Abstract
The effectivity of cancer immunotherapies is hindered by immunosuppressive tumour microenvironments that are poorly infiltrated by effector T cells and natural killer cells. In infection and autoimmune disease, the recruitment and activation of effector immune cells is coordinated by pro-inflammatory T helper 17 (TH17) cells. Here we show that pathogen-mimicking hollow nanoparticles displaying mannan (a polysaccharide that activates TH17 cells in microbial cell walls) limit the fraction of regulatory T cells and induce TH17-cell-mediated anti-tumour responses. The nanoparticles activate the pattern-recognition receptor Dectin-2 and Toll-like receptor 4 in dendritic cells, and promote the differentiation of CD4+ T cells into the TH17 phenotype. In mice, intra-tumoural administration of the nanoparticles decreased the fraction of regulatory T cells in the tumour while markedly increasing the fractions of TH17 cells (and the levels of TH17-cell-associated cytokines), CD8+ T cells, natural killer cells and M1-like macrophages. The anti-tumoural activity of the effector cells was amplified by an agonistic antibody against the co-stimulatory receptor OX40 in multiple mouse models. Nanomaterials that induce TH17-cell-mediated immune responses may have therapeutic potential.
Microbial pathogens present ‘danger’ signals at the site of infection, and confer protective immunity by inducing pro-inflammatory cytokines and mobilizing leucocytes1–3. During microbial infection, pathogen-associated molecular patterns (PAMPs) activate pattern-recognition receptors (PRRs) expressed on innate immune cells, such as dendritic cells3 (DCs), leading to the production of pro-inflammatory cytokines, including IL-1β, IL-6 and IL-23, that promote the differentiation and stabilization of T helper 17 (TH17) cells4,5. TH17 cells are a subset of CD4+ helper T cells defined by IL-17A expression, and play crucial roles in the host’s defence against infectious agents, including fungi, bacteria and viruses, by recruiting and activating effector T lymphocytes at the site of microbial infection6. In addition to their roles in infection, TH17 cells are major drivers of autoimmune diseases, which has prompted intensive research into the development of therapeutics for suppressing TH17 cells in autoimmune diseases, with several clinical trials underway7–13. In stark contrast, the role of TH17 cells in cancer progression and therapy remains under debate14,15. There are reports of adoptively transferred TH17 cells causing tumour regression in mice, and tumour-associated IL-17 correlating with survival of patients with cancer16–19. However, apart from a few studies reporting TH17-inducing immunosuppressant agents or peptide-based fibres for vaccination against infectious pathogens8,20,21, it remains largely unknown how to induce TH17 cells in vivo, without expanding their immunosuppressive counterpart (regulatory T cells; Treg cells) for successful cancer immunotherapy. In this Article, we report a synthetic ‘nano-PAMP’ that tips the balance between TH17 cells and Treg cells towards pro-inflammatory TH17 cells, with potent anti-tumour efficacy.
Importantly, fungal infection caused by the yeast Candida albicans has been shown to trigger Dectin-2 and Toll-like receptor (TLR)-4 activation in DCs and to generate TH17 immunity22,23. Dectin-2 signalling promotes IL-6, IL-1β and IL-23, which drive TH17 cell differentiation and maintenance22,23, and TLR-4-dependent maturation of DCs has been reported to contribute to TH17 immunity24. Notably, ~90% of the outer cell wall of Candida albicans is composed of polysaccharides, including mannan, glucan and chitin;3,25 among these, mannan, composed of an α-(1,6) mannose backbone with α-(1,2)- or α-(1,3)-linked oligomannose side chains, is a ligand for Dectin-2 (refs. 23,26). Therefore, we hypothesized that the multivalent display of mannan on a pathogen-mimicking system may serve as a potent stimulator of TH17 response.
We have recently reported the development of Saccharomyces cerevisiae mannan-based nanocapsules (Mann-NC) for subcutaneous delivery of messenger-RNA-based vaccines27. S. cerevisiae is a yeast widely used in winemaking, baking and brewing, and its mannan has close structural similarity to that from C. albicans. Here we show that Mann-NC composed of mannan (without any other cargo) is a potent nano-PAMP for Dectin-2 and TLR-4 activation and that the intra-tumoural administration of Mann-NC generates robust TH17 responses (Fig. 1a,b). PAMP–PRR interaction is controlled by the geometry, nanoscale arrangement and architecture of PAMP on microbes that dictate the accessibility and availability of PRR engagement28,29. Mann-NC has several favourable features as a nano-PAMP, including its bioactive ingredients without any pathogenic substance; tunable nanoscopic dimension and shape; and multivalent surface PAMP arrangement with structural flexibility for favourable PRR engagement. Intra-tumoural administration of Mann-NC induces the release of cytokines and chemokines, including CXCL10 and CCL2, resulting in the recruitment and activation of anti-tumour CD8+ T cells, natural killer (NK) cells and M1-like macrophages within the tumour microenvironment (TME) (Fig. 1a). Moreover, the anti-tumour efficacy of Mann-NC was further amplified when combined with an agonistic antibody against OX40 (αOX40), which is a co-stimulatory receptor expressed on activated effector cells, including CD4+ T cells, CD8+ T cells and NK cells, and has been shown to augment the functions of TH17 cells30,31. Overall, we present a strategy for redirecting anti-pathogen immune responses to cancer immunotherapy using a pathogen-mimicking synthetic nano-PAMP.
Fig. 1 |. Mann-NC for cancer immunotherapy.
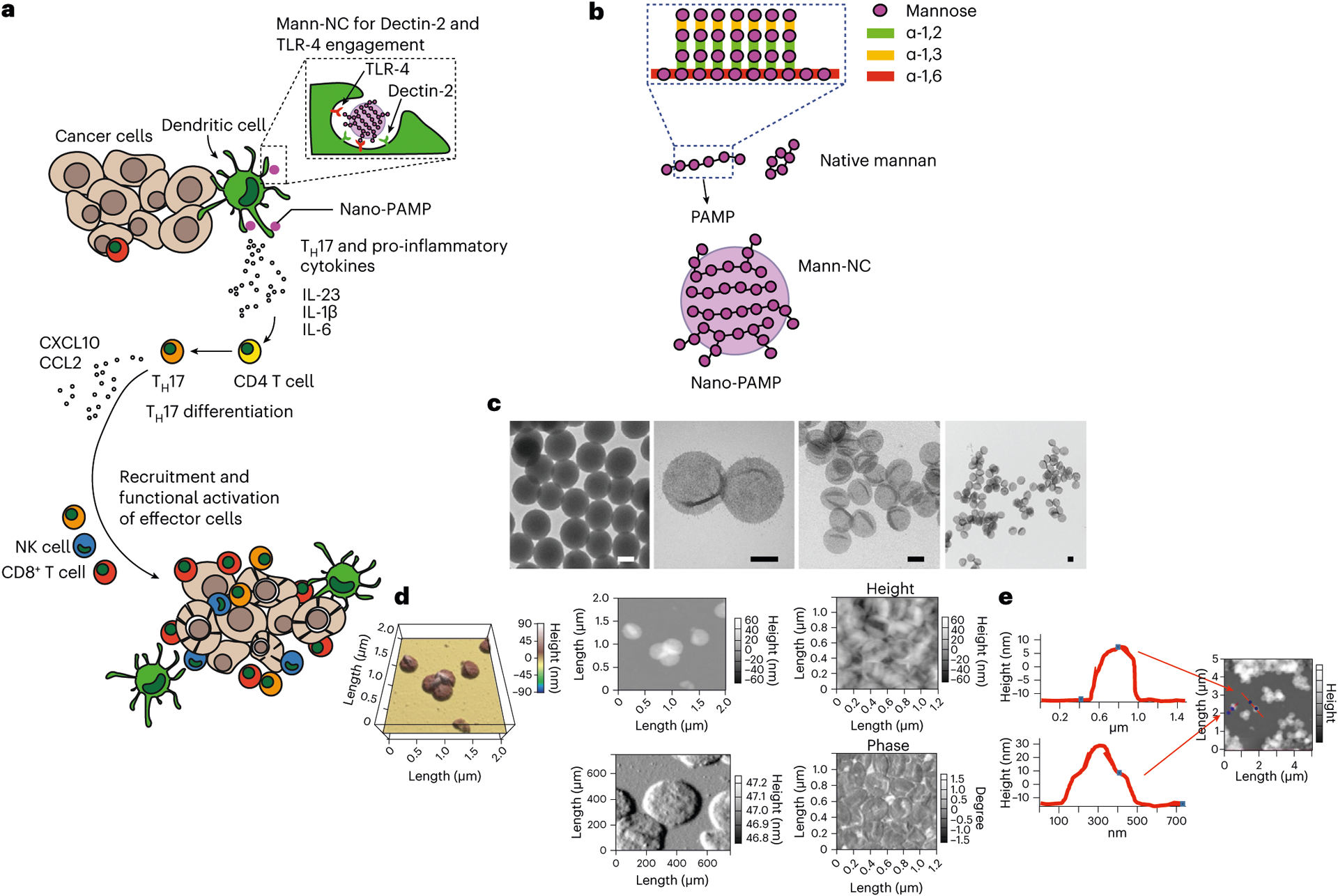
a, Mann-NC is a nano-PAMP that engages Dectin-2 and TLR-4 on DCs for TH17-skewed immune activation. TH17 cells supported by IL-23, IL-6 and IL-1β orchestrate the intra-tumoural recruitment of CD8+ T cells and NK cells via chemokines, including CXCL10 and CCL2, leading to potent anti-tumour efficacy. b, Mann-NC is composed of mannan with α-(1,6) mannoside backbone and α-(1,2) and α-(1,3) mannoside side chains. c, TEM images of a template siNP and hollow Mann-NCs. Scale bars, 100 nm. d,e, AFM images show Mann-NC in dried (top) and wet conditions (bottom) (d) and wall thickness of Mann-NC measured in dried condition (e).
Results and discussion
Preparation and characterization of Mann-NC
Mann-NC was prepared by employing silica nanoparticles (siNPs) as a sacrificial template27. Briefly, siNPs were coated with polyethyleneimine (PEI), reacted with oxidized mannan to form imine-mediated conjugates, and then removed by chemical dissolution to obtain hollow Mann-NCs (Fig. 1b and Extended Data Fig. 1). After the core templates were removed, Mann-NC was composed of mostly mannan (~93% mannan and ~7% PEI by weight), compactly packed in a thin nanoscale capsule surface for multivalent presentation and potent immune modulation. Transmission electron microscopy (TEM) images show core-empty capsule structure of Mann-NC with a size comparable to an siNP template (Fig. 1c). The hydrodynamic size and zeta potential were measured as 200 ± 3.3 nm and −46 ± 1.4 mV, and 230 ± 10.3 nm and 5 ± 0.02 mV for siNP and Mann-NC, respectively (Supplementary Fig. 1). Atomic force microscopy (AFM) analyses corroborated the deformable spherical morphology of Mann-NCs in wet or dried condition (Fig. 1d) and revealed that the well thickness of Mann-NC is approximately 10 nm in dried condition (Fig. 1e).
Mann-NC triggers robust immune activation in vitro
Mann-NC is expected to promote engagement of mannan to its target PRRs via multivalent interactions by displaying mannan on the surface with high density and structural flexibility (Fig. 2a). To test this idea, we compared Mann-NC and its native, soluble mannan counterpart (Native-Mann) for the activation of bone marrow-derived dendritic cells (BMDCs). Cy5.5-tagged Mann-NC was gradually taken up by BMDCs over 2 h of incubation, whereas only a marginal uptake was observed for Cy5.5-tagged Native-Mann (Fig. 2b). Mann-NC and Native-Mann both induced slight proliferation of BMDCs without any notable cytotoxicity (Fig. 2b). Confocal images revealed that Mann-NCs were efficiently internalized by BMDCs after 4 h, whereas Native-Mann was barely detected in the cells (Fig. 2c). BMDCs incubated with Mann-NC exhibited many outgrowing dendrites, suggesting maturation and activation. Indeed, Mann-NC induced stronger DC activation than Native-Mann, as shown by the significantly increased surface expression of CD40, CD86 and MHC-II activation markers and increased secretion of IL-12p70, TNF-α and IL-6 pro-inflammatory cytokines (Fig. 2d,e). These results showed that multivalent surface presentation of mannan on Mann-NC enhanced engagement, cellular uptake and activation of BMDCs, compared with Native-Mann.
Fig. 2 |. Mann-NC activates Dectin-2 and TLR-4 and induces IL-17+ CD4+ T cells.
