Abstract
Background:
Comparative effectiveness research between endoscopic sinus surgery(ESS) and biologic therapy for severe chronic rhinosinusitis with nasal polyposis(CRSwNP) is a nascent field as new therapeutic modalities become clinically available.
Methods:
A prospective, multi-center cohort of CRSwNP patients, undergoing ESS between 2011–2019, were compared to Phase-3 biologic trial data. Patients undergoing ESS received baseline nasal endoscopy quantified via Lund-Kennedy(LK) grading. Patients meeting inclusion criteria, modified from dupilumab-LIBERTY-NP-24&52, omalizumab-POLYP-1&2, and mepolizumab-SYNAPSE clinical trials, were included in this study. Baseline characteristics and outcome measures were compared between these cohorts at 24-weeks and 52-weeks, when possible.
Results:
One-hundred eleven CRSwNP patients met modified inclusion criteria. There were no statistically significant differences in baseline age, sex, asthma status, aspirin-exacerbated respiratory disease status, smell identification, LK-polyp score, and Lund-Mackay CT scores between ESS and biologic groups. At 24-weeks, ESS demonstrated significantly greater improvements in Sino-Nasal Outcome Test-22(SNOT-22) compared to one (of two) dupilumab trials(p<0.05) and both omalizumab trials(p<0.001). ESS associated with significantly lower nasal polyp scores(NPS) compared to dupilumab(p<0.001) and omalizumab(p<0.001), despite comparable improvements in smell identification(p>0.05). At 52-weeks, ESS resulted in statistically similar improvement in SNOT-22 scores compared to dupilumab(p=0.21), but NPS remained significantly lower in the ESS group compared to dupilumab(p<0.001) and mepolizumab(p<0.001).
Conclusions:
At 24-weeks and 52-weeks, ESS offers comparable SNOT-22 improvements compared to dupilumab. ESS and dupilumab offer comparable improvement in smell identification at 24-weeks. Compared to omalizumab, ESS offers superior SNOT-22 improvements. ESS offers significantly greater reductions in polyp size compared to omalizumab, dupilumab, and mepolizumab therapies.
Keywords: Chronic rhinosinusitis, Endoscopic sinus surgery, Eosinophilic rhinitis and nasal polyposis
MeSH Key Words: Sinusitis, chronic disease, outcome assessment (health care), quality of life
INTRODUCTION
Chronic rhinosinusitis(CRS) affects 10–15%1,2 of the population and approximately 25%3 of these patients suffer from nasal polyposis. Historically, therapeutically challenging CRS subtypes included CRS patients with nasal polyps(CRSwNP) and comorbid asthma, aspirin-exacerbated respiratory disease(AERD), and allergic fungal sinusitis.4,5 These treatment refractory subtypes fueled the development of new therapeutic options. Within the past three years, biologic medications including dupilumab, omalizumab, and mepolizumab completed phase 3 clinical trials for management of CRSwNP and have demonstrated benefits in subjective and objective measures of CRS disease severity.6–8 Additional biologics targeting the Th2 immune pathway are in the development pipeline.9–11
For severe CRSwNP refractory to standard medical therapy(topical corticosteroids and oral corticosteroids), both endoscopic sinus surgery(ESS) and biologics are treatment options. However, direct prospective comparative studies between ESS and biologics do not exist.12 In a recent study comparing Dupilumab to ESS, symptom outcomes were found to be comparable based on SNOT22 score, but this study was retrospective and the mean follow-ups for each study arm was different.13 The ideal study design would be a clinical trial that randomizes patients to either a biologic or ESS. However, it is highly unlikely a study of this design will ever be performed, given the challenge of randomizing patients to surgery, the near impossibility of truly blinding patients, and the extreme costs of such a study. Nonetheless, clinicians and patients must still weigh available outcomes data in order to make informed treatment decisions. One of the greatest challenges to comparing available data is that biologic trials are carefully restricted to patients with high polyp burden, whereas most ESS studies are more inclusive of the entire spectrum of disease.
The objective of our study was to compare the efficacy between ESS with appropriate ongoing medical management and biologics in severe nasal polyposis by applying similar inclusion criteria from the dupilumab LIBERTY NP SINUS-24&527, omalizumab POLYP-1&28, and mepolizumab SYNAPSE6 clinical trials to a prospective cohort of patients undergoing ESS.
MATERIALS and METHODS
Patient population
Adult study participants were prospectively enrolled into a multi-center observational cohort study evaluating treatment outcomes following ESS. The enrollment period was between April 2011 to August 2019. Results from this investigation have been previously described.14–16 Participants were diagnosed by fellowship-trained rhinologists with recalcitrant CRS defined by current diagnostic guidelines at the time of enrollment. Current diagnostic criteria requires the following for diagnosis of CRS: two or more cardinal symptoms(mucopurulent drainage, nasal obstruction/congestion, facial pain-pressure-fullness, or decreased sense of smell) for 12 weeks or longer and sinonasal inflammation documented on either endonasal exam (purulent mucus/edema in middle meatus or anterior ethmoid region or polyps in nasal cavity or the middle meatus) or radiographic imaging (imaging demonstrating inflammation of the paranasal sinuses).17,18 Before electing ESS as a subsequent treatment modality all participants had completed initial therapeutics including, but not limited to, daily saline irrigation, at least one course of either topical corticosteroids(≥21 days) or a 5+ day course of oral corticosteroid therapy, and at least one course(≥14 days) of broad spectrum (e.g. Augmentin, Doxycycline, etc.) or culture-directed antibiotics used at the discretion of the treating physician. The extent of ESS varied based on disease severity.
The Institutional Review Board(IRB) at each enrollment site governed investigational protocols and informed consent procedures. Enrollment locations included sinus clinics within tertiary referral, academic hospital systems in the US including: Oregon Health & Science University(OHSU; Portland, OR; IRB#7198), Stanford University(Palo Alto, CA; IRB#4947), and the University of Utah(Salt Lake City, UT; IRB#61810). Patients were assured of minimal study risk, voluntary study consent, and standard of care would not be altered due to study participation.
Enrollment procedures occurred after study participants underwent surgical counseling and voluntarily elected ESS. Study participants provided a comprehensive history and followed through the standard of postoperative care for ~18 months. Postoperative medical therapy was tailored to the extent of inflammation noted on postoperative clinic visits per the judgement of the treating surgeon. Participants were asked to complete both preoperative and postoperative evaluations at approximate 6-month intervals, either during physician-directed appointments or follow-up mailings when applicable.
Inclusion criteria
Of CRS study participants enrolled, only participants meeting inclusion criteria modified from dupilumab LIBERTY NP SINUS-24&527, omalizumab POLYP 1&28, and mepolizumab SYNAPSE6 phase 3 clinical trials were included. Modified inclusion criteria applied to this observational, prospective cohort of patients is as follows:
≥18 years of age.
Severe nasal polyposis defined as Lund Kennedy19(LK) polyp score of 4(2 on each side; 0–2 scale).
Nasal congestion score (NCS) of ≥ 2(0–3 scale).
