Abstract
The current study deals with the biological response variations on some biochemical properties of the freshwater green algae Chlorella vulgaris by exposure to different Mg2+ concentrations (5, 10, and 15) mg/L. Physiological and biochemical parameters, including growth curve, doubling time, photosynthesis pigments, total protein and carbohydrates were investigated. Moreover, enzymatic parameters such as catalase (CAT), superoxide dismutase (SOD), and reactive oxygen species (ROS) were also examined. The maximum growth rate was 0.482/day during the 9th day with 5 mg/L Mg2+, while the minimum was 0.019/day during the 13th and 14th days with 10 mg/L Mg2+. Furthermore, doubling time ranged between 15.501 during the 13th day with 10 mg/L Mg2+, and 0.624 during the 9th day with 5 mg/L. The maximum value of chlorophyll-a was 0.157 μg/mL during the 1st day with 10 mg/L Mg2+, while the minimum was 0.062 μg/mL during the 14th day with 15 mg/L Mg2+. The carotenoid ranged between 0.029 μg/mL during the 7th day with 15 mg/L Mg2+ and 0.002 μg/mL during the 14th day. The maximum protein value was 9.620 μg/L during the 1st day with 15 mg/L Mg2+, while the minimum was 1.772 μg/L during the 14th day with 15 mg/L Mg2+. The carbohydrate showed a maximum value of 0.824 μg/mL during the 1st day with 10 mg/L Mg2+, while the minimum was 0.293 μg/mL during the 14th day with 15 mg/L. Moreover, SOD ranged between 0.884 unit/L during the 1st day with 15 mg/L Mg2+, and 0.073 unit/L as the minimum value during the 14th day with 15 mg/L Mg2+. The maximum value of ROS was 3.627 mM/mL during the 1st day with 5 and 15 mg/L Mg2+, while the minimum was 1.674 mM/mL during the 14th day with 10 mg/L. The results show that values of CAT ranged between 0.200 unit/mL during the 7th day with 10 mg/L Mg2+, and 0.010 unit/mL during the 14th day with 15 mg/L. Overall, 5 mg/L for biomass production and 15 mg/L for protein and carbohydrate production were optimum doses.
Keywords: Green algae, Photosynthetic pigments, Antioxidant enzymes, Protein and carbohydrates, Metal
1. Introduction
Algae are autotrophic organisms like plants that belong to the kingdom of protists, most are single-celled, but some are large in size and multicellular [1]. Utilizing algae for protein synthesis offers various advantages over the usage of conventional high-protein crops in terms of both productivity and nutritional value. Seaweed and microalgae provide more protein per unit area (2.5–7.5 and 4–15 tons/Ha/year, respectively) than terrestrial crops, including soybean, pulse legumes, and wheat (0.6–1.2, 1–2, and 1.1 tons/Ha/year, respectively) [2]. The biosorption of metal ions relies on the unique surface features of the biomass, the concentration of these ions, and the physicochemical parameters of the solution. In recent years, microalgae have garnered great interest for their capacity to remove metals (temperature, pH, etc.) [3,4]. The optimum concentrations of desired nutrients are varied from one alga to another, even in the same algal group and based on their natural habitat. In addition, the differences in the biodiversity of algae were found to be nutritional and environmental dependent [5]. Current biotechnological applications for algae include cosmetics, animal feed, fatty acids, alginates, wastewater treatment, and biofuels [6,7]. Due to their high vitamin and mineral content, Arthrospira platensis and Chlorella vulgaris are also offered as functional foods [8].
Understanding microalgal ecophysiology is crucial not only for understanding phytoplankton fate in natural environments but also for maximizing the production of microalgal biomass at large scales, with applications in aquaculture, bioenergy, and the cosmetics industry, among others [9,10]. Not only do environmental elements such as temperature, light, pH, and nutrients impact the photosynthesis and the growth rate of algae, but they also alter the activity and content of cellular metabolism [11]. C. vulgaris is able to use a variety of organic carbon sources, including glucose and acetic acid. This alga could be stimulated to grow in the presence or absence of light by glucose and other organic substrates. Due to their capacity to digest metals, microalgal cultures have several industrial applications [12].
The United States, Japan, China, Taiwan, and Indonesia manufacture more than 2500 tons of dried chlorella annually since it is not only an essential source of nutrients, but also a functional food owing to its favorable health benefits [13]. Studies have shown that C. vulgaris is a safe source of protein for consumption and dietary supplementation with C. vulgaris may reduce high blood pressure, lower serum cholesterol and glucose levels [14]. To enhance the economics of microalgae applications, high-value co-products such as pigments, proteins, lipids, and carbohydrates should be generated alongside wastewater treatment, which is a low-cost source of nutrients [15,16]. The commercial production of microalgae-based products for human consumption focuses on high-value polar lipids such as antioxidants (astaxanthin, phycocyanin, and lutein) because they have been shown to provide multiple health benefits, such as reducing the risk of cardiovascular disease, cancer, and mental disorders [14]. Magnesium (Mg2+) ions are a key component of chlorophyll required as a growth factor for green microalgae such as Chlorella vulgaris [17] and can be found in stoichiometric ratio to chlorophyll in photosynthetic tissues. The chlorophyll content of the microalgae is known to vary with the growth conditions, such as light intensity [18] and CO2 concentration [19].
Magnesium is the most important element required for proper algal growth, and its efficiency could be maximized through nitrate form. Furthermore, biomass enriched in magnesium could have potential cosmetic applications. Magnesium is necessary for the growth and development of microalgae; it has a crucial position in the chlorophyll molecule and influences the activity of the photosynthetic enzymes [20].
This study aimed, on the one side, to establish a robust and reliable procedure for the assessment of accumulated Mg2+ ions on biomass and biochemical variations in the freshwater algae Chlorella vulgaris, and, on the other side, to construct a model for the association of Mg2+ ions with the algae; this model would serve as a tool for optimizing the bioaccumulation of Mg2+ ions in the organic biomass. Other micro-algae and ions could also be amenable to the techniques shown here.
