SUMMARY
In mammals, learning circuits play an essential role in energy balance by creating associations between sensory cues and the rewarding qualities of food. This process is altered by diet-induced obesity, but the causes and mechanisms are poorly understood. Here we exploited the relative simplicity and wealth of knowledge about the D. melanogaster reinforcement-learning network, the Mushroom Body, to study the relationship between the dietary environment, dopamine-induced plasticity, and food associations. We show that flies fed a high-sugar diet cannot make associations between sensory cues and the rewarding properties of sugar. This deficit was caused by diet exposure, not obesity, and specifically by lower dopamine-induced plasticity onto Mushroom Body Output Neurons during learning. Importantly, food memories dynamically tune the output of MBONs during eating, which remains fixed in sugar-diet animals. Interestingly, manipulating the activity of MBONs influenced eating and fat mass depending on the diet. Together, this work advances our fundamental understanding of the mechanisms, causes, and consequences of the dietary environment on reinforcement learning.
Keywords: taste, nutrition, sensory plasticity, reinforcement learning
INTRODUCTION
Learned associations between sensory cues and rewarding qualities of food, such as taste or nutrient content, are thought to affect eating by providing animals with expectations about how much to consume 1–3. In mammals, diets high in saturated fat and refined sugar – which increase eating and promote metabolic disease 4–11– are associated with impairments in food associations 3,12–21. The causes and mechanisms through which the dietary environment perturbs the neural processes that underlie the formation of food memories are poorly understood. First, we do not know if deficits in associative learning develop due to dietary exposure to drive obesity or if, instead, they are a consequence of it. Second, the extent to which diet- and obesity-related alterations in the mesolimbic circuitry, especially those in dopaminergic signaling and transmission, disrupt food associations is unclear 22–32. Here we exploited the unique advantages of the D. melanogaster model to study the relationship between the dietary environment and food reinforcement learning.
Similar to vertebrates, flies fed high sugar or fat diets consume more food during eating episodes, accumulate fat, and develop hallmarks of metabolic syndrome and chronic disease 11,33–35. our previous work found that a high sugar diet decreased the responses of dopaminergic neurons (DANs) innervating the β′2 compartment of the Mushroom Body – the associative learning center in flies; we further established that this decrease drove higher eating and fat accumulation34. Since these DANs are essential for food reinforcement learning, we reasoned that this perturbation could disrupt food associations. In flies, food associations form when animals experience a sensory cue at the same time or shortly after food; since food is a potent reward, this pairing assigns a positive value to the cue and promotes animals' interaction with it 36,37. The creation of these food associations involves neural circuits that converge around specific compartments of the Mushroom Body 38. Sensory cues are processed through the principal cells of the Mushroom Body, the Kenyon Cells, while DANs located in the Protocerebral Anterior Medial cluster signal rewarding aspects of food 39–41. The convergence of these sensory and reinforcement signals onto an ensemble of six Mushroom Body Output Neurons (MBONs) with dendrites in the β′2 and γ5 Mushroom Body compartments promotes the formation of an association between the sensory cue and food by changing the strength of the Kenyon Cell-MBON synapse 42,43. At the neural level, this DA-induced plasticity changes the neural responses of the MBONs to the cue, resulting in the fly approaching it.
In this work, we demonstrate that a high-sugar diet disrupts the formation of food associations by decreasing the strength and timing of dopamine-induced plasticity of the MBON-Kenyon Cell synapse. This effect is not caused by excess fat mass but by alterations in dopamine reinforcement that arise from changes in peripheral taste sensation with diet. These learning deficits prevent the tuning of MBONs activity during eating. Using a closed loop optogenetic system, we also reveal that MBON activity affects feeding and energy balance. Together, our experiments establish how diet-dependent deficits in dopamine plasticity affect reinforcement learning and suggest a model for how the activity of food-associative circuits may play a role in eating behavior and metabolic disease.
RESULTS
A high-sugar diet impairs the formation of food memories independently of fat accumulation.
To investigate the effects of diet on food associations in D. melanogaster, we exposed male flies to an established model of diet-induced obesity where 30% sucrose is added to the animal diet for seven days 33,35 and tested the animal’s ability to associate an odor with sucrose. Chronic exposure to this diet leads to higher feeding and fat accumulation, as well as other behavioral and metabolic changes, recently reviewed in11. To measure food associations, we employed the appetitive conditioning assay 36,37,44 using a horizontal t-maze 45. In the training phase of this assay (when the flies are being conditioned), untrained (naive) fasted flies are sequentially exposed to two odors for 2 minutes: one odor (0.1% 4-methyl cyclohexanol, MCH, conditioned stimulus, CS+) is delivered in the presence of 2M sugar (unconditioned stimulus, US), while the other odor, (0.1% 3-octanol, OCT, CS−) is delivered with water (Figure 1A, top); to ensure that this increase in performance index (PI) is not due to odor preference, the sugar pairing is reversed in different animals (odor 2=CS+, not shown in Figure 1A). In the testing phase, which occurs after 2 minutes of rest, the conditioned/trained flies are given a choice between these two odors, and a PI is calculated (Figure 1A, bottom) 39,41,43. Before conditioning, w1118CS (wCS) control untrained flies fed a control diet have no preference for either odor (Figure 1B, gray), but after training, they prefer the odor associated with sugar, CS+ (Figure 1C, gray). Although naive animals fed a sugar diet for seven days had olfactory behavioral responses to MCH and OCT identical to naive control diet flies (Figure 1B, teal), they failed to develop a preference for the CS+ after conditioning (Figure 1C, teal). To rule out the possibility that this learning deficit could be due to lower motivation, we fasted sugar diet flies for longer times (30, 36, and 48 hrs) to account for higher fat stores 33,46. These flies, however, still showed no food memory formation compared to control diet flies (Figure S1A). To test whether this deficit in food associations was due to sugar diet exposure or fat accumulation, we tested perilipin2 (plin2) mutants, which remain lean even on a sugar diet 33 (Figure S1B). plin2 mutants fed a control diet had comparable PI to control flies but failed to form a food association when fed a sugar diet (Figure 1C). Together, these observations reveal that consumption of a high-sugar diet impairs the formation of food associations and that dietary exposure even in the absence of fat accumulation is sufficient for this phenotype.
Figure 1: A high sugar diet impairs the formation of food memories independently of fat accumulation.
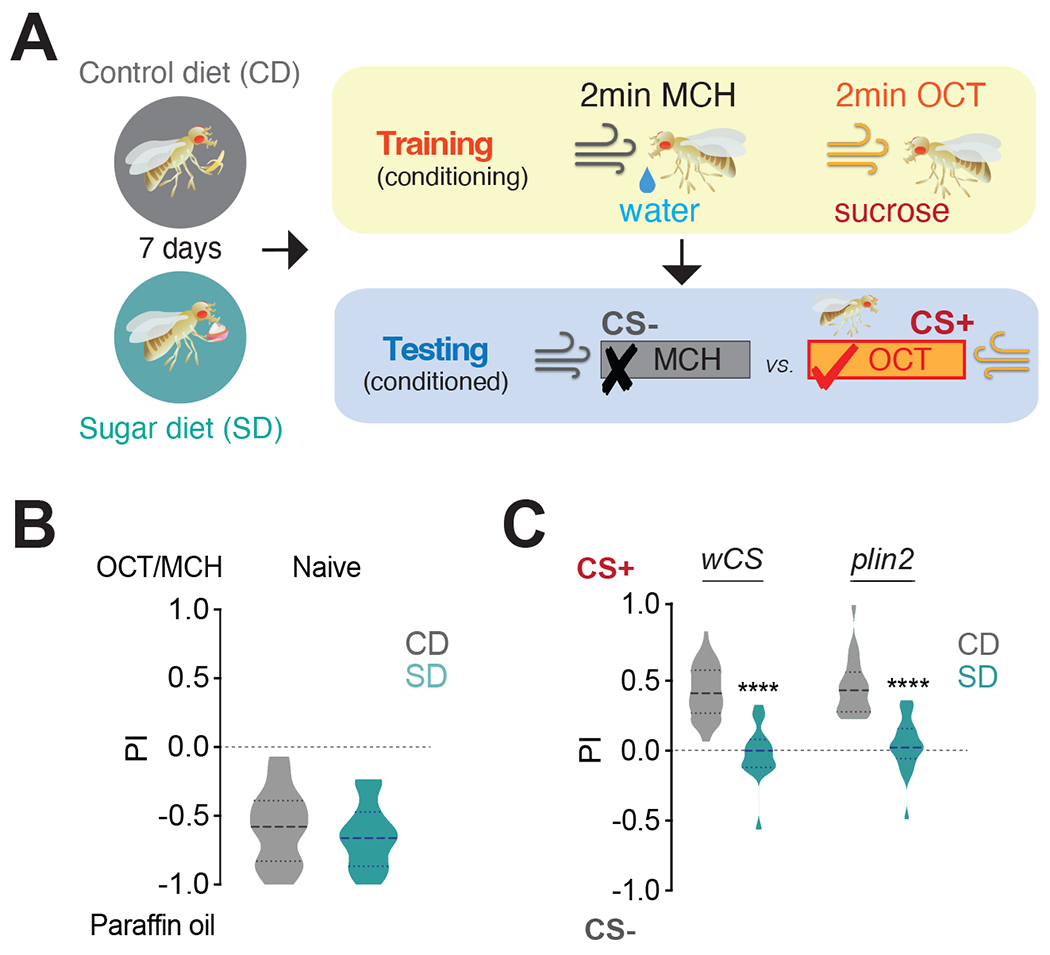
A) Schematic of appetitive conditioning: flies were fed a Control (CD) or Sugar Diet (SD) for 7 days, trained to pair an odor with either water (CS−) or sucrose (CS+), and then tested for the preference between the CS− or CS+; odors, MCH, 4-methylcyclohexanol and OCT, 3-octanol.
B) Performance index (PI) for naive olfaction between paraffin oil and MCH or OCT in wCS flies fed a CD (gray) or SD (teal). Data consist of a combined set with half of the flies tested with OCT and the other half with MCH. n=24, 1n=25 flies, Mann-Whitney test. The thicker dotted line in the violin plot shows the mean.
C) The PI of control wCS (n=53 CD, n=17 SD) and obesity-resistant plin2 mutant flies (n=22) on CD (gray) and SD (teal). Kruskal-Wallis with Dunn’s multiple comparison test, ****p<0.0001.
See also Figure S1.
Exposure to a high-sugar diet abolishes the neural signatures of food associations.
