Abstract
Objective.
To determine whether novel biomarkers of cardiometabolic health improve in response to a 12-month behavioral weight loss intervention; and to compare benefits of diet alone to diet in conjunction with physical activity on these biomarkers.
Methods.
Participants (n = 374) were randomized to either diet alone (DIET), diet plus 150 min/week of prescribed moderate intensity physical activity (DIET+PA150), or diet plus 250 min/week of prescribed moderate intensity physical activity (DIET+PA250). Biomarker concentrations were determined using nuclear magnetic resonance spectroscopy. Mixed models assessed for a time effect, group effect, or group by time interaction.
Results.
All groups significantly improved body weight (time: p<0.0001) and Lipoprotein Insulin Resistance Index (time: p<0.0001), Diabetes Risk Index (time: p<0.0001), branched-chain amino acid concentration (time: p<0.0001), and GlycA concentration (time: p<0.0001), with no group effect or group by time interactions.
Conclusions.
All intervention groups prompted a notable beneficial change among biomarkers of insulin resistance and cardiometabolic health. However, the addition of at least moderate intensity physical activity to a diet only intervention did not provide any additional benefit. These findings highlight an average weight loss of approximately 10% profoundly impacts biomarkers of insulin resistance and cardiometabolic disease in adults with overweight or obesity.
Keywords: Lifestyle modifications, exercise, obesity, lipoprotein, insulin resistance
INTRODUCTION
An estimated 1,307 million and 671 million adults over the age of 20 years worldwide are living with overweight or obesity, respectively.1 According to the World Obesity Foundation2, on current trends, one in five adults worldwide are expected to be affected by obesity by the year 2025. One third of these adults will be living with severe obesity and at greater risk for developing chronic diseases, such as cardiovascular disease and type 2 diabetes.1,2 With the increased prevalence of obesity also increasing risk for chronic disease, monitoring disease progression or response to treatment measures, such as lifestyle intervention for weight loss, via biomarkers of insulin resistance and cardiometabolic health are of clinical importance.
Insulin resistance and cardiometabolic health are traditionally characterized by increased fasting insulin, blood pressure, fasting glucose, waist circumference, and changes in lipid profile.3–5 Alternatively, 1) systemic inflammation represented by GlycA concentrations – a nuclear magnetic resonance (NMR) signal originating from glycosylated acute phase proteins6,7; 2) dysmetabolism, represented as branched-chain amino acid (BCAA) concentrations8–11; and 3) specific alterations in lipoprotein subclass and size parameters, increased large very large triglyceride-rich lipoprotein (VLTRLP), small low density lipoprotein (LDL), and decreased large high density lipoprotein (HDL), LDL size and HDL size12,13 are emerging biomarkers indicative of insulin resistance and poor cardiometabolic health. Further, NMR multimarker scores comprised of either six lipoprotein subclass and size parameters13 – Lipoprotein Insulin Resistance Index (LP-IR) – or a combination of both lipoprotein subclass and size parameters along with BCAA’s14,15 – Diabetes Risk Index – are indicative of disease risk and change in risk in response to lifestyle intervention.
Few clinical trials have explored the impact of weight loss via lifestyle intervention on these emerging biomarkers of insulin resistance and cardiometabolic health. Conventionally, weight loss of 5 to 10% achieved via lifestyle intervention is shown to be effective for improving traditional cardiovascular disease and type 2 diabetes risk factors, including: blood pressure16–18, decreased LDL cholesterol16,18,19, increased HDL cholesterol18–20, decreased TG18–21, and improved glucose tolerance.18,22
The Heart Health Study (HHS) investigated the effect of a reduced calorie diet alone compared to this same diet coupled with one of two doses of physical activity on weight loss, measures of cardiac structure, and other cardiometabolic risk factors among adults with overweight or obesity.23 The results showed all interventions significantly reduced body weight by an average of approximately 10% and improved cardiometabolic risk factors, with no difference between the intervention conditions.23 While primary results of the HHS reported on traditional cardiometabolic risk factors (total cholesterol, TG, LDL, HDL, glucose, insulin), the study did not include novel biomarkers such as LP-IR, Diabetes Risk Index, GlycA, or BCAA’s. Therefore, the use of samples from the HHS provides a unique opportunity to assess whether these additional emerging biomarkers of insulin resistance and cardiometabolic health respond to a behavioral weight loss intervention among sedentary adults with overweight or obesity.
METHODS
Study Design.
In the HHS (ClinicalTrials.gov NCT01500356, recruitment occurred between December 2011 and June 2015), participants completed assessments prior to, during, and following the end of a 12-month behavioral weight loss intervention. Participants were randomized to diet alone (DIET), diet combined with progression to 150 minutes per week of prescribed moderate-to-vigorous intensity physical activity (MVPA) (DIET+PA150), or diet combined with progression to 250 minutes per week of prescribed MVPA (DIET+PA250). As previously reported, randomization was stratified by sex and race (white or nonwhite) in randomly selected block sizes. The HHS protocol was approved by the institutional review board at the University of Pittsburgh.
Participants.
The protocol for participant recruitment has been previously reported.23 Eligibility criteria have previously been reported and include an age between 18 to 55 years and body mass index between 25 to <40 kg/m2.24 Exclusion criteria included (1) self-reporting ≥ 60 min/week of structured moderate-to-vigorous intensity physical activity; (2) weight loss of ≥ 5% within the prior 6 months or a history of bariatric surgery; (3) history of cardiometabolic disease, diabetes mellitus, or cancer; (4) taking medication that could affect heart rate or blood pressure; (5) taking medication that could influence body weight; (6) treatment for psychological conditions that included medication or counseling; (7) currently pregnant, pregnant within the prior 6 months, or planning a pregnancy within the next 12 months; (8) planning on geographical relocation outside of the region within 12 months; (9) inability to comply with the components of the interventions; or (10) had a contraindication that would prohibit cardiac magnetic resonance imaging scanning. Participants provided written informed consent and medical clearance from their physician prior to engaging in this study. Of the 383 individuals randomized to participate in this study, 374 had blood samples available at baseline to be analyzed for the additional analysis examined in this report.
Demographic Characteristics.
Information on sex, race, and ethnicity were collected via questionnaire. Age was confirmed from the birth day contained on a government issued identification card (e.g., driver’s license and passport).
Weight, height, and Body Mass Index.
