Abstract
Background
Many countries and regions have established multicenter registration studies to improve the outcomes of acute type A aortic dissection (ATAAD).
Objectives
The aims of this study were to report actual preoperative management, surgery type, and early outcomes of surgical treatment for ATAAD in China.
Methods
This cohort study uses data from the China Registry of Type A Aortic Dissection, a national clinical registry to investigate management of patients with Stanford type A aortic dissection. The data, including surgical management and outcomes of patients with ATAAD, were analyzed from January 2018 to December 2021.
Results
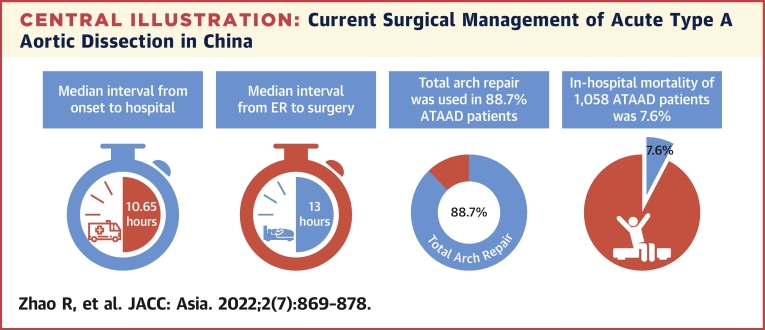
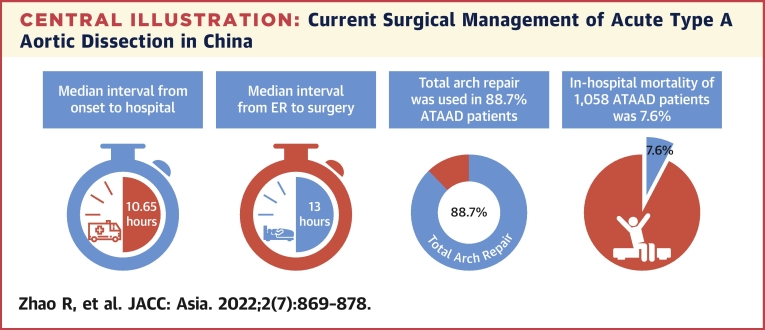
A total of 1,058 patients with ATAAD were enrolled in this study between January 2018 and December 2021. The mean age of all patients was 51.6 ±11.7 years. The median interval from onset to hospital was 10.65 hours (IQR: 6–24 hours), and the median interval from entering the emergency room to starting operation was 13 hours (IQR: 4.08–28.7 hours). Total arch repair was performed in 938 patients (88.7%), and frozen elephant trunk repair was performed in 800 patients (75.6%). The incidence of early mortality was 7.6%.
Conclusions
The population of patients with ATAAD in China experienced a longer interval from onset to arrival at the hospital, received more extensive aortic arch repair, and showed a relatively lower early mortality. These findings suggest that there may be a huge survivor bias in patients with ATAAD in China, more efforts should be made to promote prehospital emergency care and preoperative management of Chinese ATAAD patients. (A multicenter registration study of aortic dissection in China; ChiCTR1800015338).
Key Words: aortic dissection, aortic surgery, multicenter study
Abbreviations and Acronyms: ATAAD, acute type A aortic dissection; FET, frozen elephant trunk; TAAD, type A aortic dissection; TAR, total arch replacement
Central Illustration
Acute type A aortic dissection (ATAAD) is the most common aortic catastrophe and life-threatening disease associated with high morbidity and mortality rates.1, 2, 3 Many countries and regions have established multicenter registration studies.4, 5, 6, 7 The International Registry of Aortic Dissection (IRAD) established in 1996 has published a number of studies that reported a significant impact on the diagnosis and treatment of aortic dissection worldwide.8,9 In 2018, the Registry of Type A Aortic Dissection in China was established in accordance with the model of the International Registration of Aortic Dissection. The aims of this study were to report actual preoperative management, surgery type, and early outcomes of surgical treatment for ATAAD in China.
Methods
Study design and data source
We performed a retrospective cohort study of prospectively collected data from patients included in the China Registry of Type A Aortic Dissection, who underwent surgery for type A aortic dissection (TAAD) between January 2018 and December 2021. The Registry of Type A Aortic Dissection in China was launched in 2018 by Fuwai Hospital, National Center for Cardiovascular Disease, and another 9 centers in China are currently participating in the registry study, including West China Hospital, Changhai Hospital, First Hospital of China Medical University, Guangdong Provincial People’s Hospital, First Hospital of Lanzhou University, Wuhan Union Hospital, Second Hospital of Hebei Medical University, Shandong Provincial Hospital, and First Affiliated Hospital of Zhengzhou University. The study’s registry number in the Chinese Clinical Trial Registry is ChiCTR1800015338. An online database was established at the same time. Patients were identified based on imaging, surgical databases, and/or diagnostic records. The diagnosis of TAAD was based on patient history, diagnostic testing, and operative findings. This study was conducted and findings were reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cohort studies. The data and methods used for this study are available to other researchers on request. This retrospective observational study was approved by the Institutional Review Board of Fuwai Hospital, National Center for Cardiovascular Disease (2017-877).