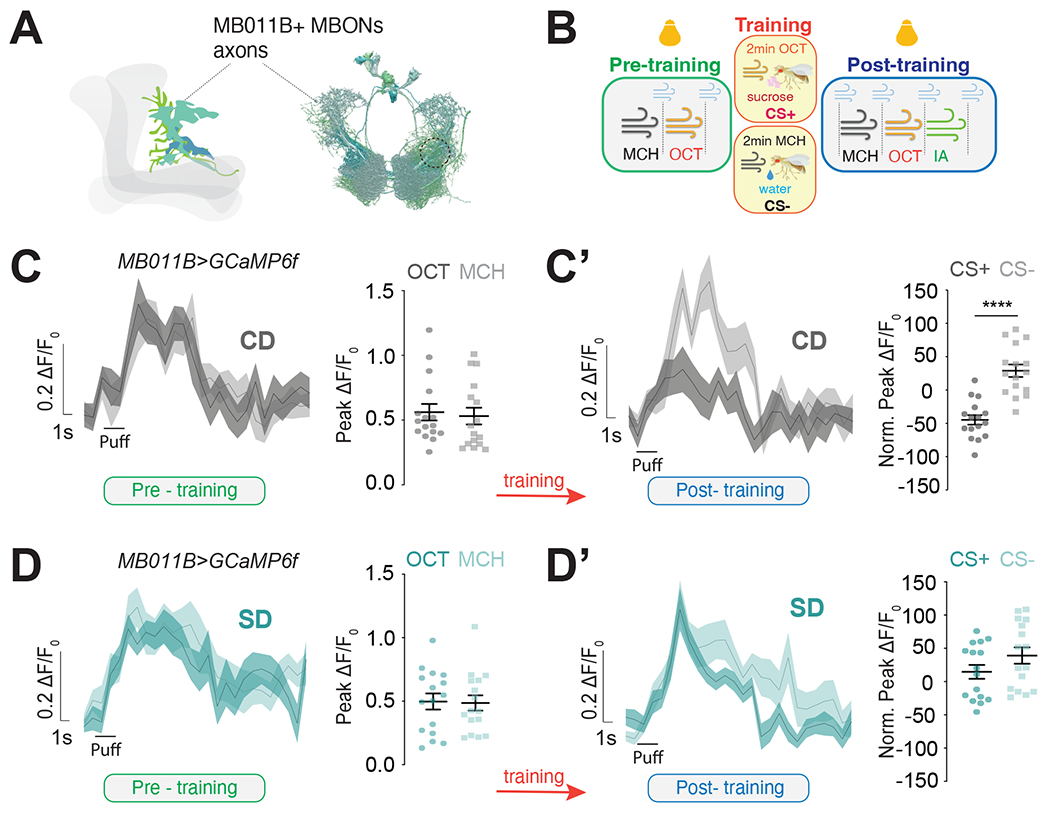
The formation of these food associations requires the activity of a group of six glutamatergic Mushroom Body Output Neurons (MBONs) that have dendrites in the γ5β′2a, β′2mp, β′2mp_bilateral compartments of the Mushroom Body and are labeled by the MB011B GAL4 (Figure 2A)47,48. These neurons receive odor information from Mushroom Body Kenyon Cells and sugar-taste reward from PAM DANs that innervate the β′2 compartment of the Mushroom Body; all of these circuits are required for this type of food association. Changes in the magnitude of β′2 MBON's dendritic responses to odors before and after appetitive conditioning underlie the formation of food associations; specifically, studies showed that conditioning lowers the dendritic responses to the CS+ while leaving responses to the CS− unchanged (or slightly increased) compared to naive flies 42,43,48. Since food associations are absent in sugar diet flies, we hypothesized that this learning-induced plasticity should also be impaired. To test this possibility, we modified an existing protocol 49 to measure the odor responses of MB011B+ β′2 dendrites (Figure 2A, mid and right) before and after conditioning in the same animal using the genetically encoded fluorescent calcium indicator UAS-GCaMP6f50. As the schematic in Figure 2B shows, the calcium responses to MCH and OCT were assessed in fasted MB011B>GCaMP6f flies before (pre-training, naive, green) and after conditioning (post-training, conditioned, blue); the protocol for sucrose conditioning was identical to that of the t-maze assay in Figure 1, with the exception that it occurred under the two-photon microscope so that the neural responses to learning could be monitored in real-time (see Methods; in Figure 2B the light bulb represents image acquisition). Naive MB011B>GCaMP6f flies fed a control diet showed robust and equal post-synaptic responses to both odors before training (Figure 2C); these were changed by conditioning with a decrease in CS+ responses and a small but significant increase in CS− after training (Figure 2C’, dark gray; see Figure S2A for pre/post training comparisons within CS+ and CS−). Naive MB011B>GCaMP6f flies fed a sugar diet also presented similar strong responses to both odors before conditioning (Figure 2D); however, there were no detectable changes between CS+ and CS− after training, and interestingly, both CS+ and CS− increased with training (Figure 2D’; Figure S2B). To directly compare the effects of diet on CS+ and CS− β′2 dendritic odor responses before (Supplement 2C and D) and after conditioning (Figure 2E and F), we plotted the difference in the magnitude of CS− and CS+ responses across the two diets. This analysis revealed that responses to CS− and CS+ odors in untrained, naive flies were identical in control diet and sugar diet flies (Figures S2C and D). Thus, high sucrose does not affect these MBONs responses before training; these observations are consistent with the finding that olfactory behavioral responses of naive flies were similar across dietary treatments (Figure 1B). In contrast, while there were no significant differences in the normalized CS− responses between diet groups (Figure 2E; also see Figures S2A and B), the normalized CS+ responses in conditioned flies were higher for the sugar diet compared to control diet flies (Figure 2F and compare S2A and B). Thus, a sugar diet affected the responses of the MBONs only after conditioning and only to the CS+, suggesting that these effects are specific and not the result of the indiscriminate action of diet on MBON activity. We also observed no changes in the calcium responses to a third, unrelated odor, isobutyl acetate, regardless of diet (Figure S2E), further showing that the absence of CS+ dendritic depression with a sugar diet was not due to nonspecific alterations in these MBONs. Together, these observations support the idea that a high-sugar diet disrupts food reinforcement learning at both the neural and behavioral levels.
Figure 2: Exposure to a high sugar diet abolishes the neural signatures of food associations.
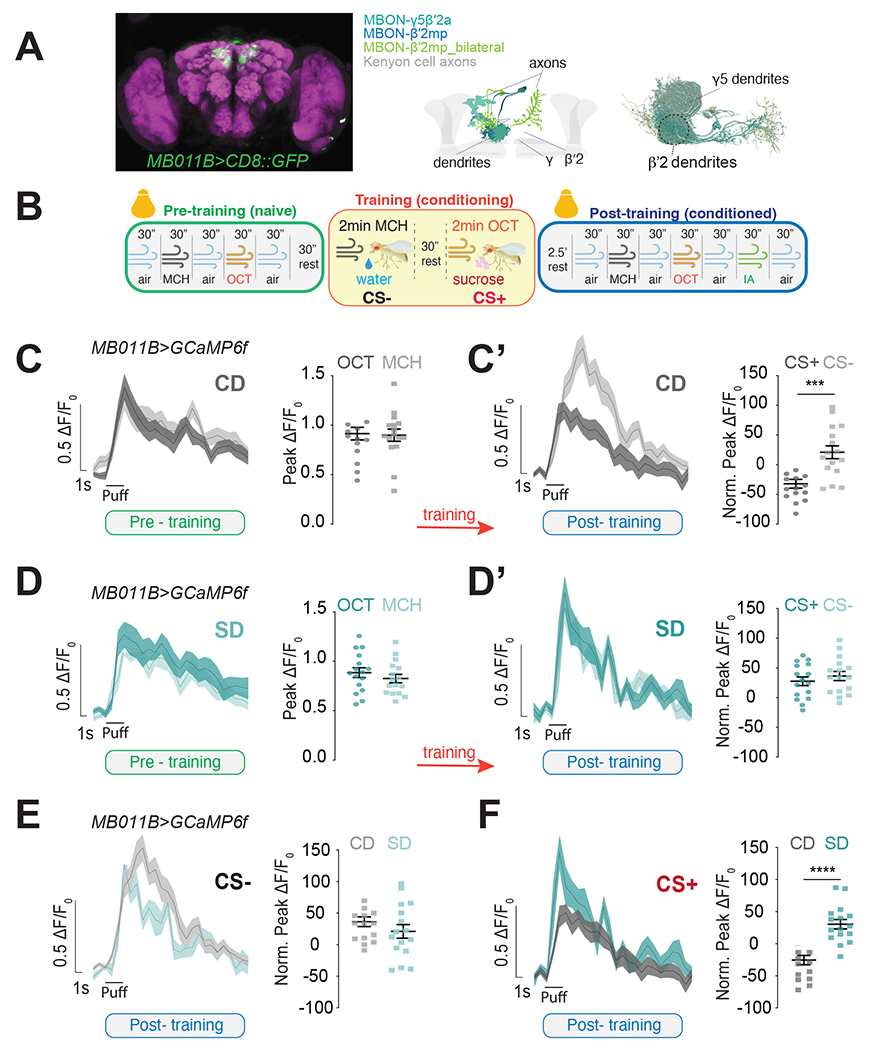
A) (left)) Maximum intensity projection showing the expression of MB011B>CD8::GFP (green) in the fly brain, co-labeled with an antibody against bruchpilot to label the presynapses (magenta). (Mid) Diagram of the different types of MBONs labeled by the MB011B-GAL4 in shades of blue (MBON-γ5β′2a, MBON-β′2mp and MBON-β′2mp_bilateral) and the Kenyon Cells (KCs) axons in gray . Connectome reconstruction of MB011B+ MBONs with region used for imaging experiments shown inside the dotted circle. Only cells in one brain hemisphere are shown for clarity.
B) Schematic of the appetitive conditioning protocol under the 2-photon microscope in pre-training (naive, green), training (yellow), and post-training (conditioned, blue) phases; bulbs represent imaging; reverse pairing not shown for clarity.
C-D’) The calcium responses to MCH and OCT (puff) in the β′2 dendrites of MB011B>GcAMP6f neurons before (C, D, green) and after (C’, D’ blue) training (orange arrow) in Control Diet, CD (C-C’) and Sugar Diet, SD (D-D’) flies.
E, F) Comparison of CS− (E) and CS+ (F) responses in the β’2 dendrites of CD (gray) and SD (teal) MB011B>GcAMP6f flies after training (data from C’ and D’).
Data are shown as mean +/− SEM, ΔF/F0 traces and quantified as maximum peak ΔF/F0 response (pre-training) or normalized to naïve responses (post-training). n=16 (n=8 with OCT as the CS+, and n=8 as MCH as the CS+); Student’s t-test; ***p<0.001, ****p<0.0001.
See also Figure S2.
A sugar diet decreases dopamine-mediated plasticity in MBONs during associative learning.
Our data indicate that the deficit in forming food associations results from the absence of MBON β′2 dendritic plasticity during learning. We next examined the causes of these impairments by asking whether they arise from alterations in Kenyon Cells or DAN transmission. To assess Kenyon Cells transmission, we expressed UAS-GCaMP6f in Kenyon Cells using OK107-GAL4 and measured their responses to MCH and OCT. The β′2 presynaptic odor responses of Kenyon Cells in OK107>GCaMP6f flies were indistinguishable between control diet and sugar diet-fed flies (Supplementary Figure 3A–C), consistent with our observation that the naive calcium responses of MB011B+ MBONs to odors were unchanged by diet (Supplementary Figure 2C and D). This corroborates the idea that there are no appreciable deficits in olfactory processing, which is also supported by data in Figure 1B showing no changes in the olfactory behavioral response to odors in sugar diet and control diet naive flies.
