1. Introduction
Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) has cause significant morbidity and mortality throughout the world. Several pharmacotherapies have been developed to decrease the progression of disease. Monoclonal antibodies have been granted emergency use authorization (EUA) by the Food and Drug Administration to decrease the progression to severe disease. Due to the recent EUA, serious or unexpected adverse reactions not previously reported, may occur.
2. Case report
A 58-year-old male with only a past medical history of obesity (BMI of 32), who was not vaccinated against SARS-CoV-2, presented for monoclonal antibody infusion due to a new COVID-19 diagnosis. He was on day 5 of symptoms, which included chills, diaphoresis, generalized weakness, and anorexia. He presented for a nurse-only appointment for infusion of casirivimab 600 mg/imdevimab 600 mg (REGN-COV2). Vitals prior to infusion included a temperature of 37.1oC, blood pressure of 98/70 mmHg, heart rate of 98/minute, respiratory rate of 18/minute, and an oxygen saturation of 98% on room air. The 21 min infusion was completed without incident. Thirty minutes later, during post-infusion monitoring, the patient was found to be hypoxic and tachypneic, with an oxygen saturation of 77% on room air and a respiratory rate of 24/minute. He was transported to the emergency department, placed on a monitor and nonrebreather with saturations improving to 95%. Over the next 40 min, he experienced progressive respiratory distress, and the patient's saturation continued to drop. He was subsequently placed on high-flow nasal cannula (HFNC) at 60 L: 58% FiO2 with saturations remaining above 92%. An arterial blood gas while on high flow revealed pH of 7.49, PCO2 of 37, PO2 of 71, HCO3 of 28, and O2 saturation of 95.9%. Laboratory values showed a white blood cell count of 9.6 with a normal differential and a troponin was normal. The D-dimer was elevated to 1.8 ug/mL and resulting CT scan showed extensive peripheral bilateral multi-lobar ground glass opacities without evidence of pulmonary embolism (Fig. 1 ). Variant sequencing was ordered which resulted as B.1.617.2, or Delta variant. He was started on dexamethasone and admitted to intensive care unit (ICU) for further care.
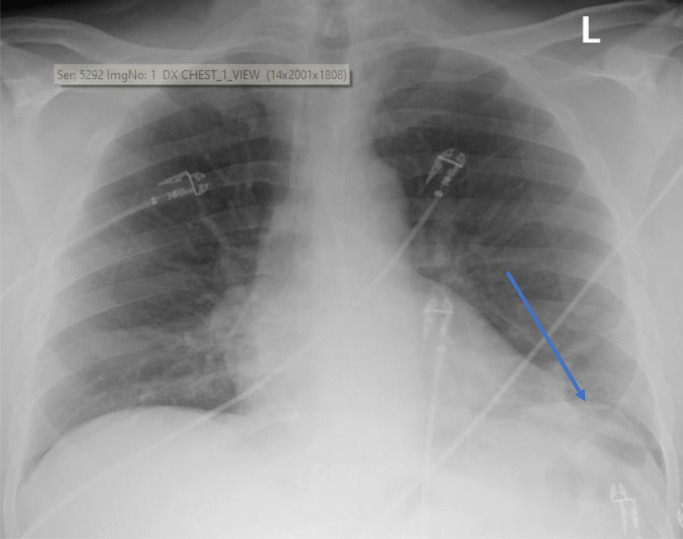
Fig. 1.
Chest x-ray taken on day prior to casirivimab/imdevimab infusion during the patient's ED admission. Imaging read from radiologist: “normal heart size. Curvilinear left lung base opacity likely reflecting atelectasis (arrow). Otherwise, lungs are clear. No effusion.”.
While in the ICU, the patient was continued on HFNC and intermittent BIPAP/CPAP for treatment of acute hypoxemic respiratory failure. Given the patient's rapid respiratory decompensation, tocilizumab (8 mg/kg) was added on hospital day 1 and administered in conjunction with an extended course of corticosteroids. Despite these interventions, he remained tachypneic and hypoxic. Repeat imaging on hospital day 7 demonstrated worsening bilateral opacities concerning for hospital-acquired pneumonia for which the patient received a 5-day course of cefepime. After 13 days in the ICU, the patient was titrated from HFNC to nasal cannula at 5 L oxygen and transferred to the general medicine floor. He was eventually weaned to room air while at rest but required 4 L of oxygen while ambulating. After 16 days, the patient was discharged from the hospital on home oxygen (Fig. 2 ).
