Abstract
Purpose.
Acute otitis media (AOM) is a common indication for antibiotics in children. We sought to characterize the frequency of non-guideline concordant antibiotic therapy for AOM in the United States, by agent and duration.
Methods.
Using national administrative claims data (2016–2019), we identified children aged 6 months to 17 years with an oral antibiotic dispensed within 3 days of a new diagnosis of suppurative AOM. Use of non-guideline concordant agents and durations, defined based on national treatment guidelines, were summarized by age, race, rurality, region, and insurance type. Subsequent oral antibiotic dispensing within the year after AOM diagnosis was also evaluated. We created sunburst diagrams to visualize longitudinal patterns of within-person antibiotic utilization for AOM, by agent and duration.
Results.
We identified 789,424 eligible commercially-insured and 502,239 Medicaid-insured children. Among commercially-insured children, 35% received non-guideline concordant agents for AOM, including cefdinir (16%), amoxicillin-clavulanate (12%), and azithromycin (7%). Fewer children age <2 years received a non-guideline concordant initial agent (27%) compared to age ≥6 years (41%). More children age <2 years received three or more antibiotics over the following year (34% vs 3% for children age ≥6 years). The most common treatment duration was 10 days for all ages; treatment duration for the initial antibiotic was non-guideline concordant for 95% and 89% of children age 2–5 years and ≥ 6 years, respectively. Patterns were similar for Medicaid-insured children.
Conclusions.
Non-guideline concordant antibiotic use is common when treating AOM in children, including use of broad-spectrum agents and longer-than-recommended antibiotic durations.
Keywords: administrative data, data visualization, otitis media, anti-bacterial agents, drug utilization, pediatrics, guideline adherence
PLAIN LANGUAGE SUMMARY
Acute otitis media (AOM) is a common indication for antibiotics in children. We aimed to study the frequency of non-guideline concordant antibiotic therapy for AOM in the U.S. Using national data from health insurance databases (2016–2019), we identified children aged 6 months to 17 years with an oral antibiotic dispensed within 3 days of a new diagnosis of suppurative AOM. Use of non-guideline concordant agents and durations, defined based on national treatment guidelines, were summarized by age, race, rurality, region, and insurance type. We created data visualizations to illustrate patterns of antibiotic utilization for AOM, by agent and duration. We identified 789,424 eligible commercially-insured and 502,239 Medicaid-insured children. Among commercially-insured children, 35% received non-guideline concordant agents for AOM. Fewer children age <2 years received a non-guideline concordant initial agent (27%) compared to age ≥6 years (41%). The most common treatment duration was 10 days for all ages; treatment duration for the initial antibiotic was non-guideline concordant for 95% and 89% of children age 2–5 years and ≥ 6 years, respectively. Patterns were similar for Medicaid-insured children. Non-guideline concordant antibiotic use is common when treating AOM in children, including use of broad-spectrum agents and longer-than-recommended antibiotic durations.
INTRODUCTION
Acute otitis media (AOM) is one of the most common indications for antibiotics in children in the United States.1 According to the American Academy of Pediatrics (AAP), 60% of children have ≥1 AOM episode by 3 years of age,2 and 95% of AOM visits result in an antibiotic prescription.3 Inappropriate antibiotic prescriptions, defined by non-first-line agents or non-guideline recommended durations, contribute to avoidable antibiotic-resistant infections,4 drug-related adverse events,5 reduced pediatric quality of life,5 and disruptions to the microbiome that increase risk for Clostridioides difficile infections and chronic diseases.6 In total, over 25% of children prescribed an oral antibiotic experience antibiotic-associated morbidity.5 Current guidelines recommend amoxicillin as first-line therapy for most children with the following durations: 10 days (<2 years), 7 days (2–5 years), and 5–7 days (≥6 years).7 These treatment guidelines apply regardless of prior history of AOM, as long as the previous AOM episode occurred >30 days before the current diagnosis.
Evidence from small, regional studies suggests that non-guideline concordant antibiotic treatment for pediatric AOM is common, including frequent use of non-first-line agents and non-guideline-recommended durations.8–10 Although antibiotic prescribing patterns vary across subgroups, including by race/ethnicity, rural-urban status, geographic region, and clinician specialty,11 non-adherence to AOM antibiotic treatment guidelines has not been well-characterized using nationally representative data. We sought to examine utilization patterns and assess guideline non-concordant antibiotic therapy for AOM, by antibiotic agent and duration, in national cohorts of commercially-insured and Medicaid-insured children. We further investigated differences in prescribing by individual- and prescriber-level characteristics, and used data visualization methods to characterize longitudinal patterns of within-person antibiotic utilization for AOM.
METHODS
Data Source
We used claims data (2016–2019) from the IBM® MarketScan® Commercial Database and IBM® MarketScan® Medicaid Database, which contain person-level, deidentified, longitudinal data on patient demographic characteristics, inpatient and outpatient procedures and services, and outpatient pharmacy-dispensed medications.12, 13 From 2016–2019, these databases contain records for >38 million commercially-insured individuals across the US,14 and >16 million Medicaid enrollees in multiple states, respectively. The institutional review board at Washington University School of Medicine deemed this study exempt from human subject review.
Study Design and Population
We included children ages 6 months to 17 years with a diagnosis of suppurative otitis media from January 1, 2017 to December 31, 2018 in an outpatient setting plus a relevant oral antibiotic dispensed on the day of, or within 3 days after the diagnosis. Suppurative otitis media was identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and ICD-10-CM diagnosis codes (Supplemental Table 1). We considered the following oral antibiotics: amoxicillin, amoxicillin-clavulanate, azithromycin, cefdinir, cefixime, cefpodoxime, ciprofloxacin, clindamycin, and levofloxacin (Supplemental Table 1). We required continuous enrollment and prescription drug coverage during the 180-day baseline period before the diagnosis date. We excluded children with an AOM diagnosis in the prior 90 days. The index date represented the first qualifying date of diagnosis of suppurative AOM during the study period for each child. Children were followed for 365 days after the index diagnosis to identify additional oral antibiotic dispensings.
AOM-related Antibiotic Prescriptions During Follow-up
We ascertained outpatient oral antibiotic prescriptions related to AOM during the 365-day follow-up period, by requiring an AOM diagnosis within 30 days prior to the antibiotic prescription. Specifically, we ascertained all commonly used oral antibiotics for AOM within 30 days after the index date. Then, starting on day 31, we required a new AOM diagnosis code to identify additional oral antibiotics during follow-up. The 30-day ascertainment window was rolling; thus, we reset the 30-day ascertainment window upon each new AOM diagnosis (Figure 1, Supplemental Figure 1). The follow-up period ended at the earliest of: 365 days of follow-up, health plan disenrollment, or 18 years of age.
Figure 1.
