Abstract
Internal impingement – or entrapment of the undersurface of the rotator cuff tendon against the glenoid during overhead activities – is believed to contribute to articular-sided tears. However, little is known about internal impingement outside athletic populations. Therefore, the objectives of this study were to: 1) describe glenoid-to-footprint distances and proximity centers during dynamic, in vivo motion in asymptomatic individuals, and 2) determine the extent to which these measures differed between individuals with and without a rotator cuff tear. Shoulder kinematics were assessed in 37 asymptomatic individuals during scapular plane abduction using a high-speed biplane radiographic system. Glenoid-to-footprint distances and proximity center locations were calculated by combining the kinematics and CT-derived bone models. Glenoid-to-footprint contact was presumed to occur when the minimum distance was less than the estimated labral thickness. The condition of the supraspinatus tendon (intact, torn) was assessed using ultrasound. Minimum distances and proximity centers were compared over humerothoracic elevation angles (90°, 110°, 130°, 150°) and between supraspinatus pathology groups using two-factor mixed model ANOVAs. The minimum distance decreased consistently across elevation angles (p<0.01) without a significant difference between groups. Contact was estimated to occur in all participants. The proximity center was generally located on the anterior half of the rotator cuff footprint and the posterosuperior glenoid.
Clinical Significance:
Internal impingement during overhead motions may be a prevalent mechanism of rotator cuff pathology as contact appears to be common and involves the region of the rotator cuff footprint where degenerative rotator cuff tears are thought to originate.
Keywords: Rotator cuff, internal impingement, kinematics
Introduction
Rotator cuff tears are common and occur with increasing prevalence after age 50.1–3 Although there are likely many factors that contribute to rotator cuff pathology, degenerative (i.e., non-traumatic) tears are often observed along the undersurface (i.e., articular surface) of the rotator cuff tendon.4–7 This finding has led some clinicians and researchers to postulate that injury may occur if the tendon’s undersurface becomes entrapped against the glenoid during overhead activities.8; 9 This phenomenon, termed internal (or posterior) impingement, was first described by Perry in 198310 and later observed arthroscopically by Walch in 1992.8 Both clinicians described tendon entrapment against the glenoid when the shoulder is placed in a combination of abduction and end-range external rotation, a position common in overhead athletics. However, recent evidence suggests that internal impingement may not be limited to overhead athletic motions but may also occur during overhead functional motions (i.e., humeral elevation without end-range axial rotation) making it a potential mechanism of rotator cuff injury in non-athletic populations.11–13
Compared to mechanical subacromial impingement, in which the rotator cuff is abraded under the coracoacromial arch,14 little is known about internal impingement. Previous research has found that the distance between the glenoid and rotator cuff tendon footprint (i.e., glenoid-to-footprint distance) decreases as the arm is elevated,11; 12 and that contact may occur in positions other than combined abduction and external rotation.11–13 Although these studies have expanded our knowledge of internal impingement, interpreting their findings is often challenging because the motion investigated was simulated,11 performed passively under anesthesia,13 or did not include the individual’s full range of motion.12 Therefore, there remains a gap in our understanding of how internal impingement risk changes across an individual’s full in vivo range of motion. Furthermore, it remains unclear to what extent internal impingement is associated with the presence of rotator cuff pathology.
In addition to glenoid-to-footprint distance, investigating the location of closest proximity (i.e., proximity centers) may help elucidate the role of internal impingement in degenerative rotator cuff pathology. For example, intraoperative studies have described “kissing” lesions, where a rotator cuff tendon tear is observed to contact the posterosuperior labrum in positions of combined abduction and external rotation.15; 16 These findings provide preliminary, albeit indirect, evidence supporting the role of internal impingement in rotator cuff and labral pathology. However, little is known about the proximity centers during less extreme overhead motions that are more common to functional tasks.
The objectives of this study were to: 1) describe glenoid-to-footprint distances and proximity centers during dynamic, in vivo motion in asymptomatic individuals, and 2) determine the extent to which these measures differed between individuals with and without a rotator cuff tear. It was hypothesized that glenoid-to-footprint distances would decrease as arm elevation increased, and that the proximity centers would be located on the anterior supraspinatus and posterosuperior glenoid.
Methods
This cross-sectional study was performed according to the procedures approved by the Institutional Review Board of Henry Ford Health System. Signed consent was obtained from all participants prior to data collection.
Participants
Thirty-seven participants were enrolled in this study as part of a larger investigation (age: 55±4 years, sex: 62.2% female, height: 170.6±9.7 cm, mass: 78.0±13.2 kg, BMI: 26.7±3.3 kg/m2). Eligible participants were 50–60 years old without any shoulder pain within the last 12 weeks. Participants were excluded if they had diabetes, currently smoked, had a BMI >30 kg/m2, a history of shoulder symptoms following trauma, significant radiation exposure (e.g., radiation therapy, recent CT scan), or shoulder corticosteroid injection(s), subluxation, dislocation, adhesive capsulitis, fracture, osteoarthritis, or surgery.
Data Collection
Shoulder motion analysis was performed using a high-speed biplane radiographic system based on methods previously described.17 Participants were seated and performed active, unconstrained, and unloaded scapular plane abduction on their dominant side while radiographic images were acquired at 60 Hz. Participants were instructed to pace their motion such that they achieved their overhead full range of motion within the two-second acquisition period.
