Summary
Background
Although several studies have reported attenuated influenza illness following influenza vaccination, results have been inconsistent and have focused predominantly on adults in the USA. This study aimed to evaluate the severity of influenza illness by vaccination status in a broad range of influenza vaccine target groups across multiple South American countries.
Methods
We analysed data from four South American countries (Argentina, Brazil, Chile, and Paraguay) participating in REVELAC-i, a multicentre, test-negative design, vaccine effectiveness network including 41 sentinel hospitals. Individuals hospitalised at one of these centres with severe acute respiratory infection were tested for influenza by real-time RT-PCR, and were included in the analysis if they had complete information about their vaccination status and outcomes of their hospital stay. We used multivariable logistic regression weighted by inverse probability of vaccination and adjusted for antiviral use, duration of illness before admission, and calendar week, to calculate the adjusted odds ratios (aORs) of intensive care unit (ICU) admission and in-hospital death (and combinations of these outcomes) among influenza-positive patients by vaccination status for three target groups: young children (aged 6–24 months), adults (aged 18–64 years) with pre-existing health conditions, and older adults (aged ≥65 years). Survival curves were used to compare length of hospital stay by vaccination status in each target group.
Findings
2747 patients hospitalised with PCR-confirmed influenza virus infection between Jan 1, 2013, and Dec 8, 2019, were included in the study: 649 children (70 [10·8%] fully vaccinated, 193 [29·7%] partially vaccinated) of whom 87 (13·4%) were admitted to ICU and 12 (1·8%) died in hospital; 520 adults with pre-existing medical conditions (118 [22·7%] vaccinated), of whom 139 (26·7%) were admitted to ICU and 55 (10·6%) died in hospital; and 1578 older adults (609 [38·6%] vaccinated), of whom 271 (17·2%) were admitted to ICU and 220 (13·9%) died in hospital. We observed earlier discharge among partially vaccinated children (adjusted hazard ratio 1·14 [95% CI 1·01–1·29]), fully vaccinated children (1·24 [1·04–1·47]), and vaccinated adults with pre-existing medical conditions (1·78 [1·18–2·69]) compared with their unvaccinated counterparts, but not among vaccinated older adults (0·82 [0·65–1·04]). Compared with unvaccinated individuals, lower odds of ICU admission were found for partially vaccinated children (aOR 0·64 [95% CI 0·44–0·92]) and fully vaccinated children (0·52 [0·28–0·98]), but not for adults with pre-existing conditions (1·25 [0·93–1·67]) or older adults (0·88 [0·72–1·08]). Lower odds of in-hospital death (0·62 [0·50–0·78]) were found in vaccinated versus unvaccinated older adults, with or without ICU admission, but did not differ significantly in partially vaccinated (1·35 [0·57–3·20]) or fully vaccinated young children (0·88 [0·16–4·82]) or adults with pre-existing medical conditions (1·09 [0·73–1·63]) compared with the respective unvaccinated patient groups.
Interpretation
Influenza vaccination was associated with illness attenuation among those hospitalised with influenza, although results differed by vaccine target group. These findings might suggest that attenuation of disease severity might be specific to certain target groups, seasons, or settings.
Funding
US Centers for Disease Control and Prevention.
Translations
For the Spanish and Portuguese translations of the abstract see Supplementary Materials section.
Research in context.
Evidence before this study
A comprehensive understanding of the value of influenza vaccines requires evaluation of the health effects of vaccination in people with breakthrough infections (eg, reduced illness severity). Based on a PubMed and MEDLINE search (using the search terms “severity” and “influenza vaccine*” and “effectiveness” with no date or language restrictions), several studies have documented a reduced risk of intensive care unit (ICU) admission, community-acquired pneumonia, invasive mechanical ventilation, and death among vaccinated patients with breakthrough infections. Although results of such studies suggest that influenza vaccination might reduce the severity of influenza illness, the heterogeneity of findings across these studies is large, and results predominantly draw upon data from the adult population in the USA. To better quantify the full value of influenza vaccines, especially in settings where health authorities might struggle to justify sustained investments in seasonal influenza vaccines, evidence across broad settings and population groups is needed.
Added value of this study
On the basis of 2747 patients hospitalised with laboratory-confirmed influenza virus infection at 41 sentinel sites, from a multicentre test-negative design network across four countries, we found that influenza vaccination was associated with reduced odds of admission to ICU, shorter lengths of hospital stay, and reduced odds of in-hospital death in some but not all patient groups. Lower odds of ICU admission were found in partially and fully vaccinated children but not in adults aged 18–64 years with pre-existing medical conditions or older adults (aged ≥65 years), whereas odds of in-hospital death were lower in vaccinated versus unvaccinated older adults but not in the other vaccine target groups. These findings might suggest that attenuation of disease severity is specific to certain target groups, seasons, or settings, potentially explaining the heterogeneity across previously published results. However, further research is needed to evaluate these specific effects.
Implications of all the available evidence
In combination with previous evidence, our findings support an attenuation of influenza illness severity associated with influenza vaccination. These results could be used to inform the global health benefits of vaccination in support of sustained investment in influenza vaccination programmes.
Introduction
Globally, influenza contributes to 9·5 million hospitalisations, 81·5 million hospital days, and 145 000 deaths each year.1 Vaccination offers the best method of preventing influenza illness, reducing illness in the general population by 40–60%.2, 3, 4, 5, 6 Growing evidence suggests that even in cases of breakthrough influenza virus infection, vaccination could confer health benefits. Several surveillance-based studies have reported reduced severity of influenza illness among hospitalised vaccinees, including lower odds of intensive care unit (ICU) admission, shorter length of stay in ICU, and shorter overall length of stay in hospital.7, 8, 9, 10, 11, 12 For example, a recent US study of 8354 older adults hospitalised with influenza showed a 52–79% reduction in the odds of in-hospital death, a 37% reduction in the odds of ICU admission, and a shorter length of stay in hospital associated with influenza vaccination.13 Nevertheless, such findings are sporadic and have not been systematically documented, especially in other vaccine target groups and in low-income and middle-income countries.7, 11 Despite a high burden of influenza-associated hospitalisations and deaths in South America,1, 14 no study has evaluated the potential health benefits of influenza vaccination among patients hospitalised with breakthrough influenza illnesses in this region. Furthermore, fewer global studies have evaluated the possibility of illness attenuation among children and adults with chronic medical conditions.
To better quantify the full value of influenza vaccines, especially in settings where health authorities might struggle to justify sustained investments in seasonal influenza vaccines, we analysed data for patients hospitalised with laboratory-confirmed influenza in four South American countries.
Methods
Overview
This study was conducted as part of the Network for the Evaluation of Vaccine Effectiveness in Latin America and the Caribbean—influenza (Red para la Evaluación de Vacunas En Latino América y el Caribe—influenza [REVELAC-i]), a multicentre, test-negative design network that annually evaluates influenza vaccine effectiveness against severe acute respiratory infection in sentinel surveillance hospitals participating in the Severe Acute Respiratory Infections Network, the Pan American Health Organization (PAHO) hospital-based influenza surveillance network in the Americas.15, 16, 17, 18 All sites apply a common, publicly available protocol for data collection for cases of severe acute respiratory infection.15 A case of severe acute respiratory infection in REVEAC-i is defined as the presence of an acute respiratory infection with a history of fever or a measured fever of 38°C or higher, cough, and onset within the past 10 days resulting in hospitalisation. Nasal or nasopharyngeal swabs are taken from all patients with severe acute respiratory infection for the detection of influenza virus by real-time RT-PCR. Demographic, clinical, and vaccine information recorded include influenza vaccination status, previous vaccination history, sex, age, smoking status, diagnosed pre-existing medical conditions, hospital admission date, date of symptom onset, antiviral medication use, and detection of other respiratory viruses. Influenza surveillance teams from each country are organised within their local area to develop case investigations, capture official data, and implement the study protocol.
Study population
We extracted data for all REVELAC-i sites contributing data during the study period and with mostly complete information on severity indicators (ie, <33% missing) in Argentina, Brazil, Chile, and Paraguay (appendix 3 p 3). We included only severe acute respiratory infection cases with real-time RT-PCR-confirmed infection with influenza A or B viruses, complete information about their vaccination status, and documented outcomes of their hospital stay, from one of 41 participating severe acute respiratory infection sentinel hospitals across the four countries, from Jan 1, 2013, to Dec 8, 2019. The study population comprised three special-risk target groups for influenza vaccination in participating countries: children aged 6–24 months, adults aged 18–64 years with pre-existing medical conditions (Chile only), and adults aged 65 years or older.19 These surveillance groups were selected on the basis of eligibility according to the Expanded Program on Immunization in participating countries.
