Abstract
Adeno-associated viral vector-mediated transfer of DNA coding for broadly neutralizing anti-HIV antibodies (bnAbs) offers an alternative to attempting to induce protection by vaccination or by repeated infusions of bnAbs. In this study, we administered a recombinant bicistronic adeno-associated virus (AAV8) vector coding for both the light and heavy chains of the potent broadly neutralizing HIV-1 antibody VRC07 (AAV8-VRC07) to eight adults living with HIV. All participants remained on effective anti-retroviral therapy (viral load (VL) <50 copies per milliliter) throughout this phase 1, dose-escalation clinical trial (NCT03374202). AAV8-VRC07 was given at doses of 5 × 1010, 5 × 1011 and 2.5 × 1012 vector genomes per kilogram by intramuscular (IM) injection. Primary endpoints of this study were to assess the safety and tolerability of AAV8-VRC07; to determine the pharmacokinetics and immunogenicity of in vivo VRC07 production; and to describe the immune response directed against AAV8-VRC07 vector and its products. Secondary endpoints were to assess the clinical effects of AAV8-VRC07 on CD4 T cell count and VL and to assess the persistence of VRC07 produced in participants. In this cohort, IM injection of AAV8-VRC07 was safe and well tolerated. No clinically significant change in CD4 T cell count or VL occurred during the 1–3 years of follow-up reported here. In participants who received AAV8-VRC07, concentrations of VRC07 were increased 6 weeks (P = 0.008) and 52 weeks (P = 0.016) after IM injection of the product. All eight individuals produced measurable amounts of serum VRC07, with maximal VRC07 concentrations >1 μg ml−1 in three individuals. In four individuals, VRC07 serum concentrations remained stable near maximal concentration for up to 3 years of follow-up. In exploratory analyses, neutralizing activity of in vivo produced VRC07 was similar to that of in vitro produced VRC07. Three of eight participants showed a non-idiotypic anti-drug antibody (ADA) response directed against the Fab portion of VRC07. This ADA response appeared to decrease the production of serum VRC07 in two of these three participants. These data represent a proof of concept that adeno-associated viral vectors can durably produce biologically active, difficult-to-induce bnAbs in vivo, which could add valuable new tools to the fight against infectious diseases.
Since the first reports of HIV infection in 1981, seven phase 2b and phase 3 HIV vaccine trials have been completed1. Only one trial showed measurable protection from HIV, with 31.2% efficacy by modified intention to treat 3.5 years after vaccination2. HIV employs many defense mechanisms involving the viral glycoprotein trimer that make vaccine design difficult, including trimer instability3,4, paucity of envelope spikes,5,6 immunodominance of non-neutralization-sensitive sites7, shifting glycan shields8,9, flexible variable loops that hide conserved sites10 and extensive sequence variability11,12. The identification and characterization of naturally occurring bnAbs marked a major advance in understanding viral vulnerability and identifying targets for vaccine-induced antibodies13-16. The development of single-cell cloning methods and the screening of large cohorts of HIV-infected individuals furthered this work by identifying antibodies with substantial breadth and potency17. bnAbs were subsequently identified that prevented simian/human immunodeficiency virus (SHIV) infection of non-human primates (NHPs)18-21 and suppressed viremia in HIV-infected individuals after a single infusion of a single bnAb for 21–28 days22,23 and for up to 30 weeks when given two potent bnAbs by intravenous (IV) infusion at 0, 3 and 6 weeks24. These discoveries led to structure-guided protein engineering and attempts to induce the production of specific bnAbs in vivo25,26. Despite these advances, the unusual features of many of these antibodies make the design of immunogens capable of eliciting bnAbs difficult.
Vectored immunoprophylaxis—the delivery of bnAbs by recombinant gene transfer using AAV vectors—has been proposed to circumvent problems associated with designing immunogens capable of inducing specific bnAbs27,28. AAV vectors have an established safety record in humans and have properties that make this approach feasible29,30. AAV transgenes persist in the nucleus as stable episomal DNA31 in post-mitotic cells32,33. Transduction occurs in vivo34,35, allowing AAV vectors to be given by IM injection and IV infusion. Capsids of multiple serotypes exist and can be bioengineered to target specific cell types and evade pre-existing immunity36,37. Results from animal studies show that AAV-mediated gene transfer of bnAbs to humanized mice and NHPs protect against high-dose IV and repeated mucosal HIV NL-4-3, JR-CSF and REJO.c challenge in mice38 and SHIV and SIVsmE660 in NHPs28,39,40. Induction of three bnAbs—3BNC117, 10-1074 and 10E8—by AAV vectors drove long-term viral suppression of SHIV AD8eo in one of four rhesus macaques41. This study41 and a study showing prolonged viral suppression in HIV-infected individuals with infusion of two bnAbs24 suggest that long-term production of multiple high-avidity bnAbs could play a role in controlling viremia in HIV-infected individuals.
A previous attempt to express an HIV-bnAb using AAV-based vectored immunoprophylaxis in humans did not demonstrate maintenance of antibody in serum42. Although intracellular production of the bnAb PG9 was demonstrated, the absence of measurable serum PG9 was attributed to production of ADAs. A relatively insensitive lower limit of PG9 quantification, 2.5 μg ml−1, also limited this study.
VRC 603 is a phase 1, dose-escalation study of AAV8-VRC07 (VRC-HIVAAV070-00GT), an AAV8 gene transfer vector carrying the genetic sequence for the CD4 binding site-directed bnAb VRC07 (Fig. 1). This vector contains a bicistronic cassette that uses an F2A DNA insert43, a nucleotide sequence that induces a ribosomal skip during translation that results in the production of separate IgG light and heavy chains from a single transcribed mRNA under the control of a single promoter and polyadenylation sequence39. Here we present preliminary primary and secondary endpoint data acquired from the time of the first enrollment in VRC 603 on 11 January 2018 through a period of 104–198 weeks after product administration for eight trial participants.
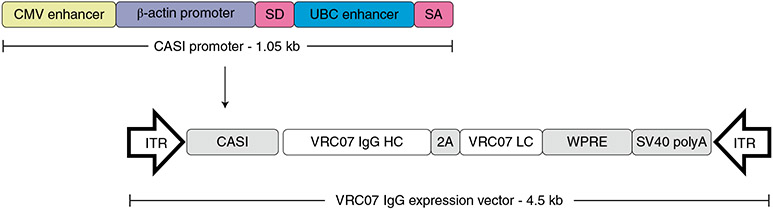
Fig. 1 ∣. Schematic representation of the AAV8-VRC07 vector cassette.
The AAV8-VRC07 vector uses a single open reading frame design packaged between two inverted terminal repeats (ITRs) with a CASI promoter, a truncated woodchuck hepatitis post-translational regulatory element (WPRE) and a single SV40 poly-A sequence (SV40 polyA). The CASI promoter is 1.05 kb and consists of a CMV enhancer; a chicken β-actin promoter; a splice donor (SD) inserted between the β-actin promoter and a human ubiquitin C enhancer element; and a splice acceptor (SA). The VRC07 coding cassette contains the VRC07 IgG heavy chain (VRC07 IgG HC) and the VRC07 IgG light chain (VRC07 LC) coding sequences. Between the heavy chain and light chain sequences is an F2A self-processing sequence (2A), which contains a picornavirus-derived ribosomal skip sequence preceded by a furin cleavage site.
Results
Trial participants.
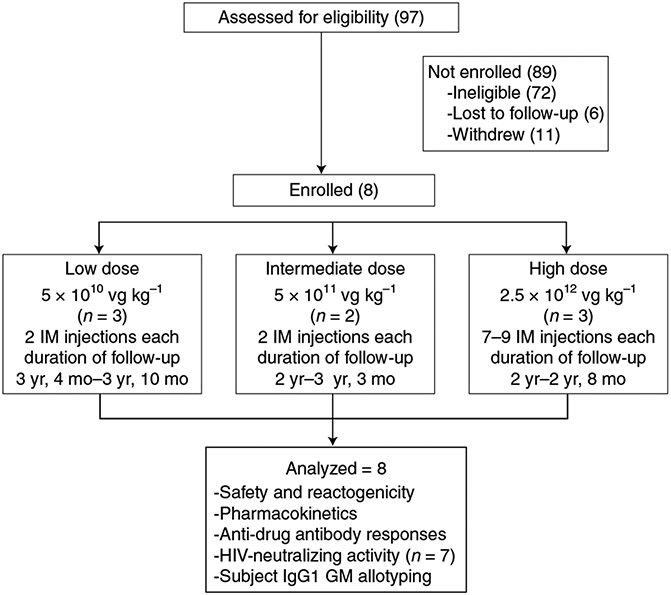
Volunteers in this report were enrolled in VRC 603 (NCT03374202) between 11 January 2018 and 21 October 2019 (Fig. 2). All participants were infected with HIV and on stable anti-retroviral (ARV) therapy for at least 3 months with a VL <50 copies per milliliter and a CD4 count ≥300 cells per microliter. All volunteers were in good health with no clinically important laboratory test abnormalities. Prior receipt of a monoclonal antibody, active liver disease, an anti-AAV8 capsid antibody titer >1:90 and any medical conditions that were judged to jeopardize the safety of the volunteer in this trial were exclusionary. Of the 97 individuals screened, 72 were ineligible for enrollment, six were lost to follow-up and 11 withdrew during screening. Reasons for ineligibility included hypertension, AAV8 seropositivity, active drug use and elevated creatinine (Supplementary Table 1). On 22 October 2021, the institutional review board (IRB) approved a change in the exclusionary criteria from evidence of pre-existing antibodies to AAV8 capsid to a serotiter of >1:90 based on data showing that low-level pre-existing seropositivity did not affect transduction in NHPs40. As a result, some individuals were disqualified from participation in this trial for AAV8 serotiters of ≤1:90. Median AAV8 titer was <1:30 (range, <1:30–1:810) in the 47 potential enrollees in which pre-existing AAV8 seropositivity was evaluated (Supplementary Fig. 1). Using an exclusionary criterion of AAV8 serotiters ≥1:90 from the beginning of this trial would have resulted in the exclusion of fewer than 5% of potential enrollees for pre-existing AAV8 seropositivity. Eight volunteers enrolled in VRC 603—six men and two women, with a median age of 52 (range, 30–60) years (Supplementary Table 2). All but one of these participants had a viral load of <20 copies per milliliter at enrollment. Median CD4 count was 528 (range, 351–950) in study participants (Supplementary Table 3). Three participants were enrolled in the 5 × 1010 vector genomes (vg) kg−1 dose group, two participants in the 5 × 1011 vg kg−1 dose group and three participants in the 2.5 × 1012 vg kg−1 dose group (Fig. 2). All participants continued ARV therapy throughout this trial.