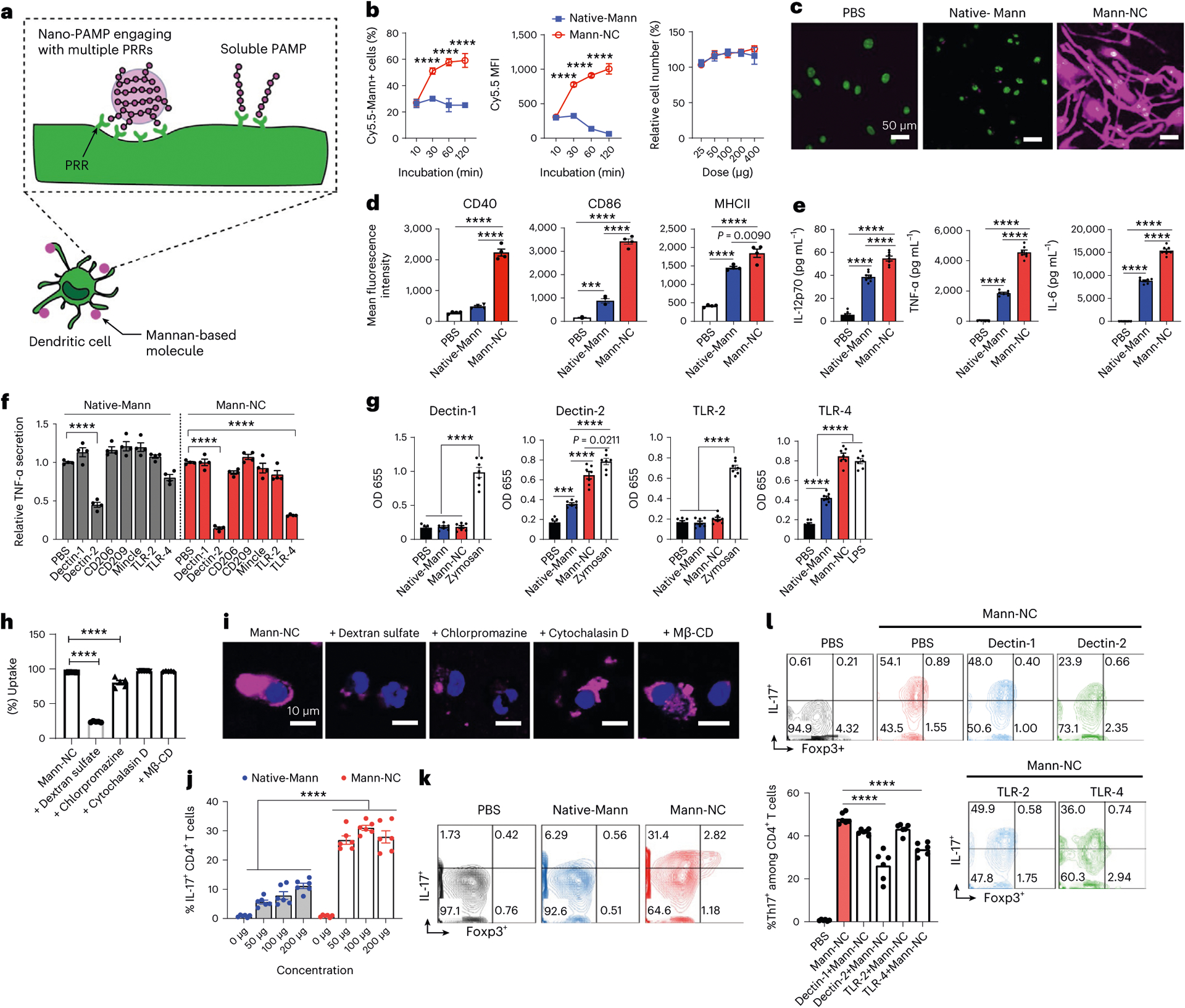
a, Schematic illustration of Mann-NC interacting with multiple PRRs for enhanced immune activation. b–e, BMDCs were incubated with Mann-NC or Native-Mann and examined for cellular uptake of Cy5.5-tagged mannan and cell number relative to the PBS control (b), confocal microscope images taken after 4 h of sample incubation (c), upregulation of activation markers on DCs (that is, CD40, CD86 and MHC-II) measured by flow cytometry (d), and secretion of pro-inflammatory cytokines (that is, IL12p70, TNF-α and IL-6) by ELISA (e). f, BMDCs were pre-treated with blocking antibodies against PRRs for 30 min and then incubated with Mann-NC or Native-Mann, followed by measurement of TNF-α release. g, HEK reporter cell lines were used to examine Dectin-1, Dectin-2, TLR-2 or TLR-4 engagement with Mann-NC. h,i, Cellular uptake of Mann-NC by BMDCs was assessed after 1 h of co-incubation of various pharmacological inhibitors, followed by examination by flow cytometry (h) and confocal microscopy (i). j,k, Conditioned media from BMDCs pulsed with Native-Mann or Mann-NC for 12 h were added to naïve CD4+ T cells. After 5 days of culture, ICS was performed by incubating CD4+ T cells with PMA, ionomycin and Brefeldin A for 4 h. Shown are the quantification of IL-17+ CD4+ T cells (j) and their representative contour plots for IL-17+ CD4+ and Foxp3+ CD4+ T cells (k). l, The frequencies of IL-17+ CD4+ T cells were measured after adding blocking antibodies against Dectin-1, Dectin-2, TLR-2 or TLR-4 during co-culture of BMDCs with Mann-NC, followed by naïve CD4+ T-cell culture as in j, and the representative contour plots are shown. Data represent mean ± standard error of the mean (s.e.m.), from a representative experiment (n = 4 (b,d–f) or n = 6 (g–l)) from two (c,h–k) or three (b,d–g,l) independent experiments. ***P < 0.001, ****P < 0.0001, analysed by one-way (d–h,l) or two-way (b,j) ANOVA with Bonferroni’s multiple comparisons test.
Mann-NC engages Dectin-2 and TLR-4 on DCs
Next, we sought to identify specific PRRs that interact with Mann-NC. Notably, mannan is known to interact with Dectin-2, Mincle, TLR-2 and TLR-4 (refs. 32,33). BMDCs were pre-treated for 30 min with blocking antibodies against Dectin-1, Dectin-2, CD206, CD209, Mincle, TLR-2 or TLR-4, and then incubated for 12 h with either Mann-NC or Native-Mann, followed by TNF-α measurement in medium as a marker for immune activation. BMDCs pre-treated with antibodies against Dectin-2 or TLR-4 produced significantly decreased amount of TNF-α after Mann-NC treatment (P < 0.0001; Fig. 2f), suggesting that Dectin-2 and TLR-4 are the major PRRs for Mann-NC. We observed a similar trend, but to a lesser degree, for Native-Mann (Fig. 2f). Corroborating these results, both Native-Mann and Mann-NC activated human embryonic kidney (HEK) reporter cells expressing Dectin-2 or TLR-4, but not Dectin-1 or TLR-2 reporter cell lines (Fig. 2g). Interestingly, compared with Native-Mann, Mann-NC induced significantly stronger activation of Dectin-2 and TLR-4 (P < 0.0001; Fig. 2g), indicating that multivalent presentation of mannan on nanocapsules significantly improved PRR activation. However, addition of antibodies against Dectin-2 or TLR-4 did not inhibit cellular uptake of Mann-NC by BMDCs (Supplementary Fig. 2). Instead, cellular uptake of Mann-NC was significantly decreased in the presence of dextran sulfate34 or chlorpromazine35, which are inhibitors of scavenger receptor class A-mediated endocytosis and clathrin-mediated endocytosis, respectively (Fig. 2h,i). In contrast, pharmacological inhibitors of micropinocytosis/phagocytosis (cytochalasin D)35 or lipid rafts/cholesterol-enriched micro-domains/caveolae (methyl-β-cyclodextrin, Mβ-CD)35 did not impact the cellular uptake of Mann-NC (Fig. 2h,i). Taken together, Mann-NC are taken up by DCs via scavenger receptor class A- and clathrin-dependent endocytosis, leading to Dectin-2- and TLR-4-mediated immune activation.
Mann-NC drives TH17 differentiation of CD4+ T cells
Next, given Mann-NC-mediated activation Dectin-2 and TLR-4, and previous reports of Dectin-2- and TLR-4-mediated DC activation of TH17 cells22,23, we examined whether Mann-NC can promote DCs to induce TH17 differentiation of CD4+ T cells. To examine this, BMDCs were incubated with Mann-NC for 12 h, and the conditioned medium was added to naïve CD4+ T cells. After 5 days of culture, CD4+ T cells were examined for their functional phenotype by intracellular cytokine staining (ICS) after restimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin. While Native-Mann induced IL-17+ CD4+ T cells in a dose-dependent manner (Fig. 2j), Mann-NC significantly increased the induction of IL-17+ CD4+ T cells across all doses tested (P < 0.0001, Fig. 2j,k). On the other hand, Mann-NC had a minimal impact on IL-4+, IFN-γ+ or Foxp3+ CD4+ T cells (Fig. 2k and Supplementary Fig. 3). We have also confirmed that Mann-NC treatment during DC-mediated priming of p31 antigen-specific BDC2.5 CD4+ T cells skewed their differentiation towards IL-17+ CD4+ T-cell phenotype (Extended Data Fig. 2a). These results suggest that during CD4+ T-cell priming by DCs, DCs stimulated by Mann-NC trigger the differentiation of CD4+ T cells towards TH17 phenotype. Next, we examined whether the interactions between Mann-NC and Dectin-2 and/or TLR-4 on DCs play any role in TH17 differentiation. BMDCs were pre-incubated with blocking antibodies against Dectin-2 or TLR-4, followed by incubation with Mann-NC. Mann-NC-conditioned media were then added to naïve CD4+ T cells. The frequency of IL-17+ CD4+ T cells was significantly reduced when T cells were supplemented with Mann-NC-conditioned media pre-treated with blocking antibodies against Dectin-2 or TLR-4 (P < 0.0001; Fig. 2l). In contrast, the presence of blocking antibodies against Dectin-1 or TLR-2 had a minimal impact. Taken together, these results show that Mann-NC activates Dectin-2 and TLR-4 on DCs, resulting in strong induction of TH17-phenotype CD4+ T cells.
Mann-NC activates innate immune cells and TH17 cells
TH17 cells are known to orchestrate the recruitment and activation of NK and CD8+ T cells by producing various cytokines and chemokines (for example, CCL2 (refs. 16,36) and CXCL10 (ref. 17)), thus leading to anti-tumour effects37. Motivated by the TH17-inducing property of Mann-NC observed in vitro, we examined the therapeutic potential of Mann-NC in murine tumour models. BALB/c mice were inoculated subcutaneously with CT26 colon carcinoma cells on day 0, followed by intra-tumoural administration of PBS, Native-Mann or Mann-NC on days 9, 12 and 15 (Fig. 3a). Mann-NC therapy significantly slowed the tumour growth and extended the animal survival (Fig. 3b,c). Interestingly, intra-tumoural administration of Native-Mann also exhibited modest anti-tumour effects (Fig. 3b,c). We examined the immune landscape of the TME on day 15 and observed that Mann-NC therapy markedly increased the frequency of CD45+ leucocytes, compared with PBS and Native-Mann (Fig. 3d). Mann-NC significantly elevated the intra-tumoural frequency of CD11c+ DCs and their maturation as shown by CD80 surface staining (Fig. 3e). In addition, Mann-NC significantly increased the frequency of CD8α+ CD103+ CD11c+ DCs (P < 0.0001; Fig. 3e), which is a critical DC subset for the induction of CD8+ cytotoxic T lymphocytes38,39. Mann-NC also increased the frequency of activated monocytes (Fig. 3f) and markedly increased the frequency of pro-inflammatory M1-like CD80+ F4/80+ macrophages without changing the frequency of M2-like macrophages (Fig. 3g). This resulted in a significantly increased M1/M2 ratio for the Mann-NC group, compared with Native-Mann (Fig. 3g). On the other hand, Mann-NC treatment did not significantly change the frequency of myeloid-derived suppressor cells (MDSCs) (Supplementary Fig. 4). Next, we investigated how Mann-NC affects CD4+ T-cell differentiation in vivo by analysing TME and tumour-draining lymph nodes (tdLNs). While Mann-NC treatment did not affect the frequency of CD4+ T cells in the tumours (Supplementary Fig. 5a), Mann-NC induced upregulation of OX40 on CD4+ T cells and Treg cells (Fig. 3h and Extended Data Fig. 2b), but not PD-1 on CD4+ T cells (Supplementary Fig. 5b). Importantly, Mann-NC markedly increased the frequency of IL-17+ CD4+ T cells on days 12 (P < 0.01) and 15 (P < 0.0001) (Fig. 3i and Supplementary Fig. 6), with a concomitant decrease in the frequency of Foxp3+ CD4+ Treg cells (Fig. 3j and Supplementary Fig. 6). This resulted in a significantly elevated TH17/Treg ratio within the TME for the Mann-NC-treated mice (Fig. 3k). We also observed a similar trend in the frequencies of CD4+ T cells, TH17+ CD4+ T cells and Foxp3+ CD4+ Treg cells and TH17/Treg ratio in tdLNs (Extended Data Fig. 2c–f). In contrast, Mann-NC did not change the frequencies of TH1 cells or TH2 cells (Extended Data Fig. 2g). Taken together, Mann-NC is a potent innate immune stimulator that shifts the TH17/Treg balance towards TH17 immunity in tumour-bearing mice.
Fig. 3 |. Mann-NC activates innate immune cells and TH17 cells and exerts anti-tumour effect.
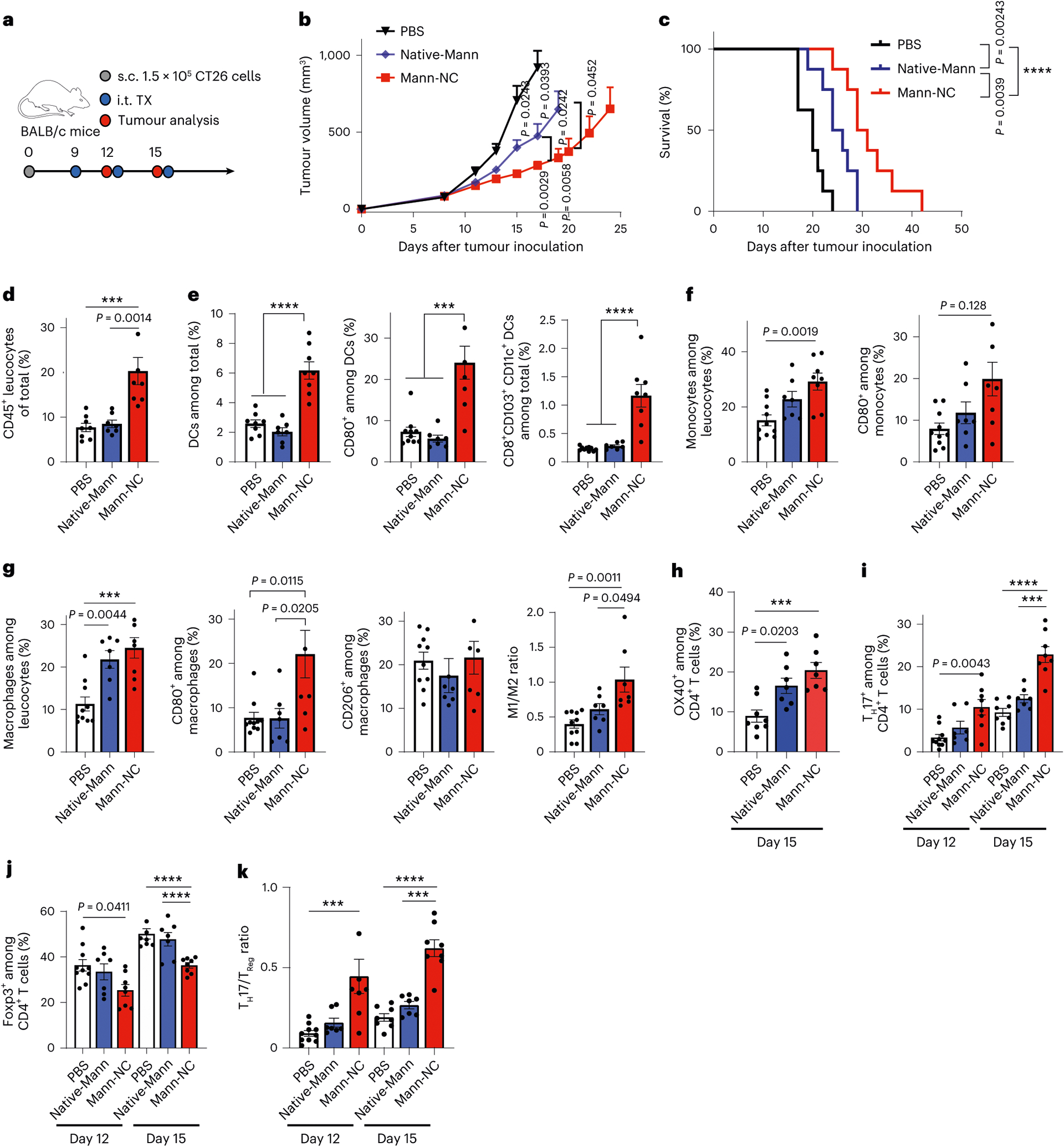
a, BALB/c mice were inoculated subcutaneously (s.c.) with 1.5 × 105 CT26 cells on day 0 and treated by intra-tumoural treatment (i.t. TX) with Mann-NC on days 9, 12 and 15. b,c, Average tumour growth (b) and survival curves (c). d–g, Tumour tissues were analysed on day 15 for CD45+ leucocytes (d), CD11c+, CD80+ CD11c+, and CD8+ CD103+ CD11c+ DCs (e), Ly6C+ and Ly6C+ CD80+ monocytes (f), and F4/80+, F4/80+ CD80+ M1-like and F4/80+ CD206+ M2-like macrophages, and the corresponding M1/M2 ratio (g). h–k, Tumour tissues were analysed for the frequencies of OX40+ CD4+ T cells on day 15 (h); the frequencies of IL-17+ CD4+ TH17 cells (i), Foxp3+ CD4+ Treg cells (j) and the corresponding TH17/Treg ratio within the TME (k). Data represent mean ± s.e.m., from a representative experiment (n = 7 for Mann-NC or 8 for PBS and Native-Mann (b,c) and n = 7 for Native-Mann or 8 for PBS and Mann-NC (d–k) biologically independent samples) from two (h) or three (b–g,i–k) independent experiments. ***P < 0.001, ****P < 0.0001, analysed by one-way (d–h) or two-way (b,h–k) ANOVA with Bonferroni’s multiple comparisons test, or log-rank (Mantel–Cox) test (c).
