Abstract
Aim:
This study aimed to perform a head-to-head comparison of changes during NASH progression throughout 6-11 weeks of an experiment to supply a faster nutritional model in mimicking NASH to decrease the duration and cost of in vivo studies.
Background:
New therapies are urgently needed because of the growing prevalence of non-alcoholic steatohepatitis (NASH) and the lack of an effective treatment approach. Currently, dietary interventions are the most efficient options.
Methods:
This study compared features of NASH in a murine model using protocol that combined special nutritional regimes based on the combination of 21.1% fat, 41% sucrose, and 1.25% cholesterol with weekly intraperitoneal injections of carbon tetrachloride (CCl4). Male C57BL/6J mice received either special compositions + CCl4 (NASH group) or standard chow diet (healthy control group) for 11 weeks. Liver histopathology based on hematoxylin and eosin (H&E) and Masson’s Trichrome (TC) staining and biochemical analyses were used to assess disease progression.
Results:
In C57BL/6J mice administered a high fat, high cholesterol, high sucrose diet and CCl4 for 8 weeks, steatohepatitis with pronounced hepatocyte ballooning, inflammation, steatosis, and fibrosis was observed. According to the NAFLD activity scoring system, the maximum NAS score was manifested after 8-9 weeks (NAS score: 6.75). Following this protocol also led to a significant increase in AST and ALT, total cholesterol, and total triglyceride serum levels in the NASH group.
Conclusion:
Following the special nutritional regime based on high fat, cholesterol, and sucrose in combination with CCL4 injections resulted in a NASH model using C57BL/6J mice in a shorter time compared to similar studies. The obtained histopathological NASH features can be advantageous for preclinical drug testing.
Key Words: Steatohepatitis, Nonalcoholic, Animal model, Liver diseases, Carbon tetrachloride
Introduction
Non-alcoholic steatohepatitis (NASH) is a more aggressive necroinflammatory form of non-alcoholic fatty liver disease (NAFLD). Sufferers are typically subjected to a variety of steatosis and hepatic inflammation and ballooning with or without fibrosis. If left untreated, NASH can progress to cirrhosis morbidity, hepatocellular cancer, and a need for liver transplantation (1-3). The heterogeneous nature of NASH is reflected by various risk factors such as obesity, diabetes, and metabolic syndromes (4). Despite the significant impact of NASH on overall health, the number of available medical interventions and pharmacological therapies for NASH is limited, making the treatment of this disease a point of contention in the medical community (5). Therefore, current therapies for NASH are confined to lifestyle modifications, such as physical activity and dietary interventions (6, 7).
As a result of creating NASH models, in addition to various in-vitro cell cultured models (e.g., human hepatic cell lines, primary human hepatocytes [PHHs], and hepatocyte-like cells [HLCs]), two-dimensional (e.g., monoculture, co-culture) and three-dimensional cell culture models (e.g., spheroids and organoids) (8), various in vivo models, and notably murine models have been suggested. Genetic techniques, chemical intervention, specific diets, and even combinations of these have been developed to emulate mouse models closely (9). Several models based on dietary approaches, including the methionine/choline-deficient (MCD) diet, high-fat diet (HFD), and western diet (WD), have been widely used to produce rodent dietary models of NASH. While the aforementioned nutritional models of NASH have been studied for decades, they cannot accurately illustrate the whole range of metabolic disorders or disease development in humans. For example, previous studies on the HFD model have reported steatohepatitis, fibrosis, and tumor progression; however, they have failed to show steatosis in the experimental group (10, 11). Other studies using the MCD diet have shown a weight reduction in the experimental mice, but did not fully report the expected metabolic syndromes in human NASH cases (12, 13). On the other hand, WD feeding has been shown to increase liver damage, steatosis, inflammation, fibrosis, insulin resistance, metabolic syndrome, and weight gain in the experimental mice, closely mimicking the pathophysiology and clinical features seen in humans. This makes WD (a diet containing high fat, cholesterol, and sucrose) the ideal diet for NASH study models (15).
Carbon tetrachloride (CCl4), a liver toxin, has been widely used for many years to cause direct hepatocyte damage and fibrosis (15-17). Moreover, previous studies have shown that different doses of CCl4 to HFD-fed mice activated steatosis, inflammation, hepatocellular ballooning, and fibrosis and also significantly elevated blood alanine aminotransferase levels after 12 weeks (18). It has further been shown that taking CCL4 in combination with WD induced severe liver histological features and increased weight gain similar to that seen in NASH patients. Nonetheless, there are limitations, and the effects of prolonged CCl4 treatment combined with WD as a potential model for NASH have not been identified (19).
