Abstract
Pancreatic ductal adenocarcinoma (PDAC) features a prominent stromal microenvironment with remarkable cellular and spatial heterogeneity that meaningfully impacts disease biology and treatment resistance. Recent advances in tissue imaging capabilities, single-cell analytics, and disease modeling have shed light on organizing principles that shape the stromal complexity of PDAC tumors. These insights into the functional and spatial dependencies that coordinate cancer cell biology and the relationships that exist between cells and extracellular matrix components present in tumors are expected to unveil therapeutic vulnerabilities. We review recent advances in the field and discuss current understandings of mechanisms by which the tumor microenvironment shapes PDAC pathogenesis and therapy resistance.
Keywords: pancreatic cancer, tumor microenvironment, tumor immunology, stroma, cancer-associated fibroblast
1. INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is the most common malignancy of the pancreas and portends a dismal 5-year survival rate of 11% (1). For patients diagnosed with localized surgically resectable disease, outcomes have steadily improved over the past 10 years (1). However, most patients present with regional or distant metastasis, and for these patients, disease progression even with treatment intervention is the norm. Chemotherapy is the mainstay of treatment for PDAC, and while some patients derive significant clinical benefit, ultimately therapeutic resistance prevails. This resistance underscores the resilience of pancreatic cancer and reflects its remarkable capacity to orchestrate a microenvironment that is tumor protective.
The microenvironment that surrounds cancer cells is a key determinant of tumor growth and metastatic potential as well as treatment resistance, an unfortunate hallmark of PDAC (2). The PDAC microenvironment is involved in both primary and acquired resistance to therapies and extends beyond cytotoxic chemotherapies to targeted therapies and immune-modulating treatments (2). As such, there is much to gain from a more thorough understanding of the components of the microenvironment in PDAC and how they impact cancer cell biology. Many of the microenvironmental features that support PDAC pathogenesis are reminiscent of those that direct the development of endoderm-derived tissues. For example, endoderm-derived tissues are shaped by interactions between the epithelium and surrounding mesenchyme (3, 4). In the pancreas, mesenchymal cells control the proliferative capacity and cell fate specification of the developing epithelium by secreting instructive cues that regulate morphogenesis and, in doing so, engage reciprocal signaling pathways. In the context of pancreatic cancer, mesenchymal cells play similar roles as regulators of paracrine signaling and epithelial cell fate but without the exquisite regulation observed in the embryo. Further, PDAC has a particularly prominent mesenchymal compartment within its stroma compared with other solid tumors, often comprising most of the tumor volume. This stromal compartment includes fibroblasts, extracellular matrix (ECM) components, immune cells, nerves, and endothelial cells, which together profoundly impact neoplastic cell phenotypes.
The complexity of the PDAC microenvironment has presented a challenge to efforts aimed at better understanding the diverse cell types and acellular features therein and how they impact tumor progression. This complexity encompasses the mechanisms and consequences of heterotypic cell–cell interactions as well as spatial heterogeneity that impacts cell function and may contribute to PDAC pathogenesis. Spatially heterogeneous features of the PDAC landscape include cellular communities surrounding tumor-infiltrating nerves, tertiary lymphoid structures (TLSs), and distinct stromal versus peritumoral regions. Each of these features may contribute to tumor progression and the prospect for tumor evolution in the context of treatment (Figure 1). However, despite this underlying appreciation for the complexity of PDAC and its surrounding tumor microenvironment, the determinants that direct heterogeneity, adaptability, and dynamics across disease states remain largely ill defined. Addressing these gaps in knowledge forms the basis for ongoing investigations to unravel key pathways and molecules that sustain critical cell–cell interactions in the heterocellular milieu of tumors and to discover spatial relationships among diverse cell types that dictate the fate and function of cancer cells. Herein, we discuss recent insights into the cellular contexture and structure of the tumor microenvironment in PDAC and how the microenvironment that surrounds cancer cells shapes tumor pathogenesis and treatment resistance.
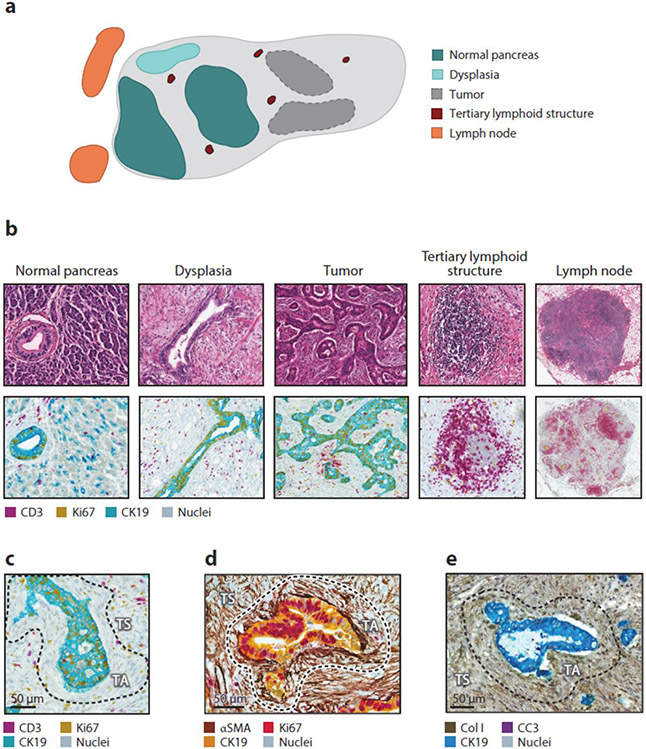
Figure 1.
The PDAC microenvironment. (a) Schematic of PDAC lesion showing discrete cellular communities and their relationship to tumor regions. (b) Representative images showing hematoxylin and eosin staining (top row) and mIHC (bottom row) of cellular communities depicted in panel a. (c)–(e) Representative mIHC images showing the spatial relationship of cells and matrix elements with cancer cells (CK19). Regions of TA and TS stroma are depicted and separated with a dashed black line. Abbreviations: CC3, cleaved caspase 3; Col I, type I collagen; mIHC, multiplex immunohistochemistry; PDAC, pancreatic ductal adenocarcinoma; SMA, smooth muscle actin; TA, tumor-adjacent; TS tumor-associated.
2. THE CELLULAR CONTEXTURE AND STRUCTURE OF THE TUMOR MICROENVIRONMENT
At first glance, the microenvironment that surrounds PDAC appears highly disorganized with a scattered arrangement of cancer cell nests. These nests are bordered by infiltrating cells that compose a stromal reaction that weaves in between. However, histological analyses of PDAC tissues have unveiled spatially distinct tumor subregions that represent unique ecosystems within tumors (5). Subregions enriched in cellular infiltrates (termed reactive) are characterized by transcriptional and proteomic signatures that are distinct from subregions wherein cellular infiltrates are sparse (termed deserted). Reactive subregions are associated with cellular stress responses, whereas deserted subregions are enriched in ECM signaling. Notably, cancer-associated fibroblasts (CAFs) isolated from these distinct subregions are phenotypically and functionally distinct. Specifically, CAFs isolated from reactive subregions appear more motile and are more supportive of tumor proliferation. Together, these findings suggest that the phenotype of the stromal reaction that surrounds cancer cells contributes to tumor cell phenotypes and varies spatially within tumor lesions. In line with this idea, deserted subregions tend to harbor more well-differentiated tumors, whereas reactive subregions are associated with tumors defined by moderate and poor differentiation.
The stromal response to PDAC produces multiple distinct histopathologic regions that culminate in communities defined by unique biological processes. These regions include the immediate tissue adjacent to tumor cells (tumor-associated stroma), the prominent stromal compartment that separates cancer cell nests (tumor-adjacent stroma), TLSs, regions of dysplasia, adjacent normal pancreas tissue, and peritumoral and peripancreatic lymph nodes (Figure 1). Within each of these tissue subregions, the contexture and spatial organization of cells is distinct. For example, quantitation of the leukocyte infiltrate in PDAC further separates tumors into hypoinflamed, myeloid enriched, and lymphoid enriched (Figure 2) (6). Patients with surgically resected tumors characterized as lymphoid enriched tend to have a more favorable prognosis consistent with findings showing that the quality and quantity of T cell infiltrates seen in surgically resected PDAC tumors correlate with survival (7). However, the leukocyte density varies significantly by histopathologic subregion. For instance, adjacent normal tissue often contains a strong leukocyte infiltrate that is not seen in normal healthy pancreatic tissue (6). The predominance of tumor-adjacent stroma also contributes to the significant spatial heterogeneity that is a hallmark of PDAC. To this end, this spatial heterogeneity represents a challenge to the interpretation of the cellular infiltrate on the basis of biopsies or tissue microarrays, which only sample a finite portion of a tumor lesion. Thus, PDAC is defined by multiple discreet cellular communities that coalesce around cancer cell nests and, in doing so, form a neoorgan that intrudes on the normal biology of adjacent healthy tissues.
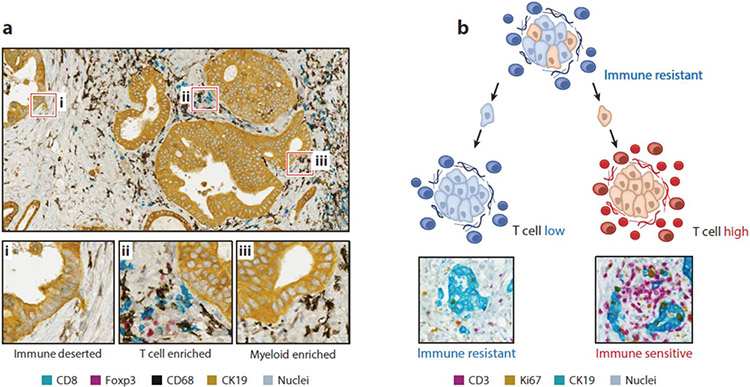
Figure 2.
Spatially defined immune heterogeneity in PDAC. (a) Representative images showing mIHC staining of human PDAC to detect cellular communities that are immune deserted, T cell enriched, and myeloid enriched. (b) Conceptual model depicting a nest of clonally heterogenous cancer cells that upon migration into the stroma give rise to either T cell low or T cell high tumor nests. Shown are representative mIHC images of T cell low and T cell high regions detected in human PDAC. Abbreviations: mIHC, multiplex immunohistochemistry; PDAC, pancreatic ductal adenocarcinoma.
Despite its well-recognized classification as an immunologically cold tumor, PDAC is commonly associated with a prominent leukocyte infiltrate (Figure 2). Notably, tumors often contain TLSs that are reminiscent of lymph node follicles (Figure 1). TLSs are lymphocyte aggregates that arise in peripheral tissues and when present within tumors, including PDAC (8-10), usually associate with a more favorable outcome (11). The maturation of TLSs is a continuum with some tumors containing early TLSs that primarily consist of T and B cells without the high-order architecture that is characteristic of mature TLSs. Overall, tumors that contain TLSs show an increased presence of lymphocytes in the tumor-adjacent stroma (8). However, tumors with mature TLSs, as compared with tumors with early TLSs, associate with more favorable survival outcomes after surgical resection (9). Studies in mouse models have revealed that TLS formation can be induced by chemokines including CXCL13 and CCL21 (10). Further, vaccines induce the formation of TLSs in resectable PDAC that associate with improved survival (12). Nonetheless, the precise mechanisms by which TLSs influence survival outcomes and the stromal response to PDAC remain poorly understood.