As previously reported23, body weight was assessed using a calibrated digital scale to the nearest 0.1 kg, with duplicate measures differing by ≤0.5 kg. Height was assessed, with shoes removed, using a wall-mounted stadiometer to the nearest 0.1 cm, and duplicate measures differing by ≤0.5 cm. Body weight and height were used to compute body mass index (BMI) (kg/m2).
Laboratory Measurements.
Fasting morning blood samples were collected at baseline, 6 months, and 12 months. Participants were instructed to fast with the exception of water, abstain from structured physical activity, and abstain from alcohol and smoking for at least 12 hours. This was confirmed by self-report prior to blood collection. Blood was collected into vacutainer tubes, and stored in 0.5 mL aliquots at −80°C. For this study, the stored plasma samples were used for NMR LipoProfile® testing.
NMR LipoProfile® testing was performed on 400 MHz NMR Profiler analyzers at LipoScience, now LabCorp (Morrisville, NC), as previously described.25,26 The lipoprotein parameters, and BCAA’s were calculated by analyzing digitally stored spectra using the newly developed LP4 algorithm.27,28 LP-IR is a composite index developed as previously described.13 LP-IR scores range from 0 (most insulin sensitive) to 100 (most insulin resistant). Diabetes Risk Index is a multimarker index composed of LP-IR, valine, and leucine.15 Diabetes Risk Index was developed using logistic regression and prospective type 2 diabetic data from MESA.29 Diabetes Risk Index scores range from 1 to 100, a higher score indicates greater risk for type 2 diabetes. The GlycA signal was quantified using proprietary deconvolution software that uses a non-negative linear least squares algorithm to fit the experimental signal to individual spectral components, including proteins and lipoproteins as well as signals representing the GlycA NMR resonance.7,26,30 The intra-assay and inter-assay variabilities for GlycA measurement are 1.9% and 2.6% respectively.7
Intervention.
As previously described, participants were randomized into DIET, DIET+PA150, and DIET+PA250 intervention conditions for a period of 12 months.23 Participants in all intervention conditions were instructed to attend weekly weight loss group sessions for weeks 1–24. For weeks 25–52 participants were instructed to attend in-person sessions approximately every other week and to also receive an individual brief telephone intervention approximately every other week. If a participant missed a group session, a brief individual make-up session was offered to allow the content to be shared with the participant.
DIET, DIET+PA150, and DIET+PA250 were prescribed the same diet to reduce energy intake to 1,200 to 1,800 kcal/d based on baseline body weight, and to reduce dietary fat intake to 20% to 30% of total daily energy intake.23 The intervention staff reviewed self-monitoring records of dietary intake and provided written feedback to the participants.
Randomization groups differed in their prescribed physical activity.23 DIET was instructed to maintain their current level of physical activity and was not provided a prescription to increase their physical activity. DIET+PA150 was prescribed a progression to 150 min/week of unsupervised MVPA, whereas DIET+PA250 was prescribed a progression to 250 min/week of unsupervised MVPA.
Statistical Analysis.
Linear mixed models were used to determine whether biomarkers of insulin resistance and cardiometabolic risk changed significantly over time, by intervention group, and group by time interaction, while controlling for sex and race (white/non-white), which were design variables adjusted in the primary analysis for the parent HHS paper. Effects of time, intervention group, and the interaction between group and time on the cardiometabolic biomarkers were examined using mixed modeling. Full models with interaction terms (intervention group × time) are included in the results. Similarly, mixed models were used to determine if weight and body mass index significantly changed over time by intervention group, while adjusting for sex and race (white/non-white). Statistical significance was defined as p < 0.05. Statistics were performed with JMP version 15.1 (SAS Institute, Cary, NC).
RESULTS
Baseline characteristics for all participants and by intervention group are displayed in Table 1. Demographic characteristics are presented for the 374 participants with data included in the current analysis. Overall, participants were 45.5 ± 8.0 years old, female (78.9%), Caucasian (71.7%), with obesity (32.3 ± 3.7 kg/m2). Body weight and body mass index significantly decreased over time (Time effect for both measures: p<0.0001), with no group effect or group by time interaction (Table 2). In addition, baseline lipoprotein, amino acid, and NMR-derived spectroscopy biomarkers are displayed in Tables 3–5. There were no significant differences among the three intervention groups at baseline with regards to the aforementioned measures.
Table 1.
Baseline demographic characteristics overall and by intervention group.
| Total (n=374) | DIET (n=124) | DIET+PA150 (N=127) | DIET+PA250 (N=123) | |
|---|---|---|---|---|
| Age, years | 45.5 (8.0) | 44.3 (8.0) | 46.9 (7.6) | 45.5 (8.2) |
| Gender, % Female | 78.9% | 79.5% | 78.7% | 78.9% |
| Race, % Caucasian/ White | 71.7% | 71.7% | 72.4% | 70.7% |
| Ethnicity, % Hispanic/Latino | 3.5% | 3.9% | 2.4% | 4.1% |
| Weight, kg | 90.8 (13.6) | 91.8 (14.5) | 89.8 (13.4) | 90.6 (13.0) |
| BMI, kg/m2 | 32.3 (3.7) | 32.6 (3.5) | 32.2 (3.8) | 32.0 (3.9) |
BMI = body mass index; values are arithmetic mean (SD).
Table 2.
Intervention and time effects on body weight and body mass index.
| LSMEAN (95% CI) | p value | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 Month | 12 Month | Group | Time | Group × Time | |
| n | ||||||
| DIET | 124 | 112 | 106 | - | - | - |
| DIET+PA150 | 127 | 111 | 103 | - | - | - |
| DIET+PA250 | 123 | 109 | 105 | - | - | - |
| Body Weight, kg | 0.222 | <0.0001*** | 0.428 | |||
| DIET | 95.6 (93.2, 98.1) | 87.0 (84.5, 89.5) | 85.1 (82.5, 87.6) | |||
| DIET+PA150 | 93.3 (90.9, 95.8) | 83.7 (81.2, 86.2) | 82.7 (80.1, 85.2) | |||
| DIET+PA250 | 94.5 (92.0, 96.9) | 85.0 (82.5, 87.5) | 85.0 (82.4, 87.5) | |||
| BMI, kg/m2 | 0.515 | <0.0001*** | 0.115 | |||
| DIET | 32.6 (31.8, 33.3) | 29.6 (28.8, 30.4) | 28.9 (28.2, 29.7) | |||
| DIET+PA150 | 32.2 (31.5, 33.0) | 28.9 (28.1, 29.6) | 28.5 (27.7, 29.3) | |||
| DIET+PA250 | 32.1 (31.3, 32.9) | 28.8 (28.1, 29.6) | 28.9 (28.1, 29.7) | |||
BMI = body mass index; All analyses were adjusted for sex and race (white/non-white).
p<0.05;
p<0.01;
p<0.0001
Table 3.