Outcome variables
We examined indicators of severity of illness among influenza-associated hospitalisations, including length of hospital stay, admission to ICU, and death in hospital. Patterns in ICU admission and in-hospital death included the following: admission to ICU and discharge from hospital; no admission to ICU and death in hospital; and admission to ICU and death in hospital. The length of hospital stay was defined as the time (in days) from admission to discharge from hospital. We also examined time to discharge as a bivariate outcome, comparing participants with a length of stay of more than 5 days versus 5 days or fewer.
Vaccination status
Information on a patient's influenza vaccination history, including whether the patient had received a vaccine (yes or no) and the date of the latest dose received, is collected through surveillance records. For children, the number of doses received when first vaccinated is also collected. Vaccination status is checked at the time of admission. Sources of information include vaccination cards and electronic registries (if available). For those with missing vaccine information, surveillance staff review the patient's clinical file and consult with the Expanded Program on Immunization team to verify vaccination status.
For the purpose of our study, we considered adults aged 18 years or older as vaccinated if they had a record of vaccination with the southern hemisphere formulation of influenza vaccine (appendix 3 p 4) at least 14 days before symptom onset. For children aged 6–24 months, we categorised participants as fully vaccinated if they had received two doses of influenza vaccine at least 14 days before symptom onset; and partially vaccinated if they had received only one dose of influenza vaccine at least 14 days before symptom onset. Participants in any age group were categorised as unvaccinated if they had no record of influenza vaccination or had a record of influenza vaccination after the onset of symptoms. Patients who developed symptoms within 14 days following vaccination (ie, indeterminate vaccination status) were excluded from the analysis.
Covariate information
Covariates were selected a priori and included country, surveillance year, calendar week of hospital admission, and participant characteristics, including sex, age, smoking status, diagnosis of pre-existing medical conditions and number of conditions, previous influenza vaccination, antiviral medication use, and duration of illness at admission. We also considered the presence of other respiratory viruses (coinfections) in a post hoc analysis for children (ie, the population group with sufficient coinfections for analysis). Missing covariate data were imputed using the mice() package in R, with multiple imputation done by chained equations with 20 imputed datasets.20
Statistical analysis
We analysed each influenza vaccine target group separately. Within each priority group, we examined the characteristics of hospitalised participants by influenza vaccination status using χ2 tests for categorical variables and Wilcoxon rank-sum tests for non-normally distributed continuous variables. To control for confounding by health-seeking behaviour, we calculated the inverse probability of treatment weights using multivariable logistic regression to estimate the predicted probability of vaccination by baseline covariates for each vaccine target group. We examined covariate balance after applying the inverse probability of treatment weights by plotting the standardised mean differences between vaccinated and unvaccinated patients for the unweighted and weighted samples (appendix 3 p 5).
To compare bivariate outcomes, we used multivariable logistic regression models weighted by the inverse probability of vaccination. Adjusted models also controlled for antiviral medication use, duration of illness before admission to hospital, and calendar week of admission (fit using a cubic spline). Pooled odds ratios (ORs) were calculated across imputed datasets using the pool() function in R, which averages the estimates across the 20 imputed datasets and calculates total variance across the repeated analyses using Rubin's rule.21
To examine time to discharge, we plotted survival curves modelling the length of hospital stay by vaccination status. To account for death as a competing event, we performed survival analyses to compare the time to discharge among vaccinated versus unvaccinated patients, accounting for death as a competing event. Like the analyses of binary outcomes, models were weighted by inverse probability of vaccination and adjusted for antiviral medication use, duration of illness before admission, and calendar week of admission.
To examine how results might vary by virus subtype, we modelled the odds of ICU admission or death by virus subtype. For analysis of influenza A(H3N2), we also did analyses restricted to years in which vaccine strains closely matched circulating virus strains (ie, when the vaccine would be most effective). To examine how results might vary by number of pre-existing health conditions, we modelled the odds of ICU admission or death by the number of pre-existing medical conditions. We also conducted sensitivity analyses of time to discharge by evaluating the effect of excluding in-hospital deaths on results.
Role of the funding source
The US Centers for Disease Control and Prevention (CDC) funded this study. CDC-affiliated authors were involved in the study design, data collection, data analysis, data interpretation, report writing, and the decision to submit the paper for publication.
Results
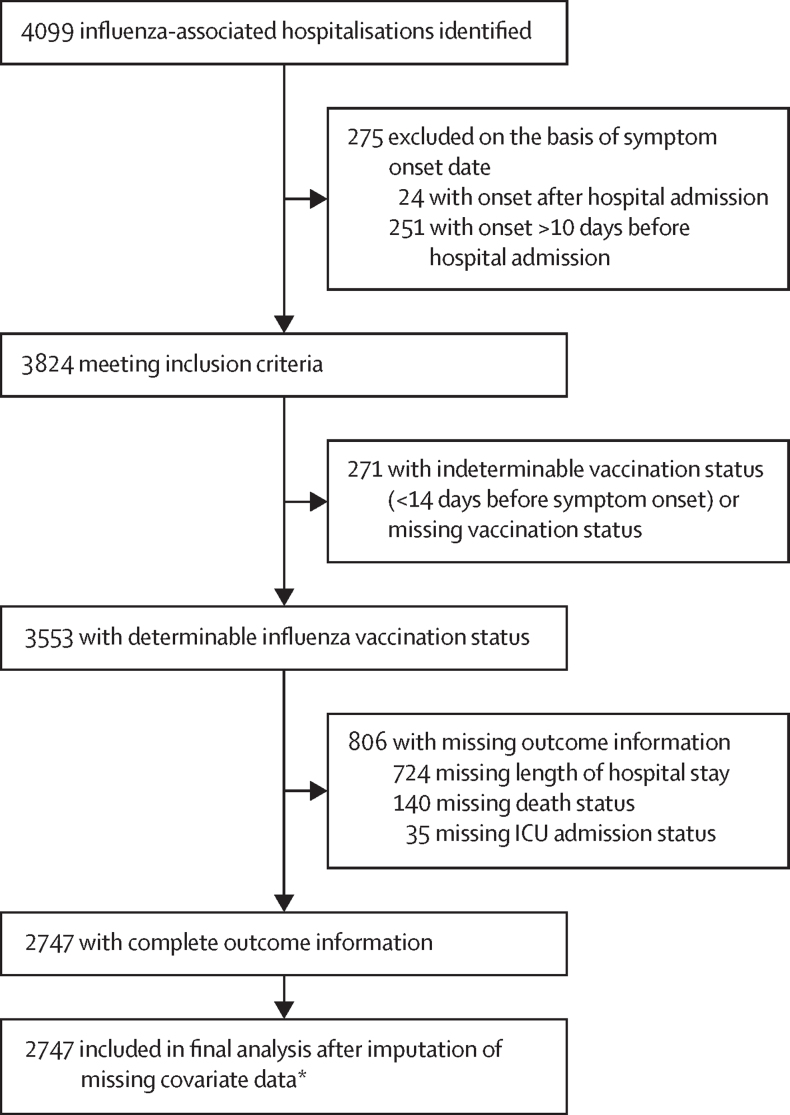
4099 hospitalised patients who were positive for influenza infection were identified from the four countries between Jan 1, 2013, and Dec 8, 2019 (figure 1). Of these cases, 2747 across all sentinel hospital sites from the four countries met the eligibility criteria and were included in the final analysis (figure 2), including 649 children aged 6–24 months, 520 adults aged 18–64 years with a pre-existing medical condition, and 1578 adults aged 65 years or older. In this final sample, we imputed information on pre-existing medical conditions (n=78), previous vaccination status (n=121), antiviral use (n=106), and smoking status (n=622).
Figure 1.
Selection of participants for inclusion in final analysis
*Missing data imputed: pre-existing condition (n=74), previous vaccination status (n=121), antiviral use (n=106), smoking status (n=388).
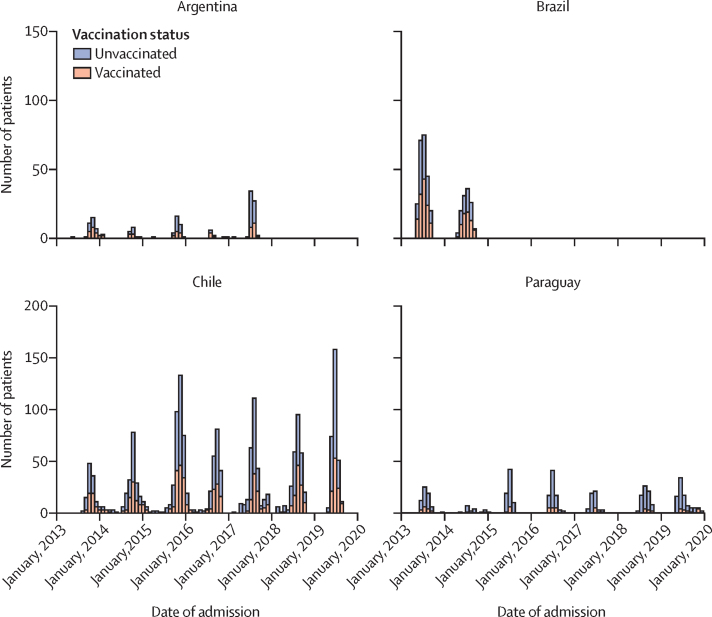
Figure 2.