PDAC displays a high proclivity for metastasis to distant tissues (e.g., liver, lung, peritoneum, and lymph nodes, among other tissues). Yet, most of our understanding of the pathophysiology of human PDAC is based on findings from primary resection of tumors arising in the pancreas. Historically, primary and metastatic lesions in PDAC were viewed as biologically distinct. However, genetic studies suggest remarkable similarity between matched primary and metastatic lesions, although metastasis may require drivers beyond those involved in primary tumor formation (13, 14). Further, matrix components of the stromal compartment that surround cancer cells appear relatively similar in primary and metastatic PDAC lesions (15). Notably, metastatic lesions acquire and sustain a dense desmoplastic reaction through the recruitment of fibroblasts and leukocytes that is typical of primary tumors (15, 16). However, the distant organ microenvironments (e.g., liver and lung) within which these metastatic lesions most commonly arise differ substantially from the pancreas. Disseminated tumor cells (DTCs) are also subjected to a Darwinian selection process as part of the metastatic cascade (17). Cancer cell–extrinsic features associated with the matrix and cellular contexture of the distant organ microenvironment are known to influence the metastatic behavior of DTCs (18). Thus, despite the apparent genetic and stromal similarities seen between primary and metastatic lesions, it is likely that individual cancer cell clones or clusters of cancer cells give rise to metastatic lesions with unique biological phenotypes that ultimately render them distinct from primary lesions. Together, these observations underscore the biological complexity of PDAC. However, further studies will be needed to better understand the cellular and matrix contexture of both primary and metastatic PDAC lesions in patients and to inform determinants that coordinate the spatial arrangement of cells and proteins within distinct tumor subregions. Addressing these gaps in knowledge is expected to provide novel insights into PDAC pathogenesis, unique therapeutic targets, and biomarkers associated with disease subsets.
3. HOW THE TUMOR MICROENVIRONMENT SHAPES TUMOR PATHOGENESIS
A local wounding environment renders a tissue permissive to tumor formation (19). Consistent with this concept, functional roles for the fibroinflammatory microenvironment in tumor progression have been well established in numerous cancer types, including PDAC. However, the functional roles of stromal cell types are complex and often exhibit context dependency with respect to stage of tumor evolution as well as anatomical site. These complex interactions between evolving tumors and their microenvironment impel careful investigation of cell types and mechanisms that shape PDAC progression, in hopes of parsing those that restrain from those that support tumor growth (Table 1). In the following sections, we discuss the composition of the PDAC microenvironment, the heterogeneity of cellular components therein, and the developing understanding of spatial relationships among these diverse cell types.
Table 1.
Tumor restraining and promoting features of the PDAC microenvironment
| Microenvironmental element | Tumor-restraining features | Tumor-promoting features |
|---|---|---|
| CAF | CAFs restrain tumor progression by depositing matrix proteins (41, 42, 48-50). | CAFs secrete metabolites that support cancer cell proliferation (53-58). CAFs can also suppress antitumor immunity (59). |
| Extracellular matrix | Type I collagen deposition suppresses tumor growth and metastasis (74, 75). | Matrix proteins engage cancer cells through integrins and support cancer cell proliferation and migration (72-74). |
| Myeloid cells (including dendritic cells, macrophages, granulocytes, and early myeloid progenitor cells) | Myeloid cells can be redirected therapeutically with antitumor (114) and antistromal (113, 114) properties. Myeloid cells can be engendered with immune stimulatory properties required for productive antitumor T cell immunity (117, 118). | CD11b+ myeloid cells facilitate PanIN development and PDAC progression (32-34). Myeloid cells also enable many of the hallmarks of cancer (114-116). |
| Treg cells | Treg cells suppress the protumor potential of CAFs (45). | Treg cells can inhibit the efficacy of immunotherapy by suppressing cytotoxic T cells (107). |
| T cells | Tumor-infiltrating cytotoxic T cells can survey and eliminate cancer cells (136, 144). | CD4 T cells secrete IL-17, which supports cancer progression (108), and IL-27, which suppresses effector activity of tumor-infiltrating T cells (25a). |
| B cells | There is insufficient evidence to support a restraining role for B cells. | B cells stimulate cancer cell proliferation via IL-35 (110) and activate tumor-promoting macrophages (111). |
| Vasculature | High expression of genes involved in vascular stability associate with a better prognosis (83). | Increased cancer cell autophagic flux is a consequence of low vascularity due to limited nutrients and supports cancer survival, immune evasion, and therapeutic resistance (53, 127, 128). |
| Peripheral nerves | There is insufficient evidence to support a restraining role for peripheral nerves. | Innervation increases during pancreatic tumorigenesis (96) and supports tumor progression (101, 102). |
Abbreviations: CAF, cancer-associated fibroblast; IL, interleukin; PDAC, pancreatic ductal adenocarcinoma; PanIN, pancreatic intraepithelial neoplasm; Treg, T regulatory cell.
3.1. Tumor Microenvironment Development and Evolution
Reciprocal interactions between transformed epithelial cells and their surrounding microenvironment influence the development and evolution of PDAC. Insights into mechanisms that coordinate this biology have been informed by genetically engineered mouse models that faithfully recapitulate the early stages of pancreatic tumorigenesis. For example, genetic mouse models incorporating epithelial-targeted Kras mutations, which are present in more than 90% of human PDAC (21), form the basis for modeling stepwise PDAC progression (22-24). Moreover, early hints that alterations in the tissue microenvironment functionally contribute to early-stage PDAC progression came from mice harboring KrasG12V, using elastase-driven Cre to target this mutation to acinar and centroacinar cells. While expression of this driver mutation in the pancreatic epithelium throughout development yielded invasive PDAC, induction of KrasG12V in the pancreas of adult mice was insufficient to drive the formation of premalignant lesions and invasive cancer. However, pancreatic inflammation combined with KrasG12V expression effectively triggered premalignant pancreatic intraepithelial neoplasia (PanINs) formation and subsequent invasive PDAC at a high penetrance (24). The inflammatory microenvironment associated with PDAC was later found to suppress oncogene-induced senescence in KrasG12V-expressing epithelial cells, thereby eliminating an important barrier to tumor progression (25). Notably, inflammatory cues induce expression of Kras and its effectors in the adult pancreas, while such expression is only marginal in noninflamed pancreatic tissue. Further, Kras activation drives oncogenic levels of mitogen-activated protein kinase (MAPK) signaling and enables tumor initiation (26). However, inflammation in the absence of an existing Kras mutation also appears to sensitize pancreatic epithelial cells to subsequent transformation by Kras (27). Specifically, pancreatic epithelial cells undergo transcriptional and epigenetic reprogramming during recovery from acute inflammation. While this adaptive response limits tissue damage from subsequent inflammatory events, it also primes epithelial cells for transformation by Kras. Thus, pancreatic tumorigenesis is an evolutionary process that is influenced by cell autonomous and paracrine factors.
Kras-transformed epithelial cells instruct and shape the stromal response in PDAC. The importance of Kras signaling in coordinating the microenvironment has been demonstrated in mouse models featuring inducible and reversible expression of KrasG12D (28, 29). Here, the early response to KrasG12D induction is associated with upregulation of Hedgehog signaling, leukocyte infiltration, and accumulation of a collagen-rich stroma. Formation of this fibroinflammatory stroma and subsequent PanIN development are accelerated when KrasG12D is induced in the context of pancreatitis. However, extinguishing mutant Kras expression in established lesions results in rapid remodeling of the tumor stroma, including reduced abundance of activated fibroblasts and resolution of inflammation (28, 29). This finding illustrates the reciprocal relationship between oncogenic signaling in the pancreatic epithelium and the fibroinflammatory microenvironment.
Oncogenic mutations in Kras promote tumorigenesis via the activation of diverse effector molecules. Kras also cooperates with Myc, a pleiotropic transcription factor involved in regulating a variety of genetic programs that support cellular proliferation (30). Genetic activation of Myc in low-grade KrasG12D-induced PanIN lesions leads to rapid accumulation of immune cells and activated stromal components that together facilitate the development of high-grade PanINs and invasive PDAC (31). Remarkably, inactivation of Myc in the epithelial compartment of established PDAC results in rapid disassembly of the tumor stroma and subsequent tumor cell death, as seen with inactivation of Kras. Thus, these findings demonstrate the dependence of PDAC on both KRAS and MYC signaling, which in tandem support a dynamic relationship between cancer cells and their surrounding microenvironment.
Kras signaling by cancer cells enforces an inflammatory reaction characterized by a robust infiltration of myeloid cells. Tumor-infiltrating myeloid cells are fundamental to establishing an immunosuppressive and protumor microenvironment. In mice, CD11b+ myeloid cells are required for Kras-driven PanIN formation. In addition, CD11b+ myeloid cells support tumor growth (32). Kras activation promotes the infiltration and polarization of myeloid cells to an immunosuppressive phenotype in part via induction of the cytokine GM-CSF (33, 34). However, multiple signals likely converge to establish the inflammatory microenvironment that initiates the early development and progression of PDAC. For example, obesity can promote inflammation and fibrosis in the pancreas, contributing to PanIN development (35, 36). This process is reversible with caloric restriction and is driven by obesity-induced adaptations in the endocrine pancreas that support early-stage PDAC. Specifically, pancreatic islets respond to obesity by upregulating cholecystokinin, which acts in a paracrine manner to promote PDAC development (36). Obesity may also alter the proinflammatory properties of adipocytes and pancreatic stellate cells within tumors that support a desmoplastic reaction (37). Further, diet-induced obesity may promote PDAC tumorigenesis via supporting COX2-dependent inflammation and fibrosis (38) or metabolic aberrations (39). Thus, cancer cell–extrinsic factors (e.g., inflammation and obesity) contribute to the formation of a microenvironment that is pliable and fundamental to pancreatic tumorigenesis.
While inflammation has been shown to be a reproducible proponent of PDAC development and progression, several other prominent stromal components have been implicated in restraining PDAC initiation. Activated fibroblasts adjacent to neoplastic lesions, or CAFs, secrete diverse cytokines, growth factors, and ECM components. CAFs were long thought to be tumor promoting in the context of PDAC. However, landmark studies published in 2014 revealed this to be an oversimplification. Epithelium-derived Hedgehog signaling was initially found to promote pancreatic fibroblast activation and the development of a desmoplastic stroma (40), prompting the expectation that Hedgehog inhibition would suppress desmoplasia and, in turn, tumor formation. Indeed, genetic or pharmacologic inhibition of Hedgehog prior to tumor formation suppresses fibroblast activation and formation of the desmoplastic reaction (41, 42). However, these interventions surprisingly accelerated PanIN formation and PDAC development. Consequently, these studies provided strong evidence for the tumor-restraining potential of CAFs, at least in the early stages of PDAC. While the precise tumor-suppressive mechanisms engaged by PDAC CAFs still remain unclear, type I collagen deletion in fibroblasts can accelerate the emergence of PanIN lesions in the context of mutant Kras and deleted p53 in the epithelial compartment (43). Several immune cell populations in addition to cytotoxic T lymphocytes can also restrain PDAC initiation, including natural killer (NK) T cells (44) and, unexpectedly, T regulatory (Treg) cells (45). The tumor-restraining potential of these immune cells stem from cross talk with other cell populations in the microenvironment, including macrophages in the case of NK T cells and fibroblasts in the case of Treg cells. Altogether, these observations illustrate functionally significant and heterotypic interactions that likely vary in consequence over the course of tumor evolution.
