greater curvature and tortuosity.3,4
The cancer risk associated with CT scans remains a controversial issue; however, the risk is generally agreed to be greatest early in life and substantially attenuated later in life.5,6 Consideration of the indication for aortic imaging, optimization of the tube settings for CT protocols, and use of alternative modalities such as MRI are all valid approaches to mitigate patient radiation exposure.7
On CT and MRI, the root diameter can be measured from the commissure to the opposite sinus, or from sinus to sinus, which results in larger dimensions (Figure 12).13 Measuring from sinus to sinus and from inner-edge to inner-edge on CT and MRI has shown good correlation with TTE for measurements of the root and ascending segments,14 as well as improved confidence in the determination of aortic root margins on MRI and lower interobserver and intraobserver variability.15 Measurement of graft material (eg, interposed surgical or endostent) may likewise include an inner-edge to inner-edge measurement for determination of the functional lumen and potential use in extension treatment planning. The use of electrocardiographic-gated images decreases motion artifact and improves edge depiction in aortic root imaging, with diminished measurement variability.16 If there are aortic wall changes (eg, atherosclerosis, mural thrombus), as is more commonly noted in the arch and distal aorta, or discrete wall thickening (eg, aortitis or IMH), the outer margins of the abnormal segments are measured.
The shape of the aortic root can be asymmetric, and the difference between the minimum (short-axis) and maximum (long-axis) root diameters can be significant, particularly in those with bicuspid valves.17 To avoid underestimation, multiple measurements should be reported, with either each of the sinus-to-sinus diameters or both short- and long-axis diameters, to avoid underestimation of the true root size.
The cross-sectional aortic area to patient height ratio has been shown to be associated with risk of aortic dissection and death in patients with tricuspid or bicuspid valves9,10 (see Section 2.3.1, “Normalizing Aortic Root and Ascending Aortic Diameters for Body Size”), and both ASI and AHI have been shown to predict risk of adverse events (rupture, dissection, or death).11
There is a wealth of historical data regarding using TTE to measure the aortic root (at end-diastole) from the leading-edge of the anterior wall to the leading-edge of the posterior wall, identifying the largest diameter.18,19 These data led to the determination of normal limits adjusted for age, sex, and body size20 and provided insight regarding the prevalence and prognostic importance of aortic dilation. Additionally, measuring from leading-edge to leading-edge on TTE has shown good correlation with inner-edge to inner-edge measurements obtained on CT and MRI.14 The method of inner-edge to inner-edge measurement on TTE images may also be considered, with some experienced investigators showing excellent measurement agreement.15
Figure 12. Aortic Imaging Techniques to Determine the Presence and Progression of Aortic Disease.
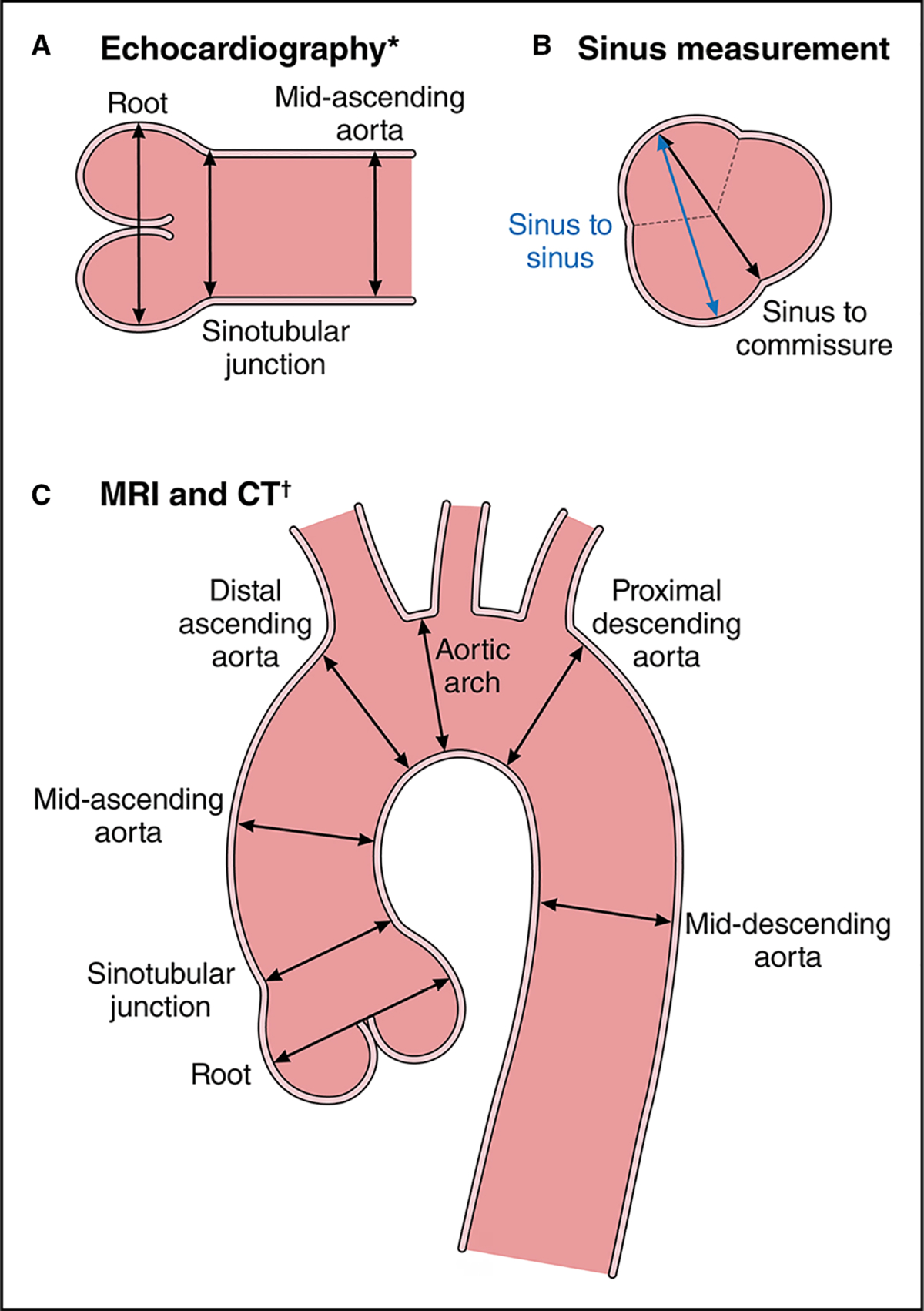
(A) Schematic shows the leading-edge to leading-edge measurement technique used in echocardiography, from left to right: measurement of the aortic root (sinuses of Valsalva), sinotubular junction, and proximal tubular ascending aorta. (B) Inner-wall to inner-wall measurements of the aortic root used in MRI and CT. In addition, a consistent approach to measuring all 3 sinuses with MRI and CT is necessary. The sinus-to-commissure and sinus-to-sinus measurements can both be used, but consistency is necessary for interval surveillance. (C) Standard measurement locations for MRI and CT with the inner-wall to inner-wall technique. Adapted from Borger et al.21 Copyright 2018, with permission from Elsevier, Inc. CT indicates computed tomography; and MRI, magnetic resonance imaging. *Leading-edge to leading-edge. †Inner-wall to inner-wall.
3.2. Conventions of Measurements
Reproducible and accurate measurements of the aorta are critical for characterizing aortic disease and guiding treatment decisions. Measurements should be obtained perpendicular to the long axis of the aorta at specified segmental locations (Figure 13),1 with measurements also taken at the location of any abnormality. Unfortunately, there is no widely accepted standard for aortic diameter measurements (eg, inner-edge to inner-edge, outer-edge to outer-edge) across imaging modalities. There is a wealth of historical data regarding using TTE to measure the aortic root (at end-diastole) from the leading-edge of the anterior wall to the leading-edge of the posterior wall, thus identifying the largest diameter.2,3 These data allowed for the creation of normal limits adjusted for age, sex, and body size4 and provided insight regarding the prevalence and prognostic importance of aortic dilation.
Figure 13. Reformatted CT Image Orthogonal to the Aortic Root at the Level of the Sinuses of Valsalva.
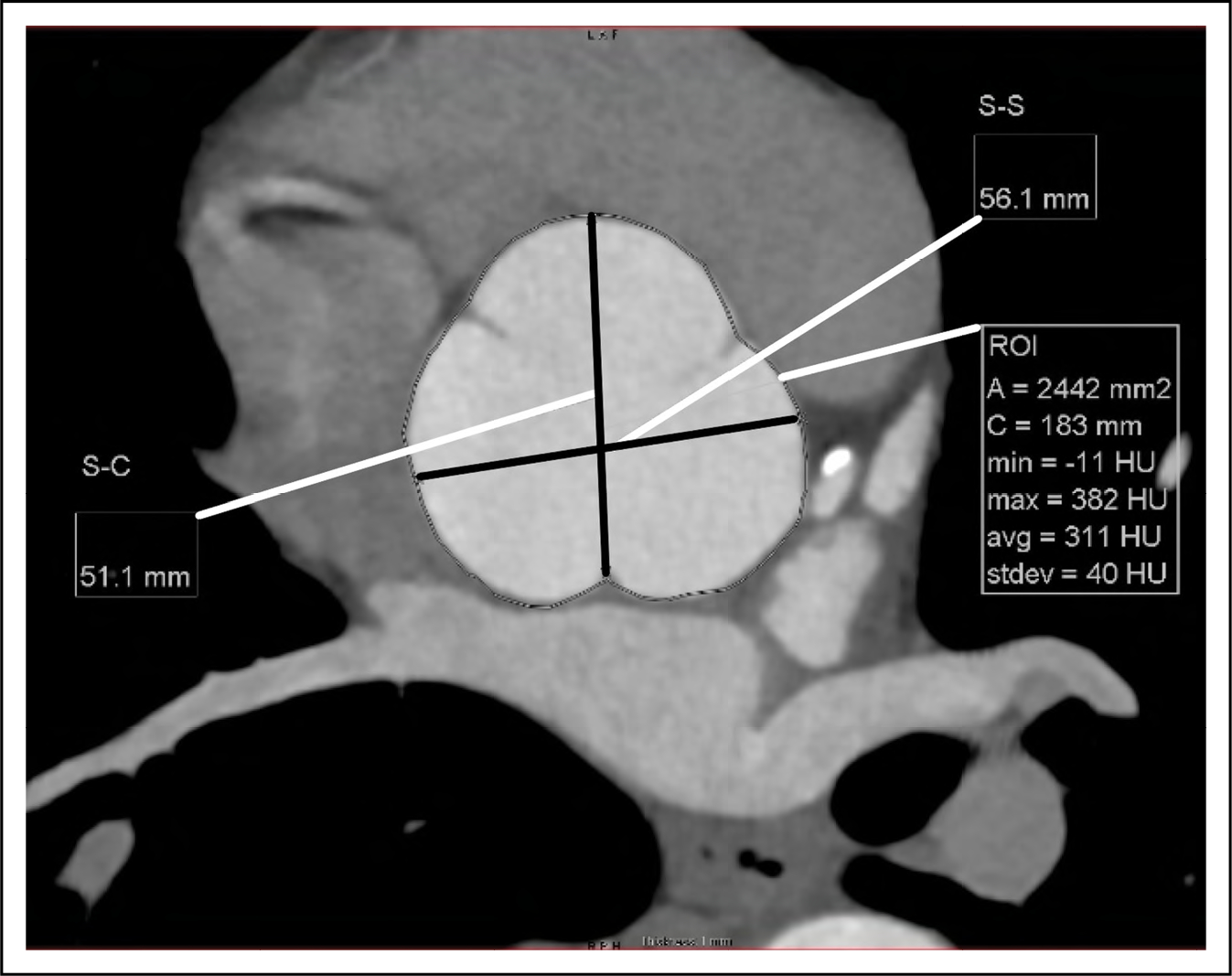
The root diameter can be measured from sinus-to-sinus (S-S) or sinus-to-commissure (S-C). The aortic root area (A) can also be measured. CT indicates computed tomography; and ROI, region-of-interest.
On CT and MRI, the root diameter can be measured from the commissure to the opposite sinus, or from sinus to sinus, which results in larger dimensions (Figure 13).5 Measuring from sinus-to-sinus and from inner-edge to inner-edge on CT and MRI has shown good correlation with TTE for measurements of the root and ascending segments,6 as well as improved confidence in the delineation of aortic root margins on MRI and lower interobserver and interobserver variability.7
Although aortic dilation as measured by diameter is a well-known risk factor for the occurrence of aortic dissection and rupture,8 most dissections occur in aortas with diameters that do not meet the threshold for preventive surgery.9 This has led investigators to search for better metrics for risk stratification and treatment guidance. For instance, research has shown that ascending aortic area indexed to height is associated with aortic dissection and adverse outcomes in patients with tricuspid or bicuspid valves.10,11 Male sex, age, height, weight, and the presence of traditional cardiovascular risk factors have also been found to correlate with increased aortic size in large population-based studies.12 Aortic length is known to increase over time; spurred by this fact, and by the observation that intimal entry tears run in a transverse direction, researchers have found that excessive elongation of the ascending aorta may be predictive of dissection and thus represents a potentially relevant measurement.13
Measurements of the arch and further distal segments should also be performed perpendicular to the aortic axis, with care taken to avoid oblique imaging that may overestimate the aortic diameter at levels of greater curvature and tortuosity. In the setting of wall changes (eg, discrete thickening from atherosclerosis, aortitis, IMH, or other processes), the abnormal wall should be measured from outer-edge to outer-edge. To assess abdominal aortic dimensions, ultrasonographic images may be obtained in a dedicated examination or as part of a surface echocardiographic examination. Several studies have shown that the volume of an AAA may progress despite a stable diameter.14,15
3.2.1. Computed Tomography
CT can image the entire aorta and its branches with high spatial resolution and fast acquisition. The use of electrocardiographic-gated technique decreases motion artifact of the root and ascending aorta,1 significantly increasing the precision of measurements and diagnostic confidence. When necessary, CT can be performed without the use of iodinated contrast, and such noncontrast imaging can still accurately provide diameter assessment of aortic aneurysms that can suffice for surveillance of patients who cannot tolerate or cooperate with MRI, although aortic wall delineation may be challenging in some instances (eg, at the aortic root level). The use of iodinated intravenous contrast allows for delineation between aortic lumen and wall and generally improves assessment of wall changes. In some instances, the potential concern of patient contrast allergy or renal toxicity may be a consideration. However, according to recent consensus statements from the American College of Radiology and the National Kidney Foundation,2 the risk of acute kidney injury developing in patients with impaired renal function after exposure to intravenous iodinated contrast media has likely been overestimated given the difficulty distinguishing coincident from contrast-induced nephropathy.
CT has a very high sensitivity and specificity for acute aortic syndromes (AAS, aortic dissection, IMH, PAU)3 and traumatic aortic injuries. Moreover, CT can identify concomitant coronary involvement,4 branch vessel involvement, and hemopericardium, and may aid in identification of dissection entry tears. In patients whose CT is negative for AAS, the images may provide insight regarding other causes of the presenting chest pain.5 When imaging patients with a suspected AAS, a noncontrast series of images is typically obtained first, to better distinguish IMH, if present, from other causes of aortic wall thickening. Then, a series of arterial phase contrast-enhanced images is obtained with thin slice to allow for reconstructions (computed tomographic angiography [CTA]), extending from the thoracic inlet to the level of the femoral arteries, to define the full extent of any dissection and thereby guide therapy. For consistency in this document, CT is used to refer to computed tomography modality broadly, with specific imaging techniques chosen dependent on a given clinical indication and patient history.
3.2.2. Magnetic Resonance Imaging
MRI provides coverage of the entire aorta and branch vessels, can characterize aortic wall changes in the setting of inflammation1 and AAS, and offers physiologic assessment of ventricular and valve function plus flow quantification. MRI uses no ionizing radiation and can often be performed without intravenous contrast. MRI is therefore often a primary option for assessing congenital aortic abnormalities and is well-suited for serial imaging in younger patients. The use of electrocardiographic-gated imaging decreases motion artifact of the aortic root2 and of 3D datasets, critical for achieving precise, repeatable measurements.3 Limitations of MRI include spatial resolution that, although good, is typically inferior to that of CT, as well as the appearance of artifacts in patients with indwelling metallic material or devices. Additionally, MRI is not as widely available as CT for aortic imaging, has a longer acquisition time, and the ability to monitor and treat unstable patients in the scanner is limited. This modality is therefore less commonly used in patients with suspected acute aortic pathology,4 especially when patients are unstable. Various MRI sequences are available for aortic depiction, including magnetic resonance angiography (MRA), which involves volumetric acquisition of aortic anatomy, with slice thickness allowing for reconstruction of images in multiple planes. Intravenous gadolinium-based contrast media are often used in MRA, although there is a very small risk of inducing nephrogenic systemic fibrosis in patients with underlying kidney disease, a risk that is particularly low with group II gadolinium-based contrast agents.5,6 Additional sequences are often used for aortic anatomic depiction that do not require intravenous contrast media, such as cine gradient echo bright blood and spin echo dark blood sequences. For consistency in this document, we use MRI to refer to the modality of magnetic resonance imaging defined broadly, which potentially includes many sequences that are often combined in complementary manner within an imaging protocol.
3.2.3. Echocardiography
Transthoracic Echocardiography (TTE)
TTE is the most common imaging modality used in the initial nonemergency assessment of the thoracic aorta.1,2 TTE is particularly useful in imaging the aortic root and ascending aorta and in delineating aortic valve anatomy and function. Although not ideal for imaging of the aortic arch, TTE often does visualize the aortic arch branch vessels and the proximal descending aorta and can aid in diagnosis of coarctation of the aorta (CoA) and patent ductus arteriosus. TTE is portable and can be performed at the bedside with a high spatial and temporal resolution. It can be useful in the evaluation of patients with AAS to detect complications, including aortic valve regurgitation, left ventricular dysfunction, and cardiac tamponade. TTE is useful in the longitudinal surveillance of aortic root and ascending aortic dilation, provided those aortic segments are well visualized.
Transesophageal Echocardiography (TEE)
TEE provides high-resolution images of most of the thoracic aorta, apart from a short segment of the distal ascending aorta just proximal to the innominate artery, attributable to acoustic shadowing from the trachea. TEE is also very useful in detailing aortic valve anatomy and function. TEE is particularly useful in the intraoperative evaluation of patients with AAS in guiding both operative and endovascular repair strategies and the assessment of true and false lumen flows before and immediately after aortic repair.1,2
3.2.4. Intravascular Ultrasound
Intravascular ultrasound is an endovascular technology designed to provide high-resolution intraluminal imaging of localized arterial and venous disease.1 Intravascular ultrasound is particularly useful in guiding the endovascular management of complex pathologies of the thoracic and abdominal aorta, because it reveals aortic size, tortuosity, plaque burden, calcification, branch vessel ostia, and intravascular filling defects (eg, thrombus, dissection flap), in addition to permitting landing zone assessment.1 Such intravascular ultrasound imaging data may help to identify patients for whom endovascular treatment is high-risk or contraindicated. Intravascular ultrasound is especially useful in the setting of aortic dissection2–4 to distinguish true and false lumen anatomy and thereby guide endovascular or open repair. Intravascular ultrasound may be used to guide deployment of endovascular stents and, during final assessment, to reduce the volume of iodinated contrast used.5 Importantly, intravascular ultrasound requires an operator who is familiar with both the acquisition and interpretation of images.
3.2.5. Abdominal Ultrasound
Vascular ultrasound is an effective and rapid imaging modality and is the recommended diagnostic tool in screening for and surveillance of AAA.1–3 The ultrasonic criterion for AAA is a diameter >3.0 cm, using primarily the outer-edge to outer-edge measurement convention in the anterior-posterior or transverse view.4–6 The sensitivity of ultrasound to detect the presence of an aneurysm approaches 100%,7 although interobserver variability exists, and successful imaging can be limited by obesity and superimposed bowel gas.8
Using B-mode imaging, color Doppler, and spectral waveform analysis, a comprehensive ultrasound evaluation of the abdominal aorta can quickly detect other aortic pathologies, such as plaque or mobile atheroma formation, arterial stenoses, mural thrombus, inflammation, dissection, pseudoaneurysm, contained rupture, and aortocaval fistulae, and these findings may prompt the need for further imaging with CT or MRI. Abdominal ultrasound can also be used for surveillance of patients who have undergone endovascular repair of AAA (EVAR); it can detect aneurysm sac expansion, which may indicate the presence of an endoleak (Figure 11), defined as abnormal flow outside of the aortic endograft, a finding that typically warrants confirmation by CT. The use of contrast-enhanced color duplex ultrasound has shown promising results in enhanced sensitivity in detection of endoleaks,9 although its use requires ongoing study.
4. MULTIDISCIPLINARY AORTIC TEAMS
Recommendations for Multidisciplinary Aortic Teams
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. For patients with acute aortic disease that requires urgent repair, a multidisciplinary team should determine the most suitable intervention. |
| 2a | C-LD | 2. For patients who are asymptomatic with extensive aortic disease, or who may benefit from complex open and endovascular aortic repairs, or with multiple comorbidities for whom intervention is considered, referral to a high-volume center (performing at least 30–40 aortic procedures annually) with experienced surgeons in a Multidisciplinary Aortic Team is reasonable to optimize treatment outcomes.1–6 |
Synopsis
Evidence-based standards for medical and surgical conditions recognize the critical relationship among both hospital and surgeon case volumes and patient outcomes. Clinical excellence is further enhanced by collaborative, multispecialty teams to foster the best treatment of patients, especially for complex presentations with multiorgan threats. Although there is no agreed on definition of a Multidisciplinary Aortic Team, an appropriate framework might be: A specialized hospital team with an exceptionally high concentration of expertise in the evaluation and management of aortic disease, in which care is delivered in a comprehensive, multidisciplinary manner.7 The concept of comprehensive heart valve centers was formally codified in the “2020 ACC/AHA Guideline for the Management of the Patient With Valvular Heart Disease,”8 which emphasized the numerous essential components of such centers, ranging from physician expertise, experience, and technical skill to data collection, research, and education, to institutional facilities and resources. Although the specific components of such teams may differ from center to center, the most common features that distinguish Multidisciplinary Aortic Teams include: Having cardiac surgical, vascular surgical, and endovascular specialists with extensive experience managing complex aortic disease at a center with a high volume of aortic interventions; having imaging specialists with expertise in aortic disease to perform and interpret CT, MRI, and echocardiography; anesthesiologists experienced in the management of acute aortic disease and cerebrospinal fluid drainage; and an intensive care unit (ICU) experienced in the management of acute aortic disease.
Recommendation-Specific Supportive Text
In cardiovascular care, we have long recognized the critical value of collaborative multidisciplinary expertise in cardiac transplantation and mechanical circulatory support conducted only at centers of excellence. More recently, we have seen the rise in multidisciplinary heart teams focused on the care of patients with complex coronary artery disease and patients with complex heart valve disease; indeed, the important role of multidisciplinary heart valve teams was emphasized in the “2020 ACC/AHA Guideline for the Management of the Patient With Valvular Heart Disease.”8 There is ample evidence that patients with complex aortic disease may similarly benefit from treatment by such multidisciplinary teams.6 Andersen et al1 compared the outcomes of patients with acute type A aortic dissection undergoing open surgical repair before and after implementation of a multidisciplinary thoracic aortic surgery program and found that operative mortality declined dramatically after implementation of the multidisciplinary team and that the significant mortality advantage persisted over a 5-year follow-up (P=0.002). Likewise, in a report from England,2 hospitals with multidisciplinary thoracic aortic programs reported significant reductions in mortality compared with hospitals without such programs.
In a study of 230 736 Medicare beneficiaries undergoing AAA repair between 2001 and 2006, in which hospital procedural volume for both open and endovascular repair was divided into quintiles, the adjusted mortality decreased as hospital volume increased, by quintile, especially among the group undergoing open surgical repair.3 The benefits of high case volume on surgical outcome apply similarly to patients with TAA. Hughes et al4 analyzed >13 000 elective aortic root and aortic valve-ascending aortic procedures performed at 741 North American hospitals from 2004 to 2007. They found a negative association between the hospital volume and the adjusted odds ratio (OR) for mortality (P<0.001), particularly at a hospital volume of <30 to 40 procedures annually (Figure 14). The inverse relationship between center case volume and mortality was shown again in a more contemporary series by Mori et al9 of >53 000 proximal thoracic aortic surgeries in the United States from 2011 to 2016 in which the risk of operative mortality decreased significantly when the annual center volume exceeded 20 to 25 cases (only 116 US centers performed >20 cases/y), and decreased significantly further still at an annual center volume of >50 cases (only 24 US centers performed >50 cases/y) (Figure 15). Perhaps the most consistent correlation between case volume and mortality rate is among patients with acute aortic dissection. In a retrospective review of 232 patients with acute type A aortic dissection who underwent urgent surgery in a single center in the United Kingdom, the 30-day mortality rate was significantly lower among those operated on by a surgeon with aortic expertise versus a nonaortic expert, at 10% versus 26%, respectively (P=0.02). Moreover, aortic specialists performed aortic root procedures significantly more often (43.0% versus 17.3%; P=0.001), and their cross-clamp times were significantly shorter.5 Finally, Umana-Pizano et al10 found that the mortality rate of acute type A aortic dissection repair was 14% versus 24% for high-volume and low-volume surgeons, respectively. Clearly not all patients with thoracic aortic disease (TAD) can be treated by Multidisciplinary Aortic Teams, especially in the setting of AAS. Nevertheless, when patients are referred for elective aortic intervention, especially at aortic diameter thresholds that are borderline, the lower surgical mortality rate with expert aortic surgeons at high-volume centers may justify early aortic repair. Similarly, when aortic procedures are relatively new or complex, the best outcomes are likely to be at centers with high-volume operators who have experience with such novel techniques. Consequently, throughout this guideline is a number of recommendations in which it is specified that certain open surgical or endovascular aortic repairs be performed by experienced operators in centers with Multidisciplinary Aortic Teams.
Figure 14. Observed Relationship Between Annual Institutional Case Volume and Risk-Adjusted Odds Ratio for Operative Mortality ±2 Standard Deviations as Assessed With Regression Analysis.
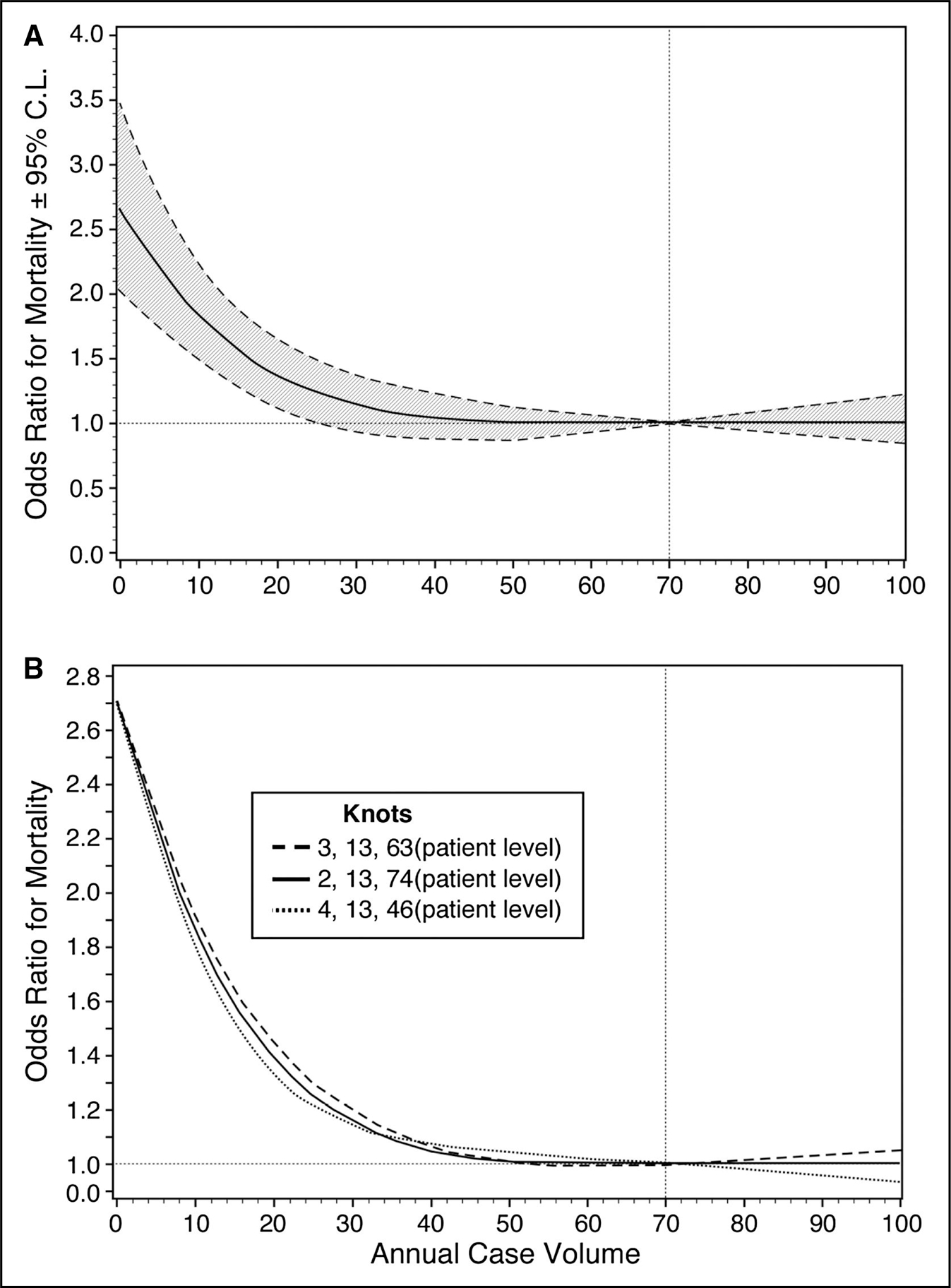
The odds ratio for operative mortality decreased as institutional case volume increased. Adapted from Hughes et al.4 Copyright 2013, with permission from Elsevier Inc.
Figure 15. Predicted Risk of Mortality Derived From the Logistic Regression Model Without Center Case Volume as a Covariate.
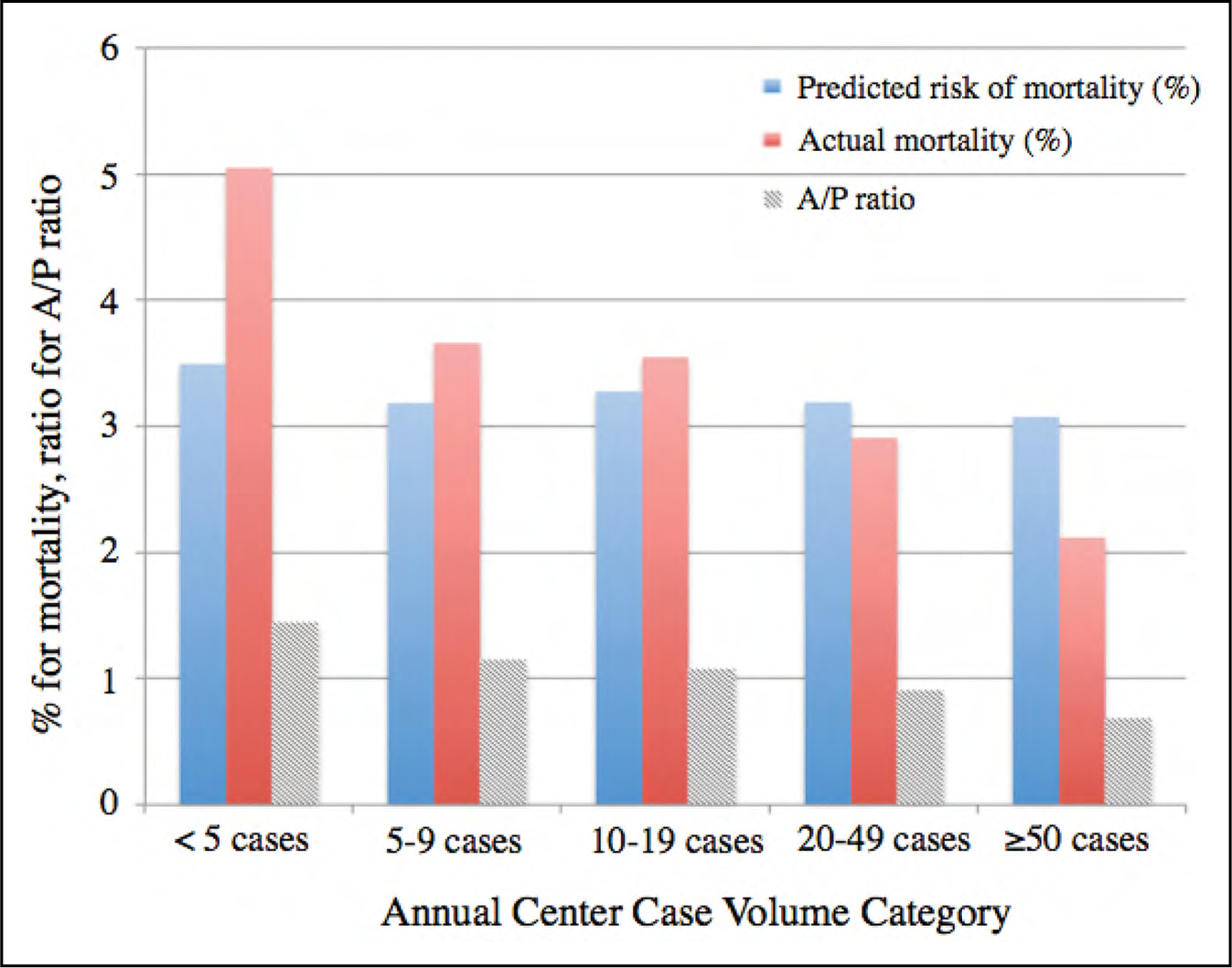
Actual mortality and the ratio of actual mortality to predicted mortality (A/P ratio, the risk-adjusted mortality rate) are also shown. A similar predicted risk of mortality across the case volume strata and a decrease in the actual mortality at higher center case volume are seen. Reprinted from Mori et al.9 Copyright 2018, with permission from Elsevier Inc.
5. SHARED DECISION-MAKING
Recommendations for Shared Decision-Making
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In patients with aortic disease, shared decision-making is recommended when determining the appropriate thresholds for intervention, deciding on the type of surgical repair, choosing between open surgical versus endovascular approaches; and in medical management and surveillance.1–6 |
| 1 | C-EO | 2. In patients with aortic disease who are contemplating pregnancy or who are pregnant, shared decision-making is recommended when considering the cardiovascular risks of pregnancy, the diameter thresholds for prophylactic aortic surgery, and the mode of delivery. |
Synopsis
Shared decision-making is increasingly used in patient-centered care as advocated by the National Academy of Medicine.7 Although no randomized trials have evaluated the value and effectiveness of shared decision-making, multiple position papers advocate strongly for the incorporation of shared decision-making in the care of patients with thoracic and AAAs.2–5 Decision aids have been developed for shared decision-making in patients with AAAs to help improve the patient understanding of the disease and treatment options.1 Shared decision-making is especially useful when considering the diameter thresholds for and the timing of intervention in addition to having an important role in considering the risks of pregnancy in patients with underlying aortic disease.
Recommendation-Specific Supportive Text
Shared decision-making is an active process in which patients and families are encouraged to share their values and preferences regarding quality of life, goals of care, and desired procedural outcomes. Formally recognizing those preferences helps physicians to better frame the risks and benefits of intervention versus conservative management. Actively involving patients in the decision-making process is especially important in situations in which there is clinical equipoise, such as: an aortic aneurysm with a diameter at the borderline of the threshold for repair; performing valve-sparing root repair rather than valved-conduit aortic root replacement; performing thoracic endovascular aortic repair (TEVAR) in a patient with an uncomplicated type B aortic dissection who is at increased risk of complications; or treating an AAA with open surgical versus endovascular repair. Shared decision-making may be used for noninterventional issues as well, such as the choice of medical therapies or the imaging modality used for surveillance.
Shared decision-making has an important role in pregnancy among those with aortic disease to determine whether to consider conception, an appropriate diameter threshold for prophylactic aortic repair, and the mode of delivery. This has particular relevance in patients with Marfan syndrome and Loeys-Dietz syndrome, and other heritable aortic disorders who are planning a pregnancy.
6. ANEURYSMS
6.1. Thoracic Aortic Aneurysm (TAA) Causes
TAAs occur in 5 to 10 per 100 000 person years.1 The natural history and treatment vary depending on the cause and location of the TAA. The size of a given segment of the thoracic aorta is influenced by age, sex, height, and body size.2 Aortic z-scores and other diameter indexing methods (see Section 2.3, “Definitions of Dilation and Aneurysm of the Aortic Root and Ascending Thoracic Aorta”) may assist with risk assessment.3 Of all TAA, aneurysms of the aortic root, ascending aorta, or both are most common (~60%), followed by those of the descending aorta (~30%) and arch (<10%). Hypertension, smoking, hypercholesterolemia, and heritable genetic variants are risk factors for TAA disease. Patients with TAA have a modestly increased incidence of AAA4 and cerebral aneurysms.5
Causes of TAA include heritable disorders, congenital conditions, multifactorial degenerative conditions, previous aortic dissection, inflammatory diseases, and infectious diseases (Table 6). Aneurysms of the aortic root and ascending thoracic aorta tend to have a heritable influence and present at younger ages, whereas aneurysms of the descending thoracic aorta tend to be degenerative and present at older ages.6 Moreover, aneurysms of the aortic root and ascending thoracic aorta are also commonly associated with BAV, although the genetic basis of BAV and why some but not all patients have a concomitant aortopathy are not well understood. Finally, many aneurysms of the root and ascending thoracic aorta are sporadic and idiopathic. Because the management of patients with aneurysms of the aortic root and ascending thoracic aorta may differ depending on the underlying cause or family history, the recommendations for medical and surgical therapy are grouped accordingly in the document, as shown in Figure 16.
Table 6.
Cause of TAA
| HTAD (see Table 7): syndromic |
| Marfan syndrome |
| Loeys-Dietz syndrome |
| Vascular Ehlers-Danlos syndrome |
| Smooth muscle dysfunction syndrome |
| Others: attributable to pathogenic variants in FLNA, BGN, LOX |
|
|
| HTAD (see Table 7): nonsyndromic |
| ACTA2, MYH11, PRKG1, MYLK, and others |
| Familial thoracic aortic aneurysm without identified pathogenic variants in a known gene for HTAD |
|
|
| Congenital conditions |
| Bicuspid aortic valve |
| Turner syndrome |
| Coarctation of the aorta |
| Complex congenital heart defects (tetralogy of Fallot, transposition of the great vessels, truncus arteriosus) |
|
|
| Hypertension |
|
|
| Atherosclerosis |
|
|
| Degenerative |
|
|
| Previous aortic dissection |
|
|
| Inflammatory aortitis |
| Giant cell arteritis |
| Takayasu arteritis |
| Behçet disease |
| Immunoglobulin G4-related disease, antineutrophil cytoplasmic antibody-related, sarcoidosis |
|
|
| Infectious aortitis |
| Bacterial, fungal, syphilitic |
|
|
| Previous traumatic aortic injury |
HTAD indicates heritable thoracic aortic diseases; and TAA, thoracic aortic aneurysms.
Figure 16.
Recommendations for Management of Aneurysms of the Aortic Root and Ascending Aorta According to Known Causative Factors.
Approximately 20% of TAA are related to a genetic or heritable condition (also referred to as heritable thoracic aortic disease [HTAD]), some of which associate with multisystem features (considered syndromic HTAD) and others with abnormalities limited to the aorta with or without its branches (known as nonsyndromic HTAD)7 (Table 7). HTAD most commonly involves the aortic root, ascending aorta, or both but may also present with distal aortic disease and aortic dissection.8 Pathogenic variants in multiple genes can lead to TAA, cerebral aneurysms, and AAA.7,8 Up to 20% of individuals with a TAA or aortic dissection have a family history of TAD, with at least 1 affected first-degree relative.8 Population studies have shown the familial nature of TAAs and dissections, with familial cases having a significantly increased risk of TAA and aortic dissection8,9 compared with sporadic cases. Therefore, among patients with aortic root and ascending aortic aneurysm or those with aortic dissection, screening of first-degree relatives with imaging is essential to detect unrecognized, asymptomatic TAD.8,10
Table 7.
TAA Syndromes and Conditions Attributable to a Heritable or Genetic Cause
| Condition | Gene | Clinical Features |
|---|---|---|
| Syndromic HTAD * | ||
| Marfan syndrome | FBN1 | Aortic root aneurysm, aortic dissection, TAA, MVP, long bone overgrowth, arachnodactyly, dolichostenomelia, scoliosis, pectus deformities, ectopia lentis, myopia, tall stature, pneumothorax, dural ectasia |
| Loeys-Dietz syndrome | TGFBR1, TGFBR2, SMAD3, † TGFB2, TGFB3 | TAA, branch vessel aneurysms, aortic dissection, arterial tortuosity, MVP, craniosynostosis, hypertelorism, bluish sclera, bifid/broad uvula, translucent skin, visible veins, club feet, dural ectasia, and premature osteoarthritis and peripheral neuropathy† |
| Vascular Ehlers-Danlos syndrome | COL3A1 | TAA, AAA, arterial rupture, aortic dissection, MVP, bowel and uterine rupture, pneumothorax, translucent skin, atrophic scars, small joint hypermobility, easy bruising, carotid-cavernous fistula |
| Arterial tortuosity syndrome | SLC2A10 | Tortuous large and medium sized arteries, aortic dilation, craniofacial, skin and skeletal features |
| Shprintzen-Goldberg syndrome | SKI | Craniosynostosis, skeletal features, aortic dilation |
| Ehlers-Danlos syndrome with periventricular nodular heterotopia | FLNA | X-linked, periventricular nodular heterotopia, TAA, BAV, MV disease, PDA, VSD, seizures, joint hypermobility |
| Meester-Loeys syndrome | BGN | X-linked, TAA, aortic dissection, MV disease |
| LOX-related TAA | LOX | TAA, BAV, aortic dissection, Marfanoid habitus in some |
| Smooth muscle dysfunction syndrome | ACTA2 | TAA, moyamoya-like cerebrovascular disease, pulmonary hypertension, pulmonary disease, hypoperistalsis, hypotonic bladder, congenital mydriasis11 |
| Nonsyndromic HTAD (Familial TAA) | ||
| FTAA | ACTA2 | TAA, aortic dissection, premature CAD and moyamoya-like cerebrovascular disease, livedo reticularis, iris flocculi |
| FTAA | MYH11 | TAA, aortic dissection, PDA |
| FTAA | MYLK | Aortic dissection at relatively small aortic size |
| FTAA | PRKG1 | Aortic dissection at young ages at small aortic sizes |
| FTAA | MAT2A | TAA, aortic dissection, BAV |
| FTAA | MFAP5 | TAA, aortic dissection, skeletal features may be present |
| FTAA | FOXE3 | TAA, aortic dissection |
| FTAA | THSD4 | TAA, aortic dissection |
| Bicuspid Aortic Valve–Associated Ascending Aortic Aneurysm | ||
| Familial BAV/AS and TAA | NOTCH1 | Aortic valve stenosis, TAA |
| BAV with TAA | TGFBR2, MAT2A, GATA5, SMAD6, LOX, ROBO4, TBX20 | Syndromic and nonsyndromic HTAD and FTAA with an increased frequency of BAV |
| Turner syndrome | XO, Xp | BAV, CoA, TAA, aortic dissection, short stature, lymphedema, webbed neck, premature ovarian failure |
Some individuals with pathogenic variants in a gene that can lead to syndromic HTAD have very few or no syndromic features, and variants in some genes causing syndromic HTAD may also lead to nonsyndromic HTAD.
SMAD3 premature osteoarthritis and peripheral neuropathy.
AAA indicates abdominal aortic aneurysm; AS, aortic stenosis; BAV, bicuspid aortic valve; CAD, coronary artery disease; CoA, coarctation of the aorta; EDS, Ehlers-Danlos syndrome; FTAA, familial thoracic aortic aneurysm (and dissection) syndrome; HTAD, heritable thoracic aortic disease; MV, mitral valve; MVP, mitra valve prolapse; PDA, patent ductus arteriosus; TAA, thoracic aortic aneurysm; and VSD, ventricular septal defect.
6.1.1. Sporadic and Degenerative TAA
Although there is a well-recognized anatomic distinction between aneurysms of the thoracic versus abdominal aorta, this should not imply that all TAA are similar in cause or natural history. Aneurysms of the aortic root and ascending aorta are typically diagnosed at younger patient ages than aneurysms of the descending thoracic aorta (60 versus 72 years, respectively).1 Even when considering just the “sporadic” aneurysms (ie, aneurysms in which there is no evidence of a syndromic, familial, or known genetic etiology), a significant difference in the ages between the 2 groups (64 versus 72 years, respectively) persists.1 In addition, typical atherosclerosis risk factors (ie, hypertension, diabetes, smoking) are significantly less common in sporadic root and ascending versus descending aortic aneurysms.2 Moreover, the prevalence of aortic calcification or atheroma (by CT or MRI) is quite low in sporadic aneurysms of the root and ascending thoracic aorta but quite high in aneurysms of the descending aorta, at 8% to 9% versus 80% to 88%, respectively.1 Collectively, these findings suggest that aneurysms of the aortic root and ascending aortic tend to have a congenital if not hereditary cause, whereas aneurysms of the descending aorta tend to have an atherosclerotic cause. Although sometimes referred to as atherosclerotic aneurysms, more often aneurysms of descending thoracic aorta (not related to connective tissue disorders) are referred to as “degenerative.” The medical management and surgical and endovascular management of sporadic and degenerative aneurysms are discussed in Sections 6.4, “Medical Management of Sporadic and Degenerative Aortic Aneurysm Disease,” and 6.5, “Surgical and Endovascular Management of Aortic Aneurysms,” respectively.
6.1.2. Genetic Aortopathies
6.1.2.1. HTAD: Genetic Testing and Screening of Family Members for TAD
Recommendations for HTAD: Genetic Testing and Screening of Family Members for TAD
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with aortic root/ascending aortic aneurysms or aortic dissection, obtaining a multigenerational family history of TAD, unexplained sudden deaths, and peripheral and intracranial aneurysms is recommended.1–3 |
| 1 | B-NR | 2. In patients with aortic root/ascending aortic aneurysms or aortic dissection and risk factors for HTAD (Table 8, Figure 17), genetic testing to identify pathogenic/likely pathogenic variants (ie, mutations) is recommended.4–6 |
| 1 | B-NR | 3. In patients with an established pathogenic or likely pathogenic variant in a gene predisposing to HTAD, it is recommended that genetic counseling be provided and the patient’s clinical management be informed by the specific gene and variant in the gene.7–9 |
| 1 | B-NR | 4. In patients with TAD who have a pathogenic/likely pathogenic variant, genetic testing of at-risk biological relatives (ie, cascade testing) is recommended.6,10,11 In family members who are found by genetic screening to have inherited the pathogenic/likely pathogenic variant, aortic imaging with TTE (if aortic root and ascending aorta are adequately visualized, otherwise with CT or MRI) is recommended.4,5,12 |
| 1 | B-NR | 5. In a family with aortic root/ascending aortic aneurysms or aortic dissection, if the disease-causing variant is not identified with genetic testing, screening aortic imaging (as per recommendation 4) of at-risk biological relatives (ie, cascade testing) is recommended.13–15 |
| 1 | C-LD | 6. In patients with aortic root/ascending aortic aneurysms or aortic dissection, in the absence of either a known family history of TAD or pathogenic/likely pathogenic variant, screening aortic imaging (as per recommendation 4) of first-degree relatives is recommended.13 |
| 1 | C-EO | 7. In patients with acute type A aortic dissection, the diameter of the aortic root and ascending aorta should be recorded in the operative note and medical record to inform the management of affected relatives. |
Table 8.
Risk Factors for Familial TAD
| TAD and syndromic features of Marfan syndrome, Loeys-Dietz syndrome, or vascular Ehlers-Danlos syndrome |
| TAD presenting at age <60 y |
| A family history of either TAD or peripheral/intracranial aneurysms in a first- or second-degree relative |
| A history of unexplained sudden death at a relatively young age in a first- or second-degree relative |
TAD indicates thoracic aortic disease.
Figure 17. Evaluation and Genetic Testing Protocol for Patients With TAD.
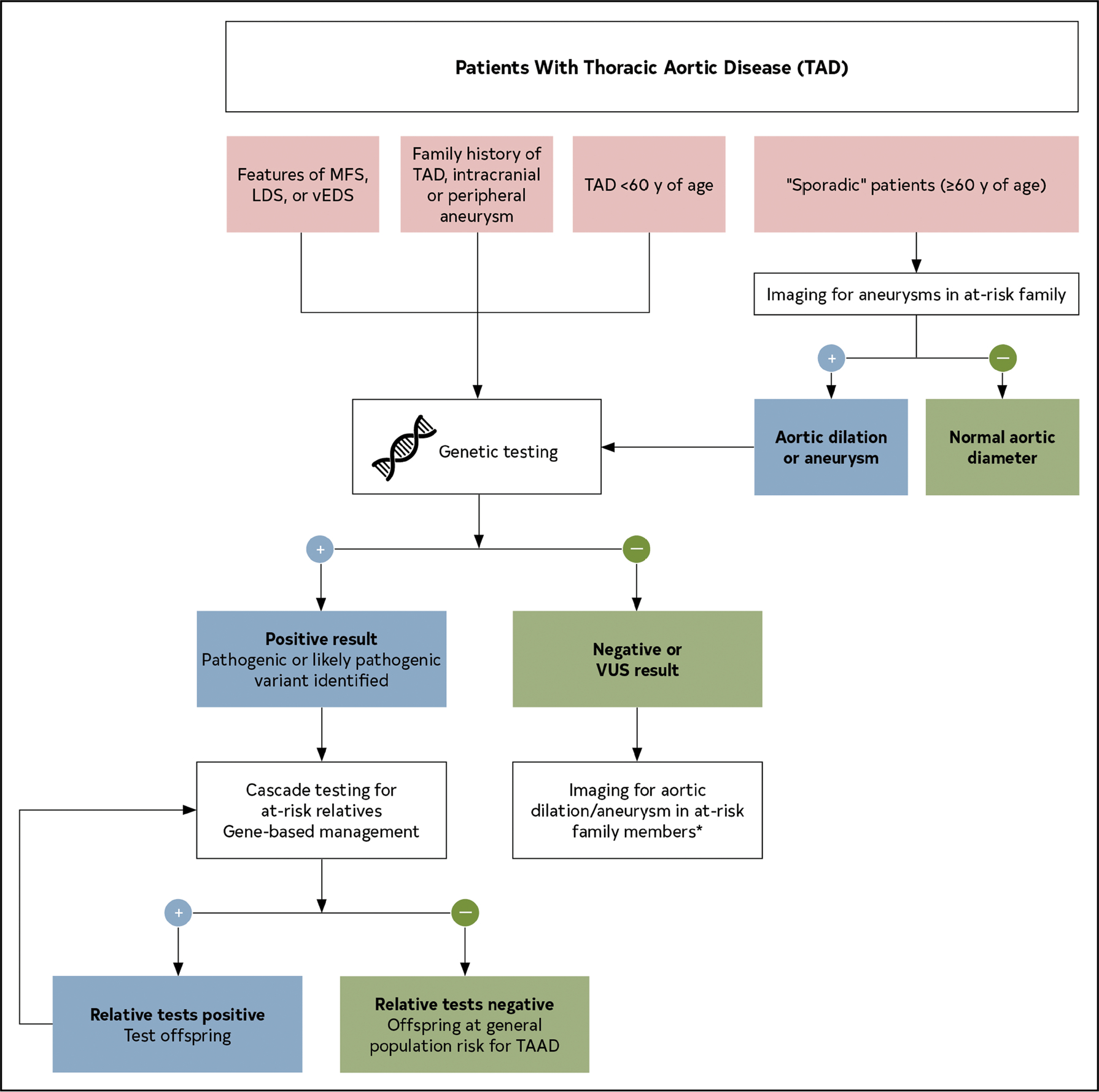
Genetic testing is recommended for individuals with syndromic features, family history of TAD, and/or early age of disease onset. Thoracic aortic imaging is recommended for first-degree relatives of all individuals with TAD, regardless of age of onset, to detect asymptomatic aneurysms. Positive genetic testing should trigger gene-based management and cascade testing of at-risk relatives. When testing is negative or reveals variants of unknown significance, first-degree relatives should undergo screening aortic imaging. Modified with permission from Milewicz et al.6 Copyright 2021, Minerva Medica. Blue (+) indicates positive; green (−), negative; LDS, Loeys-Dietz syndrome; MFS, Marfan syndrome; TAAD, thoracic aortic aneurysm and dissection; TAD, thoracic aortic disease; vEDS, vascular Ehlers-Danlos syndrome; and VUS, variants of unknown significance. *Aneurysms are typically asymptomatic.
Synopsis
A major risk factor for aortic root aneurysms, ascending aortic aneurysms, and aortic dissection is a pathogenic variant in genes predisposing to TAD. Although the recommendations focus on individuals at high risk for a single gene mutation (Table 8), genetic testing may have a role in many TAD patients. A multigene panel comprising all genes suspected to cause HTAD is the most cost-effective and clinically useful approach to testing. Only pathogenic or likely pathogenic variants are disease-causing and should be used for cascade genetic testing all relatives at risk for inheriting the disease-causing variant.15,16
In families with HTAD in which the causative gene has not been identified, the clinical features in affected family member should dictate management of other family members, including location of aneurysms; relevant clinical features include the diameter of the aortic root and ascending aorta in affected family members who have had a type A dissection (noting that the aortic root typically is not distorted by the dissection, whereas the ascending aorta may acutely enlarge17) and other vascular disease or features segregating with TAA in the family.
Recommendation-Specific Supportive Text
Current data indicate that 13% to 20% of patients with TAD and without Marfan syndrome or Loeys-Dietz syndrome features have similarly affected first-degree relatives.1,2 TAD in these families is typically inherited in an autosomal dominant manner, with decreased penetrance, particularly in women. These data suggest that heterozygous pathogenic variants in single genes are responsible for HTAD in most families.3,18 In families with HTAD, testing in an individual diagnosed with TAD should be initiated. Patients with a family history of the disease present at younger ages (average 57 years).3 These families with HTAD show variable expression of TAD, including varying age of disease onset, frequency of aortic dissection at a diameter <5.0 cm, risk for type B aortic dissection, and frequency with which dilation involves the aortic root, the tubular ascending aorta, or both.8,14 In addition, the specific altered gene impacts the risk for associated vascular conditions.
The HTAD genetic testing panels include (at the time of this writing) 11 genes that are confirmed to confer a highly penetrant risk for TAD: FBN1, LOX, COL3A1, TGFBR1, TGFBR2, SMAD3, TGFB2, ACTA2, MYH11, MYLK, and PRKG1.19 These panels also include genes that increase the risk for TAD and/or lead to systemic features that overlap with Marfan syndrome, Loeys-Dietz syndrome, or vascular Ehlers-Danlos syndrome. Clinical genetic testing is integral to the diagnostic evaluation of patients with TAD who have clinical indicators suggestive of an underlying single gene disorder (Table 8).5,20 In patients who meet the clinical diagnostic criteria for Marfan syndrome but do not have ectopia lentis (ie, dislocated lens), genetic testing is reasonable to exclude an alternative diagnosis of Loeys-Dietz syndrome. Genetic testing laboratories categorize rare variants in HTAD genes into these classes: pathogenic, likely pathogenic, variant of uncertain/unknown significance, benign, and likely benign. Variants of unknown significance have not been confirmed to cause TAD and therefore should not be used either to identify which family members are at risk or to guide clinical management. Because a subset of these variants of unknown significance may, nevertheless, be disease-causing, families with the potential to help further classify the variant of unknown significance should be evaluated in collaboration with the genetic testing company.
FBN1, TGFBR1, TGFBR2, SMAD3, and TGFB2 mutations have been identified in approximately 6% to 8% of HTAD families whose members do not have syndromic features of Marfan syndrome or Loeys-Dietz syndrome.12,20–23 Mutations in ACTA2, MYH11, MYLK, LOX, and PRKG1 have been confirmed to cause HTAD in the absence of significant features of Marfan syndrome or Loeys-Dietz syndrome.16,24 Through clinical characterization of HTAD families with pathogenic variants in novel genes, data have emerged that the underlying gene predicts not only who in the family is at risk for thoracic aortic aneurysm and dissection (TAAD) but also the aortic disease presentation, risk for aortic dissection at a given range of aortic diameters as described previously, and risk for and type of additional vascular diseases.7–9 For example, TGFBR2 mutations predispose to TAAD but also to intracranial aneurysms and aneurysms and dissections of other arteries, whereas ACTA2 mutations lead to TAAD and occlusive vascular disease, including early onset stroke and coronary artery disease. Genetic counseling is useful to explain to patients and families the genetic risk and how it is inherited, to assess the family history to determine TAD risk, to assist in cascade genetic testing and/or imaging for TAD in family members, and to offer psychosocial and ethical guidance.10
Cascade screening is the process of extending imaging to identify asymptomatic thoracic aortic enlargement to individuals at risk within a family for inheriting the pathogenic variant causing HTAD in the family; the process is repeated as family members are identified with thoracic aortic enlargement or as carriers of the pathogenic variant are identified.10 Pathogenic variants in genes for HTAD confer a high risk for TAD, so individuals found to have these pathogenic variants should be screened with aortic imaging for asymptomatic TAD.16,24
Among patients undergoing genetic testing, many will not have a pathogenic variant identified, despite other clinical evidence that the disease is likely genetically triggered (eg, extensive family history of TAD or early onset sporadic TAD with no risk factors). Despite the absence of a pathogenic variant among the currently known genes that were tested, TAD could still be inherited in the family attributable to a causative genetic variant that has yet to be identified. Consequently, multiple studies have confirmed the utility of screening aortic imaging of at-risk relatives of all TAD patients with a positive family history.13–15 If negative, repeat screening imaging might be worthwhile in 5 years of younger family members or 10 years in older family members, informed by the family history. Additionally, it is critical to obtain relevant clinical data from affected family members, including the location of the aortic dilation (ie., the aortic root versus the ascending aorta), current aortic diameter or diameter at the time of surgical repair or diameter at the time of type A aortic dissection, and the presence of other vascular diseases (eg, aneurysms in other arteries, early onset occlusive vascular diseases), as these will inform management of all affected family members. The HTADs vary in terms of the risk of other clinical cardiovascular complications that segregate with TAD; therefore, surveillance for such conditions is best guided by the family history.22,25–27
Although the data are more limited, studies also support the screening of first-degree relatives of patients with TAD who do not have a family history of the disease.13 If negative, aortic imaging may be repeated years later, depending on the relative’s age and aortic size. It should be recognized that there is no upper limit to the age at which patients present with TAD that precludes an underlying genetic cause of the disease.
Because the size at which the aortic root or ascending aorta dissects impacts the risk of aortic dissection in other affected family members, the specific aortic diameters should be recorded in the medical record (ie, operative report, discharge summary), so that the information can be readily retrieved when needed in the future.
6.1.2.1.1. Surgical Considerations for Nonsyndromic Heritable TAAs and No Identified Genetic Cause
Recommendations for Surgical Considerations for Nonsyndromic Heritable TAA and No Identified Genetic Cause
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In asymptomatic patients with aneurysms of the aortic root or ascending aorta with nonsyndromic heritable thoracic aortic disease (nsHTAD) and no identified genetic cause, determining the timing of surgical repair requires shared decision-making and is informed by known aortic diameters at the time of aortic dissection, TAA repair, or both in affected family members.1–4 |
| 1 | C-LD | 2. In asymptomatic patients with aneurysms of the aortic root or ascending aorta with nsHTAD and no identified genetic cause but no information on aortic diameters at the time of dissection or aneurysm repair in affected family members and who have no high-risk features for adverse aortic events (Table 9) it is recommended to repair the aorta when the maximal diameter reaches ≥5.0 cm.1 |
| 2a | C-LD | 3. In patients with aneurysms of the aortic root or ascending aorta with nsHTAD and no identified genetic cause and a maximal aortic diameter of ≥4.5 cm, who have high-risk features for adverse aortic events (Table 9), or who are undergoing cardiac surgery for other indications, aortic repair is reasonable when performed by experienced surgeons in a Multidisciplinary Aortic Team.5 |
Table 9.
Features Associated With an Increased Risk of Aortic Dissection in Patients With Heritable Thoracic Aortic Aneurysms
| Heritable Thoracic Aortic Aneurysms and No Identified Genetic Cause |
|---|
| Family history of aortic dissection at an aortic diameter <5.0 cm |
| Family history of unexplained sudden death at age <50 y |
| Rapid aortic growth (≥0.5 cm in 1 y or ≥0.3 cm/y in 2 consecutive y) |
Synopsis
HTAD refers to TAD caused by a highly penetrant rare variant (or mutation) in a single gene. A diagnosis of HTAD is based on ≥2 members of a family with TAD, the identification of a pathogenic variant in the gene known to cause TAD in a family member, or clinical diagnosis of syndrome that confers a risk for TAD (eg, Marfan syndrome) in a family member. Syndromic HTAD typically has systemic features with multiorgan phenotype, positive family history of aortic aneurysm or dissection, and is often caused by mutations involving extracellular matrix proteins or involved in transforming growth factor-β pathway. Such patients, including Marfan syndrome and Loeys-Dietz syndrome, are predisposed to developing aneurysms of the aortic root and ascending aorta at an early age, and have a faster rate of aortic growth than do those with sporadic aneurysms. Consequently, these patients have a higher risk of acute aortic dissection or rupture, resulting in a shorter life expectancy than those patients whose aneurysms are not genetically mediated. Prophylactic surgery to replace the aortic root and ascending aorta has dramatically improved the overall life expectancy of HTAD patients. Prophylactic elective surgery in these young patients requires a very low operative mortality with a multidisciplinary approach for genetic testing and lifelong surveillance. Surgeons in Multidisciplinary Aortic Teams have shown sufficiently low operative mortality to safely treat these patients at smaller aortic sizes. Similar to what is seen with sporadic aneurysms, aortic dissection in HTAD can occur at aortic diameters smaller than the surgical thresholds recommended in guidelines.
nsHTAD refers to a genetic predisposition to TAD running in families in the absence of systemic features. NsHTAD may be present in up to 20% of patients with TAD (based on family history), is typically inherited in an autosomal dominant manner, with a pathogenic genetic variant identified in up to 20%. When no pathogenic variant is identified in families with nsHTAD, it has often been referred to as “familial thoracic aortic aneurysm and dissection.” It tends to be more penetrant and of earlier onset in men than women within affected families. The diagnosis is often delayed until midlife but occurs earlier than for sporadic aneurysms; aneurysm growth is also typically faster than for sporadic aneurysms. Because the initial presentation is commonly acute aortic dissection, screening family members is important to guide prophylactic surgery to prevent potential aortic dissection. Clearly, elective surgery before aortic dissection yields better long-term survival with fewer aortic reinterventions than surgery after aortic dissection.4–7
Recommendation-Specific Supportive Text
The GenTAC (National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions) study found a higher risk of dissection, with most of dissection patients not having met the size criteria for prophylactic surgery.1,6 For patients with a family history of TAA, aortic dissection, or both, but with no known pathogenic variant, it is useful to determine the size at which the aorta dissected (if known) or the size at which elective aortic surgery was performed, as well as the age of the affected relative at time of the aortic event. It is appropriate to offer aortic repair based on the family member’s aortic size at dissection or elective surgery.
Patients with a family history of TAAs but with no known pathogenic variant may not have information regarding the aneurysm size at which the family members underwent either elective surgery or experienced aortic dissection. However, the GenTAC study suggested a higher risk of aortic dissection, with a large proportion of patients not having met the 5.5-cm threshold for elective repair at the time of their aortic dissection. Given that aortic dissection in this population with familial TAAs may occur at younger ages and with worse outcomes and the more frequent need for reoperations, prophylactic surgery is warranted when the maximal diameter of the aortic root or ascending aorta reaches ≥5.0 cm.1–4,8
For patients with a family history of aortic dissection at a known maximal aortic root or ascending aortic diameter <5.0 cm but with no known pathogenic variant, it is reasonable to perform prophylactic aortic repair at a maximal aortic diameter of ≥4.5 cm, because their affected relative experienced an aortic dissection at the relatively small diameter of <5.0 cm. Similarly, patients with relatives whose aortic dissection or unexplained sudden death occurred at an age <50 years are themselves at increased risk of such adverse events at ages <50 years as well. Similarly, nsHTAD patients who have documented rapid aneurysm growth are increased risk of untoward aortic events at younger ages and smaller aneurysm sizes, so prophylactic aortic surgery is reasonable when performed by experienced surgeons in Multidisciplinary Aortic Teams, with shown excellent short- and long-term outcomes.1–4,8
6.1.2.2. Marfan Syndrome
6.1.2.2.1. Diagnostic and Surveillance Aortic Imaging in Marfan Syndrome
Recommendations for Diagnostic and Surveillance Aortic Imaging in Marfan Syndrome
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| Initial Diagnosis and Surveillance Imaging | ||
| 1 | C-EO | 1. In patients with Marfan syndrome, a TTE is recommended at the time of initial diagnosis, to determine the diameters of the aortic root and ascending aorta, and 6 months thereafter, to determine the rate of aortic growth; if the aortic diameters are stable, an annual surveillance TTE is recommended.1 If the aortic root, ascending aorta, or both are not adequately visualized on TTE, a CT or MRI of the thoracic aorta is recommended.2 |
| 2a | C-EO | 2. In adults with Marfan syndrome, after the initial TTE, a CT or MRI of the thoracic aorta is reasonable to confirm the aortic diameters and assess the remainder of the thoracic aorta. |
| Imaging After Aortic Root Replacement | ||
| 1 | C-LD | 3. In patients with Marfan syndrome who have undergone aortic root replacement, surveillance imaging of the thoracic aorta by MRI (or CT) is recommended to evaluate for distal TAD, initially annually and then, if normal in diameter and unchanged after 2 years, every other year.3–6 |
| 2a | C-LD | 4. In patients with Marfan syndrome who have undergone aortic root replacement, surveillance imaging every 3 to 5 years for potential AAA is reasonable.2,6 |
Synopsis
Marfan syndrome is an autosomal dominant connective tissue disorder caused by pathogenic variants in the FBN1 gene affecting 1 in 5 000 individuals.1 Phenotypic features in the skeletal, ocular, pulmonary, cutaneous, nervous, and cardiovascular systems may be recognized. The modified Ghent criteria for diagnosis incorporate genetic testing, the systemic score, ectopia lentis, and the family history.1 Patients with Marfan syndrome develop aneurysms involving the aortic root (sinuses of Valsalva) and are at risk for aortic dissection.1 Descending aortic and AAAs are less common.6,7 Type B aortic dissection is the initial aortic event in about 10% of patients and may also occur despite previous root replacement.4 Imaging surveillance of the aorta is typically performed annually, with the frequency dependent on age, aortic diameter, rate of aortic growth, and family history.8 Prophylactic aortic root replacement for aneurysm disease prevents type A dissection and improves survival in Marfan syndrome.3,9,10
Recommendation-Specific Supportive Text
Aortic root dilation and type A aortic dissection are the leading causes of morbidity and mortality in Marfan syndrome.9,10 Aortic dilation involves the aortic root, but effacement of the sinotubular junction with enlargement of the proximal ascending aorta is often present.11 The aortic root and ascending aorta are measured by TTE and are observed annually. Nomograms accounting for age, sex, and body size (and height) assist with determining the degree to which the diameter deviates from normal in the general population.12 In patients with Marfan syndrome participating in trials of beta blockers versus angiotensin receptor blockers (ARBs), the mean growth of the aortic root was 1 mm to 1.5 mm over 3 years and 4 mm to 5 mm over 5 years. The rate of aortic dilation is faster in patients with larger aortic aneurysms. More frequent imaging is performed in patients with rapid aortic growth, in those approaching surgical thresholds, or when the diameter exceeds 4.5 cm. Patients with Marfan syndrome are at greatest risk for aneurysmal dilation of the aortic root, followed by involvement of the ascending aorta. Patient-specific factors, such as pectus deformities and lung disease, may limit the evaluation of the aortic root on TTE. When the aortic root and ascending aorta are not adequately visualized by TTE, CT or MRI should be performed to measure the aortic diameters,2 although TEE is another alternative to measure the aortic root and ascending aorta.
Patients with Marfan syndrome may develop disease of the descending aorta.9,10 In some individuals, a thorough TTE may accurately assess the diameters of aortic root, ascending aorta, aortic arch, proximal descending aorta, and distal descending aorta. For patients undergoing an initial evaluation in whom the aortic segments distal to the ascending aorta are not adequately visualized on TTE, a CT or MRI can be used to assess the more distal aortic segments.
Surgical aortic root replacement can prevent type A aortic dissection and improve longevity for patients with Marfan syndrome and aortic root aneurysms.3,9,10 Long-term complications after aortic root replacement may include graft infections, pseudoaneurysms, aneurysms in the distal aorta, and aortic dissection distal to the graft.4,13
In patients with Marfan syndrome, distal TAA and AAA (in the absence of aortic dissection) may occur but are much less common than aortic root disease. Most individuals with aortic disease distal to the root have had previous root replacement or smoke cigarettes.7,13
6.1.2.2.2. Medical Therapy in Marfan Syndrome
Recommendations for Medical Therapy in Marfan Syndrome
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A | 1. In patients with Marfan syndrome, treatment with either a beta blocker or an ARB, in maximally tolerated doses (unless contraindicated), is recommended to reduce the rate of aortic dilation.1,2 |
| 2a | C-LD | 2. In patients with Marfan syndrome, the use of both a beta blocker and an ARB, in maximally tolerated doses (unless contraindicated), is reasonable to reduce the rate of aortic dilation.3,4 |
Synopsis
Beta blockers have long been recommended for patients with Marfan syndrome to reduce heart rate and myocardial contractility and to slow aortic root growth.5–7 More recently, ARBs have also been found to be efficacious in Marfan syndrome.1–4,8
Recommendation-Specific Supportive Text
In an open-label study of patients with Marfan syndrome who were observed for >10 years, propranolol treatment was associated with a reduction in aortic root growth rate and fewer clinical events5 compared with control (no treatment). More recently, in a retrospective evaluation of children with Marfan syndrome, beta-blocker treatment was associated with a reduced aortic growth rate.6 Losartan was shown to prevent aneurysm formation in mouse models of Marfan syndrome9 and, in a small, nonrandomized open label study of children with Marfan syndrome who had previously had rapid aortic root growth, ARBs were shown to dramatically slow aortic root growth.10 However, randomized trials comparing an ARB to a beta blocker in patients with Marfan syndrome found no significant difference in the rate of either aortic root growth or clinical events (including aortic surgery or aortic dissection) between the 2 treatment groups.1,2
Multiple trials have compared the addition of an ARB to beta-blocker therapy in patients with Marfan syndrome3,4,8; in 2 studies, the addition of an ARB led to a reduction of aortic root growth rates over a 3- to 5-year follow-up,3,4 and a meta-analysis confirmed slower aortic growth rates with combination therapy.11
6.1.2.2.3. Marfan Syndrome Interventions: Replacement of the Aortic Root in Patients With Marfan Syndrome
Recommendations for Marfan Syndrome Interventions: Replacement of the Aortic Root in Patients With Marfan Syndrome
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with Marfan syndrome and an aortic root diameter of ≥5.0 cm, surgery to replace the aortic root and ascending aorta is recommended.1–4 |
| 2a | B-NR | 2. In patients with Marfan syndrome, an aortic root diameter of ≥4.5 cm, and features associated with an increased risk of aortic dissection (see Table 10), surgery to replace the aortic root and ascending aorta is reasonable, when performed by experienced surgeons in a Multidisciplinary Aortic Team.1,3,4 |
| 2a | C-LD | 3. In patients with Marfan syndrome and a maximal cross-sectional aortic root area (cm2) to patient height (m) ratio of ≥10, surgery to replace the aortic root and ascending aorta is reasonable, when performed by experienced surgeons in a Multidisciplinary Aortic Team.5 |
| 2b | C-LD | 4. In patients with Marfan syndrome and an aortic diameter approaching surgical threshold, who are candidates for valve-sparing root replacement (VSRR) and have a very low surgical risk, surgery to replace the aortic root and ascending aorta may be reasonable when performed by experienced surgeons in a Multidisciplinary Aortic Team.2–4 |
Table 10.
Features Associated With Increased Risk of Aortic Complications in Marfan Syndrome
Synopsis
Prophylactic aortic root replacement for aneurysm disease prevents type A aortic dissection and improves survival in Marfan syndrome.6–8 The size threshold for elective surgery to replace the dilated aortic root in Marfan syndrome is dependent on many factors, including the patient’s age, height and weight, family history, rate of aortic growth, and other patient-specific factors.1,3–5,9 In patients with Marfan syndrome who are managed with optimal medical therapy and whose aortic diameters are <5.0 cm, the risk of aortic dissection is low.3,4,10 However, the risk of aortic dissection increases when the aortic diameter is >5.0 cm and is greater in patients with a family history of aortic dissection or rapid aortic growth.3,4,10
Recommendation-Specific Supportive Text
In patients with Marfan syndrome and a dilated aortic root, elective aortic root and ascending aortic replacement before aortic dissection improves survival.6–8 A landmark report in 1995 documented the marked improvement in lifespan among patients with Marfan syndrome treated with elective aortic repair compared with historical controls from previous eras.6,10 Although risk of aortic dissection is low in patients with Marfan syndrome who are receiving appropriate medical care and lifestyle modifications, the risk of aortic dissection increases when the aortic diameter is >5.0 cm.3,4,11 When prophylactic surgical aortic repair is performed, both the aortic root and ascending aorta are replaced; although some centers have advocated including hemiarch replacement in patients at the time of elective root/ascending aorta replacement, data to support this approach are lacking.
In large series of patients with Marfan syndrome, about 20% have undergone elective surgery when aortic root diameters are <5.0 cm.3,4,11 Predictors of aortic dissection and other adverse aortic outcomes in Marfan syndrome are listed in Table 10. Indications for earlier aortic surgery may include rapid aortic growth (≥0.3 cm/y), family history of aortic dissection, desire for pregnancy, severe valve regurgitation, and patient preference.3,9,12 For most patients with Marfan syndrome, aortic growth rates are relatively slow, but the growth rate increases with aortic size.12
Aortic diameters vary depending on age, sex, height, and body size. Aortic event rates, including aortic dissection, increase as the aortic size indexed to height (or body size) increases. When the maximal cross-sectional area in square (cm2) of the aortic root or ascending aorta divided by the patient’s height (m) is ≥10 cm2/m, prophylactic aortic root replacement is reasonable; when this cross-sectional area to height ratio was used to guide prophylactic surgery, patients had favorable outcomes.
Aortic root replacement is associated with a very low surgical risk3,4,11 when performed by experienced surgeons in Multidisciplinary Aortic Teams. The 2 aortic root replacement procedures performed most commonly in the United States are a composite valved graft conduit and a VSRR.13 The composite valved graft conduit consists of a prosthetic aortic valve (typically mechanical but may be bioprosthetic) and aortic graft, with reimplantation of the coronary arteries (often referred to as the modified Bentall procedure). The VSRR uses the David procedure, in which the native aortic valve is reimplanted into a prosthetic aortic graft that is attached to the left ventricular outflow tract proximally and to the ascending aorta distally. The advantage of the VSRR is that, if successful, patients can potentially avoid the lifelong risks and complications associated with prosthetic valves. Consequently, early prophylactic surgery can be considered when both the procedural and late risks are low. However, durability of the spared native aortic valve is a potential concern; in one series of 239 patients with Marfan syndrome undergoing VSRR, 7% developed at least moderate AR at 1 year follow-up.13
6.1.2.2.4. Marfan Syndrome Interventions: Replacement of Primary (Nondissected) Aneurysms of the Aortic Arch, Descending, and Abdominal Aorta in Patients With Marfan Syndrome
Recommendation for Replacement of Primary (Nondissected) Aneurysms of the Aortic Arch, Descending, and Abdominal Aorta in Patients With Marfan Syndrome
| COR | LOE | Recommendation |
|---|---|---|
| 2a | C-EO | 1. In patients with Marfan syndrome and a nondissected aneurysm of the aortic arch, descending thoracic aorta, or abdominal aorta of ≥5.0 cm, surgical intervention to replace the aneurysmal segment is reasonable. |
Synopsis
Marfan syndrome most commonly leads to aneurysms of the aortic root and ascending aorta but may also affect the distal aorta and its branches.1–4 Unfortunately, there are no large datasets to inform the risk of aortic dissection or rupture in patients with Marfan syndrome with primary (nondissected) aneurysms of the aortic arch, descending, or abdominal aorta, so using a 5.0-cm diameter threshold for surgery, as is used for the aortic root, is reasonable.
Recommendation-Specific Supportive Text
Although uncommon, aortic segments distal to the aortic root and ascending aorta may dilate in Marfan syndrome, and this occurs more often after elective aortic root replacement or after a previous aortic dissection involving these segments.5 In patients at acceptable risk for operative repair or with a long life expectancy, operative intervention to resect primary (nondissected) aneurysms involving the arch, descending, or abdominal aorta is reasonable at an aortic diameter threshold of ≥5.0 cm, depending on the patient’s age, rate of aortic growth, family history, and surgical risk. Type B aortic dissection occurs in about 10% of Marfan patients, often in the absence of significant dilation of the descending aorta, and is sometimes associated with prior elective aortic root replacement,1 a previous aortic dissection elsewhere,6 or pregnancy.7
6.1.2.3. Loeys-Dietz Syndrome
6.1.2.3.1. Imaging in Loeys-Dietz Syndrome
Recommendations for Imaging in Loeys-Dietz Syndrome
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. In patients with Loeys-Dietz syndrome, a baseline TTE is recommended to determine the diameters of the aortic root and ascending aorta, and 6 months thereafter to determine the rate of aortic growth; if the aortic diameters are stable, annual surveillance TTE is recommended.1–3 |
| 1 | C-EO | 2. In patients with Loeys-Dietz syndrome and a dilated or dissected aorta and/or arterial branches at baseline, annual surveillance imaging of the affected aorta and arteries with MRI or CT is recommended.1 |
| 1 | C-LD | 3. In patients with Loeys-Dietz syndrome, a baseline MRI or CT from head to pelvis is recommended to evaluate the entire aorta and its branches for aneurysm, dissection, and tortuosity.1–4 |
| 2a | C-EO | 4. In patients with Loeys-Dietz syndrome without dilation of the aorta distal to the aortic root or ascending aorta and without dilated or dissected arterial branches, surveillance imaging from chest to pelvis with MRI (or CT) every 2 years is reasonable, but imaging may be more frequent depending on family history. |
| 2a | C-EO | 5. In patients with Loeys-Dietz syndrome without dilation of the cerebral arteries on initial screening, periodic imaging surveillance for cerebral aneurysms with MRI or CT every 2 to 3 years is reasonable. |
Synopsis
Loeys-Dietz syndrome is characterized by aortic and branch vessel aneurysms and dissections, arterial tortuosity, and skeletal features similar to those seen in Marfan syndrome but with unique craniofacial and cutaneous features.1 Pathogenic variants in 5 genes cause Loeys-Dietz syndrome, also termed transforming growth factor-β vasculopathies.1–3,5,6 Some pathogenic variants in Loeys-Dietz syndrome genes, in particular TGFBR1 and TGFBR2, may have earlier onset TAD.7 All the Loeys-Dietz syndrome genes confer a risk for aortic involvement distal to the aortic root along with branch vessel and intracranial aneurysms.1,8–11 Most clinical information is available in patients with TGFBR1 and TGFBR2 pathogenic variants.1,8 Pathogenic variants in SMAD3 are associated with premature osteoarthritis and later onset of TAD.9,12 There is much less information about the aortic and branch vessel disease in patients with variants in TGFB2 and TGFB3.13–16 Imaging with CT or MRI, from head to pelvis, is indicated to evaluate for aneurysms and arterial tortuosity.
Recommendation-Specific Supportive Text
Aortic root and ascending aortic aneurysm and aortic dissection are leading causes of morbidity and mortality in Loeys-Dietz syndrome.1,8,9,12 Aortic dissection may occur at relatively small aortic diameters in Loeys-Dietz syndrome when related to pathogenic variants in TGFBR1, TGFBR2, and SMAD3.1,2,6 The specific genetic variant and severity of extra-aortic phenotypic features, including craniofacial features, degree of arterial tortuosity, cutaneous findings, and family history inform the risk of aortic events.1,2,6 The aortic root and ascending aortic diameters are typically measured by TTE. BAV is more common in Loeys-Dietz syndrome and can be diagnosed by TTE.17 Patients with Loeys-Dietz syndrome attributable to certain pathogenic variants are at risk for aortic dissection at relatively small aortic diameters.1,8 In patients with Loeys-Dietz syndrome, the stability of the aortic size 6 months after the initial diagnosis should be determined, and then, once stability is confirmed, monitored with annual surveillance imaging.1,2
Patients with Loeys-Dietz syndrome may have variable aortic and branch vessel involvement and variable rates of dilation of involved arterial segments over time. In Loeys-Dietz syndrome patients with aortic aneurysm or previous dissection, relatively rapid arterial enlargement may occur.2,18,19
Patients with Loeys-Dietz syndrome are at risk for widespread aortic and branch vessel aneurysmal disease and dissections.1,12 In a series of 90 patients with Loeys-Dietz syndrome attributable to pathogenic variants in TGFBR1 and TGFBR2, aneurysm disease involved the ascending aorta in 78%, arch in 10%, descending aorta in 10%, abdominal aorta and branches in 17%, thoracic aortic branches in 21%, and head and neck arterial branches in 10%.1 Among patients with SMAD3-related disease, aneurysms from head to pelvis are also described.9,12 Individuals with Loeys-Dietz syndrome can be at risk for TAD and other vascular diseases in the absence of other systemic features characteristic of Marfan syndrome or Loeys-Dietz syndrome. Although there is phenotypic overlap among the genes, there is also distinct vascular disease and systemic complications associated with each gene. The physician should be cognizant of the particular gene variant in monitoring and managing patients with Loeys-Dietz syndrome.
In patients with Loeys-Dietz syndrome and aortic disease limited to segments that are well-visualized by TTE and without branch vessel disease, surveillance of the distal aorta and its branches is needed to evaluate for the possible interval occurrence of dilation (or dissection); the frequency of surveillance imaging may be influenced by the patient’s age and family history.2
Cerebral aneurysms are described in 10% to 18% of patients with Loeys-Dietz syndrome.1,9,11,20 The frequency of follow-up screening for cerebral aneurysm disease in patients without aneurysms on initial screening will depend on the patient’s age and may be informed by phenotype or other features.11
6.1.2.3.2. Medical Therapy in Loeys-Dietz Syndrome
Recommendation for Medical Therapy in Loeys-Dietz Syndrome
| COR | LOE | Recommendation |
|---|---|---|
| 2a | C-EO | 1. In patients with Loeys-Dietz syndrome, treatment with a beta blocker or an ARB (unless contraindicated), or both, in maximally tolerated doses, is reasonable. |
Synopsis
The management of individuals with Loeys-Dietz syndrome includes medical therapy, lifestyle modification, imaging surveillance, and surgical intervention. To lessen hemodynamic stress on the aorta, beta blockers are used.1 Based on studies of mouse models, ARBs have also been used.2
Recommendation-Specific Supportive Text
There are no randomized trials of medications to reduce aortic growth or the risk of aortic dissection in patients with Loeys-Dietz syndrome. Consequently, the approach to medical therapy is similar to that used for treating patients with Marfan syndrome, based on the similarities between the 2 connective tissue disorders and on data from mouse models of Loeys-Dietz syndrome.2 Thus, the use of beta blockers, ARBs, or both is reasonable.1
6.1.2.3.3. Loeys-Dietz Syndrome Surgical Interventions: Replacement of the Aorta in Patients With Loeys-Dietz Syndrome
Recommendations for Replacement of the Aorta in Patients With Loeys-Dietz Syndrome
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In patients with Loeys-Dietz syndrome and aortic dilation, the surgical threshold for prophylactic aortic root and ascending aortic replacement should be informed by the specific genetic variant, aortic diameter, aortic growth rate, extra-aortic features, family history, patient age and sex, and physician and patient preferences (Table 11).1–9 |
| 2b | C-EO | 2. In patients with Loeys-Dietz syndrome attributable to a pathogenic variant in TGFBR1, TGFBR2, or SMAD3, surgery to replace the intact aortic arch, descending aorta, or abdominal aorta at a diameter of ≥4.5 cm may be considered, with the specific genetic variant, patient age, aortic growth rate, family history, presence of high-risk features (Table 11), and surgical risk informing the decision. |
Table 11.
Surgical Thresholds for Prophylactic Aortic Root and Ascending Aortic Replacement in Loeys-Dietz Syndrome Based on Genetic Variant
| COR | LOE (references) | Genetic Variant | Presence of High-Risk Features* | Aortic Diameter (cm) |
|---|---|---|---|---|
| 1 | C-LD2 | TGFBR1 | No | ≥4.5 |
| 1 | C-LD2 | TGFBR2 | No | ≥4.5 |
| 2b | C-EO2 | TGFBR1 | Yes | ≥4.0 |
| 2a | C-LD1,2 | TGFBR2 | Yes | ≥4.0 |
| 2a | C-EO13,16 | SMAD3 | – | ≥4.5† |
| 2b | C-EO5–7 | TGFB2 ‡ | – | ≥4.5† |
| 2b | C-EO9,23 | TGFB3 | – | ≥5.0† |
Aortic surgery may be recommended at smaller aortic diameters in Loeys-Dietz syndrome attributable to TGFBR1 and TGFBR2 pathogenic variants when there are features that associate with a higher risk of aortic dissection, including: certain specific pathogenic variants; women with TGFBR2 and small body size; severe extra-aortic features (ie, craniosynostosis, cleft palate, hypertelorism, bifid uvula, marked arterial tortuosity, widened scars, and translucent skin); family history of aortic dissection (especially at young age or relatively small aortic diameter); and aortic growth rate >0.3 cm/y.
Family history, age, and aortic growth rate also inform surgical thresholds.
Pathogenic variants in the TGFB2 gene are different than variants in the TGFBR2 gene.
COR indicates class of recommendation; and LOE, level of evidence.
Colors correspond to COR and LOE in Table 2.
Synopsis
In patients with Loeys-Dietz syndrome, prophylactic aortic root replacement for aneurysm disease prevents type A aortic dissection and improves outcomes.1,2,10–12 Aortic dissection in Loeys-Dietz syndrome that is attributable to pathogenic variants in TGFBR1, TGFBR2, and SMAD3 may occur at smaller aortic diameters than in Marfan syndrome.1–3,13 Based on limited data, Loeys-Dietz syndrome attributable to pathogenic variants in TGFB25,6,14 and TGFB38,9 may have a less aggressive aortic phenotype than disease attributable to TGFBR1, TGFBR2, or SMAD3 variants.1,2,4,15,16 The size threshold for elective surgery to replace the dilated aortic root and ascending aorta in Loeys-Dietz syndrome depends on multiple factors and is informed by the specific pathogenic variant, phenotypic features, patient age, aortic growth rates, and family history (Table 11).1,2,10–12,17
There is little information about size thresholds for prophylactic surgery in Loeys-Dietz syndrome to lessen the risk of aortic dissection or rupture when there are intact aneurysms involving the aortic arch, descending, or abdominal aorta, or involving aortic branch vessels.11,12,18 After aortic dissection, progressive aneurysmal dilation commonly occurs and often requires multiple operative interventions.11,12,18
Recommendation-Specific Supportive Text
Pathogenic variants in TGFBR1, TGFBR2, SMAD3, TGFB2, and TGFB3 lead to Loeys-Dietz syndrome or may cause aortopathy with few outward features. Most information is available for TGFBR1 and TGFBR2 pathogenic variants.1,2 Patients with TGFBR1 and TGFBR2 variants are at risk of type A aortic dissection at younger ages and smaller aortic root diameters than in Marfan syndrome.1,17,19 This aggressive aortopathy, especially in those with severe craniofacial features, previously led to a recommendation for surgery at an aortic root diameter of >4.0 cm.1 The “2010 ACC/AHA Guidelines for the Management of Thoracic Aortic Disease” recommended aortic surgery at a diameter between 4.2 cm and 4.6 cm, depending on imaging modality.20 SMAD3-related Loeys-Dietz syndrome variants may lead to aortic dissection at variable diameters.4,15,16,20 Aortic dissection risk is higher in women with TGFBR2 variants who have certain extraaortic features.2 Limited data have not suggested higher aortic dissection risk at smaller aortic size in those with TGFB25,6,14 or TGFB3 variants.8,9 Marked intrafamilial variability exists for aortic disease in Loeys-Dietz syndrome.17,21,22 A shared decision about timing of prophylactic surgery to prevent type A aortic dissection in Loeys-Dietz syndrome should include consideration of the specific genetic variant, aortic diameter, aortic growth rate, age, sex, body size, family history, patient preferences, and surgical expertise.
Aneurysms of the distal ascending aorta, arch, descending aorta, and abdominal aorta may occur in Loeys-Dietz syndrome.1,2,5,8,9,11–14,16,17 At the time of aortic root replacement, the entire ascending aorta is also to be replaced because distal ascending aortic aneurysm and dissection may occur after isolated aortic root replacement.10–12,19 There is little information about aortic size thresholds at which the risk of aortic dissection warrants elective surgery in the intact aortic arch, descending, or abdominal aorta in Loeys-Dietz syndrome. A shared decision should consider the pathogenic variant, aortic diameter, rate of aortic growth, age, sex, body size, patient preference, and the surgeon’s preference and surgical expertise. Aortic interventions in Loeys-Dietz syndrome are especially common after aortic dissection.10–12
6.1.2.4. Vascular Ehlers-Danlos Syndrome: Imaging, Medical Therapy, and Surgical Intervention
Vascular Ehlers-Danlos syndrome, affecting 1 in 50 000 to 100 000 individuals, is attributable to pathogenic variants in COL3A1 and leads to spontaneous aortic and arterial dissections, aneurysms, and rupture at young ages.1,2 The onset and severity of arterial pathology correlates with the specific COL3A1 pathogenic variant.2 Imaging the aorta and branches may identify arterial segments at risk, but the frequency of screening surveillance is uncertain.1–4 Typical protocols include baseline MRI or CT from head to pelvis to evaluate the entire aorta and its branches, with annual surveillance imaging thereafter to monitor any dilated or dissected aortic or arterial segments and imaging every 2 years when the initial imaging is normal.1,2,5 Notably, the aorta and arterial branches in vascular Ehlers-Danlos syndrome may rupture (or dissect) even without significant dilation.1–3
Medical therapy of vascular Ehlers-Danlos syndrome includes education, lifestyle modification, and avoidance of invasive procedures when possible.3,6 Studies of celiprolol, a beta blocker with vasodilatory properties, have suggested a benefit in patients with vascular Ehlers-Danlos syndrome,7,8 but data were considered to be insufficient for US Food and Drug Administration (FDA) approval. In the absence of data showing efficacy in vascular Ehlers-Danlos syndrome, other beta blockers are often prescribed, with some physicians choosing alternative beta blockers with vasodilatory properties. There are no studies showing a benefit of ARBs in vascular Ehlers-Danlos syndrome.
Surgical repair in vascular Ehlers-Danlos syndrome carries an increased risk because of vascular fragility and associated bleeding complications.1–3,5 Rapid arterial aneurysm growth or the occurrence of dissection are indications for treatment,1–3,5 but no data are available to guide diameter thresholds for prophylactic surgical intervention for aortic and arterial branch vessel aneurysms in vascular Ehlers-Danlos syndrome.1–5 Consequently, the decision to intervene for aortic and branch vessel aneurysms and dissections involves a Multidisciplinary Aortic Team and shared decision-making.3,6 Open surgery requires meticulous technique to lessen vascular and tissue trauma, and interventional techniques may involve arterial embolization and endovascular therapy, depending on individual circumstances.1,3,5
Guidelines for management of pregnancy in vascular Ehlers-Danlos syndrome are limited, given the lack of data and the rarity of the condition.9 The decision to proceed with pregnancy in vascular Ehlers-Danlos syndrome is complex; for some women with specific genetic variants, null mutations, and normal vascular imaging, the risk may be lower, but shared decision-making is essential.9 Of 38 women with vascular Ehlers-Danlos syndrome completing 82 deliveries, only 13% were aware of their diagnosis before pregnancy.9 Tissue fragility complicates labor and delivery and poses risks for vascular events and wound complications.9,10 Complications may occur after vaginal or cesarean deliveries, but most women known to have vascular Ehlers-Danlos syndrome undergo cesarean delivery.9–12
6.1.2.5. Turner Syndrome
Recommendations for Diagnostic Testing, Surveillance, and Surgical Intervention for Aortic Dilation in Turner Syndrome
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with Turner syndrome, TTE and cardiac MRI are recommended at the time of diagnosis to evaluate for BAV, aortic root and ascending aortic dilation, aortic coarctation, and other congenital heart defects.1–9 |
| 1 | B-NR | 2. In patients with Turner syndrome who are ≥15 years old, the use of the ASI (ratio of aortic diameter [cm] to BSA [m2]) is recommended to define the degree of aortic dilation and assess the risk of aortic dissection.9,10,11 |
| 1 | C-LD | 3. In patients with Turner syndrome without risk factors for aortic dissection (Table 12), surveillance imaging with TTE or MRI to evaluate the aorta is recommended every 5 years in children and every 10 years in adults, as well as before planning a pregnancy.9,10,11 |
| 1 | C-EO | 4. In patients with Turner syndrome and an ASI >2.3 cm/m2, surveillance imaging of the aorta is recommended at least annually.9 |
| 1 | C-EO | 5. In patients with Turner syndrome and risk factors for aortic dissection (Table 12), surveillance aortic imaging at an interval depending on the aortic diameter, ASI, and aortic growth rate is recommended (Figure 18).9 |
| 2a | C-LD | 6. In patients with Turnery syndrome who are ≥15 years old and have an ASI of ≥2.5 cm/m2 plus risk factors for aortic dissection (Table 12), surgical intervention to replace the aortic root, ascending aorta, or both is reasonable.9,10 In those without risk factors for aortic dissection, surgical intervention to replace the aortic root, ascending aorta, or both may be considered. |
| 2b | C-EO |
Table 12.
Risk Factors for Aortic Dissection in Patients With Turner Syndrome
| Aortic coarctation |
| Aortic dilation |
| Bicuspid aortic valve |
| Hypertension |
Figure 18. Suggested Aortic Monitoring Protocol for Girls and Women With Turner Syndrome Who Are ≥15 Years of Age.
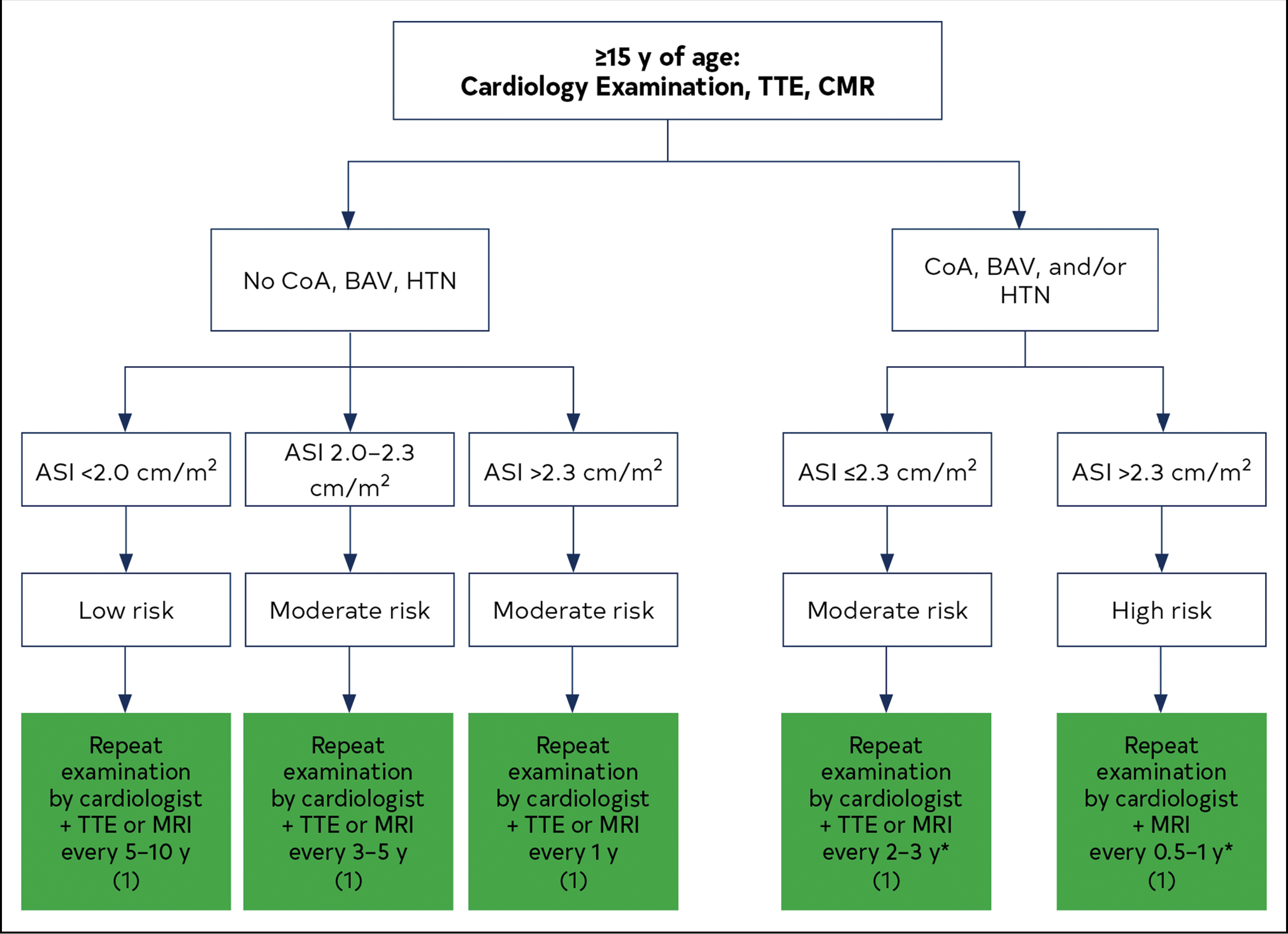
*Surveillance frequency may vary depending on disease severity (ie, aortic valve dysfunction, severity of coarctation obstruction, hypertension, and left ventricular hypertrophy). Color corresponds to Class of Recommendations in Table 2. ASI indicates aortic size index; BAV, bicuspid aortic valve; CoA, coarctation of the aorta; HTN, hypertension; MRI, magnetic resonance imaging; and TTE, transthoracic echocardiography. Modified from Silberbach et al.9 Copyright 2018, with permission from American Heart Association, Inc. Modified from Gravholt et al.12 Copyright 2017, with permission from Bioscientifica Limited.
