Abstract
Background
Tixagevimab-cilgavimab is used for pre-exposure prophylaxis of COVID-19 in immunocompromised patients, though in vitro data has shown reduced neutralizing activity against SARS-CoV-2 Omicron subvariants.
Methods
We performed genomic sequencing of SARS-CoV-2 isolated from patients diagnosed with COVID-19 following tixagevimab-cilgavimab. Resistance-associated substitutions were used to generate a predicted phenotypic susceptibility analysis to tixagevimab-cilgavimab and bebtelovimab. Clinical data collected from these patients included SARS-CoV-2 immunization status, COVID-19–directed therapies, and outcomes.
Results
SARS-CoV-2 genome sequencing was performed in 25 patients. SARS-CoV-2 Omicron BA.2 was the most common identified subvariant. All patients had viral isolates with spike codon substitutions associated with reduced susceptibility to tixagevimab-cilgavimab; their predicted phenotypic analysis showed a >2-fold reduced susceptibility to tixagevimab-cilgavimab. Two patients had viral isolates with spike codon substitutions (K444N and G446D) associated with highly reduced susceptibility to bebtelovimab, although all the viral isolates had <2-fold reduced susceptibility based on predicted phenotypic analysis. Sixteen patients received rescue therapy with bebtelovimab, but one patient with BA.2 subvariant harboring K444N mutation died of COVID-19-related complications. Five patients received other COVID-19 therapies and survived. Four had mild or asymptomatic COVID-19 with an uncomplicated course despite not receiving any additional therapy.
Discussion
Multiple SARS-CoV-2 Omicron spike codon substitutions that correlated with reduced susceptibility to tixagevimab-cilgavimab were identified in patients with COVID-19 after receiving this monoclonal antibody. Most patients had an uncomplicated course. The identification of spike codon substitutions conferring resistance to bebtelovimab highlights the importance of performing genomic surveillance to identify new resistant SARS-CoV-2 variants.
Keywords: COVID-19, SARS-CoV-2 Omicron, Monoclonal antibodies, Immunocompromised hosts
1. Background
In 2022, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron became the leading variant causing coronavirus disease 2019 (COVID-19) worldwide [1]. This variant, divided into 5 subvariant lineages (BA.1/BA.2/BA.3/BA.4/BA.5), harbors numerous mutations in the spike protein (S) that enhance transmissibility and enable escape from antibody neutralization [1,2].
Immunization is the primary preventive measure against SARS-CoV-2 infection, but immunocompromised patients have significantly lower seroconversion rates from vaccination compared to the general population, resulting in a higher risk of COVID-19 [3]. Tixagevimab-cilgavimab is a combination of two long-acting anti-spike monoclonal antibodies (mAb) that block the SARS-CoV-2 spike receptor-binding domain and received Emergent Use Authorization from the United States Food and Drug Administration for pre-exposure prophylaxis in immunocompromised individuals [4]. This authorization was based on the result of a phase 3 trial which showed that unvaccinated high-risk patients who received tixagevimab-cilgavimab had a 77% relative risk reduction in the incidence of COVID-19 [5]. However, the study only included a small number of immunocompromised persons and was conducted before the period dominated by the SARS-CoV-2 Omicron variant. In vitro data has shown substantially reduced neutralizing activity of tixagevimab-cilgavimab against these Omicron variant lineages [1,6]. Moreover, the exposure to tixagevimab-cilgavimab may predispose to the selection and appearance of novel variants with S codon substitutions that may reduce the effectiveness of other therapeutic mAb [2,7].
To monitor for its real-world effectiveness and identify the emergence of new treatment-resistant variants, we performed SARS-CoV-2 genomic sequencing on available samples from patients presenting breakthrough COVID-19 after receiving pre-exposure prophylaxis with tixagevimab-cilgavimab. Additionally, we utilized the analysis program of The Stanford Coronavirus Resistance Database (CoV-RDB; https://covdb.stanford.edu) to generate a predicted phenotypic analysis of our viral isolates. This program utilizes a large resistance database collected from different in vitro, animal, and clinical studies to provide a predicted neutralization activity data of different agents used to treat or prevent COVID-19, including mAb, based on the spike protein substitutions from a particular isolate. Herein, we describe the analyses of our recovered viral isolates.