2. Materials and methods
The C. vulgaris (SAG strain number 211-11b/sequence accession AY323465) was identified by microscopic observation [21] and incubated under controlled conditions of light intensity 286 μE/m2/s, light/dark period 16:8 h and temperature 25 ± 2 °C [22]. All equipment and medium were sterilized in an autoclave at 121 °C, 1.5 h for 15 min. Modified Chu-10 employed for the purpose of algal growth [23]. The initial cell concentration of all experimental groups of C.vulgaris was 2.67 × 10−6 cells/mL in each replicate [24]. The stocks prepared for all macro, and microelements dissolved the weight of the salt. Table S1 shows the components and concentration of the modified Chu-10 medium [25]. In one liter, 2.5 mL was gathered from the stock solution and filled up to 1 L with distilled water. Afterwards, it was sterilized with an autoclave, except stock solution (K2HPO4), which was sterilized alone and added finally to get 1 L of Chu-10. The solution pH was set to 6.4 after the sterilization using 0.01 N of NaOH or HCl. Ten milliliters of culture algal was taken in a flask containing 100 mL of Chu-10 medium and grown for 15 days. This culture was transferred into 1000 mL of medium and incubated for 14 days. The biomass of the algae increases in glass pools of 5 L [26]. The algal Chlorella vulgaris (100 mL) was cultured in 1 L of Chu-10 medium and left for at least two weeks before starting the experiment under constant laboratory conditions. Photoperiod is a key variable that regulates asexual reproduction's cell division, which proceeds throughout the light period and is increased by continuous illumination. Consequently, the photoperiod can be adjusted based on the aims of the culture: continuous illumination promotes rapid growth, while a photoperiod consisting of alternating hours of light and dark, similar to the solar photoperiod, promotes normal and healthy growth [27,28]. The culture was initiated by inoculating 100 mL of pure culture into two 500 mL Erlenmeyer flasks containing 300 mL of culture medium. Subsequently, 100 μM of a sterilized MgSO4 solution was added to the culture medium during the stationary period of the microalgae [24]. The culture medium of C. vulgaris was exposed to different concentrations of Mg2+ (5, 10, and 15) mg/L, the experiments lasted 14 days. Different physiological and biochemical parameters, including growth curve, doubling time, photosynthesis pigments (chlorophyll-a and carotenoids), total protein and carbohydrates were investigated. In addition, enzymatic parameters such as catalase (CAT), superoxide dismutase (SOD), and reactive oxygen species (ROS) were also examined.
2.1. Determination of growth rate and doubling time
The following equation [29] was used to calculate the growth constant K:
K = (log ODt – logODo) × 3.332/t.
K: growth rate t: time.
OD0: optical density at the beginning of the experiment (zero time).
ODt: optical density after (t) day.
As for the generation time of the multiplication of G, it was calculated from the following equation [30]:
G = 0.301/K G: doubling time.
2.2. Estimation of chlorophyll and carotenoid
The amount of chlorophyll-a and carotenoids were estimated based on a method reported by Ref. [31]. Briefly, 2 mL of the sample was taken and then subjected to 12,500 rpm for 5 min. Afterwards, the precipitate from the algae was taken and added 2 mL of methanol (90%). Then, it was placed in a water bath (64 °C) for 5 min after incubating for 20 h in a place Darkness at a degree of 20 and discarded at 12,500 rpm for 5 min. The filtrate was measured at three different wavelengths 470, 652 and 666 nm. The chlorophyll and carotenoids were calculated from the following equations [32,33]:
| μg Chlorophyll / mL medium = (16.29 × A665) − (8.54 × A652) |
| μg total carotenoids / mL medium = [(1000 × A470–44.76 × A666) / 221] |
2.3. Estimation of carbohydrates
Two mL of the sample was taken and dried aerobically after washing with a phosphate-buffer solution and breaking it with the sonicatre and diluted to 5 mL with distilled water. Then, 1 mL of the sample was taken and added 5 mL of sulfuric acid (96%) and 1 mL of phenol (5%) and waited for 10 min with continuous stirring. Afterwards, it was placed in a water bath (30–35 °C) for 10 min, then measured along 490 nm and compared with the standard curve of glucose prepared by dissolving 100 mg of glucose in 100 mL distilled water [9]. Figure S1 and Figure S2 show the standard curves of glucose and albumin, respectively.
2.4. Estimation of total protein
Ultrafiltration, precipitation, chromatography, dialysis, and centrifugation have all been used for the separation and concentration of microalgal proteins [13]. The total proteins were determined according to the LOWRY method modified by Ref. [21]. This was done by taking 0.5 mL of the previously prepared extract and adding 2 mL of Biuret solution after mixing it with a preheater that was heated to 30 °C for half an hour. Then, it was measured at 555 nm and compared with the standard solution depending on the Bovin serum albumin protein at concentrations (0–0.1) mL, prepared by dissolving 0.1 g of Bovin with 100 mL of puffer solution so that the concentration was 100 μg/L.
2.5. Estimation of superoxide dismutase (SOD)
It was estimated by taking 20 μL of the extract and 2 mL from the Tris-buffer. Afterwards, the sample was measured at 420 nm for 5 min. Then, the sample was measured again for the second reading (△A0), and by adding 0.2 mL of Pyralol solution with the same conditions, the second reading (△A1) was taken and calculated from the following equation.
| SOD activity (μ/mL) = [(△A0 -△A1/△A0)/50%] × Volume of sample |
To determine the dry weight content, a calibration with biomass from the stationary phase was performed to establish the absorbance-dry weight relationship. For this purpose, a homogeneous sample of the culture was centrifuged at 5000 rpm for 5 min. Then the solid phase containing biomass was dried at 105 °C overnight and allowed to cool down to room temperature inside a desiccator to obtain the weight of dry microalgae [34].
2.6. Estimation of catalase (CAT)
It was estimated by taking 20 μL of the extract and adding 1 mL of hydrogen peroxide. Then, it measured at 240 nm before and after addition. The following equation was employed to calculate the catalyze concentration.
| CAT = [(△ Abs240/Min10) × (Reaction volume20)]/0.001 |
2.7. Estimation of reactive oxygen species (ROS)
It was estimated by taking 2 mL of the sample and washing it with phosphate-buffer solution twice. Then the precipitate (algal cells) was taken, and 2 mL of perchloric acid (200 μM) was added. Afterwards, the cells were broken by a sonicator for 3 min, stopping every 20 s. Then, the sample was discarded quickly (10,000 cycles per minute) for 30 min, after which the filtrate was combined with a microbicide, and the volume was supplemented to 5 mL. Then, 1.5 mL of the extract was taken, and 0.1 mL of the working solution was added to it (it contains 19.6 mg of ammonium ferrous sulfite, 0.28 mL of Sulfuric acid, 14.3 mg of xylenol, and 3.64 g of sorbitol). The sample was incubated for 30 min at 30 °C, after which it was measured at 560 nm, and the ROS concentration was calculated from the following equation:
%inhibition ROS = △uninhibited/min(10)
2.8. Statistical analysis
The experimental data presented in this article were taken from at least three replicates for each treatment, and the results were presented as the mean and standard deviation. To determine the significance of differences, analysis of variance (ANOVA) was used, and p-values less than 0.05 were considered significant. Principal component analysis (PCA) and post-hoc analysis (Tukey) were used.
