Abstract
The objective of these studies was to determine the role of macrophage inflammatory protein 1α/CCL3 in pulmonary host defense during Klebsiella pneumoniae infection. Following intratracheal inoculation, 7-day survival of CCL3−/− mice was less than 10%, compared to 60% for CCL3+/+ mice. Survival of CCR5−/− mice was equivalent to that of controls, indicating that the enhanced susceptibility of CCL3−/− mice to K. pneumoniae is mediated via another CCL3 receptor, presumably CCR1. At day 3, CFU burden in the lungs of CCL3−/− mice was 800-fold higher than in CCL3+/+ mice, demonstrating that CCL3 is critical for control of bacterial growth in the lung. Surprisingly, CCL3−/− mice had no differences in the recruitment of monocytes/macrophages and even showed enhanced neutrophil recruitment at days 1, 2, and 3 postinfection, compared to CCL3+/+ mice. Therefore, the defect in clearance was not due to insufficient recruitment of leukocytes. No significant differences in cytokine levels of monocyte chemoattractant protein 1 (MCP-1), interleukin 12, gamma interferon, or tumor necrosis factor alpha in lung lavages were found between CCL3+/+ and CCL3−/− mice. CCL3−/− alveolar macrophages were found to have significantly lower phagocytic activity toward K. pneumoniae than CCL3+/+ alveolar macrophages. These findings demonstrate that CCL3 production is critical for activation of alveolar macrophages to control the pulmonary growth of the gram-negative bacterium K. pneumoniae.
The effective clearance of bacteria from the lung requires a coordination of proinflammatory and anti-inflammatory stages (26, 34). Initial phagocytosis by alveolar macrophages leads to the production of proinflammatory cytokines (tumor necrosis factor alpha[TNF-α] interleukin 6 [IL-6] and IL-12) and chemokines (IL-8/macrophage inflammatory protein 2 [MIP-2]/CXCL8, KC/CXCL1, IP-10/CXCL-10, Mig/CXCL9, MIP-1α/CCL3, MIP-1β/CCL4, and monocyte chemoattractant protein 1 (MCP-1)/CCL2) (30, 31). This production of cytokines and chemokines results in vigorous recruitment and activation of leukocytes. In one study, following intratracheal administration of Klebsiella pneumoniae, antibody depletion of TNF-α resulted in decreased neutrophil recruitment, increased lung bacterial burden, and decreased survival (21). Conversely, localized administration of TNF-α to the lung, through the use of an adenoviral vector or bioactive peptide, increased clearance of K. pneumoniae, with resultant increases in survival (20, 35). In another study, transgenic expression of KC/CXCL1 resulted in resistance to K. pneumoniae infection via the increased recruitment of neutrophils (38). Also important, however, is the resolution phase of the infection, in which anti-inflammatory cytokines (chiefly, IL-10) limit the systemic effects of the initial recruitment and activation phase (33). Thus, while augmentation of proinflammatory signals leads to an improved outcome, neutralization of recruitment and/or activational signals such as MIP-2 and TNF-α has a deleterious effect (17, 21, 25).
MIP-1α/CCL3, a member of the CC chemokine family, plays an important role in the development and regulation and recruitment of leukocytes. CCL3 is produced by a variety of cells, including lymphocytes, fibroblasts, and epithelial cells, as well as both resident and recruited monocytes/macrophages (3, 5, 7, 9, 10, 16). CCL3 has roles in the compartmentalization and mobilization of myeloid precursor cells (MPCs) (3–6). Through the use of CCR1 knockout mice, CCL3 has been shown to mediate the mobilization of MPCs from the bone marrow, as well as having regulatory effects on MPCs and acting to stimulate mature MPCs, but CCL3 inhibits immature cells (2, 15). CCL3 has been reported to be chemotactic for both neutrophils and monocytes in vitro and in vivo in mice (11, 29). In humans and higher primates, however, predominantly monocytic cellular infiltrates will accumulate in response to direct injection of CCL3 (12). In a number of model systems, CCL3 has been shown to play an important role in the recruitment of mononuclear cells (8, 13, 15, 18, 19, 22, 23, 28, 32). CCL3−/− mice were found to be partially protected from the accumulation of monocytes in myelocarditis and to be impaired in the ability to control the growth of coxsackievirus and influenza (8). As with other studies mentioned, these findings were attributable to defects in the efferent or recruitment phase. We have recently shown CCL3 to be involved in afferent function, as well. CCL3 was found to prevent the switch to a nonprotective Th2 response during Cryptococcus neoformans infection (27). The objective of our current studies was to determine whether CCL3 plays a role in pulmonary host defense during K. pneumoniae infection and if so, to determine the mechanism of CCL3 activity.
MATERIALS AND METHODS
Mice.
CCL3+/+ mice (B6129SF2/J and B6129PF2/J; Jackson Laboratory, Bar Harbor, Maine), CCL3−/− mice (8), and CCR5−/− mice (B6129P2-Scya3tm1Coo and B6129P-Cmkbr5tm1kuz; University of Michigan breeding colony) were housed under specific-pathogen-free conditions in enclosed filter-top cages. Clean food and water were given ad libitum. The mice were handled and were maintained using microisolator techniques with daily veterinary monitoring. Cage bedding was periodically transferred to the cages of sentinel mice, which were monitored for the presence of antibodies to murine hepatitis virus, Sendai virus, and Mycoplasma pulmonis. The CCL3−/− mice lack a promoter region, as well as exon 1 and part of exon 2 of the CCL3 gene. Male and female mice were 6 to 10 weeks of age at the time of infection, and there were no age-related or sex-related differences in the responses of these mice to K. pneumoniae infection.