2. Material and methods
2.1. Sample selection
This is a descriptive study of patients presenting with breakthrough COVID-19 after receiving pre-exposure prophylaxis with tixagevimab-cilgavimab at Mayo Clinic in Rochester, Minnesota, from January 7 through August 3, 2022. We included patients who had available SARS-CoV-2 RNA-positive upper respiratory tract swab specimens. Clinical data was collected from electronic medical records and included demographic and clinical characteristics, primary indication for tixagevimab-cilgavimab, SARS-CoV-2 immunization status (partially vaccinated, not completed primary series; fully vaccinated, completed primary series; boosted, received at least one booster dose of SARS-CoV-2 mRNA vaccine), COVID-19–directed therapies, and outcomes. We report our findings using descriptive statistics. The Mayo Clinic Institutional Review Board approved the study protocol and patient consent was waived.
3. Laboratory methods
We performed next-generation sequencing using the commercially available Ion AmpliSeq SARS-CoV-2 Research Panel (Life Technologies Corp., South San Francisco, CA) that amplified and sequenced 99% of the SARS-CoV-2 genome on the automated Genexus™ Integrated Sequencer (Life Technologies Corp.) using the Genexus™ Software version 6.2.1. Viral genomic sequences were analyzed with the web-based Pangolin COVID-19 Lineage Assigner (https://pangolin.cog-uk.io/) and the Stanford Coronavirus Antiviral and Resistance Database for determination of S codon substitutions [8], in reference to the wild-type SARS-CoV-2 Wuhan-Hu-1 sequence. Resistance-associated substitutions previously reported for tixagevimab-cilgavimab and bebtelovimab (a frequently used mAb for rescue therapy during the study period in patients with breakthrough COVID-19 after receiving tixagevimab-cilgavimab) [4,[8], [9], [10]], were analyzed according to the nearest matched in prototype subvariant lineage to generate a predicted phenotypic susceptibility to these mAb [11].
4. Results
During the study period, 1652 patients received tixagevimab-cilgavimab in our center, and 108 patients were diagnosed with COVID-19 at the Mayo Clinic Health System facilities in Minnesota and Wisconsin. The majority of patients were diagnosed with positive home antigen testing, and only 39 patients developed PCR-confirmed SARS-CoV-2 infection after receiving pre-exposure prophylaxis with tixagevimab-cilgavimab. However, only 25 patients had residual specimens (24 with PCR target cycle threshold values of <30.0) available for SARS-CoV-2 genome sequencing (Table 1 ). Eight were solid organ transplant recipients, 8 had an active hematologic malignancy, 5 were hematopoietic stem-cell transplant recipients, and 4 were patients receiving immunosuppressive treatment for an autoimmune disorder. Patients had a median Charlson comorbidity index of 6 (range, 1 – 6). The median time between tixagevimab-cilgavimab prophylaxis and the onset of COVID-19 was 59 days (range, 3–137 days). Most patients (92%) were either fully vaccinated or have been boosted. The majority of patients received the currently recommended dose of 300 mg of each component (except one patient who only received the originally recommended dose of 150 mg tixagevimab and 150 mg cilgavimab). None of the patients had received the follow-up dose of tixagevimab-cilgavimab 300 mg–300 mg which is recommended 6 months after the initial dose.
Table 1.