Patient cohort and outcome measures
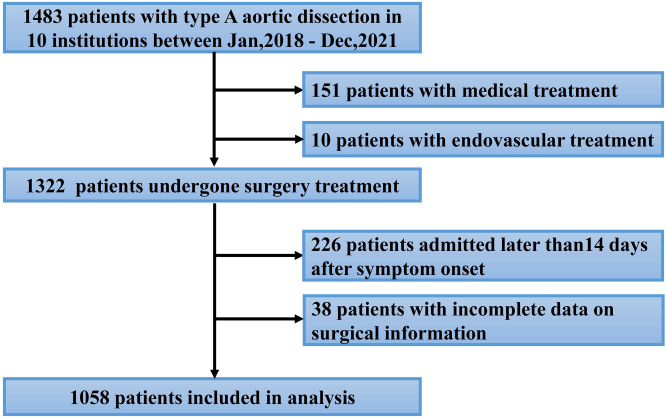
Between January 2018 and December 2021, 1,483 patients who had TAAD, including surgical treatment (n = 1,322), endovascular treatment (n = 10), and medical treatment (n = 151), were registered in the Registry of Type A Aortic Dissection in the China database. Of these patients, 38 patients were excluded because of the lack of surgical-specific information, 226 patients received surgery treatment but admitted later than14 days after symptom onset, and finally 1,058 with ATAAD were enrolled in this study (Figure 1). Data on patient demographic characteristics, medical history, symptoms and signs, management, and outcomes were collected by all 10 centers and were entered into the website case report forms. The imaging data were sent to Fuwai Hospital via CD-ROM or the Internet.
Figure 1.
Patient Selection Diagram
Between January 2018 and December 2021, 1,058 patients with acute type A aortic dissection were enrolled in this study.
ATAAD is traditionally defined as <14 days from symptom onset, and chronic aortic dissection is defined as >14 days from symptom onset according to the IRAD classification.10 The transport distance of the patient is defined as the driving distance from the patient's home address to the medical center, which is calculated using Alibaba cloud and Auto Navi Map. The interval from onset to hospital was defined as the time between onset of the first symptoms to arrival in the emergency room. The interval from entering the emergency room to starting operation was defined as the time from emergency department admission to surgery. All patients received blood pressure control treatment after entering the emergency department. We recorded the treatment drugs and blood pressure levels. Malperfusion refers to acute organ ischemia secondary to aortic branch vessel hypoperfusion.11 Drinking was defined as the consumption of an alcoholic beverage at least 3 times per week. In-hospital mortality was defined as all-cause death during hospitalization. Stroke was defined as a persistent central neurologic deficit (focal or generalized), as assessed by 1 neurologist. Re-exploration was defined as re-exploration for bleeding. Acute kidney insufficiency was defined as serum creatinine increased by >1.5 times the baseline values, a glomerular filtration rate decrease by >25%, or urine output <0.5 mL/kg/h for 6 hours, and hepatic dysfunction manifested as transient elevated hepatic enzymes by 1.5 times the upper range of normal <48 h and was self-limiting according to the International Aortic Arch Surgery Study Group.12
Statistical analysis
Normality was assessed with the Shapiro-Wilk statistic. Continuous variables with a normal distribution are expressed as the mean ± SD and were compared using the t test. Non-normally distributed continuous data are summarized as the median (IQR) and were compared using the Mann-Whitney U test. Categorical variables were expressed as counts and composition ratios and were compared using the chi-square test or Fisher exact test as appropriate. A 2-tailed P value <0.05 was regarded as statistically significant in this study. Analyses were performed using R, version 4.1.0.
Results
Demographics and history
Between January 2018 and December 2021, 1,058 patients with ATAAD were enrolled in this study (Figure 1). The mean age of all patients was 51.6 ± 11.7 years, and 806 (76.2%) of the patients were male (Table 1). A history of hypertension was elicited in 76.1% of patients, hyperlipidemia in 19.9%, diabetes in 4.1%, coronary heart disease in 6.7%, and Marfan syndrome was present in 2.6%. Approximately 8.5% of patients had previous aortic surgery, and the prevalence of cardiac surgery history was 4.2%. Nearly 40% of the patients had a history of smoking. Of all patients, 373 (39.1%) had mild aortic insufficiency, 210 (22%) had moderate aortic insufficiency, and 64 (6.7%) had severe aortic insufficiency.
Table 1.
Characteristics of 1,058 Patients With ATAAD Who Underwent Repair From January 2018 to December 2021
| Age, y | 51.6 ± 11.7 |
| Age ≥80 y | 5 (0.5) |
| Male | 806 (76.2) |
| Body mass index, kg/m2 | 26 ± 4 |
| Patient transport distance, km | 176.3 (37.7-382. 8) |
| Hypertension | 803 (76.1) |
| Hyperlipidemia | 137 (19.9) |
| Diabetes mellitus | 43 (4.1) |
| Coronary artery disease | 46 (6.7) |
| COPD | 3 (0.4) |
| Chronic renal failure | 2 (0.3) |
| Marfan syndrome | 27 (2.6) |
| Family history | 3(0.3) |
| Pervious aortic dissection | 13 (1.9) |
| Aortic surgery history | 90 (8.5) |
| Cardiac surgery history | 44 (4.2) |
| Smoker | 415 (39.4) |
| Drinking | 186 (18.0) |
| Hemoglobin, g/dL | 13.5 (12.2-14.6) |
| White blood cell, ∗109/L | 11.4 (9.2-14) |
| Platelet, ∗109/L | 173 (139-223) |
| Creatinine, μmol/L | 87.1 (70.0-109.2) |
| ALT, μ/L | 21 (14.8-36) |
| AST, μ/L | 25 (19-37) |
| D-Dimer, mg/L | 7.7 (2.5-20) |
| Aortic insufficiency | |
| None | 307 (32.2) |
| Mild | 373 (39.1) |
| Moderate | 210 (22) |
| Severe | 64 (6.7) |
| Aortic annulus diameter, mm | 25 (23-26) |
| Aortic sinus diameter, mm | 41 (37-47) |
| Ascending aortic diameter, mm | 45 (40-50) |
| Ejection fraction, % | 60 (58-64) |
| Left ventricular end diastolic diameter, mm | 50 (46-55) |
Values are mean ± SD, n (%), or median (IQR).
