Abstract
Objectives:
To establish the incidence, risk factors and correlation with survival of thrombocytopenia and thrombocytosis (T/T) among children living with HIV infection (CLWH).
Design:
A retrospective nested case control study of patients 0 – 18 years in five Baylor International Pediatric AIDS Initiative (BIPAI) centers in sub-Sahara Africa, 2004 – 2014.
Methods:
Clinical and laboratory variables including complete blood counts (CBC) were extracted from the BIPAI electronic medical record system. Incident cases of T/T were identified and frequency-matched on follow-up time with controls with normal platelets. We calculated the prevalence and incidence density of T/T and used conditional logistic regression to evaluate their association with selected clinical variables. We constructed Kaplan-Meier curves and a Cox proportional hazards model to evaluate the impact of T/T on survival.
Results:
2,109 children were sampled. The incidence density of thrombocytopenia was 1 per 57.9 (95%CI 50.3 – 66.8) CLWH-years. Thrombocytopenia was higher in children with WHO Stage III/IV, lower in children on Zidovudine, and had no association with use of lamivudine or nevirapine, CD4 suppression, age, and nutrition status. Thrombocytopenia was independently associated with 2.2-fold higher mortality (95%CI 1.62 – 3.08). The incidence density of thrombocytosis was 1 per 11.4 (95%CI 10.7 – 12.1) CLWH-years. Thrombocytosis was associated with higher CD4, younger age, and use of lamivudine or nevirapine, and did not impact survival.
Conclusions:
Platelet count is a clinically valuable biomarker of HIV clinical progression and mortality. Laboratory studies are necessary to elucidate the mechanisms of T/T.
Keywords: Children living with HIV, platelets, thrombocytopenia, thrombocytosis, combination antiretroviral therapy, biomarker, sub-Sahara Africa
Introduction
Approximately 1.8 million children live with the Human Immunodeficiency Virus (HIV) infection worldwide, most of them (over 91%) are in sub-Sahara Africa (SSA) [1]. Significant progress has been made to improve the survival of children living with HIV infection (CLWH), with over half of this population now receiving combination antiretroviral therapy (ART) and a 41% decline in new infections since 2010 [2]. However, there were still an estimated 150,000 new cases of pediatric HIV in 2019. Although ART prevents many opportunistic infections in CLWH and ensures these children survive longer, non-infectious complications have become more prominent as a cause of death and morbidity [3–5]. Non-infectious complications in people with HIV infection and on ART are putatively attributed to direct adverse effects of ART agents, residual HIV reservoirs, and chronic immune activation[4]. In view of the central role of platelets in normal and pathological immune processes, we hypothesized that quantitative platelet abnormalities are common in CLWH on ART and may serve as biomarkers of HIV clinical progression and mortality.
Platelets are myeloid lineage cells produced by the bone marrow and have a primary role in hemostasis and thrombosis. Platelets also play a central role in the immune response [6]. Platelets store, respond, and release a milieu of cytokines and chemokines that orchestrate adaptive immune responses [7]. They also interact with leukocytes and vascular endothelium through intricate adaptive mechanisms to maintain inflammatory hemostasis or respond to pathogens and tissue injury [8–10]. Multiple mechanisms may be involved in quantitative platelet abnormalities and their complications in CLWH because of the pervasive impact of HIV infection and its treatment and sequelae on the immune system [11, 12]. Immune dysregulation may cause immune complexes, anti-platelet antibodies, or cross-reactive HIV antibodies targeting platelets and cause thrombocytopenia, while persistent immune activation may result in stimulation of thrombocytosis [13]. Thrombocytopenia may also arise from bone marrow suppression by HIV and other virus co-infections or by ART agents or from consumption and depletion of platelets in the setting of severe acute bacterial infections [14, 15]. Thrombocytosis is also associated with iron-deficiency that may be common in CLWH [14].
The aim of this study was to quantify the incidence of thrombocytopenia and thrombocytosis in a population of CLWH and on ART, identify factors associated with these quantitative platelet abnormalities, and determine their association with survival of CLWH in SSA.
Materials and Methods
Study design and study population
We conducted a nested case control study of children with HIV and thrombocytopenia or thrombocytosis (T/T) (cases) and those without T/T (controls). Cases and controls were selected from an observational clinical cohort of children with HIV aged 0–18 years who initiated ART between January 2004 to December 2014 at five Baylor International Pediatric AIDS Initiative (BIPAI) centers in four SSA countries, namely Botswana (Gaborone), Malawi (Lilongwe), Tanzania (Mwanza and Mbeya), and Uganda (Kampala) [16]. Complete blood counts and other clinical data for these patients were managed using a standardized electronic medical record (EMR) system. Following diagnosis with HIV, children in the clinical cohort were initiated on ART and provided comprehensive HIV care and treatment following respective country guidelines that were developed in collaboration with BIPAI. Since 2008, the standard practice was to commence all children on ART once a diagnosis of HIV is made. Children were followed longitudinally with routine and sick clinic visits. Complete blood counts (CBC) were performed periodically as clinically indicated using automated CBC analyzers. Some patients had platelet counts only as a follow up assay following an abnormal complete blood count. We used a nested case-control design for this analysis to reduce bias from missing information on some cases and controls, particularly from the earlier periods of the clinical cohort when clinical data recording was more variable.
Cases and controls
Cases and controls were identified from the BIPAI EMR by extracting automated CBC for patients below 18 years of age. There were two case groups, namely, CLWH that had thrombocytopenia and CLWH that had thrombocytosis. Thrombocytopenia was defined as platelet counts less than 150,000 cells per μL and thrombocytosis as platelet counts more than 450,000 cells per μL, on two or more CBC/platelet checks.[17] Thrombocytopenia was further stratified by severity as follows: Mild if <150,000 and ≥100,000, Moderate for <100,000 and ≥50,000, and Severe if <50,000 cells per μL. Controls were children with HIV infection but no instance of T/T through the course of their care at the BIPAI Center.