Distribution of influenza-associated hospitalisations from 2013 to 2019, by participating country and influenza vaccination status
87 (13·4%) of 649 children were admitted to ICU, and 12 (1·8%) died in hospital (appendix 3 p 6); 72 viral coinfections were identified. 70 (10·8%) children were fully vaccinated and 193 (29·7%) were partially vaccinated. Vaccination status was associated with country (p<0·0001), asthma (p<0·0001), obesity (p=0·0329), cardiomyopathy (p=0·0342), and antiviral medication use (p<0·0001), but not with other covariates (table 1).
Table 1.
Characteristics of patients hospitalised with laboratory-confirmed influenza, by influenza vaccination status
|
Children aged 6–24 months |
Adults 18–64 years with pre-existing medical conditions* |
Older adults aged ≥65 years |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unvaccinated (n=386) | Partially vaccinated* (n=193) | Fully vaccinated* (n=70) | p value | Unvaccinated (n=402) | Vaccinated (n=118) | p value | Unvaccinated (n=969) | Vaccinated (n=609) | p value | |||
| Country | .. | .. | .. | <0·0001 | .. | .. | 0·0543 | .. | .. | <0·0001 | ||
| Argentina | 27 (7·0%) | 24 (12·4%) | 8 (11·4%) | .. | 0 | 0 | .. | 69 (7·1%) | 34 (5·6%) | .. | ||
| Brazil | 58 (15·0%) | 56 (29·0%) | 20 (28·6%) | .. | 21 (5·2%) | 11 (9·3%) | .. | 90 (9·3%) | 104 (17·1%) | .. | ||
| Chile | 146 (37·8%) | 82 (42·5%) | 36 (51·4%) | .. | 357 (88·8%) | 105 (89·0%) | .. | 611 (63·1%) | 440 (72·2%) | .. | ||
| Paraguay | 155 (40·2%) | 31 (16·1%) | 6 (8·6%) | .. | 24 (6·0%) | 2 (1·7%) | .. | 199 (20·5%) | 31 (5·1%) | .. | ||
| Year | .. | .. | .. | 0·10 | .. | .. | 0·55 | .. | .. | 0·0001 | ||
| 2013 | 86 (22·3%) | 58 (30·1%) | 25 (35·7%) | .. | 23 (5·7%) | 9 (7·6%) | .. | 143 (14·8%) | 125 (20·5%) | .. | ||
| 2014 | 43 (11·1%) | 29 (15·0%) | 13 (18·6%) | .. | 45 (11·2%) | 15 (12·7%) | .. | 113 (11·7%) | 102 (16·7%) | .. | ||
| 2015 | 66 (17·1%) | 25 (13·0%) | 8 (11·4%) | .. | 78 (19·4%) | 18 (15·3%) | .. | 174 (18·0%) | 110 (18·1%) | .. | ||
| 2016 | 55 (14·2%) | 25 (13·0%) | 7 (10·0%) | .. | 38 (9·5%) | 18 (15·3%) | .. | 111 (11·5%) | 46 (7·6%) | .. | ||
| 2017 | 29 (7·5%) | 11 (5·7%) | 2 (2·9%) | .. | 59 (14·7%) | 17 (14·4%) | .. | 194 (20·0%) | 88 (14·4%) | .. | ||
| 2018 | 39 (10·1%) | 17 (8·8%) | 9 (12·9%) | .. | 58 (14·4%) | 15 (12·7%) | .. | 135 (13·9%) | 76 (12·5%) | .. | ||
| 2019 | 68 (17·6%) | 28 (14·5%) | 6 (8·6%) | .. | 101 (25·1%) | 26 (22·0%) | .. | 99 (10·2%) | 62 (10·2%) | .. | ||
| Median age, years | 1 (0·7–1·1) | 1 (0·8–1·3) | 1 (0·9–1·2) | 0·21 | 57 (46–61) | 56 (48–62) | 0·21 | 77 (71–85) | 78 (71–84) | 0·96 | ||
| Sex | .. | .. | .. | 0·28 | .. | .. | 0·0061 | .. | .. | 0·39 | ||
| Male | 174 (45·1%) | 77 (39·9%) | 35 (50·0%) | .. | 182 (45·3%) | 71 (60·2%) | .. | 568 (58·6%) | 343 (56·3%) | .. | ||
| Female | 212 (54·9%) | 116 (60·1%) | 35 (50·0%) | .. | 220 (54·7%) | 47 (39·8%) | .. | 401 (41·4%) | 266 (43·7%) | .. | ||
| Smoking status | .. | .. | .. | NA | .. | 0·11 | .. | .. | <0·0001 | |||
| Current smoker | NA | NA | NA | .. | 121 (30·1%) | 26 (22·0%) | .. | 471 (48·6%) | 209 (34·3%) | .. | ||
| Non-smoker | NA | NA | NA | .. | 281 (69·9%) | 92 (78·0%) | .. | 498 (51·4%) | 400 (65·7%) | .. | ||
| Number of pre-existing medical conditions† | .. | .. | .. | 0·17 | .. | .. | 0·0425 | .. | .. | 0·0023 | ||
| None | 276 (71·5%) | 123 (63·7%) | 46 (65·7%) | .. | NA | NA | .. | 226 (23·3%) | 94 (15·4%) | .. | ||
| 1 | 107 (27·7%) | 65 (33·7%) | 23 (32·9%) | .. | 103 (25·6%) | 34 (28·8%) | .. | 325 (33·5%) | 222 (36·5%) | .. | ||
| 2 | 3 (0·8%) | 3 (1·6%) | 0 | .. | 90 (22·4%) | 14 (11·9%) | .. | 246 (25·4%) | 167 (27·4%) | .. | ||
| ≥3 | 0 | 2 (1·0%) | 1 (1·4%) | .. | 209 (52·0%) | 70 (59·3%) | .. | 172 (17·8%) | 126 (20·7%) | .. | ||
| At least one pre-existing medical condition† | 106 (27·5%) | 65 (33·7%) | 22 (31·4%) | 0·29 | 402 (100%) | 118 (100%) | NA | 749 (77·3%) | 516 (84·7%) | 0·0006 | ||
| Type of pre-existing medical condition | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | ||
| Obesity | 3 (0·8%) | 0 | 2 (2·9%) | 0·0329 | 95 (23·6%) | 25 (21·2%) | 0·67 | 100 (10·3%) | 58 (9·5%) | 0·67 | ||
| Asthma | 51 (13·2%) | 50 (25·9%) | 11 (15·7%) | <0·0001 | 67 (16·7%) | 38 (32·2%) | <0·0001 | 175 (18·1%) | 145 (23·8%) | 0·0079 | ||
| Diabetes | 0 | 0 | 0 | NA | 107 (26·6%) | 38 (32·2%) | 0·28 | 265 (27·3%) | 174 (28·6%) | 0·64 | ||
| Metabolic condition | 0 | 0 | 0 | NA | 91 (22·6%) | 36 (30·5%) | 0·0535 | 155 (16·0%) | 132 (21·7%) | 0·31 | ||
| Cardiomyopathy | 68 (17·6%) | 52 (26·9%) | 14 (20·0%) | 0·0342 | 88 (21·9%) | 21 (17·8%) | 0·41 | 261 (26·9%) | 156 (25·6%) | 0·60 | ||
| Immune system disorder | 7 (1·8%) | 4 (2·1%) | 1 (1·4%) | 0·94 | 58 (14·4%) | 14 (11·9%) | 0·58 | 119 (12·3%) | 71 (11·7%) | 0·77 | ||
| Immunosuppressing condition‡ | 0 | 0 | 1 (1·4%) | 0·0323 | 53 (13·2%) | 7 (5·9%) | 0·0512 | 114 (11·8%) | 69 (11·3%) | 0·85 | ||
| Neurological condition | 83 (21·5%) | 31 (16·1%) | 19 (27·1%) | 0·11 | 76 (18·9%) | 29 (24·6%) | 0·22 | 231 (23·8%) | 167 (27·4%) | 0·12 | ||
| Renal condition | 3 (0·8%) | 4 (2·1%) | 1 (1·4%) | 0·41 | 62 (15·4%) | 29 (24·6%) | 0·0218 | 90 (9·3%) | 65 (10·7%) | 0·42 | ||