K. pneumoniae.
K. pneumoniae strain 43816, serotype 2, was obtained from the American Type Culture Collection (Rockville, Md.). For infection, bacteria were grown to stationary phase (18 h) in tryptic soy broth (Soybean-Casein digest; Difco, Detroit, Mich.) in vented 50-ml conical tubes at 37°C and 5% CO2. The concentration of bacteria was determined by measuring the absorbance at 600 nm on a DU-64 Spectrophotometer (Beckman Instruments, Inc., Fullerton, Calif.), compared to a standard curve of absorbances. The bacteria were pelleted by centrifugation at 5,000 × g, were washed twice in nonpyrogenic saline (Travenol, Deerfield, Ill.), and were resuspended at a concentration of 3.3 × 104/ml. One thousand CFU was used as the inoculation dose, which was verified retrospectively by plating serial dilutions on tryptic soy agar (Soybean-Casein digest; Difco)–1% sheep blood (Colorado Serum Supply Co., Denver, Colo.).
Intratracheal inoculation of K. pneumoniae.
Mice were anesthetized by intraperitoneal injection of sodium pentobarbital (0.074 mg/g of body weight; Butler, Columbus, Ohio) and were restrained on a small board. A small incision was made through the skin over the trachea, and the underlying tissue was separated. A 30-gauge needle (Becton Dickinson, Rutherford, N.J.) was attached to a tuberculin syringe (BD & Co., Franklin Lakes, N.J.) filled with the diluted K. pneumoniae culture. The needle was inserted into the trachea, and 30 μl of inoculum was dispensed into the lungs (103 CFU). The skin was closed with cyanoacrylate adhesive. The mice recovered with minimal visible trauma. Aliquots of the inoculum were collected periodically to monitor the number of CFU being delivered.
Preparation of lung leukocytes.
The lungs from each mouse were excised, washed in phosphate-buffered saline, minced with scissors, and digested enzymatically for 30 min in 15 ml of digestion buffer medium, (RPMI medium, fetal 5% calf serum, and 1 mg of collagenase [Boehringer Mannheim Biochemical, Chicago, Ill.]/ml and 30 μg of DNase [Sigma]/ml) per lung. The cell suspension and tissue fragments were further dispersed by drawing up and down through the bore of a 10-ml syringe and were centrifuged. Erythrocytes in the pellets were lysed by the addition of 3 ml of NH4Cl buffer (0.829% NH4Cl, 0.1% KHCO3, and 0.0372% Na2EDTA, pH 7.4) for 3 min, followed by a 10-fold excess of RPMI medium. Cells were resuspended again in media containing antibiotics. A second cycle of syringe dispersion and filtration through a sterile nylon screen (Nitex, Kansas City, Mo.) were performed. The filtrate was centrifuged for 25 min at 1,500 × g n the presence of 20% Percoll (Sigma Chemical Co., St. Louis, Mo.) to separate leukocytes from cell debris and epithelial cells. Leukocyte pellets were resuspended in 10 ml of complete media and were enumerated in a hemocytometer upon dilution in trypan blue. Leukocyte recovery from uninfected CCL3+/+ and CCL3−/− mice was (21.6 ± 6.4) × 106 leukocytes (n = 5) and (20.9 ± 6.6) × 106 leukocytes (n = 7), respectively.
Assessment of leukocyte population.
For the differential count of lung cell suspensions, samples were cytospun (Shandon Cytospin, Pittsburgh, Pa.) onto glass slides and were stained using the Diff-Quik whole-blood stain (Baxter Scientific, Miami, Fla.). A total of 200 to 400 cells were counted from randomly chosen high-powered-microscope fields for each sample. The absolute number of a leukocyte subset was calculated by multiplying the percentage of each subset in an individual sample by the total number of lung leukocytes in that mouse.
Assessment of lung K. pneumoniae burden.
A 100-μl sample from each lung cell suspension was collected from lung digests, prior to erythrocyte lysis. Serial dilutions (10-fold) were plated on tryptic soy agar in duplicates. After incubation at room temperature for 20 h, CFU were counted and expressed as total CFU per lung.
BAL.
Mice were euthanatized with carbon dioxide. Mice were lavaged by cannulation of the trachea with polyethylene tubing (PE50, Intramedic; Clay Adams, Parsippany, N.J.) attached to a 25-gauge needle (Becton Dickinson) on a tuberculin syringe (Monoject, St. Louis, Mo.). Bronchoalveolar lavage (BAL) fluid was separated from cells by centrifugation at 1,500 × g and was stored at −70°C until assayed by enzyme-linked immunosorbent assay (ELISA). Each mouse was lavaged twice using 1 ml of ice-cold phosphate-buffered saline with 5 mM EDTA (Sigma) each time. Cells from BAL were added back to leukocyte preparations following enzymatic digest.
ELISA.