3.2. Organization and Heterogeneity of the Tumor Microenvironment
Interactions among cellular and acellular features of the PDAC microenvironment profoundly impact tumor phenotypes. The diverse nonneoplastic cells that constitute most of the tumor volume in PDAC exhibit inter- and intratumor heterogeneity with respect to their functions and spatial relationships. Our understanding of these microenvironmental components continues to expand, as discussed in the subsections below.
3.2.1. Cancer-associated fibroblasts.
Fibroblasts and CAFs play critical roles in development, tissue homeostasis, and tumor progression (46). However, considerable transcriptional overlap between CAFs and other stromal cell types, together with their extensive heterogeneity (47), has made CAFs challenging to study in specific and robust ways. As a result, the precise contributions of distinct CAF subsets to stepwise tumorigenesis have remained somewhat elusive. In recent years, our understanding of CAF functions in pancreatic cancer has improved dramatically. CAFs may restrain tumor growth by depositing matrix proteins that biophysically contribute to enhanced tissue stiffness and increased interstitial pressures that impede the vasculature and, in doing so, limit accessibility to serum nutrients (41, 42, 48-50). As a result, the nutrient-poor microenvironment that surrounds PDAC may act to restrain cancer cell proliferation (51, 52). However, CAFs also secrete diverse metabolites that are consumed by cancer cells and, in turn, support their proliferation within the nutrient-poor milieu (53-58). Activated pancreatic fibroblasts also release high levels of CXCL12 (59), which signals through its receptor CXCR4 to impact additional cell types in the microenvironment. To this end, CXCR4 signaling in pancreatic epithelial cells supports PanIN initiation and progression (60). However, invasive PDAC eventually develops in mice lacking CXCR4 in the epithelium, and these tumors are larger and less differentiated than CXCR4-expressing tumors. Collectively, these findings illustrate the context-dependent functional consequences of CAFs on PDAC pathogenesis.
CAF features within PDAC are spatially heterogeneous, suggesting distinct functional roles for CAFs depending on their proximity to cancer cells. For instance, CAFs located adjacent to cancer cells exhibit a classically activated, myofibroblast-like phenotype, whereas CAFs present in the surrounding stromal tissue exhibit an inflammatory phenotype characterized in part by cytokine expression (61). In this regard, single-cell genomics have provided new insights into PDAC CAF heterogeneity and revealed the existence of several subtypes including myofibroblastic and inflammatory CAFs (62-64). However, the precise functions of these CAF subtypes and how their transcriptional programs influence cancer cell fate remain unclear.
Cytokines, including interleukin 1 (IL-1) and transforming growth factor beta (TGFβ), differentially shape CAF heterogeneity (63, 65). For instance, IL-1 supports JAK/STAT (Janus kinase/signal transducer and activator of transcription) signaling and differentiation to inflammatory CAFs. In contrast, TGFβ is antagonistic to this pathway and promotes differentiation to myofibroblasts. Cancer cells also shape CAF phenotype by secreting Hedgehog ligands, which have profound, dosage-dependent effects (41, 42, 48, 66, 67). For example, reduced Hedgehog signaling in CAFs correlates with increased tumor-associated vascularity. Cancer cells with a gain-of-function mutant p53 also differentially influence CAFs and promote their capacity to establish a prometastatic environment (68). In addition to paracrine factors derived from cancer cells that may influence CAF biology, CAF heterogeneity also arises due to distinct cells of origin (69, 70). Though PDAC CAFs were previously thought to arise from a common cell of origin—the pancreatic stellate cell (PSC)—a recent fate mapping study demonstrated that PSCs give rise to a numerically minor subset of PDAC CAFs, with unique functional significance (71). Taken together, cytokines, cancer cell–derived factors, and the progenitor cell of origin are determinants of CAF heterogeneity in PDAC.
3.2.2. Extracellular matrix
The ECM that surrounds cancer cells has an active role in PDAC pathogenesis. CAFs are major producers of components of the ECM, which may suppress or support tumor progression. The ECM directly engages cancer cells through integrins and, in doing so, facilitates cancer cell proliferation and migration (72). The ECM also establishes a reservoir of proteins including cytokines and growth factors that influence the metastatic potential of cancer cells. Among the most abundant ECM proteins are fibrillar collagens (e.g., type I and III collagen) (73). Fibrillar collagens are mainly deposited in the ECM by CAFs, although cancer cells also contribute to 2–3% of fibrillar collagen deposition. However, with PDAC progression, there is an enrichment of fibrillar collagens with retained C-prodomains. Notably, cleavage of procollagen I by cancer cell–derived bone morphogenesis protein 1 (BMP1) stimulates collagen I deposition and suppression of tumor growth and metastasis (74). Consistent with this finding, type I collagen deletion in alpha smooth muscle actin (α-SMA+) cells, including CAFs, accelerates PDAC initiation and growth in mice, leading to shortened overall survival (43). In this case, type I collagen deletion causes an upregulation of Cxcl5 in cancer cells, promoting recruitment of myeloid cells, which then facilitate tumor progression (43). In the metastatic microenvironment, deletion of type I collagen in the CAF compartment also accelerates tumor progression with shortened survival (75). However, these findings contrast the effects of CAF deletion, which results in a decrease in metastatic tumor growth, suggesting that type I collagen opposes the tumor-promoting effects of CAFs. Taken together, these observations motivate careful consideration of collagen fibrillogenesis along with its abundance as a determinant of PDAC pathogenesis.
In addition to producing fibrillar collagens, cancer cells contribute to the ECM by producing matrisome proteins that promote tumor progression at various stages of the metastatic cascade. These proteins include agrin, which supports epithelial-mesenchymal transition, and cystatin B and serine protease inhibitor B5, which enhance invadopodia formation and extravasation (76). Further, matrisome proteins derived from cancer cells correlate with poor outcomes (73). Notably, this contrasts the favorable prognosis associated with individual matrisome proteins derived from stromal cells (73). This observation supports a tumor-suppressive role for the ECM in PDAC but also emphasizes the complexity of the ECM and the distinct contribution of components contributed by cancer cells, which act to support tumor pathogenesis.
Cancer cells can also regulate ECM composition and stiffness. For instance, genetic inhibition of TGFβ signaling in the epithelial compartment causes elevated mechanosignaling evidenced by activation of STAT3, MLC2, and YAP1 as well as increased matrix stiffness (77). In patients with PDAC, epithelial TGFβ signaling (assessed by SMAD4 mutation status) also associates with elevated mechanosignaling and tissue stiffness, which together correlate with a worse prognosis (77). Further, increased collagen fiber thickness, rather than abundance, correlates with a worse prognosis (77). These findings may indicate a role for β1-integrin signaling in regulation of epithelial STAT3 activation and ECM stiffness. Thus, components of the ECM are influenced by signaling pathways activated in cancer cells.
Proteoglycans and hyaluronan are additional prominent components of the ECM and contribute to water retention and increased interstitial fluid pressures. In particular, hyaluronic acid (HA) promotes a high-pressure PDAC microenvironment and impedes the vasculature, such that enzymatic digestion of HA with a PEGylated hyaluronidase (PEGPH20) reduces fluid pressures in PDAC mouse models. PEGPH20 also increases vascular perfusion and increases delivery of chemotherapeutic agents, specifically gemcitabine, to the tumor microenvironment in mice (49, 50). Consistent with these preclinical findings, PEGPH20 combined with chemotherapy in patients with PDAC increased tumor response rates. However, progression and overall survival were not improved (78). HA may also serve as a fuel source for pancreatic cancer (79). In addition, HA degradation may enhance the efficacy of immunotherapy, suggesting a role for the ECM in coordinating immune resistance in PDAC (80). Taken together, these studies highlight the complexity of the ECM and its role in shaping cancer cell, CAF, and immune biology.
3.2.3. Endothelial cells
Solid tumors commonly feature abnormal vasculature, including blood and lymphatic vessels. Poor vascularity contributes to a microenvironment characterized by low pH, hypoxia, altered metabolism, and immune evasion. In response to hypoxia, tumors usually invoke mechanisms to stimulate angiogenesis that then support tumor growth and metastasis. In this regard, leaky vessels formed during angiogenesis promote cancer cell aggressiveness at the primary site and serve as a route for hematogenous dissemination (81).
Therapeutic agents that inhibit angiogenesis and/or normalize the tumor vasculature are widely used in the treatment of patients with cancer. For example, bevacizumab is an antiangiogenic agent that binds to and inhibits vascular endothelial growth factor (VEGF). However, despite elevated levels of VEGF detected in PDAC, bevacizumab failed to improve outcomes for patients with PDAC (82). More recently, analysis of PDAC transcriptional profiles has revealed that high expression of the endothelial cell marker CD31 associates with improved overall survival (83). In a multivariate analysis, low CD31 expression and residual tumor after surgery were the only independent prognostic factors associated with worse survival in PDAC. In contrast, high expression of genes involved in vascular stability including TIE1, TIE2, ANGPT1, S1PR1, and CDH5 (VE-cadherin) associate with a better prognosis. CD31 expression also correlates with immune-related genes including CD4, CD8A, GZMB, and several Toll-like receptors, suggesting that increased vessel density may facilitate antitumor immunity. Thus, the considerable heterogeneity observed in vascular density suggests that a subset of patients may in fact benefit from an antiangiogenic agent. Consistent with this idea, mice with highly vascular PDAC showed modest but significant extension of overall survival with VEGF receptor inhibition (41).
Poor vasculature limits nutrient and oxygen delivery to tumors and restricts leukocyte recruitment. As a result, hypovascularity associates with hypoxia in PDAC. Consistent with this, hypoxia-inducible factor 1α (HIF1α), a key mediator of hypoxia, is stabilized in poorly vascularized PDAC tumors in mice (84). Stabilized HIF1α transactivates an array of genes fundamental to metabolism, angiogenesis, cell survival, and inflammation. Elevated HIF1α also associates with a poor prognosis for many malignancies (85). However, in PDAC, deletion of HIF1α accelerates tumor development in mouse models. In the absence of HIF1α, increased pancreatic tumorigenesis is facilitated by infiltrating B cells (86). This finding illustrates the resilience of PDAC and its intricate redundancies that serve to support disease progression.
In addition to the blood vasculature, lymphatics are fundamental to PDAC pathogenesis. Lymphatics not only are a major conduit for leukocytes to transport tumor antigens to draining lymph nodes for presentation to and priming of tumor-reactive T cells but also are a major route of dissemination in PDAC with cancer cells commonly detected in peritumoral lymph nodes, a finding that correlates with worse survival outcomes (87, 88). The determinants of lymphatic vasculature in PDAC and how they contribute to PDAC pathogenesis, though, are poorly understood and yet likely complex. Mechanistically, chemokines are known to contribute to lymphangiogenesis and cell migration. For example, lymphatic endothelial cells secrete CCL21, which lures dendritic cells via lymphatics to lymph nodes. Tumor cells expressing CCR7 may also co-opt this mechanism to disseminate to lymph nodes (89-91). Similarly, CXCL12 produced in lymph nodes may attract CXCR4-expressing cancer cells or leukocytes (92, 93). Thus, the lymphatic vasculature is fundamental to PDAC metastasis and may be a determinant of impaired immune surveillance.
