Abstract
Objective: To investigate the factors associated with the treatment of breast cancer with biosimilars from the perspectives of physicians and patients, and to generate evidence for promoting the uptake of biosimilars.
Methods: This study targeted trastuzumab and its indicated human epidermal growth factor receptor 2 (HER2) positive breast cancer and included female HER2 positive breast cancer patients under treatment of trastuzumab at a provincial oncology medical center in southern China from 1 January 2021, to 31 December 2021. The study extracted patients’ demographic, socioeconomic and clinical information and the basic information of their attending physicians from the hospital information system. We performed a bivariate multiple logistic regression analysis of predictive factors of the use of trastuzumab biosimilar.
Results: A total of 446 patients (aged ranging between 26 and 74, 51.4 ± 9.06) were included in the analysis, and 19.1% chose biosimilar trastuzumab. Older patients, patients enrolled in the urban and rural resident health insurance program compared with those enrolled in the urban employee health insurance program, patients who initiated treatment after January 2021 when biosimilar entered clinical use compared with those who initiated treatment before, patients with female attending physicians, younger attending physicians and with chief attending physicians compared with deputy chief attending physicians were more likely to adopt biosimilar trastuzumab for treatment (p < 05). Controlling the other factors unchanged, when the patient’s attending physician was deputy chief physician, increasing 1 year age of the patient was associated with an increased probability of adopting biosimilar by .8% (dy/dx = .008, 95%CI: .002–.01, p = .01). When the patient was aged between 26 and 60, the probability of adopting biosimilar for the patient whose attending physician was a chief physician was higher than for those whose attending physician was a deputy chief physician, and the gap was the largest when the patient was at the age of 45 (dy/dx = .20, 95%CI: .13–.27, p < .01).
Conclusion: The uptake rate of biosimilars is still low at its initial development stage in China. Educational policies and physicians making recommendations to the indicated patients at the initiation stage of treatment are helpful to avoid reduced willingness to switch to biosimilars due to non-clinical reasons. Patients with lower ability-to-pay will have better accessibility to biologic regimens through the uptake of biosimilars. Official guidelines and professional training are critical to enhancing physicians’ willingness and confidence in adopting biosimilars.
Keywords: biosimilar, breast cancer, uptake, physician, patient
Introduction
Unlike small-molecular chemical products with well-specified structures, biological agents are macromolecular substances with complex structures and manufacturing processes and are usually highly priced (Antman et al., 2017; Kirchhoff et al., 2017). Taking cancer medicines as an example, biological agents are increasingly highly priced and have a budget impact across countries, casting a shadow over the sustainability of national healthcare systems (Luzzatto et al., 2018; Godman et al., 2021). The need for a better balance between innovation and accessibility arises, hence biosimilars are raised as a potential solution to this dilemma. Biosimilars are similar to reference biologics in terms of quality, safety, and efficacy (Kirchhoff et al., 2017), they are medically equivalent to reference biologics but at a lower cost (Stebbing et al., 2017; Von Minckwitz et al., 2017; Pivot et al., 2018; Triantafyllidi and Triantafyllidis, 2022). Promoting the clinical application of biosimilars could stimulate competition, forcing down prices of reference biologics, thus to help saving health expenses and improving the accessibility of treatment based on biological regimens (Lyman et al., 2018; Chen et al., 2019; Jensen et al., 2020; Mouslim et al., 2020; Lobo and Río-Álvarez, 2021; Moorkens et al., 2021; Okoro, 2021). Higher biosimilar uptake rate is especially significant for low- and middle-income countries, where access to high-cost biological regimens is even worse (Putrik et al., 2014; Baumgart et al., 2019; Gershon et al., 2019; Al-Ziftawi et al., 2021; Tubic et al., 2021).
The European Union (EU) approved the world’s first biosimilar in 2006 and successively implemented a series of policies to promote the uptake of biosimilars, aiming to address the affordability issue of those highly-priced biological agents for both the health systems and individuals (Dylst et al., 2014; Chen et al., 2019; Diao et al., 2019; Jiang et al., 2019; Li et al., 2021a; Li et al., 2021b; Diao et al., 2021; Lobo and Río-Álvarez, 2021; Moorkens et al., 2021; Diao et al., 2022; Godman et al., 2022). These policies include biosimilar substitution (Dong et al., 2019), the requirement of a proportional volume or value of biosimilars prescribed by healthcare providers (Moorkens et al., 2017), financial incentives for clinical use of biosimilars for both patients and healthcare providers associated with health insurance reimbursement and settlement (Costa-Font and Rico, 2006; Berdud et al., 2016; Leonard et al., 2019; Gasteiger and Petrie, 2022). Researches have been conducted to prove the positive effects of these policies on national health systems across Europe, including saved budget for funding new medicines and increasing medicine volumes, gained sustainability of national health insurance system and price reduction of reference biologics (Godman et al., 2020; Godman et al., 2022).
The development of biosimilars in China is later than in the EU, but with a recent surge. China approved the first biosimilar in 2019, and the number of approved biosimilars doubled during the past 3 years with a strong pipeline (Hu et al., 2022). Many studies analyzed the factors associated with the uptake of biosimilars in the countries outside China (Costa-Font and Rico, 2006; Berdud et al., 2016; Alnahar et al., 2017; Mouslim et al., 2020; Lobo and Río-Álvarez, 2021), including physician’s and patient’s knowledge and attitudes towards biosimilars (Tubic et al., 2021), physician’s prescribing habit (Colloca et al., 2019; Okoro, 2021; Krstic et al., 2022), patient’s affordability and experience of treatment with reference biologics (Teeple et al., 2019a). While studies about biosimilars conducted in Chinese settings are mainly limited to clinical research or market entry approval (Diao et al., 2019; Xu et al., 2019; Rahalkar et al., 2021; Ren et al., 2021; Xu et al., 2021), uptake of biosimilars and studies about healthcare providers and patients are rare (Qu et al., 2021; Hu et al., 2022). This study generated evidence based on the real-world clinical data of a provincial oncology center in southern China, expecting to inform decision-making in promoting the uptake of biosimilars from the perspectives of physicians and patients.
Methods
Study design
This study was a retrospective analysis based on real-world clinical data. We divided the targeted patients into the reference and biosimilar groups and analyzed the factors associated with biosimilar uptake with a multiple regression analysis.
