Abstract
Objective:
Weight loss achieved with standard doses of GLP-1 agonists (GLP-1a) among real-world patients with type 2 diabetes has not been determined. We sought to describe the percent change in body weight 72 weeks after starting a GLP-1a.
Methods:
We conducted a retrospective cohort study of non-pregnant adults first dispensed a GLP-1a between 2011 and 2018 using electronic health record data from patients receiving care at a large health system. We used linear mixed models with a person-level random intercept controlling for baseline variables associated with missing weight data to estimate percent body weight change during follow-up.
Results:
Our cohort included 2,405 patients (mean[SD] age 48[10], 53% female), with a BMI[SD] of 37[8] kg/m2and a mean baseline weight of 238[54] lbs. Mean percent weight loss significantly increased from 1.1% (95% CI: 0.6–1.6) 8 weeks after GLP-1a dispensing to 2.2% (95% CI: 1.7–2.6) 72 weeks after GLP-1a dispensing (p for quadratic trend <.001). One third of patients lost ≥5% body weight at 72 weeks.
Conclusions:
In this real-world study of over 2,400 patients with overweight or obesity and type 2 diabetes, starting a GLP-1a at standard glycemic control dose was associated with modest weight loss through 72 weeks.
Introduction
Markedly elevated body weight is a well described risk factor for major adverse cardiovascular events and all-cause mortality among patients with type 2 diabetes.1–3 Achieving and maintaining weight loss in this group of patients may result in improved glycemic control and help patients reduce the dosage or number of glucose lowering medications.4,5 In some cases, more substantial weight loss (i.e. ≥10% to 15%) may result in disease remission.6,7
In randomized weight loss trials, patients with type 2 diabetes and overweight or obesity initiating selected GLP-1 agonists, including doses higher than typically prescribed for glycemic control, have achieved clinically significant weight loss.8–10 In the STEP 2 Trial, patients randomized to the GLP-1 agonist semaglutide 2.4mg plus a lifestyle intervention achieved a mean 9.6% weight loss from baseline at week 68 versus a mean 3.4% in the lifestyle intervention alone (a 6.2% treatment difference).9 In SCALE Diabetes, liraglutide 3.0mg achieved 6.0% weight loss compared to 2.0% in a behavioral weight loss program plus placebo control (a 4.0% treatment difference).
When subjects are taking doses of these medications in trials evaluating diabetes efficacy without a weight loss intervention in the drug or control group, we see less dramatic results. For example, in SUSTAIN-1, semaglutide 1.0mg achieved 4.5 kg weight loss (4.7%) while placebo achieved 1.0 kg (1.1%), a 3.6% treatment difference at 30 weeks.11 In LEAD-2, liraglutide 1.8mg achieved 2.8 kg weight loss (3.3%) versus 1.5 kg loss (2.0%) in control (metformin monotherapy), a 1.3% treatment difference at 26 weeks.12 In AWARD-3, dulaglutide 1.5mg achieved 2.3 kg weight loss (2.4%) at 26 weeks, an amount that was not statistically different from metformin.13
While the amounts of weight loss reported in both protocolized weight loss trials (which include calorie deficit inducing lifestyle interventions in their usual care arms)8–10 and in diabetes efficacy trials without weight loss interventions11,12 are substantial, it is not known how much weight loss can be demonstrated and sustained among real world patients with type 2 diabetes initiating a GLP-1 agonist as part of routine clinical care in the absence of a standard behavioral weight loss intervention. Using data from a large integrated healthcare system, we sought to describe the percent change in body weight from baseline among patients with overweight or obesity and type 2 diabetes 72 weeks after starting a GLP-1 agonist.
Methods
Design and Participants
Our dataset combined electronic health record and health insurance claims data for patients receiving care at a large integrated healthcare system based in Western Pennsylvania.14 We obtained these data from the PaTH Clinical Research Network, a partner network in PCORnet, the National Patient-Centered Clinical Research Network.15,16 We linked EHR and claims data using de-identified unique linkage IDs. This study was approved by the Human Research Protection Office at the University of Pittsburgh, IRB number STUDY21050064.
Non-pregnant adults (aged 18 years and older) who were first dispensed a GLP-1 agonist (liraglutide, dulaglutide, exenatide ER, semaglutide, exenatide, and albiglutide) between 2011 and 2018 were eligible (see Table S1). We only included dosages approved for glycemic control (i.e. we did not include higher dose GLP-1 agonist with approved indications for weight loss). We excluded patients without: 1) at least 72 weeks of continuous enrollment after the index dispense date, 2) at least 52 weeks of continuous enrollment before the index dispense date of a GLP-1 agonist, 3) at least one valid weight measurement within the 8 weeks before the dispense date and at least one weight measure in the 72 weeks following index dispense date of a GLP-1 agonist.