| Liver condition | 6 (1·6%) | 0 | 0 | 0·13 | 25 (6·2%) | 3 (2·5%) | 0·19 | 12 (1·2%) | 6 (1·0%) | 0·83 | ||
| Previous influenza vaccination | .. | .. | .. | NA | .. | .. | <0·0001 | .. | .. | <0·0001 | ||
| Yes | NA | NA | NA | .. | 44 (10·9%) | 84 (71·2%) | .. | 192 (19·8%) | 482 (79·1%) | .. | ||
| No | NA | NA | NA | .. | 358 (89·1%) | 34 (28·8%) | .. | 777 (80·2%) | 127 (20·9%) | .. | ||
| Antiviral medication use | .. | .. | .. | <0·0001 | .. | .. | 0·50 | .. | .. | 0·0213 | ||
| Any antiviral medication use | 106 (27·5%) | 80 (41·5%) | 28 (40·0%) | .. | 231 (57·5%) | 63 (53·4%) | .. | 493 (50·9%) | 348 (57·1%) | .. | ||
| ≤2 days after symptom onset | 24 (6·2%) | 18 (9·3%) | 7 (10·0%) | 0·0112§ | 85 (21·1%) | 25 (21·2%) | 0·16§ | 155 (16·0%) | 102 (16·7%) | 0·0931§ | ||
| ≥3 days after symptom onset | 35 (9·1%) | 27 (14·0%) | 7 (10·0%) | .. | 130 (32·3%) | 29 (24·6%) | .. | 218 (22·5%) | 155 (25·5%) | .. | ||
| No antiviral medication use | 280 (72·5%) | 113 (58·5%) | 42 (60·0%) | .. | 171 (42·5%) | 55 (46·6%) | .. | 476 (49·1%) | 261 (42·9%) | .. | ||
| Time from symptom onset to admission, days | .. | .. | .. | 0·71 | .. | .. | 0·95 | .. | .. | 0·19 | ||
| 0 | 77 (19·9%) | 40 (20·7%) | 8 (11·4%) | .. | 108 (26·9%) | 30 (25·4%) | .. | 201 (20·7%) | 153 (25·1%) | .. | ||
| 1–2 | 127 (32·9%) | 63 (32·6%) | 27 (38·6%) | .. | 132 (32·8%) | 42 (35·6%) | .. | 340 (35·1%) | 213 (35·0%) | .. | ||
| 3–4 | 98 (25·4%) | 46 (23·8%) | 20 (28·6%) | .. | 92 (22·9%) | 27 (22·9%) | .. | 235 (24·3%) | 134 (22·0%) | .. | ||
| 5–10 | 84 (21·8%) | 44 (22·8%) | 15 (21·4%) | .. | 70 (17·4%) | 19 (16·1%) | .. | 193 (19·9%) | 109 (17·9%) | .. | ||
| Influenza virus type and subtype of infection | .. | .. | .. | 0·96 | .. | .. | 0·54 | .. | .. | 0·12 | ||
| Influenza A | 311 (80·6%) | 157 (81·3%) | 56 (80·0%) | .. | 361 (89·8%) | 103 (87·3%) | .. | 853 (88·0%) | 519 (85·2%) | .. | ||
| H3N2 | 126 (32·6%) | 76 (39·4%) | 26 (37·1%) | .. | 173 (43·0%) | 53 (44·9%) | .. | 522 (53·9%) | 348 (57·1%) | .. | ||
| H1N1 | 169 (43·8%) | 74 (38·3%) | 27 (38·6%) | .. | 188 (46·8%) | 50 (42·4%) | .. | 316 (32·6%) | 162 (26·6%) | .. | ||
| No subtype available | 16 (4·1%) | 7 (3·6%) | 3 (4·3%) | .. | 0 | 0 | .. | 15 (1·5%) | 9 (1·5%) | .. | ||
| Influenza B | 75 (19·4%) | 36 (18·7%) | 14 (20·0%) | .. | 41 (10·2%) | 15 (12·7%) | .. | 116 (12·0%) | 90 (14·8%) | .. | ||
| Yamagata | 13 (3·4%) | 10 (5·2%) | 5 (7·1%) | .. | 28 (7·0%) | 12 (10·2%) | .. | 41 (4·2%) | 42 (6·9%) | .. | ||
| Victoria | 15 (3·9%) | 8 (4·1%) | 2 (2·9%) | .. | 6 (1·5%) | 2 (1·7%) | .. | 13 (1·3%) | 16 (2·6%) | .. | ||
| No lineage information | 47 (12·2%) | 18 (9·3%) | 7 (10·0%) | .. | 7 (1·7%) | 1 (0·8%) | .. | 62 (6·4%) | 32 (5·3%) | .. | ||
| Viral coinfection detected | .. | .. | .. | 0·10 | .. | .. | 0·80 | .. | .. | 0·97 | ||
| Yes | 33 (8·5%) | 25 (13·0%) | 11 (15·7%) | .. | 3 (0·7%) | 0 | .. | 11 (1·1%) | 6 (1·0%) | .. | ||
| Respiratory syncytial virus | 30 (7·8%) | 20 (10·4%) | 11 (15·7%) | .. | 3 (0·7%) | 0 | .. | 1 (0·1%) | 1 (0·2%) | .. | ||
| Parainfluenza | 1 (0·3%) | 2 (1·0%) | 0 | .. | 0 | 0 | .. | 0 | 1 (0·2%) | .. | ||
| Human metapneumovirus | 2 (0·5%) | 1 (0·5%) | 0 | .. | 0 | 0 | .. | 1 (0·1%) | 2 (0·3%) | .. | ||
| Adenovirus | 0 | 1 (0·5%) | 0 | .. | 0 | 0 | .. | 5 (0·5%) | 0 | .. | ||
| Other viruses | 0 | 1 (0·5%) | 0 | .. | 0 | 0 | .. | 4 (0·4%) | 2 (0·3%) | .. | ||
| No | 353 (91·5%) | 168 (87·0%) | 59 (84·3%) | .. | 399 (99·3%) | 118 (100%) | .. | 958 (98·9%) | 603 (99·0%) | .. | ||
Data are n (%) or median (IQR), and summarise the characteristics from a single randomly selected imputed dataset. p values are from χ2 tests for categorical variables and Wilcoxon rank sum tests for continuous variables. NA=not applicable.
Full vaccination was defined as receipt of two doses of influenza vaccine for children aged 6–24 months; partial vaccination was defined as receipt of one dose of influenza vaccine more than 14 days before symptom onset; for 22 children with no information on dose two, we assumed partial vaccination.
Diagnosis of at least one of the following pre-existing medical conditions: asthma, diabetes, metabolic disorder, immune system disorder, nervous system disorder, renal disorder, and liver disorder.
Immunosuppressing condition included use of immunosuppressing medications or receipt of transplant.
p values compare timing of antiviral medication use.
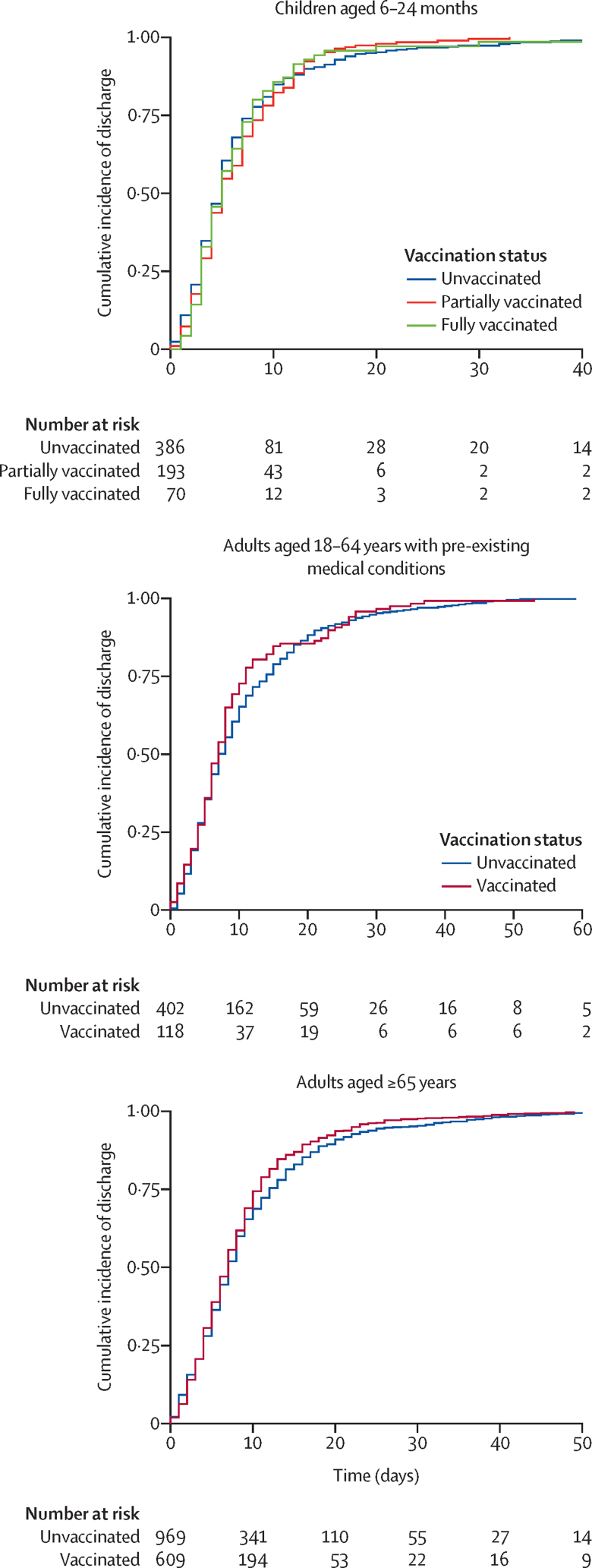
The distributions of length of hospital stay were similar for fully vaccinated, partially vaccinated, and unvaccinated children aged 6–24 months (appendix 3 p 8), with a median duration of 5 days (IQR 3–8) in the unvaccinated and fully vaccinated groups and 5 days (3–9) in the partially vaccinated group. We observed moderate evidence to support shorter hospital stay for partially vaccinated children (adjusted hazard ratio [aHR] 1·14 [95% CI 1·01–1·29]) and fully vaccinated children (1·24 [1·04–1·47]) compared with unvaccinated children (table 2; figure 3). The probability of discharge within 14 days of admission was 0·88 (95% CI 0·85–0·91) for unvaccinated children, 0·94 (0·91–0·97) for partially vaccinated children, and 0·94 (0·85–0·98) for fully vaccinated children (appendix 3 p 9).