Children who were diagnosed with T/T after 3 months were considered incident, while those that had T/T within 3 months (wash-out period) of enrolment into HIV care were considered prevalent cases and were eliminated from this analysis. All incident T/T cases were included, and they were frequency-matched to controls. Controls were randomly selected from the risk set of children without T/T that matched on follow-up time.
Study variables
All incident cases and controls were retrospectively followed for vital status from enrolment into HIV care at the BIPAI Center until 31st December 2014. Children were censored at the date of death or last attendance at the BIPAI center if they had no T/T by 31st December 2014. The maximum possible follow-up time for an individual child in the cohort was 11 years. The following covariates were automatically extracted from the EMR on all cases and controls at all time points they were available: age, sex, date of enrollment at BIPAI center, date of first incident of T/T, date of death, names of ART agents, WHO HIV/AIDS clinical Stage at diagnosis, CD4 lymphocyte counts/percentages, height, and weight.
Data cleaning and organization
Extracted data was electronically transferred and collated in Microsoft Excel version 16.16.3 (Redmond, WA) before being imported into SAS version 9.4 (Cary, NC) for further curation, organization, and analysis. Coded variables were converted to text based on a standard pre-defined variable codebook. Data were then checked for duplicates, and any duplicates were removed. New derivative variables were computed from raw data, e.g., survival time was calculated from date of enrolment into HIV care and date of death. Where applicable, continuous variables (e.g., age, CD4 counts, platelet counts) and ordinal variables (e.g., year of enrollment) were consolidated into logical categories (e.g., age groups and severity of thrombocytopenia). Particularly, CD4 lymphocyte levels were categorized as defined by the World Health Organization (Supplementary Table 1) [18]. Nutritional status was calculated and categorized using z-scores for weight-for-length/height and BMI-for-age, as defined by the World Health Organization [19].
Statistical analysis
We calculated the incidence density and risk factors associated with each of thrombocytopenia and thrombocytosis. To evaluate the association of putative risk factors and these quantitative platelet abnormalities, we used conditional logistic regression to compute odds ratios (unadjusted and adjusted – where applicable). We calculated relative risks of death at 1, 5 and 10-year time points and used Gray’s test to assess whether incidence density curves were the same or different at all time points. We also performed Kaplan-Meier survival analysis to create survival curves and a Cox proportional hazard model to estimate hazard ratios of 10-year overall survival by T/T including by severity of thrombocytopenia. To estimate the precision of associations and the robustness of the statistical models, we computed 95% confidence intervals and p-values, which were considered robust if p-value <0.05.
Ethical review
Ethical approval was obtained from the relevant institutional review boards (IRBs) in Botswana (#PPME 13/18), Malawi (#740), Tanzania (#GB.152/377 and #MR/53/100), and Uganda (#2009–090), and from Baylor College of Medicine (H-25403, H-27755, H-32491 and H-32678, and H-26616, respectively). Informed consent was waived by the respective IRBs in view of a retrospective study design using deidentified patient information.
Results
Clinical characteristics of the nested cohort
We sampled 2,109 CLWH enrolled at the five BIPAI centers between January 2004 to December 2014 and had at least one CBC result in the EMR during this period. We excluded patients that did not have any CBC in the EMR (342, 13.8%) and those that had key missing variables (130, 5.3%). The demographic and clinical characteristics of the nested case control cohort are summarized in Table 1. Cases of thrombocytosis and thrombocytopenia were adequately matched with controls on follow-up time. The incidence density of thrombocytosis was 1 per 11.4 (95%CI 10.7 – 12.1) CLWH-years, while the incidence density of thrombocytopenia was 1 per 57.9 (95%CI 50.3 – 66.8) CLWH-years, respectively. The proportion of severe thrombocytopenia was 30.5% (95%CI 24.5% - 37.8%) among those with thrombocytopenia. In this cohort, children with thrombocytopenia had the most CBC checks (median=12), followed by those with thrombocytosis (median=9); those with no platelet abnormalities had the least CBC checks (median=3). The first occurrence of T/T was mostly among children 5 – 12 years (Supplemental Table 2). In most cases, children with T/T also had other blood cell abnormalities, most commonly anemia, which occurred in 72% of those with thrombocytopenia and 68% of those with thrombocytosis.
Table 1.
Demographic and clinical characteristics of Children with Thrombocytopenia/Thrombocytosis and Controls in the BIPAI nested case control study
| Variable | ||
|---|---|---|
|
| ||
| Year of Registration in BIPAI, N (%) | 2004 – 2008 | 1046 (49.6%) |
|
| ||
| 2008 – 2014 | 1063 (50.4%) | |
|
| ||
| Sex, Male N (%) | ||
|
| ||
| All children (n=2109) | 1242 (58.9%) | |
|
| ||
| Thrombocytopenia (n=187) | 109 (58.3%) | |
|
| ||