Measures
The two outcomes of this study were: 1) percent weight change and 2) proportion of people with at least 5% weight loss in the 72 weeks following first GLP-1 agonist dispense date.9 The follow-up period was divided into 8-week intervals. When there was more than one weight measurement in a given 8-week interval, we randomly selected a weight measurement for that window.
Baseline demographic and clinical variables were measured during the 52 weeks prior to the first GLP1-a dispense date and extracted from the electronic health record portion of the dataset. These variables included age, sex, race, ethnicity, smoking status, comorbidities (i.e., Type 2 diabetes, obesity, hypertension, hyperlipidemia, coronary artery disease, coronary heart failure, stroke, and end stage renal disease) and diabetes related complications (i.e., neuropathy, retinopathy, nephropathy foot ulcers, serious hypoglycemic event, serious hyperglycemic event, and peripheral artery disease) based on ICD-9-CM and ICD-10-CM diagnostic codes, history of bariatric procedure, body weight (in pounds), body mass index (kg/m2), and laboratory measures (i.e., HbA1c, creatinine [mg/dl], low-density lipoprotein cholesterol [mg/dl], high-density lipoprotein cholesterol [mg/dl], and total cholesterol [mg/dl]). Obesity was defined as an ICD-9-CM or ICD-10-CM diagnostic code of obesity or a calculated BMI ≥30 kg/m2. To determine whether patients had undergone a bariatric procedure, we used the ICD-10-CM and CPT-4 codes described in Table S2. For patients who underwent a bariatric procedure during the 72-week follow-up period, we censored all body weight and BMI measurements that occurred following the bariatric procedure.
Statistical Analysis
Descriptive statistics summarize baseline characteristics and percent weight change and at least 5% weight loss in each 8-week follow-up interval between the index dispense date for a GLP-1 agonist and 72 weeks after the index dispense date. We used descriptive statistics to summarize the percent weight change from baseline and percent of participants who lost at least 5% of baseline body weight in each 8-week time window.
Longitudinal analyses were performed using mixed models with a person-level random intercept controlling for baseline factors associated with missing follow-up weight data (i.e., sex, smoking status, coronary artery disease, hypertension, coronary heart failure, chronic kidney disease, having at least one hospitalization and HbA1c value) as fixed effects. Mixed models were also used to test for linear and quadratic trends in weight loss and proportion with at least 5% weight loss over time. A linear mixed model was used to estimate percent weight change, with follow-up time as a discrete fixed effect (i.e., 8-week intervals from baseline to 72 weeks). Modeled means and 95% confidence intervals (CI) are reported for each follow-up time window. A Poisson mixed models with robust error variance was used to estimate the proportion of participants who lost at least 5% of their baseline body weight, with follow-up time as a discrete fixed effect. Proportions and 95% CI are reported for each follow-up time window.
To understand body weight changes at 72 weeks after index dispense date, we used descriptive statistics to compare participants with and without weight data in the 72-week time window. We examined the proportion of patients who lost at least 10% of their body weight, lost 5% to 10%, lost 3.0% to 5%, had weight maintenance (i.e., lost 3.0% to gained 3.0%), and those who gained weight (i.e., gained over 3.0%). Analyses were conducted using R (R Foundation for Statistical Computing, Vienna, Austria) and SAS version 9.4 (SAS Institute, Cary, NC, USA). All P values are two-sided. P values less than 0.05 are considered statistically significant.
Results
Seven thousand and six individuals were dispensed an initial GLP-1 agonist between 2011 and 2018. After applying exclusion criteria, the final cohort was 2,405 individuals (Figure 1). Baseline characteristics of the cohort are reported in Table 1. The mean age in the final cohort was 48 years and mean body mass index was 37 kg/m2. Approximately half of participants were female (53%), 88% were White, 92% had type 2 diabetes and 86% had obesity. Most participants were dispensed their index GLP-1 agonist in 2017 or 2018, 52.3% [n=1,258]. The most commonly dispensed GLP-1 agonists were liraglutide (52%) and dulaglutide (40%). Three (0.1%) patients had history of a bariatric surgery procedure.
Figure 1.
