Abstract
Objectives
During 2022, Omicron became the dominant SARS-CoV-2 variant in Europe. This study aims to assess the impact of such variant on severe disease from SARS-CoV-2 compared with the Delta variant in Italy.
Methods
Using surveillance data, we assessed the risk of developing severe COVID-19 with Omicron infection compared with Delta in individuals aged ≥12 years using a multilevel negative binomial model adjusting for sex, age, vaccination status, occupation, previous infection, weekly incidence, and geographical area. We also analyzed the interaction between the sequenced variant, age, and vaccination status.
Results
We included 21,645 cases of SARS-CoV-2 infection where genome sequencing found Delta (10,728) or Omicron (10,917), diagnosed from November 15, 2021 to February 01, 2022. Overall, 3,021 cases developed severe COVID-19. We found that Omicron cases had a reduced risk of severe COVID-19 compared with Delta cases (incidence rate ratio [IRR] = 0.77; 95% confidence interval [CI]: 0.70-0.86). The largest difference was observed in cases aged 40-59 (IRR = 0.66; 95% CI: 0.55-0.79), while no protective effect was found in those aged 12-39 (IRR = 1.03; 95% CI: 0.79-1.33). Vaccination was associated with a lower risk of developing severe COVID-19 in both variants.
Conclusion
The Omicron variant is associated with a lower risk of severe COVID-19 compared to infection with the Delta variant, but the degree of protection varies with age.
Keywords: SARS-CoV-2, Delta variant, Omicron variant, COVID-19, Hospitalization, Death
Background
On January 29, 2020, the first SARS-CoV-2 case was notified in Italy [1,2], and by February 21, 2022, almost 12 million confirmed cases and 150,000 deaths from COVID-19 had been recorded in the country [3].
In Italy, several variants of concern have been identified, but only three of them have become dominant. The Alpha (B.1.1.7) variant became dominant in February 2021, replacing the ancestral strain [4]. By mid-2021, the Delta (B.1.617.2) variant started to disseminate in the country, quickly becoming the most prevalent variant by July 2021 [5]. On November 2021, the Omicron variant (originally lineage B.1.1.529, then identified as sublineages BA.1, BA.2, BA.3, BA.4, BA.5, and several Omicron-Omicron recombinants) was sequenced in South Africa and Botswana. A few weeks later, the World Health Organization (WHO) declared this new variant a variant under monitoring and 2 days later a variant of concern [6]. The Omicron variant has been described to have some mutations associated with increased transmissibility with respect to previous variants [7], which could explain how, in less than a month, its prevalence grew in Italy from 0.3% (beginning of December 2021) to 81% (early January 2022) [8,9].
Omicron's mutations have also been associated with an higher probability of reinfection [10] and with reduced severity [11]. In fact, previous studies have reported a reduced risk of hospitalization [12], [13], [14] and death [15] associated with this variant compared with the Delta variant.
Despite this emerging evidence, there is wide variation in the published estimates of severe disease risk between Omicron and previous variants, and is still not well understood how individual factors such as age could modulate such risk. The aim of this paper is to strengthen the evidence around this issue.
Methods
Study design and data sources
We conducted a retrospective cohort study using individual data retrieved from the Italian National COVID-19 Integrated Surveillance System. This system collects individual demographic and clinical information on all the notified cases of SARS-CoV-2 infection tested positive in the country, including the variant responsible of infection, if the positive sample has been sequenced, and clinical outcomes (hospitalization, admission to intensive care unit [ICU], recovery, or death) [1,16]. We also used individual information about vaccination status, retrieved from the National Vaccination Registry coordinated by the Italian Ministry of Health, that was linked to the surveillance data through the individual tax code [17].
Population under study and outcomes
We considered all cases of SARS-CoV-2 infection diagnosed from November 15, 2021 (diagnosis of the first case of Omicron variant in Italy) to February 01, 2022 (to show a homogeneous period, about a month, of Delta predominance and Omicron predominance, net period of coexistence) for whom sequencing had detected the Omicron or Delta variant as the cause of infection and who were eligible for vaccination at the beginning of the study period (aged 12 years or older). Cases were followed up until February 21, 2022, and the data for both datasets was extracted on March 23, 2022, allowing for a month between the end of the follow up and the date of data extraction for possible reporting delays. We excluded from the analysis cases with missing information for the variables of interest (i.e., age and sex) and those whose date of hospital admission or date of death was recorded as preceding the diagnosis.