3.2.4. Peripheral nerves
The normal pancreas tissue is highly innervated. Autonomic innervation critically regulates development and hormone secretion by the endocrine pancreas (94, 95). Notably, innervation increases rather dramatically during pancreatic tumorigenesis. This is first observed in premalignant lesions (96). PDAC cell invasion of intrapancreatic nerves, known as perineural invasion (PNI), can promote both pain and tumor spread. While PNI is somewhat common in some tumor types, the incidence of PNI in PDAC is particularly high (>80%) (97). In a recent analysis of long-term survivors after upfront PDAC resection, absence of PNI was the only favorable independent predictor of survival greater than 5 years (98), highlighting a detrimental role for peripheral nerves in tumor progression.
Genetically engineered mouse models of PDAC also show neuroplastic changes. These neuroplastic changes are evident even at the PanIN stage, with focal areas of hyperinnervation coincident with fibrosis (96). In premalignant lesions, tumor-promoting epithelial-neuronal cross talk promotes STAT3 activation and cancer cell proliferation. This observation is attributable, at least in part, to a subpopulation of neuroendocrine PanIN cells that produce the neuropeptide substance P receptor neurokinin 1-R. Interestingly, sensory denervation in mice impairs PanIN progression to PDAC (99), suggesting a role for innervation in directing disease progression.
In invasive PDAC, large intrapancreatic nerve bundles are observed in proximity to tumor cells and vasculature (96). These nerve bundles are likely supported by expression of proinflammatory neurotrophic growth factors, such as nerve growth factor (NGF), that are released by PDAC cells. Consistent with this notion, a neutralizing antibody targeting NGF reduced neural inflammation, neural invasion, and metastasis in PDAC-bearing mice (100). More recent work suggests a feed-forward signaling loop between PDAC cells and tumor-infiltrating sympathetic nerves. For instance, nerves produce catecholamines, which stimulate PDAC development as well as NGF secretion and, subsequently, increased pancreatic nerve density (101). This observation suggests the interdependence between sensory nerves and PDAC. In line with this idea, sensory neuron ablation slows PDAC initiation and progression and prolongs survival. Further, sensory neurons appear to convey inflammatory signals from Kras-driven neoplasia to the central nervous system (102).
While tumor-promoting functions of peripheral axons in the PDAC microenvironment remain poorly understood, the functions of neural components likely extend beyond PNI and metastasis, as evidenced by a recent study suggesting that nerves secrete serine to promote PDAC cell metabolism and growth (103). However, subdiaphragmatic vagotomy has been found to accelerate PDAC progression in mice, suggesting a role in tumor suppression. In addition, cholinergic signaling from parasympathetic nerves was found to be tumor inhibitory in this disease setting (104), in part by suppressing the expansion of cancer stem-like cells. Taken together, these studies highlight the complexity and functional heterogeneity of PDAC-infiltrating nerves and impel further investigation of this prevalent element of the tumor microenvironment.
3.2.5. Leukocytes.
Leukocytes are a prominent component of PDAC tumors. Their phenotype and function contribute to the formation of a microenvironment that is ultimately permissive of tumor progression. The spatial distribution of leukocytes within PDAC tumors is vastly heterogeneous. For example, while some PDAC tumors are associated with an increased frequency of subregions containing T cells, the infiltration of T cells is by no means diffuse; rather, spatial distribution is focal and confined to unique communities that are present adjacent to tumor cells, within the stroma, or as part of TLSs (6). That said, an increased frequency of CD8+ T cells does associate with improved survival for patients with surgically resected PDAC (7, 105). However, tumors with both the highest number of neoantigens—a marker of cancer cell immunogenicity—and a strong CD8+ T cell infiltrate strongly associate with long-term survivors compared with either metric alone (7). These findings illustrate the potential for the immune infiltrate in PDAC to suppress disease progression but also highlight its variable involvement.
Single-cell RNA-sequencing analyses of surgical or fine-needle biopsy specimens have unveiled the remarkable heterogeneity of the leukocyte infiltrate in human PDAC (62, 106). Tumor-infiltrating leukocytes are composed of multiple subsets of T cells, NK cells, B cells, macrophages, and granulocytes including eosinophils, mast cells, and neutrophils. Multiplexed immunohistochemical analyses have revealed significant inter- and intrapatient spatial heterogeneity in the leukocyte infiltrate (6). Multiple leukocyte subsets have been shown to impact tumor biology in PDAC. For instance, Treg cells limit the efficacy of vaccines in mouse models (107). In contrast, depletion of Treg cells results in the differentiation of inflammatory fibroblast subsets that in turn drive myeloid cells to infiltrate the pancreas and support disease progression (45). CD4+ T cells are also recruited during early pancreatic cancer pathogenesis and support disease progression by secreting IL-17 (108). Further, B cells infiltrate PDAC lesions albeit at much lower frequencies than T cells (109). To this end, tumor-infiltrating B cell subsets can stimulate cancer cell proliferation by releasing IL-35 (110). Tumor-infiltrating B cells also activate Bruton tyrosine kinase signaling in tumor-associated macrophages, programming them with a tumor-promoting phenotype (111). Tumor-infiltrating myeloid cells are among the most abundant leukocyte subset detected in tumors. Most notably, myeloid cells are quite spatially and transcriptionally diverse (106, 112). The phenotype of myeloid cells in PDAC tumors is a determinant of treatment outcomes (113). Further, the myeloid cell phenotype is pliable such that infiltrating myeloid cells may be engendered with either tumor-promoting or tumor-suppressive features. Most often, myeloid cells support tumor progression by enabling many of the hallmarks of cancer, including tumor growth, tumor survival, invasion and metastasis, and immune evasion (114-116). However, myeloid cells can also be endowed with antitumor properties including the capacity to directly mediate tumor cell killing (114), remodel the stroma for enhanced chemotherapy efficacy (113, 114), and support productive T cell immunity (117, 118). Altogether, the leukocyte infiltrate in PDAC is dynamic and shaped by cancer cells as well as the stromal microenvironment.
3.3. Implications of the Tumor Microenvironment for Metastasis
The microenvironment that surrounds cancer cells is a critical determinant of the metastatic cascade. Stromal cells and the ECM contribute directly to metastasis by supporting cancer cell invasion and intravasation into the blood stream. In addition, the microenvironment at secondary sites influences the capacity of disseminated tumor cells to seed and form metastatic colonies (17, 119). Epithelial-to-mesenchymal transition (EMT) endows cancer cells with mesenchymal features including motility and invasiveness that are broadly associated with metastatic progression (120). EMT is triggered by a variety of factors present within the tumor microenvironment including TGFβ, leukemia inhibitory factor, hypoxia, and migration inhibitory factor (17). Remarkably, even preinvasive pancreatic lesions associate with cells undergoing EMT—notably, these cells have been shown to escape the pancreas and seed distant organs (121, 122). Induction of pancreatic inflammation dramatically increases EMT, local delamination, and mutant pancreatic cells detected in the circulation. In contrast, suppressing inflammation in mouse models of PDAC inhibits epithelial cell dissemination, thereby establishing a link between inflammation, the microenvironment, and PDAC metastasis.
Pancreatic cancer cells disseminate via multiple routes including blood and lymphatic vasculature and even by invading peripheral nerves. While the determinants that direct these distinct routes of dissemination remain elusive, it is known that hypoxia enforced by hypovascularity and defective vasculature can foster the metastatic potential of cancer cells. For instance, hypoxia induces the expression of transcription factor B lymphocyte-induced maturation protein-1 (Blimp1) in pancreatic cancer cells. Importantly, Blimp1 regulates prometastatic hypoxia-induced target genes and is required for metastatic potential (123). Microenvironmental factors are also critical at distant sites and contribute to the metastatic seeding and colonization of disseminated tumor cells (17). During cancer development, the cellular and biochemical contexture of distant organs is altered to support metastasis. This prometastatic niche that forms is characterized by the recruitment of myeloid cells and deposition of ECM proteins. In addition, disseminated tumor cells recruit and establish a desmoplastic stroma that progressively accumulates as metastatic colonies form (16). The importance of paracrine factors in supporting metastasis at distant organs is underscored by studies in which select cellular components are deleted. For instance, recent functional studies show that CAF ablation reduces the growth of PDAC liver metastases and prolongs overall survival in mice (75). Epithelial cells at secondary sites also contribute to the metastatic microenvironment and aid in formation of a prometastatic niche. In the liver, hepatocytes are activated via STAT3 signaling by IL-6 released from CAFs present in the primary PDAC tumor. Activated hepatocytes respond by producing serum amyloid proteins, which then coordinate formation of a prometastatic niche that supports liver metastasis (124). However, the role of hepatocytes is exclusive in supporting liver metastasis. As such, distinct factors coordinate the metastatic permissiveness of other distant sites (125). Nonetheless, the microenvironment is a clear and fundamental determinant of metastasis and acts to enable metastatic potential of cancer cells.
4. HOW THE TUMOR MICROENVIRONMENT SUPPORTS THERAPEUTIC RESISTANCE
The environment that surrounds cancer cells in PDAC is dynamic and pliable. This vast and heterogeneous landscape poses many barriers to the success of treatments. Notably, cancer cells co-opt elements of the stromal microenvironment to withstand insults imposed by nutrient deprivation, immune surveillance, and cytotoxic therapies. This closely knitted relationship between cancer cells and its stroma is fundamental to therapeutic resistance. To this end, multiple redundancies in cellular networks and signaling pathways thwart the efficacy of intervening on single elements of the stroma. This resilience and capacity of PDAC to endure remarkable stress underlies the nearly uniform fate of eventual progression. Here, we discuss several determinants of the microenvironment in PDAC that contribute to treatment resistance.
4.1. Hypovascularity
Tumor growth is reliant on vasculature that nourishes cancer cells with necessary nutrients and oxygen. To keep pace with cancer proliferation, small molecules, such as VEGF, promote the development of new blood vessels, or angiogenesis (81). However, PDAC is most often characterized by a low microvascular density (83). This reflects the dense fibrotic reaction that is characteristic of the PDAC microenvironment and may trap proangiogenic molecules in the stroma, thereby limiting their capacity to trigger angiogenesis. Consistent with this idea, fibrosis degradation in PDAC associates with increased vascularity (41). In a comprehensive proteogenomic analysis of PDAC, vascularity was also found to inversely correlate with cellular infiltration (126). Notably, endothelial cells and their expression of cell adhesion molecules regulate cell trafficking. PDAC tumors with decreased leukocyte infiltration show reduced endothelial cell adhesion molecules, and these tumors display activation of hypoxia pathways that may in turn influence endothelial cell biology. These observations highlight the interdependent relationships between vascularity, fibrosis, and cellular recruitment.