Clinical characteristics of 25 immunocompromised patients with genomic sequencing of SARS-CoV-2 Omicron subvariants causing breakthrough COVID-19 after receiving tixagevimab-cilgavimab prophylaxis.
| Variables | N = 25 (%) |
|---|---|
| Male | 12 (48) |
| Female | 13 (52) |
| Age in years, median (range) | 63 (25 – 93) |
| Race, Ethnicity -White, non-Hispanic - Asian, non-Hispanic - Black, non-Hispanic |
20 (80) 4 (16) 1 (4) |
| Immunocompromising condition SOT HSCT Hematologic malignancy Autoimmune disorder |
8 (32) 5 (20) 8 (32) 4 (16) |
| Other comorbidities a Chronic kidney disease Diabetes mellitus Obesity Heart failure Chronic liver disease |
11 (44) 9 (36) 7 (28) 5 (20) 3 (12) |
| Charlson comorbidity index, median (range) | 6 (1 – 12) |
| SARS-CoV-2 immunization status Boosted Fully vaccinated Partially vaccinated Not vaccinated |
13 (52) 10 (40) 1 (4) 1 (4) |
| Patients who received tixagevimab-cilgavimab 300/300 mg before COVID-19 diagnosis | 24 (96) |
| Time from last SARS-CoV-2 immunization to COVID-19 diagnosis in days, median (range) | 104 (20 – 414) |
| Median duration in days from the last dose of tixagevimab-cilgavimab to COVID-19 diagnosis (range) | 59 (3 – 137) |
| WHO clinical progression scale, median (range) b | 2 (1 – 10) |
| PCR target cycle threshold value, median (range) c | 22.1 (14.9 – 29.4) |
| SARS-CoV-2 Omicron subvariant lineages BA.1 BA.2 BA.4 BA.5 |
3 12 1 9 |
| Spike protein codon substitutions associated with reduced susceptibility to tixagevimab-cilgavimab and bebtelovimab d Q493R F486V L452R L452Q G446D K444N |
14 11 11 5 1 1 |
| Reduced susceptibility to tixagevimab-cilgavimab based on predicted phenotypic analysis e <2-fold 2-to-10-fold >10-fold |
10 12 3 |
| Reduced susceptibility to bebtelovimab based on predicted phenotypic analysis e <2-fold |
25 |
| Rescue therapy Bebtelovimab Dexamethasone alone Dexamethasone with remdesivir Dexamethasone with remdesivir and tocilizumab f Nirmatrelvir-ritonavir Sotrovimab None |
16 2 1 1 1 1 4 |
| Hospitalization due to COVID-19 | 4 |
| Mortality f | 1 |
Abbreviations: COVID-19, coronavirus disease 2019; HSCT, Hematopoietic stem-cell transplant; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOT, solid-organ transplant.
Patients frequently had one or more comorbidities. Obesity was defined as a body mass index ≥ 30.
As defined by the WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection (Lancet Infect Dis. 2020;20(8):e192-e197).
Cycle threshold values for 24 specimens tested with one of three emergency-use-authorized PCR assays (Cobas SARS-CoV-2 Qualitative, Cobas SARS-CoV-2 & Influenza A/B, and Panther Fusion SARS-CoV-2 assays).
Patients frequently had one or more spike protein codon substitutions.
Predicted phenotypic analysis performed using the Stanford University Coronavirus Antiviral and Resistance Database, SARS-CoV-2 Sequence Analysis report [11].
Patient initially received bebtelovimab after COVID-19 diagnosis but presented progressive disease requiring hospitalization. The patient received this COVID-19 regimen but eventually died.