3. Results and discussions
3.1. Growth rate
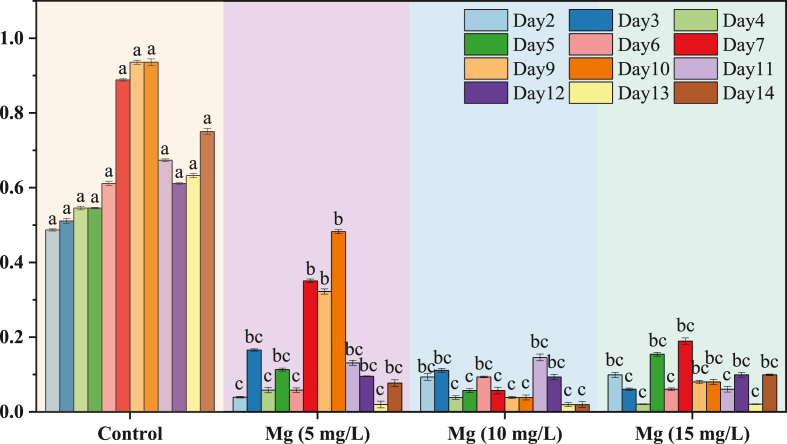
C. vulgaris consumed nitrate ions available in the medium during the autotrophic growth phase; however, it was only partially consumed even by the end of the experiment [35]. The maximum specific growth rate (μ) was determined from the absorbance data during the exponential growth phase. The maximum growth rate was 0.482 during the 9th day with 5 mg/L Mg2+, while the minimum was 0.019 during the 13th and 14th days with 10 mg/L Mg2+. Fig. 1 shows the growth rate of different concentrations of Mg2+. Another study [36] suggested that higher concentrations improved the growth of C. vulgaris. Therefore, the concentration and type of magnesium source in the growth medium influence the growth trends.
Fig. 1.
The changes in ODs of C. vulgaris by different Mg2+ levels.
Magnesium is a core atom of chlorophyll skeletal molecules and is essential for chlorophyll formation; it plays a carrier role and has contributed as an active activator to numerous enzyme responses [20]. Results showed that an increase in the Mg2+ concentrations caused a decrease in growth rates (K in control > 5 Mg2+ treatment > 10 and 15 Mg2+ treatment, p < 0.05). This was due to an increase in the concentration of magnesium, which is considered to be toxic to algae [37]. A dose of 0.1 g/L of MgSO4.7H2O was shown to be appropriate for C. vulgaris growth. However, excessive Mg2+ concentration might induce C. vulgaris flocculation and high costs [38].
3.2. Doubling time
The doubling time is the amount of time it takes for a population's size or value to double. When the relative growth rate is constant, but not the absolute growth rate, the amount grows exponentially and has a constant doubling time or period, which may be determined simply from the growth rate [39]. The maximum value of doubling time was 15.501 during the 13th day with 10 mg/L Mg2+, while the minimum was 0.624 during the 9th day with 5 mg/L. Fig. 2 shows the effect of doubling time with different Mg2+ concentrations. As shown in the current study results, the doubling time was reduced on an orderly basis with days to all treatments except Mg2+. The duplication time of Mg2+ treatment was high because Mg2+ is toxic to C. vulgaris.
Fig. 2.
Doubling time of C. vulgaris with respect to different Mg2+ concentrations.
The impact of increasing Mg2+ content in the culture medium on biomass output was favorable. Chlorophyll, the primary molecule responsible for capturing light energy, needs one magnesium ion per chlorophyll molecule for synthesis. Hence, large dosages of Mg2+ stimulated significant biomass production. As the cells proliferated, the dissolved Mg2+ concentration declined, and the Mg2+ concentration associated with the biomass rose. The quantities of absorbed and adsorbed ions grew with time and cell concentration.
3.3. Chlorophyll-a
Scientifically and economically, the generation of pigments from microalgae is vital. Pigments produced from microalgae are natural high-value chemicals with great potential. These pigments, including chlorophylls (green pigments) and carotenoids (yellow or orange pigments), provide health-promoting qualities such as vitamin precursors, antioxidants, immunological boosters, and anti-inflammatory agents [40,41].
Chlorophyll is the predominant photosynthetic pigment in C. vulgaris cells, and its concentration in the culture rose proportionally with the biomass. The thylakoids of C. vulgaris contain chlorophyll, which may reach 1–2% of the plant's dry weight. Additionally, C. vulgaris has significant levels of carotenoids [42]. The maximum value of chlorophyll-a was 0.157 μg/mL during the 1st day with 10 mg/L Mg2+, while the minimum was 0.062 μg/mL during the 14th day with 15 mg/L Mg2+. Fig. 3 shows the influence of Chlorophyll-a in different concentrations of Mg2+. Since magnesium is the center element of chlorophyll, a higher chlorophyll-a amount was expected with increasing MgSO4 concentrations.
Fig. 3.
C. vulgaris chlorophyll-a concentration in different Mg2+ levels.
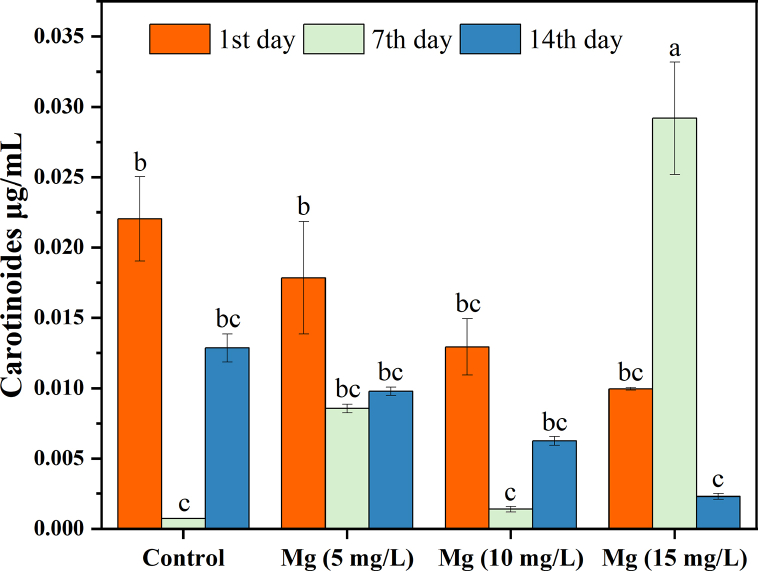
As chlorophyll, the primary molecule responsible for capturing light energy, required one magnesium ion for synthesis per chlorophyll molecule, large dosages of Mg2+ stimulated significant biomass production. C. vulgaris is not inhibited by up to 500 mg/L Mg2+ ions, and the alga can accumulate a considerable quantity of Mg2+. This metal is vital for photosynthesis because it occupies the chlorophyll molecule's center and controls the activity of several photosynthetic enzymes [43]. The maximum value of carotenoid was 0.029 μg/mL during the 7th day with 15 mg/L Mg2+, while the minimum was 0.002 μg/mL during the 14th day with 15 mg/L Mg2+ (Fig. 4).