Intervention and time effects on lipoprotein subclass concentration and size parameters.
| LSMEAN (95% CI) | p value | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 Month | 12 Month | Group | Time | Group × Time | |
| n | ||||||
| DIET | 124 | 107 | 89 | - | - | - |
| DIET+PA150 | 127 | 105 | 88 | - | - | - |
| DIET+PA250 | 123 | 107 | 86 | - | - | - |
| LDL-C, mg/dL | 0.216 | <0.0001*** | 0.262 | |||
| DIET | 95.2 (90.6, 99.7) | 89.2 (84.5, 93.9) | 87.9 (83.1, 92.7) | |||
| DIET+PA150 | 98.3 (93.8, 102.9) | 93.6 (88.9, 98.2) | 94.4 (89.4, 99.3) | |||
| DIET+PA250 | 98.9 (94.3, 103.4) | 90.1 (85.4, 94.8) | 93.3 (88.4, 98.2) | |||
| Concentration LDL particles, nmol/L | 0.280 | <0.0001*** | 0.402 | |||
| DIET | 1441.2 (1372.2, 1510.2) | 1334.0 (1263.3, 1404.8) | 1307.3 (1234.8, 1379.8) | |||
| DIET+PA150 | 1496.6 (1428.2, 1565.0) | 1390.8 (1320.2, 1461.5) | 1393.0 (1319.2, 1466.8) | |||
| DIET+PA250 | 1483.2 (1414.5, 1552.0) | 1329.9 (1258.7, 1401.2) | 1369.0 (1295.2, 1442.8) | |||
| LDL-C size, nm | 0.428 | <0.0001*** | 0.951 | |||
| DIET | 20.8 (20.7, 20.9) | 20.9 (20.8, 21.0) | 20.9 (20.8, 21.0) | |||
| DIET+PA150 | 20.8 (20.7, 20.9) | 20.9 (20.8, 21.0) | 21.0 (20.9, 21.1) | |||
| DIET+PA250 | 20.9 (20.7, 21.0) | 20.9 (20.8, 21.0) | 21.0 (20.9, 21.1) | |||
| Concentration small LDL particles, nmol/L | 0.367 | <0.0001*** | 0.623 | |||
| DIET | 851.7 (780.7,922.7) | 724.2 (649.7, 798.7) | 688.0 (609.9, 766.0) | |||
| DIET+PA150 | 851.6 (781.2, 922.0) | 753.6 (679.1, 828.0) | 764.1 (683.8, 844.5) | |||
| DIET+PA250 | 793.2 (722.3, 864.1) | 719.1 (643.8, 794.5) | 693.6 (613.2, 774.1) | |||
| HDL-C, mg/dL | 0.437 | <0.0001*** | 0.159 | |||
| DIET | 50.7 (48.3, 53.1) | 50.2 (47.8, 52.7) | 53.8 (51.3, 56.3) | |||
| DIET+PA150 | 49.7 (47.3, 52.0) | 50.2 (47.8, 52.6) | 55.2 (52.7, 57.7) | |||
| DIET+PA250 | 50.8 (48.5, 53.2) | 52.2 (49.8, 54.7) | 56.7 (54.1, 59.2) | |||
| Concentration HDL particles, μmol/L | 0.007** | <0.0001*** | 0.165 | |||
| DIET | 20.2 (19.6, 20.8) | 19.2 (18.6, 19.9) | 19.9 (19.3, 20.5) | |||
| DIET+PA150 | 20.5 (19.9, 21.1) | 19.8 (19.2, 20.4) | 20.8 (20.1, 21.4) | |||
| DIET+PA250 | 21.0 (20.4, 21.6) | 20.3 (19.6, 20.9) | 21.5 (20.8, 22.1) | |||
| HDL-C size, nm | 0.614 | <0.0001*** | 0.192 | |||
| DIET | 9.00 (8.91, 9.03) | 9.07 (9.01, 9.14) | 9.15 (9.08, 9.21) | |||
| DIET+PA150 | 8.93 (8.87, 9.00) | 9.04 (9.0, 9.11) | 9.13 (9.06, 9.20) | |||
| DIET+PA250 | 8.91 (8.84, 8.97) | 9.08 (9.01, 9.14) | 9.10 (9.03, 9.17) | |||
| Concentration large HDL particle, μmol/L | 0.820 | <0.0001*** | 0.357 | |||
| DIET | 2.0 (1.7, 2.2) | 2.3 (2.0, 2.5) | 2.7 (2.5, 3.0) | |||
| DIET+PA150 | 1.9 (1.7, 2.2) | 2.3 (2.0, 2.5) | 2.7 (2.4, 2.9) | |||
| DIET+PA250 | 1.8 (1.5, 2.0) | 2.3 (2.0, 2.6) | 2.6 (2.3, 2.9) | |||
| Concentration very large TRLP particle, nmol/L | 0.683 | <0.0001*** | 0.202 | |||
| DIET | 0.16 (0.12, 0.21) | 0.14 (0.09, 0.19) | 0.11 (0.05, 0.16) | |||
| DIET+PA150 | 0.20 (0.16, 0.24) | 0.12 (0.08, 0.17) | 0.12 (0.06, 0.17) | |||
| DIET+PA250 | 0.23 (0.19, 0.28) | 0.11 (0.06, 0.16) | 0.13 (0.07, 0.18) | |||
| Concentration Triglyceride, mg/dL | 0.982 | <0.0001*** | 0.822 | |||
| DIET | 135.8 (125.2, 146.4) | 116.5 (105.6, 127.4) | 111.2 (100.0, 122.5) | |||
| DIET+PA150 | 139.3 (128.8, 149.7) | 115.6 (104.7, 126.5) | 110.8 (99.3, 122.3) | |||
| DIET+PA250 | 138.7 (128.1, 149.2) | 112.3 (101.3, 123.3) | 111.1 (99.6, 122.6) | |||
Values are least square mean (95% confidence interval); All analyses were adjusted for sex and race (white/non-white).
p<0.05;
p<0.01;
p<0.0001
Table 5.
