Abstract
The assembly state of enzymes is gaining interest as a mechanism for regulating the function of enzymes in living cells. One of the current topics in enzymology is the relationship between enzyme activity and the assembly state due to liquid–liquid phase separation. In this study, we demonstrated enzyme activation via the formation of enzyme assemblies using L-lactate oxidase (LOX). LOX formed hundreds of nanometer-scale assemblies with poly-L-lysine (PLL). In the presence of ammonium sulfate, the LOX-PLL clusters formed micrometer-scale liquid droplets. The enzyme activities of LOX in clusters and droplets were one order of magnitude higher than those in the dispersed state, owing to a decrease in KM and an increase in kcat. Moreover, the clusters exhibited a higher activation effect than the droplets. In addition, the conformation of LOX changed in the clusters, resulting in increased enzyme activation. Understanding enzyme activation and assembly states provides important information regarding enzyme function in living cells, in addition to biotechnology applications.
Subject terms: Enzymes, Biochemistry, Molecular biology
Introduction
Enzymes are observed in various assembly states in living systems. For example, enzymes exist in aggregate1,2, fibril3,4, and condensate5,6 states in response to stress. Hence, it is assumed that the function of enzymes is precisely controlled by their assembly states. However, there is no unified view of the driving force of these assemblies and their effect on enzyme activity7. This may be because historically, several in vitro enzymatic studies have been performed under conditions that do not promote enzyme assembly, such as using diluted and purified enzymes.
In the last decade, liquid–liquid phase separation (LLPS) is one of the subjects of debate in cell biology8,9. LLPS is a phenomenon in which a one-phase solution changes to a two-phase solution according to thermodynamic equilibrium8,9. Liquid droplets formed by LLPS can regulate enzyme activities10–12. Extensive studies on intercellular droplets reveals the importance of intrinsically disordered proteins (IDPs) or nucleic acids in droplet formation13–15. IDPs comprise intrinsically disordered regions (IDRs) that lack a fixed three-dimensional structure16. Some enzymes interact favorably with IDPs and nucleic acids, known as droplet scaffold molecules. For example, RubisCO, a well-known enzyme of more than 500 kDa for carbon dioxide fixation, functions by forming droplets with a small IDP17. Multienzyme assemblies that activate multistep reactions also exhibit liquid-like properties and require IDP domains18. Furthermore, several metabolic enzymes have RNA-binding ability19,20, which are prone to forming droplets. Numerous in vitro studies have revealed that enzymes such as ribozyme21, kinase12,22, multienzyme complexes23, RNA polymerase, and ribosomes24 are activated in the formation of droplets. The droplets in these studies range in size from a few to tens of µm, making them easy to detect using an optical microscope. Moreover, several in vivo and in vitro studies report the presence of submicron-sized clusters as a precursor to micron-sized droplets25–27. Thus, the relationship between the assembly state and biological functions, such as enzymatic activation, is therefore of interest, however, this has not been demonstrated experimentally.
In this study, we investigated the relationship between assembly state and enzyme activity using L-lactate oxidase (LOX) as a model. Poly-L-lysine (PLL), which mimics the intrinsically disordered region of IDP28, was used as a scaffold molecule that electrostatically interacts with LOX. The addition of small amounts of salt (< 100 mM) has been reported to promote droplet formation, presumably by modulating electrostatic interactions29. Furthermore, kosmotropic salts tend to promote droplet formation more efficiently29. When we investigated effective salts for LOX droplet formation, ammonium sulfate (NH4)2SO4, a kosmotropic salt, caused LOX droplets to form most efficiently as expected (Supplementary Fig. S1). Thus, in this study, we employed (NH4)2SO4 salt to stabilize droplets. In the presence of PLL and absence of (NH4)2SO4, LOX formed a soluble hundreds-nm-scale assembly, called “clusterlox.” The addition of a small amount of (NH4)2SO4 formed several visible droplets in the scale of micrometers. The activities of both the assembly states were one order of magnitude higher than that of the enzyme in the dispersed state. The clusterslox and droplets have the common features of increasing kcat and decreasing KM, however, the activation effect was higher for clusterslox than for droplets.
Results
LOX assembly states in the presence of PLL and ammonium sulfate
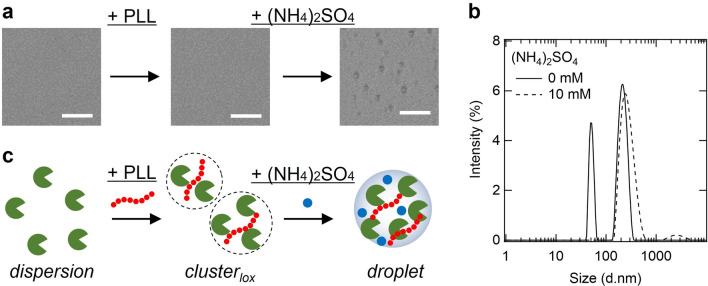
We investigated the droplet formation conditions of LOX by adding scaffold molecules and salts (Fig. 1a). LOX has an isoelectric point at approximately pH 6 (calculated); hence, it is negatively charged at the physiological pH. PLL was used as a scaffold molecule for the formation of droplets with LOX because it has an isoelectric point at approximately pH 10 (calculated), and is a disordered structure at physiological pH30. It has been shown that salts play an important role in the formation of droplets via electrostatic interactions using synthetic polymers29. We investigated the effects of (NH4)2SO4 on the formation of LOX-PLL droplets because kosmotropic salts promote droplet formation30. Microscopic images 1 h after solution preparation showed no assembly in the presence of 0.1 µM LOX and 0.02 mM PLL, whereas droplets were observed in the presence of 0.1 µM LOX, 0.02 mM PLL, and 10 mM (NH4)2SO4 (Fig. 1a). We further investigated the effects of the salts on LOX-PLL droplets (Supplementary Fig. S1). In the presence of 25 mM sodium chloride (NaCl) and sodium thiocyanate (NaSCN), LOX-PLL did not form droplets. With increasing concentrations of NaCl and NaSCN, aggregate-like assemblies were observed above 100 mM. In contrast, in the presence of 25 mM sodium sulfate (Na2SO4) and (NH4)2SO4, LOX-PLL droplets were observed (Supplementary Fig. S1). These data indicate that a small amount of sulfate ions stabilizes the LOX-PLL droplets via the kosmotropic effect, which is consistent with a previous study29. We further investigated the mixing ratios of LOX and PLL (Supplementary Fig. S2 and S3) in the presence of (NH4)2SO4, indicating that 5 µM LOX and 1 mM PLL formed well-shaped and stable droplets in the presence of 5–20 mM (NH4)2SO4. Under these conditions, the droplets contained approximately 90% of the LOX in the solution (Supplementary Fig. S4).