| Normal platelet counts (n=839) | 481 (57.3%) | |
|
| ||
| Thrombocytosis (n=953) | 560 (58.8%) | |
|
| ||
| Age at enrollment into care (Mean (SD), Median [range]) | ||
|
| ||
| All children (n=2109) | 6.6 (4.9), 5.7 [0 – 18] | |
|
| ||
| Thrombocytopenia (n=187) | 6.9 (4.6), 5.9 [0.1 – 17.3] | |
|
| ||
| Normal platelet counts (n=839) | 7.5 (5.0), 6.9 [0 – 18.0] | |
|
| ||
| Thrombocytosis (n=953) | 5.4 (4.4), 4.3 [0 – 18.0] | |
|
| ||
| Follow-up time (Years) (Mean (SD), Median, [range]) | ||
|
| ||
| Overall follow-up time (n=2109) | 5.5 (3.5), 5.5 [0 – 10] | |
|
| ||
| Thrombocytopenia (n=187) | 4.6 (3.6), 3.9 [0 – 10] | |
|
| ||
| Normal platelet counts (n=839) | 5.1 (3.6), 4.8 [0 – 10] | |
|
| ||
| Thrombocytosis (n=953) | 5.9 (3.3), 6.3 [0 – 10] | |
|
| ||
| Duration of ART (Years) (Mean (SD), Median, [range]) | ||
|
| ||
| All children (n=2109) | 4.5 (3.5), 4.2 [0 – 10] | |
|
| ||
| Thrombocytopenia (n=187) | 4.1 (3.5), 3.3 [0 – 10] | |
|
| ||
| Normal platelet counts (n=839) | 4.5 (3.5), 3.9 [0 – 10] | |
|
| ||
| Thrombocytosis (n=953) | 5.4 (3.2), 5.5 [0 – 10] | |
|
| ||
| Number of CBCs per participant (Mean (SD), Median [range]) | ||
|
| ||
| No CBC (n = 130) | - | |
|
| ||
| All children with CBC (n=1979) | 7.7 (6.9), 5.0 [1 – 56] | |
|
| ||
| Thrombocytopenia (n=187) | 13.9 (10.0), 12.0 [2 – 56] | |
|
| ||
| Normal platelet counts (n=839) | 5.4 (5.4), 3.0 [1 – 24] | |
|
| ||
| Thrombocytosis (n=953) | 9.9 (7.7), 9.0 (1 – 56) | |
|
| ||
| ART regimen, N (%) | ||
|
| ||
| All children (n=1979) | Lamivudine/Nevirapine/Stavudine | 637 (32.2%) |
| Lamivudine/Zidovudine/Efavirenz | 427 (21.6%) | |
| Lamivudine/Nevirapine/Zidovudine | 372 (18.8%) | |
| Others | 543 (27.4%) | |
|
| ||
| Thrombocytopenia (n=187) | Lamivudine/Nevirapine/Stavudine | 87 (46.5%) |
| Lamivudine/Zidovudine/Efavirenz | 27 (14.4%) | |
| Lamivudine/Nevirapine/Zidovudine | 19 (10.2%) | |
| Others | 54 (28.9%) | |
|
| ||
| Normal platelet counts (n=839) | Lamivudine/Nevirapine/Stavudine | 248 (29.6%) |
| Lamivudine/Zidovudine/Efavirenz | 184 (21.9%) | |
| Lamivudine/Nevirapine/Zidovudine | 158 (18.8%) | |
| Others | 249 (29.7%) | |
|
| ||
| Thrombocytosis (n=953) | Lamivudine/Nevirapine/Stavudine | 302 (31.7%) |
| Lamivudine/Zidovudine/Efavirenz | 216 (22.7%) | |
| Lamivudine/Nevirapine/Zidovudine | 195 (20.5%) | |
| Others | 240 (25.2%) | |
BIPAI – Baylor International Pediatric AIDS Initiative
CBC – Complete Blood Count
Risk factors of thrombocytopenia in CLWH
The association of risk factors of interest with thrombocytopenia is summarized in Table 2. Advanced WHO Stage (III/IV) was the only clinical factor associated with higher risk of thrombocytopenia (aOR 2.5, 95%CI 1.6 – 3.9, p<0.001). CD4 cell suppression, malnutrition and age were not associated with thrombocytopenia. On the other hand, use of Zidovudine had the most negative (protective) association with thrombocytopenia (aOR 0.5, 95%CI 0.3 – 0.6, p<0.001), while Nevirapine and Lamivudine had no association with thrombocytopenia.
Table 2.
Risk factors associated with thrombocytopenia in children living with HIV infection in the BIPAI cohort
| Risk Factor | Thrombocytopenia, N (%) | Normal Platelets N (%) | Odds Ratio (OR), (95%CI) | Adjusted* OR, (95%CI) |
|---|---|---|---|---|
|
| ||||
| Nutrition Status | ||||
| Normal (Ref) | 106 (56.68%) | 490 (58.40%) | - | |
| Mild | 41 (21.93%) | 161 (19.19%) | 1.28, (0.79 – 1.76) | N/A |
| Moderate | 24 (12.83%) | 88 (10.49%) | 1.261, (0.77 – 2.07) | |
| Severe | 16 (8.56%) | 100 (11.92%) | 0.74, (0.42 – 1.31) | |
|
| ||||
| CD4 Nadir | ||||
| Normal (Ref) | 25 (13.81%) | 153 (18.59%) | ||
| Mild | 25 (13.81%) | 124 (15.07%) | 1.23, (0.68 – 2.25) | 1.24, (0.68 – 2.27) |
| Moderate | 42 (23.20%) | 201 (24.42%) | 1.28, (0.75 – 2.19) | 1.28, (0.74 – 2.19) |
| Severe | 89 (49.17%) | 345 (41.92%) | 1.58, (0.97 – 2.56) | 1.58, (0.97 – 2.56) |
|
| ||||
| First WHO Stage | ||||
| I/II (Ref) | 31 (25.41%) | 279 (44.36%) | ||
| III/IV | 91 (74.59%) | 350 (55.64%) | 2.34, (1.51 – 3.62)** | 2.51, (1.61 – 3.92) ** |
|
| ||||
| Zidovudine in cART | ||||
| No Zidovudine | 133 (71.12%) | 449 (53.52%) | ||
| Zidovudine | 54 (28.88%) | 390 (46.48%) | 0.47, (0.33 – 0.66) ** | 0.46, (0.32 – 0.64)** |
|
| ||||
| Lamivudine in cART | ||||
| No Lamivudine | 16 (8.56%) | 76 (9.06%) | ||
| Lamivudine | 171 (91.44%) | 763 (90.94%) | 1.07, (0.61 – 1.87) | 1.06, (0.60 – 1.865) |
|
| ||||
| Nevirapine in cART | ||||
| No Nevirapine | 77 (41.18%) | 409 (48.75%) | ||
| Nevirapine | 110 (58.82%) | 430 (51.25%) | 1.36, (0.99 – 1.87) | 1.38, (1.00 – 1.90) |
|
| ||||
| Age at 1st Thrombocytopenia | ||||
| ≥ 12 years (Ref) | 46 (26.29%) | 207 (33.71%) | ||
| 5 – 12 years | 69 (39.43%) | 213 (34.69%) | 1.46, (0.96 – 2.22) | 1.43, (0.94 – 2.19) |
| 1 – 5 years | 49 (28.00%) | 164 (26.71%) | 1.34, (0.86 – 2.11) | 1.36, (0.87 – 2.15) |
| 0 – 1 years | 11 (6.29%) | 30 (4.89%) | 1.65, (0.77 – 3.53) | 1.79, (0.83 – 3.86) |
Adjusted for Nutritional status
p-value <0.05
In those with severe thrombocytopenia (Supplemental Table 3), WHO Stage III/IV was still linked to the presence of severe thrombocytopenia (aOR 2.9, 95%CI 1.3 – 6.6, p=0.010). Additionally, using Nevirapine, and all age groups less than 12 years were associated with severe thrombocytopenia. On the other hand, Zidovudine had an even stronger protective effect (aOR 0.3, 95%CI 0.2 – 0.6, p<0.001).