Fig. 4.
C. vulgaris carotenoids concentration in different Mg2+ concentrations.
Mg2+ could be toxic if present in high concentration, so we observed that chl-a was decreased significantly (p < 0.05) with increasing Mg2+ concentrations. Moreover, with a time of Mg2+ exposure, the chl-a decreased significantly, which was agreed with [44]. With the time of exposure, we recorded increasing in carotenoid concentrations in the control and on the 7th day; on the other hand, on the 14th day, carotenoids decreased significantly compared with the control.
Microalgal suspensions are generally stabilized by a negative surface charge on the cell, which is generated by hydroxyl, carboxyl, phosphate and/or sulphate groups. These could bind positively charged ions such as Mg2+ ions, but the absence of Mg2+ is expected to hinder cell division, cessation of chlorophyll synthesis and, hence, the growth yields [37]. Our findings showed that carotenoids were significantly increased under stress conditions, affecting photosynthesis by reducing energy efficiency by chlorophyll loss and increasing the ingredients of non-photochemically active carotenoid pigments [45]. The biomass concentration, chlorophyll content, lipid content and lipid production rate of Desmodesmus sp. WC08 were tested when the Mg2+ concentration was 0, 0.03, 0.09, 0.15 and 0.30 mmol/L. When the Mg2+ concentration was 0.30 mmol/L, the chlorophyll content of Desmodesmus sp. WC08 reached the highest value (55 mg/L) [46].
3.4. Total protein
Microalgal biomass is a prospective alternative component for the formulation of aquaculture feed due to its high protein content and balanced amino acid composition [47]. Furthermore, there has been studies and commercial interest in microalgae like Chlorella sp. as an alternate source of amino acids and proteins, even for human nutrition [48].
Proteins have an essential role in the chemistry and structure of microalgae. They participate in cellular processes such as development, repair, and maintenance. The total protein content of C. vulgaris ranged from 42 to 58% of dry biomass weight, depending on growing circumstances. The maximum protein value was 9.620 μg/L during the 1st day with 15 mg/L Mg2+, while the minimum was 1.772 μg/L during the 14th day with 15 mg/L Mg2+ (Fig. 5). Magnesium during the 14th day with 15 mg/L Mg2+ improves protein efficiency [49]; the results have shown that chlorophyll decreased due to magnesium which affected the efficacy of photosynthesis, leading to growth inhibition of Chlorella vulgaris and protein synthesis [50].
Fig. 5.
Total protein concentration of C. vulgaris for Mg2+ levels.
Karemore et al. [47] found that a higher than 10 mg/L Mg2+ condition was not helpful for accumulating Chlorococcum infudionum's biomass. Nutritional conditions, deficiency or excess, markedly limit the growth and profile of the grown alga. Mostly, such conditions are associated with a decrease in protein and chlorophyll content with a rise in carbohydrates and oils [51].
Algae have also been claimed to have high-quality proteins. However, some research has shown the contrary; seaweed proteins have been recommended as a helpful dietary supplement for better animal development, meat quality, and value addition [52]. In addition, microalgae have long been employed as the primary feed in aquaculture, particularly for the breeding of mollusks [53]. Chlorella is particularly intriguing because of its high protein content, which may reach up to 60%. Furthermore, several bioactive peptides have been extracted from C. vulgaris, exhibiting beneficial qualities such as antioxidant, antihypertensive, anti-inflammatory, anticancer, and antibacterial [54]. In addition, owing to the stiffness of the cell wall, not all proteins will be accessible when ingesting microalgae in their whole. Thus, microalgal protein hydrolysates will enhance the bioavailability of proteins/peptides/amino acids, enhancing their value as food or nutraceutical constituents [55].
3.5. Carbohydrates
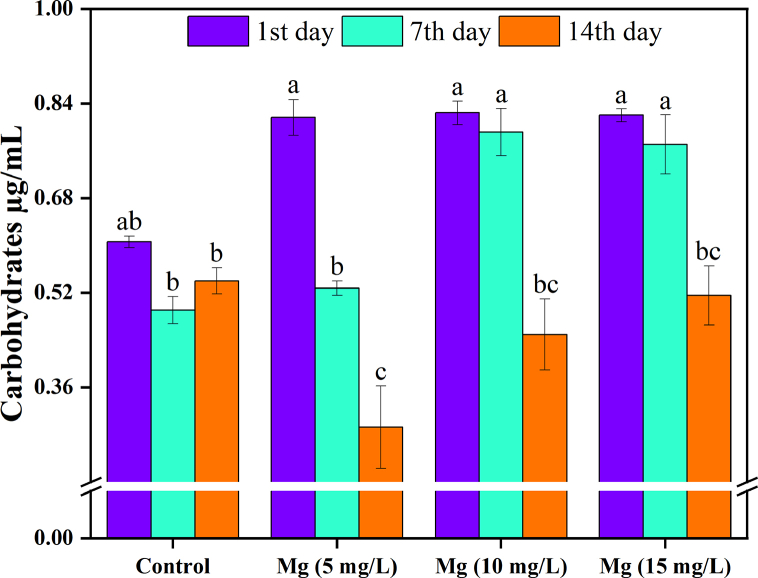
The most prevalent carbohydrate in C. vulgaris is starch. It is often found in the chloroplast and is made of amylose and amylopectin. Along with carbohydrates, it serves as the cell's energy reserve. Cellulose is a highly resistant structural polysaccharide that serves as a protective fibrous barrier on the cell wall of C. vulgaris. Moreover, one of the most significant polysaccharides in C. vulgaris is p1 3 glucan, which has many health and nutritional advantages [56]. The maximum value of carbohydrates was 0.824 μg/mL during the 1st day with 10 mg/L Mg2+, while the minimum was 0.293 μg/mL during the 14th day with 15 mg/L Mg2+ (Fig. 6).
Fig. 6.
C. vulgaris carbohydrates concentration in different Mg2+ concentrations.
For Mg2+ treatment, the results showed that carbohydrates decreased significantly by 15 mg/L on the 14th day compared to the control. At the same time, there was no significant change in carbohydrates in control and other treatments, and that was agreed that increased Mg2+ did not affect carbohydrate production.