Figure 1.
LOX assembly states in the presence of PLL and (NH4)2SO4. (a) Microscopy images of 0.1 µM LOX, 20 mM Tris–HCl (pH 8), 0 or 0.02 mM PLL (concentrations refer to lysine monomer units), 0 or 10 mM (NH4)2SO4. Scale bar, 10 µm (b) DLS data of sample solutions containing 0.1 µM LOX, 0.02 mM PLL, 20 mM Tris–HCl (pH 8), with 0 or 10 mM (NH4)2SO4. (c) Schematic image of assembly states of LOX with PLL and (NH4)2SO4.
Additionally, the presence of submicron-sized assemblies was investigated using dynamic light scattering (DLS) (Fig. 1b), because the size of these assemblies was below the detection limit of the optical microscope (~ 400 nm). In the absence of (NH4)2SO4, 0.1 µM LOX and 0.02 mM PLL formed two types of assemblies with diameters of approximately 50–60 and 200–300 nm, respectively, which could not be observed under the microscope (Fig. 1a,b). Here, we define these submicron-sized assemblies formed in the absence of (NH4)2SO4 as “clusterslox” (Fig. 1c). However, 0.1 µM LOX and 0.02 mM PLL with 10 mM (NH4)2SO4 formed broad size of droplets with diameters of approximately 200–500 nm (Fig. 1a,b), which could be observed under the microscope. The larger peak of clusterlox overlapped with the peak of the droplet, suggesting that clusterlox formed a part of the droplet-like assembly.
Features of LOX-PLL droplets
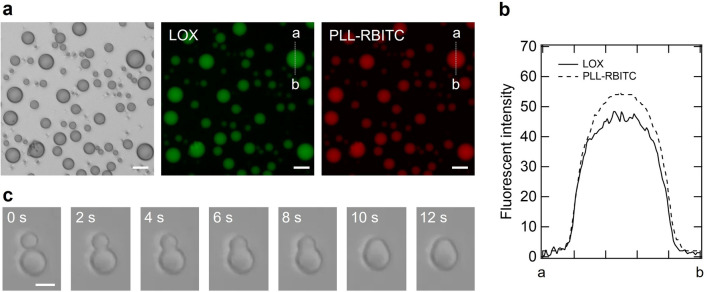
We observed the LOX-PLL droplets using bright-field and fluorescence microscopy. Figure 2 shows microscopic images of the sample containing 5 μM LOX, 1 mM PLL, and 6 mM ammonium sulfate at pH 8. PLL was monitored using chemically modified Rhodamine B isothiocyanate (RBITC) (red), and LOX was monitored using an intrinsic flavin mononucleotide (green). Bright-field microscopic images showed droplets with spherical structures and diameters of 10 µm or less (Fig. 2a), indicating the typical appearance of liquid droplets. Fluorescence microscopy revealed that the droplets contained both LOX and PLL molecules uniformly, as indicated by green and red fluorescence (Fig. 2b). Furthermore, the LOX-PLL droplets coalesced in approximately 10 s (Fig. 2c), indicating that the LOX-PLL droplets have liquid-like fluidity. This coalescence also seems to occur in small droplets formed with 0.1 µM LOX because the particle size increased in solution with time (Supplementary Fig. S5). Moreover, because clusterslox also tended to increase in particle size, it can be assumed that they have the same properties as droplets (Supplementary Fig. S5). To investigate the contribution of the electrostatic interaction between LOX and PLL in the formation of LOX-PLL droplets, we determined the effect of pH on the formation of LOX-PLL droplets (Supplementary Fig. S6). At pH 5, only amorphous aggregates were observed. At pH 6 and 7, both amorphous aggregates and liquid droplets were seen. At pH 8 and 9, however, only liquid droplets were observed. These results demonstrate that the electrostatic interaction between LOX and PLL plays a vital role in forming the liquid droplets because LOX and PLL have isoelectric points around pH 6 and 10, respectively.
Figure 2.
