Abstract
The biological events associated with mammalian reproductive processes are highly dynamic and tightly regulated by molecular, genetic, and biomechanical factors. Implementation of live imaging in reproductive research is vital for the advancement of our understanding of normal reproductive physiology and for improving the management of reproductive disorders. Optical coherence tomography (OCT) is emerging as a promising tool for dynamic volumetric imaging of various reproductive processes in mice and other animal models. In this review, we summarize recent studies employing OCT-based approaches toward the investigation of reproductive processes in both, males and females. We describe how OCT can be applied to study structural features of the male reproductive system and sperm transport through the male reproductive tract. We review OCT applications for in vitro and dynamic in vivo imaging of the female reproductive system, staging and tracking of oocytes and embryos, and investigations of the oocyte/embryo transport through the oviduct. We describe how the functional OCT approach can be applied to the analysis of cilia dynamics within the male and female reproductive systems. We also discuss the areas of research, where OCT could find potential applications to progress our understanding of normal reproductive physiology and reproductive disorders.
Keywords: in vivo imaging, cilia, oocyte, optical coherence tomography, oviduct, spermatozoa, mouse
Graphical Abstract
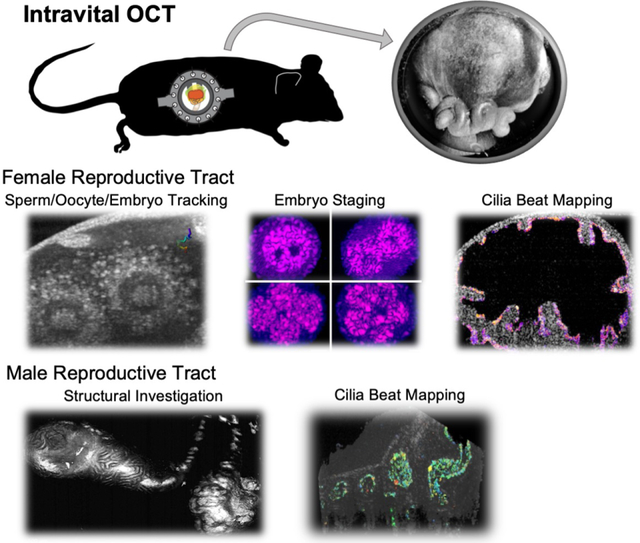
Optical coherence tomography (OCT) is a powerful tool for dynamic volumetric imaging of various reproductive processes in mammals. This review summarizes OCT applications for in vivo imaging of sperm migration, oocyte/embryo transport, embryo staging, and cilia dynamics in the oviduct, as well as structural analysis of the male reproductive system.
1. Introduction
The reproductive process is responsible for conveying genetic information to the next generation, which is essential for the survival of all species. It involves a series of highly dynamic biological events including the production, maturation, transport, fusion of male and female gametes, and embryo development. According to recent studies, infertility is estimated to affect about 15% of reproductive-age couples, or 186 million people worldwide (Krol et al., 2019); approximately 30% of those are of an unknown nature (Fainberg & Kashanian, 2019). Toward these problems, the advancement of gene manipulation techniques in animal models and biochemical approaches have provided important insights into the genetic and molecular aspects of the mammalian reproduction (Okabe, 2013, 2018). Despite the great advancement in genetic and molecular investigations, there is little information on the dynamic aspects of mammalian reproduction processes in the native environment. The major obstacle limiting the investigation of these dynamics with traditional methods is restricted imaging access to the spermatozoa, oocytes, and embryos within the reproductive tract, which is located deep inside the body.
Optical coherence tomography (OCT) is emerging as a promising technology, which can address some of the limitations of traditional imaging methods. It is uniquely suited for the volumetric and dynamic investigation of the reproductive system in mice and other animal models. OCT is a noninvasive, label-free, and depth-resolved imaging modality that provides micro-scale spatial resolution, with an imaging depth of 1 to 2 mm. OCT is often referred to as an optical analog to ultrasound (US) technology because, similarly to the US, the imaging principle is based on measuring an echo delay in backscattered signals (light for OCT, sound for US) to define spatial positions of the scatters within the sample. In OCT, this is achieved by the analysis of low-coherence interference between the light backscattered from the sample and a reference signal. A single unit of the OCT data is an in-depth spatially-resolved line, called an A-line. The two-dimensional (2D) and three-dimensional (3D) imaging is performed by transverse scanning of the imaging beam. An A-line scan rate is one of the major specifications for each OCT system defining the achievable imaging speed. In comparison to traditionally used confocal fluorescence microscopy, OCT provides higher imaging depth by about an order of magnitude, a larger field of view, and does not require exogenous contrast agents. OCT light exposure is considered to be safe by the U.S Food and Drug Administration (FDA), and is widely used in ophthalmology and cardiology clinics. It allows for dynamic volumetric imaging, which can be performed in vivo and longitudinally. These unique features allow for prolonged investigations of tissues with minimized alterations to the natural environments. Since OCT was first introduced in 1991 (Huang et al., 1991), it was extensively developed for applications in ophthalmology (Drexler et al., 2001; Ong et al., 2022), oncology (Vakoc et al., 2012; Yang et al., 2022), cardiology (Bezerra et al., 2009; Bouma et al., 2003; Gupta et al., 2022), and developmental biology (Boppart et al., 2000; Larina et al., 2011; Lopez et al., 2020). Recently, several studies extended OCT applications to reproductive biology for both, male and female, reproductive systems. This review describes the approaches for OCT imaging of different reproductive processes, such as sperm migration, oocyte/embryo transport, and cilia dynamics in the reproductive tract, in the context of other imaging studies. We highlight the findings revealed by these approaches and briefly discuss the unexplored questions and limitations for each reproductive process that OCT could potentially address in future studies.
2. Sperm Transport in Male Reproductive Tract
After spermatozoa are produced and released from the testis, they are transported through the male reproductive tract for ejaculation. Testicular spermatozoa are functionally immature and become mature during the transit through the male reproductive tract. Disrupted regulation of sperm transport can lead to incomplete sperm maturation and male infertility. For example, both the acceleration and delay of sperm transit time in the epididymis affect the sperm quality (Fernandez et al., 2008; Meistrich et al., 1975). The sperm transport begins in the seminiferous tubules inside the testes, followed by transit through the rete testis, efferent ducts, epididymal ducts, vas deferens, and urethra. Although sperm acquire motility and potential fertilizing capacity during their transit through the epididymis, they maintain a quiescent state in the male reproductive tract and become active after ejaculation (Dacheux & Dacheux, 2014; James et al., 2020). Therefore, sperm are passively transported through the male reproductive tract.
Several imaging-based investigations contributed to our understanding of this transport mechanism. Fleck et al. developed an in vivo imaging method for mouse testis and quantified the spontaneous contractions of seminiferous tubules that push immotile sperm toward the rete testis and efferent duct (Fleck et al., 2021). Through this method, the testis exposed through an incision was carefully lifted and positioned within a temperature-controlled chamber filled with medium, mounted on an imaging stage, while still attached to blood and nerve supply. In the efferent duct, both the ciliary activity and peristaltic contractions are believed to regulate sperm transport (Aprea et al., 2021; Hoque et al., 2022), but a recent paper suggested that cilia-generated movement is less powerful than smooth muscle contractions in propelling the sperm toward the epididymis (Yuan et al., 2019). For that investigation, the whole testis together with the attached efferent duct and epididymis were removed from the animal for microscopy imaging. A few other groups also approached epididymis imaging ex vivo, in freshly excised tissue that remains intact at the organ level. These time-lapse imaging studies revealed that contractions of the epididymal duct are involved in sperm transport through the epididymis (Elfgen et al., 2018; Mietens et al., 2014; Weiser et al., 2020). Collectively, it was concluded that contractions of the male reproductive tract are a major contributor to sperm transport in the testes (Fleck et al., 2021), efferent ducts (Yuan et al., 2019), epididymis (Elfgen et al., 2018; Weiser et al., 2020), and vas deferens (Koslov & Andersson, 2013).