We analyzed the association between severe COVID-19 (yes/no)—defined as a SARS-CoV-2 infection leading to hospitalization, admission to ICU, or death within 20 days from diagnosis—and variant (Delta or Omicron), identified using nasopharyngeal swab tested positive with reverse transcription-polymerase chain reaction. According to Italian guidelines, only hospitalized cases with respiratory symptoms compatible with COVID-19 should be reported as hospitalized in the surveillance system [18]. According to indications from the WHO, deaths were only considered as related to SARS-CoV-2 in the absence of a clear cause of death different from COVID-19 (e.g., road accident) and in the absence of a complete clinical recovery from the disease [19].
We classified cases according to their vaccination status as follows: unvaccinated, incompletely vaccinated, completely vaccinated (completion of the primary cycle) by 120 days or less, completely vaccinated by more than 120 days, and completely vaccinated with an additional booster dose. More information on this classification can be found in the Italian surveillance bulletin [3]. We considered as reinfections only diagnoses occurred at least 90 days after a previous positive test, according to the Italian guidelines [20].
Statistical analysis
We described the main characteristics of cases and the frequency of the outcomes of interest using counts and percentages. To estimate the incidence rate ratios (IRRs) of the dichotomous dependent variable, severe COVID-19, we used a negative binomial mixed model (model 1) where the sequenced variant was the independent variable of interest. We included in the model as covariates the sex, healthcare worker status (yes/no-not indicated), age group (12-19 years, 20-39 years, 10-year age groups from 40-49 to 70-79 years, and ≥80 years), vaccination status, information about reinfection (yes/no) and the weekly normalized regional incidence in the general population, obtained as the difference between observed incidence and mean incidence and divided by the SD of the overall incidence. We included in the model the geographical region (NUTS2 according to Eurostat) as random-effect. Given that one of the two sub-samples for sequencing was targeted at cases who had been infected after vaccination, and that Omicron cases probably are overrepresented among the vaccine breakthrough cases (due to the lower vaccine effectiveness against Omicron), this analysis was also performed restricting only to cases who were not vaccinated at the time of diagnosis. We carried out another analysis (model 2) using the same model and including the interaction between the sequenced variant and age re-coded into three groups (12-39, 40-59, and ≥60 years). In addition, we performed a third model (model 3) including the interaction between the sequenced variant and vaccination status. Estimates from all models are presented with their 95% confidence interval (CI). Finally, we conducted a sensitivity analysis for all three models. In this analysis, we used the same exclusion criteria as above to select the population under study, except for the sequencing criteria. For this reason, we added a period variable categorized as ”Delta” (Delta variant predominance, from November 15, 2021, to December 15, 2021), “Both” (Delta/Omicron variants coexistence from December 16, 2021, to January 05, 2022) and “Omicron” (Omicron variant predominance, from January 06, 2022, to February 01, 2022). Cut-off dates were decided based on the national variant bulletin [21]. Thus, using this new population, we carried out model 1, model 2, and model 3. All the analyses were carried out with RStudio 2021.09.0 under R 4.1.2 [22].
Ethics approval and consent to participate
The collection of data used for this manuscript is compulsory according to national laws on infectious diseases. The COVID-19 Italian National Working group on Bioethics has stated that consent for the collection of these data in the context of the COVID-19 emergency is not mandatory, based on guideline 12 of the WHO on ethical issues in public health surveillance [23]. The legal ordinance n. 640 [24] of February 28, 2020, explicitly declares Istituto Superiore di Sanità as entitled to collect data for COVID-19 surveillance and that such data can be used and shared, upon anonymization, to advance scientific knowledge on this new disease. In addition, the dissemination of COVID-19 surveillance data was authorized by Decree-Law n.24 of March 24, 2022 (art.13) [25] and, because of the retrospective design and the large size of the population under study, in accordance with the Authorization n. 9 released by the Italian data protection authority on December 15, 2016, individual informed consent was not requested for the conduction of this study [26].
Results
From November 15, 2021, to February 01, 2022, there were 5,482,069 cases aged 12 years or older reported to the Italian National COVID-19 Integrated Surveillance System. The percentage of cases sequenced differed throughout the study period. During the first 2 weeks (from November 15, 2021, to November 29, 2021) 4811 (3.09%) cases were sequenced whereas from December 27, 2021, to January 10, 2022, during the Omicron's wave, only 0.31% (n = 6,228) cases were sequenced. The decrease over time was homogeneous across the entire territory. Of these, 21,873 (0.40%) were found to have been infected by the Omicron or Delta variants. We excluded 228 cases with a date of hospitalization/death that preceded the date of diagnosis (1.04%), leaving 21,645 cases available for the analysis, 10,728 (49.6%) infected by the Delta variant and 10,917 (50.4%) infected by the Omicron variant. There were no records with missing information about sex or age.