Features of LOX-PLL droplets. (a) Bright-field microscopic images of droplets (left) and fluorescent microscopic images of LOX (middle) and PLL-RBITC (right). The solution contained 5 μM LOX, 1 mM PLL, 6 mM (NH4)2SO4, 20 mM Tris–HCl, and 20 mM MES (pH 8). Scale bar, 20 μm. (b) Localization of LOX and PLL-RBITC in the droplet. Fluorescence intensity along the dashed white lines was quantified from the brightness of each pixel. (c) Bright-field microscopic images of coalescing LOX-PLL droplets. The solution contained 5 μM LOX, 1 mM PLL, 6 mM (NH4)2SO4, 20 mM Tris–HCl, and 20 mM MES (pH 8). Scale bar, 10 μm.
LOX activation in clusterslox and droplets
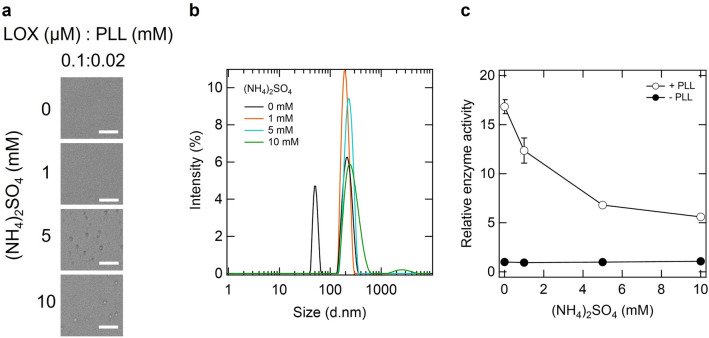
We investigated changes in the assembly state and LOX activity depending on the concentration of ammonium sulfate. Microscopic images showed the formation of several micrometer-scale droplets above approximately 5 mM ammonium sulfate (Fig. 3a). Next, we investigated the presence of the LOX assembly using DLS measurements (Fig. 3b). Two peaks appeared in the absence of ammonium sulfate, as shown in Fig. 1c; the addition of 1 mM (NH4)2SO4 changed these two peaks into one large peak. With an increase in the concentration of (NH4)2SO4, this large peak shifted to the right and broadened (Fig. 3b). This result indicates that the size of the assembly increased with increasing ammonium sulfate concentration, which is consistent with the microscopic results (Fig. 3a). Under these conditions, we investigated how the enzyme activity of LOX changed depending on ammonium sulfate concentration (Fig. 3c). In the absence of PLL, the activity slightly increased depending on the ammonium sulfate concentration. In the presence of PLL and absence of ammonium sulfate, that is, in clusterlox, the enzyme activity of LOX was approximately 15 times higher (Fig. 3c). Although the activation rate decreased as the concentration of ammonium sulfate increased, it remained approximately five times more active in the presence of 10 mM ammonium sulfate (Fig. 3c). Furthermore, we confirmed that removing clusterslox and droplets from solutions by centrifugation impaired LOX activity (Supplementary Fig. S7). The findings revealed that clusterslox and droplets both increased LOX enzyme activity, with clusterslox being more favorable for activation than droplets. The activating effects of LOX in the droplets and clusterlox remained almost unchanged after 2 h and were retained at approximately 1 and sixfold for droplets and clusterslox, respectively, even after 24 h (Supplementary Fig. S8).
Figure 3.
Assembly state and activity of LOX depend on the concentration of ammonium sulfate. (a) Bright-field microscopic images of enzyme assemblies. The solution contained 0.1 μM LOX, 0.02 mM PLL, 0–10 mM (NH4)2SO4, and 20 mM Tris–HCl (pH 8). Scale bar, 20 μm. (b) DLS data of sample solution containing 0.1 µM LOX, 0.02 mM PLL, and 20 mM Tris–HCl (pH 8), with 0–10 mM (NH4)2SO4. (c) Enzymatic activity of LOX in the presence of PLL and ammonium sulfate. Relative enzyme activity was defined as the initial reaction velocity in each condition divided by that in the absence of PLL and (NH4)2SO4.
Kinetic analysis of LOX in clusterslox and droplets
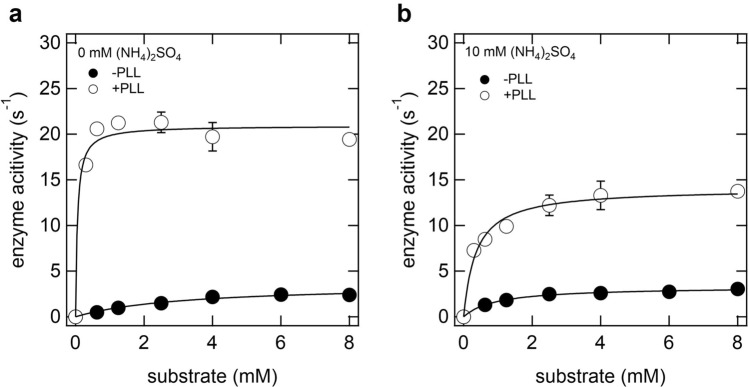
To elucidate the detailed mechanism of enzyme activation by the formation of clusterslox and droplets, we determined the kinetic parameters of LOX in 20 mM Tris–HCl (pH 8.0) at 25 °C (Fig. 4 and Table 1). The KM of LOX in clusterslox was 0.05 mM, approximately 64-fold smaller than that of LOX alone, indicating that clusterlox is favorable for binding between LOX and its substrate (Fig. 4a). Additionally, the kcat of LOX in clusterslox was approximately sixfold higher than that in the dispersed state, indicating that the turnover number of LOX increased in clusterslox (Fig. 4a). Next, the KM of LOX-PLL in the droplet was 0.36 mM, approximately threefold smaller than that without PLL, indicating that the droplet is also favorable for binding between LOX and its substrate (Fig. 4b). The kcat value of LOX in the droplets was approximately fourfold higher than that in the dispersed state (Fig. 4b). These results showed that the mechanism of enzyme activation in clusterslox and droplets is common; the synergistic effect of an increase in substrate affinity and catalytic turnover; however, clusterslox are more effective than droplets for LOX activation.