There are limited studies exploring the use of OCT in investigating the male reproductive tract in mammals. Trottmann et al. applied OCT for 2D imaging of the microstructure of the male reproductive organs in a bovine model (Trottmann et al., 2016). The imaging was performed ex vivo, and the authors suggested that OCT has the potential to diagnose the structural alterations in the reproductive tract. Ramasamy et al. applied an OCT-based approach to detect sperm within seminiferous tubules in rat testes ex vivo (Ramasamy et al., 2012). This study demonstrated that OCT could potentially be used for the evaluation of spermatogenesis within seminiferous tubules, which could aid sperm retrieval in men undergoing microdissection testicular sperm extraction.
Very recently, our group performed dynamic volumetric (3D) OCT imaging of different regions within the mouse male reproductive tract (Umezu et al., 2022). The OCT imaging was performed on freshly extracted intact reproductive organs, maintained at 37°C. With this approach, detailed structural features of the testis, efferent ducts, epididymis, and vas deferens were volumetrically visualized (Fig 1). In addition to structural details, the study demonstrated visualization of contractions of the epididymal ducts, which coincided with the flow of the luminal contents inside the duct, suggesting a potential of OCT to investigate the physiological mechanism of sperm transport through the male reproductive tract.
Figure 1.

Volumetric OCT imaging of mouse male reproductive tract. (A-D) Three-dimensional OCT reconstruction of testis (A), epididymis (B), efferent ducts (C), and vas deferens (D). (A’-D’) Cross-sectional OCT image of testis (A’), epididymis (B’), efferent ducts (C’), and vas deferens (D’). Yellow, cyan, magenta and green arrowheads indicate seminiferous tubules inside the testis, lumen of epididymal duct, muscle layers and epithelium of vas deferens, respectively. Scale bars in (A), (A’), and (B) correspond to 500 μm, and the other scale bars correspond to 200 μm. Reproduced from Umezu et al., 2022.
The afore-mentioned volumetric dynamic imaging of mouse male reproductive organs was performed ex vivo (Umezu et al., 2022); however, potentially, imaging protocols could be developed for in vivo studies. As described below in this review, the mouse female reproductive system was successfully imaged with OCT using an intravital approach, through the aperture of an implantable imaging window, which allows bypassing the skin and muscle layers (Wang & Larina, 2021). Similar methods could be implemented for OCT investigation of the male reproductive system in the future. Once the in vivo OCT-based imaging protocols are established, implementation of these methods would contribute to a better understanding of the natural sperm dynamics within the male reproductive tract. This method would also become a useful tool for functional phenotyping in genetic mouse models with abnormalities in sperm transport and tubular contractions.
3. Sperm Migration in Female Reproductive Tract
After ejaculation, spermatozoa become motile and begin their journey through the female reproductive tract. Millions or billions of sperm are deposited into the vagina, the cervix, or the uterus, depending on the animal species (Kolle, 2015). During transit, the environment of the female reproductive tract maintains sperm viability and fertilizing ability, as well as regulating sperm capacitation and migration towards the oocyte (Suarez, 2016). After coitus, the uterus exhibits active peristaltic contractions, which push dense sperm mass to the utero-tubal junction (UTJ) (Yanagimachi, 2022). Multiple proteins on the sperm plasma membrane such as ADAM3 are required to pass through the UTJ (Fujihara et al., 2018; Okabe, 2013, 2014). After passing through the UTJ, sperm reach the lower oviduct, isthmus, which is a long thin duct lined with transverse folds. Sperm are believed to form the sperm reservoir in the isthmus (or UTJ in some mammalian species) by binding their heads to the ciliated cells of the epithelium, which is documented in many mammalian species (Hunter, 2008; Kolle, 2015; Suarez, 2008). This sperm-oviduct interaction holds sperm until ovulation and serves to maintain the fertilization competence of stored sperm (Chian & Sirard, 1995; Miller, 2018; Pollard et al., 1991; Suarez, 2016). Around the time of ovulation, sperm need to detach from the epithelium and migrate towards the oviductal ampulla, where fertilization takes place. This detachment is thought to be induced by capacitation including modifications of cell surface proteins and hyperactivation of the motility (Ardon et al., 2016; Hung & Suarez, 2012; Mirihagalle et al., 2022; Suarez, 2008). Eventually, only a few sperm reach the fertilization site and fertilize an oocyte.
Multiple imaging techniques have been utilized to investigate the dynamics and mechanisms of sperm migration through the female reproductive tract in animal models. One well-established approach is microscopic imaging of fluorescently labeled sperm from genetically modified mice. Yamaguchi et al. developed an ex vivo imaging method to visualize sperm in the upper female reproductive tract using sperm with green fluorescent protein-tagged acrosin. With this approach, the authors showed that the Adam3-disrupted sperm were unable to migrate into the oviduct after the natural mating (Yamaguchi et al., 2009), supporting the idea that the UTJ serves as a major selective barrier that allows only functionally and morphologically normal sperm to enter the oviduct (Mahe et al., 2021). Chang et al. performed ex vivo imaging of mouse sperm with fluorescently labeled acrosomes in the oviduct and observed that sperm repeatedly attached to and detached from the epithelium during the migration towards the ampulla (Chang & Suarez, 2012). Similar imaging approaches were also performed in vivo to understand the dynamic process of sperm migration in the oviduct. Muro et al. demonstrated that sperm undergo the acrosome reaction around the upper isthmus before they reach the ampulla (Muro et al., 2016). Ishikawa et al. showed that both sperm motility and oviductal smooth muscle contractions mediate sperm migration in vivo (Ishikawa et al., 2016). These observations suggest that the interaction of sperm and the female reproductive tract plays an essential role in sperm migration and fertilization. However, the major limitation of studies using fluorescently labeled spermatozoa remains to be an inability to visualize sperm trajectories volumetrically in relation to the oviduct wall, which limits the application of this method for the investigation of sperm regulation by mucosal structure and complex 3D environment.
This limitation was addressed by the implementation of a functional OCT-based imaging approach (Wang & Larina, 2018), which allowed for in vivo three-dimensional tracking of sperm behaviors in the mouse oviduct (Fig 2). For the imaging, the female mouse was anesthetized, and the reproductive tract was pulled slightly out through an abdominal incision and stabilized with a surgical clamp over the course of the imaging session. The oviduct was imaged with OCT through an intact oviductal wall. Individual spermatozoa were distinguished in volumetric OCT data sets from other cells and debris in the oviduct based on their unique tortuous trajectories, produced by their active movement, and tracked volumetrically within the oviduct. This study revealed individual sperm trajectories volumetrically within the natural environment of the female reproductive tract, which were previously beyond access, revealing a great variety of never-before-seen sperm behaviors. The study demonstrated that sperm velocity changed depending on the distance to the ciliated oviductal wall. It was demonstrated that sperm exhibit a characteristic switch in swimming behavior as they approach the ciliated oviductal wall, which could be explained by the rheotactic response of sperm to cilia-generated flows. The authors also reported sperm grouping and separation behaviors with faster movement in groups as the first in vivo evidence of sperm cooperation at the site of fertilization. To our knowledge, this study is the only report of in vivo volumetric sperm imaging within the female reproductive tract with OCT; however, the reported findings set OCT as a unique tool for quantitative and volumetric analysis of sperm motility as well as microstructures of the oviduct in vivo, not achievable with other methods.