AATAD = acute type A aortic dissection; ALT = alanine aminotransferase; AST = aspartate aminotransferase; COPD = chronic obstructive pulmonary disease.
Presenting symptoms and management in ATAAD
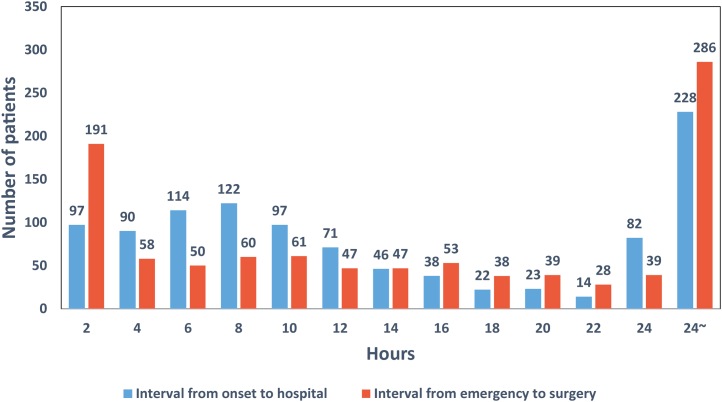
The median interval from onset to hospital was 10.65 hours (IQR: 6-24 hours), and the median interval from entering emergency room to starting operation was 13 hours (IQR: 4.08-28.7 hours) (Table 2, Central Illustration). The distribution of interval from onset to arrival to hospital and the interval from entering emergency room to starting operation by hour are shown in Figure 2. The median transport distance of patients with ATAAD was 176.3 kilometers (IQR: 37.7-382.8 kilometers). Chest pain was the most common presenting symptom (66.2%) in ATAAD, followed by back pain (52.9%) and abdominal pain (23.2%). A total of 56 (8.2%) patients with ATAAD had uncontrollable hypertension (systolic blood pressure higher than 140 mm Hg), and 41 (6%) patients had a systolic blood pressure lower than 100 mm Hg during the emergency room. Approximately 5% of patients presented with syncope, and 147 (13.9%) patients presented with malperfusion syndrome. Coma and tamponade accounted for only 0.7%, respectively (Table 2). All patients received computed tomography, and 91.2% of patients received transesophageal or transthoracic echocardiography.
Table 2.
Presenting Symptoms in ATAAD (N = 1,058)
| Interval from onset to hospital, h | 10.65 (6-24) |
| Interval from ER to surgery, h | 13.00 (4.08-28.7) |
| Blood pressure control, mm Hg | |
| ≥140 | 56 (8.2) |
| 130≤BP<140 | 148 (21.7) |
| 120≤BP<130 | 138 (20.3) |
| 110≤BP<120 | 174 (25.6) |
| 100≤BP<110 | 124 (18.2) |
| <100 | 41 (6.0) |
| Drug use | |
| Calcium channel blocker | 431 (62.2) |
| β blocker | 522 (75.3) |
| Sedatives | 127 (18.3) |
| Analgesics | 306 (44.2) |
| Urgent trachea intubation | 11 (1.7) |
| Presenting symptoms | |
| Chest pain | 689 (66.2) |
| Back pain | 551 (52.9) |
| Abdominal pain | 241 (23.2) |
| Malperfusion syndrome | 147 (13.9) |
| lower extremity malperfusion | 47 (4.5) |
| Cardiac ischemia | 18 (1.7) |
| Visceral ischemia | 23 (2.2) |
| Renal malperfusion | 69 (6.5) |
| Syncope | 50 (4.7) |
| Coma | 5 (0.7) |
| Tamponade | 7 (0.7) |
| DeBakey classification | |
| Type I | 893 (84.4) |
| Type II | 165 (15.6) |
Values are median (IQR) or n (%).
BP = blood pressure; ER = emergency room; other abbreviations as in Table 1.
Central Illustration.
Current Surgical Management of Acute Type A Aortic Dissection in China
The median interval from onset to hospital was 10.65 hours, and the median interval from entering the emergency room (ER) to starting operation was 13 hours. Total arch repair was performed in 938 patients (88.7%). The incidence of early mortality was 7.6%. ATAAD = acute type A aortic dissection.
Figure 2.
The Distribution of Interval From Onset to Surgery
The distribution of interval from onset to arrival to hospital and the interval from entering emergency room to starting operation by hour.