Population and data source
The study took trastuzumab as an example for the analysis. The reference trastuzumab was marketed in China in 2002 and covered by the national primary health insurance through national price negotiations since 2017, indicated for adjuvant and neoadjuvant therapy of human epidermal growth factor receptor 2 (HER2) positive breast cancer (China Anti Cancer Association, 2021). The first biosimilar trastuzumab was approved in China in 2020 and was immediately included in the national primary health insurance in 2021. The treatment cost of reference trastuzumab has been continuously reduced through national price negotiations from 2017 to 2019 and is now close to that of the biosimilar trastuzumab. Although there is only one biosimilar trastuzumab marketed in China by the end of July 2022, many applications are under review.
This study included female HER2 positive breast cancer patients under trastuzumab treatment from January 2021 to December 2021 in a provincial oncology center in southern China. The inclusion criteria for the targeted patients were: 1) female; 2) >18 years old; 3) diagnosed as HER2 positive breast cancer; 4) with precise tumor stage (I ∼ IV); 5) under treatment with trastuzumab (either the reference or the biosimilar); 6) under treatment of trastuzumab during January 2021 to December 2021. Patients with trastuzumab treatment contraindications, those with a history of another primary malignancy, and those with incomplete documentation of data were excluded. The inclusion criteria for the attending physicians were: 1) deputy chief or chief physicians; 2) specialized in breast cancer diagnosis and treatment; 3) with continuous clinical practice experiences in the research setting within the past 5 years (2017–2021). Since the target medical center performed team-based management for breast cancer treatment, although other members may give their advice on therapeutic measures, only the deputy chief or chief could be appointed the attending physician and make final decisions including prescription. So we excluded physicians unable to make prescription decisions (with a lower academic rank than the deputy chief) and those who did not prescribe trastuzumab (neither the reference nor the biosimilar) from January 2021 to December 2021. We extracted the targeted patients’ demographic, socioeconomic and clinical information, and their attending physicians’ basic information from the hospital information system.
Variables
The outcome variable was whether or not biosimilar was used during the observation time (used = 1, not used = 0). The explanatory variables were the characteristics of patients and their attending physicians. Patients’ demographic, socioeconomic and clinical characteristics included age, the economic development level of patient residence, residence in urban or rural areas, type of health insurance coverage, and whether they seek treatment locally. The economic development level of residence was categorized according to the lower and upper quartiles of each area’s annual average per capita disposable income (<$5924 as low, $5924–7405 as middle, and >$7405 as high). The clinical characteristics of patients included tumor stage and whether treatment with trastuzumab was initiated before January 2021, when the first biosimilar of trastuzumab started clinical use in the research setting. The characteristics of the attending physicians of patients included gender, age and academic rank.
Statistical analysis
The study firstly performed a descriptive statistical analysis of the distributions of the included patients’ demographic, socioeconomic and clinical characteristics and the characteristics of their attending physicians. We performed χ2 test and Fisher’s exact test to compare the distribution variations of the characteristics between the reference and the biosimilar groups. We had variables with statistically significant distribution variations and those supported by professional judgment included in a binary multiple logistic regression model and analyzed the predictive factors of using biosimilars from the perspectives of both physicians and patients. We also used the ‘margins’ command to analyze the interactive effect of physician and patient on the use of biosimilars. We set the significance level at .05 and completed all statistical analysis with STATA15.1 (Stebbing et al., 2017).
Results
Characteristics of patients and attending physicians
The demographic, socioeconomic and clinical characteristics of the included patients are reported in Table 1. A total of 446 patients were included in the analysis; only 85 (19.1%) were treated with the biosimilar. Patients were aged between 26 and 74 (51.4 ± 9.06). Patients diagnosed with early breast cancer (stage I ∼ II) accounted for 63.0% of all cases. Characteristics of attending physicians corresponding to the included patients are reported in Table 2. 24 physicians were included in this study. These physicians were aged between 42 and 62 (51.5 ± 5.89). Among them, 16 were male, and 12 were chief physicians.
TABLE 1.
Characteristics of the included patients.
| Characteristics | Total [n(%)] | Reference group [n(%)] | Biosimilar group [n(%)] | P value |
|---|---|---|---|---|
| 446 | 361(80.9) | 85(19.1) | ||
| Tumor stage | .18 | |||
| I∼II | 281(63.0) | 231(64.0) | 50(58.8) | |
| III | 76(17.0) | 64(17.7) | 12(14.1) | |
| IV | 89(20.0) | 66(18.3) | 23(27.1) | |
| Age a | .02 | |||
| <47 | 122(27.4) | 108(29.9) | 14(16.5) | |
| 47∼56 | 198(44.4) | 159(44.0) | 39(45.9) | |
| >56 | 126(28.3) | 94(26.1) | 32(37.6) | |
| Residence in urban or rural areas | .73 | |||
| Urban areas | 233(52.2) | 190(52.6) | 43(50.6) | |
| Rural areas | 213(47.8) | 171(47.4) | 42(49.4) | |
| The economic development level of the residence | .61 | |||
| Low | 155(34.8) | 122(33.8) | 33(38.8) | |
| Middle | 142(31.8) | 115(31.9) | 27(31.8) | |
| High | 149(33.4) | 124(34.3) | 25(29.4) | |
| Type of health insurance coverage | .03 b | |||
| Urban employee program | 163(36.5) | 142(39.3) | 21(24.7) | |
| Urban and rural resident program | 278(62.3) | 215(59.6) | 63(74.1) | |
| Others (including no insurance) | 5(1.2) | 4(1.1) | 1(1.2) | |
| Whether seeking medical treatment locally | .71 | |||
| Yes | 181(40.6) | 145(40.2) | 36(42.4) | |
| No | 265(59.4) | 216(59.8) | 49(57.6) | |
| Whether treatment with trastuzumab initiated before January 2021 | .13 | |||
| Yes | 53(11.9) | 47(13.0) | 6(7.1) | |
| No | 393(88.1) | 314(87.0) | 79(92.9) | |
| Gender of the attending physician | <.01 | |||
| Male | 344(77.1) | 291(80.6) | 53(62.4) | |
| Female | 102(22.9) | 70(19.4) | 32(37.6) | |
| Age of the attending physician c | <.01 | |||
| <50 | 135(30.3) | 119(33.0) | 16(18.8) | |
| 50∼58 | 196(43.9) | 140(38.8) | 56(65.9) | |
| >58 | 115(25.8) | 102(28.3) | 13(15.3) | |
| Academic rank of the attending physician | <.01 | |||
| Deputy chief physician | 125(28.0) | 113(31.3) | 12(14.1) | |
| Chief physician | 321(72.0) | 248(68.7) | 73(85.9) |
Patients’ age groups categorized according to the lower (47) and upper (56) quartiles.