Thrombocytopenia and survival of CLWH
Thrombocytopenia was associated with a relative risk of death of 1.9, 2.8, and 2.8 at 1 year, 5 years, and 10 years from the first occurrence of thrombocytopenia (Gray’s test p<0.001). CLWH and thrombocytopenia had 2.2-fold higher hazards of mortality (95%CI 1.6 – 3.1), p < 0.001), after adjusting for severity of CD4 nadir, WHO Stage, and use of Zidovudine (Figure 1). Stratified analysis by severity of thrombocytopenia showed increasing hazards of death with increasing severity of thrombocytopenia: aHR 3.6 (95%CI 2.4 – 5.7, p<0.001) for severe thrombocytopenia, 2.2 (95%CI 1.3 – 3.9, p=0.005) for moderate thrombocytopenia and 1.6 (95%CI 1.0 – 2.5, p=0.040) for mild thrombocytopenia (Supplemental Figure 1).
Figure 1.
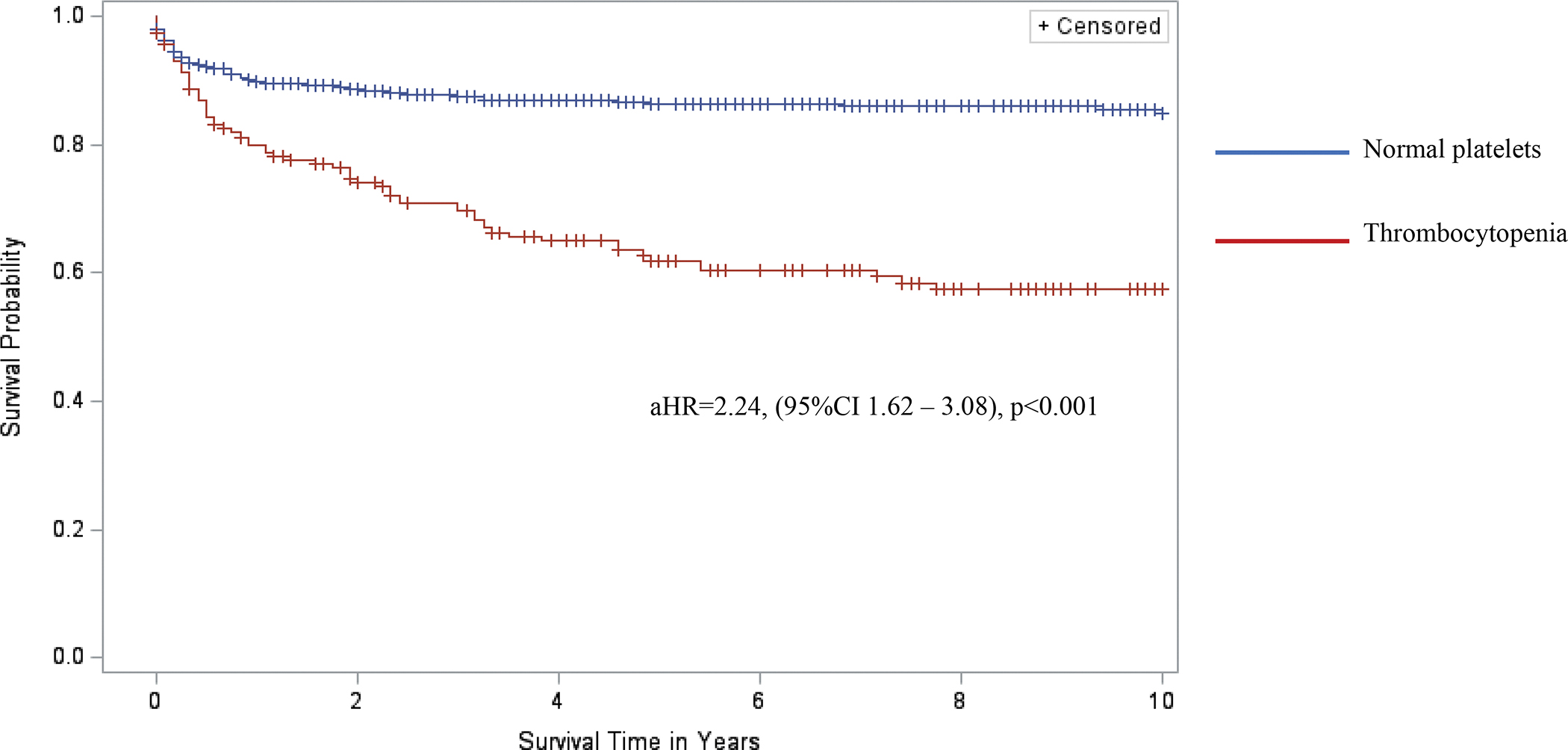
Kaplan-Meier curves comparing 10-year survival of children living with HIV infection and with and without thrombocytopenia in the BIPAI cohort. Kaplan-Meier survival analysis was used to create survival curves and a Cox proportional hazard model to estimate hazard ratios of 10-year overall survival by presence of thrombocytopenia (red font survival curve, n = 187) versus normal platelet counts (blue font survival curve, n = 839). Cross marks in the curves indicate the timing of censorship of the children upon death or loss to follow-up in the follow-up period. The adjusted hazard ratio (adjusted for nutrition status) associated with thrombocytopenia was 2.24, 95%CI 1.62 – 3.08, and p-value<0.001 (statistical model considered robust if p-value <0.05).