Surgical management
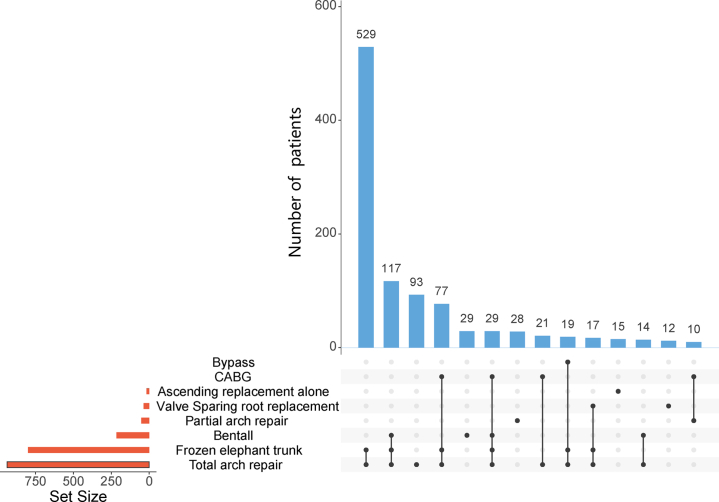
All 1,058 patients received surgical management and 893 (84.4%) patients were DeBakey I (Table 3). Bentall procedure was performed for 20.4% of all patients, valve-sparing root replacement in 3.5%. Ascending aorta replacement alone was performed in 1.6%, among which DeBakey II patients were used more than DeBakey I patients (6.7% vs 0.7%, P < 0.001). Aortic arch surgery was indicated for 93.5% of patients (partial arch replacement in 4.8% and total arch replacement [TAR] in 88.7%) (Central Illustration). Frozen elephant trunk (FET) (CRONUS, MicroPort Medical Company Limited) and hybrid aortic arch replacement were performed in 75.6% and 10.8% of patients, respectively. Concomitant procedures, including coronary artery bypass grafting and extra-anatomic bypass, were carried out in a limited number of patients (15% and 3%, respectively). The proportion and combination of all operations are shown in Figure 3. The most common surgical method is TAR combined with FET surgery, followed by Bentall surgery combined with the previously mentioned operations. The median surgery time was 6.83 hours (IQR: 5.75-8.33 hours), the median cardiopulmonary bypass time was 190 minutes (IQR: 156.25-230 minutes), the median aortic cross-clamping time was 118 minutes (IQR: 94-145 minutes), and the median hypothermia circulatory arrest time was 17 minutes (IQR: 10-22 minutes).
Table 3.
Surgical Management of 1,058 Patients With ATAAD
| Overall (N = 1,058) | DeBakey I (n = 893) | DeBakey II (n = 165) | P Value | |
|---|---|---|---|---|
| Ascending replacement alone | 17 (1.6) | 6 (0.7) | 11 (6.7) | <0.001 |
| Aortic root procedure | ||||
| Bentall | 216 (20.4) | 183 (20.5) | 33 (20.0) | 0.969 |
| VSRR | 37 (3.5) | 26 (2.9) | 11 (6.7) | 0.029 |
| Partial arch repair | 51 (4.8) | 28 (3.1) | 23 13.9) | <0.001 |
| Total arch repair | 938 (88.7) | 829 (92.8) | 109 (66.1) | <0.001 |
| Total arch repair alone | 24 (2.3) | 729 (81.6) | 95 (57.6) | <0.001 |
| Hybrid arch repair | 114 (10.8) | 100 (11.2) | 14 (8.5) | 0.37 |
| Frozen elephant trunk | 800 (75.6) | 711 (79.6) | 89 (53.9) | <0.001 |
| Concomitant surgery | ||||
| CABG | 159 (15.0) | 137 (15.4) | 22 (13.3) | 0.582 |
| Extra-anatomic bypass | 32 (3.0) | 32(3.6) | 0 (0.0) | 0.026 |
| Surgery time, h | 6.83 (5.75-8.33) | 6.96 (5.91- 8.42) | 6.38 (5.17- 7.75) | 0.001 |
| CPB time, min | 190 (156.25-230) | 195 (162- 235) | 162 (130- 196) | <0.001 |
| ACC time, min | 118 (94-145) | 120 (97- 146) | 102 (76.75- 138) | <0.001 |
| HCA time, min | 17 (10-22) | 16 (11- 21) | 17 (0.5- 26.5) | 0.779 |
| Blood loss, mL | 690 (600-900) | 750 (600- 900) | 630 (484.25- 900) | <0.001 |
| Reb blood cell input, U | 2 (0-4) | 0 (0- 4) | 1.50 (0- 4) | 0.294 |
| Plasma input, mL | 400 (0-600) | 400 (0- 600) | 400 (0- 600) | 0.705 |
| Platelet input, U | 1 (1- 2) | 1 (1- 1.75) | 1 (1- 1) | 0.526 |
Values are n (%) or median (IQR).
ACC = aortic cross clamp; CABG = coronary artery bypass graft; CPB = cardiopulmonary bypass; HCA = hypothermic circulatory arrest; VSRR = valve-sparing root replacement; other abbreviation as in Table 1.
Figure 3.
Surgical Management of 1,058 Patients With Acute Type A Aortic Dissection via Upset Plot
The combination of operations at different aortic anatomic sites. CABG = coronary artery bypass grafting.
