Abstract
Background:
Delayed cord clamping (DCC) and umbilical cord milking (UCM) provide placental transfusion to vigorous newborns. Delayed cord clamping in non-vigorous newborns may not be provided due to perceived need for immediate resuscitation. UCM is an alternative since it can be performed more quickly than DCC and may confer similar benefits.
Objective:
We hypothesized that UCM would reduce admission to the neonatal intensive care unit (NICU) compared with early cord clamping (ECC) in non-vigorous newborns born between 35–42 weeks’ gestation.
Study Design:
A pragmatic cluster-randomized crossover trial of infants born at 35–42 weeks’ gestation from 10 medical centers in 3 countries between January 2019 and May 2021. Centers were randomized to UCM or ECC for approximately one year and then crossed over for an additional year or until the required number of consented subjects was reached. Waiver of consent as obtained in all centers to implement the intervention. Infants were eligible if non-vigorous at birth (poor tone, pale color, or lack of breathing in the first 15 seconds after birth) and were assigned to UCM or ECC according to their birth hospital randomization assignment. Baseline characteristics and outcomes were collected following deferred informed consent. The primary outcome was admission to the NICU for predefined criteria. The main safety outcome was hypoxic-ischemic encephalopathy (HIE). Data were analyzed by intention to treat.
Results:
Among 16,234 screened newborns, 1780 were eligible (905 UCM, 875 ECC) and 1730 had primary outcome data for analysis (97% of eligible; 872 UCM, 858 ECC) via either informed consent (606 UCM, 601 ECC) or waiver of informed consent (266 UCM, 257 ECC). The difference in the frequency of NICU admission using predefined criteria between the UCM (23%) and ECC (28%) groups did not reach statistical significance (modeled OR 0.69, 95% CI 0.41–1.14). UCM was associated with predefined secondary outcomes including a higher hemoglobin (modeled mean difference between UCM and ECC groups 0.68 g/dL, 95% CI 0.31–1.05), lower odds of abnormal 1-minute Apgar scores (Apgar ≤3, 30% vs 34%, crude OR 0.72, 95%CI 0.56–0.92); cardiorespiratory support at delivery (61% vs 71%, modeled OR 0.57, 95% CI 0.33–0.99) and therapeutic hypothermia (3% vs 4%, crude OR 0.57, 95% CI 0.33–0.99). Moderate-severe HIE was significantly less common with UCM (1% vs 3%, crude OR 0.48, 95% CI 0.24–0.96). No significant difference was observed for normal saline bolus, phototherapy, abnormal 5-minute Apgar scores (Apgar ≤6, 15.7% vs 18.8%, crude OR 0.81, 95% CI 0.62–1.06), or a serious adverse event composite of death before discharge.
Conclusions:
Among non-vigorous infants born at 35–42 weeks’ gestation, UCM did not reduce NICU admission for predefined criteria. However, infants in the UCM arm had higher hemoglobin, received less delivery room cardiorespiratory support, had a lower incidence of moderate to severe HIE and received less therapeutic hypothermia. These data may provide the first randomized controlled trial evidence that UCM in non-vigorous infants is feasible, safe and superior to ECC.
Keywords: cord milking, non-vigorous, resuscitation, cord clamping, newborn
Introduction
Each year approximately 6 million infants worldwide require resuscitation at birth. (1) Despite improvements in the quality of resuscitation, morbidity and mortality among infants requiring resuscitation remains high. (2, 3) Epidemiologic studies identify resuscitation as a risk factor for hypoxic-ischemic encephalopathy (HIE) (4, 5), cerebral palsy (6), attention deficit hyperactive disorder (7, 8), autism (7), and neonatal stroke. (5) Optimal cord management to enhance placental transfusion might be an essential step in neonatal stabilization. (9) Several organizations currently recommend delayed cord clamping (DCC) for varying amounts of time to enable placental transfusion in vigorous infants. (9–11) However, the recommended umbilical cord management for non-vigorous infants (limp, pale, and minimal or no breathing) that might require resuscitation is to clamp the umbilical cord immediately at birth.(9, 10)
Umbilical cord milking (UCM) is an alternative to DCC for non-vigorous infants who require resuscitation since it can be accomplished quickly. Similar to DCC, UCM can improve cardiopulmonary transition, support cardiac preload, and reduce morbidity. Compared with early cord clamping (ECC), UCM and DCC improve systemic and brain perfusion, both possibly neuroprotective. (12, 13) UCM has been shown to improve heart rate, blood pressure, urine output, and cerebral oxygenation, increase hemoglobin levels, and prevent anemia in term/near-term infants. (14–16) No harm from UCM has been noted in any study among term/near-term infants. (14–24) Unlike DCC, UCM can achieve significant placental transfusion without significantly delaying resuscitation and be completed almost as quickly as ECC. (25) Despite these potential beneficial effects, neither UCM nor DCC are recommended when resuscitation at birth is needed. (10, 26, 27) The lack of investigations studying optimal placental transfusion methodology in non-vigorous newborns is a major knowledge gap identified by the American College of Obstetricians and Gynecologists (ACOG). (10)
No large randomized controlled trial (RCT) of cord management strategies among non-vigorous full term/near-term infants requiring immediate resuscitation has been published. The challenges using a traditional RCT design are difficult because the need for resuscitation in term/near-term births is unpredictable. Interventions must be performed quickly, making enrollment and randomization difficult or impossible. Pre-consenting families would require approaching virtually all eligible women prior to delivery, potentially anxiety-producing in pregnant women, time-consuming, and study-personnel expensive.
To ensure study protocol adherence and to avoid a biased patient sample related to emergency consent, we conducted a pragmatic, multicenter, cluster-randomized, crossover trial. We hypothesized that UCM would reduce admission to the NICU compared with ECC in non-vigorous newborns born between 35–42 weeks’ gestation, and that the crossover design would minimize differences between NICU sites in resuscitation and admission practices.
Methods
The Milking In Non-vigorous Infants (MINVI) trial was a cluster-randomized, crossover trial conducted at 10 multinational sites (7 in the United States, 2 in Canada and 1 in Poland). Outcomes, enrolment criteria, and cord management practices were standardized between sites. Prior to the trial investigator/coordinator meetings were held to standardize criteria for assessment of the newborn and NICU admission criteria. Investigators reviewed videos of the assessment of a non-vigorous newborn, the milking procedure, and established agreement between sites for both the milking procedure and NICU admission. All centers submitted videos of umbilical cord management procedures recorded locally. Prior to initiating interventions in Period One, each obstetric provider was trained on identification of non-vigorous infants and performing the assigned intervention (ECC or UCM). After Period One, before beginning Period Two, hospitals repeated training with the alternative intervention to ensure obstetric providers would perform the assigned intervention.
The research ethics committee or institutional review board at all participating sites approved a waiver of antenatal consent to implement the intervention and to collect data on death before discharge of any eligible infant. Nine out of 10 of the sites’ ethics boards approved a waiver of informed consent for minimal data collection to obtain delivery room cardiorespiratory support and NICU admission. At the single center that did not approve a waiver for data collection, minimal data could be obtained unless the parents refused to allow for any data collection including the primary outcome. For all eligible newborns, trained site research staff approached parents after delivery for written informed consent for collection of baseline characteristics, outcomes, and enrollment into the neurodevelopmental follow-up portion of the study. An independent data and safety monitoring board (DSMB) monitored the trial.
Eligibility
All sites were selected based on their experience with umbilical cord clamping trials, established research infrastructure, and obstetric compliance and equipoise for ECC and UCM in non-vigorous newborns. Newborns delivered between January 2019 and May 2021 were screened for eligibility. Newborns were eligible if delivered at 35–42 weeks’ gestation dated by best obstetrical estimate using earliest ultrasound or last menstrual period, and were non-vigorous at birth. Non-vigorous status included any of the following if present in the first 15 seconds after birth and prior to cord clamping, as determined by the obstetric provider: poor tone, pallor, or lack of breathing despite initial resuscitation efforts (stimulation, warmth, +/− suctioning) as per neonatal resuscitation program guidelines (Figure 1). (28)
Figure 1.