In addition to motion analysis testing, each participant underwent a diagnostic ultrasound examination of their dominant shoulder. All examinations were interpreted by a single fellowship-trained musculoskeletal radiologist with 13 years of clinical experience using established criteria.18–20 Although all visible pathology was noted, participants were classified based on the status of the supraspinatus tendon (intact, torn) as it is the rotator cuff muscle-tendon unit most found to be pathologic.21; 22 A CT scan of the full shoulder was also acquired allowing for visualization of the full humerus, scapula, and hemi-torso. Finally, all participants completed a questionnaire that inquired about their participation in overhead athletics including approximated hours per day, days per week, weeks per year, and years of participation in each sport.
Data Processing
Following data collection, each participant’s full humerus, scapula, and third rib were segmented from the CT scans using Mimics software (Materialise NV, Leuven, Belgium). Image segmentation was performed in a semi-automated manner. Specifically, initial thresholding was performed based on grey values associated with cortical bone, and the bone’s contour was subsequently refined manually to ensure continuity across the bone’s surface. Anatomical coordinates systems were constructed on the humerus and scapula based on recommendations by the International Society of Biomechanics.23 Additionally, a glenoid-based coordinate system was also defined for the scapula.24 To describe thorax kinematics – and thus humerothoracic kinematics – anatomical landmarks on the participant’s torso were visualized and digitized on the CT scan23 and described relative to the bone volume of the third rib.
Well-established methods were used to process the radiographic images and to track segmental motion.25 Segmental kinematic data were filtered using a 4th order Butterworth filter with a 5 Hz low-pass cutoff and then related to describe glenohumeral and humerothoracic kinematics using X-Z’-Y” and Y-X’-Y” rotation sequences, respectively.23; 26 All left-sided data were transformed to right-sided equivalency. To facilitate anatomical interpretation, glenohumeral anterior/posterior and superior/inferior positions were described using the glenoid-based coordinate system and were normalized to glenoid width and height, respectively.27
Each participant’s motion trial was reconstructed by combining their three-dimensional bone models with their kinematic data using a custom MATLAB code (The MathWorks, Inc, Natick, MA). For each frame of the motion trial, minimum distance maps were calculated on the glenoid and rotator cuff insertion (i.e., footprint). The rotator cuff insertion was identified on the bone models as the superior and middle facet of the greater tuberosity coinciding with the insertion of the supraspinatus and infraspinatus tendons.28 During minimum distance calculations, a constraint was imposed to prevent the minimum distance vector from penetrating the humerus or scapula. This constraint helped ensure that all minimum distances were physiologically plausible as the shortest distance vector between the surfaces often went through the humeral head at lower angles of humerothoracic elevation. From these distance maps, the minimum distance was calculated as the smallest distance between the glenoid and rotator cuff footprint (i.e., glenoid-to-footprint minimum distance).
The location of the minimum distance (i.e., proximity center) was identified on each surface and described relative to the glenoid-based or humeral coordinate system, as appropriate. As was done for glenohumeral position, these proximity centers were also normalized to facilitate anatomical interpretation. Specifically, glenoid proximity centers were normalized to glenoid height or width, as appropriate, and humeral proximity centers were normalized to the diameter of a sphere fit to the humeral articular surface using a least-squares approach. Finally, the three-dimensional distance between the biceps groove and proximity center on the rotator cuff footprint was calculated as this measure is frequently used to describe the location of rotator cuff tears.29; 30
Internal impingement during shoulder motion involves soft tissue structures (i.e., glenoid labrum) that are not visualized on the CT and therefore could not be reconstructed and included in the glenoid-to-footprint distance calculations. Therefore, to help identify potential instances of internal impingement (i.e., contact between the glenoid and rotator cuff footprint), glenoid-to-footprint minimum distances were interpreted relative to the average thickness of the posterosuperior glenoid labrum (4.3 mm).31 Specifically, glenoid-to-footprint was presumed to have occurred when the glenoid-to-footprint minimum distance fell below 4.3 mm. Finally, the prevalence of contact between the supraspinatus tendon and the acromion (i.e., subacromial contact) was also calculated using previously described methods that used subject-specific measures of rotator cuff tendon thickness to identify potential contact.32 This was done to descriptively compare the prevalence of contact between mechanical subacromial and internal impingement mechanisms and facilitate clinical interpretation.
Statistical Analysis
Although kinematic data were available for the full range of motion, data were analyzed between 90–150° coinciding with the range in which internal impingement is most likely to occur.11; 12 Differences in glenoid-to-footprint minimum distances and contact center locations were assessed over humerothoracic elevation angles (90°, 110°, 130°, 150°) and between supraspinatus pathology groups (intact, torn) using two-factor mixed model ANOVAs with a banded Toeplitz covariance structure. The prevalence of contact was compared between supraspinatus pathology groups using Chi-square tests. Finally, a sensitivity analysis was performed to determine the extent to which the estimated labral thickness impacted the determination of glenoid-to-footprint contact. Specifically, the prevalence of contact and the humeral angle at which the glenoid-to-footprint minimum distance fell below the estimated labral thickness were calculated after varying the estimation based on the standard deviation reported by Zanetti et al.31 (±1 SD, ±2 SD, ±3 SD). Statistical analysis was performed in SAS Version 9.4 (SAS Institute Inc., Cary, NC).