Early outcomes
Overall, 80 patients (7.6%) died in the hospital, the median length of hospital stay among surviving patients was 14 days (IQR: 10-20 days), the median length of intensive care unit stay was 54 hours (IQR: 6-127.81 hours), and the median mechanical ventilation time was 23 hours (IQR: 13-65 hours) (Table 4). Pneumonia was most common complication (30.1%), followed by postoperative liver dysfunction (22.9%), acute kidney insufficiency (18.2%), mental symptoms (10.7%), respiratory failure (9.1%), pleural effusion (8.3%), readmission to the intensive care unit (4.3%), stroke (3.6%), pericardial effusion (2.7%), gastrointestinal bleeding (2.3%), re-exploration for bleeding (2.3%), paraplegia (1.7%), multiple organ dysfunction syndrome (1.5%), and sternal wound infection (0.6%). Device-assisted therapy, including continuous renal replacement therapy, extracorporeal membrane oxygenation, and intra-aortic balloon pump, was implemented in a limited number of patients (8.3%, 1.5%, and 0.6%, respectively).
Table 4.
In-Hospital Death and Postoperative Complications of 1,058 Patients With ATAAD
| In-hospital death | 80 (7.6) |
| Length of stay, d, median (IQR) | 14 (10-20) |
| ICU time, d, median (IQR) | 54 (6-127.81) |
| Mechanical ventilation time, h, median (IQR) | 23 (13-65) |
| Pneumonia | 314 (30.1) |
| Postoperative liver dysfunction | 239 (22.9) |
| Acute kidney insufficiency | 190 (18.2) |
| Mental symptoms | 107 (10.7) |
| Respiratory failure | 103 (9.1) |
| Pleural effusion | 87 (8.3) |
| Readmission to the ICU | 44 (4.3) |
| Stroke | 37 (3.6) |
| Pericardial effusion | 28 (2.7) |
| Gastrointestinal bleeding | 24 (2.3) |
| Re-exploration | 23 (2.3) |
| Paraplegia | 18 (1.7) |
| Tracheotomy | 17 (1.6) |
| MODS | 16 (1.5) |
| Sternal wound infection | 6 (0.6) |
| CRRT | 86 (8.3) |
| ECMO | 16 (1.5) |
| IABP | 4 (0.6) |
Values are n (%) or median (IQR).
CRRT = continuous renal replacement therapy; ECMO = extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; ICU = intensive care unit; MODS = multiple organ dysfunction syndrome; other abbreviation as in Table 1.
Discussion
Globally, ATAAD is still considered a disease with high morbidity and mortality, and it remains a challenge to diagnose and treat. The large-scale multicenter registration study gives us a deeper understanding of the characteristics of ATAAD, and there has been a significant decrease in overall in-hospital mortality in ATAAD over the past 50 years.13 Therefore, the China Registry of Type A Aortic Dissection was established in accordance with the IRAD model in 2018 to improve the management of ATAAD in the Chinese population. We found that the population of patients with ATAAD in China undergoing surgery was younger, received more extensive aortic arch repair, and experienced a longer interval, and distance, from onset to arrival at the hospital but showed a relatively low early mortality (Central Illustration).
Compared with type B aortic dissection, the management of ATAAD is more complex, and the prognosis needs to be improved.14 Therefore, many researchers have established a separate database for ATAAD instead of grouping all types of aortic dissection, and the American Association for Thoracic Surgery has launched an expert consensus document for the surgical treatment of ATAAD.15 The German Registry for Acute Aortic Dissection Type A (GERAADA) was started in 2006 by the German Society for Thoracic and Cardiovascular Surgery, and the Nordic Consortium for Acute Type A Aortic Dissection was a collaborative effort of Nordic cardiac surgery centers to study ATAAD.4,16 Nineteen centers of cardiac surgery from 7 European countries have collaborated to create a multicenter observational registry-European registry of TAAD.7 In addition, many large multicenter cardiac surgery or aortic dissection databases also analyze patients with TAAD separately. The Society for Thoracic Surgeons National Adult Cardiac Surgery Database is the largest registry for heart surgery in the world and examines current patient characteristics, predictors, and outcomes for acute TAAD.17 The Japanese Registry of Acute Aortic Dissection (JRAD) was started in 2011 and revealed the actual clinical setting for the treatment of acute type A dissection in Japan.18 Therefore, it is necessary to establish a national multicenter database for TAAD that can summarize the disease characteristics and management omissions to improve the prognosis.
The age of patients undergoing surgery for TAAD in China is still strikingly younger. Previous studies have also shown that the Chinese population has a 1-decade disparity in age at onset compared with the IRAD database.19 We also reviewed recent data on countries and regions with similar geographic proximity and lifestyles to China, including Japan,6 South Korea,20 and Taiwan.21 The average age of onset of aortic dissection in Japan is nearly 70 years old, which is higher than the average age of the IRAD database. For South Korea and Taiwan, although their average onset ages are lower than IRAD, they are still slightly higher than the Chinese population. All the areas mentioned previously, except China, are developed countries/regions, so the difference in age may be related to the economic level, to a certain extent. Our results may also provide reference for some developing countries all over the world.