BAL was assayed for cytokine activity by ELISA. Murine IL-10, IL-12, TNF-α, MCP-1/CCL2, and gamma interferon (IFN-γ) ELISA kits (OPTEIA kits; Pharmingen, San Diego, Calif.) were used to quantify cytokine concentration in lavage samples. Reactions were performed on 96-well ELISA plates (Costal Ultra-High Binding EIA Plates; Corning, Corning, N.Y.) containing both samples and the cytokine standard in duplicates. The optical densities were read on a microplate reader (Ultra Micro EL 808; Biotek Instruments, Winooski, Vt.) at a wavelength of 510 nm. The cytokine concentration in each lavage was estimated by interpolation of sample optical densities with the cytokine standard by a four-parameter curve-fitting program. The sensitivity limit for detection was approximately 15 to 40 pg/ml.
Phagocytosis assay.
Uninfected mice aged 6 to 8 weeks were euthanatized with carbon dioxide, and BAL cells were collected as described above. Cells were pooled from multiple individual animals. BAL cells (>90% macrophages by differential staining) were plated 105 per well in eight-well Labtek chamber slides (Nunc, Inc., Naperville, Ill.). Adherent cells were washed after 1 h, and 107 CFU of live K. pneumoniae (multiplicity of infection = 100) in 2% specific immune serum (in Hanks balanced salt solution [HBSS]) were added. Slides were mixed on a plate shaker (Hoefer, San Francisco, Calif.) for 2 min and were incubated for 30 min at 37°C and 5% CO2. Extracellular bacteria were removed by washing extensively with HBSS. Slides were then air dried and stained with Diff-Quik whole-blood stain. The number of cells containing bacteria, as well as the number of intracellular bacteria, was determined for a minimum of 200 cells per well.
Calculations and statistics.
Data (mean ± standard error) for each experimental group were derived from three or more experiments. For comparisons between means, the two-sample Student t test was used. As dictated by the F test for variance, the t test assuming unequal variance was used when appropriate. Means with P of <0.05 were considered statistically significant.
RESULTS
Effect of CCL3 on survival of K. pneumoniae infection.
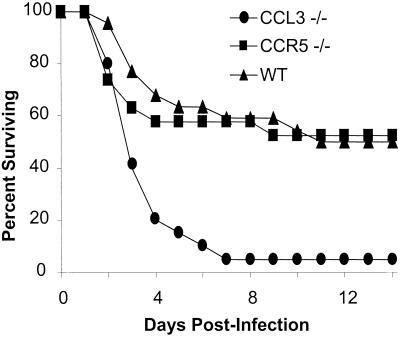
Our first objective was to assess the impact of CCL3 deletion on the outcome of pulmonary K. pneumoniae infection. The 7-day survival of CCL3−/− mice was less than 10%, compared to 60% for CCR5−/− and wild-type controls (Fig. 1). The difference in survival between CCL3+/+ and CCL3−/− mice continued through day 14, suggesting that the defect in CCL3−/− mice was not simply a shift in the kinetics of the survival curve. Furthermore, since 60% of the CCL3−/− mice were dead by day 3, CCL3 likely played an critical role in resident innate immunity. The fact that CCR5−/− mice closely matched controls in survival following K. pneumoniae infection indicates that the enhanced susceptibility of CCL3−/− mice to K. pneumoniae is mediated via another CCL3 receptor, presumably CCR1. CCR5−/− mice were created on the same 129 background as CCL3−/− mice and thus also controled for any possible contribution of the parental 129 strain. Thus, CCL3 clearly plays a role in the survival of K. pneumoniae infection which is not mediated via CCR5.
FIG. 1.
Fourteen-day survival following pulmonary infection with K. pneumoniae. CCL3−/− (n = 20), CCR5−/− (n = 19), and wild-type (WT) (B6129SF2) (n = 22) mice were infected intratracheally with 103 CFU of K. pneumoniae. The curve shown is a composite of three independent, matched infections. Additionally, survival between B6129SF2 and B6129PF2 did not differ (n = 7 per group, not shown).
Effect of MIP-1α deletion on bacterial clearance.
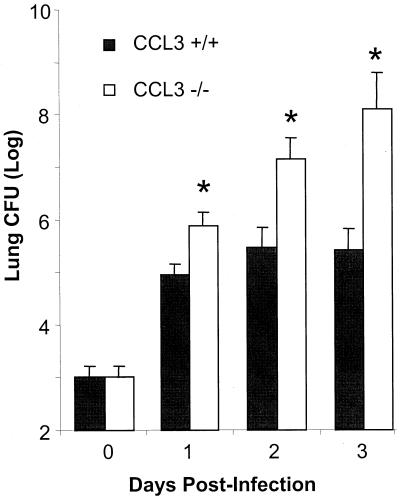
We next measured the number of lung K. pneumoniae CFU in CCL3−/− and CCL3+/+ mice to determine the role of CCL3 in control of bacterial growth in the lungs. Days 1, 2, and 3 postinfection were chosen to minimize “survivor effects.” As shown in Fig. 2, there were significantly more CFU in CCL3−/− mice at day 1 and continuing through days 2 and 3. At day 3, there was an approximately 800-fold-higher CFU burden in the lungs of CCL3−/− mice than in those of CCL3+/+ mice. Therefore, CCL3 is critical for control of bacterial growth in the lung.
FIG. 2.
Pulmonary bacterial burden following infection of K. pneumoniae. CCL3−/− and CCL3+/+ mice (n = 10 to 12 per time point) were infected intratracheally with 103 CFU (day 0) of K. pneumoniae. Lung bacterial load was determined at days 1, 2, and 3 postinfection. Data are pooled from the results of three independent, matched infections. Bars represent the mean number of CFU per lung for each group ± standard error. Additionally, note that the y axis is in logarithmic scale. ∗, P < 0.002.