Regardless of the variety in methods applied to develop NASH in mice, none of them can fully satisfy all the required criteria for an ideal human NASH model (20). Moreover, almost all existing methods progress over prolonged periods, which can cause problems such as high animal mortality and increased animal maintenance expenses. Therefore, this study aimed to decrease the duration and cost of in vivo studies and provide a dietary model that is faster and more reliable in mimicking the NASH model in humans. In this study, we provide a dynamic analysis of the progression of NASH in mice fed a WD diet, a sugar solution, and administered CCL4 injections within 6-11 weeks. Of note, WD and CCl4 treatment for 8 weeks showed higher similarity to human NASH with expected histopathological NASH features (steatosis, hepatocellular ballooning, and inflammation) along with biochemical changes.
Methods
Animals
C57BL/6J male mice (6-8 weeks of age, weighing 16-22 g) were purchased from the Pasteur Institute of Iran (Pasteur Institute, Tehran, Iran) and kept in standard cages under controlled conditions in a 10-h light – 14-h dark cycle in a 22 ± 2 °C facility. The Ethics Committee of the Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran, approved all experimental procedures of this study (Ethical code: IR.SBMU.RIGLD.REC.1399.063).
Dietary interventions
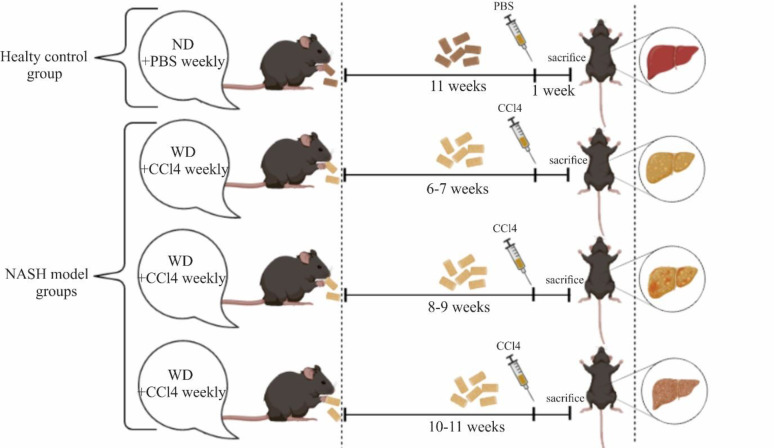
After one week of acclimatization under appointed standard terms, the C57BL/6J mice were assigned to 4 groups. Mice in the healthy control group, were fed a normal chow diet (ND) with normal water (NW). NASH experimental groups were divided into 3 subgroups: group 1 received WD for 6 and 7 weeks, group 2 received WD for 8 and 9 weeks, and group 3 received the WD for 10 and 11 weeks (from 6 weeks after initiation of dietary intervention up to 11 weeks). NASH groups consumed a WD comprised of 21.1% fat, 41% sucrose, and 1.25% cholesterol by weight and a high sugar liquid solution containing d-fructose (23.1 g/L) (Sigma-Aldrich, F0127) and d-glucose (18.9 g/L) (Sigma-Aldrich, G8270) (19). Along with the diet, the NASH model mice were administered CCl4 (99% purified, Sigma, UK) intraperitoneally every week at the dose of 0.2 µL of the mouse’s body weight (17). In the control group, phosphate-buffered saline (PBS) was administered intraperitoneally every week instead. To avoid the acute effect of CCl4 at the end of the feeding period, mice were euthanized one week after the final injection (Figure 1).
Figure 1.