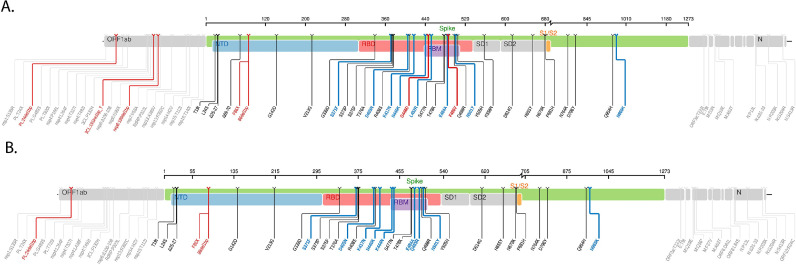
Most patients had breakthrough infection with the Omicron BA.2 subvariant, followed by BA.5 and BA.1. The specific S codon substitutions associated with reduced susceptibility to tixagevimab-cilgavimab and bebtelovimab are described in Table 2 . All patients had viral isolates with one or more S codon substitutions that were associated with reduced susceptibility to tixagevimab-cilgavimab. Two patients (a heart transplant recipient and a patient with refractory chronic lymphocytic leukemia) had viral isolates with S codon substitutions associated with reduced susceptibility to bebtelovimab (K444N substitution associated with >1901-fold reduced susceptibility and G446D substitution associated with 69-fold reduced susceptibility, respectively) (Fig. 1 A and B) [9]. The predicted phenotypic analysis showed that most of the Omicron subvariants had >2-fold reduced susceptibility to tixagevimab-cilgavimab, and all viral isolates had a predicted <2-fold reduced susceptibility to bebtelovimab.
Table 2.
Supplementary material. SARS-CoV-2 Omicron subvariants with spike protein (S) codon substitutions associated with reduced susceptibility to bebtelovimab (BEB) and tixagevimab-cilgavimab (TIX-CIL).
| S codon substitutions associated with reduced susceptibility to BEB and TIX-CIL | BA.1 | BA.1.1 | BA.1.1.529 | BA.2 (n = 6) | BA.2.12 | BA.2.12.1 (n = 4) | BA.2.3 | BA.4.6 | BA.5.1.1 | BA.5.2 | BA.5.2.1 (n = 3) | BA.5.5 (n = 3) | BA.5.6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q493Ra (TIX-CIL 3.4-fold) |
1 | 1 | 6 | 1 | 4 | 1 | |||||||
| F486Vb (TIX-CIL 10-fold) |
1 | 1 | 1 | 1 | 3 | 3 | 1 | ||||||
| L452Rc (TIX-CIL 2–5-fold) |
1 | 1 | 1 | 1 | 3 | 3 | 1 | ||||||
| L452Qc (TIX-CIL 2–5-fold) |
1 | 4 | |||||||||||
| G446Da (BEB 69-fold) |
1 | ||||||||||||
| K444Na (BEB >1901-fold) |
1 |
Abbreviations: BEB, bebtelovimab; S, spike protein; TIX-CIL, tixagevimab-cilgavimab combination.
Note: S codon substitutions associated with reduced susceptibility to either TIX or CIL alone are not listed, since these monoclonal antibodies are not used alone for therapy in clinical settings.
Based on Stanford University Coronavirus Antiviral & Resistance Database SARS-CoV-2 Mutation analysis (https://covdb.stanford.edu/sierra/sars2/by-patterns/) [11].
Based on Yamasoba, et al. report [10].
Fig. 1.
Spike protein mutation map (Stanford University Coronavirus Antiviral & Resistance Database, SARS-CoV-2 sequence analysis report, https://covdb.stanford.edu/sierra/sars2/by-patterns/) of the SARS-CoV-2 Omicron subvariants harboring the S codon substitutions G446D (BA.5.6, Fig. 1A) and K444N (BA.2, Fig. 1B).
Sixteen of 25 patients received rescue therapy with bebtelovimab and only one patient (the heart transplant recipient with BA.2 subvariant harboring the K444N substitution) required hospitalization due to acute hypoxic respiratory failure from COVID-19. This patient received remdesivir, dexamethasone, and tocilizumab with initial improvement of oxygenation, but his course was later complicated by multifactorial shock related to retroperitoneal bleeding and multiorgan failure leading to death. Three patients were diagnosed with COVID-19 at the time of presentation in the hospital due to acute hypoxic respiratory failure (two of them required mechanical ventilation). All of them received treatment with dexamethasone with or without remdesivir and survived. One patient infected with BA.1 subvariant received sotrovimab, and one infected with BA.5.1.1 subvariant received nirmatrelvir-ritonavir; both patients had mild symptoms and recovered after an uncomplicated course.