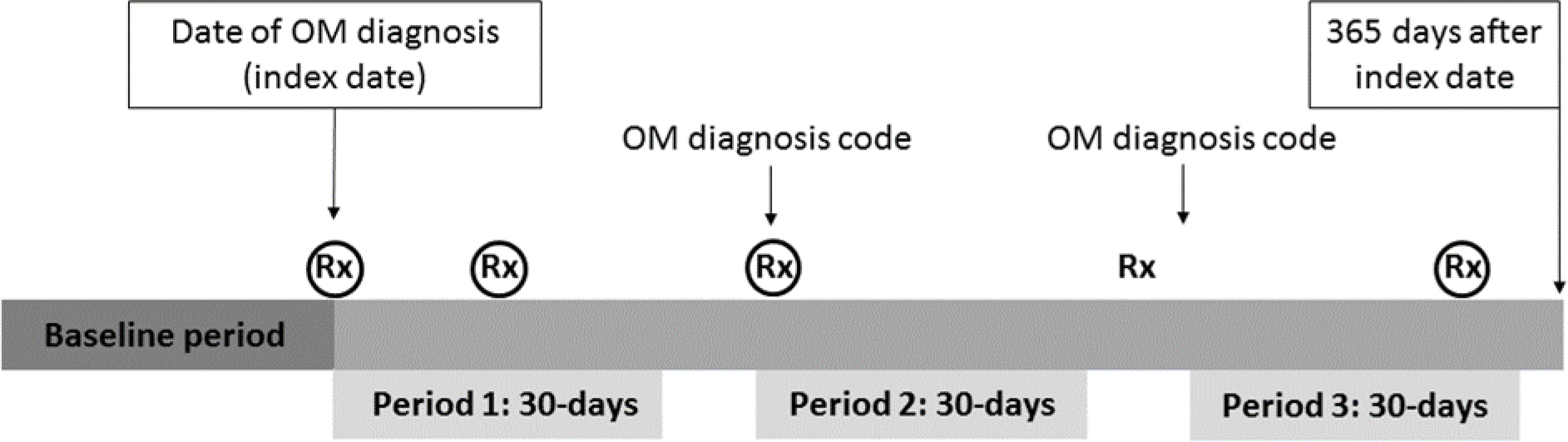
Study design schematic with longitudinal data for one hypothetical child’s history of otitis media (OM) diagnoses and relevant antibiotic prescriptions. For each child, the index date represented the first qualifying date of suppurative OM diagnosis during the study period, with a relevant oral antibiotic prescription dispensing on the day of or within 3 days after the diagnosis. The follow-up duration for subsequent relevant oral antibiotic dispensings was 365 days (primary analysis) and 30 days (secondary analysis). Black circles denote relevant antibiotic prescriptions, which were required to occur within 30 days on or after an OM diagnosis code. In this hypothetical example, the child received an antibiotic prescription on the index date (circled) as well as three subsequent relevant antibiotic prescriptions during the follow-up period (circled); one (uncircled) antibiotic prescription was not eligible to be counted because it did not occur within 30 days of a previous OM diagnosis code.
We classified antibiotic agents and treatment durations as guideline-concordant or guideline non-concordant based on pediatric AOM treatment guidelines (Supplemental Table 2).7 Amoxicillin was the only agent considered guideline-concordant for all ages. Guideline-concordant treatment durations were defined by age group at index: <2 years (10 days), 2–5 years (7 days), and ≥ 6 years (5–7 days). All other agents and durations were classified as non-guideline concordant. Multiple antibiotic prescriptions for different agents prescribed on the same date were categorized as other agent, and further classified as non-guideline concordant. Treatment duration was an approximation, since suspensions are often dispensed in a quantity slightly different from the prescription to account for measurement errors and bottle sizes. Therefore, we categorized durations as: ≤5 days included 1–6 days’ supply; 7 days included 7–9 days’ supply; 10 days included 10–13 days’ supply; and ≥14 days included ≥14 days’ supply. Most dispensings were 5, 7 or 10 days (Supplemental Figure 2).
Covariate Assessment
We ascertained several covariates, including age at index diagnosis, year of diagnosis, sex, race/ethnicity (Medicaid only), primary provider type, outpatient setting of the index AOM diagnosis, geographic region, and rural-urban status (commercial only). Patients were classified as urban if they resided in a metropolitan statistical area, according to the U.S. Census Bureau geographical mapping classification.15, 16
Statistical Analysis
We summarized individual- and prescriber-level characteristics of the commercially-insured and Medicaid-insured cohorts. We created sunburst diagrams to visualize longitudinal patterns of within-person antibiotic utilization for AOM. Within each concentric circle of the sunburst diagrams, we used different colors to illustrate the proportion of antibiotic receipt by agent or duration. The innermost concentric circle displays the distribution for the index antibiotic; each subsequent circle displays the distribution for each subsequent antibiotic dispensing. Additionally, we varied the length of the follow-up period for subsequent antibiotic prescriptions from 365 to 30 days to investigate the agents and durations used for the initial AOM episode. We conducted subgroup analyses by age group, race/ethnicity (Medicaid-insured only), rural-urban residence (commercially-insured only), provider type, and provider setting. To characterize geographic patterns, we displayed the proportion of non-guideline concordant antibiotic use on state-level maps, by agent and duration. We plotted monthly trends in utilization of non-guideline concordant antibiotic agents and durations by age group and insurance status.
RESULTS
We identified 789,424 eligible commercially-insured children and 502,239 Medicaid-insured children (Supplemental Figures 3a and 3b). Among commercially insured children, the median age at AOM diagnosis was 4 years (interquartile range [IQR], 1–8), 77% lived in an urban setting, and 54% were diagnosed by a pediatrician (Table 1). Children were diagnosed more frequently in the office than the emergency department (commercially-insured, 85% versus 4%; Medicaid-insured, 56% versus 18%). Among Medicaid-insured children, 23% were non-Hispanic Black and 16% were Hispanic/Latinx any race.
Table 1.
Characteristics of children diagnosed and treated for acute otitis media
| Commercial Insurance N = 789,424 |
Medicaid Insurance N = 502,239 |
|
|---|---|---|
| Age at diagnosis, years (median IQR) | 4 (1, 8) | 4 (2, 7) |
| < 2 | 201,717 (25.6) | 120,149 (23.9) |
| 2 to 5 | 281,989 (35.7) | 200,627 (39.9) |
| ≥ 6 | 305,718 (38.7) | 181,463 (36.1) |
| Male sex | 406,990 (51.6) | 259,004 (51.6) |
| Race/ethnicitya | ||
| Non-Hispanic or non-Latino white | -- | 239,708 (58.0) |
| Non-Hispanic or non-Latino black | -- | 94,702 (22.9) |
| Hispanic or Latino | -- | 64,234 (15.5) |
| Other | -- | 14,515 (3.5) |
| Provider type | ||
| Pediatrician | 424,685 (53.8) | 149,660 (29.8) |
| Family practice | 92,790 (11.8) | 35,637 (7.1) |
| Emergency medicine | 28,520 (3.6) | 36,215 (7.2) |
| Internal medicine | 15,414 (2.0) | 5,115 (1.0) |
| Nurse practitioner | 41,572 (5.3) | 58,540 (11.7) |
| Physician assistant | 13,840 (1.8) | 35,501 (7.1) |
| Other | 161,452 (20.5) | 80,173 (16.0) |
| Missing | 11,151 (1.4) | 101,398 (20.2) |
| Outpatient setting | ||
| Office | 673,782 (85.4) | 282,143 (56.2) |
| Urgent care | 70,300 (8.9) | 41,698 (8.3) |
| Emergency department | 27,695 (3.5) | 91,349 (18.2) |
| Outpatient hospital | 9,879 (1.3) | 23,571 (4.7) |
| Walk-in retail clinic | 2,213 (0.3) | 516 (0.1) |
| Other | 5,529 (0.7) | 62,962 (12.5) |
| Missing | 26 (0.0) | 0 (0.0) |
| Region | ||
| Northeast | 138,470 (17.5) | -- |
| Midwest | 176,951 (22.4) | -- |
| South | 368,827 (46.7) | -- |
| West | 105,176 (13.3) | -- |
| Rural-urban status | ||
| Rural | 181,694 (23.0) | -- |
| Urban | 607,730 (77.0) | -- |
Abbreviations: IQR, interquartile range; OM, otitis media.