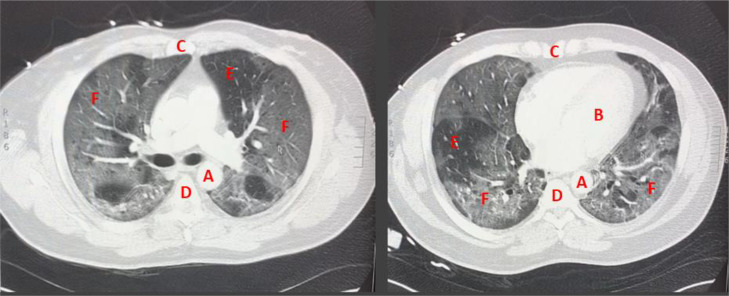
Fig. 2.
Computed tomography angiography chest (CT-PE) scan, taken post antibody infusion while patient was on high flow nasal canula, showing bilateral multi-lobar ground glass opacities without evidence of pulmonary embolism. Key: A - Aorta B - Left ventricle C - Sternum (anterior) D - Thoracic vertebrae (posterior) E - relatively normal lung tissue F - areas of ground glass opacities.
3. Discussion
On November 21st, 2020, the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) to permit the use of casirivimab/imdevimab for the treatment of mild to moderate COVID-19 in non-hospitalized adults who are at high risk for progressing to severe disease and/or hospitalization.1 This EUA defines high risk patients as those who are ≥65 years of age, have a BMI ≥25, or any of the following disease states: chronic kidney disease, diabetes mellitus, significant immunosuppression, pregnancy, cardiovascular disease, hypertension, chronic respiratory disease, neurodevelopmental disorders, or those having a medically-related technological dependence. Antibody therapy has been used to treat patients during other viral outbreaks in the absence of other effective antiviral therapeutics. In the above presented case, we hypothesize that antibody-dependent ADE occurred due to the infusion of casirivimab/imdevimab, which led to an acute increase in severity of COVID-19 pneumonia. Prior to infusion, the patient had mild COVID-19 symptoms with normal oxygen saturations. After the infusion, the patient developed acute hypoxic respiratory failure. He had no other associated signs commonly seen with anaphylaxis or other medication hypersensitivity reactions.
Trends in COVID-19 pathogenesis showed the predominant trending at the time was variant B.1.617.2 (Delta variant), which has been associated with unique viral characteristics when compared to previous variants. Wang et al. evaluated pharyngeal swabs of Delta variant vs wild type and found Delta variant had a significant higher viral load. Furthermore, they found Delta variant had a shorter incubation period of 4 days vs 6 days, when compared to the wild type.2 REGN-COV2 is a combination therapy of human immunoglobulin G-1 monoclonal antibody (mAb) with unmodified Fc regions, which binds to non-overlapping epitomes of the spike protein receptor domain. In vitro activity shows antibody dependent cell-mediated cytotoxicity via activation of natural killer cells, and antibody dependent cellular phagocytosis via activation of human macrophages.3
ADE of disease theoretically can occur in two ways: enhanced infection and enhanced immune activation. The latter is caused by excessive antibody Fc-mediated effector functions or immune complex formation. The phenotypical manifestation of this type of ADE is exacerbation of disease severity and inflammatory markers. ADE, specifically respiratory enhancement of disease, has been observed in patients with viral infections including measles and RSV.4 , 5 It has also been observed that cross reacting antibodies can enhance symptoms in patients with sequential infection with more than one serotype of Dengue.6 Gollins and Porterfield also demonstrated that West Nile viral particles will aggregate will into clusters when exposed to monoclonal human antibody.7 This provides two theoretical potential explanations for the acute decompensation in our patient. In ADE observed with sequential Dengue infection with various serotypes, it is thought that antibodies are highly cross-reactive but do not fully neutralize, alternatively rendering some non-infectious Dengue viral particles infectious.6 Given the quick deterioration in our patient, the in vitro observation with West Nile virus may provide a more plausible explanation. High SARS-CoV-2 viral loads within the lung parenchyma exposed to monoclonal antibody led to viral particle/mAb clumping and immune activation.