Fig. 2 ∣. VRC 603 CONSORT diagram.
Study enrollment, AAV8-VRC07 administration, participant follow-up and data analysis are shown for the three study groups.
The AAV8-VRC07 product was provided in individual cryovials that contained 1 ml of aqueous AAV8-VRC07 solution at a concentration of 2.84 × 1013 vg ml−1. In a stepwise, dose-escalation plan, safety was evaluated 4 weeks after initial product administration in each dose group by assessing local and systemic reactogenicity, unsolicited adverse events (AEs) and laboratory findings before administration of product to the second individual in that group. After administration of product to the second individual in the same dose group, a second 4-week safety evaluation was conducted. A serum target concentration for VRC07 of 50 μg ml−1 of sera was chosen based on pharmacokinetics data for AAV8-VRC07 from mice and NHPs40, which showed that a concentration of VRC07 of 50 μg ml−1 4 weeks after product injection results in a sustained serum concentration of 3–5 μg ml−1, a concentration shown to decrease HIV VL in humans23. If product administration to both participants was safe and VRC07 antibody concentrations were <50 μg ml−1 in both participants, dose escalation occurred. Additional participants in each dose group, up to a maximum of five participants, could be added at the principal investigator’s discretion. In the 5 × 1010 AAV8-VRC07 vg kg−1 dose group, AAV8-VRC07 was diluted with a formulation buffer to a concentration of 2.84 × 1012 vg ml−1 and given in two IM injections. Individuals in the 5 × 1011 vg kg−1 dose group and the 2.5 × 1012 vg kg−1 group received undiluted AAV8-VRC07 at a concentration of 2.84 × 1013 vg ml−1 in two and 7–9 IM injections, respectively. The time required to administer product was dictated by participant comfort. In the high-dose group, the range was between 6 and 16 minutes (median, 13 minutes) for administration of all IM injections.
Product safety.
Injection of AAV8-VRC07 was evaluated as safe and well tolerated in this cohort. Three of eight (37.5%) participants reported one or more solicited local reactogenicity symptoms. No local reactogenicity was observed in the low-dose and intermediate-dose groups. Only participants in the highest-dose group, 2.5 × 1012 vg kg−1, reported local reactogenicity during the 7 days after IM injection (Table 1). All participants in the 2.5 × 1012 vg kg−1 dose group reported the onset of mild injection site pain and tenderness on the day of product administration and again 5–6 days after injection, all resolving 8–14 days after product administration (Table 1).
Table 1 ∣.
Maximum local and systemic reactogenicity up to day 7 after IM injection of AAV8-VRC07*
| 5 × 1010 | 5 × 1011 | 2.5 × 1012 | Overall | |
|---|---|---|---|---|
| (n = 3) | (n = 2) | (n = 3) | (n = 8) | |
| n (%) | ||||
| Maximum local and systemic reactogenicity up to day 7 after IM injection of AAV8-VRC07* | ||||
| Pain/tenderness | ||||
| None | 3 (100) | 2 (100) | 0 (0) | 5 (62.5) |
| Mild | 0 (0) | 0 (0) | 3 (100) | 3 (37.5) |
| Swelling | ||||
| None | 3 (100) | 2 (100) | 3 (100) | 8 (100) |
| Mild | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Redness | ||||
| None | 3 (100) | 2 (100) | 3 (100) | 8 (100) |
| Mild | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Any local symptoms | ||||
| None | 3 (100) | 2 (100) | 0 (0) | 5 (62.5) |
| Mild | 0 (0) | 0 (0) | 3 (100) | 3 (37.5) |
| Self-reported systemic reactogenicity up to 7 days after IM injection of AAV8-VRC07* | ||||
| Malaise | ||||
| None | 3 (100) | 2 (100) | 3 (100) | 8 (100) |
| Mild | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Myalgia | ||||
| None | 0 (0) | 1 (50) | 2 (66.7) | 6 (75) |
| Mild | 0 (0) | 1 (50) | 1 (33.3) | 2 (25) |
| Headache | ||||
| None | 3 (100) | 2 (100) | 2 (66.7) | 7 (87.5) |
| Mild | 0 (0) | 0 (0) | 1 (33.3) | 1 (12.5) |
| Chills | ||||
| None | 3 (100) | 2 (100) | 3 (100) | 8 (100) |
| Mild | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Nausea | ||||
| None | 3 (100) | 2 (100) | 3 (100) | 8 (100) |
| Mild | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Temperature elevation | ||||
| None | 3 (100) | 2 (100) | 3 (100) | 8 (100) |
| Mild | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Joint Pain | ||||
| None | 3 (100) | 2 (100) | 3 (100) | 8 (100) |
| Mild | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Any systemic symptoms | ||||
| None | 3 (100) | 1 (50) | 2 (66.7) | 6 (75) |
| Mild | 0 (0) | 1 (50) | 1 (33.3) | 2 (25) |
No moderate or severe reactogenicity was reported throughout the trial.
Two of eight (25%) participants reported systemic reactogenicity 1 day after injection: one participant in the 5 × 1011 vg kg−1 dose group reported mild myalgia, and one participant in the 2.5 × 1012 vg kg−1 dose group reported mild myalgia and headache. Both resolved the same day. No joint pain, fevers or other solicited systemic reactogenicity symptoms were reported.
As of 1 November 2021, six of eight (75%) participants had one or more unsolicited AEs. Only one AE was assessed as product related. Unsolicited AEs included grade 1 aspartate aminotransferase (AST) elevation for one participant at 7 days after injection; unrelated grade 1 creatinine elevation for two participants—one in Group 2 at 14 days and 39 days after injection and one in Group 3 at 57 days after injection; and grade 3 diarrhea in one participant in Group 1 at 44 days after injection. These unsolicited AEs resolved without residual effects and were evaluated as not related to the study product. A grade 1 AST elevation in Participant C occurred 44 days after product administration and was evaluated as related to study product. This AE resolved 1 week after onset. One serious AE occurred: a drug overdose death in a participant in the intermediate-dose group 2 years after product administration. The death was evaluated as not related to study product. No study pause criteria were met. All participants will be followed for 5 years to ensure product safety. Follow-up is ongoing.
Most AAV capsids are hepatotropic, and use of AAV vectors, primarily with IV infusion, is associated with a vector-induced hepatitis44,45. Some increases above baseline AST and alanine aminotransferase (ALT) were seen in trial participants, but most of these increases were contained within normal limits, and none was greater than 2.5× the upper limit of normal (Supplementary Fig. 2). Using overlapping peptides covering the VP1, VP2 and VP3 AAV8 capsid proteins, we found no evidence of a AAV8 capsid-specific interferon γ (IFNγ) CD4 T cell response using intracellular cytokine staining. We did find an increase in AAV8 capsid-specific CD8 T cell IFNγ production after product administration in Participant C in the 5 × 1010 vg kg−1 dose group and in Participant F in the 2.5 × 1012 vg kg−1 dose group. In both cases, maximal IFNγ production occurred 8 weeks after IM injection of AAV8-VRC07 and then decreased but remained measurable at 20 weeks (Supplementary Fig. 3).
Vector persistence.
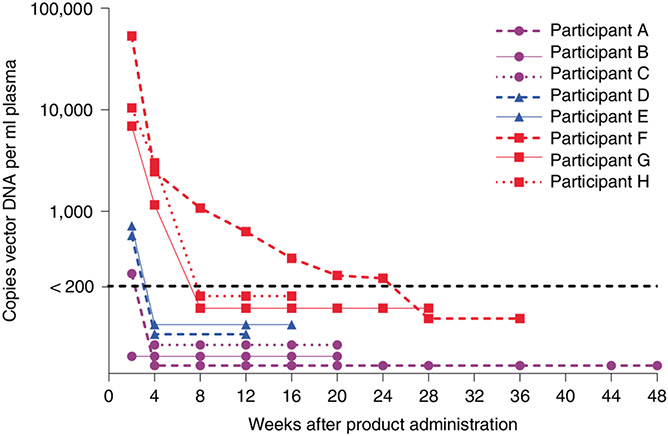
AAV8-VRC07 vector in the 5 × 1010 vg kg−1 and 5 × 1011 vg kg−1 dose groups was less than 200 copies per milliliter by week four. In the 2.5 × 1012 vg kg−1 dose group, vector DNA decreased to less than 200 copies per milliliter in all participants by week 28 (Fig. 3).