Protocol for the control and NASH experimental groups. Mice were categorized into 2 main groups: the healthy control group and the NASH group. NASH mice were separated into 3 subgroups. In each group, mice were sacrificed one week after the final injection. (ND: normal diet, PBS: phosphate-buffered saline, WD: western diet, CCl4: Carbon tetrachloride)
Histopathological experiments
The harvested liver tissues were fixed in 10% neutral buffered formalin (NBF, PH. 7.26) for 48 h, then processed and placed in paraffin. The 5-µm thick sections were prepared and stained with hematoxylin and eosin (H&E) for histology assessment and Masson’s Trichrome (TC) for fibrosis analyses. An expert pathologist evaluated the histological slides using light microscopy (Olympus BX51; Olympus, Tokyo, Japan). As shown in Table 1, steatosis grade was considered on entire H&E-stained sections and scored from 0 to 3. Lobular inflammation was evaluated by counting the number of inflammatory foci (the observation of at least 3 inflammatory cells in close proximity) per lobule in five randomly chosen lobules at 20× magnification and was graded from 0 to 3. The presence of ballooning hepatocytes was evaluated in five randomly-chosen rectangular areas of 0.3 mm2 at fields of 40× magnification in areas with steatosis. Ordinal scores of these 3 histological parameters are considered to give a NAFLD activity score (NAS) using the NASH-Clinical Research Network (CRN) criteria. According to this scoring system, the sum of steatosis (0–3), lobular inflammation (0–3), and hepatocellular ballooning degeneration (0–2) scores are considered the NAFLD activity score (NAS) (score of 0–2: not NASH; 3–4: borderline; 5–8: NASH) (21, 22). Also, the rate of hepatic fibrosis was evaluated after TC staining (23). The CRN histological scoring system was used to grade fibrosis in a different biopsy specimen (Table 2) (24).
Table 1.
NAFLD activity score (NAS) by NASH-CRN (22)
| score | steatosis | inflammation | ballooning |
|---|---|---|---|
| 0 | <5% | No foci | None |
| 1 | 5-33% | <2 foci | Few (≤4) |
| 2 | >33-66% | 2-4 foci | Many (>4) |
| 3 | >66% | >4 foci |
Table 2.
CRN histological scoring system for grading of fibrosis in NAFLD (24)
| Stage of fibrosis | CRN scoring system |
| 0 | No fibrosis |
| 1A | Mild perisinusoidal |
| 1B | Moderate perisinusoidal |
| 1C | Portal/periportal fibrosis |
| 2 | Perisinusoidal and portal/periportal |
| 3 | Bridging fibrosis |
| 4 | Cirrhosis |
Serum collection and biochemical analysis
As biomarkers of liver injury, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol, and total triglyceride levels were measured in blood samples using standard laboratory test kits to confirm pathology results at the 8th week. A 1.5-mL tube was used to collect whole blood. After centrifuging at 1000 g for 10 minutes at 4 °C, serum supernatant was collected and stored at -80 °C.
Data analysis
The statistical significance of differences between study groups was evaluated using t-test and one-way ANOVA with post hoc Tukey’s multiple comparisons test using Prism software, a statistical package, version 9 (GraphPad Software, USA). All values are means SEM. P values less than 0.05 were considered statistically significant.
Results
Features of steatohepatitis in WD/CCL4 mice
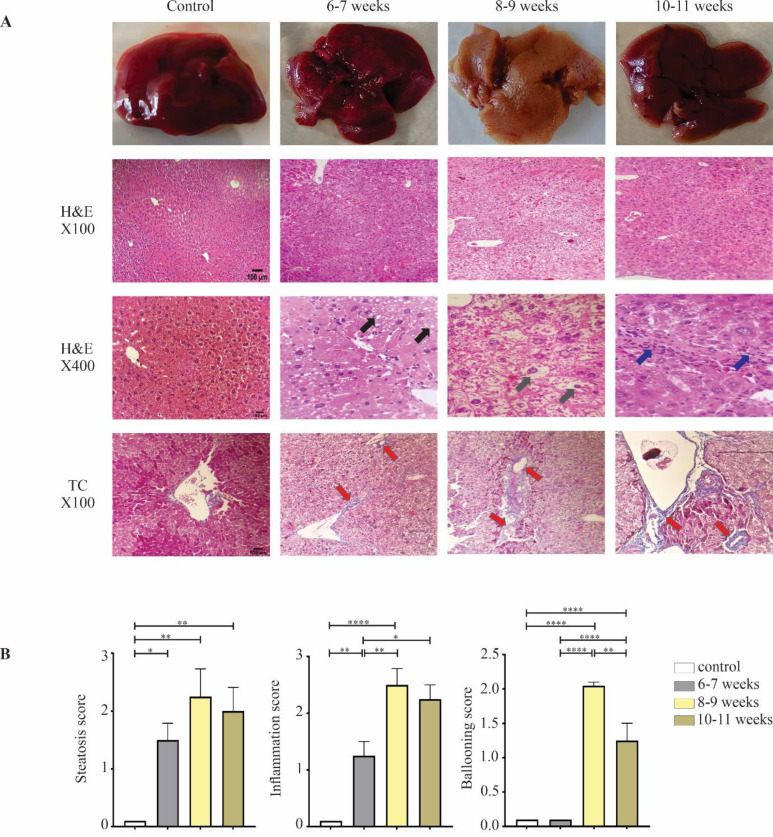