Mann-NC activates intra-tumoural CD8+ T cells and NK cells
On the basis of the strong TH17-inducing capacity of Mann-NC, we next focused on the Mann-NC formulation and studied the impact of TH17 immune response primed by Mann-NC on the cytokine and chemokine profiles and other effector immune cells (Fig. 4a). Intra-tumoural administration of Mann-NC led to significantly elevated levels of TH17-associated cytokines, including IL-6, IL-17, IL-23 and IL-1β (refs. 4,5,40) as well as pro-inflammatory chemokines, including CXCL10, CCL2, CCL3 and CCL5, within the TME (Fig. 4b,c). We also observed their increased concentrations in serum, which peaked after 6 h of Mann-NC administration (Fig. 4d). Flow cytometric analysis of tumour tissues indicated that Mann-NC was taken up mostly by intra-tumoural DCs, monocytes, macrophages and neutrophils, but not by tumour cells (Extended Data Fig. 3). Dectin-2 and TLR-4 were expressed among intra-tumoural DCs, macrophages, monocytes and neutrophils, and Mann-NC treatment did not change their expression levels (Supplementary Fig. 7). In particular, CXCL10, CCL2, CCL3 and CCL5 are important chemokines associated with tumour infiltration of effector immune cells41. Thus, we next examined the impact of Mann-NC treatment on the intra-tumoural frequencies of key effector cells, that is, CD8+ T cells and NK cells. Indeed, Mann-NC treatment significantly increased the frequency of total CD8+ T cells, CD8+ T cells against tumour-specific AH-1 antigen (H-2Ld-restricted gp70423–431, an immunodominant CD8+ T-cell epitope in CT26 tumour cells), the ratio of intra-tumoural CD8+ T cells to Treg cells, as well as IFN-γ+, TNF-α+ and granzyme B+ CD8+ T cells (Fig. 4e). Mice treated with Mann-NC also exhibited a 3.7-fold higher number of IFN-γ+ AH-1-specific CD8+ T cells in spleen (Fig. 4f). In contrast, Mann-NC treatment did not increase the frequency of IL-17+ CD8+ Tc17 cells (Supplementary Fig. 8). Moreover, Mann-NC significantly increased the intra-tumoural frequency of NK cells with elevated expression levels of CD69 and CD107a activation markers (Fig. 4g).
Fig. 4 |. Mann-NC induces TH17-associated cytokines and chemokines and promotes CD8+ T-cell and NK cell responses.
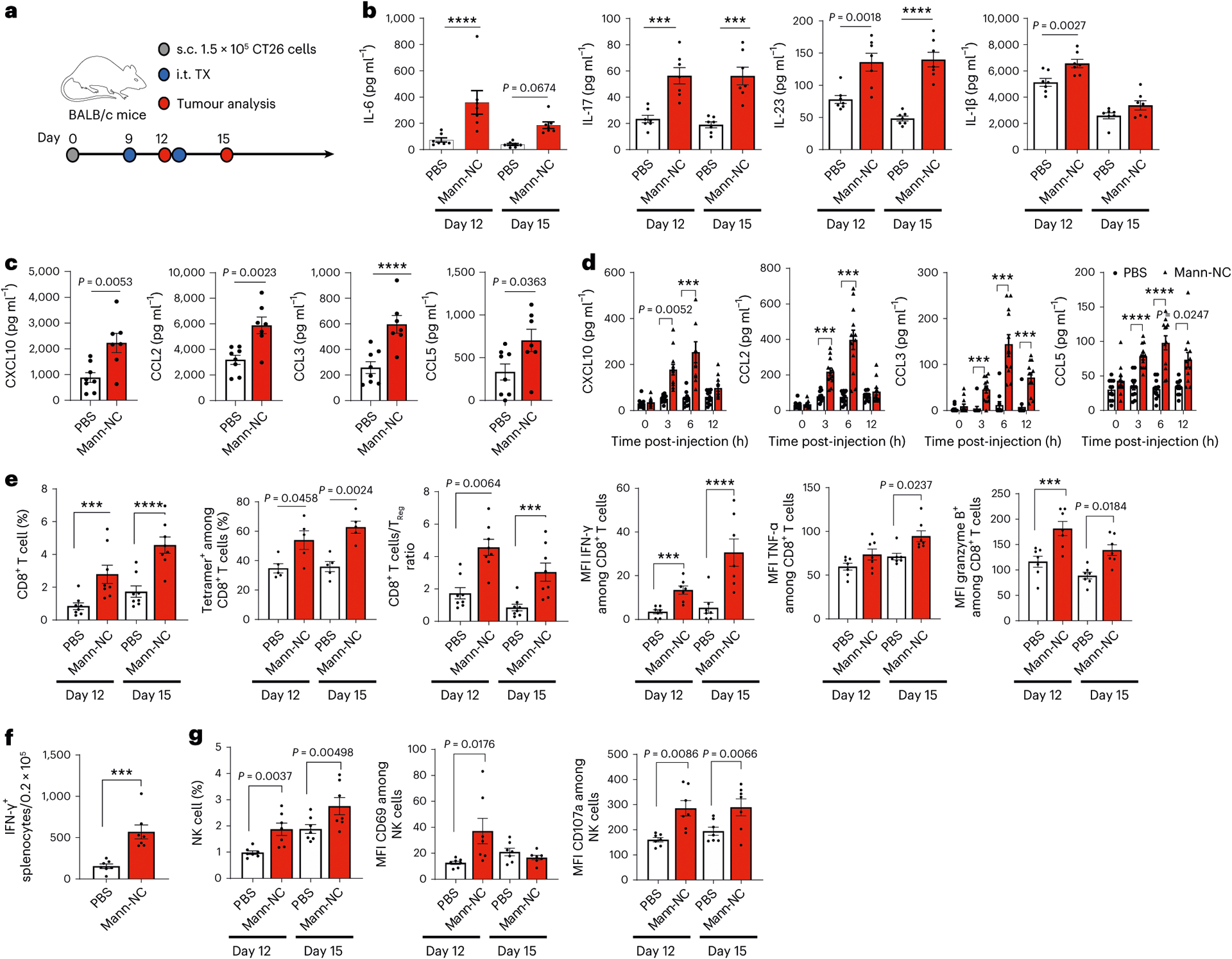
a, BALB/c mice were inoculated subcutaneously (s.c.) with 1.5 × 105 CT26 cells on day 0 and treated by intra-tumoural (i.t.) administration of Mann-NC on days 9 and 12. Tumour tissues were analysed after 3 days of each treatment. b, Intra-tumoural concentrations of IL-6, IL-17, IL-23 and IL-1β. c,d, Intra-tumoural (c) and serum (d) concentrations of CXCL10, CCL2, CCL3 and CCL5 measured on day 15 (c) and on day 9 (d). e, Intra-tumoural frequencies of CD8+ T cells, AH-1 tetramer+ CD8+ T cells, the ratio of CD8+ T cells to Treg cells, and the expression levels of IFN-γ, TNF-α and granzyme B. f, IFN-γ+ ELISPOT analysis performed on day 15 after restimulation of splenocytes with AH-1 peptide. g, Intra-tumoural frequencies of NK cells and their expression levels of CD69 and CD107a. Data represent mean ± s.e.m., from a representative experiment (n = 7 (b,c,e–g) and n = 12 (d) biologically independent samples) from two (b–d,f) or three (e,g) independent experiments. ***P < 0.001, ****P < 0.0001, analysed by two-way ANOVA with Bonferroni’s multiple comparisons test (b,d,e,g) or unpaired two-tailed Student’s t-test (c,f).
We next studied the role of each immune cell compartment on the anti-tumour efficacy of Mann-NC. Depletion antibodies against CD8+ T cells (αCD8), NK cells (α-asialo-GM1), neutrophils (αLy6G) or isotype control IgG were administered over the course of Mann-NC therapy (Fig. 5a). Depletion antibodies against CD8+ T cells or NK cells, but not neutrophils or isotype control, significantly reduced the anti-tumour efficacy of Mann-NC (Fig. 5b). As for the depletion of TH17 cells, anti-CD4 IgG would non-specifically deplete various subsets of CD4+ T cells, including TH17 cells, Treg cells and other TH1/TH2 cells. Thus, we employed anti-IL-17A IgG that neutralizes IL-17A, thereby blocking IL-17-producing effects of TH17 cells42. Administration of anti-IL-17A IgG during the Mann-NC therapy led to significantly reduced anti-tumour efficacy (Fig. 5c). Notably, Mann-NC therapy given without any antigen triggered tumour antigen-specific CD8+ and CD4+ T-cell responses, as shown by IFN-γ+ ELISPOT assay with splenocytes restimulated with CT26-derived MHC-I-restricted gp70 AH-1 epitope43 and MHC-II-restricted ME1 neo-epitope44, respectively (Fig. 5d). Co-administration of anti-IL-17A IgG during the Mann-NC therapy decreased antigen-specific CD8 + and CD4+ T-cell responses (Fig. 5d) as well as IFN-γ+ and granzyme B+ activated CD8+ T cells and NK cells from the TME (Fig. 5e,f and Extended Data Fig. 4). Collectively, these results show that Mann-NC promotes robust secretion of TH17-associated cytokines and chemokines, leading to activation and intra-tumoural infiltration of CD8+ T cells and NK cells with potent anti-tumour immune response.
Fig. 5 |. Mann-NC induces TH17-associated cytokines and chemokines and promotes CD8+ T-cell and NK cell responses.
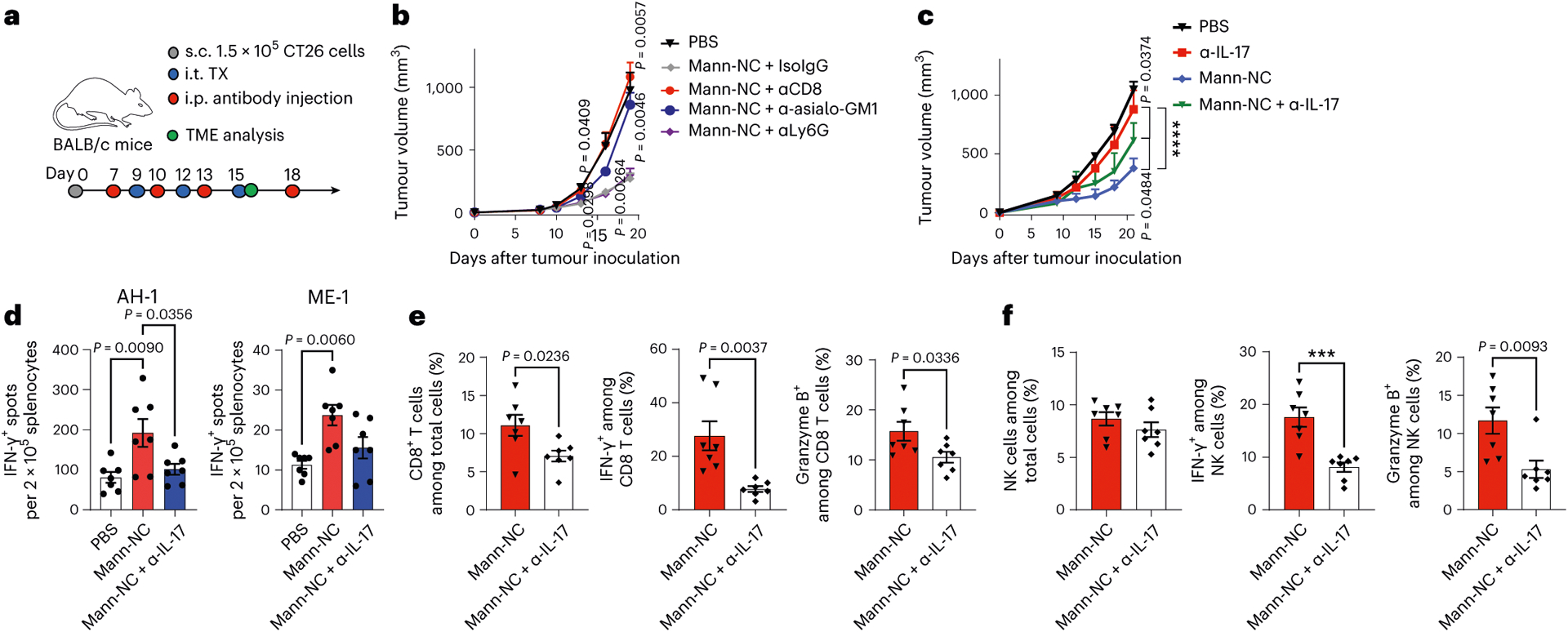
a, BALB/c mice were inoculated subcutaneously (s.c.) with 1.5 × 105 CT26 cells on day 0 and treated by intra-tumoural (i.t.) administration of Mann-NC. b,c, Isotype IgG or antibodies against αCD8, α-asialo-GM1, αLy6G (b) or α-IL-17A (c) were administered intraperitoneally (i.p.) on the indicated days, followed by tumour monitoring. d, IFN-γ+ ELISPOT analysis of splenocytes after restimulation with AH-1 or ME1 peptide. e,f, Shown are the frequency of total CD8+ T cells, IFN-γ+ CD8+ T cells and granzyme B+ CD8+ T cells (e), total NK cells, IFN-γ+ NK cells, and granzyme B+ NK cells in the TME (f). Data represent mean ± s.e.m., from a representative experiment (n = 5 for PBS or 7 for all other groups (b); n = 8 (c); or n = 7 (d–f) biologically independent samples) from one (d) or three (b,c,e,f) independent experiments. ***P < 0.001, ****P < 0.0001, analysed by one-way (d) or two-way (b,c) ANOVA with Bonferroni’s multiple comparisons test or unpaired two-tailed Student’s t-test (e,f).