Fig. 3 ∣. Concentrations of post-product administration of AAV8-VRC07 plasma DNA.
Longitudinal plasma AAV8-VRC07 DNA from 2 weeks after injection until two sequential longitudinal samples of less than 200 copies per milliliter of AAV8-VRC07 plasma DNA were found. Participants A–C received IM injections of 5 × 1010 vg kg−1 of AAV8-VRC07; Participants D and E received 5 × 1011 vg kg−1; and Participants F–H received 2.5 × 1012 vg kg−1.
Anti-capsid antibody persistence.
AAV8 capsid-specific serum antibody titers rapidly increased after injection of VRC07 AAV8 in a dose-dependent manner (Supplementary Fig. 4). In the 5 × 1010 vg kg−1 dose group, anti-AAV8 capsid titers achieved maximal serum concentrations 8–16 weeks after product administration, with titers remaining between 1:2,430 and >1:21,870 for up to 1.5 years after IM injection. In the 5 × 1011 vg kg−1 dose group, a maximal serum titer of >1:21,870 was attained in one participant 8 weeks after product administration and remained at that level for up to 1 year. In the second individual in that dose group, a maximal serum titer of 1:7,290 was attained by week eight and decreased to 1:2,430 36 weeks after product administration. In the 2.5 × 1012 vg kg−1 dose group, serum titers achieved a maximal level of >1:21,870 2–12 weeks after product administration and remained at those levels for up to 40 weeks.
Effect of AAV8-VRC07 on VL and CD4 count.
As expected for individuals on effective ARV therapy, no significant change in HIV VL was observed after AAV8-VRC07 injection (Supplementary Fig. 5). Similarly, no clinically significant change in CD4 T cell count was observed (Supplementary Fig. 6).
VRC07 in vivo production.
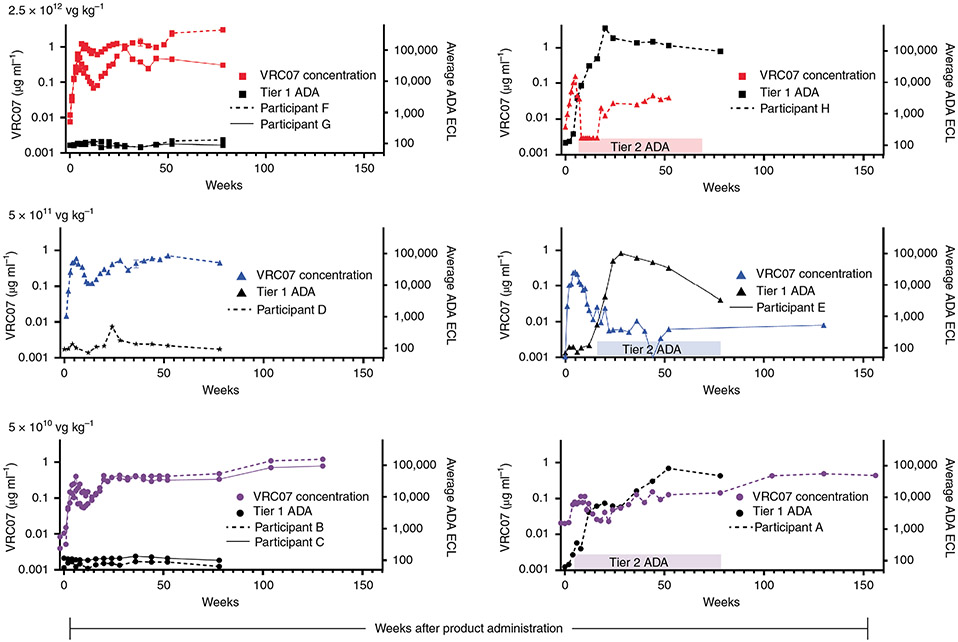
Production of measurable VRC07 occurred in all three dose groups. Except for one participant in each dose group, a characteristic expression pattern occurred. Serum VRC07 concentration achieved measurable levels 1–2 weeks after IM injection and then increased until approximately week six after IM injection. This was followed by decreasing VRC07 concentration until approximately week 14. In five of eight participants, a secondary increase in VRC07 concentration occurred from week 14 until at least week 24. In four of these five participants, VRC07 sera concentrations either established a new steady state VRC07 concentration or exhibited a gradual increase in VRC07 concentration through 2.5 years of follow-up (Fig. 4a). When participants from all dose groups are combined, the concentration of VRC07 at week 6 (P = 0.008) and at week 52 (P = 0.016) were significantly greater than pre-injection concentrations by a two-sided Wilcoxon matched-pairs signed-rank test. In one of the three participants in the 5 × 1010 vg kg−1 dose group and in two of the three participants in the 2.5 × 1012 vg kg−1 dose group, peak serum concentrations of >1 μg ml−1 were achieved. In one of the participants in the 2.5 × 1012 vg kg−1 group, the measured VRC07 concentration was 3.3 μg ml−1 1.5 years after product administration. In two of the three participants with blunted secondary increases in VRC07 concentrations, neither the primary VRC07 nor the secondary VRC07 peak concentrations were as high as was seen in the other five participants (Fig. 4b).
Fig. 4 ∣. Longitudinal serum VRC07 concentration and tier 1 and tier 2 ADA from immediately before IM injection of AAV8-VRC07 to 80–156 weeks after IM injection of 5 × 1010, 5 × 1011 and 2.5 × 1012 vg kg−1.
Participants without tier 1 and tier 2 ADA are shown in the left column; participants with tier 1 and tier 2 ADA are shown in the right column. Serum VRC07 concentrations for different participants are as shown by colored lined as indicated in the figure legend. Tier 1 ADA is as indicated by black lines, with tier 2 ADA as indicated by solid colored bars over the time at which they were identified. No tier 3 ADA was identified for any participants. For VRC07 concentration data, all data points represent the results of a single assay performed in triplicate. Tier 2 and tier 3 ADA responses represent the average of a single assay done in quadruplicate.
ADA detection.
In this study, ADA directed against VRC07 IgG was characterized using a three-tiered assay. Tier 1 assays quantitatively measure binding of serum proteins to VRC07. Tier 2 assays are qualitative competition assays in which exogenously added VRC07 displaces VRC07-binding serum proteins from the reporter protein, confirming the specificity of VRC07 binding. Tier 3 assays are qualitative assays that assess the ability of VRC07-binding serum protein to prevent VRC07-mediated neutralization of pseudovirus in vitro. In each of the three participants with blunted VRC07 serum concentrations, tier 1 and tier 2 ADA were first identified 4–12 weeks after AAV8-VRC07 IM injection and persisted for the duration of this report (Fig. 4b). In one participant in the lower-dose group, VRC07 concentration slowly increased to 0.4 μg ml−1 despite a persistent ADA response. VRC07-binding serum proteins had no effect on VRC07 neutralization activity in tier 3 assays, implying that the ADA response was not idiotypic. Samples collected from the three participants who showed tier 1 and tier 2 VRC07 ADA were then evaluated for ADA directed against the Fab fragment. In all three participants, measurable tier 1 VRC07 Fab ADA was identified. In each case, the pattern of induction of ADA directed against the Fab fragment and the entire VRC07 antibody was similar (Supplementary Fig. 7). Samples collected from three individuals without measurable VRC07 ADA showed no measurable Fab ADA.
To determine the possibility that in vivo production of VRC07 of a different allotype than that produced natively by trial participants contributed to the observed ADA response46, we determined the igG1 allotype for each participant enrolled in this trial. This was done as an exploratory objective. VRC07 produced was GM1+/GM17+. No correlation was observed between participant allotype and ADA response (Supplementary Table 4).
VRC07 viral neutralization.