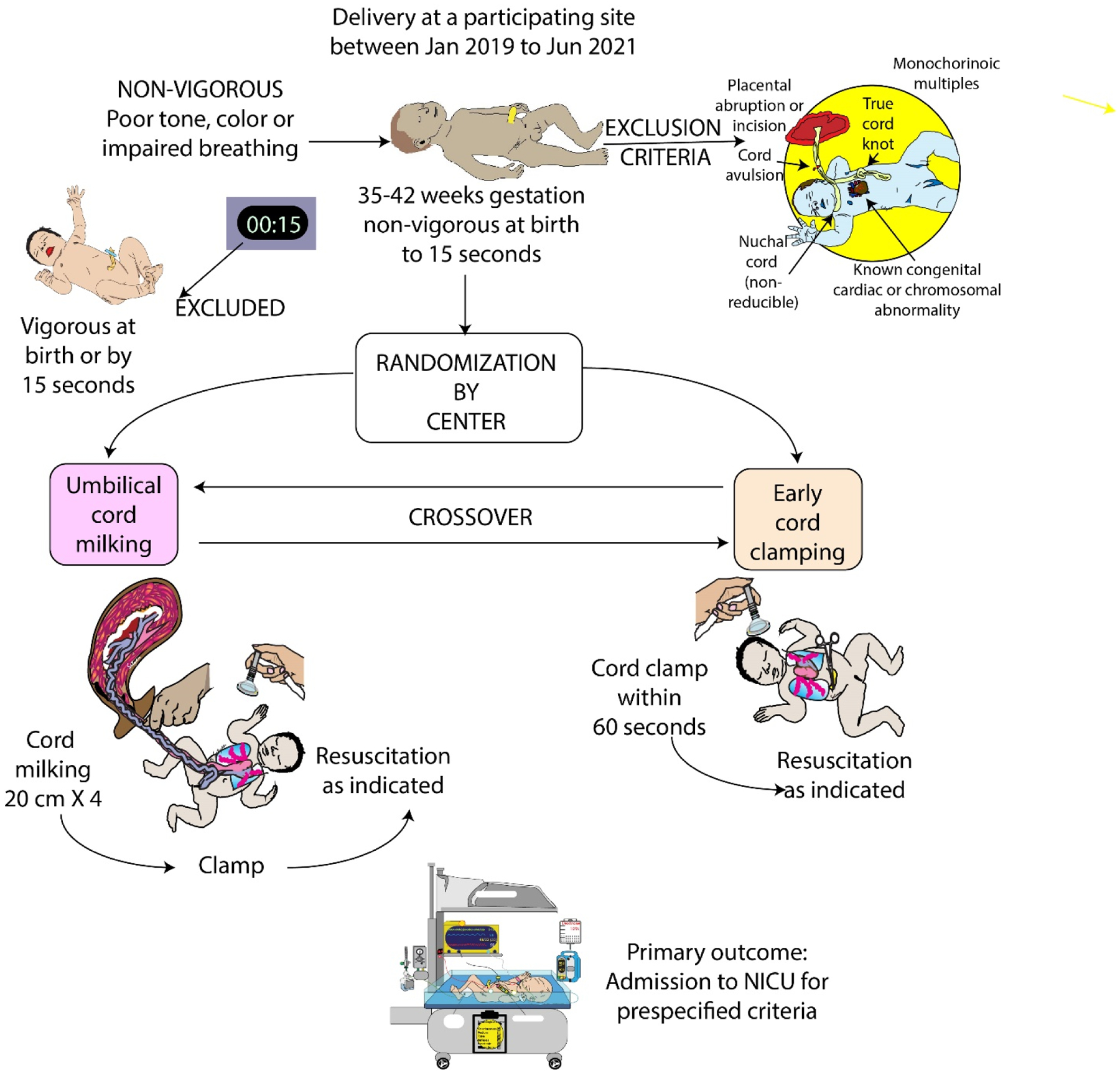
Methods for MINVI trial
Eligible infants had to be considered viable with intention to provide resuscitation if needed. Exclusion criteria included the following conditions known prior to cord clamping: major congenital or chromosomal anomalies of the newborn, cardiac defects other than small ventricular septal defects, complete placental abruption or cutting through the placenta at the time of delivery, monochorionic multiples, cord anomalies such as avulsion or true knots, presence of a non-reducible nuchal cord, and incomplete delivery data to determine eligibility. Neonates were excluded if delivered by an obstetric provider not trained in the study protocol.
Randomization and Crossover
Hospitals were randomized 1:1 (computer-generated allocation sequence) to UCM or ECC in Period One (5 hospitals per treatment group January 2019 to January 2020) until half of required enrollment was reached (N=600 consented subjects), then crossed over to the other intervention during Period Two (February 2020 to May 2021) for the remaining half of consented subjects (N=600). A 1–2 month washout period occurred after Period One, during which sites retrained personnel and assessed adherence to the new intervention. As a pragmatic trial, to minimize selection bias, all deliveries that met eligibility criteria were included for complete enumeration under a waiver of consent. Due to the nature of the intervention only the outcome assessors were blinded, and statisticians reported data as groups A and B until the data were locked.
Delivery Interventions
Infants who were vigorous at birth received usual care (i.e., delayed cord clamping without milking) and were excluded. Non-vigorous newborns received UCM or ECC according to the randomization assignment.
UCM during cesarean delivery required the obstetric provider place the newborn below the level of the incision (at the edge of the table or on a sterile blanket on the mother’s legs) while a second team member milked the cord four times. For vaginal delivery, the obstetric provider held the infant or placed the infant on the mother’s abdomen and the cord was milked four times by either the obstetric provider or a second team member.
For UCM, the provider milked 20 cm of cord over two seconds, repeating three additional times. For ECC the provider clamped the cord within 60 seconds of birth. Since both ECC and UCM occurred after a brief assessment (10–15 second assessment followed by 10 seconds of milking in UCM group) followed by cord clamping, the time of ECC was longer than previous preterm trials (15 seconds) in which the intervention was performed on all subjects regardless of whether they were vigorous. (29) Protocol deviations included infants not treated per randomized treatment group and those who had received DCC (60 seconds or greater).
Outcomes
All outcomes pertain to the individual participant. The primary outcome was admission to the NICU in the first 24 hours of life for predefined criteria: respiratory distress (tachypnea, grunting, retractions), bradycardia or tachycardia, hypotonia, lethargy or difficult to arouse, hypertonia or irritability, poor feeding or emesis, hypoglycemia, oxygen desaturations or cyanosis, need for oxygen, apnea, seizures or seizure-like activity, hyperbilirubinemia, and/or temperature instability. Admissions to the NICU for other causes not related to the intervention (e.g., need for antibiotics) or infants that were placed in the NICU for observation criteria (e.g., abnormal cord gases but well appearing) were coded as not admitted per the primary outcome. All NICU admissions were independently and blindly reviewed. The principal safety outcome was hypoxic-ischemic encephalopathy (HIE). (30) Serious adverse events included death before discharge, polycythemia, hyperbilirubinemia requiring exchange transfusion, severe intraventricular hemorrhage (grade III/IV), and pulmonary hemorrhage. HIE was categorized as mild, moderate and severe using the highest Sarnat stage documented in the first six hours of life. (30) All sites agreed to strictly follow and document the level of HIE. Predefined secondary safety and efficacy outcomes included therapeutic hypothermia, volume expanders, phototherapy, hemoglobin at 24 hours of life, and peak serum bilirubin. Exploratory outcomes included resuscitation interventions, blood pressure, and length of hospitalization.
Sample Size Calculation
A sample size of 1200 was estimated as necessary to test efficacy of UCM versus ECC for the primary outcome based on: i) a clinically meaningful 35% relative reduction in NICU admissions (16% for UCM versus 25% for ECC), ii) a two-sided type I error alpha = 0.05, 85% power, 0.02 rho (within cluster within period correlation), iii) 0.02 eta (within cluster between period correlation), and iv) a correction factor (4 × cluster size) for the small number of clusters.