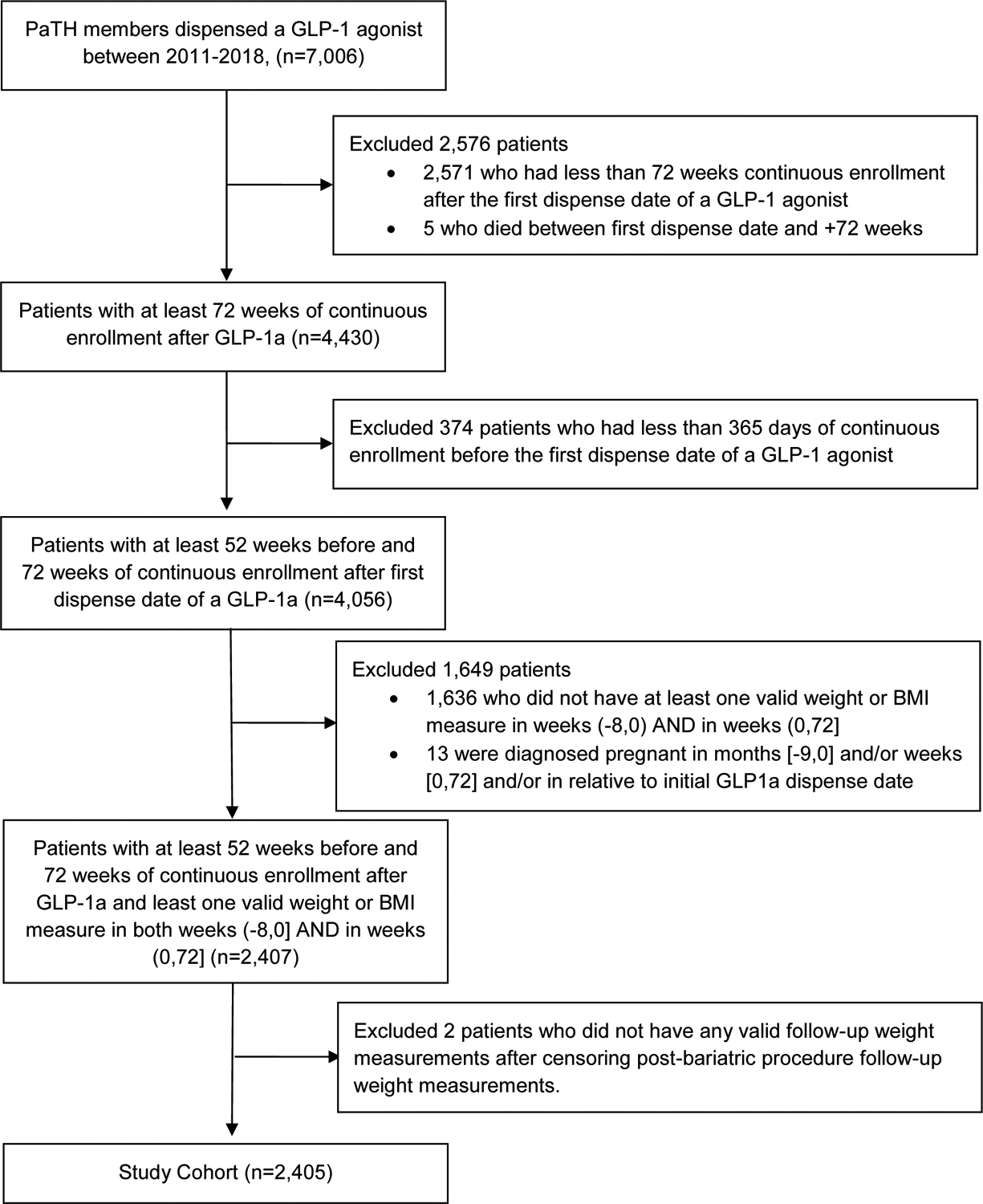
Study cohort flow diagram
Table 1.