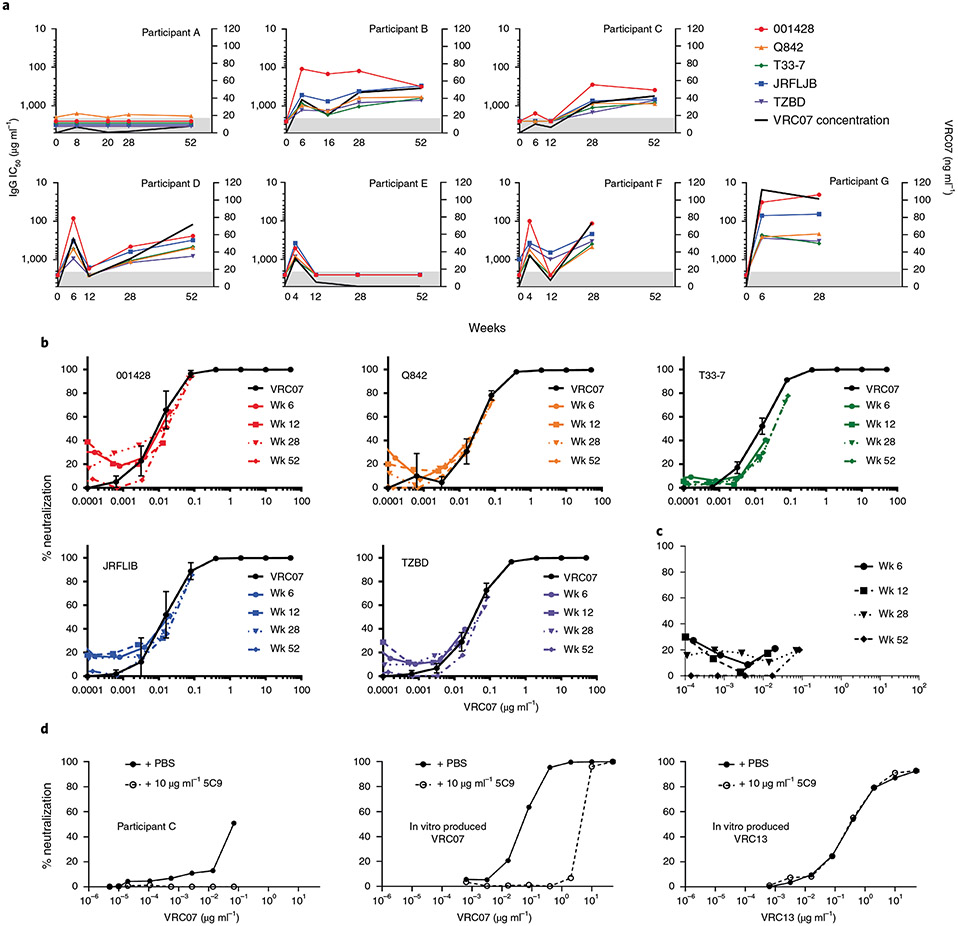
As an exploratory objective, we assessed the ability of VRC07 produced in vivo to prevent pseudovirus entry using an in vitro neutralization assay47. Use of this assay was complicated by the presence of ARVs, which cause false-positive measurements. It was, therefore, necessary to isolate and purify plasma IgG to separate them from ARVs. We used a protein A column for the purification and separation of plasma IgG away from ARVs. Purified IgG neutralized pseudotyped viruses from five tier 2 HIV viral strains that are also neutralized by purified in vitro produced VRC07: clade A Q842.G12, clade AG T33-7, clade B JRFL.JB, clade C 001428-2.42 and TZBD.02 (Fig. 5a,b). Within the precision of the assay used48, calculated pseudoviral ID50s were consistent for all seven participants studied (Supplementary Figs. 8 and 9). In 56 of 77 IC50 determinations made, IC50s for in vivo produced VRC07 were within 2 standard deviations of the geometric mean IC50s determined for ex vivo produced VRC07. Geometric means for experimentally determined IC50s for ex vivo and in vivo produced VRC07 differed by less than a factor of 2 for all but pseudovirus T33-7. For this pseudovirus, the geometric mean for in vivo produced VRC07 was 2.6-fold higher than for ex vivo produced VRC07 (Supplementary Table 5). Although VRC07 from participants’ serum did neutralize the above-mentioned pseudoviruses, it did not neutralize HIV clade C virus CAP210.E8.SG3, an isolate known to be resistant to in vitro VRC07 neutralization (Fig. 5c). This confirmed the successful purification of IgGs from ARVs. Neutralization of HIV-TZBD.02 by purified IgG from the serum of Participants B–G was inhibited by the addition of 5C9, an anti-VRC07/VRC01 idiotype antibody that blocks VRC07 binding to the viral CD4 binding site but does not block other CD4-binding site-specific antibodies, such as VRC13, further demonstrating the presence of biologically active VRC07 in these participants (Fig. 5d and Supplementary Fig. 10).
Fig. 5 ∣. Longitudinal neutralization data and the effect of VRC07 paratope binding by 5C9 on neutralization by purified IgG from study participants.
a, Neutralization of five different tier 2 pseudoviruses as shown in the figure legend by purified IgG for the first seven trial participant enrolled in VRC 603. Participants A–C received 5 × 1010 vg kg−1; Participants D and E received 5 × 1011 vg kg−1; and Participants F and G received 2.5 × 1012 vg kg−1 of AAV8-VRC07 by IM injection. Longitudinal IgG IC50s are reported as μg ml−1 of purified IgG on the left y axis for each pseudoviral strain. VRC07 concentration as determined by 5C9 titration is reported as ng ml−1 of 1 mg ml−1 of purified IgG on the right y axis. Data points represent single determinations. Data points in the shaded area are below the limits of accurate quantitation. b, Neutralization curves adjusted for the concentration of VRC07 in purified IgG serum samples are shown for Participant C at 0, 6, 12, 24 and 52 weeks after IM injection of AAV8-VRC07, as indicated in the figure legend. Neutralization curves for purified in vitro produced VRC07 for each pseudoviral species are overlaid on each plot. Data points for neutralization assays of ex vivo produced VRC07 represent the average of six replicate assays; error bars are ± s.d. Data points for in vitro produced VRC07 represent a single assay, These data are representative of data for Participants A, B and D–G. c, Representative neutralization curve showing the failure of in vitro produced VRC07 to neutralize pseudoviral strain CAP210.E8.SG3 at weeks 6,12, 28 and 52. Each data point represents a single determination. d, From left to right, neutralization of TZBD by purified IgG from Participant C containing the amount of VRC07 indicated on the x axis from week 28 purified IgG; neutralization of TZBD by purified in vitro produced VRC07; and neutralization of TZBD by in vitro produced VRC13, in the presence or absence of 10 μg ml−1 of the VRC07 paratope-binding monoclonal antibody 5C9, as shown in the figure legend. These data are representative of neutralization curves for Participants A, B and D–G. Each data point represents a single determination.
Discussion
This study demonstrates the feasibility of producing HIV-1 bnAbs in humans by vectored immunoprophylaxis. In particpants without measurable ADA, durable production of VRC07 continued for up to 3 years, with a concentration of VRC07 of >3 μg ml−1 achieved in one participant. Pseudoviral neutralization studies showed that in vivo produced VRC07 was biologically active. This study is the first, to our knowledge, to describe an intervention resulting in a statistically significant, long-lived production of a bnAb in HIV-infected individuals.
The most common means of administration of AAV vectors are by IM injection and IV infusion. Intravenous infusion of AAV vectors, which induced higher levels of product, is limited by a drug-induced hepatitis caused by a major histocompatibility class I response directed against the AAV coat protein peptides expressed on the surface of transduced cells44,49. This cytotoxic T lymphocyte (CTL) response and the associated transaminitis are first seen about 6 weeks after product administration. Although this vector-induced hepatitis can be limited with the use of moderate-dose steroids, we chose to administer AAV8 VRC07 by IM injection. An AAV8 capsid was chosen because the frequency of pre-existing seropositivity in human is less frequent than for most other AAV capsids36 and because of its ability to transduce muscle cells50. Even so, AAV8, like most AAV subtypes, is hepatotropic. Only in Participant C did we see evidence of a concurrent AAV8 capside-specific CTL response. Although we observed transient increases in transaminases during this trial, all were less than 2.5× the upper limit of normal. These data do not represent evidence of a significant vector-induced hepatitis in this trial.
Multiple reports in both NHPs and humans show that ADA can decrease the measured concentration of antibodies produced by AAV vectors40,51,52. Priddy et al.42 identified ADA in 62.5% of humans given rAAV1-PG9DP, a vector coding for PG9. In contrast, we detected ADA in three of eight individuals (38%) enrolled in VRC 603 by tier 1 and tier 2 assays. Notably, all three ADA responses failed to block pseudovirus-neutralizing activity by VRC07 in a tier 3 assay, indicating that the ADA responses produced in this trial did not prevent VRC07 binding to HIV-1 Env. Instead, these ADA responses appear to bind to regions of the Fab portion of VRC07, which do not come in direct contact with the viral envelope. Whether they increase the rate of VRC07 clearance from the blood remains unknown.
VRC07 was chosen for expression by the AAV8 coding cassette in this study for two reasons: at the time of vector design, VRC07 was the most potent bnAb available, and it is a clonal relative of VRC01. VRC01 has been studied extensively in human trials23,47,53-57. No ADA to VRC01 (refs. 23,47), VRC01LS58 or VRC07-523LS59 given by either IV infusion or subcutaneous injection, in doses ranging from 1 mg kg−1 to 40 mg kg−1 in single or multiple doses to more than 69 individuals in published phase 1 studies, was identified. There are many possible reasons for the discordance between this study and the published antibody infusion studies: differences in primary amino acid sequence, intracellular protein production compared to protein infusion and lower amounts of bnAb production in this study compared to the amount of bnAb used in infusion studies. These differences make it impossible to draw definitive conclusions about the cause of these differences.
As encouraging as these results are, we do not think that they merit an ARV treatment interruption. Although a trial simultaneously using two different bnAbs succeeded in suppressing VL during the course of treatment24, trials using a single bnAb have failed22,54,55,60. These failures were either due to not maintaining therapeutic bnAb concentration or due to either pre-existing immunity to, or viral escape from, bnAb control during the trial. Immune escape from VRC07 is less likely to occur during when new viral infection is limited by ARV therapy than during a treatment interruption. It is possible that prolonged production of a bnAb during effective ARV therapy may decrease the viral reservoir via an antibody-dependent mechanism over time, but this study was not designed specifically to test that hypothesis. Ultra-sensitive VLs will be performed on pre-enrollment and on 3-, 6- and 12-month post-enrollment samples when 1-year serum samples have been collected from all enrollees, to determine if transduction with AAV8 VRC07 results in increased viral clearance from the blood.