Figure 4.
Enzyme kinetics of LOX in clusterslox. (a) and droplets (b). The sample solution contained 0.1 µM LOX, 0 or 0.02 mM PLL, 0–8 mM L-lactic acid, 20 mM Tris–HCl (pH 8), and 0 or 10 mM (NH4)2SO4.
Table 1.
Michaelis–Menten parameters for LOX in different assembly states.
| Assembly state | 0.02 mM PLL | 10 mM (NH4)2SO4 | kcat (s-1) | KM (mM) | kcat/KM |
|---|---|---|---|---|---|
| Disperse | – | – | 3.58 ± 0.34 | 3.20 | 1.13 |
| Disperse | – | + | 3.29 ± 0.09 | 0.97 | 3.40 |
| Clusterslox | + | – | 20.92 ± 0.75 | 0.05 | 418.36 |
| Droplets | + | + | 14.06 ± 0.54 | 0.36 | 39.06 |
Secondary structure of LOX in clusterslox
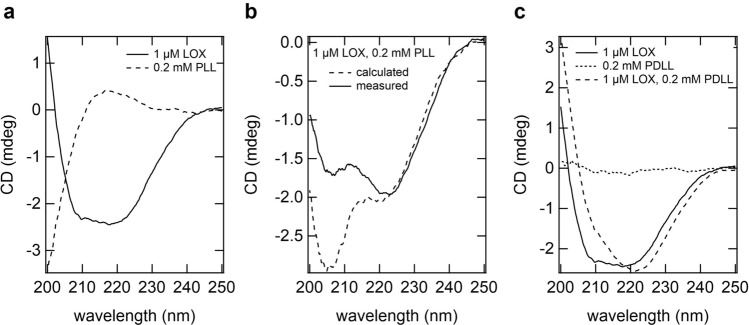
The increase in kcat may have resulted from the conformational change in LOX within clusterslox and droplets. Thus, we investigated the secondary structure of LOX via far-ultraviolet (UV) circular dichroism (CD) spectroscopy. The far-UV CD spectra of LOX showed negative peaks at 208 and 218 nm, whereas the PLL spectra showed positive peaks between 210 and 230 nm (Fig. 5a). For samples forming clusterslox, the CD spectra of the mixture of LOX and PLL did not match those calculated from the individual spectra of LOX and PLL alone (Fig. 5b). These findings indicated that some structural changes in LOX and PLL were induced by their interactions.
Figure 5.
Far-UV CD spectra of LOX and PLL in clusterslox. (a) CD spectra of 1 μM LOX (solid line) and 0.2 mM PLL (broken line). (b) CD spectrum of LOX and PLL mixture (solid line), and CD spectrum calculated from that of LOX and PLL in A (broken line). (c) CD spectra of PDLL, LOX and LOX with PDLL.
PLL and LOX alone showed large far-UV CD ellipticity (Fig. 5a). Thus, we employed poly-(D,L)-lysine (PDLL), which also induces LOX droplets and activation (Supplementary Fig. S9) but is achiral in CD measurements to detect subtle changes in the secondary structure of LOX (Fig. 5c). As expected, PDLL showed almost no far-UV CD signal owing to its lack of optical activity (Fig. 5c). The spectrum of LOX drastically changed in the presence of 0.2 mM PDLL. Notably, the intensity of LOX at 210 nm was higher than that in the absence of PDLL. These results showed that a conformational change in LOX was induced in clusterslox, which may have caused an increase in kcat. For samples containing droplets, the CD spectra could not be obtained stably within the measurement time, probably due to droplet coalescence and sedimentation (data not shown).
Interaction between substrate and PLL
Polymers with opposite charges to the substrate reduce KM31. The substrate l-lactic acid is negatively charged, hence, positively charged PLL may contribute to the decrease in KM. Therefore, we investigated the interaction of PLL and L-lactate by isothermal titration calorimetry (ITC) and nuclear magnetic resonance (NMR). The titration of L-lactate into the PLL solution showed no significant changes during titration (Supplementary Fig. S10a), indicating that L-lactate did not bind to PLL. To further confirm the binding between L-lactate and PLL, 1H-NMR spectrum of L-lactate was analyzed in the presence and absence of PLL (Supplementary Fig. S10b). L-lactate alone was assigned by three peaks ranging from 1.22–1.24, 1.34–1.38, and 1.48–1.50 ppm. The addition of PLL to the L-lactate solution did not change the intensities or chemical shifts of the peaks. These findings revealed that L-lactate does not interact with PLL. Thus, clusterlox and droplet play an essential role in increasing the affinity between the substrate and enzyme, rather than between the substrate and PLL.