Synopsis
Turner syndrome, which affects 1 in 2 500 liveborn girls, results from complete or partial loss of the second X chromosome in all or some of the cells of an individual.9,12 Approximately 50% of patients with Turner syndrome have cardiovascular defects that include BAV (15%–30%), aortic coarctation (7%–18%), and ascending aortic dilation (33%).9,12 Patients with Turner syndrome require cardiac imaging to evaluate for congenital heart and aortic defects and to determine aortic diameters. Patients with Turner syndrome are at increased risk of aortic dissection, with 85% occurring in the ascending and 15% in the descending aorta.10,11,13 Risk factors for aortic dissection include aortic dilation, hypertension, BAV, and aortic coarctation.9–11,13 Because Turner syndrome patients are of short stature, type A aortic dissection may occur at relatively small aortic diameters; consequently, indexing the aortic diameter to body size (ie, calculating an ASI) is recommended in monitoring the aorta.9,12,14
Recommendation-Specific Supportive Text
Turner syndrome may be recognized in infancy or childhood or, alternatively, go unrecognized until adolescence or adulthood. On the diagnosis of Turner syndrome, a TTE and cardiac MRI are performed to evaluate for associated congenital cardiovascular abnormalities (BAV, aortic coarctation, and others) and to measure aortic diameters.9,12
Because patients with Turner syndrome have short stature, using absolute aortic diameters alone may underestimate aortic dissection risk.9–11,13 Type A aortic dissection in Turner syndrome may occur at relatively small aortic diameters, likely reflecting the typical patient’s short stature, so indexing of aortic diameter to body size (by calculating the ASI) is performed when evaluating patients with Turner syndrome who are ≥15 years old.9,10 The ASI is calculated by dividing the maximal aortic diameter, in centimeters, by the BSA, in meters squared. An ASI >2.0 cm/m2 is considered to be abnormal, and an ASI ≥2.5 cm/m2 is associated with an increased risk of aortic disection.9–11 Using a Turner syndrome-specific z-score to assess for aortic dilation is preferred in children <15 years old.
Lifelong surveillance imaging of the aorta is used to monitor for aortic dilation: For children with Turner syndrome and no additional risk factors for aortic dissection, reevaluation at 5-year intervals is appropriate; for adults with Turner syndrome and no additional risk factors for aortic dissection, surveillance imaging of the aorta with TTE or MRI every 10 years is appropriate.9 Surveillance imaging should also be performed before planned pregnancy.9
In Turner syndrome, the risk of aortic dissection correlates with ASI,9 and an ASI ≥2.5 cm/m2 is associated with a significantly increased risk of aortic dissection. When the ASI approaches this threshold, more frequent surveillance imaging is appropriate to monitor aortic diameters.9
In Turner syndrome, risk factors for aortic dissection include aortic dilation, BAV, aortic coarctation, and hypertension.9–11,13 When these risk factors are present, surveillance imaging of the aorta is performed more frequently. For the patients with Turner syndrome who are ≥15 years old and have a stable ASI of ≤2.3 cm/m2, surveillance imaging with TTE or MRI is performed every 2 to 3 years.9 In the patients with Turner syndrome who are >15 years old with an ASI >2.3 cm/m2, at least annual surveillance imaging of the aorta is appropriate.9 The frequency of imaging should be informed by aortic diameter, aortic growth rate, severity of hypertension, and aortic valve function (Figure 18).9,12
In patients with Turner syndrome, diameter thresholds for prophylactic surgical replacement of aneurysms of the aortic root/ascending aortic replacement are based on retrospective series and case studies.10,11,13 Data from registries of aortic dissection in Turner syndrome report that the risk of dissection is significantly increased when the ASI is ≥2.5 cm/m2.9–11,13 In addition to aortic size, risk factors for aortic dissection in Turner syndrome include BAV, aortic coarctation, and hypertension.9,11,13 However, decisions using indexed calculations alone for aortic risk determination in short-statured but obese patients with Turner syndrome or those with low body weight relative to height may be less accurate. In such Turner syndrome patients who are ≥15 years old, an absolute aortic diameter of >4.0 cm may be more accurate than ASI in determining the risk of aortic disection.9 For patients with Turner syndrome who are <15 years old, a Turner syndrome-specific z-score calculation is appropriate to determine aortic risk and assess for surgical intervention.9,14 For patients with Turner syndrome without additional risk factors for aortic dissection, few data exist on the degree of aortic dilation that warrants surgical intervention.9
6.1.2.6. Pathogenic or Likely Pathogenic Variants in ACTA2, PRKG1, MYH11, MYLK, and LOX: Recommendations for Surveillance of Aorta, Medical Therapy, and Aortic Surgical Intervention
Pathogenic variants in ACTA2, PRKG1, MYH11, MYLK, and LOX confer a highly penetrant risk for TAD that is inherited in an autosomal dominant manner.1–4 In these nsHTADs, baseline imaging of the thoracic aorta with TTE, or with CT or MRI if the ascending aorta is not adequately visualized by TTE, is recommended; surveillance imaging is then performed annually, if stable. The arch and descending aorta may dilate, in which case surveillance imaging of these segments is also performed. Less frequent imaging may be considered when the aorta is normal, depending on gene variant, age, and family history. Beta-blocker therapy is used to lessen hemodynamic stress on the aorta.
Specific features associated with each gene include: Patients with ACTA2 mutations primarily present with type A or B aortic dissection, have aneurysms that involve the root and ascending aorta, and a subset of pathogenic variants predispose to occlusive vascular diseases.2,5–7 Screening for coronary artery disease and cerebrovascular disease is performed in individuals with specific pathogenic variants.5,6,8,9 Patients with ACTA2 mutations can suffer type A aortic dissection at aortic diameters <4.5 cm, and consideration of surgery at diameters <4.5 cm is informed by the presence of additional risk factors.10 PRKG1-related HTAD can present in the late teens with type A or B aortic dissection without previous aortic enlargement11–13; patients with MYH11 mutations primarily present with type A or B aortic dissection (type A aortic dissection may present at aortic diameters <5.0 cm), have aneurysms that involve the root and ascending aorta, and may have peripheral arterial disease4,14; patients with MYLK mutations present at age >40 years with type A aortic dissection with little previous enlargement of the aorta (median aortic diameter, 4.25 cm)3,15,16; patients with LOX mutations can present with aortic root aneurysms, fusiform dilation of the root and ascending aorta that can extend into the aortic arch, or type A aortic dissection, and they may have mild systemic features of Marfan syndrome.1,17,18 The decision regarding the timing of aortic repair in nsHTAD is based on the aortic diameter, age, family history, and the presence or absence of additional risk factors (Table 13).
Table 13.
Surgical Thresholds for Prophylactic Aortic Root and Ascending Aortic Replacement in Nonsyndromic Heritable Thoracic Aortic Disease Based on the Genetic Variant and Additional Risk Factors for Aortic Dissection
| COR* | LOE* | Genetic Variant | Risk Factors | Aortic Diameter (cm) |
|---|---|---|---|---|
| 2a | C-LD | ACTA2 | No | ≥4.5 |
| 2b | C-EO | ACTA2 | Yes† | ≥4.2 |
| 2b | C-LD | PRKG1 | No | ≥4.2 |
| 2b | C-EO | PRKG1 | Yes† | ≥4.0† |
Patient has risk factors for aortic dissection (family history of type A aortic dissection with minimal aortic enlargement, aortic growth rate ≥0.3 cm/y) or significant valve disease requiring surgery.
Earlier surgery may be considered in patients with a family history of type A aortic dissection in the setting of no or minimal aortic dilation, aortic growth rate ≥0.3 cm/y, or at the patient’s request.
Colors correspond to COR and LOE in Table 2.
COR indicates class of recommendation; and LOE, level of evidence.
6.1.3. BAV Aortopathy
Recommendations for BAV Aortopathy
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with a BAV, TTE is indicated to evaluate valve morphology and function, to evaluate the diameter of the aortic root and ascending aorta, and to evaluate for aortic coarctation and other associated cardiovascular defects.1–4 |
| 1 | C-LD | 2. In patients with a BAV, CT or MRI of the thoracic aorta is indicated when the diameter and morphology of the aortic root, ascending aorta, or both cannot be assessed accurately or completely by TTE.1 |
| 1 | C-LD | 3. In patients with a BAV and either HTAD or phenotypic features concerning for Loeys-Dietz syndrome, a medical genetics evaluation is recommended.5,6 |
| 1 | C-LD | 4. In patients with a BAV and a dilated aortic root or ascending aorta, screening of all first-degree relatives by TTE is recommended to evaluate for the presence of a BAV, dilation of the aortic root and ascending aorta, or both; if the diameter and morphology of the aortic root, ascending aorta, or both cannot be assessed accurately or completely by TTE, a cardiac-gated CT or MRI of the thoracic aorta is indicated.7 |
| 2a | B-NR | 5. In patients with a BAV, screening of all first-degree relatives by TTE is reasonable to evaluate for the presence of a BAV, dilation of the aortic root and ascending aorta, or both.7–10 |
Synopsis
BAV is a common congenital valve condition affecting approximately 1% of the population, with a 2 to 3:1 male-to-female predominance.3 BAV most often occurs sporadically but may be inherited in an autosomal dominant pattern with variable penetrance.5 A BAV may be isolated or associated with other congenital cardiovascular defects or aortopathy conditions.5 BAV is often associated with aortic valve dysfunction (stenosis or regurgitation) and is at risk of infective endocarditis. Patients with a BAV often have aortic dilation or aneurysms affecting the aortic root, ascending aorta, or both, with the prevalence of aortic aneurysm increasing with age.11 Distinct aortic dilation phenotypes have been described.1,12 Those with BAV and a dilated aorta are at risk for type A aortic dissection.11,13,14 Patients with BAV require lifelong surveillance imaging of the aorta, even after AVR.
Recommendation-Specific Supportive Text
Aortic dilation in BAV may affect the aortic root, the ascending aorta, or both. The ascending aorta is most commonly involved, and the dilation sometimes extends up into the arch.1–3 The prevalence of aortic dilation in BAV is reported from 20% to 84%, depending on the population studied and the definition of aortic dilation.3,12 Patients with BAV and aortic dilation are at risk for aortic dissection.3,11,13 The aortic root, ascending aorta, arch, and proximal descending aorta should be imaged by TTE to evaluate for aortic valve function, aortic dilation, and aortic coarctation.1–4 Conversely, in other patients undergoing TTE, a finding of unexplained aortic root, ascending aortic dilation, or both should prompt suspicion of an underlying BAV15; if TTE of the aortic valve is inconclusive for BAV, cardiac magnetic resonance, cardiac CTA, and TEE can be used to better visualize the aortic valve and thereby diagnose BAV.
Cardiac-gated CT or MRI provides superior images of the aortic root and ascending aorta when TTE is inadequate to visualize the full extent of the proximal aorta. The choice between CT or MRI depends on patient characteristics, institutional expertise, renal function, affordability, and radiation exposure concerns.16
Certain types of HTAD have an increased prevalence of BAV. For example, BAV is present in ~10% of patients with Loeys-Dietz syndrome (attributable to pathogenic variants in TGFBR1, TGFBR2, SMAD3, TGFB2, and TGFB3),6 and HTAD attributable to pathogenic variants in NOTCH1, ACTA2, MAT2A, SMAD6, and LOX also have an increased prevalence of BAV.5,6 Importantly, most patients with BAV and TAAs who undergo genetic testing will not be found to have a pathologic genetic variant, even when their condition is familial. Nevertheless, when the condition is familial, a medical geneticist or specialist in genetic aortopathy should evaluate, counsel, and genetically test patients with BAV and aortopathy.17
Both BAV and aortic root and ascending aortic dilation may be familial,7 and the inheritance patterns for familial BAV and aortopathy are consistent with an autosomal dominant pattern with incomplete penetrance.8–10 In families with BAV and aortic root and ascending aortic dilation, obligate carriers may have BAV, aortic dilation, both, or neither.7 In families with BAV and aortic root and ascending aortic dilation, screening of the first-degree relatives (parents, siblings, and children) with TTE to evaluate for BAV and aortic dilation identifies affected members. If a family member is discovered to have a BAV, aortic dilation, or both, cascade evaluation of other related family members is then indicated. Because families with BAV and aortic dilation may have members with aortic root and ascending aortic dilation in the absence of a BAV, if the ascending aorta is not adequately assessed by TTE, a CT or MRI should be performed to fully evaluate the size of the ascending aorta.
The prevalence of a BAV in the relatives of a patient with a BAV ranges from 9% to 20%.8–10 Family members of individuals with a BAV may also have aortic dilation. A recent analysis found that TTE screening of first-degree relatives of affected patients, to detect both BAV and aortopathy, proves to be cost-effective.18
6.1.3.1. Routine Follow-Up of BAV Disease Aortopathy
Recommendations for Routine Follow-Up of BAV Disease Aortopathy
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with a BAV who have undergone previous aortic valve repair or replacement and have a diameter of the aortic root, ascending aortic, or both of ≥4.0 cm, lifelong surveillance imaging of the aortic root and ascending aorta by TTE, CT, or MRI is recommended at an interval dependent on aortic diameter and rate of growth.1–3 |
| 1 | C-LD | 2. In patients with a BAV and a diameter of the aortic root, ascending aorta, or both of ≥4.0 cm, lifelong surveillance imaging of the aortic root and ascending aorta by TTE, CT, or MRI is recommended at an interval dependent on aortic diameter and rate of growth.4,5 |
Synopsis
Patients with BAV, with or without aortic dilation, require lifelong surveillance of the aortic root and ascending aorta because of risk of late aortic growth. The degree of aortic dilation and the progression of aortopathy may be greater in patients with aortic root phenotype and those with predominant AR.3,6 Progressive aortic growth may occur after AVR.3,7
Recommendation-Specific Supportive Text
Patients with BAV who have undergone previous isolated AVR or aortic valve repair remain at risk for future aortic dilation and dissection. In a series of 1 286 patients who underwent isolated AVR for BAV from 1960 to 1995, the 15-year freedom from aortic events (aortic dissection, aortic aneurysm of >5.0 cm, or aortic aneurysm surgery) was 89% but was lower for those with documented aortic dilation at baseline compared with those with normal diameters (85% versus 93%; P=0.001).8 Patients with BAV who have undergone isolated AVR for aortic stenosis and have only mild-to-moderate aortic dilation are at low risk for adverse aortic events at 15-year follow-up,3,9 whereas those who underwent AVR for predominant AR and those with predominant dilation of the aortic root (“root phenotype”) are at higher risk for adverse aortic events during follow-up. Among 56 patients with BAV who underwent isolated AVR for AR and had concomitant aortic root dilation (4.0–5.0 cm), adverse aortic events occurred in 34% of patients during follow-up.4 Patients with BAV who undergo isolated AVR for AR are at higher risk for late aortic dissection than patients who underwent AVR for aortic stenosis.10
In a prospective study of 90 adults with BAV, the mean increase in ascending aortic diameter was 0.47 mm/y (range, 0.2–2.3 mm/y) over a 4.8-year followup.11 Surveillance imaging can document current aortic diameters and permit calculation of aortic growth rates.2,6 Among a cohort of adult patients with BAV (mean age, 55±17 years) without a TAA at baseline (ie, the baseline aortic diameter was <4.5 cm), 13% went on to develop a TAA at 14±6 years after diagnosis, and the 25-year risk of TAA was 26%.12 For many adults, an aortic root, ascending aortic, or both diameter ≥4.0 cm is considered dilated and should therefore be monitored over time with surveillance imaging to detect progressive dilation.
6.1.3.2. BAV Aortopathy Interventions: Replacement of the Aorta in Patients With BAV
Recommendations for BAV Aortopathy Interventions: Replacement of the Aorta in Patients With BAV
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with a BAV and a diameter of the aortic root, ascending aorta, or both of ≥5.5 cm, surgery to replace the aortic root, ascending aorta, or both is recommended.1–3 |
| 2a | B-NR | 2. In patients with a BAV and a cross-sectional aortic root or ascending aortic area (cm2) to height (m) ratio of ≥10 cm2/m, surgery to replace the aortic root, ascending aorta, or both is reasonable, when performed by experienced surgeons in a Multidisciplinary Aortic Team.3,4 |
| 2a | B-NR | 3. In patients with a BAV, a diameter of the aortic root or ascending aorta of 5.0 cm to 5.4 cm, and an additional risk factor for aortic dissection (Table 14), surgery to replace the aortic root, ascending aorta, or both is reasonable, when performed by experienced surgeons in a Multidisciplinary Aortic Team.1,5 |
| 2a | B-NR | 4. In patients with a BAV who are undergoing surgical aortic valve repair or replacement, and who have a diameter of the aortic root or ascending aorta of ≥4.5 cm, concomitant replacement of the aortic root, ascending aorta, or both is reasonable, when performed by experienced surgeons in a Multidisciplinary Aortic Team.1,6 |
| 2b | B-NR | 5. In patients with a BAV, a diameter of the aortic root or ascending aorta of 5.0 cm to 5.4 cm, no other risk factors for aortic dissection (Table 14), and at low surgical risk, surgery to replace the aortic root, ascending aorta, or both may be reasonable, when performed by experienced surgeons in a Multidisciplinary Aortic Team.1,2,5 |
Table 14.
Risk Factors for Aortic Dissection
| Family history of aortic dissection |
| Aortic growth rate ≥0.3 cm/y |
| Aortic coarctation |
| “Root phenotype” aortopathy |
Synopsis
The timing of surgery to replace the aorta in BAV disease depends on the morphology and diameter of the aorta, aortic valve function, rate of aortic growth, family history, patient characteristics, patient wishes, and the expertise of the surgeon and institution.1,7
Recommendation-Specific Supportive Text
Patients with a BAV without significant aortic dilation are at low risk for type A aortic dissection,3,8 whereas those patients with BAV and aneurysmal dilation of the aortic root, ascending aorta, or both have a significantly increased risk of aortic dissection.5,8 The risk of aortic dissection rises with increasing aortic diameter, and there are “hinge points” when the ascending aorta reaches diameters >5.25 cm to 5.75 cm.9
Indexing the maximal aortic root or ascending aortic diameter to height is predictive of aortic dissection risk and therefore informs surgical thresholds.3,4 Moreover, when comparing long-term outcomes in patients with BAV and aortic root or ascending aortic dilation, survival was significantly better for those with an aortic cross-sectional area (in cm2) to height (in meters) ratio of ≥10 who underwent elective prophylactic aortic repair compared with those who did not undergo elective repair.3
There are additional risk factors for aortic dissection that may inform aortic surgical thresholds in patients with a BAV. A family history of aortic dissection10 and rapid aortic growth of ≥0.3 cm/y (when measured similarly with same technique) are both risk factors for aortic dissection. Patients with BAV and aortic coarctation have been reported to be at increased risk of aortic dissection,11 although in a recent report of 499 patients with BAV (mean age, 40±16 years), of whom 24% also had aortic coarctation, there was no difference in adverse aortic events between those with or without coarctation.12 Patients with dilation of the aortic root (“root phenotype”) represent 10% to 20% of patients with BAV and aortopathy and may have more rapid aortic growth and an increased risk of aortic complications.13,14 Because surgical aortic root replacement (and VSRR) is more complex than ascending aortic replacement, shared decision-making is often used when evaluating the risks and benefits of elective aortic root replacement at aortic diameters <5.5 cm.1,2,5,6
In patients with a BAV and indications for aortic valve intervention for stenosis or regurgitation, the data are limited regarding the degree of aortic dilation that warrants replacement of the aortic root, ascending aorta, or both at the time of AVR. Patients with a long life expectancy, low surgical risk, or with the root phenotype and predominant AR may benefit from concomitant prophylactic aortic repair. Conversely, for patients at higher surgical risk, especially those with aortic stenosis and only moderate ascending aortic dilation, the risks of concomitant aortic repair may not be warranted.
Limited data are available on the risk of aortic dissection among those with a BAV and aortic aneurysm diameter of 5.0 cm to 5.4 cm.5,15 Patient-related characteristics and surgical expertise may inform the timing of surgery, especially in low-risk patients with BAV and aortic aneurysms of 5.0 cm to 5.4 cm.1,2,5,6
6.2. AAA: Cause, Risk Factors, and Screening
Recommendations for AAA: Cause, Risk Factors, and Screening
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-R | 1. In men who are ≥65 years of age who have ever smoked, ultrasound screening for detection of AAA is recommended.1 |
| 1 | C-LD | 2. In men or women who are ≥65 years of age and who are first-degree relatives of patients with AAA, ultrasound screening for detection of AAA is recommended.2,3 |
| 2a | C-EO | 3. In women who are ≥65 years of age who have ever smoked, ultrasound screening for detection of AAA is reasonable.4,5 |
| 2b | C-LD | 4. In men or women <65 years of age and who have multiple risk factors (Table 15) or a first-degree relative with AAA, ultrasound screening for AAA may be considered.5,6 |
| 3: No Benefit | B-NR | 5. In asymptomatic men or women >75 years who have had a negative initial ultrasound screen, repeat screening for detection of AAA is not recommended.1 |
Table 15.
Risk Factors for Abdominal Aortic Aneurysm
| Strong Risk Factors | Additional Risk Factors |
|---|---|
| Smoking history | Hypertension |
| Older age | Hyperlipidemia |
| Male sex | White race |
| Family history of abdominal aortic aneurysm | Inherited vascular connective tissue disorder |
| Atherosclerotic cardiovascular disease |
Synopsis
Although AAA share risk factors with typical atherosclerosis, AAA are histopathologically distinct and characterized by medial degeneration of the aortic wall.7 Most AAA develop an intraluminal thrombus that contributes to ongoing wall degradation via oxidative stress, smooth muscle cell apoptosis, proteolysis of the extracellular matrix, and adventitial inflammation.8 A complex interplay of hereditary and environmental risk factors contributes to AAA, most notably older age, male sex, smoking, and a positive family history (Table 15).2,3,9–12 Lifetime risk for AAA is 8.2% in men and 10.5% in current smokers.11 At least 10% to 25% of patients with AAA have a family member with the same condition,2 and AAA may occur concomitantly with thoracic aortic aneurysmal disease, especially in some genetic aortopathies.11 Inflammatory aortitis is a rare cause of AAA13,14 (see Section 9.1, “Inflammatory Aortitis – Diagnosis and Treatment of Takayasu Arteritis and Giant Cell Arteritis (GCA)”). The growth of AAA is nonlinear, with a mean rate of 2.6 mm/y for AAA <5.0 cm,15 and may accelerate in the setting of smoking or a family history of AAA,16,17 and smoking may have a greater impact on growth in women than in men.4 Ultrasound screening should be targeted toward those at the greatest risk for AAA and growth (Figure 19), with the goal of preventing rupture and associated mortality.1,5,6
Figure 19. Algorithm for Identifying Patients to Screen for Abdominal Aortic Aneurysm.
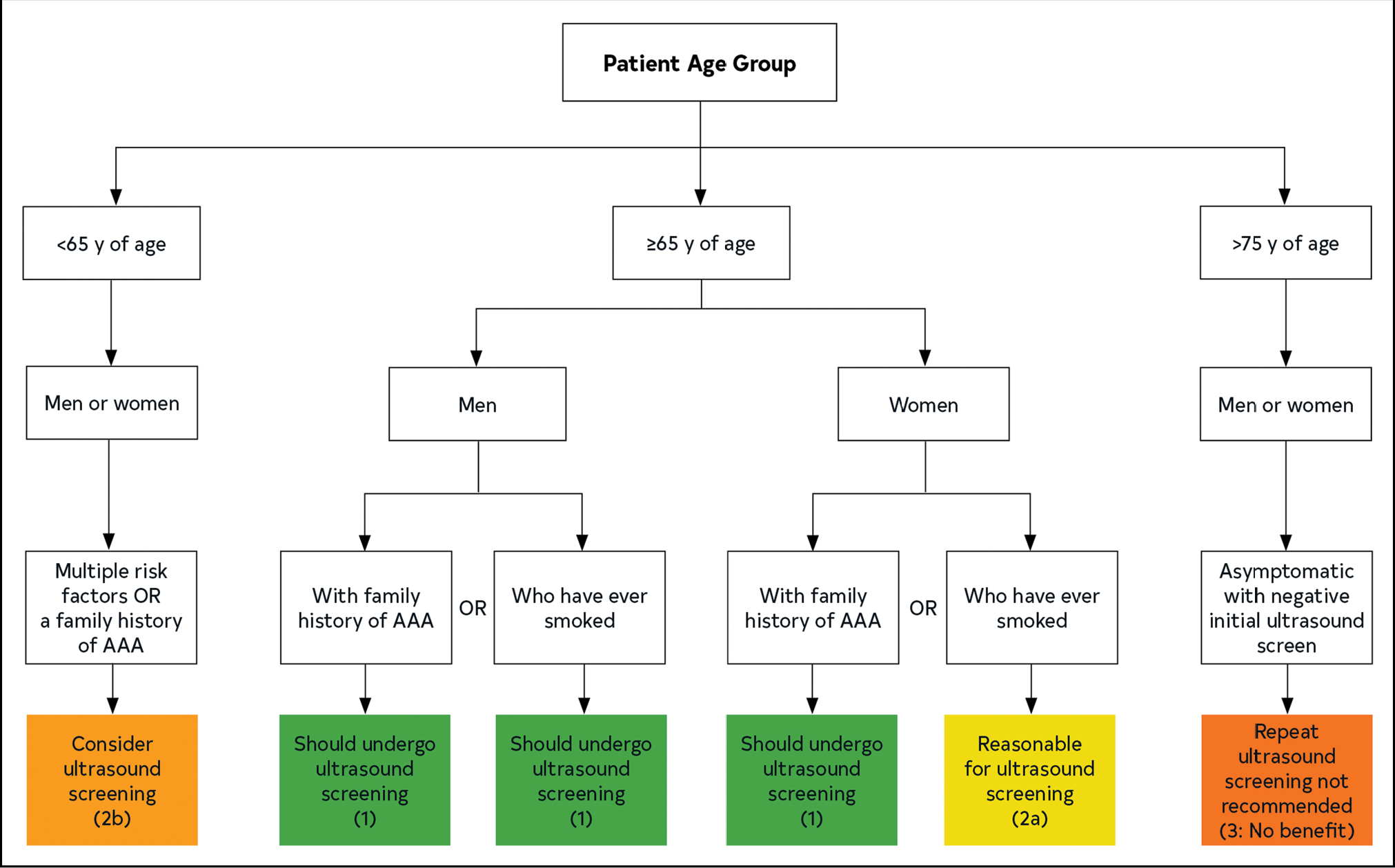
Colors correspond to Class of Recommendations in Table 2. AAA indicates abdominal aortic aneurysm.
Recommendation-Specific Supportive Text
Older age, male sex, and smoking are independent, strong risk factors for the development of AAA.9–11 Smoking history is defined as lifetime use of ≥100 cigarettes, but risk attributable to smoking varies significantly depending on use, with lowest risk of AAA in those who have lower versus higher pack-year history.9 Based on a meta-analysis of randomized clinical trials inclusive of nearly 125 000 mostly male patients, screening of men ≥65 years of age reduced long-term AAA-related mortality (4 RCTs: OR, 0.65; 95% CI, 0.57–0.74) and AAA-related ruptures (4 RCTs: OR, 0.62; 95% CI, 0.55–0.70) over 12 to 15 years.1 In a recent population-based study (of both men and women) in the United Kingdom, two-thirds of the acute AAA events occurred in those ≥75 years of age; consequently, screening to elderly patients should be offered, provided they would benefit from potential aortic repair.18
Having a first-degree relative with AAA is a well-known and well-established risk factor for development of AAA.2,3 Small cohort studies of ultrasound screening in relatives of those with AAA have identified an overall prevalence of new AAA of 10% to 20%, with the highest prevalence of 25% found among brothers. Indeed, the overall lifetime prevalence of AAA is estimated to be 32% in brothers of those with AAA,2 suggesting the need for a targeted and individualized screening approach for those who already meet age criteria within families.
Select women may be at risk for AAA and related complications.5 Randomized trials and large observational studies that evaluate outcomes of screening for AAA by ultrasound in women are lacking, as female sex has not been proven an independent risk factor for AAA,11 and overall prevalence of AAA in women is lower than in men. However, the risk of AAA may be potentiated by smoking in women; in 1 study, smoking was associated with a 15-fold increased risk of AAA among women (relative risk, 15.0; 95% CI, 13.2–17.0) versus 7-fold among men (relative risk, 7.3; 95% CI, 6.4–8.2).4 Practical implementation and outcomes of screening in women remain uncertain and warrant further study.
Select patients <65 years of age may be at increased risk of AAA rupture, and data suggest a significant proportion of those undergoing repair for ruptured AAA did not meet the standard criteria for screening based on age.5,6 In a large study from the National Inpatient Sample, 10 603 of 25 777 patients with ruptured AAA (24%) were <65 years of age.5 Notably, in patients <65 years, data are lacking on the mortality benefit of AAA screening.
Some patients may develop AAA after the age of 75 years even if they had an initial negative screen between the ages of 65 and 75 years. Although somewhat limited, data from cohort studies suggest long-term AAA-related mortality is low among patients with an initial negative screening ultrasound who had a subsequent AAA detected on repeat screening after the age of 75 years.1 However, select patients at low surgical risk who may have had borderline enlarged abdominal aorta measurements on initial screening and who have significant AAA risk factors (Table 15) may be considered for repeat screening on an individualized basis.
6.3. Growth and Natural History of Aortic Aneurysms
Aortic aneurysm growth and natural history is variable and dependent on the underlying etiology, such as HTAD (eg, Marfan syndrome and Loeys-Dietz syndrome), BAV, or sporadic aortic disease without a known genetic basis. There is significant evidence that aortic diameter correlates with aortic dissection, aortic rupture, and mortality.1–3 In patients with Marfan syndrome, the mean rate of growth of the aortic root has been reported to be 0.26 cm/y (range, 0.13–0.35 cm/y), with a tendency for larger aneurysms (>6.0 cm) to grow faster (0.46 cm/y).4 Patients with BAV have a slower rate of aortic growth, with a root predominant phenotype growing at 0.06 cm/y (0.6 mm/y) and the more common ascending aortic phenotype at 0.03 cm/y (0.3 mm/y).5 Moreover, among those with tricuspid aortic valves and sporadic ascending aortic dilation, the mean rate of growth is even slower, as low as 0.01 cm/y (0.1 mm/y).6 Aortic arch aneurysm growth has been reported to be 0.25 cm/y.7 The mean growth rate of descending and TAAA has been reported to be 0.19 cm/y, with rates increasing as the diameter increases.8 The mean rate of growth of AAA is 0.26 cm/y, with larger aneurysms growing as fast as 0.5 cm/y.9
6.4. Medical Management of Sporadic and Degenerative Aortic Aneurysm Disease
The primary goals of medical therapy in sporadic and degenerative thoracic and abdominal aneurysmal disease are to reduce growth rates, the risk of aortic-related mortality, and the need for aortic repair; a secondary goal is to decrease the risk of nonaortic cardiovascular events, given the multiple shared risk factors between aneurysmal and atherosclerotic disease.1,2 Lifestyle modification, including smoking cessation and blood pressure (BP) control, improves overall cardiovascular health and may be beneficial to patients with aortic aneurysmal disease. Pharmacotherapy specific to the treatment of aortic disease includes the use of selected antihypertensives (especially beta blockers and ARBs) that may mitigate the proteolysis pathways, leading to medial degeneration and reducing of sheer stress on the aortic wall, as well as the use of statins, which may target inflammatory and atherosclerotic pathways.3 Outcomes data from clinical trials of medical therapy in aortic aneurysms broadly are limited, as most trials have focused on cohorts of patients with either Marfan syndrome or AAA. Consequently, correlations may be imprecise when applied to other populations.
6.4.1. Medical Therapy and Risk Factor Modification in Sporadic TAA
6.4.1.1. BP Management in Sporadic TAA
Recommendations for BP Management in TAA
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with TAA and an average systolic BP (SBP) of ≥130 mm Hg or an average diastolic BP (DBP) of ≥80 mm Hg, the use of antihypertensive medications is recommended to reduce risk of cardiovascular events.1–3 |
| 2a | C-LD | 2. In patients with TAA, regardless of cause and in the absence of contraindications, use of beta blockers to achieve target BP goals is reasonable.1,4,5 |
| 2a | C-EO | 3. In patients with TAA, regardless of etiology and in the absence of contraindications, ARB therapy is a reasonable adjunct to betablocker therapy to achieve target BP goals.6 |
Synopsis
The goal of BP control in TAA is to slow growth and prevent aortic dissection, as well as to reduce nonaortic cardiovascular events, such as myocardial infarction and stroke. Uncontrolled hypertension increases the risk for aortic dissection,7 so achieving a SBP goal of ≤130 mm Hg and a DBP goal of ≤80 mm Hg, with the use of antihypertensive therapy in those with hypertension and TAA, is advised. Although data are limited, achieving a more intensive SBP goal of <120 mm Hg, if tolerated, may have added benefit in selected patients and who are not undergoing surgical repair.4 There has been significant progress in understanding the molecular basis of aneurysmal development and growth,8 and a number of clinical trials have explored the effects of beta-blocker and ARB therapy.9 A summary of these trials specific to genetic aortopathies is covered in detail in Section 6.1.2, “Genetic Aortopathies.” However, as the molecular mechanisms of aneurysm formation may have similarities between aneurysm patients with and without Marfan syndrome, data from these studies may be extrapolated in guiding the treatment of aortic disease of other causes. Further clinical trials are clearly needed.
Recommendation-Specific Supportive Text
No randomized clinical trials have evaluated the optimal threshold to which BP should be lowered in patients with TAA to reduce the risk of aortic complications (aortic growth, aortic dissection, or aortic rupture). Updated hypertension guidelines from the ACC and AHA suggest all patients with clinical cardiovascular disease should have a target SBP <130 mm Hg, DBP <80 mm Hg, or both.1 Evidence supports aggressive BP lowering to reduce vascular-related adverse events and all-cause mortality.2,3 Data from SPRINT (Systolic Blood Pressure Intervention Trial) showed that intensive BP control to a SBP <120 mm Hg, if tolerated, reduced cardiovascular events by 25% and all-cause mortality rate by 27% in patients without diabetes over a median of 3.3 years, compared with a control with a SBP target of <140 mm Hg.10,11
Prospective data on the positive effects of beta blockers in TAA based on cause are limited, with the most robust evidence derived from cohort studies of those with Marfan syndrome (see Section 6.1.2.2, “Marfan Syndrome”). In a small, open-label, randomized clinical trial of prophylactic propranolol (mean dose, 212±68 mg/d) versus placebo in adolescents and adults with Marfan syndrome, beta-adrenergic blocking drugs slowed aortic root growth and reduced aortic complications.5 In a study of 155 children <12 years of age with Marfan syndrome, beta blockers decreased the rate of aortic root growth by 0.16 mm/y, on multivariate analysis.4 In the “2017 Hypertension Clinical Practice Guideline,” beta-blocker therapy is the recommended first-line antihypertensive drug therapy for patients with hypertension and TAD.1
A meta-analysis of 1 510 randomized patients evaluating the effect of ARBs on TAA associated with Marfan syndrome showed slower growth of the aortic root with the use of ARBs compared with placebo; in a direct comparison with beta-blocker therapy, there was no difference in aortic growth; and the combination of beta blocker plus ARB led to slower aortic growth than beta blockers alone.6 In the Jikei Heart Study,12 which supported the use of ARBs in the 2010 ACC/AHA thoracic aortic disease guidelines, Japanese patients on an antihypertensive drug regimen that included valsartan had a lower rate of adverse cardiovascular events, including mortality and, in particular, a reduction was showed in the incidence of aortic dissection. However, this study was subsequently retracted13 and, consequently, the LOE for use of ARBs has been downgraded to C from B.
6.4.1.2. Treatment of TAA With Statins
Recommendations for Treatment of TAA With Statins
Synopsis
Clinical atherosclerotic cardiovascular disease (ASCVD) encompasses aortic aneurysms of atherosclerotic origin. For the purpose of this guideline, we also define aortic aneurysm with concomitant PAU or visualized atheroma as atheromatous aortic disease, even in the presence of a genetic syndrome, given some causes have shared risk factors with ASCVD. Based on the AHA/ACC “2018 Guideline on the Management of Blood Cholesterol,”1 a high-intensity statin for >50% reduction in low-density lipoprotein (LDL) for patients <75 years of age with clinical atherosclerotic cardiovascular disease was recommended to prevent adverse events (eg, myocardial infarction and stroke). If a high-intensity statin cannot be used, a moderate-intensity statin can be initiated.1 According to evidence from animal studies in nonatherosclerotic-related TAA, statin therapy may prevent growth and adverse remodeling.7 However, its use in clinical practice at this time is not fully understood.
Recommendation-Specific Supportive Text
Atherosclerotic aortic aneurysms increase risk of stroke and myocardial infarction and thus are considered a coronary artery disease equivalent according to NCEP ATP III (National Cholesterol Education Program Adult Treatment Panel III), with a >20% risk of an event within 10 years.8 The “2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease”9 gave a COR 2a recommendation for use of high-intensity statin in patients with noncoronary atherosclerotic disease to achieve an LDL goal of <70 mg/dL. From the Cholesterol Treatment Trialists’ Collaboration, when combining data from 5 RCTs of 39 612 patients over median 5.1 years, more intensive cholesterol lowering in patients with ASCVD reduced major cardiovascular events by an additional 15% beyond what was achieved with less intensive cholesterol lowering.1,2 In patients with sporadic or genetically mediated aneurysms, if there is concomitant atherosclerotic disease elsewhere, then statin therapy is still reasonable.
It has long been hypothesized that the pleiotropic effects of statins may be beneficial in preventing the adverse vascular wall remodeling associated with TAAs, thereby slowing growth, regardless of cause and whether associated atherosclerosis is present. Animal studies have shown a reduction in thoracic aneurysm growth with statin therapy, possibly via regulation of MMP activity.7,10 A study of 1348 patients with thoracic aortic ectasia showed, in a propensity-matched analysis, a possible benefit with statin therapy in the reduction of aortic growth rate as well as aortic complications.3,11 In a retrospective study that included 2267 patients who underwent TEVAR for aneurysmal disease, 1148 (64%) of whom had been treated with a statin preoperatively, preoperative statin therapy was associated with significantly lower perioperative complication rates and 5-year mortality.12 A possible benefit of statins in prevention of adverse aortic-related outcomes was also showed in a small cohort study, and slowing of aortic growth is suggested by 2 small studies in patients with BAV and aortopathy.6,8
6.4.1.3. Smoking Cessation in TAA
Recommendation for Smoking Cessation in TAA
Synopsis
Smoking cessation and avoidance of secondhand smoke exposure is considered a healthy lifestyle modification in patients with TAAs, regardless of cause. Many patients cared for in cardiovascular clinical practices have interest in smoking cessation; thus, implementation of an effective strategy using the 5 A’s (Ask, Advise, Assess, Assist, and Arrange) is worthwhile, along with a referral to dedicated programs, use of app-based tools, pharmacotherapy (which includes nicotine replacement, bupropion, or varenicline), or both.1–3 Although the use of e-cigarettes has been shown to be an effective strategy in smoking cessation,4 the efficacy and, importantly, safety of e-cigarette use in patients with TAA is not well understood.
Recommendation-Specific Supportive Text
There are many validated options for smoking cessation for patients who continue to smoke and have TAA.1–3 Although no randomized clinical trials have evaluated the effect of smoking cessation on outcomes in TAA, smoking is a risk factor for TAA expansion and, among those with atherosclerotic aortic disease, smoking cessation reduces the rates of myocardial infarction and death.5,6 The use of e-cigarettes, although an effective smoking cessation tool, has not been shown to be safe when used in patients with vascular disease, including TAA; further, small studies suggest that the flavoring chemicals in e-cigarettes may have an adverse effect on vascular endothelial function and relaxation via nitric oxide and cyclic guanosine monophosphate-mediated signaling.7,8
6.4.1.4. Antiplatelet Therapy in TAA
Recommendation for Antiplatelet Therapy in TAA
Synopsis
Aortic aneurysms of atherosclerotic origin are considered a coronary artery disease equivalent according to the NCEP ATP III, with a >20% risk of an event within 10 years.3 The 2006 updated “AHA/ACC Guidelines for Secondary Prevention for Patients With Coronary and Other Atherosclerotic Vascular Disease”1 recommend use of low-dose aspirin (75–162 mg/d) in patients with atherosclerotic aortic disease. Even in the absence of TAA, this remains true in other atherosclerotic aortic diseases, such as high-grade atheroma, PAU, or both.
Recommendation-Specific Supportive Text
In the SPARC (Stroke Prevention: Assessment of Risk in a Community) study, aortic atherosclerosis was associated with coronary artery disease (OR, 2.99; 95% CI, 1.47–6.10; P=0.003).2 In turn, in the presence of coronary artery disease, aspirin has long been recommended to reduce the risk of cardiovascular events, including stroke, death caused by coronary artery disease, and myocardial infarction.1
6.4.2. Medical Therapy and Risk Factor Modification in AAA
6.4.2.1. BP Management in AAA
Recommendation for BP Management in AAA
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
Synopsis
Reducing cardiovascular events such as myocardial infarction and stroke, as well as preventing aneurysm growth and rupture, are the main goals in antihypertensive therapy in AAA. Uncontrolled hypertension is a known risk factor for aortic rupture and dissection; therefore, achieving an SBP goal of <130 mm Hg, and a DBP goal of <80 mm Hg with the use of antihypertensive therapy in those with hypertension and AAA can reduce adverse clinical outcomes, and some patients may benefit from more intensive lowering with an SBP goal of <120 mm Hg.4 The most robust evidence of antihypertensive therapy in AAA is for beta blockers and agents that alter the renin angiotensin system; however, in prospective clinical trials in humans, no specific agent has been proven to inhibit AAA growth.
Recommendation-Specific Supportive Text
Updated hypertension guidelines from the ACC and AHA suggest all patients with clinical cardiovascular disease have a target SBP of <130 mm Hg and/or DBP <80 mm Hg.1 Evidence supports aggressive BP lowering to reduce vascular-related adverse events and all-cause mortality.2,3 A more intensive SBP goal of <120 mm Hg, if tolerated, may have added benefit in select patients without diabetes and who are not undergoing surgical aortic repair. However, data are limited to the single randomized SPRINT,4 which showed that intensive BP control to SBP <120 mm Hg reduced cardiovascular events by 25% and all-cause mortality by 27% in patients without diabetes over a median of 3.3 years, compared with a control with an SBP target of <140 mm Hg.4,5
6.4.2.2. Treatment of AAA With Statins
Recommendations for Treatment of AAA With Statins
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
Synopsis
ASCVD includes noncoronary atherosclerotic disease such as peripheral artery disease (PAD) and AAA.6 For the purpose of this guideline, we define abdominal AAA of atherosclerotic cause as those with visualized aortic wall atheroma, penetrating aortic ulceration either within the aneurysm or at another site along the aorta, or both, with a limitation being that many patients with genetically mediated AAA (see Section 6.2, “AAA: Cause, Risk Factors and Screening”) may have concomitant ASCVD. The AHA/ACC “2018 Guideline on the Management of Blood Cholesterol,”9 using evidence from the Cholesterol Treatment Trialists’ Collaboration, recommended a high-intensity statin or, in some cases, moderate-intensity, for patients with clinical ASCVD. A 50% reduction in LDL for such patients <75 years of age can prevent adverse events, such as myocardial infarction and stroke.7 Ongoing study is needed to evaluate clinical outcomes of statin therapy in patients with nonatherosclerotic AAA.
Recommendation-Specific Supportive Text
AAA of atherosclerotic cause is considered a coronary artery disease equivalent, with a >20% risk of a cardiovascular event within 10 years.8 Intensive cholesterol lowering in patients with ASCVD reduces major cardiovascular events by an additional 15% beyond what is achieved with less intensive cholesterol lowering.7,9 From a large Danish case-control study, current, but not a history of previous, statin use was associated with decreased 30-day mortality rates in patients with ruptured AAA (46.1% versus 59.3%, respectively; adjusted mortality rate, 0.80; 95% CI, 0.68–0.95).10 Retrospective data from 5 892 patients enrolled in the EUROSTAR (EUROpean collaborators on Stent-graft Techniques for abdominal aortic Aneurysm Repair) registry showed improved survival over 5 years of follow-up associated with statin use (81% for statin users versus 77% for nonusers; P=0.005).11 Additionally, in a large registry-based study of 37 950 patients undergoing repair of AAAs, those not previously on statin therapy who were started on statin before discharge had improved 1- and 5-year survival compared with those who remained off statin therapy.12
In a recent meta-analysis, in broad cohorts of patients with AAA, statin therapy was associated with slower aneurysm growth, reduced risk of rupture, and lower 30-day mortality after aortic repair4; because atherosclerosis is so prevalent among patients with AAA, it was not possible to distinguish whether statin therapy benefited those without atherosclerosis equally. The mechanisms by which statins improve survival in AAA warrant further study, as in 1 single prospective cohort study of patients undergoing long-term surveillance, statins had not been shown to slow the growth rate of AAA or have direct effect on matrix metalloproteinase-9 or interleukin-6 concentrations.5
6.4.2.3. Smoking Cessation in AAA
Recommendation for Smoking Cessation in AAA
Synopsis
Cigarette smoking is a major risk factor for the development, growth, and complications of AAA (see Section 6.2, “AAA: Cause, Risk Factors, and Screening”) and increases the risk for adverse clinical outcomes in the perioperative setting for AAA repair. Healthy lifestyle modifications in ASCVD, such as atherosclerotic AAA and PAU, include smoking cessation and avoidance of secondhand smoke. Effective strategies in those patients motivated to quit smoking use the 5 A’s (Ask, Advise, Assess, Assist, and Arrange) and may include dedicated multidisciplinary programs, app-based tools, or pharmacotherapy with nicotine replacement, bupropion, varenicline, or all 3.1–3 Although e-cigarette use has been shown to be an effective strategy in smoking cessation,4 the efficacy and safety of its use in patients with AAA has not been shown.
Recommendation-Specific Supportive Text
No randomized clinical trials have assessed the effect of smoking cessation on clinical outcomes in patients with AAA, given the inherent design limitations of such an intervention. Current guidelines and recommendations that encourage counseling and pharmacological interventions in patients motivated to quit are derived from the fact that cigarette smoking is considered the largest modifiable risk factor for AAA. The use of e-cigarettes is effective in smoking cessation; however, given its association with adverse vascular remodeling, more evidence on its safety in patients with AAA is needed.
6.4.2.4. Antithrombotic Therapy in AAA
Recommendation for Antithrombotic Therapy in AAA
| COR | LOE | Recommendation |
|---|---|---|
| 2b | C-LD | 1. In patients with AAA with concomitant atheroma and/or PAU, the use of low-dose aspirin may be considered, unless contraindicated.1 |
Synopsis
Atherosclerotic AAA are associated with a >20% risk of cardiovascular events within 10 years, as they are considered a coronary artery disease equivalent according to the NCEP ATP III.2 To reduce risk of cardiovascular events and mortality, aspirin at 75 mg to 162 mg daily for secondary prevention has been incorporated into the 2006 updated “AHA/ACC Guidelines for Secondary Prevention for Patients With Coronary and Other Atherosclerotic Vascular Disease.”3 Most AAA contain an intraluminal thrombus (see Section 6.2, “AAA: Cause, Risk Factors, and Screening”) made up of a complex matrix of platelets, inflammatory cells, and fibrin, which contributes to growth and progression, and thus antithrombotic therapy has been hypothesized to have a potential benefit in AAA. However, clinical outcomes data are limited, and further study of the efficacy of antiplatelet therapy in AAA is warranted.
Recommendation-Specific Supportive Text
Low-dose aspirin monotherapy in patients with noncoronary atherosclerosis is considered a treatment to mitigate risk of cardiovascular events, including stroke, death caused by coronary artery disease, and myocardial infarction.3 Data are limited on aortic-specific clinical outcomes in AAA. Use of low-dose aspirin has been hypothesized to reduce growth and progression of AAA attributable to the detrimental effects of platelet activation within the intraluminal thrombus. In 1 small cohort study, low-dose aspirin was associated with a reduced AAA growth rate and need for aneurysm repair at diameters of 4.0 cm to 4.9 cm but not for aneurysms <4.0 cm. However, evidence from the Danish National Registry of Patients study of 4010 age- and sex-matched subjects with AAA1 showed an increased case-fatality rate associated with preadmission aspirin use in ruptured AAA (66% in users versus 57% in nonusers; adjusted mortality rate ratio, 1.16; 95% CI, 1.06–1.27); there was no association between aspirin use and the risk of AAA rupture (adjusted OR, 0.97; 95% CI, 0.86–1.08).
6.4.3. Surveillance for Medical Management
6.4.3.1. Surveillance of Thoracic Aortic Dilation and Aneurysm
Recommendations for Surveillance of Thoracic Aortic Dilation and Aneurysm
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In patients with a dilated thoracic aorta, a TTE is recommended at the time of diagnosis to assess aortic valve anatomy, aortic valve function, and thoracic aortic diameters.1–4 |
| 2a | C-LD | 2. In patients with a dilated thoracic aorta, a CT or MRI at the time of diagnosis is reasonable to assess thoracic aortic anatomy and diameters.1,3,5–7 |
| 2a | C-LD | 3. In patients with a dilated thoracic aorta, follow-up imaging (with TTE, CT, or MRI, as appropriate based on individual anatomy) in 6 to 12 months is reasonable to determine the rate of aortic enlargement; if stable, surveillance imaging every 6 to 24 months (depending on aortic diameter) is reasonable.1,3,4 |
Synopsis
In patients with TAD, a detailed baseline assessment of all the segments of thoracic aorta, aortic valve anatomy, and aortic valve function is important. TTE, CT, and MRI are all commonly used for assessment of the thoracic aorta.
Recommendation-Specific Supportive Text
In patients with TAD not at surgical threshold, a detailed assessment with a TTE to evaluate aortic valve anatomy and aortic valve function is important for establishing a baseline. TTE usually provides clear images of the aortic root and ascending aorta, is safe and reproducible, and can be used for longitudinal surveillance. In select patients with difficult echocardiographic imaging windows, a TEE is an alternative for evaluating aortic valve anatomy and aortic dimensions.1–3
Cross-sectional imaging with CT or MRI has been established as the gold standard for assessment of all segments of thoracic aorta including arch branch vessels.5,6 Electrocardiographic-gated techniques minimize motion artifact and thus allow precise measurement of aortic root and ascending aortic dimensions.5,6
Patients with stable aortic dimensions can be observed longitudinally with TTE, CT, or MRI. The frequency of surveillance imaging should be individualized and informed by the aneurysm cause, aortic diameter, historical rate of aortic growth, how close the diameter is to the surgical threshold, and the patient’s age.8,9 In general, in patients with nongenetic and syndromic causes, the rate of aortic growth is relatively slow, so the interval for surveillance imaging may be increased.
6.4.3.2. Surveillance of Abdominal Aortic Dilation and Aneurysm
Recommendations for Surveillance of Abdominal Aortic Dilation and Aneurysm
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with an AAA of 3.0 cm to 3.9 cm, surveillance ultrasound is recommended every 3 years to assess for interval change.1–8 |
| 1 | B-NR | 2. In men with an AAA of 4.0 cm to 4.9 cm and in women with an AAA of 4.0 cm to 4.4 cm, surveillance ultrasound is recommended annually to assess for interval change.1–8 |
| 1 | B-NR | 3. In men with an AAA of ≥5.0 cm and women with an AAA of ≥4.5 cm, surveillance ultrasound is recommended every 6 months to assess for interval change.1–8 |
| 1 | C-EO | 4. In patients with an AAA that is inadequately defined with ultrasound, surveillance CT is recommended. In such patients, when there is a contraindication to CT or to lower cumulative radiation risk, surveillance MRI is reasonable.9,10 |
| 2a | C-LD | |
| 1 | C-EO | 5. In patients with an AAA that meets criteria for repair, CT is recommended for preoperative planning. |
Synopsis
In patients with AAA, imaging assessment of the abdominal aorta is important for establishing baseline diameter and determining the timing of surveillance imaging. Ultrasound imaging has been the standard for surveillance imaging of the abdominal aorta and is widely used. CT provides superior visualization of the abdominal aorta and its branches and is therefore used for preoperative planning. MRI is a reasonable alternative to CT in selected patients. Figure 20 shows a proposed general algorithm for surveillance imaging of AAA, recognizing that surveillance intervals should be individualized.
Figure 20. The Frequency of Surveillance Imaging of Abdominal Aortic Aneurysms Based on Current Aortic Diameter.
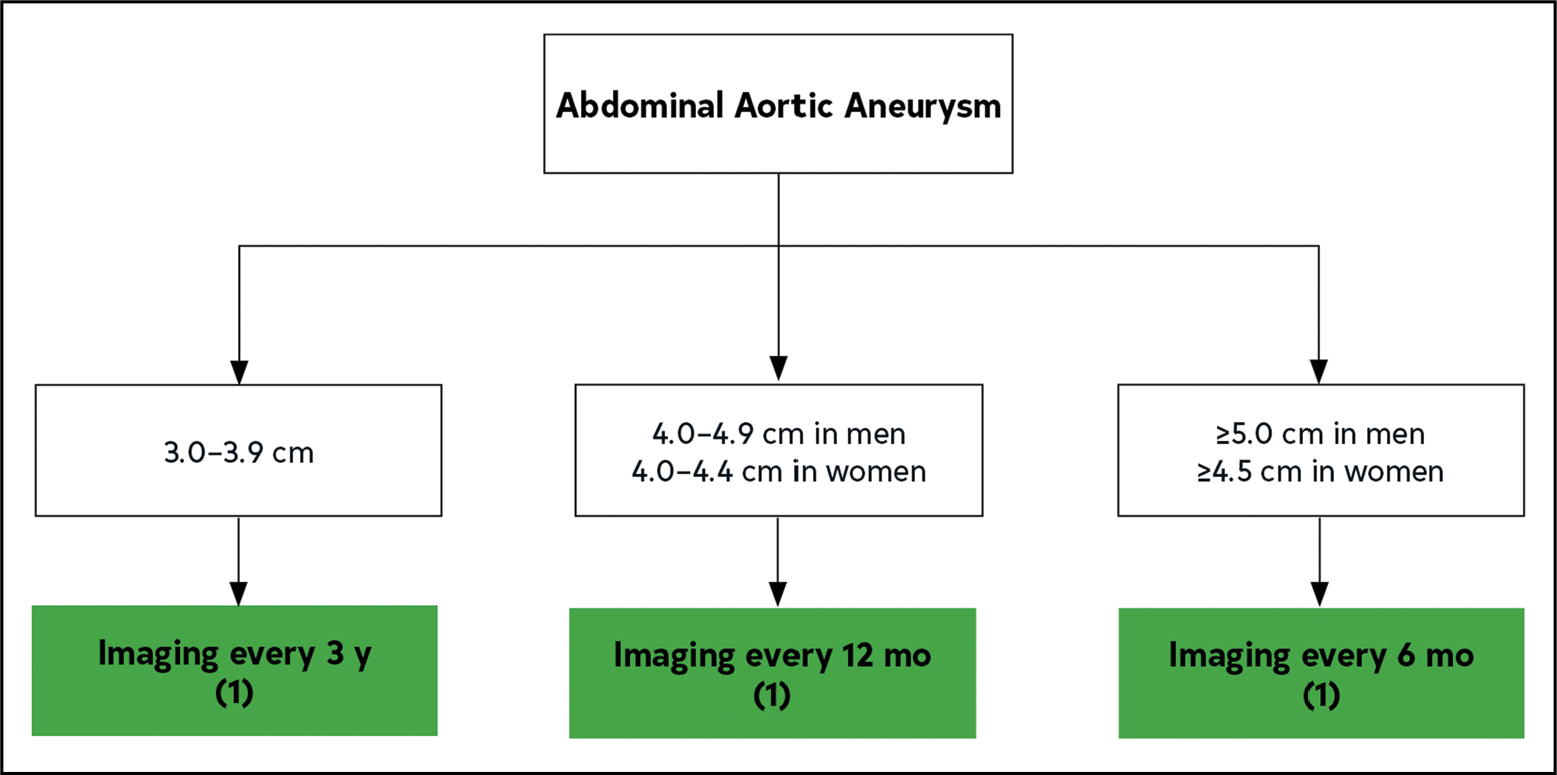
Color corresponds to Class of Recommendations in Table 2.
Recommendation-Specific Supportive Text
Multiple studies have established that ultrasound surveillance of AAAs helps to prevent rupture and mortality.2–7,11 The risk of rupture increases at an AAA diameter of >5.5 cm for men and >5.0 cm for women; accordingly, surveillance imaging should be more frequent at larger AAA diameters that approach these thresholds. Conversely, at AAA diameters of 3.0 cm to 3.9 cm, longer surveillance imaging intervals have been shown to be safe.
In patients with AAA of 4.0 cm to 4.9 cm, rates of aortic growth are higher, so annual surveillance ultrasound is recommended. Even shorter intervals are often used in those who smoke, have diabetes, or both because of their increased risk of growth.
Once the size of the AAA reaches ≥5.0 cm in men and ≥4.5 cm in women, the screening interval is shortened to every 6 months given the potential of larger aneurysms to grow more rapidly and reach the thresholds for intervention. CT provides superior visualization of the abdominal aorta and its branches and is an excellent alternative when ultrasound is inadequate. MRA is a reasonable alternative to CT. Non-IV contrast MRI techniques have also been shown to be useful in defining AAAs.9,10
CT is generally preferred when an AAA reaches the threshold for intervention, both to confirm aortic diameters and to detail the anatomy of the aorta and its branches for preoperative planning.
6.5. Surgical and Endovascular Management of Aortic Aneurysms
Most patients with TAA and AAA are asymptomatic, so the purpose of surgical or endovascular intervention is to reduce the risk of adverse aortic events (ie, aortic dissection, rupture, and aortic-related death). Consequently, determining the optimal timing of intervention requires a careful anatomic assessment, followed by weighing the risk of future adverse aortic events against the risk of intervention.The goal of open surgery is to replace the aneurysmal aortic segment with a prosthetic graft anastomosed to nonaneurysmal aortic tissues while maintaining critical aortic branch vessels. Endovascular repair leverages contiguous nonaneurysmal aortic or iliac segments for fixation of endovascular stent grafts to exclude blood flow from the aneurysmal sac. To date, the FDA has approved individual stent grafts for the treatment of aneurysms involving the descending thoracic, juxtarenal, and infrarenal aortic segments. Stent graft devices to address the ascending aorta, aortic arch, and thoracoabdominal aorta are available under investigational use in the United States, currently in physician- and industry-sponsored clinical trials. Long-term studies have shown that use of endovascular stent grafts outside of the anatomic criteria tested in their pivotal trials is associated with increased risk of aneurysm sac enlargement, underscoring the need for appropriate patient selection and for long-term surveillance after endovascular repair.1
6.5.1. Surgery for Sporadic Aneurysms of the Aortic Root and Ascending Aorta
Recommendations for Surgery for Sporadic Aneurysms of the Aortic Root and Ascending Aorta
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In patients with aneurysms of the aortic root and ascending aorta who have symptoms attributable to the aneurysm, surgery is indicated.1,2 |
| 1 | B-NR | 2. In asymptomatic patients with aneurysms of the aortic root or ascending aorta who have a maximum diameter of ≥5.5 cm, surgery is indicated.3–9 |
| 1 | C-LD | 3. In patients with an aneurysm of the aortic root or ascending aorta of <5.5 cm, whose growth rate confirmed by tomographic imaging is ≥0.3 cm/y in 2 consecutive years, or ≥0.5 cm in 1 year, surgery is indicated.10–13 |
| 2a | B-NR | 4. In asymptomatic patients with aneurysms of the aortic root or ascending aorta who have a maximum diameter of ≥5.0 cm, surgery is reasonable when performed by experienced surgeons in a Multidisciplinary Aortic Team.14–17 |
| 2a | B-NR | 5. In patients undergoing repair or replacement of a tricuspid aortic valve who have a concomitant aneurysm of the ascending aorta with a maximum diameter of ≥4.5 cm, ascending aortic replacement is reasonable when performed by experienced surgeons in a Multidisciplinary Aortic Team.18–21 In patients undergoing repair or replacement of a tricuspid aortic valve who have a concomitant aneurysm of the ascending aorta with a maximum diameter of ≥5.0 cm, ascending aortic replacement is reasonable.18–21 In patients undergoing cardiac surgery for indications other than aortic valve repair or replacement who have a concomitant aneurysm of ascending aorta with a maximum diameter of ≥5.0 cm, ascending aortic replacement may be reasonable.18 |
| 2a | B-NR | |
| 2b | C-LD | |
| 2a | C-LD | 6. In patients with a height >1 standard deviation above or below the mean who have an asymptomatic aneurysm of the aortic root or ascending aorta and a maximal cross-sectional aortic area/height ratio of ≥10 cm2/m, surgery is reasonable when performed by experienced surgeons in a Multidisciplinary Aortic Team.14,15,22 |
| 2b | C-LD | 7. In asymptomatic patients with aneurysms of the aortic root or ascending aorta who have either an ASI of ≥3.08 cm/m2 or AHI of ≥3.21 cm/m, surgery may be reasonable when performed by experienced surgeons in a Multidisciplinary Aortic Team.23 |
Synopsis
Elective surgery for aneurysms of the aortic root and ascending aorta is ideally performed when the risk of adverse events—dissection, rupture, or sudden death—outweighs the risks of surgery. No prospective multicenter observational studies have evaluated the myriad of parameters (eg, aortic diameter, length, or area, alone or indexed to height or BSA, wall stress, shear stress) proposed for predicting the risk of aortic adverse events. From a purely mechanical perspective, aortic dissection or rupture can be considered a failure event, where an imbalance exists between stresses on the aneurysm wall and the inherent strength of its tissue.24 Whether the aortic dissection is precipitated by increased wall stress or decreased wall strength, or a combination of both, is an area of active research.25–29 Maximal aortic diameter has logically been the primary criterion for elective aneurysm repair because, per LaPlace’s law, wall stress increases proportionally with aortic radius and inversely to thickness.30 The original natural history studies examined the risk of rupture or aortic dissection versus diameter and the hinge point for dissection generated the 5.5-cm threshold that has long governed clinical practice.7,8 Although a significant proportion of patients with type A aortic dissection present with diameters <5.5 cm,31,32 this surgical threshold still effectively reduces adverse events.17,33
Recommendation-Specific Supportive Text
Large aneurysms can compress nearby structures as they expand, resulting in symptoms of chest or back pain. Alternatively, pain is sometimes associated with rapid aortic growth. Consequently, the appearance of such symptoms raises concern for an increased risk of aneurysm rupture,1,2 and surgical repair is therefore indicated.
A maximum aortic diameter of ≥5.5 cm has been the primary criterion for elective surgical repair of aneurysms of the aortic root or ascending thoracic aorta,4,6 based on natural history studies that examined diameter (without centerline analysis) at the time of adverse event and an assumed operative mortality of <5%.4,7–9 The mortality rate for elective surgery is low, whereas the risk of adverse events is high when such surgery is recommended but not performed because of patient noncompliance or comorbidities.33 The same 5.5-cm diameter threshold applies regardless of whether patients have tricuspid or BAVs.5
One meta-analysis and limited observational studies have found ascending aortic aneurysm growth to be slower than previously reported, and frequently <0.5 mm/y, in patients with a tricuspid aortic valve and without a genetic aortic disorder.11,12,17,34 The meta-analysis suggested that rapid aneurysm growth is associated with an increased risk of rupture.12 Because of the inherent error in measurement as well as interobserver variability, 1 mm to 2 mm growth per year would be difficult to document consistently on surveillance imaging. Discrepancies in measurement can occur when comparing different imaging modalities or even when using the same modality when comparing images obtained with and without contrast. Ideally, growth rates are most accurate when assessed using cardiac-gated CT or MRI with centerline measurement techniques.35 Confirmed growth of ≥0.5 cm in 1 year has been, and remains, an indication for surgery.3–6 Moreover, growth of even 0.3 cm in 1 year still substantially exceeds the expected growth rate for aneurysms of the root and ascending aorta, so if that rate of growth rate is sustained for 2 consecutive years, intervention is also recommended.13
The risk of aortic dissection or rupture correlates with increasing aneurysm diameter,7,8,16 as does the rate of aortic growth.12,36 As such, aneurysms of ≥5.0 cm would be expected to have a greater risk of complications or rapid growth than would smaller aneurysms. Indeed, in a report by Paruchuri et al,37 relative to a control aortic diameter of ≤3.4 cm, a diameter of 4.0 cm to 4.4 cm conferred an 89-fold increased risk of aortic dissection, and a diameter of ≥4.5 cm conferred a 6300-fold increased risk (Figure 5). Consequently, many experienced surgeons in a Multidisciplinary Aortic Team choose to operate selectively on patients with aneurysms of 5.0 cm to 5.4 cm,17 provided the patient’s surgical risk is low,38 and they have had excellent results14–16 in doing so. However, there is an ongoing prospective multicenter RCT of patients with ascending TAAs of 5.0 cm to 5.4 cm that will compare outcomes of early elective surgery vs. medical surveillance,39 the results of which could provide further guidance.
For patients undergoing aortic valve surgery with concomitant ascending aortic aneurysm of ≥4.5 cm, guidelines have previously recommended simultaneous aortic replacement in those with BAV. On the other hand, in patients who have undergone valve surgery without concomitant aortic aneurysm surgery, whether for an underlying bicuspid or tricuspid aortic valve, the associated aneurysms have been shown to grow slowly and have low rates of aortic complications over time. Still, data have also shown the safety of performing concomitant aneurysm repair at a diameter of ≥4.5 cm by experienced surgeons working in a Multidisciplinary Aortic Team.16,18–21,40–43 Nevertheless, until there are better predictors for aortic complications, in general it is reasonable in patients undergoing aortic valve repair or replacement to offer concomitant aneurysm surgery for those with aneurysms of ≥5.0 cm, because of the faster rate of growth and higher risk of aortic dissection. Aortic root replacement should be individualized based on the type of aortic valve surgery (ie, valve repair with or without valve-sparing root versus valve replacement, mechanical versus bioprosthetic root replacement), patient condition, patient age, and comorbidities. In those undergoing cardiac surgery for indications other than aortic valve repair, concomitant prophylactic aortic replacement at a diameter of 5.0 cm may be reasonable, because it would provide a margin of safety against future aortic dissection, particularly because cardiac surgery itself becomes an additional risk factor for subsequent aortic dissection.
Data from the IRAD showed that ~60% of patients with acute type A aortic dissection had maximal aortic diameters of <5.5 cm32 at presentation, a finding that has been corroborated by others.31,44 Conversely, most patients with aneurysms <5.5 cm who are managed medically do not suffer aortic dissection or rupture. Therefore, absolute aortic diameter is far from an ideal predictor of risk. Parameters proposed to improve risk prediction include the ratio of aortic diameter to either patient height or BSA,23 the ratio of aortic area to height,14,15 the ascending aortic length (centerline, from annulus to innominate artery takeoff),14,15,45–47 aortic stiffness, and peak aortic wall stress.25,48–50 All are retrospectively promising, but none has been prospectively validated. A cross-sectional aortic area to patient height ratio of ≥10 cm2/m was found to correlate with increased mortality among unoperated patients with root or ascending aortic aneurysms and either a tricuspid15 or BAV.14 The use of the cross-sectional aortic area to height ratio is most appropriate in patients whose height is >1 standard deviation above or below the mean.
A single-center large database of TAA has grown considerably and was reevaluated with indexing of aortic diameter to BSA (ASI) or height (AHI), to improve the prediction of adverse aortic events.23 Height was preferred because the variable nature of weight and the underlying genetic contribution to height. Recommendations for prophylactic repair at aortic diameters of <5.5 cm have been proposed but not systematically tested in large-scale multicenter trials. Experienced surgeons in a Multidisciplinary Aortic Team38 may consider the use of such ratios when determining the optimal timing of intervention. This may be particular useful for female patients, but more studies are required to further evaluate surgical thresholds in women with aneurysms of the aortic root or ascending aorta.
6.5.1.1. Surgical Approach for Patients With Sporadic Aneurysms of the Aortic Root and Ascending Aorta Meeting Criteria for Surgery
Recommendations for Surgical Approach for Patients With Sporadic Aneurysms of the Aortic Root and Ascending Aorta Meeting Criteria for Surgery
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with an aneurysm isolated to the ascending aorta who meet criteria for surgery, aneurysm resection and replacement with an interposition graft should be performed.1,2 |
| 1 | B-NR | 2. In patients undergoing aortic valve repair or replacement with a concomitant ascending aortic aneurysm, a separate aortic valve intervention and ascending aortic graft is recommended.3–6 |
| 1 | B-NR | 3. In patients undergoing aortic root replacement with an aortic valve that is unsuitable for sparing or repair, a mechanical or biological valved conduit aortic root replacement is indicated.1,2,7,8 |
| 2a | B-NR | 4. In patients undergoing aortic root replacement, valve-sparing aortic root replacement is reasonable if the aortic valve is suitable for sparing or repair and when performed by experienced surgeons in a Multidisciplinary Aortic Team.9–21 |
Synopsis
The goal of prophylactic repair of aneurysms of the aortic root and ascending aorta is to prevent life-threatening complications from acute aortic events such as aortic dissection, aortic rupture, or sudden death. This goal is best achieved when the risk of future adverse aortic events is greater than the expected surgical mortality (considering both the surgeon’s and institutional experience). The STS database has clearly shown that proximal thoracic aortic surgery has a lower operative mortality when performed electively rather than emergently (2.2% versus 17.2%, respectively).1
Recommendation-Specific Supportive Text
Proximal thoracic aortic operations in the United States, including ascending thoracic aortic replacement and aortic root replacement, have an overall elective mortality rate of 2.2%. Consequently, patients who meet criteria for aneurysm repair and have low operative risk can undergo prophylactic resection and graft placement with low operative mortality risk.1 Similar results were obtained when examining the Nationwide Inpatient Sample in which operative mortality rate for proximal thoracic aortic surgery was 2.5%.2
Single-institution studies have shown that the addition of ascending TAA repair to AVR does not increase operative mortality in experienced aortic centers.22–24 However, such results may not be reproducible at low-volume centers that have a higher operative mortality rate for isolated proximal thoracic aortic surgery.1,2 Root-sparing AVR with concomitant ascending aneurysm repair is acceptable, because data suggest the aortic root dilates at a slower rate than does the ascending aorta, and studies of root-sparing surgery have shown no increase in long-term adverse aortic events.3–6,25
Surgical approaches to replace the aortic root should be guided by the aortic valve anatomy. If the aortic valve is unsuitable for sparing or repair (eg, large fenestrations, calcification), a mechanical- or biological-valved conduit aortic root replacement should be performed because, when elective, this procedure has an operative mortality rate of 2.2% in the United States based on the STS database.2,26 Single-institution series from centers with Multidisciplinary Aortic Teams have also shown excellent results both with and without concomitant hemiarch replacement.7 Long-term outcomes are similar with mechanical- versus biological-valved conduit aortic root replacements, even in patients <70 years old.8
In younger patients with an aortic valve that is amenable to sparing or repair, elective valve-sparing aortic root replacement has been performed safely by experienced surgeons in a Multidisciplinary Aortic Team.9–11,21 In patients with aortic root aneurysms without an underlying genetic disorder, valve-sparing aortic root replacement has been performed by either the reimplantation or remodeling technique with comparable survival and valve durability.12
6.5.2. Aortic Arch Aneurysms
Recommendations for Aortic Arch Aneurysms
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. In patients with an aortic arch aneurysm who have symptoms attributable to the aneurysm and are at low or intermediate operative risk, open surgical replacement is recommended. |
| 2a | B-NR | 2. In patients with an isolated aortic arch aneurysm who are asymptomatic and have a low operative risk, open surgical replacement at an arch diameter of ≥5.5 cm is reasonable.1–3 |
| 2a | C-LD | 3. In patients undergoing open surgical repair of an ascending aortic aneurysm, if the aneurysmal disease extends into the proximal aortic arch, it is reasonable to extend the repair with a hemiarch replacement.4,5 |
| 2b | C-LD | 4. In patients undergoing open surgical repair of an aortic arch aneurysm, if the aneurysmal disease extends into the proximal descending thoracic aorta, an elephant trunk procedure may be considered.6,7 |
| 2b | C-EO | 5. In patients with an aortic arch aneurysm who are asymptomatic but meet criteria for intervention, but have a high risk from open surgical repair, a hybrid or endovascular approach may be reasonable. |
Synopsis
Aortic arch aneurysms are the least common of the TAA, because <10% of aneurysms involve the arch only; in most cases, arch aneurysms are associated with adjacent pathology.1 Previous aortic dissection is the most common cause of arch aneurysms; in a large meta-analysis, only 28.3% of patients undergoing intervention on the arch had de novo aneurysmal disease, with the remainder resulting from acute or chronic aortic dissection. The risk of dissection or rupture, as related to aortic diameter, is presumed to be similar in the arch as in other thoracic locations, although no large reports consider arch dimensions alone. Additionally, because of the proximity of the aortic arch to other thoracic structures, dilation may result in symptoms before the diameter reaches a threshold typically considered for intervention. Intervention to treat arch aneurysms carries an increased risk given the need to manage the great vessels and protect the brain. Various techniques have been developed, including the use of hypothermic circulatory arrest and specialized branched grafts to aid in reconstruction. Endovascular techniques also continue to evolve.
Recommendation-Specific Supportive Text
Because of the juxtaposition of the aortic arch to other vascular structures, nerves, trachea, and the esophagus, symptoms may develop because of the mass effect from encroachment on adjoining structures. Ortner’s syndrome is unilateral hoarseness secondary to inflammation or stretching of the left recurrent laryngeal nerve.8 Dysphagia aortica can be caused by extrinsic compression of the esophagus by either fusiform or saccular aneurysms of the arch. Likewise, extrinsic compression of the trachea may result in dyspnea, and compression of the innominate vein or superior vena cava may cause superior vena cava syndrome. Nonspecific symptoms include chest pain or pressure, fatigue, and neck, jaw, or back pain.
Open replacement of the aortic arch requires the use of cardiopulmonary bypass, hypothermia, and other adjuncts for neurologic and systemic protection. Various randomized and nonrandomized trials have compared different cannulation strategies (ie, axillary, femoral, innominate),9–12 levels of hypothermia, and variations in cerebral perfusion (antegrade, select antegrade, retrograde),13–15 with no one technique dominating or being shown conclusively to be superior to another.
When proximal aneurysmal aortic disease extends to the level of the innominate artery or further into the arch, but not necessarily the whole arch, a hemiarch procedure may be able to effectively address the distal pathology. Open distal anastomosis will require the same adjuncts and approaches used in open arch replacement (as described previously), including neuroprotective strategies. Modified approaches have been described that eliminate the need for open repair whereby the ascending aorta is replaced first with a trifurcated side-branch for debranching of the arch in a sequential manner to a level that is accessible for clamping; however, no studies have yet shown the benefit of such an approach. Although it does add to the cardiopulmonary bypass time and blood loss,4,5 the addition of a hemiarch has been shown not to increase the risk of the procedure. However, this noninferiority is lost when the proximal arch is disease free, with exception of an underlying aortopathy in which the normal-sized arch will predictively enlarge or dissect at a later time; in this setting, a hemiarch is justified.16,17
The elephant trunk procedure, as originally described, extends the aortic arch graft into the proximal descending aorta, thereby facilitating the subsequent repair of diseased descending thoracic aorta (by either open repair or TEVAR).18 Either a traditional elephant trunk (an extension graft anastomosed to the distal end of an aortic arch graft at the time of arch repair that projects into the proximal descending aorta with a free distal end) or a frozen elephant trunk (a combined open aortic arch graft with an extension endovascular stent-graft extending into the descending thoracic aorta to treat extensive TAD involving both arch and descending segments via a median sternotomy) can be used.6,19 With adjunctive procedures (ie, debranching), the distal anastomosis can be moved more proximally into aortic zones 2 or 3 (Figure 3), while still proceeding with an elephant trunk and with the potential of decreasing morbidity, but data are limited on the benefits of moving the anastomotic site. A qualifying factor for considering open versus frozen elephant trunk is whether the primary distal seal will be achieved with the frozen elephant trunk. In the absence of a distal seal, the conventional approach would provide the same considerations for the second-stage procedure.
Various hybrid and endovascular techniques have been developed to address the aneurysmal arch in the setting of a high-risk patient, including open extra-anatomical bypasses (eg, left carotid-to-left subclavian artery bypass) and endovascular approaches. The Next-gen Fenestrated TEVAR trial showed the feasibility of proximal landing zone coverage, with most endografts being placed in zones 0 or 1 (Figure 3), although a landing zone of <15 mm was associated with an increased risk of a type I endoleak (Figure 11).20 The midterm follow-up showed 5-year survival of 71% with an aneurysm-related event-free survival of 77%. The most frequent reason for reoperation was type Ia endoleak (5%).21 Nonrandomized comparisons of open versus hybrid endovascular approaches have not shown significant differences in outcomes.22–24 Complete endovascular approaches have been described25,26 and may be considered by those with endovascular experience who have access to the appropriate devices, investigational devices, or both.
6.5.3. Descending TAA
6.5.3.1. Size Thresholds for Repair of Descending TAA
Recommendations for Size Thresholds for Repair of Descending TAA
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with intact descending TAA, repair is recommended when the diameter is ≥5.5 cm.1,2 |
| 2b | B-NR | 2. In patients with intact descending TAA and risk factors for rupture (Table 17), repair may be considered at a diameter of <5.5 cm.2–6 |
| 2b | B-NR | 3. In patients at increased risk for perioperative morbidity and mortality (Table 18), it may be reasonable to increase the size threshold for surgery accordingly.7 |
Table 17.
Risk Factors for Aortic Rupture Among Patients With Descending TAA
| High-Risk Features for Rupture |
|---|
| Aneurysm growth of ≥0.5 cm/y3 |
| Symptomatic aneurysm4 |
| Marfan, Loeys-Dietz, or vascular Ehlers-Danlos syndrome, or HTAD (see Section 6.1.2, “Genetic Aortopathies”)2 |
| Saccular aneurysm5 |
| Female sex2 |
| Infectious aneurysm6 |
HTAD indicates heritable thoracic aortic disease; and TAA, thoracic aortic aneurysm.
Table 18.
Patient Characteristics Associated With Increased Perioperative Morbidity and Mortality After Open and Endovascular Repair of Descending TAA
| Open Surgical Repair | Endovascular Repair |
|---|---|
| Advanced age8 | Functional dependence |
| 65–74 y (OR, 1.8; 95% CI, 1.4–2.4; P <0.001) | |
| ≥75 y (OR, 2.6; 95% CI, 2.0–3.5; P <0.001) | |
| Preoperative renal insufficiency (stage 3 or greater CKD) or hemodialysis | Thoracoabdominal aortic aneurysm extent |
| COPD and FEV1 ≤50% predicted | Pulmonary disease |
| Previous stroke9 | Need for iliac access |
| Zone 1/2 landing for thoracic stent graft7 |
CKD indicates chronic kidney disease; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; and TAA, thoracic aortic aneurysm.
Synopsis
The current aortic size threshold for repair of descending TAA is primarily based on single-center series where patients have been observed with surveillance imaging and clinical follow-up to determine the incidence of aortic-related events and deaths. Such series indicate that a descending aortic diameter of >6 cm is associated with an increased risk of adverse aortic events and mortality,1,2 as shown in Table 16. Moreover, certain patient and aneurysm features are associated with an increased risk for aortic dissection or rupture, as shown in Table 17, which may prompt consideration of earlier surgery. Conversely, some patients are at increased risk of perioperative morbidity or mortality, in which case the size threshold for aortic repair might be increased. Specifically, if the patient does not have ideal anatomy for endovascular repair, or has otherwise increased risk for contemplated open repair, close monitoring until a higher surgical threshold is reached would be justified. Advanced age,8 preoperative renal insufficiency or hemodialysis, chronic obstructive pulmonary disease, and previous stroke are harbingers of adverse outcomes or perioperative mortality after open repair (Table 18).9 Markers of frailty, pulmonary disease, thoracoabdominal extent, need for iliac access, and zone 1/2 deployment were associated with major adverse events after TEVAR (Table 18).7 A nuanced approach and detailed discussion with the patient can help guide the most reasonable treatment plan, weighing the risks of the operation against the risks of continued surveillance.
Table 16.
Adverse Aortic Events at 1 Year, Based on Baseline Aortic Diameter, Among Patients With Descending TAA
| Aortic Diameter (cm) | Definite Aortic Event* (%) | Probable Aortic Event† (%) |
|---|---|---|
| 5.0 | 5.5 | 8.0 |
| 5.5 | 7.2 | 11.2 |
| 6.0 | 9.3 | 15.6 |
| 7.0 | 15.4 | 28.1 |
Definite aortic event includes aortic dissection or rupture confirmed with imaging or intraoperative findings.
Probable aortic event includes definite aortic events as well as sudden unexplained death.
TAA indicates thoracic aortic aneurysm. Based on data from Kim JB, et al.1
Recommendation-Specific Supportive Text
There is an increased incidence of aortic-related events such as rupture or dissection with aortic diameters >6 cm, justifying intervention when the diameter is ≥5.5 cm in size.1,2
High-risk features of rupture have been previously identified, supporting repair at a smaller diameter threshold when these criteria are met. Features including rapid aortic growth (≥0.5 cm/y),3 symptomatic aneurysms,4 underlying connective tissue disorder or HTAD,2 saccular aneurysm morphology,5 female sex,2 and infected aneurysm6 have all been associated with a higher tendency for rupture.
In patients being considered for open or endovascular repair, high-risk clinical features (Table 18) have been identified that portend poor outcomes after repair. For open surgical repair, advanced age,8 preoperative renal insufficiency of stage 3 or greater, chronic obstructive pulmonary disease and forced expiratory volume in 1 second ≤50% predicted, and previous stroke9 have all been associated with increased risk of death, perioperative morbidity, or both. For endovascular repair of descending TAA, frailty indicators, pulmonary disease, as well as procedural complexity are predictive of poor outcomes after TEVAR.7 When contemplating either approach, special attention to these risk factors will allow appropriate consideration of the risks to benefits in deciding on the merits of intervention.
6.5.3.2. Endovascular Versus Open Repair of Descending TAA
Recommendations for Endovascular Versus Open Repair of Descending TAA
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients without Marfan syndrome, Loeys-Dietz syndrome, or vascular Ehlers-Danlos syndrome, who have a descending TAA that meets criteria for intervention and anatomy suitable for endovascular repair, TEVAR is recommended over open surgery.1–4 |
| 1 | B-NR | 2. In patients with a descending TAA that meets criteria for repair with TEVAR, who have smaller or diseased access vessels, considerations for alternative vascular access are recommended.5 |
| 2a | B-NR | 3. In patients with a descending TAA that meets criteria for intervention, who have anatomy unsuitable for endovascular repair, and who are without significant comorbidities and have a life expectancy of at least 10 years, open surgical repair is reasonable.6–9 |
Synopsis
Although no RCTs comparing TEVAR with open repair of descending TAA exist, the pivotal device trials1,3,10 have shown a reduced perioperative morbidity, increased clinical utility, and reduced follow-up aneurysm-related mortality compared with open surgical repair. However, reintervention after TEVAR is substantial.11 In addition, although clinical device trials showed improved perioperative and long-term outcomes with TEVAR, Medicare claims data show that the perioperative advantage was lost within the first year after intact aneurysm repair, with a 5-year survival that was significantly worse after TEVAR versus open repair, at 79% versus 89%, respectively (P<0.0001).4 Further study should be dedicated to understanding why the benefit from endovascular repair decays over time. In patients with connective tissue disorders or HTAD, or those with a longer life expectancy, open surgical repair is reasonable. Open surgical repair of descending TAA reflects a volume-outcomes relationship: Although large institutional series have shown good outcomes with open repair,6–9 these results are not replicable at lower volume centers.12 The decision to proceed with endovascular versus open repair balances the need for appropriate anatomy and access, as well as a higher reintervention rate for TEVAR versus the higher perioperative risk associated with more definitive open surgical repair.
Recommendation-Specific Supportive Text
TEVAR is associated with a reduced perioperative morbidity, reduced hospital length of stay, and better freedom from aneurysm-related mortality compared to open surgical repair, based on clinical device trial data.1,3,10 In the study by Makaroun et al in 2008,13 140 patients with fusiform aneurysms were treated with TEVAR and compared with 94 open surgical controls. At 5 years, there was a decreased aneurysm-related mortality (2.8% versus 11.7%, respectively, P=0.008), a reduced major adverse event rate (57.9% versus 78.7%, respectively, P=0.01), and decreased major aneurysm-related reintervention (3.6% versus 2.1%, respectively) in TEVAR versus open repair. In the study by Matsumura et al,10 survival was noninferior for TEVAR (98.1%) versus open surgery (94.3%) at 30 days, but the severe morbidity composite index, a marker for postoperative complications, was lower for TEVAR (0.2±0.7 versus 0.7±1.2, respectively; P<0.01). In the study by Fairman et al,11 195 TEVAR patients were compared with 189 open surgical controls, and the 30-day mortality rate was lower (2% versus 8%, respectively; P<0.01) and the major adverse event rate was lower (41% versus 84%, respectively; P<0.01) for TEVAR; at 1 year, aneurysm-related mortality rate was lower for TEVAR than for open repair (3.1% versus 11.6%, respectively; P<0.002). However, in a registry study using Medicare claims data,4 although short-term outcomes were similarly better with TEVAR compared with open repair, that survival advantage was no longer present at 1 year and, at 5 years, survival was significantly worse for TEVAR versus open repair at 79% versus 89%, respectively (P<0.0001). Overall, the data show that TEVAR is beneficial in the short- to intermediate-term in patients with appropriate anatomy for endovascular repair, but the advantage is not sustained over time.
Because of the relatively large delivery systems for thoracic endografting, iliac artery graft conduits may be required to ensure safe delivery of the endograft into the aorta. In the clinical device trials, access of vessels other than the femoral artery was required in 9.4% to 21.1% of patients because of small or diseased access vessels.1–3 In a multicenter cohort study from the GREAT (Global Registry for Endovascular Aortic Treatment) registry,5 the overall access complication rate was 2.8%, and women had a higher rate of access complications than men (4.7% versus 1.8%, respectively; P=0.013), with a higher rate of the need for iliac and aortic access or surgical conduit, as well as access vessel thrombosis irrespective of the clinical setting, type of aortic disease, and device sizing.
Open descending thoracic aortic repair can be performed with low morbidity and mortality rates in high-volume centers.6–8,14 In a multicenter retrospective study using the MEDPAR (Medicare Provider Analysis and Review) data,15 the overall mortality rate after open surgical repair of descending TAA decreased in high-volume versus low-volume centers (11% versus 15%; P<0.01). In addition, data using Medicare claims show that the benefit of TEVAR is no longer present 1 year after endovascular therapy, with a significantly worse 5-year survival compared with open repair (79% versus 89%; P<0.0001).4 In a recent retrospective, single-center study in which propensity score matching analysis was used to compare the outcomes of open and endovascular descending and TAAA repair in 278 pairs of patients,16 open repair resulted in better 10-year survival than endovascular repair (52% versus 33%; P<0.0001). Because of the lack of available long-term data on aortic-specific mortality rate in young patients after TEVAR, in patients deemed to have a life-expectancy of ≥10 years, open surgical repair is reasonable.
6.5.3.3. Left Subclavian Artery Management
Recommendations for Left Subclavian Artery Management
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with descending TAA who undergo TEVAR with planned left subclavian artery coverage, revascularization of the left subclavian artery before TEVAR is recommended to prevent spinal cord injury (SCI)1,2 and potentially to reduce stroke risk2 and prevent other ischemic complications. |
| 2b | C-LD | 2. In patients with descending TAA who have undergone TEVAR with left subclavian coverage and develop SCI that is unresponsive to an increase in BP or a cerebrospinal fluid drain, left subclavian artery revascularization may be considered.3 |
Synopsis
Left subclavian artery coverage is required in up to 40% of cases of TEVAR of descending TAAs.4 SCI and stroke remain devastating complications associated with TEVAR. Addressing these modifiable risk factors would allow for better outcomes after this less invasive treatment strategy. In addition, special considerations include the prevention of vertebrobasilar insufficiency (particularly among those with a dominant left vertebral artery), preservation of any preexisting left internal mammary artery coronary bypass graft, as well as left upper extremity dialysis access or other left upper extremity-based graft. Currently, pivotal as well as feasibility trials are ongoing for branched endografts intending to preserve flow to the left subclavian artery. Longer-term follow-up of this technology is needed, but initial results are promising.5,6
Recommendation-Specific Supportive Text
Up to 40% of patients undergoing TEVAR for thoracic aneurysm repair require left subclavian artery coverage. Preoperative left subclavian revascularization has been shown to decrease the rates of stroke2,7,8 and SCI.1,2 Vertebrobasilar insufficiency and left arm ischemia can also occur without left subclavian artery revascularization.9,10 Patients with a patent left internal mammary artery to left anterior descending artery coronary artery bypass graft, or who are otherwise reliant on inflow from the left subclavian artery (eg, for dialysis access), should undergo left subclavian revascularization to preserve flow.9
Patients undergoing TEVAR with left subclavian coverage may not be hemodynamically stable enough to undergo preemptive revascularization of the left subclavian artery. If such patients go on to develop SCI after TEVAR, there have been case reports of SCI reversal with secondary revascularization of the left subclavian artery.3
6.5.3.4. Celiac Artery Management
Recommendation for Celiac Artery Management
References that support the recommendation are included in the Online Data Supplement.
| COR | LOE | Recommendation |
|---|---|---|
| 2a | B-NR | 1. In patients with descending TAA undergoing TEVAR in whom celiac artery coverage is being considered, it is reasonable to first confirm adequate collateralization.1 |
Synopsis
Celiac artery coverage is estimated to be necessary in 15% of patients undergoing TEVAR for descending TAA repair.2 The safety and use of this practice has previously been shown with single-institution series citing low incidence of postoperative visceral ischemia. However, despite the preoperative evaluation with CTA, angiography, or both to confirm adequate collateralization between the celiac and superior mesenteric artery (SMA), a small percentage of patients still die from visceral ischemia. In addition, late distal migration of the endograft can encroach on the SMA, creating SMA stenosis and compromising flow through the SMA and celiac-based collaterals.
Recommendation-Specific Supportive Text
Migration of the endograft distally over time can cause stenosis of the SMA and decrease flow to the SMA and celiac artery-based collaterals. In patients undergoing TEVAR with celiac artery coverage who have adequate collateralization on CTA, angiography, or both, a small percentage of patients go on to develop postoperative visceral ischemia. Although the risk of visceral ischemia after celiac artery coverage with TEVAR is relatively low, there remains a finite risk (3.2% in largest clinical series)3 for visceral ischemic complications, which can lead to death.
6.5.3.5. Ruptured Descending TAA
Recommendations for Ruptured Descending TAA
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with ruptured descending TAA who are anatomic candidates for endovascular repair, TEVAR is recommended over open repair because of decreased perioperative death and morbidity.1–5 |
| 2b | B-NR | 2. In patients with ruptured descending TAA undergoing TEVAR, intentional coverage of the left subclavian artery, celiac artery, or both may be considered to increase the landing zone for endovascular repair.5–7 |
Synopsis
Ruptured TAA carry a high mortality rate. Single-center data, meta-analyses, and clinical trials have all shown the lower rates of perioperative death and complications associated with endovascular versus open surgical repair.1–5 However, the survival advantage shown in Medicare-based claims data disappears after 1.5 years,4 and single-institution series1,3 reflect the frequent need for reintervention over time. Furthermore, a meta-analysis2 showed that aneurysm-related survival was decreased in the TEVAR group over time, underscoring the importance of continued surveillance in this high-risk population.
Recommendation-Specific Supportive Text
For repair of ruptured descending TAA, TEVAR is associated with decreased perioperative morbidity and mortality compared with open repair. In 1 retrospective multi-institution study, TEVAR had a lower composite rate of death, stroke, and permanent paraplegia compared with open surgery and a trend toward lower aneurysm-related mortality at 4 years.1 Similarly, a meta-analysis showed that TEVAR was associated with a lower perioperative mortality and myocardial infarction rate compared with open repair.1 A multicenter, prospective clinical trial for aortic catastrophes—including aortic rupture—showed that TEVAR was superior with regard to the composite endpoint of mortality and paraplegia, compared with open repair.5 Although the perioperative benefit of endovascular repair of ruptured TAA was again corroborated in a Medicare-claims dataset, the survival advantage with TEVAR disappeared after 1.5 years.4
When ruptured descending TAA present, coverage of the left subclavian artery, celiac artery, or both may be necessary to gain the necessary 2 cm of seal zone for successful endovascular repair. Left subclavian artery and celiac artery coverage during thoracic aortic rupture has been associated with reasonable technical success and outcomes in single-institution series6 and 1 clinical trial5 in patients with acute rupture or complicated dissection of the descending thoracic aorta.
6.5.3.6. Access Issues for TEVAR in Descending TAA
Recommendations for Access Issues for TEVAR in Descending TAA
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with descending TAA undergoing TEVAR, review of preoperative CTA of the iliofemoral vessels should be performed to evaluate access.1,2 |
| 1 | B-NR | 2. In patients with descending TAA undergoing TEVAR, if iliac access is marginal or inadequate to prevent access-related complications, the use of alternative conduits is recommended.1,2 |
| 2a | B-NR | 3. In patients with descending TAA undergoing TEVAR who have suitable anatomy, total percutaneous femoral access is a reasonable alternative to open surgical cutdown to avoid access-related complications.3–5 |
Synopsis
Iliac artery access for stent-graft delivery systems is marginal in up to 21% of cases in which TEVAR is performed for descending TAA.1 Careful review of the CTA of the iliofemoral system is required to ensure that marginal or inadequate access is noted and properly managed. Marginal access can be successfully circumvented using surgical bypass, direct aortic or iliac exposure, or endovascular techniques to treat vessel stenosis. Percutaneous access was used successfully for endovascular abdominal aortic repair before it was applied to larger sheath devices. This technology has also been applied to TEVAR with a similarly high degree of success and reduced hospital length of stay.
Recommendation-Specific Supportive Text
Thoracic endovascular stent grafts are housed in large delivery systems, thus thorough review of the iliofemoral system is required to avoid access complications. In the clinical device trials, alternative access was required in 9.4% to 21.1% of patients because of small or diseased access vessels.6–8
Alternative access was required in up to 21.1% of patients undergoing TEVAR in the clinical device trials.8 Women have a higher incidence of smaller diameter external iliac arteries compared with men.1,2 Direct aortic or iliac artery exposure, iliac conduits, or endovascular techniques may be used to facilitate safe delivery of endografts during TEVAR.1,2 Preoperative case planning will enable safe delivery of endografts without vascular complications.