The histopathological changes in C57BL/6J mice of each group were monitored from 6-11 weeks using H&E and TC staining (Figure 2A). The histopathological evaluation of harvested liver samples in control mice showed normal structures. As expected, in WD-fed mice, H&E analyses indicated the development of steatosis, with a median steatosis score of 1.5 during 6-7 weeks compared with the control group (p<0.05). The steatosis was primarily of the microvesicular type and located in zones 1 and 2. Mild lobular inflammation was also present, with a lobular inflammation median score of 1.25 (p<0.01). However, mice in the 6-7 weeks group failed to display any prominent appearance of ballooning degeneration (median score ballooning: 0). At 8-9 weeks, more significant steatosis was observed in WD-fed mice with a median steatosis score of 2.25 (p<0.01). Both microvesicular and macrovesicular types of steatosis were seen in these samples. Moderate to severe lobular inflammation was also present with a final median score of 2.5 in this group (p<0.0001). The highest rate of ballooning degeneration was observed at 8-9 weeks (grade 2) (p<0.0001). Less significant steatosis (p<0.01) was observed in the WD-fed mice 10-11 weeks post-induction (grade 2) compared to the numbers observed in the 8-9 weeks experimental group. Furthermore, prolonged WD feeding and CCl4 administration led to moderate inflammation (grade 2.25) (p<0.0001) and an almost mild degree of hepatocellular ballooning (grade 1.25) by the end of feeding (p<0.0001) (Table 3) (Figure 2B). All TC-stained liver sections from the experimental groups were also evaluated histologically. Using the staining procedure, the extent of collagen fiber deposition was indicated. The histopathological micrographs of liver samples in the control group were normal, showing no histopathological changes; the structure of hepatocytes cords and cortical/medullary tubules were well organized (Figure 2A). Histopathological evaluation at weeks 6, 7, and 8 showed moderate centrilobular perisinusoidal fibrosis (stage 1B). At 9 weeks post-induction, delicate collagen fibers were deposited around the sinusoidal and portal areas (stage 2). At weeks 10 and 11, complex bridging fibrosis with extensive perisinusoidal fibrosis was seen in these samples (stage 3) (Table 3).
Figure 2.
Histopathological analysis of liver section at 6-11 weeks post-induction in comparison with the control group. A) Macroscopic view of the liver tissue from control and NASH mice. H&E stains of liver samples (magnification: X100 and X400) represent steatosis (black arrows), lobular inflammation (blue arrows), ballooning (gray arrows), and TC stains (magnification X100) represent fibrotic septa (red arrows) (scale bar: 100µm). B) Histological scoring for steatosis, hepatocyte ballooning, lobular inflammation. Data is expressed as mean ± SEM and compared by one-way ANOVA (*p <0.05, **p <0.01, ****p <0.0001)
Table 3.
NAFLD activity score of mice treated with WD and CCl4
| duration treatment | steatosis | inflammation | ballooning | NAS score | Fibrosis stage |
|---|---|---|---|---|---|
| control | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 6 -7 | 1.5 | 1.25 | 0.0 | 2.75 | 1B |
| 8-9 | 2.25 | 2.5 | 2 | 6.75 | 1C |
| 10-11 | 2 | 2.25 | 1.25 | 5.5 | 3 |
Serum tests for liver injury in mice treated with WD/CCl4
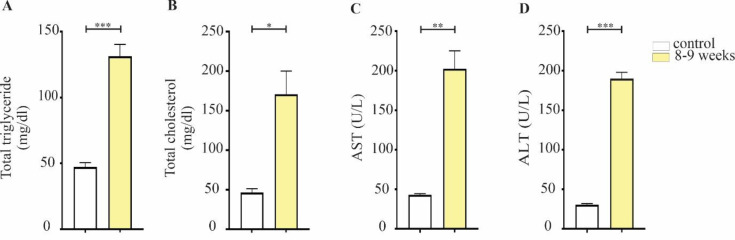
Serological analysis was performed following histopathological confirmation of the NASH model in the 8-9 weeks mice with the highest NAS score. Serum biochemical panels are another valuable tool for identifying NASH progression, as they reflect the evaluation of hepatocellular function (25, 26). In the current study, we measured serum ALT, AST, total triglyceride, and total cholesterol. Feeding a WD diet also led to a significant increase in AST and ALT serum levels (p<0.01 and p<0.001, respectively). Cholesterol has also been clinically shown to be directly involved in the development of fatty liver disease and in rodent models of NASH (27). The total serum cholesterol level in WD/CCL4 mice was significantly elevated (p<0.05). In addition, increased total TG concentrations were found in 8-9 weeks mice compared to their respective controls (p<0.001) (Figure 3).