Discussion and conclusions
In this study, we reported that the enzyme activity of LOX is increased by the formation of clusterslox and droplets induced by the addition of PLL. The mechanism of LOX activation may be derived from kinetic parameters (Fig. 4 and Table 1) and conformational properties (Fig. 5). The enzyme activity of LOX increased in both clusterslox and droplets, resulting from decreased KM and increased kcat. The kcat value of clusterslox was higher than that of the droplets. In addition, the degree of activation decreased as the droplet size increased (see Fig. 3). This suggests that the degree of activation may not be uniform throughout the droplet, such that the LOX activity is higher near the droplet surface. In contrast, the KM of the droplets decreased compared to the dispersed state, and it is likely that the compartmentalization effect of the substrate inside the droplets10. In addition to the compartmentalization effect, hundreds of nanometer-sized clusterslox are more dispersible in solution than several micrometer-sized droplets; therefore, they probably have a higher probability of collision with the substrate. This may be the reason the KM value of the clusterslox was smaller than that of the droplets.
The structural change of LOX in the clusterslox detected in CD is likely to be related to LOX activation, as LOX has been well investigated for its activity-structure relationship32–35. The LOX structure contains a core TIM barrel of eight α-helices and eight β-strands, binds FMN at the C-terminus of the β-barrel, and, importantly, has a very flexible loop and a short helix covering the active site32. Changes in this flexible loop structure and dynamics alter LOX activity by controlling substrate uptake and product release as shown in previous studies using mutations to this flexible loop33–35. The active site is open when this flexible loop adopts a disordered structure, and the active site is closed when it adopts a folded structure33–35. Specifically, in the crystal structure, α-helix decreases when active site is open compared to when active site is closed33–35. Thus, the decrease in α-helical content upon clusterslox formation may reflect structural changes and/or dynamics in the flexible loop covering the active site (Fig. 5). As no method has been established to investigate the conformational changes of folded enzymes in the enzyme assemblies, investigation of detailed conformational changes of LOX will be a future challenge.
Changes in enzyme structure and/or dynamics that accompany clusterlox and droplet formation can be attributed to the following possibilities. First, enzyme interaction with the polymer in clusterlox and droplets may change the structural stability of the native enzyme, leading to an increased kcat36,37. Second, the enzyme assemblies like droplets are highly crowded with macromolecules, resulting in the exclusion of water molecules38,39. This crowding environment may stabilize non-native structures that differ from those in dilute conditions40. Therefore, crowding may promote the transition state of the enzyme, leading to enhanced enzyme activity. Finally, droplets represent a nonpolar environment compared to a dilute solution39,41. A nonpolar solution increases the stability of hydrophobic interaction38,39,42, hydrogen bonds, and electrostatic interactions43, which change enzyme activity. Further structural analysis of the enzyme in clusterslox and droplets will be an interesting subject for future research.
The activation of LOX within the enzyme assemblies can provide valuable information for industrial applications. Currently, the primary methods of enzyme activation are protein engineering44 and directed evolution45. These methods generate favorable mutants with high activity through repeated mutagenesis and screening. However, it is time-consuming and costly to produce desirable mutants. In contrast, it is very simple to form an enzyme assembly using a polyelectrolyte to improve activity. LOX is an oxidoreductase applied to biofuel cells46,47 and biosensors48. Thus, the formation of enzyme assemblies represents a versatile approach for improving enzyme activity in practical applications using LOX or other enzymes.
Methods
Materials
Poly-L-lysine hydrobromide (MW, 70,000–150,000 Da), poly-(D,L)-lysine hydrobromide (MW, 25,000–40,000 Da), and 2,6-dichloroindophenol sodium salt hydrate (DCIP) were obtained from Sigma-Aldrich Co. (St Louis, MO, USA). NaCl, (NH4)2SO4, Na2SO4, NaSCN, and dimethyl sulfoxide (DMSO) were obtained from Kanto Chemical Co., Inc. (Tokyo, Japan). Tris(hydroxymethyl)aminomethane was obtained from Nacalai Tesque (Kyoto, Japan). 2-Morpholinoethanesulfonic acid monohydrate and 3-[4-(2-Hydroxyethyl)-1-piperazinyl] propanesulfonic acid (EPPS) were obtained from Dojindo Laboratories (Kumamoto, Japan). l-lactic acid was obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Rhodamine B isothiocyanate (RBITC) was obtained from Santa Cruz Biotechnology (Dallas, TX). LOX was prepared as previously described46.
Fluorescent labeling of PLL
PLL labeled with the amine-reactive dye RBITC (excitation/emission:555/580 nm) was prepared following the manufacturer’s instructions. Briefly, a solution of RBITC (1.77 mM) in DMSO (50 μL) was quickly added to a stirred solution of 20 mM PLL and 20 mM EPPS (950 μL; pH 8.5) at 25 °C. After the reaction mixture was gently stirred for 1.5 h, 200 mM Tris–HCl (100 μL, pH 8.5) was added. The PLL-dye conjugates were purified by filtration through Amicon Ultra-0.5 mL centrifugal filters with a molecular weight cutoff (MWCO) of 3 kDa (Millipore Sigma). The final concentration of PLL was determined using bicinchoninic acid (BCA) assay. The number of RBITC molecules conjugated to PLL in 10 mM Tris–HCl (pH 8.0) was determined from the absorbance at 556 nm, using the molar absorption coefficient ε556 = 87,000 M−1 cm−1. The number of dye molecules per PLL molecule was 0.2.