Fisher’s precision probability test.
Corresponding physician’s age groups categorized according to the lower (50) and upper (58) quartiles.
TABLE 2.
Characteristics of the attending physicians.
| Characteristics | N (%) |
|---|---|
| 24 | |
| Gender | |
| male | 16(66.7) |
| female | 8(33.3) |
| Age | |
| mean (standard deviation) | 51.5(5.89) |
| Academic rank | |
| deputy chief | 12(50.0) |
| chief | 12(50.0) |
The distribution variations of patient age (p = .02) and type of health insurance coverage (p = .03) between the reference and the biosimilar groups were statistically significant; the distribution variations of attending physicians’ gender, age and academic rank (p < .01) between the reference and biosimilar groups were statistically significant (Table 1).
Logistic regression analysis results
Firstly, we included patient variables with statistically significant distribution variations (patient’s age and type of health insurance coverage) and variables supported by professional judgment (tumor stage and whether treatment with trastuzumab initiated before 2021) in the logistic regression model (Model 1). The regression results showed that older patients (p = .02) and patients enrolled in the urban and rural resident health insurance program compared with those enrolled in the urban employee health insurance program (p = .03), were more likely to adopt biosimilar for breast cancer treatment (Table 3).
TABLE 3.
Logistic regression analysis of the associated factors of biosimilar uptake (n = 446).
| Variables | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | p-value | OR (95%CI) | p-value | Β (95%CI) | p-value | ||||
| Patient’s age | 1.04(1.01–1.07) | <0.01 | 1.03(1.01–1.06) | 0.02 | .12(.04–.20) | <0.01 | |||
| Tumor stage (Ref. I ∼ II) | |||||||||
| III | .85(.42–1.71) | 0.64 | .87(.42–1.81) | 0.71 | −.14(-.87–.59) | 0.72 | |||
| IV | 1.73(.95–3.14) | 0.07 | 1.78(.95–3.34) | 0.07 | .55(-.09–1.18) | 0.09 | |||
| Type of health insurance coverage (Ref. urban employee program) | |||||||||
| urban and rural resident program | 1.93(1.11–3.33) | 0.02 | 1.87(1.06–3.29) | 0.03 | .67(.10–1.24) | 0.02 | |||
| others | 1.74(.16–18.7) | 0.65 | 2.18(.17–28.3) | 0.56 | 1.06(-1.53–3.65) | .42 | |||
| Whether treatment with trastuzumab was initiated before January 2021 (Ref. no) | |||||||||
| yes | .41(.16–1.03) | 0.06 | .36(.14–.95) | 0.04 | −1.04(-2.00∼-.09) | 0.03 | |||
| Physician’s age | .91(.86–.97) | <0.01 | −.09(-.15∼-.04) | <0.01 | |||||
| Physician’s gender (Ref. male) | |||||||||
| female | 2.30(1.31–4.02) | <0.01 | .84(.28–1.40) | <0.01 | |||||
| Physician’s academic rank (Ref. deputy chief physician) | |||||||||
| chief physician | 4.35(2.01–9.39) | <0.01 | 1.73(.85–2.61) | <0.01 | |||||
| The interaction term of patients’ age and physicians’ academic rank | −.10(-.19∼-.01) | 0.03 | |||||||
Then we included physician variables (physician’s age, gender and academic rank) in the regression model (Model 2), and the results showed that patient with younger attending physicians (p < .01), female attending physicians compared with the male (p < .01), and patients with chief attending physicians compared with the deputy chief attending physicians (p < .01) were more likely to adopt biosimilar for breast cancer treatment (Table 3). We also found that patients initiated treatment after January 2021 were more likely to adopt biosimilars compared with those before January 2021 (p = .04), which had been on the edge of insignificance in Model 1 (p = .06). Other patient variables in Model 1 (patient’s age, tumor stage and type of health insurance coverage) remained almost the same in significance and effects in Model 2.
In order to explore how the interactions between physician and patient affected the adoption of the biosimilar, we had the characteristic variables of patients and physicians interacted and included in the regression model (Model 3). We decentralized the continuous variable (patient’s age). Only the interaction between the patient’s age and the physician’s academic rank was statistically significant (p = .03). The regression results of Model 3 are reported in Table 3. The associated factors in Model 2 (patient’s age, type of health insurance coverage and whether treatment was initiated before January 2021; physician’s age, gender and academic rank) remained statistically significant in Model 3 (all p < .05).
Marginal effects of Patient’s age and Physician’s academic rank
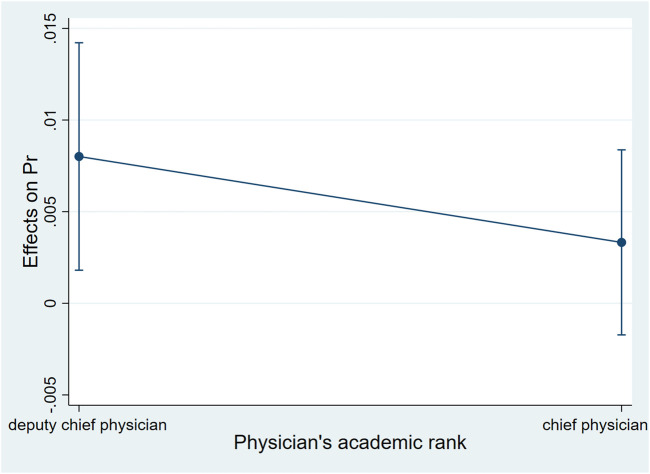
We used the ‘margins’ command to precisely estimate the marginal effects of patients’ age interacting with the academic rank of their attending physicians on the probability of adopting biosimilars. Controlling the other factors unchanged, when the academic rank of the attending physician was deputy chief physician, increasing 1 year age of the patient was associated with an increased probability of adopting biosimilar by .8% (dy/dx = .008, 95%CI: .002–.01, p = .01); when the academic rank of the attending physician was chief physician, patient’s age did not have a statistically significant association with the adoption of biosimilar (Table 4; Figure 1).