Percutaneous access for delivery of TEVAR has been performed safely and with a high degree of success, as shown in single-institution4,5 as well as multi-institution registries.3 Technical success ranged from 94.4% to 98.9%, and percutaneous access was associated with fewer complications and a shorter length of stay compared with those with surgical cutdown.
6.5.4. Thoracoabdominal Aortic Aneurysms
6.5.4.1. Size Thresholds for Open Surgical Repair of TAAA
Recommendations for Size Thresholds for Open Surgical Repair of TAAA
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with intact degenerative TAAA, repair is recommended when the diameter is ≥6.0 cm.1–3 |
| 2a | B-NR | 2. In patients with intact degenerative TAAA, repair is reasonable when the diameter is ≥5.5 cm and the repair is performed by experienced surgeons in a Multidisciplinary Aortic Team.1–3 |
| 2a | B-NR | 3. In patients with intact degenerative TAAA who have features associated with an increased risk of rupture (Table 19), repair is reasonable when the diameter is <5.5 cm.4 |
Table 19.
Features Associated With an Increased Risk of TAAA Rupture
| Rapid growth (confirmed increase in diameter of ≥0.5 cm/y) |
| Symptomatic aneurysm |
| Significant change in aneurysm appearance |
| Saccular aneurysm or presence of penetrating atherosclerotic ulcers |
TAAA indicates thoracoabdominal aortic aneurysm.
Synopsis
The data supporting aortic diameter thresholds for either open or endovascular repair of TAAA are similar to that presented for repair of descending TAA (see Section 6.5.3, “Descending Thoracic Aortic Aneurysms”). All are single-institution series with longitudinal follow-up via surveillance imaging and detection of aortic-related events and death. Intervention at diameters of <6.0 cm would reduce aortic-related events and death. There are also conditions in which intervention may be justified at smaller diameters (eg, rapid growth, symptoms, penetrating ulcers, mycotic aneurysms, connective tissue disorders). Concerns for operative death in the setting of comorbid conditions is certainly justified. However, in centers with a Multidisciplinary Aortic Team, excellent outcomes can be obtained despite the presence of such conditions, and fatal aortic events may thus be avoided.
Recommendation-Specific Supportive Text
Aortic event rates begin to rise significantly, and 5-year survival begins to fall when TAAA diameters are >6.0 cm. At this diameter, the risk of an adverse aortic event ranges from 9.3%1 to 19%,3 which is 2 to 4 times the median operative mortality rate for open TAAA repair. In patients with multiple comorbidities known to substantially increase the risk of open TAAA repair (eg, chronic obstructive pulmonary disease, advanced age, preoperative renal dysfunction, preoperative left ventricular dysfunction), it may be appropriate to continue to observe patients with TAAA diameters >6.0 cm or to refer them for endovascular repair.
In centers with a Multidisciplinary Aortic Team, despite the presence of comorbid conditions, excellent outcomes can be achieved with meticulous perioperative preparation and care as well as technically sound surgery. On multivariable analysis, patients undergoing TAAA repair with a left ventricular ejection fraction <40% were not more prone to operative death (OR, 0.28; 95% CI, 0.02–4.14; P=0.58) or long-term death (OR, 0.55; 95% CI, 0.17–1.80; P=0.23) than those with higher ejection fractions.5 Similarly, carefully selected octogenarians undergoing open TAAA repair had a similar operative mortality rate as those <80 years of age (5.2% versus 5.7%; P=0.852).6
Certain clinical factors associated with an increased risk of TAAA rupture may prompt consideration of open or endovascular intervention at a diameter below the standard surgical thresholds. In patients with intact TAAA who are being observed with surveillance imaging, confirmed rapid aneurysm growth (≥0.5 cm/y) would suggest the need for intervention regardless of absolute diameter.4 Symptoms consistent with an enlarging TAAA that are not attributable to alternative pathology portend potential rupture and also suggest the need for surgery.7 Patients with symptoms secondary to either PAU or saccular aneurysms are also at a higher risk for rupture and should be considered for intervention regardless of absolute diameter.8
6.5.4.2. Open Versus Endovascular Repair of TAAA
Recommendations for Open Versus Endovascular Repair of TAAA
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| Ruptured TAAA | ||
| 1 | B-NR | 1. In patients with ruptured TAAA requiring intervention, open repair is recommended.1–5 |
| 2b | C-LD | 2. In patients with ruptured TAAA requiring intervention, provided that the patient is hemodynamically stable, endovascular repair may be reasonable in centers with endovascular expertise and access to appropriate endovascular stent grafts.6 |
| Intact TAAA | ||
| 1 | C-LD | 3. In patients with Marfan syndrome, Loeys-Dietz syndrome, or vascular Ehlers-Danlos syndrome and intact TAAA requiring intervention, open repair is recommended over endovascular repair.7–9 |
| 2b | B-NR | 4. In patients with intact degenerative TAAA and suitable anatomy, endovascular repair with fenestrated stent grafts, branched stent grafts, or both may be considered in centers with endovascular expertise and access to appropriate endovascular stent grafts.10–13 |
Synopsis
There are no RCTs comparing early or late outcomes for open versus endovascular repair for TAAA. As of November 2022, there are no FDA-approved devices for endovascular TAAA repair. Most of the endovascular procedures currently performed are done so with customized fenestrated or branched endografts on investigational device exemption- or industry-sponsored trials. Although the number of endovascular repairs performed has been steadily increasing, follow-up remains limited, and open repair therefore remains the preferred therapy for patients with TAAA who require intervention. The results for open repair are excellent in centers with a Multidisciplinary Aortic Team. In the largest series published to date, the operative mortality rate in 3 309 patients undergoing open TAAA repair was 7.5%, including >1 000 patients undergoing repair of an extent II aneurysm, with a low risk of aortic-related reintervention. Other high-volume centers have reported similar outcomes for open repair. In 1 center, the operative mortality rate in 783 patients was 5.6%, with a low risk of SCI of 2.0% and need for postoperative hemodialysis of 5.2%. Another center, whose operators used deep hypothermic circulatory arrest, reported an operative mortality rate of 6.8% with an SCI risk of <3% and postoperative hemodialysis risk of 2.2%.
Recommendation-Specific Supportive Text
In patients with ruptured TAAA, open repair can be performed with low mortality by surgeons in centers with a Multidisciplinary Aortic Team. In a series of 100 consecutive patients with ruptured TAAA, an operative mortality rate of 14% and an SCI rate of 5% was achieved.5 Although the study population was replete with comorbid conditions, the only risk factor remaining significant after propensity matching was “shock” on arrival to the hospital. Furthermore, 5-year actuarial survival was 47.5%. Centers experienced in complex endovascular repair may opt to use this technique. In a national registry of 140 ruptured descending aneurysms, the operative mortality rate (10%) was good, but there was a disappointingly high rate of stroke (14.7%), SCI (9.6%), and need for reintervention within 30 days (19.7%). At a median follow-up of 17 months, actuarial 5-year survival rate was 31.9%. These results were similar to those reported from a device registry.1,5 Although complex endovascular repair of intact TAAA has shown promise in experienced hands and in select centers, in the setting of TAAA rupture, the endovascular approach is hampered by patient instability and the need for customized grafts (which may take several weeks to manufacture). In addition, most of the reported series of endovascular repair of ruptured TAAA are small; larger series with longer-term follow-up will be necessary to delineate the role for endovascular repair in the setting of aortic rupture.
Endovascular repair requires sequential steps for successful stenting of side branches without the ability to achieve rapid control of hemorrhage. Therefore, the role of off-the-shelf branched repair has been limited in patients with ruptured aneurysms and hemodynamic instability. However, in higher-risk patients who present with symptomatic or contained ruptured aneurysms, are hemodynamically stable, and have suitable anatomy, endovascular repair with an off-the-shelf or modified device may be considered. Kolbel et al14 reported a mortality rate of 15% for symptomatic and 30% for ruptured TAAA treated by multibranch endovascular repair.
In patients with known or suspected connective tissue disorders, such as Marfan syndrome, Loeys-Dietz syndrome, or vascular Ehlers-Danlos syndrome, open repair is recommended. Operative mortality rate is lower than in the general population undergoing open TAAA repair, as is the incidence of major complications, such as stroke and SCI. Importantly, freedom from aortic reintervention is excellent, as is long-term survival. Conversely, data are lacking on complex endovascular repair of TAAA for patients with connective tissue disorders. A small study of 17 patients treated by fenestrated-branched endovascular aortic repair (FEVAR) had no mortality rate, 100% technical success, and 1 reintervention at mean follow-up of 34 months.6 Endovascular repair may be reasonable in patients who failed previous open repair or are considered high risk and have stent-grafts placed into synthetic landing zones, or when used as a bridge to open repair in patients with hemodynamic instability.
Single- and multi-institution series of physician-sponsored investigational device exempt trials have shown the promise of fenestrated and branched endovascular stent grafts. When performed by experienced surgeons, technical success may be achieved in a high percentage of cases (92%–99.6%) with low perioperative mortality rate. At 1-year follow-up imaging, branch vessel patency was also good (96%–98%) and, at 3 years, freedom from aortic-related death was 91% and overall survival 57%.15
6.5.4.3. TAAA Spinal Cord Protection
Recommendations for TAAA Spinal Cord Protection
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A | 1. In patients undergoing open TAAA repair who are at high risk for SCI, cerebrospinal fluid drainage is recommended to reduce the incidence of temporary SCI, permanent SCI, or both.1–7 |
| 1 | B-NR | 2. In patients who experience delayed spinal cord dysfunction after either open or endovascular TAAA repair, timely measures to optimize spinal cord perfusion and decrease intrathecal pressure are recommended (Table 20).1–4,8 |
Table 20.
Measures to Optimize Spinal Cord and End-Organ Perfusion
| Cardioversion for tachyarrhythmias |
| Insertion of cerebrospinal fluid drain |
| Increase mean arterial pressure to >100 mm Hg |
| Transfuse to a hemoglobin >10 g/dL |
| Volume resuscitation |
Synopsis
SCI is a devastating complication of open and endovascular thoracoabdominal aneurysm repair, with an incidence rate of 2% to 15%, depending on aneurysm extent and cause, underlying patient comorbidities, urgency of the procedure, and surgeon and center experience. Previous ACC/AHA guidelines did not address the issue other than to suggest higher-risk populations that might benefit from adjuncts to reduce the incidence of SCI.9 The 2014 European guidelines assigned cerebrospinal fluid drainage a I B recommendation to reduce the risk of SCI.10 However, data were limited at the time to support this recommendation, and an earlier RCT11 had not shown a benefit for cerebrospinal fluid drainage in TAAA repair. A more recent RCT did show a significant reduction in SCI for a cohort undergoing repair of extensive TAAA (extent I and extent II) when cerebrospinal fluid drainage was used. Additional nonrandomized data support this recommendation.
Delayed SCI may occur up to 2 weeks after surgery. This complication has a profound impact on short- and long-term outcomes.10,12 Early recognition and aggressive management of SCI can lead to a return of lower extremity function. The reinsertion of a cerebrospinal fluid drain is a key component to salvage lower extremity function. Additional therapies, such as volume loading, increasing mean arterial pressure, and maximizing oxygen delivery to the cord through transfusion or supplemental oxygen, are also critical.
Recommendation-Specific Supportive Text
TAAA repair remains a formidable undertaking regardless of whether open or endovascular repair is performed. SCI, with either paraparesis or paraplegia, may be temporary or permanent and has a profoundly negative impact on short- and long-term survival as well as quality of life after repair. Many techniques have been suggested to reduce the incidence of this significant complication. Intraoperative management ranges from deep hypothermic circulatory arrest to left heart bypass to a “clamp-and-sew” technique, and support exists for each approach. Similarly, intraoperative and postoperative spinal cord neuromonitoring is not widespread but has support that is institutionally based. Other interventions have been also advocated as intrathecal papaverine to enhance spinal cord protection.13 Cerebrospinal fluid drainage remains the only technique proven to reduce the incidence of perioperative SCI. In an RCT examining the incidence of SCI in patients undergoing high-risk extent I and II TAAA repair, cerebrospinal fluid drainage was associated with a significant reduction in SCI compared with those having surgery without cerebrospinal fluid drainage. Over the past decade, there are few centers performing open TAAA repair without the aid of cerebrospinal fluid drainage. Furthermore, patients undergoing endovascular repair requiring extensive descending thoracic aorta coverage or in the setting of a previous infrarenal aneurysm repair also benefit from cerebrospinal fluid drainage (nonrandomized).6
In patients undergoing open TAAA repair, delayed paraplegia may account for nearly 60% of all spinal cord deficits encountered. Despite having an intact neurologic examination immediately after the procedure, patients can experience these delayed deficits anytime in the first 2 weeks postoperatively. The reported incidence of delayed SCI is approximately 5%, nearly twice that of deficits recognized immediately after surgery. Delayed deficits usually present in the setting of a hemodynamic insult (atrial fibrillation, hypovolemia, hemorrhage, infection) and may be responsive to aggressive measures to optimize spinal cord perfusion (Table 20). Cerebrospinal fluid drainage immediately reduces intrathecal pressure and increases spinal cord perfusion pressure (spinal cord perfusion pressure equals mean arterial pressure minus spinal cord fluid pressure).8,12,14 A significant proportion (57%) of patients with late deficits experience an improvement in their neurologic examination, with 17% having complete resolution of their deficits.14 The operative mortality rate for those with persistent SCI is nearly 3-fold higher than for those who recover (38% versus 13%, respectively; P<0.001). In addition, 5-year survival is significantly worse (from 75% with a return of function to 28% without; P<0.001).14
6.5.4.4. TAAA Renal and Visceral Organ Protection
Recommendations for TAAA Renal and Visceral Organ Protection
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A | 1. In patients undergoing open repair of TAAA involving the renal arteries, cold blood or crystalloid renal perfusion is recommended to provide effective protection against renal injury.1–6 |
| 1 | B-NR | 2. In patients undergoing open or endovascular TAAA repair who have end-organ ischemia or significant stenoses from atherosclerotic visceral or renal artery disease, additional revascularization procedures are recommended.7 |
Synopsis
Postoperative renal dysfunction after open TAAA repair has a significantly negative impact on short- and long-term mortality as well as quality of life. Efforts to reduce renal injury during open TAAA repair include local organ hypothermia with either cold crystalloid or cold blood-based perfusate.
Recommendation-Specific Supportive Text
Renal dysfunction after TAAA repair is defined as a doubling of the creatinine or the need for hemodialysis. When this significant complication occurs, short- and long-term survival is compromised, and the incidence of postoperative respiratory failure, SCI, and cardiac complications increase. To identify methods to reduce the incidence of postoperative renal dysfunction, 2 RCTs were performed comparing cold crystalloid renal preservation to normothermic blood perfusate and, subsequently, cold blood perfusate. When compared with normothermic blood delivered into the renal arteries directly from the left heart bypass circuit, the delivery of cold crystalloid perfusate into the renal arteries during open TAAA repair resulted in a 3-fold reduction in the incidence of postoperative renal dysfunction.7 Subsequently, cold blood perfusate delivered to the renal arteries through occlusion or perfusion catheters was found to provide the same level of renal protection as cold crystalloid perfusate during open TAAA repair.5 The results of this second RCT provided surgeons with 2 options for renal protection when open TAAA repair requires renal artery reconstruction.
In patients with renal or visceral artery stenoses or ostial obstruction secondary to chronic or acute dissection flaps, end-organ perfusion may be compromised. Improvement in perfusion to the celiac axis, SMA, and both renal arteries may be achieved by bypass, endarterectomy, or balloon angioplasty and stent placement. Patency of target vessel revascularization strategies has been documented in small series of patients having open TAAA repair with a “debranching” technique and in those undergoing endovascular TAAA repair.
6.5.5. Abdominal Aortic Aneurysms
6.5.5.1. Access During Endovascular Repair of AAA
Recommendation for Access During Endovascular Repair of AAA
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
| COR | LOE | Recommendation |
|---|---|---|
| 1 | B-R | 1. In patients undergoing endovascular repair of AAA who have suitable common femoral artery anatomy, ultrasound-guided percutaneous access and closure is recommended over open cutdown to reduce operative time, blood loss, length of stay, time to wound healing, and pain.1,2 |
Synopsis
Increased availability of percutaneous closure devices and lower profile endovascular stent grafts have made ultrasound-guided percutaneous access and closure more feasible. Two RCTs and a large national retrospective review showed favorable outcomes from percutaneous common femoral artery access and closure such as reduced operative time, reduced blood loss, and improved patient-centered outcomes, such as reduced pain.
Recommendation-Specific Supportive Text
The PEVAR trial showed the noninferiority of total percutaneous access and closure for EVAR for those with suitable common femoral artery anatomy.3 In the PiERO (Percutaneous femoral access in Endovascular Repair versus Open femoral access) study, investigators evaluated whether ultrasound-guided percutaneous access via the common femoral artery decreased the risk of surgical site infections compared with cutdown. Although the incidence of surgical site infections was too low to produce a difference in outcomes, investigators found that, compared with open cutdown for access, groins accessed and closed percutaneously healed faster and patients reported less pain.1 Although the PEVAR trial did not require ultrasound-guided femoral access, it was routine in the PiERO trial. Furthermore, a multicenter observational study of common femoral artery access showed a significant decrease in groin hematomas with routine ultrasound-guided access.4 Lastly, in an extensive comparison of different closures using data from 13 087 patients in the Vascular Quality Initiative registry, there was a significantly higher rate of cardiac complications (OR, 1.5; 95% CI, 1.14–2.05) and 30-day mortality rate (OR, 1.56; 95% CI, 1.05–2.32)2 in those undergoing cutdown versus percutaneous access.2 Operative time, estimated blood loss, and length of stay were all significantly higher in those undergoing groin cutdowns compared with percutaneous access.
6.5.5.2. Repair of Ruptured AAA
Recommendations for Repair of Ruptured AAA
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-R | 1. In patients presenting with ruptured AAA who are hemodynamically stable, CT imaging is recommended to evaluate whether the AAA is amenable to endovascular repair.1–3 |
| 1 | B-R | 2. In patients presenting with ruptured AAA who have suitable anatomy, endovascular repair is recommended over open repair to reduce the risk of morbidity and mortality.1,4–6 |
| 2a | B-NR | 3. In patients undergoing endovascular repair for ruptured AAA, local anesthesia is preferred to general anesthesia to reduce risk of perioperative mortality.7–9 |
| 2a | C-LD | 4. In patients with ruptured AAA, permissive hypotension can be beneficial to decrease the rate of bleeding.1,3,10–12 |
Synopsis
The mortality rate from ruptured abdominal aortic aneurysms (rAAA) is estimated to be 80% to 90%, with most patients never reaching the hospital.13 For those who present to a hospital, the historical mortality rate for open repair was approximately 50%. With improved team organization, prompt diagnosis, and endovascular repair options, the mortality rate after repair for rAAA has been reported to be as low as 18.5% after instituting an endovascular repair-first strategy in at least 1 observational series.1 Initial randomized trials for endovascular repair for rAAA (rEVAR) versus open repair generally showed no early survival benefit. However, shortcomings of these trials raised questions about their applicability.2,14,15 Longer-term studies of rEVAR, such as 3-year results from the IMPROVE (Immediate Management of the Patient With Rupture: Open Versus Endovascular Repair) trial, showed late survival benefit from rEVAR over open repair. Many authors have evaluated institutional experience with using rEVAR in anatomically suitable candidates and aimed to improve the process of care for rAAA by adopting “rupture protocols” that include early imaging, permissive hypotension, endovascular balloon occlusion under fluoroscopy to reduce excessive bleeding, and a team-based organization to facilitate immediate transfer of patients to the operating room for prompt hemorrhage control and repair.1,3
Recommendation-Specific Supportive Text
The IMPROVE trial was the first trial to evaluate a new paradigm in evaluating rAAA.2 Specifically, patients who were hemodynamically stable were first transported to the radiology suite for CTA to assess whether their ruptured aneurysm was amenable to endovascular repair or required open repair. This is in contrast to a strategy of transport to the operating room for open surgery without preoperative imaging. The trial did not identify any increased risk of death from a strategy of acquiring preoperative imaging and, because of the different repair options available today, such assessments can help surgeons choose appropriate therapy based on patient aneurysm anatomy and clinical status. In contemporary practice, many patients will have a CT scan, although some of these scans will not be ideally timed arterial phase imaging. Given that time is of the essence in rAAA repair, if a patient’s CT scan provides enough anatomic information to identify whether endovascular repair is feasible, another more dedicated CTA scan may add unnecessary delays to the patient’s care.
Although 3 clinical trials aimed to evaluate potential survival benefit for rEVAR over open repair, none showed significant early benefit. However, trials excluded patients who were hemodynamically unstable, thus excluding patients that may have benefitted most from an endovascular approach. It should be noted, however, that the IMPROVE trial subsequently showed that between 90 days and 3 years, rEVAR had superior survival rates compared with open repair (hazard ratio, 0.57; 95% CI, 0.36–0.9).16 Contemporary observational studies showed significant survival benefit from an endovascular approach to rAAA. For example, Wang et al6 used propensity-matched data from the Vascular Quality Initiative registry and showed that rEVAR resulted in a lower 30-day mortality rate than open repair (21% versus 34%, respectively; P<0.001) and that mortality rates after rEVAR have been steadily decreasing since 2008. Other studies have corroborated this general decline in the rEVAR mortality rate and comparatively better postoperative outcomes.4,17 Newer endovascular devices have enabled treatments of rAAA that do not necessarily meet instructions for use criteria. However, caution should be exercised, because observational studies showed increased risk of perioperative death and long-term complications when devices are used off-label in a rupture scenario.18,19
Patients presenting with rAAA often maintain adequate BPs, in part because of the body’s catecholamine responses.20 However, once induced with general anesthesia, the loss of this physiologic response—coupled with anesthetic agents that can depress BP—can lead to circulatory collapse.21–23 General anesthesia has also been shown to have deleterious effects on inflammatory and body temperature regulation.24,25 Subanalysis of the IMPROVE trial showed that patients with rAAA who underwent EVAR with only local anesthesia had lower risk of mortality compared with those who were treated under general anesthesia (adjusted OR, 0.27; 95% CI, 0.1–0.7).7 Although the trial was not designed and powered for this specific outcome, recent observational studies from large registries have corroborated this finding.8,9
Although there are no RCTs of outcomes specific to permissive hypotension in rAAA, data from the trauma literature evaluating fluid management in hemorrhagic shock show benefit in using a strategy of permissive hypotension.11,12 Many authors managing rAAA have similarly described maintaining low arterial pressures to decrease rate of bleeding in patients with rAAA.1,3,10 An SBP that allows a patient to maintain mentation, typically between 60 and 90 mm Hg, is suggested.
6.5.5.3. Threshold for AAA Repair
Recommendations for the Threshold for AAA Repair
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A | 1. In patients with unruptured AAA, repair is recommended in those with a maximal aneurysm diameter of ≥5.5 cm in men or ≥5.0 cm in women.1–6 |
| 1 | B-NR | 2. In patients with unruptured AAA who have symptoms that are attributable to the aneurysm, repair is recommended to reduce the risk of rupture.7,8 |
| 2b | C-LD | 3. In patients with unruptured saccular AAA, intervention to reduce the risk of rupture may be reasonable.9 |
| 2b | C-LD | 4. In patients with unruptured AAA and aneurysm growth of ≥0.5 cm in 6 months, repair to reduce the risk of rupture may be reasonable.1–5 |
Synopsis
One of the most significant risk factors for continued aneurysm growth and rupture is the maximum diameter. Thresholds for AAA repair must balance the expected risk of rupture against the risk of operative intervention. Historically, the risk of rupture was reported to be 0.5% to 5% for aneurysms <5 cm in maximum diameter, 3% to 15% for aneurysms 5 cm to 6.9 cm, and ≥30% for aneurysms ≥8 cm.10 Multiple trials that are now >2 decades old evaluated the use of early repair of AAAs measuring 4.0 cm to 5.4 cm via open or endovascular means. All found no survival benefit attributable to early repair and but did find an increased risk of subsequent reintervention. These studies and others have found that rupture does occur at smaller diameters for women; thus, size thresholds for men and women differ to account for these observed differences.6,11 Newer data highlight other considerations, such as aortic indexing, which may better predict aneurysm rupture risk. Lastly, although limited data exist for the natural history of saccular AAAs, available data suggest that their morphologic features may make them more likely to become symptomatic, rupture at smaller diameters, or both than fusiform AAAs.
Recommendation-Specific Supportive Text
Clinical trials conducted in the late 1990s and early 2000s, including the UKSAT (UK Small Aneurysm Trial) and ADAM (Aneurysm Detection and Management) trial for early open aneurysm repair and CAESAR (Comparison of surveillance vs. Aortic Endografting for Small Aneurysm Repair) and PIVOTAL (Positive Impact of endoVascular Options for Treating Aneurysm earLy) trials for early endovascular repair, did not find a survival benefit for repair of aortic aneurysms measuring 4.0 cm to 5.4 cm.1–5 Although long-term outcomes in the UKSAT group seemed to show better survival rates in patients in the early open surgery group, this was thought to be attributable to higher rates of smoking cessation in the early surgery group compared with the surveillance group.2,3 Based on these data, balancing the risk of intervention versus the risk of rupture, a threshold of ≥5.5 cm is acceptable for men with infrarenal AAA. In the UKSAT study, which included more women than the previous studies, women were found to have higher rates of aneurysm rupture and higher rates of aneurysm-related deaths than men.2,3 The mean maximum aneurysm diameter at rupture was 5.0 cm in women and 6.0 cm in men. More recent data highlight a different method for quantifying aneurysm rupture risk by indexing aneurysm size to the BSA (ASI equals aneurysm diameter [cm]/BSA [m2]); in women, ASI has been shown to be more predictive of rupture risk than is maximum diameter.12 Further research will help clarify whether ASI is a better metric for aneurysm repair thresholds than maximum diameter.12
Approximately 6% to 22% of treated aneurysms are symptomatic but unruptured. Symptoms that are considered high risk for impending rupture include pain in the back, abdomen, or flank, and sometimes radiating to the groin, which is attributable to the AAA. Patients presenting with such symptoms should be admitted to an ICU for arterial BP monitoring, tight BP control, medical optimization, and AAA repair, ideally in 24 to 48 hours to reduce risk of free rupture. Other symptoms that warrant expedited, although not necessarily urgent AAA repair, include tenderness to palpation overlying the AAA in the abdomen, back, or flank, embolism (eg, blue toe syndrome) or compressive symptoms (eg, obstructive uropathy). Observational studies show that patients treated for symptomatic aneurysms have higher mortality and morbidity rates than those treated electively.7,8 Although timing of repair of symptomatic aneurysms remains controversial, most studies have reported outcomes of symptomatic aneurysms repaired during a patient’s index operation, with some studies finding that performing surgery on a nonemergency basis and potentially optimizing patient’s cardiorespiratory status during their hospitalization may be advantageous.8,13–15
Saccular AAAs are rare and, consequently, there are limited natural history data. In a Dutch registry of patients treated for fusiform and saccular AAAs, researchers found that saccular aneurysms appeared more common in women and were more likely to be symptomatic at smaller sizes than fusiform aneurysms.9 Of 7 659 patients with AAA, 6.1% had saccular AAA. Of patients with saccular aneurysms and acute presentation, 25% had diameters <5.5 cm, and 8.4% had diameters <4.5 cm. In contrast, only 8.1% and 0.6% of patients with fusiform AAA presenting acutely had diameters <5.5 cm and <4.5 cm, respectively. In their 2017 guidelines on AAA,16 the Society for Vascular Surgery recommended elective repair of patients presenting with saccular AAA, although size guidance is lacking because of limited natural history data. Clearly, the decision to intervene must be informed by the patient’s individual anatomy.
Pooled analysis from thousands of patients included in AAA surveillance studies from North America, Western Europe, and East Asia showed that, although aneurysm growth is highly variable, growth rates range from 1.5 mm/y to 2 mm/y for those with AAA of 3.0 cm to 3.9 cm and from 3.3 mm/y to 5.7 mm/y in AAA of 4.0 cm to 5.9 cm at baseline.17,18 The 4 major trials evaluating efficacy of early open and endovascular treatment of AAA for small aneurysms all excluded patients with aneurysms that grew ≥7 mm in 6 months or >10 mm in 12 months, given concern for increased risk of rupture. Thus, balancing the risks, aneurysms with size increases of ≥0.5 cm in 6 months or ≥1 cm in 1 year are considered to be rapidly growing and may warrant consideration of repair.
6.5.5.4. Open Versus Endovascular Repair of AAA
Recommendations for Open Versus Endovascular Repair of AAA
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A | 1. In patients with nonruptured AAA with low to moderate operative risk and who have anatomy suitable for either open or EVAR, a shared decision-making process weighing the risks and benefits of each approach is recommended.1–11 |
| 1 | B-NR | 2. In patients undergoing elective endovascular repair for nonruptured AAA, adherence to manufacturer’s instructions for use is recommended.12–16 |
| 2a | B-NR | 3. In patients with nonruptured AAA and a high perioperative risk, EVAR is reasonable to reduce the risk of 30-day morbidity, mortality, or both.9,10 |
| 2a | B-NR | 4. For patients with nonruptured AAA, a moderate to high perioperative risk, and anatomy suitable for an FDA-approved fenestrated endovascular device, endovascular repair is reasonable over open repair to reduce the risk of perioperative complications.10,11,17,18 |
Synopsis
Options for repair of AAA have substantially grown since the first description of open repair in 1952.19 In particular, EVAR has made it possible to treat patients who may have never qualified for open surgery because of significant cardiopulmonary comorbidities, renal comorbidities, or both. With the abundance of options, clinicians must remain informed regarding empirical data that may favor one approach over another in a particular patient and consider patient preferences for surgical options when data support either approach. Historic RCTs evaluating outcomes of EVAR versus open repair showed an initial survival advantage for EVAR that dissipates at different time intervals.1,3–8 Contemporary investigations have shown a steady decline in mortality rates for EVAR in general20 and a much larger perioperative survival benefit from EVAR versus open repair.9 However, similar to historic clinical trials, these survival benefits can dissipate over time and must be weighed against suboptimal surveillance that can occur in those treated with EVAR, leading to higher rates of late rupture and associated death.21 For repair of juxtarenal aneurysms using FDA-approved fenestrated devices, available data show similar findings (ie, an initial survival benefit that may wane over time).10,11
Recommendation-Specific Supportive Text
Pooled data from 7 RCTs evaluating all-cause death after EVAR versus open surgery for infrarenal AAA repair show that the risk of perioperative mortality is much lower in those treated with EVAR (OR, 0.36; 95% CI, 0.2–0.66). This advantage persists at 6 months, after which survival from both approaches become equivalent. Moreover, after 8 years, those treated with EVAR have a higher risk of aneurysm-related death (hazard ratio, 5.12; 95% CI, 1.6–16.4), secondary intervention (hazard ratio, 2.1; 95% CI, 1.7–2.7), aneurysm rupture (OR 5; 95% CI, 1.1–23.3), and death attributable to rupture (OR 3.6; 95% CI, 1.9–6.8) compared with open repair.22 Observational studies, such as the large propensity-matched study evaluating EVAR and open repair in a Medicare population, found that the survival advantage for EVAR lasted longer among older patients.9 For complex repairs, a similar survival advantage is seen for fenestrated repair over complex open repairs in the first 30 days after surgery. More data are necessary to identify longer-term outcomes and to determine for which groups one approach may be more advantageous. Given the current clinical equipoise, engaging the patient in a process of shared decision-making is recommended, as further detailed in Section 5, “Shared Decision-Making.”
Patient-specific anatomical characteristics of the aorta, such as neck diameter, length, and angulation, and iliac seal diameter, length, and vessel access, must all be considered in endovascular repair. Some observational studies show that treating aneurysms outside of the manufacturer’s instructions for use increases failure rates, resulting in increased risks of graft migration, endoleaks, late rupture, and deaths.12,13 For example, Shanzer et al12 found that in a multicenter retrospective study of >10 000 patients undergoing EVAR between 1999 and 2008, patients with AAA treated with devices off instructions for use had significantly higher rates of sac enlargement. More recently, Herman et al13 found that any deviation from instructions for use increased risk of graft-related adverse events (hazard ratio, 1.8; 95% CI, 1.05–3.1). A meta-analysis of 17 studies found that patients treated with non-instructions for use higher overall mortality rates (hazard ratio, 1.2; 95% CI, 1.02–1.42; P=0.03).14 Given these findings, in most patients, treating off instructions for use in elective AAA repair is discouraged. Those who have been treated off instructions for use warrant closer follow-up because of higher rates of failure from endoleaks, graft migration, and late rupture.
EVAR-2 (UK Endovascular Aneurysm Repair 2) was an RCT that evaluated outcomes of EVAR in high-risk patients. Patients were enrolled if they were determined to be unfit for open surgery, with fitness assessed using cardiac, respiratory, and renal criteria.23 In these patients, the trial initially showed that EVAR did not improve survival compared with the control of no intervention; however, more than a decade later, those treated with EVAR had significantly lower aneurysm-related mortality (hazard ratio, 0.55; 95% CI, 0.34–0.91).24,25 Contemporary analyses of outcomes in high-risk patients show that perioperative death after EVAR has markedly decreased (eg, 9% in EVAR-2 versus 1.9% in the ACS national registry).26 Furthermore, in evaluating a propensity-matched Medicare population, postoperative complications that are more likely to affect high-risk patients, such as myocardial infarction, pneumonia, acute renal failure, and need for dialysis, were all significantly less likely to occur after infrarenal EVAR compared with open repair.9 In assessing which patients are “high risk” for elective AAA repair, risk calculators derived using data from the Vascular Quality Initiative and the Vascular Study Group of New England can be helpful in informing discussions with patients about repair options and potentially identify patients for which even EVAR would be of prohibitively high risk.27–29
Recent observational studies aimed to compare outcomes between open and endovascular repair for complex aortic aneurysms. Using propensity score matching, investigators found that perioperative mortality rates between patients undergoing open repair or FEVAR were similar in those enrolled in the Vascular Quality Initiatives registry (4.7% versus 3.3%, respectively, P=0.17).17 Evaluating data from the ACS, Varkevisser et al found much higher odds of 30-day death from open repair compared with FEVAR (OR, 4.9; 95% CI, 1.4–19).10 The risk of immediate postoperative complications, such as myocardial infarction, acute kidney injury, and the initiation of dialysis, is significantly higher after open complex repair compared with FEVAR.11,17,18 However, rates of late reintervention are higher after FEVAR,11,18 as are the rates of persistent renal impairment11 and 3-year mortality rate (excluding perioperative deaths) (hazard ratio, 1.7; 95% CI, 1.1–2.6).17 Thus, similar to infrarenal repair, FEVAR may be most beneficial for the moderate- to high-risk surgical candidates who are more likely to experience perioperative complications.
6.5.5.5. Treatment of Concomitant Common Iliac Aneurysms
Recommendations for the Treatment of Concomitant Common Iliac Aneurysms
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. For patients with asymptomatic small AAA and concomitant common iliac artery aneurysm(s) ≥3.5 cm, elective repair of both abdominal and iliac aneurysms is recommended.1–4 |
| 1 | B-NR | 2. When treating common iliac artery aneurysms or ectasia as part of AAA repair, preservation of at least 1 hypogastric artery is recommended, if anatomically feasible, to decrease the risk of pelvic ischemia.5,6 |
Synopsis
The prevalence of common iliac artery aneurysms in the presence of AAA has been reported to be as high as 20% to 40% in surveillance studies.1,2 In patients with both aortic and iliac aneurysms, it is common for an iliac aneurysm to reach a size appropriate for elective repair before the AAA does. Although no randomized studies for iliac aneurysm repair size thresholds exist, in large case series and registry reports, rupture of iliac aneurysms at diameters <4 cm is rare.3,7 Thus, a repair threshold of 3.5 cm seems reasonable to balance procedural risks with rupture risk. Furthermore, to achieve adequate AAA repair, repair of iliac artery ectasia or aneurysms often may be required. Consideration of pelvic perfusion is of great importance when managing concomitant iliac disease. In such cases, there is a high risk of ischemic complications from exclusion of internal iliac arteries that can lead to buttock claudication, bowel ischemia, and erectile dysfunction.5,6 For some patients, adequate treatment of diseased iliac arteries cannot be accomplished without internal iliac artery sacrifice. Thus, individualized treatment plans with shared decision-making are important when treating aorto-iliac aneurysm disease.
Recommendation-Specific Supportive Text
In a large single-center case series by Huang et al,8 438 patients with common iliac artery aneurysms were observed for an average of 3.7 years. Eighty-six percent of patients had current or previously treated AAA. Common iliac artery aneurysms grew at an average rate of 2.9 mm/y, and no iliac aneurysm ≤3.8 cm ruptured. A multinational retrospective review of patients with internal iliac artery aneurysms found that 41.7% of individuals had a concomitant AAA. Of 63 patients, 1 patient presented with a ruptured internal iliac artery aneurysm of ≤3 cm, and 4 individuals’ iliac aneurysms ruptured at diameters ≤4 cm. Recently published data from the Dutch Surgical Aneurysm Audit showed that of the 857 patients with treated iliac artery aneurysms, the median iliac artery aneurysm size at elective repair was 4.3 cm, while ruptured iliac aneurysms had a median diameter of 6.8 cm at presentation.
In a meta-analysis of studies reporting exclusion or preservation of the internal iliac artery, Kouvelos et al5 found an increased pooled occurrence of buttock claudication in those undergoing unilateral (27%) or bilateral (36%) internal iliac artery exclusion. In a separate meta-analysis, Bosanquet et al6 found similar rates of buttock claudication, as well as a 10% occurrence of erectile dysfunction in men. Other ischemic events, such as spinal, bowel, and gluteal ischemia, were rare, occurring at a rate of <1%.6 Another consideration in treating aorto-iliac disease is the risk of late intervention from growth of ectatic or aneurysmal iliac arteries. In a retrospective analysis of prospectively collected data, Gibello et al4 found that in patients with AAA undergoing EVAR, after a mean follow-up of 6.2 years, those with common iliac arteries of ≥18 mm in diameter had a significantly higher rate of type Ib endoleaks (7.2% versus 3.2%; P=0.01) and late reinterventions (19% versus 11.8%; P=0.01), leading to higher odds of composite EVAR failure (OR, 1.8; 95% CI, 1.2–2.7) and need for reintervention (OR, 1.9; 95% CI, 1.15–3.3). Hassen-Khodja et al10 and Sala et al9 found that, after open repair of AAA, common iliac arteries of ≥18 mm in diameter tended to dilate over time, warranting consideration of bifurcated grafting rather than aorto-aortic tube grafting.9,10
6.5.6. Surveillance After Aneurysm Repair
6.5.6.1. Surveillance After TAA Repair
Recommendations for Surveillance After TAA Repair
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients treated with TEVAR, surveillance imaging with CT is recommended after 1 month and 12 months and, if stable, annually thereafter.1–5 |
| 2a | B-NR | 2. In patients treated with TEVAR, longitudinal surveillance with MRI is a reasonable alternative to CT for reduction of long-term radiation exposure or avoidance of an iodinated contrast allergy.6–9 |
| 2a | B-NR | 3. In patients treated with open repair of the thoracic aorta without residual aortopathy, suiveillance imaging with a CT or MRI within 1 year postoperatively and then every 5 years thereafter is reasonable.10–14 |
| 2a | C-EO | 4. In patients treated with open repair of the thoracic aorta who have residual aortopathy or abnormal findings on surveillance imaging, annual surveillance imaging is reasonable. |
Synopsis
The role of surveillance imaging after thoracic aneurysm repair is to identify complications of the repair or monitor for progression of residual aortic pathology. CT is generally the preferred imaging modality for surveillance imaging after TEVAR7,15; MRI, although generally more limited by metallic artifact, is a reasonable alternative. Open repair of the thoracic aorta is durable.2,5,10–14 In patients undergoing TEVAR, there is a higher incidence of complications and reintervention compared with patients undergoing open repair2,4,5,10–12; TEVAR complications can include endoleak (see Section 2.6, “Classification of Endoleaks”), retrograde type A aortic dissection, stent-graft migration, stent-graft fracture or collapse, and an increase in aortic size.6,7 Complications of open repair that can be detected by surveillance imaging include graft infection and anastomotic pseudoaneurysm.10,16 Additionally, after both open repair and TEVAR, patients may develop progressive aneurysmal dilation of adjacent or remote aortic segments.
Recommendation-Specific Supportive Text
Use of TEVAR is associated with reintervention rates ranging from 7% to 23%.1,2,4,5 In the Gore TAG study,17 there was an 11% incidence of endoleak18 at 30 days, 6% at 1 year, and 9% at 2-year follow-up after TEVAR.2,17 A 6-month follow-up study may be useful in detecting a delayed retrograde type A aortic dissection.
MRI has some advantages over CT, including the avoidance of ionizing radiation and iodinated intravenous contrast administration.7,8 However, MRI is limited by its higher cost, longer acquisition times, lower resolution, and limited visualization of metallic stent graft components and adjacent structures. MRI has a potential growing role, particularly in patients who are middle aged or younger, in whom the consequences of lifelong surveillance in terms of contrast-induced nephropathy and cumulative radiation dose should be considered.9
Open repair for any segment of the thoracic aorta has proven to be durable in extended follow-up.10,11,13,14,19 Treatment failure after open repair of either the proximal or distal thoracic aorta requiring reintervention ranges from 1% to 7% in long-term (10-year) follow-up.10–12 In patients without a genetic syndrome or residual aortopathy shown on a postoperative imaging, surveillance can be done at longer intervals.
The appropriate frequency surveillance imaging in the presence of abnormal findings has neither been studied nor validated but, in such cases, annual surveillance imaging is typical. Patients requiring reintervention have a higher incidence of HTAD.10,16
6.5.6.2. Surveillance After AAA Repair
Recommendations for Surveillance After AAA Repair
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with AAA treated with EVAR, baseline surveillance imaging with CT is recommended at 1 month postoperatively1,2; if there is no evidence of endoleak or sac enlargement, continued surveillance with duplex ultrasound at 12 months and then annually thereafter is recommended.1,3,4 |
| 2a | C-LD | 2. In patients with AAA treated with EVAR who are undergoing annual surveillance imaging duplex ultrasound, additional cross-sectional imaging with CT or MRI of the abdomen and pelvis every 5 years postoperatively is reasonable.5–8 |
| 2a | C-LD | 3. In patients with AAA treated with EVAR and abnormal findings (Table 21) on any surveillance duplex ultrasound, additional cross-sectional imaging with CT or MRI is reasonable.9 |
| 2a | C-LD | 4. In patients with AAA treated with complex EVAR, a modified surveillance imaging plan that combines cross-sectional imaging and duplex ultrasound of target vessels is reasonable.10,11 |
| 2a | C-LD | 5. In patients with AAA who have undergone open repair, surveillance imaging with CT or MRI of the abdominopelvic aorta within 1 year postoperatively and then every 5 years thereafter is reasonable.5,6 |
Table 21.
Abnormal Findings on Duplex Imaging After EVAR That Should Prompt Additional Imaging
| Aneurysm sac enlargement |
| Any endoleak |
| Stent graft fracture |
| Stent graft migration |
| Stent graft separation |
EVAR indicates endovascular abdominal aortic aneurysm repair.
Synopsis
The role of routine surveillance after EVAR is to identify endoleak, sac growth, endograft migration, or endograft failure. Although the initial surveillance intervals after EVAR were at 1 month, 6 months, and 12 months postoperatively to be consistent with surveillance imaging intervals used in FDA-sponsored device trials, more recent data suggest that the 6-month interval can be eliminated if no concerning findings are observed on the 1-month imaging (Table 21).1,2
CT is the gold standard for follow-up imaging after EVAR, but it is expensive, exposes the patient to ionizing radiation, and requires the use of iodinated contrast that is potentially nephrotoxic.12,13 Duplex ultrasound, with or without contrast enhancement, has been shown to be specific for the detection of endoleaks after EVAR9,14 and complex EVAR15; however, ultrasound is limited in its ability to detect stent migration, fracture, or noncontiguous aneurysms. MRI has high diagnostic accuracy for endoleaks16 but must be accompanied by a plain abdominal radiograph to assess for endograft stent fracture, because MRI cannot accurately visualize the metallic stent struts.
The role of routine surveillance after open AAA repair is to prevent late aneurysm rupture and aneurysm-related death by detecting para-anastomotic and new aneurysms. Para-anastomotic aneurysms can occur after open AAA repair as a result of anastomotic disruption, leading to pseudoaneurysm formation or progression of aneurysmal disease in the adjacent visceral aorta or iliac arteries.17 Patients with a history of AAA are also at risk of developing an aortic aneurysm in a noncontiguous location.18
Recommendation-Specific Supportive Text
The incidence of late aortic rupture after EVAR is >5% through 8 years of follow-up.3 Significant risk factors for rupture include endoleak with associated aneurysm sac enlargement.19,20 Endoleaks may be present for 10% to 17% of EVAR at 30 days postoperatively.1,2 In patients without early (30-day) endoleak, the incidence of new endoleak at 6 and 12 months postoperatively is similar.1 Earlier detection of an endoleak at 6 vs. 12 months is not associated with improved long-term outcomes.1,2
Stent graft fracture and migration is a long-term complication after EVAR that occurs in 3% to 4% of patients by 4 years postoperatively.7,8 Duplex ultrasound has been shown to be specific for the detection of endoleaks after EVAR9,14,15 but is limited in its ability to detect stent migration, fracture, or new noncontiguous aneurysms.
Duplex ultrasound is 95% accurate for measuring aortic aneurysm sac diameter and 100% specific for the detection of type I and type III endoleaks (Figure 11) after EVAR but is insufficient for detecting type II endoleaks9 or for characterizing anatomy related to stent graft migration or failure.
Duplex ultrasound has been shown to be a useful modality for surveillance of target branch vessels11 after FEVAR. However, complex EVAR involving stenting of ≥1of the renovisceral vessels is at higher risk of type III endoleak than standard EVAR10 and may benefit from routine cross-sectional imaging for surveillance of fenestration sites, branch junctions, and adequacy of flow in the renal and mesenteric arteries.21
Para-anastomotic aneurysms after open AAA repair tend to occur late, with estimated incidence rates of 1%, 6%, and 27% to 35% at 5, 10, and 15 years postoperatively, respectively.5,6 Late aortic aneurysms in noncontiguous arterial segments from the initial aortic repair have been reported in 45% at a mean of 7 years postoperatively.18 As a result, the Society for Vascular Surgery and the European Society of Cardiology have both recommended surveillance imaging every 5 years after open AAA repair.22 No data support the use of 1 cross-sectioning imaging modality over another for the surveillance of para-anastomotic aneurysms after open AAA repair.18
7. ACUTE AORTIC SYNDROMES
7.1. Presentation
AAS, although uncommon, are associated with life-threatening complications and a mortality rate as high as 1% to 2%/h if the AAS is not rapidly identified and appropriate therapy is not instituted promptly.1 The diagnosis of AAS can be challenging, however, because the presenting symptoms overlap with other more common emergency department complaints.Although the classic textbook description of AAS is of acute “tearing” or “ripping” pain, patients more commonly report the abrupt onset of severe “sharp” or “stabbing” pain in the chest or back (and sometimes abdomen), maximal at the start, that sometimes radiates.2–5 Depending on the extent of aortic involvement, patients may present with various additional signs and symptoms (Table 22). Recording a careful history of the presenting symptoms is essential, as is obtaining a detailed family history of TAAs, genetic aortopathies, aortic dissection, or unexplained sudden death.
Table 22.
Signs and Symptoms of AAS
| Clinical Signs and Symptoms | Cause |
|---|---|
| Asymmetric blood pressure (>20 mm Hg) between limbs | Compromise of branch artery flow |
| Bowel ischemia or gastrointestinal bleed | Malperfusion of the celiac or superior mesenteric artery |
| Dysphagia | Compression of the esophagus |
| Dyspnea | Compression of trachea or bronchus, congestive heart failure from aortic regurgitation, or cardiac tamponade |
| Hemoptysis | Vascular rupture into lung parenchyma |
| Hoarseness | Compression recurrent laryngeal nerve |
| Horner’s syndrome | Compression of sympathetic chain |
| Myocardial ischemia or myocardial infarction | Coronary artery involvement by dissection or compression by aneurysm |
| New murmur of aortic regurgitation | Incomplete aortic valve closure secondary to leaflet tethering by the dilated aorta or cusp prolapse because of dissection into the aortic root |
| Oliguria or hematuria (gross) | Malperfusion of 1 or both renal arteries |
| Paraplegia | Spinal malperfusion attributable intercostal artery involvement |
| Lower extremity ischemia | Malperfusion of iliac artery |
| Shock | Cardiac tamponade, hemothorax, frank aortic rupture, acute severe aortic regurgitation, severe myocardial ischemia |
| Shortness of breath | Pericardial effusion, congestive heart failure from acute severe aortic regurgitation, or hemothorax |
| Stroke symptoms | Carotid or vertebral artery involved |
| Superior vena cava syndrome | Compression of the superior vena cava |
| Syncope | Carotid artery involvement or cardiac tamponade |
AAS indicates acute aortic syndrome.
BP should be measured in both arms and both lower extremities, to exclude a BP differential resulting from an AAS. One should auscultate for the murmurs of aortic stenosis, perhaps indicating an underlying BAV, and AR, which commonly accompanies type A aortic dissection.
7.2. AAS: Diagnostic Evaluation (Imaging, Laboratory Testing)
Recommendations for AAS: Diagnostic Evaluation (Imaging, Laboratory Testing)
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In patients with a suspected AAS, CT is recommended for initial diagnostic imaging, given its wide availability, accuracy, and speed, as well as the extent of anatomic detail it provides.1–5 |
| 2a | C-LD | 2. In patients with a suspected AAS, TEE and MRI are reasonable alternatives for initial diagnostic imaging.1–6 |
Synopsis
A plain chest x-ray is neither sufficiently sensitive nor specific for AAS to be used to be diagnostic, but certain radiographic findings (Table 23) may raise suspicion of aortic dissection or suggest an alternate diagnosis for the patient’s symptoms, in particular when there is previous radiography that shows the changes to be new in the interval.1,2,7 Fortunately, CT, TEE, and MRI are all highly accurate for the diagnosis of AAS.3 Aortography is rarely used given its invasive nature and significantly lower sensitivity than the other imaging modalities.8 Acute aortic dissection risk scoring systems (eg, aortic dissection detection risk score [AAD-RS] or aorta simplified score [AORTAs]) can aid in the diagnostic evaluation of patients presenting with AAS (Table 24 and Table 25)5,9–12 but have not been uniformly adopted.4 No biomarkers are considered diagnostic, although in patients with a low previous probability of AAS a nonelevated D-dimer (<500 ng/mL) makes the diagnosis unlikely. Consequently, integrating a low aortic dissection risk score and a low D-dimer may be a useful strategy to exclude the diagnosis of AAS.13
Table 23.
Plain Chest X-Ray Suggestive of Aortic Dissection2
| Signs of Aortic Dissection on Chest X-Ray Findings |
|---|
| Mediastinal widening |
| Disruption of the normally distinct contour of the aortic knob |
| ”Calcium sign,” which appears as a separation of the intimal calcification from the aortic wall of >5 mm |
| Double density appearance within the aorta |
| Tracheal deviation to the right |
| Deviation of the nasogastric tube to the right |
Reprinted with permission from Strayer et al.2
Table 24.
| High-Risk Conditions | High-Risk Pain Features | High-Risk Examination Features |
|---|---|---|
| Marfan syndrome or other connective tissue disease Family history of aortic disease Known aortic valve disease Recent aortic manipulation Known thoracic aortic aneurysm |
Chest, back, or abdominal pain described as: Abrupt onset Severe in intensity Ripping or tearing in quality |
Pulse deficit or systolic blood pressure differential Focal neurologic deficit (with pain) Murmur of aortic regurgitation (new, with pain) Hypotension or shock state |
For each risk category, 1 point is assigned if ≥1 risk factors are present. Consequently, the total ADD-RS will range from 0 to 3. An ADD-RS of 0 points is low risk; 1 point is moderate risk; and 2 to 3 points is high risk. Adapted with permission from Hiratzka et al.5 Copyright 2010, American Heart Association, Inc., and American College of Cardiology Foundation.
Table 25.
Aorta Simplified Score (AORTAs)11 Pretest Probability Assessment Score
| Clinical Item | Points |
|---|---|
| Hypotension/shock | 2 |
| Aneurysm | 1 |
| Pulse deficit | 1 |
| Neurologic deficit | 1 |
| Severe pain | 1 |
| Sudden-onset pain | 1 |
The patient is given the number of points corresponding to each clinical item that is positive in the patient’s presentation. The points are summed, and a total score of 0 to 1 point is low-probability of aortic dissection, where a total of ≥2 points is high probability. Reprinted with permission from Morello et al.11
Recommendation-Specific Supportive Text
Although the sensitivity and specify of CT, MRI, and TEE are all high,3 CT has become the preferred modality for evaluating most patients with suspected AAS. CT is widely available at all hours in the emergency department and is quick to perform. Not only does it diagnose the underlying AAS, it also shows the full extent of the dissection and, in some cases, the entry tear site. CT can detect the presence and mechanism of aortic branch vessel involvement as well as vessel patency, signs of malperfusion, pericardial effusion and hemopericardium, periaortic or mediastinal hematoma, and pleural effusion. Use of electrocardiographic-synchronized CT techniques should be considered when there is a need to accurately depict mediastinal structures (eg, proximal aorta, coronary ostia). When IMH is present, the extent and thickness of the hematoma can be documented and, when PAUs are present, the presence of and size of pseudoaneurysms can be easily defined.
In general, the choice of the initial imaging modality should be based on the patient’s history and clinical presentation, the specific clinical questions to be answered, and the institutional availability, experience, and expertise with each of the diagnostic imaging techniques.6 In certain clinical circumstances, for example, patients with a history of an iodinated contrast reaction or patients who are too unstable to travel to the radiology suite, CT may not be preferred. Echocardiography (TEE/TTE) is an alternative. TTE is noninvasive, can be performed at the bedside, and may be helpful in eliciting the diagnosis of AAS and quickly identifying complications of AAS, such as AR or pericardial effusion and tamponade. TEE is preferred to TTE, however, because of its higher sensitivity and better anatomic resolution; TEE can be performed at the bedside in the emergency department or, alternatively, once the patient is in the operating room. MRI is most commonly the third-choice modality, given that it is not readily available, requires skilled interpretation, and has longer acquisition times, as well as the fact it is challenging to provide clinical care to potentially unstable patients while in an MRI scanner. Consequently, MRI is most often used as a follow-up imaging modality in patients in which there is diagnostic uncertainty. Nevertheless, MRI may be the study of choice in the acute setting for a stable patient with a contraindication to iodinated contrast.
7.3. Medical Management of AAS
7.3.1. Acute Medical Management of AAS
Recommendations for Acute Medical Management of AAS
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients presenting to the hospital with AAS, prompt treatment with anti-impulse therapy with invasive monitoring of BP with an arterial line in an ICU setting is recommended as initial treatment to decrease aortic wall stress.1–5 |
| 1 | C-LD | 2. Patients with AAS should be treated to an SBP <120 mm Hg or to lowest BP that maintains adequate end-organ perfusion, as well as to a target heart rate of 60 to 80 bpm.3,6 |
| 1 | B-NR | 3. In patients with AAS, initial management should include intravenous beta blockers, except in patients with contraindications.2,5,7 In those with contraindications or intolerance to beta blockers, initial management with an intravenous non-dihydropyridine calcium channel blocker is reasonable for heart rate control.1,2,5 |
| 2a | B-NR | |
| 1 | C-LD | 4. In patients with AAS, initial management should include intravenous vasodilators if the BP is not well controlled after initiation of intravenous beta-blocker therapy.8 |
| 1 | C-EO | 5. Patients with AAS should be treated with pain control, as needed, to help with hemodynamic management. |
Synopsis
Patients presenting with AAS need to be treated promptly to prevent acute and chronic complications. In all patients with AAS, immediate medical therapy is indicated while considering urgent surgical (in patients with type A aortic dissection), endovascular intervention (in patients with type B aortic dissection), or both; medical therapy includes aggressive heart rate and BP management as well as pain control. Studies have shown that, beyond surgical and endovascular treatment, medical therapy has an important role in decreasing long-term aorta-related adverse events.1,4,9–11 Beta blockers and intravenous vasodilators are the medications most commonly studied for the initial treatment of patients with AAS, with the goal of decreasing aortic wall stress.2,8 A recent large study showed that angiotensin-converting enzyme inhibitors (ACEIs) and ARBs are beneficial in the long-term management of hypertension in patients with aortic dissection.5 Statins are used routinely in patients after aortic dissection, although the evidence is not very robust.12
Recommendation-Specific Supportive Text
There are no randomized studies that have evaluated different medical treatments in the treatment of AAS, although extensive clinical experience has established the current standard of anti-impulse therapy. This is usually accomplished with a combination of intravenous beta blockers (eg, esmolol, metoprolol, and labetalol) and vasodilators (eg, nicardipine, clevidipine, and sodium nitroprusside) with the goal of reducing both heart rate and BP to decrease aortic wall stress.2–5,7,8,11
Small, single-center studies have highlighted the importance of reducing heart rate to 60 to 80 bpm and SBP to <120 mm Hg. Experts believe that the lowest BP that does not compromise end-organ function should be targeted.3,11
Intravenous beta blockers have been the mainstay of acute medical treatment, and studies reporting benefits over the long term and emphasizing the importance of continuing this therapy at the time of hospital discharge to improve clinical outcomes.1–3,5,7,9 Caution should be used in patients with contraindications to beta blockers (eg, acute AR, heart block, or bradycardia). In patients who are intolerant to beta blockers, intravenous non-dihydropyridine calcium channel blockers (ie, verapamil or diltiazem) are typically used for initial treatment.2
Intravenous vasodilators are useful adjunctive therapy for intravenous beta blockers but should be avoided as initial treatment (before starting beta blockers or calcium channel blockers), given the potential for compensatory tachycardia.8,9
Pain related to AAS can trigger a rise in heart rate and BP, so treating the pain symptoms can help to control the patient’s BP and heart rate. Intravenous opiates are particularly efficacious in this situation. Intravenous nonsteroidal anti-inflammatory drugs, such as ketorolac, may not be suitable because of the risk of inducing hypertension as well as adverse renal effects.
7.3.2. Subsequent Medical Management of AAS
Recommendation for Subsequent Medical Management of AAS
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
| COR | LOE | Recommendation |
|---|---|---|
| 1 | B-NR | 1. In patients with AAS, it is recommended to treat with long-term beta blockers (unless contraindicated) to control heart rate and BP to reduce late aortic-related adverse events.1–7 Additional antihypertensive agents (particularly ARBs and ACEIs) should be added, as necessary, to adequately control BP. |
Synopsis
Patients with AAS with surgical or endovascular treatment need continued and long-term medical management. Controlling hypertension has consistently been shown to decrease aorta-related adverse events. Recent studies have shown long-term benefit with specific BP agents such as beta blockers, ACEIs, and ARBs.
Recommendation Supporting Text
Long-term oral antihypertensive regimens that included beta blockers, ACEIs, and ARBs have shown to improve long-term outcomes in patients with AAS treated with both surgical and endovascular treatments.1–4 Although calcium channel blockers showed some benefit in patients with type B aortic dissection, further studies in mouse models of Marfan syndrome as well as case control studies in Marfan syndrome and other inherited aortopathy patients in the GenTAC (Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions) registry showed deleterious effects of long-term calcium channel blocker use and, consequently, it may be best to avoid these agents in patients with Marfan syndrome unless necessary to achieve BP control.8
7.4. Surgical and Endovascular Management of Acute Aortic Dissection
The primary goals of open surgical or endovascular stent-graft repair for acute aortic dissection are to prevent (or treat) aortic rupture, prevent retrograde extension of the dissection into the aortic root, prevent antegrade propagation of the dissection into distal yet undissected segments, and alleviate malperfusion syndromes. Acute aortic dissection management strategies are therefore “complication specific,” guided by the patient’s signs and symptoms, the presence or absence of complications, and the specific features and constraints of the patient’s aortic and branch vessel anatomy (Figure 21).
Figure 21. Acute Aortic Dissection: Malperfusion Treatment Options.
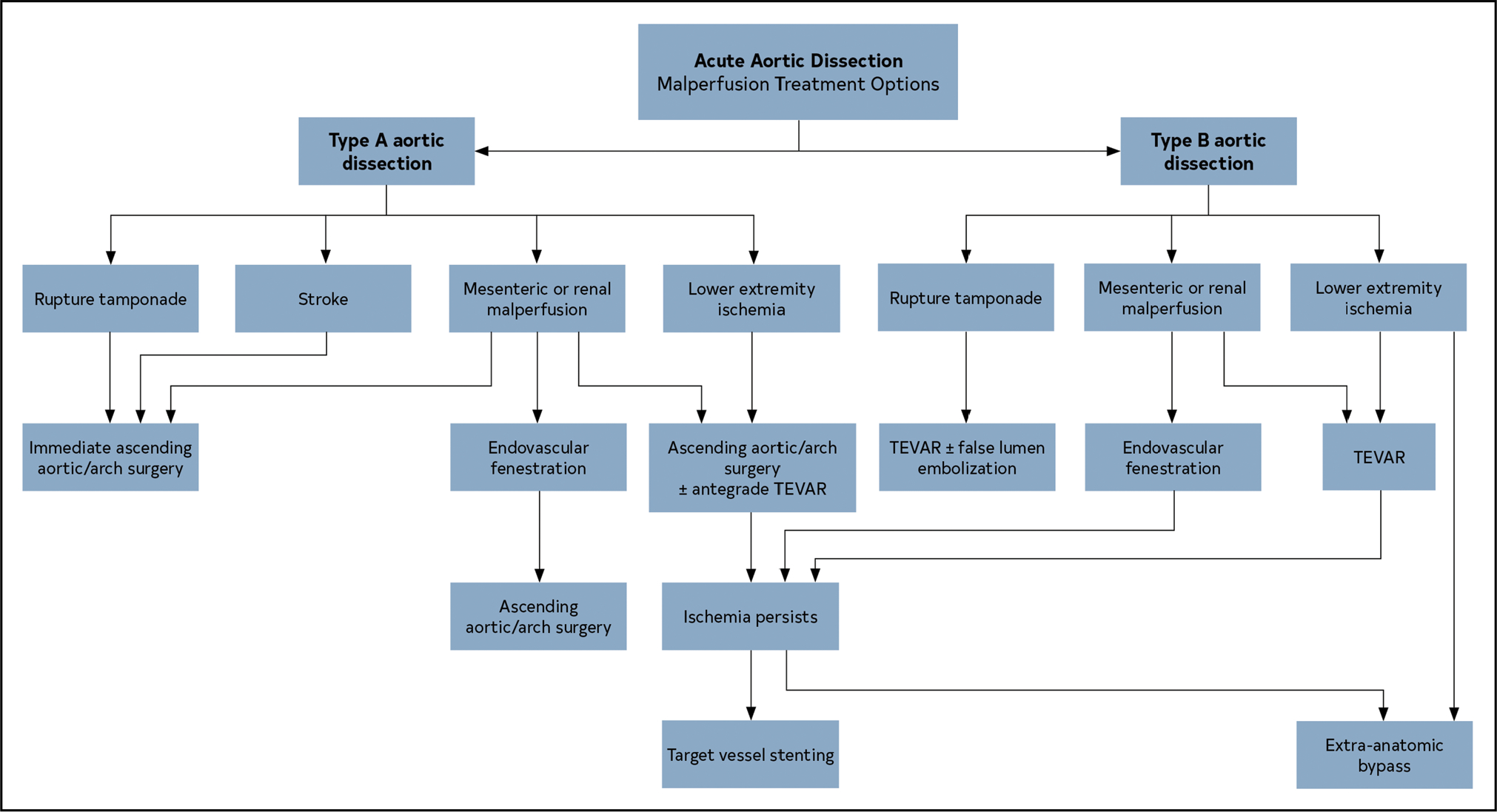
AoD indicates aortic dissection; and TEVAR, thoracic endovascular aortic repair.
7.4.1. Acute Type A Aortic Dissection
7.4.1.1. Initial Surgical Considerations in Acute Type A Aortic Dissection
Recommendations for Initial Surgical Considerations in Acute Type A Aortic Dissection
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients presenting with suspected or confirmed acute type A aortic dissection, emergency surgical consultation and evaluation and immediate surgical intervention is recommended because of the high risk of associated life-threatening complications.1,2 |
| 2a | B-NR | 2. In patients presenting with acute type A aortic dissection, who are stable enough for transfer, transfer from a low- to a high-volume aortic center is reasonable to improve survival.3,4 |
| 2a | B-NR | 3. In patients presenting with nonhemorrhagic stroke complicating acute type A aortic dissection, surgical intervention is reasonable over medical therapy to reduce mortality and improve neurologic outcomes.5,6 |
Synopsis
Acute type A aortic dissection is a life-threatening condition because of potential sequelae, including rupture that causes cardiac tamponade, acute severe AR that causes heart failure or shock, compromised coronary artery ostia causing myocardial ischemia, or malperfusion causing end-organ ischemia or infarction, all of which can all be fatal. Suspected or diagnosed acute type A aortic dissection warrants urgent surgical evaluation, because the mortality rate of medical management alone is 2 to 3 times that of surgical intervention.1 Data from IRAD showed that from 1995 to 2013, the surgical mortality rate decreased from 25% to 18%, while the medical mortality rate remained unchanged at 57%. Surgical intervention mitigates the immediate risk of aortic rupture/tamponade, corrects AR and myocardial ischemia, and reestablishes flow to malperfused vessels.
Nevertheless, the benefits of surgery must be weighed against the risks of the surgery itself (ie, a demanding, complex operation in patients who often are physiologically compromised). Universally recognized risk factors that increase the surgical mortality rate include shock and tamponade, neurologic or visceral malperfusion, and preoperative myocardial ischemia.7–9 Although age is a risk factor, elderly patients still benefit from surgery, with superior immediate and midterm outcomes compared with medical therapy.10,11 Short- and midterm outcomes can be equivalent to younger populations,12,13 with circulatory collapse being the primary predictor of long-term survival.14 In patients with significant contraindications to surgery, including frailty, clinical judgment may determine that the risk-benefit ratio favors medical management.
Recommendation-Specific Supportive Text
The potential sequelae of acute type A aortic dissection, including myocardial infarction, acute AR, cardiac tamponade, aortic rupture, and end-organ malperfusion, are associated with high rates of morbidity and mortality. Given the acuity, unpredictability, and finality of such events, immediate evaluation for surgical intervention is warranted to reverse any ongoing physiologic compromise and mitigate the risk of fatal events. The mortality rate of unoperated acute type A aortic dissection is 1%/h,15 and the time intervals between symptom onset, diagnosis, and surgery have a significant effect, with the highest mortality rate occurring in those undergoing surgery 8 to 12 hours after diagnosis.16 Patients presenting with clinical indicators of severe physiologic compromise (shock, neurologic deficits, malperfusion, myocardial ischemia) mandate the most immediate consideration for repair as the only potential option for survival.
Patients with acute type A aortic dissection who present with hemodynamic stability have an unpredictable course because of the inability to predict eventual rupture. Although some studies have suggested that night-time surgery is associated with a higher mortality rate,17,18 other studies have shown no diurnal difference in outcomes,19,20 and all studies have shown no difference with weekend surgery. Surgeon and center experience and resource availability should be considered to ensure optimal outcomes. Despite an inherent delay in the start time of surgery, transfer from low- to high-volume hospitals (one that performs ≥7 aortic root, ascending aorta, or transverse arch aortic dissection repairs per year),3 as part of regionalization of care, can result in significantly improved outcomes.3
In patients with cerebral malperfusion, survival is superior with surgery; in patients with acute type A aortic dissection and an acute stroke, the mortality rates of surgical versus medical management are 25% to 27% versus 76%, respectively.5,21 Even more strikingly, Estrera et al showed that patients with acute type A aortic dissection who had presented with stroke had an operative mortality rate of only 7% and showed no worsening of neurologic status postoperatively.6 Although their study and others,6,22 have emphasized the timeliness of the aortic repair in stroke patients, with a cutoff of ~5 to 10 hours (after which neurologic outcomes declined), Fischbein et al23 found no association between postoperative neurologic improvement and time from onset of neurologic symptoms to surgery. IRAD data revealed that cerebrovascular accident and coma resolved in 84% and 79% of patients, respectively, despite mean times to surgery of 12.3 and 13.8 hours, respectively.24 It should be noted, however, that in 1 recent report of 11 patients with acute type A aortic dissection and complete occlusion of an internal carotid artery, all died from cerebral edema and herniation, regardless of management25; consequently, this particular subset of patients may not benefit from surgical intervention.
7.4.1.2. Management of Malperfusion
Recommendations for Management of Malperfusion
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with acute type A aortic dissection presenting with renal, mesenteric, or lower extremity malperfusion, it is recommended to proceed to immediate operative repair of the ascending aorta.1,2 |
| 2a | C-LD | 2. In patients with acute type A aortic dissection presenting with clinically significant mesenteric (celiac, SMA) malperfusion, either immediate operative repair of the ascending aorta or immediate mesenteric revascularization via endovascular or open surgical intervention by those with this expertise before ascending aortic repair is reasonable.3–6 |
Synopsis
Imaging evidence of malperfusion is present in as many as 25% of patients with acute type A aortic dissection but should be distinguished from clinical evidence of end-organ ischemia, which is often referred to as malperfusion syndrome (Table 26). Malperfusion syndrome is associated with a mortality rate of 30.5%, compared with a mortality rate of only 6.2% in those without malperfusion syndrome.2 Mortality rate correlates with the number of branch artery vessels involved1 as well as the number of malperfused organs.7 The combination of pulse deficits (a marker of malperfusion) and hypotension should prompt timely interventions to reestablish vital organ perfusion, because early reperfusion predicts survival.8 The traditional approach to reestablish branch vessel perfusion has been via central aortic repair (ie, at the proximal aortic tear site). However, cardiac and visceral malperfusion portend extremely poor outcomes given the high mortality rate associated with irreversible organ damage. More recent series showed potential to improve outcomes by establishing end-organ perfusion using endovascular means, before open central aortic repair (with the timing of subsequent open repair decided on a case-by-case basis).5,8 These procedures may be performed in a hybrid operating room if the requisite resources and personnel are available.
Table 26.
Clinical Evidence of Malperfusion (“Malperfusion Syndrome”)
| End Organ | Clinical Findings |
|---|---|
| Cardiac | Electrocardiographic changes of ischemia or infarction, troponin elevation, myocardial dysfunction |
| Cerebral | Stroke and neurologic deficits, coma and altered mental status |
| Spinal | Paraplegia |
| Mesenteric | Abdominal pain, bowel ischemia, lactic acidosis, elevation of liver function test results |
| Renal | Acute kidney injury, oliguria |
| Extremity | Loss of pulses in ≥1 extremity, sensory or motor dysfunction |
Recommendation-Specific Supportive Text
In the presence of malperfusion, operative mortality rate correlates with the number of malperfused organs. Central aortic repair as the primary strategy to restore perfusion has reasonable results when renal malperfusion, extremity malperfusion, uncomplicated mesenteric malperfusion, or all of them is present.9 This strategy rapidly mitigates the risk of aortic rupture and corrects any associated coronary malperfusion, AR, and the sequelae of tamponade. After central aortic repair, any residual malperfusion should be assessed with secondary interventions, as needed.
Mesenteric malperfusion is one of the worst complications of acute type A aortic dissection, with an associated mortality rate of 63.2%.1 Consequently, such patients are often managed with medical therapy alone; yet, in IRAD, the nearly one-third of patients with mesenteric ischemia who were treated without intervention had an in-hospital mortality rate of 95%.10 For patients with acute type A aortic dissection who present with clinical evidence of mesenteric ischemia, some centers3,4 have advocated early direct reperfusion strategies (whether via endovascular or open abdominal surgery11), before central aortic repair; other centers continue to advocate for the traditional strategy of central aortic repair first.1,2 Currently, data are limited to help define the best strategy. In IRAD, a surgical and hybrid strategy appears to have superior outcome to medical or endovascular therapy alone. An institution series of endovascular therapy first showed a low aortic repair operative mortality rate of 2.1%; however, only 58% of the cohort underwent open repair, with 24% dying from organ failure and 13% from aortic rupture. Moreover, an endovascular therapy first approach requires expertise in fenestration, to treat dynamic obstruction, and branch stenting, to treat static malperfusion.5
7.4.1.3. Surgical Repair Strategies in Acute Type A Aortic Dissection
Recommendations for Surgical Repair Strategies in Acute Type A Aortic Dissection
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| Aortic Repair Strategies | ||
| 1 | B-NR | 1. In patients with acute type A aortic dissection and a partially dissected aortic root but no significant aortic valve leaflet pathology aortic valve resuspension is recommended over valve replacement.1–5 |
| 1 | B-NR | 2. In patients with acute type A aortic dissection who have extensive destruction of the aortic root, a root aneurysm, or a known genetic aortic disorder, aortic root replacement is recommended with a mechanical or biological valved conduit.6–9 In selected patients who are stable, valve-sparing root repair may be reasonable, when performed by experienced surgeons in a Multidisciplinary Aortic Team.10,11 |
| 2b | C-LD | |
| 1 | B-NR | 3. In patients with acute type A aortic dissection undergoing aortic repair, an open distal anastomosis is recommended to improve survival and increase false-lumen thrombosis rates.12–15 |
| 1 | B-NR | 4. In patients with acute type A aortic dissection without an intimal tear in the arch or a significant arch aneurysm, hemiarch repair is recommended over more extensive arch replacement.16–18 |
| 2b | C-LD | 5. In patients with acute type A aortic dissection and a dissection flap extending through the arch into the descending thoracic aorta, an extended aortic repair with antegrade stenting of the proximal descending thoracic aorta may be considered to treat malperfusion and reduce late distal aortic complications.19,20 |
| Perfusion and Cannulation Strategies | ||
| 2a | B-NR | 6. In patients with acute type A aortic dissection undergoing surgical repair, axillary cannulation, when feasible, is reasonable over femoral cannulation to reduce the risk of stroke or retrograde malperfusion.21,22 |
| 2a | B-NR | 7. In patients with acute type A aortic dissection undergoing surgical repair who require circulatory arrest, cerebral perfusion is reasonable to improve neurologic outcomes.23–25 |
| 2a | B-NR | 8. In patients with acute type A aortic dissection undergoing surgical repair, direct aortic26,27 or innominate artery28 cannulation with imaging guidance is reasonable as an alternative to femoral or axillary cannulation.29–31 |
Synopsis
To reduce the risk of late aortic complications, surgical resection should include the tear site, any aneurysmal aorta, and the proximal-most extent of the dissection. A nonresected primary tear is a risk factor for reoperation.32 A more extensive replacement that involves the aortic root, arch, or both adds operative complexity, ischemic time, and potentially circulatory arrest time but may reduce the risk of future aortic dilation, aortic insufficiency, or repeat dissection. An individualized approach to aortic root management is based on pathology and general condition. Younger patients are more likely to have proximal extension or root involvement and may have greater potential for late complications, given their longer life expectancy. VSRR has been described with excellent outcomes, but long-term reoperative risk is a concern.33
Similarly, untreated aortic arch or descending thoracic aortic tissue may be at risk of aneurysmal enlargement and the need for reintervention, particularly with acute type A aortic dissection that extends into the descending thoracic aorta. An open distal anastomosis allows direct arch inspection for intimal tears and resection of the lesser curve of the arch (ie, hemiarch technique) without increased operative death.12,13,34 In-hospital death is lower with hemiarch repair than with total arch replacement. Antegrade stenting of the proximal descending thoracic aorta may promote false-lumen thrombosis and positive remodeling,35–37 but long-term aortic-related data are scarce.
Involvement of the aortic arch by the aortic dissection can influence both interventional strategies and clinical outcomes. Various interventional approaches, such as extended open arch replacement (with or without a frozen elephant trunk),44 hybrid techniques, or endovascular stenting have been described.38–40 Aortic arch exclusion with emerging endovascular stents graft devices is a field in evolution.
Recommendation-Specific Supportive Text
Most single-center retrospective studies and an IRAD study found no difference in peri-operative mortality or survival when comparing root replacement with a more limited root repair or supracommissural replacement.2,5,7,41,42 However, a standardized and structured algorithmic approach showed a mortality rate of only 8.1% with aortic valve resuspension as the preferred approach, whenever feasible, compared with 23.1% with root replacement.43 Studies on freedom from reoperation are mixed,1,7,41,44–46 but 2 meta-analyses have shown excellent long-term durability of aortic valve resuspension, with reoperation rates 1.4% to 2.1% per patient-year and low thromboembolism and bleeding rates (1.4%/patient-year).3,4
An aneurysmal root at the time of acute type A aortic dissection repair is at long-term risk of progressive root dilation, secondary aortic insufficiency, and the need for reoperation. Specifically, an aortic root diameter of >4.5 cm has been shown to be a risk factor for late reintervention.6 A valved conduit is one option for root replacement but, if the aortic valve leaflet quality is good, the aortic insufficiency is primarily attributable to sinus dilation, and the surgeon is experienced in VSRR, a VSRR may be reasonable for younger patients.
In the development of the IRAD risk score, right hemiarch replacement was an independent predictor for a favorable surgical outcome.15 NORCAAD (Nordic Consortium for Acute Type A Aortic Dissection) found that the open-distal technique was associated with better short- and midterm survival than the clamp-on technique, although it was also associated with greater rates of cerebrovascular complications.12 Lawton et al14 found superior survival when all 3 components—no cross-clamp use, deep hypothermic circulatory arrest, and only antegrade perfusion after aortic perfusion—were used, compared with the absence of any of these components. Open distal anastomosis is also associated with higher rates of complete false-lumen thrombosis.13
Single-institution study findings that total arch replacement (TAR) is safe and promotes aortic remodeling47,48 have not been resulted in larger studies. GERAADA (German Registry for Acute Aortic Dissection Type A) found a trend toward lower mortality rates with hemiarch versus TAR (18.7% versus 25.7%; P=0.07); higher rates of excessive bleeding and rethoracotomy in the total arch group; and, in patients without preoperative neurologic deficits, lower mortality rates for hemiarch than TAR (14.1% versus 24%, respectively; P=0.02).49 A n STS database study of 12 years of acute type A aortic dissection repairs showed significantly lower operative death with hemiarch than with TAR (16% versus 27%; P<0.001).50 Two meta-analyses have found significantly lower mortality rates with partial compared with TAR.16,18 Across 3 meta-analyses, the long-term freedom from aortic reoperation does not appear to be necessarily superior with TAR.16–18
Comparative data on use of antegrade stenting of the descending thoracic aorta in the setting of surgical acute type A aortic dissection repair are limited. In several noncomparative meta-analyses, the mortality rate was ~8% to 12%, the stroke rate was 5% to 7%, and the SCI rate was 2% to 3.5%.36,51,52 False-lumen thrombosis rates appear favorable,35 but the reintervention rate was not zero, and the long-term benefit for aortic reoperation or aortic-related mortality remained undefined. In a series that included 19 patients with DeBakey type I acute type A aortic dissection and clinical malperfusion, antegrade stenting was associated with resolution of malperfusion in 16 patients (84.2%).19 In patients requiring total arch replacement, a frozen elephant trunk has higher adverse events in acute type A aortic dissection than in elective repairs. The stent length should be <15 cm and, to avoid SCI, coverage should not extend to T8.53
An STS database study50 and 2 meta-analyses21,22 have found an increased risk of stroke and short-term mortality with femoral compared with axillary cannulation. However, femoral cannulation is more expedient and is considered the primary arterial site in patients with hemodynamic instability mandating immediate cannulation, or with anatomic features precluding axillary cannulation. If initial femoral cannulation is required for these reasons, it is recommended to centrally canulate after the distal anastomosis has been completed, to maximize reestablishment of true lumen flow.
Some form of cerebral perfusion, whether antegrade or retrograde, has been shown to improve neurologic outcomes, when compared with deep hypothermic circulatory arrest alone.23 Antegrade cerebral perfusion is associated with both lower long-term mortality rates and neurologic dysfunction rates. Unilateral and bilateral antegrade cerebral perfusion appear to have similar outcomes, except in cases of prolonged circulatory arrest (>30–50 minutes), in which case bilateral antegrade cerebral perfusion may be advantageous.24,54–56
Several series for surgery for acute type A aortic dissection have reported direct aortic cannulation using a TEE-guided Seldinger technique. When performed correctly, this technique has the benefit of rapid establishment of cardiopulmonary bypass with true lumen flow. However, when the patient is stable, its safety relative to axillary cannulation is controversial,57 because stroke rates with this technique are as high as 20% in some series.29 Rosinski et al found that patients undergoing direct aortic cannulation were more hemodynamically unstable and had more extended repairs; however, even in multivariable logistic regression, it was associated with a higher risk of stroke (OR, 2.3; 95% CI, 1.05–5.1) Further, cerebral perfusion by some means other than axillary perfusion will be required for longer circulatory arrest cases. Innominate artery cannulation is another option that provides access for antegrade cerebral perfusion and appears to be safe in acute type A aortic dissection.58–60
7.4.2. Management of Acute Type B Aortic Dissection
Recommendations for the Management of Acute Type B Aortic Dissection
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In all patients with uncomplicated acute type B aortic dissection, medical therapy is recommended as the initial management strategy.1–3 |
| 1 | C-LD | 2. In patients with acute type B aortic dissection and rupture or other complications (Table 27), intervention is recommended.4–6 In patients with rupture, in the presence of suitable anatomy, endovascular stent grafting, rather than open surgical repair, is recommended. In patients with other complications, in the presence of suitable anatomy, the use of endovascular approaches, rather than open surgical repair, is reasonable.4–6,7 |
| 1 | C-EO | |
| 2a | C-LD | |
| 2b | B-R | 3. In patients with uncomplicated acute type B aortic dissection who have high-risk anatomic features (Table 28), endovascular management may be considered.8,9 |
Table 27.
Consensus Features of Complicated Acute Type B Aortic Dissection
| Feature | Comment |
|---|---|
| Aortic rupture1 | This can be either free or contained (including hemothorax, increasing periaortic hematoma, or both; or mediastinal hematoma) and should be addressed promptly. |
| Branch artery occlusion and malperfusion2 | Complete or partial occlusion of a major branch, with or without clinical evidence of ischemia; this includes visceral, renal, and peripheral arterial branches. |
| Extension of dissection3 | Extension of the dissection flap either distally or proximally (ie, retrograde type A dissection) |
| Aortic enlargement | Progressive enlargement of the true, false, or both lumens while in the acute phase may require prompt intervention. |
| Intractable pain15 | |
| Uncontrolled hypertension15 |
Table 28.
High-Risk Features in Uncomplicated Acute Type B Aortic Dissection9
| High-Risk Imaging Findings |
| Maximal aortic diameter >40 mm |
| False-lumen diameter >20–22 mm |
| Entry tear >10 mm |
| Entry tear on lesser curvature |
| Increase in total aortic diameter of >5 mm between serial imaging studies |
| Bloody pleural effusion |
| Imaging-only evidence of malperfusion |
| High-Risk Clinical Findings |
| Refractory hypertension despite >3 different classes of antihypertensive medications at maximal recommended or tolerated doses |
| Refractory pain persisting >12 h despite maximal recommended or tolerated doses |
| Need for readmission |
Synopsis
Although acute complicated type B aortic dissection historically has been treated with open repair, endovascular therapy has largely supplanted open repair given lower morbidity and mortality rates. Additionally, optimal medical management was associated with a 30-day mortality rate of 10% and midterm mortality rate of approximately 30%. The introduction of endovascular techniques has resulted in significantly lower morbidity and mortality rates when compared with optimal medical management, reported in small randomized trials including ADSORB (Acute Dissection: Stent graft OR Best medical therapy)8 and INSTEAD (Investigation of Stent Grafts in Patients with Type B Aortic Dissection)10; to date, there has not been a large RCT comparing open versus endovascular repair for either complicated or uncomplicated type B aortic dissection.
Recommendation-Specific Supportive Text
Those patients with type B aortic dissection generally have better survival than those with type A aortic dissection. In the acute uncomplicated setting, medical management is the first mode of therapy for type B aortic dissection. A review of the IRAD database showed overall in-hospital mortality rate of 13%, with those requiring open repair having higher mortality rates compared with those managed with optimal medical management and percutaneous intervention (32.1% versus 9.6% versus 6.5%, respectively; P<0.0001).1 Without intervention, risk factors for early death include shock, evidence of malperfusion, and age1–3 and can be grouped together with uncontrollable hypertension, pain, and continued growth or extension of the dissection as a complicated type B aortic dissection.
Patients presenting with complicated acute type B aortic dissection (Table 27), or developing such features after initial presentation, have an increased risk of morbidity and death, and urgent or emergency intervention may be required. For rupture, rapid coverage of the affected region of the descending aorta may be lifesaving and does not preclude subsequent further endovascular or open repair. This is an important consideration in those patients with genetically triggered aortic diseases (eg, Marfan syndrome, Loeys-Dietz syndrome). Cambria et al11 reviewed the outcomes for AAS managed with TEVAR compared with historic controls and found a 1-year survival rate of 79% for acute type B aortic dissection treated endovascularly. A subsequent single-arm study of patients treated with TEVAR found a 30-day mortality rate of 8% and 1-year survival rate of 88%.4 The VIRTUE Registry investigators5 found a benefit to early intervention but with reintervention rates of 20% to 39%. The RESTORE Patient Registry had similar results.6 Fenestration may be required if TEVAR alone does not correct the malperfusion, and visceral or renal artery stenting may also be required. When intervention is an emergency, TEVAR has a significantly lower morbidity and in-hospital mortality rates compared with open repair, with the greatest advantage among older patients.12
With medical management of uncomplicated type B aortic dissection still having a 30-day mortality rate of 10% and a decreased long-term survival, interest remains in determining if early endovascular intervention might reduce the risk of downstream complication or negative aortic remodeling, particularly in patients with high-risk features. In the ADSORB trial,8 which compared optimal medical management vs. optimal medical management plus TEVAR, there were no early deaths in either group and, at 1-year follow-up, there was just 1 death in the TEVAR group. TEVAR was superior to optimal medical management alone with significant differences in incomplete or no false-lumen thrombosis, aortic dilation, and rupture, but the primary clinical benefits are unknown. In the INSTEAD-XL (Investigation of Stent-grafts in Aortic Dissection) trial,13 in patients with uncomplicated type B aortic dissection, prophylactic TEVAR plus optimal medical was associated with improved 5-year aorta-specific survival and delayed disease progression. As the long-term mortality rate for type B aortic dissection that is managed medically and is strongly related to aortic events, the findings from the ADSORB and INSTEAD-XL trials appear promising, but larger trials with longer-term data are still needed. What remains unknown is the optimal timing for TEVAR.14 Features associated with an increased need for future intervention are summarized in Table 28.9
7.5. Management of IMH
Recommendations for Management of IMH
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with complicated (Table 29) acute type A or type B aortic IMH, urgent repair is recommended.1–3 |
| 1 | B-NR | 2. In patients with uncomplicated acute type A IMH, prompt open surgical repair is recommended.1,4–6 In selected patients with uncomplicated acute type A IMH who are at increased operative risk and do not have high-risk imaging features (Table 30), an initial or expectant approach of medical management may be considered.6–12 |
| 2b | C-LD | |
| 1 | B-NR | 3. In patients with uncomplicated acute type B IMH, medical therapy as the initial management strategy is recommended.1–3,13 |
| 2a | C-LD | 4. In patients with type B IMH who require repair of the distal aortic arch or descending thoracic aorta (zones 2–5) and have favorable anatomy, endovascular repair is reasonable when performed by surgeons with endovascular expertise.2,14 |
| 2a | C-LD | 5. In patients with type B IMH who require repair of the distal aortic arch or descending thoracic aorta (zones 2–5) and have unfavorable anatomy for endovascular repair, open surgical repair is reasonable.2,3 |
| 2b | C-LD | 6. In patients with uncomplicated type B IMH and high-risk imaging features (Table 30), intervention may be reasonable.13–16 |
Table 29.
Features of Complicated IMH
| Feature |
|---|
| Malperfusion |
| Periaortic hematoma |
| Pericardial effusion with cardiac tamponade |
| Persistent, refractory, or recurrent pain |
| Rupture |
IMH indicates intramural hematoma.
Table 30.
High-Risk Imaging Features of IMH
| For Type A IMH | For Type B IMH |
| Maximum aortic diameter >45–50 mm18,20 | Maximum aortic diameter >47–50 mm15,20 |
| Hematoma thickness ≥10 mm4 | Hematoma thickness ≥13 mm15 |
| Focal intimal disruption with ulcer-like projection involving ascending aorta or arch18,21 | Focal intimal disruption with ulcer-like projection involving the descending thoracic aorta if it develops in acute phase15,16 |
| Pericardial effusion on admission18 | Increasing or recurrent pleural effusion19,22 |
| For Both Type A and Type B IMH | |
| Progression to aortic dissection19 | |
| Increasing aortic diameter21,22 | |
| Increasing hematoma thickness21,22 | |
IMH indicates intramural hematoma.
Synopsis
Aortic IMH is a distinct pathologic entity from aortic dissection and PAU. It is characterized by hemorrhage within the media of the aortic wall and may occur with or without intimal disruption. Radiographically, IMH appears as a high-attenuation crescentic or circumferential thickening of the aorta on noncontrast imaging, with absence of blood flow through a false lumen on contrast imaging. IMH occurs more commonly in the descending thoracic aorta (60%) than in the ascending aorta (30%) or aortic arch (10%).1 Classification is the same as is used for acute aortic dissection. Symptoms at presentation are similar to aortic dissection, but patients tend to be older and more often have hypertension and atherosclerosis.1,2 Malperfusion can occur but less frequently than in aortic dissection.1,2 IMH can progress to aortic enlargement, aortic dissection, or aortic rupture; alternatively, the hematoma can sometimes be resorbed.3 Of patients presenting with AAS, the proportion who have IMH varies based by region, with reports of 6% to 23% in North America and Europe1,6 versus 26% to 44% in Asia.4,5,12
The management strategy for IMH balances patient comorbidities, the differing lethality of type A and type B IMH, mortality and death associated with open or endovascular repair in the different segments of the aorta, and the risk of aortic-related complications with medical management. Prospective randomized comparative studies are lacking, and most series and registries are limited by small sample sizes. For recommendations regarding management of IMH in association with PAU, see Section 7.6, “Management of Penetrating Atherosclerotic Ulcer (PAU).”
Recommendation-Specific Supportive Text
IMH, especially type A IMH, can be a lethal condition, complicated by rupture at presentation in 18% and, in that setting, associated with 100% mortality rate without surgical intervention.3 The features of complications of IMH are summarized in Table 29.
A nonoperative strategy for type A IMH is associated with a mortality rate as high as 40%, according to the findings of the IRAD.1 Progression to aortic dissection, rupture, or other aorta-related adverse events occurs in 14% to 37% of patients, with most events occurring within the acute or subacute phase.12,17,18 In-hospital mortality (1%–27%)1,4,6 and mid- and long-term survival4–6 after operative repair for type A IMH are reasonable and comparable to or better than survival rates reported for type A aortic dissection. There are varied approaches to timing of surgery, with low mortality rate achieved with strategies of repair within 24 hours5 and slightly delayed repair (between 24 and 72 hours), when feasible.6 The slight delay may confer an advantage by allowing the hematoma to form and the tissue quality of the aorta to improve. In addition, the extra time can allow for further diagnostic evaluation, optimization of comorbidities, or clearance of novel oral anticoagulant medications, which may improve outcomes. Delay is only reasonable in stable patients. The experience with successful medical management of type A IMH is mostly from Japan, Korea, and China, all reporting outcomes better than those reported in North America and Europe; the differences might be related to genetic or environmental factors that affect IMH natural history, so the Asian results may not be generalizable to other ethnic or geographic patient populations. The approach varies from initial medical management with planned “timely” (ranging from within a few days to before discharge) surgery to expectant medical management with surgical intervention only for complications or disease progression.7–12 One meta-analysis showed acceptable pooled proportion of an all-cause in-hospital mortality rate of 7% and 30-day mortality rate of 15%8 with initial medical management. Another meta-analysis, comparing upfront surgery to initial medical management with “timely” surgery, showed no significant difference in short-term survival (although an overall operative approach to type A IMH did show a survival benefit over medical therapy alone).9 For patients at increased operative risk (eg, advanced age, poor baseline renal function, coronary artery disease), medical management may therefore be an option. There are several high-risk imaging features (Table 30) that predict poor outcome (death, need for surgical intervention, or both) with this strategy. Shared decision-making with the patient should include discussion regarding need for an extended hospital stay of 2 to 3 weeks, including ≥3 days in the ICU on bedrest, with perhaps ≥5 CTAs during the hospitalization for close monitoring because of the dynamic disease process and moderate to high risk of progression to aortic dissection and rupture.
Type B IMH may have a more benign prognosis than type A IMH, resulting in relatively low in-hospital mortality rate (4%–6%) with medical management and 9% mortality rate at 1-year follow-up.1,2 A strategy of medical management for type B IMH with surgical intervention for severe recurrent symptoms or radiographic worsening on follow-up was associated with acceptable long-term survival.3 Intervention, whether surgical or endovascular, has associated mortality and morbidity. Although significantly less dissection and rupture may be observed during follow-up with TEVAR, compared with optimal medical therapy, this may17 or may not13,14 translate into improved aortic-related outcomes.
Literature supporting endovascular treatment of IMH is limited mainly to experience with TEVAR for IMH in the setting of PAU, or TEVAR for mixed type B AAS including IMH; the perioperative mortality rate for treatment of acute IMH ranges from 0% to 29%. Of note, in PAU with IMH or IMH with ulcer-like projection, endovascular treatment can be guided by the focal lesion. IMH with multiple ulcer-like projections may require more extensive treatment length. Favorable anatomy for TEVAR would include ideally normal aorta at both proximal and distal landing zones or, at least at the proximal landing zone, as outward tension of the stent graft transferred to abnormal aortic wall can lead to stent-induced new entry tear and subsequent aneurysmal degeneration or aortic dissection. In general, stent graft oversizing usually does not exceed 10%, and balloon aortoplasty at the landing zones is avoided. The experience with TEVAR for retrograde type A IMH associated with a distal intimal defect (ie, distal arch or descending thoracic aorta) is limited to small case reports and series. With the higher incidence of atherosclerotic disease in patients with IMH compared with aortic dissection, adequate vessel diameter for endovascular access should be determined as well.
In the IRAD experience for type B IMH, open surgical repair was performed in 5%, endovascular repair in 7%, and a hybrid approach in 1%, with no difference in results.1 Good outcomes have been reported for open repair,3,19 despite the more invasive approach. Open surgical repair may be preferable when IMH extends to the proximal landing zone of anticipated endovascular coverage, the aortic diameter at the proximal or distal extent of planned coverage is too large to accommodate existing stent graft sizes, the hematoma or aneurysm extends into the aortic arch and circulatory arrest would facilitate resection of diseased aorta, or endovascular access for stent deployment is anticipated to be inadequate.
High-risk imaging features may be present on admission or may develop in the acute, subacute, or chronic phases. Ulcer-like projections and focal intimal disruption (FID) are both terms that describe a focal outpouching of contrast arising from the lumen of the aorta in the setting of IMH with no associated atherosclerotic plaque. FID is more specifically defined by its communicating orifice measuring >3 mm, while tiny intimal disruption has a communicating orifice ≤3 mm.15 FID occurs in 32% of type B IMH and significantly predicts cardiovascular- or aorta-related death and aorta-related events,15,16,18 especially when it develops in the acute, rather than chronic, phase.15 Tiny intimal disruptions are lower risk and considered a benign finding.16 As 40% of patients can develop FID that was not present on the initial study,15 early surveillance imaging can help identify patients at risk for complications. Table 30 summarizes these and other high-risk imaging features of IMH.
7.6. Management of PAU
7.6.1. PAU With IMH, Rupture, or Both
Recommendations for PAU With IMH, Rupture, or Both
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with PAU of the aorta with rupture, urgent repair is recommended.1–3 |
| 1 | B-NR | 2. In patients with PAU of the ascending aorta with associated IMH, urgent repair is recommended.1–3 |
| 2a | C-LD | 3. In patients with PAU of the aortic arch or descending thoracic aorta with associated IMH, urgent repair is reasonable.1–3 |
| 2b | C-LD | 4. In patients with PAU of the abdominal aorta with associated IMH, urgent repair may be considered.4 |
Synopsis
A PAU is an atherosclerotic lesion of the aorta with ulceration that penetrates the internal elastic lamina and allows hematoma formation within the media of the aortic wall.5 PAUs may progress to AAS with IMH formation, aortic dissection, or rupture.1,2,6 PAU with IMH is associated with a high risk of short-term disease progression,1 particularly when localized to the ascending aorta (ie, Stanford type A).1,2 Data on outcomes for PAU with descending thoracic and abdominal aorta (ie, Stanford type B) IMH are limited to small retrospective reviews but suggest significant early disease progression among patients treated with medical management.1,2 PAUs tend to affect elderly patients with severe atherosclerotic disease and other comorbidities that put them at high surgical risk even with endovascular interventions, so the risk of repair must be weighed against the risk of severe morbidity and patient life expectancy when making decisions about appropriate management.