Results
Of the 37 participants in this study, only 4 (10.8%) reported a prior history of shoulder pain in their dominant shoulder that had lasted longer than 1 week. The results of the diagnostic ultrasound found that 20 (54.1%) did not have any evidence of a rotator cuff tear, 11 (29.7%) had a partial-thickness tear involving the supraspinatus, and 6 (16.2%) had a full-thickness tear. Approximately half of participants (51.4%) reported some involvement in overhead athletics during their life with a median total time of 912 hours (min: 52, max: 8,042) and a median hours/week per sport of 1.6 hours (min: 0.2, max: 6.8).
Kinematics
Between 90–150° humerothoracic elevation, participants generally exhibited glenohumeral elevation, anterior plane of elevation, and external rotation (Figure 1). Furthermore, the humeral head tended to move posteriorly relative to the glenoid and remained in a position slightly superior to the center of the glenoid.
Figure 1:
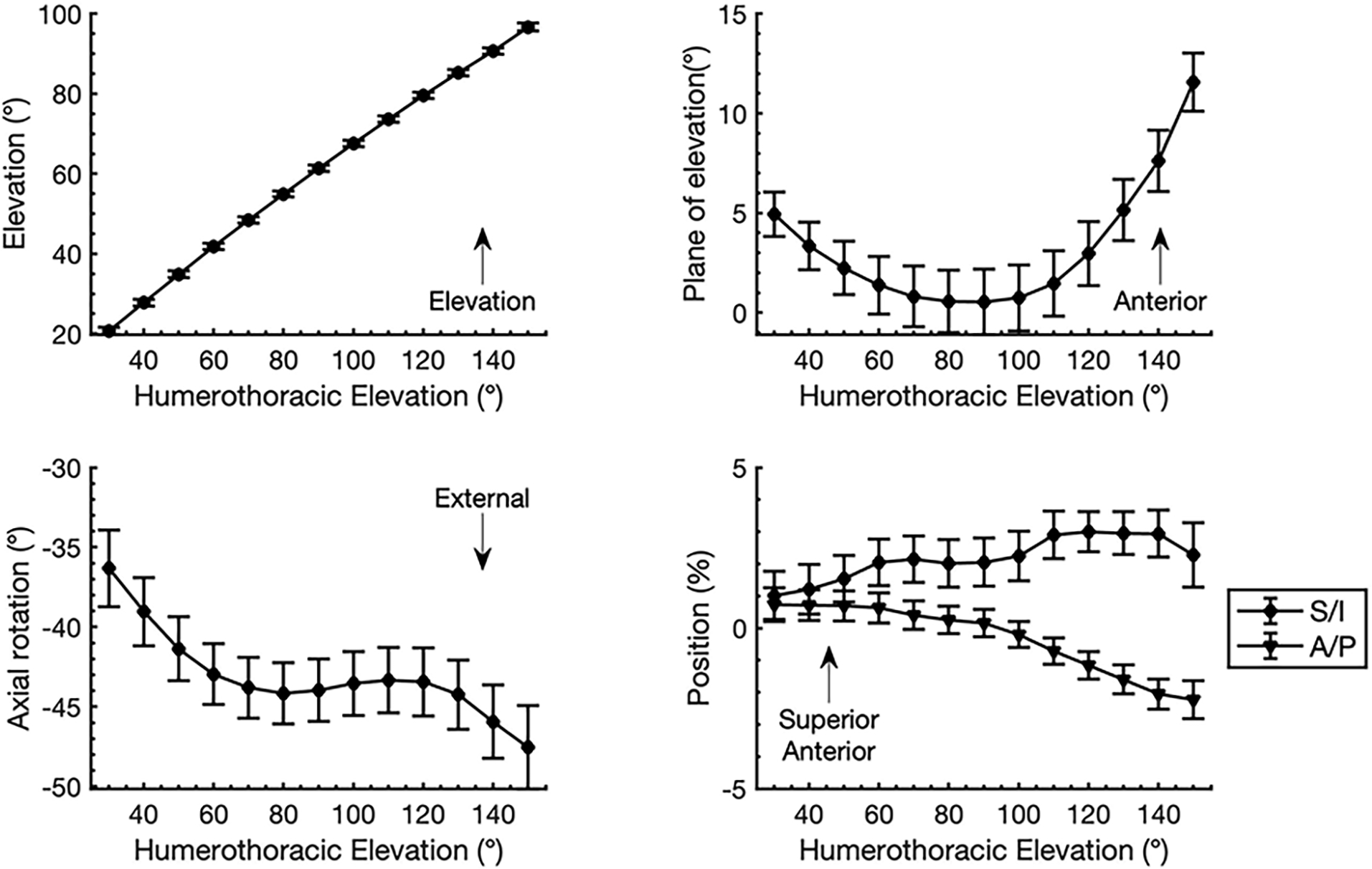
Descriptive statistics (mean ± SE) for glenohumeral kinematics across the trial of unloaded scapular plane abduction. Between 90–150° humerothoracic elevation, participants generally exhibited glenohumeral elevation, anterior plane of elevation, and external rotation. Glenohumeral orientation data are described relative to a scapular anatomical coordinate system defined based on the recommendations of the International Society of Biomechanics23 with glenohumeral elevation being transformed to positive values to facilitate interpretation. Glenohumeral anterior and superior position data are described relative to a glenoid based coordinate system and are normalized to glenoid width and height, respectively. Abbreviations: S/I = superior/inferior, A/P anterior/posterior.