VRC07 concentrations achieved in the low-dose and intermediate-dose groups were similar. These data suggest that the saturating dose of VRC07 AAV8 may be as low as, or lower than, 2.84 × 1012 vg per IM injection site. Although not statistically significant, the higher VRC07 concentration observed in the high-dose group may not be due to the use of a more concentrated vector but, instead, to multiple injections of saturating amounts of VRC07 AAV8, as Welles et al.40 have reported in NHPs. A more economical means of administering AAV8-VRC07 may be to administer a greater number of injections at lower doses per site.
The data presented here provide proof of concept that an AAV vector can be used to produce difficult-to-elicit antibodies that retain their neutralizing potency and breadth in humans. Although challenges remain for the clinical advancement of this technology, additional research is needed to further understand and classify the ADA response to AAV-induced antibody production. The practical application of vectored immunoprophylaxis will also benefit from the identification of bnAbs with lower IC80s and the introduction of methods that can increase antibody production by improved promoters, transduction of a larger number of cells or Fc modification to decrease the clearance rate from blood58. Despite these problems, vectored immunoprophylaxis/immunotherapy offers a new means to safely produce bnAbs in humans that are difficult to elicit using current vaccine methodology.
Methods
Study design and participants.
This phase 1, proof-of-principle, dose-escalation study was done at the Vaccine Evaluation Clinic as part of the Vaccine Research Center Clinical Trials Program in healthy HIV-infected individuals on effective ARV therapy at the National Institutes of Health (NIH) Clinical Center. This study conforms with all relevant ethical regulations. The National Institute of Allergy and Infectious Diseases IRB reviewed and approved the clinical trial protocol (NCT03374202). Primary endpoints of this study were to assess the safety and tolerability of AAV8-VRC07; to determine the pharmacokinetics and immunogenicity of in vivo VRC07 production; and to describe the immune response directed against AAV8-VRC07 vector and its products. Secondary endpoints were to assess the clinical effects of AAV8-VRC07 on CD4 count and VL and to assess the persistence of VRC07 produced in participants. Power calculations for groups of two and five for AEs were reported in the protocol. At full enrollment, this study is powered at an 80% confidence of identifying at least one event if the true rate is no less than 0.28 in a sample group of five. This trial was pre-registered on 15 December 2017. On 7 October 2021, this study was closed to accrual, with three individuals enrolled in the 5 × 1010 AAV8-VRC07 vg kg−1 group, three individuals enrolled in the 5 × 1011 AAV8-VRC07 vg kg−1 group and four individuals enrolled in the 2.5 × 1012 AAV8-VRC07 vg kg−1 group. With four participants in the high-dose group, this study had 80% probability of identifying at least one event in this group if the true rate of such events was no less than 0.34, and it had a 90% probability of observing no events if the true rate was no larger than 0.025.
Volunteers were recruited from the Washington, DC, area using IRB-approved written and electronic promotional media. We screened HIV-infected individuals in good health between the ages of 18 and 65 years, with a CD4 count of ≥300 cells per microliter and a VL of ≤50 copies per milliliter during the preceding 3 months. Men had to agree to condom use for all sexual activity of reproductive potential for 52 weeks after receipt of product. Women had to agree to use an effective form of birth control for 52 weeks after receipt of product and had to have a negative β-HCG at time of enrollment. Exclusion criteria included prior receipt of a monoclonal antibody or gene therapy product, active drug or alcohol abuse, an AAV8 capsid titer of >1:90, active liver disease, poorly controlled hypertension, weight >115 kg, a history of a severe allergic reaction within the preceding 2 years and any chronic or clinically significant medical condition that, in the opinion of the investigator, would jeopardize the safety or rights of the participant. Written informed consent was obtained for each participant. Before approval, all volunteers needed to pass a test that assessed understanding of the expectations of trial participants and the risks of participating in the trial. If participants did not demonstrate full understanding of the expectations of trial participants and its possible risks, the points that were not understood were discussed with the volunteer, and the test was given a second time. A score of 100% was required to pass. Two protocol amendments were made that affected enrollment criteria. On 21 March 2019, the IRB approved increasing the age of eligibility from 18–60 years to 18–65 years. On 22 October 2019, the IRB approved changing the exclusionary criteria for AAV8 serotiter from ‘pre-existing antibodies to AAV8 capsid’ to a serotiter of ‘>1:90’. Participants were provided compensation for time and travel.
Product description.
Based on earlier studies using mice and NHPs38,40,61,62, a single open reading frame design using a self-processing F2A peptide was used (Fig. 1). A 1.05-kilobase (kb) CASI promoter containing a cytomegalovirus (CMV) enhancer, a chicken β-actin promoter and a human ubiquitin C enhancer element drove transcription. A splice donor and acceptor flank the ubiquitin C enhancer. This optimizes promoter performance. The VRC07 coding cassette contains both the VRC07 light and heavy chain coding sequences separated by a F2A sequence. The F2A sequence contains a ribosomal skip sequence that prevents formation of a glycylproline bond, causing termination of the first protein and the initiation of a second. Upstream of the F2A sequence is a four-amino-acid furin cleavage site that results in the removal of the F2A peptide from the VRC07 heavy chain61,63. The proline amino acid remaining on the carboxy terminal of the light chain is upstream of the light chain signal sequence and is not on the mature protein. A truncated woodchuck post-translational regulatory element is downstream from the IgG light chain coding sequence to increase transgene expression. This truncation eliminates the X protein open reading frame64.
Solicited adverse reactions, including unusual fatigue/malaise, muscle ache, headache, chills, nausea and joint pain, were recorded daily for the first 7 days after enrollment. Participants also recorded their highest measured temperature daily. Local reactogenicity parameters, including pain/tenderness, swelling and redness at the injection site or other sites, were also recorded. All AEs were recorded for the first 8 weeks of this study; after that, only serious AEs are recorded. Individuals will be followed for 5 years after product administration.
Product manufacture and administration.
AAV8-VRC07 was manufactured by the Children’s Hospital of Philadelphia using a helper virus-free transient co-transfection of three plasmids in HEK293 cells. AAV8-VRC07 was purified by CsCl2 density centrifugation to minimize the presence of empty AAV8 capsid in purified product. Final product concentration was 2.84 × 1013 vg ml−1. Product was stored at −60 °C in 1-ml vials until time of use.
An NIH pharmacist individually prepared all doses for administration. If dilution was required, AAV8-VRC07 was mixed with a diluent containing 180 mM sodium chloride, 10 mM sodium phosphate and 0.001% Pluronic F68 at pH 7.3. AAV8-VRC07 was thawed and administered within 4 hours of thawing. No more than 1 ml of AAV8-VRC07 was given by IM injection at one site. Administration of product was weight based. If the dose to be given required more than 1 ml of product, as many 1-ml doses as needed were given, with the fractional amount of 1-ml product required given in the last injection. Injections rotated between the left deltoid to the right deltoid to the left quadriceps to the right quadriceps. All product was given by IM injections by a registered nurse using a 1-inch-long needle.
We based dose groups on previous experiments done with rhesus macaques39. In the 5 × 1010 vg kg−1 dose group and the 5 × 1011 vg kg−1 dose group, AAV8-VRC07 was given in two IM injections in between a 1.16–1.44 ml total volume, with the first injection administering 1 ml of product and the second injection the remainder of the product. For the 2.5 × 1012 dose group, AAV8-VRC07 was given in 6.28–8.01 ml of product in between seven and nine IM injections in the same manner as the 5 × 1010 vg kg−1 dose group and the 5 × 1011 vg kg−1 dose group.
A study clinician monitored all product administration and obtained safety laboratory tests from participants before administration and throughout the study. Participants recorded solicited symptoms for 7 days after injection. A study clinician monitored the site on the day of injection and at 7 days and 14 days afterwards. Clinicians recorded all AEs for 56 days after IM injection and reported serious AEs throughout the study. Participants were seen weekly for the first 12 weeks and then every other week until week 24 and then monthly until week 52. After 52 weeks, participants will be seen every 6 months through year 5.
Quantitation of AAV8 antibody levels.
Vaccine vector-matched AAV8 capsids were coated at optimized concentrations on 96-well Immulon 2 plates (Dynex Technologies) with overnight incubation at 2–8 °C. Plates were then blocked and antisera titers determined by sequential incubation of antigen with volunteer sera, followed by biotin-labeled anti-human IgG and streptavidin conjugated with horseradish peroxidase and TMB (3,3′,5,5′-tetramethylbenzidine) substrate. Color development was stopped by the addition of 1N sulfuric acid, and the plates were read with a SpectraMax microplate spectrophotometer (Molecular Devices). End-point titer was calculated as the most dilute serum concentration that gave an optical density (OD) reading that met positivity criteria. Positivity criteria were determined by the evaluation of a panel of 50 healthy adult sera and determined that ODs greater than 0.2 were indicative of the presence of AAV8 antibodies.
AAV8-VRC07 vector DNA quantitation.
Plasma AAV8-VRC07 plasmid DNA was measured by extracting DNA from plasma, concentrating and then using a real-time PCR assay to measure a 103 base sequence spanning the junction of the IgG heavy chain sequence and F2A insert. DNA was extracted from serum using an Apostle MiniMax High Efficiency cfDNA Isolation Kit, following the manufacturer’s protocol with slight modification.