αOX40 amplifies the anti-tumour efficacy of Mann-NC
OX40 is a co-stimulatory receptor expressed on activated effector immune cells31, and their immune functions can be augmented by an agonistic antibody against OX40 (αOX40) (ref. 30). As Mann-NC treatment led to marked upregulation of OX40 on CD4+ T cells (Fig. 3h), we examined whether co-administration of agonistic αOX40 IgG could further amplify the anti-tumour effects of Mann-NC. CT26 tumour-bearing mice were administered intra-tumourally on days 9, 12 and 15 with either PBS, Mann-NC, αOX40 or Mann-NC + αOX40 combination (Fig. 6a). The Mann-NC + αOX40 combination induced dramatic anti-tumour effects and eliminated established tumours in 88% of mice (Fig. 6b,c). On the other hand, Mann-NC or αOX40 mono-therapy slowed the tumour growth but did not lead to tumour eradication. We also tested Mann-NC + αPD-1 IgG combination and found that αPD-1 IgG did not enhance the anti-tumour efficacy of Mann-NC (Supplementary Fig. 9), which is in line with our observation that Mann-NC treatment did not upregulate PD-1 on CD4+ T cells (Supplementary Fig. 5b). Survivors from the Mann-NC + αOX40 combination group were protected against CT26 re-challenge performed on day 80 (Fig. 6d), suggesting the establishment of long-term immunological memory. To delineate the combination effect of Mann-NC and αOX40, we performed immune profiling of the TME. Compared with Mann-NC or αOX40 monotherapy, Mann-NC + αOX40 combination therapy further increased the frequency of CD80+ DCs and CD8α+ CD103+ CD11c+ DCs within the TME (Fig. 7a and Extended Data Fig. 5). Furthermore, TH17+ CD4+ T cells induced by Mann-NC treatment were further increased with the Mann-NC + αOX40 combination group, while the frequency of Treg cells remain similar, thus leading to an increased TH17/Treg ratio in the TME (Fig. 7b). We observed a similar trend of increased TH17+ CD4+ T cells in tdLNs with Mann-NC + αOX40 combination (Fig. 7c). Interestingly, whereas αOX40 monotherapy increased Treg cells in tdLNs, Mann-NC monotherapy as well as Mann-NC + αOX40 combination decreased the frequency of Treg cells, resulting in significantly higher TH17/Treg ratios (Fig. 7c). On the other hand, Mann-NC and/or αOX40 treatment did not change the frequencies of TH1 or TH2 cells (Supplementary Fig. 10).
Fig. 6 |. Mann-NC and αOX40 combination elicits strong immune activation with potent anti-tumour effect.
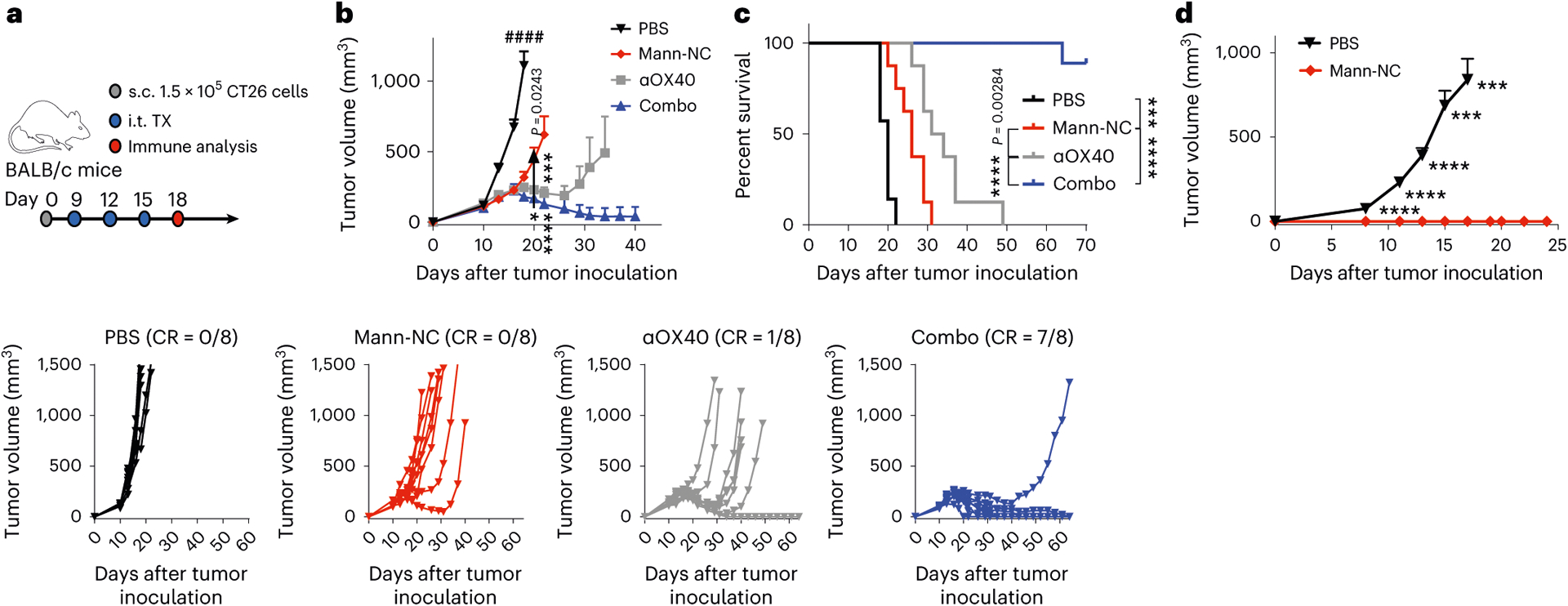
a, BALB/c mice were inoculated subcutaneously (s.c.) with 1.5 × 105 CT26 cells on day 0 and treated by intra-tumoural (i.t.) administration of Mann-NC and/or αOX40 on days 9, 12 and 15. b,c, Average and individual tumour growth (b) and survival curves (c). CR indicates complete tumor regression. d, Survivors were re-challenged with CT26 cells on day 80. Data represent mean ± s.e.m., from a representative experiment (n = 8 (b,c), or n = 7 for Mann-NC and 10 for PBS (d) biologically independent samples) from three independent experiments (b,c). ***P < 0.001, ****P < 0.0001, analysed by two-way ANOVA with Bonferroni’s multiple comparisons test (b,d); or log-rank (Mantel–Cox) test (c).
Fig. 7 |. Mann-NC and αOX40 combination elicits strong immune activation with potent anti-tumour effect.
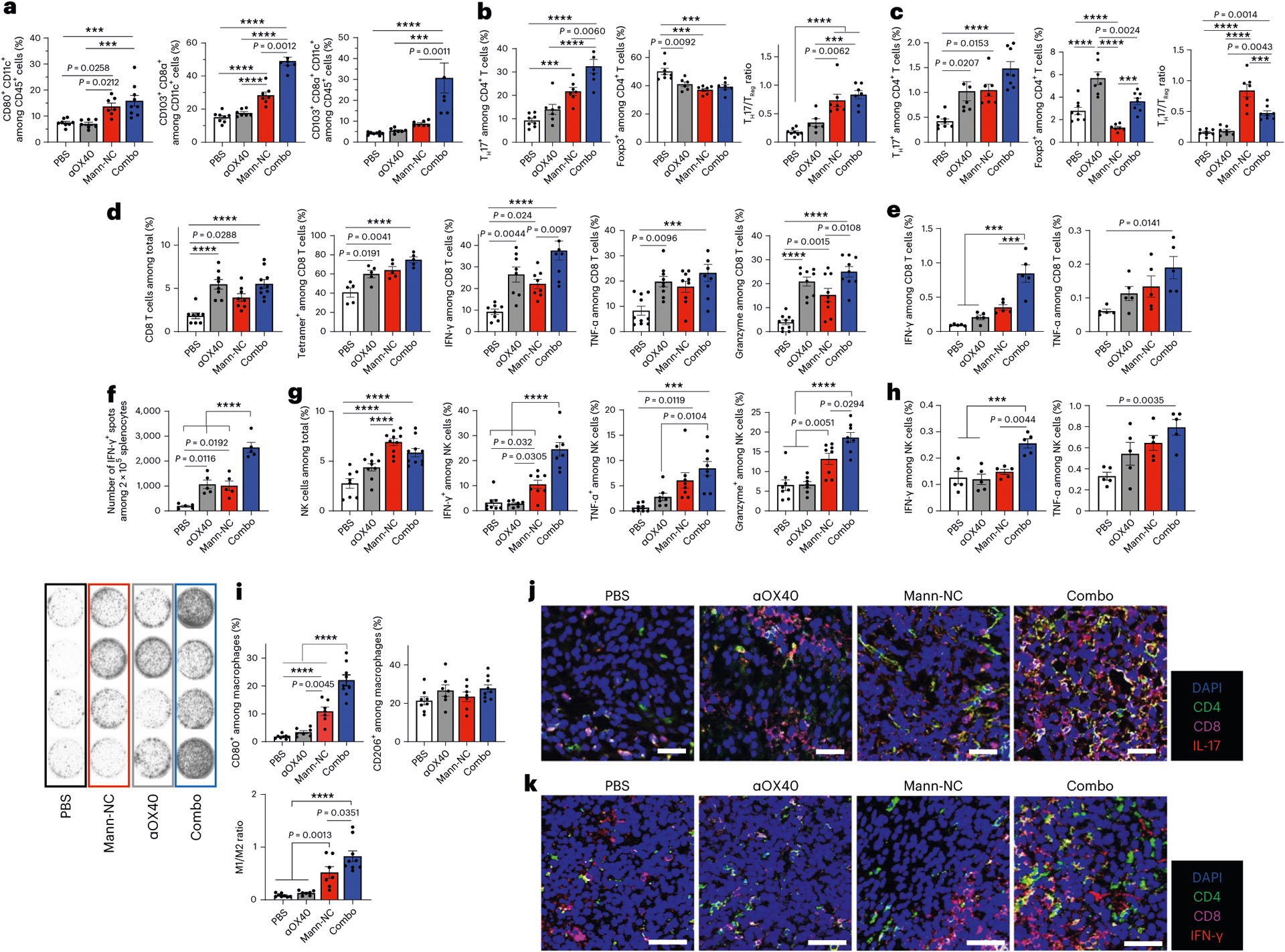
BALB/c mice were inoculated subcutaneously with 1.5 × 105 CT26 cells on day 0 and treated by intra-tumoural administration of Mann-NC and/or αOX40 on days 9, 12 and 15. a, Intra-tumoural frequencies of CD80+ CD11c+ DCs and CD8α+ CD103+ CD11c+ DCs. b,c, Frequencies of IL-17+ CD4+ TH17 cells, Foxp3+ CD4+ Treg cells and the corresponding TH17/Treg ratio within the TME (b) and tdLNs (c). d,e, Frequencies, tetramer staining and intracellular cytokine profiles of CD8+ T cells within the TME (d) and tdLNs (e). f, IFN-γ+ ELISPOT analysis of splenocytes after restimulation with AH-1 peptide. g,h, Frequencies and intracellular cytokine profiles of NK cells within the TME (g) and tdLNs (h). i, Intra-tumoural frequencies of CD80+ M1- and CD206+ M2-like macrophages and the corresponding M1/M2 ratio. j,k, Confocal microscopic images of tumour tissues excised on day 15 and stained with the indicated antibodies. Data represent mean ± s.e.m., from a representative experiment (n = 7 for PBS or 8 for αOX40, Mann-NC and the combination (a–d,f,g,i), n = 5 (e,h,j,k) biologically independent samples) from two (j,k) or three (a–i) independent experiments. ***P < 0.001, ****P < 0.0001, analysed by one-way (a–i) ANOVA with Bonferroni’s multiple comparisons test. Scale bars, 50 μm (j,k).
Mann-NC + αOX40 combination also lead to potent activation of CD8+ T cells and NK cells. Specifically, Mann-NC + αOX40 combination induced high frequencies of intra-tumoural AH-1-tetramer+CD8+ T cells, with higher levels of IFN-γ+ (P < 0.01) and granzyme B+ (P < 0.05), compared with Mann-NC alone (Fig. 7d). The Mann-NC + αOX40 combination also increased tumour-specific, IFN-γ+ CD8+ T cells in tdLN (P < 0.001) and spleen (P < 0.0001), compared with Mann-NC or αOX40 monotherapy (Fig. 7e,f). Moreover, Mann-NC + αOX40 combination significantly increased the frequency of NK cells with elevated IFN-γ, TNF-α and granzyme B expression (Fig. 7g,h). In addition, Mann-NC + αOX40 combination significantly increased the frequency of CD80+ M1-like macrophages (P < 0.0001; Fig. 7i), leading to elevated M1/M2 ratio, compared with Mann-NC or αOX40 monotherapy. In line with these results, tumour tissues analysed under confocal microscopy revealed that Mann-NC + αOX40 combination led to robust production of IL-17 and IFN-γ, which co-localized with CD4+ and CD8+ T cells, respectively (Fig. 7j,k and Extended Data Figs. 6 and 7).
To examine the contributions of TH17 cells on the anti-tumour efficacy of Mann-NC + αOX40 combination, we co-administered anti-IL-17A IgG during Mann-NC + αOX40 combination treatment; this resulted in significantly diminished anti-tumour effects (P < 0.0001; Fig. 8a), thus showing the anti-tumour effects of IL-17-producing TH17 cells. Furthermore, we assessed abscopal effects of Mann-NC + αOX40 combination using a bilateral CT26 tumour model, where only the primary tumours received treatments (Fig. 8b). Mann-NC + αOX40 combination effectively inhibited the growth of treated, primary tumours, as well as untreated, contralateral tumours (Fig. 8b), demonstrating systemic anti-tumour efficacy of local Mann-NC + αOX40 combination therapy.
Fig. 8 |. Mann-NC and αOX40 combination exhibits robust anti-tumour efficacy in murine tumour models.