Information on race/ethnicity was not available in the IBM MarketScan commercially-insured population and information on region and rurality was not available in the IBM MarketScan Medicaid Database.
Use of non-guideline concordant agents was common and more frequent among commercially-insured children (35%) than Medicaid-insured children (29%). Among children with commercial insurance, the most common non-guideline concordant agents for the initial dispensing were cefdinir (16%), amoxicillin-clavulanate (12%), and azithromycin (7%); use of non-guideline concordant agents increased with age, from 27% (<2 years) to 35% (2–5 years) to 41% (≥6 years) (Figure 2a). Patterns of non-guideline concordant agent use were similar in the Medicaid-insured population (Figure 2b). Initial use of cefdinir and azithromycin were least common among the youngest children (<2 years), irrespective of insurance type (Figure 2). The youngest children were most likely to receive multiple antibiotic prescriptions during the 365-day follow-up: 60% of commercially-insured children age <2 years had ≥2 antibiotic dispensings, and 21% had ≥4 dispensings. Among children age ≥6, only 23% and 2% had ≥2 and ≥4 dispensings, respectively (Supplemental Table 3). Within 30 days after an index dispensing of amoxicillin, cefdinir was the most common subsequent antibiotic (Supplemental Figure 4).
Figure 2.
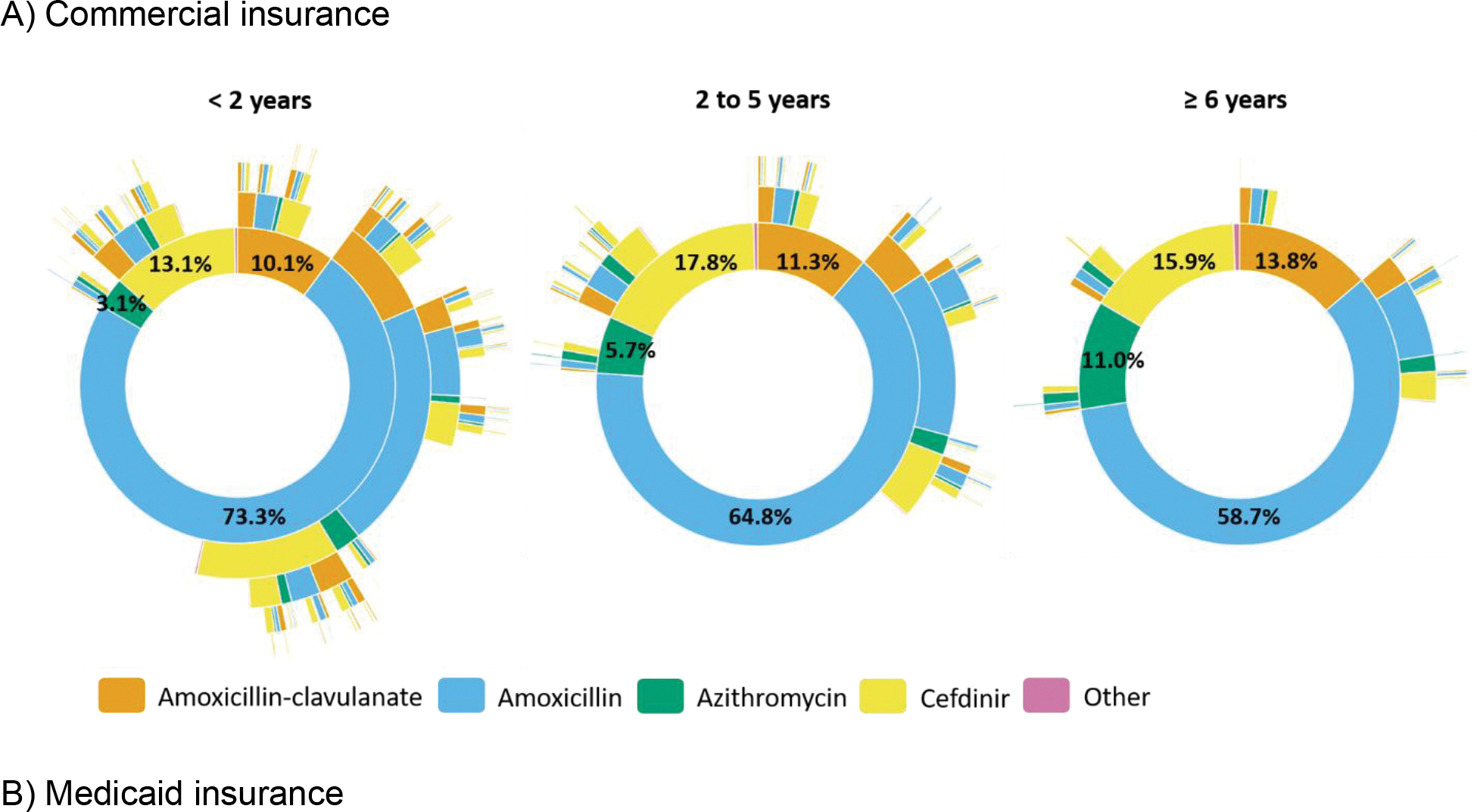
Sunburst diagrams of longitudinal patterns of within-person antibiotic treatment for otitis media by agent and age group among A) commercially-insured children and B) Medicaid-insured children, 365 days of follow-up. Each concentric circle of the sunburst diagram illustrates the proportion of antibiotic agent receipt. The innermost concentric circle displays the distribution for the index antibiotic. Each subsequent circle displays the distribution for each subsequent antibiotic dispensing. Other agents included cefixime, cefpodoxime, ciprofloxacin, clindamycin, and levofloxacin, as well as multiple prescriptions for different agents on the same date. Interactive versions of sunburst diagrams are available at https://rpubs.com/ambutler/949725.
Non-guideline concordant agent use varied across patient-level subgroups. Among commercially-insured children, use was more common in rural (40%) versus urban (34%) settings (Figure 3). State-level maps illustrate that the highest proportions of non-guideline concordant agents were prescribed in the southern region of the US (Supplemental Figure 5). Non-guideline concordant use was lowest among pediatricians (commercially-insured, 35%; Medicaid insured, 30%) and emergency departments (commercially-insured, 31%; Medicaid insured, 22%), and highest among family practice (commercially-insured, 38%; Medicaid insured, 32%) and internal medicine (commercially-insured, 40%; Medicaid insured, 35%) (Supplemental Figure 6). Antibiotic type also varied by provider location, with fewer non-guideline concordant agents dispensed in emergency departments (commercially-insured, 28%; Medicaid insured, 21%) and more frequently dispensed in an office (commercially-insured, 36%; Medicaid insured, 31%) (Supplemental Figure 7).
Figure 3.