Intervention and time effects on diabetes risk and metabolic mortality vulnerability makers.
| LSMEAN (95% CI) | p value | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 Month | 12 Month | Group | Time | Group × Time | |
| n | ||||||
| DIET | 124 | 107 | 89 | - | - | - |
| DIET+PA150 | 127 | 105 | 88 | - | - | - |
| DIET+PA250 | 123 | 107 | 86 | - | - | - |
| GlycA, μmol/L | 0.777 | <0.0001*** | 0.009** | |||
| DIET | 414.8 (402.8, 426.8) | 392.6 (380.3, 404.9) | 370.0 (357.3, 382.6) | |||
| DIET+PA150 | 411.3 (399.4, 423.2) | 394.7 (382.4, 407.0) | 378.3 (365.5, 391.1) | |||
| DIET+PA250 | 418.4 (406.5, 430.4) | 388.1 (375.6, 400.5) | 386.2 (373.4, 399.1) | |||
| LP-IR, score 0–100 | 0.934 | <0.0001*** | 0.919 | |||
| DIET | 45.3 (41.3, 49.4) | 34.9 (30.7, 39.0) | 33.5 (29.2, 37.8) | |||
| DIET+PA150 | 47.1 (43.1, 51.1) | 34.8 (30.6, 38.9) | 34.3 (29.9, 38.6) | |||
| DIET+PA250 | 46.8 (42.8, 50.8) | 34.5 (30.3, 38.7) | 34.5 (30.2, 38.9) | |||
| DRI, score 0–100 | 0.921 | <0.0001*** | 0.989 | |||
| DIET | 48.0 (45.2, 50.8) | 40.8 (37.8, 43.7) | 39.9 (36.8, 42.9) | |||
| DIET+PA150 | 47.9 (45.1, 50.7) | 40.4 (37.5, 43.3) | 39.7 (36.6, 42.8) | |||
| DIET+PA250 | 47.8 (45.0, 50.6) | 39.6 (36.6, 42.5) | 39.4 (36.3, 42.5) | |||
Values are least square mean (95% confidence interval); LP-IR = Lipoprotein Insulin Resistance Index; DRI = Diabetes Risk Index; All analyses were adjusted for sex and race (white/non-white).
p<0.05;
p<0.01;
p<0.0001
Lipoprotein Subclass and Size Parameters.
Participants had a baseline average LDL-C of 98.5 ± 23.9 mg/dL, HDL-C of 53.2 ± 12.7 mg/dL, and TG concentration of 133.1 ± 63.8 mg/dL. Intervention effects on lipoprotein subclass concentration and size parameters are displayed in Table 3. All investigated lipoprotein subclass concentration and size parameters significantly improved over time (Time effect for all measures: p <0.0001). There was a significant group effect found for concentration HDL-C, meaning the general concentration HDL-C differed across groups, specifically between DIET and DIET+PA250 (p =0.01). No other group effects or group by time interactions were observed.
Total and Specific Branched Chain Amino Acid Parameters.
At baseline, participants had an average total BCAA concentration of 406.0 ± 71.9 μmol/L, change in total BCAA concentration over time across intervention groups is displayed in Figure 1 Panel A.
Figure 1.
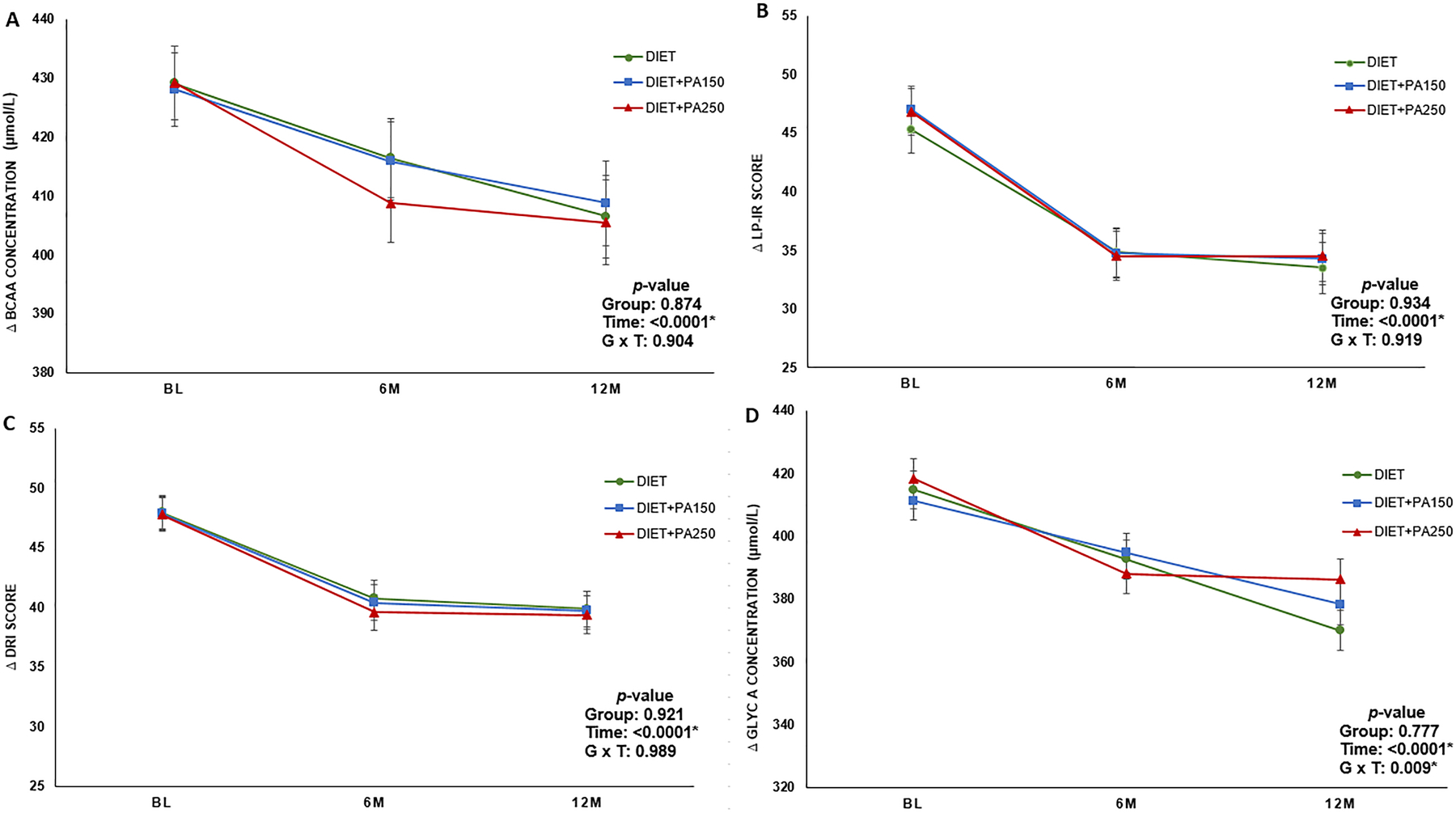
Least squares mean change in novel insulin resistance and cardiometabolic health biomarkers across randomized intervention group. Error bars represent standard error. p values are displayed in each panel describing the time effect, group effect, and group by time interaction. Panel A: Change in total branched chain amino acid concentration over time; Panel B: Change in Lipoprotein Insulin Resistance Index score over time; Panel C: Change in Diabetes Risk Index over time; Panel D: Change in GlycA concentration over time.