Figure 2.

In vivo 3D sperm tracking in the mouse oviduct using OCT. (A) The representative trajectory shows sperm swimming along the mucosa folds in the ampulla. (B) Sperm trajectory captured next to the cumulus-oocyte complexes in the ampulla. Scale bars correspond to 50 μm. Reproduced from Wang & Larina, 2018.
Potentially, the unique features of OCT imaging can enable us to address unresolved questions about mammalian sperm navigation. Based on in vitro observations, sperm behaviors can be influenced by chemical gradients (chemotaxis), temperature (thermotaxis), fluid flows (rheotaxis), and boundary conditions (Denissenko et al., 2012; Miki & Clapham, 2013; Mondal et al., 2017; Perez-Cerezales et al., 2015; Umezu et al., 2020). However, whether and how these factors control sperm migration in vivo is not known. OCT has the potential to answer these questions by defining the relationships between sperm behaviors and chemicals secreted by the female reproductive tissues, temperature gradients, oviduct smooth muscle contractions, ciliary activities, and structural features of the oviductal lumen.
4. Oocyte/Embryo Transport and Early Embryo development
Prior to ovulation, oocytes grow and mature inside ovarian follicles surrounded by somatic cells. The oocyte development is accompanied by the growth of the follicle and regulated by the responses to endocrine hormones and intraovarian factors (Liu et al., 2019). OCT is applicable to the investigations of ovarian tissue and follicular development. There have been multiple attempts to implement OCT imaging toward visualizing the ovarian microstructures including follicles, corpus luteum, stroma, epithelium, collagen, and ovarian blood vessels (Evans et al., 2009; T. Wang et al., 2015; Yang et al., 2011). OCT is also being explored as a tool for the evaluation of follicles and ovarian reserve in patients (Takae et al., 2018), as well as detecting the changes associated with neoplasia and ovarian cancer in human samples (Zeng et al., 2019). The applications for ovarian tissue evaluation are likely to expand in the future as OCT technology develops.
Following ovulation, the oocytes are transferred to the oviduct, fertilized by sperm, and the embryos are transferred to the uterus for implantation. The embryo transport through the oviduct to the uterus takes approximately 2 to 4 days in most mammals (Croxatto, 2002). The embryo transport is believed to be regulated by oviductal motile cilia, smooth muscle contractions, and secretory fluid flows (Coy et al., 2012; Ezzati et al., 2014). Biochemical and pharmacological approaches contribute to the increasing understanding of the transport mechanism at the cellular and molecular levels (Besenfelder et al., 2012; Halbert et al., 1976; Li & Winuthayanon, 2017). However, advanced imaging investigations are necessary for fully understanding this dynamic event. Multiple investigators performed OCT imaging of the oviduct with a primary focus on diagnostics of oviduct pathologies. Hermann et al. demonstrated OCT-enabled three-dimensional micro-scale imaging of the human oviductal ampulla samples obtained after the hysterectomy (Herrmann et al., 1998). Kirillin et al. established the laparoscopic OCT probe for in vivo imaging of the human oviductal wall and diagnosing inflammation (Kirillin et al., 2012). In this study, the OCT probe was inserted into the abdominal cavity and positioned above the surface of the oviductal isthmus with a holder. The majority of ovarian cancers are believed to originate in the oviduct. Therefore, there is a major effort toward the development of in vivo oviduct screening methods. Fiber-based OCT probes guided through the fallopian tube (falloposcopes) are currently being developed for the ovarian cancer diagnostics and screening (Keenan et al., 2017; Madore et al., 2017). If this trend continues, OCT fiber-based falloposcopes are expected to enter clinical trials within a few years, and have a chance to become a standard clinical practice for ovarian cancer screening within the fallopian tube.
In the mouse model, in vivo imaging of the oviduct using OCT was first demonstrated by Burton et al. (Burton et al., 2015). The reproductive organs were exposed for imaging through a dorsal incision in anesthetized female mice. To prevent movement artifacts due to animal breathing, the reproductive organs were stabilized with a clamp. This approach enabled live, high-resolution, three-dimensional visualizations of the oviductal lumen, mucosal folds, ovulated oocytes surrounded by cumulus cells in the ampulla, and distinct structural differences between different oviductal regions. Rodriguez et al. implemented OCT imaging ex vivo to analyze the nature of fertility defects in a mouse mutant Smad1/5/4-Amhr2-cre conditional knock-out (cKO) (Rodriguez et al., 2016). The imaging study revealed that cumulus-oocyte complexes in the mutant appeared morphologically similar to the controls, and the ampulla showed normal overall structure and mucosal folds, while the isthmus exhibited structural defects, which likely prevented embryo transfer to the uterus. That study demonstrated a potential for OCT in the structural phenotyping of genetic mouse models with reproductive defects.
Protocols established by Burton et al. (Burton et al., 2015) enabled short-term in vivo imaging; however, since the reproductive organs were exposed during the procedure, long-term imaging through this approach was not feasible. That limitation was resolved by Wang et al. by integrating OCT imaging with an intravital approach (Wang et al., 2018). To bypass skin and muscle layers, a 3D printed window was surgically implanted on the dorsal side of a mouse, exposing the reproductive organs for imaging through an aperture covered with a glass cover slip. With windows implanted, mice mated and embryos implanted in the uterus, suggesting a semi-physiological environment. This method allowed the authors to perform prolonged imaging of reproductive organs and visualize oviductal contractions volumetrically. It also allowed the authors to perform a longitudinal structural assessment of the reproductive system over the course of 3 days (Wang et al., 2018).
During the transit through the oviduct, preimplantation embryos undergo cellular divisions. It usually takes 3 to 4 days for human and mouse embryos to develop into an 8-cell (human) or 16-cell (mouse) stage in the oviduct (Li & Winuthayanon, 2017). Multiple studies reported static structural characterization of oocytes, zygotes, and preimplantation embryos using OCT-based technologies (Masuda et al., 2021; Xiao et al., 2012; Zarnescu et al., 2015). A major step forward came with the study by Karnowski et al. who performed a detailed, dynamic investigation of preimplantation development with a higher-resolution version of OCT, optical coherence microscopy (OCM), in vitro (Karnowski et al., 2017). That study visualized a variety of dynamic events including movements of zygotic pronuclei and first embryonic divisions. Caujolle et al. implemented speckle variance OCT to introduce additional functional contrast and assess the viability of bovine embryos (Caujolle et al., 2017). Through the implementation of intravital imaging, Moore et al. demonstrated in vivo staging of mouse preimplantation embryos within the oviduct (Moore et al., 2019). With this approach, one-cell embryos at 0.5 days post-conception (dpc), two-cell embryos at 1.5 dpc, and four-cell or higher staged embryos at 2.5 dpc were located in the ampulla, isthmus, and proximal to the UTJ, respectively. This analysis demonstrated the capability of OCT for the analysis of detailed subcellular features such as pronuclei, polar body, zona pellucida, and plasma membrane of each cell in vivo.