Baseline characteristics of study cohort
| Study cohort (N=2405) | ||
|---|---|---|
| Characteristica | n | (%)b |
| Age (years), mean[SD] | 48 | [10.3] |
| Sex | ||
| Male | 1140 | (47.4) |
| Female | 1265 | (52.6) |
| Race | ||
| White | 2112 | (87.8) |
| Black | 238 | (9.9) |
| Other | 55 | (2.3) |
| Hispanic | 18 | (0.7) |
| Comorbidityc | ||
| Type 2 diabetes | 2214 | (92.1) |
| Obesityd | 2060 | (85.7) |
| Hypertension | 1826 | (75.9) |
| Hyperlipidemia | 1365 | (56.8) |
| Smoker | 479 | (19.9) |
| Coronary artery disease | 450 | (18.7) |
| Chronic kidney disease | 251 | (10.4) |
| Neuropathy | 220 | (9.1) |
| Congestive heart failure | 120 | (5.0) |
| Foot ulcers | 76 | (3.2) |
| Stroke | 70 | (2.9) |
| Retinopathy | 68 | (2.8) |
| Nephropathy | 49 | (2.0) |
| Serious hypoglycemic event | 24 | (1.0) |
| End stage renal disease | 8 | (0.3) |
| Serious hyperglycemic event | 5 | (0.2) |
| Peripheral artery disease | 2 | (0.1) |
| History of bariatric procedure | 3 | (0.1) |
| At least one hospitalization | 263 | (10.9) |
| Body weight (lbs), mean[SD] | 237.6 | [53.7] |
| BMI, (kg/m2) mean[SD] | 37.2 | [7.5] |
| HbA1c (%), mean[SD] | 8.5 | [1.8] |
| HbA1c < 6.5% | 177 | (7.4) |
| Creatinine (mg/dl), mean[SD] | 0.99 | [0.4] |
| LDL Cholesterol (mg/dl), mean[SD] | 88.8 | [35.3] |
| HDL Cholesterol (mg/dl), mean[SD] | 42.9 | [12.3] |
| Total Cholesterol (mg/dl), mean[SD] | 169.6 | [43.8] |
| Prescribed GLP-1 agonist | ||
| Liraglutide | 1247 | (51.9) |
| Dulaglutide | 962 | (40.0) |
| Exenatide ER | 117 | (4.9) |
| Semaglutide | 39 | (1.6) |
| Exenatide | 28 | (1.2) |
| Albiglutide | 12 | (0.5) |
BMI, body mass index; GLP-1, glucagon-like peptide 1; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Baseline characteristics were measured during the 365 day baseline period (prior to the first GLP-1 agonist dispense date).
Unless otherwise specified.
Based on ICD-9-CM and ICD-10-CM diagnostic codes.
According to ICD-9-CM or ICD-10-CM diagnostic codes or calculated baseline body mass index > 30 kg/m2.
Figure 2 (and Table S3) shows the observed and modeled percent weight change in each 8-week window through 72 weeks. Mean percent weight loss significantly increased from 1.1% (95% CI: 0.6–1.6) 8 weeks after the index GLP-1 agonist dispense date to 2.2% (95% CI: 1.7–2.6) 72 weeks after the index date (p for quadratic trend <.001). The proportion of patients who lost at least 5% of their body weight significantly increased from 11.2% (95% CI: 8.4–14.1) 8 weeks after the index GLP-1 agonist dispense date to 33.3% (95% CI: 26.8–39.7) 72 weeks after the index date (p for quadratic trend <.001; Table 2). At 72 weeks, nearly half of participants (42.7%) had lost weight (Figure 3) and 10.5% had lost 10% or more of their body weight. Participants who had weight data in the 72-week window were similar to those who did not (Table S4).
Figure 2.