We thus hypothesized that learning deficits might instead arise from a decrease in DA transmission to the β′2 compartment (Figure 3A, pink), a necessary and sufficient signal to reinforce the formation of food associations 39,41–43. This hypothesis is based on our published data showing that the β′2 presynaptic responses to sucrose of a population of PAM DANs synapsing onto MB011B+ MBONs were lower in sugar diet flies 34, and in line with data from rodents showing changes in DA transmission and signaling with high fat and/or high sugar diets 14,32,51–54. To test this hypothesis, we expressed the genetically encoded fluorescent indicator of DA-transmission UAS-GRABDA 55 in MB011B+ neurons and measured the magnitude of the incoming DA signal onto the β′2 dendrites when the fly proboscis was stimulated with 30% sucrose. This revealed a substantial decrease in DA-signal onto MB011B+ β′2 dendrites in sugar diet flies compared to controls (Figure 3B), supporting the hypothesis that a sugar diet lowers the strength of the sucrose reinforcing DA signal onto these MBONs. This difference in reinforcement delivery between the two diets should affect the extent of DA-induced plasticity during learning. To directly test this idea, we measured the effects of diet on the DA-signal in MB011B+ β′2 dendrites during learning using the in vivo conditioning and imaging protocol described in Figure 2B. In untrained flies, MCH and OCT elicited robust fluorescent GRAB-DA signals, which increased after conditioning when the odor was paired with sugar (CS+) (Figure 3C vs. C’), showing potentiation of the paired odor, transfer of the reward to the odor cue, and devaluation of the odor paired with water (Figure S3D); interestingly, responses to CS+ also seem faster compared to those of the CS−. This pattern, however, was reversed in sugar diet animals: although the odors elicited DA-mediated responses in MB011B+ β′2 dendrites (Figure 3D, green), CS+ responses decreased with learning while those to the CS− did not change (Figure S3); this resulted in lower CS+ responses compared to the CS− in conditioned flies (Figure 3D’). To directly compare the effect of diet, we plotted the control and sugar diet responses before (Figure S3 F–G) and after (Figures 3E and F) conditioning across the two groups. The DA-signal before conditioning were identical between control and sugar-diet flies, but after conditioning, they showed opposite changes: relative CS− responses were higher in sugar-diet flies, while CS+ responses were higher in control-diet animals (Figures 3E and F). Responses to a third unrelated odor, Isobutyl acetate, were unaffected by diet (Figure S3H). These observations indicate that 1) the rewarding DA signal reaching the MBONs is lower in animals on a sugar diet, and 2) this decreases the reinforcing effects on the CS+. Interestingly the CS+ responses in sugar-diet flies look similar to those of the CS− in control diet animals (Figure S3D and E).
Figure 3: A sugar diet decreases dopamine-mediated plasticity onto MBONs during learning.
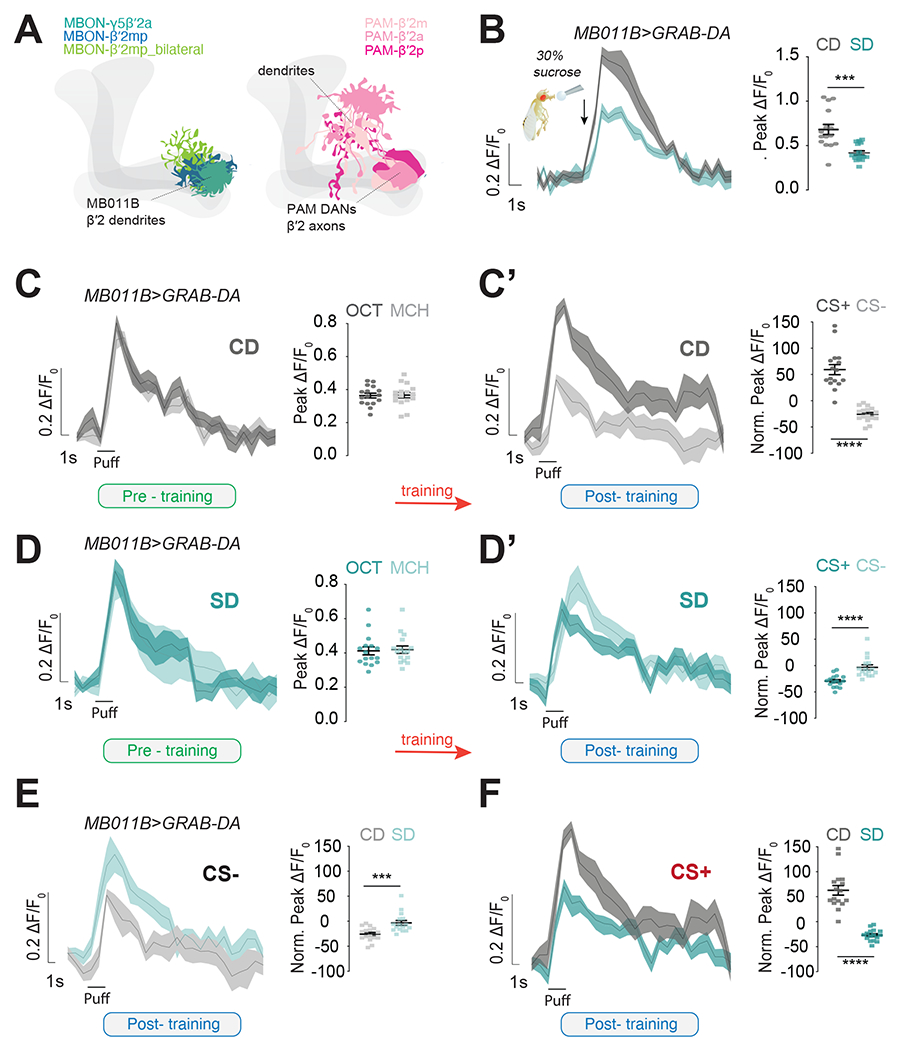
A) Graphics showing the dendrites of MB011B+ MBONs (blue/green shades) and the axons and dendrites of β’2 PAM DANs (pink shades).
B) Mean ΔF/F0 traces and quantification of maximum peak ΔF/F0 response to stimulation of the proboscis with 30% sucrose (arrow) in the β’2 dendrites of Control Diet, CD (gray) and Sugar Diet, SD (Teal) MB011B>GRAB-DA flies. n=15-16, Mann-Whitney test, ***p<0.001.
C-D’) The mean ΔF/F0 traces and quantification of maximum or normalized peak ΔF/F0 response to OCT or MCH in the β’2 dendrites of CD (C-C’, gray, Student’s t-test) and SD (D-D’, teal; left, Student’s t-test and right Mann-Whitney test) MB011B>GRAB-DA flies before training (C and D, naive, green) and after training (C’ and D’, conditioned, blue); ****p<0.0001.
E, F) Comparison of CS− (E, Mann-Whitney Test) and CS+ (F, Student’s t-test) responses in the β’2 dendrites of CD (gray) and SD (teal) MB011B>GRAB-DA flies after training (data from C’ and D’). n=16, ***p<0.001, ****p<0.0001.
Half of the flies experienced OCT=CS+ and half MCH=CS+. Data are shown as mean +/− SEM.
See also Figure S3.
The observation that less DA reaches the β′2 dendrites of MB011B+ MBONs in sugar-diet flies is consistent with our published data that this diet blunted the presynaptic calcium responses of β′2 PAM DANs to sweetness 34. This effect was caused by changes in the sensitivity of the peripheral taste system to sucrose 34 that occurs in flies and rodents fed high sucrose 33,56–60. Thus, diet-dependent perturbations in β′2 DA-to-MBON transmission may underlie the deficits in forming food associations. If that were the case, we expect that correcting β′2 PAM DAN activity in sugar-diet flies would result in normal neural responses during conditioning. We previously restored β′2 PAM DAN responses to sucrose by treating flies with an inhibitor of the metabolic enzyme O-GlcNac Transferase (OGT), which acts in the sensory neurons to decrease sweet taste sensation in response to sugar diet 33,34 (Figure 4A). MB011B>GRABDA flies treated with 10uM of the OGT small-molecule inhibitor (OSMI-1) while on a control diet showed the expected increase in CS+ and decrease in CS− post-training (Figures 4B and Figure S4A, gray) with no effect on naive responses (Figure S4B). Strikingly, supplementing the high sugar diet with OSMI-1 resulted in a potentiation of CS+ and a decrease in CS− responses with training comparable to control diet flies (Figures 4C; Figure S4A, teal) with no effect on naive responses (Figure S4D). Thus, OSMI-1 treatment rescued learning-induced DA plasticity onto the β′2 MBON dendrites; this supports the hypothesis that lower β′2 DA signals underlie learning impairments. If that were the case, we would expect OSMI-1 treatment to restore the neural signatures of learning to control-diet levels and show depression in CS+ calcium responses after conditioning. To test this possibility, we measured the responses before and after associative learning of MB011B>GCaMP6f flies on a control and sugar diet +OSMI-1. This treatment abolished the effects of the sugar diet on the post-training CS+ responses, resulting in a similar magnitude of CS+ depression in both control and sugar diet flies (Figures 4D–E; Figure S4E), without changes in the pre-training responses or to a third unrelated odor (Figure S4 F–H).
Figure 4: Correcting DA-induced plasticity restores appetitive learning in sugar-diet flies.
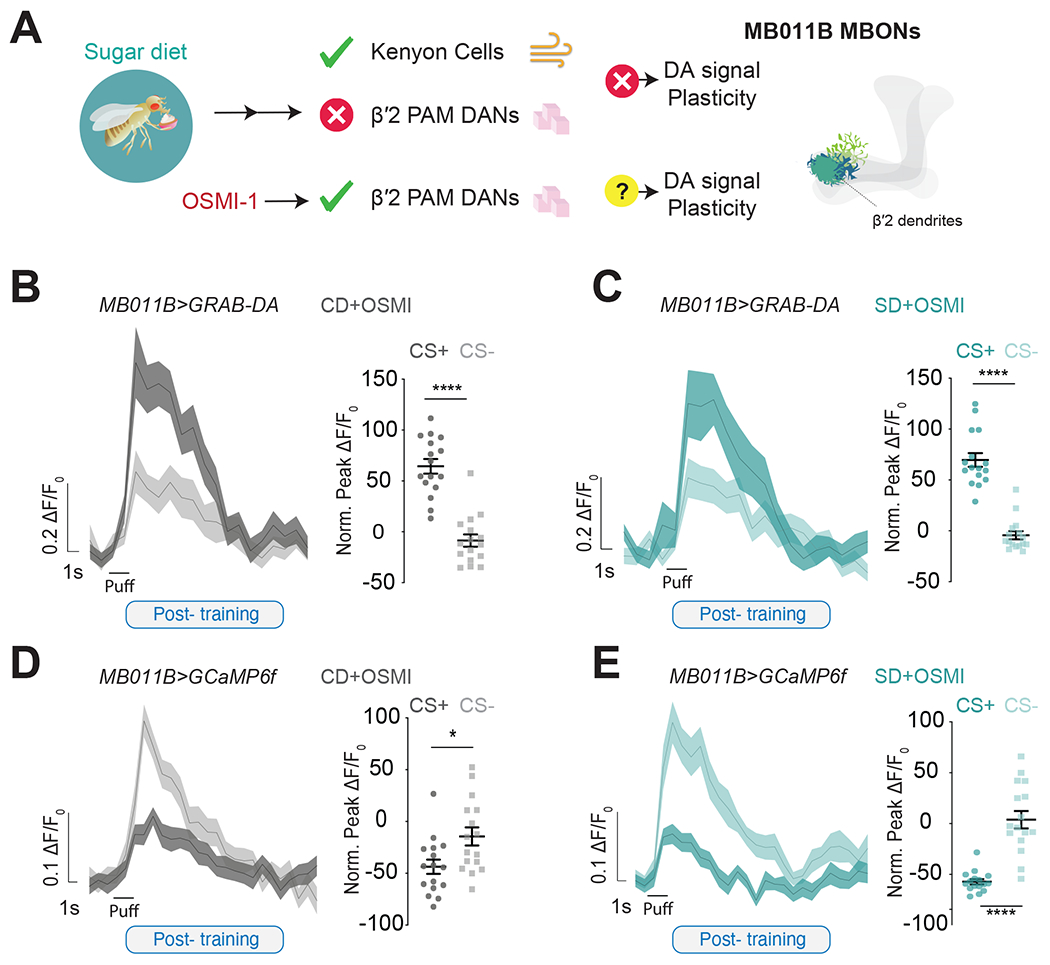
A) (left) Schematic representation of the action of OSMI-1 on the reinforcing signal carried by β’2 PAM DANs and (right) a diagram showing the dendrites in the β’2 region where imaging occurred.