Real-time PCR was performed using Invitrogen Platinum Taq real-time PCR reagents as recommended by the manufacturer. The assay contained 800 nM 5′ primer (5′-TCCAGGTCCAATGGCAAC-3′), 800 nM 3′ primer (5′-CCTGGAGACTGTGTCAACACA-3′) and 200 nM probe (5′-5/6FAM-T CTGCCGAGCCCTCTTGGAGC-BHQ1-3′) in a 25-μl volume. Primers and probes were manufactured by Integrated DNA Technologies. Real-time assays were performed on an ABI 7900HT run in 9600 emulation mode. Temperature settings were as follows: an initial 10-minute pre-incubation period at 95 °C, followed by 45 cycles of a denaturation period of 10 seconds at 95 °C, a 30-second annealing period at 57 °C and then a 10-second 72 °C extension period. Data acquisition occurred at the completion of the extension period. Standards were prepared by sub-cloning a 302-base pair (bp) sequence from AAV8-VRC07 into a pGEM-T Easy plasmid (Promega). Plasmid concentration was determined by A260/280 absorption.
T cell response to AAV8 capsid protein.
Peptides, 15mers overlapping by 11, representing the entire VP1, VP2 and VP3 protein sequences of AAV8 were manufactured by JPT Peptide Technologies and were >70% pure. Cell stimulation and staining were performed on thawed peripheral blood mononuclear cells using a modification of the method previously described65. Incubations contained monensin and brefeldin A (BD Biosciences); 1 μl of mouse anti-human CD107A BV650 (BioLegend); and 1 μg ml−1 of CD28 and 1 μg ml−1 of CD49d co-stimulation (BD Biosciences) in a 0.2-ml volume. Cells were stained with LIVE/DEAD fixable aqua dead cell stain as described by the manufacturer (Invitrogen), 0.1 μl per test of mouse anti-human CD4 cyanine5.5PE (Invitrogen), 1 μl per test of mouse anti-human CD8 BV785 (BioLegend), 0.2 μl per test of rat anti-human IL-2 APC, 0.5 ul per test of mouse anti-human TNFα Cy7PE, 0.2 μl per test of mouse anti-human IFNγ FITC and 2.5 μl per test of mouse anti-human CD3 H7APC (BD Biosciences). Validation of antibody specificity was performed by the manufacturer. Flow cytometry was done using a BD X50 flow cytometer. Flow cytometric data were acquired using FACSDiva software version 9.1 (BD Biosciences). Data were analyzed using FlowJo software version 10.6.1 (BD Biosciences). The number of cells used for each assay and the sensitivity of the assay limited the report of cytokine staining to IFNγ. Data obtained from assays in which there were fewer than 5,000 gated CD4 or CD8 cells are not reported because of a lack of sensitivity.
VRC07 Singulex quantification method.
VRC07 serum measurements were done by a modification of the method used to measure VRC07 in mucosal samples66.
Paramagnetic microparticle (MP) beads were capture antibody-labeled with 5C9 IgG2a (in-house VRC07 anti-idiotypic antibody) at 12.5 μg mg−1 (IgG/MP) using the Singulex Erenna Capture Antibody Labeling Kit (Singulex/EMD Millipore), according to the manufacturer’s protocol. The capture antibody-labeled 5C9 IgG2a MP beads were stored in frozen aliquots at −80 °C at a working concentration of 10 mg ml−1 in Coated Bead Buffer (Singulex/EMD Millipore). Similarly, unconjugated mouse anti-human IgG1 Fc secondary antibody (Thermo Fisher Scientific) was labeled according to the manufacturer’s protocol using the Singulex Erenna Detection Antibody Labeling Kit (Singulex/EMD Millipore, 03-0076-02) and stored at 4 °C.
On the day of the Singulex VRC07 quantification run, the frozen capture antibody-labeled 5C9 IgG2a MP beads were thawed and equilibrated to room temperature and re-suspended by end-over-end rotation. Test samples (serum) for VRC07 quantification were diluted (1:500 or 1:2,000) using 1× Singulex Discovery Standard Diluent (Singulex/EMD Millipore) and filtered through 96-well filter plates (Pall, 8040) into 96-well assay plates (Axygen). A VRC07 standard curve was likewise prepared with 1× Singulex Discovery Standard Diluent to produce VRC07 concentrations ranging from 29 pg ml−1 to 100,000 pg mlμ1. After transferring 100 μl of each of the prepared standard curve dilutions or test samples to a new Axygen assay plate, 100 μl of freshly prepared 5C9 IgG2a MP beads re-suspended in 1× Discovery Assay Buffer (Singulex/EMD Millipore) at 50 μg ml−1 (to deliver 5 μg per well) were added to each standard or test sample well, after which the plate was incubated on a Jitterbug Microplate Incubator Shaker (Boekel Scientific) for 2 hours at 25 °C and at shake speed 5. All test samples and standard curve samples were run in triplicate, and any outliers (% coefficient of variance (CV) > 20) within the replicates were excluded from further data analyses.
Approximately 10 minutes before the end of the capture incubation, the working stock of labeled mouse anti-human IgG1 Fc secondary detection antibody (500 ng ml−1 in Discovery Assay Buffer) was prepared and filtered into a reagent reservoir using 0.2-μm syringe filter (Pall) and syringe (VWR). After capture incubation, MP beads were allowed to settle by placing the assay plate on a magnet for 2 minutes until MP beads were amassed into pellets, after which assay plates were washed with 1× wash buffer (Singulex/EMD Millipore) using a Tecan HydroFlex 96-well format microplate washer. After this post-capture wash, 20 ×l of freshly prepared secondary detection antibody was added to all the wells of the assay plate, which was then incubated on a Jitterbug Microplate Incubator Shaker for 1 hour at 25 °C and at shake speed 5. A post-detection wash (on magnet) followed the detection incubation, after which the 96-well plate was removed from the magnet; the MP beads with bound immune complexes were manually re-suspended in 1× wash buffer; and all well contents were transferred to a new assay plate. The plate washer was used to aspirate the wash buffer from the new assay plate while it was on the magnet. After this final aspiration, the assay plate was removed from the magnet, and 11 μl of Elution Buffer B (Singulex/EMD Millipore) was added to each well. The plate was sealed and incubated on a Jitterbug Microplate Incubator Shaker at 25 °C and shake speed 5 for ~10 minutes. While elution incubation was occurring, 10 μl of Buffer D (Singulex/EMD Millipore) was added to each well in quadrants 1, 2, 3 and 4 of a 384-well plate (Nunc). After elution incubation, the assay plate was again placed on the magnet for 2 minutes to collect MP beads into pellets. Once the beads were attached to the magnet, 10 μl of the eluate per well was manually transferred from the assay plate to the corresponding wells in the 384-well plate using a multi-channel pipette. After the transfer, the 384-well plate was centrifuged to collect eluate into Buffer D and was heat-sealed using an ALPS 50 V-Manual Heat Sealer (Thermo Fisher Scientific); the plate was then read on the Singulex Erenna or SMCxPRO instrument. Sgx link version 1.4.56.39608 or SMCxPRO software version 1.1.405 with default options were used for data analysis and to back-calculate the concentration of VRC07 in the samples. The lower limit of detection for VRC07 by this assay is 11.5 ng ml−1 in this study.
VRC07 ADA assay.
A three-level tiered approach was used to screen, confirm and functionally characterize ADA in clinical serum samples. For the tier 1 screening assay, sera of the participants were pre-diluted at 1:2 with Meso Scale Discovery (MSD) assay diluent and then mixed at 1:1 with optimized concentration of SULFO-TAG therapeutic monoclonal antibody (reporter molecule) and biotinylated VRC07 (capture molecule) for a final sample dilution of 1:4 in a 384-well polypropylene plate at 50 μl per well. Samples were done in quadruplicate. These sample mixtures were incubated at 37 °C for 2 hours. During this incubation, streptavidin-coated MSD plates were incubated with MSD blocking buffer for 1 hour. After the incubation, the MSD plate was washed three times with 90 μl of wash buffer, and 25 μl of incubated sample mixture was transferred to a streptavidin-coated MSD plate and incubated at room temperature for 3 hours. Any ADA present in the serum binds both the biotinylated and SULFO-TAG-labeled VRC07, forming a bridging complex attaching to the streptavidin-coated MSD plate. After the incubation, the MSD plate was washed three times with 90 μl of wash buffer and 40 μl of MSD read buffer added. Luminescence intensity (electrochemiluminescence (ECL) unit) was measured within 20 minutes by an MSD 600 plate reader using MSD Workbench software version 4.0.12.1. The test sample is tier 1 positive if the ECL is greater than the floating positivity cutpoint (negative control ECL × 1.304).
Only tier 1 positive samples were advanced to tier 2 assays. In the tier 2 ADA assay, the sample was pre-incubated with and without the 10 μg ml−1 of unlabeled VRC07 and evaluated for the reduction (percentage) of signal in the presence of unlabeled VRC07. Serum samples from participants were pre-diluted at 1:2 with MSD assay diluent and either spiked with VRC07 at 20 μg ml−1 in MSD assay diluent or not and incubated for 1 hour at 37 °C. Samples were then processed in the same manner as tier 1 assays. The test sample is tier 2 positive if the percent reduction of signal is greater than the positivity cutpoint (33.19% reduction).