The same intravital OCT imaging approach enabled Wang & Larina to perform the first in vivo volumetric imaging of oocyte and embryo transport through the mouse oviduct (Wang & Larina, 2021). The authors reported 4D (3D + time) visualizations through different regions of the oviduct from the upper ampulla to the lower isthmus, revealing a variety of movement patterns during the oocyte and embryo transport (Fig 3). Currently, there are no alternative methods, which reveal oocyte/embryo transport volumetrically within the oviduct. In the upper ampulla, cumulus-oocyte complexes were steered along the highly-ciliated luminal wall in a repetitive circular pattern likely driven by cilia (Fig 3A). Their progression to the lower ampulla was controlled by a luminal constriction. In the lower ampulla, cumulus-oocyte complexes exhibit a short-distance pulsatile oscillatory movement (Wang & Larina, 2021), which is in agreement with the previous finding of oocyte movement in the lower ampulla of the rabbit oviduct (Halbert et al., 1976). In contrast, preimplantation embryos show long-distance, bi-directional, fast movement in the isthmus under peristaltic luminal contraction waves (Wang & Larina, 2021). Because this region of the oviduct has little motile cilia (S. Wang et al., 2015), this is likely a result of the contractile activity of the smooth muscle cells (Ezzati et al., 2014). The bi-directional movement of oocytes and embryos in the isthmus is also reported with bright-field and epi-fluorescence microscopy (Bochner et al., 2015; Ishikawa et al., 2016). Overall, the OCT based-imaging approach revealed intriguing previously hidden movements of oocytes and preimplantation embryos in their native oviductal environment. It is also suggestive of spatially dependent smooth muscle regulatory mechanisms in the oviductal transport of oocytes and embryos in vivo. These findings provide an important insight into the transport mechanism from dynamic perspective, which is not achievable with histological analysis or static reconstructions of extracted tissues. However, the molecular mechanisms and specific roles of the observed movements remain unclear and would need to be further investigated. Oviductal dysfunction is associated with reproductive disorders, including tubal ectopic pregnancy, whose etiology remains largely unknown (Shaw et al., 2010). Further understanding of oocyte/embryo transport is biologically and clinically important and might be achieved by integrating genetic or pharmacological manipulation of cilia and smooth muscle dynamics with in vivo imaging methods.
Figure 3.

In vivo 3D imaging of oocytes and embryos in the mouse oviduct. (A) The circular movement of cumulus-oocyte complexes in the upper ampulla. (B) Embryo movement and separation in the lower ampulla. Red arrows indicate the large embryo group, and purple arrowheads indicate the movement of a single embryo. Scale bars in (A) and (B) correspond to 300 μm and 200 μm, respectively. Reproduced from Wang & Larina, 2021.
5. Cilia Dynamics in Female and Male Reproductive Systems
Cilia are microtubule-based organelles located on the epithelium of a variety of tissues including both, male and female reproductive tracts. The oviduct is one of the organs that rely on ciliary activity for its functions (Halbert et al., 1976; Lyons et al., 2002). The cilia beat frequency (CBF) is widely used as a fundamental measurement of the cilia activity (Knoll et al., 1995; Liao et al., 2011; Noreikat et al., 2012; Shi et al., 2011). Bright-field microscopy and confocal microscopy are major imaging techniques that have been extensively applied for in vitro or ex vivo visualization of cilia, assessment of CBF, and studying hormonal regulation of ciliary activity (Teilmann et al., 2005). For example, using bright-field microscopy, Bylander et al. observed the rapid reduction in CBF of the dissected oviduct by nM concentrations of progesterone (Bylander et al., 2010). Liao et al. demonstrated that the presence of motile sperm increases the CBF in rat oviduct through adrenomedullin hormone, which causes an increase in the oviductal CBF (Liao et al., 2011), suggesting sperm influence on the ciliary activity, which would be interesting to investigate in vivo.
Due to the limited imaging depths of bright-field and fluorescence microscopy, the oviductal samples are usually dissected to expose the ciliated lumen for direct observation, disrupting the natural biomechanical environment. Before the implementation of OCT, in vivo investigation of ciliary dynamics through tissue layers was not feasible, and OCT is still the only technology that allows one to investigate the ciliary activity within the intact oviduct without dissection. The technology for functional mapping of cilia and CBF thorough tissue layers was developed by Wang et al. (S. Wang et al., 2015). Based on spectral analysis of temporal pixel fluctuations in OCT images, this method enables in vivo depth-resolved mapping of the cilia location and CBF in the mouse oviduct with a micro-scale spatial resolution (Fig 4A). For in vivo imaging, the reproductive tract of an anesthetized female mouse was pulled out through a small incision and stabilized with a vessel clamp. With this approach, it was demonstrated that the cilia from the different oviductal regions, isthmus and ampulla, exhibit a similar CBF range in vivo, which is supported by the in vitro experimental results of the CBF in the human oviduct (Lyons et al., 2002). In contrast, the frequency range of the cilia beat in the ampulla at 0.5 dpc is higher than that at 2.5 dpc (S. Wang et al., 2015), suggesting that the cilia activity within the oviduct is temporally regulated. In a different study implementing an intravital imaging window, Wang et al. performed a follow-up longitudinal analysis of the ciliary beating in the same location of the oviduct over the course of 3 days (Wang et al., 2018), and confirmed a significant difference in CBF between the time points. What factors regulate the oviductal cilia activity, and whether/how the cilia dynamics are coordinated with oocyte/embryo transport and fertility, is still largely unknown and would need to be further investigated.
Figure 4.