When comparing Delta and Omicron cases, no differences in the sex distribution were observed. Omicron cases were more frequently healthcare workers than Delta cases (5.0% vs 2.2%) and more frequently vaccinated with at least one dose of a COVID-19 vaccine. Omicron cases were generally younger than Delta cases and more likely to have had a previous diagnosis of SARS-CoV-2 infection (4.9% vs 1.7%) (Table 1 ).
Table 1.
Individual characteristics of SARS-CoV-2 cases included in the study.
| Delta n = 10,728 (49.6%) | Omicron n = 10,917 (50.4%) | |
|---|---|---|
| Sex | ||
| Female | 5,563 (51.9%) | 5,747 (52.6%) |
| Male | 5,165 (48.1%) | 5,170 (47.4%) |
| Health care workers | ||
| No-unknown | 10,489 (97.8%) | 10,375 (95.0%) |
| Yes | 239 (2.2%) | 542 (5.0%) |
| Vaccination status | ||
| Unvaccinated | 3,256 (30.4%) | 1,877 (17.2%) |
| Incompleted | 263 (2.5%) | 440 (4.0%) |
| Completed within 120 days | 5,071 (47.2%) | 4,445 (40.7%) |
| Completed for more than 120 days | 1,833 (17.1%) | 1,439 (13.2%) |
| Completed + booster dose | 305 (2.8%) | 2,716 (24.9%) |
| Age | ||
| 12-19 years | 845 (7.9%) | 1,033 (9.5%) |
| 20-39 years | 2,639 (24.6%) | 3,681 (33.7%) |
| 40-49 years | 2089 (19.5%) | 1,636 (15.0%) |
| 50-59 years | 1,866 (17.4%) | 1,665 (15.3%) |
| 60-69 years | 1,359 (12.6%) | 1,044 (9.5%) |
| 70-79 years | 1,150 (10.7%) | 872 (8.0%) |
| 80+ years | 780 (7.3%) | 986 (9.0%) |
| Reinfection | ||
| Yes | 184 (1.7%) | 540 (4.9%) |
| No | 10,544 (98.3%) | 10,377 (95.1%) |
During the study period, we observed 4,047 severe events in 3,021 cases (Table 2 ). We found that 12% of Omicron cases had at least one severe event vs 16% of the Delta cases. All the events analyzed—hospitalizations, ICU admissions, and deaths—were less frequent in Omicron cases than in Delta cases.
Table 2.
Incidence rate of severe COVID-19 events.
| Delta (n = 10,728) |
Omicron (n = 10,917) |
Total (n = 21,645) |
|
|---|---|---|---|
| No. cases (%) | |||
| Any severe event | 1,713 (16) | 1,308 (12) | 3,021 (14) |
| Hospitalization | 1,167 (11) | 946 (9) | 2,113 (10) |
| Intensive care unit | 89 (1) | 56 (1) | 145 (1) |
| Death | 457 (4) | 306 (3) | 763 (4) |
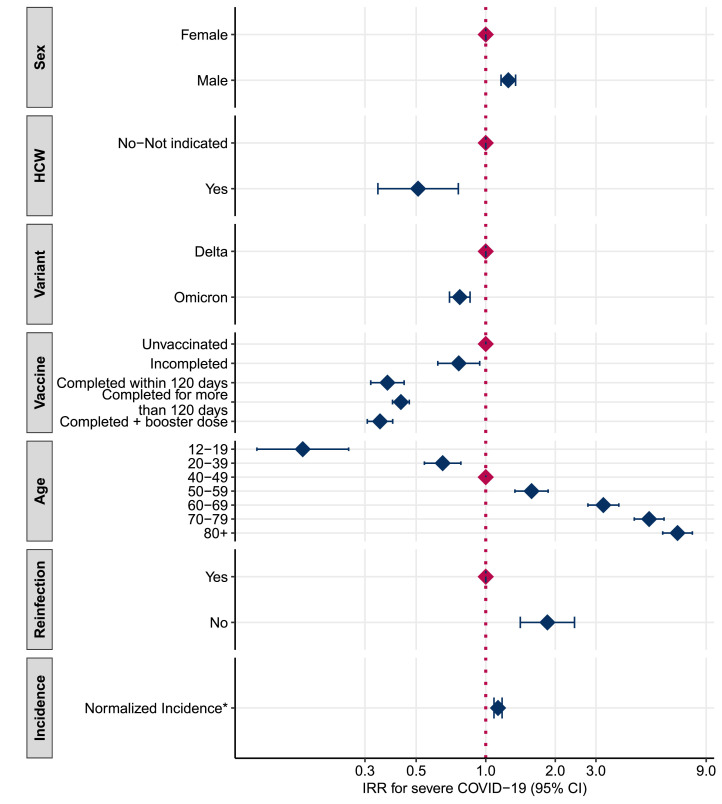
The results from the multivariable analysis (model 1) (Figure 1 and Table S1 of the Supplementary Material 1) show that cases infected with the Omicron variant were 23% less likely to develop severe COVID-19 compared with Delta cases (IRR: 0.77; 95% CI: 0.70-0.86). With regards to the other factors included in analysis, we found a higher risk of developing severe COVID-19 among male patients compared with female patients (IRR: 1.25; 95% Cl 1.16-1.35) and among unvaccinated individuals. We also found a clear age gradient where the risk of severe COVID-19 increased with age. Finally, we found a lower risk of developing severe COVID-19 among healthcare workers and among those with a previous diagnosis of SARS-CoV-2 infection. When restricting this analysis to only those unvaccinated, still those infected with the Omicron variant were at lower risk to develop severe COVID-19 compared with Delta cases (IRR:0.68; 95% CI: 0.59-0.79, data not shown in table/figure). The sensitivity analysis (Table S2 of Supplementary Material 2) shows that the risk associated with the