Recommendation-Specific Supportive Text
PAU with rupture that is not treated with intervention is associated with a high mortality rate (of 5 of 17 patients who presented with PAU with rupture who did not undergo repair, none survived).3 In contrast, in 1 small series, most patients with PAU with rupture treated by open or endovascular therapy survived to hospital discharge.1
PAU of the ascending aorta is uncommon; however, when it occurs, and in concert with IMH, the incidence rate of rupture is 33% to 75%,2,3 and progression to aortic dissection is associated with a high mortality rate.1
PAUs with type B IMH that are managed conservatively are associated with a high risk of disease progression to true aortic dissection or rupture.1,2 In a small retrospective analysis of patients presenting with PAUs and type B IMH, 3 of 17 patients (17.6%) who were managed conservatively died from progression of disease to aortic rupture at a mean of 9.3 days.1 In contrast, there was 1 death among 14 patients (7.1%) who underwent open (n=8) or endovascular (n=6) aortic repair for PAU with type B IMH.1 These data support early intervention of PAU in the setting of type B IMH in patients who are reasonable surgical candidates.
The natural history of PAU of the abdominal aorta with associated IMH is not well described, but low procedure-related and 30-day mortality rates have been described in several small series and case reports of both the endovascular and surgical treatment of abdominal aorta PAUs.7 In a literature review of 298 published cases of PAU affecting the abdominal aorta, most authors (62.0%) reported endovascular stent graft repair as the treatment of choice, followed by open surgical repair (35.4%) and conservative management (2.6%).7
7.6.2. Isolated PAU
Recommendations for Isolated PAU
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with isolated PAU who are symptomatic and have persistent pain that is clinically correlated with the radiologic findings, repair is recommended.1–3 |
| 2b | C-LD | 2. In patients with isolated PAU who are asymptomatic but have high-risk imaging features (Table 31), elective repair may be considered.1,2,4 |
Table 31.
High-Risk Imaging Features of PAUs
| Feature |
|---|
| Maximum PAU diameter ≥13–20 mm1 |
| Maximum PAU depth ≥10 mm1 |
| Significant growth of PAU diameter or depth |
| PAU associated with a saccular aneurysm5 |
| PAU with an increasing pleural effusion1 |
PAU indicates penetrating atherosclerotic ulcer.
Synopsis
Isolated PAUs are those without associated IMH, aortic dissection, or saccular aneurysm. Symptomatic isolated PAUs may herald a developing peri-ulcer hematoma, IMH, or both and are more likely to progress or result in rupture than asymptomatic PAUs.5 For patients who present with a symptomatic PAU but whose symptoms resolve with goal-directed therapy or patients who are poor operative candidates at increased risk for morbidity and death from repair, medical management has been pursued, with early and frequent surveillance imaging to assess for disease progression.4
Asymptomatic isolated PAUs are increasingly diagnosed incidentally because of the increasing use of CTA. Several series that reported mid or long-term outcomes of retrospective institutional data suggest that isolated PAUs have radiographic progression in up to 30% of patients.1–3,6,7 High-quality data evaluating thresholds for surgical repair are limited, but retrospective data have shown that PAUs with a diameter of ≥13 mm to 20 mm or depth of ≥10 mm (Figure 22) are closely associated with disease progression.1 Significant growth rates are not well defined and depend on the size of the patient, his or her aortic anatomy, and the presence of high-risk features associated with PAU (Table 31).
Figure 22. Dimensions of Penetrating Atherosclerotic Ulcers.
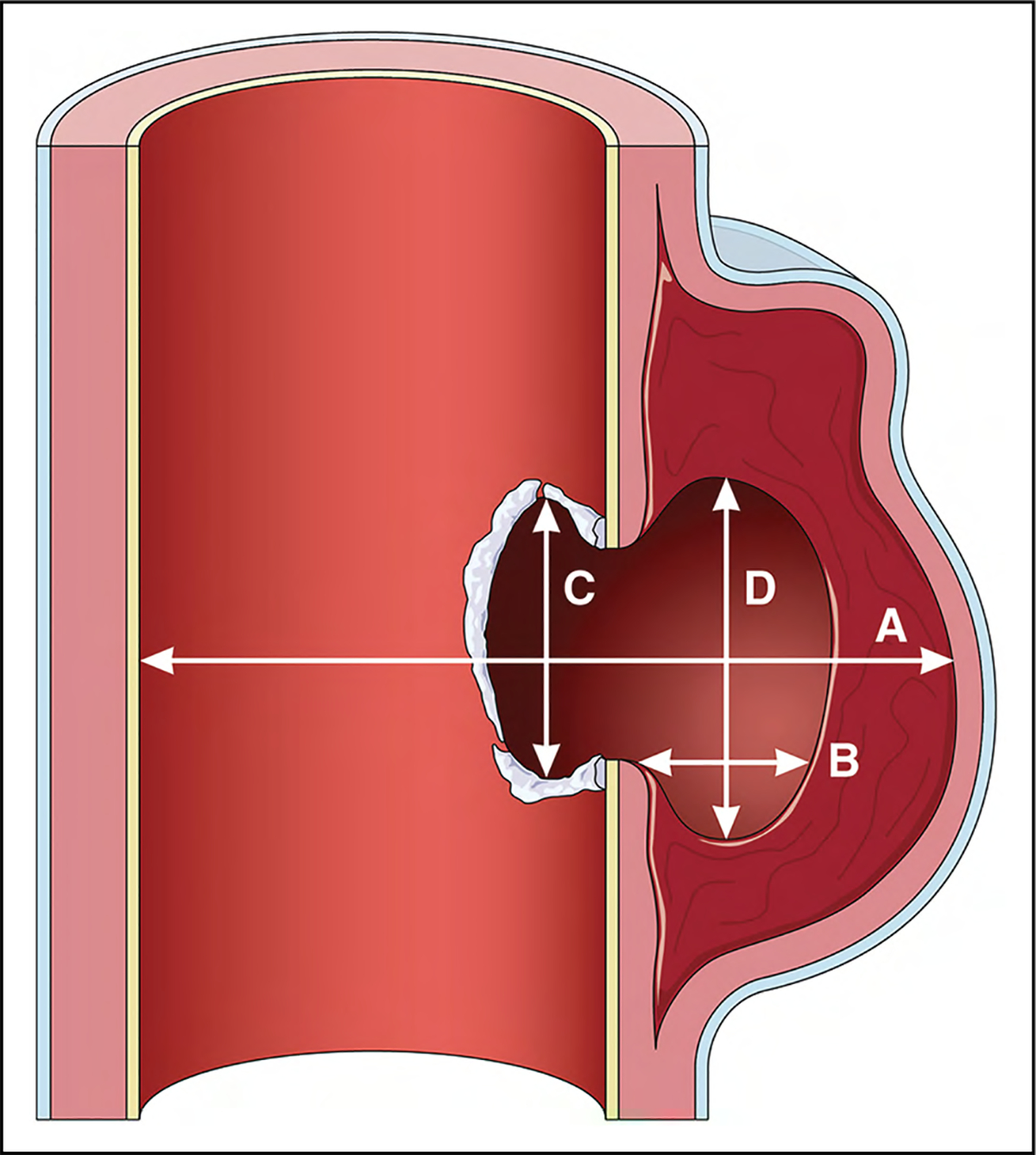
(A) Maximal aortic diameter at ulcer site diameter (from ulcer across to opposite aortic wall). (B) Depth of intramural blood pool. (C) Length of intimal defect at ulcer site. (D) Width of intramural blood pool. Adapted from Gifford et al,11 Copyright 2016, with permission from Elsevier, Inc., and from Cho et al,9 Copyright 2004 with permission from the Society for Vascular Surgery.
Recommendation-Specific Supportive Text
Symptomatic PAUs are associated with a high risk of early disease progression.2,3,5,7,8 In a small series of 25 patients presenting with symptomatic PAU managed medically, 30% had disease progression on surveillance imaging at a mean of 18 months follow-up, including expansion of the PAU and new IMH in 20% and conversion to aortic dissection in 10%.3 All patients in the series went on to require operative repair. In contemporary series, most patients with symptomatic PAU without rupture have been treated with open or endovascular repair with acceptable results (see Section 7.6.3, “PAU: Open Surgical Repair Versus Endovascular Repair”).3,7–9
Asymptomatic isolated PAU with large diameter or depth, significant growth on surveillance imaging, or other high-risk features (Table 31), are associated with disease progression.1,7 In contrast, incidental aortic PAUs that are asymptomatic and without high-risk features have a low risk of progression (3.6% and 6.5% at 5 and 10 years after diagnosis, respectively).10 Maximum depth and diameter of the PAU can be used to determine lesions that would be considered high risk and may be considered for intervention (Figure 22).4
7.6.3. PAU Open Surgical Repair Versus Endovascular Repair
Recommendations for PAU Open Surgical Repair Versus Endovascular Repair
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In patients who require repair of a PAU in the ascending aorta or proximal aortic arch (zones 0–1), open surgical repair is recommended. |
| 2a | C-LD | 2. In patients who require repair of a PAU in the distal aortic arch (zones 2–3), descending thoracic aorta, or abdominal aorta, either open surgical repair1–3 or endovascular repair is reasonable, based on anatomy and medical comorbidities.4–6 |
Synopsis
Operative repair of PAUs includes both open and endovascular treatment. Historically, most PAUs were treated with open aortic replacement, although more contemporary series have reported good technical success and short- and midterm outcomes after endovascular repair in the descending and infrarenal aorta.4–7 Comparative data are limited about the best treatment approach for a PAU but, in general, the approach depends on the location of the PAU, the patient’s aortic and branch vessel anatomy, associated pathology, and patient comorbidities (because these patients tend to be older and have significant atherosclerosis).4 Procedure-related and in-hospital death are lower for patients treated with an endovascular approach, although available data are based on small studies with a high risk of treatment bias.4 Midterm outcomes after endovascular repair of PAU have shown a 4% to 8% risk of endoleak4,7 and a 5% risk of new PAU formation.7 One-year mortality rates for patients treated with endovascular versus open repair are similar.7
Results of open surgical repair in patients with ascending aortic PAU are limited to small case series.8–11 Despite this, open repair remains the gold standard for treating AAS that involve the ascending aorta and proximal arch, with acceptable morbidity and mortality rates compared with medical therapy.12,13
Recommendation-Specific Supportive Text
Results of open surgical repair of the ascending aorta and proximal arch can be reasonably applied to PAU. Cases have been reported in which ascending aortic stenting has been performed with surgeon-modified stent-grafts or off-label use of commercially available devices, but currently there is no FDA-approved device for endovascular repair of the ascending aorta or proximal arch.
The risk of procedure-related and in-hospital death is lower for endovascular compared with open repair of PAU in the descending thoracic and abdominal aorta, although longer-term data are similar for both operative approaches.4
7.7. Traumatic Aortic Injury
7.7.1. Initial Management of Blunt Traumatic Thoracic Aortic Injury (BTTAI)
7.7.1.1. Initial Management of BTTAI in the Emergency Department
Recommendations for Initial Management of BTTAI in the Emergency Department
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. In patients with BTTAI, management and treatment at a trauma center with the facilities and expertise to treat aortic pathology is recommended. |
| 1 | C-LD | 2. In patients with BTTAI, anti-impulse therapy to reduce the risk of injury extension and rupture should be implemented, except in patients with hypotension or hypovolemic shock.1,2 |
Synopsis
BTTAI, although rare, is the second-most common cause of death in trauma patients; it results from high deceleration forces and is often associated with concomitant injuries. In the ACS National Trauma Databank, the diagnosis of BTTAI increased 196.8% from 2003 to 2013, likely attributable to more sensitive imaging.3 The mortality rate of patients with BTTAI who were treated in the emergency department was ~19%.4,5 Initial management of polytrauma at trauma centers follows Advanced Trauma Life Support protocols. However, for patients with BTTAI, special attention to BP and heart rate is warranted because of their effects on injury extension and rupture.
In stable patients, the 2011 Society for Vascular Surgery clinical practice guidelines6 suggested urgent (<24 h) repair barring other serious concomitant nonaortic injuries or immediately after treatment of other injuries. Optimal timing of intervention, however, remains unclear. In a recent study from the National Trauma Data Bank, early (<24 h) repair had increased odds of death (adjusted OR, 2.39; 95% CI, 1.01–5.67; P=0.047).7 A multicenter study showed worse adjusted mortality rate with early repair overall (adjusted OR, 7.78; 95% CI, 1.69–35.70; adjusted P=0.008) although, in subgroup analysis, mortality rate differences only trended toward favoring delayed repair (P>0.05).8
Recommendation-Specific Supportive Text
Patients with BTTAI are at elevated risk of aortic-related and overall mortality. Because of the acuity of injury and severity of concomitant polytrauma, expertise in aortic imaging and treatment at the treating facility is paramount to improve outcomes. Further, most patients will benefit from care at a level 1 trauma center with multidisciplinary expertise in treating concomitant injuries, although the risk of delayed treatment because of transport time must be weighed against the benefits of immediate treatment.
Although no randomized trials exist, historical literature shows that aortic rupture occurs in ~12% of patients with BTTAI who were awaiting repair without medical management; small studies using protocols of beta blockers as first-line therapy have reported rates of 0% rupture while awaiting repair.1,2 In the acute trauma setting, hypovolemia may result in permissive hypotension, obviating the need for administering anti-impulse medications (typically intravenous beta blockers with or without supplemental intravenous vasodilators [eg, nicardipine, clevidipine, sodium nitroprusside]) to decrease aortic wall stress. Conversely, permissive hypotension may not be tolerated with other concomitant injuries, in which adequate end-organ perfusion requires higher BPs.
7.7.1.2. Approach to the Initial Management of BTTAI
Recommendations for Approach to the Initial Management of BTTAI
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In patients with grade 1 BTTAI (Figure 23), nonoperative management and follow-up imaging are recommended.1,2 |
| 1 | C-LD | 2. In patients with grade 3 to 4 BTTAI (Figure 23) and nonprohibitive comorbidities or injuries, aortic intervention is recommended.1,3 |
| 2a | C-LD | 3. In patients with grade 2 BTTAI (Figure 23) and with high-risk imaging features (Table 32), aortic intervention is reasonable.3,4 |
| 2b | C-LD | 4. In patients with grade 2 BTTAI (Figure 23) and without high-risk imaging features (Table 32), nonoperative management and follow-up surveillance imaging may be reasonable.3,4 |
Figure 23. Classification System for BTTAIs.
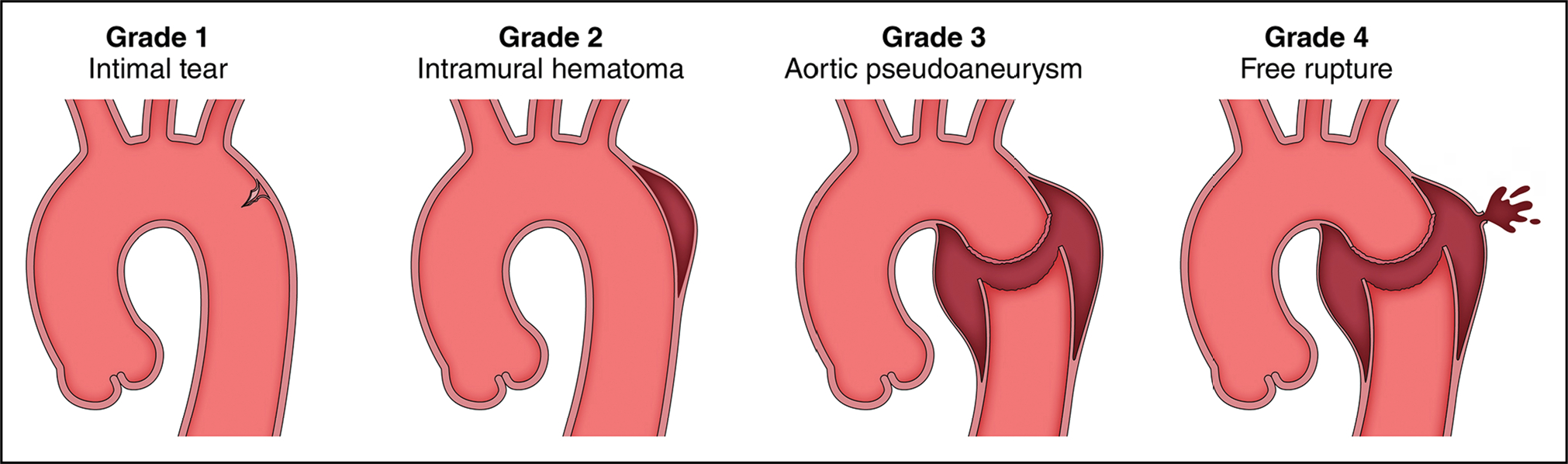
Aortic injuries are classified according to severity, based on the findings of diagnostic imaging. Grade 1, intimal tear. intimal flap, or both. Grade 2, intramural hematoma. Grade 3, aortic wall disruption with pseudoaneurysm. Grade 4, aortic wall disruption with free rupture. BTTAI indicates blunt traumatic thoracic aortic injury. Adapted from Azizzadeh et al,2 Copyright 2009, with permission from Elsevier, Inc. and the Society for Vascular Surgery.
Table 32.
High-Risk Imaging Features of BTTAI
| Posterior mediastinal hematoma >10 mm8 |
| Lesion to normal aortic diameter ratio >1.48 |
| Mediastinal hematoma causing mass effect6 |
| Pseudocoarctation of the aorta6 |
| Large left hemothorax6 |
| Ascending aortic, aortic arch, or great vessel involvement9 |
| Aortic arch hematoma7 |
BTTAI indicates blunt traumatic thoracic aortic injury.
Synopsis
The most common site of BTTAI is the aortic isthmus, because of its site as transition from the unfixed aortic arch to the fixed descending thoracic aorta and the relatively lesser tensile strength of this region. Other segments that may be involved include the proximal ascending aorta (8%–27%), aortic arch (8%–18%), and distal descending thoracic aorta (11%–21%). The most widely used grading scale is that proposed by Estrera et al and endorsed by the SVS clinical practice guideline (Figure 23).1,2 In Estrera’s original paper, all patients with grade 1 injuries were managed medically and had a 0% mortality rate.2 Current SVS guidelines recommend expectant management of grade 1 injuries and repair of all other grades.1 Trauma studies have found that 32% of BTTAIs are managed nonoperatively,3 with an associated mortality rate of 25%.5 Overall mortality rate was significantly higher in nonoperatively managed patients (35.0% versus 11.2%; P<0.001), while aortic-related mortality rate was similar (9.8% versus 5.0%; P=0.119).3
Recommendation-Specific Supportive Text: Management
The decision for nonoperative versus operative management of BTTAI includes complex and dynamic factors such as the patient’s stability, concomitant injuries, and potential imaging characteristics that may predict aortic stability. Grade 1 BTTAIs are likely to resolve and are associated with extremely low aortic-related death. Medical management and follow-up imaging to ensure resolution is appropriate.1,2
Grade 3 and 4 BTTAIs are at high risk of progression and rupture and should be treated in an urgent manner. In grade 3 injuries, nonoperative management was an independent predictor of all-cause death (OR, 29.65; 95% CI, 5.62–15.649; P<0.0001), and imaging characteristics did not predict aortic-related death.4
Although injury grade was an independent predictor of aortic-related death, outcomes of grade 1 and 2 injuries were similar between nonoperative management and TEVAR, including in-hospital and aortic-related death (P>0.05).3 A high-volume center reported no differences in mortality rates or aortic-related mortality rates between nonoperative and operative management of grade 1 and 2 injuries.4
Findings of secondary signs of injury and multiple secondary signs are more common in patients with higher-grade of aortic injury and may prompt stronger consideration for operative intervention.6 The presence of aortic arch hematoma of >15 mm in thickness was predictive of death.7
7.7.1.3. Endovascular Versus Open Surgical Repair
Recommendation for Endovascular Versus Open Surgical Repair
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
Synopsis
Endovascular therapy for BTTAI has become the predominant approach. From 2007 to 2015, rates of open repair decreased from 7.5% to 1.9%, while rates of TEVAR increased from 12.1% to 25.7%.2
No randomized trials for open versus endovascular management have been conducted.4 Rather, trauma registry data and meta-analyses have shown that, in patients with suitable anatomy, TEVAR offers superior 30-day mortality rates and lower rates of SCI and acute kidney injury. Concomitant injuries may prompt concern over procedural use of heparin, and the use of periprocedural heparin should be balanced against the overall bleeding risk for each patient. In a small study of TEVAR in patients with predominantly grade 3 BTTAI, there were no differences in bleeding, thromboembolism, or mortality rates between use of full heparin, low-dose heparin, and no heparin, although patients who received full heparin underwent repair at a time interval 3 times longer than did those who received no heparin.5
Recommendation-Specific Supportive Text
Compared with open repair, endovascular treatment of BTTAI is associated with improved procedural and 30-day mortality rates, as well as postoperative complications, including SCI and acute kidney injury.1–3,6 In a meta-analysis of 17 retrospective studies, TEVAR was associated with lower procedural and 30-day mortality rates (OR, 0.31 and 0.44, respectively) and postoperative paraplegia (OR, 0.32).1 Murad et al showed similar reductions in mortality (relative risk, 0.61) and SCI (relative risk, 0.34) in 139 studies encompassing 7768 patients.3 Studies using the National Trauma Data Bank, a multicenter registry of trauma centers, also have identified significantly improved mortality rates, shorter ICU and shorter hospital stay, and lower rates of acute kidney injury and acute respiratory distress syndrome2,6 for TEVAR compared with open repair.
7.7.2. Initial Management of Blunt Traumatic Abdominal Aortic Injury (BAAI)
Recommendations for Initial Management of BAAI
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In patients with grade 1 to 2 BAAI (Table 33) without malperfusion, anti-impulse therapy, if clinically tolerated, and repeat imaging within 24 to 48 hours of the initial scan is recommended to reduce risk of injury progression.1 |
| 1 | C-LD | 2. In patients with grade 4 BAAI (Table 33), repair should be performed to address life-threatening aortic injury.2–4 |
| 2a | C-LD | 3. In patients with grade 2 BAAI (Table 33) and associated malperfusion, it is reasonable to consider repair.1 |
| 2a | C-LD | 4. In patients with BAAI, treatment with either endovascular or open repair is reasonable and depends on degree of injury, aortic anatomy, and the patient’s overall clinical status.1–4 |
| 2b | C-LD | 5. In patients with grade 3 BAAI (Table 33), it may be reasonable to consider repair to reduce risk of progression to life-threatening injury.5 |
| 3: Harm | B-NR | 6. In patients with BAAI, the usefulness of routine application of resuscitative endovascular balloon occlusion of the aorta (REBOA) for hemorrhage control is unclear and, in some cases, may cause harm.6–8 |
Table 33.
Descriptions of Blunt Aortic Injury Grades
| Injury Grade | Descriptions |
|---|---|
| 1 | Minor intimal tear, intimal defect, or thrombus (≤10 mm) |
| 2 | Large intimal flap, intimal defect, or thrombus (≥10 mm in length or width) |
| 3 | Pseudoaneurysm |
| 4 | Aortic rupture |
In their descriptions of management of BAAIs, Shalhub et al1,2 use an aortic injury grading system described by Starnes et al13. Instead of using IMH to define grade 2 injuries, as did Azizzadeh et al,14 Starnes et al13 define grade 2 injuries based on a higher degree of intimal injury, defect, thrombus, or all of them to match radiographic findings that they deemed to be less ambiguous.
BAAI indicates blunt traumatic abdominal aortic injury; and IMH, intramural hematoma.
Synopsis
BAAI represents a rare traumatic entity, occurring in <1% of patients with blunt trauma. Patients with BAAI often have concomitant injuries such as rib fractures, abdominal visceral injury, and cardiac complications that will affect treatment decisions. Similar to BTTAI, abdominal aortic injuries are graded based on aortic contour defects, and this grading can be used to provide a framework for treatment and determination of risk of major morbidity and death from injuries (Table 33). Because BAAI is rare and symptoms are wide ranging, patients should be managed on an individual basis. In general, patients with grade 1 aortic injuries can likely be managed with antihypertensive therapy, beta blockade, and antiplatelet therapy, if not contraindicated, with repeat scan at 24 to 48 hours. Grade 2 injuries can similarly be managed nonoperatively but may progress to include end-organ vessel thrombosis or rupture. Grade 3 injuries may benefit from endovascular treatments if anatomically amenable. Grade 4 injuries are more likely to present with refractory hypotension, warranting rapid control of hemorrhage, which may be done in the emergency department (eg, antero-lateral thoracotomy with aortic cross-clamping) or operating room. Whether open or endovascular means are used for BAAI repair will depend on patient’s clinical status, hospital resources, and practitioner experience. Additionally, Shalhub et al1 propose using aortic injury zone categorization when considering options for repair, which differ from traumatic abdominal zones of injury (Figure 24). Specifically, in their multicenter experience, some zone 2 and 3 injuries could be managed endovascularly while no zone 2 injuries were managed this way. Lastly, data on the use of REBOA for hemorrhage below the diaphragm, not performed in the operating theater, and without fluoroscopic guidance are mixed, with few data showing survival benefit and some trauma registry data showing harm.
Figure 24. Abdominal Aortic Zones of Injury for Surgical Approaches and Abdominal Zones of Injury Based on Trauma Classification.
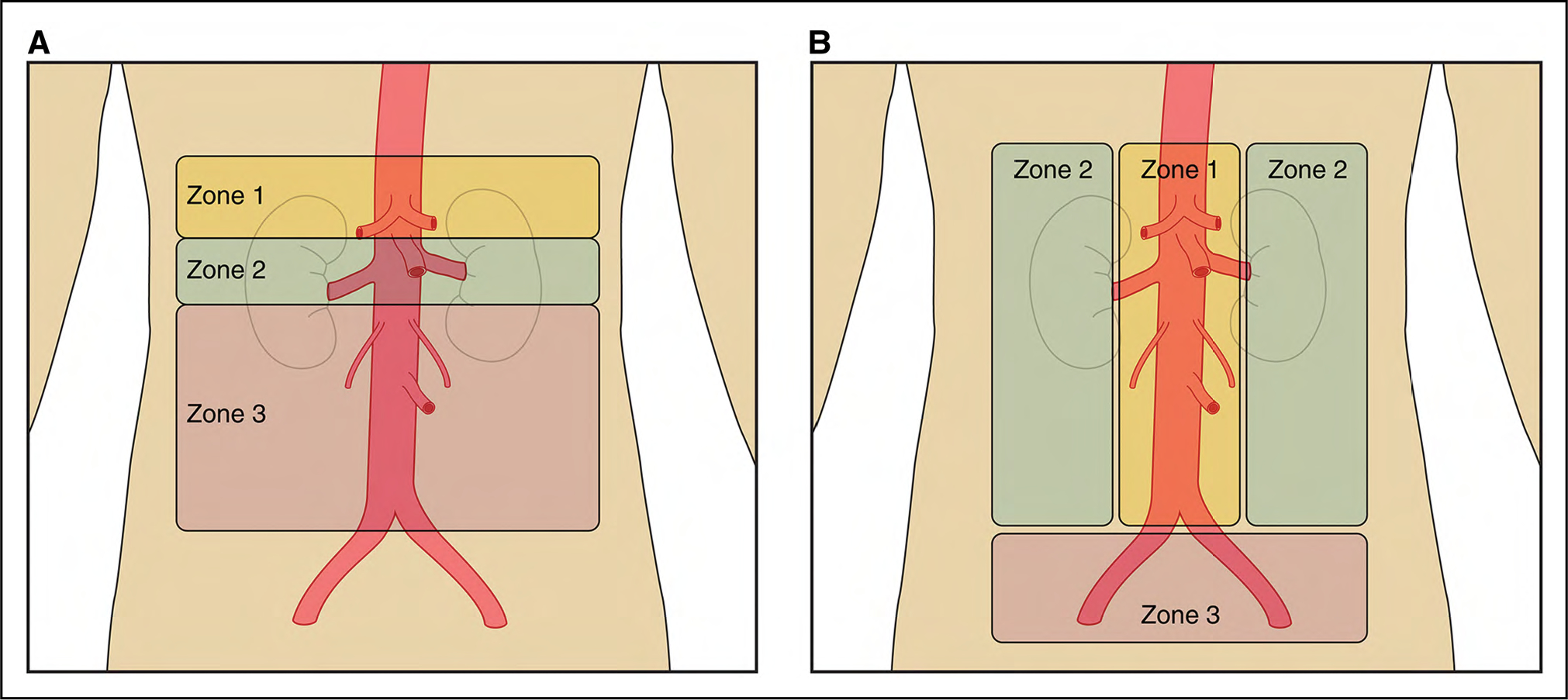
(A) The abdominal aortic zones of injury described by Shalhub et al.1 (B) The abdominal zones of injury traditionally described in trauma. The abdominal aortic zones of injury may help in prognostication and deciding whether an endovascular or open repair is feasible. Shalhub et al1 found that the mortality rate was highest in zone 2 (see panel A) grade 4 aortic injuries (Table 33). Moreover, no zone 2 aortic injuries identified in a multicenter experience were managed by endovascular means. Panel A, adapted from Shalhub et al.1 Copyright 2014, with permission from Wolters Kluwer Health, Inc.
Recommendation-Specific Supportive Text
Because BAAI is very rare, in an effort to provide clinical evidence for the management of BAAI, Shalhub et al1 aggregated data from 12 trauma centers. In the authors’ experience in treating 113 patients with BAAI, most of those with grade 1 and 2 injuries were successfully managed nonoperatively with anti-impulse therapy and repeat CTA imaging. Most of these injuries did not show progression and did not require in-hospital intervention. However, some patients will develop angiographic progression of lesions or develop symptoms from vessel occlusion, aneurysmal degeneration, or pseudoaneurysm formation. Such progression should prompt consideration of treatment to prevent further progression to symptomatic or life-threatening disease.
Patients with grade 4 injuries are more likely to present with hypotension and aortic transection as well as visceral vessel avulsion.2–4 In a single-center experience, all 8 patients with grade 4 injuries experienced cardiac arrest in the emergency department or operating room. Although all 8 patients survived to reach the operating room and 7 survived the repair, all died within days of injury. In multicenter experience, the mortality rate for grade 4 injuries was 83%. Most deaths from BAAI were within the first 24 hours of presentation and attributable to cardiac arrest from hemorrhagic shock.
Patients may present with grade 2 injuries without evidence of malperfusion and thus be managed nonoperatively. However, for patients who present with or progress to organ or limb malperfusion, endovascular or open repair may be needed to reduce morbidity and mortality rates. In their multicenter experience, Shalhub et al1 found that of the 38 patients who present with grade 2 injuries, 45% were initially managed nonoperatively, 34% were treated with open repair, and 21% were treated with endovascular repair. Of those initially managed conservatively, 3 eventually progressed to having ischemic symptoms warranting consideration of repair, with 1 patient who refused repair who died of sepsis from limb ischemia, another who died intraoperatively, and a third who successfully underwent hybrid endovascular and open repair.
Both endovascular and open approaches have been described for BAAI,1–4 and analyses of large trauma databases reveal no significant differences in mortality rates between the two. Anatomical considerations, patient clinical status and comorbid injuries, and practitioner experience will influence the choice of approach. Shalhub et al1 found that aortic zone 2 and 3 injuries appeared to be more amenable to endovascular approaches, while most grade 4 injuries were treated with open surgery.1,2 Currently, no FDA-approved devices are available specifically for treating trauma in the abdominal aorta; consequently, clinical judgment and experience are paramount in choosing an endovascular solution.
Pseudoaneurysm repair is often performed to prevent progression to uncontrolled aortic rupture, although data on characteristics associated with progression are scarce. In their multicenter study of BAAI, Shalhub et al1 found that only 30% of pseudoaneurysms were managed nonoperatively, and failure of nonoperative management occurred in 3 of these patients.
REBOA has reemerged over the past 10 years as a form of rapid hemorrhage control in trauma. Many health care centers have shown the feasibility of trauma surgeon or emergency physician placement of endovascular balloons for hemorrhage control.6,9,10 with a few studies showing significant improvement in SBP after placement11 and survival benefit compared with those who were not treated with REBOA12 or those treated with open methods of hemorrhage control.8 However, propensity-matched studies using large trauma databases showed increased mortality rate and risk of complications, such as acute kidney injury, amputation, or both, with use of REBOA.6–8 There are clinical scenarios in which REBOA is contraindicated. According to the current US Army Joint Trauma System clinical practice guidelines, REBOA is contraindicated in those with pericardial tamponade and major thoracic hemorrhage. Relative contraindications to REBOA use include cardiac arrest or shock caused by penetrating chest trauma.
7.7.3. Long-Term Management and Surveillance After Blunt Traumatic Aortic Injury (BTAI)
Recommendations for Long-Term Management and Surveillance After BTAI
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1. In patients with BTAI who have undergone aortic repair, surveillance imaging at intervals appropriate for the repair approach and location (see Section 7.8, “Long-Term Management and Surveillance Imaging Following AAS”) is reasonable.1–4 |
| 2b | C-LD | 2. In patients with BTAI who have not undergone repair, surveillance imaging with a CT at 1 month, 6 months, and 12 months after the diagnosis and, if stable, at appropriate intervals thereafter (depending on the type and extent of the injury), may be reasonable.5 |
Synopsis
In-hospital and midterm outcomes after open and endovascular repair for BTAI are good for patients who survive to hospital discharge.2–4 However, long-term data are limited that report outcomes after open or endovascular surgical repair for blunt aortic injury. The SVS clinical practice guidelines for traumatic thoracic aortic injury suggest that follow-up after TEVAR could be decreased to every 2 to 5 years in the absence of abnormalities on follow-up imaging (ie, stent graft migration, endoleak) or could follow-up standard postoperative imaging surveillance paradigms.6 No published guidelines are available for postoperative surveillance after open or endovascular abdominal aortic repair for blunt aortic injury.
Long-term data about outcomes of blunt aortic injuries managed nonoperatively are limited. A recent systemic review of nonoperative management of blunt thoracic aortic injuries showed low aortic-related event rates but injury progression in 7.6% of patients on surveillance imaging (follow-up, 1 day to 118 months).5 In published series of blunt aortic injury, patients with disease progression on repeat imaging all undergo repair.4,5
Recommendation-Specific Supportive Text
In-hospital data suggest that endograft malposition (3%) and endoleak (2%) may occur in some patients immediately after endovascular repair,1 but midterm data after open or endovascular repair of BTAIs suggest a low incidence of endoleak, stent migration, or reintervention after a mean of 52 to 60 months.2–4 Long-term data for outcomes of open or endovascular repair of BTAI are lacking.
Among patients with blunt traumatic injury who are managed nonoperatively, injury progression occurred in 7.6% of patients, and injury healing or improvement was observed in 34% of patients after a range of 1 day to 118 months of follow-up.5 Injury progression, intervention, or both occur in 0.68% of patients with grade 1 to 2 BTAI.5 Long-term data for outcomes of blunt aortic injuries managed nonoperatively are lacking.
7.8. Long-Term Management and Surveillance Imaging After AAS
7.8.1. Long-Term Surveillance Imaging After Aortic Dissection and IMH
Recommendations for Long-Term Surveillance Imaging After Aortic Dissection and IMH
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients who have had an acute aortic dissection and IMH treated with either open or endovascular aortic repair and have residual aortic disease, surveillance imaging with a CT (or MRI) is recommended after 1 month, 6 months, and 12 months and then, if stable, annually thereafter.1–6 |
| 1 | B-NR | 2. In patients who have had an acute aortic dissection and IMH that was managed with medical therapy alone, surveillance imaging with a CT (or MRI) is recommended after 1 month, 6 months, and 12 months and then, if stable, annually thereafter.7 |
Synopsis
Survival after an acute aortic dissection and IMH does not guarantee freedom from subsequent aortic events because of residual aortic dissection and risk of aneurysm formation. Ten-year survival after repair of acute type A aortic dissection is approximately 60% to 65%.1,8 Risk of reoperation is increased for the aortic valve, the aortic root, and the distal aorta,1,8,9 with an aortic root reoperation rate of approximately 15% at 15 years.9,10 The growth rate of the distal aorta is ~1 mm/y, and the risk of distal aortic reoperation ranges from 10% to 16% at 10 years.1,8,9 Although the use of TEVAR provides protection from early aortic-related death11 in acute type B aortic dissection, reintervention rates after TEVAR for can range from 27% to 39% at midterm follow-up.11,12 Surveillance imaging after thoracic aneurysm repair is critical to monitor for progression of residual aortic disease and the potential need for reintervention.
Recommendation-Specific Supportive Text
Although patients with uncomplicated type B aortic dissection who are managed medically have a favorable early prognosis, delayed aortic expansion occurs in 20% to 50% of patients over 4 years,7 so regular surveillance imaging is essential to detect midterm and late aortic growth.7
After surgical replacement of the ascending thoracic aorta in acute type A aortic dissection, patients remain at risk for progressive enlargement of unrepaired segments of residual dissected aorta, as well as potential growth of nondissected native aortic segments because of underlying medial degeneration. Consequently, repeat intervention on the aortic root, arch, or thoracoabdominal aorta may become necessary. For acute type B aortic dissection,1,2 TEVAR may leave a patent false lumen, which can lead to aneurysm growth, and can be complicated by early endoleaks in up to 35% of patients and late endoleaks in 13% of patients.5 Careful follow-up is needed to monitor for progression of disease in both dissected and nondissected aorta. In addition to using cross-sectional imaging for most of the aorta, TTE can be helpful in monitoring aortic root anatomy and aortic valve function over time.
7.8.2. Long-Term Management After Acute Aortic Dissection and IMH
Recommendation for Long-Term Management After Acute Aortic Dissection and IMH
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
Synopsis
Despite the outcomes reported for surgical repair of acute aortic dissection and IMH, a risk of ongoing growth is possible in the residually dissected as well as nondissected thoracic aortic segments. When surveillance imaging detects progression of residual aortic disease after successful treatment of acute aortic dissection and IMH, there may be a potential need for aortic reintervention.
Recommendation-Specific Supportive Text
Reoperation after acute type A aortic dissection repair is associated with low rates of complication. The primary indications for reoperation are aneurysms of the thoracic aorta, aortic anastomotic pseudoaneurysms, progressive AR, or graft infection.1 Operative mortality rate of elective repair is <10%.1–4 After TEAVR, false-lumen thrombosis can occur in 62% of extent 3B dissection and 91% of extent 3A dissection cases. Reintervention rates after TEVAR range from 15% to 26% at 5 years and are dependent on the extent of aortic dissection.4
7.8.3. Long-Term Management and Surveillance for PAUs
Recommendations for Long-Term Management and Surveillance for PAUs
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1. In patients with a PAU who have undergone aortic repair, surveillance imaging at intervals appropriate for the repair approach and location (see Section 6.5.6, “Surveillance After Aneurysm Repair”) is reasonable.1–3 |
| 2a | C-LD | 2. In patients with a PAU that is being managed medically, surveillance imaging with a CT is reasonable at 1 month after the diagnosis and, if stable, every 6 months for 2 years, and then at appropriate intervals thereafter (depending on patient age and PAU characteristics).1,4 |
Synopsis
For patients who undergo repair of a PAU, clinical failure (defined as endoleak, disease progression, graft occlusion, repeat aortic intervention, or procedure or aortic-related death) by 1 year after endovascular and open repair occur in 8.6% and 8.7%, respectively.1 No long-term data exist for outcomes after repair of PAU, but aortic-related complication rates after intervention are likely similar to those for TAA.
For patients with PAUs who are managed nonoperatively, the risk of disease progression is significant.1,5,6 Disease progression occurs more frequently in patients presenting with symptomatic versus asymptomatic PAUs7 but is >15% for both.7,8 Among patients with a PAU who have progression of disease on surveillance imaging, 73% will show continued worsening on subsequent imaging, and 46% will have progression to frank dissection after a mean of 12 months.
Recommendation-Specific Supportive Text
After open or endovascular repair of a PAU, there is a 9% risk of clinical failure by 12 months postoperatively.1 Freedom from cumulative complications and interventions is 86% at 12 months, 79% at 24 months, and 71% at 36 months postoperatively.2 After 18 months of follow-up, new PAUs are observed in approximately 5% of patients who have undergone repair of a different PAU.3
In a series of 109 patients with acute PAUs, 28% suffered from an aortic-related adverse event by 30 days of follow-up.4 Based on a systematic review of 184 patients with either thoracic or aortic PAU, 30% had radiographic evidence of disease progression on midterm follow-up.1 For patients with abdominal PAU, 23% have disease progression on CTA by 8 months of follow-up, although the risk is higher in symptomatic (43%) versus asymptomatic (17%) patients.7
8. PREGNANCY IN PATIENTS WITH AORTOPATHY
8.1. Counseling and Management of Aortic Disease in Pregnancy and Postpartum
Recommendations for Counseling and Management of Aortic Disease in Pregnancy and Postpartum
| COR | LOE | Recommendations |
|---|---|---|
| Prepregnancy | ||
| 1 | C-LD | 1. In patients with genetic aortopathies attributable to syndromic (Marfan syndrome, Loeys-Dietz syndrome, vascular Ehlers-Danlos syndrome) and nsHTAD and who are contemplating pregnancy, genetic counseling before pregnancy to discuss the heritable nature of their condition is recommended.1–4 |
| 1 | C-LD | 2. In patients with syndromic and nsHTAD, Turner syndrome, BAV with aortic dilation, and other aortopathy conditions, aortic imaging (with TTE, MRI or CT, or both as appropriate) before pregnancy is recommended to determine aortic diameters.1–3,5–13 |
| 1 | C-LD | 3. In patients with syndromic and nsHTAD, Turner syndrome, BAV with aortic dilation, and other aortopathy conditions, who are contemplating pregnancy, counseling about the risks of aortic dissection related to pregnancy is recommended.2–5,10,12,14 |
| During Pregnancy | ||
| 2a | C-EO | 4. In patients with aortic aneurysms, or at increased risk of aortic dissection, or both, it is recommended that pregnancy be managed by a multidisciplinary team including a maternal fetal medicine specialist and cardiologist, and, if logistically feasible, that delivery be planned in a hospital where the capability for emergency aortic repair is available. |
| 1 | C-LD | 5. In patients with aortopathies who are pregnant, guideline-directed treatment of hypertension is recommended.6,15,17 |
| 1 | C-EO | 6. In patients with syndromic and nsHTAD, beta-blocker therapy during pregnancy and postpartum is recommended, unless contraindicated. |
| 1 | C-LD | 7. In pregnant patients with an aortopathic condition or a dilated aortic root or ascending aorta, surveillance TTE to monitor aortic diameters and aortic valve function is recommended each trimester and again several weeks postpartum, although imaging may be more frequent depending on aortic diameter, aortic growth rate, and underlying condition.7–9,17,18 |
| 1 | C-LD | 8. In pregnant patients with aortic disease who require surveillance imaging of the aortic arch, descending, abdominal aorta, or all 3, MRI without gadolinium is recommended over CT to avoid radiation exposure to the fetus.19,20 |
Synopsis
Pregnancy leads to hemodynamic and hormonal changes and is a risk factor for aortic dissection in women with aortopathy.21 Aortic dissection may occur throughout pregnancy or several weeks postpartum, with most in the third trimester or up to 12 weeks’ postpartum.21 Women with aortopathy, including Marfan syndrome,6,7,13,14,18,19,22 Loeys-Dietz syndrome,2,22,23 vascular Ehlers-Danlos syndrome,3,24 nsHTAD,25,26 Turner syndrome,12,27 and BAV with aneurysm21,28 are at risk of pregnancy-related aortic dissection. Type A aortic dissection in pregnancy associates with aortic dilation, but type B aortic dissection may occur without aortic dilation.6,13,22
Before pregnancy, women with or at risk for aortopathy undergo TTE (and MRI or CT, as appropriate) and are counseled about risks of aortic dissection informed by specific circumstances. Aortic surveillance imaging throughout pregnancy and several weeks postpartum is performed to monitor aortic size.9
In women at low risk, vaginal delivery is performed with efforts to lessen hemodynamic stress and shorten the second stage of labor.9,14 Women at increased risk of aortic complications typically undergo cesarean delivery.9,14
In women with aortopathy, prepregnancy genetic counseling, aortic imaging, discussion about aortic dissection risk, and shared decision-making are necessary.9
Recommendation-Specific Supportive Text
HTAD encompasses conditions in which aortic disease has an underlying genetic trigger or familial occurrence.29,30 Marfan syndrome, Loeys-Dietz syndrome, vascular Ehlers-Danlos syndrome, and nsHTAD are autosomal dominant conditions with an inheritance risk of 50%.9,30 BAV may also be familial. Prepregnancy counseling by a genetic counselor, medical geneticist, or both or aortopathy specialist is recommended to discuss the heritability of these conditions and to identify at risk relatives and to discuss pregnancy concerns.9,30
Women with aortopathic conditions are at risk for aortic dilation and aortic dissection related to pregnancy.14,22,67 Evaluation of the aortic root, ascending aorta, or both by echocardiogram before pregnancy in women with aortopathy is important for prepregnancy counseling and management during pregnancy.1–3,5–7,9,11,13 In conditions that associate with aortic disease distal to the ascending aorta, prepregnancy MRI or CT is performed to evaluate for aortic disease.2,5,9,14
The risk of type A aortic dissection in pregnancy relates to the aortopathy condition and aortic diameter, but type B aortic dissection may occur without significant aortic dilation.6,22 Most dissections related to pregnancy occur in the third trimester and in the first 12 weeks’ postpartum.21 Awareness of the signs and symptoms of acute aortic dissection among stakeholders may improve diagnosis and outcomes. In Marfan syndrome, type A aortic dissection risk is very low when aortic diameters are <4.0 cm and are much higher at diameters >4.5 cm. In series of women with TGFBR1 or TGFBR2 pathogenic variants, aortic dissection was reported in 0% to 19% of pregnancies.21 Rapid aortic growth in pregnancy is reported in Loeys-Dietz syndrome.2 Limited data are available on pregnancy and SMAD3, TGFB2, or TGFB3 pathogenic variants.31,32 Maternal mortality rates in vascular Ehlers-Danlos syndrome have ranged from 4% to 25%.3 Among 283 women with vascular Ehlers-Danlos syndrome with 616 delivered pregnancies, 30 women died, with a pregnancy-related death rate of 4.9%.3 Pregnancy has typically been avoided in women with vascular Ehlers-Danlos syndrome.9 The decision to proceed with pregnancy in vascular Ehlers-Danlos syndrome is complex and, for some women with specific genetic variants, null mutations, and normal vascular imaging, the risk may be lower, and shared decision-making is required.3 Aortic dissection at small aortic diameters has been reported in some patients with nsHTAD.25,26,33 Aortic dissection related to BAV is rare and, when reported, associates with aneurysmal dilation.14,21,28
For women with aortopathic conditions, multidisciplinary evaluation before and throughout pregnancy can evaluate and manage BP, aortic diameter, and monitor pregnancy for complications. Delivery in a setting in which cardiothoracic surgery is available for urgent management of aortic dissection allows rapid treatment for this complication.25 Educating women with aortopathic conditions and their physicians about the signs and symptoms of acute aortic dissection may allow earlier diagnosis and improve outcomes.12,21,25
Hypertension is a risk factor for aortic dissection in pregnancy.6 For appropriate patients with or without hypertension, beta blockers are used throughout pregnancy and postpartum, recognizing that fetal growth may be impaired.13,15 Labetalol is suggested as the beta blocker of choice for use in pregnant women with hypertension.35 Other agents may be required as suggested by international guidelines.16,34 ARBs and ACEIs are contraindicated during pregnancy because of teratogenicity. Calcium channel blockers are generally avoided, when possible, in Marfan syndrome based on limited information and concerns raised from mouse models.34,35
Beta-blocker therapy has been shown to lessen aortic growth rates in Marfan syndrome and is recommended to lessen hemodynamic aortic stress in Marfan syndrome and related conditions.4,5,13 In the absence of controlled trials, beta blockers are used in other aortopathic conditions, and continuation of such therapy during pregnancy is recommended unless contraindicated.2,5,9 Shard decision-making is required, understanding that fetal growth and weight may be impaired when beta blockers are used in pregnancy.15 However, in ROPAC (Registry Of Pregnancy And Cardiac disease), there was no significant difference in birth weight in women treated with a beta blocker compared with untreated women (2960 g [2358–3390 g] versus 3270 g [2750–3570 g]); P=0.25).14 Because aortic dissection may occur postpartum, beta-blocker therapy is continued for at least several weeks after delivery and indefinitely for those with indications for long-term use.
Pregnancy-associated increases in maternal blood volume, heart rate, stroke volume and cardiac output, and neurohormonal activation begin in the first trimester and peak in the third trimester and peripartum period.9 In women with aortopathic conditions, the aorta may dilate during pregnancy,7 and significant dilation is a risk factor for ROPAC.8,14 Aortic imaging frequency during pregnancy is variable and is performed every trimester but may be performed more frequently depending on individual factors, including the specific aortopathic condition, aortic diameter, and rate of aortic growth.3,5,9,10,12–14,25 Evaluation of the aorta several weeks postpartum to determine aortic diameter is performed to evaluate for aortic dilation.9
In patients with aortopathy that involves the aortic arch, descending or abdominal aorta or branches, or all of them, cross-sectional imaging identifies aortic anatomy and diameters. MRI without gadolinium contrast is low-risk during pregnancy and is preferred over CT for elective imaging to avoid the risks of ionizing radiation exposure to the developing fetus.9,19,20 A TEE can be performed during pregnancy, if required, to evaluate the descending aorta.
8.2. Delivery in Pregnant Patients With Aortopathy
Recommendations for Delivery in Pregnant Patients With Aortopathy
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. In pregnant patients with a history of chronic aortic dissection, cesarean delivery is recommended. |
| 1 | C-EO | 2. In pregnant patients with an aortopathy and an aortic diameter of <4.0 cm, vaginal delivery (when otherwise appropriate) is recommended. |
| 2a | C-EO | 3. In pregnant patients with a diameter of the aortic root, ascending aorta, or both, of ≥4.5 cm, cesarean delivery is reasonable. |
| 2b | C-EO | 4. In pregnant patients with a diameter of the aortic root, ascending aorta, or both, of 4.0 cm to 4.5 cm, vaginal delivery with regional anesthesia, expedited second stage, and assisted delivery may be reasonable. |
| 2b | C-EO | 5. In pregnant patients with syndromic and nsHTAD, and a diameter of the aortic root, ascending aorta, or both, of 4.0 cm to 4.5 cm, cesarean delivery may be considered. |
Synopsis
The risk of type A aortic dissection related to pregnancy in Marfan syndrome is related to aortic root diameter, with a low risk (~1%) of aortic dissection at an aortic diameter <4.0 cm and much greater risk at aortic diameters >4.5 cm.1–3 Progressive aortic dilation and hypertension also determine dissection risk.4–6 Complex and shared decision-making is required when the aorta is between 4.0 cm and 4.5 cm in diameter, recognizing that although some series report low risk,2,7,8 aortic dissection related to pregnancy at this diameter may occur.1 Modified World Health Organization classification on cardiovascular risk places women with Marfan syndrome and moderate aortic dilation of 4.0 cm to 4.5 cm in modified World Health Organization class III and those with aortic diameter >4.5 cm in class IV.9 Because of increased risk of aortic dissection, pregnancy is avoided when the aortic root diameter is >4.5 cm.1–3,9 Type B aortic dissection is responsible for 20% to 40% of pregnancy-related dissections in Marfan syndrome, often occurring without aortic dilation1,6,8,10 and may occur after previous aortic root replacement in Marfan syndrome and Loeys-Dietz syndrome.11,12 Aortic dissection frequently occurs postpartum, with heightened risk up to 12 weeks after delivery.1 Patients at risk and their care teams should remain alert to signs and symptoms suggesting acute dissection.
Recommendation-Specific Supportive Text
Very limited information exists about pregnancy-related aortic risks in patients with chronic aortic dissection. Because of concerns for aneurysmal enlargement, recurrent dissection and aortic rupture, pregnancy is considered to be high risk in women with chronic aortic dissection. To allow optimal timing of delivery, elective cesarean delivery is usually performed in women with chronic aortic dissection.
Type A and type B aortic dissection related to pregnancy may occur in Marfan syndrome.1,2,6 Women with aortic root dilation >4.0 cm and, especially >4.5 cm, are at increased risk of type A aortic dissection during pregnancy and postpartum.1,3 Aortic dissection has been reported to be low risk in small series of women with aortic diameters between 4.0 cm and 4.5 cm,2,7,8 but aortic dissection related to pregnancy at this diameter may occur.1 Type B aortic dissection related to pregnancy may occur without significant aortic dilation and after previous aortic root replacement.1,11
In the absence of controlled trials, cesarean delivery is often performed in women with Marfan syndrome and a significantly dilated aorta to allow for a planned delivery.2,9
There are no randomized trials of delivery methods in women with aortopathy. When the aorta is not significantly dilated, vaginal delivery using methods to lessen hemodynamic stress, including regional anesthesia and an expedited second stage and assisted delivery, is often performed.2,8,9 Coexistent lumbosacral dural ectasia, spine disease, or both in women with aortopathic conditions may complicate epidural anesthesia.13,14
Cesarean delivery is often performed in women with Marfan syndrome and aortic dilation of >4.0 cm.2,8 Among 27 women with Marfan syndrome and aortic dilation, 21 of 27 women had a vaginal delivery. The cesarean delivery rate was 23.8% and 16.7% in women with diameter <4.0 cm and 4.0 cm to 4.5 cm, respectively.8
8.3. Surgery Before Pregnancy in Women With Aortic Disease
Recommendations for Surgery Before Pregnancy in Women With Aortic Disease
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In patients with Marfan syndrome and an aortic root diameter of >4.5 cm, aortic surgery before pregnancy is recommended.1–4 If the aortic root diameter is 4.0 cm to 4.5 cm, aortic surgery before pregnancy may be considered, especially if there are risk factors for aortic dissection (ie, rapid aortic growth of ≥0.3 cm/y or a family history of aortic dissection).1,2,5–8 |
| 2b | C-LD | |
| 2a | C-EO | 2. In patients with Loeys-Dietz syndrome attributable to pathogenic variants in TGFB2 or TGFB3 and an aortic diameter of ≥4.5 cm, surgery before pregnancy is reasonable. If the Loeys-Dietz syndrome is attributable to pathogenic variants in TGFBR1, TGFBR2, or SMAD3, and the aortic diameter is ≥4.0 cm, surgery before pregnancy may be considered. |
| 2b | C-EO | |
| 1 | C-EO | 3. In patients with nsHTAD and an aortic diameter of ≥4.5 cm, surgery before pregnancy is recommended. If the aortic diameter is 4.0 cm to 4.4 cm, surgery before pregnancy may be considered, depending on the molecular diagnosis, family history, and aortic growth rate. |
| 2b | C-EO | |
| 1 | C-LD | 4. In patients with Turner syndrome and ASI of ≥2.5 cm/m2, surgery before pregnancy is recommended.9–11 |
| 1 | C-EO | 5. In patients with a BAV (in the absence of Turner syndrome or an HTAD) and an aortic diameter of ≥5.0 cm, surgery before pregnancy is recommended. |
| 1 | C-EO | 6. In patients with sporadic aortic root aneurysms, ascending aortic aneurysms, or both and a diameter of ≥5.0 cm, surgery before pregnancy is recommended. |
Synopsis
The decision to proceed with operative intervention for an aortic root, ascending aortic aneurysm, or both in a woman contemplating pregnancy is complex and depends on many factors. Considerations that inform this decision include the specific disorder, genetic variant, rate of aortic growth, family history, and phenotype and include shared decision-making (Table 34). Specialists involved in this decision may include aortopathic specialists, cardiologists, medical geneticists, maternal fetal medicine specialists, and aortic surgeons at experienced centers. The risks of aortic surgery should be considered and although prophylactic aneurysm surgery will prevent proximal aortic dissection, a risk remains of pregnancy-related dissection distal to the aortic graft in HTAD, and this risk may be higher in women with Loeys-Dietz syndrome attributable to pathogenic variants in TGFBR1 and TGFBR2.12,13
Table 34.
Prophylactic Aortic Surgery Before Pregnancy in Women With Aortopathic Conditions
| Condition | Surgical Threshold Before Pregnancy* by Aortic Diameter (cm) or Aortic Size Index (cm/m2) |
|---|---|
| Marfan syndrome | >4.5 cm |
| Marfan syndrome with risk factors (rapid aortic growth of ≥0.3 cm/y; family history of aortic dissection) | 4.0–4.5 cm |
| Loeys-Dietz syndrome (attributable to pathogenic variants in TGFBR1, TGFBR2, or SMAD3) | ≥4.0 cm |
| Loeys-Dietz syndrome (attributable to pathogenic variants in TGFB2 or TGFB3) | ≥4.5 cm |
| Nonsyndromic heritable thoracic aortic disease | ≥4.5 cm† |
| Turner syndrome | >2.5 cm/m2 |
| Bicuspid aortic valve | ≥5.0 cm‡ |
Shared decision-making is required to determine the surgical threshold before elective aortic root, ascending aortic surgery, or both and is informed by the condition, specific pathogenic variant, age, body size, aortic growth rate, phenotype, and family history of aortic dissection, and surgery at smaller aortic diameters may be considered depending on individual circumstances.
Aortic dissection related to pregnancy has occurred at small aortic diameters in women with ACTA2 and MYLK pathogenic variants. Prophylactic aortic surgery before pregnancy at smaller aortic diameters may be reasonable in these conditions and other nonsyndromic heritable thoracic aortic disease and may be informed by the molecular diagnosis, family history, and aortic growth rate.
Prophylactic aortic surgery may be considered at smaller aortic diameters depending on body size, aortic growth rate, and family history.
Colors correspond to Class of Recommendations in Table 2.
Recommendation-Specific Supportive Text
Women with Marfan syndrome and aortic root dilation >40 mm and especially >4.5 cm are at increased risk of type A aortic dissection during pregnancy and postpartum.1,2,6,7,12 Pregnancy in small series of women with Marfan syndrome and aortic diameters between 4.0 cm and 4.5 cm was reported to be relatively safe in carefully monitored women,1–4 although acute type A aortic dissection may occur.5 The presence of additional risk factors for aortic dissection, including family history of aortic dissection and rapid aortic growth (≥0.3 cm/y), and patient preference may inform the shared decision for aortic surgery before pregnancy when the aortic diameter is <4.5 cm.8,14,15
Information is lacking about aortic diameters and aortic dissection risk related to pregnancy in Loeys-Dietz syndrome, because most women who were pregnant were unaware of their diagnosis before pregnancy. The size threshold for elective surgery to replace the dilated aortic root and ascending aorta in Loeys-Dietz syndrome depends on multiple factors and is informed by the specific pathogenic variant and the family history, rate of aortic growth, extra-aortic phenotypic features, and involves shared decision-making. Patients with TGFB2- and TGFB3-related Loeys-Dietz syndrome may have a lower aortic dissection risk than those with variants in TGFBR1, TGFBR2, or SMAD3.16,17 Women with aortic root diameters of >4.0 cm are likely at increased risk for pregnancy-related aortic dissection based on data from women with Marfan syndrome and the more severe aortopathy in Loeys-Dietz syndrome attributable to TGFBR1, TGFBR2, and SMAD3 variants.5,18–20 There were no pregnancy-related aortic dissection reported in a series of women with SMAD3 variants, but only 2 women had aortic diameters known before pregnancy (and both were normal).20
Because phenotypic features are absent in patients with nsHTAD because of pathogenic variants in multiple genes (eg, ACTA2, MYH11, MYLK, PRKG1, and others), the first manifestation of disease may be acute aortic dissection, including that related to pregnancy.21 In a series of patients with ACTA2 pathogenic variants, 20% of aortic dissection were related to pregnancy.21 Aortic dissection at small aortic diameters has been reported related to pregnancy in patients with ACTA2- and MYLK-related HTAD.21,22 Ruptured type B dissection has been reported.23 Individualized assessment of pregnancy risks based on the specific genetic condition and other individual factors may inform pregnancy management, recognizing that limited information is available to guide decision-making.21,22,24
Among those with Turner syndrome, an ASI >2.0 cm/m2 is considered dilated, and the risk of aortic dissection in Turner syndrome is greatest when the ASI is ≥2.5 cm/m2.9,10 When aortic dissection occurs in Turner syndrome, almost 90% of cases have an identifiable risk factor, such as underlying aortic dilation, aortic coarctation, BAV, or hypertension.11
Despite the relative frequency of BAV in the population, aortic dissection related to pregnancy in patients with a BAV (and without Turner syndrome or HTAD) is rarely reported.5,25 In 88 women with BAV and without aortic dilation, there were no cases of aortic dissection in 186 deliveries.26 In a series of 49 patients with BAV with moderate aortic dilation (median aortic diameter 42 mm) reporting pregnancy outcomes, there were no cases of aortic dissection.3 When type A aortic dissection did complicate pregnancy in isolated BAV, significant aortic dilation was noted.5,25 There is no evidence-based information regarding pregnancy outcomes in women with BAV and aortic diameters >4.5 cm to inform aortic risk. In these cases, pregnancy management and shared decisions about aortic surgery may be informed by other risk factors for dissection, including rapid aortic growth, body size, and family history. Aortic dissection risk increases in patients with BAV when the aortic diameter exceeds 5 cm.27 Because of risk of aortic dissection, pregnancy in patients with a BAV and an aortic diameter of >5.0 cm is classified to be modified World Health Organization class IV, carrying high risk of maternal morbidity and mortality.28
Aortic root and or ascending aortic dilation >4.0 cm in a woman of child-bearing age is uncommon, and its presence should trigger an evaluation for underlying genetic aortopathy.29 Even when there is clear evidence of an autosomal dominant transmission of TAA in a family, currently available molecular genetic technology only identifies a pathogenic variant in a known gene leading to TAA in about 20% to 25% of families.29 In sporadic TAA disease, genetic variants are found in even fewer cases. In young patients at low surgical risk with aortic root or ascending aortic aneurysms of 5.0 cm, surgical intervention is performed. Surgery before pregnancy at smaller aortic diameters is sometimes performed and is informed by aortic growth rate, hypertension, surgical expertise, patient wishes, and other factors involving a shared decision depending on individual circumstances.
8.4. Pregnancy in Patients With Aortopathy: Aortic Dissection and Aortic Surgery in Pregnancy
Recommendations for Pregnancy in Patients With Aortopathy: Aortic Dissection and Aortic Surgery in Pregnancy
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In patients experiencing an acute type A aortic dissection during the first or second trimester of pregnancy, urgent aortic surgery with fetal monitoring is recommended.1–3 |
| 1 | C-LD | 2. In patients experiencing an acute type A aortic dissection during the third trimester of pregnancy, urgent cesarean delivery immediately followed by aortic surgery is recommended.1–4 |
| 1 | C-EO | 3. In patients experiencing an acute type B aortic dissection during pregnancy, medical therapy is recommended, unless endovascular or surgical therapy is required to manage acute complications.5 |
| 2b | C-EO | 4. In patients with progressive aortic dilation during pregnancy prophylactic aortic surgery may be considered, depending on individual circumstances.1,2,4 |
Synopsis
During pregnancy, if marked aortic dilation is present or rapid aortic expansion occurs, risks of maternal aortic dissection or rupture must be considered. If early in pregnancy, high maternal risk of morbidity or death may warrant pregnancy termination.1,4 Prophylactic aortic surgery during pregnancy requires complex decision-making and should be individualized based on maternal and fetal risks.1,2,4 Cardiac surgery in the first trimester has risks of fetal developmental defects, while surgery in the third trimester carries risks to fetal circulation and maternal hemodynamics.1 Semi-elective surgery during pregnancy may have its lowest collective risk to fetal organogenesis and maternal hemodynamics during the second trimester.1,3,4 If type A aortic dissection occurs during pregnancy, urgent obstetric and cardiac surgical consultation is necessary, because management strategies depend on the viability of the fetus and condition of the mother. If type A aortic dissection occurs in the first 26 weeks, emergency cardiac surgery is performed, recognizing risk of fetal loss.1,2,4 When duration of pregnancy associates with higher likelihood of independent fetal survival (especially after 28 weeks), cesarean delivery followed by aortic repair provides the best chances for fetal and maternal survival.1,2,4
Recommendation-Specific Supportive Text
If type A aortic dissection occurs during the first 2 trimesters, emergency aortic surgery is performed first with fetal monitoring and modifications to anesthesia and cardiopulmonary bypass, recognizing the high risk of fetal loss.1–4 If acute type A aortic dissection occurs between 24 and 28 weeks, the care team must balance maternal and fetal risks when deciding between cesarean delivery followed by aortic surgery or aortic surgery with fetal surveillance.1,4
If type A aortic dissection occurs in the third trimester, given the increased likelihood of independent fetal survival, emergency cesarean delivery followed by maternal aortic surgery is recommended.1,2,4 In a series of 20 patients with type A aortic dissection during pregnancy, 19 underwent surgical repair and, of those at >28 weeks gestation, delivery first followed by aortic surgery achieved good fetal outcomes.2
Although uncomplicated type B aortic dissection in pregnancy is treated medically, 20% will go on to develop complications that require intervention5; in such cases, endovascular repair is preferred over open surgery, whenever feasible.5
Prophylactic aortic surgery during pregnancy requires complex decision-making, and management is individualized based on maternal and fetal risks and benefits.1,2,4
9. OTHER AORTIC CONDITIONS
9.1. Inflammatory Aortitis: Diagnosis and Treatment of Takayasu Arteritis and Giant Cell Arteritis (GCA)
Recommendations for Inflammatory Aortitis: Diagnosis and Treatment of Takayasu Arteritis and GCA
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| Diagnosis | ||
| 1 | C-LD | 1. In patients with large vessel vasculitis (LVV), prompt evaluation of the entire aorta and branch vessels with MRI or CT, with or without 18F-FDG positron emission tomography (FDG-PET), is recommended.1–6 |
| Treatment | ||
| 1 | B-NR | 2. In patients with active GCA or Takayasu arteritis, initial medical therapy should include high-dose glucocorticoids.7–12 |
| 1 | B-R | 3. In patients with GCA who have evidence of active aortitis, tocilizumab is recommended as adjunctive therapy to glucocorticoids, with methotrexate as an alternative.7,13,14 |
| 1 | C-LD | 4. In all patients with Takayasu arteritis, non-biological disease-modifying anti-rheumatic drugs (DMARD) should be given in combination with glucocorticoids.7,15,16 |
| 1 | C-LD | 5. In patients with active GCA or Takayasu arteritis, treatment efficacy should be periodically assessed by monitoring inflammatory serum markers (C-reactive protein and erythrocyte sedimentation rate), imaging with CT, MRI, or FDG-PET, and clinical symptoms.1,7,15,17–20 |
| 2a | C-LD | 6. In patients with GCA or Takayasu arteritis who are in remission, elective endovascular or open surgical intervention is reasonable to treat aortic and branch vessel complications.7,21 |
| 2a | C-EO | 7. In patients with GCA or Takayasu arteritis and aortic involvement who are in remission, annual surveillance imaging with CT, MRI, or FDG-PET is reasonable.1,7,17–19 |
Synopsis
LVV comprises Takayasu arteritis and GCA, which are the most common causes of aortitis.22,23 Other known causes of aortitis include immunoglobulin G4–related disease, antineutrophil cytoplasmic antibody-related vasculitis, sarcoidosis, Behçet’s disease, relapsing polychondritis, and granulomatosis with polyangiitis; many cases of aortitis remain idiopathic. Whereas Takayasu arteritis and GCA tend to affect the thoracic aorta, immunoglobulin G4–related disease most commonly affects the abdominal aorta. Diagnostic criteria are summarized in Table 35. Prompt diagnosis and initiation of treatment is of utmost importance, because potential complications include aortic aneurysms, aortic dissection, IMH, PAU, and rupture, as well as progressive atherosclerosis and thrombotic complications.24 18F-FDG-PET with CT is useful for both the diagnosis of suspected LVV and to evaluate anti-inflammatory treatment response, especially before planned revascularization.4,5 Initial treatment options for Takayasu arteritis and GCA include high-dose glucocorticoid therapy (prednisone at 40–60 mg/d, or equivalent) or, for select cases, intravenous pulse steroids along with adjunctive therapy, including (but not limited to) tocilizumab and methotrexate (Figures 25 and 26). Revascularization may be warranted in select cases of stable disease, as well as in AAS.
Table 35.
Diagnostic Criteria for Inflammatory Aortitis
| Names | Criteria Used in Diagnosis | When Is Diagnosis Established? |
|---|---|---|
| Takayasu arteritis | Age of onset <40 y Intermittent claudication |
≥3 criteria are present (sensitivity 90.5%; specificity 97.8%) |
| Diminished brachial artery pulse | ||
| Subclavian artery or aortic bruit | ||
| Systolic BP variation of >10 mm Hg between arms | ||
| Aortographic evidence of aorta or aortic branch stenosis | ||
| Giant cell arteritis | Age >50 y Recent-onset localized headache |
≥3 criteria are present (sensitivity >90%; specificity >90%) |
| Temporal artery tenderness or pulse attenuation | ||
| Elevated erythrocyte sedimentation rate >50 mm/h | ||
| Arterial biopsy shows necrotizing vasculitis |
Reprinted from Hiratzka et al. 2019.27
Figure 25. The 2018 European Alliance of Associations for Rheumatology (EULAR; formerly European League Against Rheumatism) Recommended Algorithms for the Pharmacological Treatment of Giant Cell Arteritis.
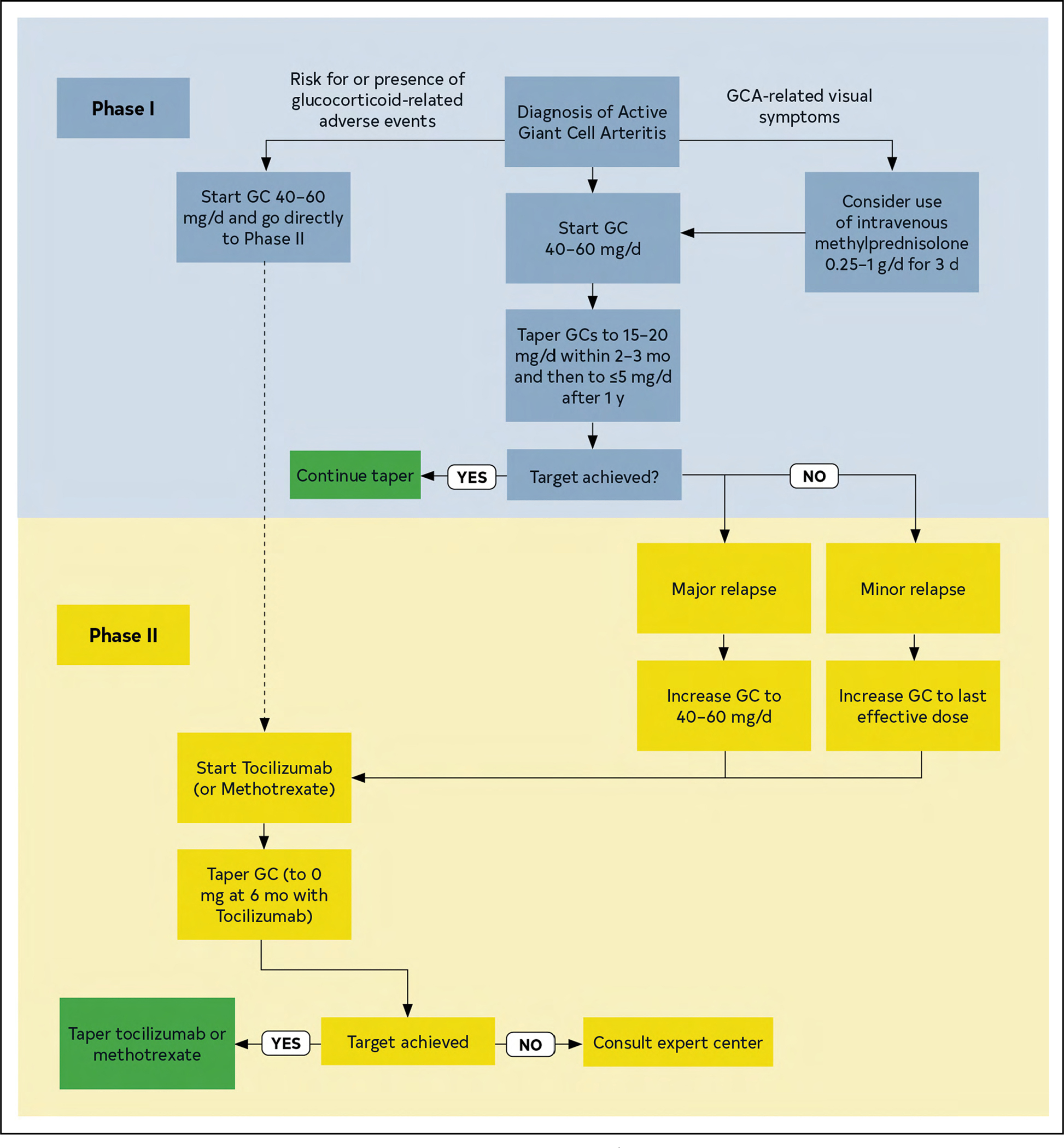
GC indicates glucocorticoids; GCA, giant cell arteritis; and TNF, tumor necrosis factor. Modified from Hellmich et al.7 Copyright 2020, with permission from BMJ Publishing Group Limited.
Figure 26. The 2018 European Alliance of Associations for Rheumatology (EULAR; formerly European League Against Rheumatism) Recommended Algorithms for the Pharmacological Treatment of Takayasu Arteritis.
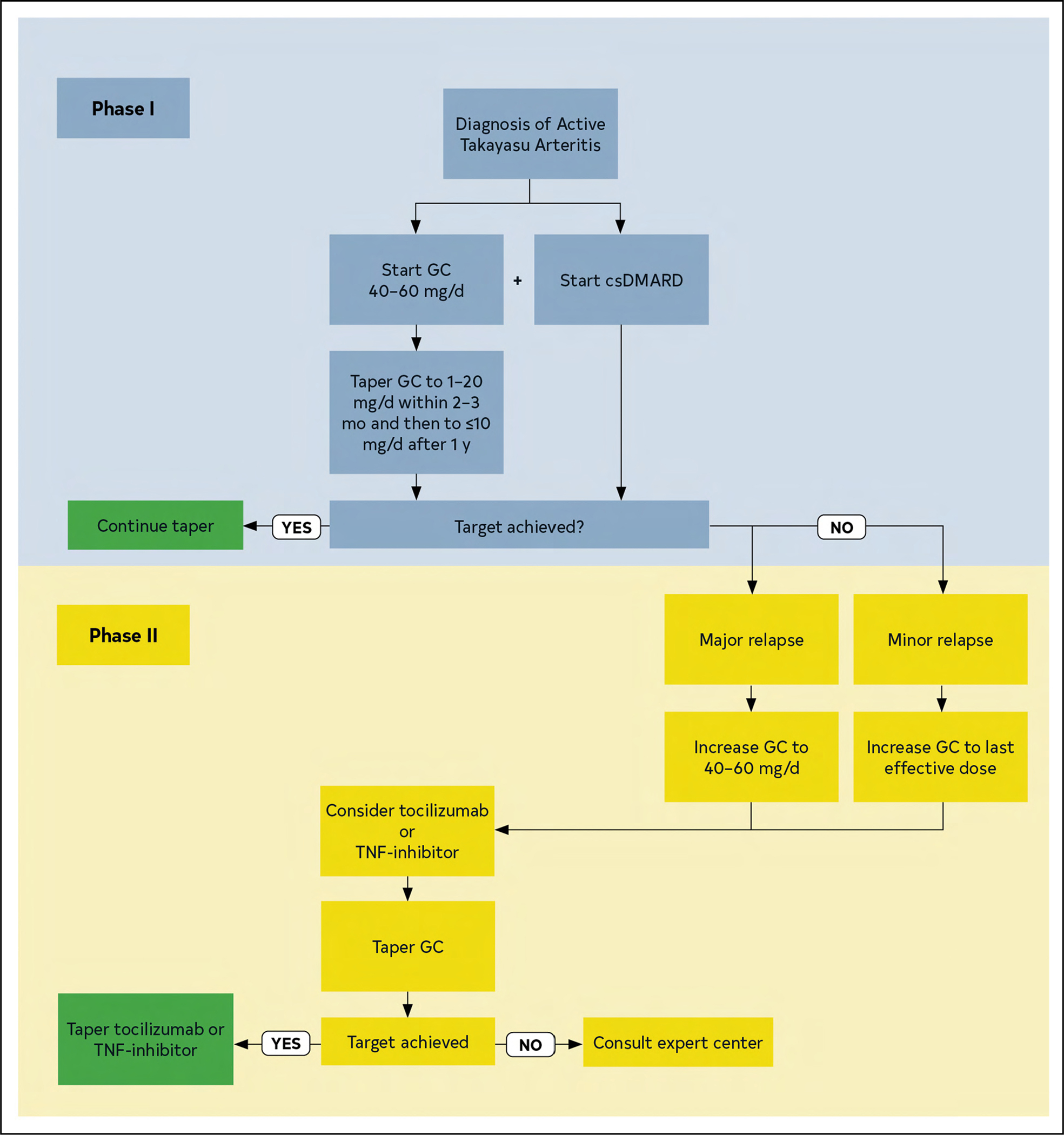
csDMARD indicates conventional synthetic disease modifying antirheumatic drug; GC, glucocorticoids; and TNF, tumor necrosis factor. Modified from Hellmich et al.7 Copyright 2020, with permission from BMJ Publishing Group Limited.
Recommendation-Specific Supportive Text
In suspected GCA or Takayasu arteritis, early imaging can confirm the diagnosis when the results complement clinical findings.1 Imaging the aorta should be performed as soon as possible so that initiation of treatment is not delayed, given the risk of complications from untreated LVV. Sensitivity of diagnostic imaging in the initial diagnosis of LVV decreases with duration of glucocorticoid treatment.2 FDG-PET has a reported specificity for GCA-related aortitis as high as 100% and CTA about 85%.5 In CT, wall thickening from inflammation may be mistaken for atherosclerosis; however, given CT’s usefulness in assessing occlusive lesions, intimal injury, ulcerative plaques, and aneurysmal disease, it is often combined with FDG-PET in LVV.1 Evidence is limited for the role of MRI in GCA, but MRI is widely used in Takayasu arteritis given the patients’ younger age at diagnosis and need for lifelong surveillance imaging.6 If proximal aortic involvement is confirmed by CT or MRI, then echocardiography may be helpful to assess aortic valve function.
Active vasculitis is diagnosed by clinical symptoms of GCA or Takayasu arteritis with evidence of inflammation by serum biomarkers, imaging, or both. High-dose glucocorticoid therapy (prednisone at 40–60 mg/d or equivalent) is standard induction therapy for GCA and Takayasu arteritis and leads to remission and control of active disease in most patients7–12 (Figures 25 and 26). Evidence supporting the efficacy of induction therapy with high-dose intravenous methylprednisolone in GCA comes only from small clinical trials, and thus the 2018 recommendations from the European Alliance of Associations for Rheumatology (EULAR; formerly the European League Against Rheumatism) limits its use to patients with severe GCA at risk for blindness in the acute setting, and administration should not delay oral glucocorticoid treatment.7–9 Once the acute phase is controlled, glucocorticoid taper should be initiated to reach a target prednisone dose of 15 to 20 mg/d within 2 to 3 months, and ≤5 mg/d for GCA and ≤10 mg/d for Takayasu arteritis after 1 year.7 Older guidelines have supported the use of antiplatelet or anticoagulants in LVV. Evidence from a meta-analysis does not support use of prophylactic antithrombotic therapy in all patients with GCA25; instead, an individualized approach to antithrombotic therapy is recommended in the acute and chronic phases of GCA and Takayasu arteritis, based on imaging and clinical findings of aortic and branch vessel complications.26
In an RCT of 251 patients with GCA, a 26-week prednisone taper combined with tocilizumab, an interleukin-6 receptor inhibitor, was superior to either a 26-week or 52-week prednisone taper plus placebo in reducing the primary outcome of glucocorticoid-free disease remission at 1 year.10 Tocilizumab gained approval for use in 2017 as adjunctive therapy for select patients with GCA, with methotrexate remaining an alternative option.7,13,14 The EULAR 2018 updated guidelines recommended limiting the use of adjunctive therapy to those with refractory or relapsing disease, those at risk of adverse effects of glucocorticoid treatment, or those at risk of cardiovascular complications (aortitis and major branch vessel involvement) from GCA7 (Figure 25).
High-quality randomized clinical trial evidence supporting the use of adjunctive therapy in Takayasu arteritis is limited. However, consensus expert opinion is to initiate DMARDs in combination with glucocorticoids in all patients with Takayasu arteritis, given high relapse rates of up to 70%.7 Nonbiological DMARDs (eg, methotrexate, hydroxychloroquine, azathioprine, sulfamethoxazole, and leflunomide) are considered first line according to the EULAR 2018 updated guidelines on Takayasu arteritis treatment, with biological DMARDs (eg, tocilizumab or tumor necrosis factor-inhibitors) as second-line agents in select patients who relapse on initial combination therapy7,15,16 (Figure 26). Optimal treatment duration in Takayasu arteritis is less well understood, because defining remission in Takayasu arteritis is challenging. Outcomes measures may include any of these: remission based on clinical criteria, normalization of inflammatory biomarkers, stabilization on serial CT or MRI, improvement on PET-CT imaging, quality of life, and presence of clinical disease relapse.15 A clear need remains for both adequately powered randomized clinical trials of Takayasu arteritis therapies and a consensus definition of treatment success.
The EULAR 2018 updated guidelines placed the greatest emphasis on both the improvement of clinical symptoms and the stability of inflammatory biomarkers in defining the remission phase of LVV. Consequently, data are limited regarding the role of surveillance imaging in those with no signs or symptoms of active disease. Currently, tomographic imaging is complementary to clinical symptoms and laboratory surveillance, and its use should be individualized, focused mostly on the evaluation of new symptoms or signs of aortic, major branch artery stenoses or aneurysms, or both.1,7,15,17–19 One prospective cohort study using FDG-PET in disease surveillance of GCA showed reduced inflammatory activity at 3 months after treatment initiation but no further change at 6 months, with most patients in clinical remission still showing positive PET findings.20 What remains unknown are the potential anatomic consequences of having a positive FDG-PET scan despite clinical remission.
In patients with LVV who are in remission and have aortic or branch artery complications that do not warrant urgent intervention, the role of elective endovascular or open surgical repair approach should be determined by a multidisciplinary team including, but not limited to, vascular surgery, vascular medicine, cardiology, and radiology specialists. The risk of such elective intervention is lowest when patients are in the remission phase of the LVV7; therefore, before intervention, imaging with 18F-FDG-PET CT is often helpful to assess treatment response and quantify the degree of ongoing active inflammation.1,4,5,18
The EULAR consensus definitions for relapse and remission have been incorporated into the 2018 updated recommendations for management of LVV.7 A major relapse of GCA and Takayasu arteritis includes recurrence of clinical features of ischemia (ie, visual loss, jaw claudication, limb claudication, stroke) or evidence of active aortic inflammation resulting in branch vessel stenosis, aortic aneurysm, or dissection. Remission of LVV is characterized by lack of new clinical symptoms, a normalization of inflammatory biomarkers, and no evidence of progressive aortic and branch artery dilation or narrowing by surveillance imaging. However, signals of vessel inflammation may persist even in the absence of clinical disease.1,6,19 For those in remission, annual surveillance imaging with CT or MRI is useful to detect disease progression in the aortic and branch arteries, even in the absence of inflammation. More frequent surveillance imaging may be necessary when evidence of active disease progression is apparent on annual imaging or if new symptoms suggestive of arterial stenosis arise.
9.2. Infectious Aortitis
9.2.1. Diagnosis and Management of Infection of the Native Aorta
Recommendations for Diagnosis and Management of Infection of the Native Aorta
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. In patients with infectious aortitis and associated aneurysms or dissection of the thoracic or abdominal aorta, open surgical repair is recommended. In select patients, treatment with endovascular repair may be considered.1–3 |
| 2b | C-LD | |
| 2a | C-EO | 2. In patients with infectious aortitis complicated by rupture, either open or endovascular repair is reasonable, based on the patient’s status at presentation and institutional expertise. |
| 2b | C-EO | 3. In patients with infectious aortitis, intravenous antimicrobial therapy of at least 6 weeks’ duration may be considered, with lifelong suppressive therapy in select cases not amenable to interventional repair or who have recurrent infection. |
Synopsis
The term “infectious aortitis” describes an infection of the aorta and has supplanted the older term “mycotic aneurysm,” which was used broadly but actually implies a fungal cause. Aortic infections arise from either contiguous spread from adjacent structures or septic emboli and hematogenous spread of microorganisms to the aortic wall via a vulnerable plaque or preexisting aneurysm.4 Staphylococcus aureus, Pneumococcus, Escherichia coli, and Salmonella are the pathogens identified in most reports.1–6 Syphilitic aortitis, which typically appears 10 to 25 years after systemic Treponema pallidum infection, is now rare. Fungal aortitis (from Candida or Aspergillus) and tuberculous aortitis are uncommon and typically arise in immunocompromised hosts.
Medical therapy is challenging because the causative organism is not always identified, but a prolonged course of antibiotics is often warranted.4 The mortality rate of infectious aortitis is high, because complications include sepsis, aneurysm formation (saccular or pseudoaneurysm), erosion and subsequent fistula, dissection, or rupture. CT and MRI can size the aneurysm, detect complications, and aid in interventional planning. TEE is especially useful for imaging involvement of the aortic root and associated complications.5 Open surgical repair is the standard treatment for infectious aortitis; however, in select patients with rupture, fistula, hemodynamic instability, or both, a hybrid or bridging approach with endovascular therapy (Table 36) may be used.2–8
Table 36.
Management of Aortic Mycotic Aneurysm: Comparison of Resection and Extra-Anatomic Reconstruction, In Situ Reconstruction, or Endovascular Device Repair
| Procedure | Potential Indications* | Advantages | Disadvantages |
|---|---|---|---|
| Extra-anatomic reconstruction | Infrarenal location with gross purulence, psoas or retroperitoneal abscess, vertebral osteomyelitis, inadequate response to antibiotic therapy, selected aortoenteric fistulae | Avoids placement of foreign body in infected area | Not technically feasible for thoracic, suprarenal, or visceral location or for emergency use Long operating time Long-term patency rates low Stump blowout Limb ischemia, amputation Reinfection rate higher than for in situ reconstruction Ischemic colitis |
| In situ reconstruction | Thoracic, suprarenal, infrarenal, or visceral location Selected aortoenteric fistulae |
More versatile than extra-anatomic: fewer long-term complications, higher patency rates, lower recurrent infection rate, shorter operating time Polyester grafts† available for emergency surgery |
Theoretical risk of infection because of interposition of foreign material in infected site |
| Endovascular device repair | Bridge procedure‡: hemodynamic instability, uncontrolled bleeding, rupture or impending rupture, selected patients with aortocentric fistulae, patients who are not fit for open surgery | Emergency stabilization Low early morbidity, mortality Less invasive No cross-clamping of aorta: spinal cord injury, reperfusion injury |
Persistent infections and device infections Higher long-term morbidity, mortality with device retention Requires device explanation, reconstruction |
Potential indication; must be individualized for each patient.
Polyester grafts, rifampin-soaked or silver-coated; less experience reported with cryopreserved arterial allografts or venous autografts.
Bridge procedure, used to stabilize patients until device explanation and arterial reconstruction.
Adapted from Wilson et al5 with permission of the American Heart Association, Inc. Copyright 2016 American Heart Association, Inc.
Recommendation-Specific Supportive Text
A diagnosis of infectious aortitis or mycotic aneurysm and its complications warrants prolonged antimicrobial therapy regardless of intervention, with 2016 scientific statement from the AHA suggesting a duration of 6 weeks to 6 months, with consideration of lifelong suppressive therapy in some cases.5 Given the high risk of rupture or contained rupture in infectious aortitis, open surgical repair is often warranted, although the data supporting open surgical repair are limited, with most evidence derived from single-institution case series and small cohort studies.6–8 Open surgical repair includes in situ reconstruction or aortic resection with extra-anatomic bypass (ie, axillobifemoral bypass or femorofemoral crossover bypass graft placement)3; surgical debridement of all infected tissue is essential to minimize the risk of persistent infection. The use of endovascular repair has been increasing in select patients with infectious aortitis.6–8 Limited data are available for comparison of open surgical versus endovascular repair; some small studies showed similar long-term survival between the 2 methods in treatment of infectious abdominal aortitis,1–4 although the evidence may have selection bias. In a nationwide Swedish retrospective population-based cohort study of 132 patients, of whom 50 (38%) presented with rupture, using propensity score analyses, 5-year survival was similar with open repair versus EVAR, at 60% versus 58%, respectively.3 Moreover, the use of EVAR was associated with improved short-term survival and was not associated with an increase in infection-related complications or a need for late reoperation.3 Use of endovascular repair in the management of infectious thoracic aneurysms, abdominal aneurysms, or both warrants ongoing study, and at present may be most appropriate as a bridge procedure in cases of instability or impending rupture, or in patients who may not be fit for open surgical intervention5 (Table 36).
The prognosis is often poor for infectious aortitis, especially if rupture has occurred.6 From a large single-institution study over 18 years of 2520 patients who underwent surgery for infectious aortic aneurysms, 24% of aneurysms had already ruptured at presentation, and 61% had penetrated into periaortic tissues.6 Open surgical treatment options include resection of infected aorta with extra-anatomic reconstruction (for abdominal aneurysm), or in situ reconstruction (for thoracic aneurysms and some aortoenteric fistulae).2–5,7,8 The choice of intervention is based on multiple factors (Table 36), including the location and extension of the aneurysm(s), the presence of fistulae, and the patient’s clinical status. In select patients with aneurysm rupture and hemodynamic instability and/or uncontrolled bleeding, endovascular repair may be used.6
Because peripheral blood cultures and surgical specimen cultures may be negative in a large proportion of patients with infectious aortitis,5 the choice of antimicrobial agents may be empiric, and infectious disease experts are usually involved in directing therapy. Treatment with antimicrobial therapy alone (ie, without intervention) is associated with high mortality rate and may not prevent aneurysm expansion or rupture6,9,10 and is thus reserved for patients who are not candidates for open or endovascular repair or for those in whom a palliative approach is appropriate. No clinical trial data are available to define the optimal duration of antimicrobial therapy, whether as solo therapy or as adjunctive therapy to aortic intervention, but expert opinion suggests a duration of at least 6 weeks, and possibly longer.5,11 Because the response of uncomplicated (without rupture or fistulae) infectious aortitis to antimicrobial therapy may influence the choice of interventional approach, it is also reasonable to have patients undergo surveillance imaging at intervals deemed appropriate by a multidisciplinary care team.
9.2.2. Diagnosis and Management of Prosthetic Aortic Graft Infection
Recommendations for Diagnosis and Management of Prosthetic Aortic Graft Infection
fmkReferenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| Diagnosis | ||
| 2a | B-NR | 1. In patients with a prosthetic aortic graft, who have signs and symptoms or culture evidence of unexplained infection or have unexplained gastrointestinal bleeding, cross-sectional imaging is reasonable to evaluate for an underlying aortic graft infection.1–6 |
| Treatment | ||
| 2a | B-NR | 2. In patients with an infected prosthetic aortic graft who are hemodynamically stable and have appropriate anatomy, it is reasonable to perform open surgery with either in situ reconstruction or extra-anatomic bypass.7–13 |
| 2a | B-NR | 3. In patients with an infected prosthetic aortic graft who are hemodynamically unstable, it is reasonable to perform open surgery with either explant or in situ reconstruction.7 |
| 2a | C-LD | 4. In patients with an infected prosthetic aortic graft, endovascular therapy is reasonable, either as bridge therapy in those with hemodynamic instability or as long-term therapy in those who are unsuitable candidates for open surgery.13–15 |
| Late Management | ||
| 1 | C-LD | 5. In patients who have undergone treatment of an acute prosthetic aortic graft infection, targeted intravenous antimicrobial therapy of at least 6 weeks’ duration, with prolonged suppressive oral therapy in select cases, plus a consultation and follow-up with an infectious disease specialist, is recommended.7,11,12,16,17 |
| 2b | C-LD | 6. In patients with an infected prosthetic aortic graft and either an extensive perigraft abscess or an infection caused by methicillin-resistant S. aureus, Pseudomonas aeruginosa, or a multidrug-resistant microorganism, or who have undergone in situ reconstruction, lifelong suppressive oral antimicrobial therapy may be considered after the initial course of therapy.14,15,18,19 |
Synopsis
Recommendations in this section apply to prosthetic aortic grafts. This includes tube grafts placed via open surgery as well as endovascular stent grafts. Although these grafts are typically made with Dacron or polytetrafluoroethylene, these recommendations also apply to allografts (eg, cryopreserved aorta) and autografts (eg, femoral vein).
Aortic graft infection is uncommon (0.3%–3%).20–22 Extension to the groin increases the risk of subsequent infection. Although some studies suggest a lower risk with endovascular versus open repair, the EVAR-1 (UK Endovascular Aneurysm Repair 1) RCT and a large Medicare analysis found equivalent rates of graft infection.23–25 Common sources of infection include: contamination at the time of implantation; graft enteric erosion or fistula to adjacent bowel, esophagus, or airway; or, rarely, hematogenous spread from remote infection. Suspicion is usually raised by symptoms, laboratory test abnormalities, or axial imaging findings. In the presence of an aortic graft infection, no surgical option is clearly superior. Basic tenets are to remove all infected tissue, including the graft and surrounding tissue, reconstruction of distal flow either as an extra-anatomic or in situ bypass, and coverage of the contaminated field with omentum, muscle flaps, or pleura. Previously, extra-anatomic bypass followed 24 to 48 hours later by graft explant and oversewing of the aortic stump was considered the gold standard for abdominal aortic infection but is usually not appropriate for the thoracic aorta. Aortic allografts, deep vein, and silver-impregnated or rifampin-soaked prosthetic grafts placed in situ have all shown good results as well, often with lower complication rates. A 6-week course of intravenous antibiotics is typically used, sometimes followed by long-term oral suppressive therapy.
Recommendation-Specific Supportive Text
Early graft infection (≤3 mo) is often associated with fever and back pain, whereas late graft infections (>3 mo) may have an insidious onset with symptoms of fatigue and malaise, or may have fever, an elevated white blood cell count, erythrocyte sedimentation rate, C-reactive protein, or advanced signs of sepsis with hemodynamic instability or frank hemorrhage from rupture or fistulae to adjacent bowel, esophagus, or airway. Because these signs and symptoms are nonspecific for site of infection, the initial workup should include basic blood work, blood cultures, and axial imaging, preferably with CTA. In those patients with bleeding, endoscopy may be used to rule out other causes and potentially temporize bleeding. Findings of graft infection on CT include peri-graft air, abscess, inflammatory changes, pseudoaneurysms, or frank hemorrhage. CTA has a sensitivity of 94% and specificity of 85% to 100% with advanced graft infection, but the sensitivity is only 64% for those with low-grade infection.1,2 The sensitivity and specificity for low-grade infection may be increased from 77% to 93% and 70% to 89%, respectively, with the use of PET-CT.3–5 MRI, tagged white blood cell scans, or both may also be useful, depending on local expertise and availability.6
Extra-anatomic bypass with subsequent graft explant, aortic stump oversewing, and omental coverage has a reasonably low rate of reinfection but a relatively high rate of amputation and occlusion and is susceptible to stump blow-out.7,8 In situ venous reconstruction has the lowest rate of reinfection but is associated with long operative times, size mismatch, and lower extremity venous morbidity.7,9 Cryopreserved allografts have a low rate of reinfection (similar to vein) but are susceptible to early and late degeneration, may have limited lengths and diameters, and have limited availability for emergencies.7,10,11 Rifampin- or silver-impregnated prosthetic grafts are more readily available and faster to implant than vein or extra-anatomic repair but are more susceptible to reinfection.7,26 None of these graft options is clearly superior to the others and, as such, in the stable patient without extensive infection with resistant organisms, the use of any of these is acceptable.27 For those with extensive peri-graft abscess, or infection with methicillin-resistant S. aureus, Pseudomonas, or multidrug resistant organisms, extra-anatomic reconstruction (when feasible) or in situ reconstruction with femoral vein or allograft may offer improved freedom from reinfection.7,13,26
Hemodynamically unstable patients require emergency proximal control with a clamp or balloon, and rapid in-line reconstruction, which is best performed with either an allograft (if immediately available) or a silver- or rifampin-impregnated prosthetic graft.7
Endovascular intervention allows relatively rapid control of hemorrhage and may improve survival in patients with an aorto-enteric fistula, when used as a bridge to definitive therapy.13–15 For patients who are not candidates for surgical graft excision, endovascular therapy may be considered for definitive therapy, in which case lifelong antibiotic suppression should be considered.