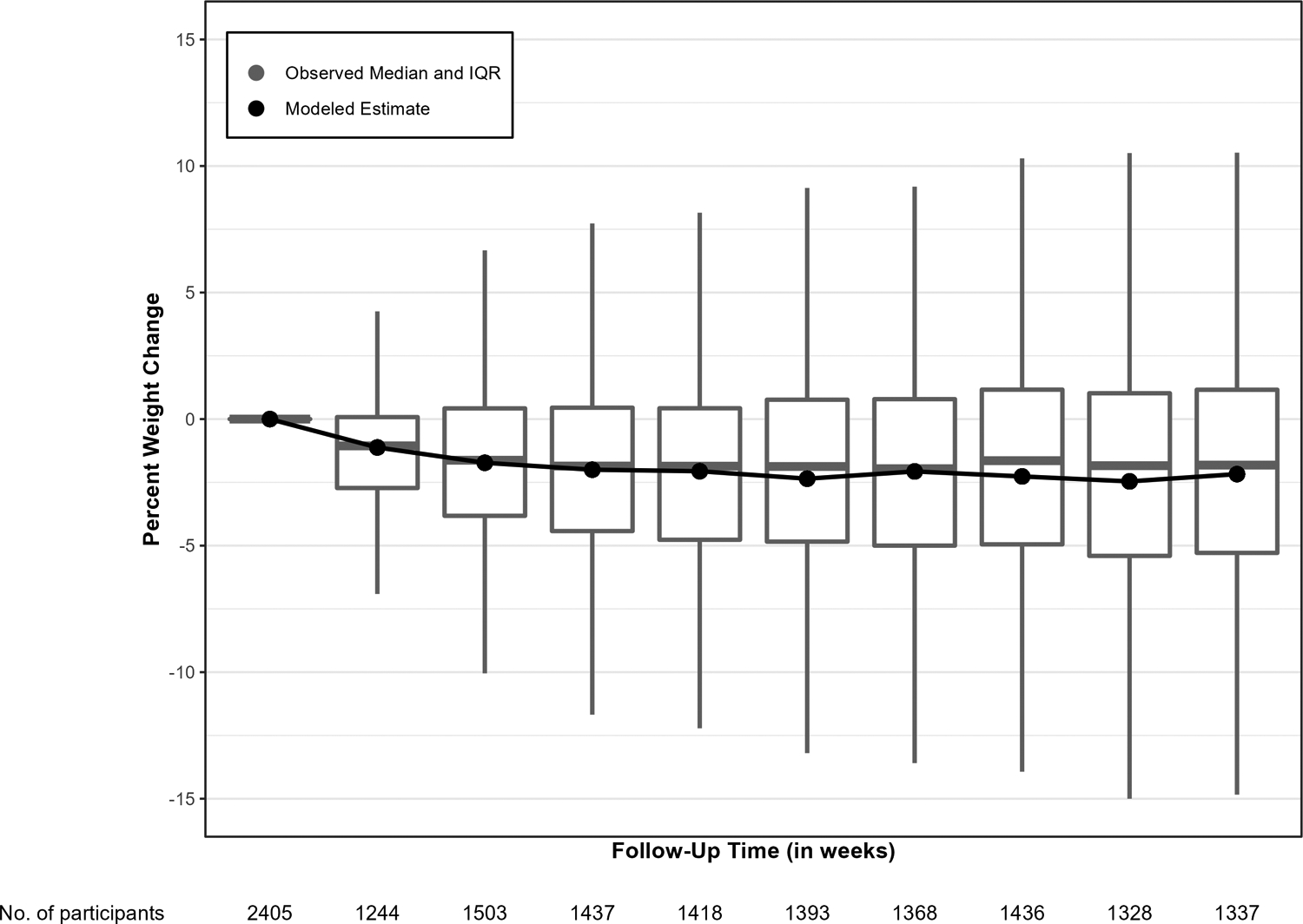
Observed and modeled percent weight change from baseline through 72 weeks after index GLP-1 agonist dispensingLines indicated modeled weight change based on linear mixed models with a person-level random intercept adjusted for baseline characteristics independently associated with missing follow-up weight (i.e.Sex, smoking status, coronary artery disease, hypertension, coronary heart failure, chronic kidney disease, having at least one hospitalization, and HbA1c value). Negative values indicate weight loss from baseline.
Table 2.
Observed and modeled percent of participants who lost at least 5% of baseline bodyweight at each 8-week time segment between the index GLP-1 agonist dispensing through week 72.
| Observed | Modeled | |||
|---|---|---|---|---|
| Time Window, weeks | n | (%) | % | (95% Confidence Interval) |
| 8 | 118 | (9.5) | 11.2 | (8.4, 14.1) |
| 16 | 265 | (17.6) | 20.2 | (16, 24.4) |
| 24 | 315 | (21.9) | 24.7 | (19.8, 29.7) |
| 32 | 351 | (24.8) | 27.7 | (22.2, 33.2) |
| 40 | 365 | (26.2) | 29.8 | (23.9, 35.6) |
| 48 | 364 | (26.6) | 30.5 | (24.6, 36.4) |
| 56 | 378 | (26.3) | 30.7 | (24.7, 36.7) |
| 64 | 386 | (29.1) | 33.0 | (26.6, 39.3) |
| 72 | 391 | (29.2) | 33.3 | (26.8, 39.7) |
p-value for quadratic trend <0.001
Proportions and 95% confidence intervals were estimated using a Poisson mixed model with robust error variance with a person-level random intercept controlling for variables associated with missing follow-up weight (i.e., sex, smoking status, coronary artery disease, hypertension, coronary heart failure, chronic kidney disease, having at least one hospitalization, and HbA1c value)
Figure 3.