Glenoid-to-Footprint Minimum Distances
Glenoid-to-footprint minimum distances decreased consistently in all participants between 90–150° humerothoracic elevation without a significant difference between supraspinatus pathology groups (interaction: p=0.95, group main effect: p=0.90, angle main effect: p<0.01) (Table 1, Figure 2). At 90° humerothoracic elevation, the average glenoid-to-footprint distance was 11.1 mm ± 0.8 mm but decreased by approximately 50% (p<0.01) during each subsequent 20° increment of humerothoracic elevation until it fell to an average of 1.3 mm ± 0.1 mm at 150° humerothoracic elevation. The glenoid-to-footprint minimum distance reached its minimum (1.1 mm ± 0.1 mm) at the maximum elevation angle achieved by the participant (153.1° ± 1.3° humerothoracic, or 99.0° ± 1.2° glenohumeral).
Table 1:
Mean glenoid-to-footprint minimum distances (± standard error) for the 2 supraspinatus pathology groups and across the 5 humerothoracic elevation angles assessed statistically. There were no significant differences between pathology groups (group-by-angle interaction: p=0.95, group main effect: p=0.90).
| Supraspinatus pathology group | ||
|---|---|---|
| Humerothoracic elevation angle | Intact (n=20) | Torn (n=17) |
| 90° | 11.0 ± 1.0 mm | 11.2 ± 1.2 mm |
| 110° | 5.2 ± 0.6 mm | 5.3 ± 0.6 mm |
| 130° | 2.2 ± 0.2 mm | 2.1 ± 0.3 mm |
| 150° | 1.3 ± 0.2 mm | 1.4 ± 0.2 mm |
Figure 2:
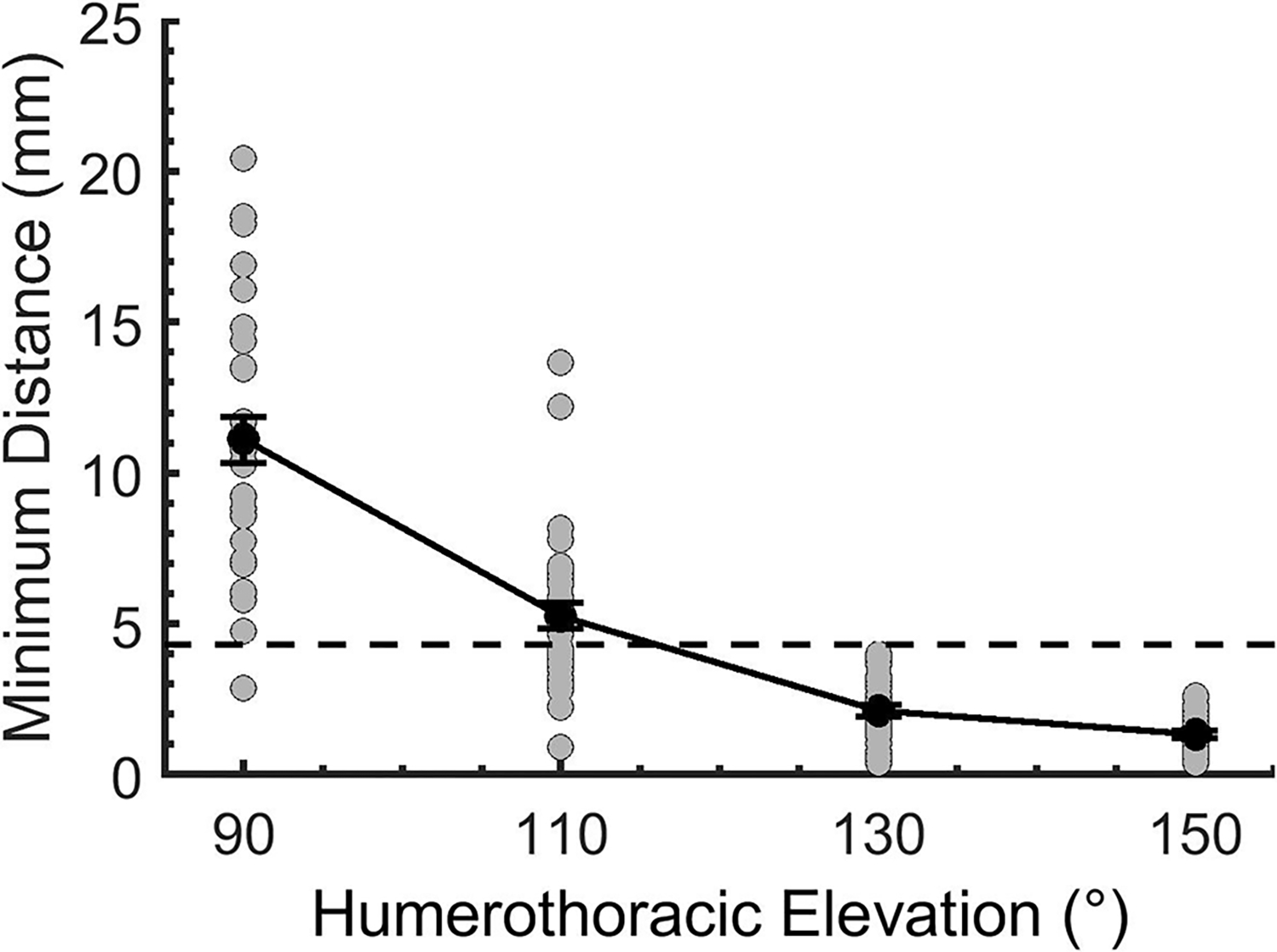
The minimum distance between the glenoid and rotator cuff footprint at the humerothoracic elevation angles assessed statistically. Glenoid-to-footprint minimum distances decreased consistently in all participants between 90–150° humerothoracic elevation and fell below the estimated labral thickness in all participants at an average angle of 114.6° ± 1.7° humerothoracic elevation. Data are presented as mean and standard error. The black line represents the mean (±SE) of all subjects, and the grey circles represent data for individual subjects. The dashed line represents an estimated average labral thickness (4.3 mm) to identify incidence of potential glenoid-to-footprint contact.