period of Omicron predominance is 40% (IRR: 0.60, 95% CI: 0.58-0.62) less than the period of Delta predominance. The risk decreases in the period of coexistence too (IRR: 0.81, 95% CI: 0.78-0.83). In general, the estimated values obtained using the whole period data agree with the result obtained in model 1 except for healthcare workers (model 1: IRR = 0.51 95% CI: 0.34-0.76, sensitivity analysis: IRR = 1.75, 95% CI: 1.63-1.89) and normalized incidence (model 1: IRR = 1.13 95% CI: 1.09-1.18, sensitivity analysis: 0.78, 95% CI: 0.76-0.81).
Figure 1.
Adjusted IRR estimated with the negative binomial linear mixed model (model 1)
*The IRR for normalized incidence, obtained as the difference between observed incidence and mean incidence and divided by the standard deviation of the overall incidence, is expressed as a unit-increase of the standard deviation. CI, confidence interval; HCW, healthcare workers; IRR, incidence rate ratio.
Results from model 2 show that cases aged 40+ years infected by the Omicron variant had a reduced risk of developing severe COVID-19 compared to cases in the same age group who were infected by the Delta variant (40-59 years: IRR = 0.66, 95% CI: 0.55-0.79; 60+ years: IRR = 0.79, 95% CI: 0.70-0.88). In contrast, the difference in the risk of severe COVID-19 according to the SARS-CoV-2 variant was found to be negligible in cases younger than 40 years (Table 3 ). The results were consistent with those of the sensitivity analysis (Table S3 of Supplementary Material 2) where we compared the period of Omicron predominance with the reference, the Delta predominance period. The IRR estimate in the 12-39 years age group significantly differs from estimates in the 40-59 years age group (P = 0.003) and 60+ years age group (P = 0.049), while the difference between the IRR estimated in the 40-59 and 60+ years age groups was not statistically significant (P = 0.057).
Table 3.
Adjusted incidence rate ratio of severe COVID-19 in Omicron cases compared to Delta cases by age (model 2).
| Age | Incidence rate ratio | 95% confidence interval | P-value | |
|---|---|---|---|---|
| Omicron vs Delta | ||||
| 12-39 years | 1.03 | 0.79 | 1.33 | 0.843 |
| 40-59 years | 0.66 | 0.55 | 0.79 | <0.001 |
| 60+ years | 0.79 | 0.70 | 0.88 | <0.001 |
Vaccination was estimated to be effective in preventing severe COVID-19 in both Delta and Omicron cases, particularly where the primary cycle was completed (Table 4 ). The only vaccination status that was not found to be a protective factor at the 95% confidence interval level was incomplete vaccination in Omicron cases (IRR = 0.87; 95% CI: 0.66-1.15). Vaccination provided a higher degree of protection in Delta cases than in Omicron cases. The only exception was cases that had received the third dose of the vaccine, for whom protection against severe COVID-19 was similar in both groups. The results of this model are confirmed by the sensitivity analysis conducted on the entire population (Table S4 of Supplementary Material 2). The estimated values confirmed that vaccination had a preventive effect against severe COVID-19 in all the periods examined. The protection was higher during the period of Delta predominance and coexistence of the two variants than in the Omicron predominance period.
Table 4.