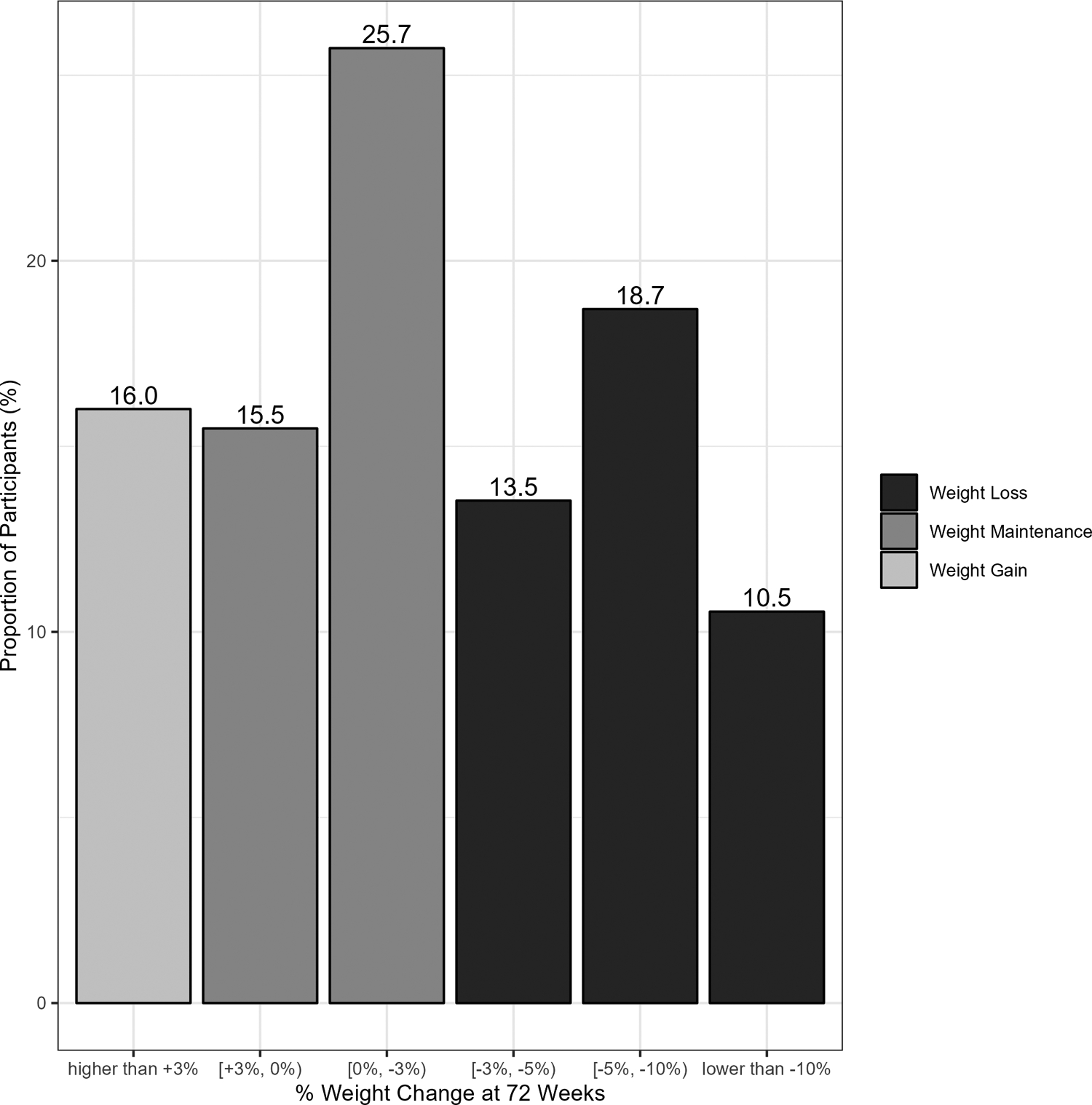
Proportion of participants at 72-week time window achieving observed body weight changes (n=1337)
Discussion
In this real-world cohort of over 2,400 patients with type 2 diabetes (baseline BMI 37 kg/m2, HbA1c 8.5%), we found that initiation of a GLP-1 agonist (predominantly dulaglutide or liraglutide) without a structured behavioral intervention was associated with a 2% (~6 lbs) weight loss at 72 weeks. One-third of patients achieved clinically significant weight loss, defined as ≥5% change from baseline. Weight loss was variable: many patients experienced no weight change compared to baseline and a few patients gained weight.