TABLE 4.
Fixing the academic rank of the attending physician, the marginal effects of patient’s age on biosimilar uptake.
| Academic rank of attending physician | dy/dx | 95%CI | p-value |
|---|---|---|---|
| deputy chief physician | .008 | .002–.01 | .01 |
| chief physician | .003 | −.002–.008 | .20 |
FIGURE 1.
Average marginal effects of patient’s age with 95%CIwhen their attending physicians were with different academic ranks.
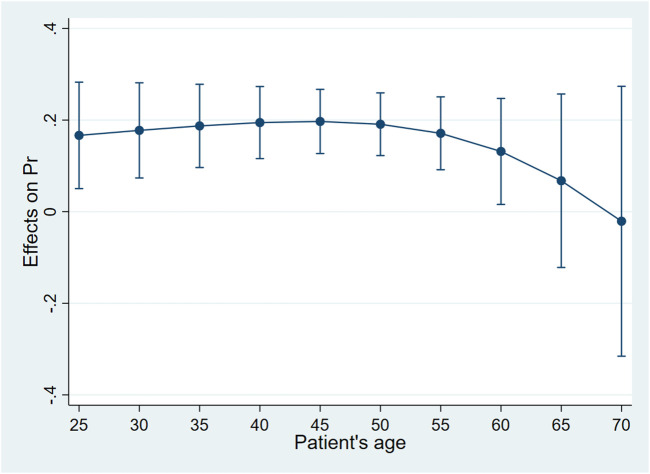
Controlling the other factors unchanged, when the patient was younger than 45 years old, the probability of choosing biosimilar for the patient whose attending physician was a chief physician was higher than for those whose attending physician was a deputy chief physician. Such a gap increased for the elder patient. When the patient was 45 years old, the gap was the largest, and the probability of adopting biosimilar for the patient whose attending physician was a chief physician was 20.0% higher than for those whose attending physician was a deputy chief physician (dy/dx = .20, 95%CI:0.13–.27, p < .01). When the patient was between 45 and 60 years old, the probability of adopting biosimilar for those whose attending physician was a chief physician was still higher than for those whose attending physician was a deputy chief physician. The gap decreased for the elder patient. When the patient reached 60 years old, the academic rank of their attending physician no longer had statistically significant associations with biosimilar uptake (Table 5; Figure 2).
TABLE 5.
Fixing patient’s age, the marginal effects of chief physician compared with the deputy chief physician on biosimilar uptake.
| Patient’s age | dy/dx | 95%CI | p-value |
|---|---|---|---|
| 25 | .17 | .05–.28 | .01 |
| 30 | .18 | .07–.28 | <.01 |
| 35 | .19 | .10–.28 | <.01 |
| 40 | .19 | .12–.27 | <.01 |
| 45 | .20 | .13–.27 | <.01 |
| 50 | .19 | .12–.26 | <.01 |
| 55 | .17 | .09–.25 | <.01 |
| 60 | .13 | .02–.25 | .03 |
| 65 | .07 | −.12–.26 | .49 |
| 70 | −.02 | −.32–.27 | .89 |
FIGURE 2.
Average marginal effects of attending physician’s academic rank with 95%CIwhen patients were of different ages.
Discussion
2021 was the first year that the trastuzumab biosimilar entered clinical use in the research setting. Less than 20% of patients included in this study under trastuzumab treatment adopted biosimilar in this year. At the initial development stage of biosimilars, the uptake rate was much lower in China than in the EU, the latter ranged from 41% to 77% in recent years and has been continuously increasing (Jarrion et al., 2022). Given the rapid development of biosimilars and immediate insurance coverage of the marketed biosimilars, we expect intense competition of trastuzumab and enhanced uptake of biosimilars in China.
This study found that the patient’s age was an essential factor associated with biosimilar uptake. The older the patients were, the higher the probability they would adopt biosimilars. This finding was consistent with the existing evidence (Shelbaya et al., 2021; Yang et al., 2022). Elderly patients may be more price sensitive and tend to use cheaper biosimilars, hoping to avoid a heavier financial burden on themselves or their children. Cancer patients with lower ability-to-pay prioritized lower treatment expenditure over treatment effects, especially for those at the terminal stage. Some patients even rejected treatment to avoid catastrophic expenditure (Meropol et al., 2008; Jenkins et al., 2013; Shrestha et al., 2019). This situation is relatively common in China, where the elderly are usually not the family’s breadwinners, and their out-of-pocket medical expenditures are usually undertaken by their children.
This study also found that only 11.3% of patients who initiated the treatment with reference trastuzumab switched to biosimilar trastuzumab when the later was available. Studies in the UK and the New Zealand also found a lower switching rate for patients who initiated the treatment with the reference product than new patients (Hemmington et al., 2017; Aladul et al., 2018). Physicians may not support switching for non-clinical reasons with a consideration of the potential psychological discomfort of patients (Teeple et al., 2019b). Information asymmetry due to lack of professional training and clinical data disclosure of biosimilars may be another reason for the non-preference of biosimilars. A survey showed that 85% of patients diagnosed with rheumatoid arthritis, Crohn’s disease, ulcerative colitis, psoriasis or psoriatic arthritis did not choose to switch to biosimilars; the primary concern of patients was the safety of biosimilars (Teeple et al., 2019a). Even if the treatment effect may not change significantly or improve after switching, patients may still be resistant to biosimilars when they have already been treated with reference biologics (Jørgensen et al., 2017; Avouac et al., 2022; Jarrion et al., 2022). Such a situation gets worse if adverse effects occur after switching. Patients may mistakenly attribute these symptoms to biosimilars, further exacerbating their mistrust of biosimilars (Gasteiger and Petrie, 2022). The weak intention of switching is a difficult question yet to be solved across countries (Dutta et al., 2020; Allocati et al., 2022). To promote the uptake of biosimilars, one appropriate solution is to enhance educational policies for both patients and physicians. Physicians recommending biosimilars to the indicated patients at the initial stage of treatment may also be available to avoid reduced willingness to switch due to non-clinical reasons. Health authorities could also be of help by formulating clinical guidelines about switching or incentivising biosimilar uptake. Health insurance coverage was associated with the choice of biosimilars. Currently, the Chinese population is universally covered by two parallel primary health insurance programs, the urban employee program and the resident program covering both urban and rural residents. The benefits packages of the former program are stronger than the latter, mainly due to the gaps in the amount of fundraising. The latter is funded heavily relying on the government subsidy for the population with a comparatively lower ability-to-pay. For patients with equivalent ability-to-pay, those enrolled in the urban employee program may be less likely to choose biosimilars than the urban and rural residents. The latter may be more concerned about higher out-of-pocket expenditure constrained by their weaker benefits packages. Physicians usually consider patients’ health insurance coverage, ability-to-pay and other economic factors when setting treatment plans. Thus, physicians are more inclined to recommend cheaper biosimilars to patients covered by a health insurance program with weaker benefits packages and lower ability-to-pay (Sullivan et al., 2017; Cook et al., 2019). Promoting the uptake of biosimilars will help to improve the accessibility of biologic agents for patients, especially for those with weaker health insurance benefits packages and lower ability-to-pay (Diao et al., 2019; Li et al., 2021a; Li et al., 2021b; Diao et al., 2021; Diao et al., 2022).