Enzyme assays
Enzyme solutions containing 0.1 μM LOX, 0–0.2 mM PLL, and 0–10 mM ammonium sulfate in 20 mM Tris–HCl (pH 8) were prepared and left standing for 20 min. A 90 μL aliquot of enzyme solution was mixed with a 10 μL aliquot of substrate solution containing 0–80 mM L-lactic acid and 1 mM DCIP solution. The initial reaction velocities (v0) were determined from the slope of the initial decrease in absorbance at 555 nm using an Ultrospec 2100 pro UV/visible spectrophotometer (Amersham Biosciences Corp, Amersham, UK). Relative enzyme activity was defined as the initial reaction velocity under each condition divided by that in the absence of PLL and (NH4)2SO4. The KM and kcat values were determined by the initial reaction velocity on a theoretical Michaelis–Menten curve by nonlinear regression.
Dynamic light scattering
Dynamic light scattering (DLS) experiments were performed using a Zetasizer Nano ZS light scattering photometer (Malvern Instruments, Worcestershire, UK) equipped with a 4 mW He–Ne ion laser (λ = 633 nm). To determine the size of LOX assemblies, solutions containing 20 nM LOX, 0–1 mM PLL, 0–10 mM (NH4)2SO4, and 20 mM Tris–HCl were placed in a 1-cm path length disposable cuvette, and DLS measurements were performed at 25 °C at a detection angle of 173°. The viscosities of the solutions were approximated using water (η = 0.87 cP). All measurements were performed in 15 min after the solution preparation to prevent a decrease in scattering intensity by the sedimentation of enzyme assemblies. All the results are presented as the mean values of three independent experiments.
Optical microscopy
Images were recorded using an all-in-one fluorescence microscope BZ-X710 (KEYENCE, Osaka, Japan) 1 h after sample preparations. Aliquots (100 μL) of the samples were placed in an ultra-low-attachment 96-well plate (Corning, NY, USA). All images were prepared using BZ-X Analyzer (KEYENCE).
Isothermal titration calorimetry
Isothermal Titration Calorimetry (ITC) was performed using a Microcal Auto-iTC200 calorimeter (Malvern Instruments). The experiments consisted of a series of 0.2 μL injections of 4 mM PLL into 200 μL of 200 μM LOX solution or 1 mM l-lactic acid in the thermostatic cell with an initial delay of 60 s, a 0.4 s duration of injection, and a spacing of 120 s between injections. In all cases, the samples were dialyzed in the same buffer containing 20 mM Tris–HCl and 20 mM MES (pH 8) to minimize interference from mixing and dilution heat signals.
Hydrogen-1 nuclear magnetic resonance spectroscopic analysis
Hydrogen-1 (1H) nuclear magnetic resonance (NMR) spectra were recorded in 20 mM Tris–HCl buffer at pH 8. The experiments were performed using a Bruker BioSpin Avance III 700 MHz NMR spectrometer at 25 °C.
Circular dichroism
Circular dichroism (CD) experiments were performed in a 1-cm path-length quartz cuvette using a spectropolarimeter (J-720 W; JASCO Co., Ltd). For clusterslox measurements, the enzyme solution containing 1 μM LOX and 20 mM Tris–HCl buffer (pH 8.0) was incubated with 0.2 mM PLL or 0.2 mM Poly-(D,L)-lysine at 25 °C for 20 min before measurement. The CD spectra of the samples were corrected by subtracting the corresponding spectra of buffers.
Precipitation rate of LOX
The formation of liquid droplets of LOX with PLL was investigated based on the precipitation rate of the LOX. The concentration dependence of the PLL on liquid droplet formation was measured as follows: The PLL stock solution was prepared using 0–2 mM PLL in 20 mM Tris–HCl (pH 8). Aliquots (100 μL) of various solutions containing 1 mM LOX in 20 mM Tris–HCl (pH 8) were mixed with 100 μL of the PLL stock solution. Furthermore, the samples were centrifuged at 18,000×g for 20 min at 25 °C. The concentration of LOX in the supernatant was determined from the absorbance at 280 nm using an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Precipitation rates were calculated as follows:
where C0 (mM) is the concentration in the supernatant without PLL and Cn (mM) is the LOX concentration in the supernatant with PLL.
Supplementary Information
Acknowledgements
This study is based on results obtained from a project, JPNP14004, commissioned by the New Energy and Industrial Technology Development Organization (NEDO). This work was also partially supported by JSPS KAKENHI (Grant No. 22H05437) to T. M., JSPS KAKENHI (Grant No. 18H02383) to K. S., JSPS Fellows (Grant No. 20J21734) and Leave a Nest grant Fundamental Biotechnology award to T.U. We would like to thank Editage (www.editage.com) for English language editing.
Author contributions
T.U., K.S., and T.M. conceived and designed the experiments. A.K. purified LOX. Y.H. performed NMR experiments and analyzed the data. T.U. performed most of the experiments and analyzed the data together with S.N. N.T., and T.K. contributed to the interpretation of results. T.U., K.S., and T.M. wrote the manuscript with input from all co-authors.
Data availability
The data supporting the findings of this study are available in the paper and Supplementary file. All other data are available from the corresponding authors upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kentaro Shiraki, Email: shiraki.kentaro.gb@u.tsukuba.ac.jp.
Tsutomu Mikawa, Email: mikawa@riken.jp.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-28040-1.