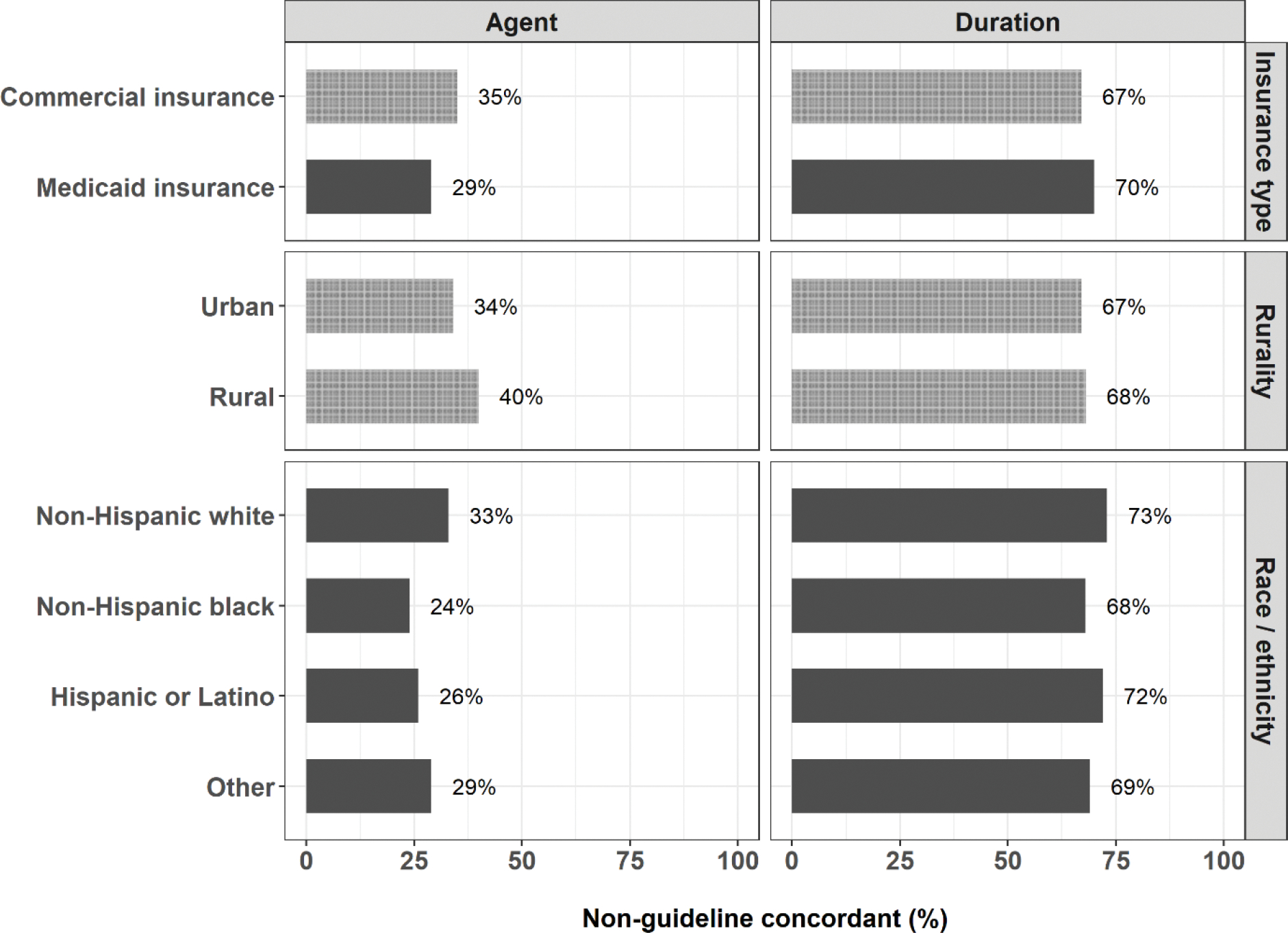
Proportion of index antibiotic dispensings for non-guideline concordant agent or duration among children with AOM, by subgroups. Due to data availability, the analysis of rurality was restricted to the commercially-insured population and the analysis of race/ethnicity was restricted to the Medicaid-insured population. Light grey denotes the commercially-insured population and dark grey denotes the Medicaid-insured population.
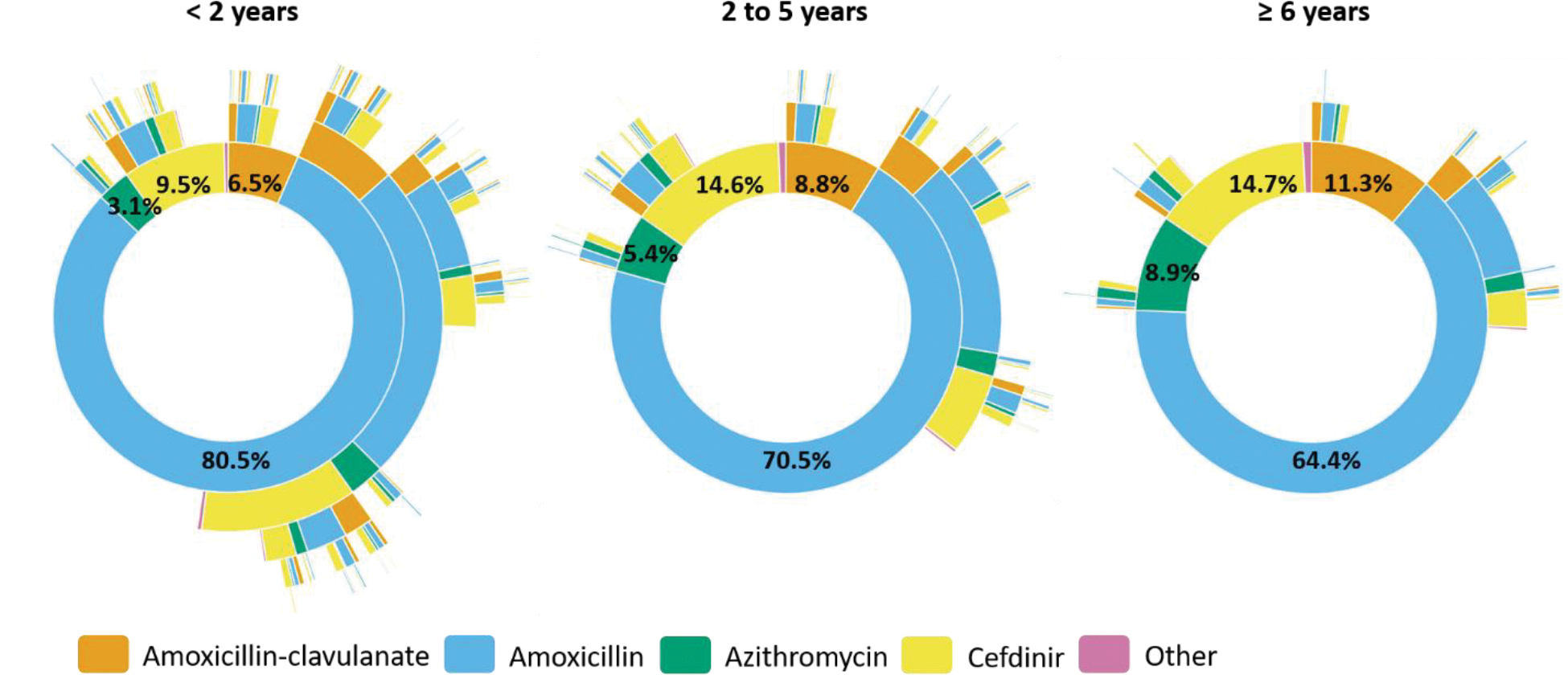
Most dispensings were 10-day prescriptions, irrespective of insurance type (Figure 4 and Supplemental Figure 8). Thus, non-guideline concordant treatment duration was uncommon for commercially-insured children <2 years (8% of initial prescriptions) but very common for children 2–5 years (90%) and ≥6 years (86%). Patterns were similar in the Medicaid-insured population. In addition, subsequent prescriptions were commonly 10-day prescriptions. For example, among children aged 2–5 years, 31% and 11% received two and three 10-day amoxicillin prescriptions within a year of the index AOM diagnosis, respectively.