Table 2.
Risk of severe influenza outcomes among individuals hospitalised with laboratory-confirmed influenza, by influenza vaccination status
|
Children aged 6–24 months |
Adults aged 18–64 years with pre-existing medical conditions |
Older adults aged ≥65 years |
|||||
|---|---|---|---|---|---|---|---|
| Unvaccinated (n=386) | Partially vaccinated (n=193) | Fully vaccinated (n=70) | Unvaccinated (n=402) | Vaccinated (n=118) | Unvaccinated (n=969) | Vaccinated (n=609) | |
| Length of hospital stay, days | |||||||
| Median (IQR) | 5 (3–8) | 5 (3–9) | 5 (3–8) | 8 (4–15) | 7 (4–11) | 7 (4–13) | 7 (4–11) |
| HR (95% CI) | 1 (ref) | 1·15 (1·03–1·30)* | 1·25 (1·05–1·48)* | 1 (ref) | 1·31 (0·89–1·92) | 1 (ref) | 0·84 (0·67–1·05) |
| aHR (95% CI) | 1 (ref) | 1·14 (1·01–1·29)* | 1·24 (1·04–1·47)* | 1 (ref) | 1·78 (1·18–2·69)* | 1 (ref) | 0·82 (0·65–1·04) |
| Length of stay >5 days | |||||||
| n (%) | 158 (40·9%) | 88 (45·6%) | 30 (42·9%) | 261 (64·9%) | 76 (64·4%) | 620 (64·0%) | 375 (61·6%) |
| OR (95% CI) | 1 (ref) | 1·15 (0·80–1·63) | 1·05 (0·63–1·78) | 1 (ref) | 0·98 (0·64–1·50) | 1 (ref) | 0·90 (0·73–1·11) |
| aOR (95% CI) | 1 (ref) | 0·98 (0·76–1·25) | 0·79 (0·55–1·14) | 1 (ref) | 0·69 (0·53–0·90)* | 1 (ref) | 0·67 (0·57–0·79)* |
| ICU admission | |||||||
| n (%) | 57 (14·8%) | 24 (12·4%) | 6 (8·6%) | 111 (27·6%) | 28 (23·7%) | 167 (17·2%) | 104 (17·1%) |
| OR (95% CI) | 1 (ref) | 0·82 (0·49–1·37) | 0·54 (0·22–1·31) | 1 (ref) | 0·82 (0·51–1·31) | 1 (ref) | 0·99 (0·76–1·29) |
| aOR (95% CI) | 1 (ref) | 0·64 (0·44–0·92)* | 0·52 (0·28–0·98)* | 1 (ref) | 1·25 (0·93–1·67) | 1 (ref) | 0·88 (0·72–1·08) |
| In-hospital death | |||||||
| n (%) | 8 (2·1%) | 3 (1·6%) | 1 (1·4%) | 45 (11·2%) | 10 (8·5%) | 163 (16·8%) | 57 (9·3%)† |
| OR (95% CI) | 1 (ref) | 0·75 (0·19–2·85) | 0·68 (0·08–5·58) | 1 (ref) | 0·73 (0·36–1·51) | 1 (ref) | 0·51 (0·37–0·70)* |
| aOR (95% CI) | 1 (ref) | 1·35 (0·57–3·20) | 0·88 (0·16–4·82) | 1 (ref) | 1·09 (0·73–1·63) | 1 (ref) | 0·62 (0·50–0·78)* |
| ICU admission and discharge | |||||||
| n (%) | 49 (12·7%) | 21 (10·9%) | 5 (7·1%) | 82 (20·4%) | 22 (18·6%) | 100 (10·3%) | 78 (12·8%) |
| OR (95% CI) | 1 (ref) | 0·83 (0·48–1·44) | 0·52 (0·20–1·37) | 1 (ref) | 0·87 (0·51–1·47) | 1 (ref) | 1·24 (0·90–1·70) |
| aOR (95% CI) | 1 (ref) | 0·56 (0·38–0·84)* | 0·50 (0·25–0·97)* | 1 (ref) | 1·20 (0·86–1·68) | 1 (ref) | 1·33 (0·93–1·51) |
| No ICU admission and in-hospital death | |||||||
| n (%) | 0 (0%) | 0 (0%) | 0 (0%) | 16 (4·0%) | 4 (3·4%) | 96 (9·9%) | 31 (5·1%)† |
| OR (95% CI) | NE (ref) | NE | NE | 1 (ref) | 0·83 (0·27–2·53) | 1 (ref) | 0·47 (0·31–0·72)* |
| aOR (95% CI) | NE (ref) | NE | NE | 1 (ref) | 1·41 (0·48–1·87) | 1 (ref) | 0·73 (0·55–0·96)* |
| ICU admission and in-hospital death | |||||||
| n (%) | 8 (2·1%) | 3 (1·6%) | 1 (1·4%) | 29 (7·2%) | 6 (5·1%) | 67 (6·9%) | 26 (4·3%)‡ |
| OR (95% CI) | 1 (ref) | 0·75 (0·19–2·85) | 0·68 (0·08–5·58) | 1 (ref) | 0·69 (0·28–1·71) | 1 (ref) | 0·60 (0·38–0·95)* |
| aOR (95% CI) | 1 (ref) | 1·35 (0·57–3·21) | 0·88 (0·16–4·83) | 1 (ref) | 1·21 (0·73–2·01) | 1 (ref) | 0·46 (0·33–0·64)* |
HRs were computed using Cox regression models with competing risk of death, weighted by inverse probability of treatment weights, and adjusted by antiviral use, duration of illness before admission, and calendar week (fit as a cubic spline). Values >1 indicate earlier discharge for vaccinated individuals compared to unvaccinated. To calculate aORs, logistic regression models were weighted by inverse probability treatment weights and adjusted for antiviral medication use, duration of illness at admission, and calendar week (fit as a cubic spline); pooled ORs were calculated using the pool() function in R, which averages the estimates across the 20 imputed datasets and calculates total variance across the repeated analyses using Rubin's rule. HR=hazard ratio. aHR=adjusted hazard ratio. OR=odds ratio. aOR=adjusted odds ratio. NE=not estimated (insufficient data).
95% CI does not overlap with 1 (significant difference compared with reference category).
Significant at p<0·001 based on χ2 test for comparisons.
Significant at p<0·05 based on χ2 test for comparisons.
Figure 3.
Survival plots comparing length of hospital stay for vaccinated and unvaccinated hospitalised patients
Compared with unvaccinated children, lower odds of ICU admission were observed for partially vaccinated children (adjusted OR [aOR] 0·64 [95% CI 0·44–0·92]) and fully vaccinated children (0·52 [0·28–0·98]) aged 6–24 months. 12 (1·8%) of 649 children aged 6–24 months died in hospital: eight (2·1%) of 386 unvaccinated children, three (1·5%) of 193 partially vaccinated children, and one (1·4%) of 70 fully vaccinated children died in hospital. We observed no strong evidence for a difference in the odds of death in partially vaccinated (aOR 1·35 [95% CI 0·57–3·20]) or fully vaccinated children (0·88 [0·16–4·82] relative to unvaccinated children (table 2). Adjusted ORs changed little (±0·01) when additionally adjusting for the presence of a coinfection.