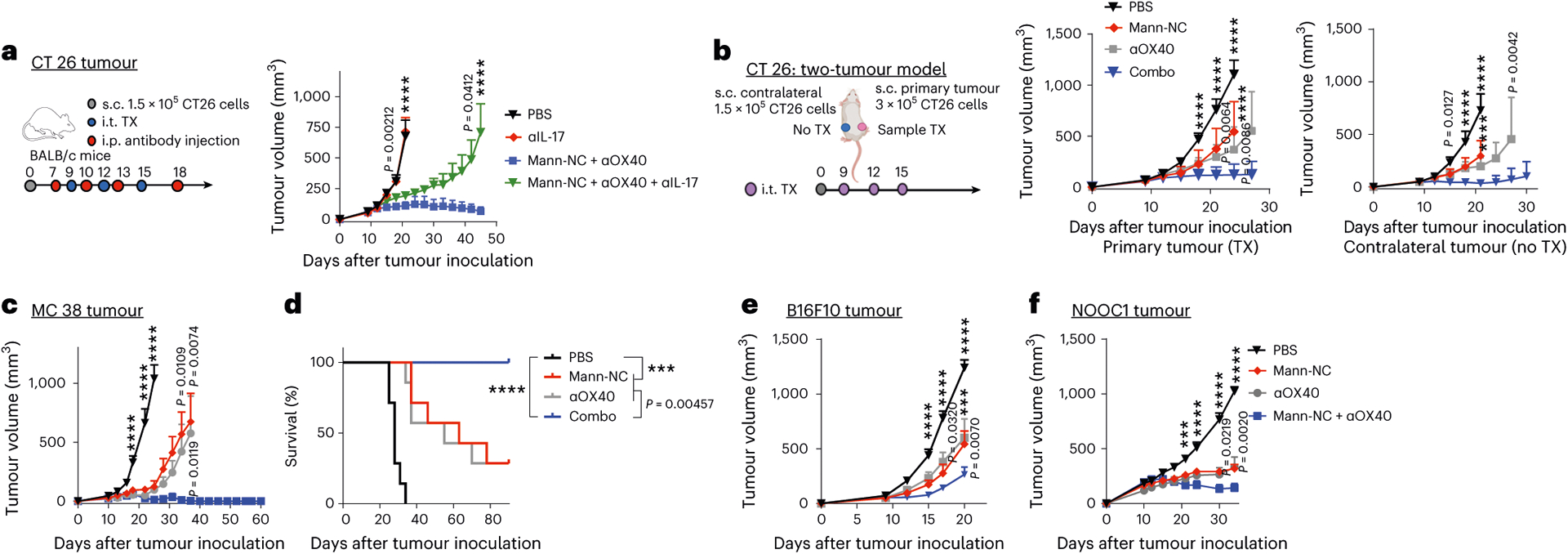
a, BALB/c mice were inoculated subcutaneously (s.c.) with 1.5 × 105 CT26 cells on day 0 and treated by intra-tumoural (i.t.) administration of Mann-NC and αOX40 on days 9, 12 and 15. A subset of animals received intraperitoneal (i.p.) administration of anti-IL-17A on days 7, 10, 13 and 18, followed by monitoring of tumour growth. b, BALB/c mice were inoculated subcutaneously with 3 × 105 CT26 cells on the right flank and 1.5 × 105 CT26 cells on the left flank on day 0, and samples were administered only into the right, primary tumours on days 9, 12 and 15. Shown are the average growth of primary and contralateral tumours. c,d, C57BL/6 mice were inoculated subcutaneously with 6 × 105 MC38 cells on day 0 and treated by intra-tumoural administration of Mann-NC and/or αOX40 on days 9, 12 and 15. Average tumour growth (c) and animal survival (d). e,f, C57BL/6 mice were inoculated subcutaneously with 2 × 105 B16F10 melanoma cells (e) or 2 × 106 NOOC1 head and neck cancer cells (f) on day 0. The treatments were given intra-tumourally starting day 8 for the B16F10 melanoma model and day 10 for the NOOC1 model with five injections 3 days apart. Data represent mean ± s.e.m., from a representative experiment (n = 7 (e,f) or 8 (a–d) biologically independent samples) from one (e) or two (a–d,f) independent experiments. ***P < 0.001, ****P < 0.0001, analysed by two-way ANOVA with Bonferroni’s multiple comparisons test (a–c,e–f); or log-rank (Mantel–Cox) test (d).
Lastly, we sought to validate the anti-tumour effect of the Mann-NC + αOX40 combination in other tumour models. In C57BL/6 mice bearing MC38 colon carcinoma, the Mann-NC + αOX40 combination therapy eradicated tumours in 100% of the animals, whereas Mann-NC alone or αOX40 monotherapy exhibited modest anti-tumour effects (P < 0.01; Fig. 8c,d). Moreover, we observed strong anti-tumour efficacy of Mann-NC + αOX40 combination therapy in the B16F10 melanoma model as well as in the tobacco-associated head-and-neck cancer model of NOOC1 tumour that we recently developed (Fig. 8e,f)45. On the other hand, intra-tumoural administration of LPS with or without αOX40 did not show anti-tumour effects in the CT26 tumour model (Supplementary Fig. 11). Collectively, these results show that the Mann-NC + αOX40 combination potently activates DCs, macrophages, TH17 cells, CD8+ T cells and NK cells, and that it exerts robust anti-tumour efficacy.
In summary, we have developed a pathogen-mimetic ‘nano-PAMP’ based on polysaccharide mannan, and presented an approach for exploiting pathogen-associated TH17 immunity to improve cancer immunotherapy. Mann-NC with hollow capsule morphology and the surface display of PAMPs mimics the yeast cell wall in nanoscale dimension (Fig. 1). Mann-NC endocytosed by DCs activates the Dectin-2 and TLR-4 signalling pathways (Fig. 2a–i), leading to the selective expansion of TH17 cells without triggering TH1, TH2 or Tc17 responses, and the release of pro-inflammatory cytokines and chemokines, including IL-17, IL-23 and CXCL10 within the TME (Figs. 2j–l, 3i and 4a–d). These immunological changes in the TME promoted tumour infiltration of activated CD8+ T cells and NK cells, resulting in increased ratios of TH17 cells:Treg cells, CD8+ T cells:Treg cells, and M1/M2 macrophages (Figs. 3g,k and 4e–g). Moreover, by combining Mann-NC with αOX40 IgG, we further amplified TH17-driven anti-tumour immune responses and achieved robust anti-tumour efficacy in multiple murine tumour models (Figs. 6–8). Overall, our study provides a biomaterial-based strategy for tipping the balance between TH17 cells and Treg cells in the TME and for exploiting TH17-based immune activation for orchestrating immune responses.
Methods
Reagents and instruments
Mannan from S. cerevisiae, triethylamine, ammonium fluoride and PEI (branched, molecular weight 25,000) were purchased from Sigma-Aldrich. siNPs (approximately 200 nm in diameter, 10 mg ml−1 in water) were purchased from nanoComposix. Amine-modified cya-nine5.5 was obtained from Lumiprobe. Sodium meta-periodate and dimethyl 3,3′-dithiobispropionimidate ٠ 2HCl (DTBP) were obtained from Thermo Fisher Scientific. UV–Vis absorption and fluorescence were measured using BioTek Synergy Neo microplate reader. TEM images were acquired using JEOL 1400-plus, and AFM was performed using Asylum-1 MFP-3D. Hydrodynamic size and zeta potential measurement were performed using Malvern Zetasizer Nano ZSP. Flow cytometry analyses were performed using Ze5 (Beckman Coulter), and the data were analysed using FlowJo 10.2 software. Confocal microscopy images were taken with Leica SP8 confocal microscope and analysed by ImageJ software (V 1.8.0).
Synthesis and characterization of Mann-NC
Mannan polymer (Mann) was first chemically modified to generate amine-reactive, aldehyde functional group by oxidation of hydroxyl groups. In detail, 200 μg of Mann was dissolved in 6 ml of ultrapure water and mixed with 6 ml of 0.01 M sodium periodate solution and reacted for 1 h with gentle shaking at room temperature in the dark. The product was purified using dialysis (molecular weight cut-off 1,000 Da, Spectrum) against de-ionized water for 3 days and lyophilized by freeze-drying in the dark for 2–3 days. The resulting aldehyde-modified Mann (Mann-CHO) was characterized using a modified hydroxylamine hydrochloride method46 for the aldehyde content and kept at 4 °C in the dark until further use.
Mann-NC was constructed using siNPs as a sacrificing template. Briefly, siNP (15 mg in 900 μl) was added with 15 μl of aqueous PEI25K solution (100 mg ml−1) and incubated for 10 min with vigorous vortex. The resulting PEI-siNP was centrifuged three times to remove excessive PEI25K, further reacted with bioreducible DTBP (0.5 mg in 1 ml 0.1 M triethylamine buffer at pH 8) for 1 h at room temperature to crosslink surface PEI, and then washed three times with ultrapure water. The outermost surface of crosslinked PEI-siNP was incubated with Mann-CHO (1,000 μl, 2 mg ml−1 ultrapure water) for 12 h at room temperature to prepare Mann-coated siNP. Finally, Mann-NC was obtained by selectively dissolving the solid siNP core using ammonium fluoride, followed by successive washing with ultrapure water three times and PBS twice. Hydrodynamic size and zeta potential of Mann-NC were measured using Malvern Zetasizer Nano ZS. Mann-NC (5 mg ml−1 in PBS) was stored at 4 °C until further use. The endotoxin level for each in vivo dose of Mann-NC and native Mann was measured to be <0.05 endotoxin units (EU) per dose, as determined by the LAL Chromogenic Endotoxin Quantitation Kit (Pierce).
Mann-NC uptake studies with BMDCs
BMDCs were prepared according to the literature47. Briefly, aseptically isolated bone-marrow cells from femurs and tibia of C57BL/6 mice were cultured in complete DC medium (RPMI-1640 (Gibco) supplemented with 10% foetal bovine serum (FBS, Corning), 1% penicillin–streptomycin (Gibco), 55 μM β-mercaptoethanol (Gibco) and 20 ng ml−1 granulocyte–macrophage colony-stimulating factor (GenScript)) at 37 °C with 5% CO2. Fresh medium was supplemented on day 3, and the medium was replaced on days 6 and 8. The cultured BMDCs were used between days 7 and 12 before being fully matured.
For time-dependent uptake study, immature BMDCs in complete DC medium were plated at 105 cells per well in 96-well round-bottom plate and cultured 12 h with the Mann-NC or Native-Mann for the indicated duration. For the Mann-NC uptake inhibition studies, BMDCs were pre-treated for 30 min with 20 μl of pharmacological inhibitors, including dextran sulfate (4 mg ml−1), chlorpromazine (50 μM), cytochalasin D (2.5 μM) and Mβ-CD (32 μM). BMDCs were then collected with cell dissociation reagent (StemPro Accutase, Gibco) and washed twice with PBS for flow cytometry analysis. For antibody staining, BMDCs were treated with anti-CD16/32 Fcɣ blocking antibody (eBioscience) in 1:20 dilution for 10 min and further stained with anti-CD11c antibody (eBioscience) in 1:100 dilution for 30 min at room temperature. After washing twice with FACS buffer (1% BSA in PBS), cells were dispersed in FACS buffer with 4,6-diamidino-2-phenylindole (DAPI, 300 nM) for running flow cytometry. For confocal microscopy analysis, 3 × 105 BMDCs were plated on the coverslip-bottom 12-well plate and treated for 6 h with Mann-NC or Native-Mann prepared using Cy5.5-conjugated mannan. BMDCs were then washed three times with cold PBS, stained with DAPI in PBS (300 nM) for 30 min, and fixed with 4% formaldehyde for 30 min. After mounting coverslips with a mounting medium (Molecular Probes), cells were imaged by a confocal microscope.
BMDC activation and cytokine release
A total of 105 BMDCs were plated per well in 96-well round-bottom plate and treated for 12 h with Native-Mann or Mann-NC. Supernatants were collected for cytokine analysis, including IL-12p70, TNF-α and IL-6, while BMDCs were stained with anti-CD16/32 blocking antibody in 1:20 dilution for 10 min, followed by fluorophore-labelled antibodies in 1:100 dilution for 30 min against CD11c (FITC, eBioscience), CD40 (PE, eBioscience), CD86 (APC, eBioscience) and MHC-II (PE-CY7, BioLegend). Cells were analysed by flow cytometry.
PRR inhibition study using blocking antibodies
A total of 105 BMDCs were seeded on 24-well plate and incubated for 30 min with blocking antibodies (2 μg ml−1) against PRRs on BMDCs surface, including Dectin-1, Dectin-2, CD206, CD209 (DC-SIGN), Mincle, TLR-2 and TLR-4, followed by treatment with Native-Mann or Mann-NC for 12 h. Supernatants were collected for TNF-α analysis by enzyme-linked immunosorbent assay (ELISA; R&D system).
Incubation with HEK-blue Dectin and TLR cells
A total of 2 × 105 HEK-blue cells (Dectin-1, Dectin-2, TLR-2 and TLR-4 cells from Invivogen) were incubated with Mann formulations in HEK-blue detection medium. Zymosan, and LPS (1 μg ml−1) were employed as positive controls for Dectin-1/2, TLR-2 and TLR-4, respectively. Activation of HEK-blue cells was quantified after 12 h by the absorbance at 650 nm.
In vitro CD4+ T-cell differentiation
TH1, TH2, iTreg and TH17 cells were generated by activating naïve CD4+ T cells with anti-CD3 and anti-CD28 monoclonal antibodies in the presence of cytokines and neutralizing antibodies. Briefly, naïve CD4+ T cells were isolated from spleen and lymph nodes using naïve CD4+ T-cell isolation kit (STEMCELL Technology) according to the manufacturer’s protocol. Isolated naïve CD4+ T cells were then resuspended in conditioned BMDC medium obtained by incubating BMDCs with Mann-NC or Native-Mann for 12 h, and stimulated for 3–5 days with pre-coated anti-CD3 (1 μg ml−1, BioLegend) and anti-CD28 (1 μg ml−1, BioLegend) in 96-well plate.
For ICS by flow cytometry, cells were restimulated with PMA (10 ng ml−1) and ionomycine (1,000 ng ml−1) for 4 h in the presence of Brefeldin A (5 μg ml−1). After washing twice with FACS buffer, antibody staining was performed in 1:100 dilution against surface markers CD4+ (APC) and CD25 (BV605), followed by lineage-specific, intracellular cytokine staining after fixation and permeabilization; IFN-γ (PE-CY7), IL-4 (BV605), Foxp3 (PE) and IL-17 (FITC). Supernatant collected from each well was analysed for cytokines by ELISA.
BMDC and BDC2.5 CD4+ T-cell co-culture studies
A total of 105 BMDCs isolated from BDC2.5 transgenic mice were seeded on 96-well plate and treated overnight with 100 ng of MHC-II-restricted p31 epitope (the cognate antigen for BDC2.5 transgenic CD4+ T cells) in the presence or absence of Mann-NC. BDC2.5 CD4+ T cells were isolated from spleen and lymph nodes of BDC2.5 T cell receptor transgenic mice using CD4+ T-cell isolation kit and added to p31-pulsed BMDCs (2 × 104 cells per well). After 3 days, CD4+ T cells were analysed by ICS as described above, and the cell medium from each well was analysed for IL-17A by ELISA.