B, C) The mean ΔF/F0 traces and quantification of normalized peak ΔF/F0 response to CS+ or CS− in the β’2 dendrites of Control Diet, CD+OSMI-! (B, gray, Student’s t-test, ****p<0.001) and Sugar Diet, SD +OSMI-1 (C, teal, Mann-Whitney test, ****p<0.001) MB011B>GRAB-DA flies after training. n=16, (half of the flies experienced OCT=CS+ and half MCH=CS+).
D, E) The mean ΔF/F0 traces and quantification of normalized peak ΔF/F0 response to CS+ or CS− in the β’2 dendrites of CD+OSMI-1 (D, gray, Student’s t-test, *p<0.01) and SD+OSMI-1 (E, Student’s t-test, ****p<0.001) MB011B>GCaMP6f flies after training. n=16, (half of the flies experienced OCT=CS+ and half MCH=CS+).
Data shown as mean +/− SEM.
We next tested whether the deficit in food associations was linked to diet-dependent changes in the activity of the ~12 β′2 PAM DANs labeled by MB301B-GAL4. Optogenetic activation during conditioning of experimental MB301B>Chrimson flies had no effect on associative learning of control-diet flies but restored that of sugar-diet flies to control levels; this was in contrast to the lack of food associations seen in the no retinol controls (Figure S5A). Thus, pharmacological and optogenetic correction of the DA-transmission onto MB011B+ neurons restored the neural and behavioral signatures of STM in sugar diet-flies. Together, these data support the idea that alterations in DA-plasticity during learning cause deficits in food associations.
Food associations shape the output of MBONs during eating.
According to the satiety cascade framework, food associations provide animals with salient cues during eating 2,61,62. Learning changes the postsynaptic responses of MB011B+ MBONs to guide the animal's behavior based on experience 41–43,48,63; we thus reasoned that food associations might shape the output of MB011B+ MBONs during eating. To test this hypothesis, we first recorded odor responses from MB011B>GCaMP6f axons during the same conditioning protocol used above (Figure 5A and B). Similar to our measurements in the dendrites, the neural signatures of associative learning were visible in the MB011B+ axons as a decrease in CS+ and a slight increase in CS− responses after conditioning, which resulted in a relative change between the two stimuli (Figure 5C vs. C’; Figure S5B). Exposure to a sugar diet abolished the decrease in presynaptic CS+ responses and the relative change between CS+ and CS− with associative learning, consistent with the absence of DA-induced plasticity (Figure 5D vs. D’; Figure S5B). Significantly, when directly compared to the control diet, the sugar diet only affected presynaptic responses after conditioning (Figure S5E–F) without any effects on the odor responses of naive animals or conditioned animals to a third odor (Figure S5C–D, G).
Figure 5: Learning changes the presynaptic responses of the MB011B+ MBONs to cues.
A) Graphic (left) and connectome reconstruction (right) of MB011B+ β’2mp axons; in the graphic only cells in one hemisphere are shown; dotted circle shows the region imaged.
B) Schematic of the appetitive conditioning protocol under the 2-photon microscope in pre-training (naive, green), training (yellow), and post-training (testing, blue) phases; yellow bulbs represent imaging.
C-D) The calcium responses to MCH and OCT (puff) in the β′2mp axons of MB011B>GcAMP6f neurons before (C and D, green) and after (C’ and D’, blue) training (red arrow) in Control Diet, CD (C-C’) and Sugar Diet, SD (D-D’) flies. Data are shown as mean +/− SEM ΔF/F0 traces and quantified as maximum peak ΔF/F0 response (pre-training) or normalized to naive responses (post-training). C-C’) left Mann-Whitney and right student t-test, D-D’) left student t-test and right Mann-Whitney test; ****p<0.0001.
See also Figure S5.
To then examine the effects of food learning on MBONs activity during eating, we used the experimental design of Figure 5 to measure the MBON's responses to odors both before and after animals ate 2M sucrose for 30 minutes (Figure 6A). We confirmed that appetitive conditioning resulted in a decrease in CS+ with respect to CS− after training in control diet fasted flies (Figure 6B, fasted, light pink); however, the difference between CS+ and CS− disappeared after flies consumed sucrose for 30 minutes (Figure 6B, fed, dark pink). This argues that, although learning shapes the output of the MBONs, this activity is tuned during eating. Since flies on a sugar diet cannot make associations between sugar and odor cues, we would expect this to also prevent shifts in MBON activity during eating. Indeed, we observed no changes in odor responses between fasted and sated sugar-diet flies (Figure 6C), despite ascertaining that they consumed food to similar levels to control diet flies (Figure S6C). Comparing CS− and CS+ responses between diets revealed that only the CS+ responses were changed, while CS− were identical (Figure S6A and B). We also did not observe any changes in the baseline responses to CS+ and CS− before and after eating regardless of diet treatment (Two-way ANOVA, control diet, p=0.9952 and sugar diet, p=0.7071). Together these results indicate that learning changes the activity of MBONs during eating but that a sugar diet prevents this dynamic change.
Figure 6: Learning shapes the output of MBONs during eating.
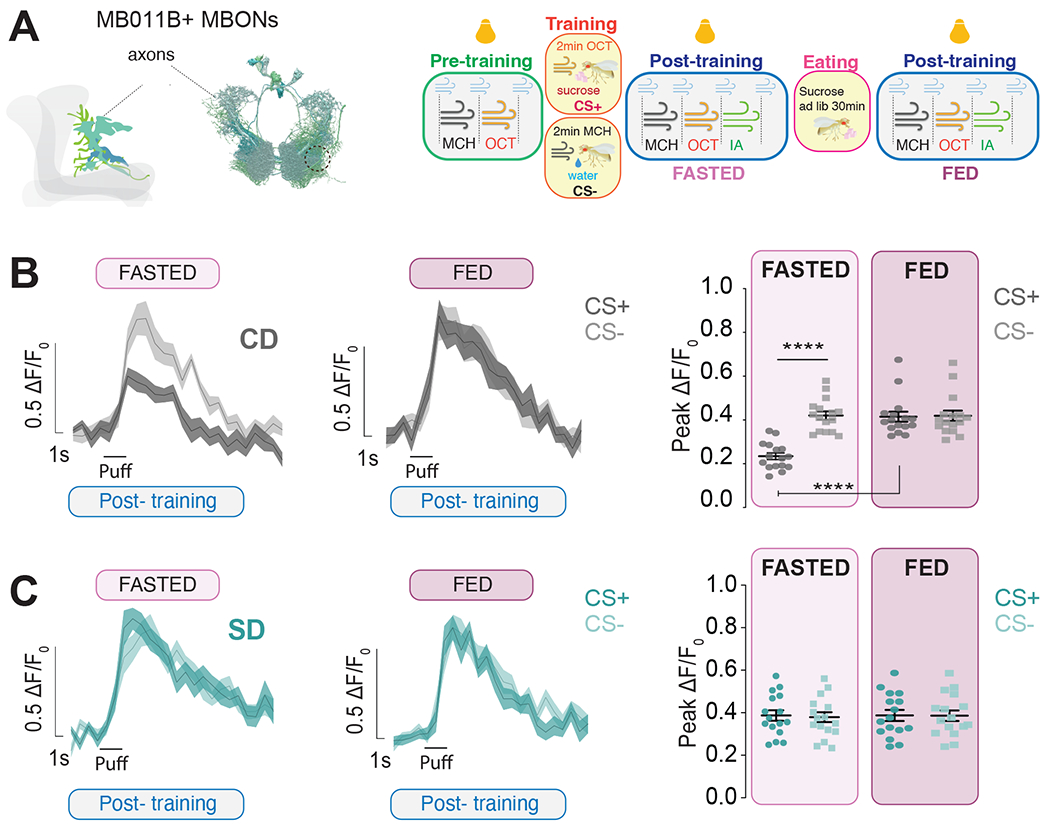
A) Left, Graphic and connectome reconstruction of β’mp axons of MB011B MBONs; in the cartoon, only cells in one hemisphere are shown; dotted line shows the region imaged. Right, Schematic of the appetitive conditioning protocol under the 2-photon microscope in pre-training (naive, green), training (yellow), and post-training (testing, blue) phases, 30 minutes apart, one before (light pink) and one after eating 2M sucrose (dark pink); yellow bulbs represent imaging and blue puffs air.
B-C) The calcium responses to MCH and OCT (puff) in the β′2mp axons of MB011B>GcAMP6f neurons post training but before (light pink, fasted) or after (dark pink, fed) consuming sucrose in Control Diet, CD (B) and Sugar Diet, SD (C) flies; n=16, data are shown as mean +/− SEM, B) ****p<0.0001 CD fasted CS+ vs fasted CS− (student’s t-test) and CD fasted CS+ vs fed CS+ (Wilcoxon test); ns, CD fed CS+ vs fed CS− (Mann-Whitney test), CD fed CS+ vs fed CS− (Wilcoxon test); C) respective comparisons ns, (Student’s t-test).
See also Figure S6.
The activity of MB011B+ MBONs influences eating and energy balance.
Since we observed changes in the presynaptic responses of MBONs to the cues between fasted and refed flies, we asked whether activating or inhibiting the MBONs affected feeding behavior. To manipulate the activity of MBONs only when the fly was eating, we used a closed-loop light delivery system in the Fly-to-Liquid-Food Interaction Counter (FLIC), which records the interactions of individual animals with food 33,34. Optogenetic activation with the light-gated cation channel UAS-CsChrimson 64 while flies are a control diet (5% sucrose) did not affect eating (Figure S7A and B). However, activating the neurons while the flies ate a high sugar diet (20% sucrose) resulted in lower food interactions (licks) in MB011B>CsChrimson +retinal experimental flies (blue) compared to no retinal (gray) (Figure 7A) and UAS-CsChrimson alone controls (Figure 7B). In contrast, optogenetic inhibition of activity with the anion opsin UAS-GtAcR1 65 on the control diet led to MB011B>GtAcR1 +retinal experimental flies licking more compared to no retinal (Figure 7C, blue vs. gray) and UAS-GtAcR1 controls (Figure 7D). Thus, manipulating the activity of MB011B+ MBONs influenced feeding depending on the dietary experience of the animal. To test if the activity of MBONs also affected energy homeostasis, we measured the fat and lean mass of flies with activated or inactivated MB011B+ neurons. Consistent with the feeding behavior data, activating MB011B+ neurons in flies fed a control diet did not affect the fat/lean mass of experimental MB011B>NaChBac flies compared to controls; however, this manipulation protected MB011B>NaChBac flies from diet-induced obesity while on a sugar diet (Figure 7E). In contrast, MB011B+ inhibition resulted in flies with higher fat-to-lean mass than controls (Figure 7F). Together, these findings establish that the activity of MB011b+ neurons affects eating and energy balance depending on the dietary condition.
Figure 7: The activity of MB011B+ MBONs affects eating and energy balance.