Figure 3.
(A) total triglyceride, (B) total cholesterol, (C) serum AST, and (D) ALT were measured in the 8-9 weeks treatment group in comparison with controls. Data is expressed as mean ± SEM and compared by t-test (*p<0.05, **p<0.01, ***p<0.001; ALT, alanine aminotransferase; AST, aspartate aminotransferase)
Discussion
NASH, a progressive stage of NAFLD, is a crucial hazard to the health of the general population. Subsequent diseases that follow NASH, such as fibrosis, cirrhosis, and liver cancer, cannot be treated until their underlying disorder is improved. As NASH lacks an approved pharmacotherapy, a trustworthy animal model for simulating the disease in humans is required for the evaluation of healing applications and preclinical testing before launching expensive human clinical trials (28, 29). Current NASH animal models focusing on dietary modifications have successfully shown the effects of diet on disease progression (30); however, studies on these models differ in the feeding periods used, which ultimately leads to drastic differences in the NASH characteristics reported in each study. These differences are mainly noticed in the range of steatosis, inflammatory response, and hepatic fibrosis development (31). It is important to note that the protocol described herein is simple, affordable, and achievable in a relatively short time.
It might be difficult to decide which diet to utilize for the NASH study, because so many options are currently available (MCD, HFD, WD). A common feature of these diets is their ability to induce steatohepatitis, obesity, and insulin resistance in mice, showing a close resemblance to the disease progression in humans. That being said, the WD, characterized by foods that are high fat, fructose (or sucrose), and cholesterol, is the most commonly used diet in studies, as it appears to more closely emulate NASH in humans (12, 32-35). In addition, adding a hepatotoxin such as CCl4 increases WD-induced hepatocyte ballooning, inflammation, fibrosis stage, and hepatic stellate cell (HSC) activation (36). As a result, CCl4 and WD may have a synergistic influence on NASH pathogenesis. Furthermore, a combination of fructose and glucose in drinking water may be more effective in promoting hepatic steatosis (37).
The current study has illustrated that CCl4 injections and WD feeding in C57BL/6J mice correlate with the NASH model of illness in humans. We carried out a head-to-head comparison of dynamic changes that occur during the evolution of NASH throughout 6-11 weeks of the experiment. In our screening, C57BL/6J mice that continued a WD for 8 weeks manifested the maximum score of our NASH model's histological features (steatosis, lobular inflammation, and ballooning) according to the NAFLD activity scoring system.
After 6 weeks of the WD, the mice had mild steatosis and inflammation with no signs of ballooning degeneration. This may demonstrate an insufficient period of WD feeding. In the 8–9-week groups, the increase of the NAS score in mice on the WD regimen to 6.75 reflects the progression of NASH in these mouse models. According to Chheda et al., the fast food diet (FFD)-CCl4 rat model exhibited NAFLD histological characteristics, including steatosis, inflammation, and fibrosis, with a NAS score of 6 in 8 weeks.38 This may point to the greater influence of WD in raising the NAS score. We also observed an increase in ALT and AST levels as hepatocyte injury indicators at 8 weeks, which is consistent with the findings of Chheda et al (38). There is also some evidence that total cholesterol and triglyceride levels can rise as a result of a high fat diet. In line with prior reports (39), our investigations also indicated higher levels of total triglyceride and cholesterol in NASH mice after 8 weeks of intervention compared to the healthy control group. Surprisingly, in mice remaining on a diet for more than 9 weeks, the NAS scoring dropped to 5.5 in the 10–11-week groups.
The goal of this study was not to create a new model for NASH with a unique disease development mechanism. Instead, our demonstrated NASH model aimed to reinforce previous studies while encompassing the physiological, metabolic, and histological aspects of the disease in humans. Previous studies have shown the development of a NASH model in mice that mimics the human condition after several weeks, with most studies showing this after 12 weeks. Marcher et al. suggested that feeding WD to male C57BL/6J mice along with fructose-supplemented drinking water can create a relevant mode of human NASH with steatosis after 12 weeks (40). Another study by Tsuchiya et al. combined WD with CCl4 and once again showed the development of NASH in the models, similar to the human disease, after 12 weeks (19).