Statistics
Analyses were performed at the individual participant level and intention-to-treat among neonates with primary outcome data. Descriptive analyses used the Wilcoxon rank-sum test for continuous variables and the chi-square or Fisher’s exact tests for categorical variables. For the primary outcome and secondary outcomes with adequate numbers (i.e., cardiorespiratory support, Apgar score at 1 minute ≤ 3, and Apgar score at 5 minute ≤ 6) hierarchical generalized linear mixed models were used to account for the cluster randomized crossover study design, with fixed treatment group effect, fixed period effect, random cluster effect and random cluster*period effect. Odds ratio (OR) and 95% confidence intervals (CI) were estimated. A similar mixed model accounting for the cluster randomized crossover study design using least squared means was employed for continuous outcomes to estimate the least square means difference and 95% CI. For multinomial outcomes (1-minute and 5-minute Apgar scores with three categories), multinomial logistic regression was used, with fixed treatment group effect only due to reduced sample size in strata. For all rare secondary binomial outcomes, logistic regression was used with fixed treatment group effect only due to small number of outcomes. No additional adjustment was warranted. Two sets of twins were included, too few to account for clustering within a pregnancy, so each neonate was considered independent. No interim analysis for efficacy was performed, a p-value < 0.05 was used to define statistical significance, and all tests were two tailed. P-values for secondary outcomes were not adjusted for multiple testing for safety reasons, as multiple testing may discount a potential harm. Analyses were performed using software (SAS, version 9.4; SAS Institute Inc., Cary, NC).
Subgroup analyses
Prior studies offered little basis for assuming a priori interactions between baseline characteristics and treatment group. Preplanned tests for interactions with treatment assignment were not warranted and not powered by the sample size. However, in accordance with National Institutes of Health (NIH) guidelines, an evaluation of the consistency of the primary outcome across racial-ethnic and neonatal sex subgroups was performed. An evaluation of treatment by site interaction was included.
Results
Enrollment in the trial continued until at least 1200 were consented per the protocol (with a final N=1207). To minimize bias, the sites included all eligible neonates that met criteria if the local IRB allowed primary outcome data via waiver of consent. Together there were 16,234 newborns screened at 10 hospitals; 1780 were eligible (905 UCM, 875 ECC), and 1730 had primary outcome data for analysis (97% of eligible; 872 UCM, 858 ECC). Of the 1730 infants with primary outcome data, 523 infants had minimal demographic and delivery room data obtained using only the waiver of informed consent (266 UCM, 257 ECC). (Supplemental Figure 1). The two treatment groups were similar at baseline except infants in the UCM group were less likely to have delivered in the first period (42% vs 58%, p<0.001), and more likely to have maternal hypertension (20% vs 16%, p=0.04) (Table 1). 94% were treated per protocol UCM group, 96% in the ECC group (Table 2). Median clamping times were 29 seconds (IQR 20, 30) in the UCM group, 20 seconds (IQR 10, 20), in the ECC group.
Table 1.
Maternal characteristics at baseline
| Characteristic | Umbilical cord milking (N=872) | Early cord clamping (N=858) | P-value |
|---|---|---|---|
| Consented | 606 (69.5) | 601 (70.1) | 0.80 |
| Delivered during period 1 | 366 (42.0) | 494 (57.6) | <0.001 |
| Maternal | |||
| Race-ethnicity | 0.03 | ||
| Hispanic | 97 (11.1) | 136 (15.8) | |
| Non-Hispanic Black | 55 (6.3) | 53 (6.2) | |
| Non-Hispanic Asian | 49 (5.6) | 56 (6.5) | |
| Non-Hispanic White | 338 (38.8) | 303 (35.3) | |
| Non-Hispanic Native American | 14 (1.6) | 5 (0.6) | |
| Non-Hispanic Pacific Islander | 6 (0.6) | 9 (1.1) | |
| Non-Hispanic Multiracial | 16 (1.8) | 22 (2.6) | |
| Not stated or unknown | 297 (34.1) | 274(31.9) | |
| Age, yr | 31 (27–35) n=606 | 30 (27–35) n=612 | 0.21 |
| At least some college education | 404/536 (75.4) | 384/508 (75.6) | 0.93 |
| Any diabetes | 106/757 (14.0) | 92/727 (12.7) | 0.45 |
| Any hypertension | 154/757 (20.3) | 118/727 (16.2) | 0.04 |
| Intrauterine inflammation or infection | 84/757 (11.1) | 96/728 (13.2) | 0.22 |
| GBS | 0.17 | ||
| Positive | 164 (21.7) | 139 (19.1) | |
| Negative | 542 (71.7) | 525 (72.1) | |
| Not done or unknown | 50 (6.6) | 64 (8.8) | |
| Rupture of membranes before delivery, hr | 7 (1–14) n=752 | 6 (1–14) n=719 | 0.16 |
| Intravenous or oral narcotic or CNS depressant medication within 2 hours prior to delivery | 63 (10.4) | 66 (10.8) | 0.84 |
| General anesthesia | 41/606 (6.8) | 41/612 (6.7) | 0.96 |
| Multiple gestation* | 21/752 (2.8) | 29/730 (4.0) | 0.21 |
Data are n (%), or median (IQR), unless otherwise noted.
Two sets of twins were included (one set was delivered in period 1 and one set was delivered in period 2; both sets were randomized to umbilical cord milking).
Table 2.
Neonatal and delivery characteristics
| Characteristic | Umbilical cord milking (N=872) | Early cord clamping (N=858) | P-value |
|---|---|---|---|
| Neonatal | |||
| Race-ethnicity | 0.10 | ||
| Hispanic | 113 (13.0) | 152 (17.7) | |
| Non-Hispanic Black | 50 (5.7) | 47 (5.5) | |
| Non-Hispanic Asian | 39 (4.5) | 45 (5.2) | |
| Non-Hispanic White | 298 (34.2) | 273 (31.8) | |
| Non-Hispanic Native American | 8 (0.9) | 4 (0.5) | |
| Non-Hispanic Pacific Islander | 2 (0.2) | 6 (0.7) | |
| Non-Hispanic Multiracial | 61 (6.9) | 54 (6.3) | |
| Not stated or unknown | 301 (34.5) | 277 (32.3) | |
| Mode of delivery | 0.09 | ||
| Vaginal | 395 (45.3) | 397 (46.3) | |
| Cesarean | 362 (41.5) | 321 (37.4) | |
| Unknown | 115 (13.2) | 140 (16.3) | |
| Location of infant during cord treatment | 0.72 | ||
| Mothers abdomen | 463 (53.1) | 429 (50.0) | |
| Mothers thigh | 115 (13.2) | 119 (13.9) | |
| OB held at level of introitus | 101 (11.6) | 100 (11.7) | |
| Below level of introitus | 22 (2.5) | 22 (2.6) | |
| Unknown | 171 (19.6) | 188 (21.9) | |
| Gestational age at delivery, wk | 39.3 (38.1–40.3) | 39.4 (38.1–40.3) | 0.55 |
| Male sex | 460/835 (55.1) | 455/810 (56.2) | 0.66 |
| Birthweight, g | 3355 (3000–3705) n=757 | 3395 (2960–3775) n=730 | 0.55 |
| Poor tone at birth* | 700/756 (92.6) | 682/731 (93.3) | 0.60 |
| Poor breathing at birth* | 694/756 (91.8) | 697/731 (95.4) | 0.005 |
| Pale color at birth* | 702/756 (92.9) | 693/731 (94.8) | 0.12 |
| Breathing or crying before clamping | 165/747 (22.1) | 142/715 (19.9) | 0.29 |
| Treated per protocol | <0.001 | ||
| No | 40 (4.6) | 11 (1.3) | |
| Yes | 815 (93.5) | 819 (95.5) | |
| Unknown | 17 (2.0) | 28 (3.3) | |
| Times milked | 4 (4–4) n=817 | 0 (0–0) n=830 | <0.001 |
| Clamping time, sec | 29 (20–30) n=835 | 20 (10–30) n=792 | <0.001 |
| Clamping time was less than 60 seconds | 816/835 (97.7) | 791/792 (99.9) | <0.001 |
Data are n (%), or median (IQR), unless otherwise noted.