Proximity Center Location
Between 90–150° humerothoracic elevation, the anterior half of the rotator cuff footprint was generally in close proximity with the posterosuperior aspect of the glenoid (Figure 3). The proximity center location was not significantly different between supraspinatus pathology groups at any angle of humerothoracic elevation (interaction: p>0.35, group main effect: p>0.36). Therefore, the movement of the proximity pattern location on the glenoid and rotator cuff footprint will be described on average across all subjects.
Figure 3:
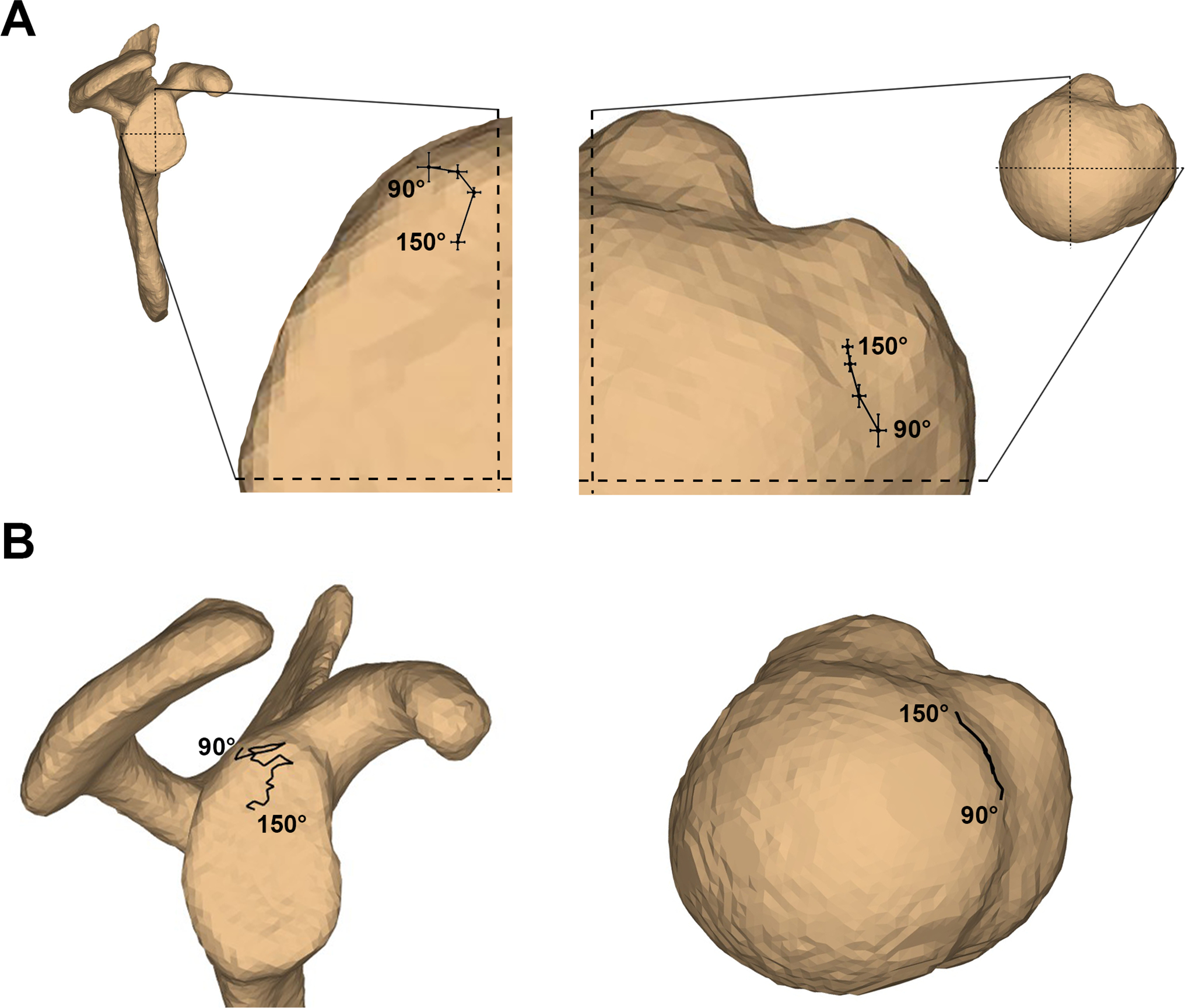
The proximity center locations on the glenoid (lateral view) and footprint (superior view) illustrated as A) the average ± standard error across all participants at the angles assessed statistically (90°, 110°, 130°, and 150° humerothoracic elevation), and B) for a representative subject for all frames of the motion trial between 90–150° humerothoracic elevation. In A, the average proximity center location is superimposed over an approximately average-sized glenoid and humeral head to facilitate interpretation. The dashed lines coincide with the anterior/posterior and superior/inferior axes of the glenoid coordinate system, and the anterior/posterior and medial/lateral axes of the humeral coordinate system. In B, the proximity center path location is superimposed over the participant’s actual anatomy.