Assessment of monocyte/macrophage recruitment following intratracheal challenge with K. pneumoniae.
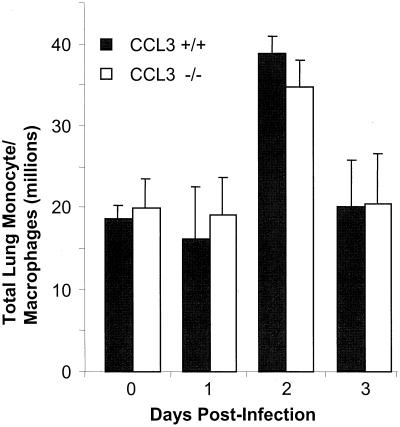
To establish whether the decrease in clearance observed in CCL3−/− mice was due to a lack of monocyte/macrophage recruitment, lung leukocytes from CCL3−/− and CCL3+/+ mice (n = 10 to 12 per time point) were recovered by enzymatic digest of whole lungs at days 1 to 3 postinfection. The percentage of monocytes/macrophages was determined by differential stain, and total numbers of cells were determined by multiplying the percentage of monocytes/macrophages by total leukocyte number, as described in Materials and Methods. Following intratracheal challenge with K. pneumoniae, CCL3+/+ mice had a transient influx of monocytes/macrophages between days 1 and 2 (Fig. 3). CCL3−/− mice had magnitude and kinetics of lung monocyte/macrophage recruitment similar to those of CCL3+/+ mice. Therefore, the decreased clearance observed in CCL3−/− mice was not due to a decrease in lung monocyte/macrophage recruitment.
FIG. 3.
Total monocytes/macrophages from the lungs of CCL3−/− and CCL3+/+ mice (n = 10 to 12 per group per time point), following intratracheal challenge with K. pneumoniae. Lung monocytes/macrophages were isolated from enzymatically digested lungs as described in Materials and Methods. Monocyte/macrophage recovery from uninfected CCL3−/− mice (n = 5) was (19.9 ± 6.1) × 106 and was (18.6 ± 5.6) × 106 from CCL3+/+ mice (n = 7). Data are from three independent, matched experiments.
Assessment of recruitment of other leukocyte subsets following intratracheal challenge with K. pneumoniae.
Total numbers of lung neutrophils, lymphocytes, and eosinophils were determined to establish whether the decrease in clearance observed in CCL3−/− mice was due to a lack of recruitment of other leukocyte cell types. Compared to uninfected controls, no increase in lymphocytes or eosinophils was observed at days 1 to 3 in CCL3−/− or CCL3+/+ mice (data not shown). In contrast, neutrophil recruitment was apparent as early as day 1 in CCL3+/+ and increased slightly at days 2 and 3 to a peak of approximately 12 million cells (Fig. 4). CCL3−/− mice actually showed enhanced neutrophil recruitment at days 1, 2, and 3 postinfection. This difference approached significance at day 1 (P = 0.068) and was significant at days 2 and 3. These data demonstrate that CCL3−/− mice have no defect in the ability to recruit neutrophils or other leukocyte subsets.
FIG. 4.
Neutrophils from the lungs of CCL3−/− and CCL3+/+ mice following intratracheal challenge with K. pneumoniae. Lung neutrophils were isolated from enzymatically digested lungs as described in Materials and Methods (n = 10 to 12 per group per time point). Neutrophil recovery from uninfected CCL3−/− mice (n = 5) was (0.8 ± 0.4) × 106 and was (1.1 ± 0.5) × 106 from CCL3+/+ mice (n = 7). Data are from three independent, matched experiments. ∗, P < 0.03.
Assessment of lung cytokine profile following intratracheal challenge with K. pneumoniae.
BAL fluid was assayed by ELISA for cytokine levels to determine if differences in lung cytokine production were responsible for the observed differences in clearance of K. pneumoniae. Begining at day 2 postinfection, cytokine levels were elevated above those of uninfected lung lavages for MCP-1, IL-12, IFN-γ, and TNF-α in both CCL3+/+ and CCL3−/− mice. No significant differences, however, were found between CCL3−/− and CCL3+/+ mice (Table 1). Thus, as measured by the presence of cytokines in BAL, CCL3−/− mice develop an inflammatory response which is similar to that of CCL3+/+ mice.
TABLE 1.
Cytokine level in BAL fluid of K. pneumoniae-infected and uninfected (day 0) micea
| Day | Type of mice | Concn (pg/ml) of
|
||||
|---|---|---|---|---|---|---|
| MCP-1 | IL-12 | IFN-γ | TNF-α | IL-10 | ||
| 0 | CCL3+/+ | <18 | <12 | 73 ± 5 | 97 ± 14 | 60 ± 6 |
| CCL3−/− | <18 | <12 | 59 ± 5 | 76 ± 6 | 42 ± 12 | |
| 1 | CCL3+/+ | 22 ± 9 | 15 ± 10 | 82 ± 31 | 98 ± 30 | 38 ± 10 |
| CCL3−/− | 49 ± 20 | 73 ± 54 | 34 ± 11 | 82 ± 23 | 19 ± 10 | |
| 2 | CCL3+/+ | 311 ± 131* | 192 ± 79 | 375 ± 211* | 219 ± 76* | 66 ± 23 |
| CCL3−/− | 649 ± 159* | 281 ± 205* | 508 ± 320* | 785 ± 297* | 47 ± 19 | |
| 3 | CCL3+/+ | 201 ± 120* | 1,166 ± 838* | 784 ± 497* | 386 ± 239* | 34 ± 14 |
| CCL3−/− | 405 ± 106* | 900 ± 663* | 1,193 ± 597* | 512 ± 278* | 25 ± 15 | |
Cytokine level in BAL fluid of K. pneumoniae-infected CCL3+/+ and CCL3−/− mice (n = 5 to 7 per time point, from two independent experiments). *, P < 0.05, compared to day 0. Infected CCL3−/− animals did not differ significantly from infected CCL3+/+ animals at any time point.