Compared with other databases, the interval from the onset of symptoms to arrival at the hospital and the interval from entering the emergency room to starting operation were longer, which could reflect geographic and emergency medical factors. Therefore, we also analyzed the transport distance of patients for the first time, but there are no other studies to compare with these data. According to the IRAD data, the interval from diagnosis to surgical intervention is 4.3 hours.22 The median referral interval from onset of symptoms to arrival at JRAD centers was only 199 minutes.18 However, our data found that the preoperative transport time was more than twice that of IRAD, and even many patients did not receive timely treatment. This situation was particularly prominent in the outbreak of COVID-19 in 2020.23 Even if a simple comparison of intervals between different studies is difficult, this result means that we still have much room for improvement, and this result will also promote the medical management department to pay attention to the management of prehospital first aid in China.
The management of the aortic arch in the context of ATAAD has also been under constant debate.24, 25, 26 Recent data from IRAD showed that TAR is not as widely used as hemiarch or partial arch replacement.27 The GERAADA showed that a more aggressive approach of aortic arch treatment can be applied without higher perioperative risk even in the onset of ATAAD.28 In our study, TAR combined with FET accounted for a large proportion and achieved relatively low in-hospital mortality. This may be because TAR combined with FET has been used in China for nearly 20 years and was once considered a standard treatment for TAAD.29,30 Most DeBakey II patients with ATAAD received a more limited procedure. In DeBakey I patients, 92.8% of the patients received TAR, whereas in DeBakey II patients, the proportion decreased to 66.1% with statistical difference. We believe that one-half of the patients with DeBakey II may have a widened aortic arch, and we have carried out more extensive repair, which is also the focus of our future research. In addition, it can also be seen from our study that Chinese patients with ATAAD are relatively younger, and postoperative patent false lumen is present at a high rate in young patients despite entry resection.31 The FET procedure remains an increasingly popular approach to address complex multisegmental aortic pathologies owing to its ability to promote false lumen thrombosis and reduce the need for second-stage operations.32 Based on this theory and the age of Chinese patients with ATAAD, the aortic surgeons chose a more extensive repair method in China. Some new prostheses, including Thoraflex Hybrid33 and E-Vita Open prostheses,34 are also undergoing clinical trials, which conforms to the trend of aggressive aortic arch treatment worldwide. At present, the medical center of the China Registry of Type A Aortic Dissection is also working closely to complete the clinical trial of sutureless integrated stented grafts.35 This is also the first multicenter study to show the main surgical strategies of ATAAD treatment in China, and subsequent studies will determine the long-term results of this treatment strategy.
Data from our database revealed relatively lower in-hospital mortality rates among surgically treated patients than among IRAD, GERAADA, and JRAD patients. It is strange that patients with a more aggressive approach of aortic arch treatment have lower mortality. A previous study considered that this might have resulted from an inadequate medical system. In China, medical resources are unevenly distributed in different areas. The cardiovascular centers included in the study were mainly located in large cities.19 Hospitals in large cities have more experienced surgeons and better medical equipment, which can be explained to a certain extent, but our results show that it is very likely to be a huge survivor bias in Chinese patients with ATAAD. They were relatively younger, and they were transported for a long time and over a long distance before surgery. Patients with severe complications might have died before they could receive surgical treatment. The mortality rate tended to be higher during the hyperacute (0-24 hours) stage after the onset of symptoms than during the time after that stage according to the IRAD study.10 Therefore, patients who undergo surgical management in our studies have already been selected to some extent. The low postoperative in-hospital mortality is not a satisfactory outcome of surgical management, but the embodiment of insufficient prehospital management. We should let more people pay attention to those patients who cannot be treated in time, and strengthen the management of prehospital emergency care in China. This is also the most important message in our finding and how we should change our management according to the results.
Study limitations
First, some patients were unable to be included in the study because of the lack of baseline data. Second, because our cohort is based on a multicenter study, and the follow-up data of some centers are incomplete, we can only take the in-hospital mortality as the endpoint. Therefore, we will invest more effort to improve the follow-up of patients. Our findings also suggest that future research should focus on evaluating long-term survival.
Conclusions
We found that the population of patients with ATAAD in China undergoing surgery was younger, experienced a longer interval and distance from onset to arrival at the hospital, received more extensive aortic arch repair, and showed a relatively lower early mortality. More effort is needed to promote prehospital emergency care and preoperative management of Chinese patients with ATAAD and longer follow-up to determine the prognosis of extended aortic arch surgery in younger patients.
Perspectives.
COMPETENCY IN MEDICALKNOWLEDGE: Many countries and regions have established multicenter registration studies to improve the outcomes of ATAAD; however, the actual clinical outcomes of surgical treatment for ATAAD in China are unclear. Our findings highlight the differences in the management of ATAAD in different countries.
TRANSLATIONAL OUTLOOK: The findings of this study suggest that there may be a huge survivor bias in patients with ATAAD in China. More effort is needed to promote prehospital emergency care and preoperative management of Chinese patients with ATAAD and longer follow-up to determine the prognosis of extensive aortic arch surgery in younger patients.