At least one other complaint of smell loss or nasal discharge.
Exclusion criteria
Study participants were considered lost to follow-up and excluded if they did not provide any postoperative follow-up evaluation(≥24 weeks) during the study duration. Additional participants with comorbid cystic fibrosis or primary ciliary dyskinesia were excluded from final analysis due to variation in global health status and differential treatment considerations in the standard of care.
Biologic studies
Data from five recently published phase 3 randomized-controlled clinical trials investigating use of dupilumab, omalizumab, and mepolizumab in severe CRSwNP were reviewed. Table 1 outlines the biologic brand names, generic names, associated clinical trials, and each study’s inclusion criteria.
Table 1.
Biologics, associated clinical trials, and inclusion criteria
| Brand name | Generic | CRSwNP Clinical Trials | Abbreviated study inclusion criteria |
|---|---|---|---|
| Dupixent | dupilumab |
|
|
| Xolair | omalizumab |
|
|
| Nucala | mepolizumab |
|
|
| Endoscopic sinus surgery cohort |
|
||
Two dupilumab studies7 – LIBERTY NP SINUS-24(Dupi-24) and LIBERTY NP SINUS-52(Dupi-52) – were reviewed. In Dupi-24, CRSwNP patients received 300 mg. of dupilumab by subcutaneous injection every 2 weeks and primary outcomes were assessed at 24-weeks. In Dupi-52, CRSwNP patients received dupilumab 300 mg. every 2 weeks for 52-weeks(n=150) or 300 mg. every 2 weeks for first 24-weeks followed by 300 mg. every 4 weeks till week 52 (n=145). Outcomes for Dupi-52 were assessed at 24-weeks and 52-weeks.
Two omalizumab studies8 – POLYP 1(Oma-1)(n=72) & POLYP 2(Oma-2)(n=62) – were reviewed. These were identically designed studies in which treatment arms were given 75–600 mg. of omalizumab by subcutaneous injection every 2 or 4 weeks depending on the pretreatment serum total IgE level and body weight. Outcomes were assessed at 24-weeks.
A single mepolizumab study6 – SYNAPSE(Mepo) – was reviewed. Patients in the treatment arm received 100 mg. of mepolizumab by subcutaneous injection every 4 weeks for 52-weeks. Outcomes were assessed only at 52-weeks.
Baseline patient characteristics
Baseline characteristics were analyzed for the prospective, ESS cohort and extracted from the aforementioned published phase 3 clinical trials. Baseline characteristics included patient demographics, objective measures of disease severity, a patient reported outcome measure(PROM), and smell identification. Patient characteristics and demographics included age, sex, asthma status, AERD status, and prior surgery status. Objective measures of disease severity included endoscopic NPS20, LK polyp score, and Lund MacKay CT21 score when available. The PROM included the Sino-Nasal Outcome Test-22(SNOT-22).
Baseline smell identification was assessed using Smell Identification Test-40(SIT-40; previously referred to as the UPSIT-40)22 in Dupi-24&52 and in Oma-1&2 studies. In the ESS cohort, either SIT-40 or Sniffin’ Sticks23 were used to assess baseline olfaction. Olfactory category distributions (i.e. anosmic, hyposmic, and normosmic) were determined when possible based on published normative data.22,24
Outcome measures
Outcome measures assessed included NPS, LK polyp score, SNOT-22 score, NCS, loss of smell score, and smell identification (using SIT-40 or SS). For the ESS cohort, NCS and loss of smell scores were taken from corresponding SNOT-22 questions and converted to a 0–3 scale (i.e. same scale employed in biologic clinical trials).
For the ESS cohort, baseline, 24-week, and 52-week outcomes were determined and compared to available biologic trial data when available at these time points.
An analysis of NPS distributions at 24-weeks and 52-weeks following treatment (by either ESS or biologic therapy) was performed. NPS is on a 0–8 scale (0–4 on each side). For biologic groups (dupilumab, omalizumab, and mepolizumab), a best-case scenario was assumed with biologic patients starting at a baseline NPS of 5, the minimum based on inclusion criteria for all biologic studies. The aforementioned phase 3 biologic trials reported (in variable detail) mean change in NPS and in what portion of patients (i.e. % no improvement, % improved 1 point, % improved 2 points, etc.). Biologic groups’ post-treatment NPS distributions were then calculated by applying reported improvements to the baseline NPS value of 5. ESS 24-week and 52-week NPS were determined by converting from LK polyp scores. LK polyp score and NPS grading systems are outlined in table 5. Based on the scoring schemes a LK 0 and LK 1 are equivalent to a NPS 0 and NPS 1, respectively. However, a LK 2 encompasses NPS 2–4. Based on these definitions, the NPS score of patients undergoing ESS can be determined for those that received a LK score of 0 or 1 on either side (i.e. LK 0 on right and LK 0 on left = total NPS 0; LK 0 on right and LK 1 on left = total NPS 1; LK 1 on right and LK 1 on left = total NPS2).
Table 5.
Nasal polyp grading systems
| Nasal Polyp Score (NPS) | Lund Kennedy Polyp Score (LK-NP) | ||
|---|---|---|---|
| NPS 0 | No polyps | LK 0 | No polyps |
| NPS 1 | Polyps not reaching below inferior border of middle turbinate/confined to middle meatus | LK 1 | Polyps confined/restricted to the middle meatus |
| NPS 2 | Polyps reaching below the lower border of the middle turbinate | LK 2 | Polyps extending beyond the middle meatus |
| NPS 3 | Large polyps reaching the lower border of the inferior turbinate | ||
| NPS 4 | Large polyps causing complete obstruction of the inferior nasal cavity | ||
NPS = Nasal Polyp Score; LK-NP = Lund Kennedy Nasal Polyp Score
For Dupi-24&52 and Oma-1&2 clinical trials, smell identification was determined at baseline and at 24-weeks post-treatment using SIT-40. For the ESS cohort, baseline and 24-weeks post-treatment and 52-weeks post-treatment smell identification was determined with SIT-40 or Sniffin’ Sticks. Using normative data, patients were grouped into olfactory categories (anosmics, hyposmics, and normosmics) when possible. Baseline and 24-week distributions of olfactory categories were determined for the ESS, Dupi-24, and Dupi-52 groups and compared to each other.
Data Management and Statistical Analysis
All descriptive analyses and statistical comparisons were completed using SPSS software (version 28.0; IBM Corporation, Armonk, NY.). Summary statistics derived from data reported within each biologic study was utilized as the basis for between-group statistical comparisons. Descriptive measures of data variance, including standard deviation[±SD] and standard errors(±SE), from the ESS cohort were elected to facilitate highest comparability between studies. Pearson’s chi-square test statistics, with various contingency table dimensions, were used for between-group comparisons of prevalence (%) while two-sided independent sample t-testing was used to compare continuous measures, where applicable. Relative mean improvement(RMI) was defined as the percentage improvement/change compared to baseline summary measures provided by each biologic study to account for baseline differences. Type-I error probabilities(p-values) are reported for differences below the conventional 0.050 α-level. For outcome measures analyses, a decision was made to perform statistical analyses for only SNOT22 and NCS as these were primary outcomes in many of the biologic phase-3 clinical trials.