CCL3−/− alveolar macrophages have impaired phagocytic activity toward K. pneumoniae.
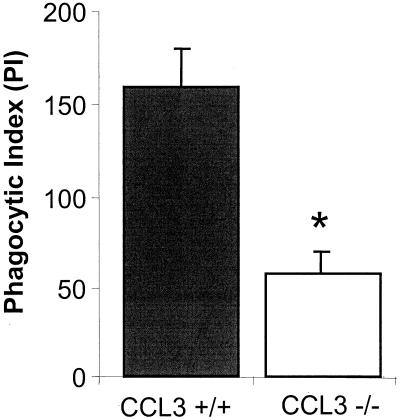
To determine if the increased pulmonary bacterial burden observed in CCL3−/− mice was the result of defective phagocytosis, the K. pneumoniae-specific phagocytic activities of CCL3−/− and CCL3+/+ alveolar macrophages were assayed. Alveolar macrophages were lavaged from uninfected CCL3+/+ and CCL3−/− mice. Adherent cells were incubated in vitro with live, opsonized K. pneumoniae. As shown in Fig. 5, CCL3−/− alveolar macrophages have a significantly lower phagocytic index (PI) than do CCL3+/+ alveolar macrophages. PI is a measure which takes into account both the frequency and magnitude of phagocytosis. The difference in PI was predominantly due to differences in phagocytic frequency (33.7% ± 3% for CCL3+/+ alveolar macrophages versus 12.0% ± 2.5% for CCL3−/− alveolar macrophages). Thus, CCL3 plays an important role in promoting the phagocytic activity of alveolar macrophages towards K. pneumoniae.
FIG. 5.
Phagocytic activity of alveolar macrophages from CCL3−/− and CCL3+/+ mice toward K. pneumoniae. Alveolar macrophages from CCL3−/− and CCL3+/+ mice were cultured with live, opsonized K. pneumoniae (Materials and Methods). PI = % macrophages containing phagocytozed bacterium × mean number of bacteria per positive cell. Bars represent the mean for each group ± standard error. In each case, data are pooled from the results of three separate experiments (n ≥ 4 for each; total n = 14 for each group). *, P < 0.0001.
DISCUSSION
In this study, we sought to examine the role of CCL3 in a murine model of acute bacterial pneumonia. Intratracheal inoculation of K. pneumoniae resulted in a 7-day survival of less than 10% in CCL3−/− mice, compared to approximately 60% for CCL3+/+ controls. This survival defect correlated with significantly higher bacterial loads in the lungs of CCL3−/− mice. The recruitment of monocytes/macrophages was not defective in CCL3−/− mice. Enhanced recruitment of polymorphonuclear leukocytes was found in the lungs of CCL3−/− mice in response to K. pneumoniae infection, although this recruitment was not protective. In an in vitro assay of phagocytic function, macrophages from CCL3−/− mice were found to be defective in their phagocytic activity toward K. pneumoniae. These findings suggest that the survival defect in CCL3−/− mice is due to inadequate activation of alveolar macrophages in CCL3−/− mice, leading to unchecked bacterial growth.
CCL3 plays an important role in the survival of acute bacterial pneumonia. Following intratracheal K. pneumoniae administration, the 7-day survival of CCL3−/− mice was <10%, compared to greater than 60% for CCL3+/+ controls. The fact that CCR5−/− mice are similar to wild-type controls suggests that the protective role of CCL3 is mediated through CCR1 (the other functional receptor for CCL3). In response to intravenous Aspergillus fumigatus, CCR1−/− mice were found to have accelerated lethality (15). We would predict that the survival of CCR1−/− mice in response to K. pneumoniae infection would be similar to that of CCL3−/− mice. Thus, CCL3 plays an essential role in survival of acute bacterial pneumonia, and this effect is most likely mediated via CCR1.
There was no defect in the recruitment of monocytes/macrophages in CCL3−/− mice or production of inflammatory cytokines following intratracheal K. pneumoniae infection. It may be expected that lack of CCL3 would impair the recruitment of monocytes and macrophages. CCR1−/− mice had impaired granuloma formation induced by Schistosoma mansoni injection (15). CCL3−/− mice had an impaired inflammatory response to influenza virus and were protected from virus-induced myocarditis (8). These findings support a role for CCL3 in leukocyte trafficking. However, CCR1−/− mice had enhanced recruitment of macrophages and T cells in a nephrotoxic nephritis model (37). Therefore, the requirement for CCL3 in leukocyte trafficking is pathogen and stimulus dependent, and in this model of acute Klebsiella pneumonia, CCL3 is not required for the recruitment of monocytes/macrophages into the lungs.