Adjusted incidence rate ratio of severe COVID-19 in vaccinated vs unvaccinated cases by SARS-CoV-2 variant (model 3).
| Vaccination status | Incidence rate ratio | 95% confidence interval | P-value | |
|---|---|---|---|---|
| Unvaccinated | Reference | |||
| Delta | ||||
| Incompleted | 0.71 | 0.52 | 0.96 | 0.027 |
| Completed within 120 days | 0.28 | 0.22 | 0.35 | <0.001 |
| Completed for more than 120 days | 0.38 | 0.34 | 0.42 | <0.001 |
| Completed + booster dose | 0.42 | 0.33 | 0.54 | <0.001 |
| Omicron | ||||
| Incompleted | 0.87 | 0.66 | 1.15 | 0.316 |
| Completed within 120 days | 0.60 | 0.47 | 0.77 | <0.001 |
| Completed for more than 120 days | 0.53 | 0.46 | 0.60 | <0.001 |
| Completed + booster dose | 0.37 | 0.32 | 0.43 | <0.001 |
Discussion
Main findings
Since the beginning of 2022, most of the new SARS-CoV-2 infections in Italy have been caused by the Omicron variant, which was found to be predominant in the country according to the national representative surveys conducted from January 03, 2022, onwards [9]. Our results suggest that SARS-CoV-2 cases infected by the Omicron variant were 23% less likely to develop severe COVID-19 compared to those infected by the Delta variant. Infections due to the Omicron variant were less severe than those due to the Delta variant particularly in the oldest age groups (40-59 and 60+ years), while the difference was negligible in the youngest cases aged 12-39 years. Finally, we found that vaccination was effective in preventing severe COVID-19 in all cases, especially in people infected with the Delta variant.
Comparison with other studies
Our results are in line with findings reported previously in the literature suggesting a reduction in the risk of severe COVID-19 in Omicron cases compared with Delta case. They also coincide with animal data showing a decreased lung infectivity and pathogenic effects of Omicron compared to Delta and ancestral SARS-CoV-2 [27]. However, the risk reduction estimated in our study is lower than that reported in other countries. Studies conducted in England have estimated that the Omicron variant reduces the risk of hospitalization from COVID-19 by over 50% and the risk of death by 69% compared with the Delta variant [15,28]. In Portugal, the reduction of the risk of hospitalization and death in Omicron cases compared to Delta cases was estimated at 75% and 86%, respectively [12]. There are several factors that could partly explain the differences between these studies and our results. One possible influential factor could be differences in hospital attendance and/or admission across countries, given that not all healthcare systems suffered equal pressure during the Omicron wave. Furthermore, during Omicron it became more challenging to discriminate between hospitalizations where Omicron was the reason for admission and those where it was an incidental finding. Thus, models across countries should be compared with caution when they use severe COVID-19 as the outcome of interest.
Estimating the age-specific IRRs of severe COVID-19 in Omicron cases relative to Delta cases, we found no differences in the youngest age group but a reduced risk of severe COVID-19 associated with the Omicron variant in adults aged 40-59 years and elderly cases aged 60+ years. A previous study reporting as well a slightly higher likelihood of severe disease with Omicron infection in the younger age groups, suggests that this finding could be due to differences in the clinical presentation between Omicron and Delta infections [28]. More specifically, that children infected with Omicron may be more likely to present with fever and upper respiratory symptoms, which could lower the threshold for hospital admission [29]. In any case, the lack of significant risk differences between Delta and Omicron in the younger population, together with the increased protection against severe diseases found in people vaccinated with the booster dose, confirm the role of age and immunization in preventing severe diseases.
In Italy, 90% of vaccine administrations were performed using messenger RNA vaccines (approximately 65% Comirnaty and 25% Spikevax) [30]. In this context, in line with other studies, we found that vaccination provided a protective effect against severe COVID-19, both in Delta and in Omicron cases [12,28]. The fact that we found a lower degree of vaccine-induced protection in Omicron cases compared with Delta are consistent with findings from studies suggesting that Omicron's spike mutations confer a higher probability of evading acquired immunity compared with Delta [31]. The only vaccinated group showing similar degree of protection against severe COVID-19 due to the Omicron and Delta variants consisted of cases who received a booster dose of vaccine. However, this comparison might be affected by selection bias, given that most of the Delta cases included in our study occurred early in the study period, when only the most fragile population was eligible to receive a booster dose. Finally, besides the protection conferred by vaccine-induced immunity, we also found that immunity induced by a previous infection was a protective factor against severe COVID-19, in line with findings from another study [32].
Strengths and limitations
This study provides valuable information for public health authorities. We carried out an analysis on a relatively large nationally representative sample of cases and we were able to adjust for several individual variables, reducing the probability of residual confounding.