RESULTS
Baseline patient characteristics and demographics
A total of 165 patients with CRSwNP undergoing ESS were enrolled and 111 patients met inclusion criteria. As detailed in Table 2, the ESS cohort had a mean[±SD] age of 51.9 years [±15.8]. Just over half of patients (53%) were men, and 59% had a history of prior surgery. Regarding comorbidities, 61% had asthma and 33% had AERD. NPS was not available for this cohort of patients, but LK polyp score based on inclusion criteria was 4(i.e. 2 on each side) for all patients. Mean Lund-Mackay CT score was 18.7[±4.2] and mean SNOT-22 score was 56.1[±19.6].
Table 2.
Baseline patient demographics
| ESS(n=111) | Dupi-24 (n=143) | Statistics (ESS vs Dupi) |
Dupi-52 (n = 295) |
Statistics (ESS vs Dupi) | Oma-1 (n=72) | Statistics (ESS vs Oma) |
Oma-2 (n= 62) |
Statistics (ESS vs Oma) | Mepo(n=206) | Statistics (ESS vs Mepo) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 51.9 [±15.8] | 52 (39–61) | -- | 52.0 (42–63) | -- | 50.0 [±14.5] | NS | 49.0 [±11.9] | NS | 48.6 [±13.6] | NS |
| Men (%) | 59 (53.2%) | 88 (62.0%) | NS | 184 (62.4%) | NS | 47 (65.3%) | NS | 39 (62.9%) | NS | 139 (67%) | NS |
| Asthma (%) | 68 (61.3%) | 82 (57.0%) | NS | 176 (59.7%) | NS | 42 (58.3%) | NS | 38 (61.3%) | NS | 140 (68%) | NS |
| AERD (%) | 37 (33.3 %) | 46 (32.0%) | NS | 76 (25.8%) | NS | 16 (22.2%) | NS | 24 (38.7%) | NS | 45 (22%) | NS |
| ≥1 previous ESS (%) | 66 (59.4%) | 99 (69.0%) | NS | 173 (58.6%) | NS | 39 (54.1%) | NS | 22 (35.5%) | (p=0.002) | 206 (100%) | (p<0.0001) |
| NPS (0–8) | ---- | 5.64 [±1.23] | ---- | 6.18 [±1.10] | ---- | 6.2 [±1.0] | ---- | 6.4 [±0.9] | ---- | 5.4 [±1.2] | ---- |
| LK polyp score (0–4) | 4.0 [±0.0] | 4.0 [±0.0]* | NS | 4.0 [±0.0]* | NS | 4.0 [±0.0]* | NS | 4.0 [±0.0]* | NS | 4.0 [±0.0]* | NS |
| Lund-Mackay CT total score (0–24) | 18.7 [±4.2] | 18.55 [±4.55] | NS | 18.12 [±2.00] | NS | ---- | ---- | ---- | ---- | ---- | ---- |
| SNOT-22 total score (mean±SD) | 56.1 [±19.6] | 48.0 [±20.16] | p=0.002 | 51.0 [±4.67] | p<0.001 | 59.8 [±19.7] | NS | 59.2 [±20.5] | NS | 63.7 [±17.6] | p<0.001 |
| Sniffin Sticks (mean±SD) (n=48) | 14.6 [±7.4] | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| SIT-40 score (mean±SD) (n=4) | 23.3 [±12.5] | 14.68 [±8.66] | NS | 13.53 [±2.75] | NS | 12.8 [±7.9] | NS | 12.8 [±7.6] | NS | ---- | ---- |
LEGEND:--- = comparisons were unable to be made due to unavailable data; ESS = Endoscopic sinus surgery; Dupi-24= Dupilumab Liberty NP SINUS-24; Dupi-52= Dupilumab Liberty NP SINUS-52; Mepo = mepolizumab-SYNAPSE; Oma-1 = Omalizumab POLYP-1; Oma-2 = Omalizumab POLYP-2; SNOT-22 = Sinonasal outcome test 22; NPS = Nasal polyp score; AERD = Aspirin exacerbated respiratory disease; LK = Lund-Kennedy; PROM = patient reported outcome measure; SIT-40 = Smell identification test-40; NS=Not significant;
based on clinical trial inclusion criteria of NPS≥5 with minimum of 2 on each sides by definition means all patients are LK-NP of 4
There were no statistically significant differences in age, sex distribution, asthma status, AERD status, smell identification based on SIT-40, or LK polyp score between ESS cohort and any of the biologic treatment arms. Lund MacKay CT scores were also compared between ESS and Dupi-24 and Dupi-52 treatment arms and were nearly identical without any statistically differences(p>0.05). Prior surgery status was statistically similar between ESS, Dupi-24, Dupi-52, and Oma-1(p>0.05). SNOT-22 scores were statistically similar between ESS, Oma-1, and Oma-2(p>0.05).
The portion of prior surgery patients was significantly lower in Oma-2 compared to ESS(p<0.001). Inclusion criteria for mepolizumab study required prior surgery, thus it had higher rates compared to the ESS cohort(p<0.001). Compared to ESS cohort, baseline SNOT-22 was significantly lower in Dupi-24(p=0.002) and Dupi-52(p<0.001). Compared to Mepo, SNOT-22 for ESS was significantly higher(p<0.001).
Twenty-four-week PROM outcomes
Results for ESS cohort, Dupi-24, Dupi-52, Oma-1, and Oma-2 are presented (Table 3). At 24 weeks, ESS resulted in a mean(±SE) SNOT-22 improvement of 33(±18.7) (59% relative improvement compared to baseline), a mean NCS improvement of 1.9(±0.9) (66% relative improvement compared to baseline), and a mean loss of smell score of 1.5(±1.2) (44% relative improvement compared to baseline).
Table 3.