Between 90–150° humerothoracic elevation, the proximity center on the glenoid consistently shifted inferiorly by an average of 16.2% ± 3.9% of the glenoid’s height (p<0.01). However, in the anterior/posterior direction, the proximity center first shifted anteriorly by an average of 13.1% ± 2.9% of the glenoid’s width between 90°–130° humerothoracic elevation (p<0.01) before shifting posteriorly by 4.7% ± 1.8% between 130°–150° humerothoracic elevation (p<0.01). On the rotator cuff footprint, the proximity center shifted anteriorly by 15.6% ± 2.8% of the humeral head diameter (p<0.01) and medially by an average of 5.7% ± 1.3% of the humeral head diameter (p<0.01). The proximity center on the footprint was located an average of 16.6 mm ± 0.7 mm posterior to the biceps groove when the glenoid-to-footprint minimum distance fell below the estimated labral thickness (i.e., the angle of initial contact) (range: 8.1–28.0 mm). At the participants’ highest humerothoracic elevation angle (153.1° ± 1.3°), the proximity center had shifted anteriorly such that it was now located 12.2 mm ± 0.4 mm posterior to the biceps groove (range: 6.1–18.2 mm).
Contact Analysis
The glenoid-to-footprint minimum distance fell below the estimated labral thickness in all participants (suggesting glenoid-to-footprint contact) at an average angle of 114.6° ± 1.7° humerothoracic elevation (range: 90.1°–130.1°) – or 76.4° ± 1.2° glenohumeral elevation (range: 59.4°–91.2°) – without a significant difference between supraspinatus pathology groups (p=0.92; intact: 114.7°±2.4°, torn: 114.4°±2.5°). The acromion-to-footprint minimum distance fell below the measured rotator cuff thickness (suggesting acromion-to-footprint contact) in 51.4% of participants without a significant difference between supraspinatus pathology groups (p=0.84). Glenoid-to-acromion contact occurred at an average angle of 27.6° ± 2.0° humerothoracic elevation (range: 15.0°–44.9°) – or 18.9° ± 2.1° glenohumeral elevation (range: 2.5°–34.5°) – without a significant difference between supraspinatus pathology groups (p=0.84; intact: 28.0°±3.0°, torn: 27.2°±3.0°).
Sensitivity Analysis for Labral Thickness
Varying the estimated labral thickness did not substantially change the prevalence of estimated contact (Table 2). In general, increasing the estimated labral thickness from the mean value decreased the angle of initial contact, while decreasing the estimated labral thickness from the mean value increased the angle of initial contact. However, decreasing the labral thickness by at least 1 SD only resulted in a change in contact classification (i.e., contact, no contact) for only 1 participant.
Table 2:
The results of the sensitivity analysis in which the prevalence of contact and the humeral angle at which the glenoid-to-footprint minimum distance fell below the estimated labral thickness were calculated after varying the estimation based on the standard deviation reported by Zanetti et al (0.46 mm).31 The angle of initial contact refers to the humerothoracic and glenohumeral angle at which the glenoid-to-footprint minimum distance fell below the estimated labral thickness, and are presented as mean ± SE.
| Angle of Initial Contact | ||||
|---|---|---|---|---|
| SD from mean | Labral thickness | Contact Prevalence | Humerothoracic | Glenohumeral |
| +3 | 5.68 mm | 100% | 108.4° ± 1.6° | 72.6° ± 1.2° |
| +2 | 5.22 mm | 100% | 110.3° ± 1.7° | 73.8° ± 1.2° |
| +1 | 4.76 mm | 100% | 112.5° ± 1.7° | 75.1° ± 1.3° |
| 0 | 4.3 mm | 100% | 114.6° ± 1.7° | 76.4° ± 1.2° |
| −1 | 3.84 mm | 97.3% | 117.7° ± 1.7° | 78.3° ± 1.2° |
| −2 | 3.38 mm | 97.3% | 119.9° ± 1.8° | 79.4° ± 1.2° |
| −3 | 2.92 mm | 97.3% | 124.6° ± 2.1° | 82.0° ± 1.2° |
Discussion
The primary results of this study suggest that contact between the glenoid and rotator cuff footprint is likely common during scapular plane abduction and typically occurred well within a participant’s functional range of motion. For example, the glenoid-to-footprint minimum distance fell below the estimated labral thickness (suggesting glenoid-to-footprint contact) at an average humerothoracic elevation angle of 115°, which is well below the average maximal range of motion achieved by participants in this study (153°). Although this finding may be surprising given internal impingement is typically considered relative to competitive overhead athletics,8; 15; 33 it is consistent with previous studies that have investigated the proximity between the glenoid and rotator cuff tendon during planar arm elevation.11–13 When combined with proximity center locations, this information may be helpful to understand the role of internal impingement in the etiology of degenerative rotator cuff tears.