Table 4 displays the intervention effects on amino acid concentrations. All BCAA parameters significantly improved over time (Time effect for all measures: p values ranged from 0.003 to <0.001). No significant group effects or group by time interactions were found.
Table 4.
Intervention and time effects on amino acid concentrations.
| LSMEAN (95% CI) | p value | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 Month | 12 Month | Group | Time | Group × Time | |
| n | ||||||
| DIET | 124 | 107 | 89 | - | - | - |
| DIET+PA150 | 127 | 105 | 88 | - | - | - |
| DIET+PA250 | 123 | 107 | 86 | - | - | - |
| BCAA concentration, μmol/L | 0.874 | <0.0001*** | 0.904 | |||
| DIET | 429.2 (416.8, 441.6) | 416.4 (403.3, 429.5) | 406.5 (392.8, 420.2) | |||
| DIET+PA150 | 428.1 (415.8, 440.4) | 415.9 (402.8, 428.9) | 408.8 (394.6, 423.0) | |||
| DIET+PA250 | 429.2 (416.8, 441.6) | 408.8 (395.6, 422.0) | 405.5 (391.3, 419.6) | |||
| Valine concentration, μmol/L | 0.793 | <0.0001*** | 0.984 | |||
| DIET | 228.4 (221.6, 235.3) | 220.9 (213.7, 228.1) | 215.8 (208.3, 223.4) | |||
| DIET+PA150 | 227.6 (220.8, 234.4) | 221.5 (214.3, 228.7) | 217.2 (209.4, 225.0) | |||
| DIET+PA250 | 226.9 (220.0, 233.7) | 218.0 (210.7, 225.3) | 214.2 (206.4, 222.1) | |||
| Leucine concentration, μmol/L | 0.456 | 0.001*** | 0.9801 | |||
| DIET | 151.5 (146.0, 156.9) | 145.0 (139.2, 150.8) | 145.6 (139.5, 151.7) | |||
| DIET+PA150 | 148.0 (142.6, 153.4) | 142.6 (136.8, 148.3) | 143.6 (137.2, 149.9) | |||
| DIET+PA250 | 149.2 (143.8, 154.7) | 141.3 (135.4, 147.1) | 141.9 (135.6, 148.3) | |||
| Isoleucine concentration, μmol/L | 0.154 | 0.003** | 0.436 | |||
| DIET | 49.3 (46.2, 52.4) | 50.4 (47.1, 53.7) | 44.8 (41.3, 48.4) | |||
| DIET+PA150 | 52.5 (49.4, 55.6) | 52.0 (48.7, 55.3) | 48.3 (44.7, 52.0) | |||
| DIET+PA250 | 52.8 (49.6, 55.9) | 49.0 (45.6, 52.4) | 48.7 (45.0, 52.3) | |||
| Glycine concentration, μmol/L | 0.134 | <0.0001*** | 0.192 | |||
| DIET | 250.6 (233.9, 267.3) | 273.2 (256.2, 290.3) | 270.1 (252.7, 287.6) | |||
| DIET+PA150 | 251.0 (234.5, 267.6) | 269.9 (252.8, 286.9) | 259.2 (241.4, 276.9) | |||
| DIET+PA250 | 262.0 (245.4, 278.6) | 285.0 (267.8, 302.2) | 291.2 (273.4, 308.9) | |||
| Glucose concentration, mg/dL | 0.150 | <0.0001*** | 0.425 | |||
| DIET | 79.8 (78.1, 81.6) | 77.7 (75.9, 79.5) | 76.0 (74.1, 77.9) | |||
| DIET+PA150 | 80.7 (78.9, 82.4) | 78.0 (76.2, 79.8) | 78.4 (76.5, 80.3) | |||
| DIET+PA250 | 81.5 (79.8, 83.3) | 79.3 (77.5, 81.2) | 78.7 (76.7, 80.6) | |||
Values are least square mean (95% confidence interval); All analyses were adjusted for sex and race (white/non-white).
p<0.05;
p<0.01;
p<0.0001
Diabetes Risk and Metabolic Mortality Vulnerability Markers.
At baseline, participants had an average LP-IR score of 42.9 ± 23.5, Diabetes Risk Index of 43.6 ± 16.8, and GlycA level of 423.9 ± 65.0 μmol/L. Intervention effects on diabetes risk and metabolic mortality vulnerability biomarkers are displayed in Table 5 and Figure 1 Panels B, C, and D. All diabetes risk and metabolic mortality biomarkers significantly improved over time (Time effect for all measures: p <0.0001). A significant group by time interaction was observed for GlycA concentration (p = 0.009), meaning the change in GlycA across time was significantly different among the three intervention groups. Figure 1 Panel D shows DIET+PA250 had the greatest reduction in GlycA concentration from baseline to 6 months, whereas DIET and DIET+PA150 had a steady linear reduction in concentration over the 12-month intervention. No other group effects or group by time interactions were found.
DISCUSSION
The purpose of this secondary analysis was to extend beyond the findings from our parent study by assessing whether emerging biomarkers of insulin resistance and cardiometabolic health respond to a behavioral weight loss intervention among sedentary adults with overweight or obesity. We found all intervention groups prompted a notable beneficial change among biomarkers of insulin resistance and cardiometabolic health. However, the addition of moderate intensity physical activity in a dose-response approach to a diet only intervention did not provide any additional benefit among these biomarkers. These findings highlight the impact weight loss of an average of approximately 10% through dietary modification, alone or in combination with physical activity, achieved at 6 months and maintained at 12 months, can have on markers of insulin resistance and cardiometabolic health. An important finding from these secondary analyses are the observed changes in emerging markers of insulin resistance and cardiometabolic health, which were not included in the parent study, did not differ between interventions involving modest dietary restriction alone or with the combination of dietary restriction and physical activity; suggesting the important contribution of weight loss to these beneficial improvements.