We found modest evidence that odds of the composite outcome of ICU admission or in-hospital death were lower in vaccinated versus unvaccinated children aged 6–24 months infected with influenza A(H1N1), but not in those with influenza A(H3N2) infections (table 3). Among children aged 6–24 months with no pre-existing health conditions, we observed modest evidence of lower odds of influenza-associated ICU admission or death among those who were partially or fully vaccinated compared with those who were unvaccinated (appendix 3 p 10). Because pre-existing health conditions were present in only a small number of children, we were unable to assess whether multiple pre-existing conditions affected the association between vaccination status and influenza severity.
Table 3.
Risk of intensive care unit admission or in-hospital death among individuals hospitalised with influenza, by influenza vaccination status and influenza virus type and subtype
|
Children aged 6–24 months |
Adults aged 18–64 years with pre-existing medical conditions |
Older adults aged ≥65 years | |||||
|---|---|---|---|---|---|---|---|
| Unvaccinated | Partially vaccinated | Fully vaccinated | Unvaccinated | Vaccinated | Unvaccinated | Vaccinated | |
| Influenza A(H3N2), 2013–19 | |||||||
| n/N (%) | 15/126 (11·9%) | 11/76 (14·5%) | 3/26 (11·5%) | 52/173 (30·1%) | 17/53 (32·1%) | 113/522 (21·6%) | 78/348 (22·4%) |
| OR (95% CI) | 1 (ref) | 1·25 (0·54–2·90) | 0·97 (0·26–3·63) | 1 (ref) | 1·10 (0·56–2·14) | 1 (ref) | 1·04 (0·75–1·45) |
| aOR (95% CI) | 1 (ref) | 1·29 (0·71–2·33) | 1·05 (0·41–2·70) | 1 (ref) | 1·49 (0·99–2·24) | 1 (ref) | 0·98 (0·76–1·27) |
| Influenza A(H3N2), 2013–19 (excluding 2014 and 2017) | |||||||
| n/N (%) | 9/76 (11·8%) | 6/44 (13·6%) | 3/13 (23·1%) | 21/82 (25·6%) | 5/28 (17·9%) | 58/259 (22·4%) | 44/188 (23·4%) |
| OR (95% CI) | 1 (ref) | 1·17 (0·38–3·59) | NE | 1 (ref) | 0·63 (0·21–1·89) | 1 (ref) | 1·06 (0·68–1·66) |
| aOR (95% CI) | 1 (ref) | 1·28 (0·57–2·88) | NE | 1 (ref) | 0·53 (0·17–1·71) | 1 (ref) | 1·13 (0·80–1·61) |
| Influenza A(H1N1), 2013–19 | |||||||
| n/N (%) | 29/169 (17·1%) | 9/74 (12·2%) | 2/27 (7·4%) | 66/188 (35·1%) | 12/50 (24·0%) | 107/316 (33·9%) | 38/162 (23·5%) |
| OR (95% CI) | 1 (ref) | 0·67 (0·30–1·50) | 0·39 (0·09–1·73) | 1 (ref) | 0·58 (0·28–1·20) | 1 (ref) | 0·60 (0·39–0·92)* |
| aOR (95% CI) | 1 (ref) | 0·40 (0·21–0·74)* | 0·38 (0·12–1·19) | 1 (ref) | 0·53 (0·32–0·88)* | 1 (ref) | 0·75 (0·56–1·01) |
| Influenza B, 2013–19 | |||||||
| n/N (%) | 10/75 (13·3%) | 4/36 (11·1%) | 1/14 (7·1%) | 9/41 (21·9%) | 3/15 (20·0%) | 33/116 (28·4%) | 16/90 (17·8%) |
| OR (95% CI) | 1 (ref) | 0·81 (0·23–2·83) | 0·50 (0·06–4·34) | 1 (ref) | 0·89 (0·20–3·98) | 1 (ref) | 0·54 (0·28–1·07) |
| aOR (95% CI) | 1 (ref) | 0·40 (0·14–1·11) | 0·32 (0·06–1·61) | 1 (ref) | 1·01 (0·21–4·89) | 1 (ref) | 0·58 (0·34–0·98)* |
To calculate aORs, logistic regression models were weighted by inverse probability treatment weights and adjusted for antiviral medication use, duration of illness at admission, and calendar week (fit as a cubic spline); pooled ORs were calculated using the pool() function in R, which averages the estimates across the 20 imputed datasets and calculates total variance across the repeated analyses using Rubin's rule. OR=odds ratio. aOR=adjusted odds ratio. NE=not estimated (insufficient data).
95% CI does not overlap with 1 (significant difference compared with reference category).
Of the 520 adults aged 18–64 years with pre-existing medical conditions and hospitalised with influenza, 139 (26·7%) were admitted to ICU and 55 (10·6%) died in hospital (appendix 3 p 6). 118 (22·7%) were vaccinated against influenza. Vaccination status was associated with sex (p=0·0061), pre-existing asthma (p<0·0001) or renal conditions (p=0·0218), previous vaccination against influenza (p<0·0001), and number of pre-existing medical conditions (p=0·0425), with the vaccinated group having a higher proportion of patients with 3 or more pre-existing medical conditions than the unvaccinated group (table 1).
The distributions of and median lengths of hospital stay were similar for vaccinated (median 7 days [IQR 4–11]) and unvaccinated adults with pre-existing medical conditions (8 days [4–15]; appendix 3 p 8). We observed moderate evidence of a shorter length of stay for vaccinated compared with unvaccinated adults with pre-existing conditions (aHR 1·78 [95% CI 1·18–2·69]; table 2). The probability of discharge within 14 days of admission was 0·75 (95% CI 0·70–0·79) for unvaccinated and 0·84 (0·76–0·89) for vaccinated adults with pre-existing conditions (appendix 3 p 11). After excluding patients who died before hospital discharge from the analysis of length of hospital stay, the effect estimate was attenuated (aHR 1·36 [95% CI 1·19–1·56]). We observed no strong indication that the odds of ICU admission (aOR 1·25 [95% CI 0·93–1·67]) and in-hospital death (1·09 [0·73–1·63]) differed between groups.
Among adults with pre-existing medical conditions, we observed moderate to strong evidence for a reduction in the odds of ICU admission or death in vaccinated versus unvaccinated patients with influenza A(H1N1) infection (aOR 0·53 [95% CI 0·32–0·88]), but weak evidence for such a reduction among those with influenza A(H3N2) infection (1·49 [0·99–2·24]) or influenza B infection (1·01 [0·21–4·89]; table 3). When we excluded seasons with known mismatch for vaccine strains (ie, 2014 and 2017), the aOR for ICU admission or death in vaccinated versus unvaccinated patients with influenza A(H3N2) infection was reduced to 0·53 (0·17–1·71) but no statistically significant difference was found between groups. Odds of ICU admission or death in adults hospitalised with influenza infection did not differ significantly between vaccinated and unvaccinated patients, regardless of number of pre-existing medical conditions (appendix 3 p 10).
Of the 1578 older adults (aged ≥65 years) hospitalised with influenza, 271 (17·2%) were admitted to ICU, and 220 (13·9%) died in hospital (appendix 3 p 6). 609 (38·6%) were vaccinated. Vaccination status was associated with country (p<0·0001), surveillance year (p<0·0001), smoking status (p<0·0001), number of pre-existing medical conditions (p=0·0023), receipt of previous influenza vaccine (p<0·0001), and antiviral medication use (p=0·0213; table 1).
Median length of hospital stay was the same for vaccinated (7 days [IQR 4–11]) and unvaccinated (7 days [4–13]) adults aged 65 years and older, with similar distributions between groups (appendix 3 p 8). Survival analyses showed no significant difference in length of hospital stay in vaccinated versus unvaccinated patients (aHR 0·82 [95% CI 0·65–1·04]; table 2). However, when we excluded patients who died in hospital from the analysis, we observed moderate evidence to support a shorter length of stay among vaccinated older adults (1·25 [1·15–1·35]) compared with unvaccinated older adults. The probability of discharge within 14 days of admission was 0·81 (95% CI 0·78–0·83) for unvaccinated and 0·85 (0·82–0·88) for vaccinated older adults (appendix 3 p 11).
Among older adults hospitalised with influenza, we observed weak evidence in support of lower odds of ICU admission in vaccinated versus unvaccinated patients (aOR 0·88 [95% CI 0·72–1·08]), whereas odds of in-hospital death were lower in vaccinated patients versus unvaccinated patients (0·62 [0·50–0·78]). The odds of in-hospital death with no ICU admission (0·73 [0·55–0·96]) and in-hospital death with ICU admission (0·46 [0·33–0·64]) were lower for vaccinated older adults compared with unvaccinated older adults.