Yet, our analysis has several limitations. The sampling strategy foresaw two separate sub-samples [33,34]. The first is a randomly drawn sample from the total diagnosed cases; and the second is drawn from cases with particular characteristics (e.g., having contracted SARS-CoV-2 after vaccination). Although the choice of sample components is random with respect to the severity of the disease at the time of testing, it is possible that in periods with high incidence, severe cases were more likely selected for sequencing than milder ones, thus, inducing a selection bias. Another limitation is that the percentage of sequenced cases changed during the study period. We carried out a sensitivity analysis on all notified cases in Italy (whether sequenced or not) during the same period and classified them as Delta or Omicron based on the prevalence of the variant on the day they were diagnosed. The results show that the risk of severe disease in the Omicron-prevalent period was 40% lower than the risk associated with Delta (Table S2 of Supplementary Material 2). The results of this sensitivity analysis suggest that the reduction in the clinical risk associated with Omicron is larger than the one estimated in our main analysis. We were not able to account for this possible bias, as we did not have information on cases’ comorbidities. Due to this bias, the estimated IRR for normalized incidence obtained with the sensitivity analysis does not agree with the estimated value obtained with model 1. As previously mentioned, the Omicron variant is associated with higher transmissibility but also lower severity; therefore, if there were no selection bias, it would not be strange to think that as incidence increases, disease severity decreases, as per the sensitivity analysis. Instead, in model 1, with only sequenced cases, as incidence increases, the risk of severe disease also increases. Another limitation is that, as for all studies based on surveillance data, an ascertainment bias might have affected our estimates, given that part of all SARS-CoV-2 infections occurring in the country might not have been notified. The under-ascertainment and under-reporting, due to the introduction of house testing at the end of 2021, could have played a role in our estimates. Unfortunately, we do not have a precise estimate of this, but, if the under-ascertainment-and under-reporting increased during the Omicron predominance period, our estimates of the reduction of COVID-19 severity could be underestimated. In fact, an individual with only a positive home test will not be reported to the Italian National COVID-19 Integrated Surveillance System unless he needs to be hospitalized or dies; in such cases, he will be swabbed at the hospital entrance or following determination of the cause of death. Finally, compared to other studies, given the relatively low number of admissions to the ICU and deaths, we were not able to provide robust outcome-specific estimates.
Conclusion
Omicron cases were less likely to develop severe COVID-19 compared to Delta cases, in particular those aged 40 years or more. Immunity induced by a previous infection or vaccination provided protection against severe disease, both in Omicron and Delta cases.
Acknowledgments
Declaration of competing interests
The authors have no competing interests to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
Italian Integrated Surveillance of COVID-19
Italian Integrated Surveillance (ISS) coordination group: Antonino Bella, Stefano Boros, Marco Bressi, Fortunato (Paolo) D'Ancona, Martina Del Manso, Corrado Di Benedetto, Massimo Fabiani, Antonietta Filia, Alberto Mateo Urdiales, Daniele Petrone, Patrizio Pezzotti, Flavia Riccardo, Maria Cristina Rota, Chiara Sacco, Paola Stefanelli, Marco Tallon, Maria Fenicia Vescio.
Regional representatives: Antonia Petrucci (Abruzzo); Michele La Bianca (Basilicata); Anna Domenica Mignuoli (Calabria); Pietro Buono (Campania); Erika Massimiliani (Emilia-Romagna); Fabio Barbone (Friuli Venezia Giulia); Francesco Vairo (Lazio); Camilla Sticchi (Liguria); Danilo Cereda (Lombardia); Marco Pompili (Marche); Francesco Sforza (Molise); Pierpaolo Bertoli (P.A. Bolzano); Pier Paolo Benetollo (P.A. Trento); Chiara Pasqualini (Piemonte); Lucia Bisceglia (Puglia); Maria Antonietta Palmas (Sardegna); Sebastiano Pollina Addario (Sicilia); Emanuela Balocchini (Toscana); Anna Tosti (Umbria); Mauro Ruffier (Valle D'Aosta); Filippo Da Re (Veneto).
Author contributions
PP, CS, AMU, and DP designed the paper. AB, MDM, and MF ensured quality of COVID-19 surveillance. LA, ALP, ADM, PS, and ATP ensured quality of sequencing data of COVID-19 cases. DP, supported by CS and EC, carried out the analysis. DP, AMU, MF, FR, and PP wrote the manuscript which was reviewed and approved by the other authors.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.01.027.
Appendix. Supplementary materials
References
- 1.Riccardo F, Ajelli M, Andrianou XD, Bella A, Manso MD, Fabiani M, et al. Epidemiological characteristics of COVID-19 cases and estimates of the reproductive numbers 1 month into the epidemic, Italy, 28 January to 31 March 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.49.2000790. 2000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Istituto Superiore di Sanità. I primi due casi confermati in Italia, https://www.iss.it/covid-19-primo-piano/-/asset_publisher/yX1afjCDBkWH/content/i-primi-due-casi-confermati-in-italia; 2022 [accessed on 04 July 2022].