It is crucial to evaluate how the current study differs from previous NASH animal model studies as well as its potential implications. Our NASH model is relevant in many respects to that reported by Tsuchiya et al., but it is distinguished from it by the head-to-head screening of changes during 6-11 weeks of NASH evolution as well as timeframes needed for NASH development. As existing diet-induced mouse models take a longer time to develop this hallmark of NASH, we were able to create NASH in a reasonable time frame, based on 6-11 weeks follow-up, in an attempt to reduce the length and expense of in vivo investigations. The rapid development of a NASH animal model might speed up mechanistic studies of NAFLD progression and provide a reliable in vivo system for screening and evaluating drugs to treat this widespread liver disease (41).
The advantage of the introduced model is the rapid development of NASH within 8 weeks of diet in contrast with other dietary NASH models, which need an induction period of at least 15 weeks or even longer (11, 32, 42-44). Therefore, this model has the benefits of being economical, having a high degree of resemblance to human disease, and in the availability of diet ingredients. However, there are limitations to our study. We detected steatosis, inflammation, and fibrosis associated with NASH in the WD-CCl4 model after 8 weeks, despite the fact that we were unable to assess insulin resistance or hyperinsulinemia in cases that needed to be measured.
Conclusion
To conclude, while the optimum animal model for NASH has yet to be established, the current dietary models offer complementary tools for studying this main human disease. The study's most noteworthy finding is the ability to correlate the histological development of NASH with the serological alterations that characterize disease progression in only 8-9 weeks. Not only might the fast establishment of animal models for NASH therapy hasten mechanistic research on NAFLD development, but it may also produce influential in vivo system screening/testing agents which act contrary to frequent liver illnesses. Genetic and epigenetic alterations, altered gene and protein expression, and pathophysiologic pathways that have been discovered in NASH should all be included in future investigations.
Conflict of interests
The authors declare that they have no conflict of interest.
References
- 1.Dufour JF, Scherer R, Balp MM, McKenna SJ, Janssens N, Lopez P, et al. The global epidemiology of nonalcoholic steatohepatitis (NASH) and associated risk factors–A targeted literature review. Endocr Metab Sci. 2021;3:100089. [Google Scholar]
- 2.Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. Jama. 2020;323:1175–1183. doi: 10.1001/jama.2020.2298. [DOI] [PubMed] [Google Scholar]
- 3.Abdelmalek MF. Nonalcoholic fatty liver disease: another leap forward. Nat Rev Gastroenterol Hepatol. 2021;18:85–86. doi: 10.1038/s41575-020-00406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thrasher T, Abdelmalek MF. Nonalcoholic fatty liver disease. N C Med J. 2016;77:216–219. doi: 10.18043/ncm.77.3.216. [DOI] [PubMed] [Google Scholar]
- 5.Dufour JF, Caussy C, Loomba R. Combination therapy for non-alcoholic steatohepatitis: rationale, opportunities and challenges. Gut. 2020;69:1877–1884. doi: 10.1136/gutjnl-2019-319104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geier A, Rau M. Emerging therapies for NASH - the future is now. Expert Rev Clin Pharmacol. 2017;10:467–469. doi: 10.1080/17512433.2017.1305269. [DOI] [PubMed] [Google Scholar]
- 7.Campbell P, Symonds A, Barritt ASt. Therapy for nonalcoholic fatty liver disease: current options and future directions. Clin Ther. 2021;43:500–517. doi: 10.1016/j.clinthera.2021.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Soret PA, Magusto J, Housset C, Gautheron J. In vitro and in vivo models of non-alcoholic fatty liver disease: a critical appraisal. J Clin Med. 2020;10 doi: 10.3390/jcm10010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carreres L, Jílková ZM, Vial G, Marche PN, Decaens T, Lerat H. Modeling diet-induced NAFLD and NASH in rats: a comprehensive review. Biomedicines. 2021;9:378. doi: 10.3390/biomedicines9040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura A, Tajima K, Zolzaya K, Sato K, Inoue R, Yoneda M, et al. Protection from non-alcoholic steatohepatitis and liver tumourigenesis in high fat-fed insulin receptor substrate-1-knockout mice despite insulin resistance. Diabetologia. 2012;55:3382–91. doi: 10.1007/s00125-012-2703-1. [DOI] [PubMed] [Google Scholar]