Hypovascularity is a determinant of therapeutic resistance. Most notably, hypovascularity hinders the penetration and distribution of drugs into the tumor microenvironment, thereby limiting their efficacy. However, poor vascularization also produces a deprived nutrient milieu that invokes cancer cell autophagy to support metabolic processes and tumor growth. Autophagy is a conserved self-degradation process and associates with increased therapeutic resistance to cytotoxic therapies (127). In addition, autophagy has been found to promote immune evasion by causing degradation of major histocompatibility complex (MHC) class I molecules in pancreatic cancer cells, which are necessary for recognition by CD8+ cytotoxic T cells (128). Inhibiting autophagy reverses this process and, in mouse models, sensitizes PDAC to immunotherapy (128). Autophagy is also activated in stromal cells including PSCs. Notably, PSC autophagy is triggered by cancer cells and causes PSCs to release nonessential amino acids (e.g., alanine) that, in turn, are utilized by cancer cells as a fuel source (53). Thus, hypovascularity induces a concerted response by pancreatic cancer cells and their surrounding stromal microenvironment that acts to promote tumor survival and thwart the efficacy of cytotoxic- and immune-based therapies.
4.2. Fibrosis
Dense fibrosis is commonly referred to as a physical barrier to treatment efficacy in PDAC. Fibroblasts contribute to fibrosis by producing components of the ECM. CAFs also shape and remodel the stromal microenvironment by mediating collagen cross-linking and, in doing so, modulate tumor stiffness, which supports tumor progression and cancer cell migration. Single-cell analyses have revealed multiple CAF subsets present within PDAC tumors that are defined by unique transcriptional signatures (62-64). The spatial arrangement of CAF subsets is also distinct. For instance, CAFs appear to associate with cancer cells on the basis of their differentiation status. For instance, α-SMA+ CAFs are found juxtaposed to well-differentiated cancer cell nests, whereas fibroblast activation protein (FAP+) CAFs tend to localize adjacent to poorly differentiated regions of tumor (61). Further, these markers of CAF subsets have been used to define the stromal microenvironment in PDAC as enriched in collagen, FAP, or α-SMA. Notably, these unique microenvironments associate with distinct survival and molecular features (61).
CAFs may be engendered with tumor permissive or restrictive functions. For instance, CD105+ CAFs. including both myofibroblastic and inflammatory CAFs, are tumor permissive, whereas CD105neg CAFs restrict tumor growth (129). CD105neg CAFs show reduced FAP expression and uniquely express MHC class II antigen machinery corresponding to antigen-presenting CAFs (62). This CAF subset has been implicated in supporting the tumor-suppressive potential of adaptive immunity, whereas other CAF populations restrict antitumor immune responses.
CAFs and their associated fibrosis may be barriers to treatment efficacy. Mouse modeling has shown the potential benefit of disrupting elements of stromal fibrosis for enhancing the efficacy of cytotoxic chemotherapy (49). For instance, depletion of HA facilitates enhanced efficacy of gemcitabine. This finding reflects improved vascular perfusion in tumors leading to a shorter diffusion time for gemcitabine, which has a short half-life and is readily metabolized in tumors (130). However, for other chemotherapeutics with longer half-lives, the benefit of fibrosis degradation may not be as readily apparent.
4.3. Immune Evasion
A hallmark of PDAC is its remarkable resistance to immunotherapy. Primary immune resistance in cancer can involve multiple mechanisms (131). For instance, cancer cells may evade immune recognition because of a lack of potent immunogenic tumor antigens (antigenicity). Importantly, neoantigens resulting from gene mutations are less abundant in PDAC compared with many other immune-sensitive cancers (132). Poorly immunogenic tumors may also escape immune recognition due to poor antigen presentation required for effective priming of tumor-reactive T cells (immunogenicity). Together, the poor antigenicity and immunogenicity of PDAC forms the basis for ongoing studies investigating vaccines and drugs targeting molecules (e.g., CD40 and CTLA4) that regulate T cell priming (133-135). However, the microenvironment that surrounds PDAC is also fundamental to productive immune surveillance (microenvironment). Exclusion of leukocyte populations (e.g., T cells and dendritic cells) that are necessary for immune recognition of cancer is a central theme in PDAC (136, 137). In contrast, PDAC is known for its recruitment of myeloid and fibroblast populations that possess immune suppressive and tumor-promoting features. Finally, the functional competency of leukocyte populations in patients may be compromised (immune health). For instance, PDAC is commonly associated with systemic inflammation (138), a deficiency of circulating dendritic cells (117, 118), and peripheral T cells that are terminally differentiated (139). Notably, systemic inflammation marked by an elevated neutrophil-to-lymphocyte ratio in the peripheral blood associates with reduced efficacy of cytotoxic chemotherapy (138, 140). Altogether, the immune evasiveness of PDAC is multifactorial and influenced by cancer cell–intrinsic and –extrinsic mechanisms.
Leukocytes are a predominant component of the PDAC microenvironment and a determinant of therapeutic outcomes. Within tumors, the leukocyte infiltrate is remarkably heterogenous. This variability in the localization and density of leukocytes is particularly pronounced for T cells, which tend to congregate in the stroma and less commonly interact directly with cancer cells. In this regard, T cells appear to be drawn to some cancer cell nests while being excluded from others (Figure 2). T cell infiltration correlates with improved outcomes in patients with surgically resected PDAC, and in mouse models T cells suppress metastasis (7, 141). However, the precise determinants that direct T cell intratumoral heterogeneity are unclear. Genetic mouse models suggest that pancreatic tumors may be composed of cancer cell clones with vastly distinct immunogenicity and responsiveness to immunotherapy (141). In these studies, clonal cell lines generated from tumors arising spontaneously in KPC mice were found to produce tumors with either a strong or weak T cell infiltrate when transplanted into syngeneic mice. In addition, these clones responded differentially to immunotherapy, with strong responses observed for T cell–enriched tumors. However, upon mixing of the cancer cell clones, the phenotype of T cell–poor tumors was shown to dominate. Taken together, these observations suggest that immune heterogeneity in PDAC is directed by cancer cell–intrinsic features, with individual clones cooperating to fine-tune elements of the tumor microenvironment that support a fitness advantage for tumor progression and immune evasion (Figure 2).
4.4. Remodeling of the Tumor Microenvironment
The tumor microenvironment is a well-recognized therapeutic barrier in PDAC. However, the microenvironment that surrounds cancer cells is pliable, and this suggests the potential to shift its polarity from treatment resistant to treatment sensitize. Disrupting specific elements of the microenvironment, such as myeloid cells, fibroblasts, or ECM components, can enhance the sensitivity of cytotoxic- and immune-based therapies. For instance, depletion of CSF1R+ myeloid cells in mouse models and in some patients with PDAC improves outcomes to cytotoxic chemotherapy by augmenting the infiltration of T cells and their antitumor activity (142). Similarly, inhibiting focal adhesion kinase (FAK) causes a reduction in immunosuppressive leukocyte populations including myeloid cells and Treg cells and, in doing so, sensitizes PDAC to both chemotherapy and immunotherapy in mice (143). Together, these findings suggest that multiple mechanisms underlie therapeutic resistance in PDAC.
An alternative strategy to remodeling tumors for enhanced treatment sensitivity involves harnessing the therapeutic potential of tumor-infiltrating leukocytes. For instance, activation of the CD40 pathway using an agonistic antibody redirects tumor-infiltrating inflammatory monocytes to remodel the ECM of tumors and sensitize PDAC to chemotherapy (113, 114). Engineered T cells recognizing mesothelin also trigger remodeling of the ECM in PDAC, suggesting that immune activation may be a generalized mechanism for altering the contexture of the microenvironment in PDAC (144). Another approach to remodeling the tumor microenvironment involves restoring deficits in immune health associated with PDAC. For example, Fms-like tyrosine kinase 3 ligand triggers dendritic cell mobilization, survival, and infiltration into PDAC tumors and combines with a CD40 agonist to promote T cell infiltration and antitumor activity in mouse models (117, 118). Partial activation of CD11b using a small molecule agonist can also redirect tumor-infiltrating myeloid cells and reduce resistance to immunotherapy in PDAC models (145). Thus, treatment strategies designed to induce tumor-suppressive mechanisms, as opposed to removing tumor-promoting elements, offer an approach for leveraging the tumor microenvironment for therapeutic benefit.
4.5. Pliability
PDAC shows remarkable resilience in response to therapeutic intervention. Determinants that contribute to this resilience are multifactorial and include the cellular and spatial heterogeneity of stromal cell populations as well as cancer cell clonal heterogeneity, which supports tumor evolution. Pancreatic cancer cells adapt to interventions by invoking compensatory signaling pathways. For instance, inhibiting autophagy in pancreatic cancer cells prompts their upregulation of alternative pathways, such as macropinocytosis, for extracting nutrients from the microenvironment (146). However, blocking both autophagy and macropinocytosis deprives cancer cells of essential metabolic needs and induces cell death. Similarly, pancreatic cancer cells respond to suppression of pathways downstream of KRAS, including ERK/MAPK and RAF/MEK/ERK, by increasing autophagic flux (147, 148). This establishes a vulnerability in cancer cells such that intervening on these downstream KRAS signaling pathways in combination with autophagy inhibition causes synergistic antiproliferative effects.
Cancer cells coevolve with their surrounding microenvironment such that insults sustained by cancer cells provoke reciprocal responses in the microenvironment, and vice versa. For instance, cancer cells respond to cytotoxic stress by inducing the recruitment of myeloid cell populations that support neovascularization and tumor survival (149). Dying cancer cells also release ATP into the extracellular milieu, acting as a potent danger signal for the recruitment of inflammatory cells, which then degrade ATP by converting it to adenosine, which, in turn, is immunosuppressive (150). Intervening on these compensatory inflammatory responses by blocking chemokines/chemokine receptors (e.g., CCL2, CCR2, and CXCR2) or inhibiting adenosine formation (e.g., CD73) has been found to improve survival outcomes in mouse models (149, 151). However, intervening on a single determinant of inflammation provoked by cytotoxic therapy triggers compensatory responses. For instance, combining chemotherapy with CCR2-blocking antibodies to prevent monocyte recruitment to tumors triggers a compensatory increase in neutrophil recruitment (152). Conversely, blocking neutrophil recruitment using CXCR2-blocking antibodies induces the recruitment of monocytes to tumors in the setting of chemotherapy. To this end, combined blockade of both CCR2 and CXCR2 improves survival in mouse models of PDAC. Similarly, inhibition of FAK remodels PDAC to be more sensitive to cytotoxic chemotherapy and immunotherapy, but ultimately resistance is observed (153). This compensatory survival pathway reflects a remodeling of the tumor microenvironment. Specifically, FAK inhibition reduces the antagonistic effects of TGFβ on tumor-intrinsic STAT3 signaling such that combined inhibition of FAK and STAT3 synergizes to suppress PDAC growth in mouse models (153). Thus, multiple nonredundant and compensatory pathways may need to be targeted to prevent cancer cells from undermining the efficacy of cytotoxic and targeted therapies.
While PDAC cell biology is fundamentally defined by oncogenic drivers (e.g., KRAS) and other mutations (e.g., TP53, INK4A, and SMAD4, among others), cancer cells are continuously influenced by their surrounding microenvironment. To this end, cancer cells must adapt to immune pressure or succumb to elimination. Secretion of cytokines (e.g., interferons) by infiltrating T cells triggers upregulation of antigen presentation machinery in cancer cells and, in doing so, sensitizes them to recognition and elimination by cytotoxic T cells (131). However, this pressure imposed by the immune system invokes a Darwinian selection process whereby cancer cells with increased fitness ultimately emerge. For instance, adoptive cell therapy with T cells engineered to recognize mesothelin provokes the emergence of cancer cells that have lost mesothelin expression (144). In addition, tumor-infiltrating T cells are ultimately coaxed into an exhausted state by resident myeloid cells. These findings illustrate the adaptability of PDAC and emphasize that a multipronged approach that addresses both primary and acquired resistance mechanisms to treatment intervention will need to be considered.