Risk factors of thrombocytosis in CLWH
The association of risk factors of interest with thrombocytosis is summarized in Table 3. Use of Lamivudine or Nevirapine in the ART regimen was associated with thrombocytosis. Compared to adolescents ≥12 years old, the incidence of thrombocytosis increased with lower age, i.e., aOR 3.3 (95%CI 2.1 – 5.3, p<0.001) for infants 0 – 1 year, aOR 2.6 (95%CI 1.8 – 3.5, p<0.001) for 1 – 5 years old, and aOR 1.6 (95%CI 1.2 – 2.2, p<0.001) for 5 – 12 years old. All levels of CD4 suppression had a negative association with thrombocytosis. Use of Zidovudine, WHO Stage, and nutrition status all had no association with thrombocytosis.
Table 3.
Risk factors associated with thrombocytosis in children living with HIV infection in the BIPAI cohort
| Risk Factor | Thrombocytosis, N (%) | Normal Platelets N (%) | Odds Ratio (OR), (95CI) | Adjusted* OR, (95CI) |
|---|---|---|---|---|
|
| ||||
| Nutrition | ||||
| Adequate (Ref) | 603 (63.27%) | 490 (58.40%) | ||
| Mild | 188 (19.73%) | 161 (19.19%) | 1.06, (0.83 – 1.34) | |
| Moderate | 83 (8.71%) | 88 (10.49%) | 1.17, (0.87 – 1.59) | N/A |
| Severe | 79 (8.29%) | 100 (11.92%) | 0.83, (0.61 – 1.13) | |
|
| ||||
| CD4 Nadir | ||||
| Normal (Ref) | 232 (24.55%) | 153 (18.59%) | ||
| Mild | 120 (12.70%) | 124 (15.07%) | 0.64, (0.46 – 0.88)** | 0.64, (0.46 – 0.88)** |
| Moderate | 198 (20.95%) | 201 (24.42%) | 0.65, (0.49 – 0.86)** | 0.65, (0.49 – 0.87)** |
| Severe | 395 (41.80%) | 345 (41.92%) | 0.76, (0.59 – 0.97)** | 0.75, (0.59 – 0.97)** |
|
| ||||
| First WHO Stage | ||||
| I/II | 264 (40.55%) | 279 (44.36%) | ||
| III/IV | 387 (59.45%) | 350 (55.64%) | 1.17, (0.94 – 1.46) | 1.20, (0.96 – 1.51) |
|
| ||||
| Zidovudine in cART | ||||
| No Zidovudine | 476 (49.95%) | 449 (53.52%) | ||
| Zidovudine | 477 (50.05%) | 390 (46.48%) | 1.15, (0.96 – 1.39) | 1.15, (0.96 – 1.39) |
|
| ||||
| Lamivudine in cART | ||||
| No Lamivudine | 39 (4.09%) | 76 (9.06%) | ||
| Lamivudine | 914 (95.91%) | 763 (90.94%) | 2.33, (1.57 – 3.47)** | 2.31, (1.55 – 3.45)** |
|
| ||||
| Nevirapine in cART | ||||
| No Nevirapine | 419 (43.97%) | 409 (48.75%) | ||
| Nevirapine | 534 (56.03%) | 430 (51.25%) | 1.21, (1.01 – 1.46)** | 1.21, (1.01 – 1.46)** |
|
| ||||
| Age at 1st Thrombocytosis | ||||
| ≥ 12 years (Ref) | 161 (18.99%) | 207 (33.71%) | ||
| 5 – 12 years | 279 (32.90%) | 213 (34.69%) | 1.68, (1.28 – 2.21)** | 1.64, (1.25 – 2.16)** |
| 1 – 5 years | 333 (39.27%) | 164 (26.71%) | 2.61, (1.98 – 3.45)** | 2.62, (1.98 – 3.46)** |
| 0 – 1 years | 75 (8.84%) | 30 (4.89%) | 3.21, (2.01 – 5.15)** | 3.31, (2.06 – 5.31)** |
Adjusted for Nutritional status
p-value <0.05
Thrombocytosis and survival of CLWH
Thrombocytosis was associated with 52% lower risk of death at 1 year but had no longer term survival advantage compared to children with normal platelet counts. Overall, after adjusting for severity of CD4 nadir, WHO HIV stage, and use of Zidovudine and Lamivudine, thrombocytosis did not significantly affect the hazards of 10-year survival (Figure 2).
Figure 2.