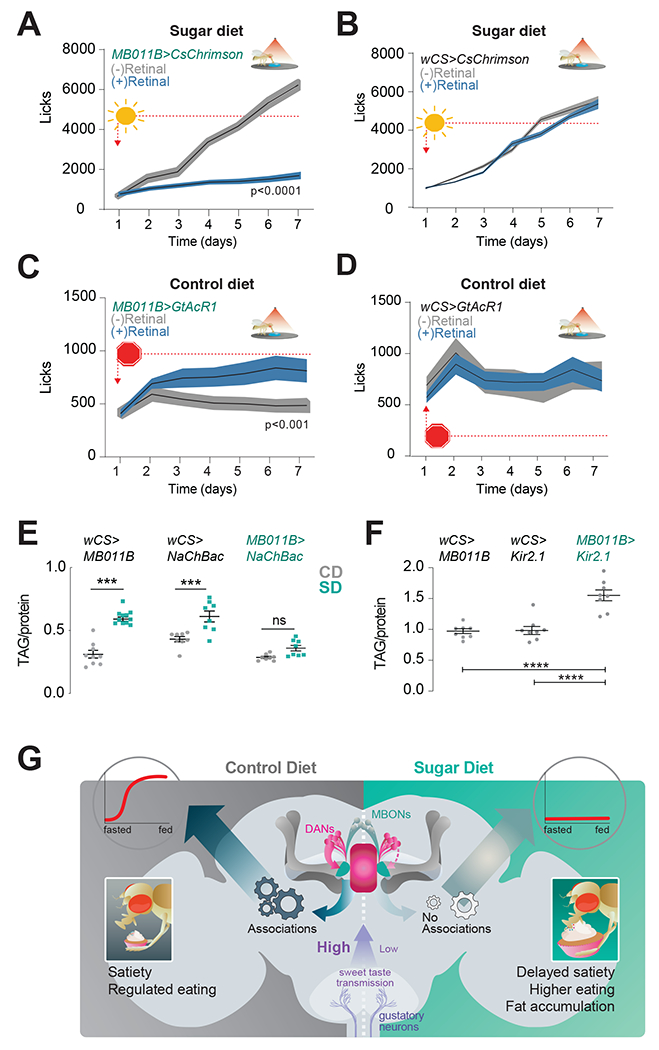
A) The number of food interactions (licks) per day on a high sugar diet in experimental MB011B>CSChrimson +retinal (blue) flies or control MB011B>CSChrimson - retinal (gray) flies. n=27 flies, Two-way Repeated Measure ANOVA with Sidak’s test, p<0.0001.
B) The number of food interactions (licks) per day on a high sugar diet in control CSChrimson>wCS + retinal (blue) or - retinal (gray) flies. n=27 flies, Two-way Repeated Measure ANOVA with Sidak’s test, p>0.05.
C) The number of food interactions (licks) per day on a control diet in experimental MB011B>GtAcR1 +retinal (blue) flies or control n MB011B>GtAcR1-retinal (gray) flies n=24-25, Two-way ANOVA with Sidak’s test, **p<0.001.
D) The number of food interactions (licks) per day on a 20% sucrose diet in control GtAcR1>wCS +retinal (blue) or - retinal (gray) flies. n=24 flies, Two-way Repeated Measure ANOVA with Sidak’s test, p>0.05.
E) Triglyceride levels normalized to protein in age-matched male MB011B>NaChBac and control flies on Control Diet, CD (gray) or Sugar Diet, SD (teal). n=8-11, one-way ANOVA with Tukey’s test, comparisons to control diet within genotype, ***p<0.001, ns, not significant. Additional comparisons, within control diet: MB011B>wcs vs. MB011B>NaChBac (p=0.9864), NaChBac>wCS vs. MB011B>wcs and NaChBac>wCS vs MB011B>NaChBac p<0.05; within sugar diet, MB011B>wCS vs. MB011B>NaChBac (p<0.05), NaChBac>wcs vs. MB011B>wcs, p<0.01, and NaChBac>wCS vs. MB011B>NaChBac p<0.0001.
F) Triglyceride levels normalized to protein in age-matched male MB011B>Kir2.1 and control flies on a CD (containg 10% sucrose in food). n=8, One-way ANOVA with Tukey’s test, ****p<0.001 and MB011B>wCS vs. NaChBac>wCS p=0.9919.
G) A circuit model for how the dietary environment affects food reinforcement learning.
Data shown as mean +/− SEM.
See also Figure S7.
DISCUSSION
In this study, we took advantage of the simplicity of the associative learning circuits in D. melanogaster to investigate how the dietary environment affects food learning. We show that consumption of a high-sugar diet disrupts associative learning by decreasing the reinforcing power of DA signals onto the MBONs (Figure 7G). This decrease in DA transmission results from changes in sweetness sensation that develop in the taste neurons with exposure to dietary sugar, even in the absence of fat accumulation. Thus, these impairments in food memories result from diet, not obesity. These findings establish the critical role of nutrients in brain processes and support accumulating evidence from mammalian studies on the effects of diet exposure on reinforcement learning 3,66,67.
Dopamine transmission is essential to the neuromodulation of MBON activity that underlies the learning process; here, we demonstrate that the decrease in DA-signal with high dietary sugar is insufficient to drive the neurophysiological changes that link a sensory cue with reward, preventing the formation of the food memory. To understand how this was linked to the output of MBONs, we examined the presynaptic activity of this circuit in response to sensory cues before and after learning. We found that the formation of food associations shifts MBON output and that, interestingly, responses to cues change dynamically during eating (fasted vs. 30 minutes refed) (Figure 7G). In the absence of learning in the sugar-diet animals, however, the output of MBONs remains static during eating. When we examined the effects of MBON activity on eating and energy balance, we found no effect of activating MBONs when flies were fed a control diet. However, activation while the animals were on a sugar diet, corrected eating and fat accumulation. Importantly, inhibiting MBON activity promoted higher eating and fat accumulation. We thus propose a model where components of processed food contribute to deficits in food associations independently of weight gain by decreasing the reinforcing power of DA signals (Figure 7G). We also show that the activity of associative learning circuits affects eating in diet-dependent contexts. These results are consistent with the known impairments in reinforcement learning that occur with obesogenic diets in mammals 12–21 and provide causes and mechanisms for these effects. Beyond this, our data also support the satiety cascade's theoretical framework, where cognitive circuits and processes are postulated to affect intake 1,3,62,67,68.
How does a high sucrose diet change DA-induced plasticity during learning?
The etiology of alterations in DA-neuron activity with diet-induced obesity in mammals remains unresolved. Our work demonstrates that changes in β’2 PAM DANs arise from changes in the peripheral sensory processing of sweetness 34. In previous studies, we found that the responses of the sweet gustatory neurons to sucrose and the transmission of the sweetness signal were reduced by exposure to a high-sugar diet 33,34,56. Correcting these sensory deficits with opto- and neurogenetic tools or pharmacological interventions restored normal DAN responses to sucrose as well as feeding behavior and fat mass in flies fed a high sucrose diet 33,34. Here, the same pharmacological manipulation corrected the neural signatures of learning, suggesting that sensory changes in response to the dietary environment play an essential role in deregulating food associations and eating. Of note, similar sensory alterations have been observed in mammals and humans exposed to high fat and sugar diets or with high Body-Mass index58,59,69,70, raising the possibility that these chemosensory alterations may contribute to changes in DA-induced plasticity and higher food intake with some diets. This result is interesting because we still do not understand how sensory processing promotes satiety, although sensory components of food play an important role 71–73. Thus, diet-induced chemosensory plasticity could provide a new lens to explain how some food environments, or even how diseases that impact the chemosensory system, like COVID-19 74, affect food intake.
Perturbations in DA-plasticity, however, could also arise from changes in the expression or function of DA receptors or transporters or even in DA synthesis, all of which have been described with diet-induced obesity in mammals 21,24,25,75–82. In flies the DA Receptor 1 and Receptor 2 (D2R) play important roles in associative learning 38,63,83 and food-seeking 84,85. To the best of our knowledge, the effects of diet on the expression or activity of these receptors have not been investigated in flies, but if they occur, these could also underlie some of the phenotypes observed here. Finally, plasticity at the MBON synapse could also reflect changes in Mushroom Body network activity, especially the contributions of antagonistic MBON and DAN circuits 49,83,86, some of which may also be important for energy homeostasis and foraging 87,88.
Food reinforcement learning and eating:
A growing body of data in mammals supports the model that obesity arises from changes in food learning rather than innate “food pleasure or liking,” 32,67 because manipulations of circuits necessary for reinforcement learning and memory influence eating and fat mass12–21. In the current work, we also found a strong effect of MBON activity on feeding behavior and fat accumulation. In flies, the activity of MBONs affects the innate animal’s preference (avoidance vs. approach) for cues depending on experience 41–43,48,89. Because of this, the feeding and obesity phenotypes we observed with manipulations of MBONs activity could be due to their effect on innate or learned preferences. Although our experiments, like those in rodents, do not provide a direct causal link between food associations and eating, we favor the second interpretation. First, if MBONs affected feeding solely through their innate regulation of avoidance/approach, we would expect activation of these neurons to decrease eating and fat mass under control-diet conditions. However, this is not what we observed: closed-loop activation of MBONs while flies were on a control diet did not affect feeding or fat mass. Only when MBONs were activated in animals eating a sugar diet did we observe a reduction in eating and protection from diet-induced obesity. In our opinion, the most parsimonious explanation for these results is that on a sugar diet, the activity of the neurons is lower, and activating them corrects this deficit. This interpretation is also consistent with the observation that inhibiting the activity of MBONs recapitulated the higher eating and obesity found in sugar-diet flies. Thus, although we cannot rule out the possibility that MBONs affect eating exclusively through learning or prove that learning deficits drive eating, we believe that the weight of the evidence better aligns with the idea that diet-driven impairments in associative learning contribute to at least some of the escalation of sugar intake.
MBONs project to sensory-motor integration areas in the fly Central Complex 86,90,91 involved in motor aspects of the eating program, such as foraging, proboscis extension, and eating rate 92–95. This part of the fly brain is genetically, functionally, and anatomically related to the mammalian Basal Ganglia 96, which receives input from the limbic circuitry involved in reinforcement learning. This connection between reinforcements, associative learning, and pre-motor areas provides a neural pathway to turn food associations into actions. In flies, the dynamic changes in the responses of MBONs to cues we observed during eating could control the activity of downstream pre-motor circuits and the animal’s interaction with food. In the absence of these, as seen in the sugar diet condition, the animal may stay “locked” in its interaction with food until pre- and post-absorptive signals disengage it from eating 97,98. Another possibility is that a mismatch between the different rewards contributing to food associations creates incentives to eat more 32,66,99. in humans, rodents, and flies, a mismatch created by giving animals non-caloric sweeteners along with sugars (or other carbohydrate-containing foods) changes food associations and central responses to sugar 36,37,40,66,99–104. In a similar way, high-sugar diet may uncouple taste and nutrient rewards by degrading the sweetness-reinforced CS+, or generalizing the reward to the CS−, which may result in a higher-than-expected reward from nutrients and drive intake 32,105.
In summary, our work sheds light on the causes and mechanisms through which processed food components impair the formation of food memories. Future functional dissections of the circuits in this network in flies and preclinical models, as well as investigations of the molecular and cellular mechanisms involved, will provide new insights into understanding the connection between food memories and eating.
STAR METHODS
RESOURCE AVAILABILITY
Lead Contact:
Further information and reasonable requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Monica Dus (mdus@umich.edu).
Materials Availability:
No new reagents or materials were generated by this study. The reagents used are listed in the Key Resource Table and are commercially available.