Growth of Chlorella vulgaris under alkaline conditions increases the lipid and carbohydrate content, as cells accumulate oils as a defense mechanism against stress conditions [57]. The culture that removed Mg2+ could increase the lipid content of Chlorella protothecoides UTEX 250 from 4.4% to 9.5%, and a heavy metal ion was helpful for the increase of some of the microalgae's lipid content [58]. The micro-organism showed a preference for autotrophic growth even in the presence of glucose. Later on, during the experiment, there was no evidence for mixotrophic growth, although this could not be excluded either.
3.6. SOD
The maximum value of SOD was 0.884 unit/L during the 1st day with 15 mg/L Mg2+, while the minimum was 0.073 unit/L during the 14th day with 15 mg/L Mg2+ (Fig. 7). Usually, the synthesis of oxidant-antioxidant molecules is balanced within the cell; if oxidant production is increased due to stressful conditions, the cell avoids the harmful effect of cumulative oxidant molecules by overconsumption of antioxidant compounds [59]. The results showed that SOD concentrations decreased with the time of the control Mg2+ experiments, with the exception of 15 mg/L Mg2+ treatment on the 14th day.
Fig. 7.
C. vulgaris SOD concentration in different Mg2+concentrations.
The role of Mg2+ ions in activating the enzyme acetyl-CoA carboxylase, catalyzing the first step of fatty acid biosynthesis, is well established [60], and fatty acid synthesis has a very high requirement for Mg2+ ions.
3.7. ROS
ROS works as an indicator molecule for activating acclimatory/protection responses through transduction pathways, environmental stress such as a high concentration of Mg2+ induces excess ROS that can injure algal cells by oxidation of cellular components such as proteins, inactivate metabolic enzymes, DNA and lipids. As a result, defenses against ROS are activated by an array of nonenzymatic antioxidants, such as SOD work together for the detoxification of ROS [61].
The maximum value of ROS was 3.627 mu/mL during the 1st day with 5 and 15 mg/L Mg2+, while the minimum was 1.674 mu/mL during the 14th day with 10 mg/L Mg2+. ROS results showed a slight decrease with the time for all Mg2+ treatments (Fig. 8). The antioxidant action of protein-rich Chlorella compounds is supplied by functional groups or amino acid residues. The antioxidant activity of C. vulgaris in acetone was 57.25 mg/L, indicating that it may serve as a scavenger of free radicals and reduce the quantity of reactive oxygen species (ROS) [62]. Due to its instability and susceptibility to heat, oxygen, pH, and acid destruction, the antioxidant from Chlorella is susceptible to color change [63]. C. vulgaris is often used in cosmetics because it contains bioactive compounds such as chlorophyll, which as an antioxidant, are able to reduce the ROS content [64].
Fig. 8.
C. vulgaris ROS concentration in different Mg2+ levels.
3.8. CAT
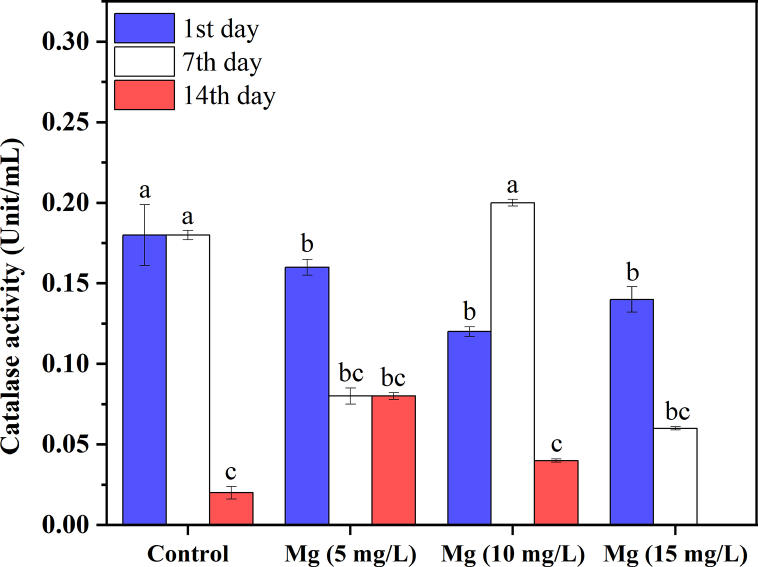
The maximum value of CAT was 0.200 unit/mL during the 7th day with 10 mg/L Mg2+, while the minimum was 0.010 unit/mL during the 14th day with 15 mg/L Mg2+. The CAT decreased with the time of Mg2+ treatments and control, except on the 7th day of Mg2+ treatment with 10 mg/L (Fig. 9).The mechanism of Mg2+ bioaccumulation results from the combination of metabolic processes and mass transfer. The hydrodynamics govern the external transport of Mg2+ from the solvent to the algae, whilst the physiology of the cells and their growth restrictions govern the biochemical processes (light). A higher amount of development would provide a wider surface area across which transfers may occur. Chlorella has a thick cellulose wall; hence, proteins derived from whole chlorella cells provide poor human outcomes. Enzymatic hydrolysis has been proposed as a promising technique for enhancing the digestibility of proteins.
Fig. 9.
C. vulgaris CAT concentration in different Mg2+ levels.
The activity of catalase (CAT) decreased with the increase in concentrations of Mg2+ as a defiance mechanism and CAT change in the green microalga, which may be attributable to the inhibition of CAT enzyme synthesis or the change in the assembly of enzyme subunits at an extremely high concentration of metals [65].
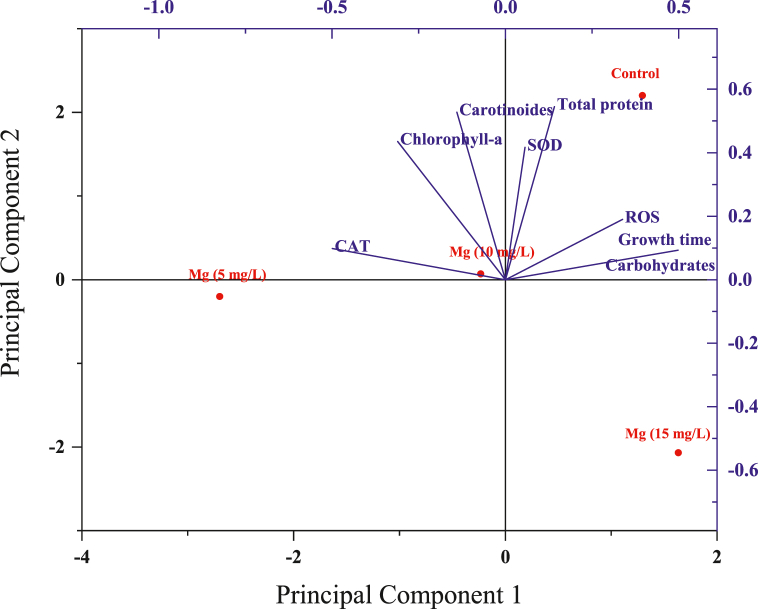
Using principal component analysis (PCA), which is beneficial for identifying patterns within the specie viability data, the relationship between the measured parameters and C. vulgaris was examined. The effects of the applied magnesium treatments on different physiological and biochemical parameters (carbohydrates, growth time, carotinoides, chlorophyll-a, total protein, CAT, SOD and ROS) have been plotted (Fig. 10). Principal component analysis showed that the first two components altogether accounted for 86.87% of the total variation. PC1 explained 48.67% of the variance for different concentrations of Mg2+ exposure, whereas PC2 covered 38.20% of the variance. The PCA analysis confirmed and illustrated well our detailed results, such as (i) the inhibitory effect of magnesium ion at the higher level on the different physiological and biochemical parameters of C. vulgaris, and (ii) its optimal concentration, which is close to the ‘control’ conditions. This could explain that proteins transporting magnesium are required to recognize the large hydrated cation, strip off its hydration shell and deliver the bare (i.e. dehydrated) ion to the transmembrane transport pathway through the membrane.