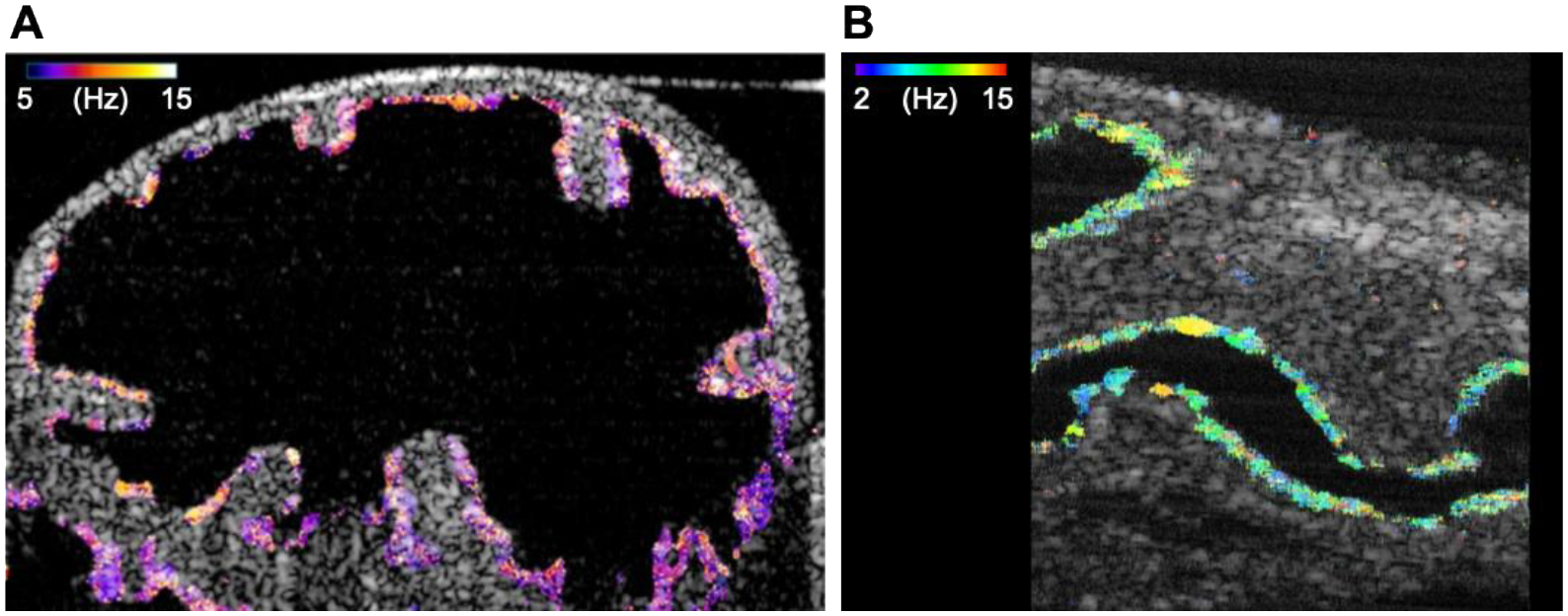
CBF mapping of female and male reproductive tracts with functional OCT. (A) Mapping of CBF in the mouse oviduct. (B) Cross-sectional image with OCT mapping of the CBF in the mouse efferent ducts. Reproduced from Wang et al., 2015 and Umezu et al., 2022.
In the male reproductive tract, the efferent duct is a very unique region that harbors ciliated cells (Hoque et al., 2022; Terre et al., 2019). Motile cilia of the efferent duct play an essential role in sperm passage through the reproductive tract. Loss of cilia beating can cause sperm aggregation, sperm granulomas, oligozoospermia, and male infertility (Aprea et al., 2021; Yuan et al., 2019). Despite the biological importance of the cilia dynamics in this organ, there are very few quantitative studies of ciliary activity in the efferent ducts due to the lack of suitable imaging tools. Very recently, our group reported a volumetric OCT imaging approach with the spectral analysis of the speckle variation, which allowed us to capture the cilia dynamics of mouse efferent ducts (Umezu et al., 2022). Quantitative volumetric mapping of cilia and CBF was performed. The 3D CBF mapping showed that the ciliated cells were equally distributed across the luminal epithelium of the efferent ducts (Fig 4B). While that study was performed ex vivo, intravital imaging protocols can potentially be implemented, similar to those described above for female reproductive organs. These methods are likely to be established in the near future, enabling in vivo dynamic and longitudinal analysis of the ciliary activity in the male reproductive system. Considering that motile cilia play an important role in efferent duct function, such as the regulation of sperm transport and prevention of sperm aggregation, this approach can be very useful in defining mechanistic aspects of the process in genetic mouse models.
6. Summary
Over recent years, OCT is gaining recognition in reproductive research due to its unique technical characteristics. Cellular-level resolution, millimeter-level imaging depth, large field of view, reliance on natural tissue contrast, and ability of dynamic volumetric imaging make this technology very attractive for investigation of various dynamic processes. Taking advantage of these features, OCT already contributes to a better understanding of sperm migration, oocyte/embryo transport, cilia dynamics, and the interplay between these processes in the native state.
As all imaging methods, OCT is not ideal for all applications. One of the major limitations of OCT is inability to label particular cells of interest. Therefore, when cellular or molecular specificity is important, fluorescence microscopy would be an imaging technology of choice. For applications where in vivo dynamic aspects are not critical, micro-computed tomography (microCT) or light-sheet microscopy in combination with tissue clearing might be a better choice in comparison to OCT since these technologies allow for higher spatial resolution. However, OCT is a superior technology when it comes to volumetric dynamic investigations of reproductive processes without exogenous labeling, particularly in mouse models.
There is a great spectrum of opportunities for OCT technology investigations in the future. Much of our understanding regarding mammalian reproduction is still largely derived from the extrapolation of in vitro and ex vivo experiments and lacks in vivo evidence. Specifically, it remains uncertain whether chemotaxis, thermotaxis, and rheotaxis contribute to in vivo sperm migration, where/when sperm induce hyperactivation in vivo, how cilia dynamics and muscle contractions contribute to preimplantation embryo transport, what really happens in vivo in numerous animal models of infertility, all of which would highly benefit from dynamic imaging assessment. Since many molecules affecting male and female fertility have been identified through genetic manipulation in mice (Okabe, 2013, 2018; Satouh & Ikawa, 2018), dynamic imaging investigations under functionally disrupted conditions might be useful to address unresolved questions in mammalian reproduction. Such a combination of the advanced imaging technique and genetic, molecular, or pharmacological approach would hopefully provide important insights and better strategies for the management of human infertility and other reproductive pathologies.
Acknowledgments
We would like to thank Dr. Deirdre Scully for her critical reading of the manuscript. This study was financially supported by a postdoctoral fellowship from the Japan Society for the Promotion of Science and grants from the National Institutes of Health R01EB027099 and R01HD096335.
Footnotes
Conflict of Interests
The authors declare no conflicts of interest.