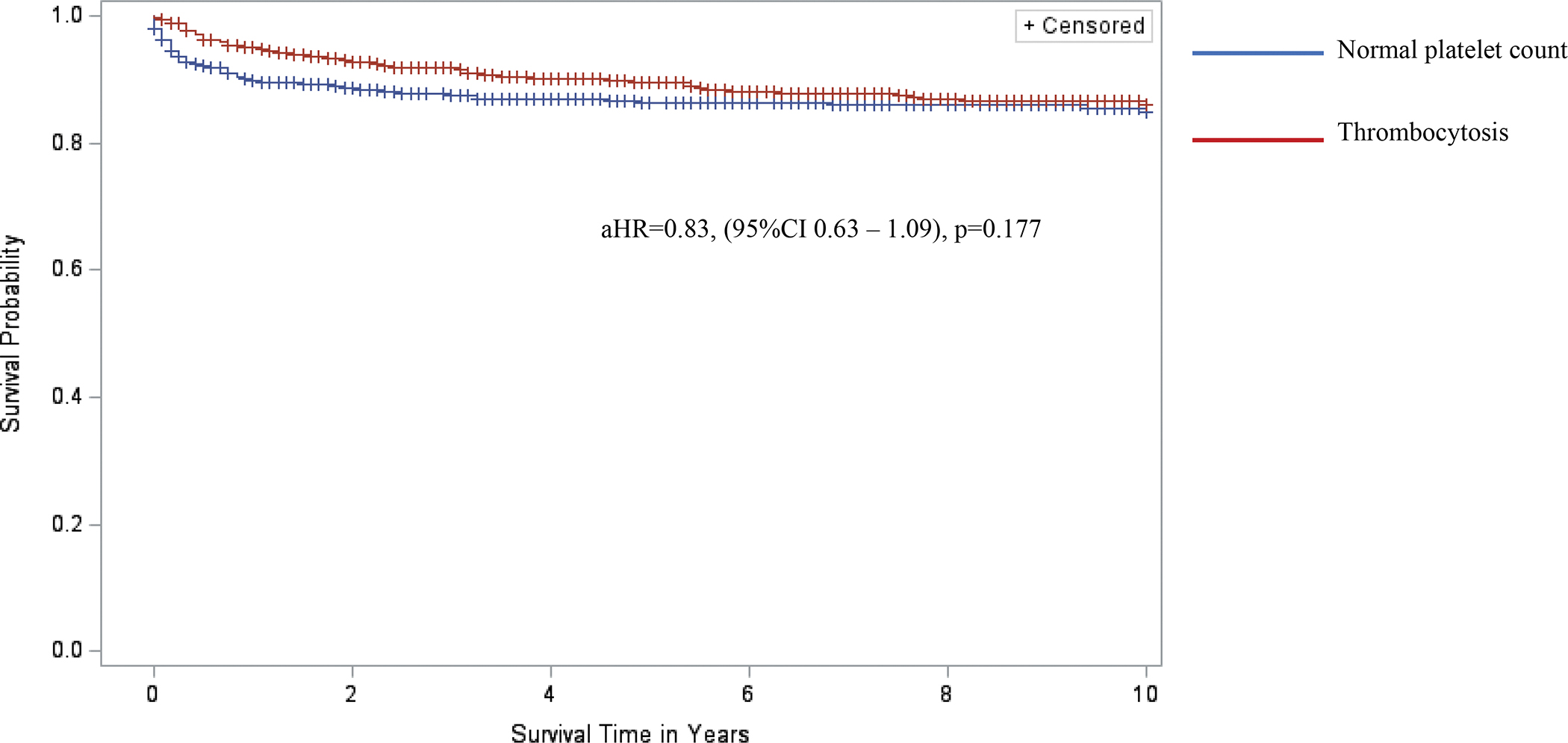
Kaplan-Meier curves comparing 10-year survival of children living with HIV infection and with and without thrombocytosis in the BIPAI cohort. Kaplan-Meier survival analysis was used to create survival curves and a Cox proportional hazard model to estimate hazard ratios of 10-year overall survival by presence of thrombocytosis (red font survival curve, n = 953) versus normal platelet counts (blue font survival curve, n = 839). Cross marks in the curves indicate the timing of censorship of the children upon death or loss to follow-up in the follow-up period. The adjusted hazard ratio (adjusted for nutrition status) associated with thrombocytosis was 0.83, 95%CI 0.63 – 1.09, and p-value=0.177 (statistical model considered robust if p-value <0.05).
Discussion
The aim of this study was to quantify the incidence of thrombocytopenia and thrombocytosis in a population of pediatric HIV patients on ART, identify factors associated with these quantitative platelet abnormalities in CLWH, and determine their association with survival of CLWH in SSA.
The incidence of thrombocytopenia in the general population of children without HIV infection is unknown because thrombocytopenia can be a clinically inapparent feature of a diverse variety of childhood diseases. However, the most common cause of isolated thrombocytopenia in children is immune thrombocytopenia (ITP) that has an incidence of 1 case per 23,810 person-years. [20] A few prior studies have reported the prevalence but not incidence of thrombocytopenia in children with HIV infection: Ellaurie M. et al (1986) reported that 13% of CLWH before the ART era had thrombocytopenia [21]. Other studies, including adolescents and adults with HIV infection have reported a prevalence of thrombocytopenia of 10 – 30% [5, 22], with thrombocytopenia associated with lower CD4 levels while thrombocytosis was associated with ART [23, 24].
Thrombocytopenia was associated with higher short- and long-term mortality among CLWH, suggesting it can serve as a key biomarker of HIV-related opportunistic diseases or immune dysfunction that is readily available in clinical settings worldwide. The association of thrombocytopenia with WHO Stage III/IV and not CD4 suppression suggests that thrombocytopenia in CLWH is secondary to other AIDS-defining conditions, such as opportunistic infections and cancers, and not necessarily a marker of the prevailing underlying immune status. However, thrombocytopenia may still be an indicator of abnormal immune function that is not measurable by quantitative CD4 levels as used in this study. Hence, in future studies it is important to determine the mechanisms of thrombocytopenia (e.g., chronic ITP vs. consumptive destruction vs. bone marrow suppression) and their associations. The protective effect of Zidovudine (AZT) against thrombocytopenia is likely indicative of the high potency of AZT in controlling HIV load, restoring immune function and preventing HIV-related thrombocytopenia [25, 26].
Regarding thrombocytosis, it has been shown to occur in 14% of children with various infections in an ambulatory setting and is associated with younger age[27]. Ellaurie M. (2004) reported a prevalence of 6% in a cohort of 400 CLWH that were not on ART [13]. This study is the first to quantify the incidence of thrombocytosis in CLWH on ART, demonstrating that it is a very common feature, i.e., 1 per 11.4 CLWH-years.
Thrombocytosis had no association with mortality in this cohort of CLWH. The tendency for younger children to develop thrombocytosis as part of an inflammatory response is an established phenomenon and was observed in this cohort as well [27]. Also, thrombocytosis was associated with favorable factors, including less CD4 suppression and ART i.e., Lamivudine and Nevirapine. The myelosuppressive effect of AZT may explain why this agent was not particularly associated with thrombocytosis [28]. Overall, these findings suggest that thrombocytosis may indicate preservation or restoration of a robust immune response to chronic antigenic stimulation in CLWH. However, although this may be a favorable effect with regards to immune surveillance against opportunistic diseases, it is possible that these CLWH and thrombocytosis are at increased risk for delayed morbidity and mortality from diseases associated with chronic immune hyperactivation in adulthood, such as malignancies and cardiovascular disease [29, 30].