Between 90–150° humerothoracic elevation, the posterosuperior glenoid was predominantly in close proximity with the anterior portion of the rotator cuff footprint (Figure 3). The proximity center on the glenoid is consistent with previous studies that have investigated internal impingement arthroscopically.8; 15; 16 However, the location of contact on the rotator cuff tendon has not been well described. In this study, the proximity center was found to be located predominantly in the region of the greater tuberosity occupied by the supraspinatus tendon insertion.28 As humerothoracic elevation angle increased, the proximity center location moved anteriorly and medially such that it was located an average of 12.2–16.6 mm posterior to the biceps groove when the footprint was in presumed contact with the glenoid (i.e., between the angle of initial contact and the participant’s end range of motion). Interesting, this location appears to be consistent with where degenerative rotator cuff tears are believed to originate (10–15 mm posterior to the biceps groove).29; 30 When considered in combination with the evidence that approximately 30–62% of rotator cuff tears are articular-sided partial-thickness tears,4–7 these findings offer indirect although intriguing evidence that internal impingement during overhead functional motions may play a larger role in the development of rotator cuff pathology than previously thought.
The lack of significant difference in glenoid-to-footprint minimum distances between supraspinatus pathology groups is interesting with implications that are not yet clear. It is possible that changes in glenoid-to-footprint minimum distances occurs only in the presence of symptoms when glenohumeral kinematics are often impaired.34–36 For example, previous research suggests that the humerus translates more inferiorly during humeral abduction in individuals with shoulder pain than asymptomatic controls.34 Presumably, a more inferior position of the humerus on the glenoid would result in smaller glenoid-to-footprint minimum distances. However, this is speculative as it remains unclear the extent to which differences in glenohumeral kinematics impacts glenoid-to-footprint distances. Future work will expand the investigation to symptomatic individuals to understand the interaction between rotator cuff pathology, symptom presentation, kinematics, and potential mechanisms of rotator cuff pathology.
The findings that contact was estimated to occur in all participants regardless of supraspinatus pathology status raises the question of whether internal impingement is pathological or physiological. Intraoperative studies in overhead athletes have described “kissing” lesions, where a rotator cuff tendon tear is observed to contact the posterosuperior labrum in positions of combined abduction and external rotation as support for the pathoanatomical consequences of internal impingement.15; 16 Using this logic, the prevalence of concomitant superior labral degeneration in individuals with articular-sided partial-thickness rotator cuff tears may provide additional support for the pathological consequences of internal impingement. Unfortunately, concomitant labral pathology was not assessed in the current study. However, Budoff et al. reported that 73% of individuals with an articular-sided partial thickness tear had Type I labral fraying superior to the glenoid equatorial (i.e., anterior-posterior) axis.7 Furthermore, 29% had lesions in the posterosuperior labrum in the region where the glenoid-to-footprint minimum distances were found to be smallest in the current study. Although causation cannot be inferred by these findings, our results provide compelling evidence to consider internal impingement as a potential mechanism of rotator cuff injury even in non-athletic populations.
Ultimately, the pathological consequence of contact is likely influenced by many other factors.37; 38 For example, factors such as the velocity of movement and the frequency, magnitude, and duration of contact are likely important considerations, as is the tendon’s ability to tolerate the associated tissue stresses.38 Within the participants in this study, the angle of initial contact varied widely with contact occurring at as low as 90° humerothoracic elevation in one individual and as high as 130° humerothoracic elevation in another. Theoretically, contact occurring earlier in the range of motion may be more detrimental as it is likely to be experienced more frequently during functional activities. Furthermore, the extent to which other factors such as glenohumeral kinematics and morphology impacts the prevalence and nature of glenoid-to-footprint contact remains unclear. More research is needed to understand these factors to help clarify the potential role of internal impingement in rotator cuff pathology.
In addition to characterizing glenoid-to-footprint contact during functional motion, more research is needed to identify and understand the extent to which contact results in rotator cuff tendon deformation. Based on visual inspections of the models and the presumed course of the supraspinatus muscle-tendon unit from the insertional footprint (i.e., enthesis) to the supraspinatus fossa (Figure 4), it appears the tendon may be deformed in a way that places the articular surface under tension (e.g., bending). Therefore, it is possible that internal impingement may be associated with other types of deformation (e.g., torsion) or forces that may act on the tendon at the enthesis (e.g., shear). However, this remains highly speculative as the tendon could not be directly visualized in the current study. Future research that aims to directly assess the soft tissue forces and deformations associated with internal impingement would be invaluable in the effort to understand its potential role in the etiology of rotator cuff tears.