Our analysis from the perspective of the patient’s attending physician found that older physicians were less likely to have their patients treated with biosimilars. This may be associated with the tendency of older physicians to be more conservative. Conversely, young physicians may be more willing to accept newly developed medical technologies and are more open to follow cutting-edge evidence (Ren et al., 2021; Hu et al., 2022). A study of physicians showed that clinicians over 50 became more conservative about switching to biosimilars (57%); they thought biosimilars were newly marketed, lacking long-term clinical use evidence compared with the references (Leonard et al., 2019). Female physicians were more likely to adopt biosimilars than males. Studies found that female physicians show more empathy as well as a stronger willingness to communicate with patients, so they may be more likely to prescribe biosimilars to reduce medical expenditure or perform better explanations to patients about biosimilar regimens (Cooper-Patrick et al., 1999; Sandhu et al., 2009; Chaitoff et al., 2017). Chief physicians were more likely to adopt biosimilars than deputy chief physicians. This may be because physicians with senior academic ranks may have a better understanding and confidence in biosimilars. Studies showed that physicians with less clinical experience tended to choose more expensive imported medicines to satisfy patients or avoid medical responsibilities caused by poor treatment effects in Chinese healthcare settings (Zhu, 2012; Hu et al., 2022). Foreign studies also reported that physicians with more clinical experience or higher academic ranks were more confident in choosing biosimilars for patients with complex symptoms (Cook et al., 2019; Poon et al., 2021).
Although the price sensitivity of patients and the lower price of biosimilars may be the point of considering switching, this was not always the case like in eastern European countries, where were absent of substitution policy and appropriate incentives for the switch (Tubic et al., 2021). To enhance physicians’ willingness to switch to biosimilars, health authorities and healthcare associations may play an essential role in formulating guidelines for clinical application and switching of biosimilars, as well as strengthening professional training to make physicians fully understand the equivalence and interchangeability of biosimilars. Studies have found that demand-side policies like educational measures and official guidelines could enhance the confidence of physicians in biosimilars by avoiding information asymmetry (Kim et al., 2020; Godman et al., 2022). Financial incentives implemented in the EU to promote the uptake of biosimilars could also be considered in China when more biosimilars are available and more clinical safety and efficacy evidence is generated in the real world.
Limitations and future perspectives
This study had several limitations. First, this was a single-center study based on observations only 1 year after the availability of the first biosimilar in China. The study only focused on a single indication and one biological agent. The findings should be carefully interpreted for expanded indications and biological products. A small sample size may limit the efficiency of statistical analysis. Secondly, some essential socioeconomic characteristics of both physicians and patients (such as the patient’s educational background, occupation and income, physician’s length of clinical experience and professional training background, etc.) may also affect the uptake of biosimilars. With constraints of the retrospective data extracted from the hospital information system in the real clinical world, we were not able to collect information on all these variables. The only available socioeconomic characteristic of patients was insurance coverage. The study calculated each region’s annual average per capita disposable income to indicate patients’ ability-to-pay and analyzed the attending physicians’ academic rank to indicate their clinical experience and professional training background. The missing measurement should be considered through comprehensive investigations in future studies.
Conclusion
The uptake rate of the biosimilar is still low at its initial development stage in China. Educational policies on biosimilars and physicians making recommendations of biosimilars to the indicated patients at the initial stage of treatment are helpful to avoid reduced willingness to switch due to non-clinical reasons. Patients with a weak ability-to-pay will have better accessibility to biological regimens through the uptake of biosimilars. Official guidelines and professional training are critical to enhancing physicians’ willingness and confidence in adopting biosimilars.
Acknowledgments
The authors appreciate the health information system staff of Fujian Cancer Hospital, who provided valuable support to data extraction.