When we examined the odds of severe influenza by virus subtype, we observe weak evidence for a reduced odds of ICU admission or death in vaccinated versus unvaccinated older adults with influenza A(H1N1) infection (aOR 0·75 [95% CI 0·56–1·01]) and influenza A(H3N2) infection (0·98 [0·76–1·27]; table 3). After removing seasons with known mismatch between influenza vaccines and circulating wildtype virus, we did not observe evidence for a difference in the odds of ICU admission or death between vaccinated and unvaccinated older adults with influenza A(H3N2) infection (1·13 [0·80–1·61]). Odds of ICU admission or in-hospital death were not significantly associated with influenza vaccination among older adults when analysed by number of pre-existing medical conditions (appendix 3 p 10).
Discussion
Based on sentinel surveillance data from four countries, our results indicate that influenza vaccination could offer health benefits to some patients hospitalised with breakthrough influenza virus infections. We observed shorter lengths of hospital stay among partially and fully vaccinated children aged 6–24 months and vaccinated adults aged 18–64 years with pre-existing medical conditions compared with unvaccinated individuals in the respective age groups. Length of hospital stay was also shorter among vaccinated older adults (aged ≥65 years) who did not die in hospital. Additionally, the odds of ICU admission were lower among vaccinated versus unvaccinated young children, and the odds of in-hospital death were lower among vaccinated versus unvaccinated older adults. Although the primary aim of annual influenza vaccination is to prevent influenza illness and subsequent complications, our results suggest that influenza vaccination could also attenuate the severity of influenza illness, even when breakthrough infection occurs. In combination with the existing literature,12, 17, 18, 22, 23 these results have important implications for understanding the health benefits of influenza vaccination programmes globally.
Our findings align with those from previously published investigations, indicating that even when breakthrough infection occurs, illness might be less severe for vaccinated patients.7, 13 A 2021 narrative review and pooled meta-analysis of eight studies of influenza-hospitalised adults reported a 26% reduction in the odds of ICU admission associated with influenza vaccination, and five studies showed a 31% reduction in the odds of death among vaccinated compared with unvaccinated hospitalised adults.11 Although the existing literature supports the hypothesis that vaccination can attenuate the course of illness among those with breakthrough infections, heterogeneity has been shown in the results of previous studies11 as well as our own. Although several hypotheses have been put forward, including heterogeneity in outcome definitions and measurement, our findings might suggest that population and infection-related factors influence the ability of influenza vaccination to modify influenza severity. Our study indicated that on average, between 2013 and 2019, influenza vaccination was associated with lower odds of severe influenza A(H1N1) virus illness among all target groups, but not lower odds of severe illness with influenza A(H3N2) virus. Previous studies have documented lower severity for influenza A(H1N1) virus but not influenza A(H3N2) virus associated with influenza vaccination.13 A number of studies conducted during the 2009 influenza A(H1N1) pandemic showed that vaccination was associated with a lower odds of severe influenza A(H1N1) virus illness.24, 25, 26 Furthermore, with the exception of a shorter length of stay, we observed indications of less severe disease in vaccinated young children and older adults, but not among vaccinated adults aged 18–64 years with pre-existing medical conditions. This could suggest that attenuation of disease severity could be specific to certain target groups, potentially explaining the heterogeneity in previously published results. However, further research is needed to investigate these associations.
This study provides additional evidence in support of influenza illness attenuation among vaccinated, previously healthy, young children aged 6–24 months. Although previous studies have documented disease attenuation following influenza vaccination in adults, fewer studies have evaluated this in young children with breakthrough infection. Most previous paediatric studies have focused on estimating the effectiveness of vaccines for preventing admission to hospital, admission to ICU, or death.17, 18, 22, 23 A US case-cohort analysis showed that influenza vaccination was associated with a 65% reduction in the odds of paediatric death.27 One randomised controlled trial of a quadrivalent inactivated influenza vaccine among children aged 6–35 months across 13 countries showed that paediatric influenza vaccination resulted in lower odds of fever and moderate-to-severe illness.28 A 2013 US study indicated that influenza vaccination was associated with a 75% reduction the odds of in-hospital death or invasive ventilation among children younger than 18 years admitted to ICU.23 However, these studies did not explicitly evaluate breakthrough infections in children. We observed a 36–48% reduction in the odds of ICU admission among partially or fully vaccinated, influenza-infected children aged 6–24 months, most of whom did not have diagnosed pre-existing medical conditions, indicating that influenza vaccination might attenuate the severity of influenza among previously healthy young children.
We drew from health and medical information collected through a large, pre-existing sentinel surveillance network, which allowed us to gather information from a large, 7 year cohort of hospitalised patients with PCR-confirmed influenza virus infection across 41 sites from four South American countries. Data collection conformed to a common protocol, reducing heterogeneity in measurements. As a result, we were able to evaluate the health effects associated with influenza vaccination in a large sample of three priority groups, including children aged 6–24 months. Our large sample size also enabled analyses by influenza virus subtype and by number of pre-existing health conditions. Second, we applied propensity score analyses to reduce the potential confounding influence of vaccine-seeking behaviour (a common concern in observational studies of influenza vaccination) and achieve covariate balance between vaccinated and unvaccinated groups (similar to a randomised controlled trial). Despite these strengths, however, this study relied on observational data that were collected for surveillance purposes, and although we applied robust techniques to account for confounding, we cannot exclude the possibility of residual confounding, particularly due to differential health-seeking behaviour between vaccination groups (the so-called healthy vaccinee effect). Furthermore, we did not have consistently collected information about influenza symptoms or discharge diagnoses, which made it impractical to evaluate these outcomes. These data limitations highlight the importance of complete, high-quality surveillance data for research and surveillance. Future research should also consider a wider spectrum of outcomes and the potential influence of residual confounding on findings. Furthermore, although these results help to extend the evidence documenting benefits of influenza vaccine programmes to South America, the findings might not be generalisable to other continents with different circulating types or subtypes of influenza viruses and different health systems. Finally, we did not have adequate information to identify individual hospitals for study records, which precluded us from adjusting for possible variations in standard of care or hospital practices across sites. We do not believe this would strongly influence our results, because there is no reason to believe an inverse association exists between a clinician's likelihood of admitting patients to ICU and vaccination status. However, we cannot discount potential confounding by individual hospital clinical practices.
In summary, these results from the surveillance of young children and adults hospitalised with laboratory-confirmed influenza virus infection at 41 sentinel sites across Argentina, Brazil, Chile, and Paraguay suggest that influenza vaccination is associated with illness attenuation among this population. These results could be used to inform the global health benefits of annual influenza vaccination among individuals with risk factors for severe influenza.
Data sharing
Surveillance data collected for the study are not publicly available and the research team does not have permission to make these data available to others. The protocol used for this project is publicly available and can be downloaded from the PAHO website.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This work was supported by a grant from the US CDC through cooperative agreements with PAHO and WHO. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US CDC. We thank the national epidemiological and virological influenza surveillance and immunisation teams in participating countries including, among others, Teresa Varela (Ministry of Health, Argentina); Ernesto Issac Montenegro Renoiner, Ana Carolina de Lacerda Sousa, Carla Domingues, Felipe Cotrim de Carvalho, and Swamy Lima Palmeira (Ministry of Health, Brazil); Leticia Garay Martins (Health Department of Rio Grande do Sul, Brazil); Janaina Almeida (Health Department of Minas Gerais, Brazil); Patricia Marques Ferreira (Health Department of São Paulo, Brazil); Fernanda Crosewski and Laurina Tanabe (Health Department of Paraná, Brazil); Eduardo Marques Macario (Health Department of Santa Catarina, Brazil); Alice Rodovalho (Health Department of Pernambuco, Brazil); Mirleide Santos (Evandro Chagas Institute, National Influenza Center (NIC), Brazil; Terezinha Paiva (Adolfo Lutz Institute, National Influenza Center (NIC), Brazil; Marilda Agudo Mendonça Teixeira de Siqueira (Oswaldo Cruz Foundation, National Influenza Center (NIC), Brazil); Sonia Arza Fernández, and José Sánchez (Ministry of Health, Paraguay); María Fernanda Olivares Barraza, and Reinaldo Rosas (Ministry of Health, Chile); Olga López Muñoz (Hospital Iquique, Chile); Alberto Fica (Hospital Militar, Chile); Claudia Aguayo (Hospital G G Benavente, Chile); Tania Campos (Hospital Temuco, Chile); Carolina Nuñez (Hospital de Puerto Montt, Chile); Camila Bolados Zumelzu (Hospital Dr. Eduardo Shutz Schroeder, Chile); Marta Werne Canales (Hospital Guillermo Grant Benavnete Concepcion, Chile); Jeannette Dabanch Peña (Hospital Clinico Universidad de Chile, Chile); Juliana Leite and Angel Rodriguez (PAHO influenza team, Washington, DC, USA); and the PAHO immunisation focal points for their support in the implementation of the REVELAC-i network (Mirta Magariños and Tamara Mancero [Argentina], Claudio Marcelo Canales and Mario Cruz-Penate [Chile], Maria Almiron, Priscila Leal e Leite, and Lely Guzman [Brazil], and Fabiana Michel Romeo Montoya [Paraguay]). We also thank Nathalie El Omeiri (PAHO) for assistance in implementing the REVELAC-i network; the US CDC for their technical and financial support; and Sara Mirza and Victor Veguilla for their management of the cooperative agreement.