5. CHALLENGES AND OPPORTUNITIES FOR STUDYING AND INTERVENING ON THE TUMOR MICROENVIRONMENT
Genomic studies have revealed unique patient subsets in PDAC that derive benefit from cytotoxic-, targeted-, and immune-based therapies. For instance, PARP inhibitors show activity in patients with germline BRCA mutations who have not progressed on platinum-based chemotherapy (154, 155). In addition, patients with mismatch repair deficient PDAC have an increased likelihood of responding to immune checkpoint inhibitors (156). Given the remarkable inter- and intrapatient heterogeneity seen in the tumor microenvironment of PDAC, similar approaches to patient selection may be needed to realize the benefit of strategies that intervene on elements of the tumor microenvironment. One approach to patient selection involves the use of genetic determinants (e.g., mutations in Kras, p53, SMAD4, and BRCA) that represent significant patient subsets. Another approach is to consider elements of immune health that may influence the therapeutic potential of a treatment strategy. For example, patients with a low neutrophil-to-lymphocyte ratio show improved outcomes to treatment with gemcitabine and a CD40 agonist (138). Lastly, baseline and on-treatment tumor analyses may inform patient selection. For example, patients might be selected for treatment on the basis of the phenotype of the tumor, such as (a) deserted or reactive (6), (b) SMA, FAP, or collagen enriched (61), (c) T cell or myeloid cell enriched or poor (6), or (d) classical or basal (157, 158). On-treatment biopsies may also be needed to assess whether treatments focally or diffusely alter the tumor microenvironment (159). However, with these approaches, stringent criteria will be necessary given the potential for tissue sampling bias. Specifically, interpretation of the tumor microenvironment using biopsies is challenged by the remarkable cellular and spatial heterogeneity of PDAC. Thus, results from biopsies could be misleading if this heterogeneity is not appropriately considered.
Determinants of spatial and cellular heterogeneity in PDAC are poorly understood. Current tissue analyses offer only a snapshot of this biology. Given the impact that the intra- and extratumoral elements can have on tumor pathogenesis, it is expected that the microenvironment is dynamic and is continuously shaping the evolution of cancer cells. Future efforts will need to identify determinants of this heterogeneity and better understand how cancer cell–intrinsic signaling pathways of mutational landscapes instruct the contexture and arrangement of cells within the microenvironment. Such analyses have the potential to unveil novel therapeutic targets and approaches to dismantle the complex PDAC ecosystem.
The microenvironment that surrounds PDAC holds potentially key insights into treatment vulnerabilities. Preclinical models will be fundamental to complimenting observations made from human tissue analyses. Organoid and mouse models have demonstrated value in defining functional relationships between cell subsets within tumors and compensatory mechanisms of resistance to treatment interventions. These model systems hold promise for addressing fundamental questions in PDAC. For instance, how do genomic alterations (e.g., mutations in p53, BRCA, etc.) affect the cellular contexture of the microenvironment in PDAC? Are intercellular relationships involving cancer cells and stromal cells similarly observed in primary and metastatic lesions? How does the microenvironment regulate the transition from low-grade dysplasia to invasive cancer? These questions and more will begin to unravel unique patient subsets with therapeutic implications and may reveal strategies for early disease intervention in high-risk patients.
6. CONCLUDING REMARKS
Over the past two decades, great strides have been made in understanding the complex stromal microenvironment of PDAC and its impact on disease biology and treatment resistance. Although seemingly disorganized, the tumor microenvironment in PDAC is intricate and interwoven with many dynamic elements that govern disease progression, immune evasion, metastasis, and therapeutic resistance. Reciprocal interactions between cancer cell–intrinsic and –extrinsic mechanisms shape the tumor microenvironment and form the basis of PDAC’s remarkable resilience. Emerging and existing state-of-the-art technologies offer unprecedented opportunities to discover fundamental biology underlying the contexture, functional status, and spatial organization of the tumor microenvironment in PDAC. To this end, many key gaps in knowledge exist including understudied components of the microenvironment (e.g., peripheral nerves, adipocytes, and endothelial cells) and the dynamics of intercellular relationships in tumors. The conceptual advances described herein serve to provide a framework for future investigations. Ultimately, it is expected that this knowledge will inform novel strategies and therapeutic targets for improving patient outcomes and advancing a personalized medicine approach to PDAC.
ACKNOWLEDGMENTS
M.H.S. is funded in part by grants from the National Institutes of Health/National Cancer Institute (R01 CA229580, R01 CA250917). G.L.B. reports funding support from the National Institutes of Health/National Cancer Institute (R01 CA197916, R01 CA245323, and U01 CA224193), 2017 Pancreatic Cancer Action Network Precision Medicine Targeted Grant 17-85-BEAT, and a research grant from the Lustgarten Foundation, Inc.
Footnotes
DISCLOSURE STATEMENT
M.H.S. is a paid consultant for Autobahn Labs, Palo Alto, California. G.L.B. reports prior or active roles as a consultant/advisory board member for Adicet Bio, Aduro Biotech, AstraZeneca, BiolineRx, BioMarin Pharmaceuticals, Boehinger Ingelheim, Bristol-Myers Squibb, Cantargia, Cour Pharmaceuticals, Genmab, HotSpot Therapeutics, Incyte, Janssen, Merck, Molecular Partners, Monopteros, Nano Ghosts, Opsona, Pancreatic Cancer Action Network, Seagen, Shattuck Laboratories, and Verastem and reports receiving commercial research grants from Incyte, Bristol-Myers Squibb, Verastem, Halozyme, Biothera, Hibercell, Newlink, Novartis, Arcus, and Janssen. G.L.B. is an inventor of intellectual property (U.S. patent numbers 10,640,569 and 10,577,417) that is licensed by the University of Pennsylvania to Novartis and Tmunity Therapeutics and is a recipient of royalties related to CAR T cells.
LITERATURE CITED
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. 2022. Cancer statistics, 2022. CA Cancer J. Clin 72:7–33 [DOI] [PubMed] [Google Scholar]
- 2.Beatty GL, Werba G, Lyssiotis CA, Simeone DM. 2021. The biological underpinnings of therapeutic resistance in pancreatic cancer. Genes Dev. 35:940–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiello NM, Stanger BZ. 2016. Echoes of the embryo: using the developmental biology toolkit to study cancer. Dis. Model. Mech 9:105–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landsman L, Nijagal A, Whitchurch TJ, Vanderlaan RL, Zimmer WE, et al. 2011. Pancreatic mesenchyme regulates epithelial organogenesis throughout development. PLOS Biol. 9:e1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grunwald BT, Devisme A, Andrieux G, Vyas F, Aliar K, et al. 2021. Spatially confined sub-tumor microenvironments in pancreatic cancer. Cell 184:5577–92.e18 [DOI] [PubMed] [Google Scholar]
- 6.Liudahl SM, Betts CB, Sivagnanam S, Morales-Oyarvide V, da Silva A, et al. 2021. Leukocyte heterogeneity in pancreatic ductal adenocarcinoma: phenotypic and spatial features associated with clinical outcome. Cancer Discov. 11:2014–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balachandran VP, Luksza M, Zhao JN, Makarov V, Moral JA, et al. 2017. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 551:512–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiraoka N, Ino Y, Yamazaki-Itoh R, Kanai Y, Kosuge T, Shimada K. 2015. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br. J. Cancer 112:1782–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunderson AJ, Rajamanickam V, Bui C, Bernard B, Pucilowska J, et al. 2021. Germinal center reactions in tertiary lymphoid structures associate with neoantigen burden, humoral immunity and long-term survivorship in pancreatic cancer. Oncoimmunology 10:1900635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delvecchio FR, Fincham REA, Spear S, Clear A, Roy-Luzarraga M, et al. 2021. Pancreatic cancer chemotherapy is potentiated by induction of tertiary lymphoid structures in mice. Cell. Mol. Gastroenterol. Hepatol. 12:1543–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schumacher TN, Thommen DS. 2022. Tertiary lymphoid structures in cancer. Science 375:eabf9419. [DOI] [PubMed] [Google Scholar]
- 12.Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, et al. 2014. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol. Res 2:616–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yachida S, Jones S, Bozic I, Antal T, Leary R, et al. 2010. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467:1114–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, et al. 2010. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 467:1109–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whatcott CJ, Diep CH, Jiang P, Watanabe A, LoBello J, et al. 2015. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin. Cancer Res 21:3561–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aiello NM, Bajor DL, Norgard RJ, Sahmoud A, Bhagwat N, et al. 2016. Metastatic progression is associated with dynamic changes in the local microenvironment. Nat. Commun 7:12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas SK, Lee J, Beatty GL. 2020. Paracrine and cell autonomous signalling in pancreatic cancer progression and metastasis. EbioMedicine 53:102662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JW, Beatty GL. 2020. Inflammatory networks cultivate cancer cell metastasis to the liver. Cell Cycle 19:642–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolberg DS, Hollingsworth R, Hertle M, Bissell MJ. 1985. Wounding and its role in RSV-mediated tumor formation. Science 230:676–78 [DOI] [PubMed] [Google Scholar]
- 20.Burrack AL, Rollins MR, Spartz EJ, Mesojednik TD, Schmiechen ZC, et al. 2021. CD40 agonist overcomes T cell exhaustion induced by chronic myeloid cell IL-27 production in a pancreatic cancer preclinical model. J. Immunol 206:1372–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ying H, Dey P, Yao W, Kimmelman AC, Draetta GF, et al. 2016. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 30:355–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, et al. 2003. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4:437–50 [DOI] [PubMed] [Google Scholar]
- 23.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, et al. 2005. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7:469–83 [DOI] [PubMed] [Google Scholar]
- 24.Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, et al. 2007. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 11:291–302 [DOI] [PubMed] [Google Scholar]
- 25.Guerra C, Collado M, Navas C, Schuhmacher AJ, Hernandez-Porras I, et al. 2011. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell 19:728–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assi M, Achouri Y, Loriot A, Dauguet N, Dahou H, et al. 2021. Dynamic regulation of expression of KRAS and its effectors determines the ability to initiate tumorigenesis in pancreatic acinar cells. Cancer Res. 81:2679–89 [DOI] [PubMed] [Google Scholar]