References
- Aprea I, Nothe-Menchen T, Dougherty GW, Raidt J, Loges NT, Kaiser T, Wallmeier J, Olbrich H, Strunker T, Kliesch S, Pennekamp P, & Omran H (2021). Motility of efferent duct cilia aids passage of sperm cells through the male reproductive system. Mol Hum Reprod, 27(3). 10.1093/molehr/gaab009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardon F, Markello RD, Hu L, Deutsch ZI, Tung CK, Wu M, & Suarez SS (2016). Dynamics of Bovine Sperm Interaction with Epithelium Differ Between Oviductal Isthmus and Ampulla. Biol Reprod, 95(4), 90. 10.1095/biolreprod.116.140632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besenfelder U, Havlicek V, & Brem G (2012). Role of the oviduct in early embryo development. Reprod Domest Anim, 47 Suppl 4, 156–163. 10.1111/j.1439-0531.2012.02070.x [DOI] [PubMed] [Google Scholar]
- Bezerra HG, Costa MA, Guagliumi G, Rollins AM, & Simon DI (2009). Intracoronary optical coherence tomography: a comprehensive review clinical and research applications. JACC Cardiovasc Interv, 2(11), 1035–1046. 10.1016/j.jcin.2009.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner F, Fellus-Alyagor L, Kalchenko V, Shinar S, & Neeman M (2015). A Novel Intravital Imaging Window for Longitudinal Microscopy of the Mouse Ovary. Sci Rep, 5, 12446. 10.1038/srep12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boppart SA, Brezinski ME, & Fujimoto JG (2000). Optical coherence tomography imaging in developmental biology. Methods Mol Biol, 135, 217–233. 10.1385/1-59259-685-1:217 [DOI] [PubMed] [Google Scholar]
- Bouma BE, Tearney GJ, Yabushita H, Shishkov M, Kauffman CR, DeJoseph Gauthier D, MacNeill BD, Houser SL, Aretz HT, Halpern EF, & Jang IK (2003). Evaluation of intracoronary stenting by intravascular optical coherence tomography. Heart, 89(3), 317–320. 10.1136/heart.89.3.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton JC, Wang S, Stewart CA, Behringer RR, & Larina IV (2015). High-resolution three-dimensional in vivo imaging of mouse oviduct using optical coherence tomography. Biomed Opt Express, 6(7), 2713–2723. 10.1364/BOE.6.002713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylander A, Nutu M, Wellander R, Goksor M, Billig H, & Larsson DG (2010). Rapid effects of progesterone on ciliary beat frequency in the mouse fallopian tube. Reprod Biol Endocrinol, 8, 48. 10.1186/1477-7827-8-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caujolle S, Cernat R, Silvestri G, Marques MJ, Bradu A, Feuchter T, Robinson G, Griffin DK, & Podoleanu A (2017). Speckle variance OCT for depth resolved assessment of the viability of bovine embryos. Biomedical Optics Express, 8(11), 5139–5150. 10.1364/BOE.8.005139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, & Suarez SS (2012). Unexpected flagellar movement patterns and epithelial binding behavior of mouse sperm in the oviduct. Biol Reprod, 86(5), 140, 141–148. 10.1095/biolreprod.111.096578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chian RC, & Sirard MA (1995). Fertilizing ability of bovine spermatozoa cocultured with oviduct epithelial cells. Biol Reprod, 52(1), 156–162. 10.1095/biolreprod52.1.156 [DOI] [PubMed] [Google Scholar]
- Coy P, Garcia-Vazquez FA, Visconti PE, & Aviles M (2012). Roles of the oviduct in mammalian fertilization. Reproduction, 144(6), 649–660. 10.1530/REP-12-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxatto HB (2002). Physiology of gamete and embryo transport through the fallopian tube. Reprod Biomed Online, 4(2), 160–169. 10.1016/s1472-6483(10)61935-9 [DOI] [PubMed] [Google Scholar]
- Dacheux JL, & Dacheux F (2014). New insights into epididymal function in relation to sperm maturation. Reproduction, 147(2), R27–42. 10.1530/REP-13-0420 [DOI] [PubMed] [Google Scholar]
- Denissenko P, Kantsler V, Smith DJ, & Kirkman-Brown J (2012). Human spermatozoa migration in microchannels reveals boundary-following navigation. Proc Natl Acad Sci U S A, 109(21), 8007–8010. 10.1073/pnas.1202934109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler W, Morgner U, Ghanta RK, Kartner FX, Schuman JS, & Fujimoto JG (2001). Ultrahigh-resolution ophthalmic optical coherence tomography. Nat Med, 7(4), 502–507. 10.1038/86589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfgen V, Mietens A, Mewe M, Hau T, & Middendorff R (2018). Contractility of the epididymal duct - function, regulation and potential drug effects. Reproduction. 10.1530/rep-17-0754 [DOI] [PubMed] [Google Scholar]
- Evans CL, Rizvi I, Hasan T, & de Boer JF (2009). In vitro ovarian tumor growth and treatment response dynamics visualized with time-lapse OCT imaging. Opt Express, 17(11), 8892–8906. 10.1364/oe.17.008892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati M, Djahanbakhch O, Arian S, & Carr BR (2014). Tubal transport of gametes and embryos: a review of physiology and pathophysiology. J Assist Reprod Genet, 31(10), 1337–1347. 10.1007/s10815-014-0309-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainberg J, & Kashanian JA (2019). Recent advances in understanding and managing male infertility. F1000Res, 8. 10.12688/f1000research.17076.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez CD, Porto EM, Arena AC, & Kempinas Wde G (2008). Effects of altered epididymal sperm transit time on sperm quality. Int J Androl, 31(4), 427–437. 10.1111/j.1365-2605.2007.00788.x [DOI] [PubMed] [Google Scholar]
- Fleck D, Kenzler L, Mundt N, Strauch M, Uesaka N, Moosmann R, Bruentgens F, Missel A, Mayerhofer A, Merhof D, Spehr J, & Spehr M (2021). ATP activation of peritubular cells drives testicular sperm transport. Elife, 10. 10.7554/eLife.62885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara Y, Miyata H, & Ikawa M (2018). Factors controlling sperm migration through the oviduct revealed by gene-modified mouse models. Exp Anim, 67(2), 91–104. 10.1538/expanim.17-0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Shrivastava A, Vijayvergiya R, Chhikara S, Datta R, Aziz A, Singh Meena D, Nath RK, & Kumar JR (2022). Optical Coherence Tomography: An Eye Into the Coronary Artery. Front Cardiovasc Med, 9, 854554. 10.3389/fcvm.2022.854554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert SA, Tam PY, & Blandau RJ (1976). Egg transport in the rabbit oviduct: the roles of cilia and muscle. Science, 191(4231), 1052–1053. 10.1126/science.1251215 [DOI] [PubMed] [Google Scholar]
- Herrmann JM, Brezinski ME, Bouma BE, Boppart SA, Pitris C, Southern JF, & Fujimoto JG (1998). Two- and three-dimensional high-resolution imaging of the human oviduct with optical coherence tomography. Fertil Steril, 70(1), 155–158. 10.1016/s0015-0282(98)00097-1 [DOI] [PubMed] [Google Scholar]
- Hoque M, Kim EN, Chen D, Li FQ, & Takemaru KI (2022). Essential Roles of Efferent Duct Multicilia in Male Fertility. Cells, 11(3). 10.3390/cells11030341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, & et al. (1991). Optical coherence tomography. Science, 254(5035), 1178–1181. 10.1126/science.1957169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung PH, & Suarez SS (2012). Alterations to the bull sperm surface proteins that bind sperm to oviductal epithelium. Biol Reprod, 87(4), 88. 10.1095/biolreprod.112.099721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RH (2008). Sperm release from oviduct epithelial binding is controlled hormonally by peri-ovulatory graafian follicles. Mol Reprod Dev, 75(1), 167–174. 10.1002/mrd.20776 [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Usui T, Yamashita M, Kanemori Y, & Baba T (2016). Surfing and Swimming of Ejaculated Sperm in the Mouse Oviduct. Biol Reprod, 94(4), 89. 10.1095/biolreprod.115.135418 [DOI] [PubMed] [Google Scholar]