A tier 3 confirmatory HIV neutralization assay using a pseudovirus with an ARV-resistant backbone was used to functionally characterize tier 2 positive ADA67. In the confirmatory HIV neutralization assay, VRC07 is spiked at IC80 concentration (80% neutralization of target virus) into growth medium used in the assay. The test sample was diluted at 1:10, followed by nine serial dilutions of 1:5 for a total of ten dilution points in duplicate wells. The diluted sample in VRC07-spiked medium was incubated at 37 °C for 45 minutes. Diluted ART-resistant pseudotyped virus was added to the sample and incubated at 37 °C for 45–90 minutes. Mycoplasma-free TZM-bl cells were then added to the sample–virus mixture and incubated at 37 °C for 48–56 hours. At the end of the incubation, the luciferase activity was measured after addition of Britelite Plus luciferase substrate using a luminometer. The percent reduction in luminescence signal was calculated with baseline wells, and 5PL non-linear regression analysis was used to interpolate the ID50 (50% neutralization titer). Any neutralization readout equal to or lower than 50% (reportable ID50 titer) neutralized by the spiked sample was reported as ADA positive, indicating that the ADA in the sample interferes with the function of the therapeutic monoclonal antibody. If the therapeutic monoclonal antibody-spiked sample had a neutralization readout that is higher than 50%, this suggested that the ADA present in the test sample did not inhibit the functionality of the therapeutic monoclonal antibody. Luminescence was acquired using a Molecular Devices Paradigm Multi-Mode Microplate Reader using Software Pro. Tier 3 VRC07 ADA was analyzed using LabKey neutralization analysis tool version 19.2.
IgG1 allotyping.
IgG1 allotypes GM1, GM3 and GM17 were determined by a two-step nested PCR and TaqMan genotyping assay from Applied Biosystems. The determination of IgG1 marker GM1 was done by a two-step nested PC and TaqMan genotyping assay from Applied Biosystems, using the following primers and probes: initial PCR forward primer 5′-TTGTGACAAAACTCACACATGC-3′ and reverse primer 5′-GATGTCGCTGGGATAGAAGC-3′, followed by a nested qPCR using forward primer 5′-AGTGACCGCTGTACCAACCT-3′, reverse primer 5′-GATGTCGCTGGGATAGAAGC-3′, probe 1 (GM1-specific) 5′-CCGGGATG AGCTGACCAA-3′ and probe 2 (GM1-specific) 5′-CCGGGAGGAGATGACC AA-3′. IgG1 markers GM3 and GM17 were determined by a pre-designed TaqMan genotyping assay from Applied Biosystems employing the following primers and probes: forward primer 5′-CCCAGACCTACATCTGCAACGTGA-3′, reverse primer 5′-CTGCCCTGGACTGGGACTGCAT-3′, probe 1 (GM17-specific) VIC-CTCTCACCAACTTTCTTGT-NFQ and probe 2 (GM3-specific) FAM-CTCTCACCAACTCTCTTGT-NFQ68. The presence/absence of GM1 was also confirmed serologically by a standard hemagglutination-inhibition assay69.
Purification of IgG from plasma.
Plasma was heat-inactivated at 56 °C for 1 hour and then centrifuged at 16,000g for 10 minutes. Plasma was diluted to 10 ml total volume in Pierce Protein A Binding Buffer (Thermo Fisher Sciwentific) and applied to a column containing a 1-ml bed of Protein A Sepharose Fast Flow (GE Healthcare) equilibrated in Protein A Binding Buffer. After being allowed to drain, the column was washed three times with 10-ml volumes of Protein A Binding Buffer. IgG was eluted with Pierce Protein A Elution Buffer (Thermo Fisher Scientific) and collected in a 1:10 volume of 1 M Tris-HCL pH 8.0. IgG was buffer exchanged in PBS using 10,000 MWCO Amicon Ultra-15 centifugal filter units (EMD Millipore) over three rounds of spinning. IgGs were diluted to a final concentration of 10 mg ml−1 with PBS.
TZM-bl pseudovirus neutralization assay.
For standard pseudovirus neutralization assays, 10 μl of five-fold serially diluted IgG or monoclonal antibody in complete DMEM (supplemented with 1× penicillin–streptomycin and 10% FBS) was incubated with 40 μl of diluted HIV-1 Env-pseudotyped virus and incubated for 30 minutes at 37 °C in a black 96-well tissue culture plate. Next, 20 μl of TZM-bl cells (10,000 cells per well) (NIH AIDS Reagent Program) with 70 μg ml−1 of DEAE-Dextran were then added and incubated overnight at 37 °C. Each experiment plate also had a column of wells containing cells only (no antibody or virus) and a column of wells containing cells and virus only (no antibody) as controls for background TZM-bl luciferase activity and maximal viral entry, respectively. The following day, all wells received 80 μl of fresh complete DMEM (cDMEM) and were incubated overnight at 37 °C. The next day, 40 μl of steadylight plus luciferase reagent (PerkinElmer) was added to cells, followed by plate shaking at 600 r.p.m. for 15 minutes. Luminometry was performed on a SpectraMax L luminometer (Molecular Devices). Background luciferase activity of TZM-bl cells was subtracted from all test wells using the average relative light units (RLU) from the uninfected control wells (cells only) before calculating the percent neutralization. Percent neutralization was determined by calculating the difference in average RLU between virus-only wells (cells + virus) and test wells (cells + virus + IgG), dividing this result by the average RLU of virus-only wells (cells + virus) and multiplying by 100. Neutralizing IgG titers are expressed as the antibody concentration required to achieve 50% neutralization and calculated using a dose–response curve fit with a five-parameter non-linear function.
For neutralization assays assessing 5C9 competition with VRC07, the standard neutralization assay was performed with the following modification. Before the addition of virus, 5 μl of 100 μg ml−1 5C9 antibody was pre-incubated with 10 μl of serially diluted IgG at 37 °C for 30 minutes before adding 35 μl of diluted HIV-1 Env-pseudotyped virus (effectively diluting 5C9 to a final concentration of 10 μg ml−1) and incubation at 37 °C for 30 minutes.
Statistics.
Statistical analysis was performed using GraphPad Prism version 9.0 and R version 4.0.2.
Supplementary Material
Acknowledgements
We would like to acknowledge J. Gilly and C. Case of Science Applications International Corporation for their contributions to study product manufacturing as well as P. Johnson and F. Wright of Children’s Hospital of Philadelphia for providing critical AAV expertise. We thank R. Kothera for technical assistance in GM allotyping. We would like to thank our trial volunteers for their contribution and commitment to developing an effective clinical intervention for the prevention and control of HIV. This work was supported by intramural funding from the National Institute of Allergy and Infectious Diseases through the National Institutes of Health Intramural Research Program. A.B.B. is supported by National Institutes for Drug Abuse Avenir New Innovator Award DP2DA040254, the MGH Transformative Scholars Program as well as funding from the Charles H. Hood Foundation. J.P.P. received funding from Leidos Biomedical Research, Inc. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
the VRC 603 Study Team
Charla Andrews1, Anita Arthur1, Seemal F. Awan1, Allison Beck1, Eugeania Burch1, Maria C. Burgos Florez1, Nina M. Berkowitz1, Eli A. Boritz1, Kevin Carlton1, Cora T. Cartagena1, Christina Carter1, Grace L. Chen1, Pamela Costner1, Jennifer Cunningham1, Daniel C. Douek1, Aba M. Eshun1, Catina Evans1, Renunda Hicks1, Katherine V. Houser1, Justine Jones1, Brenda Larkin1, Lam Le1, Floreliz Mendoza1, Stephen Migueles1, John Misasi1, Thuy A. Nguyen1, Abidemi Ola1, Karen Parker1, Iris Pittman1, La’ Shawn Requilman1, Ro Shauna Rothwell1, Gretchen L. Schieber1, Jamie Saunders1, Sandra Sitar1, Colin Tran1, Olga Trofymenko1, Olga Vasilenko1, Sana Waheed1, Lingshu Wang1, Xiaolin Wang1, William Whalen1, Pernell Williams1, Richard L. Wu1 and Kathy Zephir1
Footnotes
Reporting Summary. Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Competing interests
A.B. and D.B. are named inventors on patent US9527904B2 held by the California Institute of Technology describing the vector used in this study. J.M. and G.N. are named inventors on patents US 61/568,520, 14/363,740 and 15/612,846 held by the National Institutes of Health describing the ex vivo production of VRC07. The remaining authors declare no competing interests.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41591-022-01762-x.
A list of authors and their affiliations appears at the end of the paper.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41591-022-01762-x.
Data availability
Data generated in this study, including the study protocol, statistical analysis plan and informed consent form, will be available as de-identified data on ClinicalTrials.gov (NCT03186781) within 1 year from the primary completion date of the study. Individual de-identified participant data that underlie the results reported in this article are available, after de-identification, in the Supplementary Information section immediately after publication with no end date. Requests for additional data or materials will be promptly reviewed by the corresponding author (J.C.) to determine if these are subject to intellectual property, confidentiality or ethical obligations. Any data and materials that can be shared will be released via a material transfer agreement. Personal data underlying this article cannot be shared publicly as they are sensitive. Inquiries regarding data or material availability should be directed to jcasazza@mail.nih.gov.