Although the magnitude of weight loss reported in our observational study is not as impressive as what is described in the weight-loss specific GLP-1 agonist trials (e.g. 9.6% for semaglutide 2.4mg in STEP 2), our results are similar in magnitude to the results seen among participants of diabetes trials testing GLP-1 agonist at standard dosages for HbA1c reduction. In addition, our results are also in line with the mean 1.6% to 4.6% differential weight loss demonstrated in placebo-controlled cardiovascular outcome trials for liraglutide 1.8mg (LEADER),17 semaglutide 1.0mg (SUSTAIN-6)18 and dulaglutide 1.5mg (REWIND).19
Prior studies have examined real-world weight change among patients with type 2 diabetes initiating a GLP-1 agonist in other settings. For example, one cohort study used United Kingdom claims data to examine body weight changes among 589 patients with T2D initiating a GLP-1 agonist.22 In that sample, 33% achieved weight loss ≥5% at 12 months. These results are nearly identical to ours despite the fact that the study took place in the United Kingdom.
There are several limitations to our study. First, our findings may have limited generalizability since they were derived from patients who predominately identify as white and are receiving care at a single large regional healthcare system. Second, claims data have inherent limitations such as incomplete or missing data, inaccurate data, and lack of specificity of some billing codes. Given the date range utilized to achieve numbers and duration of follow-up, there was very little use of semaglutide which did not become available in the US market until its approval in December 201721. Similarly, we could not examine effects of dulaglutide 3.0 or 4.5 mg in our sample due to the timing of FDA approval for these two dosages (September 2020). Despite these limitations, our sample size is large and the baseline demographic and clinical characteristics of our patients may be very similar to patients receiving care in other US-based clinical settings. Additionally, we did not examine changes in body weight among the subset of patients with high medication adherence. We made this choice a priori and deliberately because we aimed to describe real world results. However, this means that our weight loss results may be more modest than what can be expected in patients who are fully adherent to GLP-1 agonist therapy. Moreover, we did not exclude participants who had undergone bariatric procedures before their GLP-1 agonist index date. However, there were only 3 patients and we censored all weight data following bariatric procedures for the 5 patients who underwent a bariatric procedure during the 72 weeks of follow-up.
These findings therefore highlight the added value of combining pharmacologic treatments (i.e. GLP-1 agonist at standard diabetes dosages) with structured weight loss interventions (e.g. 500kcal deficit and increased physical activity and monthly follow-up contact), which patients in our cohort did not receive. Since structured weight loss programs are not typically covered by commercial or government health plans, findings that combine pharmacologic treatments with structured weight loss interventions may not reflect real world weight loss. Because our study did not include patients using structured weight loss interventions, our findings may better reflect the expected range of weight loss in the real world.20
In this real-world study using data from over 2,400 patients with overweight or obesity and type 2 diabetes, most patients who started a GLP-1 agonist lost weight through 72 weeks and one third lost a clinically meaningful amount (at least 5% of body weight). This suggests that GLP-1 agonists may be used in patients who would benefit from weight loss in real-world settings. Future research should examine changes in body weight among patients who are fully adherent to GLP-1 agonist therapy and identify factors associated with less observed weight loss in real world patients living with diabetes.
Supplementary Material
Study Importance.
What is already known about this subject?
In randomized weight loss trials, patients with type 2 diabetes and overweight or obesity initiating selected GLP-1 agonists have achieved clinically significant weight loss.
It is not known how much weight loss can be demonstrated and sustained among real world patients with type 2 diabetes initiating a GLP-1 agonist as part of routine clinical care in the absence of a standard behavioral weight loss intervention.
What are the new findings in your manuscript?
Mean percent weight loss significantly increased from 1.1% (95% CI: 0.6–1.6) 8 weeks after GLP-1a dispensing to 2.2% (95% CI: 1.7–2.6) 72 weeks after GLP-1a dispensing (p for quadratic trend <.001).
One third of patients lost ≥5% body weight at 72 weeks.
How might your results change the direction of research or the focus of clinical practice?
These findings suggest that GLP-1 agonists be used in patients who would benefit from weight loss in real-world settings.
Acknowledgements:
Study protocol and statistical analysis plan will be available upon request. Individual participant data will not be available.