Fig. 10.
PCA biplot for various parameters measured for exposed C. vulgaris to different Mg2+ concentrations.
4. Conclusion
This research aimed to determine how C. vulgaris altered and its effect by magnesium supplementation. In the present study, we confirmed the possibility of magnesium bioaccumulation by C. vulgaris in a batch system and determined the effects of Mg2+ on biomass and biochemical properties and repartition of the metal ions between the cell wall and the cell interior; however, the rate of accumulation can be improved before possible industrial applications. The value of prospects for this microalga for various applications is presented about its biochemical components. The mechanism of Mg2+ bioaccumulation results from the combination of metabolic processes and mass transfer. The hydrodynamics govern the external transport of Mg2+ from the solvent to the algae, whilst the physiology of the cells and their growth restrictions govern the biochemical processes. Environmental stress, such as high concentration of Mg2+, induces excess ROS that can injure algal cells by oxidation of cellular components such as proteins, inactivating metabolic enzymes, DNA and lipids. As a result, defenses against ROS are activated by an array of nonenzymatic antioxidants, such as SOD work together for the detoxification of ROS. This work provides encouraging findings for the industrial development of C. vulgaris cultures with excellent cell output and Mg2+ ion absorption capacity. Autotrophic development removal of Mg2+ ions from the growing medium by biomass was directly proportional to cell concentration and physiology.
Author contribution statement
Jasim M. Salman: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper. Ruqayah Ali Grmasha: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Csilla Stenger-Kovács: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper. Edina Lengyel: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Osamah J. Al-sareji: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Ahed M. A. AR. AL-Cheban: Analyzed and interpreted the data; Performed the experiments. Mónika Meiczinger: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Ruqayah Ali Grmasha was supported by New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation [ÚNKP-22-3-I-PE-5], Stipendium Hungaricum Scholarship [gk7don].
Data availability statement
No data was used for the research described in the article.
Declaration of interest's statement
The authors declare no conflict of interest.
Acknowledgement
We are grateful to the anonymous reviewers and the Editor for improving the paper's quality.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e13072.
Contributor Information
Ruqayah Ali Grmasha, Email: ruqayah.grmasha@unswalumni.com, ruqayah.grmasha@uobabylon.edu.iq.
Osamah J. Al-sareji, Email: osamah.al-sareji@unswalumni.com, eng.osama.jaber@uobabylon.edu.iq.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Granéli E. Algal growth potential and limiting nutrients for phytoplankton production in Öresund water of Baltic and Kattegat origin. Limnologica. 1984;15(2):563–569. [Google Scholar]
- 2.Van Krimpen M.M., Bikker P., Van der Meer I.M., Van der Peet-Schwering C.M.C., Vereijken J.M. Cultivation, Processing and Nutritional Aspects for Pigs and Poultry of European Protein Sources as Alternatives for Imported Soybean Products. Wageningen UR Livestock Research; 2013. No. 662) [Google Scholar]
- 3.Sabzi S., Mehrgan M.S., Islami H.R., Shekarabi S.P.H. Changes in biochemical composition and fatty acid accumulation of Nannochloropsis oculata in response to different iron concentrations. Biofuels. 2018 doi: 10.1080/17597269.2018.1489672. [DOI] [Google Scholar]
- 4.Al-Hussieny A.A., Hussein H.T., Hmood A.H. Increase algae culture by using various ways by different media culture. J. Coll. Basic Educ. 2014;20(84):121–143. [Google Scholar]
- 5.Hiwase P.D., Hajare H.V. Review of environmental assessment methods. Int. J. Res. Eng. Sci. Technol. 2017;3(5):7–10. [Google Scholar]
- 6.El-Sheekh M.M., Salman J.M., Grmasha R.A., Abdul-Adel E., Saleh M.M., Al-sareji O.J. Biomass Conversion and Biorefinery; 2022. Influence of Fe+ 2 on the Biomass, Pigments, and Essential Fatty Acids Of Arthrospira Platensis; pp. 1–9. [Google Scholar]
- 7.Rizwan M., Mujtaba G., Memon S.A., Lee K., Rashid N. Exploring the potential of microalgae for new biotechnology applications and beyond: a review. Renew. Sustain. Energy Rev. 2018;92:394–404. [Google Scholar]
- 8.Lafarga T., Acién G. Microalgae for the food industry: from biomass production to the development of functional foods. Foods. 2022;11(5):765. doi: 10.3390/foods11050765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chia M.A., Lombardi A.T., MelãO M.D. Growth and biochemical composition of Chlorella vulgaris in different growth media. An Acad. Bras Ciências. 2013;85:1427–1438. doi: 10.1590/0001-3765201393312. [DOI] [PubMed] [Google Scholar]
- 10.Salman J.M., Grmasha R.A., Al-sareji O.J. AIP Conference Proceedings. Vol. 2386. AIP Publishing LLC; 2022, January. Potential efficiency of macroalgae Cladophora sp. to remove toxic industrial dye (safranin O) from aqueous solution; p. 20048. [Google Scholar]
- 11.Kassim T.I., Sabri A.W., Al‐Lami A.A., Abood S.M. The impacts of sewage treatment plant on phytoplankton of Diyala and Tigris Rivers. J. Environ. Sci. Health, Part A. 1996;31(5):1067–1088. [Google Scholar]
- 12.Priya A.K., Jalil A.A., Vadivel S., Dutta K., Rajendran S., Fujii M., Soto-Moscoso M. Heavy metal remediation from wastewater using microalgae: recent advances and future trends. Chemosphere. 2022;305 doi: 10.1016/j.chemosphere.2022.135375. [DOI] [PubMed] [Google Scholar]
- 13.Bishop W.M., Zubeck H.M. Evaluation of microalgae for use as nutraceuticals and nutritional supplements. J. Nutr. Food Sci. 2012;2(5):1–6. [Google Scholar]