Tone, breathing and color status was not reported due to IRB constraints in 243 non-vigorous neonates (UCM 116 [13.3%], ECC 127 [14.8%])
In order to qualify as non-vigorous obstetricians had to document whether the infants had poor color, tone or breathing. 84% of infants had all three criteria in the UCM group, 87% in the ECC group. Poor tone was reported in 93% of infants in both groups. 93% of the UCM infants had poor color, 95% of the ECC infants. 92% of the UCM infants had poor respiratory effort, 95% of the ECC group (Table 2).
The frequency of NICU admission between the UCM (199/872, 23%) and ECC (239/858, 28%) groups was different (crude OR 0.77 CI 0.62–0.95, Table 3). However, after accounting for study design by site, it was not statistically significant (OR 0.69, 95% CI 0.41–1.14) (Figure 2, Table 3). The most common cause for NICU admission was respiratory distress (UCM N=167 (19%) vs ECC N=189 (22%)). An additional 105 neonates in the UCM (53/872, 6%) and ECC (52/858, 6%) groups were admitted to the NICU for non-study criteria, the most common being observation for 6 hours due to abnormal cord gas without abnormal neurological exam (20 in UCM, 23 in the ECC group).
Table 3.
Primary outcome
| Primary outcome | Umbilical cord milking (N=872) | Early cord clamping (N=858) | Crude odds ratio (95% CI) | Odds ratio (95% CI) accounting for study design |
|---|---|---|---|---|
| NICU admission by predefined criteria* | 199 (22.8) | 239 (27.9) | 0.77 (0.62–0.95) | 0.69 (0.41–1.14) |
| Respiratory distress | 167 (19.2) | 189 (22.0) | ||
| Oxygen desaturation | 40 (4.6) | 53 (6.2) | ||
| Hypoglycemia | 20 (2.3) | 27 (3.2) | ||
| Hypotonia | 15 (1.7) | 29 (3.4) | ||
| Apnea | 14 (1.6) | 17 (2.0) | ||
| Lethargy or difficult to arouse | 5 (0.6) | 13 (1.5) | ||
| Hypertonia or irritability | 4 (0.5) | 5 (0.6) | ||
| Temperature instability | 2 (0.2) | 7 (0.8) | ||
| Bradycardia or tachycardia | 4 (0.5) | 4 (0.5) | ||
| Seizures or seizure-like activity | 3 (0.3) | 3 (0.4) | ||
| Poor feeding or emesis | 2 (0.2) | 3 (0.4) | ||
| Hyperbilirubinemia | 2 (0.2) | 0 | ||
| Death prior to NICU admission† | 0 | 1 (0.1) |
Data are n (%), unless otherwise noted.
Could have met more than one criterion.
Cause of death was hypoxic-ischemic encephalopathy and consistent with the respiratory distress criterion.
Figure 2.
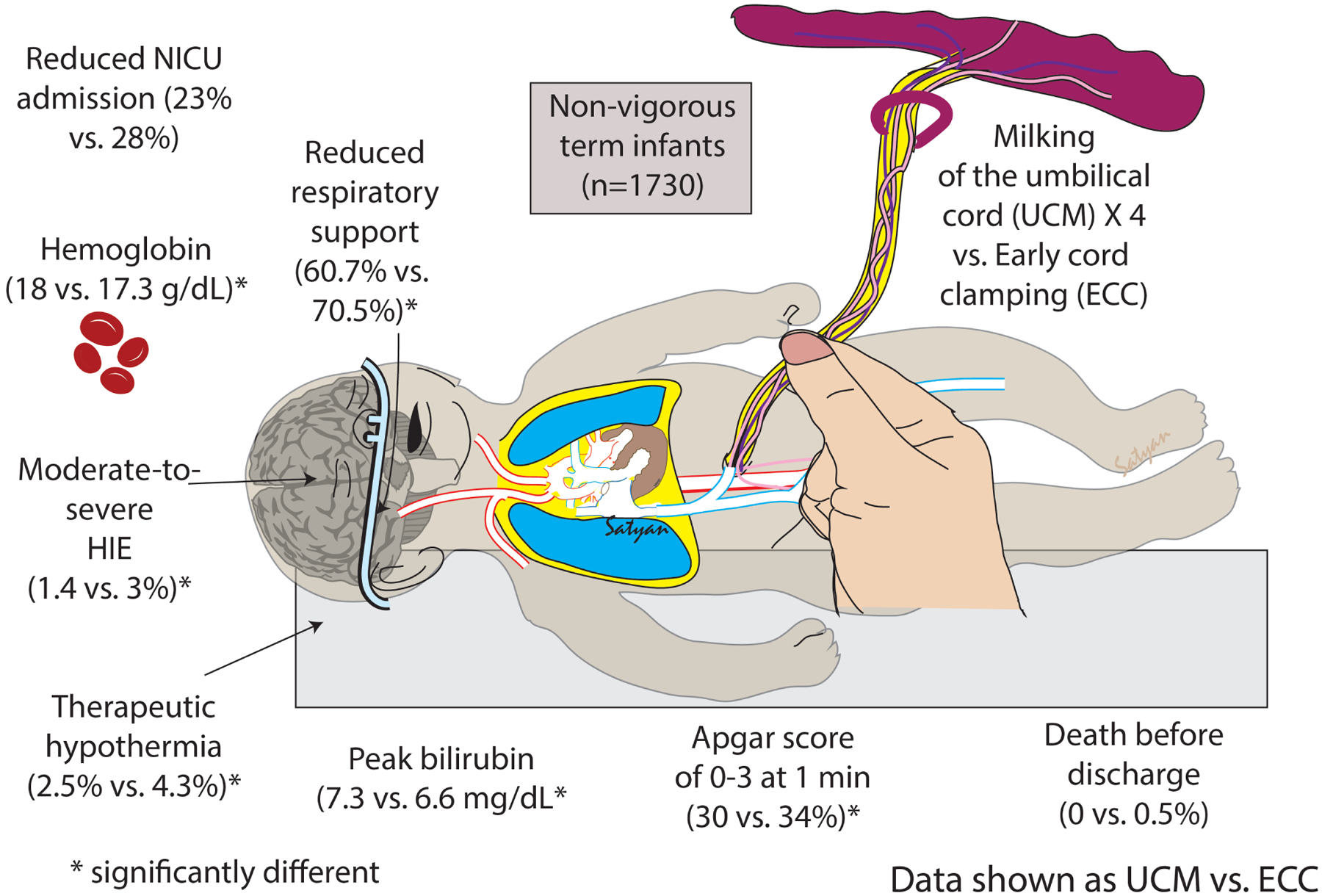
MINVI Trial Results
There was one death in the delivery room in the ECC group. This was a live-born infant not successfully resuscitated with death at 20 minutes after birth and a postmortem diagnosis of HIE.
UCM was associated with lower odds of abnormal 1-minute Apgar scores compared with ECC (Apgar ≤3, 30% vs 34%, crude OR 0.72, 95%CI 0.56–0.92; Apgar 4–6, 33% vs 36%, crude OR 0.74, 95%CI 0.58–0.95) (Table 4), receipt of delivery room cardiorespiratory support (61% vs 71%, modeled OR 0.57, 95% CI 0.33–0.99) and therapeutic hypothermia (3% vs 4%, crude OR 0.57, 95% CI 0.33–0.99). The difference between groups in any grade of HIE was not significant, but moderate-severe HIE was less common with UCM (1% vs 3%, crude OR 0.48, 95% CI 0.24–0.96). No significant difference was observed in 5-minute Apgar, volume expanders, phototherapy, or the serious adverse event composite of death before discharge or severe IVH. There were no cases of polycythemia, hyperbilirubinemia requiring an exchange transfusion, or pulmonary hemorrhage in either group.