Contributors
AKR and CSA conceived the study. AKR did the formal analysis, wrote the original draft of the manuscript, and was responsible for data visualisation. CSA supervised the study. CSA, EA, and AMR acquired funding for the study. CSA, LD, and EA contributed to reviewing and editing the manuscript. AKR, CSA, LD, EA, PC, SL, FN, WAFdA, JA, SA, MAAV, SB, IB, PB, MEF, RF, CMG, CIGC, MvH, MVJ, NK, MFO, DAdS, ETdS, VS, and NV contributed to methodology. PC, SL, FN, WAFdA, JA, SA, MAAV, SB, IB, PB, MEF, RF, CMG, CIGC, MvH, MVJ, NK, MFO, DAdS, ETdS, VS, AMR, and NV contributed to data curation and reviewing editing the manuscript. AKR and CSA had access to the data. AKR was responsible for the decision to submit the manuscript.
Contributor Information
Annette K Regan, Email: akregan@usfca.edu.
Eduardo Azziz-Baumgartner, Email: eha9@cdc.gov.
Supplementary Material
References
- 1.GBD 2017 Influenza Collaborators Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2019;7:69–89. doi: 10.1016/S2213-2600(18)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flannery B, Chung JR, Monto AS, et al. Influenza vaccine effectiveness in the United States during the 2016–2017 season. Clin Infect Dis. 2019;68:1798–1806. doi: 10.1093/cid/ciy775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flannery B, Kondor RJG, Chung JR, et al. Spread of antigenically drifted influenza A(H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 season. J Infect Dis. 2020;221:8–15. doi: 10.1093/infdis/jiz543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson ML, Chung JR, Jackson LA, et al. Influenza vaccine effectiveness in the United States during the 2015–2016 season. N Engl J Med. 2017;377:534–543. doi: 10.1056/NEJMoa1700153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rolfes MA, Flannery B, Chung JR, et al. Effects of influenza vaccination in the United States during the 2017–2018 influenza season. Clin Infect Dis. 2019;69:1845–1853. doi: 10.1093/cid/ciz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tenforde MW, Kondor RJG, Chung JR, et al. Effect of antigenic drift on influenza vaccine effectiveness in the United States—2019–2020. Clin Infect Dis. 2021;73:e4244–e4250. doi: 10.1093/cid/ciaa1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arriola CS, Anderson EJ, Baumbach J, et al. Does influenza vaccination modify influenza severity? Data on older adults hospitalized with influenza during the 2012–2013 season in the United States. J Infect Dis. 2015;212:1200–1208. doi: 10.1093/infdis/jiv200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patriarca PA, Weber JA, Parker RA, et al. Efficacy of influenza vaccine in nursing homes. Reduction in illness and complications during an influenza A (H3N2) epidemic. JAMA. 1985;253:1136–1139. [PubMed] [Google Scholar]
- 9.Casado I, Domínguez A, Toledo D, et al. Effect of influenza vaccination on the prognosis of hospitalized influenza patients. Expert Rev Vaccines. 2016;15:425–432. doi: 10.1586/14760584.2016.1134328. [DOI] [PubMed] [Google Scholar]
- 10.Deiss RG, Arnold JC, Chen WJ, et al. Vaccine-associated reduction in symptom severity among patients with influenza A/H3N2 disease. Vaccine. 2015;33:7160–7167. doi: 10.1016/j.vaccine.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferdinands JM, Thompson MG, Blanton L, Spencer S, Grant L, Fry AM. Does influenza vaccination attenuate the severity of breakthrough infections? A narrative review and recommendations for further research. Vaccine. 2021;39:3678–3695. doi: 10.1016/j.vaccine.2021.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Thompson MG, Pierse N, Sue Huang Q, et al. Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe disease among adults in New Zealand 2012-2015. Vaccine. 2018;36:5916–5925. doi: 10.1016/j.vaccine.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 13.Arriola C, Garg S, Anderson EJ, et al. Influenza vaccination modifies disease severity among community-dwelling adults hospitalized with influenza. Clin Infect Dis. 2017;65:1289–1297. doi: 10.1093/cid/cix468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palekar RS, Rolfes MA, Arriola CS, et al. Burden of influenza-associated respiratory hospitalizations in the Americas, 2010–2015. PLoS One. 2019;14:e0221479. doi: 10.1371/journal.pone.0221479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan American Health Organization Network for the evaluation of vaccine effectiveness in Latin America and the Caribbean—influenza, (REVELAC-i) 2022. https://www.paho.org/en/network-evaluation-vaccine-effectiveness-latin-america-and-caribbean-influenza-revelac-i
- 16.El Omeiri N, Azziz-Baumgartner E, Clará W, et al. Pilot to evaluate the feasibility of measuring seasonal influenza vaccine effectiveness using surveillance platforms in Central-America, 2012. BMC Public Health. 2015;15:673. doi: 10.1186/s12889-015-2001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Omeiri N, Azziz-Baumgartner E, Thompson MG, et al. Seasonal influenza vaccine effectiveness against laboratory-confirmed influenza hospitalizations—Latin America, 2013. Vaccine. 2018;36:3555–3566. doi: 10.1016/j.vaccine.2017.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sofia Arriola C, El Omeiri N, Azziz-Baumgartner E, et al. Influenza vaccine effectiveness against hospitalizations in children and older adults—data from South America, 2013–2017. A test negative design. Vaccine X. 2019;3:100047. doi: 10.1016/j.jvacx.2019.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ropero-Álvarez AM, El Omeiri N, Kurtis HJ, Danovaro-Holliday MC, Ruiz-Matus C. Influenza vaccination in the Americas: progress and challenges after the 2009 A(H1N1) influenza pandemic. Hum Vaccin Immunother. 2016;12:2206–2214. doi: 10.1080/21645515.2016.1157240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.RDocumentation mice: mice: multivariate imputation by chained equations. 2022. https://www.rdocumentation.org/packages/mice/versions/3.14.0/topics/mice
- 21.CRAN pool: combine estimates by pooling rules. 2022. https://rdrr.io/cran/mice/man/pool.html
- 22.Boddington NL, Pearson I, Whitaker H, Mangtani P, Pebody RG. Effectiveness of influenza vaccination in preventing hospitalization due to influenza in children: a systematic review and meta-analysis. Clin Infect Dis. 2021;73:1722–1732. doi: 10.1093/cid/ciab270. [DOI] [PubMed] [Google Scholar]
- 23.Olson SM, Newhams MM, Halasa NB, et al. Vaccine effectiveness against life-threatening influenza illness in US children. Clin Infect Dis. 2022 doi: 10.1093/cid/ciab931. published online Jan 13. [DOI] [PubMed] [Google Scholar]
- 24.Mahmud SM, Bozat-Emre S, Hammond G, Elliot L, Caeseel PV. Did the H1N1 vaccine reduce the risk of admission with influenza and pneumonia during the pandemic? PLoS One. 2015;10:e0142754. doi: 10.1371/journal.pone.0142754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castilla J, Godoy P, Domínguez A, et al. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis. 2013;57:167–175. doi: 10.1093/cid/cit194. [DOI] [PubMed] [Google Scholar]
- 26.Orellano PW, Reynoso JI, Carlino O, Uez O. Protection of trivalent inactivated influenza vaccine against hospitalizations among pandemic influenza A (H1N1) cases in Argentina. Vaccine. 2010;28:5288–5291. doi: 10.1016/j.vaccine.2010.05.051. [DOI] [PubMed] [Google Scholar]
- 27.Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010–2014. Pediatrics. 2017;139:e20164244. doi: 10.1542/peds.2016-4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danier J, Rivera L, Claeys C, et al. Clinical presentation of influenza in children 6 to 35 months of age: findings from a randomized clinical trial of inactivated quadrivalent influenza vaccine. Pediatr Infect Dis J. 2019;38:866–872. doi: 10.1097/INF.0000000000002387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Surveillance data collected for the study are not publicly available and the research team does not have permission to make these data available to others. The protocol used for this project is publicly available and can be downloaded from the PAHO website.