- 11.Kohli R, Kirby M, Xanthakos SA, Softic S, Feldstein AE, Saxena V, et al. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology. 2010;52:934–44. doi: 10.1002/hep.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machado MV, Michelotti GA, Xie G, Almeida Pereira T, Boursier J, Bohnic B, et al. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS One. 2015;10:0127991. doi: 10.1371/journal.pone.0127991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itagaki H, Shimizu K, Morikawa S, Ogawa K, Ezaki T. Morphological and functional characterization of non-alcoholic fatty liver disease induced by a methionine-choline-deficient diet in C57BL/6 mice. Int J Clin Exp Pathol. 2013;6:2683–96. [PMC free article] [PubMed] [Google Scholar]
- 14.Stephenson K, Kennedy L, Hargrove L, Demieville J, Thomson J, Alpini G, et al. Updates on dietary models of nonalcoholic fatty liver disease: current studies and insights. Gene Expr. 2018;18:5–17. doi: 10.3727/105221617X15093707969658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marques TG, Chaib E, da Fonseca JH, Lourenço AC, Silva FD, Ribeiro MA Jr, et al. Review of experimental models for inducing hepatic cirrhosis by bile duct ligation and carbon tetrachloride injection. Acta Cir Bras. 2012;27:589–94. doi: 10.1590/s0102-86502012000800013. [DOI] [PubMed] [Google Scholar]
- 16.Liedtke C, Luedde T, Sauerbruch T, Scholten D, Streetz K, Tacke F, et al. Experimental liver fibrosis research: update on animal models, legal issues and translational aspects. Fibrogenesis Tissue Repair. 2013;6:19. doi: 10.1186/1755-1536-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholten D, Trebicka J, Liedtke C, Weiskirchen R. The carbon tetrachloride model in mice. Lab Anim. 2015;49:4–11. doi: 10.1177/0023677215571192. [DOI] [PubMed] [Google Scholar]
- 18.Kubota N, Kado S, Kano M, Masuoka N, Nagata Y, Kobayashi T, et al. A high-fat diet and multiple administration of carbon tetrachloride induces liver injury and pathological features associated with non-alcoholic steatohepatitis in mice. Clin Exp Pharmacol Physiol. 2013;40:422–30. doi: 10.1111/1440-1681.12102. [DOI] [PubMed] [Google Scholar]
- 19.Tsuchida T, Lee YA, Fujiwara N, Ybanez M, Allen B, Martins S, et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J Hepatol. 2018;69:385–395. doi: 10.1016/j.jhep.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen HH, Feigh M, Veidal SS, Rigbolt KT, Vrang N, Fosgerau K. Mouse models of nonalcoholic steatohepatitis in preclinical drug development. Drug Discov Today. 2017;22:1707–1718. doi: 10.1016/j.drudis.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Savari F, Mard SA, Badavi M, Rezaie A, Gharib-Naseri MK. A new method to induce nonalcoholic steatohepatitis (NASH) in mice. BMC Gastroenterol. 2019;19:125. doi: 10.1186/s12876-019-1041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang W, Menke AL, Driessen A, Koek GH, Lindeman JH, Stoop R, et al. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS One. 2014;9:115922. doi: 10.1371/journal.pone.0115922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu G, Ye J, Liu XJ, Zhang NP, Zhao YM, Fan J, et al. Activation of pluripotent genes in hepatic progenitor cells in the transition of nonalcoholic steatohepatitis to pre-malignant lesions. Lab Invest. 2017;97:1201–1217. doi: 10.1038/labinvest.2017.84. [DOI] [PubMed] [Google Scholar]
- 24.Iqbal U, Perumpail BJ, Akhtar D, Kim D, Ahmed A. The epidemiology, risk profiling and diagnostic challenges of nonalcoholic fatty liver disease. Medicines. 2019;6:41. doi: 10.3390/medicines6010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. Cmaj. 2005;172:367–79. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–70. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 27.Farrell GC, van Rooyen D. Liver cholesterol: is it playing possum in NASH? Am J Physiol Gastrointest Liver Physiol. 2012;303:9–11. doi: 10.1152/ajpgi.00008.2012. [DOI] [PubMed] [Google Scholar]