- James ER, Carrell DT, Aston KI, Jenkins TG, Yeste M, & Salas-Huetos A (2020). The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction. Int J Mol Sci, 21(15). 10.3390/ijms21155377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnowski K, Ajduk A, Wieloch B, Tamborski S, Krawiec K, Wojtkowski M, & Szkulmowski M (2017). Optical coherence microscopy as a novel, non-invasive method for the 4D live imaging of early mammalian embryos. Sci Rep, 7(1), 4165. 10.1038/s41598-017-04220-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan M, Tate TH, Kieu K, Black JF, Utzinger U, & Barton JK (2017). Design and characterization of a combined OCT and wide field imaging falloposcope for ovarian cancer detection. Biomed Opt Express, 8(1), 124–136. 10.1364/BOE.8.000124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirillin M, Panteleeva O, Yunusova E, Donchenko E, & Shakhova N (2012). Criteria for pathology recognition in optical coherence tomography of fallopian tubes. J Biomed Opt, 17(8), 081413–081411. 10.1117/1.JBO.17.8.081413 [DOI] [PubMed] [Google Scholar]
- Knoll M, Shaoulian R, Magers T, & Talbot P (1995). Ciliary beat frequency of hamster oviducts is decreased in vitro by exposure to solutions of mainstream and sidestream cigarette smoke. Biol Reprod, 53(1), 29–37. 10.1095/biolreprod53.1.29 [DOI] [PubMed] [Google Scholar]
- Kolle S (2015). Transport, Distribution and Elimination of Mammalian Sperm Following Natural Mating and Insemination. Reprod Domest Anim, 50 Suppl 3, 2–6. 10.1111/rda.12576 [DOI] [PubMed] [Google Scholar]
- Koslov DS, & Andersson KE (2013). Physiological and pharmacological aspects of the vas deferens-an update. Front Pharmacol, 4, 101. 10.3389/fphar.2013.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol M, Nap A, Michels R, Veraart C, & Goossens L (2019). Health state utilities for infertility and subfertility. Reprod Health, 16(1), 47. 10.1186/s12978-019-0706-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larina IV, Larin KV, Justice MJ, & Dickinson ME (2011). Optical Coherence Tomography for live imaging of mammalian development. Curr Opin Genet Dev, 21(5), 579–584. 10.1016/j.gde.2011.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, & Winuthayanon W (2017). Oviduct: roles in fertilization and early embryo development. J Endocrinol, 232(1), R1–R26. 10.1530/JOE-16-0302 [DOI] [PubMed] [Google Scholar]
- Liao SB, Ho JC, Tang F, & O WS (2011). Adrenomedullin increases ciliary beat frequency and decreases muscular contraction in the rat oviduct. Reproduction, 141(3), 367–372. 10.1530/REP-10-0230 [DOI] [PubMed] [Google Scholar]
- Liu YX, Zhang Y, Li YY, Liu XM, Wang XX, Zhang CL, Hao CF, & Deng SL (2019). Regulation of follicular development and differentiation by intra-ovarian factors and endocrine hormones. Front Biosci (Landmark Ed), 24(5), 983–993. 10.2741/4763 [DOI] [PubMed] [Google Scholar]
- Lopez AL 3rd, Wang S, & Larina IV (2020). Embryonic Mouse Cardiodynamic OCT Imaging. J Cardiovasc Dev Dis, 7(4). 10.3390/jcdd7040042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons RA, Djahanbakhch O, Mahmood T, Saridogan E, Sattar S, Sheaff MT, Naftalin AA, & Chenoy R (2002). Fallopian tube ciliary beat frequency in relation to the stage of menstrual cycle and anatomical site. Hum Reprod, 17(3), 584–588. 10.1093/humrep/17.3.584 [DOI] [PubMed] [Google Scholar]
- Madore WJ, De Montigny E, Deschenes A, Benboujja F, Leduc M, Mes-Masson AM, Provencher DM, Rahimi K, Boudoux C, & Godbout N (2017). Morphologic three-dimensional scanning of fallopian tubes to assist ovarian cancer diagnosis. J Biomed Opt, 22(7), 76012. 10.1117/1.JBO.22.7.076012 [DOI] [PubMed] [Google Scholar]
- Mahe C, Zlotkowska AM, Reynaud K, Tsikis G, Mermillod P, Druart X, Schoen J, & Saint-Dizier M (2021). Sperm migration, selection, survival, and fertilizing ability in the mammalian oviductdagger. Biol Reprod, 105(2), 317–331. 10.1093/biolre/ioab105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Y, Hasebe R, Kuromi Y, Kobayashi M, Iwamoto M, Hishinuma M, Ohbayashi T, & Nishimura R (2021). Three-dimensional live imaging of bovine embryos by optical coherence tomography. J Reprod Dev, 67(2), 149–154. 10.1262/jrd.2020-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meistrich ML, Hughes TH, & Bruce WR (1975). Alteration of epididymal sperm transport and maturation in mice by oestrogen and testosterone. Nature, 258(5531), 145–147. 10.1038/258145a0 [DOI] [PubMed] [Google Scholar]
- Mietens A, Tasch S, Stammler A, Konrad L, Feuerstacke C, & Middendorff R (2014). Time-lapse imaging as a tool to investigate contractility of the epididymal duct--effects of cGMP signaling. PLoS One, 9(3), e92603. 10.1371/journal.pone.0092603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K, & Clapham DE (2013). Rheotaxis guides mammalian sperm. Curr Biol, 23(6), 443–452. 10.1016/j.cub.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ (2018). Review: The epic journey of sperm through the female reproductive tract. Animal, 12(s1), s110–s120. 10.1017/S1751731118000526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirihagalle S, Hughes JR, & Miller DJ (2022). Progesterone-Induced Sperm Release from the Oviduct Sperm Reservoir. Cells, 11(10). 10.3390/cells11101622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal MA, Takagi Y, Baba SA, & Hamano KI (2017). Involvement of calcium channels and intracellular calcium in bull sperm thermotaxis. J Reprod Dev, 63(2), 143–148. 10.1262/jrd.2016-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EL, Wang S, & Larina IV (2019). Staging mouse preimplantation development in vivo using optical coherence microscopy. J Biophotonics, 12(5), e201800364. 10.1002/jbio.201800364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro Y, Hasuwa H, Isotani A, Miyata H, Yamagata K, Ikawa M, Yanagimachi R, & Okabe M (2016). Behavior of Mouse Spermatozoa in the Female Reproductive Tract from Soon after Mating to the Beginning of Fertilization. Biol Reprod, 94(4), 80. 10.1095/biolreprod.115.135368 [DOI] [PubMed] [Google Scholar]
- Noreikat K, Wolff M, Kummer W, & Kolle S (2012). Ciliary activity in the oviduct of cycling, pregnant, and muscarinic receptor knockout mice. Biol Reprod, 86(4), 120. 10.1095/biolreprod.111.096339 [DOI] [PubMed] [Google Scholar]
- Okabe M (2013). The cell biology of mammalian fertilization. Development, 140(22), 4471–4479. 10.1242/dev.090613 [DOI] [PubMed] [Google Scholar]
- Okabe M (2014). Mechanism of fertilization: a modern view. Exp Anim, 63(4), 357–365. 10.1538/expanim.63.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M (2018). Sperm-egg interaction and fertilization: past, present, and future. Biol Reprod, 99(1), 134–146. 10.1093/biolre/ioy028 [DOI] [PubMed] [Google Scholar]