Consultation with an infectious disease specialist is recommended for all patients with aortic graft infection. A 6-week course of intravenous antimicrobial therapy has been recommended in multiple reports from high-volume centers and in scientific statements.7,11,16,17,26,28 For Pseudomonas or multidrug resistant organisms, multiple antimicrobial agents may be needed. A subsequent course of oral antimicrobial therapy for 3 to 6 months may be considered depending on the specific organism, the extent of infection, and the type of repair.
Lifelong suppressive oral antimicrobial therapy has been suggested for selected patients, such as those with extensive infection, aggressive organisms, in situ prosthetic replacement, or endovascular coverage without resection.14,15,18,19 Axial imaging is typically continued long term to identify evidence of reinfection, such as inflammatory changes, fluid or air collections, or pseudoaneurysm formation.
9.3. Atherosclerotic Disease
Recommendations for Atherosclerotic Disease
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In patients with aortic atherosclerotic disease and concomitant coronary artery disease, PAD or both, it is recommended to prescribe antiplatelet therapy, anticoagulant therapy or both, guided by the clinical setting.1–3 |
| 2a | C-LD | 2. In patients with aortic atherosclerotic disease and risk factors for confirmed coronary artery disease, it is reasonable to prescribe a moderate- or high-intensity statin.4–6 |
| 2b | C-LD | 3. In patients with aortic atheromas of a thickness ≥4 mm, statin therapy may be reasonable.1,7–9 |
Synopsis
Atherosclerosis is a chronic immunoinflammatory, fibroproliferative disease of the aorta and its branches that is propagated by lipids.10 This disease process has multiple risk factors and begins early in life so that the aorta may develop extensive disease over many decades.11 The diagnosis of aortic atherosclerosis may occur incidentally, during the evaluation of symptomatic vascular events, or both. The size and location of aortic plaques have been associated with embolic complications.4,7,8,12–16 The presence of aortic atheromas has been significantly associated with all-cause death.9 The management of aortic atherosclerosis includes, in general, control of risk factors, lifestyle modification, and appropriate pharmacological therapies. Although lifestyle changes may be the most important treatment strategy, compliance may be challenging.17,18
Recommendation-Specific Supporting Text
Patients with aortic atherosclerosis often have concomitant cardiovascular diseases such as coronary artery disease, atrial fibrillation, and PAD. These concomitant conditions frequently determine the selection of guideline-based antiplatelet agents, anticoagulant agents, or both.1–3
The indications for statin therapy in patients with a history of coronary artery disease, myocardial infarction, and stroke are well established.5,6 The data for statin therapy specific to aortic atherosclerosis alone are very limited. Therefore, this recommendation has been made for those patients at risk for or with confirmed coronary artery disease because the available data best support statin therapy in this cohort.
Atherosclerotic disease of the aortic arch is a potential source of emboli to the brain.1,2,9 A prospective study (N=500) showed that the OR for stroke among patients with aortic atheromatous plaques (atheromas) of ≥4 mm versus controls was 9.1 (95% CI, 3.3–25.2; P<0.001).7 Moreover, in a clinical trial of 519 patients with severe thoracic aortic plaques, multivariate analysis showed that statin therapy was protective against strokes (P=0.0001).8 (The data from these 2 studies relate specifically to atheroma thickness of ≥4 mm, which does not align precisely with the most commonly used grading systems for severity of aortic atherosclerosis, which define severe atheromas by a thickness of >5 mm.) Although antiplatelet therapy is commonly used in patients with aortic atheromas, there is no evidence to support the use of prophylactic anticoagulation in this population.
9.3.1. Aortic Thrombus
Aortic mural thrombus is typically associated with underlying aortic pathology, such as aneurysm, aortitis, atherosclerosis, dissection, and aortic graft material.1–3 Because such thrombi arise in the setting of underlying aortic pathology, the thrombi can be considered “secondary,” and they most often appear in the descending thoracic and abdominal aorta.1–3 In contrast, “primary” thrombus occurs in a normal or minimally atherosclerotic aorta and, rather than being mural, are often pedunculated and protrude into the aortic lumen. Most often, primary aortic thrombi are idiopathic, but some have been associated with hypercoagulable states (eg, malignancy, heparin-induced thrombocytopenia, and the antiphospholipid syndrome).2–6
Aortic thrombus is most often asymptomatic but may present with limb ischemia, visceral ischemia, or stroke2–7 from embolization. The diagnosis is often typically confirmed by either CTA or TEE.8,9 Asymptomatic patients with secondary mural thrombus are usually managed conservatively, but patients with primary aortic thrombus or those presenting with embolic events are often managed with anticoagulation, endovascular intervention, or open surgical therapy; such treatments are informed by the patient’s history and the location, size, and mobility of the thrombus.2–7,10,11 Long-term anticoagulation is most often considered in patients with thrombus in the ascending aorta and aortic arch, because of the increased risk of stroke from potential embolization should aortic thrombus recur.5–7,10
9.3.2. Aortic Occlusion
Aortic occlusion, which occurs most often secondary to extensive atherosclerotic disease, can present along a spectrum of acute and chronic clinical courses. CTA is most useful in identifying the occlusion, determining its cause, and defining the extent of associated aortic and branch arterial disease. Aortic occlusion typically occurs below the renal arteries but rarely can arise above this level, leading to renal and possibly visceral malperfusion.
Treatment in acute presentations is typically surgical, including open embolectomy in the setting of embolus or aorto-iliac and femoral reconstruction for atherosclerotic occlusion.1 Chronic aortic occlusion can occasionally be asymptomatic because collateral circulation has developed, in which case intervention may not be required. More commonly, patients with chronic aortic occlusion present with lower extremity claudication that may be accompanied by buttock claudication, central core muscle weakness, and impotence in males caused by pelvic malperfusion. These patients often have cardiopulmonary comorbidities and multifocal atherosclerotic disease, and these issues should be addressed preoperatively to mitigate potential complications.
Revascularization options include endovascular,2 open aortic (eg, aortobifemoral bypass),3 or extra-anatomic (eg, axillofemoral bypass), and hybrid options (eg, iliofemoral endarterectomy and patch plus iliac stenting). The preferred revascularization strategy is informed by the arterial anatomy, the severity of disease and symptoms, the patient’s substrate, and the expected procedural durability. No RCTs have shown an advantage for any given revascularization procedure, and all perform well in early follow-up. Open aortic reconstruction has improved long-term patency compared with less invasive options3 but at a cost of a higher risk of perioperative complications.
9.3.3. Porcelain Aorta
“Porcelain” aorta refers to the extensive, eggshell-like, near-circumferential or circumferential calcification of the intima or media of the aortic wall in the ascending aorta or aortic arch. It is most often associated with late-stage atherosclerosis, although it can also be a late consequence of aortitis. It generally occurs in older patients with atherosclerotic disease elsewhere and carries an increased risk for cardiovascular events and mortality.1 Porcelain aorta is best seen on a noncontrast CT scan, although very thin calcification may only be detected intraoperatively with epi-aortic ultrasound or manual palpation.Impenetrable ascending aortic calcification makes it difficult, if not impossible, to perform central aortic cannulation for cardiopulmonary bypass, the anastomosis of proximal coronary bypass grafts to the aorta, aortotomy during aortic valve replacement, and graft-aorta anastomoses during aortic replacement. Additionally, performing aortic cross-clamping for cardiopulmonary bypass can crack the calcified wall, increasing the risk of stroke from embolization, or immediate exsanguination. Surgical management strategies have included use of alternative sites for cannulation and proximal bypass grafts with off-pump or beating heart techniques,2–4 balloon occlusion of the aorta,5 and the use of circulatory arrest with ascending aortic replacement.6
9.4. Coarctation of the Aorta (CoA) and Congenital Abnormalities of the Arch
CoA is a narrowing of the aorta occurring most often just distal to the left subclavian artery, typically with an aneurysmal aortic segment immediately beyond the stenosis, but variants are frequent.1 Significant CoA presents with upper extremity hypertension and lower extremity hypotension (Table 37). MRI and CT are both useful to evaluate the extent of aortic narrowing and dilation, as well as the presence of collaterals,2 whereas TTE is useful for evaluating the gradient across the CoA, as well as identifying a coexisting BAV (present in 50%) and other potential congenital defects.3 Untreated CoA may be complicated by aortic dissection, heart failure, ruptured cerebral aneurysm, distal hypoperfusion, or the consequences of significant hypertension. Late complications following surgical or endovascular CoA repair may include undersized grafts, recurrent stenosis, aneurysm or pseudoaneurysm formation, and rupture, which are typically treated with endovascular procedures unless anatomic features dictate open or hybrid surgery.4–11 Hypertension is common after CoA repair, especially during exercise, and when the repair is performed in adults.12,13 Ambulatory BP monitoring and exercise testing are useful in diagnosis and management.12,13 Patients with CoA undergo lifelong follow-up and imaging because of the associated cardiovascular risks and the potential requirement for repeat intervention.6,14
Table 37.
| The presence of significant CoA is based on evidence of upper extremity hypertension (at rest, on ambulatory BP monitoring, or with pathologic blood pressure response to exercise) or left ventricular hypertrophy and evidence for 1 of these gradient measurements: |
| 1. A noninvasive blood pressure difference of >20 mm Hg between the upper and lower extremities |
| 2. A peak-to-peak gradient of >20 mm Hg across the coarct by catheterization; or a peak-to-peak gradient of >10 mm Hg across the coarct by catheterization in the setting of decreased left ventricular systolic function or significant collateral flow |
| 3. A mean gradient of >20 mm Hg across the coarct by Doppler echocardiography; or a mean gradient of >10 mm Hg across the coarct by Doppler echocardiography in the setting of decreased left ventricular systolic function or significant collateral flow |
CoA indicates coarctation of the aorta.
An aberrant subclavian artery (ASCA) is commonly an incidental finding but may present with compressive symptoms (including dysphagia and dyspnea) because it courses posterior to the esophagus and trachea and may associate with aneurysm disevase.15–18 A normal left aortic arch with a right ASCA occurs in ~1% of the population, whereas a right aortic arch with a left ASCA is much rarer and may form a vascular ring.17,18 Dilation of the origin of either a right or left ASCA occurs in 20% to 60% of cases and is known as a Kommerell diverticulum.15,18 Such Kommerell diverticula may lead to aortic dissection, rupture, or embolization.18–20 Indications for treatment of ASCA relate to symptoms and aneurysm size.
9.4.1. Coarctation of the Aorta
Recommendations for CoA
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with CoA, including those who have undergone surgical or endovascular intervention, an MRI or CT is recommended for initial, surveillance, and follow-up aortic imaging.1–4 |
| 1 | C-EO | 2. In patients with CoA, BPs should be measured in both arms and one of the lower extremities. |
| 1 | B-NR | 3. In patients with significant native or recurrent CoA (Table 37) and hypertension, endovascular stenting or open surgical repair of the coarctation is recommended.2,3,5–12 |
| 1 | C-EO | 4. In patients with CoA, guideline-directed medical therapy is recommended for the treatment of hypertension.13 |
| 2b | B-NR | 5. In adult patients with CoA, screening for intracranial aneurysms by MRI or CT may be reasonable.14–18 |
Synopsis
CoA may have many anatomic variants and occurs most commonly at the level of the ductus arteriosus and distal to the left subclavian artery. Echocardiogram is indicated in the evaluation of patients with CoA because a BAV coexists in at least 50% of cases, and CoA may associate with complex congenital heart disease.4 Upper extremity hypertension and lower extremity hypoperfusion are the hallmarks of CoA. Intracranial aneurysms may occur in adults with CoA.14–16 Ascending aortic aneurysms may occur in those with BAV, and aneurysms may be present in the distal arch and descending aorta.2,11,19,20 Untreated CoA may be complicated by aortic dissection, heart failure, ruptured cerebral aneurysm, or complications from hypertension. Repair of CoA is performed by endovascular, open surgical, and hybrid procedures, depending on patient-specific and anatomic features.2,3,5,8–12 In patients with previous procedures, late complications may include recurrent stenosis, aneurysm or pseudoaneurysm formation, rupture, and persistent hypertension.2,3,6,8,12,21 Hypertension is common after CoA repair, especially during exercise, and ambulatory monitoring and exercise testing may be useful in diagnosis and management.3,6,7,22–24 Lifelong clinical and imaging follow-up is important to evaluate for hypertension, recurrent coarctation, and aortic wall abnormalities after repair.1,2,6,24
Recommendation-Specific Supportive Text
In patients with CoA, both MRI and CT are can detect coexistent BAV, examine the full thoracic aorta for coexistent aneurysm disease or arch abnormalities, and assist in treatment planning.4,25 TTE is also can detect the gradients across the site of the coarctation and assess for recoarctation (recurrence of a significant coarct). After repair of CoA, complications may occur, including recoarctation, aortic aneurysm, pseudoaneurysm, and aortic dissection.2,3,11,12,26 Arch and descending aortic complications are better visualized by MRI or CT than TTE. The optimal imaging frequency after repair of CoA is not well established and is best individualized based on the type of repair, physical examination findings, and previous imaging findings.27 After establishing stable aortic imaging after CoA repair, surveillance imaging is often obtained every 3 to 5 years.20,28–30 Recoarctation occurs in about 10%6,8 after surgical repair and about 8% after balloon dilation.21 After endovascular repair of CoA, MRI or CT can evaluate for complications, recoarctation, or endoleaks.2,3,11
Patients with a significant CoA typically have hypertension in the upper extremities and a reduction in BP in the lower extremities. The location of the CoA will inform any BP differential between the left and right arms. Physical examination may reveal a delay in timing and a decreased amplitude of the femoral pulse. After CoA repair, recurrent coarctation may occur. Obtaining the BP in the upper and lower extremities assesses for native and recurrent coarctation.
CoA presents with upper extremity hypertension, lower extremity hypoperfusion, and imaging confirmation of narrowing of the aorta that may include collateral formation.2,3,6,11 Significant native or recoarctation has been variably defined, but commonly used criteria are listed in Table 37.7,11 The presence of left ventricular hypertrophy is an important marker of disease.28 In addition to abnormal aortic gradients, anatomic evidence for CoA is necessary and is well characterized by MRI or CT. Adult congenital guidelines have reported the best evidence to proceed with intervention to correct CoA, including hypertension, BP differential between upper and lower extremities, and TTE-derived gradients across the coarctation.11 For individuals with native or recurrent CoA and appropriate anatomic characteristics, endovascular treatment with stenting is typically performed.2,3,6,9–12,29 Open surgical repair of CoA may include subclavian flap aortoplasty, resection and end-to-end anastomosis, interposition grafting, or bypass grafting, with the choice of procedure informed by patient- and anatomic-specific characteristics.5,11 In adults who have undergone a previous open surgical CoA repair and develop recoarctation, aneurysm, or pseudoaneurysm, an endovascular approach (assuming there is adequate iliofemoral access and absence of involvement of the supra-aortic trunks) avoids the need for reoperation.2,3,9,12,29
Patients with CoA are at risk for complications of hypertension, including heart failure, stroke, coronary artery disease, and aortic complications, so hypertension should be assessed and in accordance with current guidelines.13 Multiple studies have shown that persistent hypertension is common after CoA correction.3,6,7,23,24 Ambulatory BP monitoring and exercise testing may be useful in the evaluation and treatment of hypertension in patients with native CoA and after repair.3,22,24
Screening studies suggest that adults with CoA have an 10% prevalence of intracranial aneurysms (compared with a prevalence of 2% in the normal adult population), with the greatest risk among older adults and those with hypertension.14–16,18 Cost-effective analysis supports screening for intracranial aneurysms in adults with CoA, but preferred screening strategies remain unknown.17 Because many of the intracranial aneurysms detected by screening will be very small and not require treatment, shared decision-making about screening may be informed by age, risk factors, and anticoagulation considerations.18,30
9.4.2. Other Arch Abnormalities
9.4.2.1. Aberrant Subclavian Artery, Kommerell’s Diverticulum
Recommendations for Aberrant Subclavian Artery, Kommerell’s Diverticulum
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1. In patients discovered to have an ASCA in the absence of thoracic aortic imaging, dedicated imaging to assess for TAA is reasonable.1,2 |
| 2b | C-LD | 2. In patients with Kommerell’s diverticulum, depending on patient anatomy and comorbidities, repair may be reasonable when the diverticulum orifice is >3.0 cm, the combined diameter of the diverticulum and adjacent descending aorta is >5.0 cm, or both (Figure 27).3 |
Figure 27. Measurements of Kommerell’s Diverticulum.
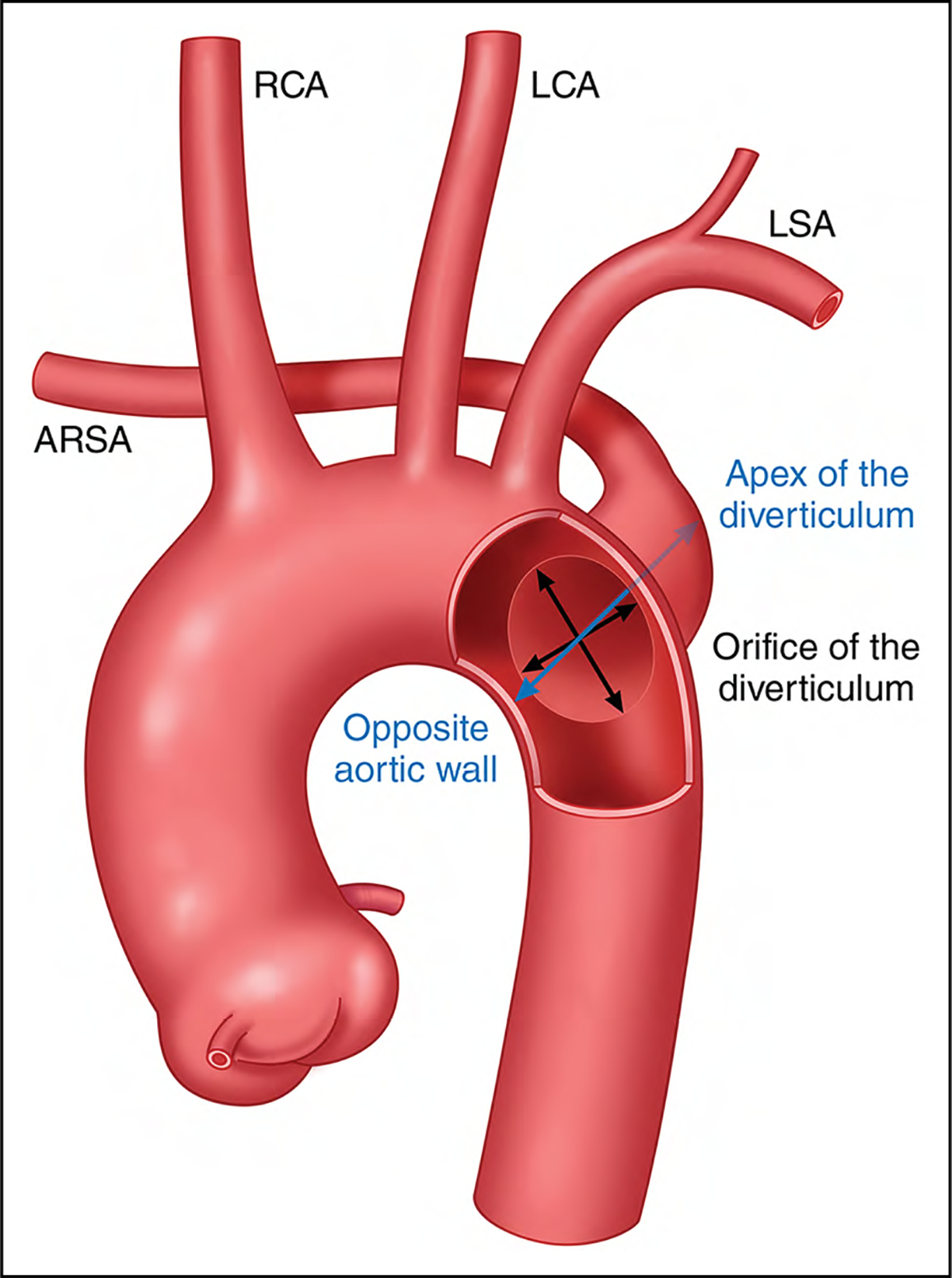
Two diameter measurements should be obtained using cross-sectional imaging: the diverticulum orifice (radially and longitudinally at the aortic wall) and the combined diameter of the diverticulum and adjacent descending thoracic aorta (measured from the apex of the diverticulum to the opposite aortic wall). ARSA indicates aberrant right subclavian artery; LCA, left common carotid artery; LSA, left subclavian artery; and RCA, right common carotid artery. Adapted from Erben et al,7 Copyright 2020, with permission from Elsevier, Inc., and the Society for Vascular Surgery.
Synopsis
Anomalies of the aortic arch are usually detected incidentally on a CT of the chest or neck ordered for other reasons. An ASCA arises as the fourth branch from the aorta, distal to the left subclavian artery (or right subclavian artery in the case of a right-sided aortic arch). It courses through the posterior mediastinum behind the esophagus in its path to perfuse the arm and can cause a vascular ring around the trachea and esophagus that results in dysphagia, respiratory symptoms, or recurrent laryngeal nerve palsy. Kommerell’s diverticulum is a persistent remnant of the fourth primitive dorsal aortic arch because of failed regression3 and may be present in 20% to 60% of patients with an aberrant right or left subclavian artery. The risk of rupture or dissection of a Kommerell’s diverticulum has been reported to be as high as 50% in case series, although high-quality data on the natural history are very limited. The 2020 SVS clinical practice guidelines recommend surgical intervention for Kommerell’s diverticulum when the diverticulum orifice is >3.0 cm, the combined diameter of the diverticulum and adjacent descending aorta is >5.0 cm, or both.4 Successful repair has been described using open, endovascular, and hybrid approaches depending on patient anatomy and comorbidities.3
Recommendation-Specific Supportive Text
Variant aortic arch anatomy has been found to be significantly associated with TAA in several single-center retrospective observational series,1 with 33% of patients with right-sided aortic arch having concomitant TAA.2 Left-sided aortic arch with aberrant right subclavian artery was also significantly associated with TAD but only occurred in 2% to 8% of those patients.1 Consequently, if the imaging study that detected the ASCA did not include imaging of the thoracic aorta, then a dedicated CT or MRI to evaluate for an associated aortic aneurysm is reasonable.
Case series have reported rupture and/or dissection of Kommerell’s diverticulum for diverticula ranging from 4.0 cm to 10 cm (mean size, 5.0 cm).3 The measurement of the Kommerell’s diverticulum may be difficult, and various strategies to standardize measures have been proposed.3 Based on CT, 2 diameter measurements should be obtained (Figure 27) using cross-sectional imaging: the diverticulum orifice (radially and longitudinally at the aortic wall) and the combined diameter of the diverticulum and adjacent descending thoracic aorta (measured from the tip of the diverticulum to the opposite aortic wall6). Repair of Kommerell’s diverticulum has been suggested when the orifice diameter is >3 cm or the combined diameter of the diverticulum and adjacent descending thoracic aorta is >5.0 cm.3,4,7
9.4.2.2. Aberrant Left Vertebral Artery Origin
Recommendation for Aberrant Left Vertebral Artery Origin
| COR | LOE | Recommendation |
|---|---|---|
| 2a | C-EO | 1. In patients with an aberrant left vertebral artery origin arising directly from the thoracic aorta who require aortic repair involving reconstruction or coverage of the vertebral artery origin, revascularization of the vertebral artery is reasonable. |
Synopsis
The most common anatomic variant for the left vertebral artery is arising directly from the aortic arch; 6% of adults have a left vertebral artery that arises from the arch between the left carotid and left subclavian arteries,1,2 rather than of a branch of the left subclavian artery. There is a paucity of data on the management of the left vertebral artery arising from the aortic arch in patients undergoing thoracic aortic repair. For patients undergoing elective open surgical partial or total arch repair or undergoing TEVAR for TAA or dissection, revascularization of the left subclavian artery is recommended to preserve left vertebral artery perfusion and reduce the risk of symptomatic vertebrobasilar insufficiency, SCI, and stroke.3 This may be particularly important in patients with a dominant left vertebral artery or a nonintact circle of Willis. Vertebral artery revascularization via either an open bypass or transposition technique can be accomplished with good outcomes.3,4
Recommendation-Specific Supportive Text
In patients undergoing elective TEVAR with planned left subclavian artery coverage, preoperative revascularization of the left subclavian artery has been shown to decrease the risk of stroke and SCI,5–8 presumably by maintaining perfusion through the posterior circulation via the left vertebral artery. In a small series of 9 patients with an aberrant left vertebral artery origin undergoing open aortic arch replacement, no neurologic complications were reported among patients who first underwent revascularization of the left vertebral artery.4
9.4.2.3. Bovine Arch (Common Innominate and Left Carotid Artery)
Recommendation for Bovine Arch (Common Innominate and Left Carotid Artery)
Synopsis
The most common anatomic pattern of great vessel origin, occurring in approximately 70% of adults, is a type I arch, in which the 3 great vessels originate directly from the aorta.4 Bovine arch variants are the most common arch anomalies, and 2 types are described: In type II-A, found in 9% of the population, the left common carotid artery arises directly from the innominate artery (Figure 28); in type II-B, found in 13% of the population, the innominate and left common carotid arteries arise from a common origin (Figure 28).5 The term “bovine arch” is a misnomer, because the arch vasculature in cattle has a single, large brachiocephalic vessel that subsequently trifurcates into 2 subclavian arteries and a bicarotid trunk.5 Others have referred to the bovine aortic arch pattern as an aortic arch with a common origin of the innominate and left carotid artery.
Figure 28. Normal and Bovine Aortic Arch Configurations.
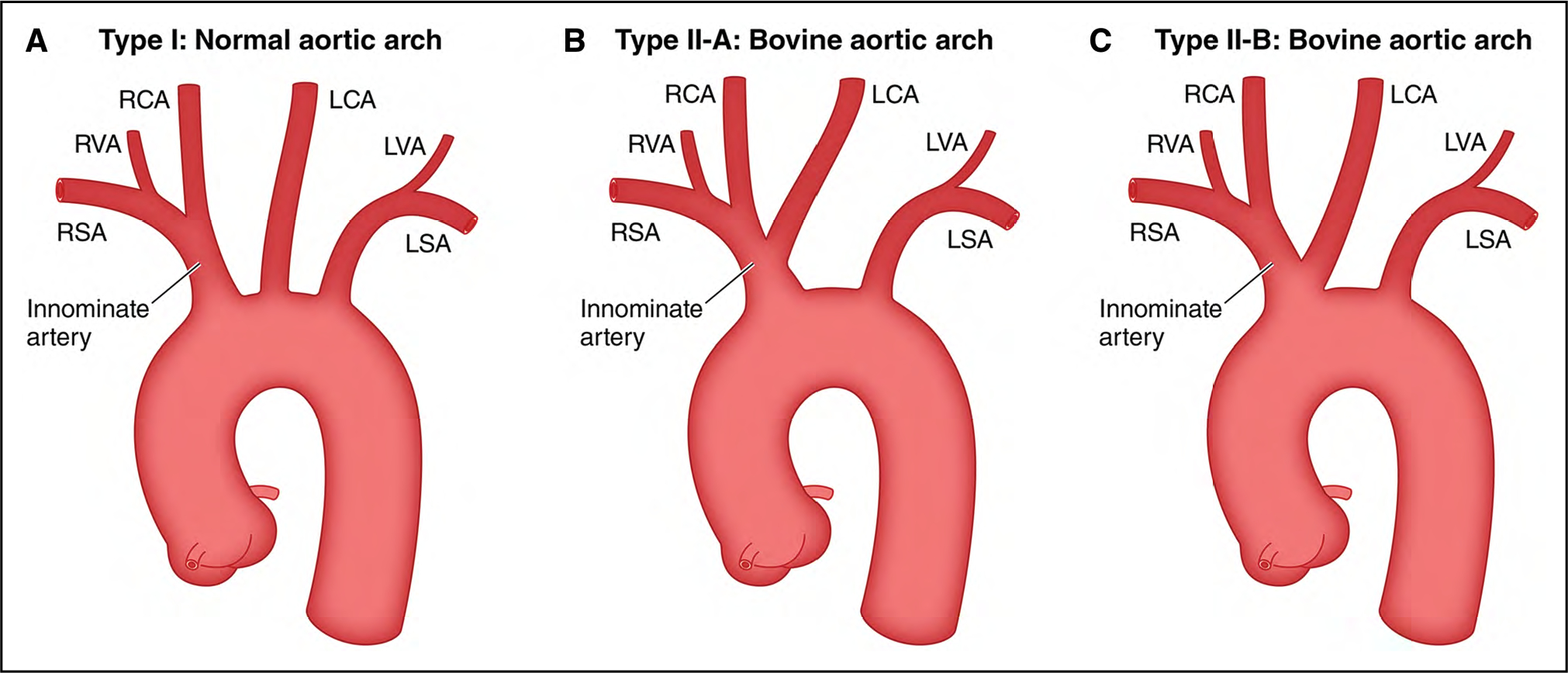
(A) Type I aortic arch: The normal aortic arch configuration. (B) Type II-A aortic arch: The left common carotid artery originates from the innominate artery. (C) Type II-B aortic arch: The innominate and left common carotid arteries share a common origin. LCA indicates left common carotid artery; LSA, left subclavian artery; LVA, left vertebral artery; RCA, right common carotid artery; RSA, right subclavian artery; and RVA, right vertebral artery. Adapted from Layton et al.5 Copyright 2006, American Society of Neuroradiology. Used with permission from Mayo Foundation for Medical Education and Research, all rights reserved.
Some authors have suggested that a bovine arch increases the risk of aortic dissection, but the data are limited.2,6 Among patients with acute type A aortic dissection, a bovine arch was highly predictive of an arch tear (OR, 5.9; 95% CI, 2.89–12.04; P<0.001) and increased perioperative stroke (OR, 2.69; 95% CI, 1.2–6.0; P=0.016) based on multivariable analysis, although it was not associated with worse long-term survival.7
Recommendation-Specific Supportive Text
A bovine aortic arch appears to be a marker for TAD and more rapid aortic expansion.1 Among patients with TAD, the prevalence of a bovine arch was 26.3%, compared with 16.4% in controls (P<0.001). Moreover, among patients with TAA, the annual aortic growth rate was 0.29 cm/y among those with a bovine arch versus 0.09 cm/y among those with normal arch anatomy. A recent meta-analysis found that the proportion of TAD among patients with bovine arch was 41.5%, compared with 34.0% among patients with standard arch configuration.3 If aortic dilation or aneurysm is found on imaging, subsequent surveillance imaging may be obtained.
9.5. Tumors
Tumors of the thoracic aorta are usually secondary, resulting from contiguous or metastatic spread of primary malignancies, especially lung and esophageal.1,2 Primary malignant tumors of the aorta, which are extremely rare, are most often primary sarcomas that protrude into the lumen but leave the aortic wall intact. Aortic sarcomas are aggressive tumors with a propensity for arterial embolization, disseminated metastases, and rapid clinical deterioration,3,4 usually with limited survival after initial diagnosis.5,6 Tumors of the thoracoabdominal aorta may exhibit nonspecific symptoms. On imaging, aortic tumors are often initially mistaken for atherosclerosis or aneurysmal disease7 (although PET imaging may suggest tumor metabolic activity over metabolically quiescent atherosclerosis), so the diagnosis is often made by histologic examination of embolic debris or surgical specimens8–10; in some cases, the diagnosis is made postmortem. Combined therapy with surgery (resection and reconstruction of the segment of aorta containing the neoplasm) and chemoradiotherapy provide the best survival results, although the overall prognosis remains poor.
10. PHYSICAL ACTIVITY AND QUALITY OF LIFE
Recommendations for Physical Activity and Quality of Life
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. For patients with significant aortic disease, education and guidance should be provided about avoiding intense isometric exercises (eg, heavy weightlifting or activities requiring the Valsalva maneuver), burst exertion and activities, and collision sports.1,2 |
| 1 | C-EO | 2. For patients who have undergone surgery for aortic aneurysm or dissection, postoperative cardiac rehabilitation is recommended.3,4 |
| 2a | C-LD | 3. In patients with thoracic or abdominal aortic aneurysms whose BP is adequately controlled, it is reasonable to encourage 30 to 60 minutes of mild-to-moderate intensity aerobic activity at least 3 to 4 days per week.5,6 |
| 2a | C-LD | 4. For patients with clinically significant aortic disease, it is reasonable to screen for anxiety, depression, and posttraumatic stress disorder and, when indicated, provide resources for support7,8; it is also reasonable to provide education and resources to minimize patients’ concerns, support optimal decision-making, and enhance quality of life.5,9–11 |
Synopsis
As surgical outcomes for aortic disease improve, a focus on patient-reported health-related quality of life (HRQOL) outcomes is becoming increasingly important,10 because patients have become increasingly concerned about HRQOL issues such as returning to work, pain management, risk of infection, activity recommendations and restrictions, and neurologic complications. The most common measures of HRQOL are generic patient-reported outcome measures (eg, SF-36), although validated aneurysm-specific measures have been developed.7,12,13
In patients with Marfan syndrome in the GenTAC registry, HRQOL was slightly below the population norm. Better HRQOL was independently associated with socioeconomic factors (eg, private insurance, active employment) but not factors related to disease severity or comorbidities.14,15 Although aneurysms are usually asymptomatic before diagnosis, surgical aortic repair is associated with an initial deterioration in HRQOL at 3 months, including decreased physical, cognitive, and social function that generally returns to preoperative levels after 6 to 12 months.11 Standardized reporting of preoperative and postoperative HRQOL measures is needed to guide further improvements in interventional strategies and improve the overall patient experience.16
Patients with aortic aneurysms, who have adequate BP control, may have improvements in overall cardiovascular health when undertaking moderate intensity aerobic activity at least 3 to 4 days per week, 30 to 60 minutes per session.17–19 Although resistance training may be beneficial to patients with cardiovascular disease, it increases central aortic BP and, therefore, benefits for those with aortic aneurysm are less well understood because, theoretically, increases in BP could contribute to subsequent aortic growth, complications, or both. Further longitudinal study is warranted.20–22
Recommendation-Specific Supportive Text
In patients with aortic disease, limited data are available to guide recommendations regarding the forms of exercise that are safe and promote cardiovascular health versus those that pose an acute or long-term risk of aortic growth or rupture. But evidence exists regarding the physiologic benefits of exercise and the hemodynamic consequences of various form of exercise and exertion in case series and relevant animal models. There has been a uniform consensus among numerous expert committees on aortic disease that it is wise to avoid intense isometric exertion or exercises that require the Valsalva maneuver, given that heavy lifting with Valsalva can produce acute increases in SBP to >300 mm Hg. There is also a consensus that light weightlifting and low-intensity aerobic exercise are safe and likely improve both physical and mental health. No uniform consensus exists about the safety of intermediate-level static and aerobic exercise. Recommendations for exercise intensity are best individualized, informed by multiple factors that include underlying aortic pathology, aortic diameter and ASI, aortic growth rate, age, family history, and any other high-risk features (eg, uncontrolled hypertension). Ongoing investigation is needed to better define the levels of resistance activities that would be considered low-risk for adverse aortic events, favoring greater exercise restrictions among patients at higher risk of dissection.17,23–26,27
Although data are limited, cardiac rehabilitation has been shown to be useful and safe for patients after aortic surgery.4,5,27,28 A randomized trial of exercise-based cardiac rehabilitation in patients who have undergone surgery for type A aortic dissection showed improved peak oxygen uptake, maximal workload, and HRQOL.3 Fear of a repeat cardiac event can cause patients who are post–aortic dissection to decrease or stop exercise and sexual activity, but mild-to-moderate intensity exercise may be cardioprotective. Because of deconditioning, patients may be unable to exercise initially at the recommended level.27 An intensity of 3 to 5 metabolic equivalents of task is recommended, while avoiding strenuous lifting, lifting to the point of exhaustion, or other activities that entail maximal exertion.6,29 In a retrospective study, patients with small AAA went through a modified cardiac rehabilitation program before surgery, and the rate of aortic growth was slower in the rehabilitation group.28
High-intensity athletic training in 1 study has been shown to be an independent predictor of aortic growth, although these data were limited to the aortic root and did not include AAA.30 In a recent study in 442 athletes of mean age 61 years, aortic root enlargement by z-score was present in 24% of participants and, after multivariate analysis, elite competitor status was found to be an independent predictor of aortic growth.31 Less is known about the potential effects of mild-to-moderate intensity aerobic activity on aortic growth, but it is known to improve overall cardiovascular health, including among patients with TAA32–34 and AAA.20,35,36 A recent meta-analysis suggests that that higher physical activity is associated with a reduced risk of AAA.37 In 1 study of a mouse model of Marfan syndrome, rates of aortic root growth were significant slower in mice that exercised daily on a treadmill compared with sedentary mice.38 In another study of mice with Marfan syndrome, both mild and moderate—but not strenuous—aerobic exercise protected the structural integrity of the aortic wall, as evidenced by reduced elastin fragmentation and reduced expression of matrix metalloproteinases 2 and 9 within the aortic wall, compared with sedentary controls.39
Depression and anxiety often occur in patients with aortic disease, regardless of surgical status. Posttraumatic stress disorder after dissection is a particular risk.8 Screening patients and providing resources for assistance may prevent mental health issues from becoming more severe and lead to an increased HRQOL.9,40 The SF-36 is a common tool for assessing mental health for these patients7,11,12,41 but may not cover all patient concerns, such as activity restriction, family life, and losing ability to earn income.16 More studies are needed with both pre- and postoperative HRQOL data to improve shared decision-making and patient outcomes.12,13,41 Exercise may decrease depression.9 Education before procedures helps most patients feel more satisfied with their procedures16 and improve postoperative HRQOL.41 Patients and clinicians can define surgery success differently, showing the importance of discussing expectations and risks.
11. COST AND VALUE CONSIDERATIONS
Although assessment of cost and value in development of guidelines is of growing importance, studies are limited on the cost-effectiveness of aortic disease treatment and lack standard methods for comparison.1
Screening for AAA among men ≥65 years of age has been shown to be cost-effective,2,3 although data for screening women are less clear. Women have a lower incidence of AAA but higher risk of rupture and longer life expectancy, so incremental cost-effectiveness is similar to men and may justify screening, especially in those with a history of smoking.4
In patients with AAA, studies comparing EVAR to open surgical repair generally show lower initial costs for EVAR based on shorter hospital stays; however, ongoing expenses for EVAR surveillance and reinterventions may minimize long-term cost advantages after 2 to 5 years.5–9 In addition, significant variability in costs across organizations and countries, and changing efficiencies in techniques, makes it difficult to make recommendations on preferred interventional approaches based primarily on relative costs.6,10,11
Findings are mixed but similar for descending TAA, with trends toward lower initial hospital costs with TEVAR compared with open surgery stemming from shorter length of stay, but the long-term results are more neutral.12–14
Few data examine the cost-effectiveness of management strategies of TAA. For the management of AAS, the costs are not easily modifiable. However, for management of chronic TAD, patients often see a host of specialists, including both cardiologists and surgeons, have follow-up visits with specialists in both the community and at tertiary or quaternary centers, or both. Moreover, diagnostic imaging is often duplicated because of differences in imaging protocols or quality, or simply because images are not readily transferrable. Consequently, there are likely opportunities for significant cost savings if redundant clinician visits and imaging could be reduced through common protocols, common imaging platforms, and coordinated care.15
12. EVIDENCE GAPS AND FUTURE DIRECTIONS
Most of the current recommendations for patients with aortic disease are based on expert opinion and data from observational studies, large registries, and prospective studies, but few are from randomized clinical trials. More data are needed from basic science studies and RCTs to guide prevention, early diagnosis, and advanced treatment for aortic disease. In the future, precision medicine and patient-centered approaches will enable clinicians to develop care plans to optimize outcomes for each patient. Future research should include diverse populations and examine race, ethnicity, and sex differences to ensure that all patient groups are represented and that questions pertinent to their aortic health are answered.
12.1. Biomarker Studies
Although interest in using circulating biomarkers for risk stratification of patients with aortopathy has increased, biomarker expression has not been clearly associated with relevant clinical aortic events. Most studies have focused on protein-based biomarkers and noncoding RNAs in patients with bicuspid aortopathy. These emerging biomarkers and other better, early-stage biomarkers, along with advanced noninvasive imaging modalities, may help us precisely identify the risk associated with adverse outcomes in these patients. In addition, noncoding RNAs such as microRNA are biological molecules whose expression can be modified through targeted mechanisms and present opportunities to identify new treatment options for patients with aortic disease.1–7 In addition, developing image-based cardiac and aortic markers derived from large-scale imaging studies with automated machine learning–based analysis might provide a wealth of information for guiding the optimal care of these patients.
12.2. Genetic and Nongenetic Factors
Various genes have been associated with and linked to TAA and dissection. Consequently, genetic testing can identify pathogenic mutations in specific genes that increase a patient’s risk of aneurysm, dissection, or both and may inform the optimal timing of aortic repair. As the prevalence of genetic testing increases, the discovery of more genes will help in the earlier diagnosis of asymptomatic nonsyndromic TAA. In addition to the contribution of genetic variants, environmental factors and lifestyle habits may contribute to aortic aneurysm formation. Further research on these factors may provide evidence to guide lifestyle modifications that could reduce a patient’s lifelong aortic risk. Recent evidence suggests that fluoroquinolone use is associated with an increased risk of aortic aneurysm and dissection, but the pathways through which this effect is mediated are unknown. Future research investigating the potentially protective or harmful effects of other pharmacologic agents on aortic health might further elucidate the pathophysiology of aortic disease.1–10
12.3. Biomechanics of the Aorta
Emerging evidence suggests that aortic diameter alone is an insufficient predictor of risk for aortic dissection. Understanding the distribution of biomechanical wall stress in the various anatomic locations of the aorta, as well as potential contributions of hemodynamic flow disturbances such as those from aortic valve stenosis or regurgitation, or even from a well-functioning BAV, may improve risk stratification strategies and, in turn, patient outcomes, filling a knowledge gap on wall stress distribution in patients with aortic aneurysms.1–4
12.4. Sex, Race, and Ethnicity
Conflicting data exist in the literature on the association between sex and outcome in patients with aortic disease. Studies have shown different rates of aortic aneurysm growth and dissection risk in male versus female patients. Nevertheless, the data are inconsistent, because some outcome studies indicate that sex affects prognosis, whereas others show no impact of sex. Clearly, further research is needed to elucidate the impact of sex on the incidence and progression of aortic disease, the risk of aortic dissection, and the outcomes of intervention. Even more challenging is the fact that few studies have been published on racial and ethnic disparities among patients with aortic disease and those undergoing aortic intervention. Moreover, it is unclear that all patients with aortic disease have equal access to skilled practitioners to care for them, so it is imperative that we seek ways to actively minimize such health care disparities.1–17 Similarly, efforts should be made to broaden clinical trials to represent the diverse populations that we treat; study design, methodology, reporting, and implementation should be designed to be more inclusive.18,19
12.5. Quality of Life in Patients With Aortic Disease
Baseline HRQOL assessment in patients with aortic disease is lacking, and the few studies that have targeted HRQOL have been conducted only in patients receiving endovascular or open aortic repair. The impact of physical, mental, emotional, sexual, and professional status on the psychosocial well-being, tolerance of medical therapies, and recovery from aortic intervention has not been well studied. The long-term effects on physical and mental HRQOL after aortic repair are unknown. In addition, evidence-based knowledge on studies targeting quality of life in patients with heritable TAA is narrow or limited only to patients with Marfan syndrome; almost no studies have been performed in patients with Loeys-Dietz syndrome or vascular Ehlers-Danlos syndrome, for example. Furthermore, only scattered studies have examined strategies for boosting the psychological health of patients with aortic disease and those undergoing aortic surgery. Aortic diseases require a lifetime of treatment and surveillance, so research is needed on ways to improve and sustain patient engagement, especially among those who are disadvantaged or at a lower educational level.1–8
12.6. New Endovascular Technology
Advances in endovascular technology have dramatically impacted treatment strategies in patients with aortic disease requiring intervention. Despite this significant progress, current endovascular designs are limited in their application because of the differing hemodynamic and anatomic challenges presented by each segment of the aorta and individual differences in aortic anatomy. In addition, operator knowledge and experience, as well as methodical patient selection, are important for obtaining optimal outcomes from endovascular procedures. Continued evolution in stent-graft design, focused on flexibility and durability, improved vascular imaging technology, and advances in simulation training for operators, will likely further reduce the risk of reinterventions and improve long-term outcomes.1–6
12.7. Optimal Exercise and Rehabilitation Protocols
Very limited research has been conducted on optimal exercise in patients with aortic disease. Moreover, no specific rehabilitation strategies exist for patients who still have untreated diseased aortic segments after surgical aortic repair and who do not meet the surgical threshold for intervention. Developing patient-centric rehabilitation protocols and individualized exercise programs for patients with aortic disease is an unmet need that requires further study.1–4
12.8. Equitable Care and Training Opportunities
Sociodemographic disparities can pose challenges to patients and clinicians who seek and offer cardiovascular and aortic care. Market competition, a relatively modern phenomenon, and physician market concentration can drive decision-making and subsequently affect optimal care. Providing optimal cardiovascular and aortic care will depend on widespread regional quality improvement projects to determine best practices, minimize variations in areas where evidence-based medicine has finite benchmarks, and standardize patient selection and case management. Physician participation in these programs should be encouraged, and educational interventions and training should be provided to disseminate knowledge and improve performance, which will help increase awareness for patients and physicians in less-populated, underserved areas.1–3
Supplementary Material
1.6. Abbreviations
- 3D
3-dimensional
- AAA
abdominal aortic aneurysm
- AAS
acute aortic syndrome
- ACEI
angiotensin-converting enzyme inhibitor
- AHI
aortic height index
- AR
aortic regurgitation
- ARB
angiotensin receptor blocker
- ASCA
aberrant subclavian artery
- ASCVD
atherosclerotic cardiovascular disease
- ASI
aortic size index
- AVR
aortic valve replacement
- BAAI
blunt traumatic abdominal aortic injury
- BAV
bicuspid aortic valve
- BP
blood pressure
- BSA
body surface area
- BTAI
blunt traumatic aortic injury
- BTTAI
blunt traumatic thoracic aortic injury
- CMR
cardiac magnetic resonance
- CoA
coarctation of the aorta
- CT
computed tomography
- CTA
computed tomographic angiography
- DBP
diastolic blood pressure
- DMARD
disease-modifying anti-rheumatic drug
- ECG
electrocardiogram
- EVAR
endovascular abdominal aortic aneurysm repair
- FID
focal intimal disruption
- FDA
US Food and Drug Administration
- FDG-PET
fluorodeoxyglucose–positron emission tomography
- FEVAR
fenestrated endovascular aortic repair
- GCA
giant cell arteriti
- HRQOL
health-related quality of life
- HTAD
heritable thoracic aortic disease
- ICU
intensive care unit
- IMH
intramural hematoma
- IRAD
International Registry of Acute Aortic Dissection
- LDL
low-density lipoprotein
- LVV
large vessel vasculitis
- MR
magnetic resonance
- MRA
magnetic resonance angiography
- MRI
magnetic resonance imaging
- nsHTAD
nonsyndromic heritable thoracic aortic disease
- PAD
peripheral artery disease
- PAU
penetrating atherosclerotic ulcer
- PET
positron emission tomography
- rAAA
ruptured abdominal aortic aneurysm
- RCT
randomized controlled trial
- REBOA
resuscitative endovascular balloon occlusion of the aorta
- rEVAR
endovascular repair for rAAA
- SMA
superior mesenteric artery
- SBP
systolic blood pressure
- SCI
spinal cord injury
- TAA
thoracic aortic aneurysm
- TAAA
thoracoabdominal aortic aneurysm
- TAAD
thoracic aortic aneurysm and dissection
- TAD
thoracic aortic disease
- TAR
total arch replacement
- TEE
transesophageal echocardiography
- TEVAR
thoracic endovascular aortic repair
- TTE
transthoracic echocardiography
- VSRR
valve-sparing root replacement
Appendix 1. Author Relationships With Industry and Other Entities (Relevant)–2022 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease
| Committee Member | Employment | Consultant | Speakers Bureau | Ownership/Partnership/Principal | Personal Research | Institutional, Organizational, or Other Financial Benefit | Expert Witness |
|---|---|---|---|---|---|---|---|
| Eric M. Isselbacher, Chair | Massachusetts General Hospital–Director, Healthcare Transformation Lab; Co-Director, MGH Thoracic Aortic Center; Harvard Medical School–Associate Professor of Medicine | None | None | None | None | None | None |
| James Hamilton Black III, Vice Chair | Johns Hopkins Medicine–Professor of Surgery | None | None | None | None | None | None |
| Ourania Preventza, Vice Chair | Baylor College of Medicine; Baylor St. Luke’s Medical Center–Professor of Surgery, Division of Cardiothoracic Surgery | • Terumo Aortic (Bolton Medical) • W.L. Gore & Associates |
None | None | None | None | None |
| John G. Augoustides | University of Pennsylvania–Professor, Department of Anesthesiology and Critical Care | None | None | None | None | None | None |
| Adam W. Beck | University of Alabama at Birmingham–Professor and Director of Vascular Surgery and Endovascular Therapy, Department of Surgery | • Cook Medical • Cryolife • Endologix • Philips • Terumo Aortic (Bolton Medical) |
None | None | • Cook Medical • Medtronic • Terumo Aortic (Bolton Medical) • W.L. Gore & Associates |
None | None |
| Michael A. Bolen | Cleveland Clinic, Main Campus–Associate Professor of Radiology, Division of Radiology | None | None | None | None | None | None |
| Alan C. Braverman | Washington University School of Medicine–Alumni Endowed Professor in Cardiovascular Diseases; Director, Marfan Syndrome and Aortopathy Clinic Cardiovascular Division, Department of Medicine | None | None | None | None | None | None |
| Bruce E. Bray | University of Utah–Professor, Department of Internal Medicine and Biomedical Informatics | None | None | None | None | None | None |
| Maya M. Brown-Zimmerman | Patient advocate | None | None | None | None | None | None |
| Edward P. Chen | Duke University Medical Center–Professor and Division Chief, Division of Cardiovascular and Thoracic Surgery | None | None | None | • Bolton Medical* | • Bolton Medical† | None |
| Tyrone J. Collins | Ochsner Medical Center–Section Head, Interventional Cardiology, Co-Director, Cardiac Catheterization Laboratory, Department of Interventional Cardiology | • InspireMD | None | None | None | • W.L. Gore & Associates† | None |
| Abe DeAnda Jr | UTMB-Galveston–Professor and Chief, Division of Cardiovascular and Thoracic Surgery, Division of Surgery | None | None | None | None | None | None |
| Christina L. Fanola | University of Minnesota–Assistant Professor, Department of Cardiovascular Medicine | • Janssen Pharmaceuticals | None | None | None | None | None |
| Leonard N. Girardi | Weill Cornell Medicine–Professor and Chairman, Department of Cardiothoracic Surgery | None | None | None | None | None | None |
| Caitlin W. Hicks | Johns Hopkins University School of Medicine–Associate Professor of Surgery, Division of Surgery | None | None | None | None | None | None |
| Dawn S. Hui | University of Texas Health San Antonio– Associate Professor, Department of Cardiothoracic Surgery | None | None | None | • Astellas Pharma* | None | None |
| William Schuyler Jones | Duke University–Associate Professor of Medicine & Director of Cardiac Catheterization Laboratory, Division of Medicine/Cardiology | • Bayer‡ • Janssen Pharmaceuticals‡ |
None | None | • Boehringer Ingelheim • Bristol-Myers Squibb |
None | None |
| Vidyasagar Kalahasti | Cleveland Clinic–Assistant Professor, Cleveland Clinic Lerner College of Medicine of the Case Western Reserve University; Director, Marfan Syndrome & Connective Tissue Disorder Clinic, Aortic Center, Heart, Vascular and Thoracic Institute | None | None | None | None | None | None |
| Karen M. Kim | University of Michigan–Assistant Professor, Department of Cardiac Surgery | None | None | None | None | None | None |
| Dianna M. Milewicz | University of Texas Health Science Center at Houston–President George H.W. Bush Chair of Cardiovascular Medicine Vice Chair, Department of Internal Medicine Director, Division of Medical Genetics Director, Division of Internal Medicine | None | None | None | None | None | None |
| Gustavo S. Oderich | The University of Texas Health Science Center at Houston, McGovern Medical School–Professor of Surgery and Chief of Vascular and Endovascular Surgery, Director of Aortic Center, Department of Cardio-Thoracic and Vascular Surgery | • Cook Medical • GE Healthcare • W.L. Gore & Associates |
None | None | • Cook Medical‡ • GE Healthcare‡ • W.L. Gore & Associates‡ |
• Centerline Biomedical, Advisory Board Member* • Cook Medical† • Philips, Advisory Board Member • W.L. Gore & Associates† |
None |
| Laura Ogbechie | Baylor College of Medicine–Nurse Practitioner, Department of Cardiothoracic Surgery | None | None | None | None | None | None |
| Susan B. Promes | Penn State Health Hershey Medical Center–Professor and Chair, Department of Emergency Medicine | None | None | None | None | None | None |
| Elsie Gyang Ross | Stanford University School of Medicine– Assistant Professor, Department of Surgery and Medicine | None | None | None | None | None | None |
| Marc L. Schermerhorn | Beth Israel Deaconess Medical Center– George H. A. Clowes Jr. Professor of Surgery, Chief, Division of Vascular and Endovascular Surgery, Department of Surgery | None | None | None | None | • Medtronic, Scientific Advisory Board Member* • Philips, Scientific Advisory Board Member* |
None |
| Sabrina Singleton Times§ | American Heart Association/American College of Cardiology Guideline Advisor | None | None | None | None | None | None |
| Elaine E. Tseng | University of California San Francisco; San Francisco VA Medical Center–Professor of Surgery University of California San Francisco; Chief of Cardiothoracic Surgery, San Francisco VA Department of Surgery | None | None | None | None | • Cryolife†‡ | None |
| Grace J. Wang | University of Pennsylvania Hospital–Associate Professor of Surgery, Division of Vascular Surgery and Endovascular Therapy | None | None | None | None | None | None |
| Y. Joseph Woo | Stanford University–Chair, Division of Cardiothoracic Surgery | None | None | None | None | None | None |
This table represents the relationships of committee members with industry and other entities that were determined to be relevant to this document. These relationships were reviewed and updated in conjunction with all meetings and/or conference calls of the writing committee during the document development process. The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of ≥5% of the voting stock or share of the business entity, or ownership of ≥$5000 of the fair market value of the business entity; or if funds received by the person from the business entity exceed 5% of the person’s gross income for the previous year. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted.
According to the ACC/AHA, a person has a relevant relationship IF: a) the relationship or interest relates to the same or similar subject matter, intellectual property or asset, topic, or issue addressed in the document; or b) the company/entity (with whom the relationship exists) makes a drug, drug class, or device addressed in the document or makes a competing drug or device addressed in the document; or c) the person or a member of the person’s household, has a reasonable potential for financial, professional or other personal gain or loss as a result of the issues/content addressed in the document.
No financial benefit.
This disclosure was entered under the Clinical Trial Enroller category in the ACC’s disclosure system. To appear in this category, the author acknowledges that there is no direct or institutional relationship with the trial sponsor as defined in the (ACCF or AHA/ACC) Disclosure Policy for Writing Committees.
Significant relationship.
Sabrina Singleton Times is an AHA/ACC joint staff member and acts as the Guideline Advisor for the “2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease.” No relevant relationships to report. Nonvoting author on measures and not included/counted in the RWI balance for this committee.
ACC indicates American College of Cardiology; ACCF, American College of Cardiology Foundation; AHA, American Heart Association; MGH, Massachusetts General Hospital; RWI, relationships with industry and other entities; UTMB, University of Texas Medical Branch; and VA, Veterans Affairs.
Appendix 2. Reviewer Relationships With Industry and Other Entities (Comprehensive)–2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease
| Reviewer | Employment | Consultant | Speakers Bureau | Ownership/Partnership/Principal | Personal Research | Institutional, Organizational, or Other Financial Benefit | Expert Witness |
|---|---|---|---|---|---|---|---|
| David P. Faxon, Peer Review Committee Chair | Harvard University; Brigham and Women’s Hospital | None | None | • REVA Medical | • Boston Scientific (DSMB) • CSL Behring (DSMB) • Medtronic (DSMB)* |
None | None |
| Gilbert R. Upchurch Jr, Peer Review Committee Vice Chair | University of Florida College of Medicine | None | None | • Antyllus* | • NIH, PI* | • Bolton† • Cook† • Gore† • Medtronic† |
None |
| Aaron W. Aday | Vanderbilt University Medical Center | • OptumCare‡ • TransThera Sciences |
None | None | • Janssen Pharmaceuticals† • University of Manitoba† |
None | |
| Ali Azizzadeh | Cedars Sinai | None | None | None | None | • Gore† | None |
| Michael Boisen (representing SCA) | University of Pittsburgh | None | None | None | None | None | None |
| Mohammad H. Eslami (representing SVS) | University of Pittsburgh | None | None | None | None | None | None |
| Beau Hawkins (representing SVM) | The University of Oklahoma College of Medicine | • Baim Institute for Clinical Research | None | None | None | • Behring† • Boston Scientific† • Hemostemix† • NIH† |
None |
| Christopher M. Kramer | University of Virginia | • Bristol Myers Squibb‡ • Cytokinetics • Eli Lilly • Xencor |
None | None | • NHLBI‡ • NIBIB‡ |
• Cytokinetics† • MyoKardia† |
None |
| Jessica G. Y. Luc | University of British Columbia | None | None | None | None | None | None |
| Thomas E. MacGillivary (representing STS) | Houston Methodist | None | None | None | None | • Xylocor† | None |
| S. Christopher Malaisrie (representing AATS) | Northwestern University | • Artivion • Medtronic |
• Edwards Lifesciences‡ • Terumo |
None | • Artivion‡ • Atricure • Edwards Lifesciences‡ • Terumo‡ |
• Artivion† • Edwards Lifesciences† • Medtronic† |
None |
| Kathryn Osteen | Baylor University Louise Herrington School of Nursing | None | None | None | None | • AHA* • Congenital Heart Public Health Consortium* • Sigma Theta Tau-Eta Gamma Chapter* • The Children’s Heart Foundation* |
None |
| Himanshu J. Patel | University of Michigan Health | • Edwards Lifesciences • Gore • Medtronic‡ • Terumo |
None | None | None | • Edwards Lifesciences† • Gore† • Medtronic† • Nexus† |
None |
| Parag J. Patel (representing SIR) | Medical College of Wisconsin | None | None | None | None | • AHA* • SIR* |
None |
| Wanda M. Popescu (representing SCA) | Yale School of Medicine | None | None | None | None | • Ambu, Advisory Board Member | None |
| Evelio Rodriguez | Ascension Tennessee Saint Thomas Hospital | • Abbott‡ • Edwards Lifesciences‡ |
• Abbott‡ • Edwards Lifesciences‡ • Philips‡ |
None | • Abbott • Atricure • Boston Scientific • Claret • Direct Flow • Edwards Lifesciences‡ • Medtronic |
• Abbott† • Atricure† • Boston Scientific† • Edwards Lifesciences† • Medtronic† |
None |
| Rebecca Sorber | Johns Hopkins University School of Medicine | None | None | None | None | None | None |
| Philip S. Tsao | VA Palo Alto Health Care System; Stanford University School of Medicine | None | None | None | None | None | None |
| Annabelle Santos Volgman | Rush College of Medicine | • Bristol Myers Squibb, DCICDP • Janssen Pharmaceuticals • Merck • Pfizer • Sanofi‡ |
None | None | • NIH‡ | • Apple* • Novartis† |
None |
This table represents all reviewers’ relationships with industry and other entities that were reported at the time of peer review, including those not deemed to be relevant to this document, at the time this document was under review. The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of 5% of the voting stock or share of the business entity, or ownership of $5000 of the fair market value of the business entity; or if funds received by the person from the business entity exceed 5% of the person’s gross income for the previous year. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted. Names are listed in alphabetical order within each category of review. Please refer to https://www.acc.org/guidelines/about-guidelines-and-clinical-documents/ relationships-with-industry-policy for definitions of disclosure categories or additional information about the ACC/AHA Disclosure Policy for Writing Committees.
No financial benefit.
This disclosure was entered under the Clinical Trial Enroller category in the ACC’s disclosure system. To appear in this category, the author acknowledges that there is no direct or institutional relationship with the trial sponsor as defined in the (ACCF or AHA/ACC) Disclosure Policy for Writing Committees.
Significant relationship.
AATS indicates American Association for Thoracic Surgery; ACC, American College of Cardiology; ACCF, American College of Cardiology Foundation; AHA, American Heart Association; DCICDP, Diverse Clinical Investigator Career Development Program; DSMB, data and safety monitoring board; NIH, National Institutes of Health; NHLBI, National Heart, Lung, and Blood Institute; NIBIB, National Institute of Biomedical Imaging and Bioengineering; PRC, Peer Review Committee; PI, principal investigator; SCA, Society of Cardiovascular Anesthesiologists; SIR, Society of Interventional Radiology; STS, Society of Thoracic Surgeons; SVM, Society for Vascular Medicine; SVS, Society for Vascular Surgery; and VA, Veterans Affairs.
Footnotes
Writing committee members are required to recuse themselves from voting on sections to which their specific relationships with industry may apply; see Appendix 1 for detailed information.
SCA representative.
ACR representative.
AHA/ACC Joint Committee on Clinical Data Standards liaison.
Lay stakeholder representative.
SCAI representative.
AATS representative.
ACC/AHA Joint Committee on Performance Measures liaison.
AHA/ACC Joint Committee on Clinical Practice Guidelines liaison.
STS representative.
SVS representative.
AHA/ACC staff representative.
SCA representative.
SCAI representative.
AHA/ACC Joint Committee on Clinical Practice Guidelines liaison.
AATS representative.
SIR representative.
Former Joint Committee on Clinical Practice Guideline member, current member during the writing effort.
Eric M. Isselbacher, MD, MSc, FACC, Chair; Ourania Preventza, MD, MBA, Vice Chair; James Hamilton Black III, MD, DFSVS, Vice Chair; John G. Augoustides, MD, FAHA†; Adam W. Beck, MD, DFSVS; Michael A. Bolen, MD‡; Alan C. Braverman, MD, FACC; Bruce E. Bray, MD, FACC§; Maya M. Brown-Zimmerman, MPH∥; Edward P. Chen, MD, FAHA; Tyrone J. Collins, MD, MSCAI, FACC, FAHA, FSVM¶; Abe DeAnda Jr, MD, FAHA; Christina L. Fanola, MD, MSc; Leonard N. Girardi, MD, FAHA#; Caitlin W. Hicks, MD, MS, FSVS**; Dawn S. Hui, MD; William Schuyler Jones, MD, FACC††; Vidyasagar Kalahasti, MD, FACC; Karen M. Kim, MD, MS‡‡; Dianna M. Milewicz, MD, PhD; Gustavo S. Oderich, MD; Laura Ogbechie, MSN; Susan B. Promes, MD, MBA; Elsie Gyang Ross, MD, MSc; Marc L. Schermerhorn, MD, DFSVS§§; Sabrina Singleton Times, DHSc, MPH∥∥; Elaine E. Tseng, MD, FAHA; Grace J. Wang, MD, MSCE; Y. Joseph Woo, MD, FACC, FAHA††
PEER REVIEW COMMITTEE MEMBERS
David P. Faxon, MD, FACC, Chair; Gilbert R. Upchurch Jr, MD, FAHA, FACC, Vice Chair; Aaron W. Aday, MD, MSc, FAHA; Ali Azizzadeh, MD; Michael Boisen, MD*; Beau Hawkins, MD, FACC†; Christopher M. Kramer, MD, FACC, FAHA; Jessica G.Y. Luc, MD; Thomas E. MacGillivray, MD‡; S. Christopher Malaisrie, MD, FACC§; Kathryn Osteen, PhD, RN, CMSRN, CNE; Himanshu J. Patel, MD, FACC, FAHA; Parag J. Patel, MD∥; Wanda M. Popescu, MD; Evelio Rodriguez, MD, FACC; Rebecca Sorber, MD; Philip S. Tsao, PhD; Annabelle Santos Volgman, MD, FACC, FAHA
AHA/ACC JOINT COMMITTEE MEMBERS
Joshua A. Beckman, MD, MS, FAHA, FACC, Chair; Catherine M. Otto, MD, FAHA, FACC, Chair-Elect; Patrick T. O’Gara, MD, MACC, FAHA, Immediate Past Chair¶; Anastasia Armbruster, PharmD, FACC; Kim K. Birtcher, PharmD, MS, FACC¶; Lisa de las Fuentes, MD, MS, FAHA; Anita Deswal, MD, MPH, FACC, FAHA; Dave L. Dixon, PharmD, FACC, FAHA¶; Bulent Gorenek, MD, FACC; Norrisa Haynes, MD, MPH; Adrian F. Hernandez, MD, MHS, FAHA; José A. Joglar, MD, FACC, FAHA¶; W. Schuyler Jones, MD, FACC; Daniel Mark, MD, MPH, FACC, FAHA¶; Debabrata Mukherjee, MD, FACC, FAHA, FSCAI; Latha Palaniappan, MD, MS, FACC, FAHA; Mariann R. Piano, RN, PhD, FAHA; Tanveer Rab, MD, FACC; Erica S. Spatz, MD, MS, FACC; Jacqueline E. TamisHolland, MD, FAHA, FACC, FSCAI; Y. Joseph Woo, MD, FACC, FAHA
PRESIDENTS AND STAFF
American College of Cardiology
Edward T.A. Fry, MD, FACC, President
Cathleen C. Gates, Chief Executive Officer
Richard J. Kovacs, MD, MACC, Chief Medical Adviser
MaryAnne Elma, MPH, Senior Director, Enterprise Content and Digital Strategy
Grace D. Ronan, Team Leader, Clinical Policy Publications
Leah Patterson, Project Manager, Clinical Content Development
American College of Cardiology/American Heart Association
Thomas S.D. Getchius, Director, Guideline Strategy and Operations
Abdul R. Abdullah, MD, Director, Guideline Science and Methodology
American Heart Association
Donald M. Lloyd-Jones, MD, ScM, FAHA, President
Nancy Brown, Chief Executive Officer
Mariell Jessup, MD, FAHA, Chief Science and Medical Officer
Radhika Rajgopal Singh, PhD, Senior Vice President, Office of Science and Medicine
Johanna A. Sharp, MSN, RN, Science and Medicine Advisor, Office of Science, Medicine and Health
Jody Hundley, Production and Operations Manager, Scientific Publications, Office of Science Operations
Developed in collaboration with and endorsed by the American Association for Thoracic Surgery, American College of Radiology, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, and Society for Vascular Surgery
Endorsed by the Society of Interventional Radiology and Society for Vascular Medicine
REFERENCES
PREAMBLE
- 1.Committee on Standards for Developing Trustworthy Clinical Practice Guidelines, Institute of Medicine (US). Clinical Practice Guidelines We Can Trust. National Academies Press; 2011. [Google Scholar]
- 2.Committee on Standards for Systematic Reviews of Comparative Effectiveness Research, Institute of Medicine (US). Finding What Works in Health Care: Standards for Systematic Reviews. National Academies Press; 2011. [PubMed] [Google Scholar]
- 3.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129:2329–2345. [DOI] [PubMed] [Google Scholar]
- 4.ACCF/AHA Task Force on Practice Guidelines. Methodology Manual and Policies from the ACCF/AHA Task Force on Practice Guidelines. American College of Cardiology and American Heart Association. 2010. Accessed September 23, 2019. https://www.acc.org/Guidelines/About-Guidelines-and-Clinical-Documents/Methodology and https://professional.heart.org/-/media/phd-files/guidelines-and-statements/methodology_manual_and_policies_ucm_319826.pdf [Google Scholar]
- 5.Halperin JL, Levine GN, Al-Khatib SM, et al. Further evolution of the ACC/AHA clinical practice guideline recommendation classification system: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2014;129:2329–2345.24677315 [Google Scholar]
- 6.Arnett DK, Goodman RA, Halperin JL, et al. AHA/ACC/HHS strategies to enhance application of clinical practice guidelines in patients with cardiovascular disease and comorbid conditions: from the American Heart Association, American College of Cardiology, and U.S. Department of Health and Human Services. Circulation. 2014;130:1662–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine GN, O’Gara PT, Beckman JA, et al. Recent innovations, modifications, and evolution of ACC/AHA clinical practice guidelines: an update for our constituencies: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e879–e886. [DOI] [PubMed] [Google Scholar]
1.4. Scope of the Guideline
- 1.Upchurch GR Jr, Escobar GA, Azizzdeh A, et al. Society for Vascular Surgery clinical practice guidelines of thoracic endovascular aortic repair for descending thoracic aortic aneurysms. J Vasc Surg. 2021;73:55S–83S. [DOI] [PubMed] [Google Scholar]
- 2.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–e227. [DOI] [PubMed] [Google Scholar]
- 3.Hellmich B, Agueda A, Monti S, et al. 2018 update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2020;79:19–30. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e698–e800. [DOI] [PubMed] [Google Scholar]
- 6.Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77.e72. [DOI] [PubMed] [Google Scholar]
- 7.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 8.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–e725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riambau V, Bockler D, Brunkwall J, et al. Editor’s choice - management of descending thoracic aorta diseases: clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017;53:4–52. [DOI] [PubMed] [Google Scholar]
- 10.Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg. 2016;151:959–966. [DOI] [PubMed] [Google Scholar]
- 11.Wilson WR, Bower TC, Creager MA, et al. Vascular graft infections, mycotic aneurysms, and endovascular infections: a scientific statement from the American Heart Association. Circulation. 2016;134:e412–e460. [DOI] [PubMed] [Google Scholar]
- 12.Lee WA, Matsumura JS, Mitchell RS, et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53:187–192. [DOI] [PubMed] [Google Scholar]
- 13.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. [DOI] [PubMed] [Google Scholar]
- 14.Smith SC Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation. 2006;113:2363–2372. [DOI] [PubMed] [Google Scholar]
- 15.Malaisrie SC, Szeto WY, Halas M, et al. 2021 The American Association for Thoracic Surgery expert consensus document: surgical treatment of acute type A aortic dissection. J Thorac Cardiovasc Surg. 2021;162:735–758.e2. [DOI] [PubMed] [Google Scholar]
- 16.MacGillivray TE, Gleason TG, Patel HJ, et al. The Society of Thoracic Surgeons/American Association for Thoracic Surgery clinical practice guidelines on the management of type B aortic dissection. Ann Thorac Surg. 2022;113:1073–1092. [DOI] [PubMed] [Google Scholar]
2.2. Aortic Landing Zones
- 1.Czerny M, Schmidli J, Adler S, et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS). Eur J Cardiothorac Surg. 2019;55:133–162. [DOI] [PubMed] [Google Scholar]
- 2.Roselli EE, Idrees JJ, Johnston DR, et al. Zone zero thoracic endovascular aortic repair: a proposed modification to the classification of landing zones. J Thorac Cardiovasc Surg. 2018;155:1381–1389. [DOI] [PubMed] [Google Scholar]
- 3.Johnston KW, Rutherford RB, Tilson MD, et al. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg. 1991;13:452–458. [DOI] [PubMed] [Google Scholar]
- 4.Borger MA, Preston M, Ivanov J, et al. Should the ascending aorta be replaced more frequently in patients with bicuspid aortic valve disease? J Thorac Cardiovasc Surg. 2004;128:677–683. [DOI] [PubMed] [Google Scholar]
- 5.Paruchuri V, Salhab KF, Kuzmik G, et al. Aortic size distribution in the general population: explaining the size paradox in aortic dissection. Cardiology. 2015;131:265–272. [DOI] [PubMed] [Google Scholar]
- 6.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 7.Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111:816–828. [DOI] [PubMed] [Google Scholar]
- 8.Kruger T, Sandoval Boburg R, Lescan M, et al. Aortic elongation in aortic aneurysm and dissection: the Tubingen Aortic Pathoanatomy (TAIPAN) project. Eur J Cardiothorac Surg. 2018;54:26–33. [DOI] [PubMed] [Google Scholar]
- 9.Kawsara A, Nunez Gil IJ, Alqahtani F, et al. Management of coronary artery aneurysms. J Am Coll Cardiol Intv. 2018;11:1211–1223. [Google Scholar]
- 10.van Kimmenade RR, Kempers M, de Boer MJ, et al. A clinical appraisal of different Z-score equations for aortic root assessment in the diagnostic evaluation of Marfan syndrome. Genet Med. 2013;15:528–532. [DOI] [PubMed] [Google Scholar]
- 11.Davies RR, Gallo A, Coady MA, et al. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg. 2006;81:169–177. [DOI] [PubMed] [Google Scholar]
- 12.Zafar MA, Li Y, Rizzo JA, et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J Thorac Cardiovasc Surg. 2018;155:1938–1950. [DOI] [PubMed] [Google Scholar]
- 13.Svensson LG, Khitin L. Aortic cross-sectional area/height ratio timing of aortic surgery in asymptomatic patients with Marfan syndrome. J Thorac Cardiovasc Surg. 2002;123:360–361. [DOI] [PubMed] [Google Scholar]
- 14.Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation. 2016;134:1724–1737. [DOI] [PubMed] [Google Scholar]
- 15.Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with bicuspid aortic valve and a dilated ascending aorta. Circ Cardiovasc Imaging. 2017;10:e006249. [DOI] [PubMed] [Google Scholar]
2.4. Definitions and Classification of Acute Aortic Syndrome (AAS)
- 1.Clough RE, Nienaber CA. Management of acute aortic syndrome. Nat Rev Cardiol. 2015;12:103–114. [DOI] [PubMed] [Google Scholar]
- 2.Booher AM, Isselbacher EM, Nienaber CA, et al. The IRAD classification system for characterizing survival after aortic dissection. Am J Med. 2013;126:730.e719–730.e724. [DOI] [PubMed] [Google Scholar]
- 3.Tsai TT, Nienaber CA, Eagle KA. Acute aortic syndromes. Circulation. 2005;112:3802–3813. [DOI] [PubMed] [Google Scholar]
- 4.Czerny M, Schmidli J, Adler S, et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for CardioThoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS). Eur J Cardiothorac Surg. 2019;55:133–162. [DOI] [PubMed] [Google Scholar]
- 5.Lombardi JV, Hughes GC, Appoo JJ, et al. Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) reporting standards for type B aortic dissections. J Vasc Surg. 2020;71:723–747. [DOI] [PubMed] [Google Scholar]
- 6.Grewal S, Contrella BN, Sherk WM, et al. Endovascular Management of Malperfusion Syndromes in Aortic Dissection. Tech Vasc Interv Radiol. 2021;24:100751. [DOI] [PubMed] [Google Scholar]
- 7.Mussa FF, Horton JD, Moridzadeh R, et al. Acute aortic dissection and intramural hematoma: a systematic review. JAMA. 2016;316:754–763. [DOI] [PubMed] [Google Scholar]
- 8.Oderich GS, Karkkainen JM, Reed NR, et al. Penetrating aortic ulcer and intramural hematoma. Cardiovasc Intervent Radiol. 2019;42:321–334. [DOI] [PubMed] [Google Scholar]
- 9.Harris KM, Braverman AC, Eagle KA, et al. Acute aortic intramural hematoma: an analysis from the International Registry of Acute Aortic Dissection. Circulation. 2012;126:S91–S96. [DOI] [PubMed] [Google Scholar]
- 10.Bossone E, LaBounty TM, Eagle KA. Acute aortic syndromes: diagnosis and management, an update. Eur Heart J. 2018;39:739d–749d. [DOI] [PubMed] [Google Scholar]
- 11.Song JK, Kim HS, Kang DH, et al. Different clinical features of aortic intramural hematoma versus dissection involving the ascending aorta. J Am Coll Cardiol. 2001;37:1604–1610. [DOI] [PubMed] [Google Scholar]
- 12.Brown JA, Arnaoutakis GJ, Kilic A, et al. Current trends in the management of acute type A aortic intramural hematoma. J Card Surg. 2020;35:2331–2337. [DOI] [PubMed] [Google Scholar]
- 13.Bischoff MS, Geisbusch P, Peters AS, et al. Penetrating aortic ulcer: defining risks and therapeutic strategies. Herz. 2011;36:498–504. [DOI] [PubMed] [Google Scholar]
2.5. Classification of Thoracoabdominal Aortic Aneurysm (TAAA)
- 1.Safi HJ, Winnerkvist A, Miller CC 3rd, et al. Effect of extended cross-clamp time during thoracoabdominal aortic aneurysm repair. Ann Thorac Surg. 1998;66:1204–1209. [DOI] [PubMed] [Google Scholar]
- 2.Riambau V, Bockler D, Brunkwall J, et al. Editor’s choice - management of descending thoracic aorta diseases: clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017;53:4–52. [DOI] [PubMed] [Google Scholar]
- 3.Conrad MF, Cambria RP. Contemporary management of descending thoracic and thoracoabdominal aortic aneurysms: endovascular versus open. Circulation. 2008;117:841–852. [DOI] [PubMed] [Google Scholar]
3.1. Aortic Imaging Techniques to Determine Presence and Progression of Aortic Disease
- 1.Sternbergh WC 3rd, Greenberg RK, Chuter TA, et al. Redefining postoperative surveillance after endovascular aneurysm repair: recommendations based on 5-year follow-up in the US Zenith multicenter trial. J Vasc Surg. 2008;48:278–284; discussion 84–85. [DOI] [PubMed] [Google Scholar]
- 2.Rokosh RS, Wu WW, Schermerhorn M, et al. Society for Vascular Surgery implementation of clinical practice guidelines for patients with an abdominal aortic aneurysm: Postoperative surveillance after abdominal aortic aneurysm repair. J Vasc Surg. 2021;74:1438–1439. [DOI] [PubMed] [Google Scholar]
- 3.Mendoza DD, Kochar M, Devereux RB, et al. Impact of image analysis methodology on diagnostic and surgical classification of patients with thoracic aortic aneurysms. Ann Thorac Surg. 2011;92:904–912. [DOI] [PubMed] [Google Scholar]
- 4.Rudarakanchana N, Bicknell CD, Cheshire NJ, et al. Variation in maximum diameter measurements of descending thoracic aortic aneurysms using unformatted planes versus images corrected to aortic centerline. Eur J Vasc Endovasc Surg. 2014;47:19–26. [DOI] [PubMed] [Google Scholar]
- 5.Amis ES Jr, Butler PF, American College of Radiology. ACR white paper on radiation dose in medicine: three years later. J Am Coll Radiol. 2010;7:865–870. [DOI] [PubMed] [Google Scholar]
- 6.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. [DOI] [PubMed] [Google Scholar]
- 7.Costello JE, Cecava ND, Tucker JE, et al. CT radiation dose: current controversies and dose reduction strategies. AJR Am J Roentgenol. 2013;201:1283–1290. [DOI] [PubMed] [Google Scholar]
- 8.Svensson LG, Kim KH, Lytle BW, et al. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg. 2003;126:892–893. [DOI] [PubMed] [Google Scholar]
- 9.Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation. 2016;134:1724–1737. [DOI] [PubMed] [Google Scholar]
- 10.Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with bicuspid aortic valve and a dilated ascending aorta. Circ Cardiovasc Imaging. 2017;10:e006249. [DOI] [PubMed] [Google Scholar]
- 11.Zafar MA, Li Y, Rizzo JA, et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J Thorac Cardiovasc Surg. 2018;155:1938–1950. [DOI] [PubMed] [Google Scholar]
- 12.Beebe HG, Kritpracha B, Serres S, et al. Endograft planning without preoperative arteriography: a clinical feasibility study. J Endovasc Ther. 2000;7:8–15. [DOI] [PubMed] [Google Scholar]
- 13.Vriz O, Driussi C, Bettio M, et al. Aortic root dimensions and stiffness in healthy subjects. Am J Cardiol. 2013;112:1224–1229. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Palomares JF, Teixido-Tura G, Galuppo V, et al. Multimodality Assessment of Ascending Aortic Diameters: Comparison of Different Measurement Methods. J Am Soc Echocardiogr. 2016;29:819–826.e814. [DOI] [PubMed] [Google Scholar]
- 15.Asch FM, Yuriditsky E, Prakash SK, et al. The need for standardized methods for measuring the aorta: multimodality core lab experience from the GenTAC Registry. J Am Coll Cardiol Img. 2016;9:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potthast S, Mitsumori L, Stanescu LA, et al. Measuring aortic diameter with different MR techniques: comparison of three-dimensional (3D) navigated steady-state free-precession (SSFP), 3D contrast-enhanced magnetic resonance angiography (CE-MRA), 2D T2 black blood, and 2D cine SSFP. J Magn Reson Imaging. 2010;31:177–184. [DOI] [PubMed] [Google Scholar]
- 17.Plonek T, Berezowski M, Bochenek M, et al. A comparison of aortic root measurements by echocardiography and computed tomography. J Thorac Cardiovasc Surg. 2019;157:479–486. [DOI] [PubMed] [Google Scholar]
- 18.Vasan RS, Larson MG, Benjamin EJ, et al. Echocardiographic reference values for aortic root size: the Framingham Heart Study. J Am Soc Echocardiogr. 1995;8:793–800. [DOI] [PubMed] [Google Scholar]
- 19.Vasan RS, Larson MG, Levy D. Determinants of echocardiographic aortic root size. The Framingham Heart Study. Circulation. 1995;91:734–740. [DOI] [PubMed] [Google Scholar]
- 20.Devereux RB, de Simone G, Arnett DK, et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am J Cardiol. 2012;110:1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borger MA, Fedak PWM, Stephens EH, et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: full online-only version. J Thorac Cardiovasc Surg. 2018;156:e41–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
3.2. Conventions of Measurements
- 1.Lombardi JV, Hughes GC, Appoo JJ, et al. Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) reporting standards for type B aortic dissections. J Vasc Surg. 2020;71:723–747. [DOI] [PubMed] [Google Scholar]
- 2.Vasan RS, Larson MG, Levy D. Determinants of echocardiographic aortic root size. The Framingham Heart Study. Circulation. 1995;91:734–740. [DOI] [PubMed] [Google Scholar]
- 3.Vasan RS, Larson MG, Benjamin EJ, et al. Echocardiographic reference values for aortic root size: the Framingham Heart Study. J Am Soc Echocardiogr. 1995;8:793–800. [DOI] [PubMed] [Google Scholar]
- 4.Devereux RB, de Simone G, Arnett DK, et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am J Cardiol. 2012;110:1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vriz O, Driussi C, Bettio M, et al. Aortic root dimensions and stiffness in healthy subjects. Am J Cardiol. 2013;112:1224–1229. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Palomares JF, Teixido-Tura G, Galuppo V, et al. Multimodality Assessment of Ascending Aortic Diameters: Comparison of Different Measurement Methods. J Am Soc Echocardiogr. 2016;29:819–826.e814. [DOI] [PubMed] [Google Scholar]
- 7.Weinrich JM, Avanesov M, Lenz A, et al. Reliability of non-contrast magnetic resonance angiography-derived aortic diameters in Marfan patients: comparison of inner vs. outer vessel wall measurements. Int J Cardiovasc Imaging. 2020;36:1533–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17–27; discussion 27–28. [DOI] [PubMed] [Google Scholar]
- 9.Kim EK, Choi SH, Sung K, et al. Aortic diameter predicts acute type A aortic dissection in patients with Marfan syndrome but not in patients without Marfan syndrome. J Thorac Cardiovasc Surg. 2014;147:1505–1510. [DOI] [PubMed] [Google Scholar]
- 10.Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation. 2016;134:1724–1737. [DOI] [PubMed] [Google Scholar]
- 11.Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with bicuspid aortic valve and a dilated ascending aorta. Circ Cardiovasc Imaging. 2017;10:e006249. [DOI] [PubMed] [Google Scholar]
- 12.Bons LR, Rueda-Ochoa OL, El Ghoul K, et al. Sex-specific distributions and determinants of thoracic aortic diameters in the elderly. Heart. 2020;106:133–139. [DOI] [PubMed] [Google Scholar]
- 13.Wu J, Zafar MA, Li Y, et al. Ascending aortic length and risk of aortic adverse events: the neglected dimension. J Am Coll Cardiol. 2019;74:1883–1894. [DOI] [PubMed] [Google Scholar]
- 14.Renapurkar RD, Setser RM, O’Donnell TP, et al. Aortic volume as an indicator of disease progression in patients with untreated infrarenal abdominal aneurysm. Eur J Radiol. 2012;81:e87–e93. [DOI] [PubMed] [Google Scholar]
- 15.den Hartog AW, Franken R, de Witte P, et al. Aortic disease in patients with Marfan syndrome: aortic volume assessment for surveillance. Radiology. 2013;269:370–377. [DOI] [PubMed] [Google Scholar]
3.2.1. Computed Tomography
- 1.Lu TL, Huber CH, Rizzo E, et al. Ascending aorta measurements as assessed by ECG-gated multi-detector computed tomography: a pilot study to establish normative values for transcatheter therapies. Eur Radiol. 2009;19:664–669. [DOI] [PubMed] [Google Scholar]
- 2.Davenport MS, Perazella MA, Yee J, et al. Use of Intravenous Iodinated Contrast Media in Patients With Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Kidney Med. 2020;2:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshida S, Akiba H, Tamakawa M, et al. Thoracic involvement of type A aortic dissection and intramural hematoma: diagnostic accuracy--comparison of emergency helical CT and surgical findings. Radiology. 2003;228:430–435. [DOI] [PubMed] [Google Scholar]
- 4.Imoto K, Uchida K, Karube N, et al. Risk analysis and improvement of strategies in patients who have acute type A aortic dissection with coronary artery dissection. Eur J Cardiothorac Surg. 2013;44:419–424; discussion 424–425. [DOI] [PubMed] [Google Scholar]
- 5.Thoongsuwan N, Stern EJ. Chest CT scanning for clinical suspected thoracic aortic dissection: beware the alternate diagnosis. Emerg Radiol. 2002;9:257–261. [DOI] [PubMed] [Google Scholar]
3.2.2. Magnetic Resonance Imaging (MRI)
- 1.Barra L, Kanji T, Malette J, et al. Imaging modalities for the diagnosis and disease activity assessment of Takayasu’s arteritis: a systematic review and meta-analysis. Autoimmun Rev. 2018;17:175–187. [DOI] [PubMed] [Google Scholar]
- 2.Potthast S, Mitsumori L, Stanescu LA, et al. Measuring aortic diameter with different MR techniques: comparison of three-dimensional (3D) navigated steady-state free-precession (SSFP), 3D contrast-enhanced magnetic resonance angiography (CE-MRA), 2D T2 black blood, and 2D cine SSFP. J Magn Reson Imaging. 2010;31:177–184. [DOI] [PubMed] [Google Scholar]
- 3.Krishnam MS, Tomasian A, Malik S, et al. Image quality and diagnostic accuracy of unenhanced SSFP MR angiography compared with conventional contrast-enhanced MR angiography for the assessment of thoracic aortic diseases. Eur Radiol. 2010;20:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore AG, Eagle KA, Bruckman D, et al. Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD). Am J Cardiol. 2002;89:1235–1238. [DOI] [PubMed] [Google Scholar]
- 5.Lunyera J, Mohottige D, Alexopoulos AS, et al. Risk for nephrogenic systemic fibrosis after exposure to newer gadolinium agents: a systematic review. Ann Intern Med. 2020;173:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinreb JC, Rodby RA, Yee J, et al. Use of intravenous gadolinium-based contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology. 2021;298:28–35. [DOI] [PubMed] [Google Scholar]
3.2.3. Echocardiography
- 1.Evangelista A, Flachskampf FA, Erbel R, et al. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur J Echocardiogr. 2010;11:645–658. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein SA, Evangelista A, Abbara S, et al. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:119–182. [DOI] [PubMed] [Google Scholar]
3.2.4. Intravascular Ultrasound
- 1.Kpodonu J, Ramaiah VG, Diethrich EB. Intravascular ultrasound imaging as applied to the aorta: a new tool for the cardiovascular surgeon. Ann Thorac Surg. 2008;86:1391–1398. [DOI] [PubMed] [Google Scholar]
- 2.Martin ZL, Mastracci TM. The evaluation of aortic dissections with intravascular ultrasonography. Vasc Dis Manag. 2011;8:E93–E97. [Google Scholar]
- 3.Lortz J, Tsagakis K, Rammos C, et al. Intravascular ultrasound assisted sizing in thoracic endovascular aortic repair improves aortic remodeling in type B aortic dissection. PLoS One. 2018;13:e0196180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lortz J, Papathanasiou M, Rammos C, et al. High intimal flap mobility assessed by intravascular ultrasound is associated with better short-term results after TEVAR in chronic aortic dissection. Sci Rep. 2019;9:7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Illuminati G, Pacile MA, Ceccanei G, et al. Peroperative intravascular ultrasound for endovascular aneurysm repair versus peroperative angiography: a pilot study in fit patients with favorable anatomy. Ann Vasc Surg. 2020;64:54–61. [DOI] [PubMed] [Google Scholar]
3.2.5. Abdominal Ultrasound
- 1.Harter LP, Gross BH, Callen PW, et al. Ultrasonic evaluation of abdominal aortic thrombus. J Ultrasound Med. 1982;1:315–318. [DOI] [PubMed] [Google Scholar]
- 2.Steiner E, Rubens D, Weiss SL, et al. Sonographic examination of the abdominal aorta through the left flank: a prospective study. J Ultrasound Med. 1986;5:499–502. [DOI] [PubMed] [Google Scholar]
- 3.Owens DK, Davidson KW, Krist AH, et al. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:2211–2218. [DOI] [PubMed] [Google Scholar]
- 4.Ellis M, Powell JT, Greenhalgh RM. Limitations of ultrasonography in surveillance of small abdominal aortic aneurysms. Br J Surg. 1991;78:614–616. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease). Circulation. 2006;113:e463–e654. [DOI] [PubMed] [Google Scholar]
- 6.The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998;352:1649–1655. [PubMed] [Google Scholar]
- 7.Wilmink AB, Forshaw M, Quick CR, et al. Accuracy of serial screening for abdominal aortic aneurysms by ultrasound. J Med Screen. 2002;9:125–127. [DOI] [PubMed] [Google Scholar]
- 8.Long A, Rouet L, Lindholt JS, et al. Measuring the maximum diameter of native abdominal aortic aneurysms: review and critical analysis. Eur J Vasc Endovasc Surg. 2012;43:515–524. [DOI] [PubMed] [Google Scholar]
- 9.Abraha I, Luchetta ML, De Florio R, et al. Ultrasonography for endoleak detection after endoluminal abdominal aortic aneurysm repair. Cochrane Database Syst Rev. 2017;(6):CD010296. [DOI] [PMC free article] [PubMed] [Google Scholar]
4. Multidisciplinary Aortic Teams
- 1.Andersen ND, Ganapathi AM, Hanna JM, et al. Outcomes of acute type A dissection repair before and after implementation of a multidisciplinary thoracic aortic surgery program. J Am Coll Cardiol. 2014;63:1796–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottle A, Mariscalco G, Shaw MA, et al. Unwarranted variation in the quality of care for patients with diseases of the thoracic aorta. J Am Heart Assoc. 2017;6:e004913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landon BE, O’Malley AJ, Giles K, et al. Volume-outcome relationships and abdominal aortic aneurysm repair. Circulation. 2010;122:1290–1297. [DOI] [PubMed] [Google Scholar]
- 4.Hughes GC, Zhao Y, Rankin JS, et al. Effects of institutional volumes on operative outcomes for aortic root replacement in North America. J Thorac Cardiovasc Surg. 2013;145:166–170. [DOI] [PubMed] [Google Scholar]
- 5.Khan H, Hussain A, Chaubey S, et al. Acute aortic dissection type A: impact of aortic specialists on short and long term outcomes. J Card Surg. 2021;36:952–958. [DOI] [PubMed] [Google Scholar]
- 6.Preventza O In type A aortic dissection repair, an effective team approach and relational coordination are more important for patients’ outcomes than surgeon volume. J Thorac Cardiovasc Surg. 2017;154:407–408. [DOI] [PubMed] [Google Scholar]
- 7.Elrod JK, Fortenberry JL Jr. Centers of excellence in healthcare institutions: what they are and how to assemble them. BMC Health Serv Res. 2017;17:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–e227. [DOI] [PubMed] [Google Scholar]
- 9.Mori M, Shioda K, Wang X, et al. Perioperative risk profiles and volume-outcome relationships in proximal thoracic aortic surgery. Ann Thorac Surg. 2018;106:1095–1104. [DOI] [PubMed] [Google Scholar]
- 10.Umana-Pizano JB, Nissen AP, Sandhu HK, et al. Acute type A dissection repair by high-volume vs low-volume surgeons at a high-volume aortic center. Ann Thorac Surg. 2019;108:1330–1336. [DOI] [PubMed] [Google Scholar]
5. Shared Decision-Making
- 1.Ubbink DT, Knops AM, Molenaar S, et al. Design and development of a decision aid to enhance shared decision making by patients with an asymptomatic abdominal aortic aneurysm. Patient Prefer Adherence. 2008;2:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hess EP, Coylewright M, Frosch DL, et al. Implementation of shared decision making in cardiovascular care: past, present, and future. Circ Cardiovasc Qual Outcomes. 2014;7:797–803. [DOI] [PubMed] [Google Scholar]
- 3.Lin GA, Fagerlin A. Shared decision making: state of the science. Circ Cardiovasc Qual Outcomes. 2014;7:328–334. [DOI] [PubMed] [Google Scholar]
- 4.Treasure T, King A, Hidalgo Lemp L, et al. Developing a shared decision support framework for aortic root surgery in Marfan syndrome. Heart. 2018;104:480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gokalp AL, Takkenberg JJM. Decision-making in thoracic aortic aneurysm surgery-clinician and patient view. Semin Thorac Cardiovasc Surg. 2019;31:638–642. [DOI] [PubMed] [Google Scholar]
- 6.Swart M, McCarthy R. Shared decision making for elective abdominal aortic aneurysm surgery. Clin Med (Lond). 2019;19:473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alston CZ, Berger S, Brownlee G, et al. Shared decision-making strategies for best care: patient decision aids NAM Perspectives. Discussion paper. National Academy of Medicine; 2014. [Google Scholar]
6.1. Thoracic Aortic Aneurysm (TAA) Cause
- 1.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. [DOI] [PubMed] [Google Scholar]
- 2.Biaggi P, Matthews F, Braun J, et al. Gender, age, and body surface area are the major determinants of ascending aorta dimensions in subjects with apparently normal echocardiograms. J Am Soc Echocardiogr. 2009;22:720–725. [DOI] [PubMed] [Google Scholar]
- 3.Zafar MA, Li Y, Rizzo JA, et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J Thorac Cardiovasc Surg. 2018;155:1938–1950. [DOI] [PubMed] [Google Scholar]
- 4.Obel LM, Diederichsen AC, Steffensen FH, et al. Population-based risk factors for ascending, arch, descending, and abdominal aortic dilations for 60–74-year-old individuals. J Am Coll Cardiol. 2021;78:201–211. [DOI] [PubMed] [Google Scholar]
- 5.Rouchaud A, Brandt MD, Rydberg AM, et al. Prevalence of intracranial aneurysms in patients with aortic aneurysms. AJNR Am J Neuroradiol. 2016;37:1664–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vapnik JS, Kim JB, Isselbacher EM, et al. Characteristics and outcomes of ascending versus descending thoracic aortic aneurysms. Am J Cardiol. 2016;117:1683–1690. [DOI] [PubMed] [Google Scholar]
- 7.Pinard A, Jones GT, Milewicz DM. Genetics of thoracic and abdominal aortic diseases. Circ Res. 2019;124:588–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thakker PD, Braverman AC. Cardiogenetics: genetic testing in the diagnosis and management of patients with aortic disease. Heart. 2021;107:619–626. [DOI] [PubMed] [Google Scholar]
- 9.Raunso J, Song RJ, Vasan RS, et al. Familial clustering of aortic size, aneurysms, and dissections in the community. Circulation. 2020;142:920–928. [DOI] [PubMed] [Google Scholar]
- 10.Verhagen JMA, Kempers M, Cozijnsen L, et al. Expert consensus recommendations on the cardiogenetic care for patients with thoracic aortic disease and their first-degree relatives. Int J Cardiol. 2018;258:243–248. [DOI] [PubMed] [Google Scholar]
- 11.Milewicz DM, Ostergaard JR, la-Kokko LM, et al. De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am J Med Genet A. 2010;152A:2437–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
6.1.1. Sporadic and Degenerative TAA
- 1.Vapnik JS, Kim JB, Isselbacher EM, et al. Characteristics and outcomes of ascending versus descending thoracic aortic aneurysms. Am J Cardiol. 2016;117:1683–1690. [DOI] [PubMed] [Google Scholar]
- 2.Upchurch GR Jr, Escobar GC, Azizzdeh A, et al. Society for Vascular Surgery clinical practice guidelines for thoracic endovascular aneurysm repair (Tevar). J Vasc Surg. 2021;73:55S–83S. [DOI] [PubMed] [Google Scholar]
6.1.2.1. HTAD: Genetic Testing and Screening of Family Members for TAD
- 1.Biddinger A, Rocklin M, Coselli J, et al. Familial thoracic aortic dilatations and dissections: a case control study. J Vasc Surg. 1997;25:506–511. [DOI] [PubMed] [Google Scholar]
- 2.Coady MA, Davies RR, Roberts M, et al. Familial patterns of thoracic aortic aneurysms. Arch Surg. 1999;134:361–367. [DOI] [PubMed] [Google Scholar]
- 3.Albornoz G, Coady MA, Roberts M, et al. Familial thoracic aortic aneurysms and dissections-incidence, modes of inheritance, and phenotypic patterns. Ann Thorac Surg. 2006;82:1400–1405. [DOI] [PubMed] [Google Scholar]