Four patients with mild or asymptomatic COVID-19 did not receive further therapy; two were out of the eligibility period for receiving therapy, and two declined the offer for additional treatment.
5. Discussion
The genotypic analysis of SARS-CoV-2 Omicron subvariants of our patients with breakthrough COVID-19 showed that all isolates had S codon substitutions that were associated with reduced susceptibility to tixagevimab-cilgavimab. Likewise, the predicted phenotypic analysis of these isolates showed that most of them had a predicted >2-fold reduction in susceptibility to tixagevimab-cilgavimab. These findings are consistent with in-vitro studies [1,6] and a recent publication that reported on the reduced neutralizing activity of tixagevimab-cilgavimab (dose 150 mg–150 mg only) against the Omicron variant in kidney transplant recipients who received this pre-exposure prophylaxis [12].
The majority of our patients with breakthrough COVID-19 received rescue treatment with bebtelovimab. The predicted phenotypic analysis of all viral isolates in this study showed only a <2-fold reduction in susceptibility to bebtelovimab. However, two patients had S codon substitutions that correlated with a highly reduced susceptibility to bebtelovimab, including one who progressed to severe COVID-19 respiratory failure and eventual death. The finding of discordance between genotypic and predicted phenotypic susceptibility in this study is likely due to the exclusion of certain S codon substitutions (K444N and G446D) associated with bebtelovimab resistance in the predicted phenotypic analysis. At present, bebtelovimab is no longer authorized by the U.S. Food and Drug Administration for the treatment of COVID-19 as in vitro data is showing that currently emerging SARS-CoV-2 Omicron subvariants BQ.1 and BQ1.1 are resistant to this mAb [9,13].
A recent study evaluated the presence of S codon substitutions in viral isolates from patients who received tixagevimab-cilgavimab (300 mg–300 mg) for the treatment of mild COVID-19 [7]. All patients were infected with the Omicron BA.2 subvariant. Multiple S codon substitutions were detected, including the substitution K444N, between 7 and 14 days after receiving tixagevimab-cilgavimab. Whether the occurrence of K444N substitution in one of our cases is induced by tixagevimab-cilgavimab is unclear, but a cause-and-effect association needs corroboration with further in vivo and clinical studies. The correlation of real-time genomic surveillance data will help to define the clinical significance and therapeutic implications of this S codon substitution since, at the time of this study, bebtelovimab had been preferred for use in immunosuppressed patients for whom other treatment options were less favorable due to inconvenience (remdesivir), lower efficacy (molnupiravir) or drug-drug interactions (nirmatrelvir/ritonavir).
Our study has several limitations. The relatively small number of analyzed viral sequences did not allow us to make definitive associations between the observed substitutions and clinical outcomes. Secondly, the inference of reduced susceptibility to mAb is based on in vitro studies without considering other host factors such as immunization status and pharmacologic immunosuppression. In addition, the genotypic analyses revealed the presence of some S codon substitutions of unknown clinical significance, but such substitutions may later be found to confer reduced susceptibility to mAb treatment.
In summary, our study found that breakthrough COVID-19 in patients who received pre-exposure prophylaxis was caused by SARS-CoV-2 Omicron subvariants with S codon substitutions associated with genotypic and/or predicted phenotypic reduced susceptibility to tixagevimab-cilgavimab. Most patients with breakthrough infection did not require hospitalization, probably due to multi-layered additive protection afforded by vaccination, tixagevimab-cilgavimab, and rescue treatment with mAb or antiviral drugs. Importantly, the identification of S codon substitutions conferring resistance to bebtelovimab highlights the importance of continued correlation of genomic surveillance for SARS-CoV-2 variants with the clinical outcomes of COVID-19 directed therapies, especially among immunocompromised hosts in whom these passive immunotherapies are commonly administered.