References
- 1.Fuchs SP & Desrosiers RC Promise and problems associated with the use of recombinant AAV for the delivery of anti-HIV antibodies. Mol. Ther. Methods Clin. Dev 3, 16068 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rerks-Ngarm S et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med 361, 2209–2220 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Gift SK, Leaman DP, Zhang L, Kim AS & Zwick MB Functional stability of HIV-1 envelope trimer affects accessibility to broadly neutralizing antibodies at its apex. J. Virol 91, e01216–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torrents de la Pena A et al. Improving the immunogenicity of native-like HIV-1 envelope trimers by hyperstabilization. Cell Rep. 20, 1805–1817 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein JS & Bjorkman PJ Few and far between: how HIV may be evading antibody avidity. PLoS Pathog. 6, e1000908 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiller J & Chackerian B Why HIV virions have low numbers of envelope spikes: implications for vaccine development. PLoS Pathog. 10, e1004254 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton DR & Mascola JR Antibody responses to envelope glycoproteins in HIV-1 infection. Nat. Immunol 16, 571–576 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pancera M et al. Structural basis for diverse N-glycan recognition by HIV-1-neutralizing V1–V2-directed antibody PG16. Nat. Struct. Mol. Biol 20, 804–813 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei X et al. Antibody neutralization and escape by HIV-1. Nature 422, 307–312 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Hartley O, Klasse PJ, Sattentau QJ & Moore JP V3: HIV’s switch-hitter. AIDS Res Hum. Retroviruses 21, 171–189 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Bonsignori M et al. Antibody-virus co-evolution in HIV infection: paths for HIV vaccine development. Immunol. Rev 275, 145–160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korber B et al. Evolutionary and immunological implications of contemporary HIV-1 variation. Br. Med. Bull 58, 19–42 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Huang J et al. Identification of a CD4-binding-site antibody to HIV that evolved near-pan neutralization breadth. Immunity 45, 1108–1121 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491, 406–412 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mouquet H et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc. Natl Acad. Sci. USA 109, E3268–3277 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sok D et al. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc. Natl Acad. Sci. USA 111, 17624–17629 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X HIV broadly neutralizing antibodies: VRC01 and beyond. Adv. Exp. Med. Biol 1075, 53–72 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Liu J et al. Antibody-mediated protection against SHIV challenge includes systemic clearance of distal virus. Science 353, 1045–1049 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mascola JR et al. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol 73, 4009–4018 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudicell RS et al. Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. J. Virol 88, 12669–12682 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saunders KO et al. Sustained delivery of a broadly neutralizing antibody in nonhuman primates confers long-term protection against simian/human immunodeficiency virus infection. J. Virol 89, 5895–5903 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caskey M et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522, 487–491 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lynch RM et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci. Transl. Med 7, 319ra206 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Mendoza P et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 561, 479–484 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burton DR & Hangartner L Broadly neutralizing antibodies to HIV and their role in vaccine design. Annu Rev. Immunol 34, 635–659 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwong PD, Mascola JR & Nabel GJ Rational design of vaccines to elicit broadly neutralizing antibodies to HIV-1. Cold Spring Harb. Perspect. Med 1, a007278 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balazs AB et al. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 481, 81–84 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson PR et al. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat. Med 15, 901–906 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharon D & Kamen A Advancements in the design and scalable production of viral gene transfer vectors. Biotechnol. Bioeng 115, 25–40 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Daya S & Berns KI Gene therapy using adeno-associated virus vectors. Clin. Microbiol. Rev 21, 583–593 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan D et al. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J. Virol 72, 8568–8577 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nowrouzi A et al. Integration frequency and intermolecular recombination of rAAV vectors in non-human primate skeletal muscle and liver. Mol. Ther 20, 1177–1186 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penaud-Budloo M et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J. Virol 82, 7875–7885 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brady JM, Baltimore D & Balazs AB Antibody gene transfer with adeno-associated viral vectors as a method for HIV prevention. Immunol. Rev 275, 324–333 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnepp BC & Johnson PR Adeno-associated virus delivery of broadly neutralizing antibodies. Curr. Opin. HIV AIDS 9, 250–256 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calcedo R, Vandenberghe LH, Gao G, Lin J & Wilson JM Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis 199, 381–390 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srivastava A In vivo tissue-tropism of adeno-associated viral vectors. Curr. Opin. Virol 21, 75–80 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balazs AB et al. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat. Med 20, 296–300 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saunders KO et al. Broadly neutralizing human immunodeficiency virus type 1 antibody gene transfer protects nonhuman primates from mucosal simian-human immunodeficiency virus infection. J. Virol 89, 8334–8345 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welles HC et al. Vectored delivery of anti-SIV envelope targeting mAb via AAV8 protects rhesus macaques from repeated limiting dose intrarectal swarm SIVsmE660 challenge. PLoS Pathog. 14, e1007395 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez-Navio JM et al. Adeno-associated virus delivery of anti-HIV monoclonal antibodies can drive long-term virologic suppression. Immunity 50, 567–575 e565 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Priddy FH et al. Adeno-associated virus vectored immunoprophylaxis to prevent HIV in healthy adults: a phase 1 randomised controlled trial. Lancet HIV 6, e230–e239 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szymczak AL et al. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat. Biotechnol 22, 589–594 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Manno CS et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med 12, 342–347 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Rangarajan S et al. AAV5-Factor VIII gene transfer in severe hemophilia A. N. Engl. J. Med 377, 2519–2530 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Jefferis R & Lefranc MP Human immunoglobulin allotypes: possible implications for immunogenicity. MAbs 1, 332–338 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ledgerwood JE et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin. Exp. Immunol 182, 289–301 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarzotti-Kelsoe M et al. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J. Immunol. Methods 409, 131–146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nathwani AC et al. Long-term safety and efficacy of Factor IX gene therapy in hemophilia B. N. Engl. J. Med 371, 1994–2004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lisowski L, Tay SS & Alexander IE Adeno-associated virus serotypes for gene therapeutics. Curr. Opin. Pharm 24, 59–67 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Fuchs SP et al. AAV-delivered antibody mediates significant protective effects against SIVmac239 challenge in the absence of neutralizing activity. PLoS Pathog. 11, e1005090 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuchs SP, Martinez-Navio JM, Rakasz EG, Gao G & Desrosiers RC Liver-directed but not muscle-directed AAV-antibody gene transfer limits humoral immune responses in rhesus monkeys. Mol. Ther. Methods Clin. Dev 16, 94–102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bar KJ et al. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N. Engl. J. Med 375, 2037–2050 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cale EM et al. Neutralizing antibody VRC01 failed to select for HIV-1 mutations upon viral rebound. J. Clin. Invest 130, 3299–3304 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crowell TA et al. Safety and efficacy of VRC01 broadly neutralising antibodies in adults with acutely treated HIV (RV397): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet HIV 6, e297–e306 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cunningham CK et al. Safety, tolerability, and pharmacokinetics of the broadly neutralizing human immunodeficiency virus (HIV)-1 monoclonal antibody VRC01 in HIV-exposed newborn infants. J. Infect. Dis 222, 628–636 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riddler SA et al. Randomized clinical trial to assess the impact of the broadly neutralizing HIV-1 monoclonal antibody VRC01 on HIV-1 persistence in individuals on effective ART. Open Forum Infect. Dis 5, ofy242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaudinski MR et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults. PLoS Med. 15, e1002493 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaudinski MR et al. Safety and pharmacokinetics of broadly neutralising human monoclonal antibody VRC07-523LS in healthy adults: a phase 1 dose-escalation clinical trial. Lancet HIV 6, e667–e679 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caskey M et al. Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat. Med 23, 185–191 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fang J et al. Stable antibody expression at therapeutic levels using the 2A peptide. Nat. Biotechnol 23, 584–590 (2005). [DOI] [PubMed] [Google Scholar]
- 62.Zhou T et al. Multidonor analysis reveals structural elements, genetic determinants, and maturation pathway for HIV-1 neutralization by VRC01-class antibodies. Immunity 39, 245–258 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fang J et al. An antibody delivery system for regulated expression of therapeutic levels of monoclonal antibodies in vivo. Mol. Ther 15, 1153–1159 (2007). [DOI] [PubMed] [Google Scholar]
- 64.Schambach A et al. Woodchuck hepatitis virus post-transcriptional regulatory element deleted from X protein and promoter sequences enhances retroviral vector titer and expression. Gene Ther. 13, 641–645 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Casazza JP et al. Therapeutic vaccination expands and improves the function of the HIV-specific memory T-cell repertoire. J. Infect. Dis 207, 1829–1840 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prabhakaran M et al. A sensitive method to quantify HIV-1 antibodies in mucosal samples. J. Immunol. Methods 491, 112995 (2021). [DOI] [PubMed] [Google Scholar]
- 67.Seaman MS et al. Optimization and qualification of a functional anti-drug antibody assay for HIV-1 bnAbs. J. Immunol. Methods 479, 112736 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pandey JP et al. Immunoglobulin genes and immunity to HSV1 in Alzheimer’s disease. J. Alzheimers Dis 70, 917–924 (2019). [DOI] [PubMed] [Google Scholar]
- 69.Schanfield M & van Logem E in Handbook of Experimental Immunology Vol. 94 (ed. Weir D) 1–18 (Blackwell, 1986). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated in this study, including the study protocol, statistical analysis plan and informed consent form, will be available as de-identified data on ClinicalTrials.gov (NCT03186781) within 1 year from the primary completion date of the study. Individual de-identified participant data that underlie the results reported in this article are available, after de-identification, in the Supplementary Information section immediately after publication with no end date. Requests for additional data or materials will be promptly reviewed by the corresponding author (J.C.) to determine if these are subject to intellectual property, confidentiality or ethical obligations. Any data and materials that can be shared will be released via a material transfer agreement. Personal data underlying this article cannot be shared publicly as they are sensitive. Inquiries regarding data or material availability should be directed to jcasazza@mail.nih.gov.