Lipoprotein Subclass and Size Parameters
Elevated VLTRLP, small LDL, and decreased large HDL, LDL size and HDL size are associated with insulin resistance and poor cardiometabolic health, therefore important measures to target in lifestyle interventions.12,13 We found all investigated lipoprotein subclass and size parameters significantly improved across the behavioral weight loss intervention, regardless of randomized intervention group; leading us to conclude weight loss is a key factor contributing to improvements in lipid profiles among adults with overweight or obesity.
Previously published work supports our findings in that lifestyle interventions can lead to beneficial changes in insulin resistance and cardiometabolic health. However, studies have primarily targeted older adults and/or have not focused specifically on weight loss for improvements in these biomarkers. The Growing Old Together (GOTO) study investigated the effects of a lifestyle intervention in older adults by both clinical and metabolomic profiles.31 The lifestyle intervention corresponded to one of the three CALERIE-Phase 1 interventions, having participants reduce energy balance by 25% for 13 weeks, targeted by 12.5% reduction in caloric intake and 12.5% increase in physical activity. Following the intervention, significant reductions in VLTRLP and small LDL in both men and women. Further, significant improvements in large HDL and HDL size were reported, however this was only among male participants. The investigators concluded, despite considerable heterogeneity in response, metabolic health was generally beneficially influenced following the 13-week intervention.31 Although our current study did not conduct sex-specific comparisons, we similarly found a beneficial response among insulin resistance and cardiometabolic health biomarkers among individuals. However, our findings add the importance of weight loss, regardless of how weight loss is achieved, for significant improvements in lipid subclass and size parameters among adults with overweight or obesity.
BCAA, LP-IR, and Diabetes Risk Index
Elevated BCAA’s, as a measure of dysmetabolism, are associated with worse metabolic health and future insulin resistance or type 2 diabetes.8–11,32 Similarly, LP-IR is strongly associated with measures of insulin resistance13 and predicts incidence of type 2 diabetes.15,29,33,34 The combination of LP-IR and BCAA’s valine and leucine, produces the Diabetes Risk Index, of which has been found to enhance type 2 diabetes risk stratification among individuals in normoglycemic range and prediabetic range.14,15 We found all BCAA parameters, LP-IR and Diabetes Risk Index significantly improved across the behavioral weight loss intervention, regardless of randomized intervention group.
Previously published work has been conducted in physical activity only interventions, as well as weight loss via diet and physical activity interventions across individuals varying in disease severity. The Studies of a Targeted Risk Reduction Intervention through Defined Exercise (STRRIDE) randomized trials investigated the effects of different amounts, intensities, and modes of exercise in LP-IR and Diabetes Risk Index. Investigators found significant improvement in insulin action measures35–37 and LP-IR, with limited improvement in Diabetes Risk Index.38 Additionally, weight loss was also shown to be an important contributor for changes in BCAA concentrations, which in turn led to significant improvement in DRI among the Clinical Lifestyle group (consisting of Diet + Low-Amount/Moderate-Intensity exercise). Moreover, null findings were reported across all exercise-only interventions groups for change in BCAA concentrations. Investigators concluded a weight change of greater than 7.0% may be required in a lifestyle intervention to elicit a significant reduction in BCAAs.38 Our findings are consistent with STRRIDE, in which an average of approximately 10% weight loss resulted in significant improvements in LP-IR, Diabetes Risk Index, and BCAA parameters.
Among non-supervised conditions, Ellsworth and colleagues39 investigated if clinical lifestyle programs – two programs differing in dietary stringency, exercise intensity, and time commitment - improved LP-IR among individuals with varying severity of metabolic dysfunction. Following the one-year long programs, both groups lost weight, with the moderate program reducing LP-IR score by 8.8% and the intensive program by 13.3%. They concluded both programs were effective for improving insulin resistance, measured by change in LP-IR score. Similarly, we found all intervention groups significantly improved total BCAA concentration, LP-IR score, and Diabetes Risk Index from baseline to 12 months.
GlycA
GlycA is a relatively new marker of systemic inflammation, with increased GlycA found to be positively correlated with BMI, insulin resistance, and markers of metabolic syndrome.40,41 Moreover, GlycA is shown to be associated with cardiovascular disease risk42 and cardiovascular disease events in numerous studies.43–45 We found GlycA significantly improved across all of the intervention conditions in this study, which included dietary restriction alone or in combination with physical activity.
Few studies have investigated the effects of lifestyle interventions on GlycA levels. The STRRIDE-PD trial is one of the first to investigate the effect of an exercise-based lifestyle intervention on GlycA.30 They aimed to evaluate how exercise-based lifestyle interventions modulate GlycA in persons with prediabetes. Participants (n=169) were randomized to one of four, six-month interventions: 1) Low-Amount/Moderate-Intensity, 2) High-Amount/Moderate-Intensity, 3) High-Amount/Vigorous-Intensity, and 4) Clinical Lifestyle (Diet + Low-Amount/Moderate-Intensity). They found GlycA was significantly reduced on average by 3% in the High-Amount/Vigorous-Intensity group and by 4% in the Clinical Lifestyle intervention.30 Similarly, we found among sedentary, adults with overweight or obesity, and normal GlycA levels, dietary restriction alone and in combination with exercise significantly reduced GlycA in a 12-month intervention. Given GlycA is a relatively new marker of systemic inflammation, our study provides further evidence that weight loss, induced by diet alone or diet in combination with physical activity, provides a beneficial impact on GlycA levels among adults with overweight or obesity.
Strengths and Limitations
Strengths of this study include having a randomized design and being one of the first lifestyle interventions that compares a diet only intervention to diet in conjunction with various amounts of moderate intensity exercise on novel measures of insulin resistance and cardiometabolic disease. Further, this study was a 12-month long behavioral weight loss intervention, with measures collected at baseline, 6-, and 12 months. Lastly, the behavioral weight loss intervention resulted in an average weight loss of approximately 10%, which is substantial enough to result in health benefits.
This study has limitations that are important to recognize. This is a secondary analysis, meaning the Heart Health study was not specifically designed to test these specific variables. This study included participants with a BMI range from 25 to <40 kg/m2, which may limit generalizability to individuals with higher or lower BMIs. Further, aside from having overweight or obesity, and engaging in low amounts of physical activity, this sample of participants was relatively healthy. Thus, whether similar findings would be observed in participants who have other risk factors or known cardiometabolic diseases is unable to be determined. Moreover, the NMR-based lipoprotein measures investigated were correlated within our sample; therefore other, unrelated, measures of cardiometabolic health should be investigated in a similar fashion in the future. Lastly, this study prescribed physical activity to be at least moderate intensity aerobic exercise and therefore lacks the ability to differentiate based on intensity or mode of exercise in conjunction with diet on novel biomarkers of insulin resistance and cardiometabolic disease.