Endoscopic sinus surgery and biologic outcomes at 24-weeks
| Endpoints | Baseline(mean±SD) | Week 24 (mean±SD) | LS mean change from baseline (±SE) | Relative change | ||
|---|---|---|---|---|---|---|
| ESS | NPS | ---- | ---- | ---- | ---- | |
| (n=111) | NCS | 2.9 [±0.3] | 0.9 [±0.9] | −1.9 [±0.09] | −66% | |
| LK-NP | 4.0 [±--] | 0.9 [±1.2] | −3.1 [±0.14] | −80% | ||
| Loss of smell score | 2.7 [±0.7] | 1.5 [±1.2] | −1.2 [±0.13] | −44% | ||
| SNOT 22 score | 56.1 [±19.6] | 22.9 [±19.6] | −33.3 [±1.8] | −59% | ||
| SIT-40 (n=4) | 23.3 [±12.5] | 31.8 [±5.2) | 8.5 [±5.2] | 36% | ||
| Sniffin’ sticks total (n=34) | 13.9 [±6.9] | 21.1 [±8.3] | 7.1 [±1.4] | 51% | ||
| Endpoints | Baseline(mean±SD) | Week 24mean±SD) | LS mean change from baseline (±SE) | Relative change | Statistics (comparison of mean Δ to ESS) | |
| Dupi-24 | NPS | 5.64 [±1.23] | 3.75 [±1.98] | −1.89 [±0.14] | −34% | ---- |
| (n=143) | NCS | 2.26 [±0.57] | 0.94 [±0.75] | −1.34 [±0.07] | −59% | p<0.001 |
| Loss of smell score | 2.70 [±0.57] | 1.35 [±0.99] | −1.41 [±0.07] | −52% | NS | |
| SNOT 22 score | 48.00 [±20.16] | 18.58 [±14.92] | −30.43 [±1.54] | −63% | NS | |
| SIT-40 | 14.68 [±8.66] | 25.39 [±9.49] | 11.26 [±0.67] | 77% | NS | |
| Endpoints | Baseline(mean±SD) | Week 24 (mean±SD) | LS mean change from baseline (±SE) | Relative change | Statistics (comparison of mean Δ to ESS) | |
| Dupi-52 | NPS | 6.18 [±1.10] | 4.46 [±1.89] | −1.71 [±0.11] | −28% | ---- |
| (n=295) | NCS | 2.46 [±0.77] | 1.19 [±0.90] | −1.25 [±0.06] | −51% | p<0.001 |
| Loss of smell score | 2.77 [±0.77] | 1.55 [±1.02] | −1.21 [±0.06] | −44% | NS | |
| SNOT 22 score | 51.0 [±4.67] | 23.89 [±18.77] | −27.77 [±1.26] | −54% | p=0.018 | |
| SIT-40 | 13.53 [±2.75] | 23.89 [±9.21] | 9.71 [±0.56] | 72% | NS | |
| Endpoints | Baseline(mean±SD) | Week 24 (mean) (Unable to calculate SD) | LS mean change from baseline (±SE) | Relative change | Statistics (comparison of mean Δ to ESS) | |
| Oma-1 | NPS | 6.2 [±1.0] | 5.12 | −1.08 [±0.16] | −17% | ---- |
| (n=72) | NCS | 2.4 [±0.7] | 1.51 | −0.89 [±0.10] | −37% | p<0.001 |
| Loss of smell score | 2.5 [±0.8] | 1.94 | −0.56 [±0.09] | −22% | P<0.001 | |
| SNOT 22 score | 59.8 [±19.7] | 35.1 | −24.70 [±2.01] | −41% | p=0.002 | |
| SIT-40 | 12.8 [±7.9] | 17.24 | 4.44 [±0.84] | 35% | NS | |
| Endpoints | Baseline(mean±SD) | Week 24 (mean) (Unable to calculate SD) | LS mean change from baseline (±SE) | Relative change | Statistics (comparison of mean Δ to ESS) | |
| Oma-2 | NPS | 6.4 [±0.9] | 5.5 | −0.90 [±0.17] | −14% | ---- |
| (n=62) | NCS | 2.3 [±0.7] | 1.6 | −0.70 [±0.11] | −30% | p<0.001 |
| Loss of smell score | 2.6 [±0.8] | 2.02 | −0.58 [±0.10] | −22% | p=0.001 | |
| SNOT 22 score | 59.2 [±20.5] | 37.61 | −21.59 [±2.25] | −36% | p<0.001 | |
| SIT-40 | 12.8 [±7.6] | 17.11 | 4.31 [±0.83] | 34% | NS |
LEGEND:--- = comparisons were unable to be made due to unavailable data, therefore comparisons could not be made; ESS = Endoscopic sinus surgery; Dupi-24= Dupilumab Liberty NP SINUS-24; Dupi-52= Dupilumab Liberty NP SINUS-52; Oma-1 = Omalizumab POLYP-1; Oma-2 = Omalizumab POLYP-2; LS = Least square; NPS = Nasal polyp score; NCS = Nasal congestion score; LK-NP = Lund-Kennedy Polyp score; SNOT22 = Sinonasal Outcome Test 22; SIT-40 = Smell Identification Test-40; SD = Standard deviation; SE = Standard error; SIT-40 = Smell identification test-40; NS = Not significant
ESS resulted in significantly greater improvements in SNOT-22 and NCS compared to Dupi-52 (p<0.05), but improvement in loss of smell score was similar between groups(p=0.937). Compared to Dupi-24, ESS resulted in significantly greater improvement in NCS(p<0.001), but improvements in SNOT-22(p=0.225) and loss of smell score(p=0.133) were statistically similar. Compared to Oma-1 and Oma-2, ESS resulted in significantly greater improvements in SNOT-22, NCS, and loss of smell score (p<0.001).
Fifty-two-week PROM outcomes
Results for ESS cohort, Dupi-52 and Mepo are presented (Table 4). At 52-weeks, ESS resulted in a mean(±SE) SNOT-22 improvement of 33.9(±21.1) (59% relative improvement compared to baseline), a mean NCS improvement of 1.7(±1.3) (59% relative improvement compared to baseline), and a mean loss of smell score of 1.2(±0.16) (43% relative improvement compared to baseline). ESS resulted in significantly greater improvements in NCS(p<0.001) and loss of smell score(p>0.05) compared to Mepo, but statistically similar improvements in SNOT-22(p=0.244). Compared to Dupi-52, ESS resulted in statistically similar improvements in SNOT-22 scores(p=0.105). However, ESS resulted in statistically greater improvement in NCS compared to Dupi-52(p<0.001).
Table 4.