- 3.Istituto Superiore di Sanità. REPORT ESTESO ISS: COVID-19: Sorveglianza, Impatto Delle Infezioni Ed Efficacia Vaccinale, https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_2-marzo-2022.pdf; 2022 [accessed on 11 April 2022].
- 4.Istituto Superiore di Sanità. Prevalenza delle varianti VOC 202012/01 (lineage B.1.1.7), P.1, e 501.V2 (lineage B.1.351) in Italia, https://www.epicentro.iss.it/coronavirus/pdf/sars-cov-2-monitoraggio-varianti-indagini-rapide-18-febbraio-2021.pdf; 2021 [accessed on 11 April 2022].
- 5.Istituto Superiore di Sanità. Stima della prevalenza delle varianti VOC (Variants of Concern) in Italia: B.1.1.7, B.1.351, P.1 e B.1.617.2, e altre varianti di SARS-CoV-2, https://www.epicentro.iss.it/coronavirus/pdf/sars-cov-2-monitoraggio-varianti-indagini-rapide-20-luglio-2021.pdf; 2021 [accessed on 11 April 2022].
- 6.World Health Organization. Tracking SARS-CoV-2 variants, https://www.who.int/activities/tracking-SARS-CoV-2-variants; 2022 [accessed on 11 April 2022].
- 7.Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398:2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Istituto Superiore di Sanità. Stima della prevalenza delle varianti VOC (Variants of Concern) in Italia: beta, gamma, delta, omicron e altre varianti di SARS-CoV-2, https://www.epicentro.iss.it/coronavirus/pdf/sars-cov-2-monitoraggio-varianti-indagini-rapide-6-dicembre-2021.pdf; 2021 [accessed on 11 April 2022].
- 9.Istituto Superiore di Sanità. Stima della prevalenza delle varianti VOC (Variant Of Concern) e di altre varianti di SARS-CoV-2 in Italia, https://www.epicentro.iss.it/coronavirus/pdf/sars-cov-2-monitoraggio-varianti-indagini-rapide-3-gennaio-2022.pdf; 2022 [accessed on 11 April 2022].
- 10.Pulliam JRC, van Schalkwyk C, Govender N, von Gottberg A, Cohen C, Groome MJ, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science. 2022;376(eabn4947) doi: 10.1126/science.abn4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bager P, Wohlfahrt J, Bhatt S, Stegger M, Legarth R, Møller CH, et al. Risk of hospitalisation associated with infection with SARS-CoV-2 omicron variant versus delta variant in Denmark: an observational cohort study. Lancet Infect Dis. 2022;22:967–976. doi: 10.1016/S1473-3099(22)00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peralta-Santos A, Rodrigues EF, Moreno J, Ricoca V, Casaca P, Fernandes E, et al. Omicron (BA.1) SARS-CoV-2 variant is associated with reduced risk of hospitalization and length of stay compared with Delta (B.1.617.2) medRxiv. 25 January. 2022 https://www.medrxiv.org/content/10.1101/2022.01.20.22269406v2 [accessed on 12 April 2022] [Google Scholar]
- 13.Paredes MI, Lunn SM, Famulare M, Frisbie LA, Painter I, Burstein R, et al. Associations between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants and risk of coronavirus disease 2019 (COVID-19) hospitalization among confirmed cases in Washington State: a retrospective cohort study. Clin Infect Dis. 2022;75:e536–e544. doi: 10.1093/cid/ciac279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheikh A, Kerr S, Woolhouse M, McMenamin J, Robertson C. EAVE II Collaborators, et al. Severity of omicron variant of concern and effectiveness of vaccine boosters against symptomatic disease in Scotland (EAVE II): a national cohort study with nested test-negative design. Lancet Infect Dis. 2022;22:959–966. doi: 10.1016/S1473-3099(22)00141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward IL, Bermingham C, Ayoubkhani D, Gethings OJ, Pouwels KB, Yates T, et al. Risk of covid-19 related deaths for SARS-CoV-2 omicron (B.1.1.529) compared with delta (B.1.617.2): retrospective cohort study. BMJ. 2022;378:e070695. doi: 10.1136/bmj-2022-070695. [DOI] [PMC free article] [PubMed] [Google Scholar]; https://www.bmj.com/content/378/bmj-2022-070695.
- 16.Istituto Superiore di Sanità. Prevalenza e distribuzione delle varianti di SARS-CoV-2 di interesse per la sanità pubblica in Italia, https://www.epicentro.iss.it/coronavirus/pdf/sars-cov-2-monitoraggio-varianti-rapporti-periodici-18-febbraio-2022.pdf; 2022 [accessed on 27 April 2022].