- 27.Del Poggetto E, Ho IL, Balestrieri C, Yen EY, Zhang S, et al. 2021. Epithelial memory of inflammation limits tissue damage while promoting pancreatic tumorigenesis. Science 373:eabj0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins MA, Bednar F, Zhang Y, Brisset JC, Galban S, et al. 2012. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J. Clin. Investig 122:639–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, et al. 2012. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149:656–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR. 2000. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 14:2501–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sodir NM, Kortlever RM, Barthet VJA, Campos T, Pellegrinet L, et al. 2020. MYC instructs and maintains pancreatic adenocarcinoma phenotype. Cancer Discov. 10:588–607 [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Velez-Delgado A, Mathew E, Li D, Mendez FM, et al. 2017. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut 66:124–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. 2012. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 21:836–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, et al. 2012. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 21:822–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawson DW, Hertzer K, Moro A, Donald G, Chang HH, et al. 2013. High-fat, high-calorie diet promotes early pancreatic neoplasia in the conditional KrasG12D mouse model. Cancer Prev. Res 6:1064–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung KM, Singh J, Lawres L, Dorans KJ, Garcia C, et al. 2020. Endocrine-exocrine signaling drives obesity-associated pancreatic ductal adenocarcinoma. Cell 181:832–47.e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Incio J, Liu H, Suboj P, Chin SM, Chen IX, et al. 2016. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 6:852–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Philip B, Roland CL, Daniluk J, Liu Y, Chatterjee D, et al. 2013. A high-fat diet activates oncogenic Kras and COX2 to induce development of pancreatic ductal adenocarcinoma in mice. Gastroenterology 145:1449–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaytouni T, Tsai PY, Hitchcock DS, DuBois CD, Freinkman E, et al. 2017. Critical role for arginase 2 in obesity-associated pancreatic cancer. Nat. Commun 8:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, et al. 2008. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin. Cancer Res 14:5995–6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, et al. 2014. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25:735–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JJ, Perera RM, Wang H, Wu DC, Liu XS, et al. 2014. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. PNAS 111:E3091–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Kim J, Yang S, Wang H, Wu CJ, et al. 2021. Type I collagen deletion in αSMA+ myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell 39:548–65.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janakiram NB, Mohammed A, Bryant T, Ritchie R, Stratton N, et al. 2017. Loss of natural killer T cells promotes pancreatic cancer in LSL-KrasG12D/+ mice. Immunology 152:36–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Lazarus J, Steele NG, Yan W, Lee HJ, et al. 2020. Regulatory T-cell depletion alters the tumor microenvironment and accelerates pancreatic carcinogenesis. Cancer Discov. 10:422–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalluri R 2016. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16:582–98 [DOI] [PubMed] [Google Scholar]
- 47.Buechler MB, Pradhan RN, Krishnamurty AT, Cox C, Calviello AK, et al. 2021. Cross-tissue organization of the fibroblast lineage. Nature 593:575–79 [DOI] [PubMed] [Google Scholar]
- 48.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, et al. 2009. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324:1457–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. 2012. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21:418–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, et al. 2013. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62:112–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, et al. 2015. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 75:544–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sullivan MR, Danai LV, Lewis CA, Chan SH, Gui DY, et al. 2019. Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. Elife 8:e44235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, et al. 2016. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536:479–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sherman MH, Yu RT, Tseng TW, Sousa CM, Liu S, et al. 2017. Stromal cues regulate the pancreatic cancer epigenome and metabolome. PNAS 114:1129–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao H, Yang L, Baddour J, Achreja A, Bernard V, et al. 2016. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife 5:e10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Auciello FR, Bulusu V, Oon C, Tait-Mulder J, Berry M, et al. 2019. A stromal lysolipid-autotaxin signaling axis promotes pancreatic tumor progression. Cancer Discov. 9:617–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Francescone R, Barbosa Vendramini-Costa D, Franco-Barraza J, Wagner J, Muir A, et al. 2021. Netrin G1 promotes pancreatic tumorigenesis through cancer-associated fibroblast-driven nutritional support and immunosuppression. Cancer Discov. 11:446–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Recouvreux MV, Jung M, Galenkamp KMO, Li Y, et al. 2021. Macropinocytosis in cancer-associated fibroblasts is dependent on CaMKK2/ARHGEF2 signaling and functions to support tumor and stromal cell fitness. Cancer Discov. 11:1808–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, et al. 2013. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. PNAS 110:20212–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morita T, Kodama Y, Shiokawa M, Kuriyama K, Marui S, et al. 2020. CXCR4 in tumor epithelial cells mediates desmoplastic reaction in pancreatic ductal adenocarcinoma. Cancer Res. 80:4058–70 [DOI] [PubMed] [Google Scholar]
- 61.Ohlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, et al. 2017. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med 214:579–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, et al. 2019. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 9:1102–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dominguez CX, Muller S, Keerthivasan S, Koeppen H, Hung J, et al. 2020. Single-cell RNA sequencing reveals stromal evolution into LRRC15+ myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov. 10:232–53 [DOI] [PubMed] [Google Scholar]
- 64.Hosein AN, Huang H, Wang Z, Parmar K, Du W, et al. 2019. Cellular heterogeneity during mouse pancreatic ductal adenocarcinoma progression at single-cell resolution. JCI Insight 5:e129212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Biffi G, Oni TE, Spielman B, Hao Y, Elyada E, et al. 2019. IL1-induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 9:282–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steele NG, Biffi G, Kemp SB, Zhang Y, Drouillard D, et al. 2021. Inhibition of Hedgehog signaling alters fibroblast composition in pancreatic cancer. Clin. Cancer Res 27:2023–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mathew E, Zhang Y, Holtz AM, Kane KT, Song JY, et al. 2014. Dosage-dependent regulation of pancreatic cancer growth and angiogenesis by Hedgehog signaling. Cell Rep. 9:484–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vennin C, Melenec P, Rouet R, Nobis M, Cazet AS, et al. 2019. CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat. Commun 10:3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Waghray M, Yalamanchili M, Dziubinski M, Zeinali M, Erkkinen M, et al. 2016. GM-CSF mediates mesenchymal-epithelial cross-talk in pancreatic cancer. Cancer Discov. 6:886–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garcia PE, Adoumie M, Kim EC, Zhang Y, Scales MK, et al. 2020. Differential contribution of pancreatic fibroblast subsets to the pancreatic cancer stroma. Cell. Mol. Gastroenterol. Hepatol 10:581–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Helms EJ, Berry MW, Chaw RC, DuFort CC, Sun D, et al. 2022. Mesenchymal lineage heterogeneity underlies nonredundant functions of pancreatic cancer-associated fibroblasts. Cancer Discov. 12:484–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kai F, Drain AP, Weaver VM. 2019. The extracellular matrix modulates the metastatic journey. Dev. Cell 49:332–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian C, Clauser KR, Ohlund D, Rickelt S, Huang Y, et al. 2019. Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. PNAS 116:19609–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tian C, Huang Y, Clauser KR, Rickelt S, Lau AN, et al. 2021. Suppression of pancreatic ductal adenocarcinoma growth and metastasis by fibrillar collagens produced selectively by tumor cells. Nat. Commun 12:2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhattacharjee S, Hamberger F, Ravichandra A, Miller M, Nair A, et al. 2021. Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts. J. Clin. Investig 131:e146987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tian C, Ohlund D, Rickelt S, Lidstrom T, Huang Y, et al. 2020. Cancer cell-derived matrisome proteins promote metastasis in pancreatic ductal adenocarcinoma. Cancer Res. 80:1461–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laklai H, Miroshnikova YA, Pickup MW, Collisson EA, Kim GE, et al. 2016. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat. Med 22:497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van Cutsem E, Tempero MA, Sigal D, Oh DY, Fazio N, et al. 2020. Randomized phase III trial of pegvorhyaluronidase alfa with nab-paclitaxel plus gemcitabine for patients with hyaluronan-high metastatic pancreatic adenocarcinoma. J. Clin. Oncol 38:3185–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim PK, Halbrook CJ, Kerk SA, Radyk M, Wisner S, et al. 2021. Hyaluronic acid fuels pancreatic cancer cell growth. Elife 10:e62645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blair AB, Kim VM, Muth ST, Saung MT, Lokker N, et al. 2019. Dissecting the stromal signaling and regulation of myeloid cells and memory effector T cells in pancreatic cancer. Clin. Cancer Res 25:5351–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin JD, Seano G, Jain RK. 2019. Normalizing function of tumor vessels: progress, opportunities, and challenges. Annu. Rev. Physiol 81:505–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, et al. 2010. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J. Clin. Oncol 28:3617–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Katsuta E, Qi Q, Peng X, Hochwald SN, Yan L, Takabe K. 2019. Pancreatic adenocarcinomas with mature blood vessels have better overall survival. Sci. Rep. 9:1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rankin EB, Giaccia AJ. 2008. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 15:678–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keith B, Johnson RS, Simon MC. 2011. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 12:9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee KE, Spata M, Bayne LJ, Buza EL, Durham AC, et al. 2016. Hif1a deletion reveals pro-neoplastic function of B cells in pancreatic neoplasia. Cancer Discov. 6:256–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fink DM, Steele MM, Hollingsworth MA. 2016. The lymphatic system and pancreatic cancer. Cancer Lett. 381:217–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shen CN, Goh KS, Huang CR, Chiang TC, Lee CY, et al. 2019. Lymphatic vessel remodeling and invasion in pancreatic cancer progression. EbioMedicine 47:98–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao B, Cui K, Wang CL, Wang AL, Zhang B, et al. 2011. The chemotactic interaction between CCL21 and its receptor, CCR7, facilitates the progression of pancreatic cancer via induction of angiogenesis and lymphangiogenesis. J. Hepatobiliary Pancreat. Sci 18:821–28 [DOI] [PubMed] [Google Scholar]
- 90.Guo J, Lou W, Ji Y, Zhang S. 2013. Effect of CCR7, CXCR4 and VEGF-C on the lymph node metastasis of human pancreatic ductal adenocarcinoma. Oncol. Lett 5:1572–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sperveslage J, Frank S, Heneweer C, Egberts J, Schniewind B, et al. 2012. Lack of CCR7 expression is rate limiting for lymphatic spread of pancreatic ductal adenocarcinoma. Int. J. Cancer 131:E371–81 [DOI] [PubMed] [Google Scholar]
- 92.Wehler T, Wolfert F, Schimanski CC, Gockel I, Herr W, et al. 2006. Strong expression of chemokine receptor CXCR4 by pancreatic cancer correlates with advanced disease. Oncol. Rep 16:1159–64 [PubMed] [Google Scholar]
- 93.Cui K, Zhao W, Wang C, Wang A, Zhang B, et al. 2011. The CXCR4-CXCL12 pathway facilitates the progression of pancreatic cancer via induction of angiogenesis and lymphangiogenesis. J. Surg. Res 171:143–50 [DOI] [PubMed] [Google Scholar]
- 94.Ahren B 2000. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia 43:393–410 [DOI] [PubMed] [Google Scholar]
- 95.Borden P, Houtz J, Leach SD, Kuruvilla R. 2013. Sympathetic innervation during development is necessary for pancreatic islet architecture and functional maturation. Cell Rep. 4:287–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stopczynski RE, Normolle DP, Hartman DJ, Ying H, DeBerry JJ, et al. 2014. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 74:1718–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bapat AA, Hostetter G, Von Hoff DD, Han H. 2011. Perineural invasion and associated pain in pancreatic cancer. Nat. Rev. Cancer 11:695–707 [DOI] [PubMed] [Google Scholar]
- 98.Belfiori G, Crippa S, Francesca A, Pagnanelli M, Tamburrino D, et al. 2021. Long-term survivors after upfront resection for pancreatic ductal adenocarcinoma: an actual 5-year analysis of disease-specific and post-recurrence survival. Ann. Surg. Oncol 28:8249–60 [DOI] [PubMed] [Google Scholar]