Flow cytometry and antibodies
Single-cell suspensions were incubated with Fc receptor-blocking anti-CD16/32 antibody (eBioscience) at room temperature for 10 min, and stained with Fixable viability Dye eFluor 450 (eBioscience) at 37 °C for 30 min. After washing, surface markers were stained for 30 min at 4 °C. To detect cytokine production upon ex vivo restimulation, cells were resuspended in RPMI-1640 with 10% FBS and then cultured on plates pre-coated with 1 μg ml−1 anti-CD3 antibody (clone 145–2C11; BioLegend) and 1 μg ml−1 anti-CD28 antibody (clone 37.51; BioLegend) for 5 h at 37 °C in the presence of 2.5 μg ml−1 Brefeldin A Solution (BioLegend). Cells were processed for intracellular cytokine staining as described above.
Animal experiments
Animals were cared for following the federal, state, and local guidelines. All work performed on animals was in accordance with and approved by the Institutional Animal Care and Use Committee at the University of Michigan, Ann Arbor. For in vivo studies, 6–8-week-old female BALB/c mice (Jackson Laboratory, 18–20 g) and C57BL/6 mice (Jackson Laboratory, 18–20 g) were used. NOD.BDC2.5 transgenic mice (5–7-week-old female or male, 15–20 g) were obtained from Jackson Laboratory. Mice were housed in 12 h light–dark cycle, at 72 F, and about 30% humidity. For studies with CT26 colon carcinoma, BALB/c mice were subcutaneously inoculated on the right flank with 1.5 × 105 CT26 cells on day 0 and administered with samples on days 9, 12 and 15 by intra-tumoural injection. The dose of mannan-based formulations (Native-Mann and Mann-NC) were matched at 1 mg mannan per injection. For the combination study, anti-mouse OX40 (5 or 20 μg per injection) was co-administered with Mann-NC via intra-tumoural (i.t.) route on days 9, 12 and 15. After 80 days of the tumour inoculation, surviving mice were re-challenged with 5 × 105 CT26 cells on the left flank to evaluate immunological memory effect. For the antibody depletion study, depletion antibodies (200 μg per injection) against CD8α (BioXcell, clone 2.43, #BP0061), CD4 (Bioxcell, clone GK1.5, #BP0003–1), NK (Wako chemicals USA, anti-asialo GM1, #986–10001), Ly6G (BioXcell, clone 1A8, #BP0075–1), IL-17A (Bioxcell, clone 17F3, #BE0173) and isotype control immunoglobulin G 2b (BioXcell, clone LTF-2, #BP0090) were administered intraperitoneally on days 7, 10, 13 and 18. For combination studies in MC38 colon carcinoma model, C57BL/6 mice were subcutaneously inoculated with 5 × 105 MC38 cells on the right flank on day 0, followed by i.t. administration of Mann-NC and/or αOX40 (20 μg per injection) on days 9, 12 and 15.
For the therapeutic studies, C57BL/6 mice were inoculated with 1 × 105 B16F10 cells (ATCC) per mouse on the right flank by subcutaneous injection on day 0 and treated on the indicated days. Immune checkpoint blocker-resistant NOOC1 head-and-neck cells were maintained as reported previously45 in Iscove’s modified Dulbecco’s medium (Gibco, 12440053) supplemented with with F-12 nutrient mix (Gibco, 11765054), FBS (Hyclone, SH3039603), penicillin–streptomycin (Thermo Fisher, 15–140-122), insulin (Invitrogen, 12585014), hydro-cortisone (Sigma-Aldrich, H0888–1G) and epidermal growth factor (EMD Millipore, 01–107). For in vivo inoculation, NOOC1 was washed once with PBS and mixed with Matrigel (Thermo Fisher, CB-40230) to reach the cell density of 2 × 107 cells ml−1. A total of 2 × 106 NOOC1 cells were implanted on the right flank of each mouse. Tumour growth was monitored every other day, and the tumour volume was calculated by (width)2 × length × 0.52. Animals were killed when the tumour masses reached 1.5 cm in one dimension or when animals became moribund or exhibited severe weight loss or tumour ulceration.
ELISPOT
For analysis of tumour antigen-specific CD8α+ T-cell response, ELISPOT assay was performed with splenocytes from immunized mice. Spleens were collected aseptically, processed into single-cell suspension, and plated with 5 × 105 splenocytes per well in 96-well PVDF plates (EMD Millipore) pre-coated with IFN-γ antibody (R&D Systems) overnight. Splenocytes were then restimulated with antigen peptides (2 μg ml−1) or controls for 24 h. Assays were completed using sequential incubations with biotinylated secondary antibody, streptavidin alkaline phosphatase (Sigma Chemical) and NBT/BCIP substrate (Surmodics). Developed spots were analysed using an AID iSpot Reader (Autoimmun Diagnostika GmbH).
Tissue processing and cell staining for flow cytometry
Tumours and tumour draining LNs were excised and homogenized using pestle motor and treated with collagenase type IV (Sigma-Aldrich, 1 mg ml−1) and DNase I (Sigma-Aldrich, 100 U ml−1) in 1 ml of serum-free RPMI medium for 30 min at 37 °C with gentle shaking. For cytokine/chemokine analysis, supernatants were collected and kept at −70 °C. The cell suspension was then filtered through a cell strainer (70 μm) and washed with FACS buffer three times. Cells were incubated with CD16/32 blocking antibody in 1:20 dilution for 10 min, and then stained with antibodies for 30 min at room temperature. For the TME analysis, CD8, CD4, NK cells, DCs, monocytes, MDSCs and macrophages were stained with the following antibodies in 1:100 dilution: CD8+ T cells (CD45-FITC, CD8α-APC and CD3-PE-CY7), CD4+ T cells (CD45-FITC, CD4-APC and CD3-PE-CY7), DCs (CD45-FITC, CD11c-PE and CD86-PE-CY7), NK cells (CD45-FITC, CD49b-PE and CD3-PE-CY7), MDSCs (Ly6G-PE, Ly6C-APC and CD11b-FITC) and others (Dectin-2/CLEC6A α-APC and CD284 (TLR-4)-PE). Cells were washed twice with FACS buffer, resuspended in 2 μg ml−1 DAPI solution, and analysed by flow cytometry.
Immunofluorescence staining
For immunofluorescence staining, tumour tissues were excised and cut into small pieces. Tumour tissues were embedded in OCT compound and flash-frozen in liquid nitrogen. The frozen blocks were sectioned into 6 μm thickness and attached to slide glasses. Sectioned tumours were kept in room temperature for 10 min and washed with DPBS. Washed tissue sections were permeabilized with 0.3% Triton X100 in PBS (PBS-T) for 1 h, and blocked with 1% BSA in PBST for 1 h. Tissue sections were stained at 4 °C for 24 h with primary antibodies diluted to 1:100 in 1% BSA–PBST, followed by staining at room temperature for 2 h with fluorophore-conjugated secondary antibodies diluted to 1:500 in 1% BSA–PBST. CD8+ and CD4+ T cells were stained with the following antibodies: primary antibodies (CD3 (R&D Systems, #MAB4841–100), IFN-γ (R&D Systems, #AF-585-NA), IL-17A (R&D Systems, #AF-421-NA), integrin α 2/CD49b (R&D Systems, #AF1740), CD4 (Novus Biologicals, #NBP1–19371), CD8 (Novus Biologicals, #NBP1–49045)); secondary antibodies (goat anti-rabbit IgG H&L (Alexa Flour 488, Abcam, #ab150077), donkey anti-sheep IgG H&L (Alexa Flour 488, Abcam, #ab150177), donkey anti-goat IgG H&L (Cy3, Abcam, #ab6949) and goat anti-rat IgG H&L (Alexa Flour 647, Invitrogen, #A-21247)). Then, the tissue sections were stained with DAPI and mounted on slide glass using Prolong Diamond Antifade mountant (Invitrogen). Stained tumours were analysed with confocal microscopy (Nikon A1Rsi).
Statistical analysis
Sample sizes were chosen on the basis of preliminary data from pilot experiments and previously published results in the literature. All animal studies were performed after randomization. Experiments were not performed in a blinded fashion. Statistical analysis was performed with Prism 6.0 software (GraphPad Software) by one-way or two-way analysis of variance (ANOVA) with Bonferroni multiple comparisons post-test. Statistical significance for the survival curve was calculated by the log-rank test. Data were approximately normally distributed, and variance between groups was similar. Statistical significance was indicated as *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Extended Data
Extended Data Fig. 1 |. A schematic illustration of Mann-NC synthesis.
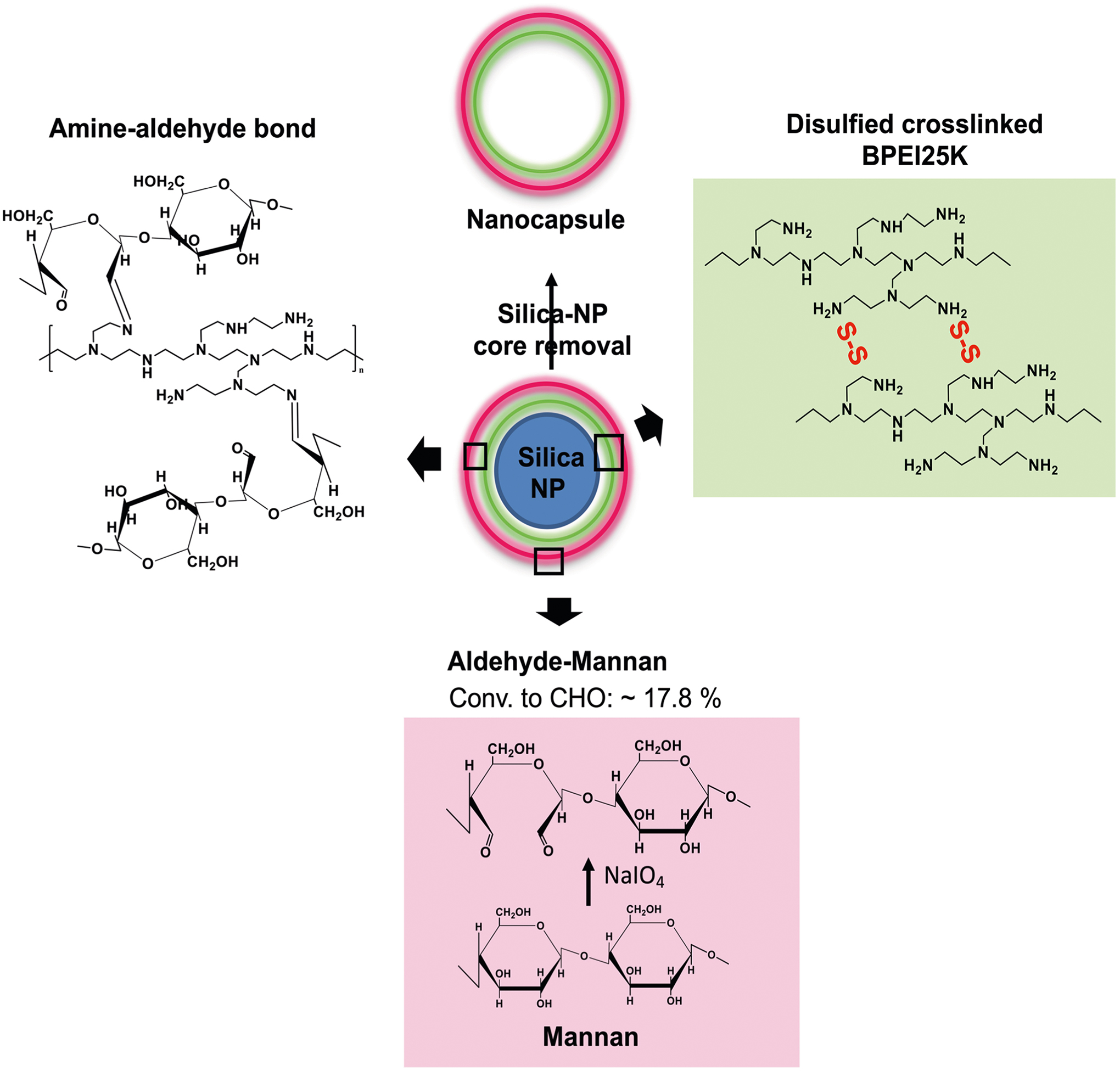
A negatively charged template silica nanoparticle was coated with branched polyethyleneimine (PEI, 25 kDa) polymer in ultra-pure water, followed by a chemical crosslinking step. The surface of PEI-coated silica nanoparticle was reacted with oxidized mannan polymers by the aldehyde-amine reaction. Then the silica nanoparticle core was selectively removed, resulting in hollow Mann-NC entirely composed of mannan polymers.
Extended Data Fig. 2 |. Phenotype and activity of CD4 T cells induced by Mann-NC.