Funding Statement
This research was funded by Peking Union Medical College. The findings and conclusions made by the authors do not necessarily reflect the opinions of Peking Union Medical College.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Fujian Cancer Hospital. Written informed consent from the (patients/ participants OR patients/participants legal guardian/next of kin) was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
All authors contributed to the study design, data analysis and results interpretation, and meet the requirements for authorship and submissions. JS and CC led the study, contributed to the methodology, and provided critical comments on the article. QW was responsible for data collection and cleaning, statistical analysis, and writing the first draft of the manuscript. ZL, XW, and HC participated in data collection and analysis, and revised the draft. HY, GZ, FW, and JL supported data collection and interpretation and provided comments on the overall article with intellectual content and professional knowledge.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Al-Ziftawi N. H., Shafie A. A., Mohamed Ibrahim M. I. (2021). Cost-effectiveness analyses of breast cancer medications use in developing countries: A systematic review. Expert Rev. Pharmacoecon Outcomes Res. 21 (4), 655–666. 10.1080/14737167.2020.1794826 [DOI] [PubMed] [Google Scholar]
- Aladul M. I., Fitzpatrick R. W., Chapman S. R. (2018). Healthcare professionals’ perceptions and perspectives on biosimilar medicines and the barriers and facilitators to their prescribing in UK: A qualitative study. BMJ Open 8 (11), e023603. 10.1136/bmjopen-2018-023603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allocati E., Godman B., Gobbi M., Garattini S., Banzi R. (2022). Switching among biosimilars: A review of clinical evidence. Front. Pharmacol. 13, 917814. 10.3389/fphar.2022.917814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnahar S., Elliott R. A., Smith M. D. (2017). Biosimilar uptake by British local formularies: A cross sectional study. Int. J. Clin. Pharm. 39 (5), 1055–1060. 10.1007/s11096-017-0523-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anti Cancer Association China. (2021). China anti cancer association guidelines for diagnosis and treatment of breast cancer (2021). China Oncol. 31 (10), 954–1040. 10.19401/j.cnki.1007-3639.2021.10.013 [DOI] [Google Scholar]
- Antman E. M., Creager M. A., Houser S. R., Warner J. J., Konig M. (2017). American heart association principles on the accessibility and affordability of drugs and biologics: A presidential advisory from the American heart association. Circulation 136 (24), e441–e447. 10.1161/CIR.0000000000000551 [DOI] [PubMed] [Google Scholar]
- Avouac J., Murail R. C., Goulvestre C., Dumas S., Molto A., Miceli-Richard C., et al. (2022). Immunogenicity of Rituximab biosimilar GP2013 in chronic inflammatory rheumatic disorders in daily clinical practice. Semin. Arthritis Rheu 52, 151951. 10.1016/j.semarthrit.2022.151951 [DOI] [PubMed] [Google Scholar]
- Baumgart D. C., Misery L., Naeyaert S., Taylor P. C. (2019). Biological therapies in immune-mediated inflammatory diseases: Can biosimilars reduce access inequities? Front. Pharmacol. 10, 279. 10.3389/fphar.2019.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdud M., Cabasés J. M., Nieto J. (2016). Incentives and intrinsic motivation in healthcare. Gac. Sanit. 30 (6), 408–414. 10.1016/j.gaceta.2016.04.013 [DOI] [PubMed] [Google Scholar]
- Chaitoff A., Sun B., Windover A., Bokar D., Featherall J., Rothberg M. B., et al. (2017). Associations between physician empathy, physician characteristics, and standardized measures of patient experience. Acad. Med. 92 (10), 1464–1471. 10.1097/ACM.0000000000001671 [DOI] [PubMed] [Google Scholar]
- Chen B., Nagai S., Armitage J. O., Witherspoon B., Nabhan C., Godwin A. C., et al. (2019). Regulatory and clinical experiences with biosimilar filgrastim in the U.S., the European union, Japan, and Canada. Oncologist 24 (4), 537–548. 10.1634/theoncologist.2018-0341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L., Panaccione R., Murphy T. K. (2019). The clinical implications of nocebo effects for biosimilar therapy. Front. Pharmacol. 10, 1372. 10.3389/fphar.2019.01372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. W., McGrath M. K., Dixon M. D., Switchenko J. M., Harvey R. D., Pentz R. D. (2019). Academic oncology clinicians’ understanding of biosimilars and information needed before prescribing. Ther. Adv. Med. Oncol. 11, 1758835918818335. 10.1177/1758835918818335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper-Patrick L., Gallo J. J., Gonzales J. J., Vu H. T., Powe N. R., Nelson C., et al. (1999). Race, gender, and partnership in the patient-physician relationship. JAMA 282 (6), 583–589. 10.1001/jama.282.6.583 [DOI] [PubMed] [Google Scholar]
- Costa-Font J., Rico A. (2006). Vertical competition in the Spanish national health system (NHS). Public Choice 128 (3), 477–498. 10.1007/s11127-005-9011-y [DOI] [Google Scholar]
- Diao Y., Lin M., Xu K., Huang J., Wu X., Li M., et al. (2021). How government health insurance coverage of novel anti-cancer medicines benefited patients in China–a retrospective analysis of hospital clinical data. BMC Health Serv. Res. 21 (1), 856–859. 10.1186/s12913-021-06840-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao Y., Lin M., Xu K., Huang J., Wu X., Li M., et al. (2022). Impact of public health insurance coverage of novel anticancer medication on medical expenditure and patient affordability in a provincial medical centre of China: A propensity score-matching analysis with the quasi-experimental design. BMJ Open 12 (2), e054713. 10.1136/bmjopen-2021-054713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao Y., Qian J., Liu Y., Zhou Y., Wang Y., Ma H., et al. (2019). How government insurance coverage changed the utilization and affordability of expensive targeted anti-cancer medicines in China: An interrupted time-series study. J. Glob. Health 9 (2), 020702. 10.7189/jogh.09.020702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Jiang R., Shao R. (2019). Medical insurance access and clinical use policy of biosimilars in European Union and its enlightenment in China. Chin. J. Hosp. Pharm. 39 (19), 1915–1919. 10.13286/j.cnki.chinhosppharmacyj.2019.19.01 [DOI] [Google Scholar]