References
- 1.Saad S, et al. Reversible protein aggregation is a protective mechanism to ensure cell cycle restart after stress. Nat. Cell Biol. 2017;19:1202–1213. doi: 10.1038/ncb3600. [DOI] [PubMed] [Google Scholar]
- 2.Narayanaswamy R, et al. Widespread reorganization of metabolic enzymes into reversible assemblies upon nutrient starvation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:10147–10152. doi: 10.1073/pnas.0812771106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cereghetti G, et al. Reversible amyloids of pyruvate kinase couple cell metabolism and stress granule disassembly. Nat. Cell Biol. 2021;23:1085–1094. doi: 10.1038/s41556-021-00760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonet JC, Burrell AL, Kollman JM, Peterson JR. Freedom of assembly: Metabolic enzymes come together. Mol. Biol. Cell. 2020;31:1201–1205. doi: 10.1091/mbc.E18-10-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miura N, et al. Spatial reorganization of Saccharomyces cerevisiae enolase to alter carbon metabolism under hypoxia. Eukaryot. Cell. 2013;12:1106–1119. doi: 10.1128/EC.00093-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An S, Kumar R, Sheets ED, Benkovic SJ. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science. 2008;320:103–106. doi: 10.1126/science.1152241. [DOI] [PubMed] [Google Scholar]
- 7.Schmitt DL, An S. Spatial organization of metabolic enzyme complexes in cells. Biochemistry. 2017;56:3184–3196. doi: 10.1021/acs.biochem.7b00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin Y, Brangwynne CP. Liquid phase condensation in cell physiology and disease. Science. 2017;357:1253. doi: 10.1126/science.aaf4382. [DOI] [PubMed] [Google Scholar]
- 9.Banani SF, Lee HO, Hyman AA, Rosen MK. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017;18:285–298. doi: 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peeples W, Rosen MK. Mechanistic dissection of increased enzymatic rate in a phase-separated compartment. Nat. Chem. Biol. 2021;17:693–702. doi: 10.1038/s41589-021-00801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakashima KK, Vibhute MA, Spruijt E. Biomolecular chemistry in liquid phase separated compartments. Front. Mol. Biosci. 2019;6:21. doi: 10.3389/fmolb.2019.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ura T, Tomita S, Shiraki K. Dynamic behavior of liquid droplets with enzyme compartmentalization triggered by sequential glycolytic enzyme reactions. Chem. Commun. (Camb) 2021;57:12544–12547. doi: 10.1039/D1CC04596B. [DOI] [PubMed] [Google Scholar]
- 13.Oldfield CJ, Dunker AK. Intrinsically disordered proteins and intrinsically disordered protein regions. Annu. Rev. Biochem. 2014;83:553–584. doi: 10.1146/annurev-biochem-072711-164947. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro DM, Ney M, Eghtesadi SA, Chilkoti A. Protein phase separation arising from intrinsic disorder: first-principles to bespoke applications. J. Phys. Chem. B. 2021;25:6740–6759. doi: 10.1021/acs.jpcb.1c01146. [DOI] [PubMed] [Google Scholar]
- 15.Martin EW, Holehouse AS. Intrinsically disordered protein regions and phase separation: Sequence determinants of assembly or lack thereof. Emerg. Top Life Sci. 2020;4:307–329. doi: 10.1042/ETLS20190164. [DOI] [PubMed] [Google Scholar]
- 16.Uversky VN. Intrinsically disordered proteins in overcrowded milieu: Membrane-less organelles, phase separation, and intrinsic disorder. Curr. Opin. Struct. Biol. 2017;44:18–30. doi: 10.1016/j.sbi.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, et al. RuBisCO condensate formation by CcmM in β-carboxysome biogenesis. Nature. 2019;566:131–135. doi: 10.1038/s41586-019-0880-5. [DOI] [PubMed] [Google Scholar]
- 18.Jin M, et al. Glycolytic enzymes coalesce in G bodies under hypoxic stress. Cell Rep. 2017;20:895–908. doi: 10.1016/j.celrep.2017.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castello A, Hentze MW, Preiss T. Metabolic enzymes enjoying new partnerships as RNA-binding proteins. Trends Endocrinol. Metab. 2015;26:746–757. doi: 10.1016/j.tem.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckmann BM, et al. The RNA-binding proteomes from yeast to man harbour conserved enigmRBPs. Nat. Commun. 2015;6:10127. doi: 10.1038/ncomms10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strulson CA, Molden RC, Keating CD, Bevilacqua PC. RNA catalysis through compartmentalization. Nat. Chem. 2012;4:941–946. doi: 10.1038/nchem.1466. [DOI] [PubMed] [Google Scholar]
- 22.Koga S, Williams DS, Perriman AW, Mann S. Peptide-nucleotide microdroplets as a step towards a membrane-free protocell model. Nat. Chem. 2011;3:720–724. doi: 10.1038/nchem.1110. [DOI] [PubMed] [Google Scholar]
- 23.Crosby J, et al. Stabilization and enhanced reactivity of actinorhodin polyketide synthase minimal complex in polymer-nucleotide coacervate droplets. Chem. Commun. (Camb) 2012;48:11832–11834. doi: 10.1039/c2cc36533b. [DOI] [PubMed] [Google Scholar]