An enhanced recruitment of polymorphonuclear leukocytes was found in the lungs of CCL3−/− mice in response to K. pneumoniae infection. The protective role of neutrophils in the effector phase of the immune response to K. pneumoniae is well documented (17, 20, 21, 24–26, 33, 36, 38). In this study, however, an increase in neutrophil recruitment correlated with a decrease in survival. Since the significant increase in the lung count of CFU precedes neutrophilia, we conclude that the increase in neutrophil recruitment may be the result of increased bacterial load. This phenomenon was also observed when mice were depleted of alveolar macrophages prior to K. pneumoniae infection (1). Thus, this previous study and the present study reported here demonstrate that the recruitment of neutrophils alone is not sufficient for bacterial clearance.
In this study, we demonstrate that CCL3 plays a role in macrophage phagocytosis of K. pneumoniae. Following intratracheal inoculation, an increase in the lung count of CFU in CCL3−/− mice was apparent as early as 24 h postinfection. This finding suggests that early events in the phagocytosis and/or activation of phagocytes may be involved. The alveolar macrophage plays a critical role in the early/innate phase of this response. In vitro, CCL3−/− alveolar macrophages have a significantly lower PI than do CCL3+/+ alveolar macrophages. In vivo, at day 1 postinfection, CCL3−/− mice have nearly 10-fold more bacteria in the lungs. However, alveolar macrophages lavaged from the lungs of infected CCL3−/− mice do not have significantly more ingested bacteria than do wild-type alveolar macrophages. Therefore, the observation that CCL3−/− and wild-type macrophages have equivalent numbers of phagocytozed bacteria is still consistent with a phagocytic defect in CCL3−/− macrophages. By day 3 postinfection, there is a trend toward fewer Klebsiella-positive macrophages in both lavage and total lung leukocytes. Depletion of alveolar macrophages using dichloromethylene diphosphonate-encapsulated liposomes resulted in enhanced bacterial burden in the lungs and the death of 100% of K. pneumoniae-infected mice (versus 0% of infected, nondepleted controls) (1). In contrast, the number of neutrophils which have phagocytozed bacteria and the magnitude of phagocytosis were similar for the two groups (data not shown). Thus, the phagocytic activity of macrophages plays a significant role in the clearance and survival of an acute K. pneumoniae pulmonary infection.
These studies demonstrate that CCL3 may play a significant role in phagocyte activation during antibacterial host defense. In preliminary studies, the addition of exogenous CCL3 is able to augment the PI of alveolar macrophages (data not shown). CCL3 can increase Trypanosoma cruzi uptake and parasite killing by human macrophages in a nitric oxide-dependent manner (39). It has also been shown previously that incubation of peritoneal macrophages with CCL3 peptide stimulated the release of TNF-α, IL-1α, and IL-6 (14). Thus, in a K. pneumoniae infection, CCL3 may promote phagocytosis directly or indirectly via the induction of proinflammatory cytokines and augment the killing of intracellular K. pneumoniae.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants ROI-HL63670 (G.B.H.), ROI-HL57243 (T.J.S.), ROI-HL58200 (T.J.S.), and P50-HL60289 (T.J.S.).
We thank Donald Cook for providing CCL3−/− mice, John Lee and Andrea Bediako for their technical contributions to this project, and Michal Olszewski for helpful discussions.
REFERENCES
- 1.Broug-Holub E, Toews G B, Van Iwaarden J F, Strieter R M, Kunkel S L, Paine III R, Standiford T J. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun. 1997;65:1139–1146. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broxmeyer H E, Cooper S, Hangoe G, Gao J L, Murphy P M. Dominant myelopoietic effector functions mediated by chemokine receptor CCRI. J Exp Med. 1999;189:1987–1992. doi: 10.1084/jem.189.12.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broxmeyer H E, Sherry B, Cooper S, Lu L, Maze R, Beckmann M P, Cerami A, Ralph P. Comparative analysis of the human macrophage inflammatory protein family of cytokines (chemokines) on proliferation of human myeloid progenitor cells. Interacting effects involving suppression, synergistic suppression, and blocking of suppression. J Immunol. 1993;150:3448–3458. [PubMed] [Google Scholar]
- 4.Broxmeyer H E, Sherry B, Cooper S, Ruscetti F W, Williams D E, Arosio P, Kwon B S, Cerami A. Macrophage inflammatory protein (MIP)-1 beta abrogates the capacity of MIP-1 alpha to suppress myeloid progenitor cell growth. J Immunol. 1991;147:2586–2594. [PubMed] [Google Scholar]