- 17.Fabiani M, Puopolo M, Morciano C, Spuri M, Alegiani SS, Filia A, et al. Effectiveness of mRNA vaccines and waning of protection against SARS-CoV-2 infection and severe Covid-19 during predominant circulation of the delta variant in Italy: retrospective cohort study. BMJ. 2022;376 doi: 10.1136/bmj-2021-069052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Istituto Superiore di Sanità. Piattaforma Web della Sorveglianza Integrata dei casi di COVID-19: Manuale Utente (ver. 1.0), https://covid-19.iss.it/files/manuale_utente_v1_sorveglianza_covid19.pdf; 2022 [accessed on 19 April 2022].
- 19.World Health Organization. International Guidelines for Certification and Classification (Coding) of COVID-19 as Cause of Death, https://www,who.int/publications/m/item/international-guidelines-for-certification-and-classification-(coding)-of-covid-19-as-cause-of-death; 2020 [accessed on 11 May 2022].
- 20.Ministero della Salute. OGGETTO: Flusso dati aggregati Ministero della Salute/Protezione Civile: aggiornamento sulla possibilità di inserimento delle reinfezioni da SARS-CoV-2, https://www,seremi.it/sites/default/files/GR3917-000127.pdf. 2021 [accessed on 12 April 2022].
- 21.Istituto Superiore di Sanità. Prevalenza e distribuzione delle varianti di SARS-CoV-2 di interesse per la sanità pubblica in Italia, https://www.epicentro.iss.it/coronavirus/pdf/sars-cov-2-monitoraggio-varianti-rapporti-periodici-25-marzo-2022.pdf; 2022 [accessed on 17 October 2022].
- 22.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/; 2022 [accessed on 19 April 2022]
- 23.World Health Organization. Ethics and governance of artificial intelligence for health: WHO guidance, https://apps.who.int/iris/handle/10665/341996; 2021 [accessed on 23 May 2022].
- 24.Presidenza del consiglio dei ministri dipartimento della protezione civile, Italy. Ulteriori interventi urgenti di protezione civile in relazione all'emergenza relativa al rischio sanitario connesso all'insorgenza di patologie derivanti da agenti virali trasmissibili, (Ordinanza n 640), https://www.gazzettaufficiale.it/eli/id/2020/02/28/20A01348/sg; 2020 [accessed on 23 May 2022].
- 25.Presidenza del consiglio dei ministri, Italy. Disposizioni urgenti per il superamento delle misure di contrasto alla diffusione dell'epidemia da COVID-19, in conseguenza della cessazione dello stato di emergenza, https://www.gazzettaufficiale.it/eli/id/2022/03/24/22G00034/sg; 2022 [accessed on 01 June 2022].
- 26.Garante per la Protezione dei Dati Personali, GPDP, Italy. Autorizzazione n. 9/2016 - Autorizzazione generale al trattamento dei dati personali effettuato per scopi di ricerca scientifica - 15 dicembre 2016 [5805552], https://www.garanteprivacy.it:443/home/docweb/-/docweb-display/docweb/5805552; 2016 [accessed on 01 June 2022].
- 27.McMahan K, Giffin V, Tostanoski LH, Chung B, Siamatu M, Suthar MS, et al. Reduced pathogenicity of the SARS-CoV-2 omicron variant in hamsters. Med. 2022;3:262–268. doi: 10.1016/j.medj.2022.03.004. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark M, Walker B, Bennett E, Herrick A, Kenny S, Gent N. Clinical characteristics of SARS-CoV-2 omicron infection in children under one year. SSRN Journal 15 February 2022 [PREPRINT journal]. 10.2139/ssrn.4013461 [accessed on 11 May 2022]. [DOI]
- 30.Governo Italiano. Report vaccini anti Covid-19, https://www,governo.it/it/cscovid19/report-vaccini/; 2022 [accessed on 19 April 2022].
- 31.Hu J, Peng P, Cao X, Wu K, Chen J, Wang K, et al. Increased immune escape of the new SARS-CoV-2 variant of concern Omicron. Cell Mol Immunol. 2022;19:293–295. doi: 10.1038/s41423-021-00836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399:437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Istituto Superiore di Sanità. Come funziona il monitoraggio delle varianti in Italia, https://iss.it/cov19-cosa-fa-iss-varianti/-/asset_publisher/yJS4xO2fauqM/content/come-funziona-il-monitoraggio-delle-varianti-in-italia-; 2022 [accessed on 20 October 2022]
- 34.Ministero della Salute. OGGETTO: Indicazioni operative relative al rischio di diffusione di nuove varianti SARS-CoV-2 in Unione europea/Spazio Economico Europeo (UE/SEE): misure di prevenzione per iviaggiatori e sorveglianza di laboratorio, https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&codLeg=78153&parte=1%20&serie=null; 2021 [accessed on 20 October 2022].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.