- 24.Sokolova E, et al. Enhanced transcription rates in membrane-free protocells formed by coacervation of cell lysate. Proc. Natl Acad. Sci. U. S. A. 2013;110:11692–11697. doi: 10.1073/pnas.1222321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forman-Kay JD, Ditlev JA, Nosella ML, Lee HO. What are the distinguishing features and size requirements of biomolecular condensates and their implications for RNA-containing condensates? RNA. 2022;28:36–47. doi: 10.1261/rna.079026.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kar M, et al. Phase-separating RNA-binding proteins form heterogeneous distributions of clusters in subsaturated solutions. Proc. Natl. Acad. Sci. U. S. A. 2022;119:e2202222119. doi: 10.1073/pnas.2202222119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penga S, Lia W, Yaoa Y, Xinga W, Lia P, Chen C, et al. Phase separation at the nanoscale quantified by dcFCCS. Proc. Natl. Acad. Sci. (PNAS) 2020;117(44):27124–27131. doi: 10.1073/pnas.2008447117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher RS, Elbaum-Garfinkle S. Tunable multiphase dynamics of arginine and lysine liquid condensates. Nat. Commun. 2020;11:4628. doi: 10.1038/s41467-020-18224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry SL, Li Y, Priftis D, Leon L, Tirrell M. The effect of salt on the complex coacervation of vinyl polyelectrolytes. Polymers. 2014;6:1756–1772. doi: 10.3390/polym6061756. [DOI] [Google Scholar]
- 30.Perry SL, et al. Chirality-selected phase behaviour in ionic polypeptide complexes. Nat. Commun. 2015;6:6052. doi: 10.1038/ncomms7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurinomaru T, Tomita S, Hagihara Y, Shiraki K. Enzyme hyperactivation system based on a complementary charged pair of polyelectrolytes and substrates. Langmuir. 2014;30:3826–3831. doi: 10.1021/la500575c. [DOI] [PubMed] [Google Scholar]
- 32.Leiros I, et al. The 2.1 Å structure of Aerococcus viridans L-lactate oxidase (LOX) Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006;F62:1185–1190. doi: 10.1107/S1744309106044678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiraka K, et al. Structure of lactate oxidase from Enterococcus hirae revealed new aspects of active site loop function: Product- inhibition mechanism and oxygen gatekeeper. Protein Sci. 2022;10:c4434. doi: 10.1002/pro.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoisser T, Brunsteiner M, Wilson DK, Nidetzky B. Conformational flexibility related to enzyme activity: evidence for a dynamic active-site gatekeeper function of Tyr(215) in Aerococcus viridans lactate oxidase. Sci. Rep. 2016;6:27892. doi: 10.1038/srep27892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoisser T, Klimacek M, Wilson DK, Nidetzky B. Speeding up the product release: A second-sphere contribution from Tyr191 to the reactivity of L-lactate oxidase revealed in crystallographic and kinetic studies of site-directed variants. FEBS J. 2015;282:4130–4140. doi: 10.1111/febs.13409. [DOI] [PubMed] [Google Scholar]
- 36.Thiele JM, et al. Enzyme–polyelectrolyte complexes boost the catalytic performance of enzymes. ACS Catal. 2018;8:10876–10887. doi: 10.1021/acscatal.8b02935. [DOI] [Google Scholar]
- 37.Lee E-H, et al. Enhancement of enzyme activity and stability by poly(γ-glutamic acid) Polym. J. 2010;42:818–822. doi: 10.1038/pj.2010.71. [DOI] [Google Scholar]
- 38.Brady JP, et al. Structural and hydrodynamic properties of an intrinsically disordered region of a germ cell-specific protein on phase separation. Proc. Natl Acad. Sci. U. S. A. 2017;114:E8194–E8203. doi: 10.1073/pnas.1706197114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nott TJ, et al. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell. 2015;57:936–947. doi: 10.1016/j.molcel.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harada R, Tochio N, Kigawa T, Sugita Y, Feig M. Reduced native state stability in crowded cellular environment due to protein-protein interactions. J. Am. Chem. Soc. 2013;135:3696–3701. doi: 10.1021/ja3126992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tibble RW, Depaix A, Kowalska J, Jemielity J, Gross JD. Biomolecular condensates amplify mRNA decapping by biasing enzyme conformation. Nat. Chem. Biol. 2021;17:615–623. doi: 10.1038/s41589-021-00774-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Privalov PL, Gill SJ. Stability of protein structure and hydrophobic interaction. Adv. Protein Chem. 1988;39:191–234. doi: 10.1016/S0065-3233(08)60377-0. [DOI] [PubMed] [Google Scholar]
- 43.Pace CN, Treviño S, Prabhakaran E, Scholtz JM. Protein structure, stability and solubility in water and other solvents. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004;359:1225–1234. doi: 10.1098/rstb.2004.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldsmith M, Tawfik DS. Enzyme engineering: Reaching the maximal catalytic efficiency peak. Curr. Opin. Struct. Biol. 2017;47:140–150. doi: 10.1016/j.sbi.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Bornscheuer UT, Pohl M. Improved biocatalysts by directed evolution and rational protein design. Curr. Opin. Chem. Biol. 2001;5:137–143. doi: 10.1016/S1367-5931(00)00182-4. [DOI] [PubMed] [Google Scholar]
- 46.Shitanda I, et al. High-power lactate/O2 enzymatic biofuel cell based on carbon cloth electrodes modified with MgO-templated carbon. J. Power Sources. 2019;436:226844. doi: 10.1016/j.jpowsour.2019.226844. [DOI] [Google Scholar]
- 47.Shitanda I, et al. High-performance, two-step/Bi-enzyme lactate biofuel cell with lactate oxidase and pyruvate oxidase. J. Power Sources. 2021;498:229935. doi: 10.1016/j.jpowsour.2021.229935. [DOI] [Google Scholar]
- 48.Bollella P, Sharma S, Cass AEG, Antiochia R. Microneedle-based biosensor for minimally-invasive lactate detection. Biosens. Bioelectron. 2019;123:152–159. doi: 10.1016/j.bios.2018.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available in the paper and Supplementary file. All other data are available from the corresponding authors upon request.