- 4.Renard M, Francis C, Ghosh R, et al. Clinical validity of genes for heritable thoracic aortic aneurysm and dissection. J Am Coll Cardiol. 2018;72:605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolford BN, Hornsby WE, Guo D, et al. Clinical implications of identifying pathogenic variants in individuals with thoracic aortic dissection. Circ Genom Precis Med. 2019;12:e002476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milewicz DM, Guo D, Hostetler E, et al. Update on the genetic risk for thoracic aortic aneurysms and acute aortic dissections: implications for clinical care. J Cardiovasc Surg (Torino). 2021;62:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace SE, Regalado ES, Gong L, et al. MYLK pathogenic variants aortic disease presentation, pregnancy risk, and characterization of pathogenic missense variants. Genet Med. 2019;21:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regalado ES, Guo DC, Prakash S, et al. Aortic disease presentation and outcome associated with ACTA2 mutations. Circ Cardiovasc Genet. 2015;8:457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo DC, Regalado E, Casteel DE, et al. Recurrent gain-of-function mutation in PRKG1 causes thoracic aortic aneurysms and acute aortic dissections. Am J Hum Genet. 2013;93:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris SL, Lindsay ME. Role of clinical genetic testing in the management of aortopathies. Curr Cardiol Rep. 2021;23:10. [DOI] [PubMed] [Google Scholar]
- 11.Demo E, Rigelsky C, Rideout AL, et al. Genetics and precision medicine: heritable thoracic aortic disease. Med Clin North Am. 2019;103:1005–1019. [DOI] [PubMed] [Google Scholar]
- 12.Regalado ES, Guo DC, Villamizar C, et al. Exome sequencing identifies SMAD3 mutations as a cause of familial thoracic aortic aneurysm and dissection with intracranial and other arterial aneurysms. Circ Res. 2011;109:680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson EN, van der Linde D, Sherrah AG, et al. Familial non-syndromal thoracic aortic aneurysms and dissections-incidence and family screening outcomes. Int J Cardiol. 2016;220:43–51. [DOI] [PubMed] [Google Scholar]
- 14.Hannuksela M, Stattin EL, Johansson B, et al. Screening for familial thoracic aortic aneurysms with aortic imaging does not detect all potential carriers of the disease. Aorta (Stamford). 2015;3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mariscalco G, Debiec R, Elefteriades JA, et al. Systematic review of studies that have evaluated screening tests in relatives of patients affected by nonsyndromic thoracic aortic disease. J Am Heart Assoc. 2018;7:e009302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo DC, Pannu H, Papke CL, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39:1488–1493. [DOI] [PubMed] [Google Scholar]
- 17.Rylski B, Blanke P, Beyersdorf F, et al. How does the ascending aorta geometry change when it dissects? J Am Coll Cardiol. 2014;63:1311–1319. [DOI] [PubMed] [Google Scholar]
- 18.Milewicz DM, Chen H, Park ES, et al. Reduced penetrance and variable expressivity of familial thoracic aortic aneurysms/dissections. Am J Cardiol. 1998;82:474–479. [DOI] [PubMed] [Google Scholar]
- 19.Renard M, Francis C, Ghosh R, et al. Clinical validity of genes for heritable thoracic aortic aneurysm and dissection. J Am Coll Cardiol. 2018;72:605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo DC, Hostetler EM, Fan Y, et al. Heritable thoracic aortic disease genes in sporadic aortic dissection. J Am Coll Cardiol. 2017;70:2728–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boileau C, Guo DC, Hanna N, et al. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat Genet. 2012;44:916–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran-Fadulu V, Pannu H, Kim DH, et al. Analysis of multigenerational families with thoracic aortic aneurysms and dissections due to TGFBR1 or TGFBR2 mutations. J Med Genet. 2009;46:607–613. [DOI] [PubMed] [Google Scholar]
- 23.Villamizar C, Regalado ES, Fadulu VT, et al. Paucity of skeletal manifestations in hispanic families with FBN1 mutations. Eur J Med Genet. 2010;53:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morisaki H, Akutsu K, Ogino H, et al. Mutation of ACTA2 gene as an important cause of familial and nonfamilial nonsyndromatic thoracic aortic aneurysm and/or dissection (TAAD). Hum Mutat. 2009;30:1406–1411. [DOI] [PubMed] [Google Scholar]
- 25.Guo DC, Papke CL, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and moyamoya disease, along with thoracic aortic disease. Am J Hum Genet. 2009;84:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loscalzo ML, Goh DL, Loeys B, et al. Familial thoracic aortic dilation and bicommissural aortic valve: a prospective analysis of natural history and inheritance. Am J Med Genet A. 2007;143:1960–1967. [DOI] [PubMed] [Google Scholar]
- 27.Zhu L, Vranckx R, Khau Van KP, et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet. 2006;38:343–349. [DOI] [PubMed] [Google Scholar]
6.1.2.1.1. Surgical Considerations for Nonsyndromic Heritable TAAs and No Identified Genetic Cause
- 1.Weinsaft JW, Devereux RB, Preiss LR, et al. Aortic Dissection in Patients With Genetically Mediated Aneurysms: Incidence and Predictors in the GenTAC Registry. J Am Coll Cardiol. 2016;67:2744–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albornoz G, Coady MA, Roberts M, et al. Familial thoracic aortic aneurysms and dissections--incidence, modes of inheritance, and phenotypic patterns. Ann Thorac Surg. 2006;82:1400–1405. [DOI] [PubMed] [Google Scholar]
- 3.Robertson EN, van der Linde D, Sherrah AG, et al. Familial non-syndromal thoracic aortic aneurysms and dissections - Incidence and family screening outcomes. Int J Cardiol. 2016;220:43–51. [DOI] [PubMed] [Google Scholar]
- 4.Brownstein AJ, Kostiuk V, Ziganshin BA, et al. Genes associated with thoracic aortic aneurysm and dissection: 2018 update and clinical implications. Aorta (Stamford). 2018;6:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson EN, van der Linde D, Sherrah AG, et al. Familial non-syndromal thoracic aortic aneurysms and dissections-incidence and family screening outcomes. Int J Cardiol. 2016;220:43–51. [DOI] [PubMed] [Google Scholar]
- 6.Weinsaft JW, Devereux RB, Preiss LR, et al. Aortic dissection in patients with genetically mediated aneurysms: incidence and predictors in the GenTAC registry. J Am Coll Cardiol. 2016;67:2744–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albornoz G, Coady MA, Roberts M, et al. Familial thoracic aortic aneurysms and dissections-incidence, modes of inheritance, and phenotypic patterns. Ann Thorac Surg. 2006;82:1400–1405. [DOI] [PubMed] [Google Scholar]
- 8.Saeyeldin A, Zafar MA, Li Y, et al. Decision-making algorithm for ascending aortic aneurysm: effectiveness in clinical application? J Thorac Cardiovasc Surg. 2019;157:1733–1745. [DOI] [PubMed] [Google Scholar]
6.1.2.2.1. Diagnostic and Surveillance Aortic Imaging in Marfan Syndrome
- 1.Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476–485. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein SA, Evangelista A, Abbara S, et al. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:119–182. [DOI] [PubMed] [Google Scholar]
- 3.Finkbohner R, Johnston D, Crawford ES, et al. Marfan syndrome. Long-term survival and complications after aortic aneurysm repair. Circulation. 1995;91:728–733. [DOI] [PubMed] [Google Scholar]
- 4.den Hartog AW, Franken R, Zwinderman AH, et al. The risk for type B aortic dissection in Marfan syndrome. J Am Coll Cardiol. 2015;65:246–254. [DOI] [PubMed] [Google Scholar]
- 5.Girdauskas E, Kuntze T, Borger MA, et al. Distal aortic reinterventions after root surgery in Marfan patients. Ann Thorac Surg. 2008;86:1815–1819. [DOI] [PubMed] [Google Scholar]
- 6.LeMaire SA, Carter SA, Volguina IV, et al. Spectrum of aortic operations in 300 patients with confirmed or suspected Marfan syndrome. Ann Thorac Surg. 2006;81:2063–2078; discussion 2078. [DOI] [PubMed] [Google Scholar]
- 7.Hagerty T, Geraghty P, Braverman AC. Abdominal aortic aneurysm in Marfan syndrome. Ann Vasc Surg. 2017;40:294.e1–294.6. [DOI] [PubMed] [Google Scholar]
- 8.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. [DOI] [PubMed] [Google Scholar]
- 9.Gott VL, Greene PS, Alejo DE, et al. Replacement of the aortic root in patients with Marfan’s syndrome. N Engl J Med. 1999;340:1307–1313. [DOI] [PubMed] [Google Scholar]
- 10.Silverman DI, Burton KJ, Gray J, et al. Life expectancy in the Marfan syndrome. Am J Cardiol. 1995;75:157–160. [DOI] [PubMed] [Google Scholar]
- 11.Roman MJ, Rosen SE, Kramer-Fox R, et al. Prognostic significance of the pattern of aortic root dilation in the Marfan syndrome. J Am Coll Cardiol. 1993;22:1470–1476. [DOI] [PubMed] [Google Scholar]
- 12.Devereux RB, de Simone G, Arnett DK, et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am J Cardiol. 2012;110:1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeMaire SA, Carter SA, Volguina IV, et al. Spectrum of aortic operations in 300 patients with confirmed or suspected Marfan syndrome. Ann Thorac Surg. 2006;81:2063–2078; discussion 2078. [DOI] [PubMed] [Google Scholar]
6.1.2.2.2. Medical Therapy in Marfan Syndrome
- 1.Forteza A, Evangelista A, Sanchez V, et al. Efficacy of losartan vs. atenolol for the prevention of aortic dilation in Marfan syndrome: a randomized clinical trial. Eur Heart J. 2016;37:978–985. [DOI] [PubMed] [Google Scholar]
- 2.Lacro RV, Dietz HC, Sleeper LA, et al. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med. 2014;371:2061–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groenink M, den Hartog AW, Franken R, et al. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J. 2013;34:3491–3500. [DOI] [PubMed] [Google Scholar]
- 4.Mullen M, Jin XY, Child A, et al. Irbesartan in Marfan syndrome (AIMS): a double-blind, placebo-controlled randomised trial. Lancet. 2019;394:2263–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shores J, Berger KR, Murphy EA, et al. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan’s syndrome. N Engl J Med. 1994;330:1335–1341. [DOI] [PubMed] [Google Scholar]
- 6.Ladouceur M, Fermanian C, Lupoglazoff JM, et al. Effect of beta-blockade on ascending aortic dilatation in children with the Marfan syndrome. Am J Cardiol. 2007;99:406–409. [DOI] [PubMed] [Google Scholar]
- 7.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. [DOI] [PubMed] [Google Scholar]
- 8.Milleron O, Arnoult F, Ropers J, et al. Marfan Sartan: a randomized, double-blind, placebo-controlled trial. Eur Heart J. 2015;36:2160–2166. [DOI] [PubMed] [Google Scholar]
- 9.Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooke BS, Habashi JP, Judge DP, et al. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med. 2008;358:2787–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Abcha A, Saleh Y, Mujer M, et al. Meta-analysis examining the usefulness of angiotensin receptor blockers for the prevention of aortic root dilation in patients with the Marfan syndrome. Am J Cardiol. 2020;128:101–106. [DOI] [PubMed] [Google Scholar]
6.1.2.2.3. Marfan Syndrome Interventions: Replacement of the Aortic Root in Patients With Marfan Syndrome
- 1.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. [DOI] [PubMed] [Google Scholar]
- 2.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 3.Milleron O, Arnoult F, Delorme G, et al. Pathogenic FBN1 genetic variation and aortic dissection in patients with Marfan syndrome. J Am Coll Cardiol. 2020;75:843–853. [DOI] [PubMed] [Google Scholar]
- 4.Martin C, Evangelista A, Serrano-Fiz S, et al. Aortic complications in Marfan syndrome: should we anticipate preventive aortic root surgery? Ann Thorac Surg. 2020;109:1850–1857. [DOI] [PubMed] [Google Scholar]
- 5.Svensson LG, Khitin L. Aortic cross-sectional area/height ratio timing of aortic surgery in asymptomatic patients with Marfan syndrome. J Thorac Cardiovasc Surg. 2002;123:360–361. [DOI] [PubMed] [Google Scholar]
- 6.Silverman DI, Gray J, Roman MJ, et al. Family history of severe cardiovascular disease in Marfan syndrome is associated with increased aortic diameter and decreased survival. J Am Coll Cardiol. 1995;26:1062–1067. [DOI] [PubMed] [Google Scholar]
- 7.Finkbohner R, Johnston D, Crawford ES, et al. Marfan syndrome. Long-term survival and complications after aortic aneurysm repair. Circulation. 1995;91:728–733. [DOI] [PubMed] [Google Scholar]
- 8.Gott VL, Greene PS, Alejo DE, et al. Replacement of the aortic root in patients with Marfan’s syndrome. N Engl J Med. 1999;340:1307–1313. [DOI] [PubMed] [Google Scholar]
- 9.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 10.Silverman DI, Burton KJ, Gray J, et al. Life expectancy in the Marfan syndrome. Am J Cardiol. 1995;75:157–160. [DOI] [PubMed] [Google Scholar]
- 11.Jondeau G, Detaint D, Tubach F, et al. Aortic event rate in the Marfan population: a cohort study. Circulation. 2012;125:226–232. [DOI] [PubMed] [Google Scholar]
- 12.Saeyeldin A, Zafar MA, Velasquez CA, et al. Natural history of aortic root aneurysms in Marfan syndrome. Ann Cardiothorac Surg. 2017;6:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coselli JS, Volguina IV, LeMaire SA, et al. Early and 1-year outcomes of aortic root surgery in patients with Marfan syndrome: a prospective, multicenter, comparative study. J Thorac Cardiovasc Surg. 2014;147:1758–1766, 1767.e1–4. [DOI] [PubMed] [Google Scholar]
- 14.Roman MJ, Rosen SE, Kramer-Fox R, et al. Prognostic significance of the pattern of aortic root dilation in the Marfan syndrome. J Am Coll Cardiol. 1993;22:1470–1476. [DOI] [PubMed] [Google Scholar]
- 15.Morris SA, Orbach DB, Geva T, et al. Increased vertebral artery tortuosity index is associated with adverse outcomes in children and young adults with connective tissue disorders. Circulation. 2011;124:388–396. [DOI] [PubMed] [Google Scholar]
6.1.2.2.4. Marfan Syndrome Interventions: Replacement of Primary (Nondissected) Aneurysms of the Aortic Arch, Descending, and Abdominal Aorta in Patients With Marfan Syndrome
- 1.den Hartog AW, Franken R, Zwinderman AH, et al. The risk for type B aortic dissection in Marfan syndrome. J Am Coll Cardiol. 2015;65:246–254. [DOI] [PubMed] [Google Scholar]
- 2.LeMaire SA, Carter SA, Volguina IV, et al. Spectrum of aortic operations in 300 patients with confirmed or suspected Marfan syndrome. Ann Thorac Surg. 2006;81:2063–2078; discussion 2078. [DOI] [PubMed] [Google Scholar]
- 3.Roselli EE, Idrees JJ, Lowry AM, et al. Beyond the aortic root: staged open and endovascular repair of arch and descending aorta in patients with connective tissue disorders. Ann Thorac Surg. 2016;101:906–912. [DOI] [PubMed] [Google Scholar]
- 4.Schoenhoff FS, Yildiz M, Langhammer B, et al. The fate of nonaortic arterial segments in Marfan patients. J Thorac Cardiovasc Surg. 2019;157:2150–2156. [DOI] [PubMed] [Google Scholar]
- 5.Engelfriet PM, Boersma E, Tijssen JG, et al. Beyond the root: dilatation of the distal aorta in Marfan’s syndrome. Heart. 2006;92:1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JB, Spotnitz M, Lindsay ME, et al. Risk of aortic dissection in the moderately dilated ascending aorta. J Am Coll Cardiol. 2016;68:1209–1219. [DOI] [PubMed] [Google Scholar]
- 7.Braverman AC, Mittauer E, Harris KM, et al. Clinical features and outcomes of pregnancy-related acute aortic dissection. JAMA Cardiol. 2021;6:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
6.1.2.3.1. Imaging in Loeys-Dietz Syndrome
- 1.Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–798. [DOI] [PubMed] [Google Scholar]
- 2.MacCarrick G, Black JH 3rd, Bowdin S, et al. Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med. 2014;16:576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. [DOI] [PubMed] [Google Scholar]
- 4.van der Linde D, van de Laar IM, Bertoli-Avella AM, et al. Aggressive cardiovascular phenotype of aneurysms-osteoarthritis syndrome caused by pathogenic SMAD3 variants. J Am Coll Cardiol. 2012;60:397–403. [DOI] [PubMed] [Google Scholar]
- 5.Bulder RMA, Eefting D, Vriens P, et al. Editor’s choice - a systemic evaluation of the costs of elective EVAR and open abdominal aortic aneurysm repair implies cost equivalence. Eur J Vasc Endovasc Surg. 2020;60:655–662. [DOI] [PubMed] [Google Scholar]
- 6.Jondeau G, Ropers J, Regalado E, et al. International Registry of Patients Carrying TGFBR1 or TGFBR2 Mutations: results of the MAC (Montalcino Aortic Consortium). Circ Cardiovasc Genet. 2016;9:548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitagawa A, Greenberg RK, Eagleton MJ, et al. Fenestrated and branched endovascular aortic repair for chronic type B aortic dissection with thoracoabdominal aneurysms. J Vasc Surg. 2013;58:625–634. [DOI] [PubMed] [Google Scholar]
- 8.Jondeau G, Ropers J, Regalado E, et al. International Registry of Patients Carrying TGFBR1 or TGFBR2 Mutations: results of the MAC (Montalcino Aortic Consortium). Circ Cardiovasc Genet. 2016;9:548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hostetler EM, Regalado ES, Guo DC, et al. SMAD3 pathogenic variants: risk for thoracic aortic disease and associated complications from the Montalcino Aortic Consortium. J Med Genet. 2019;56:252–260. [DOI] [PubMed] [Google Scholar]
- 10.van de Laar IM, van der Linde D, Oei EH, et al. Phenotypic spectrum of the SMAD3-related aneurysms-osteoarthritis syndrome. J Med Genet. 2012;49:47–57. [DOI] [PubMed] [Google Scholar]
- 11.Huguenard AL, Gupta VP, Braverman AC, et al. Genetic and heritable considerations in patients or families with both intracranial and extracranial aneurysms. J Neurosurg. 2021;134:1999–2006. [DOI] [PubMed] [Google Scholar]
- 12.van der LD, van d LI, Bertoli-Avella AM, et al. Aggressive cardiovascular phenotype of aneurysms-osteoarthritis syndrome caused by pathogenic SMAD3 variants. J Am Coll Cardiol. 2012;60:397–403. [DOI] [PubMed] [Google Scholar]
- 13.Bertoli-Avella AM, Gillis E, Morisaki H, et al. Mutations in a TGF-beta ligand, TGFB3, cause syndromic aortic aneurysms and dissections. J Am Coll Cardiol. 2015;65:1324–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boileau C, Guo DC, Hanna N, et al. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat Genet. 2012;44:916–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindsay ME, Schepers D, Bolar NA, et al. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet. 2012;44:922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schepers D, Tortora G, Morisaki H, et al. A mutation update on the LDS-associated genes TGFB2/3 and SMAD2/3. Hum Mutat. 2018;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thakker PD, Braverman AC. Cardiogenetics: genetic testing in the diagnosis and management of patients with aortic disease. Heart. 2021;107:619–626. [DOI] [PubMed] [Google Scholar]
- 18.Patel ND, Alejo D, Crawford T, et al. Aortic root replacement for children with Loeys-Dietz syndrome. Ann Thorac Surg. 2017;103:1513–1518. [DOI] [PubMed] [Google Scholar]
- 19.Schoenhoff FS, Alejo DE, Black JH, et al. Management of the aortic arch in patients with Loeys-Dietz syndrome. J Thorac Cardiovasc Surg. 2020;160:1166–1175. [DOI] [PubMed] [Google Scholar]
- 20.Regalado ES, Guo DC, Villamizar C, et al. Exome sequencing identifies SMAD3 mutations as a cause of familial thoracic aortic aneurysm and dissection with intracranial and other arterial aneurysms. Circ Res. 2011;109:680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
6.1.2.3.2. Medical Therapy in Loeys-Dietz Syndrome
- 1.MacCarrick G, Black JH 3rd, Bowdin S, et al. Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med. 2014;16:576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallo EM, Loch DC, Habashi JP, et al. Angiotensin II-dependent TGF-beta signaling contributes to Loeys-Dietz syndrome vascular pathogenesis. J Clin Invest. 2014;124:448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
6.1.2.3.3. Loeys-Dietz Syndrome Surgical Interventions: Replacement of the Aorta in Patients With Loeys-Dietz Syndrome
- 1.Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–798. [DOI] [PubMed] [Google Scholar]
- 2.Jondeau G, Ropers J, Regalado E, et al. International Registry of Patients Carrying TGFBR1 or TGFBR2 Mutations: results of the MAC (Montalcino Aortic Consortium). Circ Cardiovasc Genet. 2016;9:548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der LD, van d LI, Bertoli-Avella AM, et al. Aggressive cardiovascular phenotype of aneurysms-osteoarthritis syndrome caused by pathogenic SMAD3 variants. J Am Coll Cardiol. 2012;60:397–403. [DOI] [PubMed] [Google Scholar]
- 4.Hostetler EM, Regalado ES, Guo DC, et al. SMAD3 pathogenic variants: risk for thoracic aortic disease and associated complications from the Montalcino Aortic Consortium. J Med Genet. 2019;56:252–260. [DOI] [PubMed] [Google Scholar]
- 5.Boileau C, Guo DC, Hanna N, et al. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat Genet. 2012;44:916–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindsay ME, Schepers D, Bolar NA, et al. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet. 2012;44:922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renard M, Callewaert B, Baetens M, et al. Novel MYH11 and ACTA2 mutations reveal a role for enhanced TGFss signaling in FTAAD. Int J Cardiol. 2013;165:314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertoli-Avella AM, Gillis E, Morisaki H, et al. Mutations in a TGF-beta ligand, TGFB3, cause syndromic aortic aneurysms and dissections. J Am Coll Cardiol. 2015;65:1324–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsili L, Overwater E, Hanna N, et al. Phenotypic spectrum of TGFB3 disease-causing variants in a Dutch-French cohort and first report of a homozygous patient. Clin Genet. 2020;97:723–730. [DOI] [PubMed] [Google Scholar]
- 10.MacCarrick G, Black JH 3rd, Bowdin S, et al. Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med. 2014;16:576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel ND, Crawford T, Magruder JT, et al. Cardiovascular operations for Loeys-Dietz syndrome: intermediate-term results. J Thorac Cardiovasc Surg. 2017;153:406–412. [DOI] [PubMed] [Google Scholar]
- 12.Aftab M, Cikach FS, Zhu Y, et al. Loeys-Dietz syndrome: intermediate-term outcomes of medically and surgically managed patients. J Thorac Cardiovasc Surg. 2019;157:439–450.e5. [DOI] [PubMed] [Google Scholar]
- 13.Hostetler EM, Regalado ES, Guo DC, et al. SMAD3 pathogenic variants: risk for thoracic aortic disease and associated complications from the Montalcino Aortic Consortium. J Med Genet. 2019;56:252–260. [DOI] [PubMed] [Google Scholar]
- 14.Renard M, Callewaert B, Malfait F, et al. Thoracic aortic-aneurysm and dissection in association with significant mitral valve disease caused by mutations in TGFB2. Int J Cardiol. 2013;165:584–587. [DOI] [PubMed] [Google Scholar]
- 15.van de Laar IM, Oldenburg RA, Pals G, et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet. 2011;43:121–126. [DOI] [PubMed] [Google Scholar]
- 16.van der Linde D, van de Laar IM, Bertoli-Avella AM, et al. Aggressive cardiovascular phenotype of aneurysms-osteoarthritis syndrome caused by pathogenic SMAD3 variants. J Am Coll Cardiol. 2012;60:397–403. [DOI] [PubMed] [Google Scholar]
- 17.Tran-Fadulu V, Pannu H, Kim DH, et al. Analysis of multigenerational families with thoracic aortic aneurysms and dissections due to TGFBR1 or TGFBR2 mutations. J Med Genet. 2009;46:607–613. [DOI] [PubMed] [Google Scholar]
- 18.Schoenhoff FS, Alejo DE, Black JH, et al. Management of the aortic arch in patients with Loeys-Dietz syndrome. J Thorac Cardiovasc Surg. 2020;160:1166–1175. [DOI] [PubMed] [Google Scholar]
- 19.Jondeau G, Ropers J, Regalado E, et al. International Registry of Patients Carrying TGFBR1 or TGFBR2 Mutations: results of the MAC (Montalcino Aortic Consortium). Circ Cardiovasc Genet. 2016;9:548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. [DOI] [PubMed] [Google Scholar]
- 21.Singh KK, Rommel K, Mishra A, et al. TGFBR1 and TGFBR2 mutations in patients with features of Marfan syndrome and Loeys-Dietz syndrome. Hum Mutat. 2006;27:770–777. [DOI] [PubMed] [Google Scholar]
- 22.Skeik N, Golden M, Berg A, et al. Type A aortic dissection caused by Loeys-Dietz syndrome with novel variation. Ann Vasc Surg. 2020;68:567.e1–567.4. [DOI] [PubMed] [Google Scholar]
- 23.Bertoli-Avella AM, Gillis E, Morisaki H, et al. Mutations in a TGF-beta ligand, TGFB3, cause syndromic aortic aneurysms and dissections. J Am Coll Cardiol. 2015;65:1324–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
6.1.2.4. Vascular Ehlers-Danlos Syndrome: Imaging, Medical Therapy, and Surgical Intervention
- 1.Shalhub S, Byers PH, Hicks KL, et al. A multi-institutional experience in the aortic and arterial pathology in individuals with genetically confirmed vascular Ehlers-Danlos syndrome. J Vasc Surg. 2019;70:1543–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shalhub S, Black JH 3rd, Cecchi AC, et al. Molecular diagnosis in vascular Ehlers-Danlos syndrome predicts pattern of arterial involvement and outcomes. J Vasc Surg. 2014;60:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benrashid E, Ohman JW. Current management of the vascular subtype of Ehlers-Danlos syndrome. Curr Opin Cardiol. 2020;35:603–609. [DOI] [PubMed] [Google Scholar]
- 4.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 5.Brooke BS, Arnaoutakis G, McDonnell NB, et al. Contemporary management of vascular complications associated with Ehlers-Danlos syndrome. J Vasc Surg. 2010;51:131–138; discussion 138–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sage L, Russo ML, Byers PH, et al. Setting a research agenda for vascular Ehlers-Danlos syndrome using a patient and stakeholder engagement model. J Vasc Surg. 2020;72:1436–1444.e2. [DOI] [PubMed] [Google Scholar]
- 7.Ong KT, Perdu J, De Backer J, et al. Effect of celiprolol on prevention of cardiovascular events in vascular Ehlers-Danlos syndrome: a prospective randomised, open, blinded-endpoints trial. Lancet. 2010;376:1476–1484. [DOI] [PubMed] [Google Scholar]
- 8.Frank M, Adham S, Seigle S, et al. Vascular Ehlers-Danlos syndrome: long-term observational study. J Am Coll Cardiol. 2019;73:1948–1957. [DOI] [PubMed] [Google Scholar]
- 9.Murray ML, Pepin M, Peterson S, et al. Pregnancy-related deaths and complications in women with vascular Ehlers-Danlos syndrome. Genet Med. 2014;16:874–880. [DOI] [PubMed] [Google Scholar]
- 10.Byers PH, Belmont J, Black J, et al. Diagnosis, natural history, and management in vascular Ehlers-Danlos syndrome. Am J Med Genet C Semin Med Genet. 2017;175:40–47. [DOI] [PubMed] [Google Scholar]
- 11.Campens L, Baris L, Scott NS, et al. Pregnancy outcome in thoracic aortic disease data from the Registry of Pregnancy and Cardiac Disease. Heart. 2021;107:1704–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–3241. [DOI] [PubMed] [Google Scholar]
6.1.2.5. Turner Syndrome
- 1.Mazzanti L, Cacciari E. Congenital heart disease in patients with Turner’s syndrome. Italian Study Group for Turner Syndrome (ISGTS). J Pediatr. 1998;133:688–692. [DOI] [PubMed] [Google Scholar]
- 2.Sybert VP. Cardiovascular malformations and complications in Turner syndrome. Pediatrics. 1998;101:E11. [DOI] [PubMed] [Google Scholar]
- 3.Volkl TM, Degenhardt K, Koch A, et al. Cardiovascular anomalies in children and young adults with Ullrich-Turner syndrome the Erlangen experience. Clin Cardiol. 2005;28:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gotzsche CO, Krag-Olsen B, Nielsen J, et al. Prevalence of cardiovascular malformations and association with karyotypes in Turner’s syndrome. Arch Dis Child. 1994;71:433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzanti L, Prandstraller D, Tassinari D, et al. Heart disease in Turner’s syndrome. Helv Paediatr Acta. 1988;43:25–31. [PubMed] [Google Scholar]
- 6.Kim HK, Gottliebson W, Hor K, et al. Cardiovascular anomalies in Turner syndrome: spectrum, prevalence, and cardiac MRI findings in a pediatric and young adult population. AJR Am J Roentgenol. 2011;196:454–460. [DOI] [PubMed] [Google Scholar]
- 7.Ho VB, Bakalov VK, Cooley M, et al. Major vascular anomalies in Turner syndrome - prevalence and magnetic resonance angiographic features. Circulation. 2004;110:1694–1700. [DOI] [PubMed] [Google Scholar]
- 8.Loscalzo ML, Van PL, Ho VB, et al. Association between fetal lymphedema and congenital cardiovascular defects in Turner syndrome. Pediatrics. 2005;115:732–735. [DOI] [PubMed] [Google Scholar]
- 9.Silberbach M, Roos-Hesselink JW, Andersen NH, et al. Cardiovascular health in Turner syndrome: a scientific statement from the American Heart Association. Circ Genom Precis Med. 2018;11:e000048. [DOI] [PubMed] [Google Scholar]
- 10.Matura LA, Ho VB, Rosing DR, et al. Aortic dilatation and dissection in Turner syndrome. Circulation. 2007;116:1663–1670. [DOI] [PubMed] [Google Scholar]
- 11.Carlson M, Airhart N, Lopez L, et al. Moderate aortic enlargement and bicuspid aortic valve are associated with aortic dissection in Turner syndrome: report of the international turner syndrome aortic dissection registry. Circulation. 2012;126:2220–2226. [DOI] [PubMed] [Google Scholar]
- 12.Gravholt CH, Andersen NH, Conway GS, et al. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol. 2017;177:G1–G70. [DOI] [PubMed] [Google Scholar]
- 13.Carlson M, Silberbach M. Dissection of the aorta in Turner syndrome: two cases and review of 85 cases in the literature. J Med Genet. 2007;44:745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quezada E, Lapidus J, Shaughnessy R, et al. Aortic dimensions in Turner syndrome. Am J Med Genet A. 2015;167A:2527–2532. [DOI] [PubMed] [Google Scholar]
6.1.2.6. Pathogenic or Likely Pathogenic Variants in ACTA2, PRKG1, MYH11, MYLK, and LOX: Recommendations for Surveillance of Aorta, Medical Therapy, and Aortic Surgical Intervention
- 1.Guo DC, Regalado ES, Gong L, et al. LOX Mutations Predispose to Thoracic Aortic Aneurysms and Dissections. Circ Res. 2016;118:928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo DC, Pannu H, Papke CL, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39:1488–1493. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Guo DC, Cao J, et al. Mutations in Myosin light chain kinase cause familial aortic dissections. Am J Hum Genet. 2010;87:701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pannu H, Tran-Fadulu V, Papke CL, et al. MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Hum Mol Genet. 2007;16:3453–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo DC, Papke CL, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and moyamoya disease, along with thoracic aortic disease. Am J Hum Genet. 2009;84:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milewicz DM, Ostergaard JR, la-Kokko LM, et al. De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am J Med Genet A. 2010;152A:2437–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regalado ES, Guo DC, Prakash S, et al. Aortic disease presentation and outcome associated with ACTA2 mutations. Circ Cardiovasc Genet. 2015;8:457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauer A, Speroni SL, Patel JB, et al. Cerebrovascular disease progression in patients with ACTA2 Arg179 pathogenic variants. Neurology. 2021;96:e538–e552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regalado ES, Guo DC, Prakash S, et al. Aortic disease presentation and outcome associated with ACTA2 mutations. Circ Cardiovasc Genet. 2015;8:457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galian-Gay L, Carro Hevia A, Teixido-Tura G, et al. Familial clustering of bicuspid aortic valve and its relationship with aortic dilation in first-degree relatives. Heart. 2019;105:603–608. [DOI] [PubMed] [Google Scholar]
- 11.Guo DC, Regalado E, Casteel DE, et al. Recurrent gain-of-function mutation in PRKG1 causes thoracic aortic aneurysms and acute aortic dissections. Am J Hum Genet. 2013;93:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shalhub S, Regalado ES, Guo DC, et al. The natural history of type B aortic dissection in patients with PRKG1 mutation c.530G>A (p.Arg177Gln). J Vasc Surg. 2019;70:718–723. [DOI] [PubMed] [Google Scholar]
- 13.Gago-Diaz M, Blanco-Verea A, Teixido G, et al. PRKG1 and genetic diagnosis of early-onset thoracic aortic disease. Eur J Clin Invest. 2016;46:787–794. [DOI] [PubMed] [Google Scholar]
- 14.Zhu L, Vranckx R, Khau Van KP, et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet. 2006;38:343–349. [DOI] [PubMed] [Google Scholar]
- 15.Wallace SE, Regalado ES, Gong L, et al. MYLK pathogenic variants aortic disease presentation, pregnancy risk, and characterization of pathogenic missense variants. Genet Med. 2019;21:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luyckx I, Proost D, Hendriks JM, et al. Two novel MYLK nonsense mutations causing thoracic aortic aneurysms/dissections in patients without apparent family history. Clin Genet. 2017;92:444–446. [DOI] [PubMed] [Google Scholar]
- 17.Lee VS, Halabi CM, Hoffman EP, et al. Loss of function mutation in LOX causes thoracic aortic aneurysm and dissection in humans. Proc Natl Acad Sci U S A. 2016;113:8759–8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cirnu A, Kolokotronis K, Walz K, et al. Novel mutation in LOX associates with a complex aneurysmal vascular and cardiac phenotype. Circ Genom Precis Med. 2021;14:e003217. [DOI] [PubMed] [Google Scholar]
6.1.3. BAV Aortopathy
- 1.Kang JW, Song HG, Yang DH, et al. Association between bicuspid aortic valve phenotype and patterns of valvular dysfunction and bicuspid aortopathy: comprehensive evaluation using MDCT and echocardiography. J Am Coll Cardiol Img. 2013;6:150–161. [DOI] [PubMed] [Google Scholar]
- 2.Della Corte A, Bancone C, Buonocore M, et al. Pattern of ascending aortic dimensions predicts the growth rate of the aorta in patients with bicuspid aortic valve. J Am Coll Cardiol Img. 2013;6:1301–1310. [DOI] [PubMed] [Google Scholar]
- 3.Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with bicuspid aortic valve and a dilated ascending aorta. Circ Cardiovasc Imaging. 2017;10:e006249. [DOI] [PubMed] [Google Scholar]
- 4.Schaefer BM, Lewin MB, Stout KK, et al. The bicuspid aortic valve: an integrated phenotypic classification of leaflet morphology and aortic root shape. Heart. 2008;94:1634–1638. [DOI] [PubMed] [Google Scholar]
- 5.Bravo-Jaimes K, Prakash SK. Genetics in bicuspid aortic valve disease: where are we? Prog Cardiovasc Dis. 2020;63:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillis E, Kumar AA, Luyckx I, et al. Candidate gene resequencing in a large bicuspid aortic valve-associated thoracic aortic aneurysm cohort: SMAD6 as an important contributor. Front Physiol. 2017;8:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loscalzo ML, Goh DL, Loeys B, et al. Familial thoracic aortic dilation and bicommissural aortic valve: a prospective analysis of natural history and inheritance. Am J Med Genet A. 2007;143:1960–1967. [DOI] [PubMed] [Google Scholar]
- 8.Galian-Gay L, Carro Hevia A, Teixido-Tura G, et al. Familial clustering of bicuspid aortic valve and its relationship with aortic dilation in first-degree relatives. Heart. 2019;105:603–608. [DOI] [PubMed] [Google Scholar]
- 9.Kerstjens-Frederikse WS, Du Marchie Sarvaas GJ, Ruiter JS, et al. Left ventricular outflow tract obstruction: should cardiac screening be offered to first-degree relatives? Heart. 2011;97:1228–1232. [DOI] [PubMed] [Google Scholar]
- 10.Cripe L, Andelfinger G, Martin LJ, et al. Bicuspid aortic valve is heritable. J Am Coll Cardiol. 2004;44:138–143. [DOI] [PubMed] [Google Scholar]
- 11.Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–1112. [DOI] [PubMed] [Google Scholar]
- 12.Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. 2014;370:1920–1929. [DOI] [PubMed] [Google Scholar]
- 13.Wojnarski CM, Svensson LG, Roselli EE, et al. Aortic dissection in patients with bicuspid aortic valve-associated aneurysms. Ann Thorac Surg. 2015;100:1666–1673; discussion 1673–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKellar SH, Michelena HI, Li Z, et al. Long-term risk of aortic events following aortic valve replacement in patients with bicuspid aortic valves. Am J Cardiol. 2010;106:1626–1633. [DOI] [PubMed] [Google Scholar]
- 15.Sperling JS, Lubat E. Forme fruste or ‘incomplete’ bicuspid aortic valves with very small raphes: the prevalence of bicuspid valve and its significance may be underestimated. Int J Cardiol. 2015;184:1–5. [DOI] [PubMed] [Google Scholar]
- 16.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–e227. [DOI] [PubMed] [Google Scholar]
- 17.Musunuru K, Hershberger RE, Day SM, et al. Genetic testing for inherited cardiovascular diseases: a scientific statement from the American Heart Association. Circ Genom Precis Med. 2020;13:e000067. [DOI] [PubMed] [Google Scholar]
- 18.Tessler I, Leshno M, Shmueli A, et al. Cost-effectiveness analysis of screening for first-degree relatives of patients with bicuspid aortic valve. Eur Heart J Qual Care Clin Outcomes. 2021;7:447–457. [DOI] [PubMed] [Google Scholar]
6.1.3.1. Routine Follow-Up of BAV Disease Aortopathy
- 1.Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation. 2008;117:2776–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–1112. [DOI] [PubMed] [Google Scholar]
- 3.Girdauskas E, Disha K, Raisin HH, et al. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg. 2012;42:832–837; discussion 837–838. [DOI] [PubMed] [Google Scholar]
- 4.Girdauskas E, Disha K, Rouman M, et al. Aortic events after isolated aortic valve replacement for bicuspid aortic valve root phenotype: echocardiographic follow-up study. Eur J Cardiothorac Surg. 2015;48:e71–e76. [DOI] [PubMed] [Google Scholar]
- 5.Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. 2014;370:1920–1929. [DOI] [PubMed] [Google Scholar]
- 6.Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with bicuspid aortic valve and a dilated ascending aorta. Circ Cardiovasc Imaging. 2017;10:e006249. [DOI] [PubMed] [Google Scholar]
- 7.Borger MA, Fedak PWM, Stephens EH, et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: full online-only version. J Thorac Cardiovasc Surg. 2018;156:e41–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKellar SH, Michelena HI, Li Z, et al. Long-term risk of aortic events following aortic valve replacement in patients with bicuspid aortic valves. Am J Cardiol. 2010;106:1626–1633. [DOI] [PubMed] [Google Scholar]
- 9.Girdauskas E, Disha K, Borger MA, et al. Long-term prognosis of ascending aortic aneurysm after aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Thorac Cardiovasc Surg. 2014;147:276–282. [DOI] [PubMed] [Google Scholar]
- 10.Girdauskas E, Rouman M, Disha K, et al. Aortic dissection after previous aortic valve replacement for bicuspid aortic valve disease. J Am Coll Cardiol. 2015;66:1409–1411. [DOI] [PubMed] [Google Scholar]
- 11.Avadhani SA, Martin-Doyle W, Shaikh AY, et al. Predictors of ascending aortic dilation in bicuspid aortic valve disease: a five-year prospective study. Am J Med. 2015;128:647–652. [DOI] [PubMed] [Google Scholar]
- 12.Devereux RB, de Simone G, Arnett DK, et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am J Cardiol. 2012;110:1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
6.1.3.2. BAV Aortopathy Interventions: Replacement of the Aorta in Patients With BAV
- 1.Borger MA, Fedak PWM, Stephens EH, et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: full online-only version. J Thorac Cardiovasc Surg. 2018;156:e41–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–e227. [DOI] [PubMed] [Google Scholar]
- 3.Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with bicuspid aortic valve and a dilated ascending aorta. Circ Cardiovasc Imaging. 2017;10:e006249. [DOI] [PubMed] [Google Scholar]
- 4.Svensson LG, Blackstone EH, Cosgrove DM III. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol. 2003;28:417–480. [DOI] [PubMed] [Google Scholar]
- 5.Wojnarski CM, Svensson LG, Roselli EE, et al. Aortic dissection in patients with bicuspid aortic valve-associated aneurysms. Ann Thorac Surg. 2015;100:1666–1673; discussion 1673–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borger MA, Preston M, Ivanov J, et al. Should the ascending aorta be replaced more frequently in patients with bicuspid aortic valve disease? J Thorac Cardiovasc Surg. 2004;128:677–683. [DOI] [PubMed] [Google Scholar]
- 7.Braverman AC. Aortic replacement for bicuspid aortic valve aortopathy: when and why? J Thorac Cardiovasc Surg. 2019;157:520–525. [DOI] [PubMed] [Google Scholar]
- 8.Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–1112. [DOI] [PubMed] [Google Scholar]
- 9.Zafar MA, Li Y, Rizzo JA, et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J Thorac Cardiovasc Surg. 2018;155:1938–1950. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Lin Y, Zhang H, et al. Relationship of platelet counts and inflammatory markers to 30-day mortality risk in patients with acute type A aortic dissection. Biomed Res Int. 2020;2020:1057496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver JM, Alonso-Gonzalez R, Gonzalez AE, et al. Risk of aortic root or ascending aorta complications in patients with bicuspid aortic valve with and without coarctation of the aorta. Am J Cardiol. 2009;104:1001–1006. [DOI] [PubMed] [Google Scholar]
- 12.Duijnhouwer A, van den Hoven A, Merkx R, et al. Differences in aortopathy in patients with a bicuspid aortic valve with or without aortic coarctation. J Clin Med. 2020;9:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girdauskas E, Disha K, Raisin HH, et al. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg. 2012;42:832–837; discussion 837–838. [DOI] [PubMed] [Google Scholar]
- 14.Girdauskas E, Disha K, Rouman M, et al. Aortic events after isolated aortic valve replacement for bicuspid aortic valve root phenotype: echocardiographic follow-up study. Eur J Cardiothorac Surg. 2015;48:e71–e76. [DOI] [PubMed] [Google Scholar]
- 15.Kim JB, Spotnitz M, Lindsay ME, et al. Risk of aortic dissection in the moderately dilated ascending aorta. J Am Coll Cardiol. 2016;68:1209–1219. [DOI] [PubMed] [Google Scholar]
6.2. AAA: Cause, Risk Factors, and Screening
- 1.Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219–2238. [DOI] [PubMed] [Google Scholar]
- 2.Sakalihasan N, Defraigne JO, Kerstenne MA, et al. Family members of patients with abdominal aortic aneurysms are at increased risk for aneurysms: analysis of 618 probands and their families from the Liege AAA Family Study. Ann Vasc Surg. 2014;28:787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuivaniemi H, Shibamura H, Arthur C, et al. Familial abdominal aortic aneurysms: collection of 233 multiplex families. J Vasc Surg. 2003;37:340–345. [DOI] [PubMed] [Google Scholar]
- 4.Carter JL, Morris DR, Sherliker P, et al. Sex-specific associations of vascular risk factors with abdominal aortic aneurysm: findings from 1.5 million women and 0.8 million men in the United States and United Kingdom. J Am Heart Assoc. 2020;9:e014748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dansey KD, Varkevisser RRB, Swerdlow NJ, et al. Epidemiology of endovascular and open repair for abdominal aortic aneurysms in the United States from 2004–2015 and implications for screening. J Vasc Surg. 2021;74:414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linne A, Forsberg J, Lindstrom D, et al. Age at detection of abdominal aortic aneurysms in siblings of patients with abdominal aortic aneurysms. J Vasc Surg. 2016;63:883–887. [DOI] [PubMed] [Google Scholar]
- 7.Davies MJ. Aortic aneurysm formation: lessons from human studies and experimental models. Circulation. 1998;98:193–195. [DOI] [PubMed] [Google Scholar]
- 8.Sakalihasan N, Michel JB, Katsargyris A, et al. Abdominal aortic aneurysms. Nat Rev Dis Primers. 2018;4:34. [DOI] [PubMed] [Google Scholar]
- 9.Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539–548. [DOI] [PubMed] [Google Scholar]
- 10.Altobelli E, Rapacchietta L, Profeta VF, et al. Risk factors for abdominal aortic aneurysm in population-based studies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2018;15:2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang W, Yao L, Roetker NS, et al. Lifetime risk and risk factors for abdominal aortic aneurysm in a 24-year prospective study: the ARIC Study (Atherosclerosis Risk in Communities). Arterioscler Thromb Vasc Biol. 2016;36:2468–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson E, Granath F, Swedenborg J, et al. A population-based case-control study of the familial risk of abdominal aortic aneurysm. J Vasc Surg. 2009;49:47–50; discussion 51. [DOI] [PubMed] [Google Scholar]
- 13.Saadoun D, Vautier M, Cacoub P. Medium- and large-vessel vasculitis. Circulation. 2021;143:267–282. [DOI] [PubMed] [Google Scholar]
- 14.Sorelius K, Budtz-Lilly J, Mani K, et al. Systematic review of the management of mycotic aortic aneurysms. Eur J Vasc Endovasc Surg. 2019;58:426–435. [DOI] [PubMed] [Google Scholar]
- 15.Brady AR, Thompson SG, Fowkes FG, et al. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation. 2004;110:16–21. [DOI] [PubMed] [Google Scholar]
- 16.Sweeting MJ, Thompson SG, Brown LC, et al. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655–665. [DOI] [PubMed] [Google Scholar]
- 17.Akai A, Watanabe Y, Hoshina K, et al. Family history of aortic aneurysm is an independent risk factor for more rapid growth of small abdominal aortic aneurysms in Japan. J Vasc Surg. 2015;61:287–290. [DOI] [PubMed] [Google Scholar]
- 18.Howard DP, Banerjee A, Fairhead JF, et al. Population-based study of incidence of acute abdominal aortic aneurysms with projected impact of screening strategy. J Am Heart Assoc. 2015;4:e001926. [DOI] [PMC free article] [PubMed] [Google Scholar]
6.3. Growth and Natural History of Aortic Aneurysms
- 1.Bashir M, Fok M, Hammoud I, et al. A perspective on natural history and survival in nonoperated thoracic aortic aneurysm patients. Aorta (Stamford). 2013;1:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harky A, Bashir M, Antoniou A, et al. Size and dissection: what is the relation? Indian J Thorac Cardiovasc Surg. 2019;35:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuzmik GA, Sang AX, Elefteriades JA. Natural history of thoracic aortic aneurysms. J Vasc Surg. 2012;56:565–571. [DOI] [PubMed] [Google Scholar]
- 4.Saeyeldin A, Zafar MA, Velasquez CA, et al. Natural history of aortic root aneurysms in Marfan syndrome. Ann Cardiothorac Surg. 2017;6:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Della Corte A, Bancone C, Buonocore M, et al. Pattern of ascending aortic dimensions predicts the growth rate of the aorta in patients with bicuspid aortic valve. J Am Coll Cardiol Img. 2013;6:1301–1310. [DOI] [PubMed] [Google Scholar]
- 6.Kim JB, Spotnitz M, Lindsay ME, et al. Risk of aortic dissection in the moderately dilated ascending aorta. J Am Coll Cardiol. 2016;68:1209–1219. [DOI] [PubMed] [Google Scholar]
- 7.Yiu RS, Cheng SW. Natural history and risk factors for rupture of thoracic aortic arch aneurysms. J Vasc Surg. 2016;63:1189–1194. [DOI] [PubMed] [Google Scholar]
- 8.Zafar MA, Chen JF, Wu J, et al. Natural history of descending thoracic and thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2021;161:498–511.e1. [DOI] [PubMed] [Google Scholar]
- 9.Hendy K, Gunnarson R, Golledge J. Growth rates of small abdominal aortic aneurysms assessed by computerised tomography-a systematic literature review. Atherosclerosis. 2014;235:182–188. [DOI] [PubMed] [Google Scholar]
6.4. Medical Management of Sporadic and Degenerative Aortic Aneurysm Disease
- 1.Smith SC Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation. 2006;113:2363–2372. [DOI] [PubMed] [Google Scholar]
- 2.Davies MJ. Aortic aneurysm formation: lessons from human studies and experimental models. Circulation. 1998;98:193–195. [DOI] [PubMed] [Google Scholar]
- 3.Danyi P, Elefteriades JA, Jovin IS. Medical therapy of thoracic aortic aneurysms: are we there yet? Circulation. 2011;124:1469–1476. [DOI] [PubMed] [Google Scholar]
6.4.1.1. BP Management in Sporadic TAA
- 1.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 2.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 3.Ogden LG, He J, Lydick E, et al. Long-term absolute benefit of lowering blood pressure in hypertensive patients according to the JNC VI risk stratification. Hypertension. 2000;35:539–543. [DOI] [PubMed] [Google Scholar]
- 4.Ladouceur M, Fermanian C, Lupoglazoff JM, et al. Effect of beta-blockade on ascending aortic dilatation in children with the Marfan syndrome. Am J Cardiol. 2007;99:406–409. [DOI] [PubMed] [Google Scholar]
- 5.Shores J, Berger KR, Murphy EA, et al. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan’s syndrome. N Engl J Med. 1994;330:1335–1341. [DOI] [PubMed] [Google Scholar]
- 6.Al-Abcha A, Saleh Y, Mujer M, et al. Meta-analysis examining the usefulness of angiotensin receptor blockers for the prevention of aortic root dilation in patients with the Marfan syndrome. Am J Cardiol. 2020;128:101–106. [DOI] [PubMed] [Google Scholar]
- 7.Howard DP, Banerjee A, Fairhead JF, et al. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation. 2013;127:2031–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danyi P, Elefteriades JA, Jovin IS. Medical therapy of thoracic aortic aneurysms: are we there yet? Circulation. 2011;124:1469–1476. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann Bowman MA, Eagle KA, Milewicz DM. Update on clinical trials of losartan with and without β-blockers to block aneurysm growth in patients with Marfan syndrome: a review. JAMA Cardiol. 2019;4:702–707. [DOI] [PubMed] [Google Scholar]
- 10.Group SR, Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaduganathan M, Claggett BL, Juraschek SP, et al. Assessment of long-term benefit of intensive blood pressure control on residual life span: secondary analysis of the Systolic Blood Pressure Intervention Trial (SPRINT). JAMA Cardiol. 2020;5:576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mochizuki S, Shimizu M, Taniguchi I, et al. JIKEI HEART Study-a morbi-mortality and remodeling study with valsartan in Japanese patients with hypertension and cardiovascular disease. Cardiovasc Drugs Ther. 2004;18:305–309. [DOI] [PubMed] [Google Scholar]
- 13.Mochizuki S, Dahlof B, Shimizu M, et al. Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open-label, blinded endpoint morbidity-mortality study. Lancet. 2007;369:1431–1439. [DOI] [PubMed] [Google Scholar]
6.4.1.2. Treatment of TAA With Statins
- 1.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angeloni E, Vitaterna A, Pirelli M, et al. Effects of statin therapy on ascending aorta aneurysms growth: a propensity-matched analysis. Int J Cardiol. 2015;191:52–55. [DOI] [PubMed] [Google Scholar]
- 4.Goel SS, Tuzcu EM, Agarwal S, et al. Comparison of ascending aortic size in patients with severe bicuspid aortic valve stenosis treated with versus without a statin drug. Am J Cardiol. 2011;108:1458–1462. [DOI] [PubMed] [Google Scholar]
- 5.Stein LH, Berger J, Tranquilli M, et al. Effect of statin drugs on thoracic aortic aneurysms. Am J Cardiol. 2013;112:1240–1245. [DOI] [PubMed] [Google Scholar]
- 6.Taylor AP, Yadlapati A, Andrei AC, et al. Statin use and aneurysm risk in patients with bicuspid aortic valve disease. Clin Cardiol. 2016;39:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato T, Arakawa M, Tashima Y, et al. Statins Reduce Thoracic Aortic Aneurysm Growth in Marfan Syndrome Mice via Inhibition of the Ras-Induced ERK (Extracellular Signal-Regulated Kinase) Signaling Pathway. J Am Heart Assoc. 2018;7:e008543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 9.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–e725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans J, Powell JT, Schwalbe E, et al. Simvastatin attenuates the activity of matrix metalloprotease-9 in aneurysmal aortic tissue. Eur J Vasc Endovasc Surg. 2007;34:302–303. [DOI] [PubMed] [Google Scholar]
- 11.Diehm N, Becker G, Katzen B, et al. Statins are associated with decreased mortality in abdominal, but not in thoracic aortic aneurysm patients undergoing endovascular repair: propensity score-adjusted analysis. Vasa. 2008;37:241–249. [DOI] [PubMed] [Google Scholar]
- 12.Allar BG, Swerdlow NJ, de Guerre L, et al. Preoperative statin therapy is associated with higher 5-year survival after thoracic endovascular aortic repair. J Vasc Surg. 2021;74:1996–2005. [DOI] [PubMed] [Google Scholar]
6.4.1.3. Smoking Cessation in TAA
- 1.Katz DA, Holman JE, Nugent AS, et al. The emergency department action in smoking cessation (EDASC) trial: impact on cessation outcomes. Nicotine Tob Res. 2013;15:1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitlock EP, Orleans CT, Pender N, et al. Evaluating primary care behavioral counseling interventions: an evidence-based approach. Am J Prev Med. 2002;22:267–284. [DOI] [PubMed] [Google Scholar]
- 3.Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. US Dept. of Health and Human Services. Public Health Service. May 2008. [Google Scholar]
- 4.Berry KM, Reynolds LM, Collins JM, et al. E-cigarette initiation and associated changes in smoking cessation and reduction: the Population Assessment of Tobacco and Health Study, 2013–2015. Tob Control. 2019;28:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dapunt OE, Galla JD, Sadeghi AM, et al. The natural history of thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 1994;107:1323–1332; discussion 1332–1333. [PubMed] [Google Scholar]
- 6.Gu BH, Choi JC, Shen YH, et al. Elastin-specific autoimmunity in smokers with thoracic aortic aneurysm and dissection is independent of chronic obstructive pulmonary disease. J Am Heart Assoc. 2019;8:e011671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fetterman JL, Keith RJ, Palmisano JN, et al. Alterations in vascular function associated with the use of combustible and electronic cigarettes. J Am Heart Assoc. 2020;9:e014570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fetterman JL, Weisbrod RM, Feng B, et al. Flavorings in tobacco products induce endothelial cell dysfunction. Arterioscler Thromb Vasc Biol. 2018;38:1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
6.4.1.4. Antiplatelet Therapy in TAA
- 1.Smith SC, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation. 2006;113:2363–2372. [DOI] [PubMed] [Google Scholar]
- 2.Agmon Y, Khandheria BK, Meissner I, et al. Relation of coronary artery disease and cerebrovascular disease with atherosclerosis of the thoracic aorta in the general population. Am J Cardiol. 2002;89:262–267. [DOI] [PubMed] [Google Scholar]
- 3.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
6.4.2.1. BP Management in AAA
- 1.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 2.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 3.Ogden LG, He J, Lydick E, et al. Long-term absolute benefit of lowering blood pressure in hypertensive patients according to the JNC VI risk stratification. Hypertension. 2000;35:539–543. [DOI] [PubMed] [Google Scholar]
- 4.Group SR, Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaduganathan M, Claggett BL, Juraschek SP, et al. Assessment of long-term benefit of intensive blood pressure control on residual life span: secondary analysis of the Systolic Blood Pressure Intervention Trial (SPRINT). JAMA Cardiol. 2020;5:576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
6.4.2.2. Treatment of AAA With Statins
- 1.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 2.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 3.Ogden LG, He J, Lydick E, et al. Long-term absolute benefit of lowering blood pressure in hypertensive patients according to the JNC VI risk stratification. Hypertension. 2000;35:539–543. [DOI] [PubMed] [Google Scholar]
- 4.Salata K, Syed M, Hussain MA, et al. Statins Reduce Abdominal Aortic Aneurysm Growth, Rupture, and Perioperative Mortality: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2018;7:e008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson CD, Clancy P, Bourke B, et al. Association of statin prescription with small abdominal aortic aneurysm progression. Am Heart J. 2010;159:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 7.Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regalado ES, Mellor-Crummey L, De BJ, et al. Clinical history and management recommendations of the smooth muscle dysfunction syndrome due to ACTA2 arginine 179 alterations. Genet Med. 2018;20:1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wemmelund H, Hogh A, Hundborg HH, et al. Statin use and rupture of abdominal aortic aneurysm. Br J Surg. 2014;101:966–975. [DOI] [PubMed] [Google Scholar]
- 11.Leurs LJ, Visser P, Laheij RJ, et al. Statin use is associated with reduced all-cause mortality after endovascular abdominal aortic aneurysm repair. Vascular. 2006;14:1–8. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell TFX, Deery SE, Shean KE, et al. Statin therapy is associated with higher long-term but not perioperative survival after abdominal aortic aneurysm repair. J Vasc Surg. 2018;68:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
6.4.2.3. Smoking Cessation in AAA
- 1.Katz DA, Holman JE, Nugent AS, et al. The emergency department action in smoking cessation (EDASC) trial: impact on cessation outcomes. Nicotine Tob Res. 2013;15:1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitlock EP, Orleans CT, Pender N, et al. Evaluating primary care behavioral counseling interventions: an evidence-based approach. Am J Prev Med. 2002;22:267–284. [DOI] [PubMed] [Google Scholar]
- 3.Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. US Dept. of Health and Human Services. Public Health Service. May 2008. [Google Scholar]
- 4.Berry KM, Reynolds LM, Collins JM, et al. E-cigarette initiation and associated changes in smoking cessation and reduction: the Population Assessment of Tobacco and Health Study, 2013–2015. Tob Control. 2019;28:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
6.4.2.4. Antithrombotic Therapy in AAA
- 1.Wemmelund H, Jorgensen TM, Hogh A, et al. Low-dose aspirin and rupture of abdominal aortic aneurysm. J Vasc Surg. 2017;65:616–625.e4. [DOI] [PubMed] [Google Scholar]
- 2.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 3.Smith SC Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation. 2006;113:2363–2372. [DOI] [PubMed] [Google Scholar]
6.4.3.1. Surveillance of Thoracic Aortic Dilation and Aneurysm
- 1.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. [DOI] [PubMed] [Google Scholar]
- 2.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein SA, Evangelista A, Abbara S, et al. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:119–182. [DOI] [PubMed] [Google Scholar]
- 4.Wang TKM, Desai MY. Thoracic aortic aneurysm: optimal surveillance and treatment. Cleve Clin J Med. 2020;87:557–568. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal PP, Chughtai A, Matzinger FR, et al. Multidetector CT of thoracic aortic aneurysms. Radiographics. 2009;29:537–552. [DOI] [PubMed] [Google Scholar]
- 6.Freeman LA, Young PM, Foley TA, et al. CT and MRI assessment of the aortic root and ascending aorta. AJR Am J Roentgenol. 2013;200:W581–W592. [DOI] [PubMed] [Google Scholar]
- 7.Poskaite P, Pamminger M, Kranewitter C, et al. Self-navigated 3D whole-heart MRA for non-enhanced surveillance of thoracic aortic dilation: a comparison to CTA. Magn Reson Imaging. 2021;76:123–130. [DOI] [PubMed] [Google Scholar]
- 8.Kuzmik GA, Sang AX, Elefteriades JA. Natural history of thoracic aortic aneurysms. J Vasc Surg. 2012;56:565–571. [DOI] [PubMed] [Google Scholar]
- 9.Gagne-Loranger M, Dumont E, Voisine P, et al. Natural history of 40–50 mm root/ascending aortic aneurysms in the current era of dedicated thoracic aortic clinics. Eur J Cardiothorac Surg. 2016;50:562–566. [DOI] [PubMed] [Google Scholar]
6.4.3.2. Surveillance of Abdominal Aortic Dilation and Aneurysm
- 1.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 2.Brady AR, Thompson SG, Fowkes FG, et al. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation. 2004;110:16–21. [DOI] [PubMed] [Google Scholar]
- 3.Marcaccio CL, Schermerhorn ML. Epidemiology of abdominal aortic aneurysms. Semin Vasc Surg. 2021;34:29–37. [DOI] [PubMed] [Google Scholar]
- 4.RESCAN Collaborators, Bown MJ, Sweeting MJ, et al. Surveillance intervals for small abdominal aortic aneurysms: a meta-analysis. JAMA. 2013;309:806–813. [DOI] [PubMed] [Google Scholar]
- 5.Badger SA, Jones C, McClements J, et al. Surveillance strategies according to the rate of growth of small abdominal aortic aneurysms. Vasc Med. 2011;16:415–421. [DOI] [PubMed] [Google Scholar]
- 6.Hendy K, Gunnarson R, Golledge J. Growth rates of small abdominal aortic aneurysms assessed by computerised tomography-a systematic literature review. Atherosclerosis. 2014;235:182–188. [DOI] [PubMed] [Google Scholar]
- 7.Oliver-Williams C, Sweeting MJ, Jacomelli J, et al. Safety of men with small and medium abdominal aortic aneurysms under surveillance in the NAAASP. Circulation. 2019;139:1371–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson S, Brown L, Sweeting M, et al. Systematic review and meta-analysis of the growth and rupture rates of small abdominal aortic aneurysms: implications for surveillance intervals and their cost-effectiveness. Health Technol Assess. 2013;17:1–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salehi Ravesh M, Langguth P, Pfarr JA, et al. Non-contrast-enhanced magnetic resonance imaging for visualization and quantification of endovascular aortic prosthesis, their endoleaks and aneurysm sacs at 1.5T. Magn Reson Imaging. 2019;60:164–172. [DOI] [PubMed] [Google Scholar]
- 10.Kawada H, Goshima S, Sakurai K, et al. Utility of noncontrast magnetic resonance angiography for aneurysm follow-up and detection of endoleaks after endovascular aortic repair. Korean J Radiol. 2021;22:513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77.e72. [DOI] [PubMed] [Google Scholar]
6.5. Surgical and Endovascular Management of Aortic Aneurysms
- 1.Schanzer A, Greenberg RK, Hevelone N, et al. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation. 2011;123:2848–2855. [DOI] [PubMed] [Google Scholar]
6.5.1. Surgery for Sporadic Aneurysms of the Aortic Root and Ascending Aorta
- 1.Kouchoukos NT. Composite graft replacement of the ascending aorta and aortic valve with the inclusion-wrap and open techniques. Semin Thorac Cardiovasc Surg. 1991;3:171–176. [PubMed] [Google Scholar]
- 2.Mehta RH, Suzuki T, Hagan PG, et al. Predicting death in patients with acute type A aortic dissection. Circulation. 2002;105:200–206. [DOI] [PubMed] [Google Scholar]
- 3.Borger MA, Fedak PWM, Stephens EH, et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: executive summary. J Thorac Cardiovasc Surg. 2018;156:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. [DOI] [PubMed] [Google Scholar]
- 5.Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2016;133:680–686. [DOI] [PubMed] [Google Scholar]
- 6.Svensson LG, Adams DH, Bonow RO, et al. Aortic valve and ascending aorta guidelines for management and quality measures. Ann Thorac Surg. 2013;95:S1–S66. [DOI] [PubMed] [Google Scholar]
- 7.Coady MA, Rizzo JA, Hammond GL, et al. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg. 1997;113:476–491. [DOI] [PubMed] [Google Scholar]
- 8.Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17–27; discussion 27–28. [DOI] [PubMed] [Google Scholar]
- 9.Ziganshin BA, Zafar MA, Elefteriades JA. Descending threshold for ascending aortic aneurysmectomy: is it time for a “left-shift” in guidelines? J Thorac Cardiovasc Surg. 2019;157:37–42. [DOI] [PubMed] [Google Scholar]
- 10.Avadhani SA, Martin-Doyle W, Shaikh AY, et al. Predictors of ascending aortic dilation in bicuspid aortic valve disease: a five-year prospective study. Am J Med. 2015;128:647–652. [DOI] [PubMed] [Google Scholar]
- 11.Kerneis C, Pasi N, Arangalage D, et al. Ascending aorta dilatation rates in patients with tricuspid and bicuspid aortic stenosis: the COFRASA/GENERAC study. Eur Heart J Cardiovasc Imaging. 2018;19:792–799. [DOI] [PubMed] [Google Scholar]
- 12.Oladokun D, Patterson BO, Sobocinski J, et al. Systematic review of the growth rates and influencing factors in thoracic aortic aneurysms. Eur J Vasc Endovasc Surg. 2016;51:674–681. [DOI] [PubMed] [Google Scholar]
- 13.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 14.Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with bicuspid aortic valve and a dilated ascending aorta. Circ Cardiovasc Imaging. 2017;10:e006249. [DOI] [PubMed] [Google Scholar]
- 15.Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation. 2016;134:1724–1737. [DOI] [PubMed] [Google Scholar]
- 16.Wojnarski CM, Svensson LG, Roselli EE, et al. Aortic dissection in patients with bicuspid aortic valve-associated aneurysms. Ann Thorac Surg. 2015;100:1666–1673; discussion 1673–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JB, Spotnitz M, Lindsay ME, et al. Risk of aortic dissection in the moderately dilated ascending aorta. J Am Coll Cardiol. 2016;68:1209–1219. [DOI] [PubMed] [Google Scholar]
- 18.Idrees JJ, Roselli EE, Blackstone EH, et al. Risk of adding prophylactic aorta replacement to a cardiac operation. J Thorac Cardiovasc Surg. 2020;159:1669–1678.e10. [DOI] [PubMed] [Google Scholar]
- 19.Hui SK, Fan CS, Christie S, et al. The aortic root does not dilate over time after replacement of the aortic valve and ascending aorta in patients with bicuspid or tricuspid aortic valves. J Thorac Cardiovasc Surg. 2018;156:5–13.e11. [DOI] [PubMed] [Google Scholar]
- 20.Peterss S, Bhandari R, Rizzo JA, et al. The aortic root: natural history after root-sparing ascending replacement in nonsyndromic aneurysmal patients. Ann Thorac Surg. 2017;103:828–833. [DOI] [PubMed] [Google Scholar]
- 21.Peterss S, Charilaou P, Dumfarth J, et al. Aortic valve disease with ascending aortic aneurysm: Impact of concomitant root-sparing (supracoronary) aortic replacement in nonsyndromic patients. J Thorac Cardiovasc Surg. 2016;152:791–798.e1. [DOI] [PubMed] [Google Scholar]
- 22.Acharya MN, Youssefi P, Soppa G, et al. Analysis of aortic area/height ratio in patients with thoracic aortic aneurysm and type A dissection. Eur J Cardiothorac Surg. 2018;54:696–701. [DOI] [PubMed] [Google Scholar]
- 23.Zafar MA, Li Y, Rizzo JA, et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J Thorac Cardiovasc Surg. 2018;155:1938–1950. [DOI] [PubMed] [Google Scholar]
- 24.Thubrikar MJ, Agali P, Robicsek F. Wall stress as a possible mechanism for the development of transverse intimal tears in aortic dissections. J Med Eng Technol. 1999;23:127–134. [DOI] [PubMed] [Google Scholar]
- 25.Emerel L, Thunes J, Kickliter T, et al. Predissection-derived geometric and distensibility indices reveal increased peak longitudinal stress and stiffness in patients sustaining acute type A aortic dissection: implications for predicting dissection. J Thorac Cardiovasc Surg. 2019;158:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plonek T, Zak M, Rylski B, et al. Wall stress correlates with intimal entry tear localization in type A aortic dissection. Interact Cardiovasc Thorac Surg. 2018;27:797–801. [DOI] [PubMed] [Google Scholar]
- 27.Gomez A, Wang Z, Xuan Y, et al. Abstract 16886: wall stress profiles in tricuspid aortic valve associated ascending thoracic aortic aneurysms: the effect of surgical threshold diameter ≥5.5cm. Circulation. 2020;142(suppl 3):A16886. [Google Scholar]
- 28.Pichamuthu JE, Phillippi JA, Cleary DA, et al. Differential tensile strength and collagen composition in ascending aortic aneurysms by aortic valve phenotype. Ann Thorac Surg. 2013;96:2147–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bollache E, Guzzardi DG, Sattari S, et al. Aortic valve-mediated wall shear stress is heterogeneous and predicts regional aortic elastic fiber thinning in bicuspid aortic valve-associated aortopathy. J Thorac Cardiovasc Surg. 2018;156:2112–2120.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emmott A, Garcia J, Chung J, et al. Biomechanics of the ascending thoracic aorta: a clinical perspective on engineering data. Can J Cardiol. 2016;32:35–47. [DOI] [PubMed] [Google Scholar]
- 31.Rylski B, Blanke P, Beyersdorf F, et al. How does the ascending aorta geometry change when it dissects? J Am Coll Cardiol. 2014;63:1311–1319. [DOI] [PubMed] [Google Scholar]
- 32.Pape LA, Tsai TT, Isselbacher EM, et al. Aortic diameter > or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2007;116:1120–1127. [DOI] [PubMed] [Google Scholar]
- 33.Saeyeldin A, Zafar MA, Li Y, et al. Decision-making algorithm for ascending aortic aneurysm: effectiveness in clinical application? J Thorac Cardiovasc Surg. 2019;157:1733–1745. [DOI] [PubMed] [Google Scholar]
- 34.Gagne-Loranger M, Dumont E, Voisine P, et al. Natural history of 40–50 mm root/ascending aortic aneurysms in the current era of dedicated thoracic aortic clinics. Eur J Cardiothorac Surg. 2016;50:562–566. [DOI] [PubMed] [Google Scholar]
- 35.Elefteriades JA, Mukherjee SK, Mojibian H. Discrepancies in measurement of the thoracic aorta: JACC review topic of the week. J Am Coll Cardiol. 2020;76:201–217. [DOI] [PubMed] [Google Scholar]
- 36.Guo MH, Appoo JJ, Saczkowski R, et al. Association of mortality and acute aortic events with ascending aortic aneurysm: a systematic review and meta-analysis. JAMA Netw Open. 2018;1:e181281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paruchuri V, Salhab KF, Kuzmik G, et al. Aortic size distribution in the general population: explaining the size paradox in aortic dissection. Cardiology. 2015;131:265–272. [DOI] [PubMed] [Google Scholar]
- 38.Mori M, Shioda K, Wang X, et al. Perioperative risk profiles and volume-outcome relationships in proximal thoracic aortic surgery. Ann Thorac Surg. 2018;106:1095–1104. [DOI] [PubMed] [Google Scholar]
- 39.Guo MH, Appoo JJ, Wells GA, et al. Protocol for a randomised controlled trial for Treatment in Thoracic Aortic Aneurysm: Surgery versus Surveillance (TITAN: SvS). BMJ Open. 2021;11:e052070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Girdauskas E, Rouman M, Disha K, et al. Aortic dissection after previous aortic valve replacement for bicuspid aortic valve disease. J Am Coll Cardiol. 2015;66:1409–1411. [DOI] [PubMed] [Google Scholar]
- 41.Kaneko T, Shekar P, Ivkovic V, et al. Should the dilated ascending aorta be repaired at the time of bicuspid aortic valve replacement? Eur J Cardiothorac Surg. 2018;53:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svensson LG, Kim KH, Blackstone EH, et al. Bicuspid aortic valve surgery with proactive ascending aorta repair. J Thorac Cardiovasc Surg. 2011;142:622–629, 629.e1–3. [DOI] [PubMed] [Google Scholar]
- 43.Borger MA, Preston M, Ivanov J, et al. Should the ascending aorta be replaced more frequently in patients with bicuspid aortic valve disease? J Thorac Cardiovasc Surg. 2004;128:677–683. [DOI] [PubMed] [Google Scholar]
- 44.Mansour AM, Peterss S, Zafar MA, et al. Prevention of aortic dissection suggests a diameter shift to a lower aortic size threshold for intervention. Cardiology. 2018;139:139–146. [DOI] [PubMed] [Google Scholar]
- 45.Heuts S, Adriaans BP, Gerretsen S, et al. Aortic elongation part II: the risk of acute type A aortic dissection. Heart. 2018;104:1778–1782. [DOI] [PubMed] [Google Scholar]
- 46.Kruger T, Sandoval Boburg R, Lescan M, et al. Aortic elongation in aortic aneurysm and dissection: the Tubingen Aortic Pathoanatomy (TAIPAN) project. Eur J Cardiothorac Surg. 2018;54:26–33. [DOI] [PubMed] [Google Scholar]
- 47.Wu J, Zafar MA, Li Y, et al. Ascending aortic length and risk of aortic adverse events: the neglected dimension. J Am Coll Cardiol. 2019;74:1883–1894. [DOI] [PubMed] [Google Scholar]
- 48.Gomez A, Wang Z, Xuan Y, et al. Wall stress distribution in bicuspid aortic valve-associated ascending thoracic aortic aneurysms. Ann Thorac Surg. 2020;110:807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, Flores N, Lum M, et al. Wall stress analyses in patients with ≥5 cm versus <5 cm ascending thoracic aortic aneurysm. J Thorac Cardiovasc Surg. 2021;162:1452–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xuan Y, Wang Z, Liu R, et al. Wall stress on ascending thoracic aortic aneurysms with bicuspid compared with tricuspid aortic valve. J Thorac Cardiovasc Surg. 2018;156:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
6.5.1.1. Surgical Approach for Patients With Sporadic Aneurysms of the Aortic Root and Ascending Aorta Meeting Criteria for Surgery
- 1.Mori M, Shioda K, Wang X, et al. Perioperative risk profiles and volume-outcome relationships in proximal thoracic aortic surgery. Ann Thorac Surg. 2018;106:1095–1104. [DOI] [PubMed] [Google Scholar]
- 2.Mullan CW, Mori M, Bin Mahmood SU, et al. Incidence and characteristics of hospitalization for proximal aortic surgery for acute syndromes and for aneurysms in the USA from 2005 to 2014. Eur J Cardiothorac Surg. 2020;58:583–589. [DOI] [PubMed] [Google Scholar]
- 3.Girdauskas E, Rouman M, Disha K, et al. The fate of mild-to-moderate proximal aortic dilatation after isolated aortic valve replacement for bicuspid aortic valve stenosis: a magnetic resonance imaging follow-up study. Eur J Cardiothorac Surg. 2016;49:e80–e86; discussion e86-e87. [DOI] [PubMed] [Google Scholar]
- 4.Hui SK, Fan CS, Christie S, et al. The aortic root does not dilate over time after replacement of the aortic valve and ascending aorta in patients with bicuspid or tricuspid aortic valves. J Thorac Cardiovasc Surg. 2018;156:5–13.e11. [DOI] [PubMed] [Google Scholar]
- 5.Peterss S, Bhandari R, Rizzo JA, et al. The aortic root: natural history after root-sparing ascending replacement in nonsyndromic aneurysmal patients. Ann Thorac Surg. 2017;103:828–833. [DOI] [PubMed] [Google Scholar]
- 6.Vendramin I, Meneguzzi M, Sponga S, et al. Bicuspid aortic valve disease and ascending aortic aneurysm: should an aortic root replacement be mandatory? Eur J Cardiothorac Surg. 2016;49:103–109. [DOI] [PubMed] [Google Scholar]
- 7.Preventza O, Coselli JS, Price MD, et al. Elective primary aortic root replacement with and without hemiarch repair in patients with no previous cardiac surgery. J Thorac Cardiovasc Surg. 2017;153:1402–1408. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy FH, Bavaria JE, McDermott KM, et al. At the root of the repair debate: outcomes after elective aortic root replacements for aortic insufficiency with aneurysm. Ann Thorac Surg. 2016;102:1199–1205. [DOI] [PubMed] [Google Scholar]
- 9.David TE, David CM, Ouzounian M, et al. A progress report on reimplantation of the aortic valve. J Thorac Cardiovasc Surg. 2021;161:890–899.e1. [DOI] [PubMed] [Google Scholar]
- 10.Kari FA, Doll KN, Hemmer W, et al. Survival and freedom from aortic valve-related reoperation after valve-sparing aortic root replacement in 1015 patients. Interact Cardiovasc Thorac Surg. 2016;22:431–438. [DOI] [PubMed] [Google Scholar]
- 11.Lenoir M, Maesen B, Stevens LM, et al. Reimplantation versus remodelling with ring annuloplasty: comparison of mid-term outcomes after valve-sparing aortic root replacement. Eur J Cardiothorac Surg. 2018;54:48–54. [DOI] [PubMed] [Google Scholar]
- 12.Arabkhani B, Mookhoek A, Di Centa I, et al. Reported outcome after valve-sparing aortic root replacement for aortic root aneurysm: a systematic review and meta-analysis. Ann Thorac Surg. 2015;100:1126–1131. [DOI] [PubMed] [Google Scholar]
- 13.Flynn CD, Tian DH, Wilson-Smith A, et al. Systematic review and meta-analysis of surgical outcomes in Marfan patients undergoing aortic root surgery by composite-valve graft or valve sparing root replacement. Ann Cardiothorac Surg. 2017;6:570–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kari FA, Russe MF, Peter P, et al. Late complications and distal growth rates of Marfan aortas after proximal aortic repair. Eur J Cardiothorac Surg. 2013;44:163–171. [DOI] [PubMed] [Google Scholar]
- 15.Price J, Magruder JT, Young A, et al. Long-term outcomes of aortic root operations for Marfan syndrome: a comparison of Bentall versus aortic valve-sparing procedures. J Thorac Cardiovasc Surg. 2016;151:330–336. [DOI] [PubMed] [Google Scholar]
- 16.Schoenhoff FS, Jungi S, Czerny M, et al. Acute aortic dissection determines the fate of initially untreated aortic segments in Marfan syndrome. Circulation. 2013;127:1569–1575. [DOI] [PubMed] [Google Scholar]
- 17.Schoenhoff FS, Kadner A, Czerny M, et al. Should aortic arch replacement be performed during initial surgery for aortic root aneurysm in patients with Marfan syndrome? Eur J Cardiothorac Surg. 2013;44:346–351; discussion 351. [DOI] [PubMed] [Google Scholar]
- 18.Schoenhoff FS, Langhammer B, Wustmann K, et al. Decision-making in aortic root surgery in Marfan syndrome: bleeding, thromboembolism and risk of reintervention after valve-sparing or mechanical aortic root replacement. Eur J Cardiothorac Surg. 2015;48:931–935; discussion 935–936. [DOI] [PubMed] [Google Scholar]
- 19.Aftab M, Cikach FS, Zhu Y, et al. Loeys-Dietz syndrome: intermediate-term outcomes of medically and surgically managed patients. J Thorac Cardiovasc Surg. 2019;157:439–450.e5. [DOI] [PubMed] [Google Scholar]
- 20.Patel ND, Crawford T, Magruder JT, et al. Cardiovascular operations for Loeys-Dietz syndrome: intermediate-term results. J Thorac Cardiovasc Surg. 2017;153:406–412. [DOI] [PubMed] [Google Scholar]
- 21.Coselli JS, Hughes MS, Green SY, et al. Valve-sparing aortic root replacement: early and midterm outcomes in 83 patients. Ann Thorac Surg. 2014;97:1267–1273; discussion 1273–1274. [DOI] [PubMed] [Google Scholar]
- 22.Idrees JJ, Roselli EE, Blackstone EH, et al. Risk of adding prophylactic aorta replacement to a cardiac operation. J Thorac Cardiovasc Surg. 2020;159:1669–1678.e10. [DOI] [PubMed] [Google Scholar]
- 23.Peterss S, Charilaou P, Dumfarth J, et al. Aortic valve disease with ascending aortic aneurysm: Impact of concomitant root-sparing (supracoronary) aortic replacement in nonsyndromic patients. J Thorac Cardiovasc Surg. 2016;152:791–798.e1. [DOI] [PubMed] [Google Scholar]
- 24.Rinewalt D, McCarthy PM, Malaisrie SC, et al. Effect of aortic aneurysm replacement on outcomes after bicuspid aortic valve surgery: validation of contemporary guidelines. J Thorac Cardiovasc Surg. 2014;148:2060–2069. [DOI] [PubMed] [Google Scholar]
- 25.Girdauskas E, Disha K, Raisin HH, et al. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg. 2012;42:832–837; discussion 837–838. [DOI] [PubMed] [Google Scholar]
- 26.Wallen T, Habertheuer A, Bavaria JE, et al. Elective aortic root replacement in North America: analysis of STS Adult Cardiac Surgery Database. Ann Thorac Surg. 2019;107:1307–1312. [DOI] [PubMed] [Google Scholar]
6.5.2. Aortic Arch Aneurysms
- 1.Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111:816–828. [DOI] [PubMed] [Google Scholar]
- 2.Coady MA, Rizzo JA, Hammond GL, et al. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg. 1997;113:476–491. [DOI] [PubMed] [Google Scholar]
- 3.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 4.Sultan S, Kavanagh EP, Diethrich E, et al. A clinical review of early outcomes from contemporary flow modulation versus open, fenestrated and branch technologies in the management of thoracoabdominal aortic aneurysm. Vascular. 2018;26:209–215. [DOI] [PubMed] [Google Scholar]
- 5.Malaisrie SC, Duncan BF, Mehta CK, et al. The addition of hemiarch replacement to aortic root surgery does not affect safety. J Thorac Cardiovasc Surg. 2015;150:118–124.e2. [DOI] [PubMed] [Google Scholar]
- 6.Shrestha M, Bachet J, Bavaria J, et al. Current status and recommendations for use of the frozen elephant trunk technique: a position paper by the Vascular Domain of EACTS. Eur J Cardiothorac Surg. 2015;47:759–769. [DOI] [PubMed] [Google Scholar]
- 7.Hanif H, Dubois L, Ouzounian M, et al. Aortic arch reconstructive surgery with conventional techniques vs frozen elephant trunk: a systematic review and meta-analysis. Can J Cardiol. 2018;34:262–273. [DOI] [PubMed] [Google Scholar]
- 8.Yuan SM. Hoarseness due to aortic arch aneurysms. Braz J Cardiovasc Surg. 2020;35:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson MD, Garg V, Mazer CD, et al. A randomized trial comparing axillary versus innominate artery cannulation for aortic arch surgery. J Thorac Cardiovasc Surg. 2020;S0022–5223:33143–3. [DOI] [PubMed] [Google Scholar]
- 10.Harky A, Grafton-Clarke C, Hadlett M, et al. In thoracic aortic surgery, is innominate artery cannulation a safe and effective alternative to axillary artery cannulation? Interact Cardiovasc Thorac Surg. 2019;29:604–607. [DOI] [PubMed] [Google Scholar]
- 11.Preventza O, Price MD, Spiliotopoulos K, et al. In elective arch surgery with circulatory arrest, does the arterial cannulation site really matter? A propensity score analysis of right axillary and innominate artery cannulation. J Thorac Cardiovasc Surg. 2018;155:1953–1960.e4. [DOI] [PubMed] [Google Scholar]
- 12.Svensson LG, Blackstone EH, Rajeswaran J, et al. Does the arterial cannulation site for circulatory arrest influence stroke risk? Ann Thorac Surg. 2004;78:1274–1284. [DOI] [PubMed] [Google Scholar]
- 13.Tian WZ, Er JX, Liu L, et al. Effects of autologous platelet rich plasma on intraoperative transfusion and short-term outcomes in total arch replacement (Sun’s procedure): a prospective, randomized trial. J Cardiothorac Vasc Anesth. 2019;33:2163–2169. [DOI] [PubMed] [Google Scholar]
- 14.Tian DH, Weller J, Hasmat S, et al. Temperature selection in antegrade cerebral perfusion for aortic arch surgery: a meta-analysis. Ann Thorac Surg. 2019;108:283–291. [DOI] [PubMed] [Google Scholar]
- 15.Svensson LG, Blackstone EH, Apperson-Hansen C, et al. Implications from neurologic assessment of brain protection for total arch replacement from a randomized trial. J Thorac Cardiovasc Surg. 2015;150:1140–1147.e11. [DOI] [PubMed] [Google Scholar]
- 16.Regalado ES, Mellor-Crummey L, De BJ, et al. Clinical history and management recommendations of the smooth muscle dysfunction syndrome due to ACTA2 arginine 179 alterations. Genet Med. 2018;20:1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacCarrick G, Black JH 3rd, Bowdin S, et al. Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med. 2014;16:576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svensson LG, Rushing GD, Valenzuela ES, et al. Modifications, classifications, and outcomes of elephant-trunk procedures. Ann Thorac Surg. 2013;96:548–558. [DOI] [PubMed] [Google Scholar]
- 19.Czerny M, Schmidli J, Adler S, et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS). Eur J Cardiothorac Surg. 2019;55:133–162. [DOI] [PubMed] [Google Scholar]
- 20.Azuma T, Yokoi Y, Yamazaki K. The next generation of fenestrated endografts: results of a clinical trial to support an expanded indication for aortic arch aneurysm treatment. Eur J Cardiothorac Surg. 2013;44:e156–e163; discussion e163. [DOI] [PubMed] [Google Scholar]
- 21.Furuta A, Azuma T, Yokoi Y, et al. The midterm results of thoracic endovascular aortic repair with a precurved fenestrated endograft in zone 0–1. Eur J Cardiothorac Surg. 2020;58:722–729. [DOI] [PubMed] [Google Scholar]
- 22.Bavaria J, Vallabhajosyula P, Moeller P, et al. Hybrid approaches in the treatment of aortic arch aneurysms: postoperative and midterm outcomes. J Thorac Cardiovasc Surg. 2013;145:S85–S90. [DOI] [PubMed] [Google Scholar]
- 23.Preventza O, Tan CW, Orozco-Sevilla V, et al. Zone zero hybrid arch exclusion versus open total arch replacement. Ann Cardiothorac Surg. 2018;7:372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preventza O, Garcia A, Cooley DA, et al. Total aortic arch replacement: a comparative study of zone 0 hybrid arch exclusion versus traditional open repair. J Thorac Cardiovasc Surg. 2015;150:1591–1598; discussion 1598–1600. [DOI] [PubMed] [Google Scholar]
- 25.Patel R, Sweeting MJ, Powell JT, et al. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366–2374. [DOI] [PubMed] [Google Scholar]
- 26.Haulon S, Greenberg RK, Spear R, et al. Global experience with an inner branched arch endograft. J Thorac Cardiovasc Surg. 2014;148:1709–1716. [DOI] [PubMed] [Google Scholar]
6.5.3.1. Size Thresholds for Repair of Descending TAA
- 1.Kim JB, Kim K, Lindsay ME, et al. Risk of rupture or dissection in descending thoracic aortic aneurysm. Circulation. 2015;132:1620–1629. [DOI] [PubMed] [Google Scholar]
- 2.Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17–27; discussion 27–28. [DOI] [PubMed] [Google Scholar]
- 3.Lobato AC, Puech-Leao P. Predictive factors for rupture of thoracoabdominal aortic aneurysm. J Vasc Surg. 1998;27:446–453. [DOI] [PubMed] [Google Scholar]
- 4.Juvonen T, Ergin MA, Galla JD, et al. Prospective study of the natural history of thoracic aortic aneurysms. Ann Thorac Surg. 1997;63:1533–1545. [DOI] [PubMed] [Google Scholar]
- 5.Nathan DP, Xu C, Pouch AM, et al. Increased wall stress of saccular versus fusiform aneurysms of the descending thoracic aorta. Ann Vasc Surg. 2011;25:1129–1137. [DOI] [PubMed] [Google Scholar]
- 6.Oderich GS, Panneton JM, Bower TC, et al. Infected aortic aneurysms: aggressive presentation, complicated early outcome, but durable results. J Vasc Surg. 2001;34:900–908. [DOI] [PubMed] [Google Scholar]
- 7.Harris DG, Olson SL, Panthofer AM, et al. A frailty-based risk score predicts morbidity and mortality after elective endovascular repair of descending thoracic aortic aneurysms. Ann Vasc Surg. 2020;67:90–99. [DOI] [PubMed] [Google Scholar]
- 8.Schermerhorn ML, Giles KA, Hamdan AD, et al. Population-based outcomes of open descending thoracic aortic aneurysm repair. J Vasc Surg. 2008;48:821–827. [DOI] [PubMed] [Google Scholar]
- 9.Girardi LN, Leonard JR, Lau C, et al. Gender-related outcomes after open repair of descending thoracic and thoracoabdominal aortic aneurysms. J Vasc Surg. 2019;69:1028–1035.e1. [DOI] [PubMed] [Google Scholar]
6.5.3.2. Endovascular Versus Open Repair of Descending TAA
- 1.Makaroun MS, Dillavou ED, Kee ST, et al. Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg. 2005;41:1–9. [DOI] [PubMed] [Google Scholar]
- 2.Matsumura JS, Cambria RP, Dake MD, et al. International controlled clinical trial of thoracic endovascular aneurysm repair with the Zenith TX2 endovascular graft: 1-year results. J Vasc Surg. 2008;47:247–257; discussion 257. [DOI] [PubMed] [Google Scholar]
- 3.Fairman RM, Criado F, Farber M, et al. Pivotal results of the Medtronic Vascular Talent Thoracic Stent Graft System: the VALOR trial. J Vasc Surg. 2008;48:546–554. [DOI] [PubMed] [Google Scholar]
- 4.Goodney PP, Travis L, Lucas FL, et al. Survival after open versus endovascular thoracic aortic aneurysm repair in an observational study of the Medicare population. Circulation. 2011;124:2661–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lomazzi C, Mascoli C, de Beaufort HWL, et al. Gender related access complications after TEVAR: analysis from the Retrospective Multicentre Cohort GORE® GREAT Registry Study. Eur J Vasc Endovasc Surg. 2020;60:203–209. [DOI] [PubMed] [Google Scholar]
- 6.Coselli JS, Bozinovski J, LeMaire SA. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg. 2007;83:S862–S864; discussion S890-S892. [DOI] [PubMed] [Google Scholar]
- 7.Girardi LN, Leonard JR, Lau C, et al. Gender-related outcomes after open repair of descending thoracic and thoracoabdominal aortic aneurysms. J Vasc Surg. 2019;69:1028–1035.e1. [DOI] [PubMed] [Google Scholar]
- 8.Estrera AL, Jan A, Sandhu H, et al. Outcomes of open repair for chronic descending thoracic aortic dissection. Ann Thorac Surg. 2015;99:786–793; discussion 794. [DOI] [PubMed] [Google Scholar]
- 9.Pujara AC, Roselli EE, Hernandez AV, et al. Open repair of chronic distal aortic dissection in the endovascular era: implications for disease management. J Thorac Cardiovasc Surg. 2012;144:866–873. [DOI] [PubMed] [Google Scholar]
- 10.Matsumura JS, Cambria RP, Dake MD, et al. International controlled clinical trial of thoracic endovascular aneurysm repair with the Zenith TX2 endovascular graft: 1-year results. J Vasc Surg. 2008;47:247–257; discussion 257. [DOI] [PubMed] [Google Scholar]
- 11.Fairman AS, Beck AW, Malas MB, et al. Reinterventions in the modern era of thoracic endovascular aortic repair. J Vasc Surg. 2020;71:408–422. [DOI] [PubMed] [Google Scholar]
- 12.Patel VI, Mukhopadhyay S, Ergul E, et al. Impact of hospital volume and type on outcomes of open and endovascular repair of descending thoracic aneurysms in the United States Medicare population. J Vasc Surg. 2013;58:346–354. [DOI] [PubMed] [Google Scholar]
- 13.Makaroun MS, Dillavou ED, Wheatley GH, et al. Five-year results of endovascular treatment with the Gore TAG device compared with open repair of thoracic aortic aneurysms. J Vasc Surg. 2008;47:912–918. [DOI] [PubMed] [Google Scholar]
- 14.Coselli JS, LeMaire SA, Preventza O, et al. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg. 2016;151:1323–1337. [DOI] [PubMed] [Google Scholar]
- 15.Patel HJ, Dake MD, Bavaria JE, et al. Branched endovascular therapy of the distal aortic arch: preliminary results of the feasibility multicenter trial of the Gore Thoracic Branch Endoprosthesis. Ann Thorac Surg. 2016;102:1190–1198. [DOI] [PubMed] [Google Scholar]
- 16.Tong MZ, Eagleton MJ, Roselli EE, et al. Outcomes of open versus endovascular repair of descending thoracic and thoracoabdominal aortic aneurysms. Ann Thorac Surg. 2022;113:1144–1152. [DOI] [PubMed] [Google Scholar]
6.5.3.3. Left Subclavian Artery Management
- 1.Cooper DG, Walsh SR, Sadat U, et al. Neurological complications after left subclavian artery coverage during thoracic endovascular aortic repair: a systematic review and meta-analysis. J Vasc Surg. 2009;49:1594–1601. [DOI] [PubMed] [Google Scholar]
- 2.Buth J, Harris PL, Hobo R, et al. Neurologic complications associated with endovascular repair of thoracic aortic pathology: Incidence and risk factors. a study from the European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) registry. J Vasc Surg. 2007;46:1103–1110; discussion 1110–1111. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura E, Nakamura K, Furukawa K, et al. Left subclavian artery revascularization for delayed paralysis after thoracic endovascular aortic repair. Ann Vasc Dis. 2019;12:233–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotelis D, Geisbusch P, Hinz U, et al. Short and midterm results after left subclavian artery coverage during endovascular repair of the thoracic aorta. J Vasc Surg. 2009;50:1285–1292. [DOI] [PubMed] [Google Scholar]
- 5.Patel HJ, Dake MD, Bavaria JE, et al. Branched endovascular therapy of the distal aortic arch: preliminary results of the feasibility multicenter trial of the Gore Thoracic Branch Endoprosthesis. Ann Thorac Surg. 2016;102:1190–1198. [DOI] [PubMed] [Google Scholar]
- 6.Roselli EE, Arko FR 3rd, Thompson MM, et al. Results of the Valiant Mona LSA early feasibility study for descending thoracic aneurysms. J Vasc Surg. 2015;62:1465–1471.e3. [DOI] [PubMed] [Google Scholar]
- 7.Waterford SD, Chou D, Bombien R, et al. Left subclavian arterial coverage and stroke during thoracic aortic endografting: a systematic review. Ann Thorac Surg. 2016;101:381–389. [DOI] [PubMed] [Google Scholar]
- 8.von Allmen RS, Gahl B, Powell JT. Editor’s choice - incidence of stroke following thoracic endovascular aortic repair for descending aortic aneurysm: a systematic review of the literature with meta-analysis. Eur J Vasc Endovasc Surg. 2017;53:176–184. [DOI] [PubMed] [Google Scholar]
- 9.Woo EY, Carpenter JP, Jackson BM, et al. Left subclavian artery coverage during thoracic endovascular aortic repair: a single-center experience. J Vasc Surg. 2008;48:555–560. [DOI] [PubMed] [Google Scholar]
- 10.Rizvi AZ, Murad MH, Fairman RM, et al. The effect of left subclavian artery coverage on morbidity and mortality in patients undergoing endovascular thoracic aortic interventions: a systematic review and meta-analysis. J Vasc Surg. 2009;50:1159–1169. [DOI] [PubMed] [Google Scholar]
6.5.3.4. Celiac Artery Management
- 1.Rose MK, Pearce BJ, Matthews TC, et al. Outcomes after celiac artery coverage during thoracic endovascular aortic aneurysm repair. J Vasc Surg. 2015;62:36–42. [DOI] [PubMed] [Google Scholar]
- 2.Vaddineni SK, Taylor SM, Patterson MA, et al. Outcome after celiac artery coverage during endovascular thoracic aortic aneurysm repair: preliminary results. J Vasc Surg. 2007;45:467–471. [DOI] [PubMed] [Google Scholar]
- 3.Mehta M, Darling RC 3rd, Taggert JB, et al. Outcomes of planned celiac artery coverage during TEVAR. J Vasc Surg. 2010;52:1153–1158. [DOI] [PubMed] [Google Scholar]
6.5.3.5. Ruptured Descending TAA
- 1.Jonker FH, Verhagen HJ, Lin PH, et al. Open surgery versus endovascular repair of ruptured thoracic aortic aneurysms. J Vasc Surg. 2011;53:1210–1216. [DOI] [PubMed] [Google Scholar]
- 2.Jonker FH, Trimarchi S, Verhagen HJ, et al. Meta-analysis of open versus endovascular repair for ruptured descending thoracic aortic aneurysm. J Vasc Surg. 2010;51:1026–1032, 1032.e1–1032.e2. [DOI] [PubMed] [Google Scholar]
- 3.Patel HJ, Williams DM, Upchurch GR Jr, et al. A comparative analysis of open and endovascular repair for the ruptured descending thoracic aorta. J Vasc Surg. 2009;50:1265–1270. [DOI] [PubMed] [Google Scholar]
- 4.Goodney PP, Travis L, Lucas FL, et al. Survival after open versus endovascular thoracic aortic aneurysm repair in an observational study of the Medicare population. Circulation. 2011;124:2661–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cambria RP, Crawford RS, Cho JS, et al. A multicenter clinical trial of endovascular stent graft repair of acute catastrophes of the descending thoracic aorta. J Vasc Surg. 2009;50:1255–1264.e1-4. [DOI] [PubMed] [Google Scholar]
- 6.Banno H, Ikeda S, Kawai Y, et al. Early and midterm outcomes of celiac artery coverage during thoracic endovascular aortic repair. J Vasc Surg. 2020;72:1552–1557. [DOI] [PubMed] [Google Scholar]
- 7.Vaddineni SK, Taylor SM, Patterson MA, et al. Outcome after celiac artery coverage during endovascular thoracic aortic aneurysm repair: preliminary results. J Vasc Surg. 2007;45:467–471. [DOI] [PubMed] [Google Scholar]
6.5.3.6. Access Issues for TEVAR in Descending TAA
- 1.Jackson BM, Woo EY, Bavaria JE, et al. Gender analysis of the pivotal results of the Medtronic Talent Thoracic Stent Graft System (VALOR) trial. J Vasc Surg. 2011;54:358–363, 363.e1. [DOI] [PubMed] [Google Scholar]
- 2.Lomazzi C, Mascoli C, de Beaufort HWL, et al. Gender related access complications after TEVAR: analysis from the Retrospective Multicentre Cohort GORE® GREAT Registry Study. Eur J Vasc Endovasc Surg. 2020;60:203–209. [DOI] [PubMed] [Google Scholar]
- 3.Baxter RD, Hansen SK, Gable CE, et al. Outcomes of open versus percutaneous access for patients enrolled in the GREAT registry. Ann Vasc Surg. 2021;70:370–377. [DOI] [PubMed] [Google Scholar]
- 4.Lee WA, Brown MP, Nelson PR, et al. Midterm outcomes of femoral arteries after percutaneous endovascular aortic repair using the Preclose technique. J Vasc Surg. 2008;47:919–923. [DOI] [PubMed] [Google Scholar]
- 5.de Souza LR, Oderich GS, Banga PV, et al. Outcomes of total percutaneous endovascular aortic repair for thoracic, fenestrated, and branched endografts. J Vasc Surg. 2015;62:1442–1449.e3. [DOI] [PubMed] [Google Scholar]
- 6.Makaroun MS, Dillavou ED, Kee ST, et al. Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg. 2005;41:1–9. [DOI] [PubMed] [Google Scholar]
- 7.Matsumura JS, Cambria RP, Dake MD, et al. International controlled clinical trial of thoracic endovascular aneurysm repair with the Zenith TX2 endovascular graft: 1-year results. J Vasc Surg. 2008;47:247–257; discussion 257. [DOI] [PubMed] [Google Scholar]
- 8.Fairman RM, Criado F, Farber M, et al. Pivotal results of the Medtronic Vascular Talent Thoracic Stent Graft System: the VALOR trial. J Vasc Surg. 2008;48:546–554. [DOI] [PubMed] [Google Scholar]
6.5.4.1. Size Thresholds for Open Surgical Repair of TAAA
- 1.Kim JB, Kim K, Lindsay ME, et al. Risk of rupture or dissection in descending thoracic aortic aneurysm. Circulation. 2015;132:1620–1629. [DOI] [PubMed] [Google Scholar]
- 2.Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17–27; discussion 27–28. [DOI] [PubMed] [Google Scholar]
- 3.Zafar MA, Chen JF, Wu J, et al. Natural history of descending thoracic and thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2021;161:498–511.e1. [DOI] [PubMed] [Google Scholar]
- 4.Lobato AC, Puech-Leao P. Predictive factors for rupture of thoracoabdominal aortic aneurysm. J Vasc Surg. 1998;27:446–453. [DOI] [PubMed] [Google Scholar]
- 5.Gambardella I, Gaudino MFL, Rahouma M, et al. Impact of left ventricular ejection fraction on the outcomes of open repair of descending thoracic and thoracoabdominal aneurysms. J Thorac Cardiovasc Surg. 2021;161:534–541.e5. [DOI] [PubMed] [Google Scholar]
- 6.Girardi LN, Lau C, Ohmes LB, et al. Open repair of descending and thoracoabdominal aortic aneurysms in octogenarians. J Vasc Surg. 2018;68:1287–1296.e3. [DOI] [PubMed] [Google Scholar]
- 7.Juvonen J, Surcel HM, Satta J, et al. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1997;17:2843–2847. [DOI] [PubMed] [Google Scholar]
- 8.Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74:S1877–S1880. [DOI] [PubMed] [Google Scholar]
6.5.4.2. Open Versus Endovascular Repair of TAAA
- 1.Walsh K, O’Connor DJ, Weaver F, et al. Survival after endovascular therapy in patients with ruptured thoracic aortic diseases: results from the Global Registry for Endovascular Aortic Treatment Registry. J Vasc Surg. 2020;72:1544–1551. [DOI] [PubMed] [Google Scholar]