Table 4.
Categorical secondary outcomes
| Outcome | Umbilical cord milking (N=872) | Early cord clamping (N=858) | Crude odds ratio (95%CI) | Odds ratio (95% CI) accounting for study design |
|---|---|---|---|---|
| Interventions | ||||
| Cardiorespiratory support* | 460/758 (60.7) | 515/730 (70.5) | 0.64 (0.52–0.80) | 0.57 (0.33–0.99) |
| Supplemental oxygen | 267 (30.6) | 337 (39.3) | ||
| Continuous positive airway pressure | 295 (33.8) | 305 (35.6) | ||
| Positive pressure ventilation (mask or endotracheal tube) | 313 (35.9) | 377 (43.9) | ||
| Intubation | 26 (3.0) | 35 (4.1) | ||
| Compressions | 9 (1.0) | 7 (0.8) | ||
| Medications (e.g. epinephrine, volume) | 2 (0.2) | 3 (0.4) | ||
| Therapeutic hypothermia† | 21/829 (2.5) | 35/806 (4.3) | 0.57 (0.33–0.99) | ‡ |
| Volume bolus (i.e. normal saline) | 58/823 (7.1) | 56/790 (7.1) | 0.99 (0.68–1.45) | ‡ |
| Phototherapy | 88/606 (14.5) | 77/613 (12.6) | 1.18 (0.85–1.64) | ‡ |
| Outcomes | ||||
| Hypoxic-ischemic encephalopathy | 27/828 (3.3) | 38/806 (4.7) | 0.68 (0.41–1.13) | ‡ |
| Mild | 15 (1.8) | 14 (1.7) | ||
| Moderate | 8 (1.0) | 19 (2.4) | ||
| Severe | 4 (0.5) | 5 (0.6) | ||
| Moderate-severe hypoxic-ischemic encephalopathy | 12/828 (1.4) | 24/806 (3.0) | 0.48 (0.24–0.96) | ‡ |
| Any serious adverse event below | 1 (0.1) | 6 (0.7) | 0.16 (0.02–1.36) | ‡ |
| Death before discharge | 0 | 4 (0.5) | ||
| Polycythemia | 0 | 0 | ||
| Hyperbilirubinemia requiring an exchange transfusion | 0 | 0 | ||
| Severe intraventricular hemorrhage§ | 1 (0.1) | 2 (0.2) | ||
| Pulmonary hemorrhage | 0 | 0 | ||
| Apgar score at 1 minute ≤ 3 | 227/757 (30.0) | 248/730 (34.0) | 0.83 (0.67–1.04) | 0.79 (0.53–1.18) |
| Apgar score at 1 minute | ||||
| 0–3 | 227 (30.0) | 248 (34.0) | 0.72 (0.56–0.92) | ‡ |
| 4–6 | 246 (32.5) | 260 (35.6) | 0.74 (0.58–0.95) | ‡ |
| 7–10 | 284 (37.5) | 222 (30.4) | Ref | |
| Apgar score at 5 minute ≤ 6 | 119/756 (15.7) | 137/730 (18.8) | 0.81 (0.62–1.06) | 0.77 (0.58–1.03) |
| Apgar score at 5 minute | ||||
| 0–3 | 24 (3.2) | 19 (2.6) | 1.18 (0.64–2.17) | ‡ |
| 4–6 | 95 (12.6) | 118 (16.2) | 0.75 (0.56–1.00) | ‡ |
| 7–10 | 637 (84.3) | 593 (81.2) | Ref |
Data are n (%), unless otherwise noted.
Could have received more than one type of support.
Infants with mild hypoxic-ischemic encephalopathy at some centers received therapeutic hypothermia.
Did not account for study design due to too few outcomes or reduced sample size in strata.
One case in umbilical cord milking group was discovered during outpatient follow-up of in-hospital grade 2 intraventricular hemorrhage diagnosis.
The UCM group had higher hemoglobin (modeled mean difference between UCM and ECC groups 0.68 g/dL, 95% CI 0.31–1.05), and peak serum bilirubin (modeled mean difference between UCM and ECC groups 1.4 mg/dL, 95% CI 0.5–2.2) (Table 5). No differences were observed for mean blood pressure or length of hospitalization.
Table 5.
Continuous secondary outcomes
| Outcome | Umbilical cord milking (N=872) | Early cord clamping (N=858) | Crude least squares mean difference (95%CI)* | Least squares mean difference (95%CI)* accounting for study design |
|---|---|---|---|---|
| Hemoglobin, g/dL† | 18.0 (16.3–19.4) n=298 | 17.3 (15.7–18.8) n=382 | 0.6 (0.2– 0.97) | 0.7 (0.3– 1.1) |
| Peak serum bilirubin, mg/dL† | 8.4 (5.9–11.5) n=370 | 7.0 (5.5–10.1) n=450 | 0.9 (0.4– 1.4) | 1.4 (0.5– 2.2) |
| Transcutaneous bilirubin, mg/dL† | 7.1 (5.2–9.5) n=393 | 6.5 (4.7–8.8) n=319 | 0.7 (0.1– 1.2) | 0.1 (−1.1– 1.3) |
| Mean blood pressure, mmHg† | 48 (43–56) n=182 | 46 (41–54) n=196 | 2 (−0– 4) | 2 (−0– 4) |
| Length of hospitalization among those discharged alive, d | 3 (3–5) n=606 | 3 (3–4) n=613 | 0.3 (−0.4– 0.9) | 0.3 (−0.4– 0.9) |
Data are median (IQR), unless otherwise noted.
The 95% CIs in this table are for least squares mean differences. A zero would indicate no difference. Non-significant results are represented by confidence intervals that include zero (i.e., range is from a negative value to a positive value). Significant results are represented by confidence intervals that do not include zero.
Among subset (n provided for each measurement) of neonates with data for that measurement.
No significant interaction was observed between treatment assignment and maternal race-ethnicity or neonatal sex. A significant interaction was observed for site (p-value for interaction = 0.008) suggesting the frequency of NICU admission varied across sites and treatment assignment. Approximately 22% of the total variation (from covariance parameter estimates) in the primary outcome was hospital of birth (site). The inter-cluster coefficients (ICC) indicate that part of the total variation in the probability of NICU admission was accounted for by differences across sites (ICC=0.16), and to a lesser degree, by the differences between periods within each site (ICC=0.10). Furthermore, Supplemental Figure 2 provides a visual summary of the differences in NICU admission by site and also shows that NICU admissions were higher at the hospitals that performed UCM first.
Post hoc analyses were conducted to evaluate the primary outcome across hospital subgroups defined by delivery volume (low <5000 deliveries per year or high ≥5000 deliveries per year) and by hospital type (community or university). A significant interaction was observed between treatment group and delivery volume (p-value for interaction delivery volume*treatment group= 0.02). There was a large primary treatment effect in NICU admission by treatment arm (UCM 22.4% vs ECC 36.7% OR 0.42 (0.26–0.70)) for hospitals with ≥5000 births per year) compared with hospitals with < 5000 births per year (UCM 23.1% vs ECC 23.1% OR 1.04 (0.44–1.41), Supplemental Table 1). No significant interaction was observed between treatment assignment and hospital type.
Comment
Principal Findings
UCM in the near-term/term non-vigorous newborn was not associated with a reduction in NICU admissions compared with ECC. UCM was associated with a reduction in cardiorespiratory support in the delivery room, fewer cases of moderate to severe hypoxic-ischemic encephalopathy, lower use of therapeutic hypothermia, and higher hemoglobin. There was no evidence of harm associated with UCM compared with ECC.