CONCLUSION
In conclusion, the HHS was successful in improving biomarkers of insulin resistance and cardiometabolic health among sedentary adults with overweight or obesity. Further, the addition of at least moderate intensity physical activity in conjunction with diet did not provide any further beneficial changes among these biomarkers compared to a diet only intervention. These findings highlight the impact weight loss of at least 10% through dietary modification, achieved at 6 months and maintained at 12 months, can have on markers of insulin resistance and cardiometabolic health, regardless of physical activity participation.
Study Importance Questions.
- What is already known about this subject
- Weight loss of 5% to 10% achieved via lifestyle intervention is effective for improving traditional cardiovascular disease and type 2 diabetes risk factors including, blood pressure, LDL cholesterol, HDL cholesterol, triglycerides, and glucose tolerance.
- What are the new findings in the manuscript?
- All intervention groups prompted a notable beneficial change among biomarkers of insulin resistance and cardiometabolic health, including LP-IR score, Diabetes Risk Index score, Total BCAA’s, and GlycA.
- The addition of at least moderate intensity physical activity to a diet only intervention did not provide any additional benefit on these novel biomarkers.
- How might your results change the direction of research or the focus of clinical practice?
- These findings highlight an average weight loss of approximately 10% profoundly impacts biomarkers of insulin resistance and cardiometabolic disease in adults with overweight or obesity.
- Therefore, if improving cardiometabolic disease risk is necessary for an individual, emphasizing weight loss, regardless of how the weight loss is achieved may be an important direction to focus research.
ACKNOWLEDGMENTS
We recognize the contribution of the staff and graduation students at the Physical Activity and Weight Management Research Center at the University of Pittsburgh who contributed to this project. We would also like to acknowledge and thank all participants.
Funding
Support for the parent project was provided by R01 HL096770 and UL1 TR001857. KAC is supported by the National Human Genome Research Institute - 1 T32 HG008955-01.
Footnotes
Clinical Trial Registration
ClinicalTrials.gov identifier NCT01500356
Conflict of Interest
JMJ currently receives compensation for serving on the Scientific Advisory Board for Wondr Health, Inc. and previously received compensation for serving on the Scientific Advisory Board for WW International, Inc., has received remuneration for professional presentations, and serves as a volunteer in a professional leadership role to the American College of Sports Medicine. RJR has received remuneration for professional presentations, and serves as a volunteer in a professional leadership role to the American College of Sports Medicine. EBS serves on the Scientific Advisory Board for Hay Therapeutics. The other authors declared no conflict of interest.
Data Availability
Data sharing for this secondary analysis may be made available upon request that meet the approved process contained within the informed consent and other institutional policies. These data will not be provided in a public-use data set, but rather may require approval through a material transfer agreement, and only deidentified data may be made available.
REFERENCES
- 1.NCD Risk Factor Collaboration. Worldwide trends in body mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017(390):2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Obesity Federation. Obesity: missing the 2025 global targets. 2020.
- 3.Ginsberg HN. Insulin resistance and cardiovascular disease. The Journal of Clinical Investigation. 2000;106(4):453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egan BM, Greene EL, Goodfriend TL. Insulin resistance and cardiovascular disease. American Journal of Hypertension. 2001;14(S3):116S–125S. [DOI] [PubMed] [Google Scholar]
- 5.D’Agostino RB, Hamman RF, Karter AJ, Mykkanen L, Wagenknecht LE, Haffner SM. Cardiovascular disease risk factors predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes Care. 2004;27(9):2234–2240. [DOI] [PubMed] [Google Scholar]
- 6.Bell JD, Brown JC, Nicholson JK, Sadler PJ. Assignment of resonances for ‘acute-phase’glycoproteins in high resolution proton NMR spectra of human blood plasma. FEBS Letters. 1987;215(2):311–315. [DOI] [PubMed] [Google Scholar]
- 7.Otvos JD, Shalaurova I, Wolak-Dinsmore J, et al. GlycA: a composite nuclear magnetic resonance biomarker of systemic inflammation. Clinical Chemistry. 2015;61(5):714–723. [DOI] [PubMed] [Google Scholar]
- 8.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metabolism. 2012;15(5):606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nature Reviews Endocrinology. 2014;10(12):723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon M-S. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients. 2016;8(7):405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holeček M. Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutrition & Metabolism. 2018;15(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frazier-Wood AC, Garvey WT, Dall T, Honigberg R, Pourfarzib R. Opportunities for using lipoprotein subclass profile by nuclear magnetic resonance spectroscopy in assessing insulin resistance and diabetes prediction. Metabolic Syndrome and Related Disorders. 2012;10(4):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shalaurova I, Connelly MA, Garvey WT, Otvos JD. Lipoprotein Insulin Resistance Index: a lipoprotein particle–derived measure of insulin resistance. Metabolic Syndrome and Related Disorders. 2014;12(8):422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flores-Guerrero JL, Osté MC, Kieneker LM, et al. Plasma branched-chain amino acids and risk of incident type 2 diabetes: results from the PREVEND prospective cohort study. Journal of Clinical Medicine. 2018;7(12):513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flores-Guerrero JL, Connelly MA, Shalaurova I, et al. Lipoprotein insulin resistance index, a high-throughput measure of insulin resistance, is associated with incident type II diabetes mellitus in the prevention of renal and vascular end-stage disease study. Journal of Clinical Lipidology. 2019;13(1):129–137. e121. [DOI] [PubMed] [Google Scholar]
- 16.Lalonde L, Gray-Donald K, Lowensteyn I, et al. Comparing the benefits of diet and exercise in the treatment of dyslipidemia. Preventive Medicine. 2002;35(1):16–24. [DOI] [PubMed] [Google Scholar]
- 17.Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Annals of Internal Medicine. 2001;134(1):1–11. [DOI] [PubMed] [Google Scholar]
- 18.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Medicine & Science in Sports & Exercise. 2009;41(2):459–471. [DOI] [PubMed] [Google Scholar]
- 19.Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. The American Journal of Clinical Nutrition. 1992;56(2):320–328. [DOI] [PubMed] [Google Scholar]