The high rate of co-occurrence of anemia, leukopenia and/or leukocytosis with quantitative platelet abnormalities in CLWH also highlights the systemic impact of HIV and its treatment on the hematopoietic system. Many of the potential pathogenesis mechanisms of thrombocytopenia or thrombocytosis including infections, ART agents, immune dysregulation, and micronutrient deficiencies are known to affect multiple hematopoietic progenitors [22].
Limitations
This was a retrospective nested case control study that may have the limitations of retrospective designs, particularly bias due to missing data. We used a nested case-control instead of a retrospective cohort design to mitigate this potential bias and applied the most common sparse data-fitting methods for stratified logistic models, conditional logistic regression, to reduce sparse data bias.
Children with T/T had significantly more CBC checks than those without. It is possible that healthier children did not have CBCs checked to pick up occult platelet abnormalities. However, our primary independent variable of interest was the first incident of T/T. We also excluded from analysis the children (13.8%) who did not have any CBC in the EMR, and it is possible that these children were dissimilar from those that we studied. Additional contemporaneous clinical variables (e.g., viral loads, presence or absence of acute opportunistic infections) would be relevant to understand the likely underlying mechanisms and significance of the platelet abnormalities. However, due to absence of data on viral loads for most of the children and ambiguity or variability in the methods of diagnosis of opportunistic infections in the cohort we were unable to include these variables in this analysis.
We were unable to interrogate the causes of mortality in this cohort because the information was not available in the EMR. This would help to identify the possible links between thrombocytopenia and mortality in CLWH.
Conclusion
Children with thrombocytopenia should be identified and specifically investigated to evaluate the underlying cause of the thrombocytopenia because it is associated with higher mortality. Biomarkers for long-term mortality are critically needed in children than adults since the latter are exposed to HIV and ART for longer periods (typically from birth) and at a time of many organ systems, including the hematopoietic system. Thrombocytopenia is associated with poor prognosis in the short- and long-term while thrombocytosis is not associated with increased mortality during childhood. Following up CLWH into adulthood will be important to determine the longer-term effect of thrombocytosis on morbidity and mortality related to sequelae of longstanding immune hyperactivation. Studies to examine the hematological effects of newer ART agents and regimens are also important. Most importantly, to guide the use of platelet counts as biomarkers for interventions that may reduce or prevent long-term morbidity and mortality in CLWH, it is critical to conduct laboratory studies to elucidate the pathophysiology mechanisms for thrombocytopenia and thrombocytosis and their sequelae in CLWH.
Supplementary Material
Supplementary Figure 1
Kaplan-Meier curves comparing 10-year survival of children living with HIV infection and by severity of thrombocytopenia in the BIPAI cohort. Kaplan-Meier survival analysis was used to create survival curves and a Cox proportional hazard model to estimate hazard ratios of 10-year overall survival by presence of severity of thrombocytopenia normal platelet counts – 150,000 – 450,000/μL (green font survival curve, n = 839), mild thrombocytopenia – platelets 50,000 – 150,000 (blue font survival curve, n = 82), moderate thrombocytopenia – platelets 20,000 – 50,000/μL (red font survival curve, n = 48), and severe thrombocytopenia – platelets <20,000/μL (brown font survival curve, n = 57). Cross marks in the curves indicate the timing of censorship of the children upon death or loss to follow-up in the follow-up period. Using normal platelet counts as the reference group, the adjusted hazard ratios (adjusted for nutrition status) associated with mild, moderate, and severe thrombocytopenia were 1.60 (95%CI 1.02 – 2.49, p-value=0.039), 2.23 (95%CI 1.27 – 3.91, p=0.005), and 3.67 (95%CI 2.36 – 5.70, p<0.001). The statistical model was considered robust if p-value <0.05.
Acknowledgements
The authors wish to thank the children and families of the BIPAI clinical centers and all the front-line clinical staff who have cared for them over the years. We also acknowledge the contributions of the following individuals to this work: Mr. Mike Mizwa and Mr. John Dudley.
This research was funded by National Institutes of Health/National Cancer Institute, P30 CA125123-09S3.
Funding
3P30CA125123-09S3, National Institutes of Health, National Cancer Institute, P30 Supplement for HIV-related Malignancies
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
Disclaimers: None
References
- 1.UNAIDS. Global HIV & AIDS statistics — 2020 fact sheet. In; 2020.
- 2.UNICEF. HIV/AIDS: Paediatric care and treatment. In; 2020.