Figure 4.
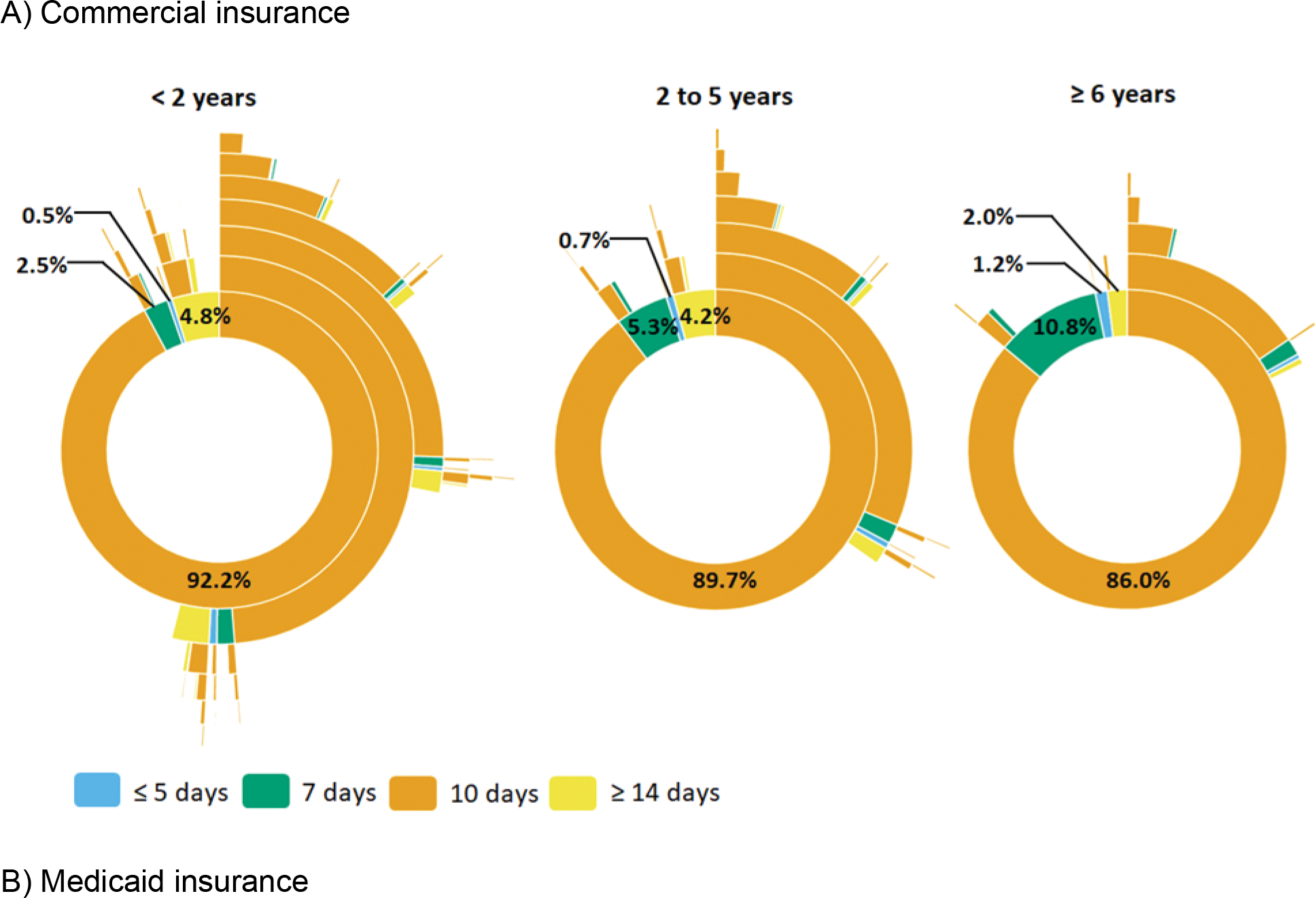
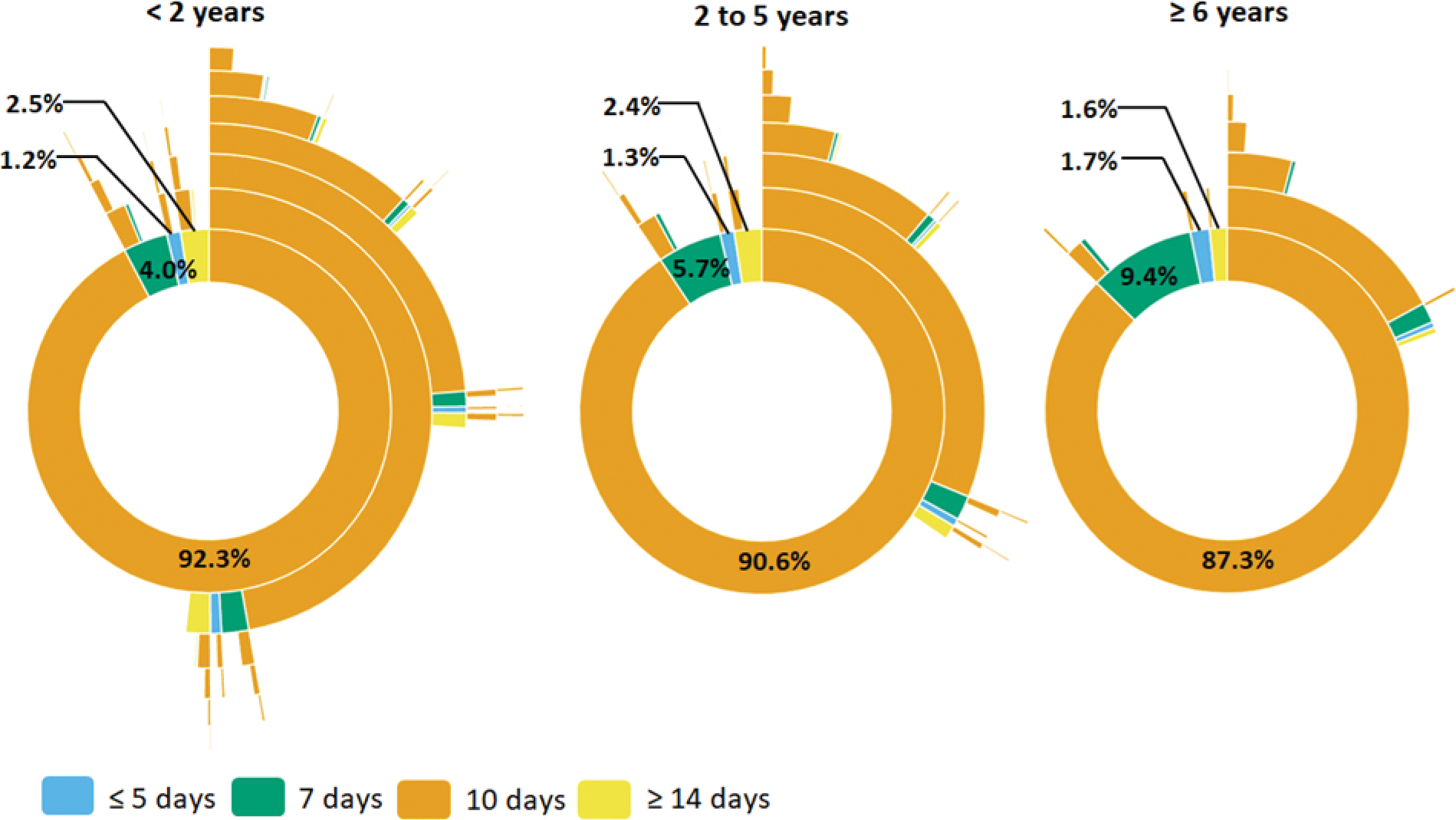
Sunburst diagrams of longitudinal patterns of within-person antibiotic treatment for otitis media by treatment duration and age group among A) commercially-insured children and B) Medicaid-insured children, 365 days of follow-up. Children prescribed azithromycin were excluded from this analysis. Each concentric circle of the sunburst diagram illustrates the proportion of antibiotic treatment duration. The innermost concentric circle displays the distribution for the index antibiotic. Each subsequent circle displays the distribution for each subsequent antibiotic dispensing. Interactive versions of sunburst diagrams are available at https://rpubs.com/ambutler/949725.