Results in the Context of What is Known
ACOG acknowledges that “infants requiring resuscitation may benefit considerably from placental transfusion, but their need for immediate attention raises questions about whether they should undergo [early] cord clamping or whether umbilical cord milking may offer unique benefits.” (31) UCM is currently not recommended by any national or international body of experts for healthy or non-vigorous newborns. (28, 32, 33) This study provides evidence that UCM is safe for the non-vigorous term/near-term newborn and provides possible newborn health advantages compared with ECC.
Clinical Implications
When the umbilical cord is cut immediately after birth, the infant has no access to approximately 30 percent of the total fetal-placental blood volume. (34) Fetal-placental cord blood contains billions of stem cells (35), 15 mL/kg of red blood cell volume to improve oxygen carrying capacity and sustain iron stores, neuroprotective agents such as progesterone (36), as well as essential cytokines, growth factors, and messenger cells that can improve transition. (37) Denying this additional blood volume by ECC can increase the vulnerability of infants to inflammatory processes and ischemia due to blood loss. (38) In older physiologic studies comparing UCM or DCC with ECC, ECC resulted in less favorable outcomes: hypovolemia, lower blood pressures, increased vascular resistance, decreased red cell flow to brain and intestines, less renal blood flow, lower urine output, increased sodium excretion, lower red cell volume, hematocrit, and hemoglobin levels. (39–44) Data from animal studies suggest clamping the cord before the onset of breathing leads to decreases in heart rate, right ventricular output, and pulmonary blood flow, while causing a spike in carotid artery blood flow. (45) ECC results in an increase in afterload and a decrease in preload, which causes a significant reduction in cardiac output. (45)
These data may provide the first evidence that milking the intact cord prior to cord clamping not only reduces the negative impacts of early cord clamping but enhances additional blood volume that can improve newborn health. McAdams et al measured collected blood after four cord milkings (46) and Mercer et al after five and reported ~15 mL/kg and ~17 mL/kg, respectively.(47) Our observed greater hemoglobin with UCM compared to ECC is consistent with these findings. UCM may provide a stimulatory effect promoting earlier initiation of breathing, heart rate, and tone by the first minute of life. This is supported by the observed improvement in 1-minute Apgar score and need for fewer cardiorespiratory interventions, oxygen administration and positive pressure ventilation. The subsequent benefits of additional fetal hemoglobin, progesterone, stem cells, and other key factors may be protective against brain injury as suggested by the lower incidence of moderate-severe HIE and decreased need for therapeutic hypothermia. Serum bilirubin was increased in the UCM infants but was not associated with increased phototherapy. Bilirubin, an abundant antioxidant in the newborn, may be associated with improved neurodevelopmental outcomes in newborns with HIE. (48) Increasing neonatal blood volume with resultant additional antioxidants may alleviate or prevent potential oxidative stress as well as hypoperfusion and ischemia. Higher fetal hemoglobin provided by UCM or DCC can result in additional oxygen delivery to organs that have suffered hypoxic injury. The MINVI study infants are being followed for 2-year neurodevelopmental outcomes (NCT03621943).
Research Implications
Our study suggests that UCM reduces the need for cardiorespiratory support in the delivery room, the incidence of moderate to severe HIE and the use of therapeutic hypothermia conceivably by enhancing placental transfusion to the infant. Low- and middle-income countries with fewer resources report higher incidence of HIE, so UCM studies in these settings may have a greater impact on neonatal morbidity and mortality. It is also critical to determine whether the short-term benefits of UCM are linked to improved long term neurodevelopmental outcomes.
Strengths and Limitations
This is the largest study of UCM to date and the biggest examining non-vigorous newborns at birth. A prior single center pilot study using UCM for non-vigorous term/near-term infants from India (N=101 infants) compared with ECC did not show any differences in neonatal outcomes. (49)
Several potential biases were addressed. All potentially eligible pregnancies between 35 and 42 weeks’ gestational age were screened and logged so that all eligible infants could be enrolled ensuring the generalizability of our results. The primary outcome of NICU admission was based on pre-defined criteria. All admissions were independently reviewed to confirm the primary outcome. The pre-specified inclusion criteria minimized selection bias since infant appearance at birth rather than actual cord management determined which infants were included. Allocation bias was minimized by randomly assigning all non-vigorous infants in each period to one type of cord management. It was impossible to blind obstetric providers and parents to the assigned treatment arm, but documentation of the intervention (UCM or ECC) in the medical record was discouraged. Members of the data center team were blinded to the aggregate data allocation until data collection was complete. Some data were missing due to parents declining any data collection, but was obtained for the majority of participants for the primary outcome.
The difference in NICU admissions between the treatment arms was not statistically significant after accounting for the study design, however site delivery volume was a significant effect modifier. Lower delivery centers (<5000 deliveries per year) did not see a significant treatment effect, however a significant lower odds of NICU admission was observed with UCM in large delivery hospitals (≥5000 deliveries per year). There may be several plausible explanations for the variable results associated with delivery volumes or size of the NICU that we are unable to analyze in a robust manner. Whether UCM of non-vigorous infants might have differential effects depending upon the number of a hospitals newborn deliveries per year needs further testing.
Conclusions
In non-vigorous infants born at 35–42 weeks’ gestation, UCM did not reduce NICU admission for predefined criteria. UCM infants received less delivery room cardiorespiratory support, had a lower incidence of moderate to severe HIE, received less therapeutic hypothermia, and had a higher hemoglobin. UCM appears to be safe and feasible, with no obvious harms. These data provide the first RCT evidence that milking the intact umbilical cord prior to clamping in non-vigorous infants may well be an important, affordable, and easy potentially better practice.
Supplementary Material
AJOG at a Glance.
A. Why was the study conducted?
Delayed cord clamping (DCC) and umbilical cord milking (UCM) provide a placental transfusion to vigorous newborns. However, delayed cord clamping in non-vigorous newborns might not be performed due to perceived need for immediate resuscitation. UCM is an alternative since placental transfusion can be accomplished more quickly than delayed cord clamping and may confer similar benefits. This study assessed the safety and efficacy of UCM in non-vigorous term/near-term newborns compared with traditional early cord clamping (ECC) at birth.
B. What are the key findings?
Among non-vigorous infants born at 35–42 weeks’ gestation, UCM did not reduce NICU admission for predefined criteria. However, infants in the UCM arm had higher hemoglobin, received less delivery room cardiorespiratory support, and had a lower incidence of moderate to severe hypoxic-ischemic encephalopathy with decreased use of therapeutic hypothermia. Milking the intact umbilical cord prior to clamping in non-vigorous infants is feasible, safe and may improve certain outcomes compared with ECC.
Acknowledgments:
The members that participated in the MINV trials are as follows:
Sharp Mary Birch Hospital for Women & Newborns, Neonatal Research Institute, San Diego, CA, United States of America- Anup Katheria, MD, Yvonne Gollin, MD, Paul Wozinak, MD, Katherine Baker, RN, Wade Rich, RCP, Ana Morales, MPH, Debra Poeltler, PhD, Yvonne Vaucher, MD, MPH, Judith Mercer, PhD, Neil Finer, MD, Sarah Steen, RN, Kathy Arnell, RN, Shashank Sanjay, MS, Elizabeth Chan, Alona Diem and Marcie Portillo.
University of Utah School of Medicine, Maternal Fetal Medicine & Neonatology, Salt Lake City, United States of America- Bradley Yoder, MD, Erin Clark, MD, Carrie, Rau, RN, Kandace McGrath, Dawn Bentley, MSN, RN.
University of Alberta, Neonatology, Alberta, Canada-Georg Schmoelzer, MD, PhD, Brenda Hiu Yan Law, MD, PhD, Caroline Fray, RN, Melba Athaide.
Dalhousie University, Neonatology & Obstetrics, Halifax, AB, Canada- Walid El-Naggar, MD, FRCPC, David Rittenberg, MD, Tara Hatfield, Cari-Lee Carnell.
LLUCH, Loma Linda, Loma Linda, CA, United States of America- Courtney Martin, MD, Farha Vora, MD, Jackie Lopez, Daisy Sekly, Brenda Gonzalez, LVN.