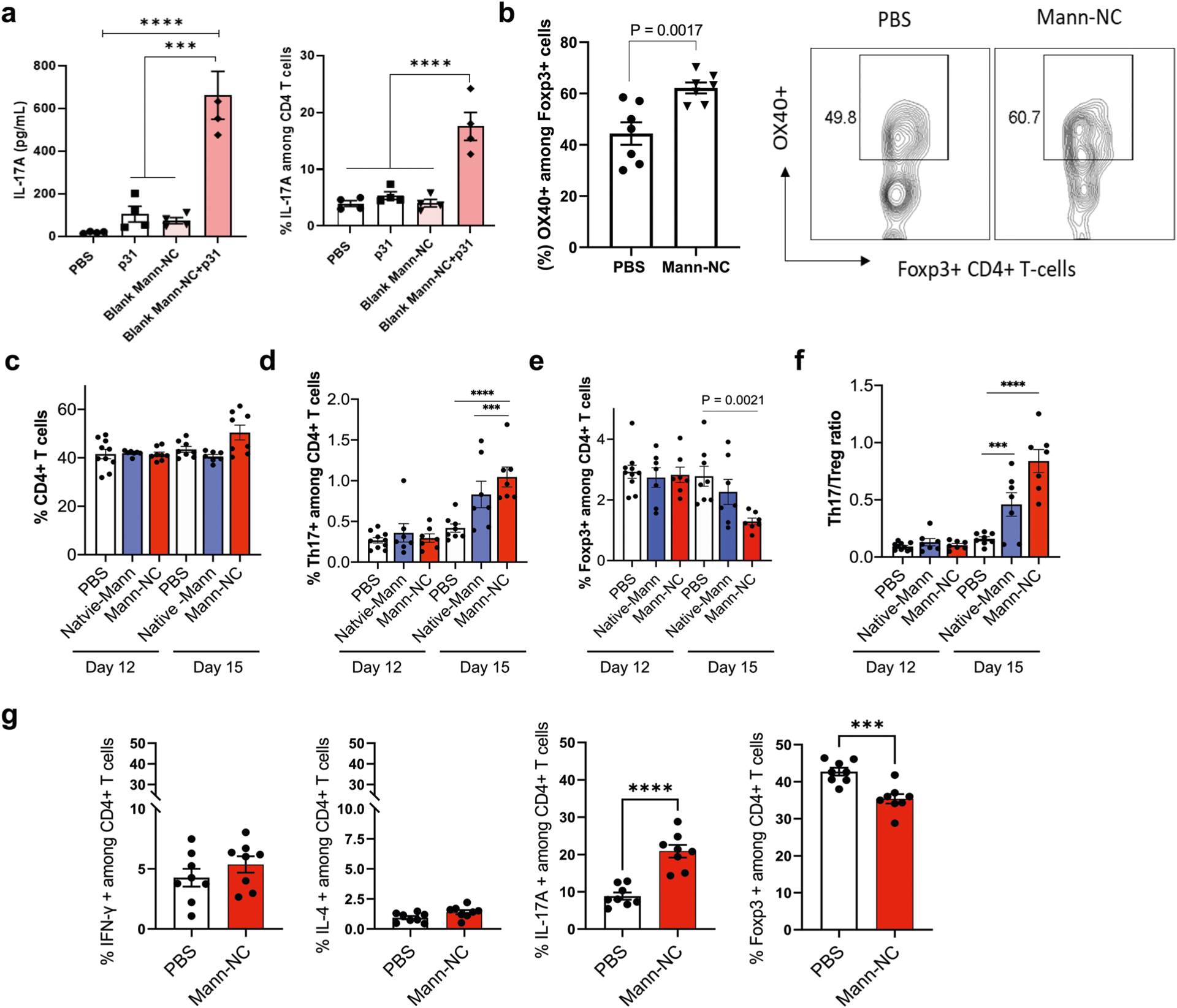
(a) DCs stimulated by Mann-NC trigger the differentiation of CD4 + T-cells toward Th17 phenotype. BMDCs isolated from BDC2.5 TCR transgenic mice were treated with p31 MHC-II epitope with and without Mann-NC. After overnight incubation, p31-specific CD4 + T-cells from BDC2.5 TCR transgenic mice were added and cultured for 3 days. IL-17A released into the media was measured by ELISA, and CD4 + T-cells expressing IL-17A was measured by intracellular cytokine staining. (b) Mann-NC-inducing OX40 expression levels of Foxp3 + CD4 + T-cells and representative contour plots. (c-f) Tumor-draining lymph nodes were analysed for the frequencies of (c) CD4 + T-cells, (d) IL17 + CD4 + Th17 cells, (e) Foxp3 + CD4 + Tregs, and (f) the corresponding Th17/Treg ratio. (g) CT26 tumor-bearing mice were treated with Mann-NC as in Fig. 3a, and CD4 + T-cells in the TME on day 15 were analysed for their subsets. Data represent mean ± SEM, from a representative experiment (n = 4 (a); n = 7 (b); n = 7 for Native-Mann and Mann-NC, or 8 for PBS on day 15, or 10 for PBS on day 12 (c-f); or n = 8 (g) biologically independent samples) from two (a,b,g) or three (c-f) independent experiments. ***P < 0.001, ****P < 0.0001, analysed by one-way (a) or two-way (c-f) ANOVA with Bonferroni’s multiple comparisons test or unpaired two-tailed Student’s t-test (b, g).
Extended Data Fig. 3 |. Cellular uptake of Mann-NC among tumor cells and innate immune cells in local tumors.
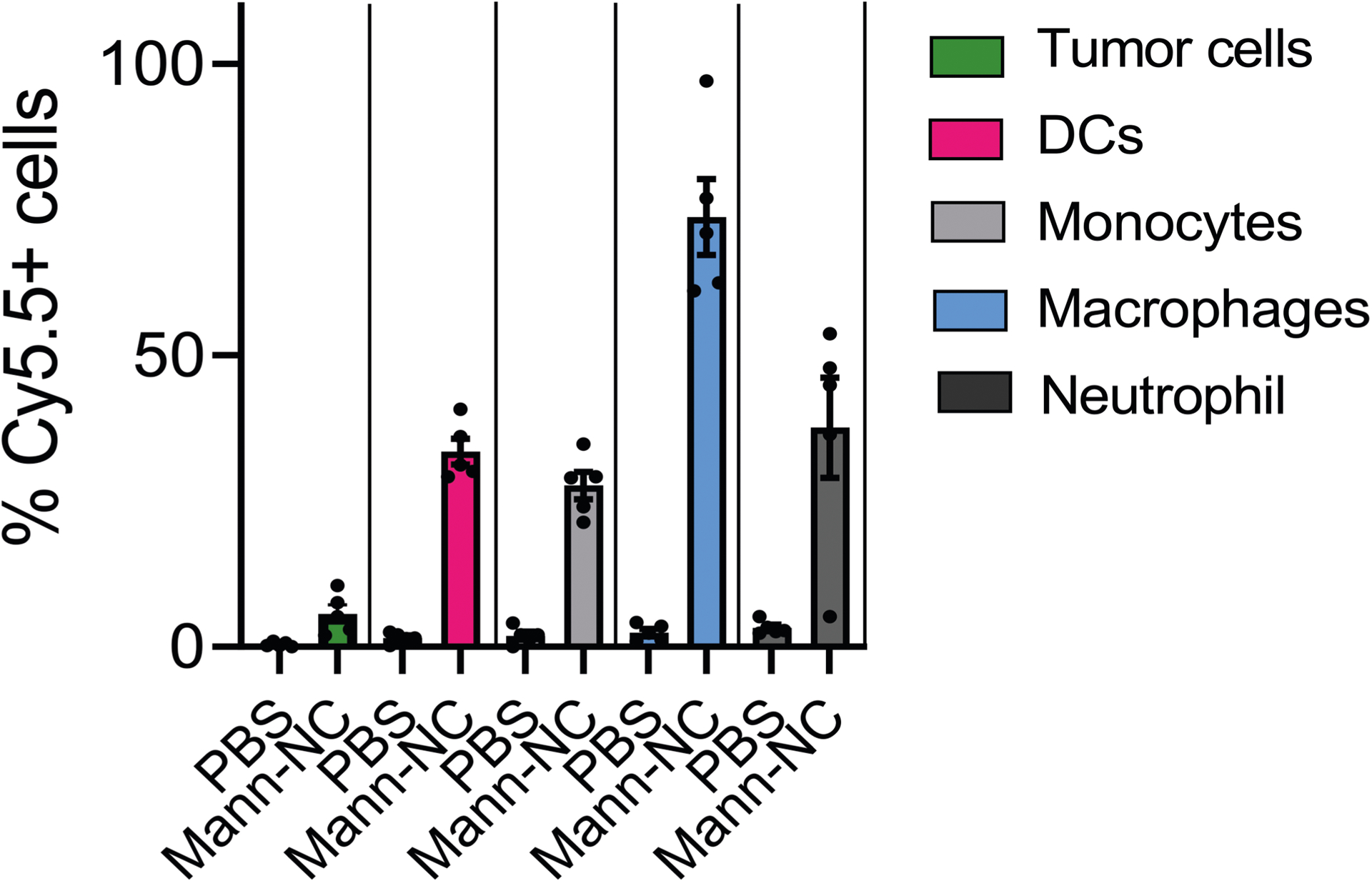
BALB/c mice were inoculated subcutaneously with 1.5 × 105 CT26 cells on day 0 and treated by intratumoral administration of Cy5.5-Mann-NC on day 9. After 3 days, tumor tissues were analysed for Cy5.5+ cells among CD45- tumor cells, CD11c + DCs, Ly6C+ monocytes, F4/80+ macrophages, and Ly6G+ neutrophils. Data represent mean ± SEM, from a representative experiment with n = 5 biologically independent samples from two independent experiments.
Extended Data Fig. 4 |. Representative contour plots.
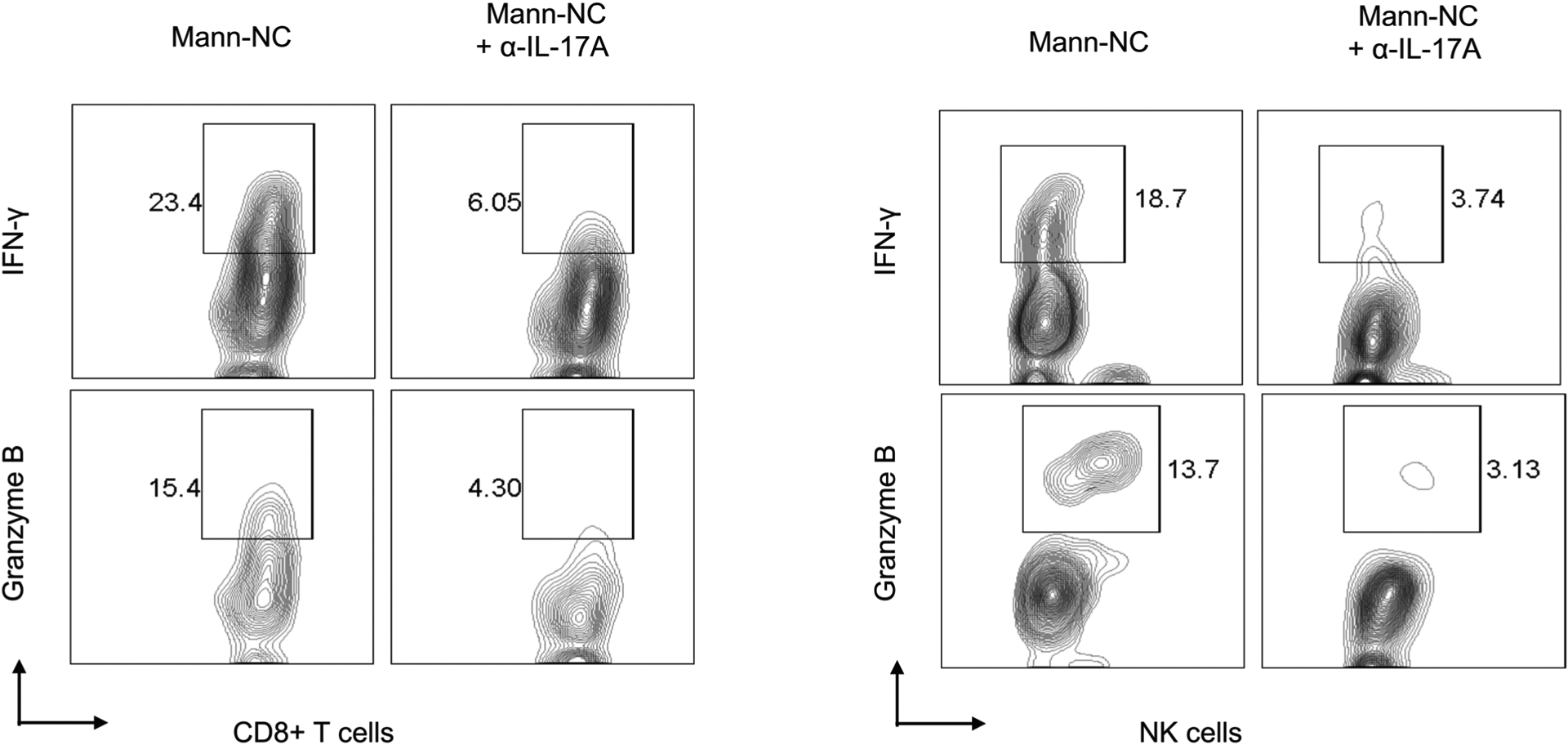
Shown are representative contour plots of IFN-γ + CD8 T-cells, granzyme B + CD8 + T-cells, IFN-γ + NK cells, and granzyme B + NK cells for the dataset shown in the main Fig. 5e–f.
Extended Data Fig. 5 |. Representative contour plots.
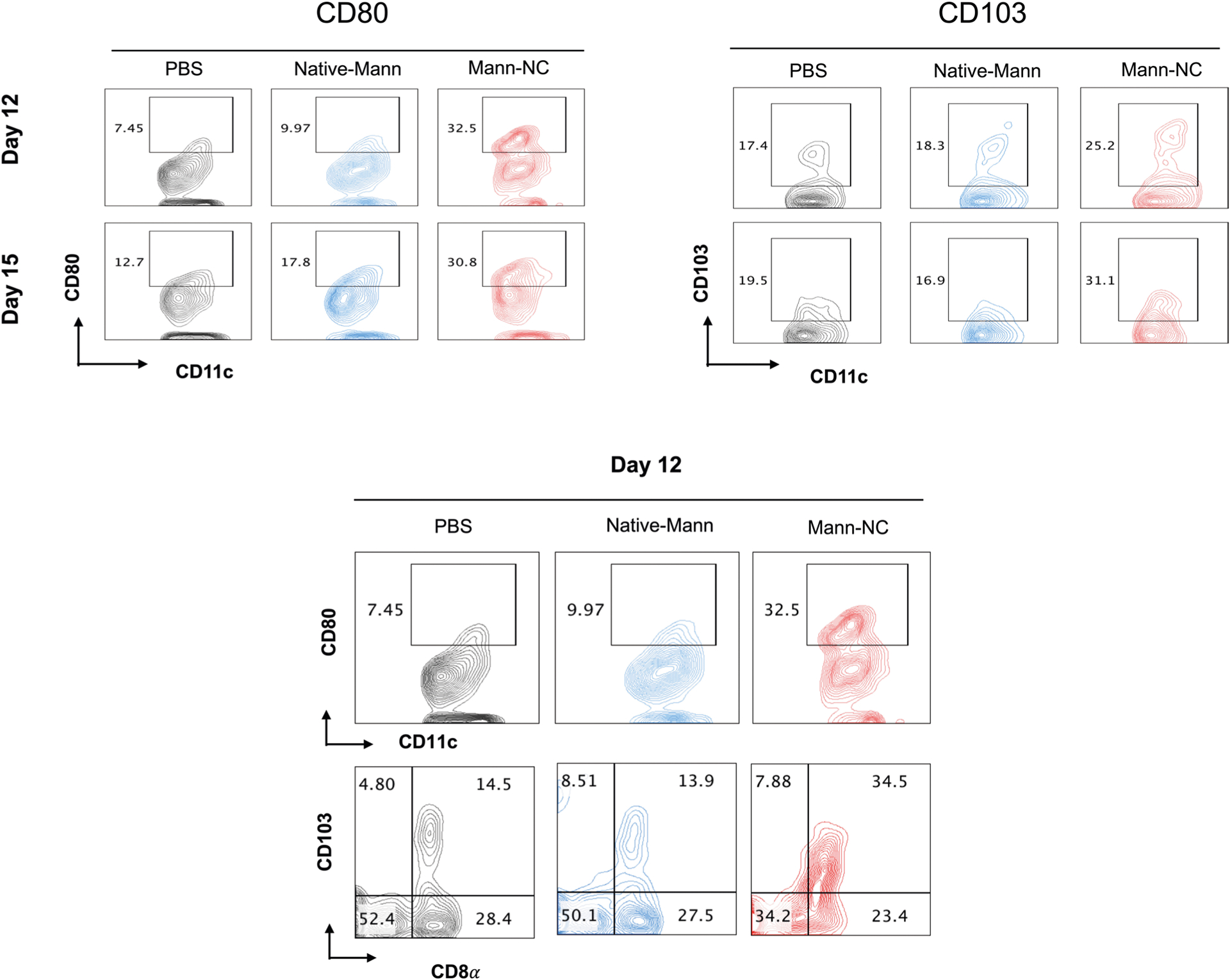
Shown are the representative contour plots of CD80 + CD11c + DCs, CD103 + CD11c + DCs, and CD8α + CD103 + CD11c + DCs for the dataset shown in the main Fig. 7a.