Funding Support and Author Disclosures
This study was supported by the CAMS Initiative for Innovative Medicine (CAMS-I2M) [2016-I2M-1-016], Special Subject Development Foundation of Fuwai Hospital (NO.2015-FWTS01), Beijing Science and Technology Program (NO. Z191100007619042), and China Scholarship Council (No. [2021]070). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank the Chinese surgeons who have put a lot of effort into this research from all centers, including but not limited to the following colleagues: Qing Xue, Changjiang Yu, Weitao Liang, Xuan Jiang, Long Wu, Jiuwei Liu, Qi Tan, Yong Li, Suhua Zang, Yang Liu, Lihua Chen, Ruisheng Liu, Fushuo Zhou, Wei Sun, and Jue Yang.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Leonard J.C. Thomas Bevill Peacock and the early history of dissecting aneurysm. Br Med J. 1979;2:260–262. doi: 10.1136/bmj.2.6184.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta R.H., O'Gara P.T., Bossone E., et al. Acute type A aortic dissection in the elderly: clinical characteristics, management, and outcomes in the current era. J Am Coll Cardiol. 2002;40:685–692. doi: 10.1016/s0735-1097(02)02005-3. [DOI] [PubMed] [Google Scholar]
- 3.Collins J.S., Evangelista A., Nienaber C.A., et al. Differences in clinical presentation, management, and outcomes of acute type a aortic dissection in patients with and without previous cardiac surgery. Circulation. 2004;110:II237–II242. doi: 10.1161/01.CIR.0000138219.67028.2a. [DOI] [PubMed] [Google Scholar]
- 4.Geirsson A., Ahlsson A., Franco-Cereceda A., et al. The Nordic Consortium for Acute type A Aortic Dissection (NORCAAD): objectives and design. Scand Cardiovasc J. 2016;50:334–340. doi: 10.1080/14017431.2016.1235284. [DOI] [PubMed] [Google Scholar]
- 5.Weigang E., Conzelmann L.O., Kallenbach K., Dapunt O., Karck M. German registry for acute aortic dissection type A (GERAADA)--lessons learned from the registry. Thorac Cardiovasc Surg. 2010;58:154–158. doi: 10.1055/s-0029-1240806. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi T., Nakai M., Sumita Y., et al. Current status of the management and outcomes of acute aortic dissection in Japan: Analyses of nationwide Japanese Registry of All Cardiac and Vascular Diseases-Diagnostic Procedure Combination data. Eur Heart J Acute Cardiovasc Care. 2020;9:S21–S31. doi: 10.1177/2048872619872847. [DOI] [PubMed] [Google Scholar]
- 7.Biancari F., Mariscalco G., Yusuff H., et al. European registry of type A aortic dissection (ERTAAD) - rationale, design and definition criteria. J Cardiothorac Surg. 2021;16:171. doi: 10.1186/s13019-021-01536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagan P.G., Nienaber C.A., Isselbacher E.M., et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]
- 9.Evangelista A., Isselbacher E.M., Bossone E., et al. Insights from the International Registry of Acute Aortic Dissection: a 20-year experience of collaborative clinical research. Circulation. 2018;137:1846–1860. doi: 10.1161/CIRCULATIONAHA.117.031264. [DOI] [PubMed] [Google Scholar]
- 10.Booher A.M., Isselbacher E.M., Nienaber C.A., et al. The IRAD classification system for characterizing survival after aortic dissection. Am J Med. 2013;126:730.e19–730.e24. doi: 10.1016/j.amjmed.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Augoustides J.G.T., Geirsson A., Szeto W.Y., et al. Observational study of mortality risk stratification by ischemic presentation in patients with acute type A aortic dissection: the Penn classification. Nat Clin Pract Cardiovasc Med. 2009;6:140–146. doi: 10.1038/ncpcardio1417. [DOI] [PubMed] [Google Scholar]
- 12.Yan T.D., Tian D.H., LeMaire S.A., et al. Standardizing clinical end points in aortic arch surgery: a consensus statement from the International Aortic Arch Surgery Study Group. Circulation. 2014;129:1610–1616. doi: 10.1161/CIRCULATIONAHA.113.006421. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y., Lingala B., Baiocchi M., et al. Type A aortic dissection-experience over 5 decades: JACC Historical Breakthroughs in Perspective. J Am Coll Cardiol. 2020;76:1703–1713. doi: 10.1016/j.jacc.2020.07.061. [DOI] [PubMed] [Google Scholar]
- 14.Pape L.A., Awais M., Woznicki E.M., et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol. 2015;66:350–358. doi: 10.1016/j.jacc.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 15.Malaisrie S.C., Szeto W.Y., Halas M., et al. 2021 The American Association for Thoracic Surgery expert consensus document: surgical treatment of acute type A aortic dissection. J Thorac Cardiovasc Surg. 2021;162:735–758.e2. doi: 10.1016/j.jtcvs.2021.04.053. [DOI] [PubMed] [Google Scholar]
- 16.Boening A., Karck M., Conzelmann L.O., et al. German Registry for Acute Aortic Dissection Type A: structure, results, and future perspectives. Thorax Cardiovasc Surg. 2017;65:77–84. doi: 10.1055/s-0036-1572436. [DOI] [PubMed] [Google Scholar]