- Dutta B., Huys I., Vulto A. G., Simoens S. (2020). Identifying key benefits in European off-patent biologics and biosimilar markets: It is not only about price. BioDrugs 34 (2), 159–170. 10.1007/s40259-019-00395-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dylst P., Vulto A., Simoens S. (2014). Barriers to the uptake of biosimilars and possible solutions: A Belgian case study. Pharmacoeconomics 32 (7), 681–691. 10.1007/s40273-014-0163-9 [DOI] [PubMed] [Google Scholar]
- Gasteiger C., Petrie K. J. (2022). Moving forward: Implementing health psychology research to improve patient acceptance of biosimilars. Res. Soc. Admin Pharm. 18 (10), 3860–3863. 10.1016/j.sapharm.2022.03.009 [DOI] [PubMed] [Google Scholar]
- Gershon N., Berchenko Y., Hall P. S., Goldstein D. A. (2019). Cost effectiveness and affordability of trastuzumab in sub-Saharan Africa for early stage HER2-positive breast cancer. Cost. Eff. Resour. A 17 (1), 5–10. 10.1186/s12962-019-0174-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godman B., Allocati E., Moorkens E., Kwon H. Y. (2020). Can local policies on biosimilars optimize the use of freed resources–experiences from Italy. GaBI J. 9 (4), 183–187. 10.5639/gabij.2020.0904.029 [DOI] [Google Scholar]
- Godman B., Hill A., Simoens S., Selke G., Selke Krulichová I., Zampirolli Dias C., et al. (2021). Potential approaches for the pricing of cancer medicines across Europe to enhance the sustainability of healthcare systems and the implications. Expert Rev. Pharmacoecon Outcomes Res. 21 (4), 527–540. 10.1080/14737167.2021.1884546 [DOI] [PubMed] [Google Scholar]
- Godman B., Tubic B., Allocati E., Wladysiuk M., McTaggart S., Kurdi A., et al. (2022). Biosimilars are essential for sustainable healthcare systems; however, key challenges remain as seen with long-acting insulin analogues. J. Appl. Pharm. Sci. 12 (3), 55–72. 10.7324/JAPS.2022.120306 [DOI] [Google Scholar]
- Hemmington A., Dalbeth N., Jarrett P., Fraser A. G., Broom R., Browett P., et al. (2017). Medical specialists’ attitudes to prescribing biosimilars. Pharmacoepidem Dr. S 26 (5), 570–577. 10.1002/pds.4186 [DOI] [PubMed] [Google Scholar]
- Hu Y., Song Z., Jiang D., Zhuo L., Cheng Y., Zhao R. (2022). Knowledge, attitudes and practice of healthcare providers, healthcare regulatory practitioners and patients toward biosimilars in China: Insights from a nationwide survey. Front. Pharmacol. 13, 876503. 10.3389/fphar.2022.876503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrion Q., Azzouz B., Robinson J., Jolly D., Vallet C., Trenque T. (2022). Penetration rate of anti-TNF biosimilars and savings at 5 years after their introduction in French hospitals. Ther 77 (4), 467–475. 10.1016/j.therap.2021.10.012 [DOI] [PubMed] [Google Scholar]
- Jenkins V., Catt S., Banerjee S., Gourley C., Montes A., Solis-Trapala I., et al. (2013). Patients’ and oncologists’ views on the treatment and care of advanced ovarian cancer in the UK: Results from the ADVOCATE study. Brit J. Cancer 108 (11), 2264–2271. 10.1038/bjc.2013.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T. B., Kim S. C., Jimenez-Solem E., Bartels D., Christensen H. R., Andersen J. T. (2020). Shift from adalimumab originator to biosimilars in Denmark. JAMA Intern Med. 180 (6), 902–903. 10.1001/jamainternmed.2020.0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R., Dong X., Shao R. (2019). Analysis on the policy measures of clinical use incentives and risk control for biosimilars in the European union. Chin. Pharm. J. 54 (22), 1895–1900. [Google Scholar]
- Jørgensen K. K., Olsen I. C., Goll G. L., Lorentzen M., Bolstad N., Haavardsholm E. A., et al. (2017). Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): A 52-week, randomised, double-blind, non-inferiority trial. Lancet 389 (10086), 2304–2316. 10.1016/S0140-6736(17)30068-5 [DOI] [PubMed] [Google Scholar]
- Kim Y., Kwon H. Y., Godman B., Moorkens E., Simoens S., Bae S. (2020). Uptake of biosimilar infliximab in the UK, France, Japan, and korea: Budget savings or market expansion across countries? Front. Pharmacol. 11, 970. 10.3389/fphar.2020.00970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff C. F., Wang X. Z. M., Conlon H. D., Anderson S., Ryan A. M., Bose A. (2017). Biosimilars: Key regulatory considerations and similarity assessment tools. Biotechnol. Bioeng. 114 (12), 2696–2705. 10.1002/bit.26438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krstic M., Devaud J. C., Marti J., Sadeghipour F. (2022). Exploring the reasons behind the substantial discontinuation rate among patients taking ct-P13 in a large tertiary hospital in western Switzerland: A retrospective cohort study using routinely collected medical data. Drugs-Real Wor Outc 9 (3), 425–436. 10.1007/s40801-022-00299-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard E., Wascovich M., Oskouei S., Gurz P., Carpenter D. (2019). Factors affecting health care provider knowledge and acceptance of biosimilar medicines: A systematic review. J. Manag. Care Spec. P. H. 25 (1), 102–112. 10.18553/jmcp.2019.25.1.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Diao Y., Ye J., Sun J., Jiang Y. (2021b). Analysis of how public health insurance coverage of targeted anti-breast cancer medicines through national medicines price negotiation Benefited patient. Chin. J. Pharmacoepidemiol 30 (6), 53–59. [Google Scholar]
- Li M., Diao Y., Ye J., Sun J., Jiang Y. (2021a). The public health insurance coverage of novel targeted anticancer medicines in China—in favor of whom? A retrospective analysis of the insurance claim data. Front. Pharmacol. 12, 778940. 10.3389/fphar.2021.778940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo F., Río-Álvarez I. (2021). Barriers to biosimilar prescribing incentives in the context of clinical governance in Spain. Pharmaceuticals 14 (3), 283. 10.3390/ph14030283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzatto L., Hyry H. I., Schieppati A., Costa E., Simoens S., Schaefer F., et al. (2018). Outrageous prices of orphan drugs: A call for collaboration. Lancet 392 (10149), 791–794. 10.1016/S0140-6736(18)31069-9 [DOI] [PubMed] [Google Scholar]
- Lyman G. H., Balaban E., Diaz M., Ferris A., Tsao A., Voest E., et al. (2018). American society of clinical oncology statement: Biosimilars in oncology. J. Clin. Oncol. 36 (12), 1260–1265. 10.1200/JCO.2017.77.4893 [DOI] [PubMed] [Google Scholar]