The frequency of non-guideline concordant treatment duration varied across subgroups, including race/ethnicity, geographic region, provider type, and provider location. Among Medicaid-insured patients, those who identified as non-Hispanic white were more likely to be prescribed non-guideline concordant durations than other children (Figure 3). Non-guideline concordant durations were dispensed most commonly in the northeastern and western regions of the US (Supplemental Figure 9) but did not differ by urban-rural status (Supplemental Figure 10). Non-guideline concordant duration differed by provider type (i.e., lowest among pediatricians and emergency medicine specialists) and provider location (i.e., highest at walk-in retail clinics and urgent care) (Supplemental Figures 6 & 7). Interactive versions of sunburst diagrams are available at https://rpubs.com/ambutler/949725 (primary) and https://rpubs.com/ambutler/949730 (supplemental). Utilization of non-guideline concordant antibiotic agents and durations did not vary considerably over time by age group or insurance status (Supplemental Figure 11).
DISCUSSION
Using administrative claims data from almost 1.3 million commercially-insured or Medicaid-insured children diagnosed and treated with AOM, we documented that 40% of index prescriptions were non-first-line agents and 95% of prescriptions for children ≥2 years of age were longer-than-recommended durations. Antibiotic selection and duration varied by individual-level characteristics including age group, race/ethnicity, insurance type, rural-urban status, and geographic region and provider-level characteristics including specialty. A substantial proportion of children received additional AOM-related antibiotic prescriptions in the year following the initial AOM diagnosis.
Approximately two-thirds of initial antibiotic agents were amoxicillin – which AAP recommends as first-line therapy for most children with AOM due to high effectiveness against common AOM bacterial pathogens as well as narrow microbiologic spectrum, good safety profile, acceptable taste, and low cost.7, 17, 18 The remaining one-third of children with AOM initially used broad-spectrum agents, which are associated with increased risk of adverse drug events.5 Of these broad-spectrum agents, cefdinir (non-first-line agent recommended only for penicillin allergy) was used more commonly than amoxicillin-clavulanate (second-line agent), consistent with a previous national study.8 Given that ~10% of patients have documented penicillin allergy—of which true allergies are rare—we observed higher than expected use of agents other than amoxicillin or amoxicillin-clavulanate.7, 19 Azithromycin, which is never recommended for AOM therapy, was used initially by 3–11% of commercially- and Medicaid-insured children, with older children receiving it more frequently. Furthermore, subsequent agents prescribed within 30-days of initial AOM diagnosis were not predominantly second-line amoxicillin-clavulanate agents.
Nearly all prescriptions for children ≥2 years were longer than recommended. Although current pediatric treatment guidelines recommend 7 days (2–5 years) or 5–7 days (≥6 years) of antibiotic therapy for most children with mild/moderate symptoms, 10-day prescriptions were nearly universal, irrespective of age. Our findings are consistent with a recent national study of outpatient antibiotic therapy for common outpatient infections (including pediatric AOM) which reported that clinicians frequently defaulted to 10-day durations even when guidelines recommend shorter durations.20 Similarly, in another recent study conducted at a university-affiliated, community-based health care system, more than half of pediatric patients with AOM received ≥10 days of therapy.9 Given that longer antibiotic courses have been associated with increased risks of adverse events21–23 and antibiotic-resistant infections,24, 25 interventions are needed to encourage minimum effective durations in children.
Future antimicrobial stewardship interventions ought to consider individual-level characteristics associated with non-guideline concordant outpatient antibiotic prescribing. Our findings on race-based differences in antibiotic use are consistent with previous findings that black children receive a lower proportion of broad-spectrum antibiotic prescriptions than white children,26, 27 and Hispanic children receive shorter durations of antibiotics than non-Hispanic children.9 Our study extends the scope of prior knowledge by demonstrating similar race-based patterns related to treatment duration. These differences in treatment choice for racial and ethnic minorities may indicate race-based differences in physician practice patterns and parental preferences for children with AOM,26, 27 and research is currently ongoing to understand these patterns. While these racial and ethnic minority populations experience slightly lower non-guideline concordant prescribing by agent and duration, they still experience substantial inappropriate prescribing. Improving health equity for racial and ethnic minority populations will require education and policy solutions, including the recognition and reduction of implicit bias among healthcare providers.28, 29
We also observed geographic variability in non-guideline concordant antibiotic use for AOM, including more frequent use among rural versus urban patients. Given distance-to-healthcare barriers for rural patients, it is possible that clinicians may justify prescribing broad-spectrum agents to avoid future treatment failure-related healthcare encounters. This rationale has also been proposed to explain rural-urban differences in outpatient antibiotic prescribing for uncomplicated urinary tract infection (but for inappropriate duration rather than agent).30 Separately, our results confirmed previous reports that the highest proportion of non-guideline concordant antibiotic agents for pediatric AOM occurred in the South;26 this finding is particularly concerning from a public health perspective since the South is the highest prescribing region in the U.S.11 Conversely, the most frequent use of non-guideline concordant antibiotic duration occurred in the Northeast and West, rather than the South.
Importantly, many children received more than one AOM-related antibiotic prescription in the year following the initial AOM diagnosis (e.g., 60%, 40%, and 23% of commercially-insured children aged >2 years, 2–5 years, and ≥6 years, respectively). Some children may have acquired a first-line antibiotic-resistant pathogen31 and required a different antibiotic for the initial infection. Our analyses limited to 30-day follow-up illustrates patterns of use for the initial AOM episode, however, this clearly does not explain all future dispensings. Another explanation is that children who have antibiotic exposures (particularly early in life) may be more likely to have future infections requiring antibiotic use or other comorbid conditions that predispose to infections.32 This subgroup of children may be of particular interest to focus stewardship programs; future studies that account for cumulative antibiotic exposure in early life are needed.