Endoscopic sinus surgery and biologic outcomes at 52-weeks
| Endpoints | Baseline (mean±SD) | Week 52 (mean±SD) | LS mean from baseline (±SE) | Relative change compared to baseline | ||
|---|---|---|---|---|---|---|
| ESS | NPS | ---- | ---- | ---- | ---- | |
| NCS (n=48) | 2.9 [±0.3] | 0.8 [±0.8] | −2.1 [±0.13] | −72% | ||
| LK-NP (n=20) | 4.0 [±--] | 1.0 [±1.1] | −3.0 [±0.25] | −75% | ||
| Loss of smell score (n=48) | 2.8 [±0.5] | 1.6 [±1.2] | −1.2 [±0.16] | −43% | ||
| SNOT-22 score (n=48) | 57.7 [±18.9] | 23.8 [±19.1] | −33.9 [±3.04] | −59% | ||
| SIT-40 (n=3) | 19.7 [±12.5] | 26.7 [±13.3] | 7.0 [±8.6] | 36% | ||
| Sniffin’ sticks total (n=48) | 14.6 [±7.4] | ---- | ---- | ---- | ||
| Endpoints | Baseline (mean±SD) | Week 52 (mean±SD) | LS mean from baseline (±SE) | Relative change compared to baseline | Statistics (comparison to ESS) | |
| Dupi-52 (group A q2w dosing) | NPS | 6.07 [±1.22] | 3.76 [±2.20] | −2.24 [+0.15] | −37% | ---- |
| (n=150) | NCS | 2.48 [±0.62] | 1.10 [±0.92] | −1.35 [+0.07] | −54% | p<0.001 |
| Loss of smell score | 2.81 [±0.46] | ---- | ---- | ---- | ---- | |
| SNOT 22 score | 50.16 [±19.72] | 21.67 [±19.16] | −29.84 [+1.63] | −59% | NS | |
| SIT-40 | 13.46 [±8.20] | ---- | ---- | ---- | ---- | |
| Endpoints | Baseline (mean±SD) | Week 52 (mean; Unable to calculate SD) | LS mean from baseline (±SD) | Relative change compared to baseline | Statistics (comparison to ESS) | |
| Mepo | NPS | 5.4 [±1.2] | 4.5 | −0.9 [±1.90] | −17% | ---- |
| (n=206) | NCS | 2.67 [±0.24] | 1.4 | −1.26 [±1.03] | −47% | p<0.001 |
| Loss of smell score | 2.88 [±0.24] | 2 | −0.84 [±1.08] | −29% | p=0.039 | |
| SNOT 22 score | 63.7 [±17.6] | 34.3 | −29.4 [±24.67] | −46% | NS | |
| SIT-40 | ---- | ---- | ---- | ---- | ---- |
LEGEND:--- = comparisons were unable to be made due to unavailable data, therefore comparisons could not be made; ESS = Endoscopic sinus surgery; Dupi-52= Dupilumab Liberty NP-SINUS 52;Mepo = mepolizumab-SYNAPSE; LS = Least square; NPS = Nasal polyp score; NCS = Nasal congestion score; LK-NP = Lund-Kennedy Polyp score; SNOT22 = Sinonasal Outcome Test 22; SIT-40 = Smell identification test-40; SD = Standard deviation; SE = Standard error; SIT-40 = Smell identification test-40; NS = Not significant
Nasal polyp scores
Twenty-four-week and 52-week post-treatment NPS distributions are presented in Tables 6 and 7, respectively. A detailed analysis is included as supplemental material. Distributions in NPS were determined for biologic groups in as granular a fashion as possible based upon available data from the respective clinical trials. For the ESS group, LK polyp scores were converted to NPS in as granular fashion as possible using conversions in table 5.. Statistical analysis revealed that ESS resulted in significantly lower NPS distributions compared to all biologic therapies at 24-weeks and 52-weeks(p<0.001). In summary, at least 54% of patients receiving biologic therapies had post-treatment NPS of 4 or greater compared to 13% or fewer patients who underwent ESS.
Table 6.
Distribution of nasal polyp scores at 24-weeks
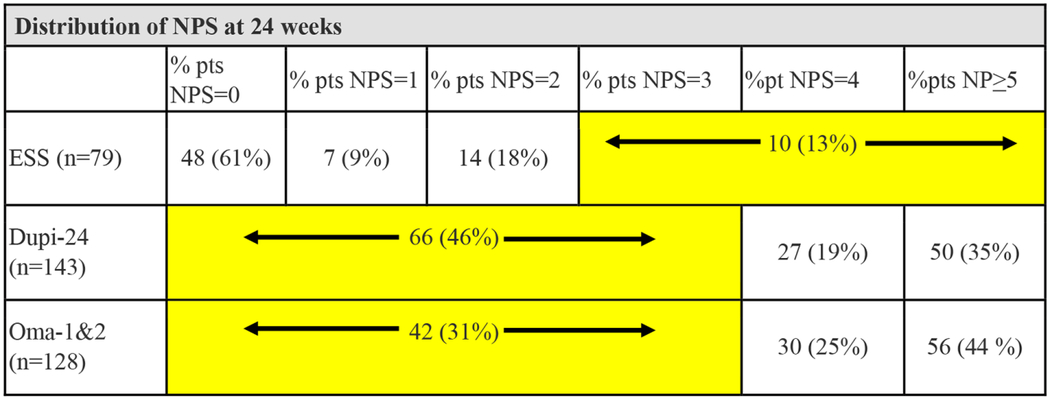
|
LEGEND:There is a significantly higher prevalence of lower NPS scores in the ESS group compared to both the Dupi 24W cohort (χ2 =36.2; p<0.001) and Oma 24W cohort study (χ2 =58.4; p<0.001). NPS = Nasal polyp score; ESS = endoscopic sinus surgery; Dupi-24 = dupilumab LIBERTY NP SINUS-24; Oma-1&2 = omalizumab POLYP-1&2
Table 7.
Distribution of nasal polyp scores at 52-weeks
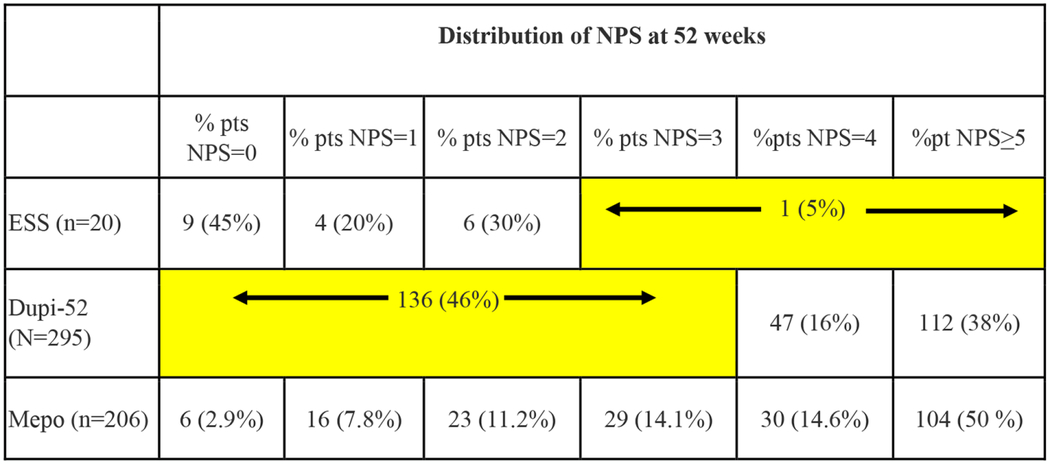
|
LEGEND:There is a significantly higher prevalence of lower NPS scores in the ESS group compared to both the Dupi 52W cohort (χ2 =17.9; p<;0.001) and Mepo 52W cohort study ((χ2 =26.7; p<0.001). NPS = Nasal polyp score; ESS = endoscopic sinus surgery; Dupi-52 = dupilumab LIBERTY NP SINUS-52; Mepo = mepolizumab-SYNAPSE
Smell Identification
At 24-weeks, compared to the ESS cohort, Dupi-24&52 and Oma-1&2 experienced statistically similar improvements in SIT-40 scores compared to baseline(p>0.05). At baseline and 24-weeks, olfactory category distributions(i.e. anosmia, hyposmia, and normosmia) were determined for Dupi-24 and Dupi-52 based on published study data.7 At baseline and 24 weeks, patients in the ESS cohort were similarly grouped into appropriate olfactory categories using SIT-40 and Sniffin’ Sticks normative data.22,23 Baseline and 24-week olfactory distributions were compared between ESS cohort, Dupi-24, and Dupi-52 study arms and no statistically significant differences were noted(p>0.05; Table 8).