- 5.Broxmeyer H E, Sherry B, Lu L, Cooper S, Carow C, Wolpe S D, Cerami A. Myelopoietic enhancing effects of murine macrophage inflammatory proteins 1 and 2 on colony formation in vitro by murine and human bone marrow granulocyte/macrophage progenitor cells. J Exp Med. 1989;170:1583–1594. doi: 10.1084/jem.170.5.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broxmeyer H E, Sherry B, Lu L, Cooper S, Oh K O, Tekamp-Olson P, Kwon B S, Cerami A. Enhancing and suppressing effects of recombinant murine macrophage inflammatory proteins on colony formation in vitro by bone marrow myeloid progenitor cells. Blood. 1990;76:1110–1116. [PubMed] [Google Scholar]
- 7.Cipriani B, Borsellino G, Poccia F, Placido R, Tramonti D, Bach S, Battistini L, Brosnan C F. Activation of C-C beta-chemokines in human peripheral blood gammadelta T cells by isopentenyl pyrophosphate and regulation by cytokines. Blood. 2000;95:39–47. [PubMed] [Google Scholar]
- 8.Cook D N, Beck M A, Coffman T M, Kirby S L, Sheridan J F, Pragnell I B, Smithies O. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science. 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 9.Cook D N, Smithies O, Strieter R M, Frelinger J A, Serody J S. CD8+ T cells are a biologically relevant source of macrophage inflammatory protein-1 alpha in vivo. J Immunol. 1999;162:5423–5428. [PubMed] [Google Scholar]
- 10.Danforth J M, Strieter R M, Kunkel S L, Arenberg D A, VanOtteren G M, Standiford T J. Macrophage inflammatory protein-1 alpha expression in vivo and in vitro: the role of lipoteichoic acid. Clin Immunol Immunopathol. 1995;74:77–83. doi: 10.1006/clin.1995.1011. [DOI] [PubMed] [Google Scholar]
- 11.Davatelis G, Tekamp-Olson P, Wolpe S D, Hermsen K, Luedke C, Gallegos C, Coit D, Merryweather J, Cerami A. Cloning and characterization of a cDNA for murine macrophage inflammatory protein (MIP), a novel monokine with inflammatory and chemokinetic properties. J Exp Med. 1988;167:1939–1944. doi: 10.1084/jem.167.6.1939. . (Erratum, 170:2189, 1989.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Didier P J, Paradis T J, Gladue R P. The CC chemokine MIP-1alpha induces a selective monocyte infiltration following intradermal injection into nonhuman primates. Inflammation. 1999;23:75–86. doi: 10.1023/a:1020243701890. [DOI] [PubMed] [Google Scholar]
- 13.Doyle H A, Murphy J W. MIP-1 alpha contributes to the anticryptococcal delayed-type hypersensitivity reaction and protection against Cryptococcus neoformans. J Leukoc Biol. 1997;61:147–155. doi: 10.1002/jlb.61.2.147. [DOI] [PubMed] [Google Scholar]
- 14.Fahey T J, III, Tracey K J, Tekamp-Olson P, Cousens L S, Jones W G, Shires G T, Cerami A, Sherry B. Macrophage inflammatory protein 1 modulates macrophage function. J Immunol. 1992;148:2764–2769. [PubMed] [Google Scholar]
- 15.Gao J L, Wynn T A, Chang Y, Lee E J, Broxmeyer H E, Cooper S, Tiffany H L, Westphal H, Kwon-Chung J, Murphy P M. Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med. 1997;185:1959–1968. doi: 10.1084/jem.185.11.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham G J, Wright E G, Hewick R, Wolpe S D, Wilkie N M, Donaldson D, Lorimore S, Pragnell I B. Identification and characterization of an inhibitor of haemopoietic stem cell proliferation. Nature. 1990;344:442–444. doi: 10.1038/344442a0. [DOI] [PubMed] [Google Scholar]
- 17.Greenberger M J, Strieter R M, Kunkel S L, Danforth J M, Laichalk L L, McGillicuddy D C, Standiford T J. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J Infect Dis. 1996;173:159–165. doi: 10.1093/infdis/173.1.159. [DOI] [PubMed] [Google Scholar]
- 18.Huffnagle G B, Strieter R M, McNeil L K, McDonald R A, Burdick M D, Kunkel S L, Toews G B. Macrophage inflammatory protein-1alpha (MIP-1alpha) is required for the efferent phase of pulmonary cell-mediated immunity to a Cryptococcus neoformans infection. J Immunol. 1997;159:318–327. [PubMed] [Google Scholar]
- 19.Karpus W J, Lukacs N W, McRae B L, Strieter R M, Kunkel S L, Miller S D. An important role for the chemokine macrophage inflammatory protein-1 alpha in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J Immunol. 1995;155:5003–5010. [PubMed] [Google Scholar]
- 20.Laichalk L L, Bucknell K A, Huffnagle G B, Wilkowski J M, Moore T A, Romanelli R J, Standiford T J. Intrapulmonary delivery of tumor necrosis factor agonist peptide augments host defense in murine gram-negative bacterial pneumonia. Infect Immun. 1998;66:2822–2826. doi: 10.1128/iai.66.6.2822-2826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laichalk L L, Kunkel S L, Strieter R M, Danforth J M, Bailie M B, Standiford T J. Tumor necrosis factor mediates lung antibacterial host defense in murine Klebsiella pneumonia. Infect Immun. 1996;64:5211–5218. doi: 10.1128/iai.64.12.5211-5218.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukacs N W, Kunkel S L, Strieter R M, Warmington K, Chensue S W. The role of macrophage inflammatory protein 1 alpha in Schistosoma mansoni egg-induced granulomatous inflammation. J Exp Med. 1993;177:1551–1559. doi: 10.1084/jem.177.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehrad B, Moore T A, Standiford T J. Macrophage inflammatory protein-1 alpha is a critical mediator of host defense against invasive pulmonary aspergillosis in neutropenic hosts. J Immunol. 2000;165:962–968. doi: 10.4049/jimmunol.165.2.962. [DOI] [PubMed] [Google Scholar]