- 28.Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063–2072. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- 29.Ibrahim SH, Hirsova P, Malhi H, Gores GJ. Animal models of nonalcoholic steatohepatitis: eat, delete, and inflame. Dig Dis Sci. 2016;61:1325–36. doi: 10.1007/s10620-015-3977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuchiya T, Naitoh T, Nagao M, Tanaka N, Watanabe K, Imoto H, et al. Increased bile acid signals after duodenal-jejunal bypass improve non-alcoholic steatohepatitis (NASH) in a rodent model of diet-induced NASH. Obes Surg. 2018;28:1643–1652. doi: 10.1007/s11695-017-3065-z. [DOI] [PubMed] [Google Scholar]
- 31.Liu XJ, Duan NN, Liu C, Niu C, Liu XP, Wu J. Characterization of a murine nonalcoholic steatohepatitis model induced by high fat high calorie diet plus fructose and glucose in drinking water. Lab Invest. 2018;98:1184–1199. doi: 10.1038/s41374-018-0074-z. [DOI] [PubMed] [Google Scholar]
- 32.Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, et al. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol. 2011;301:825–34. doi: 10.1152/ajpgi.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asgharpour A, Cazanave SC, Pacana T, Seneshaw M, Vincent R, Banini BA, et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J Hepatol. 2016;65:579–588. doi: 10.1016/j.jhep.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clapper JR, Hendricks MD, Gu G, Wittmer C, Dolman CS, Herich J, et al. Diet-induced mouse model of fatty liver disease and nonalcoholic steatohepatitis reflecting clinical disease progression and methods of assessment. Am J Physiol Gastrointest Liver Physiol. 2013;305:483–95. doi: 10.1152/ajpgi.00079.2013. [DOI] [PubMed] [Google Scholar]
- 35.Dowman JK, Hopkins LJ, Reynolds GM, Nikolaou N, Armstrong MJ, Shaw JC, et al. Development of hepatocellular carcinoma in a murine model of nonalcoholic steatohepatitis induced by use of a high-fat/fructose diet and sedentary lifestyle. Am J Pathol. 2014;184:1550–1561. doi: 10.1016/j.ajpath.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urtasun R, Lopategi A, George J, Leung TM, Lu Y, Wang X, et al. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin α(V)β(3) engagement and PI3K/pAkt/NFκB signaling. Hepatology. 2012;55:594–608. doi: 10.1002/hep.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eng JM, Estall JL. Diet-induced models of non-alcoholic fatty liver disease: food for thought on sugar, fat, and cholesterol. Cell. 2021:10. doi: 10.3390/cells10071805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chheda TK, Shivakumar P, Sadasivan SK, Chanderasekharan H, Moolemath Y, Oommen AM, et al. Fast food diet with CCl4 micro-dose induced hepatic-fibrosis--a novel animal model. BMC Gastroenterol. 2014;14 doi: 10.1186/1471-230X-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosada M, Wasityastuti W, Pratama YY, Siwi K, Widasari DI, Wahyuni TS. The effects of high-fat diet and CCl₄ administration on liver function and lipid profile in non-alcoholic fatty liver disease rat model. 7th International Conference on Biological Science (ICBS 2021), Atlantis Press. 2022 [Google Scholar]
- 40.Marcher AB, Bendixen SM, Terkelsen MK, Hohmann SS, Hansen MH, Larsen BD, et al. Transcriptional regulation of hepatic stellate cell activation in NASH. Sci Rep. 2019;9:2324–2324. doi: 10.1038/s41598-019-39112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng C, Stewart AG, Woodman OL, Ritchie RH, Qin CX. Non-alcoholic steatohepatitis: a review of its mechanism, models and medical treatments. Front Pharmacol. 2020;11:603926. doi: 10.3389/fphar.2020.603926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito M, Suzuki J, Tsujioka S, Sasaki M, Gomori A, Shirakura T, et al. Longitudinal analysis of murine steatohepatitis model induced by chronic exposure to high-fat diet. Hepatol Res. 2007;37:50–7. doi: 10.1111/j.1872-034X.2007.00008.x. [DOI] [PubMed] [Google Scholar]
- 43.Van Rooyen DM, Gan LT, Yeh MM, Haigh WG, Larter CZ, Ioannou G, et al. Pharmacological cholesterol lowering reverses fibrotic NASH in obese, diabetic mice with metabolic syndrome. J Hepatol. 2013;59:144–52. doi: 10.1016/j.jhep.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 44.Bieghs V, Van Gorp PJ, Wouters K, Hendrikx T, Gijbels MJ, van Bilsen M, et al. LDL receptor knock-out mice are a physiological model particularly vulnerable to study the onset of inflammation in non-alcoholic fatty liver disease. PLoS One. 2012;7:30668. doi: 10.1371/journal.pone.0030668. [DOI] [PMC free article] [PubMed] [Google Scholar]