Table 8.
Smell identification categories
| ESS | Dupi-24 | Statistics (ESS vs Dupi-24) | Dupi-52 | Statistics (ESS vs Dupi-52) | |
|---|---|---|---|---|---|
| Baseline | |||||
| N | 52 | 140 | NS | 287 | NS |
| Anosmia | 36 (69%) | 104 (74%) | 228 (79%) | ||
| Hyposmia | 13 (25%) | 32 (23%) | 54 (19%) | ||
| Normal | 3 (6%) | 4 (3%) | 5 (2%) | ||
| Week 24 | |||||
| N | 38 | 138 | NS | 280 | NS |
| Anosmia | 9 (24%) | 33 (24%) | 84 (30%) | ||
| Hyposmia | 20 (53%) | 82 (59%) | 163 (58%) | ||
| Normal | 9 (24%) | 23 (17%) | 33 (12%) | ||
LEGEND:At baseline, the prevalence of all olfactory categories between the ESS cohort and Dupi-24 cohort (χ2 =1.09; p=0.580) and Dupi-52 cohort (χ2 =4.47; p=0.107) was statistically similar. At the 24W follow-up, the prevalence of all olfactory categories between the ESS cohort and Dupi Sinus 24 cohort (χ2 =1.05; p=0.593) and the Dupi Sinus 52 (χ2 =4.22; p=0.121) were also statistically similar. Dupi-24= Dupilumab Liberty NP SINUS-24; Dupi-52= Dupilumab Liberty NP SINUS-52; ESS = Endoscopic sinus surgery;
DISCUSSION
We present an analysis comparing a prospective real-world cohort of patients with severe CRSwNP undergoing ESS to recently published biologic clinical trials involving dupilumab, omalizumab, and mepolizumab. While each biologic study had slightly differing inclusion criteria, the included ESS cohort had remarkably similar baseline patient characteristics to biologic cohorts, including demographics, asthma status, AERD status, LK polyp score, smell identification(i.e. SIT-40 and olfactory category distributions), and Lund-MacKay CT score. This suggests that very similar populations are present across these cohorts prior to treatment. Of note, there were a few significant baseline differences across cohorts. The SNOT-22 score was significantly lower(better) in Dupi-24&52 and higher(worse) in Mepo studies compared to ESS, however it is unlikely these differences are clinically significant as all cohorts started with mean SNOT-22 scores in the severe range. Prior reports demonstrate that the relative improvements and percent achieving an minimal clinically important difference on SNOT-22 following ESS is similar in patients with baseline SNOT-22 scores of 40–49, 50–59, and 60–69.27 Additionally, Oma-2 had a significantly lower portion of patients with prior surgery whereas Mepo had a significantly higher portion of prior surgery patients(100%) compared to ESS, a reflection of the Mepo study’s inclusion criteria requiring surgical failure.
Overall, the ESS cohort had significantly better quality of life and symptom benefit in terms of SNOT-22, loss of smell score, and NCS than most biologic study arms at both timepoints. Compared to Oma-1&2 studies, the ESS cohort had significantly greater improvements in SNOT-22, NCS, and loss of smell score at 24-weeks. Dupi-24&52 appears to fare better than Oma 1&2 at 24-weeks, but the ESS cohort still had significantly greater benefit compared to Dupi-24&52 in many PROMs. However, at 52-weeks, most PROM outcomes were equivalent between ESS and Dupi-52 study arms. Compared to Mepo, the ESS cohort had numerically greater SNOT-22 improvements at 52-weeks, but this comparison did not reach significance. NCS and loss of smell score, on the other hand, did demonstrate significantly greater improvements in ESS group compared to mepolizumab at 52-weeks. A recent randomized control trial found that at 12-month follow-up, CRSwNP patients achieved greater symptom benefit from ESS plus medical therapy compared to medical therapy alone (which only included nasal steroids, nasal rinsing, systematic corticosteroids or systematic antibiotics) -- although the minimal clinically important difference between treatment arms was not met. This study also noted that the surgery treatment arm scored better in terms of systemic medication usage, nasal polyp score, and nasal obstructions at 12 months.27 These results are in line with prior systematic reviews and prospective cohort studies demonstrating low revision ESS rates and sustained benefits in PROMs.28,29
A recent randomized control trial investigating Benralizumab (Fasenra) in CRSwNP demonstrated a statistically significant benefit over placebo at 40 weeks and 56 weeks. Unfortunately, the endpoint times were different in this Benralizumab study than the biologic studies presented in this work making comparisons difficult. Additionally, measures of variance (i.e. standard deviations) were not reported in the published study making statistical comparisons to the ESS cohort impossible. Of note, SNOT-22 relative improvement in this study was 23% at 40 weeks which is worse than the relative SNOT-22 improvement for Dupi-24&52, Oma-1&2, and ESS groups at 24 weeks.
Smell identification improvements at 24 weeks were comparable between ESS, Dupi-24&52, and Oma-1&2. When looking at smell identification between Dupi-24&52 and ESS, both groups had approximately 70% of patients with anosmia at baseline. At 24 weeks, both treatments still had approximately 25% of patients that remained anosmic. There were no significant differences between baseline and 24-week olfactory category distributions between ESS and Dupi-24&52.
Not unexpectedly, NP scores were better in ESS patients following surgery. Presumably after ESS and complete removal of polyps, patients have an NPS of 0. This is in contrast to biologics which pharmacologically reduce polyp burden to a varying degree. While difficult to extrapolate, the Mepo study did stratify NPS precisely and approximately 10% of patients had near perfect endoscopy with a NPS of 0 or 1. Across all biologics, it is interesting to note that over 50% of patients still have significant NP burden with NPS of 4 or 5. This indicates shrinkage of 1 point or less in half of patients. This suggests that benefits of treatment are not simply tied to reduction in polyp size alone. This apparent disparity between NPS and both PROMs and olfaction suggests that there is a complex, nonlinear relationship between NP size, symptoms and olfaction. If one considers that NP size alone is likely an incomplete measure of mucosal inflammation in CRSwNP, then it should not be surprising that change in symptoms does not correlate perfectly with change in NP size. There remains much to be learned with regard to the specific mechanisms by which ESS and biologics achieve clinical impacts.
Several national societies have published updated CRSwNP treatment algorithms that include biologic therapy.12–15,28 Many of these national society and multidisciplinary consensus recommendations advocate for complete surgery prior to consideration of a biologic. Support for this approach has been garnered by recent cost-utility analyses and a meta-analysis showing a 16.2% revision rate for ESS over an 89.6 month mean follow-up period.4 The current study evaluating ESS and biologics outcomes adds further support by demonstrating that ESS provides either superior or equivalent quality-of-life and clinical outcomes compared to biologics, supporting its role as primary treatment when initial medications fail.