Extended Data Fig. 6 |. Immunofluorescence analysis of IL-17A expression.
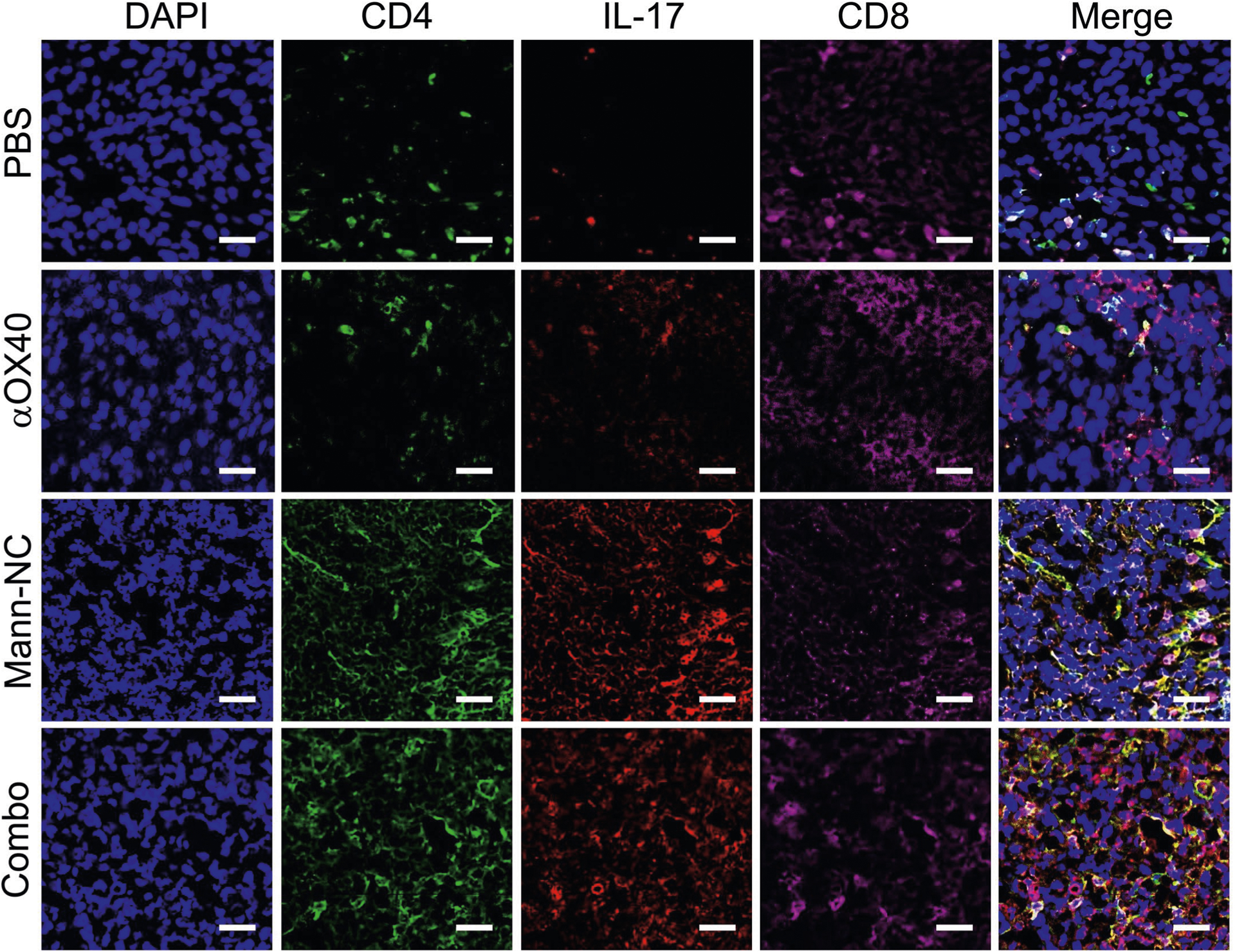
Immunofluorescence images of IL-17A expression among CD4 T-cells and CD8 T-cells in TME on day 15. Scale bar represents 50 μm.
Extended Data Fig. 7 |. Immunofluorescence analysis of IFN-γ expression.
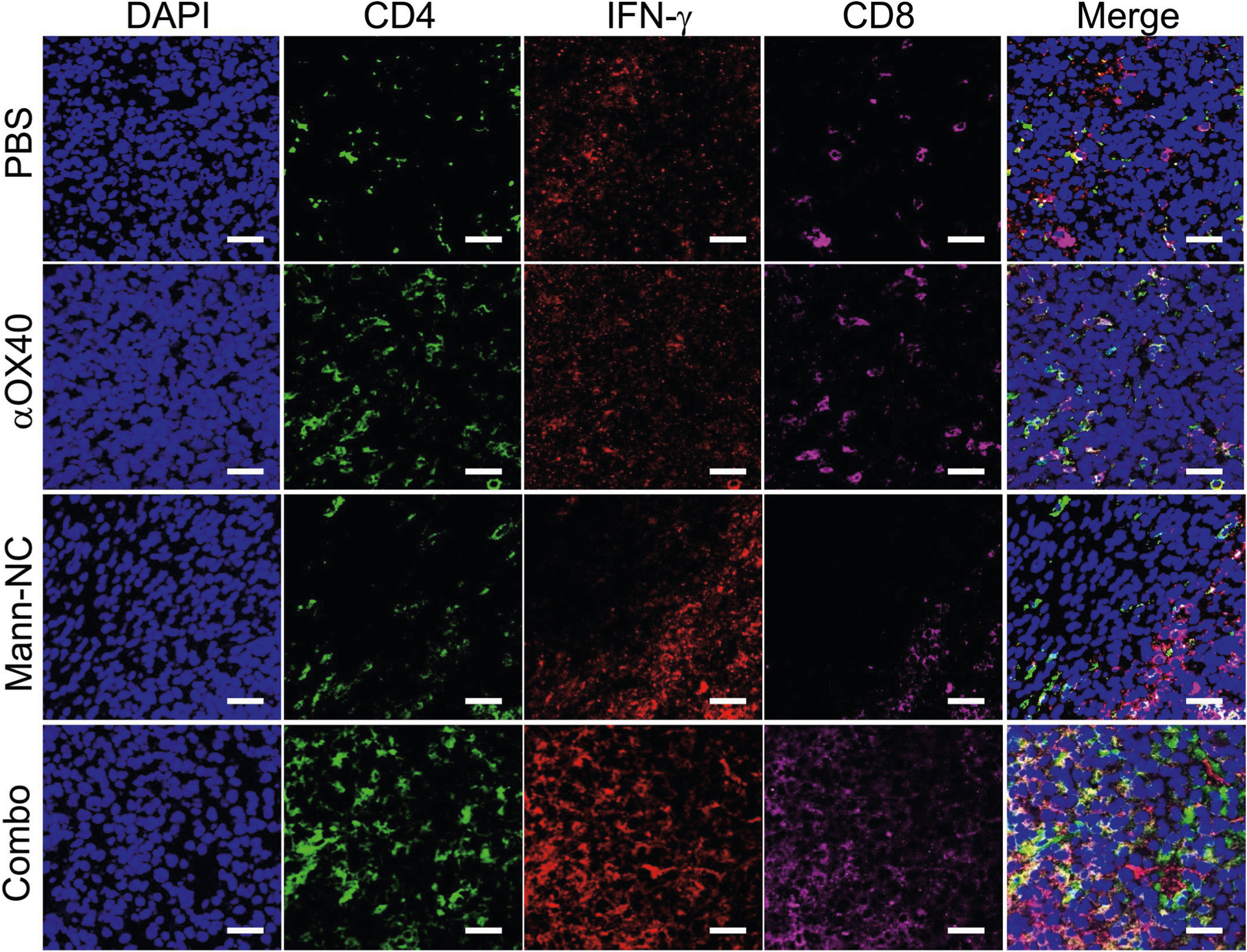
Immunofluorescence images of IFN-γ expression among CD4 T-cells and CD8 T-cells in TME on day 15. Scale bar represents 50 μm.
Supplementary Material
Acknowledgements
This work was supported in part by NIH (R01DE030691, R01DE031951, R01DK125087, R01CA271799, R01NS122536, U01CA210152 and P30CA046592), David Koch-Prostate Cancer Foundation Award in Nanotherapeutics and National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2022R1F1A1075064 and 2022R1A2C1006643). A.S.K. acknowledges financial support from the UM CBTP Training Program (NIH T32GM008353). K.S.P. acknowledges financial support from the UM TEAM Training Program (NIH T32DE007057). We thank the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of MHC-I tetramers; and the University of Michigan Cancer Center Immunology Core for ELISA analysis.
Footnotes
Competing interests
A patent application for particles for the delivery of biomolecules has been filed, with S.S., O.C.F. and J.J.M. as inventors. O.C.F. has financial interests in Selecta Biosciences, Tarveda Therapeutics, and Seer. J.J.M. declares financial interests for board membership, as a paid consultant, for research funding, and/or as equity holder in EVOQ Therapeutics, Saros Therapeutics and Intrinsic Medicine.
Extended data is available for this paper at https://doi.org/10.1038/s41551-022-00973-4.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41551-022-00973-4.
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. Source data are provided with this paper. All data generated in this study are available from the corresponding authors on reasonable request.
References
- 1.Medzhitov R & Janeway C Jr Innate immunity. N. Engl. J. Med 343, 338–344 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Matzinger P The danger model: a renewed sense of self. Science 296, 301–305 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Mogensen TH Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev 22, 240–273 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou W & Restifo NP TH17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol 10, 248–256 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noack M & Miossec P Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev 13, 668–677 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Khader SA et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol 8, 369–377 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Bettelli E, Korn T, Oukka M & Kuchroo VK Induction and effector functions of TH17 cells. Nature 453, 1051–1057 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quintana FJ et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Korn T, Bettelli E, Oukka M & Kuchroo VK IL-17 and Th17 cells. Annu. Rev. Immunol 27, 485–517 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Solt LA et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 472, 491–494 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esplugues E et al. Control of TH17 cells occurs in the small intestine. Nature 475, 514–518 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu T et al. Metabolic control of TH17 and induced Treg cell balance by an epigenetic mechanism. Nature 548, 228–233 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hang S et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 576, 143–148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilke CM, Bishop K, Fox D & Zou W Deciphering the role of Th17 cells in human disease. Trends Immunol. 32, 603–611 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knochelmann HM et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell. Mol. Immunol 15, 458–469 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin-Orozco N et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31, 787–798 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kryczek I et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 114, 1141–1149 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Y et al. Naive tumor-specific CD4+ T cells differentiated in vivo eradicate established melanoma. J. Exp. Med 207, 651–667 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muranski P et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 35, 972–985 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viaud S et al. Cyclophosphamide induces differentiation of Th17 cells in cancer patients. Cancer Res. 71, 661–665 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Si Y et al. Adjuvant-free nanofiber vaccine induces in situ lung dendritic cell activation and TH17 responses. Sci. Adv 6, eaba0995 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson MJ et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J. Exp. Med 206, 2037–2051 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saijo S et al. Dectin-2 recognition of α-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32, 681–691 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Netea MG et al. Variable recognition of Candida albicans strains by TLR4 and lectin recognition receptors. Med Mycol. 48, 897–903 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Kawai T & Akira S The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol 11, 373 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Rubio R, de Oliveira HC, Rivera J & Trevijano-Contador N The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front. Microbiol 10, 2993 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Son S et al. Sugar-nanocapsules imprinted with microbial molecular patterns for mRNA vaccination. Nano Lett. 20, 1499–1509 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pradhan A et al. Non-canonical signalling mediates changes in fungal cell wall PAMPs that drive immune evasion. Nat. Commun 10, 5315 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graus MS et al. Mannan molecular substructures control nanoscale glucan exposure in Candida. Cell Rep. 24, 2432–2442. e2435 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Croft M Control of immunity by the TNFR-related molecule OX40 (CD134). Annu. Rev. Immunol 28, 57–78 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aspeslagh S et al. Rationale for anti-OX40 cancer immunotherapy. Eur. J. Cancer 52, 50–66 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Korolenko TA, Bgatova NP & Vetvicka V Glucan and mannan—two peas in a pod. Int J. Mol. Sci 20, 3189 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vendele I et al. Mannan detecting C-type lectin receptor probes recognise immune epitopes with diverse chemical, spatial and phylogenetic heterogeneity in fungal cell walls. PLoS Pathog. 16, e1007927 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou Y et al. Co-delivery of antigen and dual adjuvants by aluminum hydroxide nanoparticles for enhanced immune responses. J. Control. Release 326, 120–130 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Rennick JJ, Johnston AP & Parton RG Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol 16, 266–276 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Chen C & Gao F-H Th17 cells paradoxical roles in melanoma and potential application in immunotherapy. Front. Immunol 10, 187 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ankathatti Munegowda M, Deng Y, Mulligan SJ & Xiang J Th17 and Th17-stimulated CD8+ T cells play a distinct role in Th17-induced preventive and therapeutic antitumor immunity. Cancer Immunol. Immunother 60, 1473–1484 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shortman K & Heath WR The CD8+ dendritic cell subset. Immunological Rev. 234, 18–31 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Cerovic V et al. Lymph-borne CD8α+ dendritic cells are uniquely able to cross-prime CD8+ T cells with antigen acquired from intestinal epithelial cells. Mucosal Immunol. 8, 38–48 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGeachy MJ et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol 10, 314–324 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bromley SK, Mempel TR & Luster AD Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat. Immunol 9, 970–980 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Xin L et al. Commensal microbes drive intestinal inflammation by IL-17-producing CD4+ T cells through ICOSL and OX40L costimulation in the absence of B7–1 and B7–2. Proc. Natl Acad. Sci. USA 111, 10672–10677 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang AY et al. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc. Natl Acad. Sci. USA 93, 9730–9735 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kreiter S et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 520, 692–696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun X et al. Amplifying STING activation by cyclic dinucleotide-manganese particles for local and systemic cancer metalloimmunotherapy. Nat. Nanotechnol 16, 1260–1270 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao H & Heindel ND Determination of degree of substitution of formyl groups in polyaldehyde dextran by the hydroxylamine hydrochloride method. Pharm. Res 8, 400–402 (1991). [DOI] [PubMed] [Google Scholar]
- 47.Lutz MB et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 (1999). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The main data supporting the results in this study are available within the paper and its Supplementary Information. Source data are provided with this paper. All data generated in this study are available from the corresponding authors on reasonable request.