- 24.Mehrad B, Strieter R M, Moore T A, Tsai W C, Lira S A, Standiford T J. CXC chemokine receptor-2 ligands are necessary components of neutrophil-mediated host defense in invasive pulmonary aspergillosis. J Immunol. 1999;163:6086–6094. [PubMed] [Google Scholar]
- 25.Moore T A, Newstead M W, Strieter R M, Mehrad B, Beaman B L, Standiford T J. Bacterial clearance and survival are dependent on CXC chemokine receptor-2 ligands in a murine model of pulmonary Nocardia asteroides infection. J Immunol. 2000;164:908–915. doi: 10.4049/jimmunol.164.2.908. [DOI] [PubMed] [Google Scholar]
- 26.Moore T A, Standiford T J. The role of cytokines in bacterial pneumonia: an inflammatory balancing act. Proc Assoc Am Physicians. 1998;110:297–305. [PubMed] [Google Scholar]
- 27.Olszewski M A, Huffnagle G B, McDonald R A, Lindell D M, Moore B B, Cook D N, Toews G B. The role of macrophage inflammatory protein-1alpha/CCL3 in regulation of T cell-mediated immunity to Cryptococcus neoformans infection. J Immunol. 2000;165:6429–6436. doi: 10.4049/jimmunol.165.11.6429. [DOI] [PubMed] [Google Scholar]
- 28.Seebach J, Bartholdi D, Frei K, Spanaus K S, Ferrero E, Widmer U, Isenmann S, Strieter R M, Schwab M, Pfister H, et al. Experimental Listeria meningoencephalitis. Macrophage inflammatory protein-1 alpha and -2 are produced intrathecally and mediate chemotactic activity in cerebrospinal fluid of infected mice. J Immunol. 1995;155:4367–4375. [PubMed] [Google Scholar]
- 29.Sherry B, Tekamp-Olson P, Gallegos C, Bauer D, Davatelis G, Wolpe S D, Masiarz F, Coit D, Cerami A. Resolution of the two components of macrophage inflammatory protein 1, and cloning and characterization of one of those components, macrophage inflammatory protein 1 beta. J Exp Med. 1988;168:2251–2259. doi: 10.1084/jem.168.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Standiford T J, Kunkel S L, Greenberger M J, Laichalk L L, Strieter R M. Expression and regulation of chemokines in bacterial pneumonia. J Leukoc Biol. 1996;59:24–28. doi: 10.1002/jlb.59.1.24. [DOI] [PubMed] [Google Scholar]
- 31.Standiford T J, Kunkel S L, Lukacs N W, Greenberger M J, Danforth J M, Kunkel R G, Strieter R M. Macrophage inflammatory protein-1 alpha mediates lung leukocyte recruitment, lung capillary leak, and early mortality in murine endotoxemia. J Immunol. 1995;155:1515–1524. [PubMed] [Google Scholar]
- 32.Standiford T J, Rolfe M W, Kunkel S L, Lynch III J P, Burdick M D, Gilbert A R, Orringer M B, Whyte R I, Strieter R M. Macrophage inflammatory protein-1 alpha expression in interstitial lung disease. J Immunol. 1993;151:2852–2863. [PubMed] [Google Scholar]
- 33.Standiford T J, Strieter R M, Lukacs N W, Kunkel S L. Neutralization of IL-10 increases lethality in endotoxemia. Cooperative effects of macrophage inflammatory protein-2 and tumor necrosis factor. J Immunol. 1995;155:2222–2229. [PubMed] [Google Scholar]
- 34.Standiford T J, Tsai W C, Mehrad B, Moore T A. Cytokines as targets of immunotherapy in bacterial pneumonia. J Lab Clin Med. 2000;135:129–138. doi: 10.1067/mlc.2000.103196. [DOI] [PubMed] [Google Scholar]
- 35.Standiford T J, Wilkowski J M, Sisson T H, Hattori N, Mehrad B, Bucknell K A, Moore T A. Intrapulmonary tumor necrosis factor gene therapy increases bacterial clearance and survival in murine gram-negative pneumonia. Hum Gene Ther. 1999;10:899–909. doi: 10.1089/10430349950018300. [DOI] [PubMed] [Google Scholar]
- 36.Tessier P A, Naccache P H, Clark-Lewis I, Gladue R P, Neote K S, McColl S R. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J Immunol. 1997;159:3595–3602. [PubMed] [Google Scholar]
- 37.Topham P S, Csizmadia V, Soler D, Hines D, Gerard C J, Salant D J, Hancock W W. Lack of chemokine receptor CCR1 enhances Th1 responses and glomerular injury during nephrotoxic nephritis. J Clin Investig. 1999;104:1549–1557. doi: 10.1172/JCI7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai W C, Strieter R M, Wilkowski J M, Bucknell K A, Burdick M D, Lira S A, Standiford T J. Lung-specific transgenic expression of KC enhances resistance to Klebsiella pneumoniae in mice. J Immunol. 1998;161:2435–2440. [PubMed] [Google Scholar]
- 39.Villalta F, Zhang Y, Bibb K E, Kappes J C, Lima M F. The cysteine-cysteine family of chemokines RANTES, MIP-1α, and MIP-β induce trypanocidal activity in human macrophages via nitric oxide. Infect Immun. 1998;66:4690–4695. doi: 10.1128/iai.66.10.4690-4695.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]