Ultimately, the decision to pursue ESS or biologic should be centered on shared decision-making that best maximizes patient goals. In patients with significant comorbidities who are unsafe for general anesthesia, biologics are, to date, low-risk and can provide significant quality-of-life benefits. In patients with specific comorbid indications, such as those with severe asthma or atopic dermatitis, biologics may provide quality of life benefits across multiple health domains. It is important to note, however, that the impact of ESS upon asthma has been examined and studies demonstrate that ESS improves asthma control and asthma-specific quality-of-life, and has been associated with decreases in asthma attacks, steroid use and hospitalizations.29,30 Certainly, for patients who have failed a comprehensive surgery with adequate post-operative topical steroid therapy, consideration of a biologic is a logical next step. For patients wishing to avoid surgery altogether, biologic therapy may help fulfill this aim. Studies investigating use of dupilumab in surgically naïve patients found that patients receiving mometasone sprays and dupilumab had a nearly 10-fold less likelihood of progressing to surgery compared to patients receiving mometasone alone.31 It is important to note that long-term treatment is needed to sustain benefits from biologics, as loss of efficacy with symptom recurrence appears to begin within 1–2 months of stopping biologics.7 Additionally, this need for long-term and sustained biologic therapy has high costs associated with it. In a recent cost utility analysis of dupilumab versus ESS for CRSwNP, the ESS strategy proved to be more cost effective for upfront treatment of CRSwNP. Over a 36-year time horizon, the dupilumab treatment strategy cost over 10x that of ESS.32 It is important to note that this study did not take into the effects of comorbid asthma. Ultimately, qualitative multi-criteria decision analysis looking at disease severity, age, and socioeconomic status at the health system level may be a better strategy for promoting better decision making, equity, and transparency than using cost effective analysis alone.37 A comprehensive discussion between provider and patient that includes information highlighting treatment efficacy, treatment costs, treatment duration, treatment risks, and patient preference will aid in patient-centered decision making.
There are several limitations to this study. In order to select a similar patient population, modified inclusion criteria taken from dupilumab LIBERTY NP SINUS-24&52 and omalizumab POLYP-1&2 phase 3 clinical trial selection criteria were used and applied to a prospective, multiinstitutional cohort of patients undergoing ESS. A LK polyp grading system was used for the ESS cohort due to ease of use in a busy clinical practice and because this prospective study began before the biologic NP scales were widely adopted. Dupi-24&52 and Oma-1&2 studies used the more granular NPS grading system. Theoretically, there may be patients within our ESS cohort with a NPS of 4 total – i.e., 2 on each side – that were included who would have otherwise been excluded in the biologic trials leading to an ESS cohort with slightly lower objective disease severity and perhaps decreasing the potential for relative improvement in objective outcomes and PROMs. However, it is reassuring that the baseline Lund-MacKay CT scores did not differ between ESS and Dupi-24&52 trials, suggesting that objective disease severity was comparable. Another limitation of the study was loss of follow-up for patients in the ESS cohort which may introduce selection bias. Furthermore, precise perioperative treatment regimens in the ESS group (i.e. postop antibiotics and steroids) were not available for this study, but routine practice for providers in the ESS group was to prescribe 2–3 weeks of postoperative prednisone for CRSwNP patients. Lastly, in an ideal world, comparisons are drawn between a group of patients randomized to real surgery with a sham biologic versus another group undergoing sham surgery with real biologic therapy. However, there are numerous ethical and logistical barriers to such a study. Therefore, studies such as this one and other real-world observational investigations will likely represent the most feasible and highest-quality evidence comparing biologic and ESS outcomes.
CONCLUSIONS
Endoscopic sinus surgery offers comparable symptom benefits compared to dupilumab at 24-weeks and 52-weeks. In regards to smell identification, ESS and dupilumab offer comparable benefits at 24-weeks. ESS appears to be more effective in symptom improvement than omalizumab(at 24-weeks) and mepolizumab(at 52-weeks). ESS offers significantly greater reductions in polyp size compared to all biologics suggesting that a more complex relationship exists between polyp-size and symptom burden. While further comparative studies are needed, this information may assist with personalized decision making for patients with severe, refractory CRSwNP.
Supplementary Material
Funding Source:
Timothy L. Smith, Zachary M. Soler, Jess C. Mace, and Rodney J. Schlosser were supported by a grant for this investigation from a grant from the National Institute on Deafness and Other Communication Disorders (NIDCD; R01-DC005805). This funding organization did not contribute to the design or conduct of this study; preparation, review, approval or decision to submit this manuscript for publication.
Footnotes
Potential Conflicts of Interest: The authors declare that they have no potential conflict of interests or financial disclosures related to this submission. This material has never been published and is not currently under evaluation in any other peer-reviewed publication.
RJS disclosures: Consultant for GSK, Sanofi, Genentech, Optinose, Stryker
ZMS: Consultant for GSK, Novartis, Optinose, and Lyra. Medical director, Sinusonic
REFERENCES
- 1.Halawi AM, Smith SS, Chandra RK. Chronic rhinosinusitis: epidemiology and cost. Allergy asthma Proc. 2013;34(4):328–334. doi: 10.2500/aap.2013.34.3675 [DOI] [PubMed] [Google Scholar]
- 2.DeConde AS, Soler ZM. Chronic rhinosinusitis: Epidemiology and burden of disease. Am J Rhinol Allergy. 2016;30(2):134–139. doi: 10.2500/ajra.2016.30.4297 [DOI] [PubMed] [Google Scholar]
- 3.Stevens WW, Schleimer RP, Kern RC. Chronic Rhinosinusitis with Nasal Polyps. doi: 10.1016/j.jaip.2016.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loftus CA, Soler ZM, Koochakzadeh S, et al. Revision surgery rates in chronic rhinosinusitis with nasal polyps: meta-analysis of risk factors. Int Forum Allergy Rhinol. 2020;10(2):199–207. doi: 10.1002/alr.22487 [DOI] [PubMed] [Google Scholar]
- 5.Miglani A, Divekar RD, Azar A, Rank MA, Lal D. Revision endoscopic sinus surgery rates by chronic rhinosinusitis subtype. Int Forum Allergy Rhinol. 2018;8(9):1047–1051. doi: 10.1002/alr.22146 [DOI] [PubMed] [Google Scholar]
- 6.Han JK, Bachert C, Fokkens W, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;2600(21). doi: 10.1016/S2213-2600(21)00097-7 [DOI] [PubMed] [Google Scholar]