University of California Davis Children’s Hospital, Sacramento, United States of America- Satyan Lakshminrusimha, MD, Mark Underwood MD, Deb Wright, MD, Iesha Miller, Rosa Pesavento.
Poznan University of Medical Science, Poznan University of Medical Science, Ponzan, Poland¬- Jan Mazela, MD, Agnieszka Basiukajc, MD, Joanna Powiertowska.
Providence St. Vincent Medical Center, Providence St. Vincent Medical Center, Oregon, United States of America- Joseph Kaempf, MD, Mark Tomilson MD, Alexis Young, Jennifer Geranios, Jesse Wallace, Abigail Kernan-Schloss.
Sharp Grossmont Hospital, La Mesa, CA, United States of America, La Mesa, CA, United States of America- MD, Kevin Fulford, MD, Yvonne Goff, MD, Felix Ines, RCP, Gabi Aliyev, RN.
George Washington Medical Faculty Associates & Obstetrics, Washington, DC- Sheetal Sheth, MD, Mohamed A Mohamed, MD, Leslie, Mayri, PhD, Dinan Abdelatif.
George Washington University Biostatistics Center, Washington, DC - Madeline Murguia Rice, PhD, Elizabeth A. Thom, PhD, Laure El ghormli, MS.
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under Award Number R01HD096023. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflict of interest.
Data sharing statements:
Data will be available after the primary follow-up paper is published. Individual participant data (including data dictionaries) will be available upon review by the study PI.
REFERENCES
- 1.Wall SN, Lee ACC, Niermeyer S, English M, Keenan WJ, Carlo W, Bhutta ZA, Bang A, Narayanan I, Ariawan I, Lawn JE. Neonatal resuscitation in low-resource settings: What, who, and how to overcome challenges to scale up? International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2009;107(Suppl 1):S47–S64. doi: 10.1016/j.ijgo.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mercer J, Erickson-Owens D, Skovgaard R. Cardiac asystole at birth: Is hypovolemic shock the cause? Med Hypotheses. 2009;72(4):458–63. Epub 2009/01/06. doi: 10.1016/j.mehy.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Menticoglou S, Schneider C. Resuscitating the Baby after Shoulder Dystocia2016;2016:8674167. doi: 10.1155/2016/8674167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez A, Ritter S, Brotschi B, Werner H, Caflisch J, Martin E, Latal B. Long-term neurodevelopmental outcome with hypoxic-ischemic encephalopathy. J Pediatr. 2013;163(2):454–9. Epub 2013/03/19. doi: 10.1016/j.jpeds.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Biarge M, Cheong JL, Diez-Sebastian J, Mercuri E, Dubowitz LM, Cowan FM. Risk Factors for Neonatal Arterial Ischemic Stroke: The Importance of the Intrapartum Period. J Pediatr. 2016;173:62–8.e1. Epub 2016/04/07. doi: 10.s6/j.jpeds.2016.02.064. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen LF, Schendel D, Grove J, Hvidtjorn D, Jacobsson B, Josiassen T, Vestergaard M, Uldall P, Thorsen P. Asphyxia-related risk factors and their timing in spastic cerebral palsy. Bjog. 2008;115(12):1518–28. Epub 2008/11/28. doi: 10.1111/j.1471-0528.2008.01896.x. [DOI] [PubMed] [Google Scholar]
- 7.Getahun D, Rhoads GG, Demissie K, Lu SE, Quinn VP, Fassett MJ, Wing DA, Jacobsen SJ. In utero exposure to ischemic-hypoxic conditions and attention-deficit/hyperactivity disorder. Pediatrics. 2013;131(1):e53–61. Epub 2012/12/12. doi: 10.1542/peds.2012-1298. [DOI] [PubMed] [Google Scholar]
- 8.Zhu T, Gan J, Huang J, Li Y, Qu Y, Mu D. Association Between Perinatal Hypoxic-Ischemic Conditions and Attention-Deficit/Hyperactivity Disorder: A Meta-Analysis. J Child Neurol. 2016;31(10):1235–44. Epub 2016/05/28. doi: 10.1177/0883073816650039. [DOI] [PubMed] [Google Scholar]
- 9.Textbook of Neonatal Resuscitation (NRP), 7th Ed. Weiner GM, Zaichkin J, editors 2016. 326 p. [Google Scholar]
- 10.Committee Opinion No. 684: Delayed Umbilical Cord Clamping After Birth. Obstet Gynecol. 2017;129(1):e5–e10. Epub 2016/12/22. doi: 10.1097/aog.0000000000001860. [DOI] [PubMed] [Google Scholar]
- 11.WHO Guidelines Approved by the Guidelines Review Committee. Guidelines on Basic Newborn Resuscitation. Geneva: World Health Organization.; 2012. [PubMed] [Google Scholar]
- 12.Katheria AC. The effects of umbilical cord milking on hemodynamics and neonatal outcomes in premature neonates. American journal of perinatology. 2014;164(5):1045–50.e1. Epub 2013/10/11. doi: 10.1055/s-0033-135726410.1016/j.jpeds.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 13.Takami T, Suganami Y, Sunohara D, Kondo A, Mizukaki N, Fujioka T, Hoshika A, Akutagawa O, Isaka K. Umbilical cord milking stabilizes cerebral oxygenation and perfusion in infants born before 29 weeks of gestation. J Pediatr. 2012;161(4):742–7. Epub 2012/05/15. doi: 10.1016/j.jpeds.2012.03.053. [DOI] [PubMed] [Google Scholar]
- 14.Colozzi AE. Clamping of the umbilical cord; its effect on the placental transfusion. N Engl J Med. 1954;250(15):629–32. Epub 1954/04/15. doi: 10.1056/nejm195404152501502. [DOI] [PubMed] [Google Scholar]
- 15.Upadhyay A, Gothwal S, Parihar R, Garg A, Gupta A, Chawla D, Gulati IK. Effect of umbilical cord milking in term and near term infants: randomized control trial. Am J Obstet Gynecol. 2013;208(2):120.e1–6. Epub 2012/11/06. doi: 10.1016/j.ajog.2012.10.884. [DOI] [PubMed] [Google Scholar]
- 16.Erickson-Owens DA, Mercer JS, Oh W. Umbilical cord milking in term infants delivered by cesarean section: a randomized controlled trial. J Perinatol. 2012;32(8):580–4. Epub 2011/11/19. doi: 10.1038/jp.2011.159. [DOI] [PubMed] [Google Scholar]
- 17.Walsh SZ. Early clamping versus stripping of card: comparative study of electrocardiogram in neonatal period. British Heart Journal. 1969;31(1):122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaiswal P, Upadhyay A, Gothwal S, Chaudhary H, Tandon A. Comparison of Umbilical Cord Milking and Delayed Cord Clamping on Cerebral Blood Flow in Term Neonates. Indian J Pediatr. 2015. Epub 2015/05/27. doi: 10.1007/s12098-015-1734-2. [DOI] [PubMed] [Google Scholar]
- 19.Al-Wassia H, Shah PS. Efficacy and safety of umbilical cord milking at birth: a systematic review and meta-analysis. JAMA Pediatr. 2015;169(1):18–25. Epub 2014/11/05. doi: 10.1001/jamapediatrics.2014.1906. [DOI] [PubMed] [Google Scholar]
- 20.Katheria AT, Cousins L, Oshiro B, Finer N. A randomized controlled trial of umbilical cord milking compared to delayed cord clamping in premature infants. 2015. [Google Scholar]
- 21.Katheria A, Rich W, Finer N. Development of a strategic process using checklists to facilitate team preparation and improve communication during neonatal resuscitation. Resuscitation. 2013;84(11):1552–7. doi: 10.1016/j.resuscitation.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Katheria A, Blank D, Rich W, Finer N. Umbilical cord milking improves transition in premature infants at birth. PLoS One. 2014;9(4):e94085. doi: 10.1371/journal.pone.0094085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosono S, Mugishima H, Fujita H, Hosono A, Minato M, Okada T, Takahashi S, Harada K. Umbilical cord milking reduces the need for red cell transfusions and improves neonatal adaptation in infants born at less than 29 weeks’ gestation: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2008;93(1):F14–9. Epub 2007/01/20. doi: 10.1136/adc.2006.108902. [DOI] [PubMed] [Google Scholar]