- 2.Hammo S, Larzon T, Hultgren R, et al. Outcome after endovascular repair of ruptured descending thoracic aortic aneurysm: a national multicentre study. Eur J Vasc Endovasc Surg. 2019;57:788–794. [DOI] [PubMed] [Google Scholar]
- 3.Goodney PP, Travis L, Lucas FL, et al. Survival after open versus endovascular thoracic aortic aneurysm repair in an observational study of the Medicare population. Circulation. 2011;124:2661–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogawa Y, Watkins AC, Lingala B, et al. Improved midterm outcomes after endovascular repair of nontraumatic descending thoracic aortic rupture compared with open surgery. J Thorac Cardiovasc Surg. 2021;161:2004–2012. [DOI] [PubMed] [Google Scholar]
- 5.Gaudino M, Lau C, Munjal M, et al. Open repair of ruptured descending thoracic and thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2015;150:814–821. [DOI] [PubMed] [Google Scholar]
- 6.Clough RE, Martin-Gonzalez T, Van Calster K, et al. Endovascular Repair of Thoracoabdominal and Arch Aneurysms in Patients with Connective Tissue Disease Using Branched and Fenestrated Devices. Ann Vasc Surg. 2017;44:158–163. [DOI] [PubMed] [Google Scholar]
- 7.Pacini D, Parolari A, Berretta P, et al. Endovascular treatment for type B dissection in Marfan syndrome: is it worthwhile? Ann Thorac Surg. 2013;95:737–749. [DOI] [PubMed] [Google Scholar]
- 8.Coselli JS, Green SY, Price MD, et al. Results of open surgical repair in patients with Marfan syndrome and distal aortic dissection. Ann Thorac Surg. 2016;101:2193–2201. [DOI] [PubMed] [Google Scholar]
- 9.Waterford SD, Gardner RL, Moon MR. Extent of aortic replacement in type A dissection: current answers for an endless debate. Ann Thorac Surg. 2018;106:1246–1250. [DOI] [PubMed] [Google Scholar]
- 10.Aucoin VJ, Eagleton MJ, Farber MA, et al. Spinal cord protection practices used during endovascular repair of complex aortic aneurysms by the U.S. Aortic Research Consortium. J Vasc Surg. 2021;73:323–330. [DOI] [PubMed] [Google Scholar]
- 11.Scali ST, Kim M, Kubilis P, et al. Implementation of a bundled protocol significantly reduces risk of spinal cord ischemia after branched or fenestrated endovascular aortic repair. J Vasc Surg. 2018;67:409–423.e4. [DOI] [PubMed] [Google Scholar]
- 12.Banga PV, Oderich GS, Reis de Souza L, et al. Neuromonitoring, cerebrospinal fluid drainage, and selective use of iliofemoral conduits to minimize risk of spinal cord injury during complex endovascular aortic repair. J Endovasc Ther. 2016;23:139–149. [DOI] [PubMed] [Google Scholar]
- 13.Oderich GS, Tenorio ER, Mendes BC, et al. Midterm outcomes of a prospective, nonrandomized study to evaluate endovascular repair of complex aortic aneurysms using fenestrated-branched endografts. Ann Surg. 2021;274:491–499. [DOI] [PubMed] [Google Scholar]
- 14.Kolbel T, Spanos K, Jama K, et al. Early outcomes of the t-Branch off-the-shelf multi-branched stent graft in 542 patients for elective and urgent aortic pathologies - a retrospective observational study. J Vasc Surg. 2021;S0741–5214:00916–2. [DOI] [PubMed] [Google Scholar]
- 15.Eagleton MJ, Follansbee M, Wolski K, et al. Fenestrated and branched endovascular aneurysm repair outcomes for type II and III thoracoabdominal aortic aneurysms. J Vasc Surg. 2016;63:930–942. [DOI] [PubMed] [Google Scholar]
6.5.4.3. TAAA Spinal Cord Protection
- 1.Coselli JS, LeMaire SA, Preventza O, et al. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg. 2016;151:1323–1337. [DOI] [PubMed] [Google Scholar]
- 2.Coselli JS, LeMaire SA, Koksoy C, et al. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg. 2002;35:631–639. [DOI] [PubMed] [Google Scholar]
- 3.Girardi LN, Lau C, Ohmes LB, et al. Open repair of descending and thoracoabdominal aortic aneurysms in octogenarians. J Vasc Surg. 2018;68:1287–1296.e3. [DOI] [PubMed] [Google Scholar]
- 4.Khan FM, Naik A, Hameed I, et al. Open repair of descending thoracic and thoracoabdominal aortic aneurysms: a meta-analysis. Ann Thorac Surg. 2020;110:1941–1949. [DOI] [PubMed] [Google Scholar]
- 5.Estrera AL, Miller CC 3rd, Huynh TT, et al. Neurologic outcome after thoracic and thoracoabdominal aortic aneurysm repair. Ann Thorac Surg. 2001;72:1225–1230; discussion 1230–1231. [DOI] [PubMed] [Google Scholar]
- 6.Wong CS, Healy D, Canning C, et al. A systematic review of spinal cord injury and cerebrospinal fluid drainage after thoracic aortic endografting. J Vasc Surg. 2012;56:1438–1447. [DOI] [PubMed] [Google Scholar]
- 7.Wynn MM, Sebranek J, Marks E, et al. Complications of spinal fluid drainage in thoracic and thoracoabdominal aortic aneurysm surgery in 724 patients treated from 1987 to 2013. J Cardiothorac Vasc Anesth. 2015;29:342–350. [DOI] [PubMed] [Google Scholar]
- 8.Sandhu HK, Evans JD, Tanaka A, et al. Fluctuations in Spinal Cord Perfusion Pressure: A Harbinger of Delayed Paraplegia After Thoracoabdominal Aortic Repair. Semin Thorac Cardiovasc Surg. 2017;29:451–459. [DOI] [PubMed] [Google Scholar]
- 9.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. [DOI] [PubMed] [Google Scholar]
- 10.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 11.Crawford ES, Svensson LG, Hess KR, et al. A prospective randomized study of cerebrospinal fluid drainage to prevent paraplegia after high-risk surgery on the thoracoabdominal aorta. J Vasc Surg. 1991;13:36–45; discussion 45–46. [PubMed] [Google Scholar]
- 12.Chatterjee S, Casar JG, LeMaire SA, et al. Perioperative care after thoracoabdominal aortic aneurysm repair: The Baylor College of Medicine experience. Part 2: postoperative management. J Thorac Cardiovasc Surg. 2021;161:699–705. [DOI] [PubMed] [Google Scholar]
- 13.Lima B, Nowicki ER, Blackstone EH, et al. Spinal cord protective strategies during descending and thoracoabdominal aortic aneurysm repair in the modern era: the role of intrathecal papaverine. J Thorac Cardiovasc Surg. 2012;143:945–952.e941. [DOI] [PubMed] [Google Scholar]
- 14.Estrera AL, Miller CC 3rd, Huynh TT, et al. Preoperative and operative predictors of delayed neurologic deficit following repair of thoracoabdominal aortic aneurysm. J Thorac Cardiovasc Surg. 2003;126:1288–1294. [DOI] [PubMed] [Google Scholar]
6.5.4.4. TAAA Renal and Visceral Organ Protection
- 1.Moulakakis KG, Karaolanis G, Antonopoulos CN, et al. Open repair of thoracoabdominal aortic aneurysms in experienced centers. J Vasc Surg. 2018;68:634–645.e612. [DOI] [PubMed] [Google Scholar]
- 2.Coselli JS, LeMaire SA, Preventza O, et al. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg. 2016;151:1323–1337. [DOI] [PubMed] [Google Scholar]
- 3.Murana G, Castrovinci S, Kloppenburg G, et al. Open thoracoabdominal aortic aneurysm repair in the modern era: results from a 20-year single-centre experience. Eur J Cardiothorac Surg. 2016;49:1374–1381. [DOI] [PubMed] [Google Scholar]
- 4.Khan FM, Naik A, Hameed I, et al. Open repair of descending thoracic and thoracoabdominal aortic aneurysms: a meta-analysis. Ann Thorac Surg. 2020;110:1941–1949. [DOI] [PubMed] [Google Scholar]
- 5.Lemaire SA, Jones MM, Conklin LD, et al. Randomized comparison of cold blood and cold crystalloid renal perfusion for renal protection during thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2009;49:11–19; discussion 19. [DOI] [PubMed] [Google Scholar]
- 6.Koksoy C, LeMaire SA, Curling PE, et al. Renal perfusion during thoracoabdominal aortic operations: cold crystalloid is superior to normothermic blood. Ann Thorac Surg. 2002;73:730–738. [DOI] [PubMed] [Google Scholar]
- 7.Svensson LG, Crawford ES, Hess KR, et al. Thoracoabdominal aortic aneurysms associated with celiac, superior mesenteric, and renal artery occlusive disease: methods and analysis of results in 271 patients. J Vasc Surg. 1992;16:378–389; discussion 389–390. [PubMed] [Google Scholar]
6.5.5.1. Access During Endovascular Repair of AAA
- 1.Vierhout BP, Pol RA, Ott MA, et al. Randomized multicenter trial on percutaneous versus open access in endovascular aneurysm repair (PiERO). J Vasc Surg. 2019;69:1429–1436. [DOI] [PubMed] [Google Scholar]
- 2.Siracuse JJ, Farber A, Kalish JA, et al. Comparison of access type on peri-operative outcomes after endovascular aortic aneurysm repair. J Vasc Surg. 2018;68:91–99. [DOI] [PubMed] [Google Scholar]
- 3.Nelson PR, Kracjer Z, Kansal N, et al. A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). J Vasc Surg. 2014;59:1181–1193. [DOI] [PubMed] [Google Scholar]
- 4.Kalish J, Eslami M, Gillespie D, et al. Routine use of ultrasound guidance in femoral arterial access for peripheral vascular intervention decreases groin hematoma rates. J Vasc Surg. 2015;61:1231–1238. [DOI] [PubMed] [Google Scholar]
6.5.5.2. Repair of Ruptured AAA
- 1.Starnes BW, Quiroga E, Hutter C, et al. Management of ruptured abdominal aortic aneurysm in the endovascular era. J Vasc Surg. 2010;51:9–17; discussion 17–18. [DOI] [PubMed] [Google Scholar]
- 2.Powell JT, Sweeting MJ, Thompson MM, et al. Endovascular or open repair strategy for ruptured abdominal aortic aneurysm: 30 day outcomes from IMPROVE randomised trial. BMJ. 2014;348:f7661. [DOI] [PubMed] [Google Scholar]
- 3.Mehta M, Taggert J, Darling RC 3rd, et al. Establishing a protocol for endovascular treatment of ruptured abdominal aortic aneurysms: outcomes of a prospective analysis. J Vasc Surg. 2006;44:1–8; discussion 8. [DOI] [PubMed] [Google Scholar]
- 4.Kontopodis N, Galanakis N, Antoniou SA, et al. Meta-analysis and meta-regression analysis of outcomes of endovascular and open repair for ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2020;59:399–410. [DOI] [PubMed] [Google Scholar]
- 5.Tan TW, Eslami M, Rybin D, et al. Outcomes of endovascular and open surgical repair of ruptured abdominal aortic aneurysms in elderly patients. J Vasc Surg. 2017;66:64–70. [DOI] [PubMed] [Google Scholar]
- 6.Wang LJ, Locham S, Al-Nouri O, et al. Endovascular repair of ruptured abdominal aortic aneurysm is superior to open repair: propensity-matched analysis in the Vascular Quality Initiative. J Vasc Surg. 2020;72:498–507. [DOI] [PubMed] [Google Scholar]
- 7.Powell JT, Hinchliffe RJ, Thompson MM, et al. Observations from the IM-PROVE trial concerning the clinical care of patients with ruptured abdominal aortic aneurysm. Br J Surg. 2014;101:216–224; discussion 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faizer R, Weinhandl E, El Hag S, et al. Decreased mortality with local versus general anesthesia in endovascular aneurysm repair for ruptured abdominal aortic aneurysm in the Vascular Quality Initiative database. J Vasc Surg. 2019;70:92–101.e101. [DOI] [PubMed] [Google Scholar]
- 9.Mouton R, Rogers CA, Harris RA, et al. Local anaesthesia for endovascular repair of ruptured abdominal aortic aneurysm. Br J Surg. 2019;106:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veith FJ, Ohki T, Lipsitz EC, et al. Endovascular grafts and other catheter-directed techniques in the management of ruptured abdominal aortic aneurysms. Semin Vasc Surg. 2003;16:326–331. [DOI] [PubMed] [Google Scholar]
- 11.Bickell WH, Wall MJ Jr, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105–1109. [DOI] [PubMed] [Google Scholar]
- 12.Hambly PR, Dutton RP. Excess mortality associated with the use of a rapid infusion system at a level 1 trauma center. Resuscitation. 1996;31:127–133. [DOI] [PubMed] [Google Scholar]
- 13.Hoornweg LL, Storm-Versloot MN, Ubbink DT, et al. Meta analysis on mortality of ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2008;35:558–570. [DOI] [PubMed] [Google Scholar]
- 14.Reimerink JJ, Hoornweg LL, Vahl AC, et al. Endovascular repair versus open repair of ruptured abdominal aortic aneurysms: a multicenter randomized controlled trial. Ann Surg. 2013;258:248–256. [DOI] [PubMed] [Google Scholar]
- 15.Desgranges P, Kobeiter H, Katsahian S, et al. Editor’s choice - ECAR (Endovasculaire ou Chirurgie dans les Anevrysmes aorto-iliaques Rompus): a French randomized controlled trial of endovascular versus open surgical repair of ruptured aorto-iliac aneurysms. Eur J Vasc Endovasc Surg. 2015;50:303–310. [DOI] [PubMed] [Google Scholar]
- 16.IMPROVE Trial Investigators. Comparative clinical effectiveness and cost effectiveness of endovascular strategy v open repair for ruptured abdominal aortic aneurysm: three year results of the IMPROVE randomised trial. BMJ. 2017;359:j4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varkevisser RRB, Swerdlow NJ, de Guerre L, et al. Five-year survival following endovascular repair of ruptured abdominal aortic aneurysms is improving. J Vasc Surg. 2020;72:105–113.e4. [DOI] [PubMed] [Google Scholar]
- 18.Baderkhan H, Goncalves FM, Oliveira NG, et al. Challenging anatomy predicts mortality and complications after endovascular treatment of ruptured abdominal aortic aneurysm. J Endovasc Ther. 2016;23:919–927. [DOI] [PubMed] [Google Scholar]
- 19.Zarkowsky DS, Sorber R, Ramirez JL, et al. Aortic neck IFU violations during EVAR for ruptured infrarenal aortic aneurysms are associated with increased in-hospital mortality. Ann Vasc Surg. 2021;75:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Runciman WB, Skowronski GA. Pathophysiology of haemorrhagic shock. Anaesth Intensive Care. 1984;12:193–205. [DOI] [PubMed] [Google Scholar]
- 21.Henry X, Verhaeghe P, Canarelli JP, et al. [General anesthesia in rabbits for digestive surgical research. A spontaneous ventilation technic by Nembutal-diazepam combination]. J Chir (Paris). 1977;113:437–440. [PubMed] [Google Scholar]
- 22.Hug CC Jr, McLeskey CH, Nahrwold ML, et al. Hemodynamic effects of propofol: data from over 25, 000 patients. Anesth Analg. 1993;77(suppl 4):S21–S29. [PubMed] [Google Scholar]
- 23.Crosby ET, Miller DR, Hamilton PP, et al. A randomized double-blind comparison of fentanyl- and sufentanil-oxygen anesthesia for abdominal aortic surgery. J Cardiothorac Anesth. 1990;4:168–176. [DOI] [PubMed] [Google Scholar]
- 24.Schneemilch CE, Schilling T, Bank U. Effects of general anaesthesia on inflammation. Best Pract Res Clin Anaesthesiol. 2004;18:493–507. [DOI] [PubMed] [Google Scholar]
- 25.Lenhardt R The effect of anesthesia on body temperature control. Front Biosci (Schol Ed). 2010;2:1145–1154. [DOI] [PubMed] [Google Scholar]
6.5.5.3. Threshold for AAA Repair
- 1.Lederle FA, Wilson SE, Johnson GR, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437–1444. [DOI] [PubMed] [Google Scholar]
- 2.The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998;352:1649–1655. [PubMed] [Google Scholar]
- 3.Powell JT, Brady AR, Brown LC, et al. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445–1452. [DOI] [PubMed] [Google Scholar]
- 4.Cao P, De Rango P, Verzini F, et al. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg. 2011;41:13–25. [DOI] [PubMed] [Google Scholar]
- 5.Ouriel K, Clair DG, Kent KC, et al. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg. 2010;51:1081–1087. [DOI] [PubMed] [Google Scholar]
- 6.Brown LC, Powell JT, et al. UK Small Aneurysm Trial Participants, Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. Ann Surg. 1999;230:289–296; discussion 296–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soden PA, Zettervall SL, Ultee KH, et al. Outcomes for symptomatic abdominal aortic aneurysms in the American College of Surgeons National Surgical Quality Improvement Program. J Vasc Surg. 2016;64:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Martino RR, Nolan BW, Goodney PP, et al. Outcomes of symptomatic abdominal aortic aneurysm repair. J Vasc Surg. 2010;52:5–12.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karthaus EG, Tong TML, Vahl A, et al. Saccular abdominal aortic aneurysms: patient characteristics, clinical presentation, treatment, and outcomes in the Netherlands. Ann Surg. 2019;270:852–858. [DOI] [PubMed] [Google Scholar]
- 10.Brewster DC, Cronenwett JL, Hallett JW Jr, et al. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003;37:1106–1117. [DOI] [PubMed] [Google Scholar]
- 11.Sweeting MJ, Thompson SG, Brown LC, et al. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655–665. [DOI] [PubMed] [Google Scholar]
- 12.Lo RC, Lu B, Fokkema MT, et al. Relative importance of aneurysm diameter and body size for predicting abdominal aortic aneurysm rupture in men and women. J Vasc Surg. 2014;59:1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tambyraja AL, Raza Z, Stuart WP, et al. Does immediate operation for symptomatic non-ruptured abdominal aortic aneurysm compromise outcome? Eur J Vasc Endovasc Surg. 2004;28:543–546. [DOI] [PubMed] [Google Scholar]
- 14.Cambria RA, Gloviczki P, Stanson AW, et al. Symptomatic, nonruptured abdominal aortic aneurysms: are emergent operations necessary? Ann Vasc Surg. 1994;8:121–126. [DOI] [PubMed] [Google Scholar]
- 15.Abdulrasak M, Sonesson BJ, Vaccarino R, et al. Endovascular aneurysm repair for symptomatic abdominal aortic aneurysms has comparable results to elective repair in the long term. J Vasc Surg. 2020;72:1927–1937.e1921. [DOI] [PubMed] [Google Scholar]
- 16.Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77.e72. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka A, Leonard SD, Sandhu HK, et al. Open descending and thoracoabdominal aortic repairs in patients younger than 50 years old. Ann Thorac Surg. 2019;108:693–699. [DOI] [PubMed] [Google Scholar]
- 18.Ameli-Renani S, Das R, Morgan RA. Thoracic endovascular aortic repair for the treatment of aortic dissection: post-operative imaging, complications and secondary interventions. Cardiovasc Intervent Radiol. 2015;38:1391–1404. [DOI] [PubMed] [Google Scholar]
6.5.5.4. Open Versus Endovascular Repair of AAA
- 1.De Bruin JL, Baas AF, Buth J, et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2010;362:1881–1889. [DOI] [PubMed] [Google Scholar]
- 2.Becquemin JP, Pillet JC, Lescalie F, et al. A randomized controlled trial of endovascular aneurysm repair versus open surgery for abdominal aortic aneurysms in low- to moderate-risk patients. J Vasc Surg. 2011;53:1167–1173.e1161. [DOI] [PubMed] [Google Scholar]
- 3.Lederle FA, Freischlag JA, Kyriakides TC, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302:1535–1542. [DOI] [PubMed] [Google Scholar]
- 4.Lederle FA, Freischlag JA, Kyriakides TC, et al. Long-term comparison of endovascular and open repair of abdominal aortic aneurysm. N Engl J Med. 2012;367:1988–1997. [DOI] [PubMed] [Google Scholar]
- 5.Lederle FA, Kyriakides TC, Stroupe KT, et al. Open versus endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2019;380:2126–2135. [DOI] [PubMed] [Google Scholar]
- 6.EVAR Trial Participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005;365:2179–2186. [DOI] [PubMed] [Google Scholar]
- 7.Greenhalgh RM, Brown LC, et al. ; United Kingdom EVAR Trial Investigators, Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010;362:1863–1871. [DOI] [PubMed] [Google Scholar]
- 8.Patel R, Sweeting MJ, Powell JT, et al. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366–2374. [DOI] [PubMed] [Google Scholar]
- 9.Schermerhorn ML, O’Malley AJ, Jhaveri A, et al. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008;358:464–474. [DOI] [PubMed] [Google Scholar]
- 10.Varkevisser RRB, O’Donnell TFX, Swerdlow NJ, et al. Fenestrated endovascular aneurysm repair is associated with lower perioperative morbidity and mortality compared with open repair for complex abdominal aortic aneurysms. J Vasc Surg. 2019;69:1670–1678. [DOI] [PubMed] [Google Scholar]
- 11.Rao R, Lane TR, Franklin IJ, et al. Open repair versus fenestrated endovascular aneurysm repair of juxtarenal aneurysms. J Vasc Surg. 2015;61:242–255. [DOI] [PubMed] [Google Scholar]
- 12.Schanzer A, Greenberg RK, Hevelone N, et al. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation. 2011;123:2848–2855. [DOI] [PubMed] [Google Scholar]
- 13.Herman CR, Charbonneau P, Hongku K, et al. Any nonadherence to instructions for use predicts graft-related adverse events in patients undergoing elective endovascular aneurysm repair. J Vasc Surg. 2018;67:126–133. [DOI] [PubMed] [Google Scholar]
- 14.Antoniou GA, Juszczak MT, Nasr H, et al. Prognosis review and time-to-event data meta-analysis of endovascular aneurysm repair outside versus within instructions for use of aortic endograft devices. J Vasc Surg. 2020;71:1415–1431.e1415. [DOI] [PubMed] [Google Scholar]
- 15.Walker J, Tucker LY, Goodney P, et al. Adherence to endovascular aortic aneurysm repair device instructions for use guidelines has no impact on outcomes. J Vasc Surg. 2015;61:1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beckerman WE, Tadros RO, Faries PL, et al. No major difference in outcomes for endovascular aneurysm repair stent grafts placed outside of instructions for use. J Vasc Surg. 2016;64:63–74.e62. [DOI] [PubMed] [Google Scholar]
- 17.O’Donnell TFX, Boitano LT, Deery SE, et al. Open versus fenestrated endovascular repair of complex abdominal aortic aneurysms. Ann Surg. 2020;271:969–977. [DOI] [PubMed] [Google Scholar]
- 18.Jones AD, Waduud MA, Walker P, et al. Meta-analysis of fenestrated endovascular aneurysm repair versus open surgical repair of juxtarenal abdominal aortic aneurysms over the last 10 years. BJS Open. 2019;3:572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubost C, Allary M, Oeconomos N. Resection of an aneurysm of the abdominal aorta: reestablishment of the continuity by a preserved human arterial graft, with result after five months. AMA Arch Surg. 1952;64:405–408. [PubMed] [Google Scholar]
- 20.Varkevisser RRB, Swerdlow NJ, de Guerre L, et al. Five-year survival following endovascular repair of ruptured abdominal aortic aneurysms is improving. J Vasc Surg. 2020;72:105–113.e4. [DOI] [PubMed] [Google Scholar]
- 21.Li B, Khan S, Salata K, et al. A systematic review and meta-analysis of the long-term outcomes of endovascular versus open repair of abdominal aortic aneurysm. J Vasc Surg. 2019;70:954–969.e930. [DOI] [PubMed] [Google Scholar]
- 22.Antoniou GA, Antoniou SA, Torella F. Editor’s choice-endovascular vs. open repair for abdominal aortic aneurysm: systematic review and meta-analysis of updated peri-operative and long term data of randomised controlled trials. Eur J Vasc Endovasc Surg. 2020;59:385–397. [DOI] [PubMed] [Google Scholar]
- 23.Brown LC, Epstein D, Manca A, et al. The UK Endovascular Aneurysm Repair (EVAR) trials: design, methodology and progress. Eur J Vasc Endovasc Surg. 2004;27:372–381. [DOI] [PubMed] [Google Scholar]
- 24.EVAR trial participants. Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet. 2005;365:2187–2192. [DOI] [PubMed] [Google Scholar]
- 25.Sweeting MJ, Patel R, Powell JT, et al. Endovascular repair of abdominal aortic aneurysm in patients physically ineligible for open repair: very long-term follow-up in the EVAR-2 randomized controlled trial. Ann Surg. 2017;266:713–719. [DOI] [PubMed] [Google Scholar]
- 26.Adkar SS, Turner MC, Leraas HJ, et al. Low mortality rates after endovascular aortic repair expand use to high-risk patients. J Vasc Surg. 2018;67:424–432.e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertges DJ, Neal D, Schanzer A, et al. The Vascular Quality Initiative Cardiac Risk Index for prediction of myocardial infarction after vascular surgery. J Vasc Surg. 2016;64:1411–1421.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eslami MH, Rybin D, Doros G, et al. Comparison of a Vascular Study Group of New England risk prediction model with established risk prediction models of in-hospital mortality after elective abdominal aortic aneurysm repair. J Vasc Surg. 2015;62:1125–1133.e1122. [DOI] [PubMed] [Google Scholar]
- 29.Eslami MH, Rybin DV, Doros G, et al. External validation of Vascular Study Group of New England risk predictive model of mortality after elective abdominal aorta aneurysm repair in the Vascular Quality Initiative and comparison against established models. J Vasc Surg. 2018;67:143–150. [DOI] [PubMed] [Google Scholar]
6.5.5.5. Treatment of Concomitant Common Iliac Aneurysms
- 1.Richards T, Dharmadasa A, Davies R, et al. Natural history of the common iliac artery in the presence of an abdominal aortic aneurysm. J Vasc Surg. 2009;49:881–885. [DOI] [PubMed] [Google Scholar]
- 2.Armon MP, Wenham PW, Whitaker SC, et al. Common iliac artery aneurysms in patients with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 1998;15:255–257. [DOI] [PubMed] [Google Scholar]
- 3.Jalalzadeh H, Indrakusuma R, Koelemay MJW, et al. Editor’s choice - nationwide analysis of patients undergoing iliac artery aneurysm repair in the Netherlands. Eur J Vasc Endovasc Surg. 2020;60:49–55. [DOI] [PubMed] [Google Scholar]
- 4.Gibello L, Varetto G, Ruffino MA, et al. Long term outcomes of endovascular aortic repair in patients with abdominal aortic aneurysm and ectatic common iliac arteries. Eur J Vasc Endovasc Surg. 2020;60:356–364. [DOI] [PubMed] [Google Scholar]
- 5.Kouvelos GN, Katsargyris A, Antoniou GA, et al. Outcome after interruption or preservation of internal iliac artery flow during endovascular repair of abdominal aorto-iliac aneurysms. Eur J Vasc Endovasc Surg. 2016;52:621–634. [DOI] [PubMed] [Google Scholar]
- 6.Bosanquet DC, Wilcox C, Whitehurst L, et al. Systematic review and meta-analysis of the effect of internal iliac artery exclusion for patients undergoing EVAR. Eur J Vasc Endovasc Surg. 2017;53:534–548. [DOI] [PubMed] [Google Scholar]
- 7.Laine MT, Bjorck M, Beiles CB, et al. Few internal iliac artery aneurysms rupture under 4 cm. J Vasc Surg. 2017;65:76–81. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Gloviczki P, Duncan AA, et al. Common iliac artery aneurysm: expansion rate and results of open surgical and endovascular repair. J Vasc Surg. 2008;47:1203–1210; discussion 1210–1211. [DOI] [PubMed] [Google Scholar]
- 9.Sala F, Hassen-Khodja R, Branchereau P, et al. Outcome of common iliac arteries after aortoaortic graft placement during elective repair of infrarenal abdominal aortic aneurysms. J Vasc Surg. 2002;36:982–987. [DOI] [PubMed] [Google Scholar]
- 10.Hassen-Khodja R, Feugier P, Favre JP, et al. Outcome of common iliac arteries after straight aortic tube-graft placement during elective repair of infrarenal abdominal aortic aneurysms. J Vasc Surg. 2006;44:943–948. [DOI] [PubMed] [Google Scholar]
6.5.6.1. Surveillance After TAA Repair
- 1.Meena RA, Benarroch-Gampel J, Leshnower BG, et al. Surveillance recommendations after thoracic endovascular aortic repair should be based on initial indication for repair. Ann Vasc Surg. 2019;57:51–59. [DOI] [PubMed] [Google Scholar]
- 2.Bavaria JE, Appoo JJ, Makaroun MS, et al. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg. 2007;133:369–377. [DOI] [PubMed] [Google Scholar]
- 3.Ranney DN, Cox ML, Yerokun BA, et al. Long-term results of endovascular repair for descending thoracic aortic aneurysms. J Vasc Surg. 2018;67:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Allmen RS, Anjum A, Powell JT. Outcomes after endovascular or open repair for degenerative descending thoracic aortic aneurysm using linked hospital data. Br J Surg. 2014;101:1244–1251. [DOI] [PubMed] [Google Scholar]
- 5.Roselli EE, Subramanian S, Sun Z, et al. Endovascular versus open elephant trunk completion for extensive aortic disease. J Thorac Cardiovasc Surg. 2013;146:1408–1416; discussion 1416–1417. [DOI] [PubMed] [Google Scholar]
- 6.Daye D, Walker TG. Complications of endovascular aneurysm repair of the thoracic and abdominal aorta: evaluation and management. Cardiovasc Diagn Ther. 2018;8:S138–S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ameli-Renani S, Das R, Morgan RA. Thoracic endovascular aortic repair for the treatment of aortic dissection: post-operative imaging, complications and secondary interventions. Cardiovasc Intervent Radiol. 2015;38:1391–1404. [DOI] [PubMed] [Google Scholar]
- 8.Hellinger JC. Endovascular repair of thoracic and abdominal aortic aneurysms: pre- and postprocedural imaging. Tech Vasc Interv Radiol. 2005;8:2–15. [DOI] [PubMed] [Google Scholar]
- 9.Weigel S, Tombach B, Maintz D, et al. Thoracic aortic stent graft: comparison of contrast-enhanced MR angiography and CT angiography in the follow-up: initial results. Eur Radiol. 2003;13:1628–1634. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka A, Leonard SD, Sandhu HK, et al. Open descending and thoracoabdominal aortic repairs in patients younger than 50 years old. Ann Thorac Surg. 2019;108:693–699. [DOI] [PubMed] [Google Scholar]
- 11.Schaffer JM, Lingala B, Fischbein MP, et al. Midterm Outcomes of Open Descending Thoracic Aortic Repair in More Than 5, 000 Medicare Patients. Ann Thorac Surg. 2015;100:2087–2094; discussion 2094. [DOI] [PubMed] [Google Scholar]
- 12.Sadek M, Abjigitova D, Pellet Y, et al. Operative outcomes after open repair of descending thoracic aortic aneurysms in the era of endovascular surgery. Ann Thorac Surg. 2014;97:1562–1567. [DOI] [PubMed] [Google Scholar]
- 13.Peterss S, Charilaou P, Dumfarth J, et al. Aortic valve disease with ascending aortic aneurysm: Impact of concomitant root-sparing (supracoronary) aortic replacement in nonsyndromic patients. J Thorac Cardiovasc Surg. 2016;152:791–798.e1. [DOI] [PubMed] [Google Scholar]
- 14.Gaudino M, Lau C, Munjal M, et al. Contemporary outcomes of surgery for aortic root aneurysms: a propensity-matched comparison of valve-sparing and composite valve graft replacement. J Thorac Cardiovasc Surg. 2015;150:1120–1129.e1. [DOI] [PubMed] [Google Scholar]
- 15.Wang TKM, Desai MY. Thoracic aortic aneurysm: optimal surveillance and treatment. Cleve Clin J Med. 2020;87:557–568. [DOI] [PubMed] [Google Scholar]
- 16.Groner LK, Lau C, Devereux RB, et al. Imaging of the postsurgical aorta in Marfan syndrome. Curr Treat Options Cardiovasc Med. 2018;20:80. [DOI] [PubMed] [Google Scholar]
- 17.Makaroun MS, Dillavou ED, Wheatley GH, et al. Five-year results of endovascular treatment with the Gore TAG device compared with open repair of thoracic aortic aneurysms. J Vasc Surg. 2008;47:912–918. [DOI] [PubMed] [Google Scholar]
- 18.Chen SW, Chan YH, Lin CP, et al. Association of long-term use of antihypertensive medications with late outcomes among patients with aortic dissection. JAMA Netw Open. 2021;4:e210469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill RA. Do short-term changes in white matter structure indicate learning-induced myelin plasticity? J Neurosci. 2013;33:19393–19395. [DOI] [PMC free article] [PubMed] [Google Scholar]
6.5.6.2. Surveillance After AAA Repair
- 1.Sternbergh WC 3rd, Greenberg RK, Chuter TA, et al. Redefining postoperative surveillance after endovascular aneurysm repair: recommendations based on 5-year follow-up in the US Zenith multicenter trial. J Vasc Surg. 2008;48:278–284; discussion 84–85. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Magid DJ, Wells B, et al. The cardiovascular research network: a new paradigm for cardiovascular quality and outcomes research. Circ Cardiovasc Qual Outcomes. 2008;1:138–147. [DOI] [PubMed] [Google Scholar]
- 3.Schermerhorn ML, Buck DB, O’Malley AJ, et al. Long-Term Outcomes of Abdominal Aortic Aneurysm in the Medicare Population. N Engl J Med. 2015;373:328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go MR, Barbato JE, Rhee RY, et al. What is the clinical utility of a 6-month computed tomography in the follow-up of endovascular aneurysm repair patients? J Vasc Surg. 2008;47:1181–1186; discussion 1186–1187. [DOI] [PubMed] [Google Scholar]
- 5.Mii S, Mori A, Sakata H, et al. Para-anastomotic aneurysms: incidence, risk factors, treatment and prognosis. J Cardiovasc Surg (Torino). 1998;39:259–266. [PubMed] [Google Scholar]
- 6.Edwards JM, Teefey SA, Zierler RE, et al. Intraabdominal paraanastomotic aneurysms after aortic bypass grafting. J Vasc Surg. 1992;15:344–350; discussion 351–353. [PubMed] [Google Scholar]
- 7.Fransen GA, Desgranges P, Laheij RJ, et al. Frequency, predictive factors, and consequences of stent-graft kink following endovascular AAA repair. J Endovasc Ther. 2003;10:913–918. [DOI] [PubMed] [Google Scholar]
- 8.Tonnessen BH, Sternbergh WC 3rd, Money SR. Mid- and long-term device migration after endovascular abdominal aortic aneurysm repair: a comparison of AneuRx and Zenith endografts. J Vasc Surg. 2005;42:392–400; discussion 400–401. [DOI] [PubMed] [Google Scholar]
- 9.Iscan HZ, Unal EU, Akkaya B, et al. Color Doppler ultrasound for surveillance following EVAR as the primary tool. J Card Surg. 2021;36:111–117. [DOI] [PubMed] [Google Scholar]
- 10.Swerdlow NJ, McCallum JC, Liang P, et al. Select type I and type III endoleaks at the completion of fenestrated endovascular aneurysm repair resolve spontaneously. J Vasc Surg. 2019;70:381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran K, McFarland G, Sgroi M, et al. Duplex ultrasound surveillance of renal branch grafts after fenestrated endovascular aneurysm repair. J Vasc Surg. 2019;70:1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noll RE Jr, Tonnessen BH, Mannava K, et al. Long-term postplacement cost after endovascular aneurysm repair. J Vasc Surg. 2007;46:9–15, discussion 15. [DOI] [PubMed] [Google Scholar]
- 13.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. [DOI] [PubMed] [Google Scholar]
- 14.Johnsen L, Hisdal J, Jonung T, et al. Contrast-enhanced ultrasound detects type II endoleaks during follow-up for endovascular aneurysm repair. J Vasc Surg. 2020;72:1952–1959. [DOI] [PubMed] [Google Scholar]
- 15.George J, Tadros RO, Rao A, et al. Duplex ultrasound can successfully identify endoleaks and renovisceral stent patency in patients undergoing complex endovascular aneurysm repair. Vasc Endovascular Surg. 2021;55:234–238. [DOI] [PubMed] [Google Scholar]
- 16.Kawada H, Goshima S, Sakurai K, et al. Utility of noncontrast magnetic resonance angiography for aneurysm follow-up and detection of endoleaks after endovascular aortic repair. Korean J Radiol. 2021;22:513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen RC, Schneider J, Longenecker L, et al. Paraanastomotic aneurysms of the abdominal aorta. J Vasc Surg. 1993;18:424–431; discussion 431–432. [PubMed] [Google Scholar]
- 18.Conrad MF, Crawford RS, Pedraza JD, et al. Long-term durability of open abdominal aortic aneurysm repair. J Vasc Surg. 2007;46:669–675. [DOI] [PubMed] [Google Scholar]
- 19.Dingemans SA, Jonker FH, Moll FL, et al. Aneurysm sac enlargement after endovascular abdominal aortic aneurysm repair. Ann Vasc Surg. 2016;31:229–238. [DOI] [PubMed] [Google Scholar]
- 20.Candell L, Tucker LY, Goodney P, et al. Early and delayed rupture after endovascular abdominal aortic aneurysm repair in a 10-year multicenter registry. J Vasc Surg. 2014;60:1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zierler RE. Duplex ultrasound follow-up after fenestrated and branched endovascular aneurysm repair (FEVAR and BEVAR). Semin Vasc Surg. 2020;33:60–64. [DOI] [PubMed] [Google Scholar]
- 22.Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77.e72. [DOI] [PubMed] [Google Scholar]
7.1. Presentation
- 1.Meszaros I, Morocz J, Szlavi J, et al. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117:1271–1278. [DOI] [PubMed] [Google Scholar]
- 2.Evangelista A, Isselbacher EM, Bossone E, et al. Insights from the International Registry of Acute Aortic Dissection: a 20-year experience of collaborative clinical research. Circulation. 2018;137:1846–1860. [DOI] [PubMed] [Google Scholar]
- 3.Bossone E, Pyeritz RE, O’Gara P, et al. Acute aortic dissection in blacks: insights from the International Registry of Acute Aortic Dissection. Am J Med. 2013;126:909–915. [DOI] [PubMed] [Google Scholar]
- 4.Bossone E, LaBounty TM, Eagle KA. Acute aortic syndromes: diagnosis and management, an update. Eur Heart J. 2018;39:739d–749d. [DOI] [PubMed] [Google Scholar]
- 5.Nienaber CA, Fattori R, Mehta RH, et al. Gender-related differences in acute aortic dissection. Circulation. 2004;109:3014–3021. [DOI] [PubMed] [Google Scholar]
7.2. AAS: Diagnostic Evaluation (Imaging, Laboratory Testing)
- 1.Bhave NM, Nienaber CA, Clough RE, et al. Multimodality imaging of thoracic aortic diseases in adults. J Am Coll Cardiol Img. 2018;11:902–919. [DOI] [PubMed] [Google Scholar]
- 2.Strayer RJ, Shearer PL, Hermann LK. Screening, evaluation, and early management of acute aortic dissection in the ED. Curr Cardiol Rev. 2012;8:152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiga T, Wajima Z, Apfel CC, et al. Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: systematic review and meta-analysis. Arch Intern Med. 2006;166:1350–1356. [DOI] [PubMed] [Google Scholar]
- 4.Diercks DB, Promes SB, Schuur JD, et al. Clinical policy: critical issues in the evaluation and management of adult patients with suspected acute nontraumatic thoracic aortic dissection. Ann Emerg Med. 2015;65:32–42.e12. [DOI] [PubMed] [Google Scholar]
- 5.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. [DOI] [PubMed] [Google Scholar]
- 6.Kicska GA, Hurwitz Koweek LM, et al. Expert Panel on Cardiac Imaging, ACR Appropriateness Criteria® Suspected Acute Aortic Syndrome. J Am Coll Radiol. 2021;18:S474–S481. [DOI] [PubMed] [Google Scholar]
- 7.Nazerian P, Pivetta E, Veglia S, et al. Integrated use of conventional chest radiography cannot rule out acute aortic syndromes in emergency department patients at low clinical probability. Acad Emerg Med. 2019;26:1255–1265. [DOI] [PubMed] [Google Scholar]
- 8.Svensson LG, Labib SB, Eisenhauer AC, et al. Intimal tear without hematoma: an important variant of aortic dissection that can elude current imaging techniques. Circulation. 1999;99:1331–1336. [DOI] [PubMed] [Google Scholar]
- 9.Bima P, Pivetta E, Nazerian P, et al. Systematic review of aortic dissection detection risk score plus D-dimer for diagnostic rule-out of suspected acute aortic syndromes. Acad Emerg Med. 2020;27:1013–1027. [DOI] [PubMed] [Google Scholar]
- 10.Hiestand BC, Ohle R, Anjum O, et al. What is the specificity of the aortic dissection detection risk score in a low-prevalence population? Acad Emerg Med. 2019;26:632–638. [DOI] [PubMed] [Google Scholar]
- 11.Morello F, Bima P, Pivetta E, et al. Development and validation of a simplified probability assessment score integrated with age-adjusted d-Dimer for diagnosis of acute aortic syndromes. J Am Heart Assoc. 2021;10:e018425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki T, Distante A, Zizza A, et al. Diagnosis of acute aortic dissection by D-dimer: the International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) experience. Circulation. 2009;119:2702–2707. [DOI] [PubMed] [Google Scholar]
- 13.Nazerian P, Mueller C, de Matos Soeiro A, et al. Diagnostic accuracy of the aortic dissection detection risk score plus D-dimer for acute aortic syndromes: the ADvISED prospective multicenter study. Circulation. 2018;137:250–258. [DOI] [PubMed] [Google Scholar]
- 14.Rogers AM, Hermann LK, Booher AM, et al. Sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection at initial presentation: results from the international registry of acute aortic dissection. Circulation. 2011;123:2213–2218. [DOI] [PubMed] [Google Scholar]
7.3.1. Acute Medical Management of AAS
- 1.Jonker FH, Trimarchi S, Rampoldi V, et al. Aortic expansion after acute type B aortic dissection. Ann Thorac Surg. 2012;94:1223–1229. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki T, Isselbacher EM, Nienaber CA, et al. Type-selective benefits of medications in treatment of acute aortic dissection (from the International Registry of Acute Aortic Dissection [IRAD]). Am J Cardiol. 2012;109:122–127. [DOI] [PubMed] [Google Scholar]
- 3.Kumar KU, Zhao Q, Bai X, et al. Controlled heart rate and blood pressure reduce the life threatening aortic events and increase survival in patients with type B aortic dissection: a single center experience. Int J Cardiol Heart Vasc. 2015;8:73–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Ge M, Chen T, et al. Impact of hypertension on short- and long-term survival of patients who underwent emergency surgery for type A acute aortic dissection. J Thorac Dis. 2020;12:6618–6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen SW, Chan YH, Lin CP, et al. Association of long-term use of antihypertensive medications with late outcomes among patients with aortic dissection. JAMA Netw Open. 2021;4:e210469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin YL, Wang F, Li TX, et al. Endovascular repair compared with medical management of patients with uncomplicated type B acute aortic dissection. J Am Coll Cardiol. 2016;67:2835–2842. [DOI] [PubMed] [Google Scholar]
- 7.Grubb BP, Sirio C, Zelis R. Intravenous labetalol in acute aortic dissection. JAMA. 1987;258:78–79. [PubMed] [Google Scholar]
- 8.Ulici A, Jancik J, Lam TS, et al. Clevidipine versus sodium nitroprusside in acute aortic dissection: a retrospective chart review. Am J Emerg Med. 2017;35:1514–1518. [DOI] [PubMed] [Google Scholar]
- 9.Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol. 2013;61:1661–1678. [DOI] [PubMed] [Google Scholar]
- 10.Qin YL, Wang F, Li TX, et al. Endovascular Repair Compared With Medical Management of Patients With Uncomplicated Type B Acute Aortic Dissection. J Am Coll Cardiol. 2016;67:2835–2842. [DOI] [PubMed] [Google Scholar]
- 11.Lu N, Ma X, Xu T, et al. Optimal blood pressure control for patients after thoracic endovascular aortic repair of type B aortic dissection. BMC Cardiovasc Disord. 2019;19:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masaki N, Kumagai K, Sasaki K, et al. Suppressive effect of pitavastatin on aortic arch dilatation in acute Stanford type B aortic dissection: analysis of STANP trial. Gen Thorac Cardiovasc Surg. 2018;66:334–343. [DOI] [PubMed] [Google Scholar]
7.3.2. Subsequent Medical Management of AAS
- 1.Suzuki T, Isselbacher EM, Nienaber CA, et al. Type-selective benefits of medications in treatment of acute aortic dissection (from the International Registry of Acute Aortic Dissection [IRAD]). Am J Cardiol. 2012;109:122–127. [DOI] [PubMed] [Google Scholar]
- 2.Lu N, Ma X, Xu T, et al. Optimal blood pressure control for patients after thoracic endovascular aortic repair of type B aortic dissection. BMC Cardiovasc Disord. 2019;19:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, Ge M, Chen T, et al. Impact of hypertension on short- and long-term survival of patients who underwent emergency surgery for type A acute aortic dissection. J Thorac Dis. 2020;12:6618–6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen SW, Chan YH, Lin CP, et al. Association of long-term use of antihypertensive medications with late outcomes among patients with aortic dissection. JAMA Netw Open. 2021;4:e210469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol. 2013;61:1661–1678. [DOI] [PubMed] [Google Scholar]
- 6.Kumar KU, Zhao Q, Bai X, et al. Controlled heart rate and blood pressure reduce the life threatening aortic events and increase survival in patients with type B aortic dissection: a single center experience. Int J Cardiol Heart Vasc. 2015;8:73–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin YL, Wang F, Li TX, et al. Endovascular Repair Compared With Medical Management of Patients With Uncomplicated Type B Acute Aortic Dissection. J Am Coll Cardiol. 2016;67:2835–2842. [DOI] [PubMed] [Google Scholar]
- 8.Doyle JJ, Doyle AJ, Wilson NK, et al. A deleterious gene-by-environment interaction imposed by calcium channel blockers in Marfan syndrome. Elife. 2015;4:e08648. [DOI] [PMC free article] [PubMed] [Google Scholar]
7.4.1.1. Initial Surgical Considerations in Acute Type A Aortic Dissection
- 1.Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. [DOI] [PubMed] [Google Scholar]
- 2.Pape LA, Awais M, Woznicki EM, et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol. 2015;66:350–358. [DOI] [PubMed] [Google Scholar]
- 3.Goldstone AB, Chiu P, Baiocchi M, et al. Interfacility transfer of Medicare beneficiaries with acute type A aortic dissection and regionalization of care in the United States. Circulation. 2019;140:1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umana-Pizano JB, Nissen AP, Sandhu HK, et al. Acute type A dissection repair by high-volume vs low-volume surgeons at a high-volume aortic center. Ann Thorac Surg. 2019;108:1330–1336. [DOI] [PubMed] [Google Scholar]
- 5.Di Eusanio M, Patel HJ, Nienaber CA, et al. Patients with type A acute aortic dissection presenting with major brain injury: should we operate on them? J Thorac Cardiovasc Surg. 2013;145:S213–S221.e1. [DOI] [PubMed] [Google Scholar]
- 6.Estrera AL, Garami Z, Miller CC, et al. Acute type A aortic dissection complicated by stroke: can immediate repair be performed safely? J Thorac Cardiovasc Surg. 2006;132:1404–1408. [DOI] [PubMed] [Google Scholar]
- 7.Rampoldi V, Trimarchi S, Eagle KA, et al. Simple risk models to predict surgical mortality in acute type A aortic dissection: the International Registry of Acute Aortic Dissection score. Ann Thorac Surg. 2007;83:55–61. [DOI] [PubMed] [Google Scholar]
- 8.Trimarchi S, Nienaber CA, Rampoldi V, et al. Contemporary results of surgery in acute type A aortic dissection: the International Registry of Acute Aortic Dissection Experience. J Thorac Cardiovasc Surg. 2005;129:112–122. [DOI] [PubMed] [Google Scholar]
- 9.Long SM, Tribble CG, Raymond DP, et al. Preoperative shock determines outcome for acute type A aortic dissection. Ann Thorac Surg. 2003;75:520–524. [DOI] [PubMed] [Google Scholar]
- 10.Hattori S, Noguchi K, Gunji Y, et al. Acute type A aortic dissection in nonagenarians: to cut or not. Interact Cardiovasc Thorac Surg. 2020;31:102–107. [DOI] [PubMed] [Google Scholar]
- 11.Biancari F, Vasques F, Benenati V, et al. Contemporary results after surgical repair of type A aortic dissection in patients aged 80 years and older: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2011;40:1058–1063. [DOI] [PubMed] [Google Scholar]
- 12.Kawahito K, Kimura N, Yamaguchi A, et al. Early and late surgical outcomes of acute type A aortic dissection in octogenarians. Ann Thorac Surg. 2018;105:137–143. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki T, Asai T, Kinoshita T. Emergency surgery for acute type A aortic dissection in octogenarians without patient selection. Ann Thorac Surg. 2019;107:1146–1153. [DOI] [PubMed] [Google Scholar]
- 14.Zindovic I, Sjogren J, Bjursten H, et al. Impact of hemodynamic instability and organ malperfusion in elderly surgical patients treated for acute type A aortic dissection. J Card Surg. 2015;30:822–829. [DOI] [PubMed] [Google Scholar]
- 15.Braverman AC. Acute aortic dissection: clinician update. Circulation. 2010;122:184–188. [DOI] [PubMed] [Google Scholar]
- 16.Matthews CR, Madison M, Timsina LR, et al. Impact of time between diagnosis to treatment in acute type A aortic dissection. Sci Rep. 2021;11:3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu J, Zhang L, Luo X, et al. Higher mortality in patients undergoing night-time surgical procedures for acute type A aortic dissection. Ann Thorac Surg. 2018;106:1164–1170. [DOI] [PubMed] [Google Scholar]
- 18.Ahlsson A, Wickbom A, Geirsson A, et al. Is there a weekend effect in surgery for type A dissection?: results from the Nordic Consortium for Acute type A Aortic Dissection database. Ann Thorac Surg. 2019;108:770–776. [DOI] [PubMed] [Google Scholar]
- 19.Gokalp O, Yilik L, Besir Y, et al. “Overtime hours effect” on emergency surgery of acute type A aortic dissection. Braz J Cardiovasc Surg. 2019;34:680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasser S, Stastny L, Kofler M, et al. Surgery out of office hours for type A aortic dissection: does night-time and weekend surgery worsen outcome? Interact Cardiovasc Thorac Surg. 2020;31:806–812. [DOI] [PubMed] [Google Scholar]
- 21.Sultan I, Bianco V, Patel HJ, et al. Surgery for type A aortic dissection in patients with cerebral malperfusion: results from the International Registry of Acute Aortic Dissection. J Thorac Cardiovasc Surg. 2021;161:1713–1720.e1. [DOI] [PubMed] [Google Scholar]
- 22.Tsukube T, Hayashi T, Kawahira T, et al. Neurological outcomes after immediate aortic repair for acute type A aortic dissection complicated by coma. Circulation. 2011;124:S163–S167. [DOI] [PubMed] [Google Scholar]
- 23.Chiu P, Rotto TJ, Goldstone AB, et al. Time-to-operation does not predict outcome in acute type A aortic dissection complicated by neurologic injury at presentation. J Thorac Cardiovasc Surg. 2019;158:665–672. [DOI] [PubMed] [Google Scholar]
- 24.Berretta P, Trimarchi S, Patel HJ, et al. Malperfusion syndromes in type A aortic dissection: what we have learned from IRAD. J Vis Surg. 2018;4:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuhara S, Norton EL, Chaudhary N, et al. Type A aortic dissection with cerebral malperfusion: new insights. Ann Thorac Surg. 2020;112:501–509. [DOI] [PubMed] [Google Scholar]
7.4.1.2. Management of Malperfusion
- 1.Berretta P, Trimarchi S, Patel HJ, et al. Malperfusion syndromes in type A aortic dissection: what we have learned from IRAD. J Vis Surg. 2018;4:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geirsson A, Szeto WY, Pochettino A, et al. Significance of malperfusion syndromes prior to contemporary surgical repair for acute type A dissection: outcomes and need for additional revascularizations. Eur J Cardiothorac Surg. 2007;32:255–262. [DOI] [PubMed] [Google Scholar]
- 3.Leshnower BG, Keeling WB, Duwayri YM, et al. The “thoracic endovascular aortic repair-first” strategy for acute type A dissection with mesenteric malperfusion: Initial results compared with conventional algorithms. J Thorac Cardiovasc Surg. 2019;158:1516–1524. [DOI] [PubMed] [Google Scholar]
- 4.Yang B, Rosati CM, Norton EL, et al. Endovascular fenestration/stenting first followed by delayed open aortic repair for acute type A aortic dissection with malperfusion syndrome. Circulation. 2018;138:2091–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang B, Norton EL, Rosati CM, et al. Managing patients with acute type A aortic dissection and mesenteric malperfusion syndrome: a 20-year experience. J Thorac Cardiovasc Surg. 2019;158:675–687.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawton JS, Moon MR, Liu J, et al. The profound impact of combined severe acidosis and malperfusion on operative mortality in the surgical treatment of type A aortic dissection. J Thorac Cardiovasc Surg. 2018;155:897–904. [DOI] [PubMed] [Google Scholar]
- 7.Zindovic I, Gudbjartsson T, Ahlsson A, et al. Malperfusion in acute type A aortic dissection: an update from the Nordic Consortium for Acute Type A Aortic Dissection. J Thorac Cardiovasc Surg. 2019;157:1324–1333.e1326. [DOI] [PubMed] [Google Scholar]
- 8.Uchida K, Karube N, Kasama K, et al. Early reperfusion strategy improves the outcomes of surgery for type A acute aortic dissection with malperfusion. J Thorac Cardiovasc Surg. 2018;156:483–489. [DOI] [PubMed] [Google Scholar]
- 9.Kawahito K, Kimura N, Yamaguchi A, et al. Malperfusion in type A aortic dissection: results of emergency central aortic repair. Gen Thorac Cardiovasc Surg. 2019;67:594–601. [DOI] [PubMed] [Google Scholar]
- 10.Evangelista A, Isselbacher EM, Bossone E, et al. Insights from the International Registry of Acute Aortic Dissection: a 20-year experience of collaborative clinical research. Circulation. 2018;137:1846–1860. [DOI] [PubMed] [Google Scholar]
- 11.Yamashiro S, Arakaki R, Kise Y, et al. Management of visceral malperfusion complicated with acute type A aortic dissection. Interact Cardiovasc Thorac Surg. 2015;21:346–351. [DOI] [PubMed] [Google Scholar]
7.4.1.3. Surgical Repair Strategies in Acute Type A Aortic Dissection
- 1.Hysi I, Juthier F, Fabre O, et al. Aortic root surgery improves long-term survival after acute type A aortic dissection. Int J Cardiol. 2015;184:285–290. [DOI] [PubMed] [Google Scholar]
- 2.Peterss S, Dumfarth J, Rizzo JA, et al. Sparing the aortic root in acute aortic dissection type A: risk reduction and restored integrity of the untouched root. Eur J Cardiothorac Surg. 2016;50:232–239. [DOI] [PubMed] [Google Scholar]
- 3.Chen SK, Qiu ZH, Fang GH, et al. Reported outcomes after aortic valve resuspension for acute type A aortic dissection: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2019;29:331–338. [DOI] [PubMed] [Google Scholar]
- 4.Saczkowski R, Malas T, Mesana T, et al. Aortic valve preservation and repair in acute type A aortic dissection. Eur J Cardiothorac Surg. 2014;45:e220–e226. [DOI] [PubMed] [Google Scholar]
- 5.Qiu J, Wu J, Xie E, et al. Surgical management and outcomes of the aortic root in acute type A aortic dissection. Ann Thorac Surg. 2020;110:136–143. [DOI] [PubMed] [Google Scholar]
- 6.Vendramin I, Lechiancole A, Piani D, et al. Type A acute aortic dissection with ≥40-mm aortic root: results of conservative and replacement strategies at long-term follow-up. Eur J Cardiothorac Surg. 2021;59:1115–1122. [DOI] [PubMed] [Google Scholar]
- 7.Di Eusanio M, Trimarchi S, Peterson MD, et al. Root replacement surgery versus more conservative management during type A acute aortic dissection repair. Ann Thorac Surg. 2014;98:2078–2084. [DOI] [PubMed] [Google Scholar]
- 8.Yang B, Norton EL, Hobbs R, et al. Short- and long-term outcomes of aortic root repair and replacement in patients undergoing acute type A aortic dissection repair: twenty-year experience. J Thorac Cardiovasc Surg. 2019;157:2125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malaisrie SC, Szeto WY, Halas M, et al. 2021 The American Association for Thoracic Surgery expert consensus document: surgical treatment of acute type A aortic dissection. J Thorac Cardiovasc Surg. 2021;162:735–758.e2. [DOI] [PubMed] [Google Scholar]
- 10.Beckmann E, Martens A, Pertz J, et al. Valve-sparing David I procedure in acute aortic type A dissection: a 20-year experience with more than 100 patients. Eur J Cardiothorac Surg. 2017;52:319–324. [DOI] [PubMed] [Google Scholar]
- 11.Mosbahi S, Stak D, Gravestock I, et al. A systemic review and meta-analysis: Bentall versus David procedure in acute type A aortic dissection. Eur J Cardiothorac Surg. 2019;55:201–209. [DOI] [PubMed] [Google Scholar]
- 12.Geirsson A, Shioda K, Olsson C, et al. Differential outcomes of open and clamp-on distal anastomosis techniques in acute type A aortic dissection. J Thorac Cardiovasc Surg. 2019;157:1750–1758. [DOI] [PubMed] [Google Scholar]
- 13.Malvindi PG, Modi A, Miskolczi S, et al. Open and closed distal anastomosis for acute type A aortic dissection repair. Interact Cardiovasc Thorac Surg. 2016;22:776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawton JS, Liu J, Kulshrestha K, et al. The impact of surgical strategy on survival after repair of type A aortic dissection. J Thorac Cardiovasc Surg. 2015;150:294–301.e291. [DOI] [PubMed] [Google Scholar]
- 15.Rampoldi V, Trimarchi S, Eagle KA, et al. Simple risk models to predict surgical mortality in acute type A aortic dissection: the International Registry of Acute Aortic Dissection score. Ann Thorac Surg. 2007;83:55–61. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh WC, Kan CD, Yu HC, et al. Ascending aorta replacement vs. total aortic arch replacement in the treatment of acute type A dissection: a meta-analysis. Eur Rev Med Pharmacol Sci. 2019;23:9590–9611. [DOI] [PubMed] [Google Scholar]
- 17.Poon SS, Theologou T, Harrington D, et al. Hemiarch versus total aortic arch replacement in acute type A dissection: a systematic review and meta-analysis. Ann Cardiothorac Surg. 2016;5:156–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan Y, Xu L, Zhang H, et al. Proximal aortic repair versus extensive aortic repair in the treatment of acute type A aortic dissection: a meta-analysis. Eur J Cardiothorac Surg. 2016;49:1392–1401. [DOI] [PubMed] [Google Scholar]
- 19.Preventza O, Cervera R, Cooley DA, et al. Acute type I aortic dissection: traditional versus hybrid repair with antegrade stent delivery to the descending thoracic aorta. J Thorac Cardiovasc Surg. 2014;148:119–125. [DOI] [PubMed] [Google Scholar]
- 20.Roselli EE, Idrees JJ, Bakaeen FG, et al. Evolution of Simplified Frozen Elephant Trunk Repair for Acute DeBakey Type I Dissection: Midterm Outcomes. Ann Thorac Surg. 2018;105:749–755. [DOI] [PubMed] [Google Scholar]
- 21.Benedetto U, Mohamed H, Vitulli P, et al. Axillary versus femoral arterial cannulation in type A acute aortic dissection: evidence from a meta-analysis of comparative studies and adjusted risk estimates. Eur J Cardiothorac Surg. 2015;48:953–959. [DOI] [PubMed] [Google Scholar]
- 22.Ren Z, Wang Z, Hu R, et al. Which cannulation (axillary cannulation or femoral cannulation) is better for acute type A aortic dissection repair? A meta-analysis of nine clinical studies. Eur J Cardiothorac Surg. 2015;47:408–415. [DOI] [PubMed] [Google Scholar]
- 23.Wiedemann D, Kocher A, Dorfmeister M, et al. Effect of cerebral protection strategy on outcome of patients with Stanford type A aortic dissection. J Thorac Cardiovasc Surg. 2013;146:647–655.e641. [DOI] [PubMed] [Google Scholar]
- 24.Preventza O, Simpson KH, Cooley DA, et al. Unilateral versus bilateral cerebral perfusion for acute type A aortic dissection. Ann Thorac Surg. 2015;99:80–87. [DOI] [PubMed] [Google Scholar]
- 25.Samanidis G, Kanakis M, Khoury M, et al. Antegrade and retrograde cerebral perfusion during acute type A aortic dissection repair in 290 patients. Heart Lung Circ. 2021;30:1075–1083. [DOI] [PubMed] [Google Scholar]
- 26.Jormalainen M, Raivio P, Mustonen C, et al. Direct Aortic Versus Peripheral Arterial Cannulation in Surgery for Type A Aortic Dissection. Ann Thorac Surg. 2020;110:1251–1258. [DOI] [PubMed] [Google Scholar]
- 27.Kreibich M, Chen Z, Rylski B, et al. Outcome after aortic, axillary, or femoral cannulation for acute type A aortic dissection. J Thorac Cardiovasc Surg. 2019;158:27–34.e29. [DOI] [PubMed] [Google Scholar]
- 28.Preventza O, Bakaeen FG, Stephens EH, et al. Innominate artery cannulation: an alternative to femoral or axillary cannulation for arterial inflow in proximal aortic surgery. J Thorac Cardiovasc Surg. 2013;145:S191–S196. [DOI] [PubMed] [Google Scholar]
- 29.Reece TB, Tribble CG, Smith RL, et al. Central cannulation is safe in acute aortic dissection repair. J Thorac Cardiovasc Surg. 2007;133:428–434. [DOI] [PubMed] [Google Scholar]
- 30.Khaladj N, Shrestha M, Peterss S, et al. Ascending aortic cannulation in acute aortic dissection type A: the Hannover experience. Eur J Cardiothorac Surg. 2008;34:792–796; discussion 796. [DOI] [PubMed] [Google Scholar]
- 31.Frederick JR, Yang E, Trubelja A, et al. Ascending aortic cannulation in acute type A dissection repair. Ann Thorac Surg. 2013;95:1808–1811. [DOI] [PubMed] [Google Scholar]
- 32.Zierer A, Voeller RK, Hill KE, et al. Aortic enlargement and late reoperation after repair of acute type A aortic dissection. Ann Thorac Surg. 2007;84:479–486; discussion 486–487. [DOI] [PubMed] [Google Scholar]
- 33.Wu J, Huang Y, Qiu J, et al. Is valve-sparing root replacement a safe option in acute type A aortic dissection? A systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2019;29:766–775. [DOI] [PubMed] [Google Scholar]
- 34.Moon MR, Sundt TM 3rd, Pasque MK, et al. Does the extent of proximal or distal resection influence outcome for type A dissections? Ann Thorac Surg. 2001;71:1244–1249; discussion 1249–1250. [DOI] [PubMed] [Google Scholar]
- 35.Preventza O, Olive JK, Liao JL, et al. Acute type I aortic dissection with or without antegrade stent delivery: mid-term outcomes. J Thorac Cardiovasc Surg. 2019;158:1273–1281. [DOI] [PubMed] [Google Scholar]
- 36.Takagi H, Umemoto T. A meta-analysis of total arch replacement with frozen elephant trunk in acute type A aortic dissection. Vasc Endovascular Surg. 2016;50:33–46. [DOI] [PubMed] [Google Scholar]
- 37.Vallabhajosyula P, Szeto WY, Pulsipher A, et al. Antegrade thoracic stent grafting during repair of acute Debakey type I dissection promotes distal aortic remodeling and reduces late open distal reoperation rate. J Thorac Cardiovasc Surg. 2014;147:942–948. [DOI] [PubMed] [Google Scholar]
- 38.Chen LW, Lu L, Dai XF, et al. Total arch repair with open triple-branched stent graft placement for acute type A aortic dissection: experience with 122 patients. J Thorac Cardiovasc Surg. 2014;148:521–528. [DOI] [PubMed] [Google Scholar]
- 39.Rylski B, Perez M, Beyersdorf F, et al. Acute non-A non-B aortic dissection: incidence, treatment and outcome. Eur J Cardiothorac Surg. 2017;52:1111–1117. [DOI] [PubMed] [Google Scholar]
- 40.Takagi H, Umemoto T. A Meta-Analysis of the Association of Chronic Obstructive Pulmonary Disease with Abdominal Aortic Aneurysm Presence. Ann Vasc Surg. 2016;34:84–94. [DOI] [PubMed] [Google Scholar]
- 41.Chiu P, Trojan J, Tsou S, et al. Limited root repair in acute type A aortic dissection is safe but results in increased risk of reoperation. J Thorac Cardiovasc Surg. 2018;155:1–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halstead JC, Spielvogel D, Meier DM, et al. Composite aortic root replacement in acute type A dissection: time to rethink the indications? Eur J Cardiothorac Surg. 2005;27:626–632; discussion 632–633. [DOI] [PubMed] [Google Scholar]
- 43.Geirsson A, Szeto WY, Pochettino A, et al. Significance of malperfusion syndromes prior to contemporary surgical repair for acute type A dissection: outcomes and need for additional revascularizations. Eur J Cardiothorac Surg. 2007;32:255–262. [DOI] [PubMed] [Google Scholar]
- 44.Castrovinci S, Pacini D, Di Marco L, et al. Surgical management of aortic root in type A acute aortic dissection: a propensity-score analysis. Eur J Cardiothorac Surg. 2016;50:223–229. [DOI] [PubMed] [Google Scholar]
- 45.Nishida H, Tabata M, Fukui T, et al. Surgical strategy and outcome for aortic root in patients undergoing repair of acute type A aortic dissection. Ann Thorac Surg. 2016;101:1464–1469. [DOI] [PubMed] [Google Scholar]
- 46.Ergin MA, McCullough J, Galla JD, et al. Radical replacement of the aortic root in acute type A dissection: indications and outcome. Eur J Cardiothorac Surg. 1996;10:840–844; discussion 845. [DOI] [PubMed] [Google Scholar]
- 47.Fichadiya A, Gregory AJ, Kotha VK, et al. Extended-arch repair for acute type-A aortic dissection: perioperative and mid-term results. Eur J Cardiothorac Surg. 2019;56:714–721. [DOI] [PubMed] [Google Scholar]
- 48.Kazui T, Yamashita K, Washiyama N, et al. Impact of an aggressive surgical approach on surgical outcome in type A aortic dissection. Ann Thorac Surg. 2002;74:S1844–S1847;discussion S1857–S1863. [DOI] [PubMed] [Google Scholar]
- 49.Easo J, Weigang E, Holzl PP, et al. Influence of operative strategy for the aortic arch in DeBakey type I aortic dissection: analysis of the German Registry for Acute Aortic Dissection Type A. J Thorac Cardiovasc Surg. 2012;144:617–623. [DOI] [PubMed] [Google Scholar]
- 50.Helder MRK, Schaff HV, Day CN, et al. Regional and temporal trends in the outcomes of repairs for acute type A aortic dissections. Ann Thorac Surg. 2020;109:26–33. [DOI] [PubMed] [Google Scholar]
- 51.Lin HH, Liao SF, Wu CF, et al. Outcome of frozen elephant trunk technique for acute type A aortic dissection: as systematic review and meta-analysis. Medicine (Baltimore). 2015;94:e694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith HN, Boodhwani M, Ouzounian M, et al. Classification and outcomes of extended arch repair for acute type A aortic dissection: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2017;24:450–459. [DOI] [PubMed] [Google Scholar]
- 53.Preventza O, Liao JL, Olive JK, et al. Neurologic complications after the frozen elephant trunk procedure: a meta-analysis of more than 3000 patients. J Thorac Cardiovasc Surg. 2020;160:20–33.e4. [DOI] [PubMed] [Google Scholar]
- 54.Tong G, Zhang B, Zhou X, et al. Bilateral versus unilateral antegrade cerebral perfusion in total arch replacement for type A aortic dissection. J Thorac Cardiovasc Surg. 2017;154:767–775. [DOI] [PubMed] [Google Scholar]
- 55.Liu Z, Wang C, Zhang X, et al. Effect of different types of cerebral perfusion for acute type A aortic dissection undergoing aortic arch procedure, unilateral versus bilateral. BMC Surg. 2020;20:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Angleitner P, Stelzmueller ME, Mahr S, et al. Bilateral or unilateral antegrade cerebral perfusion during surgery for acute type A dissection. J Thorac Cardiovasc Surg. 2020;159:2159–2167.e2152. [DOI] [PubMed] [Google Scholar]
- 57.Tiwari KK, Murzi M, Bevilacqua S, et al. Which cannulation (ascending aortic cannulation or peripheral arterial cannulation) is better for acute type A aortic dissection surgery? Interact Cardiovasc Thorac Surg. 2010;10:797–802. [DOI] [PubMed] [Google Scholar]
- 58.Preventza O, Garcia A, Tuluca A, et al. Innominate artery cannulation for proximal aortic surgery: outcomes and neurological events in 263 patients. Eur J Cardiothorac Surg. 2015;48:937–942; discussion 942. [DOI] [PubMed] [Google Scholar]
- 59.Payabyab EC, Hemli JM, Mattia A, et al. The use of innominate artery cannulation for antegrade cerebral perfusion in aortic dissection. J Cardiothorac Surg. 2020;15:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harky A, Wong CHM, Chan JSK, et al. Innominate artery cannulation in aortic surgery: a systematic review. J Card Surg. 2018;33:818–825. [DOI] [PubMed] [Google Scholar]
7.4.2. Management of Acute Type B Aortic Dissection
- 1.Suzuki T, Mehta RH, Ince H, et al. Clinical profiles and outcomes of acute type B aortic dissection in the current era: lessons from the International Registry of Aortic Dissection (IRAD). Circulation. 2003;108 (Suppl 1):II312–317. [DOI] [PubMed] [Google Scholar]
- 2.Estrera AL, Miller CC 3rd, Safi HJ, et al. Outcomes of medical management of acute type B aortic dissection. Circulation. 2006;114:I384–I389. [DOI] [PubMed] [Google Scholar]
- 3.Umana JP, Lai DT, Mitchell RS, et al. Is medical therapy still the optimal treatment strategy for patients with acute type B aortic dissections? J Thorac Cardiovasc Surg. 2002;124:896–910. [DOI] [PubMed] [Google Scholar]
- 4.Cambria RP, Conrad MF, Matsumoto AH, et al. Multicenter clinical trial of the conformable stent graft for the treatment of acute, complicated type B dissection. J Vasc Surg. 2015;62:271–278. [DOI] [PubMed] [Google Scholar]
- 5.The VIRTUE Registry Investigators. Mid-term outcomes and aortic remodeling after thoracic endovascular repair for acute, subacute, and chronic aortic dissection: the VIRTUE Registry. Eur J Vasc Endovasc Surg. 2014;48:363–371. [DOI] [PubMed] [Google Scholar]
- 6.Zipfel B, Czerny M, Funovics M, et al. Endovascular treatment of patients with types A and B thoracic aortic dissection using Relay thoracic stent-grafts: results from the RESTORE Patient Registry. J Endovasc Ther. 2011;18:131–143. [DOI] [PubMed] [Google Scholar]
- 7.Norton EL, Williams DM, Kim KM, et al. Management of acute type B aortic dissection with malperfusion via endovascular fenestration/stenting. J Thorac Cardiovasc Surg. 2020;160:1151–1161.e1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunkwall J, Kasprzak P, Verhoeven E, et al. Endovascular repair of acute uncomplicated aortic type B dissection promotes aortic remodelling: 1 year results of the ADSORB trial. Eur J Vasc Endovasc Surg. 2014;48:285–291. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz SI, Durham C, Clouse WD, et al. Predictors of late aortic intervention in patients with medically treated type B aortic dissection. J Vasc Surg. 2018;67:78–84. [DOI] [PubMed] [Google Scholar]
- 10.Nienaber CA, Kische S, Akin I, et al. Strategies for subacute/chronic type B aortic dissection: the Investigation Of Stent Grafts in Patients with type B Aortic Dissection (INSTEAD) trial 1-year outcome. J Thorac Cardiovasc Surg. 2010;140(suppl 6):S101–S108; discussion S142–S146. [DOI] [PubMed] [Google Scholar]
- 11.Cambria RP, Crawford RS, Cho JS, et al. A multicenter clinical trial of endovascular stent graft repair of acute catastrophes of the descending thoracic aorta. J Vasc Surg. 2009;50:1255–1264.e1-4. [DOI] [PubMed] [Google Scholar]
- 12.Sachs T, Pomposelli F, Hagberg R, et al. Open and endovascular repair of type B aortic dissection in the Nationwide Inpatient Sample. J Vasc Surg. 2010;52:860–866; discussion 866. [DOI] [PubMed] [Google Scholar]
- 13.Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;6:407–416. [DOI] [PubMed] [Google Scholar]
- 14.Torrent DJ, McFarland GE, Wang G, et al. Timing of thoracic endovascular aortic repair for uncomplicated acute type B aortic dissection and the association with complications. J Vasc Surg. 2021;73:826–835. [DOI] [PubMed] [Google Scholar]
- 15.Trimarchi S, Eagle KA, Nienaber CA, et al. Importance of refractory pain and hypertension in acute type B aortic dissection. Insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2010;122:1283–1289. [DOI] [PubMed] [Google Scholar]
7.5. Management of IMH
- 1.Harris KM, Braverman AC, Eagle KA, et al. Acute aortic intramural hematoma: an analysis from the International Registry of Acute Aortic Dissection. Circulation. 2012;126:S91–S96. [DOI] [PubMed] [Google Scholar]
- 2.Tolenaar JL, Harris KM, Upchurch GR Jr, et al. The differences and similarities between intramural hematoma of the descending aorta and acute type B dissection. J Vasc Surg. 2013;58:1498–1504. [DOI] [PubMed] [Google Scholar]
- 3.Chou AS, Ziganshin BA, Charilaou P, et al. Long-term behavior of aortic intramural hematomas and penetrating ulcers. J Thorac Cardiovasc Surg. 2016;151:361–373.e1. [DOI] [PubMed] [Google Scholar]
- 4.Hata M, Hata H, Sezai A, et al. Optimal treatment strategy for type A acute aortic dissection with intramural hematoma. J Thorac Cardiovasc Surg. 2014;147:307–311. [DOI] [PubMed] [Google Scholar]
- 5.Matsushita A, Fukui T, Tabata M, et al. Preoperative characteristics and surgical outcomes of acute intramural hematoma involving the ascending aorta: a propensity score-matched analysis. J Thorac Cardiovasc Surg. 2016;151:351–358. [DOI] [PubMed] [Google Scholar]
- 6.Sandhu HK, Tanaka A, Charlton-Ouw KM, et al. Outcomes and management of type A intramural hematoma. Ann Cardiothorac Surg. 2016;5:317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi YJ, Son JW, Lee SH, et al. Treatment patterns and their outcomes of acute aortic intramural hematoma in real world: multicenter registry for aortic intramural hematoma. BMC Cardiovasc Disord. 2014;14:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wee I, Varughese RS, Syn N, et al. Non-operative management of type A acute aortic syndromes: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2019;58:41–51. [DOI] [PubMed] [Google Scholar]
- 9.Chow SCY, Wong RHL, Lakhani I, et al. Management of acute type A intramural hematoma: upfront surgery or individualized approach? A retrospective analysis and meta-analysis. J Thorac Dis. 2020;12:680–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Y, Dun Y, Guo H, et al. Clinical features and surgical outcomes of type A intramural hematoma. J Thorac Dis. 2020;12:3964–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitamura T, Torii S, Miyamoto T, et al. Watch-and-wait strategy for type A intramural haematoma and acute aortic dissection with thrombosed false lumen of the ascending aorta: a Japanese single-centre experience. Eur J Cardiothorac Surg. 2020;58:590–597. [DOI] [PubMed] [Google Scholar]
- 12.Song JK, Yim JH, Ahn JM, et al. Outcomes of patients with acute type A aortic intramural hematoma. Circulation. 2009;120:2046–2052. [DOI] [PubMed] [Google Scholar]
- 13.Schoenhoff FS, Zanchin C, Czerny M, et al. Aorta Related and All-cause Mortality in Patients with Aortic Intramural Haematoma. Eur J Vasc Endovasc Surg. 2017;54:447–453. [DOI] [PubMed] [Google Scholar]
- 14.Chakos A, Twindyawardhani T, Evangelista A, et al. Endovascular versus medical management of type B intramural hematoma: a meta-analysis. Ann Cardiothorac Surg. 2019;8:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moral S, Cuellar H, Avegliano G, et al. Clinical Implications of Focal Intimal Disruption in Patients With Type B Intramural Hematoma. Journal of the American College of Cardiology (JACC). 2017;69:28–39. [DOI] [PubMed] [Google Scholar]
- 16.Moral S, Ballesteros E, Roque M, et al. Intimal disruption in type B aortic intramural hematoma. Does size matter? A systematic review and meta-analysis. Int J Cardiol. 2018;269:298–303. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Jiao Y, Zou J, et al. Thoracic endovascular aortic repair versus best medical treatment for high-risk type B intramural hematoma: a systematic review of clinical studies. Ann Vasc Surg. 2018;52:273–279. [DOI] [PubMed] [Google Scholar]
- 18.Kageyama S, Mitake H, Nakajima A, et al. A novel risk score on admission for predicting death or need for surgery in patients with acute type A intramural hematoma receiving medical therapy. Heart Vessels. 2020;35:1164–1170. [DOI] [PubMed] [Google Scholar]
- 19.Ganaha F, Miller DC, Sugimoto K, et al. Prognosis of aortic intramural hematoma with and without penetrating atherosclerotic ulcer: a clinical and radiological analysis. Circulation. 2002;106:342–348. [DOI] [PubMed] [Google Scholar]
- 20.Evangelista A, Dominguez R, Sebastia C, et al. Prognostic value of clinical and morphologic findings in short-term evolution of aortic intramural haematoma. Therapeutic implications. Eur Heart J. 2004;25:81–87. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Chen Y, Guo J, et al. Prediction of adverse events in patients with initially medically treated type A intramural hematoma. Int J Cardiol. 2020;313:114–120. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Lu B, Chen Y, et al. Acute type B aortic intramural hematoma: the added prognostic value of a follow-up CT. Eur Radiol. 2019;29:6571–6580. [DOI] [PubMed] [Google Scholar]
7.6.1. PAU With IMH, Rupture, or Both
- 1.Ganaha F, Miller DC, Sugimoto K, et al. Prognosis of aortic intramural hematoma with and without penetrating atherosclerotic ulcer: a clinical and radiological analysis. Circulation. 2002;106:342–348. [DOI] [PubMed] [Google Scholar]
- 2.Tittle SL, Lynch RJ, Cole PE, et al. Midterm follow-up of penetrating ulcer and intramural hematoma of the aorta. J Thorac Cardiovasc Surg. 2002;123:1051–1059. [DOI] [PubMed] [Google Scholar]
- 3.Chou AS, Ziganshin BA, Charilaou P, et al. Long-term behavior of aortic intramural hematomas and penetrating ulcers. J Thorac Cardiovasc Surg. 2016;151:361–373.e1. [DOI] [PubMed] [Google Scholar]
- 4.Georgiadis GS, Antoniou GA, Georgakarakos EI, et al. Surgical or endovascular therapy of abdominal penetrating aortic ulcers and their natural history: a systematic review. J Vasc Interv Radiol. 2013;24:1437–1449.e1433. [DOI] [PubMed] [Google Scholar]
- 5.Nathan DP, Boonn W, Lai E, et al. Presentation, complications, and natural history of penetrating atherosclerotic ulcer disease. J Vasc Surg. 2012;55:10–15. [DOI] [PubMed] [Google Scholar]
- 6.Cho KR, Stanson AW, Potter DD, et al. Penetrating atherosclerotic ulcer of the descending thoracic aorta and arch. J Thorac Cardiovasc Surg. 2004;127:1393–1399; discussion 1399–1401. [DOI] [PubMed] [Google Scholar]
- 7.Kotsis T, Spyropoulos BG, Asaloumidis N, et al. Penetrating atherosclerotic ulcers of the abdominal aorta: a case report and review of the literature. Vasc Spec Int. 2019;35:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
7.6.2. Isolated PAU
- 1.Ganaha F, Miller DC, Sugimoto K, et al. Prognosis of aortic intramural hematoma with and without penetrating atherosclerotic ulcer: a clinical and radiological analysis. Circulation. 2002;106:342–348. [DOI] [PubMed] [Google Scholar]
- 2.Tittle SL, Lynch RJ, Cole PE, et al. Midterm follow-up of penetrating ulcer and intramural hematoma of the aorta. J Thorac Cardiovasc Surg. 2002;123:1051–1059. [DOI] [PubMed] [Google Scholar]
- 3.Chou AS, Ziganshin BA, Charilaou P, et al. Long-term behavior of aortic intramural hematomas and penetrating ulcers. J Thorac Cardiovasc Surg. 2016;151:361–373.e1. [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Zhang QY, Wang XZ, et al. Long-term imaging evolution and clinical prognosis among patients with acute penetrating aortic ulcers: a retrospective observational study. J Am Heart Assoc. 2020;9:e014505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan DP, Boonn W, Lai E, et al. Presentation, complications, and natural history of penetrating atherosclerotic ulcer disease. J Vasc Surg. 2012;55:10–15. [DOI] [PubMed] [Google Scholar]
- 6.Georgiadis GS, Antoniou GA, Georgakarakos EI, et al. Surgical or endovascular therapy of abdominal penetrating aortic ulcers and their natural history: a systematic review. J Vasc Interv Radiol. 2013;24:1437–1449.e1433. [DOI] [PubMed] [Google Scholar]
- 7.Salim S, Locci R, Martin G, et al. Short- and long-term outcomes in isolated penetrating aortic ulcer disease. J Vasc Surg. 2020;72:84–91. [DOI] [PubMed] [Google Scholar]
- 8.D’Annoville T, Ozdemir BA, Alric P, et al. ; Thoracic endovascular aortic repair for penetrating aortic ulcer: literature review. Ann Thorac Surg. 2016;101:2272–2278. [DOI] [PubMed] [Google Scholar]
- 9.Cho KR, Stanson AW, Potter DD, et al. Penetrating atherosclerotic ulcer of the descending thoracic aorta and arch. J Thorac Cardiovasc Surg. 2004;127:1393–1399; discussion 1399–1401. [DOI] [PubMed] [Google Scholar]
- 10.DeCarlo C, Latz CA, Boitano LT, et al. Prognostication of asymptomatic penetrating aortic ulcers: a modern approach. Circulation. 2021;144:1091–1101. [DOI] [PubMed] [Google Scholar]
- 11.Gifford SM, Duncan AA, Greiten LE, et al. The natural history and outcomes for thoracic and abdominal penetrating aortic ulcers. J Vasc Surg. 2016;63:1182–1188. [DOI] [PubMed] [Google Scholar]
7.6.3. PAU Open Surgical Repair Versus Endovascular Repair
- 1.Amis ES Jr, Butler PF, American College of Radiology. ACR white paper on radiation dose in medicine: three years later. J Am Coll Radiol. 2010;7:865–870. [DOI] [PubMed] [Google Scholar]
- 2.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. [DOI] [PubMed] [Google Scholar]
- 3.Costello JE, Cecava ND, Tucker JE, et al. CT radiation dose: current controversies and dose reduction strategies. AJR Am J Roentgenol. 2013;201:1283–1290. [DOI] [PubMed] [Google Scholar]
- 4.Georgiadis GS, Antoniou GA, Georgakarakos EI, et al. Surgical or endovascular therapy of abdominal penetrating aortic ulcers and their natural history: a systematic review. J Vasc Interv Radiol. 2013;24:1437–1449.e1433. [DOI] [PubMed] [Google Scholar]
- 5.Georgiadis GS, Trellopoulos G, Antoniou GA, et al. Endovascular therapy for penetrating ulcers of the infrarenal aorta. ANZ J Surg. 2013;83:758–763. [DOI] [PubMed] [Google Scholar]
- 6.Salim S, Locci R, Martin G, et al. Short- and long-term outcomes in isolated penetrating aortic ulcer disease. J Vasc Surg. 2020;72:84–91. [DOI] [PubMed] [Google Scholar]
- 7.D’Annoville T, Ozdemir BA, Alric P, et al. ; Thoracic endovascular aortic repair for penetrating aortic ulcer: literature review. Ann Thorac Surg. 2016;101:2272–2278. [DOI] [PubMed] [Google Scholar]
- 8.Ganaha F, Miller DC, Sugimoto K, et al. Prognosis of aortic intramural hematoma with and without penetrating atherosclerotic ulcer: a clinical and radiological analysis. Circulation. 2002;106:342–348. [DOI] [PubMed] [Google Scholar]
- 9.Tittle SL, Lynch RJ, Cole PE, et al. Midterm follow-up of penetrating ulcer and intramural hematoma of the aorta. J Thorac Cardiovasc Surg. 2002;123:1051–1059. [DOI] [PubMed] [Google Scholar]
- 10.Chou AS, Ziganshin BA, Charilaou P, et al. Long-term behavior of aortic intramural hematomas and penetrating ulcers. J Thorac Cardiovasc Surg. 2016;151:361–373.e1. [DOI] [PubMed] [Google Scholar]
- 11.Troxler M, Mavor AI, Homer-Vanniasinkam S. Penetrating atherosclerotic ulcers of the aorta. Br J Surg. 2001;88:1169–1177. [DOI] [PubMed] [Google Scholar]
- 12.Pape LA, Awais M, Woznicki EM, et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol. 2015;66:350–358. [DOI] [PubMed] [Google Scholar]
- 13.Harris KM, Braverman AC, Eagle KA, et al. Acute aortic intramural hematoma: an analysis from the International Registry of Acute Aortic Dissection. Circulation. 2012;126:S91–S96. [DOI] [PubMed] [Google Scholar]
7.7.1.1. Initial Management of BTTAI in the Emergency Department
- 1.Pate JW, Gavant ML, Weiman DS, et al. Traumatic rupture of the aortic isthmus: program of selective management. World J Surg. 1999;23:59–63. [DOI] [PubMed] [Google Scholar]
- 2.Fabian TC, Davis KA, Gavant ML, et al. Prospective study of blunt aortic injury: helical CT is diagnostic and antihypertensive therapy reduces rupture. Ann Surg. 1998;227:666–676; discussion 676–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scalea TM, Feliciano DV, DuBose JJ, et al. Blunt Thoracic Aortic Injury: Endovascular Repair Is Now the Standard. J Am Coll Surg. 2019;228:605–610. [DOI] [PubMed] [Google Scholar]
- 4.Fabian TC, Richardson JD, Croce MA, et al. Prospective study of blunt aortic injury: Multicenter Trial of the American Association for the Surgery of Trauma. J Trauma. 1997;42:374–380; discussion 380–383. [DOI] [PubMed] [Google Scholar]
- 5.Gombert A, Barbati ME, Storck M, et al. Treatment of blunt thoracic aortic injury in Germany-assessment of the TraumaRegister DGU®. PLoS One. 2017;12:e0171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee WA, Matsumura JS, Mitchell RS, et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53:187–192. [DOI] [PubMed] [Google Scholar]
- 7.Marcaccio CL, Dumas RP, Huang Y, et al. Delayed endovascular aortic repair is associated with reduced in-hospital mortality in patients with blunt thoracic aortic injury. J Vasc Surg. 2018;68:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demetriades D, Velmahos GC, Scalea TM, et al. Blunt traumatic thoracic aortic injuries: early or delayed repair-results of an American Association for the Surgery of Trauma prospective study. J Trauma. 2009;66:967–973. [DOI] [PubMed] [Google Scholar]
7.7.1.2. Approach to the Initial Management of BTTAI
- 1.Lee WA, Matsumura JS, Mitchell RS, et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53:187–192. [DOI] [PubMed] [Google Scholar]
- 2.Azizzadeh A, Keyhani K, Miller CC 3rd, et al. Blunt traumatic aortic injury: initial experience with endovascular repair. J Vasc Surg. 2009;49:1403–1408. [DOI] [PubMed] [Google Scholar]
- 3.DuBose JJ, Leake SS, Brenner M, et al. Contemporary management and outcomes of blunt thoracic aortic injury: a multicenter retrospective study. J Trauma Acute Care Surg. 2015;78:360–369. [DOI] [PubMed] [Google Scholar]
- 4.Fortuna GR Jr, Perlick A, DuBose JJ, et al. Injury grade is a predictor of aortic-related death among patients with blunt thoracic aortic injury. J Vasc Surg. 2016;63:1225–1231. [DOI] [PubMed] [Google Scholar]
- 5.Grigorian A, Spencer D, Donayre C, et al. National trends of thoracic endovascular aortic repair versus open repair in blunt thoracic aortic injury. Ann Vasc Surg. 2018;52:72–78. [DOI] [PubMed] [Google Scholar]
- 6.Rabin J, DuBose J, Sliker CW, et al. Parameters for successful nonoperative management of traumatic aortic injury. J Thorac Cardiovasc Surg. 2014;147:143–149. [DOI] [PubMed] [Google Scholar]
- 7.Starnes BW, Lundgren RS, Gunn M, et al. A new classification scheme for treating blunt aortic injury. J Vasc Surg. 2012;55:47–54. [DOI] [PubMed] [Google Scholar]
- 8.Harris DG, Rabin J, Starnes BW, et al. Evolution of lesion-specific management of blunt thoracic aortic injury. J Vasc Surg. 2016;64:500–505. [DOI] [PubMed] [Google Scholar]
- 9.Gavant ML. Helical CT grading of traumatic aortic injuries. Impact on clinical guidelines for medical and surgical management. Radiol Clin North Am. 1999;37:553–574, vi. [DOI] [PubMed] [Google Scholar]
7.7.1.3. Endovascular Versus Open Surgical Repair
- 1.Xenos ES, Abedi NN, Davenport DL, et al. Meta-analysis of endovascular vs open repair for traumatic descending thoracic aortic rupture. J Vasc Surg. 2008;48:1343–1351. [DOI] [PubMed] [Google Scholar]
- 2.Grigorian A, Spencer D, Donayre C, et al. National trends of thoracic endovascular aortic repair versus open repair in blunt thoracic aortic injury. Ann Vasc Surg. 2018;52:72–78. [DOI] [PubMed] [Google Scholar]
- 3.Murad MH, Rizvi AZ, Malgor R, et al. Comparative effectiveness of the treatments for thoracic aortic transection [corrected]. J Vasc Surg. 2011;53:193–199.e1–21. [DOI] [PubMed] [Google Scholar]
- 4.Pang D, Hildebrand D, Bachoo P. Thoracic endovascular repair (TEVAR) versus open surgery for blunt traumatic thoracic aortic injury. Cochrane Database Syst Rev. 2019;CD006642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenel-Pierre S, Ramos Duran E, Abi-Chaker A, et al. The role of heparin in endovascular repair of blunt thoracic aortic injury. J Vasc Surg. 2019;70:1809–1815. [DOI] [PubMed] [Google Scholar]
- 6.Scalea TM, Feliciano DV, DuBose JJ, et al. Blunt Thoracic Aortic Injury: Endovascular Repair Is Now the Standard. J Am Coll Surg. 2019;228:605–610. [DOI] [PubMed] [Google Scholar]
7.7.2. Initial Management of Blunt Traumatic Abdominal Aortic Injury (BAAI)
- 1.Shalhub S, Starnes BW, Brenner ML, et al. Blunt abdominal aortic injury: a Western Trauma Association multicenter study. J Trauma Acute Care Surg. 2014;77:879–885; discussion 885. [DOI] [PubMed] [Google Scholar]
- 2.Shalhub S, Starnes BW, Tran NT, et al. Blunt abdominal aortic injury. J Vasc Surg. 2012;55:1277–1285. [DOI] [PubMed] [Google Scholar]
- 3.Sheehan BM, Grigorian A, de Virgilio C, et al. Predictors of blunt abdominal aortic injury in trauma patients and mortality analysis. J Vasc Surg. 2020;71:1858–1866. [DOI] [PubMed] [Google Scholar]
- 4.de Mestral C, Dueck AD, Gomez D, et al. Associated injuries, management, and outcomes of blunt abdominal aortic injury. J Vasc Surg. 2012;56:656–660. [DOI] [PubMed] [Google Scholar]
- 5.Borovcanin Z Anesthesia for abdominal major vascular injury. In: Papadakos PJ, Gestring ML, eds. Encyclopedia of Trauma Care. Springer; Berlin Heidelberg; 2015:15–20. [Google Scholar]
- 6.Norii T, Crandall C, Terasaka Y. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score-adjusted untreated patients. J Trauma Acute Care Surg. 2015;78:721–728. [DOI] [PubMed] [Google Scholar]
- 7.Joseph B, Zeeshan M, Sakran JV, et al. Nationwide analysis of resuscitative endovascular balloon occlusion of the aorta in civilian trauma. JAMA Surg. 2019;154:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manzano-Nunez R, Orlas CP, Herrera-Escobar JP, et al. A meta-analysis of the incidence of complications associated with groin access after the use of resuscitative endovascular balloon occlusion of the aorta in trauma patients. J Trauma Acute Care Surg. 2018;85:626–634. [DOI] [PubMed] [Google Scholar]
- 9.Ordonez CA, Rodriguez F, Parra M, et al. Resuscitative endovascular balloon of the aorta is feasible in penetrating chest trauma with major hemorrhage: proposal of a new institutional deployment algorithm. J Trauma Acute Care Surg. 2020;89:311–319. [DOI] [PubMed] [Google Scholar]
- 10.Darrabie MD, Croft CA, Brakenridge SC, et al. Resuscitative endovascular balloon occlusion of the aorta: implementation and preliminary results at an academic level I trauma center. J Am Coll Surg. 2018;227:127–133. [DOI] [PubMed] [Google Scholar]
- 11.Moore LJ, Martin CD, Harvin JA, et al. Resuscitative endovascular balloon occlusion of the aorta for control of noncompressible truncal hemorrhage in the abdomen and pelvis. Am J Surg. 2016;212:1222–1230. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto R, Cestero RF, Suzuki M, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA) is associated with improved survival in severely injured patients: a propensity score matching analysis. Am J Surg. 2019;218:1162–1168. [DOI] [PubMed] [Google Scholar]
- 13.Starnes BW, Lundgren RS, Gunn M, et al. A new classification scheme for treating blunt aortic injury. J Vasc Surg. 2012;55:47–54. [DOI] [PubMed] [Google Scholar]
- 14.Azizzadeh A, Keyhani K, Miller CC 3rd, et al. Blunt traumatic aortic injury: initial experience with endovascular repair. J Vasc Surg. 2009;49:1403–1408. [DOI] [PubMed] [Google Scholar]
7.7.3. Long-Term Management and Surveillance After Blunt Traumatic Aortic Injury (BTAI)
- 1.DuBose JJ, Leake SS, Brenner M, et al. Contemporary management and outcomes of blunt thoracic aortic injury: a multicenter retrospective study. J Trauma Acute Care Surg. 2015;78:360–369. [DOI] [PubMed] [Google Scholar]
- 2.Topcu AC, Ozeren-Topcu K, Bolukcu A, et al. Blunt traumatic aortic injury: 10-year single-center experience. Aorta (Stamford). 2020;8:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charlton-Ouw KM, DuBose JJ, Leake SS, et al. Observation may be safe in selected cases of blunt traumatic abdominal aortic injury. Ann Vasc Surg. 2016;30:34–39. [DOI] [PubMed] [Google Scholar]
- 4.Shalhub S, Starnes BW, Brenner ML, et al. Blunt abdominal aortic injury: a Western Trauma Association multicenter study. J Trauma Acute Care Surg. 2014;77:879–885; discussion 885. [DOI] [PubMed] [Google Scholar]
- 5.Jacob-Brassard J, Salata K, Kayssi A, et al. A systematic review of nonoperative management in blunt thoracic aortic injury. J Vasc Surg. 2019;70:1675–1681.e6. [DOI] [PubMed] [Google Scholar]
- 6.Lee WA, Matsumura JS, Mitchell RS, et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53:187–192. [DOI] [PubMed] [Google Scholar]
7.8.1. Long-Term Surveillance Imaging After Aortic Dissection and IMH
- 1.Aizawa K, Kawahito K, Misawa Y. Long-term outcomes of tear-oriented ascending/hemiarch replacements for acute type A aortic dissection. Gen Thorac Cardiovasc Surg. 2016;64:403–408. [DOI] [PubMed] [Google Scholar]
- 2.Bajona P, Quintana E, Schaff HV, et al. Aortic arch surgery after previous type A dissection repair: results up to 5 years. Interact Cardiovasc Thorac Surg. 2015;21:81–85; discussion 85–86. [DOI] [PubMed] [Google Scholar]
- 3.Kimura N, Itoh S, Yuri K, et al. Reoperation for enlargement of the distal aorta after initial surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg. 2015;149(suppl 2):S91–S98.e1. [DOI] [PubMed] [Google Scholar]
- 4.Arafat A, Roselli EE, Idrees JJ, et al. Stent grafting acute aortic dissection: comparison of DeBakey extent IIIA versus IIIB. Ann Thorac Surg. 2016;102:1473–1481. [DOI] [PubMed] [Google Scholar]
- 5.Stelzmueller ME, Nolz R, Mahr S, et al. Thoracic endovascular repair for acute complicated type B aortic dissections. J Vasc Surg. 2019;69:318–326. [DOI] [PubMed] [Google Scholar]
- 6.Huang W, Yang F, Luo J, et al. Outcomes and morphologic changes of immediate type IA endoleak following endovascular repair of acute type B aortic dissection. Ann Vasc Surg. 2015;29:174–182. [DOI] [PubMed] [Google Scholar]
- 7.Akin I, Kische S, Rehders TC, et al. Thoracic endovascular stent-graft therapy in aortic dissection. Curr Opin Cardiol. 2010;25:552–559. [DOI] [PubMed] [Google Scholar]
- 8.Halstead JC, Meier M, Etz C, et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg. 2007;133:127–135. [DOI] [PubMed] [Google Scholar]
- 9.Sievers HH, Richardt D, Diwoky M, et al. Survival and reoperation after valve-sparing root replacement and root repair in acute type A dissection. J Thorac Cardiovasc Surg. 2018;156:2076–2082.e2072. [DOI] [PubMed] [Google Scholar]
- 10.Chiu P, Trojan J, Tsou S, et al. Limited root repair in acute type A aortic dissection is safe but results in increased risk of reoperation. J Thorac Cardiovasc Surg. 2018;155:1–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The VIRTUE Registry Investigators. Mid-term outcomes and aortic remodeling after thoracic endovascular repair for acute, subacute, and chronic aortic dissection: the VIRTUE Registry. Eur J Vasc Endovasc Surg. 2014;48:363–371. [DOI] [PubMed] [Google Scholar]
- 12.Giles KA, Beck AW, Lala S, et al. Implications of secondary aortic intervention after thoracic endovascular aortic repair for acute and chronic type B dissection. J Vasc Surg. 2019;69:1367–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
7.8.2. Long-Term Management After Acute Aortic Dissection and IMH
- 1.Wang H, Wagner M, Benrashid E, et al. Outcomes of reoperation after acute type A aortic dissection: implications for index repair strategy. J Am Heart Assoc. 2017;6:e006376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajona P, Quintana E, Schaff HV, et al. Aortic arch surgery after previous type A dissection repair: results up to 5 years. Interact Cardiovasc Thorac Surg. 2015;21:81–85; discussion 85–86. [DOI] [PubMed] [Google Scholar]
- 3.Malvindi PG, van Putte BP, Sonker U, et al. Reoperation after acute type A aortic dissection repair: a series of 104 patients. Ann Thorac Surg. 2013;95:922–927. [DOI] [PubMed] [Google Scholar]
- 4.Arafat A, Roselli EE, Idrees JJ, et al. Stent grafting acute aortic dissection: comparison of DeBakey extent IIIA versus IIIB. Ann Thorac Surg. 2016;102:1473–1481. [DOI] [PubMed] [Google Scholar]
7.8.3. Long-Term Management and Surveillance for PAUs
- 1.Georgiadis GS, Antoniou GA, Georgakarakos EI, et al. Surgical or endovascular therapy of abdominal penetrating aortic ulcers and their natural history: a systematic review. J Vasc Interv Radiol. 2013;24:1437–1449.e1433. [DOI] [PubMed] [Google Scholar]
- 2.Georgiadis GS, Trellopoulos G, Antoniou GA, et al. Endovascular therapy for penetrating ulcers of the infrarenal aorta. ANZ J Surg. 2013;83:758–763. [DOI] [PubMed] [Google Scholar]
- 3.D’Annoville T, Ozdemir BA, Alric P, et al. ; Thoracic endovascular aortic repair for penetrating aortic ulcer: literature review. Ann Thorac Surg. 2016;101:2272–2278. [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Zhang QY, Wang XZ, et al. Long-term imaging evolution and clinical prognosis among patients with acute penetrating aortic ulcers: a retrospective observational study. J Am Heart Assoc. 2020;9:e014505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou AS, Ziganshin BA, Charilaou P, et al. Long-term behavior of aortic intramural hematomas and penetrating ulcers. J Thorac Cardiovasc Surg. 2016;151:361–373.e1. [DOI] [PubMed] [Google Scholar]
- 6.Cho KR, Stanson AW, Potter DD, et al. Penetrating atherosclerotic ulcer of the descending thoracic aorta and arch. J Thorac Cardiovasc Surg. 2004;127:1393–1399; discussion 1399–1401. [DOI] [PubMed] [Google Scholar]
- 7.Nathan DP, Boonn W, Lai E, et al. Presentation, complications, and natural history of penetrating atherosclerotic ulcer disease. J Vasc Surg. 2012;55:10–15. [DOI] [PubMed] [Google Scholar]
- 8.Salim S, Locci R, Martin G, et al. Short- and long-term outcomes in isolated penetrating aortic ulcer disease. J Vasc Surg. 2020;72:84–91. [DOI] [PubMed] [Google Scholar]
8.1. Counseling and Management of Aortic Disease in Pregnancy and Postpartum
- 1.Roos-Hesselink JW, Budts W, Walker F, et al. Organisation of care for pregnancy in patients with congenital heart disease. Heart. 2017;103:1854–1859. [DOI] [PubMed] [Google Scholar]
- 2.Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–798. [DOI] [PubMed] [Google Scholar]
- 3.Murray ML, Pepin M, Peterson S, et al. Pregnancy-related deaths and complications in women with vascular Ehlers-Danlos syndrome. Genet Med. 2014;16:874–880. [DOI] [PubMed] [Google Scholar]
- 4.Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476–485. [DOI] [PubMed] [Google Scholar]
- 5.MacCarrick G, Black JH 3rd, Bowdin S, et al. Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med. 2014;16:576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roman MJ, Pugh NL, Hendershot TP, et al. Aortic complications associated with pregnancy in Marfan syndrome: the NHLBI National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC). J Am Heart Assoc. 2016;5:e004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnelly RT, Pinto NM, Kocolas I, et al. The immediate and long-term impact of pregnancy on aortic growth rate and mortality in women with Marfan syndrome. J Am Coll Cardiol. 2012;60:224–229. [DOI] [PubMed] [Google Scholar]
- 8.Kuperstein R, Cahan T, Yoeli-Ullman R, et al. Risk of aortic dissection in pregnant patients with the Marfan syndrome. Am J Cardiol. 2017;119:132–137. [DOI] [PubMed] [Google Scholar]
- 9.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–3241. [DOI] [PubMed] [Google Scholar]
- 10.Cauldwell M, Steer PJ, Curtis S, et al. Maternal and fetal outcomes in pregnancies complicated by the inherited aortopathy Loeys-Dietz syndrome. BJOG. 2019;126:1025–1031. [DOI] [PubMed] [Google Scholar]
- 11.Pyeritz RE. Maternal and fetal complications of pregnancy in the Marfan syndrome. Am J Med. 1981;71:784–790. [DOI] [PubMed] [Google Scholar]
- 12.Silberbach M, Roos-Hesselink JW, Andersen NH, et al. Cardiovascular health in Turner syndrome: a scientific statement from the American Heart Association. Circ Genom Precis Med. 2018;11:e000048. [DOI] [PubMed] [Google Scholar]
- 13.Cauldwell M, Steer PJ, Curtis SL, et al. Maternal and fetal outcomes in pregnancies complicated by Marfan syndrome. Heart. 2019;105:1725–1731. [DOI] [PubMed] [Google Scholar]
- 14.Campens L, Baris L, Scott NS, et al. Pregnancy outcome in thoracic aortic disease data from the Registry of Pregnancy and Cardiac Disease. Heart. 2021;107:1704–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cauldwell M, Steer P, Sterrenburg M, et al. Birth weight in pregnancies complicated by maternal heart disease. Heart. 2019;105:391–398. [DOI] [PubMed] [Google Scholar]
- 16. Deleted in press.