- 3.Njuguna IN, Cranmer LM, Otieno VO, Mugo C, Okinyi HM, Benki-Nugent S, et al. Urgent versus post-stabilisation antiretroviral treatment in hospitalised HIV-infected children in Kenya (PUSH): a randomised controlled trial. Lancet HIV 2018; 5(1):e12–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frigati L, Archary M, Rabie H, Penazzato M, Ford N. Priorities for Decreasing Morbidity and Mortality in Children With Advanced HIV Disease. Clin Infect Dis 2018; 66(suppl_2):S147–s151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scaradavou A HIV-related thrombocytopenia. Blood Rev 2002; 16(1):73–76. [DOI] [PubMed] [Google Scholar]
- 6.Ali RA, Wuescher LM, Worth RG. Platelets: essential components of the immune system. Curr Trends Immunol 2015; 16:65–78. [PMC free article] [PubMed] [Google Scholar]
- 7.Gear AR, Camerini D. Platelet chemokines and chemokine receptors: linking hemostasis, inflammation, and host defense. Microcirculation 2003; 10(3–4):335–350. [DOI] [PubMed] [Google Scholar]
- 8.Gawaz M, Neumann FJ, Dickfeld T, Reininger A, Adelsberger H, Gebhardt A, et al. Vitronectin receptor (alpha(v)beta3) mediates platelet adhesion to the luminal aspect of endothelial cells: implications for reperfusion in acute myocardial infarction. Circulation 1997; 96(6):1809–1818. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa M, Cooper D, Arumugam TV, Zhang JH, Nanda A, Granger DN. Platelet-leukocyte-endothelial cell interactions after middle cerebral artery occlusion and reperfusion. J Cereb Blood Flow Metab 2004; 24(8):907–915. [DOI] [PubMed] [Google Scholar]
- 10.Andre P, Denis CV, Ware J, Saffaripour S, Hynes RO, Ruggeri ZM, et al. Platelets adhere to and translocate on von Willebrand factor presented by endothelium in stimulated veins. Blood 2000; 96(10):3322–3328. [PubMed] [Google Scholar]
- 11.Ellaurie M, Burns ER, Bernstein LJ, Shah K, Rubinstein A. Thrombocytopenia and human immunodeficiency virus in children. Pediatrics 1988; 82(6):905–908. [PubMed] [Google Scholar]
- 12.Passos AM, Treitinger A, Spada C. An overview of the mechanisms of HIV-related thrombocytopenia. Acta Haematol 2010; 124(1):13–18. [DOI] [PubMed] [Google Scholar]
- 13.Ellaurie M Thrombocytosis in pediatric HIV infection. Clin Pediatr (Phila) 2004; 43(7):627–629. [DOI] [PubMed] [Google Scholar]
- 14.Evans RH, Scadden DT. Haematological aspects of HIV infection. Baillieres Best Pract Res Clin Haematol 2000; 13(2):215–230. [DOI] [PubMed] [Google Scholar]
- 15.Landonio G, Nosari AM, Vigorelli R, Spinelli F, Schlacht I, Caggese L. More than one form of HIV-related thrombocytopenia. Haematologica 1990; 75(6):589. [PubMed] [Google Scholar]
- 16.Damonti J, Doykos P, Wanless RS, Kline M. HIV/AIDS in African children: the Bristol-Myers Squibb Foundation and Baylor response. Health Aff (Millwood) 2012; 31(7):1636–1642. [DOI] [PubMed] [Google Scholar]
- 17.Goodnight S, Hathaway W. Disorders of Hemostasis & Thrombosis: A Clinical Guide. Second ed. Lancaster, PA: McGraw-Hill, Inc; 2001. [Google Scholar]
- 18.Organization WH. WHO case definitions of HIV for surveillance and revised clinical staging and immunologic classification of HIV-related disease in adults and children. In; 2007. pp. 1–48. [Google Scholar]
- 19.Organization WH. Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. In: WHO Child Growth Standards: methods and development. Geneva: World Health Organization; 2006. [Google Scholar]
- 20.Yong M, Schoonen WM, Li L, Kanas G, Coalson J, Mowat F, et al. Epidemiology of paediatric immune thrombocytopenia in the General Practice Research Database. Br J Haematol 2010; 149(6):855–864. [DOI] [PubMed] [Google Scholar]
- 21.Ellaurie M, Bernstein LJ, Shah K Thrombocytopenia in pediatric AIDS. Blood 1986; (68). [Google Scholar]
- 22.Teachey DT, Lambert MP. Diagnosis and management of autoimmune cytopenias in childhood. Pediatr Clin North Am 2013; 60(6):1489–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Getawa S, Aynalem M, Bayleyegn B, Adane T. The global prevalence of thrombocytopenia among HIV-infected adults: A systematic review and meta-analysis. International Journal of Infectious Diseases 2021; 105:495–504. [DOI] [PubMed] [Google Scholar]
- 24.Li B, Zhang L, Liu Y, Xiao J, Wang X, Wei Y, et al. Manifestations and Related Risk Factors of Thrombocyte Abnormalities in HIV-Positive Patients Before and After the Initiation of ART. Infect Drug Resist 2021; 14:4809–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckhardt BJ, Gulick RM. 152 - Drugs for HIV Infection. In: Infectious Diseases (Fourth Edition). Cohen J, Powderly WG, Opal SM (editors): Elsevier; 2017. pp. 1293–1308.e1292. [Google Scholar]
- 26.Research OoA. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. In. Edited by Health NIo. ROCKVILLE MD; 2020. [Google Scholar]
- 27.Heath HW, Pearson HA. Thrombocytosis in pediatric outpatients. J Pediatr 1989; 114(5):805–807. [DOI] [PubMed] [Google Scholar]
- 28.Brogan KL, Zell SC. Hematologic toxicity of zidovudine in HIV-infected patients. Am Fam Physician 1990; 41(5):1521–1528. [PubMed] [Google Scholar]
- 29.O’Byrne KJ, Dalgleish AG. Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer 2001; 85(4):473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Longenecker CT, Sullivan C, Baker JV. Immune activation and cardiovascular disease in chronic HIV infection. Curr Opin HIV AIDS 2016; 11(2):216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Kaplan-Meier curves comparing 10-year survival of children living with HIV infection and by severity of thrombocytopenia in the BIPAI cohort. Kaplan-Meier survival analysis was used to create survival curves and a Cox proportional hazard model to estimate hazard ratios of 10-year overall survival by presence of severity of thrombocytopenia normal platelet counts – 150,000 – 450,000/μL (green font survival curve, n = 839), mild thrombocytopenia – platelets 50,000 – 150,000 (blue font survival curve, n = 82), moderate thrombocytopenia – platelets 20,000 – 50,000/μL (red font survival curve, n = 48), and severe thrombocytopenia – platelets <20,000/μL (brown font survival curve, n = 57). Cross marks in the curves indicate the timing of censorship of the children upon death or loss to follow-up in the follow-up period. Using normal platelet counts as the reference group, the adjusted hazard ratios (adjusted for nutrition status) associated with mild, moderate, and severe thrombocytopenia were 1.60 (95%CI 1.02 – 2.49, p-value=0.039), 2.23 (95%CI 1.27 – 3.91, p=0.005), and 3.67 (95%CI 2.36 – 5.70, p<0.001). The statistical model was considered robust if p-value <0.05.