Given that AOM is among the most common indication for antibiotics in children,11 efforts to improve antibiotic prescribing have the capability to substantially reduce inappropriate antibiotic exposure on a population-level. To assist health systems and practices with addressing inappropriate antibiotic use in the outpatient setting, the Centers for Disease Control and Prevention released the Core Elements of Outpatient Antibiotic Stewardship33 in 2016 and the American Academy of Pediatrics released a Change Package for Antibiotic Prescribing in 2019.34 These publications provide effective, pragmatic tools to reduce inappropriate prescribing. Additionally, AOM-specific stewardship interventions are effective in decreasing non-guideline concordant antibiotic prescriptions (i.e., immediate, non-first-line, longer-than-recommended). The most effective interventions include multi-faceted approaches that incorporate provider education, individualized audit-and-feedback to providers on prescribing compared to peers, and electronic health record changes.35–38 Small electronic health record changes—even when not coupled with other interventions—can also substantially improve prescribing for AOM.35, 39 Future research is warranted to study the implementation of stewardship interventions for AOM in different clinical settings and among diverse patient populations.
Our results are subject to several limitations. First, insurance claims data do not directly link diagnoses to prescription medication dispensings. While we required an AOM diagnosis followed by an antibiotic within 3 days for the index prescription or within 30 days for subsequent prescriptions, it is possible that the antibiotic was prescribed for another condition. Second, we used inclusive otitis media diagnostic code definitions to ensure broad capture of potential AOM patients (including misclassified patients or diagnostic drift to justify broader-spectrum antibiotic use). However, inclusion of patients coded for chronic otitis media was rare (N=284 (0.02%)), and possible differences in treatment patterns were unlikely to impact the results considerably. Third, recent exposure to amoxicillin may influence antibiotic selection for AOM infection; although we excluded children with an AOM diagnosis in the prior 90 days, we did not account for amoxicillin prescriptions within the prior 30 days. Previous work using the IBM MarketScan Commercial Database indicates that up to 9% of children with AOM may have had prior antibiotic exposure within 30 days.40 Even if we had excluded these children, our results still would have indicated excessive broad-spectrum antibiotic use. Fourth, claims data do not provide information on the accuracy or severity of AOM diagnoses. We classified 10-day antibiotic prescriptions as non-guideline concordant for children aged ≥ 2 years under the assumption that AOM infections were mild/moderate; however, 10-day prescriptions are recommended for severe AOM infections and other select circumstances, which would have resulted in misclassification for some patients.7 Fifth, claims data do not indicate reasons for prescribers’ choice of antibiotic agents or durations, and it is possible that some non-first-line agent use was prescribed due to history of allergy or prior adverse events. However, even though ~10% of patients report a history of penicillin allergy, up to 90% of these individuals are able to tolerate penicillin and are designated as having “penicillin allergy” unnecessarily.7, 41 Therefore, it is unlikely that penicillin allergy can fully explain the increased use of other types of antibiotics observed in this study. Sixth, we are not able to quantify whether the “watch-and-wait” strategy was employed after a prescription dispensing. It is possible that parents filled but did not administer a prescription, which would not be captured in claims data. Lastly, Medicaid data were only available for a subset of states. Therefore, these results may not generalize to all Medicaid-insured children in the U.S.
Conclusions
Non-guideline concordant antibiotic use is very common when treating AOM among children in the U.S., including use of broad-spectrum antibiotic agents and longer-than-recommended antibiotic durations. Providers and health systems should critically evaluate prescribing patterns for AOM and implement programs to reduce inappropriate prescribing, which will ultimately reduce antibiotic-associated morbidity in the pediatric population.
Supplementary Material
KEY POINTS.
Evidence is limited about the frequency of non-guideline concordant antibiotic treatment for acute otitis media among children in the U.S, by agent and duration.
Use of longitudinal, patient-level, health insurance claims data with capture of outpatient pharmacy-dispensed medications allowed examination of antibiotic utilization patterns for acute otitis media in commercially- and Medicaid-insured children.
Non-guideline recommended initial antibiotic use (e.g., cefdinir, azithromycin) for treatment of acute otitis media was common, particularly for children age ≥6 years (commercial insurance, 41%; Medicaid insurance, 36%). Treatment duration was longer than recommended for about 90% of children ≥2 years.
Data visualization techniques can be useful to characterize longitudinal patterns of drug utilization.
FUNDING INFORMATION:
A.M.B. received salary support from the National Center for Advancing Translational Sciences (NCATS), NIH under award number KL2 TR002346. Data programming for this study was conducted by the Center for Administrative Data Research, which is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH), Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality (AHRQ). H.F. received salary support from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K23HD099925. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
POTENTIAL CONFLICTS OF INTEREST: Dr. Butler reported receiving investigator-initiated research funds from Merck. Dr. McGrath was employed by and held equity in NoviSci/Target RWE and is currently employed by Pfizer. Dr. Newland reported receiving investigator-initiated research funds from Pfizer, Merck, AHRQ, and NIH and serving as an advisor for the Joint Commission. No other authors have financial relationships or conflicts of interest relevant to this study.
PRIOR PRESENTATION: Results were presented as an oral presentation at the 2021 International Conference on Pharmacoepidemiology & Therapeutic Risk Management (virtual format).
ETHICS STATEMENT: The institutional review board at Washington University School of Medicine deemed this study exempt from human subject review.
AUTHOR ACCESS TO DATA: Dr. Butler had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Vaz LE, Kleinman KP, Raebel MA, et al. Recent trends in outpatient antibiotic use in children. Pediatrics. Mar 2014;133(3):375–85. doi: 10.1542/peds.2013-2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaur R, Morris M, Pichichero ME. Epidemiology of Acute Otitis Media in the Postpneumococcal Conjugate Vaccine Era. Pediatrics. Sep 2017;140(3)doi: 10.1542/peds.2017-0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venekamp RP, Sanders SL, Glasziou PP, Del Mar CB, Rovers MM. Antibiotics for acute otitis media in children. The Cochrane database of systematic reviews. Jun 23 2015;2015(6):Cd000219. doi: 10.1002/14651858.CD000219.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. 2019.
- 5.Gerber JS, Ross RK, Bryan M, et al. Association of Broad- vs Narrow-Spectrum Antibiotics With Treatment Failure, Adverse Events, and Quality of Life in Children With Acute Respiratory Tract Infections. Jama. Dec 19 2017;318(23):2325–2336. doi: 10.1001/jama.2017.18715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kronman MP, Zaoutis TE, Haynes K, Feng R, Coffin SE. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics. Oct 2012;130(4):e794–803. doi: 10.1542/peds.2011-3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. Mar 2013;131(3):e964–99. doi: 10.1542/peds.2012-3488 [DOI] [PubMed] [Google Scholar]
- 8.Palms DL, Hicks LA, Bartoces M, et al. First-Line Antibiotic Selection in Outpatient Settings. Antimicrobial agents and chemotherapy. Nov 2019;63(11)doi: 10.1128/aac.01060-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frost HM, Becker LF, Knepper BC, Shihadeh KC, Jenkins TC. Antibiotic Prescribing Patterns for Acute Otitis Media for Children 2 Years and Older. The Journal of pediatrics. May 2020;220:109–115.e1. doi: 10.1016/j.jpeds.2020.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrera SC, Cancino RS, Barreto TW. The impact of continuity of care on antibiotic prescribing in acute otitis media. International journal of pediatric otorhinolaryngology. Nov 2019;126:109616. doi: 10.1016/j.ijporl.2019.109616 [DOI] [PubMed] [Google Scholar]
- 11.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011. Jama. May 03 2016;315(17):1864–73. doi: 10.1001/jama.2016.4151 [DOI] [PubMed] [Google Scholar]
- 12.IBM Watson Health. IBM MarketScan Research Databases for life sciences researchers. 2020
- 13.Butler AM, Nickel KB, Overman RA, Brookhart MA. IBM MarketScan Research Databases. In: Sturkenboom MC, Schink T, eds. Databases for Pharmacoepidemiological Research. Springer; 2021:243–251. [Google Scholar]
- 14.Truven Health MarketScan® Research Databases. MarketScan user guide, commercial claims and encounters, medical supplemental and coordination of benefits. 2015
- 15.Hansen LG. White Paper: IBM MarketScan Research Databases for Life Sciences Researchers. 2018.