- 17.Seely EW, Ecker J. Chronic hypertension in pregnancy. Circulation. 2014;129:1254–1261. [DOI] [PubMed] [Google Scholar]
- 18.Meijboom LJ, Vos FE, Timmermans J, et al. Pregnancy and aortic root growth in the Marfan syndrome: a prospective study. Eur Heart J. 2005;26:914–920. [DOI] [PubMed] [Google Scholar]
- 19.Rossiter JP, Repke JT, Morales AJ, et al. A prospective longitudinal evaluation of pregnancy in the Marfan syndrome. Am J Obstet Gynecol. 1995;173:1599–1606. [DOI] [PubMed] [Google Scholar]
- 20.Ray JG, Vermeulen MJ, Bharatha A, et al. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA. 2016;316:952–961. [DOI] [PubMed] [Google Scholar]
- 21.Chartier AL, Bouvier MJ, McPherson DR, et al. The safety of maternal and fetal MRI at 3 T. AJR Am J Roentgenol. 2019;213:1170–1173. [DOI] [PubMed] [Google Scholar]
- 22.Braverman AC, Mittauer E, Harris KM, et al. Clinical features and outcomes of pregnancy-related acute aortic dissection. JAMA Cardiol. 2021;6:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran-Fadulu V, Pannu H, Kim DH, et al. Analysis of multigenerational families with thoracic aortic aneurysms and dissections due to TGFBR1 or TGFBR2 mutations. J Med Genet. 2009;46:607–613. [DOI] [PubMed] [Google Scholar]
- 24.Jondeau G, Ropers J, Regalado E, et al. International Registry of Patients Carrying TGFBR1 or TGFBR2 Mutations: results of the MAC (Montalcino Aortic Consortium). Circ Cardiovasc Genet. 2016;9:548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byers PH, Belmont J, Black J, et al. Diagnosis, natural history, and management in vascular Ehlers-Danlos syndrome. Am J Med Genet C Semin Med Genet. 2017;175:40–47. [DOI] [PubMed] [Google Scholar]
- 26.Regalado ES, Guo DC, Estrera AL, et al. Acute aortic dissections with pregnancy in women with ACTA2 mutations. Am J Med Genet A. 2014;164:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace SE, Regalado ES, Gong L, et al. MYLK pathogenic variants aortic disease presentation, pregnancy risk, and characterization of pathogenic missense variants. Genet Med. 2019;21:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlson M, Silberbach M. Dissection of the aorta in Turner syndrome: two cases and review of 85 cases in the literature. J Med Genet. 2007;44:745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Immer FF, Bansi AG, Immer-Bansi AS, et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg. 2003;76:309–314. [DOI] [PubMed] [Google Scholar]
- 30.Thakker PD, Braverman AC. Cardiogenetics: genetic testing in the diagnosis and management of patients with aortic disease. Heart. 2021;107:619–626. [DOI] [PubMed] [Google Scholar]
- 31.Pinard A, Jones GT, Milewicz DM. Genetics of thoracic and abdominal aortic diseases. Circ Res. 2019;124:588–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hostetler EM, Regalado ES, Guo DC, et al. SMAD3 pathogenic variants: risk for thoracic aortic disease and associated complications from the Montalcino Aortic Consortium. J Med Genet. 2019;56:252–260. [DOI] [PubMed] [Google Scholar]
- 33.van de Luijtgaarden KM, Bastos Goncalves F, Hoeks SE, et al. Higher 30 day mortality in patients with familial abdominal aortic aneurysm after EVAR. Eur J Vasc Endovasc Surg. 2017;54:142–149. [DOI] [PubMed] [Google Scholar]
- 34.Shalhub S, Regalado ES, Guo DC, et al. The natural history of type B aortic dissection in patients with PRKG1 mutation c.530G>A (p.Arg177Gln). J Vasc Surg. 2019;70:718–723. [DOI] [PubMed] [Google Scholar]
- 35.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 36.Doyle JJ, Doyle AJ, Wilson NK, et al. A deleterious gene-by-environment interaction imposed by calcium channel blockers in Marfan syndrome. Elife. 2015;4:e08648. [DOI] [PMC free article] [PubMed] [Google Scholar]
8.2. Delivery in Pregnant Patients With Aortopathy
- 1.Braverman AC, Mittauer E, Harris KM, et al. Clinical features and outcomes of pregnancy-related acute aortic dissection. JAMA Cardiol. 2021;6:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campens L, Baris L, Scott NS, et al. Pregnancy outcome in thoracic aortic disease data from the Registry of Pregnancy and Cardiac Disease. Heart. 2021;107:1704–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pyeritz RE. Maternal and fetal complications of pregnancy in the Marfan syndrome. Am J Med. 1981;71:784–790. [DOI] [PubMed] [Google Scholar]
- 4.Kamel H, Roman MJ, Pitcher A, et al. Pregnancy and the risk of aortic dissection or rupture: a cohort-crossover analysis. Circulation. 2016;134:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuperstein R, Cahan T, Yoeli-Ullman R, et al. Risk of aortic dissection in pregnant patients with the Marfan syndrome. Am J Cardiol. 2017;119:132–137. [DOI] [PubMed] [Google Scholar]
- 6.Roman MJ, Pugh NL, Hendershot TP, et al. Aortic complications associated with pregnancy in Marfan syndrome: the NHLBI National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC). J Am Heart Assoc. 2016;5:e004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meijboom LJ, Vos FE, Timmermans J, et al. Pregnancy and aortic root growth in the Marfan syndrome: a prospective study. Eur Heart J. 2005;26:914–920. [DOI] [PubMed] [Google Scholar]
- 8.Minsart AF, Mongeon FP, Laberge AM, et al. Obstetric and cardiac outcomes in women with Marfan syndrome and an aortic root diameter ≤ 45mm. Eur J Obstet Gynecol Reprod Biol. 2018;230:68–72. [DOI] [PubMed] [Google Scholar]
- 9.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–3241. [DOI] [PubMed] [Google Scholar]
- 10.Cauldwell M, Steer PJ, Curtis SL, et al. Maternal and fetal outcomes in pregnancies complicated by Marfan syndrome. Heart. 2019;105:1725–1731. [DOI] [PubMed] [Google Scholar]
- 11.Williams D, Lindley KJ, Russo M, et al. Pregnancy after aortic root replacement in Marfan’s syndrome: a case series and review of the literature. AJP Rep. 2018;8:e234–e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braverman AC, Moon MR, Geraghty P, et al. Pregnancy after aortic root replacement in Loeys-Dietz syndrome: high risk of aortic dissection. Am J Med Genet A. 2016;170:2177–2180. [DOI] [PubMed] [Google Scholar]
- 13.Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476–485. [DOI] [PubMed] [Google Scholar]
- 14.McNeilly G, Nicholl A, Broadway J, et al. Dural ectasia in Marfan’s syndrome: magnetic resonance imaging appearances and anaesthetic experience of three deliveries. Int J Obstet Anesth. 2013;22:337–339. [DOI] [PubMed] [Google Scholar]
8.3. Surgery Before Pregnancy in Women With Aortic Disease
- 1.Meijboom LJ, Vos FE, Timmermans J, et al. Pregnancy and aortic root growth in the Marfan syndrome: a prospective study. Eur Heart J. 2005;26:914–920. [DOI] [PubMed] [Google Scholar]
- 2.Minsart AF, Mongeon FP, Laberge AM, et al. Obstetric and cardiac outcomes in women with Marfan syndrome and an aortic root diameter ≤ 45mm. Eur J Obstet Gynecol Reprod Biol. 2018;230:68–72. [DOI] [PubMed] [Google Scholar]
- 3.Campens L, Baris L, Scott NS, et al. Pregnancy outcome in thoracic aortic disease data from the Registry of Pregnancy and Cardiac Disease. Heart. 2021;107:1704–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narula N, Devereux RB, Malonga GP, et al. Pregnancy-related aortic complications in women with Marfan syndrome. J Am Coll Cardiol. 2021;78:870–879. [DOI] [PubMed] [Google Scholar]
- 5.Braverman AC, Mittauer E, Harris KM, et al. Clinical features and outcomes of pregnancy-related acute aortic dissection. JAMA Cardiol. 2021;6:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pyeritz RE. Maternal and fetal complications of pregnancy in the Marfan syndrome. Am J Med. 1981;71:784–790. [DOI] [PubMed] [Google Scholar]
- 7.Roman MJ, Pugh NL, Hendershot TP, et al. Aortic complications associated with pregnancy in Marfan syndrome: the NHLBI National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC). J Am Heart Assoc. 2016;5:e004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 9.Matura LA, Ho VB, Rosing DR, et al. Aortic dilatation and dissection in Turner syndrome. Circulation. 2007;116:1663–1670. [DOI] [PubMed] [Google Scholar]
- 10.Silberbach M, Roos-Hesselink JW, Andersen NH, et al. Cardiovascular health in Turner syndrome: a scientific statement from the American Heart Association. Circ Genom Precis Med. 2018;11:e000048. [DOI] [PubMed] [Google Scholar]
- 11.Carlson M, Silberbach M. Dissection of the aorta in Turner syndrome: two cases and review of 85 cases in the literature. J Med Genet. 2007;44:745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braverman AC, Moon MR, Geraghty P, et al. Pregnancy after aortic root replacement in Loeys-Dietz syndrome: high risk of aortic dissection. Am J Med Genet A. 2016;170:2177–2180. [DOI] [PubMed] [Google Scholar]
- 13.Williams D, Lindley KJ, Russo M, et al. Pregnancy after aortic root replacement in Marfan’s syndrome: a case series and review of the literature. AJP Rep. 2018;8:e234–e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. [DOI] [PubMed] [Google Scholar]
- 15.Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476–485. [DOI] [PubMed] [Google Scholar]
- 16.Boileau C, Guo DC, Hanna N, et al. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat Genet. 2012;44:916–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsili L, Overwater E, Hanna N, et al. Phenotypic spectrum of TGFB3 disease-causing variants in a Dutch-French cohort and first report of a homozygous patient. Clin Genet. 2020;97:723–730. [DOI] [PubMed] [Google Scholar]
- 18.Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–798. [DOI] [PubMed] [Google Scholar]
- 19.Jondeau G, Ropers J, Regalado E, et al. International Registry of Patients Carrying TGFBR1 or TGFBR2 Mutations: results of the MAC (Montalcino Aortic Consortium). Circ Cardiovasc Genet. 2016;9:548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Hagen IM, van der Linde D, van de Laar IM, et al. Pregnancy in women with SMAD3 mutation. J Am Coll Cardiol. 2017;69:1356–1358. [DOI] [PubMed] [Google Scholar]
- 21.Regalado ES, Guo DC, Estrera AL, et al. Acute aortic dissections with pregnancy in women with ACTA2 mutations. Am J Med Genet A. 2014;164:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace SE, Regalado ES, Gong L, et al. MYLK pathogenic variants aortic disease presentation, pregnancy risk, and characterization of pathogenic missense variants. Genet Med. 2019;21:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shalhub S, Regalado ES, Guo DC, et al. The natural history of type B aortic dissection in patients with PRKG1 mutation c.530G>A (p.Arg177Gln). J Vasc Surg. 2019;70:718–723. [DOI] [PubMed] [Google Scholar]
- 24.Milewicz DM, Regalado ES. Use of genetics for personalized management of heritable thoracic aortic disease: how do we get there? J Thorac Cardiovasc Surg. 2015;149:S3–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Immer FF, Krahenbuhl E, Hagen U, et al. Large area of the false lumen favors secondary dilatation of the aorta after acute type A aortic dissection. Circulation. 2005;112:I249–I252. [DOI] [PubMed] [Google Scholar]
- 26.McKellar SH, MacDonald RJ, Michelena HI, et al. Frequency of cardiovascular events in women with a congenitally bicuspid aortic valve in a single community and effect of pregnancy on events. Am J Cardiol. 2011;107:96–99. [DOI] [PubMed] [Google Scholar]
- 27.Wojnarski CM, Svensson LG, Roselli EE, et al. Aortic dissection in patients with bicuspid aortic valve-associated aneurysms. Ann Thorac Surg. 2015;100:1666–1673; discussion 1673–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–3241. [DOI] [PubMed] [Google Scholar]
- 29.Thakker PD, Braverman AC. Cardiogenetics: genetic testing in the diagnosis and management of patients with aortic disease. Heart. 2021;107:619–626. [DOI] [PubMed] [Google Scholar]
8.4. Pregnancy in Patients With Aortopathy: Aortic Dissection and Aortic Surgery in Pregnancy
- 1.Lansman SL, Goldberg JB, Kai M, et al. Aortic surgery in pregnancy. J Thorac Cardiovasc Surg. 2017;153:S44–S48. [DOI] [PubMed] [Google Scholar]
- 2.Zhu JM, Ma WG, Peterss S, et al. Aortic dissection in pregnancy: management strategy and outcomes. Ann Thorac Surg. 2017;103:1199–1206. [DOI] [PubMed] [Google Scholar]
- 3.Yates MT, Soppa G, Smelt J, et al. Perioperative management and outcomes of aortic surgery during pregnancy. J Thorac Cardiovasc Surg. 2015;149:607–610. [DOI] [PubMed] [Google Scholar]
- 4.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–3241. [DOI] [PubMed] [Google Scholar]
- 5.Tadros RO, Tang GHL, Barnes HJ, et al. Optimal treatment of uncomplicated type B aortic dissection: JACC review topic of the week. J Am Coll Cardiol. 2019;74:1494–1504. [DOI] [PubMed] [Google Scholar]
9.1. Inflammatory Aortitis: Diagnosis and Treatment of Takayasu Arteritis and Giant Cell Arteritis
- 1.Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77:636–643. [DOI] [PubMed] [Google Scholar]
- 2.Hauenstein C, Reinhard M, Geiger J, et al. Effects of early corticosteroid treatment on magnetic resonance imaging and ultrasonography findings in giant cell arteritis. Rheumatology (Oxford). 2012;51:1999–2003. [DOI] [PubMed] [Google Scholar]
- 3.Prieto-Gonzalez S, Arguis P, Garcia-Martinez A, et al. Large vessel involvement in biopsy-proven giant cell arteritis: prospective study in 40 newly diagnosed patients using CT angiography. Ann Rheum Dis. 2012;71:1170–1176. [DOI] [PubMed] [Google Scholar]
- 4.Blockmans D, Stroobants S, Maes A, et al. Positron emission tomography in giant cell arteritis and polymyalgia rheumatica: evidence for inflammation of the aortic arch. Am J Med. 2000;108:246–249. [DOI] [PubMed] [Google Scholar]
- 5.Lariviere D, Benali K, Coustet B, et al. Positron emission tomography and computed tomography angiography for the diagnosis of giant cell arteritis: a real-life prospective study. Medicine (Baltimore). 2016;95:e4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamada I, Nakagawa T, Himeno Y, et al. Takayasu arteritis: diagnosis with breath-hold contrast-enhanced three-dimensional MR angiography. J Magn Reson Imaging. 2000;11:481–487. [DOI] [PubMed] [Google Scholar]
- 7.Hellmich B, Agueda A, Monti S, et al. 2018 update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2020;79:19–30. [DOI] [PubMed] [Google Scholar]
- 8.Mazlumzadeh M, Hunder GG, Easley KA, et al. Treatment of giant cell arteritis using induction therapy with high-dose glucocorticoids: a double-blind, placebo-controlled, randomized prospective clinical trial. Arthritis Rheum. 2006;54:3310–3318. [DOI] [PubMed] [Google Scholar]
- 9.Chevalet P, Barrier JH, Pottier P, et al. A randomized, multicenter, controlled trial using intravenous pulses of methylprednisolone in the initial treatment of simple forms of giant cell arteritis: a one year followup study of 164 patients. J Rheumatol. 2000;27:1484–1491. [PubMed] [Google Scholar]
- 10.Raine C, Stapleton PP, Merinopoulos D, et al. A 26-week feasibility study comparing the efficacy and safety of modified-release prednisone with immediate-release prednisolone in newly diagnosed cases of giant cell arteritis. Int J Rheum Dis. 2018;21:285–291. [DOI] [PubMed] [Google Scholar]
- 11.Kyle V, Hazleman BL. Treatment of polymyalgia rheumatica and giant cell arteritis. II. Relation between steroid dose and steroid associated side effects. Ann Rheum Dis. 1989;48:662–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Les I, Pijoan JI, Rodriguez-Alvarez R, et al. Effectiveness and safety of medium-dose prednisone in giant cell arteritis: a retrospective cohort study of 103 patients. Clin Exp Rheumatol. 2015;33:S90–S97. [PubMed] [Google Scholar]
- 13.Stone JH, Klearman M, Collinson N. Trial of tocilizumab in giant-cell arteritis. N Engl J Med. 2017;377:1494–1495. [DOI] [PubMed] [Google Scholar]
- 14.Jover JA, Hernandez-Garcia C, Morado IC, et al. Combined treatment of giant-cell arteritis with methotrexate and prednisone. a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;134:106–114. [DOI] [PubMed] [Google Scholar]
- 15.Misra DP, Rathore U, Patro P, et al. Disease-modifying anti-rheumatic drugs for the management of Takayasu arteritis-a systematic review and meta-analysis. Clin Rheumatol. 2021;40:4391–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mekinian A, Comarmond C, Resche-Rigon M, et al. Efficacy of biological-targeted treatments in Takayasu arteritis: multicenter, retrospective study of 49 patients. Circulation. 2015;132:1693–1700. [DOI] [PubMed] [Google Scholar]
- 17.Reichenbach S, Adler S, Bonel H, et al. Magnetic resonance angiography in giant cell arteritis: results of a randomized controlled trial of tocilizumab in giant cell arteritis. Rheumatology (Oxford). 2018;57:982–986. [DOI] [PubMed] [Google Scholar]
- 18.Zerizer I, Tan K, Khan S, et al. Role of FDG-PET and PET/CT in the diagnosis and management of vasculitis. Eur J Radiol. 2010;73:504–509. [DOI] [PubMed] [Google Scholar]
- 19.Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4:e000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blockmans D, de Ceuninck L, Vanderschueren S, et al. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum. 2006;55:131–137. [DOI] [PubMed] [Google Scholar]
- 21.Saadoun D, Lambert M, Mirault T, et al. Retrospective analysis of surgery versus endovascular intervention in Takayasu arteritis: a multicenter experience. Circulation. 2012;125:813–819. [DOI] [PubMed] [Google Scholar]
- 22.Saadoun D, Vautier M, Cacoub P. Medium- and large-vessel vasculitis. Circulation. 2021;143:267–282. [DOI] [PubMed] [Google Scholar]
- 23.Svensson LG, Arafat A, Roselli EE, et al. Inflammatory disease of the aorta: patterns and classification of giant cell aortitis, Takayasu arteritis, and nonsyndromic aortitis. J Thorac Cardiovasc Surg. 2015;149:S170–S175. [DOI] [PubMed] [Google Scholar]
- 24.Nuenninghoff DM, Hunder GG, Christianson TJ, et al. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum. 2003;48:3522–3531. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Taboada VM, Lopez-Hoyos M, Narvaez J, et al. Effect of antiplatelet/anticoagulant therapy on severe ischemic complications in patients with giant cell arteritis: a cumulative meta-analysis. Autoimmun Rev. 2014;13:788–794. [DOI] [PubMed] [Google Scholar]
- 26.Smith SC Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation. 2006;113:2363–2372. [DOI] [PubMed] [Google Scholar]
- 27.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. [DOI] [PubMed] [Google Scholar]
9.2.1. Diagnosis and Management of Infection of the Native Aorta
- 1.Vallejo N, Picardo NE, Bourke P, et al. The changing management of primary mycotic aortic aneurysms. J Vasc Surg. 2011;54:334–340. [DOI] [PubMed] [Google Scholar]
- 2.Kan CD, Lee HL, Luo CY, et al. The efficacy of aortic stent grafts in the management of mycotic abdominal aortic aneurysm-institute case management with systemic literature comparison. Ann Vasc Surg. 2010;24:433–440. [DOI] [PubMed] [Google Scholar]
- 3.Sorelius K, Wanhainen A, Furebring M, et al. Nationwide study of the treatment of mycotic abdominal aortic aneurysms comparing open and endovascular repair. Circulation. 2016;134:1822–1832. [DOI] [PubMed] [Google Scholar]
- 4.Sakalihasan N, Michel JB, Katsargyris A, et al. Abdominal aortic aneurysms. Nat Rev Dis Primers. 2018;4:34. [DOI] [PubMed] [Google Scholar]
- 5.Wilson WR, Bower TC, Creager MA, et al. Vascular graft infections, mycotic aneurysms, and endovascular infections: a scientific statement from the American Heart Association. Circulation. 2016;134:e412–e460. [DOI] [PubMed] [Google Scholar]
- 6.Muller BT, Wegener OR, Grabitz K, et al. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: experience with anatomic and extra-anatomic repair in 33 cases. J Vasc Surg. 2001;33:106–113. [DOI] [PubMed] [Google Scholar]
- 7.Oz MC, Brener BJ, Buda JA, et al. A ten-year experience with bacterial aortitis. J Vasc Surg. 1989;10:439–449. [DOI] [PubMed] [Google Scholar]
- 8.Cina CS, Arena GO, Fiture AO, et al. Ruptured mycotic thoracoabdominal aortic aneurysms: a report of three cases and a systematic review. J Vasc Surg. 2001;33:861–867. [DOI] [PubMed] [Google Scholar]
- 9.Yu SY, Lee CH, Hsieh HC, et al. Treatment of primary infected aortic aneurysm without aortic resection. J Vasc Surg. 2012;56:943–950. [DOI] [PubMed] [Google Scholar]
- 10.Weis-Muller BT, Rascanu C, Sagban A, et al. Single-center experience with open surgical treatment of 36 infected aneurysms of the thoracic, thoracoabdominal, and abdominal aorta. Ann Vasc Surg. 2011;25:1020–1025. [DOI] [PubMed] [Google Scholar]
- 11.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435–1486. [DOI] [PubMed] [Google Scholar]
9.2.2. Diagnosis and Management of Prosthetic Aortic Graft Infection
- 1.Low RN, Wall SD, Jeffrey RB Jr, et al. Aortoenteric fistula and perigraft infection: evaluation with CT. Radiology. 1990;175:157–162. [DOI] [PubMed] [Google Scholar]
- 2.Mark A, Moss AA, Lusby R, et al. CT evaluation of complications of abdominal aortic surgery. Radiology. 1982;145:409–414. [DOI] [PubMed] [Google Scholar]
- 3.Fukuchi K, Ishida Y, Higashi M, et al. Detection of aortic graft infection by fluorodeoxyglucose positron emission tomography: comparison with computed tomographic findings. J Vasc Surg. 2005;42:919–925. [DOI] [PubMed] [Google Scholar]
- 4.Berger P, Vaartjes I, Scholtens A, et al. Differential FDG-PET uptake patterns in uninfected and infected central prosthetic vascular grafts. Eur J Vasc Endovasc Surg. 2015;50:376–383. [DOI] [PubMed] [Google Scholar]
- 5.Sah BR, Husmann L, Mayer D, et al. Diagnostic performance of 18F-FDG-PET/CT in vascular graft infections. Eur J Vasc Endovasc Surg. 2015;49:455–464. [DOI] [PubMed] [Google Scholar]
- 6.Shahidi S, Eskil A, Lundof E, et al. Detection of abdominal aortic graft infection: comparison of magnetic resonance imaging and indium-labeled white blood cell scanning. Ann Vasc Surg. 2007;21:586–592. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor S, Andrew P, Batt M, et al. A systematic review and meta-analysis of treatments for aortic graft infection. J Vasc Surg. 2006;44:38–45. [DOI] [PubMed] [Google Scholar]
- 8.Reilly LM, Stoney RJ, Goldstone J, et al. Improved management of aortic graft infection: the influence of operation sequence and staging. J Vasc Surg. 1987;5:421–431. [DOI] [PubMed] [Google Scholar]
- 9.Clagett GP, Valentine RJ, Hagino RT. Autogenous aortoiliac/femoral reconstruction from superficial femoral-popliteal veins: feasibility and durability. J Vasc Surg. 1997;25:255–266; discussion 267–270. [DOI] [PubMed] [Google Scholar]
- 10.Harlander-Locke MP, Harmon LK, Lawrence PF, et al. The use of cryopreserved aortoiliac allograft for aortic reconstruction in the United States. J Vasc Surg. 2014;59:669–674. [DOI] [PubMed] [Google Scholar]
- 11.Kieffer E, Gomes D, Chiche L, et al. Allograft replacement for infrarenal aortic graft infection: early and late results in 179 patients. J Vasc Surg. 2004;39:1009–1017. [DOI] [PubMed] [Google Scholar]
- 12.Oderich GS, Farber MA, Sanchez LA. Urgent endovascular treatment of symptomatic or contained ruptured aneurysms with modified stent grafts. Perspect Vasc Surg Endovasc Ther. 2011;23:186–194. [DOI] [PubMed] [Google Scholar]
- 13.Kakkos SK, Bicknell CD, Tsolakis IA, et al. Editor’s choice - management of secondary aorto-enteric and other abdominal arterio-enteric fistulas: a review and pooled data analysis. Eur J Vasc Endovasc Surg. 2016;52:770–786. [DOI] [PubMed] [Google Scholar]
- 14.Haidar GM, Hicks TD, Strosberg DS, et al. “In situ” endografting in the treatment of arterial and graft infections. J Vasc Surg. 2017;65:1824–1829. [DOI] [PubMed] [Google Scholar]
- 15.Plotkin A, Magee GA, Elsayed RS, et al. Methicillin-resistant Staphylococcus aureus portends a poor prognosis after endovascular repair of mycotic aortic aneurysms and aortic graft infections. J Vasc Surg. 2020;72:276–285. [DOI] [PubMed] [Google Scholar]
- 16.Chiesa R, Astore D, Frigerio S, et al. Vascular prosthetic graft infection: epidemiology, bacteriology, pathogenesis and treatment. Acta Chir Belg. 2002;102:238–247. [DOI] [PubMed] [Google Scholar]
- 17.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435–1486. [DOI] [PubMed] [Google Scholar]
- 18.Lau C, Gaudino M, de Biasi AR, et al. Outcomes of open repair of mycotic descending thoracic and thoracoabdominal aortic aneurysms. Ann Thorac Surg. 2015;100:1712–1717. [DOI] [PubMed] [Google Scholar]
- 19.Antonello RM, D’Oria M, Cavallaro M, et al. Management of abdominal aortic prosthetic graft and endograft infections. A multidisciplinary update. J Infect Chemother. 2019;25:669–680. [DOI] [PubMed] [Google Scholar]
- 20.Hallett JW Jr, Marshall DM, Petterson TM, et al. Graft-related complications after abdominal aortic aneurysm repair: reassurance from a 36-year population-based experience. J Vasc Surg. 1997;25:277–284; discussion 285–286. [DOI] [PubMed] [Google Scholar]
- 21.Lehnert T, Gruber HP, Maeder N, et al. Management of primary aortic graft infection by extra-anatomic bypass reconstruction. Eur J Vasc Surg. 1993;7:301–307. [DOI] [PubMed] [Google Scholar]
- 22.Vogel TR, Symons R, Flum DR. The incidence and factors associated with graft infection after aortic aneurysm repair. J Vasc Surg. 2008;47:264–269. [DOI] [PubMed] [Google Scholar]
- 23.Schermerhorn ML, O’Malley AJ, Jhaveri A, et al. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008;358:464–474. [DOI] [PubMed] [Google Scholar]
- 24.Hobo R, Buth J, EUROSTAR collaborators. Secondary interventions following endovascular abdominal aortic aneurysm repair using current endografts. A EUROSTAR report. J Vasc Surg. 2006;43:896–902.e1. [DOI] [PubMed] [Google Scholar]
- 25.EVAR Trial Participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005;365:2179–2186. [DOI] [PubMed] [Google Scholar]
- 26.Oderich GS, Bower TC, Hofer J, et al. In situ rifampin-soaked grafts with omental coverage and antibiotic suppression are durable with low reinfection rates in patients with aortic graft enteric erosion or fistula. J Vasc Surg. 2011;53:99–106, 107.e1–7; discussion 106–107. [DOI] [PubMed] [Google Scholar]
- 27.Janko MR, Woo K, Hacker RI, et al. In situ bypass and extra-anatomic bypass procedures result in similar survival in patients with secondary aortoenteric fistulas. J Vasc Surg. 2021;73:210–221.e1. [DOI] [PubMed] [Google Scholar]
- 28.Bandyk DF, Novotney ML, Back MR, et al. Expanded application of in situ replacement for prosthetic graft infection. J Vasc Surg. 2001;34:411–419; discussion 419–420. [DOI] [PubMed] [Google Scholar]
9.3. Atherosclerotic Disease
- 1.Agmon Y, Khandheria BK, Meissner I, et al. Relation of coronary artery disease and cerebrovascular disease with atherosclerosis of the thoracic aorta in the general population. Am J Cardiol. 2002;89:262–267. [DOI] [PubMed] [Google Scholar]
- 2.Caron F, Anand SS. Antithrombotic therapy in aortic diseases: a narrative review. Vasc Med. 2017;22:57–65. [DOI] [PubMed] [Google Scholar]
- 3.Jones WS, Mulder H, Wruck LM, et al. Comparative effectiveness of aspirin dosing in cardiovascular disease. N Engl J Med. 2021;384:1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tunick PA, Rosenzweig BP, Katz ES, et al. High risk for vascular events in patients with protruding aortic atheromas: a prospective study. J Am Coll Cardiol. 1994;23:1085–1090. [DOI] [PubMed] [Google Scholar]
- 5.Plehn JF, Davis BR, Sacks FM, et al. Reduction of stroke incidence after myocardial infarction with pravastatin: the Cholesterol and Recurrent Events (CARE) study. The Care Investigators. Circulation. 1999;99:216–223. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 7.Amarenco P, Cohen A, Tzourio C, et al. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331:1474–1479. [DOI] [PubMed] [Google Scholar]
- 8.Tunick PA, Nayar AC, Goodkin GM, et al. Effect of treatment on the incidence of stroke and other emboli in 519 patients with severe thoracic aortic plaque. Am J Cardiol. 2002;90:1320–1325. [DOI] [PubMed] [Google Scholar]
- 9.Butler CG, Ho Luxford JM, Huang CC, et al. Aortic atheroma increases the risk of long-term mortality in 20, 000 patients. Ann Thorac Surg. 2017;104:1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMahan CA, Gidding SS, Fayad ZA, et al. Risk scores predict atherosclerotic lesions in young people. Arch Intern Med. 2005;165:883–890. [DOI] [PubMed] [Google Scholar]
- 11.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–1531. [DOI] [PubMed] [Google Scholar]
- 12.Karalis DG, Chandrasekaran K, Victor MF, et al. Recognition and embolic potential of intraaortic atherosclerotic debris. J Am Coll Cardiol. 1991;17:73–78. [DOI] [PubMed] [Google Scholar]
- 13.Jones EF, Kalman JM, Calafiore P, et al. Proximal aortic atheroma. An independent risk factor for cerebral ischemia. Stroke. 1995;26:218–224. [PubMed] [Google Scholar]
- 14.Khatibzadeh M, Mitusch R, Stierle U, et al. Aortic atherosclerotic plaques as a source of systemic embolism. J Am Coll Cardiol. 1996;27:664–669. [DOI] [PubMed] [Google Scholar]
- 15.Mitusch R, Doherty C, Wucherpfennig H, et al. Vascular events during follow-up in patients with aortic arch atherosclerosis. Stroke. 1997;28:36–39. [DOI] [PubMed] [Google Scholar]
- 16.Kronzon I, Tunick PA. Aortic atherosclerotic disease and stroke. Circulation. 2006;114:63–75. [DOI] [PubMed] [Google Scholar]
- 17.Qvist I, Soegaard R, Lindholt JS, et al. Adherence to prescribed drugs among 65–74 year old men diagnosed with abdominal aortic aneurysm or peripheral arterial disease in a screening trial: a VIVA substudy. Eur J Vasc Endovasc Surg. 2019;57:442–450. [DOI] [PubMed] [Google Scholar]
- 18.Phrommintikul A, Krittayaphong R, Wongcharoen W, et al. Management of atherosclerosis risk factors for patients at high cardiovascular risk in real-world practice: a multicentre study. Singapore Med J. 2017;58:535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
9.3.1. Aortic Thrombus
- 1.Yang S, Yu J, Zeng W, et al. Aortic floating thrombus detected by computed tomography angiography incidentally: five cases and a literature review. J Thorac Cardiovasc Surg. 2017;153:791–803. [DOI] [PubMed] [Google Scholar]
- 2.Caron F, Anand SS. Antithrombotic therapy in aortic diseases: a narrative review. Vasc Med. 2017;22:57–65. [DOI] [PubMed] [Google Scholar]
- 3.Fayad ZY, Semaan E, Fahoum B, et al. Aortic mural thrombus in the normal or minimally atherosclerotic aorta. Ann Vasc Surg. 2013;27:282–290. [DOI] [PubMed] [Google Scholar]
- 4.Arima T, Muroya K, Kawamoto K, et al. Aortic thrombosis in a patient with malignant disease: a literature review and case presentation. Vasc Endovascular Surg. 2019;53:139–144. [DOI] [PubMed] [Google Scholar]
- 5.Morris ME, Galinanes EL, Nichols WK, et al. Thoracic mural thrombi: a case series and literature review. Ann Vasc Surg. 2011;25:1140.e1117–1140.e1121. [DOI] [PubMed] [Google Scholar]
- 6.Kadoya Y, Zen K, Oda Y, et al. Successful endovascular treatment for aortic thrombosis due to primary antiphospholipid syndrome: a case report and literature review. Vasc Endovascular Surg. 2019;53:51–57. [DOI] [PubMed] [Google Scholar]
- 7.Meyermann K, Trani J, Caputo FJ, et al. Descending thoracic aortic mural thrombus presentation and treatment strategies. J Vasc Surg. 2017;66:931–936. [DOI] [PubMed] [Google Scholar]
- 8.Barbato VA, Castro R, Goertz A. Case report and review of the literature: floating aortic thrombus. Am J Med. 2014;127:e3–e4. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein SA, Evangelista A, Abbara S, et al. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:119–182. [DOI] [PubMed] [Google Scholar]
- 10.Bowdish ME, Weaver FA, Liebman HA, et al. Anticoagulation is an effective treatment for aortic mural thrombi. J Vasc Surg. 2002;36:713–719. [PubMed] [Google Scholar]
- 11.Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. 2014;370:1920–1929. [DOI] [PubMed] [Google Scholar]
9.3.2. Aortic Occlusion
- 1.Grip O, Wanhainen A, Bjorck M. Acute aortic occlusion. Circulation. 2019;139:292–294. [DOI] [PubMed] [Google Scholar]
- 2.Fang L, Lai Z, Qiu C, et al. Endovascular treatment for infrarenal aortic occlusion: a systematic review and meta-analysis. Ann Vasc Surg. 2020;62:432–441.e13. [DOI] [PubMed] [Google Scholar]
- 3.Mayor J, Branco BC, Chung J, et al. Outcome comparison between open and endovascular management of TASC II D aortoiliac occlusive disease. Ann Vasc Surg. 2019;61:65–71.e3. [DOI] [PubMed] [Google Scholar]
9.3.3. Porcelain Aorta
- 1.Eisen A, Tenenbaum A, Koren-Morag N, et al. Calcification of the thoracic aorta as detected by spiral computed tomography among stable angina pectoris patients: association with cardiovascular events and death. Circulation. 2008;118:1328–1334. [DOI] [PubMed] [Google Scholar]
- 2.Mills NL, Everson CT. Atherosclerosis of the ascending aorta and coronary artery bypass. Pathology, clinical correlates, and operative management. J Thorac Cardiovasc Surg. 1991;102:546–553. [PubMed] [Google Scholar]
- 3.Lev-Ran O, Ben-Gal Y, Matsa M, et al. ‘No touch’ techniques for porcelain ascending aorta: comparison between cardiopulmonary bypass with femoral artery cannulation and off-pump myocardial revascularization. J Card Surg. 2002;17:370–376. [DOI] [PubMed] [Google Scholar]
- 4.Sirin G, Sarkislali K, Konakci M, et al. Extraanatomical coronary artery bypass grafting in patients with severely atherosclerotic (porcelain) aorta. J Cardiothorac Surg. 2013;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greelish JP, Soltesz EG, Byrne JG. Valve surgery in octogenarians with a “porcelain” aorta and aortic insufficiency. J Card Surg. 2002;17:285–288. [DOI] [PubMed] [Google Scholar]
- 6.Girardi LN, Krieger KH, Mack CA, et al. No-clamp technique for valve repair or replacement in patients with a porcelain aorta. Ann Thorac Surg. 2005;80:1688–1692. [DOI] [PubMed] [Google Scholar]
9.4. Coarctation of the Aorta (CoA) and Congenital Abnormalities of the Arch
- 1.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. [DOI] [PubMed] [Google Scholar]
- 2.Muzzarelli S, Meadows AK, Ordovas KG, et al. Usefulness of cardiovascular magnetic resonance imaging to predict the need for intervention in patients with coarctation of the aorta. Am J Cardiol. 2012;109:861–865. [DOI] [PubMed] [Google Scholar]
- 3.Teo LL, Cannell T, Babu-Narayan SV, et al. Prevalence of associated cardiovascular abnormalities in 500 patients with aortic coarctation referred for cardiovascular magnetic resonance imaging to a tertiary center. Pediatr Cardiol. 2011;32:1120–1127. [DOI] [PubMed] [Google Scholar]
- 4.Attenhofer Jost CH, Schaff HV, Connolly HM, et al. Spectrum of reoperations after repair of aortic coarctation: importance of an individualized approach because of coexistent cardiovascular disease. Mayo Clin Proc. 2002;77:646–653. [DOI] [PubMed] [Google Scholar]
- 5.Erben Y, Oderich GS, Verhagen HJM, et al. Multicenter experience with endovascular treatment of aortic coarctation in adults. J Vasc Surg. 2019;69:671–679.e1. [DOI] [PubMed] [Google Scholar]
- 6.Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e698–e800. [DOI] [PubMed] [Google Scholar]
- 7.Carr JA. The results of catheter-based therapy compared with surgical repair of adult aortic coarctation. J Am Coll Cardiol. 2006;47:1101–1107. [DOI] [PubMed] [Google Scholar]
- 8.Lala S, Scali ST, Feezor RJ, et al. Outcomes of thoracic endovascular aortic repair in adult coarctation patients. J Vasc Surg. 2018;67:369–381.e2. [DOI] [PubMed] [Google Scholar]
- 9.Meadows J, Minahan M, McElhinney DB, et al. Intermediate outcomes in the prospective, multicenter Coarctation of the Aorta Stent Trial (COAST). Circulation. 2015;131:1656–1664. [DOI] [PubMed] [Google Scholar]
- 10.Roselli EE, Qureshi A, Idrees J, et al. Open, hybrid, and endovascular treatment for aortic coarctation and postrepair aneurysm in adolescents and adults. Ann Thorac Surg. 2012;94:751–756; discussion 757–758. [DOI] [PubMed] [Google Scholar]
- 11.Taggart NW, Minahan M, Cabalka AK, et al. Immediate outcomes of covered stent placement for treatment or prevention of aortic wall injury associated with coarctation of the aorta (COAST II). JACC Cardiovasc Interv. 2016;9:484–493. [DOI] [PubMed] [Google Scholar]
- 12.Egbe AC, Miranda WR, Bonnichsen CR, et al. Potential benefits of ambulatory blood pressure monitoring in coarctation of aorta. J Am Coll Cardiol. 2020;75:2089–2090. [DOI] [PubMed] [Google Scholar]
- 13.Meijs TA, Warmerdam EG, Slieker MG, et al. Medium-term systemic blood pressure after stenting of aortic coarctation: a systematic review and meta-analysis. Heart. 2019;105:1464–1470. [DOI] [PubMed] [Google Scholar]
- 14.Puranik R, Tsang VT, Puranik S, et al. Late magnetic resonance surveillance of repaired coarctation of the aorta. Eur J Cardiothorac Surg. 2009;36:91–95; discussion 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karangelis D, Loggos S, Tzifa A, et al. The aberrant subclavian artery: approach to management. Curr Opin Cardiol. 2020;35:636–642. [DOI] [PubMed] [Google Scholar]
- 16.Plotkin A, Ng B, Han SM, et al. Association of aberrant subclavian arteries with aortic pathology and proposed classification system. J Vasc Surg. 2020;72:1534–1543. [DOI] [PubMed] [Google Scholar]
- 17.Priya S, Thomas R, Nagpal P, et al. Congenital anomalies of the aortic arch. Cardiovasc Diagn Ther. 2018;8(suppl 1):S26–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka A, Milner R, Ota T. Kommerell’s diverticulum in the current era: a comprehensive review. Gen Thorac Cardiovasc Surg. 2015;63:245–259. [DOI] [PubMed] [Google Scholar]
- 19.Kim KM, Cambria RP, Isselbacher EM, et al. Contemporary surgical approaches and outcomes in adults with Kommerell diverticulum. Ann Thorac Surg. 2014;98:1347–1354. [DOI] [PubMed] [Google Scholar]
- 20.Kouchoukos NT, Masetti P. Aberrant subclavian artery and Kommerell aneurysm: surgical treatment with a standard approach. J Thorac Cardiovasc Surg. 2007;133:888–892. [DOI] [PubMed] [Google Scholar]
9.4.1. Coarctation of the Aorta
- 1.Brown ML, Burkhart HM, Connolly HM, et al. Coarctation of the aorta: lifelong surveillance is mandatory following surgical repair. J Am Coll Cardiol. 2013;62:1020–1025. [DOI] [PubMed] [Google Scholar]
- 2.Erben Y, Oderich GS, Verhagen HJM, et al. Multicenter experience with endovascular treatment of aortic coarctation in adults. J Vasc Surg. 2019;69:671–679.e1. [DOI] [PubMed] [Google Scholar]
- 3.Meadows J, Minahan M, McElhinney DB, et al. Intermediate outcomes in the prospective, multicenter Coarctation of the Aorta Stent Trial (COAST). Circulation. 2015;131:1656–1664. [DOI] [PubMed] [Google Scholar]
- 4.Teo LL, Cannell T, Babu-Narayan SV, et al. Prevalence of associated cardiovascular abnormalities in 500 patients with aortic coarctation referred for cardiovascular magnetic resonance imaging to a tertiary center. Pediatr Cardiol. 2011;32:1120–1127. [DOI] [PubMed] [Google Scholar]
- 5.Carr JA. The results of catheter-based therapy compared with surgical repair of adult aortic coarctation. J Am Coll Cardiol. 2006;47:1101–1107. [DOI] [PubMed] [Google Scholar]
- 6.Hager A, Kanz S, Kaemmerer H, et al. Coarctation Long-term Assessment (COALA): significance of arterial hypertension in a cohort of 404 patients up to 27 years after surgical repair of isolated coarctation of the aorta, even in the absence of restenosis and prosthetic material. J Thorac Cardiovasc Surg. 2007;134:738–745. [DOI] [PubMed] [Google Scholar]
- 7.Holzer R, Qureshi S, Ghasemi A, et al. Stenting of aortic coarctation: acute, intermediate, and long-term results of a prospective multi-institutional registry-Congenital Cardiovascular Interventional Study Consortium (CCISC). Catheter Cardiovasc Interv. 2010;76:553–563. [DOI] [PubMed] [Google Scholar]
- 8.Kaushal S, Backer CL, Patel JN, et al. Coarctation of the aorta: midterm outcomes of resection with extended end-to-end anastomosis. Ann Thorac Surg. 2009;88:1932–1938. [DOI] [PubMed] [Google Scholar]
- 9.Lala S, Scali ST, Feezor RJ, et al. Outcomes of thoracic endovascular aortic repair in adult coarctation patients. J Vasc Surg. 2018;67:369–381.e2. [DOI] [PubMed] [Google Scholar]
- 10.Sohrabi B, Jamshidi P, Yaghoubi A, et al. Comparison between covered and bare Cheatham-Platinum stents for endovascular treatment of patients with native post-ductal aortic coarctation: immediate and intermediate-term results. J Am Coll Cardiol Intv. 2014;7:416–423. [DOI] [PubMed] [Google Scholar]
- 11.Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e698–e800. [DOI] [PubMed] [Google Scholar]
- 12.Taggart NW, Minahan M, Cabalka AK, et al. Immediate outcomes of covered stent placement for treatment or prevention of aortic wall injury associated with coarctation of the aorta (COAST II). J Am Coll Cardiol Intv. 2016;9:484–493. [DOI] [PubMed] [Google Scholar]
- 13.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 14.Connolly HM, Huston J 3rd, Brown RD Jr, et al. Intracranial aneurysms in patients with coarctation of the aorta: a prospective magnetic resonance angiographic study of 100 patients. Mayo Clin Proc. 2003;78:1491–1499. [DOI] [PubMed] [Google Scholar]
- 15.Cook SC, Hickey J, Maul TM, et al. Assessment of the cerebral circulation in adults with coarctation of the aorta. Congenit Heart Dis. 2013;8:289–295. [DOI] [PubMed] [Google Scholar]
- 16.Curtis SL, Bradley M, Wilde P, et al. Results of screening for intracranial aneurysms in patients with coarctation of the aorta. AJNR Am J Neuroradiol. 2012;33:1182–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickard SS, Prakash A, Newburger JW, et al. Screening for intracranial aneurysms in coarctation of the aorta: a decision and cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2020;13:e006406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrade L, Hoskoppal A, Hunt Martin M, et al. Intracranial aneurysm and coarctation of the aorta: prevalence in the current era. Cardiol Young. 2021;31:229–232. [DOI] [PubMed] [Google Scholar]
- 19.Attenhofer Jost CH, Schaff HV, Connolly HM, et al. Spectrum of reoperations after repair of aortic coarctation: importance of an individualized approach because of coexistent cardiovascular disease. Mayo Clin Proc. 2002;77:646–653. [DOI] [PubMed] [Google Scholar]
- 20.Oliver JM, Alonso-Gonzalez R, Gonzalez AE, et al. Risk of aortic root or ascending aorta complications in patients with bicuspid aortic valve with and without coarctation of the aorta. Am J Cardiol. 2009;104:1001–1006. [DOI] [PubMed] [Google Scholar]
- 21.Fawzy ME, Fathala A, Osman A, et al. Twenty-two years of follow-up results of balloon angioplasty for discreet native coarctation of the aorta in adolescents and adults. Am Heart J. 2008;156:910–917. [DOI] [PubMed] [Google Scholar]
- 22.Egbe AC, Miranda WR, Bonnichsen CR, et al. Potential benefits of ambulatory blood pressure monitoring in coarctation of aorta. J Am Coll Cardiol. 2020;75:2089–2090. [DOI] [PubMed] [Google Scholar]
- 23.Luitingh TL, Lee MGY, Jones B, et al. A cross-sectional study of the prevalence of exercise-induced hypertension in childhood following repair of coarctation of the aorta. Heart Lung Circ. 2019;28:792–799. [DOI] [PubMed] [Google Scholar]
- 24.Meijs TA, Warmerdam EG, Slieker MG, et al. Medium-term systemic blood pressure after stenting of aortic coarctation: a systematic review and meta-analysis. Heart. 2019;105:1464–1470. [DOI] [PubMed] [Google Scholar]
- 25.Muzzarelli S, Meadows AK, Ordovas KG, et al. Usefulness of cardiovascular magnetic resonance imaging to predict the need for intervention in patients with coarctation of the aorta. Am J Cardiol. 2012;109:861–865. [DOI] [PubMed] [Google Scholar]
- 26.Preventza O, Livesay JJ, Cooley DA, et al. Coarctation-associated aneurysms: a localized disease or diffuse aortopathy. Ann Thorac Surg. 2013;95:1961–1967; discussion 1967. [DOI] [PubMed] [Google Scholar]
- 27.Therrien J, Thorne SA, Wright A, et al. Repaired coarctation: a “cost-effective” approach to identify complications in adults. J Am Coll Cardiol. 2000;35:997–1002. [DOI] [PubMed] [Google Scholar]
- 28.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 29.Roselli EE, Qureshi A, Idrees J, et al. Open, hybrid, and endovascular treatment for aortic coarctation and postrepair aneurysm in adolescents and adults. Ann Thorac Surg. 2012;94:751–756; discussion 757–758. [DOI] [PubMed] [Google Scholar]
- 30.Kim YY, Andrade L, Cook SC. Aortic coarctation. Cardiol Clin. 2020;38:337–351. [DOI] [PubMed] [Google Scholar]
9.4.2.1. ASCA, Kommerell’s Diverticulum
- 1.Dumfarth J, Chou AS, Ziganshin BA, et al. Atypical aortic arch branching variants: a novel marker for thoracic aortic disease. J Thorac Cardiovasc Surg. 2015;149:1586–1592. [DOI] [PubMed] [Google Scholar]
- 2.Yousef S, Singh S, Alkukhun A, et al. Variants of the aortic arch in adult general population and their association with thoracic aortic aneurysm disease. J Card Surg. 2021;36:2348–2354. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka A, Milner R, Ota T. Kommerell’s diverticulum in the current era: a comprehensive review. Gen Thorac Cardiovasc Surg. 2015;63:245–259. [DOI] [PubMed] [Google Scholar]
- 4.Upchurch GR Jr, Escobar GA, Azizzdeh A, et al. Society for Vascular Surgery clinical practice guidelines of thoracic endovascular aortic repair for descending thoracic aortic aneurysms. J Vasc Surg. 2021;73:55S–83S. [DOI] [PubMed] [Google Scholar]
- 5.Vinnakota A, Idrees JJ, Rosinski BF, et al. Outcomes of repair of Kommerell diverticulum. Ann Thorac Surg. 2019;108:1745–1750. [DOI] [PubMed] [Google Scholar]
- 6.Idrees J, Arafat A, Svensson LG, et al. Hybrid repair of aortic aneurysm in patients with previous coarctation. J Thorac Cardiovasc Surg. 2014;148:60–64. [DOI] [PubMed] [Google Scholar]
- 7.Czerny M, Schmidli J, Adler S, et al. Editor’s choice - current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic Surgery (EACTS) & the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2019;57:165–198. [DOI] [PubMed] [Google Scholar]
- 8.Erben Y, Brownstein AJ, Velasquez CA, et al. Natural history and management of Kommerell’s diverticulum in a single tertiary referral center. J Vasc Surg. 2020;71:2004–2011. [DOI] [PubMed] [Google Scholar]
9.4.2.2. Aberrant Left Vertebral Artery Origin
- 1.Magklara EP, Pantelia ET, Solia E, et al. Vertebral artery variations revised: origin, course, branches and embryonic development. Folia Morphol (Warsz). 2021;80:1–12. [DOI] [PubMed] [Google Scholar]
- 2.Rylski B, Schofer F, Beyersdorf F, et al. Aortic arch anatomy in candidates for aortic arch repair. Semin Thorac Cardiovasc Surg. 2021;S1043–0679:00113–1. [DOI] [PubMed] [Google Scholar]
- 3.Rangel-Castilla L, Kalani MY, Cronk K, et al. Vertebral artery transposition for revascularization of the posterior circulation: a critical assessment of temporary and permanent complications and outcomes. J Neurosurg. 2015;122:671–677. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki K, Kazui T, Bashar AH, et al. Total aortic arch replacement in patients with arch vessel anomalies. Ann Thorac Surg. 2006;81:2079–2083. [DOI] [PubMed] [Google Scholar]
- 5.Cooper DG, Walsh SR, Sadat U, et al. Neurological complications after left subclavian artery coverage during thoracic endovascular aortic repair: a systematic review and meta-analysis. J Vasc Surg. 2009;49:1594–1601. [DOI] [PubMed] [Google Scholar]
- 6.Buth J, Harris PL, Hobo R, et al. Neurologic complications associated with endovascular repair of thoracic aortic pathology: Incidence and risk factors. a study from the European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) registry. J Vasc Surg. 2007;46:1103–1110; discussion 1110–1111. [DOI] [PubMed] [Google Scholar]
- 7.Waterford SD, Chou D, Bombien R, et al. Left subclavian arterial coverage and stroke during thoracic aortic endografting: a systematic review. Ann Thorac Surg. 2016;101:381–389. [DOI] [PubMed] [Google Scholar]
- 8.von Allmen RS, Gahl B, Powell JT. Editor’s choice - incidence of stroke following thoracic endovascular aortic repair for descending aortic aneurysm: a systematic review of the literature with meta-analysis. Eur J Vasc Endovasc Surg. 2017;53:176–184. [DOI] [PubMed] [Google Scholar]
9.4.2.3. Bovine Arch (Common Innominate and Left Carotid Artery)
- 1.Hornick M, Moomiaie R, Mojibian H, et al. ‘Bovine’ aortic arch - a marker for thoracic aortic disease. Cardiology. 2012;123:116–124. [DOI] [PubMed] [Google Scholar]
- 2.Wanamaker KM, Amadi CC, Mueller JS, et al. Incidence of aortic arch anomalies in patients with thoracic aortic dissections. J Card Surg. 2013;28:151–154. [DOI] [PubMed] [Google Scholar]
- 3.Marrocco-Trischitta MM, Alaidroos M, Romarowski RM, et al. Aortic arch variant with a common origin of the innominate and left carotid artery as a determinant of thoracic aortic disease: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2020;57:422–427. [DOI] [PubMed] [Google Scholar]
- 4.Berko NS, Jain VR, Godelman A, et al. Variants and anomalies of thoracic vasculature on computed tomographic angiography in adults. J Comput Assist Tomogr. 2009;33:523–528. [DOI] [PubMed] [Google Scholar]
- 5.Layton KF, Kallmes DF, Cloft HJ, et al. Bovine aortic arch variant in humans: clarification of a common misnomer. AJNR Am J Neuroradiol. 2006;27:1541–1542. [PMC free article] [PubMed] [Google Scholar]
- 6.Ye W, Zheng C, Yu D, et al. Lipoxin A4 ameliorates acute pancreatitis-associated acute lung injury through the antioxidative and anti-inflammatory effects of the Nrf2 pathway. Oxid Med Cell Longev. 2019;2019:2197017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumfarth J, Peterss S, Kofler M, et al. In DeBakey type I aortic dissection, bovine aortic arch is associated with arch tears and stroke. Ann Thorac Surg. 2017;104:2001–2008. [DOI] [PubMed] [Google Scholar]
9.5. Tumors
- 1.Diaconu R, Florescu R, Cornelissen A, et al. An unusual case of aortic metastasis from lung cancer. Discoveries (Craiova). 2020;8:e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd C, Silvestri GA. Mediastinal staging of non-small-cell lung cancer. Cancer Control. 2001;8:311–317. [DOI] [PubMed] [Google Scholar]
- 3.Rusthoven CG, Liu AK, Bui MM, et al. Sarcomas of the aorta: a systematic review and pooled analysis of published reports. Ann Vasc Surg. 2014;28:515–525. [DOI] [PubMed] [Google Scholar]
- 4.Fatima J, Duncan AA, Maleszewski JJ, et al. Primary angiosarcoma of the aorta, great vessels, and the heart. J Vasc Surg. 2013;57:756–764. [DOI] [PubMed] [Google Scholar]
- 5.Bohner H, Luther B, Braunstein S, et al. Primary malignant tumors of the aorta: clinical presentation, treatment, and course of different entities. J Vasc Surg. 2003;38:1430–1433. [DOI] [PubMed] [Google Scholar]
- 6.Vacirca A, Faggioli G, Pini R, et al. Predictors of survival in malignant aortic tumors. J Vasc Surg. 2018;67:e100. [DOI] [PubMed] [Google Scholar]
- 7.Chiche L, Mongredien B, Brocheriou I, et al. Primary tumors of the thoracoabdominal aorta: surgical treatment of 5 patients and review of the literature. Ann Vasc Surg. 2003;17:354–364. [DOI] [PubMed] [Google Scholar]
- 8.Salm R Primary fibrosarcoma of aorta. Cancer. 1972;29:73–83. [DOI] [PubMed] [Google Scholar]
- 9.Bendel EC, Maleszewski JJ, Araoz PA. Imaging sarcomas of the great vessels and heart. Semin Ultrasound CT MR. 2011;32:377–404. [DOI] [PubMed] [Google Scholar]
- 10.von Falck C, Meyer B, Fegbeutel C, et al. Imaging features of primary sarcomas of the great vessels in CT, MRI and PET/CT: a single-center experience. BMC Med Imaging. 2013;13:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
10. Physical Activity and Quality of Life
- 1.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 2.Thijssen CGE, Bons LR, Gokalp AL, et al. Exercise and sports participation in patients with thoracic aortic disease: a review. Expert Rev Cardiovasc Ther. 2019;17:251–266. [DOI] [PubMed] [Google Scholar]
- 3.Fuglsang S, Heiberg J, Hjortdal VE, et al. Exercise-based cardiac rehabilitation in surgically treated type-A aortic dissection patients. Scand Cardiovasc J. 2017;51:99–105. [DOI] [PubMed] [Google Scholar]
- 4.Hornsby WE, Norton EL, Fink S, et al. Cardiopulmonary exercise testing following open repair for a proximal thoracic aortic aneurysm or dissection. J Cardiopulm Rehabil Prev. 2020;40:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaddha A, Eagle KA, Braverman AC, et al. Exercise and physical activity for the post-aortic dissection patient: the clinician’s conundrum. Clin Cardiol. 2015;38:647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaddha A, Kline-Rogers E, Woznicki EM, et al. Cardiology patient page. Activity recommendations for postaortic dissection patients. Circulation. 2014;130:e140–e142. [DOI] [PubMed] [Google Scholar]
- 7.Franke UFW, Isecke A, Nagib R, et al. Quality of life after aortic root surgery: reimplantation technique versus composite replacement. Ann Thorac Surg. 2010;90:1869–1875. [DOI] [PubMed] [Google Scholar]
- 8.Pasadyn SR, Roselli EE, Artis AS, et al. From tear to fear: posttraumatic stress disorder in patients with acute type A aortic dissection. J Am Heart Assoc. 2020;9:e015060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaddha A, Kline-Rogers E, Braverman AC, et al. Survivors of aortic dissection: activity, mental health, and sexual function. Clin Cardiol. 2015;38:652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coughlin PA, Jackson D, White AD, et al. Meta-analysis of prospective trials determining the short- and mid-term effect of elective open and endovascular repair of abdominal aortic aneurysms on quality of life. Br J Surg. 2013;100:448–455. [DOI] [PubMed] [Google Scholar]
- 11.Lohse F, Lang N, Schiller W, et al. Quality of life after replacement of the ascending aorta in patients with true aneurysms. Tex Heart Inst J. 2009;36:104–110. [PMC free article] [PubMed] [Google Scholar]
- 12.de Heer F, Gokalp AL, Kluin J, et al. Measuring what matters to the patient: health related quality of life after aortic valve and thoracic aortic surgery. Gen Thorac Cardiovasc Surg. 2019;67:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duncan R, Essat M, Jones G, et al. Systematic review and qualitative evidence synthesis of patient-reported outcome measures for abdominal aortic aneurysm. Br J Surg. 2017;104:317–327. [DOI] [PubMed] [Google Scholar]
- 14.Gokalp AL, Takkenberg JJM. Decision-making in thoracic aortic aneurysm surgery-clinician and patient view. Semin Thorac Cardiovasc Surg. 2019;31:638–642. [DOI] [PubMed] [Google Scholar]
- 15.Tan MK, Jarral OA, Thong EH, et al. Quality of life after mitral valve intervention. Interact Cardiovasc Thorac Surg. 2017;24:265–272. [DOI] [PubMed] [Google Scholar]
- 16.Peach G, Romaine J, Wilson A, et al. Design of new patient-reported outcome measures to assess quality of life, symptoms and treatment satisfaction in patients with abdominal aortic aneurysm. Br J Surg. 2016;103:1003–1011. [DOI] [PubMed] [Google Scholar]
- 17.Brady AR, Thompson SG, Fowkes FG, et al. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation. 2004;110:16–21. [DOI] [PubMed] [Google Scholar]
- 18.Badger SA, Jones C, McClements J, et al. Surveillance strategies according to the rate of growth of small abdominal aortic aneurysms. Vasc Med. 2011;16:415–421. [DOI] [PubMed] [Google Scholar]
- 19.Bown MJ, Sweeting MJ, et al. RESCAN Collaborators, Surveillance intervals for small abdominal aortic aneurysms: a meta-analysis. JAMA. 2013;309:806–813. [DOI] [PubMed] [Google Scholar]
- 20.Lomazzi C, Mascoli C, de Beaufort HWL, et al. Gender related access complications after TEVAR: analysis from the Retrospective Multicentre Cohort GORE® GREAT Registry Study. Eur J Vasc Endovasc Surg. 2020;60:203–209. [DOI] [PubMed] [Google Scholar]
- 21.Hendy K, Gunnarson R, Golledge J. Growth rates of small abdominal aortic aneurysms assessed by computerised tomography-a systematic literature review. Atherosclerosis. 2014;235:182–188. [DOI] [PubMed] [Google Scholar]
- 22.Oliver-Williams C, Sweeting MJ, Jacomelli J, et al. Safety of men with small and medium abdominal aortic aneurysms under surveillance in the NAAASP. Circulation. 2019;139:1371–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maron BJ, Chaitman BR, Ackerman MJ, et al. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation. 2004;109:2807–2816. [DOI] [PubMed] [Google Scholar]
- 24.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. [DOI] [PubMed] [Google Scholar]
- 25.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 26.Cheng A, Owens D. Marfan syndrome, inherited aortopathies and exercise: what is the right answer? Heart. 2015;101:752–757. [DOI] [PubMed] [Google Scholar]
- 27.Delsart P, Maldonado-Kauffmann P, Bic M, et al. Post aortic dissection: gap between activity recommendation and real life patients aerobic capacities. Int J Cardiol. 2016;219:271–276. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama A, Morita H, Nagayama M, et al. Cardiac rehabilitation protects against the expansion of abdominal aortic aneurysm. J Am Heart Assoc. 2018;7:e007959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatzaras IS, Bible JE, Koullias GJ, et al. Role of exertion or emotion as inciting events for acute aortic dissection. Am J Cardiol. 2007;100:1470–1472. [DOI] [PubMed] [Google Scholar]
- 30.Ulug P, Powell JT, Warschkow R, et al. Editor’s choice - sex specific differences in the management of descending thoracic aortic aneurysms: systematic review with meta-analysis. Eur J Vasc Endovasc Surg. 2019;58:503–511. [DOI] [PubMed] [Google Scholar]
- 31.Marcaccio CL, Schermerhorn ML. Epidemiology of abdominal aortic aneurysms. Semin Vasc Surg. 2021;34:29–37. [DOI] [PubMed] [Google Scholar]
- 32.Thompson S, Brown L, Sweeting M, et al. Systematic review and meta-analysis of the growth and rupture rates of small abdominal aortic aneurysms: implications for surveillance intervals and their cost-effectiveness. Health Technol Assess. 2013;17:1–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salehi Ravesh M, Langguth P, Pfarr JA, et al. Non-contrast-enhanced magnetic resonance imaging for visualization and quantification of endovascular aortic prosthesis, their endoleaks and aneurysm sacs at 1.5T. Magn Reson Imaging. 2019;60:164–172. [DOI] [PubMed] [Google Scholar]
- 34.Kawada H, Goshima S, Sakurai K, et al. Utility of noncontrast magnetic resonance angiography for aneurysm follow-up and detection of endoleaks after endovascular aortic repair. Korean J Radiol. 2021;22:513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fairman AS, Beck AW, Malas MB, et al. Reinterventions in the modern era of thoracic endovascular aortic repair. J Vasc Surg. 2020;71:408–422. [DOI] [PubMed] [Google Scholar]
- 36.Coselli JS, Bozinovski J, LeMaire SA. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg. 2007;83:S862–S864; discussion S890–S892. [DOI] [PubMed] [Google Scholar]
- 37.Aune D, Sen A, Kobeissi E, et al. Physical activity and the risk of abdominal aortic aneurysm: a systematic review and meta-analysis of prospective studies. Sci Rep. 2020;10:22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mas-Stachurska A, Siegert AM, Batlle M, et al. Cardiovascular benefits of moderate exercise training in Marfan syndrome: insights from an animal model. J Am Heart Assoc. 2017;6:e006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibson C, Nielsen C, Alex R, et al. Mild aerobic exercise blocks elastin fiber fragmentation and aortic dilatation in a mouse model of Marfan syndrome associated aortic aneurysm. J Appl Physiol (1985). 2017;123:147–160. [DOI] [PubMed] [Google Scholar]
- 40.Thijssen CGE, Dekker S, Bons LR, et al. Health-related quality of life and lived experiences in males and females with thoracic aortic disease and their partners. Open Heart. 2020;7:e001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jarral OA, Kidher E, Patel VM, et al. Quality of life after intervention on the thoracic aorta. Eur J Cardiothorac Surg. 2016;49:369–389. [DOI] [PubMed] [Google Scholar]
11. Cost and Value Considerations
- 1.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129:2329–2345. [DOI] [PubMed] [Google Scholar]
- 2.Thompson SG, Ashton HA, Gao L, et al. Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomised Multicentre Aneurysm Screening Study. BMJ. 2009;338:b2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarrouk M, Lundqvist A, Holst J, et al. Cost-effectiveness of screening for abdominal aortic aneurysm in combination with medical intervention in patients with small aneurysms. Eur J Vasc Endovasc Surg. 2016;51:766–773. [DOI] [PubMed] [Google Scholar]
- 4.Wanhainen A, Lundkvist J, Bergqvist D, et al. Cost-effectiveness of screening women for abdominal aortic aneurysm. J Vasc Surg. 2006;43:908–914; discussion 914. [DOI] [PubMed] [Google Scholar]
- 5.Nargesi S, Abutorabi A, Alipour V, et al. Cost-effectiveness of endovascular versus open repair of abdominal aortic aneurysm: a systematic review. Cardiovasc Drugs Ther. 2021;35:829–839. [DOI] [PubMed] [Google Scholar]
- 6.Trooboff SW, Wanken ZJ, Gladders B, et al. Longitudinal spending on endovascular and open abdominal aortic aneurysm repair. Circ Cardiovasc Qual Outcomes. 2020;13:e006249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulug P, Hinchliffe RJ, Sweeting MJ, et al. Strategy of endovascular versus open repair for patients with clinical diagnosis of ruptured abdominal aortic aneurysm: the IMPROVE RCT. Health Technol Assess. 2018;22:1–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IMPROVE Trial Investigators. Comparative clinical effectiveness and cost effectiveness of endovascular strategy v open repair for ruptured abdominal aortic aneurysm: three year results of the IMPROVE randomised trial. BMJ. 2017;359:j4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stroupe KT, Lederle FA, Matsumura JS, et al. Cost-effectiveness of open versus endovascular repair of abdominal aortic aneurysm in the OVER trial. J Vasc Surg. 2012;56:901–909.e902. [DOI] [PubMed] [Google Scholar]
- 10.Epstein D, Sculpher MJ, Powell JT, et al. Long-term cost-effectiveness analysis of endovascular versus open repair for abdominal aortic aneurysm based on four randomized clinical trials. Br J Surg. 2014;101:623–631. [DOI] [PubMed] [Google Scholar]
- 11.van Bochove CA, Burgers LT, Vahl AC, et al. Cost-effectiveness of open versus endovascular repair of abdominal aortic aneurysm. J Vasc Surg. 2016;63:827–838.e822. [DOI] [PubMed] [Google Scholar]
- 12.Arnaoutakis GJ, Hundt JA, Shah AS, et al. Comparative analysis of hospital costs of open and endovascular thoracic aortic repair. Vasc Endovascular Surg. 2011;45:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillen JR, Schaheen BW, Yount KW, et al. Cost analysis of endovascular versus open repair in the treatment of thoracic aortic aneurysms. J Vasc Surg. 2015;61:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Locham S, Dakour-Aridi H, Nejim B, et al. Outcomes and cost of open versus endovascular repair of intact thoracoabdominal aortic aneurysm. J Vasc Surg. 2018;68:948–955.e1. [DOI] [PubMed] [Google Scholar]
- 15.McClure RS, Brogly SB, Lajkosz K, et al. Economic burden and healthcare resource use for thoracic aortic dissections and thoracic aortic aneurysms-a population-based cost-of-illness analysis. J Am Heart Assoc. 2020;9:e014981. [DOI] [PMC free article] [PubMed] [Google Scholar]
12.1. Biomarker Studies
- 1.Girdauskas E, Petersen J, Neumann N, et al. Novel approaches for BAV aortopathy prediction-is there a need for cohort studies and biomarkers? Biomolecules. 2018;8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai W, Suzuki H, Huang J, et al. A population-based phenome-wide association study of cardiac and aortic structure and function. Nat Med. 2020;26:1654–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lesiak M, Augusciak-Duma A, Stepien KL, et al. Searching for new molecular markers for cells obtained from abdominal aortic aneurysm. J Appl Genet. 2021;62:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maredia AK, Greenway SC, Verma S, et al. Bicuspid aortic valve-associated aortopathy: update on biomarkers. Curr Opin Cardiol. 2018;33:134–139. [DOI] [PubMed] [Google Scholar]
- 5.Michelena HI, Della Corte A, Evangelista A, et al. International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and its aortopathy, for clinical, surgical, interventional and research purposes. Ann Thorac Surg. 2021;112:e203–e235. [DOI] [PubMed] [Google Scholar]
- 6.Portelli SS, Robertson EN, Malecki C, et al. Epigenetic influences on genetically triggered thoracic aortic aneurysm. Biophys Rev. 2018;10:1241–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu S, Zhu K, Li J, et al. Nano-biomaterials for the delivery of therapeutic and monitoring cues for aortic diseases. Front Bioeng Biotechnol. 2020;8:583879. [DOI] [PMC free article] [PubMed] [Google Scholar]
12.2. Genetic and Nongenetic Factors
- 1.Ashvetiya T, Fan SX, Chen YJ, et al. Identification of novel genetic susceptibility loci for thoracic and abdominal aortic aneurysms via genome-wide association study using the UK Biobank Cohort. PLoS One. 2021;16:e0247287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faggion Vinholo T, Zafar MA, Ziganshin BA, et al. Nonsyndromic thoracic aortic aneurysms and dissections-is screening possible? Semin Thorac Cardiovasc Surg. 2019;31:628–634. [DOI] [PubMed] [Google Scholar]
- 3.Kaluza J, Stackelberg O, Harris HR, et al. Mediterranean diet is associated with reduced risk of abdominal aortic aneurysm in smokers: results of two prospective cohort studies. Eur J Vasc Endovasc Surg. 2021;62:284–293. [DOI] [PubMed] [Google Scholar]
- 4.Kaluza J, Stackelberg O, Harris HR, et al. Anti-inflammatory diet and risk of abdominal aortic aneurysm in two Swedish cohorts. Heart. 2019;105:1876–1883. [DOI] [PubMed] [Google Scholar]
- 5.Lai CC, Lu CT, Kao KC, et al. Association of fluoroquinolones use with the risk of aortic aneurysm or aortic dissection: facts and myths. J Microbiol Immunol Infect. 2021;54:182–184. [DOI] [PubMed] [Google Scholar]
- 6.Milewicz DM, Regalado ES. Use of genetics for personalized management of heritable thoracic aortic disease: how do we get there? J Thorac Cardiovasc Surg. 2015;149:S3–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mordi IR, Forsythe RO, Gellatly C, et al. Plasma desmosine and abdominal aortic aneurysm disease. J Am Heart Assoc. 2019;8:e013743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newton ER, Akerman AW, Strassle PD, et al. Association of fluoroquinolone use with short-term risk of development of aortic aneurysm. JAMA Surg. 2021;156:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stackelberg O, Bjorck M, Larsson SC, et al. Fruit and vegetable consumption with risk of abdominal aortic aneurysm. Circulation. 2013;128:795–802. [DOI] [PubMed] [Google Scholar]
- 10.Stackelberg O, Bjorck M, Larsson SC, et al. Alcohol consumption, specific alcoholic beverages, and abdominal aortic aneurysm. Circulation. 2014;130:646–652. [DOI] [PubMed] [Google Scholar]
12.3. Biomechanics of the Aorta
- 1.Cebull HL, Rayz VL, Goergen CJ. Recent advances in biomechanical characterization of thoracic aortic aneurysms. Front Cardiovasc Med. 2020; 7:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xuan Y, Wisneski AD, Wang Z, et al. Regional biomechanical and failure properties of healthy human ascending aorta and root. J Mech Behav Biomed Mater. 2021;123:104705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xuan Y, Wang Z, Liu R, et al. Wall stress on ascending thoracic aortic aneurysms with bicuspid compared with tricuspid aortic valve. J Thorac Cardiovasc Surg. 2018;156:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xuan Y, D’Souza SN, Wang Z, et al. Patient-specific biomechanics in Marfan ascending thoracic aortic aneurysms. Ann Thorac Surg. Published online August 17, 2021. doi: 10.1016/j.athoracsur.2021.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
12.4. Sex, Race, and Ethnicity
- 1.Chen JF, Zafar MA, Wu J, et al. Increased virulence of descending thoracic and thoracoabdominal aortic aneurysms in women. Ann Thorac Surg. 2021;112:45–52. [DOI] [PubMed] [Google Scholar]
- 2.Cheung K, Boodhwani M, Chan KL, et al. Thoracic aortic aneurysm growth: role of sex and aneurysm etiology. J Am Heart Assoc. 2017;6:e003792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou EL, Pettinger M, Haring B, et al. Association of premature menopause with risk of abdominal aortic aneurysm in the Women’s Health Initiative. Ann Surg. Published online November 4, 2020. doi: 10.1097/SLA.0000000000004581. [DOI] [PubMed] [Google Scholar]
- 4.Deery SE, Soden PA, Zettervall SL, et al. Sex differences in mortality and morbidity following repair of intact abdominal aortic aneurysms. J Vasc Surg. 2017;65:1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo RC, Schermerhorn ML. Abdominal aortic aneurysms in women. J Vasc Surg. 2016;63:839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicolini F, Vezzani A, Corradi F, et al. Gender differences in outcomes after aortic aneurysm surgery should foster further research to improve screening and prevention programmes. Eur J Prev Cardiol. 2018;25:32–41. [DOI] [PubMed] [Google Scholar]
- 7.Nienaber CA, Fattori R, Mehta RH, et al. Gender-related differences in acute aortic dissection. Circulation. 2004;109:3014–3021. [DOI] [PubMed] [Google Scholar]
- 8.Olson SL, Wijesinha MA, Panthofer AM, et al. Evaluating growth patterns of abdominal aortic aneurysm diameter with serial computed tomography surveillance. JAMA Surg. 2021;156:363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preventza O, Cekmecelioglu D, Chatterjee S, et al. Sex differences in ascending aortic and arch surgery: a propensity-matched comparison of 1153 pairs. Ann Thorac Surg. 2022;113:1153–1158. [DOI] [PubMed] [Google Scholar]
- 10.Rylski B, Georgieva N, Beyersdorf F, et al. Gender-related differences in patients with acute aortic dissection type A. J Thorac Cardiovasc Surg. 2021;162:528–535.e1. [DOI] [PubMed] [Google Scholar]
- 11.Spiliotopoulos K, Price MD, Amarasekara HS, et al. Are outcomes of thoracoabdominal aortic aneurysm repair different in men versus women? A propensity-matched comparison. J Thorac Cardiovasc Surg. 2017;154:1203–1214.e1206. [DOI] [PubMed] [Google Scholar]
- 12.Tomee SM, Lijftogt N, Vahl A, et al. A registry-based rationale for discrete intervention thresholds for open and endovascular elective abdominal aortic aneurysm repair in female patients. J Vasc Surg. 2018;67:735–739. [DOI] [PubMed] [Google Scholar]
- 13.Tumer NB, Askin G, Akkaya BB, et al. Outcomes after EVAR in females are similar to males. BMC Cardiovasc Disord. 2021;21:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vervoort D, Canner JK, Haut ER, et al. Racial disparities associated with reinterventions after elective endovascular aortic aneurysm repair. J Surg Res. 2021;268:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Lehman EB, Aziz F. African Americans are less likely to have elective endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2019;70:462–470. [DOI] [PubMed] [Google Scholar]
- 16.Yin K, AlHajri N, Rizwan M, et al. Black patients have a higher burden of comorbidities but a lower risk of 30-day and 1-year mortality after thoracic endovascular aortic repair. J Vasc Surg. 2021;73:2071–2080.e2. [DOI] [PubMed] [Google Scholar]
- 17.Cho L, Kibbe MR, Bakaeen F, et al. Cardiac Surgery in Women in the Current Era: What Are the Gaps in Care? Circulation. 2021;144:1172–1185. [DOI] [PubMed] [Google Scholar]
- 18.Mayor JM, Preventza O, McGinigle K, et al. Persistent under-representation of female patients in United States trials of common vascular diseases from 2008 to 2020. J Vasc Surg. 2022;75:30–36. [DOI] [PubMed] [Google Scholar]
- 19.Preventza O, Critsinelis A, Simpson K, et al. Sex, racial, and ethnic disparities in U.S. cardiovascular trials in more than 230, 000 patients. Ann Thorac Surg. 2021;112:726–735. [DOI] [PubMed] [Google Scholar]
12.5. Quality of Life in Patients With Aortic Disease
- 1.Archer S, Pinto A, Vuik S, et al. Surgery, complications, and quality of life: a longitudinal cohort study exploring the role of psychosocial factors. Ann Surg. 2019;270:95–101. [DOI] [PubMed] [Google Scholar]
- 2.Blakeslee-Carter J, Menon AJ, Novak Z, et al. Association of mental health disorders and aortic dissection. Ann Vasc Surg. 2021;77:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghanta RK, Green SY, Price MD, et al. Midterm survival and quality of life after extent II thoracoabdominal aortic repair in Marfan syndrome. Ann Thorac Surg. 2016;101:1402–1409; discussion 1409. [DOI] [PubMed] [Google Scholar]
- 4.Luo ZR, Liao DS, Chen LW. Comparative analysis of postoperative sexual dysfunction and quality of life in type A aortic dissection patients of different ages. J Cardiothorac Surg. 2021;16:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nilsson O, Stenman M, Letterstal A, et al. A randomized clinical trial of an eHealth intervention on anxiety in patients undergoing abdominal aortic aneurysm surgery. Br J Surg. 2021;108:917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smolock CJ, Xiang F, Roselli EE, et al. Health-related quality of life after extensive aortic replacement. Semin Thorac Cardiovasc Surg. 2021;S1043–0679:00318–X. [DOI] [PubMed] [Google Scholar]
- 7.Thijssen CGE, Doze DE, Gokalp AL, et al. Male-female differences in quality of life and coping style in patients with Marfan syndrome and hereditary thoracic aortic diseases. J Genet Couns. 2020;29:1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velvin G, Wilhelmsen JE, Johansen H, et al. Systematic review of quality of life in persons with hereditary thoracic aortic aneurysm and dissection diagnoses. Clin Genet. 2019;95:661–676. [DOI] [PubMed] [Google Scholar]
12.6. New Endovascular Technology
- 1.Czerny M, Rylski B, Morlock J, et al. Orthotopic branched endovascular aortic arch repair in patients who cannot undergo classical surgery. Eur J Cardiothorac Surg. 2018;53:1007–1012. [DOI] [PubMed] [Google Scholar]
- 2.D’Onofrio A, Cibin G, Antonello M, et al. Endovascular exclusion of the entire aortic arch with branched stent-grafts after surgery for acute type A aortic dissection. JTCVS Tech. 2020;3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haulon S, Greenberg RK, Spear R, et al. Global experience with an inner branched arch endograft. J Thorac Cardiovasc Surg. 2014;148:1709–1716. [DOI] [PubMed] [Google Scholar]
- 4.Kan X, Ma T, Lin J, et al. Patient-specific simulation of stent-graft deployment in type B aortic dissection: model development and validation. Biomech Model Mechanobiol. 2021;20:2247–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z, Wu L, Yang J, et al. Thoracic aorta stent grafts design in terms of biomechanical investigations into flexibility. Math Biosci Eng. 2020;18:800–816. [DOI] [PubMed] [Google Scholar]
- 6.Oderich GS, Tenorio ER, Mendes BC, et al. Midterm outcomes of a prospective, nonrandomized study to evaluate endovascular repair of complex aortic aneurysms using fenestrated-branched endografts. Ann Surg. 2021;274:491–499. [DOI] [PubMed] [Google Scholar]
12.7. Optimal Exercise and Rehabilitation Protocols
- 1.Castaneda-Lopez J, Vasquez-Jimenez C, Medina-Soto L, et al. Cardiac rehabilitation in a patient with DeBakey IIIb aortic disecction case report and literature review. Arch Cardiol Mex. 2020;90:309–312. [DOI] [PubMed] [Google Scholar]
- 2.DeFabio DC, DeFabio CJ. Exercise parameters for the chronic type B aortic dissection patient: a literature review and case report. Postgrad Med. 2021;133:217–222. [DOI] [PubMed] [Google Scholar]
- 3.Delsart P, Maldonado-Kauffmann P, Bic M, et al. Post aortic dissection: gap between activity recommendation and real life patients aerobic capacities. Int J Cardiol. 2016;219:271–276. [DOI] [PubMed] [Google Scholar]
- 4.Fuglsang S, Heiberg J, Hjortdal VE, et al. Exercise-based cardiac rehabilitation in surgically treated type-A aortic dissection patients. Scand Cardiovasc J. 2017;51:99–105. [DOI] [PubMed] [Google Scholar]
12.8. Equitable Care and Training Opportunities
- 1.Holscher CM, Weaver ML, Black JH 3rd, et al. Regional market competition is associated with aneurysm diameter at the time of EVAR. Ann Vasc Surg. 2021;70:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullan CW, Mori M, Bin Mahmood SU, et al. Incidence and characteristics of hospitalization for proximal aortic surgery for acute syndromes and for aneurysms in the USA from 2005 to 2014. Eur J Cardiothorac Surg. 2020;58:583–589. [DOI] [PubMed] [Google Scholar]
- 3.Zettervall SL, Buck DB, Soden PA, et al. Regional variation exists in patient selection and treatment of abdominal aortic aneurysms. J Vasc Surg. 2016;64:921–927.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