- Ong J, Zarnegar A, Corradetti G, Singh SR, & Chhablani J (2022). Advances in Optical Coherence Tomography Imaging Technology and Techniques for Choroidal and Retinal Disorders. J Clin Med, 11(17). 10.3390/jcm11175139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Cerezales S, Boryshpolets S, & Eisenbach M (2015). Behavioral mechanisms of mammalian sperm guidance. Asian J Androl, 17(4), 628–632. 10.4103/1008-682X.154308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW, Plante C, King WA, Hansen PJ, Betteridge KJ, & Suarez SS (1991). Fertilizing capacity of bovine sperm may be maintained by binding of oviductal epithelial cells. Biol Reprod, 44(1), 102–107. 10.1095/biolreprod44.1.102 [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Sterling J, Manzoor M, Salamoon B, Jain M, Fisher E, Li PS, Schlegel PN, & Mukherjee S (2012). Full field optical coherence tomography can identify spermatogenesis in a rodent sertoli-cell only model. J Pathol Inform, 3, 4. 10.4103/2153-3539.93401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Tripurani SK, Burton JC, Clementi C, Larina I, & Pangas SA (2016). SMAD Signaling Is Required for Structural Integrity of the Female Reproductive Tract and Uterine Function During Early Pregnancy in Mice. Biol Reprod, 95(2), 44. 10.1095/biolreprod.116.139477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satouh Y, & Ikawa M (2018). New Insights into the Molecular Events of Mammalian Fertilization. Trends Biochem Sci, 43(10), 818–828. 10.1016/j.tibs.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JL, Dey SK, Critchley HO, & Horne AW (2010). Current knowledge of the aetiology of human tubal ectopic pregnancy. Hum Reprod Update, 16(4), 432–444. 10.1093/humupd/dmp057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D, Komatsu K, Uemura T, & Fujimori T (2011). Analysis of ciliary beat frequency and ovum transport ability in the mouse oviduct. Genes Cells, 16(3), 282–290. 10.1111/j.1365-2443.2011.01484.x [DOI] [PubMed] [Google Scholar]
- Suarez SS (2008). Regulation of sperm storage and movement in the mammalian oviduct. Int J Dev Biol, 52(5–6), 455–462. 10.1387/ijdb.072527ss [DOI] [PubMed] [Google Scholar]
- Suarez SS (2016). Mammalian sperm interactions with the female reproductive tract. Cell Tissue Res, 363(1), 185–194. 10.1007/s00441-015-2244-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takae S, Tsukada K, Maeda I, Okamoto N, Sato Y, Kondo H, Shinya K, Motani Y, & Suzuki N (2018). Preliminary human application of optical coherence tomography for quantification and localization of primordial follicles aimed at effective ovarian tissue transplantation. J Assist Reprod Genet, 35(4), 627–636. 10.1007/s10815-018-1166-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teilmann SC, Byskov AG, Pedersen PA, Wheatley DN, Pazour GJ, & Christensen ST (2005). Localization of transient receptor potential ion channels in primary and motile cilia of the female murine reproductive organs. Mol Reprod Dev, 71(4), 444–452. 10.1002/mrd.20312 [DOI] [PubMed] [Google Scholar]
- Terre B, Lewis M, Gil-Gomez G, Han Z, Lu H, Aguilera M, Prats N, Roy S, Zhao H, & Stracker TH (2019). Defects in efferent duct multiciliogenesis underlie male infertility in GEMC1-, MCIDAS- or CCNO-deficient mice. Development, 146(8). 10.1242/dev.162628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottmann M, Kolle S, Leeb R, Doering D, Reese S, Stief CG, Dulohery K, Leavy M, Kuznetsova J, Homann C, & Sroka R (2016). Ex vivo investigations on the potential of optical coherence tomography (OCT) as a diagnostic tool for reproductive medicine in a bovine model. J Biophotonics, 9(1–2), 129–137. 10.1002/jbio.201500009 [DOI] [PubMed] [Google Scholar]
- Umezu K, Hara K, Hiradate Y, Numabe T, & Tanemura K (2020). Stromal cell-derived factor 1 regulates in vitro sperm migration towards the cumulus-oocyte complex in cattle. PLoS One, 15(4), e0232536. 10.1371/journal.pone.0232536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezu K, Xia T, & Larina IV (2022). Dynamic volumetric imaging and cilia beat mapping in the mouse male reproductive tract with optical coherence tomography. Biomed Opt Express, 13(6), 3672–3684. 10.1364/BOE.459937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakoc BJ, Fukumura D, Jain RK, & Bouma BE (2012). Cancer imaging by optical coherence tomography: preclinical progress and clinical potential. Nat Rev Cancer, 12(5), 363–368. 10.1038/nrc3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Burton JC, Behringer RR, & Larina IV (2015). In vivo micro-scale tomography of ciliary behavior in the mammalian oviduct. Sci Rep, 5, 13216. 10.1038/srep13216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, & Larina IV (2018). In vivo three-dimensional tracking of sperm behaviors in the mouse oviduct. Development, 145(6). 10.1242/dev.157685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, & Larina IV (2021). In vivo dynamic 3D imaging of oocytes and embryos in the mouse oviduct. Cell Rep, 36(2), 109382. 10.1016/j.celrep.2021.109382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Syed R, Grishina OA, & Larina IV (2018). Prolonged in vivo functional assessment of the mouse oviduct using optical coherence tomography through a dorsal imaging window. J Biophotonics, 11(5), e201700316. 10.1002/jbio.201700316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Brewer M, & Zhu Q (2015). An overview of optical coherence tomography for ovarian tissue imaging and characterization. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 7(1), 1–16. 10.1002/wnan.1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser D, Mietens A, Stadler B, Ježek D, Schuler G, & Middendorff R (2020). Contractions transport exfoliated epithelial cells through the neonatal epididymis. Reproduction, 160(1), 109–116. 10.1530/rep-19-0617 [DOI] [PubMed] [Google Scholar]
- Xiao J, Wang B, Lu G, Zhu Z, & Huang Y (2012). Imaging of oocyte development using ultrahigh-resolution full-field optical coherence tomography. Appl Opt, 51(16), 3650–3654. 10.1364/AO.51.003650 [DOI] [PubMed] [Google Scholar]
- Yamaguchi R, Muro Y, Isotani A, Tokuhiro K, Takumi K, Adham I, Ikawa M, & Okabe M (2009). Disruption of ADAM3 impairs the migration of sperm into oviduct in mouse. Biol Reprod, 81(1), 142–146. 10.1095/biolreprod.108.074021 [DOI] [PubMed] [Google Scholar]
- Yanagimachi R (2022). Mysteries and unsolved problems of mammalian fertilization and related topics. Biol Reprod, 106(4), 644–675. 10.1093/biolre/ioac037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Chen Y, Ling S, Wang J, Wang G, Zhang B, Zhao H, Zhao Q, & Mao J (2022). Research progress on the application of optical coherence tomography in the field of oncology. Front Oncol, 12, 953934. 10.3389/fonc.2022.953934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li X, Wang T, Kumavor PD, Aguirre A, Shung KK, Zhou Q, Sanders M, Brewer M, & Zhu Q (2011). Integrated optical coherence tomography, ultrasound and photoacoustic imaging for ovarian tissue characterization. Biomed Opt Express, 2(9), 2551–2561. 10.1364/BOE.2.002551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Liu Y, Peng H, Tang C, Hennig GW, Wang Z, Wang L, Yu T, Klukovich R, Zhang Y, Zheng H, Xu C, Wu J, Hess RA, & Yan W (2019). Motile cilia of the male reproductive system require miR-34/miR-449 for development and function to generate luminal turbulence. Proc Natl Acad Sci U S A, 116(9), 3584–3593. 10.1073/pnas.1817018116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnescu L, Leung MC, Abeyta M, Sudkamp H, Baer T, Behr B, & Ellerbee AK (2015). Label-free characterization of vitrification-induced morphology changes in single-cell embryos with full-field optical coherence tomography. J Biomed Opt, 20(9), 096004. 10.1117/1.JBO.20.9.096004 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Nandy S, Rao B, Li S, Hagemann AR, Kuroki LK, McCourt C, Mutch DG, Powell MA, Hagemann IS, & Zhu Q (2019). Histogram analysis of en face scattering coefficient map predicts malignancy in human ovarian tissue. J Biophotonics, 12(11), e201900115. 10.1002/jbio.201900115 [DOI] [PMC free article] [PubMed] [Google Scholar]