- 20.Wadden TA, Anderson DA, Foster GD. Two-year changes in lipids and lipoproteins associated with the maintenance of a 5% to 10% reduction in initial weight: some findings and some questions. Obesity Research. 1999;7(2):170–178. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez ML, Metghalchi S, Vega-López S, Conde-Knape K, Lohman TG, Cordero-Macintyre ZR. Beneficial effects of weight loss on plasma apolipoproteins in postmenopausal women. The Journal of Nutritional Biochemistry. 2004;15(12):717–721. [DOI] [PubMed] [Google Scholar]
- 22.Ditschuneit HH, Flechtner-Mors M, Johnson TD, Adler G. Metabolic and weight-loss effects of a long-term dietary intervention in obese patients. The American Journal of Clinical Nutrition. 1999;69(2):198–204. [DOI] [PubMed] [Google Scholar]
- 23.Jakicic JM, Rogers RJ, Lang W, et al. Impact of weight loss with diet or diet plus physical activity on cardiac magnetic resonance imaging and cardiovascular disease risk factors: Heart Health Study randomized trial. Obesity. 2022;30(5):1039–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers RJ, Schelbert EB, Lang W, Fridman Y, Yuan N, Jakicic JM. Association of fitness and body fatness with left ventricular mass: The heart health study. Obesity Science & Practice. 2020;6(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clinics in Laboratory Medicine. 2006;26(4):847–870. [DOI] [PubMed] [Google Scholar]
- 26.Ormseth MJ, Chung CP, Oeser AM, et al. Utility of a novel inflammatory marker, GlycA, for assessment of rheumatoid arthritis disease activity and coronary atherosclerosis. Arthritis Res Ther. 2015;17(1):117–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolak-Dinsmore J, Gruppen EG, Shalaurova I, et al. A novel NMR-based assay to measure circulating concentrations of branched-chain amino acids: Elevation in subjects with type 2 diabetes mellitus and association with carotid intima media thickness. Clinical Biochemistry. 2018;54:92–99. [DOI] [PubMed] [Google Scholar]
- 28.Kinzer AB, Shamburek RD, Lightbourne M, Muniyappa R, Brown RJ. Advanced lipoprotein analysis shows atherogenic lipid profile that improves after metreleptin in patients with lipodystrophy. Journal of the Endocrine Society. 2019;3(8):1503–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackey RH, Mora S, Bertoni AG, et al. Lipoprotein particles and incident type 2 diabetes in the multi-ethnic study of atherosclerosis. Diabetes Care. 2015;38(4):628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartlett DB, Slentz CA, Connelly MA, et al. Association of the composite inflammatory biomarker GlycA, with exercise-induced changes in body habitus in men and women with prediabetes. Oxidative Medicine and Cellular Longevity. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Rest O, Schutte BAM, Deelen J, et al. Metabolic effects of a 13-weeks lifestyle intervention in older adults: The Growing Old Together Study. Aging (Albany NY). 2016;8(1):111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metabolism. 2009;9(4):311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dugani SB, Akinkuolie AO, Paynter N, Glynn RJ, Ridker PM, Mora S. Association of lipoproteins, insulin resistance, and rosuvastatin with incident type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiology. 2016;1(2):136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harada PH, Demler OV, Dugani SB, et al. Lipoprotein insulin resistance score and risk of incident diabetes during extended follow-up of 20 years: The Women’s Health Study. Journal of Clinical Lipidology. 2017;11(5):1257–1267. e1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. Journal of Applied Physiology. 2004;96(1):101–106. [DOI] [PubMed] [Google Scholar]
- 36.AbouAssi H, Slentz CA, Mikus CR, et al. The effects of aerobic, resistance, and combination training on insulin sensitivity and secretion in overweight adults from STRRIDE AT/RT: a randomized trial. Journal of Applied Physiology. 2015;118(12):1474–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slentz CA, Bateman LA, Willis LH, et al. Effects of exercise training alone vs a combined exercise and nutritional lifestyle intervention on glucose homeostasis in prediabetic individuals: a randomised controlled trial. Diabetologia. 2016;59(10):2088–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross LM, Slentz CA, Zidek AM, et al. Effects of Amount, Intensity, and Mode of Exercise Training on Insulin Resistance and Type 2 Diabetes Risk in the STRRIDE Randomized Trials. Frontiers in Physiology. 2021;12(67). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellsworth D, Costantino N, Blackburn H, Engler R, Kashani M, Vernalis M. Lifestyle modification interventions differing in intensity and dietary stringency improve insulin resistance through changes in lipoprotein profiles. Obesity Science & Practice. 2016;2(3):282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dullaart RP, Gruppen EG, Connelly MA, Otvos JD, Lefrandt JD. GlycA, a biomarker of inflammatory glycoproteins, is more closely related to the leptin/adiponectin ratio than to glucose tolerance status. Clinical Biochemistry. 2015;48(12):811–814. [DOI] [PubMed] [Google Scholar]
- 41.Lorenzo C, Festa A, Hanley AJ, Rewers MJ, Escalante A, Haffner SM. Novel Protein Glycan–Derived Markers of Systemic Inflammation and C-Reactive Protein in Relation to Glycemia, Insulin Resistance, and Insulin Secretion. Diabetes Care. 2017;40(3):375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Connelly MA, Gruppen EG, Otvos JD, Dullaart RP. Inflammatory glycoproteins in cardiometabolic disorders, autoimmune diseases and cancer. Clinica Chimica Acta. 2016;459:177–186. [DOI] [PubMed] [Google Scholar]
- 43.Akinkuolie AO, Buring JE, Ridker PM, Mora S. A novel protein glycan biomarker and future cardiovascular disease events. Journal of the American Heart Association. 2014;3(5):e001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duprez DA, Otvos J, Sanchez OA, Mackey RH, Tracy R, Jacobs DR Jr. Comparison of the predictive value of GlycA and other biomarkers of inflammation for total death, incident cardiovascular events, noncardiovascular and noncancer inflammatory-related events, and total cancer events. Clinical Chemistry. 2016;62(7):1020–1031. [DOI] [PubMed] [Google Scholar]
- 45.Gruppen EG, Riphagen IJ, Connelly MA, Otvos JD, Bakker SJ, Dullaart RP. GlycA, a pro-inflammatory glycoprotein biomarker, and incident cardiovascular disease: relationship with C-reactive protein and renal function. PloS One. 2015;10(9):e0139057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing for this secondary analysis may be made available upon request that meet the approved process contained within the informed consent and other institutional policies. These data will not be provided in a public-use data set, but rather may require approval through a material transfer agreement, and only deidentified data may be made available.