Key Resources Table
| Key Resources | ||||
|---|---|---|---|---|
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
| genetic reagent (D. melanogaster) | MB011B - GAL4 | Bloomington Drosophila Stock Center; Aso et al. 2014b | RRID: BDSC_57669 | Flybase symbol: P{Gr64f-GAL4.9.7}5 |
| genetic reagent (D. melanogaster) | UAS-CaMP6f | Bloomington Drosophila Stock Center; Chen et al. 2013 | RRID: BDSC_42747 | Flybase symbol: P{20XUAS-IVS-GCaMP6f} |
| genetic reagent (D. melanogaster) | UAS-NaChBac | M. Nitabach; Nitabach et al. 2006 | RRID: BDSC_9469 | Flybase symbol: P{UAS-NaChBac}2 |
| genetic reagent (D. melanogaster) | UAS-Kir2.1 | M. Nitabach; Nitabach et al. 2002 | n/a | UAS-Kir2.1 |
| genetic reagent (D. melanogaster) | Plin2−/− | R. Kuhnlein | RKF610 | y*plin2[51]/FM7i; P{w[+mc] = ActGFP}JMR3 or hom or Dp(1;Y) y[+] |
| genetic reagent (D. melanogaster) | UAS-GRABDA | Bloomington Drosophila Stock Center; Sun et al. 2018 | BDSC_80047 | P{UAS-GRAB(DA1m)} |
| genetic reagent (D. melanogaster) | UAS-Chrimson | Bloomington Drosophila Stock Center; Klapoetke et al. 2014 | BDSC_55135 | P{20XUAS-IVS-CsChrimson.mVenus} |
| genetic reagent (D. melanogaster) | UAS-GtACR1 | A Clardige–Change; Mohammad et al. 2017 | BDSC_92983 | P{UAS-GtACR1.d.EYFP} |
| genetic reagent (D. melanogaster) | w1118-CS | A. Simon S. Benzer | n/a | |
| genetic reagent (D. melanogaster) | UAS-mCD8-GFP | A.-S. Chiang; Dus et al. 2015 | n/a | |
| commercial assay or kit | Pierce BCA Protein Assay Kit | Thermo Scientific | Cat. #23225 | |
| commercial assay or kit | Triglyceride LiquiColor Test (Enzymatic) | Stanbio | Ref. # 2100-430 | |
| chemical compound, drug | D-sucrose | Fisher Scientific | BP220-10 | |
| Chemical compound, drug | all-trans-retinal | Sigma-Aldrich | R2500-100MG, CAS: 116-31-4 | |
| Chemical compound, drug | 3-octanol | Sigma Aldrich | 218405-50G CAS: 589-98-0 | |
| Chemical compound, drug | 4-methylcyclohexanol | Sigma Aldrich | 153095-250ML CAS: 589-91-3 | |
| Chemical compound, drug | OSMI-1 OGT inhibitor | Sigma-Aldrich | SML1621-5MG CAS: 1681056-61-0 | |
| Software, algorithm | FLIC Monitor | FLIC support; Ro et al., 2014 | RRID:SCR_018387 | |
| Software, algorithm | Olympus FluoView FV1200-ASW 4.2 | Olympus Life Science | RRID:SCR_014215 | |
| Software, algorithm | Suite2p | (Pachitariu et al., 2017) | ||
| Software, algorithm | RStudio | RStudio, Inc | RRID:SCR_000432 | |
| Software, algorithm | FLIC analysis R code | FLIC support; Ro et al., 2014; May et al., 2019 | RRID:SCR_018386 | |
| Software, algorithm | Fiji | ImageJ | RRID:SCR_002285 | |
| Other | UV Glue | Loctite | 3106 | |
| Other | Electra Waxer | Almore | 66000 | |
| Other | Gulfwax Paraffin | Hardware store | C0130 | |
Data Availability:
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
All flies were maintained at 25°C in a humidity-controlled incubator with a 12:12 hr light/dark cycle. For all experiments, flies were collected under CO2 anesthesia 2–4 days following eclosion and housed in groups of 20–30 within culture vials to age until 4-5 days old. The stocks used are listed in the Key Resource Table. As a control, we used w1118Canton-S (wCS) flies (gift from Anne Simon, University of Western Ontario), which were obtained by backcrossing a w1118 strain (Benzer lab, Caltech) to Canton-S (CS) (Benzer lab, Caltech) for 10 generations.
Dietary manipulations:
Flies were transferred to vials containing respective diets 4-5 days after eclosion and left on their food for 7 days; fresh food was provided every other day. The composition and caloric amount of each diet were as follows:
‘Control Diet/CD’ was a standard cornmeal food (Bloomington Food B recipe), with approx. 0.6 cal/g.
‘Sugar Diet/SD’ was 30 g of table sugar added to 89 g Control Diet for 100 mL final volume of 30% sucrose w/v, with approx. 1.4 cal/g.
For diets supplemented with OSMI-1, the inhibitor was added to control diet or sugar diet food at a concentration of 10 μM.
For diets supplemented with all-trans-retinal, the final concentration was 200 mM in food.
The sucrose for the optoFLIC was dissolved in 4 mg/L MgCl2, and consisted of 10% (control diet) or 20% (sugar diet).
METHOD DETAILS
Appetitive conditioning on the T-maze
Adult age-matched male flies, following 7 days of control diet or sugar diet, were fasted on a wet Kimwipe for 24hrs or 36hrs, respectively, before the behavioral assay. Flies were then allowed to acclimatize for 2 hours in a dark behavior room at a temperature of 24°C and humidity of 50%. We utilized a horizontal T-maze described in 45. Following acclimatization, appetitive training was performed as described 89. Briefly, flies were exposed to 0.1% of the CS− for 2 min, followed by 30 seconds of air and then to 0.1% of the CS+ in the presence of 2M dry sucrose for 2 min. All behavioral experiments were performed in reciprocal (averaged between alternative order of odors), testing dietary conditions and genotypes in parallel. For testing, flies were given 2 min to choose between the CS+ and CS− in the T-maze. Performance index (PI) was calculated as the number of flies approaching the conditioned odor minus the number of flies going the other direction, divided by the total number of flies in the experiment . A single PI value is an average score from flies of the identical genotype tested with the reciprocal reinforced/non-reinforced odor combination, as shown in 89,106.
To assay naïve responses to odors, fasted flies were exposed to two 0.1% of MCH or 0.1% OCT vs. paraffin oil in the absence of conditioning; the arms in which the odors were presented were switched in half of the experiments. The preference index was calculated as the number of flies approaching the odor minus the number approaching paraffin oil, divided by the total number of flies in the experiment .
For the optogenetic experiment, the T-maze was operated essentially in the same manner as above, but during the conditioning phase, 627nm light (60Hz) was delivered along with sugar for 2 minutes. Flies were kept on retinal with a control or sugar diet in the dark for 5 days before the start of the experiment.
Two-Photon in vivo imaging
Adult age-matched male flies on 7 days of control diet or sugar diet exposure were fasted on a wet Kimwipe for 24hrs or 30hrs, respectively, before conducting the imaging. Flies were prepared for imaging with their head tilted forward under a small Petri dish with an empty space on top of the head cuticle to expose the brain to the artificial hemolymph and to access the antennae and proboscis for odor and sucrose delivery as described in 107. The fly’s legs were removed to avoid interference with odor and sucrose delivery. Artificial hemolymph consisted of 51.5 mM NaCl, 2 mM MgCl2, 13 mM NaHCO3, 0.5 mM NaH2PO4, 0.75 mM CaCl2-2H20, 1.5 mM KCl, 2.5 mM HEPES, pH to 7.00 - 7.10, prepared 24 hrs before and allowed to reach room temperature before use.
Conditioning:
Flies were placed under the objective and presented with a modified conditioning protocol based on 43,49: 1) Testing Pre-training responses: head-fixed, naive flies were exposed to 2” of 0.1% 4-methyl cyclohexanol (MCH) and then to 2” of 0.1% 3-octanol (OCT) (with 30” of air stream in between); 2) Training for Appetitive Conditioning: After 30” of rest from the end of the sequence in 1), one odor was presented for 2min (CS−); after 30” of rest, the second odor was presented for 2min while 2M sucrose (US) was offered (CS+); odor pairing was reversed in half of the animals; 3) Testing post-training responses: After 2.5min of rest, the post-conditioning calcium responses to the two odors were measured while animals were exposed to the same odor sequence as 1, with the addition of a new odor (0.1% isobutyl acetate, IA) as control. The odor presentation before and after conditioning was done twice, and responses averaged when available, except for refeeding assay, where, due to limited time, it was done once. To test responses to cues after feeding, flies were conditioned as described above; after the last odor presentation after training, they were fed 2M sucrose for 30 minutes as AHL was replaced manually. After 30 minutes, the odor sequence (1) was presented again. Imaging data were acquired with a two-photon microscope, 20x water immersion objective, and at a rate of 15Hz, with a resolution of 1024 x 1024 utilizing a resonant scanner. The odor delivery and imaging system were as 42,107.
Kenyon Cells responses:
Same method as described above under “testing pre-training responses”.
Sweet responses:
Under similar preparations before training, flies were placed under the two-photon
Imaging data analysis
Fiji was used to manually draw regions of interest around the β′2 MB011B dendrites or axons in both hemispheres and quantify the relative difference in fluorescence intensity (ΔF/F0) before (F0 5 frames) and after stimulus onset (F). To measure the difference in CS+ and CS− responses with conditioning (Norm peak ΔF/F) we normalized the peak responses after conditioning to those of naive flies [(ΔFpost/F0- ΣΔFnaive/F0)/ΣΔFnaive/F0 *100]. Not normalized responses are in supplementary data.
Closed-loop optogenetic stimulation in Fly-to-Liquid-food Interaction Counter (OptoFLIC)
The feeding behavior was measured using the Fly Liquid-Food Interaction Counter (FLIC), described previously 108. Briefly, adult flies were placed on +/− retinal food and kept in the dark for 5 days until the start of the experiment. The feeding behaviors were recorded inside a 12-hour dark/dark cycle incubator with 40-50% humidity and 25°C. The LED activation protocols were as follows and microscope and were given 30% sucrose to taste for 1 second using a manipulator. activated only when the fly interacted with the food: For experiments with MB110B>Chrimson, 100 ms of red (~627 nm) light pulsing at a frequency of 60 Hz and with a pulse width of 11 ms was triggered by every food interaction signal over 10. For experiments with MB011B>GtACR1, 100ms of green (~530 nm) light pulsing at a frequency of 20 Hz and with a pulse width of 11ms was triggered by every food interaction signal over 10._Analysis of daily food interactions was as previously described in 34; the R code used can be found on Github (https://github.com/chrismayumich/May_et_al_optoFLIC; copy archived at https://github.com/elifesciences-publications/ May_et_al_optoFLIC).
Triacylglyceride (TAGs) Assay
The levels of TAG and protein were measured as previously described in 109, n=1 equals 2 flies.
Immunofluorescence
The expression of MB011B>CD8::GFP was visualized as previously described 110.
QUANTIFICATION AND STATISTICAL ANALYSIS
Prism GraphPad was used to analyze the normal distribution (Shapiro-Wilk test) and the significance of the data. Statistical tests and sample size can be found in each figure legend. Details of how each assay was quantified are found under each method.
Supplementary Material
Pardo Garcia et al. show that a high-sugar diet disrupts the creation of food memories by decreasing the reinforcing power of dopamine signals onto the Mushroom Body Output Neurons (MBONs). In the absence of learning signals, the activity of MBONs is static during eating, affecting intake and energy homeostasis.
Sugar consumption impairs food associations independently of obesity
Weaker DA-induced plasticity causes deficits in associative learning
Food associations change the responses of associative learning circuits during eating
The activity of associative learning circuits affects eating and energy balance
Acknowledgments:
We thank the University of Indiana at Bloomington stock collection and all the investigators who kindly shared fly lines with us. We thank Dr. Karla Kaun and her lab for their direction with behavioral experiments. We thank the Clowney lab for allowing us to use their equipment for imaging experiments. We thank Dr. Manaswini Sarangi and previous members of the Dus lab for their help with the experiments and manuscript. We thank Drs. Manuel Perisse and David Oswald for assistance with conditioning and imaging experiments. We are thankful to Dr. Scott Pletcher for assistance with the OPTO-FLIC. Julia Kuhn designed the graphics for the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest:
The authors declare no conflict of interest.