- 14.Bito T., Okumura E., Fujishima M., Watanabe F. Potential of Chlorella as a dietary supplement to promote human health. Nutrients. 2020;12(9):2524. doi: 10.3390/nu12092524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alam T., Najam L., Al-Harrasi A. Extraction of natural pigments from marine algae. J. Agric. Marine Sci. 2018;23(1):81–91. [Google Scholar]
- 16.Khani M., Soltani M., Mehrjan M.S., Foroudi F., Ghaeni M. The effect of Chlorella vulgaris (Chlorophyta, Volvocales) microalga on some hematological and immune system parameters of Koi carp (Cyprinus carpio) Iran. J. Ichthyol. 2017;4(1):62–68. [Google Scholar]
- 17.Ayed H.B.A.B., Taidi B., Ayadi H., Pareau D., Stambouli M. Effect of magnesium ion concentration in autotrophic cultures of Chlorella vulgaris. Algal Res. 2015;9:291–296. [Google Scholar]
- 18.Yeesang C., Cheirsilp B. Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour. Technol. 2011;102(3):3034–3040. doi: 10.1016/j.biortech.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Donella M. Earth scan; London-UK: 2008. Thinking in Systems: A Primer; p. 33Pp. [Google Scholar]
- 20.Carvalho A.P., Silva S.O., Baptista J.M., Malcata F.X. Light requirements in microalgal photobioreactors: an overview of biophotonic aspects. Appl. Microbiol. Biotechnol. 2011;89(5):1275–1288. doi: 10.1007/s00253-010-3047-8. [DOI] [PubMed] [Google Scholar]
- 21.Ermis H., Guven-Gulhan U., Cakir T., Altinbas M. Effect of iron and magnesium addition on population dynamics and high value product of microalgae grown in anaerobic liquid digestate. Sci. Rep. 2020;10(1):1–12. doi: 10.1038/s41598-020-60622-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S., Zhao S.X., Wei C.L., Yu S.Y., Shi J.P., Zhang B.G. Effect of magnesium deficiency on photosynthetic physiology and triacylglyceride (TAG) accumulation of Chlorella vulgaris. Huan Jing ke Xue. 2014;35(4):1462–1467. [PubMed] [Google Scholar]
- 23.Barsanti L., Coltelli P., Evangelista V., Frassanito A.M., Passarelli V., Vesentini N. In: Algal Toxins: Nature, Occurrence, Effect and Detection. Evangelista V., Barsanti L., Frassanito A.M., editors. Springer; 2015. Oddities and curiosities in the algal world; pp. 353–3591Pp. [Google Scholar]
- 24.Huang L., Xu J., Li T., Wang L., Deng T., Yu X. Effects of additional Mg2+ on the growth, lipid production, and fatty acid composition of Monoraphidium sp. FXY-10 under different culture conditions. Ann. Microbiol. 2014;64(3):1247–1256. [Google Scholar]
- 25.Bleakley S., Hayes M. Algal proteins: extraction, application, and challenges concerning production. Foods. 2017;6(5):33. doi: 10.3390/foods6050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marzouk M.A., El-Sayed A.E.K., El-Gamal A.D. Differential growth of Amphora coffeaeformis under different treatments of nitrogen sources. Egy. J. Phycol. 2021;22(1):31–47. [Google Scholar]
- 27.Sánchez-Bayo A., Morales V., Rodríguez R., Vicente G., Bautista L.F. Cultivation of microalgae and cyanobacteria: effect of operating conditions on growth and biomass composition. Molecules. 2020;25(12):2834. doi: 10.3390/molecules25122834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen J.F. State transitions--a question of balance. Science. 2003;299(5612):1530–1532. doi: 10.1126/science.1082833. [DOI] [PubMed] [Google Scholar]
- 29.Ayed H.B.A.B., Taidi B., Ayadi H., Pareau D., Stambouli M. Magnesium uptake by the green microalga Chlorella vulgaris in batch cultures. J. Microbiol. Biotechnol. 2016;26(3):503–510. doi: 10.4014/jmb.1507.07039. [DOI] [PubMed] [Google Scholar]
- 30.Ayed H.B.A.B., Taidi B., Ayadi H., Pareau D., Stambouli M. The use of Chlorella vulgaris to accumulate magnesium under different culture conditions. J. Appl. Biotechnol. Bioeng. 2017;2(5):43–48. [Google Scholar]
- 31.Juneja A., Ceballos R.M., Murthy G.S. Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: a review. Energies. 2013;6(9):4607–4638. [Google Scholar]
- 32.Chacón‐Lee T.L., González‐Mariño G.E. Microalgae for “healthy” foods—possibilities and challenges. Compr. Rev. Food Sci. Food Saf. 2010;9(6):655–675. doi: 10.1111/j.1541-4337.2010.00132.x. [DOI] [PubMed] [Google Scholar]
- 33.Brennan L., Owende P. Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010;14(2):557–577. [Google Scholar]
- 34.Lam M.K., Yusoff M.I., Uemura Y., Lim J.W., Khoo C.G., Lee K.T., Ong H.C. Cultivation of Chlorella vulgaris using nutrients source from domestic wastewater for biodiesel production: growth condition and kinetic studies. Renew. Energy. 2017;103:197–207. [Google Scholar]
- 35.Soto-Sierra L., Wilken L.R., Mallawarachchi S., Nikolov Z.L. Process development of enzymatically-generated algal protein hydrolysates for specialty food applications. Algal Res. 2021;55 [Google Scholar]
- 36.Li J. Research progress on potassium, calcium and magnesium nutrients in plants. Fujian Sci. Technol. Rice Wheat. 2007;25:39–42. [Google Scholar]
- 37.Sarma S.J., Das R.K., Brar S.K., Le Bihan Y., Buelna G., Verma M., Soccol C.R. Application of magnesium sulfate and its nanoparticles for enhanced lipid production by mixotrophic cultivation of algae using biodiesel waste. Energy. 2014;78:16–22. [Google Scholar]
- 38.Leonardos N., Harris G.N. Comparative effects of light on pigments of two strains of emiliania huxleyi (haptophyta) 1. J. Phycol. 2006;42(6):1217–1224. [Google Scholar]
- 39.Clément‐Larosière B., Lopes F., Gonçalves A., Taidi B., Benedetti M., Minier M., Pareau D. Carbon dioxide biofixation by Chlorella vulgaris at different CO2 concentrations and light intensities. Eng. Life Sci. 2014;14(5):509–519. [Google Scholar]
- 40.Waghmare A.G., Salve M.K., LeBlanc J.G., Arya S.S. Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa. Biores. Bioproc. 2016;3(1):1–11. [Google Scholar]