- 99.Sinha S, Fu YY, Grimont A, Ketcham M, Lafaro K, et al. 2017. PanIN neuroendocrine cells promote tumorigenesis via neuronal cross-talk. Cancer Res. 77:1868–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saloman JL, Singhi AD, Hartman DJ, Normolle DP, Albers KM, Davis BM. 2018. Systemic depletion of nerve growth factor inhibits disease progression in a genetically engineered model of pancreatic ductal adenocarcinoma. Pancreas 47:856–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, et al. 2018. β2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 33:75–90.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saloman JL, Albers KM, Li D, Hartman DJ, Crawford HC, et al. 2016. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. PNAS 113:3078–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Banh RS, Biancur DE, Yamamoto K, Sohn ASW, Walters B, et al. 2020. Neurons release serine to support mRNA translation in pancreatic cancer. Cell 183:1202–18.e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Renz BW, Tanaka T, Sunagawa M, Takahashi R, Jiang Z, et al. 2018. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov. 8:1458–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, et al. 2019. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 178:795–806.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Steele NG, Carpenter ES, Kemp SB, Sirihorachai V, The S, et al. 2020. Multimodal mapping of the tumor and peripheral blood immune landscape in human pancreatic cancer. Nat. Cancer 1:1097–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Keenan BP, Saenger Y, Kafrouni MI, Leubner A, Lauer P, et al. 2014. A Listeria vaccine and depletion of T-regulatory cells activate immunity against early stage pancreatic intraepithelial neoplasms and prolong survival of mice. Gastroenterology 146:1784–94.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Y, Yan W, Mathew E, Bednar F, Wan S, et al. 2014. CD4+ T lymphocyte ablation prevents pancreatic carcinogenesis in mice. Cancer Immunol. Res 2:423–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ko AH, Jordan AC, Tooker E, Lacey SF, Chang RB, et al. 2020. Dual targeting of mesothelin and CD19 with chimeric antigen receptor-modified T cells in patients with metastatic pancreatic cancer. Mol. Ther 28:2367–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pylayeva-Gupta Y, Das S, Handler JS, Hajdu CH, Coffre M, et al. 2016. IL35-producing B cells promote the development of pancreatic neoplasia. Cancer Discov. 6:247–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, et al. 2016. Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov. 6:270–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vayrynen SA, Zhang J, Yuan C, Vayrynen JP, Dias Costa A, et al. 2021. Composition, spatial characteristics, and prognostic significance of myeloid cell infiltration in pancreatic cancer. Clin. Cancer Res 27:1069–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Long KB, Gladney WL, Tooker GM, Graham K, Fraietta JA, Beatty GL. 2016. IFNγ and CCL2 cooperate to redirect tumor-infiltrating monocytes to degrade fibrosis and enhance chemotherapy efficacy in pancreatic carcinoma. Cancer Discov. 6:400–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, et al. 2011. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331:1612–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Long KB, Collier AI, Beatty GL. 2019. Macrophages: key orchestrators of a tumor microenvironment defined by therapeutic resistance. Mol. Immunol 110:3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144:646–74 [DOI] [PubMed] [Google Scholar]
- 117.Lin JH, Huffman AP, Wattenberg MM, Walter DM, Carpenter EL, et al. 2020. Type 1 conventional dendritic cells are systemically dysregulated early in pancreatic carcinogenesis. J. Exp. Med 217:e20190673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hegde S, Krisnawan VE, Herzog BH, Zuo C, Breden MA, et al. 2020. Dendritic cell paucity leads to dysfunctional immune surveillance in pancreatic cancer. Cancer Cell 37:289–307.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Quail DF, Joyce JA. 2013. Microenvironmental regulation of tumor progression and metastasis. Nat. Med 19:1423–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lambert AW, Pattabiraman DR, Weinberg RA. 2017. Emerging biological principles of metastasis. Cell 168:670–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, et al. 2012. EMT and dissemination precede pancreatic tumor formation. Cell 148:349–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rhim AD, Thege FI, Santana SM, Lannin TB, Saha TN, et al. 2014. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology 146:647–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chiou SH, Risca VI, Wang GX, Yang D, Gruner BM, et al. 2017. BLIMP1 induces transient metastatic heterogeneity in pancreatic cancer. Cancer Discov. 7:1184–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee JW, Stone ML, Porrett PM, Thomas SK, Komar CA, et al. 2019. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature 567:249–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stone ML, Beatty GL. 2019. Cellular determinants and therapeutic implications of inflammation in pancreatic cancer. Pharmacol. Ther 201:202–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cao L, Huang C, Cui Zhou D, Hu Y, Lih TM, et al. 2021. Proteogenomic characterization of pancreatic ductal adenocarcinoma. Cell 184:5031–52.e26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.White E 2012. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 12:401–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, et al. 2020. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature 581:100–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hutton C, Heider F, Blanco-Gomez A, Banyard A, Kononov A, et al. 2021. Single-cell analysis defines a pancreatic fibroblast lineage that supports anti-tumor immunity. Cancer Cell 39:1227–44.e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cattel L, Airoldi M, Delprino L, Passera R, Milla P, Pedani F. 2006. Pharmacokinetic evaluation of gemcitabine and 2',2'-difluorodeoxycytidine-5'-triphosphate after prolonged infusion in patients affected by different solid tumors. Ann. Oncol 17(Suppl. 5):v142–47 [DOI] [PubMed] [Google Scholar]
- 131.Beatty GL, Gladney WL. 2015. Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res 21:687–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schumacher TN, Schreiber RD. 2015. Neoantigens in cancer immunotherapy. Science 348:69–74 [DOI] [PubMed] [Google Scholar]
- 133.Beatty GL, Li Y, Long KB. 2017. Cancer immunotherapy: activating innate and adaptive immunity through CD40 agonists. Expert Rev. Anticancer Ther 17:175–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bengsch F, Knoblock DM, Liu A, McAllister F, Beatty GL. 2017. CTLA-4/CD80 pathway regulates T cell infiltration into pancreatic cancer. Cancer Immunol. Immunother 66:1609–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sánchez-Paulete AR, Teijeira A, Cueto FJ, Garasa S, Pérez-Gracia JL, et al. 2017. Antigen cross-presentation and T-cell cross-priming in cancer immunology and immunotherapy. Ann. Oncol 28(Suppl. 12):xii44–55 [DOI] [PubMed] [Google Scholar]
- 136.Beatty GL, Winograd R, Evans RA, Long KB, Luque SL, et al. 2015. Exclusion of T cells from pancreatic carcinomas in mice is regulated by Ly6Clow F4/80+ extratumoral macrophages. Gastroenterology 149:201–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Joyce JA, Fearon DT. 2015. T cell exclusion, immune privilege, and the tumor microenvironment. Science 348:74–80 [DOI] [PubMed] [Google Scholar]
- 138.Wattenberg MM, Herrera VM, Giannone MA, Gladney WL, Carpenter EL, Beatty GL. 2021. Systemic inflammation is a determinant of outcomes of CD40 agonist-based therapy in pancreatic cancer patients. JCI Insight 6:e145389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xu J, Sai H, Li Y, Jordan AC, McGettigan SE, et al. 2019. Peripheral blood T-cell fitness is diminished in patients with pancreatic carcinoma but can be improved with homeostatic cytokines. Cell. Mol. Gastroenterol. Hepatol 8:656–58.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, et al. 2015. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J. Natl. Cancer Inst 107:dju413. [DOI] [PubMed] [Google Scholar]
- 141.Li J, Byrne KT, Yan F, Yamazoe T, Chen Z, et al. 2018. Tumor cell-intrinsic factors underlie heterogeneity of immune cell infiltration and response to immunotherapy. Immunity 49:178–93.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wainberg Z, Piha-Paul S, Luke J, Kim E, Thompson J, et al. 2017. First-in-human phase 1 dose escalation and expansion of a novel combination, anti–CSF-1 receptor (cabiralizumab) plus anti–PD-1 (nivolumab), in patients with advanced solid tumors. In Proceedings of the 32nd SITC Annual Meeting, National Harbor, MD, USA, November 8–12, 2017, Abstract O4. Milwaukee, WI: Soc. Immunother. Cancer [Google Scholar]
- 143.Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, et al. 2016. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med 22:851–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stromnes IM, Schmitt TM, Hulbert A, Brockenbrough JS, Nguyen H, et al. 2015. T cells engineered against a native antigen can surmount immunologic and physical barriers to treat pancreatic ductal adenocarcinoma. Cancer Cell 28:638–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Panni RZ, Herndon JM, Zuo C, Hegde S, Hogg GD, et al. 2019. Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies. Sci. Transl. Med 11:eaau9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Su H, Yang F, Fu R, Li X, French R, et al. 2021. Cancer cells escape autophagy inhibition via NRF2-induced macropinocytosis. Cancer Cell 39:678–93.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bryant KL, Stalnecker CA, Zeitouni D, Klomp JE, Peng S, et al. 2019. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat. Med 25:628–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kinsey CG, Camolotto SA, Boespflug AM, Guillen KP, Foth M, et al. 2019. Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat. Med 25:620–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kalbasi A, Komar C, Tooker GM, Liu M, Lee JW, et al. 2017. Tumor-derived CCL2 mediates resistance to radiotherapy in pancreatic ductal adenocarcinoma. Clin. Cancer Res 23:137–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vijayan D, Young A, Teng MWL, Smyth MJ. 2017. Targeting immunosuppressive adenosine in cancer. Nat. Rev. Cancer 17:709–24 [DOI] [PubMed] [Google Scholar]
- 151.King RJ, Shukla SK, He C, Vernucci E, Thakur R, et al. 2022. CD73 induces GM-CSF/MDSC-mediated suppression of T cells to accelerate pancreatic cancer pathogenesis. Oncogene 41:971–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nywening TM, Belt BA, Cullinan DR, Panni RZ, Han BJ, et al. 2018. Targeting both tumour-associated CXCR2+ neutrophils and CCR2+ macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 67:1112–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jiang H, Liu X, Knolhoff BL, Hegde S, Lee KB, et al. 2020. Development of resistance to FAK inhibition in pancreatic cancer is linked to stromal depletion. Gut 69:122–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, et al. 2019. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N. Engl. J. Med 381:317–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Reiss KA, Mick R, O'Hara MH, Teitelbaum U, Karasic TB, et al. 2021. Phase II study of maintenance rucaparib in patients with platinum-sensitive advanced pancreatic cancer and a pathogenic germline or somatic variant in BRCA1, BRCA2, or PALB2. J. Clin. Oncol 39:2497–505 [DOI] [PubMed] [Google Scholar]
- 156.Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, et al. 2020. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J. Clin. Oncol 38:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.O'Kane GM, Grunwald BT, Jang GH, Masoomian M, Picardo S, et al. 2020. GATA6 expression distinguishes classical and basal-like subtypes in advanced pancreatic cancer. Clin. Cancer Res 26:4901–10 [DOI] [PubMed] [Google Scholar]
- 158.Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, et al. 2011. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med 17:500–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Balachandran VP, Beatty GL, Dougan SK. 2019. Broadening the impact of immunotherapy to pancreatic cancer: challenges and opportunities. Gastroenterology 156:2056–72 [DOI] [PMC free article] [PubMed] [Google Scholar]