Funding:
Research reported in this publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR001856 and the National Institute of Diabetes and Digestive and Kidney Diseases (Luo) under Award Number K23DK120956. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The research reported in this article was conducted using PCORnet®, the National Patient-Centered Clinical Research Network. PCORnet® has been developed with funding from the Patient-Centered Outcomes Research Institute® (PCORI®). This work is partially supported through the Patient-Centered Outcomes Research Institute Program Award RI-CRN-2020-006. The views presented in this work are solely the responsibility of the author(s) and do not necessarily represent the views of organizations participating in, collaborating with, or funding PCORnet® or of the Patient-Centered Outcomes Research Institute® (PCORI®).
Disclosures:
Dr. White, Ms. Shu, Dr. Arnold, and Dr. Korytkowski have no conflicts to disclose. Dr. Luo received consulting fees from Health Action International and Alosa Health outside the scope of this work. Dr. Rometo received consulting fees from Nestle Health Sciences outside the scope of this work.
References
- 1.Zhang C, Rexrode KM, Van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117(13):1658–1667. [DOI] [PubMed] [Google Scholar]
- 2.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968–977. [DOI] [PubMed] [Google Scholar]
- 3.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. Journal of the American college of cardiology. 2009;53(21):1925–1932. [DOI] [PubMed] [Google Scholar]
- 4.The Look Ahead Research Group. Reduction in Weight and Cardiovascular Disease Risk Factors in Individuals With Type 2 Diabetes: One-year results of the Look AHEAD trial. Diabetes care. 2007;30(6):1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Committee ADAPP, Committee: ADAPP. 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(Supplement_1):S113–S124. [DOI] [PubMed] [Google Scholar]
- 6.Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. The Lancet. 2018;391(10120):541–551. [DOI] [PubMed] [Google Scholar]
- 7.Lean ME, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. The lancet Diabetes & endocrinology. 2019;7(5):344–355. [DOI] [PubMed] [Google Scholar]
- 8.Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. Jama. 2015;314(7):687–699. [DOI] [PubMed] [Google Scholar]
- 9.Davies M, Færch L, Jeppesen OK, et al. Semaglutide 2· 4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. The Lancet. 2021;397(10278):971–984. [DOI] [PubMed] [Google Scholar]
- 10.Frias JP, Bonora E, Nevarez Ruiz L, et al. Efficacy and safety of dulaglutide 3.0 mg and 4.5 mg versus dulaglutide 1.5 mg in metformin-treated patients with type 2 diabetes in a randomized controlled trial (AWARD-11). Diabetes Care. 2021;44(3):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. Apr 2017;5(4):251–260. doi: 10.1016/s2213-8587(17)30013-x [DOI] [PubMed] [Google Scholar]
- 12.Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. Jan 2009;32(1):84–90. doi: 10.2337/dc08-1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umpierrez G, Tofé Povedano S, Pérez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care. Aug 2014;37(8):2168–76. doi: 10.2337/dc13-2759 [DOI] [PubMed] [Google Scholar]
- 14.Luo J, Feldman R, Rothenberger S, Korytkowski M, Fischer MA, Gellad WF. Incidence and Predictors of Primary Nonadherence to Sodium Glucose Co-transporter 2 Inhibitors and Glucagon-Like Peptide 1 Agonists in a Large Integrated Healthcare System. J Gen Intern Med. Jan 19 2022;doi: 10.1007/s11606-021-07331-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleurence RL, Curtis LH, Califf RM, Platt R, Selby JV, Brown JS. Launching PCORnet, a national patient-centered clinical research network. Journal of the American Medical Informatics Association. 2014;21(4):578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McTigue KM, Wellman R, Nauman E, et al. Comparing the 5-year diabetes outcomes of sleeve gastrectomy and gastric bypass: the National Patient-Centered Clinical Research Network (PCORNet) Bariatric Study. JAMA surgery. 2020;155(5):e200087–e200087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine. 2016;375(4):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 19.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. The Lancet. 2019;394(10193):121–130. [DOI] [PubMed] [Google Scholar]
- 20.Kushner RF, Ryan DH. Assessment and lifestyle management of patients with obesity: clinical recommendations from systematic reviews. Jama. 2014;312(9):943–952. [DOI] [PubMed] [Google Scholar]
- 21.Dhillon S. Semaglutide: First Global Approval. Drugs. Feb 2018;78(2):275–284. doi: 10.1007/s40265-018-0871-0 [DOI] [PubMed] [Google Scholar]
- 22.Weiss T, Yang L, Carr RD, et al. Real-world weight change, adherence, and discontinuation among patients with type 2 diabetes initiating glucagon-like peptide-1 receptor agonists in the UK. BMJ Open Diabetes Research and Care. 2022;10(1):e002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