There are previous reports of ADE after both the COVID-19 vaccine and monoclonal antibody treatment and vaccine.8 , 9 Because of the temporal relationship of the reaction following the infusion, the adverse event confirmed by objective evidence on the CT scan, a Naranjo Adverse Drug Reaction Probability Scale reveals the monoclonal antibody was the possible cause of the patients deterioration.10 As for alternative causes for the acute hypoxia, the patient had no other signs or symptoms of an acute allergic reaction—such as hives or flushing. Also, the CT scan ruled out pulmonary embolism. A cardiac abnormality was unlikely due to the absence of EKG changes, troponin or BNP elevations.
4. Conclusion
Due to the rapidly evolving nature of the SARS-CoV-2 virus and therapeutics used to treat this disease, many of which have been granted EUA status by the FDA, clinicians should be aware that serious adverse events, such as those present in our patient, can occur at any time. Antibody dependent enhancement can lead to more severe illness, result in cytokine storm and further tissue damage.
Question 1
Question Type True & False
Question Text Patients must have a high viral load in order to experience potential antibody dependent enhancement?
Answer Options
-
(a)
True
-
(b)
False
Correct Answer = b
False. While this adverse event appears to be rare, it is important that providers are aware of this potential, life-threatening adverse reaction. While prophylactic use of monoclonal antibodies in high-risk individuals, without a diagnosis of COVID-19 may be beneficial because of low antigen load, the use of these same monoclonal antibodies in patients with confirmed disease may put patients at higher risk of ADE due to an increased amount of antigen in the body. A recent study showed that viral shedding in the upper respiratory tract was indistinguishable between patients with asymptomatic and symptomatic COVID-19. Symptomatic patients showed higher anti-SARS-CoV-2 antibody titres and cleared the virus from the upper respiratory tract more quickly, contradicting a simpler hypothesis that antibody titres are simply caused by higher viral loads
Ref: Long, Q. X. et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 26, 1200–1204 (2020).
Question 2
Question Type True & False
Question Text Antibody dependent enhancement can occur after the treatment of a disease with the administration of antibody therapy, but not with the preventative therapeutic such as a vaccine?
Answer Options
-
(a)
True
-
(b)
False
Correct Answer = b
False. Antibody dependent enhancement has been reported with both the use of vaccines and monoclonal antibody administration.
Ref:
-
1
Hegazy, A.N., Krönke, J., Angermair, S. et al. Anti-SARS-CoV2 antibody-mediated cytokine release syndrome in a patient with acute promyelocytic leukemia. BMC Infect Dis 22, 537 (2022).
-
2
Sridhar P, Singh A, Salomon N, Steiger D. Vaccine-induced antibody dependent enhancement in COVID-19. Chest 2022 Oct;162(4):A646–7. doi: 10.1016/jchest.2022.08.506.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.FDA, Fact sheet for healthcare providers: emergency use authorization (EUA) of REGEN-COV (casirivimab and imdevimab). (2020).
- 2.Wang Y., et al. Transmission, viral kinetics and clinical characteristics of the emergent SARS-CoV-2 Delta VOC in Guangzhou, China. EClinicalMedicine. 2021;40 doi: 10.1016/j.eclinm.2021.101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuzmina N.A., et al. Antibody-dependent enhancement of ebola virus infection by human antibodies isolated from survivors. Cell Rep. 2018;24:1802–1815. doi: 10.1016/j.celrep.2018.07.035. e1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nader P.R., Horwitz M.S., Rousseau J. Atypical exanthem following exposure to natural measles: eleven cases in children previously inoculated with killed vaccine. J Pediatr. 1968;72:22–28. doi: 10.1016/j.jpeds.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 5.Kim H.W., et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 6.Dejnirattisai W., et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–748. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gollins S.W., Porterfield J.S. Flavivirus infection enhancement in macrophages: an electron microscopic study of viral cellular entry. J Gen Virol. 1985;66(Pt 9):1969–1982. doi: 10.1099/0022-1317-66-9-1969. [DOI] [PubMed] [Google Scholar]
- 8.Hegazy A.N., Krönke J., Angermair S., et al. Anti-SARS-CoV2 antibody-mediated cytokine release syndrome in a patient with acute promyelocytic leukemia. BMC Infect Dis. 2022;22:537. doi: 10.1186/s12879-022-07513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sridhar P., Singh A., Salomon N., Steiger D. Vaccine-induced antibody dependent enhancement in COVID-19. Chest. 2022;162(4):A646–A647. doi: 10.1016/jchest.2022.08.506. Oct. [DOI] [Google Scholar]
- 10.Naranjo C.A., et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30 doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]