Funding
This work was supported by the Mayo Clinic, Division of Public Health, Infectious Diseases and Occupational Medicine, and the Department of Development.
CRediT authorship contribution statement
Eloy E. Ordaya: Conceptualization, Data curation, Formal analysis, Visualization, Writing – original draft, Funding acquisition. Paschalis Vergidis: Conceptualization, Investigation, Supervision, Writing – review & editing. Raymund R. Razonable: Conceptualization, Investigation, Supervision, Writing – review & editing. Joseph D. Yao: Investigation, Supervision, Resources, Writing – review & editing. Elena Beam: Conceptualization, Investigation, Supervision, Writing – review & editing, Funding acquisition.
Declaration of Competing Interest
Raymund R. Razonable has received grants from Regeneron, Roche, and Gilead for research not directly related to this study. Paschalis Vergidis has received research grants from Ansun, Scynexis, and Cidara and has served on the data and safety monitoring board for AbbVie, Vanda, and Algernon Pharmaceuticals (all fees paid to Mayo Clinic). All other authors report no potential conflicts of interest.
Acknowledgments
We thank Erin Fischer MS, RN from the Division of Clinical Informatics at Mayo Clinic for helping us to identify the cases from the electronic medical records.
References
- 1.Planas D., Saunders N., Maes P., et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602(7898):671–675. doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
- 2.Vellas C., Trémeaux P., Del Bello A., et al. Resistance mutations in SARS-CoV-2 Omicron variant in patients treated with sotrovimab. Clin. Microbiol. Infect. 2022;28(9):1297–1299. doi: 10.1016/j.cmi.2022.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee A., Wong S.Y., Chai L.Y.A., et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376 doi: 10.1136/bmj-2021-068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization for Evusheld (tixagevimab co-packaged with cilgavimab). https://www.fda.gov/media/154701/download. Accessed 23 Aug 2022.
- 5.Levin M.J., Ustianowski A., De Wit S., et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of Covid-19. N. Engl. J. Med. 2022;386(23):2188–2200. doi: 10.1056/NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iketani S., Liu L., Guo Y., et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604(7906):553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vellas C., Kamar N., Izopet J. Resistance mutations in SARS-CoV-2 omicron variant after tixagevimab-cilgavimab treatment. J. Infect. 2022;85(5):e162–e163. doi: 10.1016/j.jinf.2022.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzou P.L., Tao K., Pond S.L.K., Shafer R.W. Coronavirus resistance database (CoV-RDB): SARS-CoV-2 susceptibility to monoclonal antibodies, convalescent plasma, and plasma from vaccinated persons. PLoS ONE. 2022;17 doi: 10.1371/journal.pone.0261045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization for bebtelovimab. https://www.fda.gov/media/156152/download. Accessed 13 Dec 2022.
- 10.Yamasoba D., Kosugi Y., Kimura I., et al. Neutralisation sensitivity of SARS-CoV-2 Omicron subvariants to therapeutic monoclonal antibodies. Lancet Infect. Dis.. 20;22(7):942–943, doi:10.1016/S1473-3099(22)00365-6. [DOI] [PMC free article] [PubMed]
- 11.Stanford University Coronavirus antiviral & Resistance Database . 2022. SARS-CoV-2 Mutations Analysis, MAb Susceptibility Summary.https://covdb.stanford.edu/sierra/sars2/by-patterns/ Accessed 10 Sept. [Google Scholar]
- 12.Benotmane I., Velay A., Gautier-Vargas G., et al. Pre-exposure prophylaxis with 300 mg Evusheld elicits limited neutralizing activity against the Omicron variant. Kidney Int. 2022;102(2):442–444. doi: 10.1016/j.kint.2022.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Planas D., Bruel T., Staropoli I., et al. Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies. bioRxiv [Preprint] 2022 doi: 10.1101/2022.11.17.516888. Nov 21:2022.11.17.516888. [DOI] [PMC free article] [PubMed] [Google Scholar]