- 16.Office of Management and Budget. 2010 standards for delineating metropolitan and micropolitan statistical areas. Federal Register 2010:37245–37252. [Google Scholar]
- 17.Klein JO. Microbiologic efficacy of antibacterial drugs for acute otitis media. Pediatr Infect Dis J. Dec 1993;12(12):973–5. doi: 10.1097/00006454-199312000-00001 [DOI] [PubMed] [Google Scholar]
- 18.Piglansky L, Leibovitz E, Raiz S, et al. Bacteriologic and clinical efficacy of high dose amoxicillin for therapy of acute otitis media in children. Pediatr Infect Dis J. May 2003;22(5):405–13. doi: 10.1097/01.inf.0000065688.21336.fa [DOI] [PubMed] [Google Scholar]
- 19.Pichichero ME, Casey JR. Safe use of selected cephalosporins in penicillin-allergic patients: a meta-analysis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. Mar 2007;136(3):340–7. doi: 10.1016/j.otohns.2006.10.007 [DOI] [PubMed] [Google Scholar]
- 20.King LM, Hersh AL, Hicks LA, Fleming-Dutra KE. Duration of Outpatient Antibiotic Therapy for Common Outpatient Infections, 2017. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. May 18 2021;72(10):e663–e666. doi: 10.1093/cid/ciaa1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozyrskyj A, Klassen TP, Moffatt M, Harvey K. Short-course antibiotics for acute otitis media. The Cochrane database of systematic reviews. Sep 8 2010;2010(9):Cd001095. doi: 10.1002/14651858.CD001095.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. Mar 01 2011;52(5):e103–20. doi: 10.1093/cid/ciq257 [DOI] [PubMed] [Google Scholar]
- 23.Tansarli GS, Mylonakis E. Systematic Review and Meta-analysis of the Efficacy of Short-Course Antibiotic Treatments for Community-Acquired Pneumonia in Adults. Antimicrobial agents and chemotherapy. Sep 2018;62(9)doi: 10.1128/aac.00635-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. Oct 2011;53(7):e25–76. doi: 10.1093/cid/cir531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gouliouris T, Warne B, Cartwright EJP, et al. Duration of exposure to multiple antibiotics is associated with increased risk of VRE bacteraemia: a nested case-control study. The Journal of antimicrobial chemotherapy. Jun 1 2018;73(6):1692–1699. doi: 10.1093/jac/dky075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleming-Dutra KE, Shapiro DJ, Hicks LA, Gerber JS, Hersh AL. Race, otitis media, and antibiotic selection. Pediatrics. Dec 2014;134(6):1059–66. doi: 10.1542/peds.2014-1781 [DOI] [PubMed] [Google Scholar]
- 27.Gerber JS, Prasad PA, Localio AR, et al. Racial Differences in Antibiotic Prescribing by Primary Care Pediatricians. Pediatrics. 2013;131(4):677–684. doi: 10.1542/peds.2012-2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joynt Maddox KE, James CV. How the Biden Administration Can Improve Health Equity for Racial and Ethnic Minority Populations. Jama. Apr 13 2021;325(14):1387–1388. doi: 10.1001/jama.2021.3046 [DOI] [PubMed] [Google Scholar]
- 29.Satcher D, Dawes DE. Race and the Patient-Physician Relationship in 2021. Jama. Aug 17 2021;326(7):595–596. doi: 10.1001/jama.2021.12454 [DOI] [PubMed] [Google Scholar]
- 30.Clark AW, Durkin MJ, Olsen MA, et al. Rural-urban differences in antibiotic prescribing for uncomplicated urinary tract infection. Infection control and hospital epidemiology. Feb 24 2021:1–8. doi: 10.1017/ice.2021.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hullegie S, Venekamp RP, van Dongen TMA, et al. Prevalence and Antimicrobial Resistance of Bacteria in Children With Acute Otitis Media and Ear Discharge: A Systematic Review. Pediatr Infect Dis J. Aug 1 2021;40(8):756–762. doi: 10.1097/inf.0000000000003134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aversa Z, Atkinson EJ, Schafer MJ, et al. Association of Infant Antibiotic Exposure With Childhood Health Outcomes. Mayo Clinic proceedings. Jan 2021;96(1):66–77. doi: 10.1016/j.mayocp.2020.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core Elements of Outpatient Antibiotic Stewardship. MMWR Recomm Rep. Nov 11 2016;65(6):1–12. doi: 10.15585/mmwr.rr6506a1 [DOI] [PubMed] [Google Scholar]
- 34.American Academy of Pediatrics CQNC. Chapter Quality Network Improving Antibiotic Prescribing for Children | Change Package. Accessed December 27, 2021, https://downloads.aap.org/DOCCSA/CQN%20ABX%20Change%20Package%20Final%20October%202019.pdf
- 35.Frost HM, Lou Y, Keith A, Byars A, Jenkins TC. Increasing Guideline-Concordant Durations of Antibiotic Therapy for Acute Otitis Media. The Journal of pediatrics. Jan 2022;240:221–227.e9. doi: 10.1016/j.jpeds.2021.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frost HM, Monti JD, Andersen LM, et al. Improving Delayed Antibiotic Prescribing for Acute Otitis Media. Pediatrics. 2021;147(6):e2020026062. doi: 10.1542/peds.2020-026062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norlin C, Fleming-Dutra K, Mapp J, et al. A Learning Collaborative to Improve Antibiotic Prescribing in Primary Care Pediatric Practices. Clin Pediatr (Phila). May 2021;60(4–5):230–240. doi: 10.1177/00099228211001623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. Jama. Jun 12 2013;309(22):2345–52. doi: 10.1001/jama.2013.6287 [DOI] [PubMed] [Google Scholar]
- 39.Forrest CB, Fiks AG, Bailey LC, et al. Improving adherence to otitis media guidelines with clinical decision support and physician feedback. Pediatrics. Apr 2013;131(4):e1071–81. doi: 10.1542/peds.2012-1988 [DOI] [PubMed] [Google Scholar]
- 40.Frost HM, Bizune D, Gerber JS, Hersh AL, Hicks LA, Tsay SV. Amoxicillin versus other antibiotic agents for the treatment of acute otitis media in children. The Journal of pediatrics. Aug 6 2022;doi: 10.1016/j.jpeds.2022.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. Oct 2010;105(4):259–273. doi: 10.1016/j.anai.2010.08.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