- 17.Lee T.C., Kon Z., Cheema F.H., et al. Contemporary management and outcomes of acute type A aortic dissection: an analysis of the STS adult cardiac surgery database. J Card Surg. 2018;33:7–18. doi: 10.1111/jocs.13511. [DOI] [PubMed] [Google Scholar]
- 18.Inoue Y., Matsuda H., Uchida K., et al. Analysis of acute type A aortic dissection in Japan Registry of Aortic Dissection (JRAD) Ann Thorac Surg. 2020;110:790–798. doi: 10.1016/j.athoracsur.2019.12.051. [DOI] [PubMed] [Google Scholar]
- 19.Wang W., Duan W., Xue Y., et al. Clinical features of acute aortic dissection from the Registry of Aortic Dissection in China. J Thorac Cardiovasc Surg. 2014;148:2995–3000. doi: 10.1016/j.jtcvs.2014.07.068. [DOI] [PubMed] [Google Scholar]
- 20.Ahn J.-M., Kim H., Kwon O., et al. Differential clinical features and long-term prognosis of acute aortic syndrome according to disease entity. Eur Heart J. 2019;40:2727–2736. doi: 10.1093/eurheartj/ehz153. [DOI] [PubMed] [Google Scholar]
- 21.Yeh T.-Y., Chen C.-Y., Huang J.-W., Chiu C.-C., Lai W.-T., Huang Y.-B. Epidemiology and medication utilization pattern of aortic dissection in Taiwan: a population-based study. Medicine (Baltimore) 2015;94:e1522. doi: 10.1097/MD.0000000000001522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris K.M., Strauss C.E., Eagle K.A., et al. Correlates of delayed recognition and treatment of acute type A aortic dissection: the International Registry of Acute Aortic Dissection (IRAD) Circulation. 2011;124:1911–1918. doi: 10.1161/CIRCULATIONAHA.110.006320. [DOI] [PubMed] [Google Scholar]
- 23.Zhao R., Xu W., Wang Z., Yu C., Yang Y. Impact of COVID-19 on emergency management of acute type A aortic dissection: a single-center historic control study. Rev Cardiovasc Med. 2022;23 doi: 10.31083/j.rcm2306200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Eusanio M., Berretta P., Cefarelli M., et al. Total arch replacement versus more conservative management in type A acute aortic dissection. Ann Thorac Surg. 2015;100:88–94. doi: 10.1016/j.athoracsur.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 25.Trivedi D., Navid F., Balzer J.R., et al. Aggressive aortic arch and carotid replacement strategy for type A aortic dissection improves neurologic outcomes. Ann Thorac Surg. 2016;101:896–903. doi: 10.1016/j.athoracsur.2015.08.073. [DOI] [PubMed] [Google Scholar]
- 26.Frankel W.C., Green S.Y., Orozco-Sevilla V., Preventza O., Coselli J.S. Contemporary surgical strategies for acute type A aortic dissection. Semin Thorac Cardiovasc Surg. 2020;32:617–629. doi: 10.1053/j.semtcvs.2020.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Larsen M., Trimarchi S., Patel H.J., et al. Extended versus limited arch replacement in acute Type A aortic dissection. Eur J Cardiothorac Surg. 2017;52:1104–1110. doi: 10.1093/ejcts/ezx214. [DOI] [PubMed] [Google Scholar]
- 28.Easo J., Weigang E., Hölzl P.P.F., et al. Influence of operative strategy for the aortic arch in DeBakey type I aortic dissection: analysis of the German Registry for Acute Aortic Dissection Type A. J Thorac Cardiovasc Surg. 2012;144:617–623. doi: 10.1016/j.jtcvs.2011.07.066. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z.-G., Sun L.-Z., Chang Q., et al. Should the "elephant trunk" be skeletonized? Total arch replacement combined with stented elephant trunk implantation for Stanford type A aortic dissection. J Thorac Cardiovasc Surg. 2006;131:107–113. doi: 10.1016/j.jtcvs.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Sun L., Qi R., Zhu J., Liu Y., Zheng J. Total arch replacement combined with stented elephant trunk implantation: a new "standard" therapy for type a dissection involving repair of the aortic arch? Circulation. 2011;123:971–978. doi: 10.1161/CIRCULATIONAHA.110.015081. [DOI] [PubMed] [Google Scholar]
- 31.Tamura K., Chikazawa G., Hiraoka A., Totsugawa T., Yoshitaka H. Characteristics and surgical results of acute type A aortic dissection in patients younger than 50 years of age. Ann Vasc Dis. 2019;12:507–513. doi: 10.3400/avd.oa.19-00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian D.H., Ha H., Joshi Y., Yan T.D. Long-term outcomes of the frozen elephant trunk procedure: a systematic review. Ann Cardiothorac Surg. 2020;9:144–151. doi: 10.21037/acs.2020.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shrestha M., Pichlmaier M., Martens A., Hagl C., Khaladj N., Haverich A. Total aortic arch replacement with a novel four-branched frozen elephant trunk graft: first-in-man results. Eur J Cardiothorac Surg. 2013;43:406–410. doi: 10.1093/ejcts/ezs296. [DOI] [PubMed] [Google Scholar]
- 34.Tsagakis K., Pacini D., Grabenwöger M., et al. Results of frozen elephant trunk from the international E-vita Open registry. Ann Cardiothorac Surg. 2020;9:178–188. doi: 10.21037/acs-2020-fet-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J., Qiu J., Qiu J., et al. A new graft for total arch replacement with frozen elephant trunk in type A dissection. Semin Thorac Cardiovasc Surg. 2020;32:840–842. doi: 10.1053/j.semtcvs.2020.02.022. [DOI] [PubMed] [Google Scholar]