- Meropol N. J., Egleston B. L., Buzaglo J. S., Benson A. B., III, Cegala D. J., Diefenbach M. A., et al. (2008). Cancer patient preferences for quality and length of life. Cancer 113 (12), 3459–3466. 10.1002/cncr.23968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorkens E., Godman B., Huys I., Hoxha I., Malaj A., Keuerleber S., et al. (2021). The expiry of Humira® market exclusivity and the entry of adalimumab biosimilars in Europe: An overview of pricing and national policy measures. Front. Pharmacol. 11, 591134. 10.3389/fphar.2020.591134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorkens E., Vulto A. G., Huys I., Dylst P., Godman B., Keuerleber S., et al. (2017). Policies for biosimilar uptake in Europe: An overview. PLOS ONE 12 (12), e0190147. 10.1371/journal.pone.0190147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouslim M. C., Trujillo A. J., Alexander G. C., Segal J. B. (2020). Association between filgrastim biosimilar availability and changes in claim payments and patient out-of-pocket costs for biologic filgrastim products. Value Health 23 (12), 1599–1605. 10.1016/j.jval.2020.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoro R. N. (2021). Biosimilar medicines uptake: The role of the clinical pharmacist. Explor Res. Clin. Soc. Pharm. 1, 100008. 10.1016/j.rcsop.2021.100008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivot X., Bondarenko I., Nowecki Z., Dvorkin M., Trishkina E., Ahn J. H., et al. (2018). Phase III, randomized, double-blind study comparing the efficacy, safety, and immunogenicity of SB3 (trastuzumab biosimilar) and reference trastuzumab in patients treated with neoadjuvant therapy for human epidermal growth factor receptor 2–positive early breast cancer. J. Clin. Oncol. 36 (10), 968–974. 10.1200/JCO.2017.74.0126 [DOI] [PubMed] [Google Scholar]
- Poon S. Y. K., Hsu J. C., Ko Y., Chiang S. C. (2021). Assessing knowledge and attitude of healthcare professionals on biosimilars: A national survey for pharmacists and physicians in taiwan. Healthcare 9 (11), 1600. 10.3390/healthcare9111600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putrik P., Ramiro S., Kvien T. K., Sokka T., Pavlova M., Uhlig T., et al. (2014). Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann. Rheum. Dis. 73 (1), 198–206. 10.1136/annrheumdis-2012-202603 [DOI] [PubMed] [Google Scholar]
- Qu J., Zuo W., Wang S., Du L., Liu X., Gao Y., et al. (2021). Knowledge, perceptions and practices of pharmacists regarding generic substitution in China: A cross-sectional study. BMJ Open 11 (10), e051277. 10.1136/bmjopen-2021-051277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahalkar H., Sheppard A., Santos G. M. L., Dasgupta C., Perez-Tapia S. M., Lopez-Morales C. A., et al. (2021). Current regulatory requirements for biosimilars in six member countries of BRICS-TM: Challenges and opportunities. Front. Med. 8, 726660. 10.3389/fmed.2021.726660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z., Xu J., Bai Y., Xu A., Cang S., Du C., et al. (2021). Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study. Lancet Oncol. 22 (7), 977–990. 10.1016/S1470-2045(21)00252-7 [DOI] [PubMed] [Google Scholar]
- Sandhu H., Adams A., Singleton L., Clark-Carter D., Kidd J. (2009). The impact of gender dyads on doctor–patient communication: A systematic review. Patient Educ. Couns. 76 (3), 348–355. 10.1016/j.pec.2009.07.010 [DOI] [PubMed] [Google Scholar]
- Shelbaya A., Kelton J. M., Thompson J., Alvir J. M., Maculaitis M. C., Yang J. (2021). Real-world use and acceptance of biosimilar monoclonal antibodies of rituximab in oncology practice in the USA. Future Oncol. 17 (30), 3941–3950. 10.2217/fon-2021-0618 [DOI] [PubMed] [Google Scholar]
- Shrestha A., Martin C., Burton M., Walters S., Collins K., Wyld L. (2019). Quality of life versus length of life considerations in cancer patients: A systematic literature review. Psychooncology 28 (7), 1367–1380. 10.1002/pon.5054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing J., Baranau Y. V., Baryash V., Manikhas A., Moiseyenko V., Dzagnidze G., et al. (2017). Double-blind, randomized phase III study to compare the efficacy and safety of CT-P6, trastuzumab biosimilar candidate versus trastuzumab as neoadjuvant treatment in HER2 positive early breast cancer (EBC). J. Clin. Oncol. 35 (15), 510. 10.1200/JCO.2017.35.15_suppl.510 [DOI] [Google Scholar]
- Sullivan E., Piercy J., Waller J., Black C. M., Kachroo S. (2017). Assessing gastroenterologist and patient acceptance of biosimilars in ulcerative colitis and Crohn’s disease across Germany. PLOS ONE 12 (4), e0175826. 10.1371/journal.pone.0175826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeple A., Ellis L. A., Huff L., Reynolds C., Ginsburg S., Howard L., et al. (2019b). Physician attitudes about non-medical switching to biosimilars: Results from an online physician survey in the United States. Curr. Med. Res. Opin. 35 (4), 611–617. 10.1080/03007995.2019.1571296 [DOI] [PubMed] [Google Scholar]
- Teeple A., Ginsburg S., Howard L., Huff L., Reynolds C., Walls D., et al. (2019a). Patient attitudes about non-medical switching to biosimilars: Results from an online patient survey in the United States. Curr. Med. Res. Opin. 35 (4), 603–609. 10.1080/03007995.2018.1560221 [DOI] [PubMed] [Google Scholar]
- Triantafyllidi E., Triantafillidis J. K. (2022). Systematic review on the use of biosimilars of trastuzumab in HER2+ breast cancer. Biomedicines 10 (8), 2045. 10.3390/biomedicines10082045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubic B., Marković-Peković V., Jungić S., Allocati E., Godman B. (2021). Availability and accessibility of monoclonal antibodies in Bosnia and Herzegovina: Findings and implications. Med. Access @ Point Care 5, 239920262110276–239920262110277. 10.1177/23992026211027692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Minckwitz G., Ponomarova O., Morales S., Zhang N., Hanes V. (2017). Efficacy and safety of biosimilar ABP 980 compared with trastuzumab in HER2 positive early breast cancer. Ann. Oncol. 28, v44. 10.1093/annonc/mdx362.002 [DOI] [PubMed] [Google Scholar]
- Xu Y., Xie L., Zhang E., Gao W., Wang L., Cao Y., et al. (2019). Physicochemical and functional assessments demonstrating analytical similarity between rituximab biosimilar HLX01 and the MabThera®. mAbs. 11 (3), 606–620. doi: 10.1080/19420862.2019.1578147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Han S., Guan X., Shi L. (2021). Consideration and suggestion on clinical drug switching of biosimilars in China. Chin. J. New Drugs 30 (17), 1554–1558. [Google Scholar]
- Yang J., Kelton J. M., Thompson J., Jimenez Alvir J. M., Maculaitis M. C., Shelbaya A. (2022). Real-world usage of bevacizumab-bvzr biosimilar in US oncology practice. Am. J. Manag. Care 28 (4), 160–166. 10.37765/ajmc.2022.88831 [DOI] [PubMed] [Google Scholar]
- Zhu Y. (2012). Analysis of physician prescribing behavior based on the model of theory of planned behavior. Drug Eval. 9 (17), 16–19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.