- 41.Sehnal L., Váczi P., Barták M. Effect of temperature and increased concentration of CO2 on growth and photosynthetic activity of polar alga Trebouxia sp. Czech Polar Rep. 2014;4(1):47–56. [Google Scholar]
- 42.Vandamme D., Foubert I., Fraeye I., Meesschaert B., Muylaert K. Flocculation of Chlorella vulgaris induced by high pH: role of magnesium and calcium and practical implications. Bioresour. Technol. 2012;105:114–119. doi: 10.1016/j.biortech.2011.11.105. [DOI] [PubMed] [Google Scholar]
- 43.Wellburn A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994;144(3):307–313. [Google Scholar]
- 44.Salomon E.P., Berg L.R., Martin D.W. fifth ed. Saunders College Publishing; Fort Worth: 1999. Biology. [Google Scholar]
- 45.Ru I.T.K., Sung Y.Y., Jusoh M., Wahid M.E.A., Nagappan T. Chlorella vulgaris: a perspective on its potential for combining high biomass with high value bioproducts. Appl. Phycol. 2020;1(1):2–11. [Google Scholar]
- 46.Polat E., Yüksel E., Altınbaş M. Effect of different iron sources on sustainable microalgae-based biodiesel production using Auxenochlorella protothecoides. Renew. Energy. 2020;162:1970–1978. [Google Scholar]
- 47.Karemore A., Pal R., Sen R. Strategic enhancement of algal biomass and lipid in Chlorococcum infusionum as bioenergy feedstock. Algal Res. 2013;2(2):113–121. [Google Scholar]
- 48.El-Sayed A.B. Carotenoids accumulation in the green alga Scenedesmus sp. incubated with industrial citrate waste and different induction stresses. Nat. Sci. 2010;8(10):34–40. [Google Scholar]
- 49.Dierick N., Ovyn A., De Smet S. Effect of feeding intact brown seaweed Ascophyllum nodosum on some digestive parameters and on iodine content in edible tissues in pigs. J. Sci. Food Agric. 2009;89(4):584–594. [Google Scholar]
- 50.Becker W. In: Handbook of Microalgal Culture: Biotechnology and Applied Phycology. Richmond A., editor. Wiley-Blackwell; Hoboken, NJ, USA: 2004. Microalgae in human and animal nutrition; p. 312. [Google Scholar]
- 51.Kose A., Ozen M.O., Elibol M., Oncel S.S. Investigation of in vitro digestibility of dietary microalga Chlorella vulgaris and cyanobacterium Spirulina platensis as a nutritional supplement. 3 Biotech. 2017;7(3):1–7. doi: 10.1007/s13205-017-0832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lordan S., Ross R.P., Stanton C. Marine bioactives as functional food ingredients: potential to reduce the incidence of chronic diseases. Mar. Drugs. 2011;9(6):1056–1100. doi: 10.3390/md9061056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Almutairi A.W., El-Sayed A.E.K.B., Reda M.M. Combined effect of salinity and pH on lipid content and fatty acid composition of Tisochrysis lutea. Saudi J. Biol. Sci. 2020;27(12):3553–3558. doi: 10.1016/j.sjbs.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baalamurugan J., Kumar V.G., Chandrasekaran S., Balasundar S., Venkatraman B., Padmapriya R., Raja V.B. Utilization of induction furnace steel slag in concrete as coarse aggregate for gamma radiation shielding. J. Hazard Mater. 2019;369:561–568. doi: 10.1016/j.jhazmat.2019.02.064. [DOI] [PubMed] [Google Scholar]
- 55.Al‐Zuhair S., Ashraf S., Hisaindee S., Darmaki N.A., Battah S., Svistunenko D., Reeder B., Stanway G., Chaudhary A. Enzymatic pre‐treatment of microalgae cells for enhanced extraction of proteins. Eng. Life Sci. 2017;17(2):175–185. doi: 10.1002/elsc.201600127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson D.L., Cox M.M. Principles of Biochemistry. fourth ed. W. H. Freeman and Company; New York: 2008. Lipid biosynthesis; pp. 805–845. [Google Scholar]
- 57.Agustina S., Aidha N.N., Oktarina E. IOP Conference Series: Materials Science and Engineering. Vol. 1011. IOP Publishing; 2021. The extraction of antioxidants from Chlorella vulgaris for cosmetics. No. 1. [Google Scholar]
- 58.Kumar R.R., Rao P.H., Subramanian V.V., Sivasubramanian V. Enzymatic and non-enzymatic antioxidant potentials of Chlorella vulgaris grown in effluent of a confectionery industry. J. Food Sci. Technol. 2014;51(2):322–328. doi: 10.1007/s13197-011-0501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morris H.J., Carrillo O.V., Almarales Á., Bermúdez R.C., Alonso M.E., Borges L., Quintana M.M., Fontaine R., Llauradó G., Hernández M. Protein hydrolysates from the alga Chlorella vulgaris 87/1 with potentialities in immunonutrition. Biotecnol. Apl. 2009;26(2):162–165. [Google Scholar]
- 60.Cunha S.A., Coscueta E.R., Nova P., Silva J.L., Pintado M.M. Bioactive hydrolysates from chlorella vulgaris: optimal process and bioactive properties. Molecules. 2022;27(8):2505. doi: 10.3390/molecules27082505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sarker U., Oba S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018;8(1):1–12. doi: 10.1038/s41598-018-34944-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Safafar H., Uldall Nørregaard P., Ljubic A., Møller P., Løvstad Holdt S., Jacobsen C. Enhancement of protein and pigment content in two Chlorella species cultivated on industrial process water. J. Mar. Sci. Eng. 2016;4(4):84. [Google Scholar]
- 63.Griffiths M.J., van Hille R.P., Harrison S.T. The effect of nitrogen limitation on lipid productivity and cell composition in Chlorella vulgaris. Appl. Microbiol. Biotechnol. 2014;98(5):2345–2356. doi: 10.1007/s00253-013-5442-4. [DOI] [PubMed] [Google Scholar]
- 64.Samek D., Mišurcová L., Machů L., Buňková L., Minařík A., Fišera M. Whole-cell protein profiles of disintegrated freshwater green algae and cyanobacterium. J. Aquat. Food Prod. Technol. 2016;25(1):15–23. [Google Scholar]
- 65.Qiu C., Wang W., Zhang Y., Zhou G.J., Bi Y. Response of antioxidant enzyme activities of the green microalga Chlorococcum sp. AZHB to Cu2+ and Cd2+ stress. Sustainability. 2022;14(16) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.