References
- 1.Benelam B (2009). Satiation, satiety and their effects on eating behaviour. Nutr. Bull 34, 126–173. [Google Scholar]
- 2.Bellisle F, and Blundell JE (2013). Satiation, satiety: concepts and organisation of behaviour. Satiation, Satiety and the Control of Food Intake, 3–11. 10.1533/9780857098719.1.3. [DOI] [Google Scholar]
- 3.Davidson TL, and Martin AA (2014). Obesity: Cognitive impairment and the failure to “eat right.” Curr. Biol 24, R685–7. [DOI] [PubMed] [Google Scholar]
- 4.Melhorn SJ, Krause EG, Scott KA, Mooney MR, Johnson JD, Woods SC, and Sakai RR (2010). Acute exposure to a high-fat diet alters meal patterns and body composition. Physiol. Behav 99, 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, Chung ST, Costa E, Courville A, Darcey V, et al. (2019). Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metabolism 30, 67–77.e3. 10.1016/j.cmet.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.la Fleur SE, Luijendijk MCM, van der Zwaal EM, Brans MAD, and Adan RAH (2014). The snacking rat as model of human obesity: effects of a free-choice high-fat high-sugar diet on meal patterns. Int. J. Obes 38, 643–649. [DOI] [PubMed] [Google Scholar]
- 7.Warwick ZS, Synowski SJ, Rice KD, and Smart AB (2003). Independent effects of diet palatability and fat content on bout size and daily intake in rats. Physiol. Behav 80, 253–258. [DOI] [PubMed] [Google Scholar]
- 8.Furnes MW, Zhao C-M, and Chen D (2009). Development of obesity is associated with increased calories per meal rather than per day. A study of high-fat diet-induced obesity in young rats. Obes. Surg 19, 1430–1438. [DOI] [PubMed] [Google Scholar]
- 9.Warwick ZS, McGuire CM, Bowen KJ, and Synowski SJ (2000). Behavioral components of high-fat diet hyperphagia: meal size and postprandial satiety. Am. J. Physiol. Regul. Integr. Comp. Physiol 278, R196–200. [DOI] [PubMed] [Google Scholar]
- 10.Treesukosol Y, and Moran TH (2014). Analyses of meal patterns across dietary shifts. Appetite 75, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarangi M, and Dus M (2021). Crème de la Créature: Dietary Influences on Behavior in Animal Models. Frontiers in Behavioral Neuroscience; Lausanne. 10.3389/fnbeh.2021.746299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z, Manson KF, Schiller D, and Levy I (2014). Impaired associative learning with food rewards in obese women. Curr. Biol 24, 1731–1736. [DOI] [PubMed] [Google Scholar]
- 13.van den Akker K, Schyns G, and Jansen A (2017). Altered appetitive conditioning in overweight and obese women. Behav. Res. Ther 99, 78–88. [DOI] [PubMed] [Google Scholar]
- 14.Coppin G, Nolan-Poupart S, Jones-Gotman M, and Small DM (2014). Working memory and reward association learning impairments in obesity. Neuropsychologia 65, 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Akker K, Schyns G, Breuer S, van den Broek M, and Jansen A (2019). Acquisition and generalization of appetitive responding in obese and healthy weight females. Behav. Res. Ther 123, 103500. [DOI] [PubMed] [Google Scholar]
- 16.Kanoski SE, Meisel RL, Mullins AJ, and Davidson TL (2007). The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav. Brain Res 182, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Akker K, Schyns G, and Jansen A (2018). Learned Overeating: Applying Principles of Pavlovian Conditioning to Explain and Treat Overeating. Curr Addict Rep 5, 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanoski SE, Zhang Y, Zheng W, and Davidson TL (2010). The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J. Alzheimers. Dis 21, 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reichelt AC, Morris MJ, and Westbrook RF (2014). Cafeteria diet impairs expression of sensory-specific satiety and stimulus-outcome learning. Front. Psychol 5, 852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson MJF, Burghardt PR, Patterson CM, Nobile CW, Akil H, Watson SJ, Berridge KC, and Ferrario CR (2015). Individual Differences in Cue-Induced Motivation and Striatal Systems in Rats Susceptible to Diet-Induced Obesity. Neuropsychopharmacology 40, 2113–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadler JR, Shearrer GE, Papantoni A, Yokum ST, Stice E, and Burger KS (2021). Correlates of neural adaptation to food cues and taste: the role of obesity risk factors. Soc. Cogn. Affect. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, and Pothos EN (2009). Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience 159, 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narayanaswami V, Thompson AC, Cassis LA, Bardo MT, and Dwoskin LP (2013). Diet-induced obesity: dopamine transporter function, impulsivity and motivation. Int. J. Obes 37, 1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van de Giessen E, la Fleur SE, Eggels L, de Bruin K, van den Brink W, and Booij J (2013). High fat/carbohydrate ratio but not total energy intake induces lower striatal dopamine D2/3 receptor availability in diet-induced obesity. Int. J. Obes 37, 754–757. [DOI] [PubMed] [Google Scholar]
- 25.Johnson PM, and Kenny PJ (2010). Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci 13, 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee Y, Kroemer NB, Oehme L, Beuthien-Baumann B, Goschke T, and Smolka MN (2018). Lower dopamine tone in the striatum is associated with higher body mass index. Eur. Neuropsychopharmacol 28, 719–731. [DOI] [PubMed] [Google Scholar]
- 27.Barry RL, Byun NE, Williams JM, Siuta MA, Tantawy MN, Speed NK, Saunders C, Galli A, Niswender KD, and Avison MJ (2018). Brief exposure to obesogenic diet disrupts brain dopamine networks. PLoS One 13, e0191299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokum S, and Stice E (2019). Weight gain is associated with changes in neural response to palatable food tastes varying in sugar and fat and palatable food images: a repeated-measures fMRI study. Am. J. Clin. Nutr 110, 1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis JF, Tracy AL, Schurdak JD, Tschöp MH, Lipton JW, Clegg DJ, and Benoit SC (2008). Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav. Neurosci 122, 1257–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiFeliceantonio AG, and Small DM (2019). Dopamine and diet-induced obesity. Nat. Neurosci 22, 1–2. [DOI] [PubMed] [Google Scholar]
- 31.Tellez LA, Medina S, Han W, Ferreira JG, Licona-Limón P, Ren X, Lam TT, Schwartz GJ, and de Araujo IE (2013). A gut lipid messenger links excess dietary fat to dopamine deficiency. Science 341, 800–802. [DOI] [PubMed] [Google Scholar]
- 32.Robinson MJF, Robinson TE, and Berridge KC (2014). Incentive Salience in Addiction and Over-Consumption. The Interdisciplinary Science of Consumption, 185–198. 10.7551/mitpress/9780262027670.003.0010. [DOI] [Google Scholar]
- 33.May CE, Vaziri A, Lin YQ, Grushko O, Khabiri M, Wang Q-P, Holme KJ, Pletcher SD, Freddolino PL, Neely GG, et al. (2019). High Dietary Sugar Reshapes Sweet Taste to Promote Feeding Behavior in Drosophila melanogaster. Cell Rep 27, 1675–1685.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.May CE, Rosander J, Gottfried J, Dennis E, and Dus M (2020). Dietary sugar inhibits satiation by decreasing the central processing of sweet taste. Elife 9. 10.7554/eLife.54530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musselman LP, and Kühnlein RP (2018). Drosophila as a model to study obesity and metabolic disease. J. Exp. Biol 221. 10.1242/jeb.163881. [DOI] [PubMed] [Google Scholar]
- 36.Burke CJ, and Waddell S (2011). Remembering Nutrient Quality of Sugar in Drosophila. Current Biology 21, 746–750. 10.1016/j.cub.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujita M, and Tanimura T (2011). Drosophila evaluates and learns the nutritional value of sugars. Curr. Biol 21, 751–755. [DOI] [PubMed] [Google Scholar]
- 38.Modi MN, Shuai Y, and Turner GC (2020). The Drosophila Mushroom Body: From Architecture to Algorithm in a Learning Circuit. Annu. Rev. Neurosci 43, 465–484. [DOI] [PubMed] [Google Scholar]
- 39.Yamagata N, Ichinose T, Aso Y, Plaçais P-Y, Friedrich AB, Sima RJ, Preat T, Rubin GM, and Tanimoto H (2015). Distinct dopamine neurons mediate reward signals for short- and long-term memories. Proc. Natl. Acad. Sci. U. S. A 112, 578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huetteroth W, Perisse E, Lin S, Klappenbach M, Burke C, and Waddell S (2015). Sweet taste and nutrient value subdivide rewarding dopaminergic neurons in Drosophila. Curr. Biol 25, 751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aso Y, and Rubin GM (2016). Dopaminergic neurons write and update memories with cell-type-specific rules. Elife 5. 10.7554/eLife.16135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohn R, Morantte I, and Ruta V (2015). Coordinated and Compartmentalized Neuromodulation Shapes Sensory Processing in Drosophila. Cell 163, 1742–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Owald D, Felsenberg J, Talbot CB, Das G, Perisse E, Huetteroth W, and Waddell S (2015). Activity of defined mushroom body output neurons underlies learned olfactory behavior in Drosophila. Neuron 86, 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burke CJ, Huetteroth W, Owald D, Perisse E, Krashes MJ, Das G, Gohl D, Silies M, Certel S, and Waddell S (2012). Layered reward signalling through octopamine and dopamine in Drosophila. Nature 492, 433–437. 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ichinose T, and Tanimoto H (2016). Dynamics of memory-guided choice behavior in Drosophila. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci 92, 346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilinski D, Winzeler J, Duren W, Persons JL, Holme KJ, Mosquera J, Khabiri M, Kinchen JM, Freddolino PL, Karnovsky A, et al. (2019). Rapid metabolic shifts occur during the transition between hunger and satiety in Drosophila melanogaster. Nat. Commun 10, 4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aso Y, Hattori D, Yu Y, Johnston RM, Iyer NA, Ngo T-TB, Dionne H, Abbott LF, Axel R, Tanimoto H, et al. (2014). The neuronal architecture of the mushroom body provides a logic for associative learning. Elife 3, e04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aso Y, Sitaraman D, Ichinose T, Kaun KR, Vogt K, Belliart-Guérin G, Plaçais P-Y, Robie AA, Yamagata N, Schnaitmann C, et al. (2014). Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. Elife 3, e04580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Felsenberg J, Jacob PF, Walker T, Barnstedt O, Edmondson-Stait AJ, Pleijzier MW, Otto N, Schlegel P, Sharifi N, Perisse E, et al. (2018). Integration of Parallel Opposing Memories Underlies Memory Extinction. Cell 175, 709–722.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. (2013). Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reichelt AC, Westbrook RF, and Morris MJ (2015). Integration of reward signalling and appetite regulating peptide systems in the control of food-cue responses. Br. J. Pharmacol 172, 5225–5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stice E, and Burger K (2019). Neural vulnerability factors for obesity. Clin. Psychol. Rev 68, 38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stice E, Figlewicz DP, Gosnell BA, Levine AS, and Pratt WE (2013). The contribution of brain reward circuits to the obesity epidemic. Neurosci. Biobehav. Rev 37, 2047–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kenny PJ (2011). Reward mechanisms in obesity: new insights and future directions. Neuron 69, 664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF, Luo Y, Li F, Wang H, Yamaguchi T, et al. (2018). A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell 174, 481–496.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ganguly A, Dey M, Scott C, Duong V-K, and Dahanukar A (2021). Dietary Macronutrient Imbalances Lead to Compensatory Changes in Peripheral Taste via Independent Signaling Pathways. J. Neurosci 10.1523/JNEUROSCI.2154-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Q-P, Lin YQ, Lai M-L, Su Z, Oyston LJ, Clark T, Park SJ, Khuong TM, Lau M-T, Shenton V, et al. (2020). PGC1α Controls Sucrose Taste Sensitization in Drosophila. Cell Rep 31, 107480. [DOI] [PubMed] [Google Scholar]