- 24.Katheria AC, Leone TA, Woelkers D, Garey DM, Rich W, Finer NN. The effects of umbilical cord milking on hemodynamics and neonatal outcomes in premature neonates. The Journal of pediatrics. 2014;164(5):1045–50 e1. doi: 10.1016/j.jpeds.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 25.Mercer JS, Erickson-Owens DA. Rethinking placental transfusion and cord clamping issues. J Perinat Neonatal Nurs. 2012;26(3):202–17; quiz 18–9. Epub 2012/07/31. doi: 10.1097/JPN.0b013e31825d2d9a. [DOI] [PubMed] [Google Scholar]
- 26.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early human development. 2010;86(6):329–38. Epub 2010/06/18. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Lee AC, Kozuki N, Blencowe H, Vos T, Bahalim A, Darmstadt GL, Niermeyer S, Ellis M, Robertson NJ, Cousens S, Lawn JE. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatric research. 2013;74 Suppl 1:50–72. Epub 2013/12/25. doi: 10.1038/pr.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyckoff MH, Aziz K, Escobedo MB, Kapadia VS, Kattwinkel J, Perlman JM, Simon WM, Weiner GM, Zaichkin JG. Part 13: Neonatal Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S543–60. doi: 10.1161/CIR.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 29.Katheria AC, Leone TA, Woelkers D, Garey DM, Rich W, Finer NN. The effects of umbilical cord milking on hemodynamics and neonatal outcomes in premature neonates. J Pediatr. 2014;164(5):1045–50.e1. Epub 2014/02/25. doi: 10.1016/j.jpeds.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 30.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33(10):696–705. Epub 1976/10/01. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 31.Delayed Umbilical Cord Clamping After Birth: ACOG Committee Opinion Summary, Number 814. Obstetrics & Gynecology. 2020;136(6):1238–9. doi: 10.1097/aog.0000000000004168. [DOI] [PubMed] [Google Scholar]
- 32.Perlman JM, Wyllie J, Kattwinkel J, Wyckoff MH, Aziz K, Guinsburg R, Kim HS, Liley HG, Mildenhall L, Simon WM, Szyld E, Tamura M, Velaphi S. Part 7: Neonatal Resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations (Reprint). Pediatrics. 2015;136 Suppl 2:S120–66. Epub 2015/10/17. doi: 10.1542/peds.2015-3373D. [DOI] [PubMed] [Google Scholar]
- 33.Finer NN, Katheria A. Slow to change: new cord clamping policies for premature infants. Journal of neonatal-perinatal medicine. 2014;7(2):85–7. Epub 2014/08/12. doi: 10.3233/npm-14814013. [DOI] [PubMed] [Google Scholar]
- 34.Yao AC, Moinian M, Lind J. Distribution of blood between infant and placenta after birth. Lancet. 1969;2(7626):871–3. Epub 1969/10/25. [DOI] [PubMed] [Google Scholar]
- 35.Lawton C, Acosta S, Watson N, Gonzales-Portillo C, Diamandis T, Tajiri N, Kaneko Y, Sanberg PR, Borlongan CV. Enhancing endogenous stem cells in the newborn via delayed umbilical cord clamping. Neural Regeneration Research. 2015;10(9):1359–62. doi: 10.4103/1673-5374.165218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.González-Orozco JC, Camacho-Arroyo I. Progesterone Actions During Central Nervous System Development. Frontiers in Neuroscience. 2019;13. doi: 10.3389/fnins.2019.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhury S, Saqibuddin J, Birkett R, Falcon-Girard K, Kraus M, Ernst LM, Grobman W, Mestan KK. Variations in Umbilical Cord Hematopoietic and Mesenchymal Stem Cells With Bronchopulmonary Dysplasia. Frontiers in Pediatrics. 2019;7. doi: 10.3389/fped.2019.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajnik M, Salkowski CA, Thomas KE, Li YY, Rollwagen FM, Vogel SN. Induction of early inflammatory gene expression in a murine model of nonresuscitated, fixed-volume hemorrhage. Shock. 2002;17(4):322–8. Epub 2002/04/17. [DOI] [PubMed] [Google Scholar]
- 39.Nelle M, Zilow EP, Kraus M, Bastert G, Linderkamp O. The effect of Leboyer delivery on blood viscosity and other hemorheologic parameters in term neonates. Am J Obstet Gynecol. 1993;169(1):189–93. Epub 1993/07/01. [DOI] [PubMed] [Google Scholar]
- 40.Oh W, Lind J. Venous and Capillary Hematocrit in Newborn Infants and Placental Transfusion. Acta Pædiatrica. 1966;55(1):38–48. doi: 10.1111/j.1651-2227.1966.tb15207.x. [DOI] [PubMed] [Google Scholar]
- 41.Oh W, Oh MA, Lind J. Renal Function and Blood Volume in Newborn Infant Related to Placental Transfusion. Acta Pædiatrica. 1966;55(2):197–210. doi: 10.1111/j.1651-2227.1966.tb15226.x. [DOI] [Google Scholar]
- 42.Oh W, Carlo WA, Fanaroff AA, McDonald S, Donovan EF, Poole K, et al. Delayed cord clamping in extremely low birth weight infants - a pilot randomized controlled trial. Pediatric Research. 2002;51((4 Suppl)):365–6. [Google Scholar]
- 43.Nelle M, Fischer S, Conze S, Beedgen B, Brischke EM, Linderkamp O. Effects of later cord clamping on circulation in prematures (Abstract). Pediatr Res. 1998;44:420. [Google Scholar]
- 44.Nelle M, Zilow EP, Bastert G, Linderkamp O. Effect of Leboyer childbirth on cardiac output, cerebral and gastrointestinal blood flow velocities in full-term neonates. Am J Perinatol. 1995;12(3):212–6. Epub 1995/05/01. doi: 10.1055/s-2007-994455. [DOI] [PubMed] [Google Scholar]
- 45.Bhatt S, Alison BJ, Wallace EM, Crossley KJ, Gill AW, Kluckow M, te Pas AB, Morley CJ, Polglase GR, Hooper SB. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J Physiol. 2013;591(Pt 8):2113–26. doi: 10.1113/jphysiol.2012.250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAdams RM, Fay E, Delaney S. Whole blood volumes associated with milking intact and cut umbilical cords in term newborns. J Perinatol. 2018;38(3):245–50. Epub 2017/12/14. doi: 10.1038/s41372-017-0002-x. [DOI] [PubMed] [Google Scholar]
- 47.Mercer JS, Erickson-Owens DA, Collins J, Barcelos MO, Parker AB, Padbury JF. Effects of delayed cord clamping on residual placental blood volume, hemoglobin and bilirubin levels in term infants: a randomized controlled trial. J Perinatol. 2017;37(3):260–4. Epub 2016/12/09. doi: 10.1038/jp.2016.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bin-Nun A, Mimouni FB, Kasirer Y, Schors I, Schimmel MS, Kaplan M, Hammerman C. Might Bilirubin Serve as a Natural Antioxidant in Response to Neonatal Encephalopathy? Am J Perinatol. 2018;35(11):1107–12. Epub 2018/04/11. doi: 10.1055/s-0038-1641746. [DOI] [PubMed] [Google Scholar]
- 49.Girish M, Jain V, Dhokane R, Gondhali SB, Vaidya A, Aghai ZH. Umbilical cord milking for neonates who are depressed at birth: a randomized trial of feasibility. Journal of Perinatology. 2018;38(9):1190–6. doi: 10.1038/s41372-018-0161-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available after the primary follow-up paper is published. Individual participant data (including data dictionaries) will be available upon review by the study PI.