- 7.Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. Published online 2019. doi: 10.1016/S0140-6736(19)31881-1 [DOI] [PubMed] [Google Scholar]
- 8.Gevaert P, Omachi TA, Corren J, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol. Published online 2020. doi: 10.1016/j.jaci.2020.05.032 [DOI] [PubMed] [Google Scholar]
- 9.Kuruvilla ME, Levy J. Efficacy of Reslizumab in Eosinophilic Chronic Sinusitis with Nasal Polyposis. J Allergy Clin Immunol. 2018;141(2):AB270. doi: 10.1016/j.jaci.2017.12.860 [DOI] [Google Scholar]
- 10.Weinstein SF, Katial RK, Bardin P, et al. Effects of Reslizumab on Asthma Outcomes in a Subgroup of Eosinophilic Asthma Patients with Self-Reported Chronic Rhinosinusitis with Nasal Polyps. J Allergy Clin Immunol Pract. 2019;7(2):589–596.e3. doi: 10.1016/j.jaip.2018.08.021 [DOI] [PubMed] [Google Scholar]
- 11.Laidlaw TM, Buchheit KM. Biologics in chronic rhinosinusitis with nasal polyposis. Ann Allergy, Asthma Immunol. 2020;124(4):326–332. doi: 10.1016/j.anai.2019.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naclerio R, Baroody F, Bachert C, et al. Clinical Research Needs for the Management of Chronic Rhinosinusitis with Nasal Polyps in the New Era of Biologics: A National Institute of Allergy and Infectious Diseases Workshop. J allergy Clin Immunol Pract. 2020;8(5):1532–1549.e1. doi: 10.1016/j.jaip.2020.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dharmarajan H, Falade O, Lee SE, Wang EW. Outcomes of dupilumab treatment versus endoscopic sinus surgery for chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2021;(September 2021):1–10. doi: 10.1002/alr.22951 [DOI] [PubMed] [Google Scholar]
- 14.Alt JA, Orlandi RR, Mace JC, Soler ZM, Smith TL. Does Delaying Endoscopic Sinus Surgery Adversely Impact Quality-of-Life Outcomes? Laryngoscope. 2019;129(2):303–311. doi: 10.1002/lary.27473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chowdhury NI, Mace JC, Bodner TE, et al. Investigating the minimal clinically important difference for SNOT-22 symptom domains in surgically managed chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017;7(12):1149–1155. doi: 10.1002/alr.22028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy JM, Mace JC, Bodner TE, Alt JA, Smith TL. Defining the minimal clinically important difference for olfactory outcomes in the surgical treatment of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017;7(8):821–826. doi: 10.1002/alr.21964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: Adult sinusitis. Otolaryngol - Head Neck Surg. 2007;137(3 SUPPL.):1–31. doi: 10.1016/j.otohns.2007.06.726 [DOI] [PubMed] [Google Scholar]
- 18.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): Adult sinusitis. Otolaryngol - Head Neck Surg (United States). 2015;152:S1–S39. doi: 10.1177/0194599815572097 [DOI] [PubMed] [Google Scholar]
- 19.Lund VJ, Kennedy DW. Quantification for staging sinusitis. The Staging and Therapy Group. Ann Otol Rhinol Laryngol Suppl. 1995;167:17–21. [PubMed] [Google Scholar]
- 20.Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: Developing guidance for clinical trials. J Allergy Clin Immunol. 2006;118(5 SUPPL.):17–61. doi: 10.1016/j.jaci.2006.09.005 [DOI] [PubMed] [Google Scholar]
- 21.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31(4):183–184. [PubMed] [Google Scholar]
- 22.Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94(2 Pt 1):176–178. doi: 10.1288/00005537-198402000-00004 [DOI] [PubMed] [Google Scholar]
- 23.Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. “Sniffin” sticks’. Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. Published online 1997. doi: 10.1093/chemse/22.1.39 [DOI] [PubMed] [Google Scholar]
- 24.Oleszkiewicz A, Schriever VA, Croy I, Hähner A, Hummel T. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Oto-Rhino-Laryngology. 2019;276(3):719–728. doi: 10.1007/s00405-018-5248-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–1650. doi: 10.1016/S0140-6736(19)31881-1 [DOI] [PubMed] [Google Scholar]
- 26.Rudmik L, Soler ZM, Mace JC, Deconde AS, Schlosser RJ, Smith TL. Using preoperative SNOT-22 score to inform patient decision for Endoscopic sinus surgery. Laryngoscope. 2015;125(7):1517–1522. doi: 10.1002/lary.25108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lourijsen ES, Reitsma S, Vleming M, et al. Endoscopic sinus surgery with medical therapy versus medical therapy for chronic rhinosinusitis with nasal polyps: a multicentre, randomised, controlled trial. Lancet Respir Med. 2022;2600(21):1–10. doi: 10.1016/s2213-2600(21)00457-4 [DOI] [PubMed] [Google Scholar]
- 28.Smith TL, Schlosser RJ, Mace JC, et al. Long-term outcomes of endoscopic sinus surgery in the management of adult chronic rhinosinusitis. Int Forum Allergy Rhinol. 2019;9(8):831–841. doi: 10.1002/alr.22369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loftus CA, Soler ZM, Desiato VM, et al. Factors impacting revision surgery in patients with chronic rhinosinusitis with nasal polyposis. Int Forum Allergy Rhinol. 2020;10(3):289–302. doi: 10.1002/alr.22505 [DOI] [PubMed] [Google Scholar]
- 30.Thamboo A, Kilty S, Witterick I, et al. Canadian Rhinology Working Group consensus statement: biologic therapies for chronic rhinosinusitis. J Otolaryngol - Head Neck Surg. 2021;50(1):1–9. doi: 10.1186/s40463-021-00493-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roland LT, Smith TL, Schlosser RJ, et al. Guidance for contemporary use of biologics in management of chronic rhinosinusitis with nasal polyps: discussion from a National Institutes of Health–sponsored workshop. Int Forum Allergy Rhinol. 2020;10(9):1037–1042. doi: 10.1002/alr.22633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fokkens WJ, Lund V, Bachert C, et al. EUFOREA consensus on biologics for CRSwNP with or without asthma. Allergy Eur J Allergy Clin Immunol. 2019;74(12):2312–2319. doi: 10.1111/all.13875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orlandi RR, Kingdom TT, Smith TL, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021;11(3):213–739. doi: 10.1002/alr.22741 [DOI] [PubMed] [Google Scholar]
- 34.Vashishta R, Soler ZM, Nguyen SA, Schlosser RJ. A systematic review and meta-analysis of asthma outcomes following endoscopic sinus surgery for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013;3(10):788–794. doi: 10.1002/alr.21182 [DOI] [PubMed] [Google Scholar]
- 35.Bachert C, Hellings PW, Mullol J, et al. Dupilumab improves patient-reported outcomes in patients with chronic rhinosinusitis with nasal polyps and comorbid asthma. J Allergy Clin Immunol Pract. 2019;7(7):2447–2449.e2. doi: 10.1016/j.jaip.2019.03.023 [DOI] [PubMed] [Google Scholar]
- 36.Scangas GA, Wu AW, Ting JY, et al. Cost Utility Analysis of Dupilumab Versus Endoscopic Sinus Surgery for Chronic Rhinosinusitis With Nasal Polyps. Laryngoscope. 2021;131(1):E26–E33. doi: 10.1002/lary.28648 [DOI] [PubMed] [Google Scholar]
- 37.DiStefano MJ, Levin JS. Does incorporating cost-effectiveness analysis into prescribing decisions promote drug access equity? AMA J Ethics. 2019;21(8):679–685. doi: 10.1001/amajethics.2019.679 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