- 58.McCluskey LP, He L, Dong G, and Harris R (2020). Chronic exposure to liquid sucrose and dry sucrose diet have differential effects on peripheral taste responses in female rats. Appetite 145, 104499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sung H, Vesela I, Driks H, Ferrario CR, Mistretta CM, Bradley RM, and Dus M (2022). High-sucrose diet exposure is associated with selective and reversible alterations in the rat peripheral taste system. Curr. Biol 10.1016/j.cub.2022.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaziri A, Khabiri M, Genaw BT, May CE, Freddolino PL, and Dus M (2020). Persistent epigenetic reprogramming of sweet taste by diet. Sci Adv 6. 10.1126/sciadv.abc8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blundell JE, Rogers PJ, and Hill AJ (1987). Evaluating the satiating power of foods: implications for acceptance and consumption. Food acceptance and nutrition / edited by J. Solms … [et al. ]. [Google Scholar]
- 62.Cunningham PM, and Rolls BJ (2021). The Satiation Framework: Exploring processes that contribute to satiation. Physiol. Behav 236, 113419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Handler A, Graham TGW, Cohn R, Morantte I, Siliciano AF, Zeng J, Li Y, and Ruta V (2019). Distinct Dopamine Receptor Pathways Underlie the Temporal Sensitivity of Associative Learning. Cell 178, 60–75.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, et al. (2014). Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mohammad F, Stewart JC, Ott S, Chlebikova K, Chua JY, Koh T-W, Ho J, and Claridge-Chang A (2017). Optogenetic inhibition of behavior with anion channelrhodopsins. Nat. Methods 14, 271–274. [DOI] [PubMed] [Google Scholar]
- 66.Davidson TL, Sample CH, and Swithers SE (2014). An application of Pavlovian principles to the problems of obesity and cognitive decline. Neurobiol. Learn. Mem 108, 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kroemer NB, and Small DM (2016). Fuel not fun: Reinterpreting attenuated brain responses to reward in obesity. Physiol. Behav 162, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeomans MR (2017). Introducing sensory and cognitive influences on satiation and satiety. In Flavor, Satiety and Food Intake (John Wiley & Sons, Ltd; ), pp. 1–12. [Google Scholar]
- 69.May CE, and Dus M (2020). Confection Confusion: Interplay Between Diet, Taste, and Nutrition. Trends Endocrinol. Metab 10.1016/j.tem.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Donaldson LF, Bennett L, Baic S, and Melichar JK (2009). Taste and weight: is there a link? Am. J. Clin. Nutr 90, 800S–803S. [DOI] [PubMed] [Google Scholar]
- 71.Forde CG, van Kuijk N, Thaler T, de Graaf C, and Martin N (2013). Oral processing characteristics of solid savoury meal components, and relationship with food composition, sensory attributes and expected satiation. Appetite 60, 208–219. [DOI] [PubMed] [Google Scholar]
- 72.Weijzen PLG, Zandstra EH, Alfieri C, and de Graaf C (2008). Effects of complexity and intensity on sensory specific satiety and food acceptance after repeated consumption. Food Qual. Prefer 19, 349–359. [Google Scholar]
- 73.Wittekind A, Higgins K, McGale L, Schwartz C, Stamataki NS, Beauchamp GK, Bonnema A, Dussort P, Gibson S, de Graaf C, et al. (2018). A workshop on “Dietary Sweetness—Is It an Issue?” Int. J. Obes 42, 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cooper KW, Brann DH, Farruggia MC, Bhutani S, Pellegrino R, Tsukahara T, Weinreb C, Joseph PV, Larson ED, Parma V, et al. (2020). COVID-19 and the Chemical Senses: Supporting Players Take Center Stage. Neuron 107, 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vucetic Z, Carlin JL, Totoki K, and Reyes TM (2012). Epigenetic dysregulation of the dopamine system in diet-induced obesity. J. Neurochem 120, 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bello NT, Lucas LR, and Hajnal A (2002). Repeated sucrose access influences dopamine D2 receptor density in the striatum. Neuroreport 13, 1575–1578. 10.1097/00001756-200208270-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pepino MY, Eisenstein SA, Bischoff AN, Klein S, Moerlein SM, Perlmutter JS, Black KJ, and Hershey T (2016). Sweet Dopamine: Sucrose Preferences Relate Differentially to Striatal D2 Receptor Binding and Age in Obesity. Diabetes 65, 2618–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stice E, Spoor S, Bohon C, and Small DM (2008). Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 322, 449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.South T, and Huang X-F (2008). High-fat diet exposure increases dopamine D2 receptor and decreases dopamine transporter receptor binding density in the nucleus accumbens and caudate putamen of mice. Neurochem. Res 33, 598–605. [DOI] [PubMed] [Google Scholar]
- 80.Kessler RM, Zald DH, Ansari MS, Li R, and Cowan RL (2014). Changes in dopamine release and dopamine D2/3 receptor levels with the development of mild obesity. Synapse 68, 317–320. [DOI] [PubMed] [Google Scholar]
- 81.Fritz BM, Muñoz B, Yin F, Bauchle C, and Atwood BK (2018). A High-fat, High-sugar “Western” Diet Alters Dorsal Striatal Glutamate, Opioid, and Dopamine Transmission in Mice. Neuroscience 372, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Janssen LK, Herzog N, Waltmann M, Breuer N, Wiencke K, Rausch F, Hartmann H, Poessel M, and Horstmann A (2019). Lost in translation? On the need for convergence in animal and human studies on the role of dopamine in diet-induced obesity. Curr. Addict. Rep 6, 229–257. [Google Scholar]
- 83.Cognigni P, Felsenberg J, and Waddell S (2018). Do the right thing: neural network mechanisms of memory formation, expression and update in Drosophila. Curr. Opin. Neurobiol 49, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Landayan D, Feldman DS, and Wolf FW (2018). Satiation state-dependent dopaminergic control of foraging in Drosophila. Sci. Rep 8, 5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Landayan D, and Wolf FW (2015). Shared neurocircuitry underlying feeding and drugs of abuse in Drosophila. Biomed. J 38, 496–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li F, Lindsey JW, Marin EC, Otto N, Dreher M, Dempsey G, Stark I, Bates AS, Pleijzier MW, Schlegel P, et al. (2020). The connectome of the adult Drosophila mushroom body provides insights into function. Elife 9. 10.7554/eLife.62576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Al-Anzi B, and Zinn K (2018). Identification and characterization of mushroom body neurons that regulate fat storage in Drosophila. Neural Dev 13, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsao C-H, Chen C-C, Lin C-H, Yang H-Y, and Lin S (2018). Drosophila mushroom bodies integrate hunger and satiety signals to control innate food-seeking behavior. Elife 7. 10.7554/eLife.35264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perisse E, Yin Y, Lin AC, Lin S, Huetteroth W, and Waddell S (2013). Different kenyon cell populations drive learned approach and avoidance in Drosophila. Neuron 79, 945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scheffer LK, Xu CS, Januszewski M, Lu Z, Takemura S-Y, Hayworth KJ, Huang GB, Shinomiya K, Maitlin-Shepard J, Berg S, et al. (2020). A connectome and analysis of the adult Drosophila central brain. Elife 9. 10.7554/eLife.57443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scaplen KM, Talay M, Fisher JD, Cohn R, Sorkaç A, Aso Y, Barnea G, and Kaun KR (2020). Transsynaptic mapping of Drosophila mushroom body output neurons Cold Spring Harbor Laboratory, 2020.09.22.309021. 10.1101/2020.09.22.309021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chia J, and Scott K (2020). Activation of specific mushroom body output neurons inhibits proboscis extension and sucrose consumption. PLoS One 15, e0223034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dus M, Ai M, and Suh GSB (2013). Taste-independent nutrient selection is mediated by a brain-specific Na+ /solute co-transporter in Drosophila. Nat. Neurosci 16, 526–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fisher YE (2022). Flexible navigational computations in the Drosophila central complex. Curr. Opin. Neurobiol 73, 102514. [DOI] [PubMed] [Google Scholar]
- 95.Sareen PF, McCurdy LY, and Nitabach MN (2021). A neuronal ensemble encoding adaptive choice during sensory conflict in Drosophila. Nat. Commun 12, 4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Strausfeld NJ, and Hirth F (2013). Deep homology of arthropod central complex and vertebrate basal ganglia. Science 340, 157–161. [DOI] [PubMed] [Google Scholar]
- 97.Chambers AP, Sandoval DA, and Seeley RJ (2013). Integration of satiety signals by the central nervous system. Curr. Biol 23, R379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sandoval DA, and Seeley RJ (2017). Physiology: Gut feeling for food choice. Nature 542, 302–303. [DOI] [PubMed] [Google Scholar]
- 99.Swithers SE, and Davidson TL (2008). A role for sweet taste: calorie predictive relations in energy regulation by rats. Behav. Neurosci 122, 161–173. [DOI] [PubMed] [Google Scholar]
- 100.Dalenberg JR, Patel BP, Denis R, Veldhuizen MG, Nakamura Y, Vinke PC, Luquet S, and Small DM (2020). Short-Term Consumption of Sucralose with, but Not without, Carbohydrate Impairs Neural and Metabolic Sensitivity to Sugar in Humans. Cell Metab 31, 493–502.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beeler JA, McCutcheon JE, Cao ZFH, Murakami M, Alexander E, Roitman MF, and Zhuang X (2012). Taste uncoupled from nutrition fails to sustain the reinforcing properties of food. Eur. J. Neurosci 36, 2533–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davidson TL, Martin AA, Clark K, and Swithers SE (2011). Intake of high-intensity sweeteners alters the ability of sweet taste to signal caloric consequences: implications for the learned control of energy and body weight regulation. Q. J. Exp. Psychol 64, 1430–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Musso P-Y, Lampin-Saint-Amaux A, Tchenio P, and Preat T (2017). Ingestion of artificial sweeteners leads to caloric frustration memory in Drosophila. Nat. Commun 8, 1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Casperson SL, Johnson L, and Roemmich JN (2017). The relative reinforcing value of sweet versus savory snack foods after consumption of sugar- or non-nutritive sweetened beverages. Appetite 112, 143–149. [DOI] [PubMed] [Google Scholar]
- 105.Berridge KC, and Robinson TE (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev 28, 309–369. [DOI] [PubMed] [Google Scholar]
- 106.Tully T, and Quinn WG (1985). Classical conditioning and retention in normal and mutant Drosophila melanogaster. J. Comp. Physiol. A 157, 263–277. [DOI] [PubMed] [Google Scholar]
- 107.Elkahlah NA, Rogow JA, Ahmed M, and Clowney EJ (2020). Presynaptic developmental plasticity allows robust sparse wiring of the Drosophila mushroom body. Elife 9. 10.7554/eLife.52278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ro J, Harvanek ZM, and Pletcher SD (2014). FLIC: high-throughput, continuous analysis of feeding behaviors in Drosophila. PLoS One 9, e101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tennessen JM, Barry WE, Cox J, and Thummel CS (2014). Methods for studying metabolism in Drosophila. Methods 68, 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dus M, Lai JS-Y, Gunapala KM, Min S, Tayler TD, Hergarden AC, Geraud E, Joseph CM, and Suh GSB (2015). Nutrient Sensor in the Brain Directs the Action of the Brain-Gut Axis in Drosophila. Neuron 87, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