Figure 4:
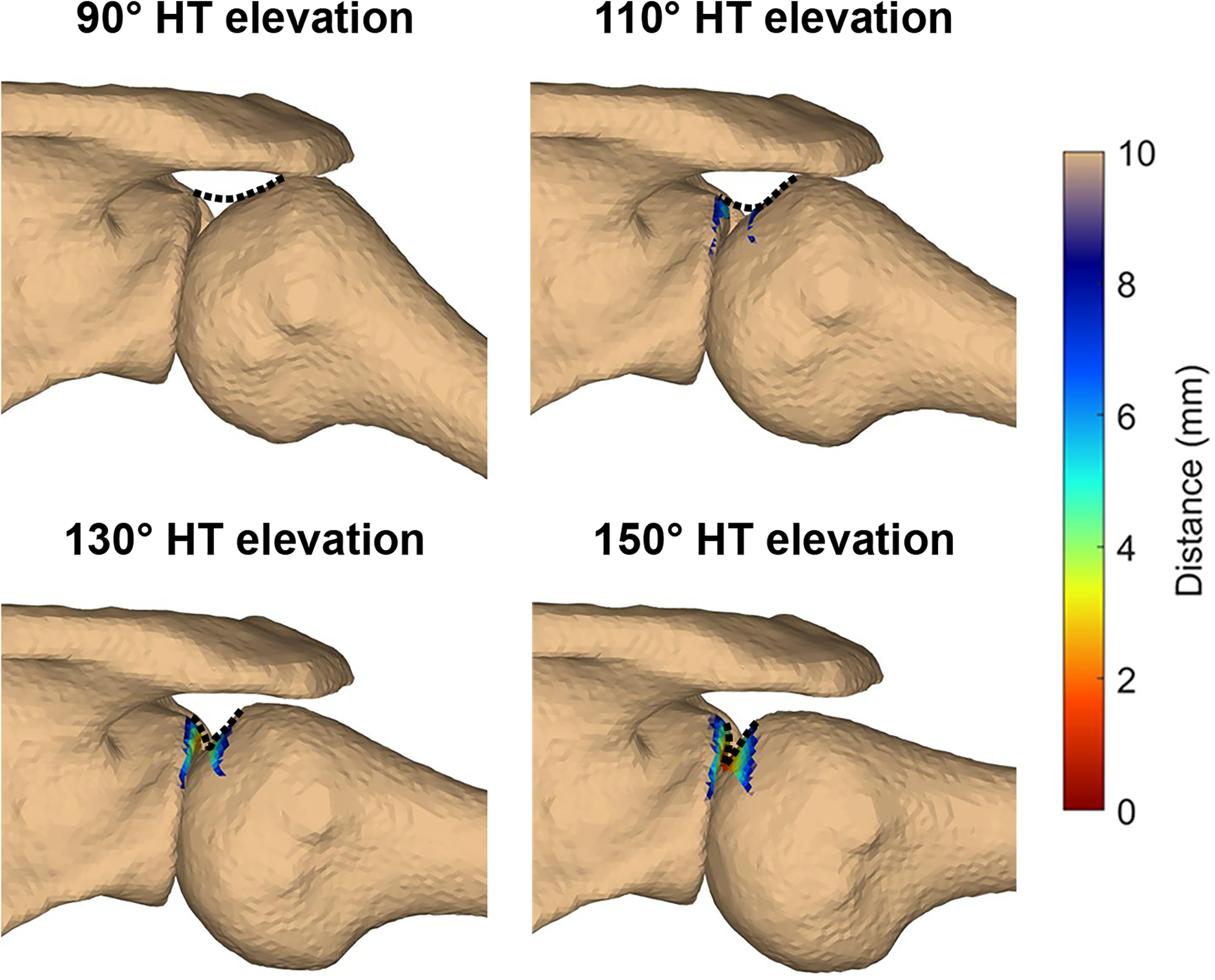
The minimum distance between the glenoid and rotator cuff footprint at the humerothoracic elevation angles assessed statistically in a representative participant. The colormap corresponds to the distance between the glenoid and rotator cuff footprint. The dashed line represents the estimated undersurface of the rotator cuff in the free tendon and insertional footprint regions. Abbreviation: HT = humerothoracic.
Another interesting finding of this study was the high prevalence of glenoid-to-footprint contact (100%) compared to acromion-to-footprint contact (51.4%), without a significant difference between supraspinatus pathology groups. This finding is consistent with growing evidence that subacromial contact occurs in only about 50% of individuals during humeral elevation.32; 39; 40 Further, the relative prevalence of subacromial and internal contact found in this study agree with those of a recent investigation,11 and together suggest that internal impingement may occur often than subacromial impingement during humeral elevation. Taken together, these findings are contrary to historical clinical dogma in which mechanical subacromial impingement has been the primary focus of “extrinsic” mechanisms of rotator cuff pathology37; 41 while internal impingement has been associated almost exclusively with overhead athletes.8; 9 Ultimately, it remains critical that clinicians and researchers test our assumptions and clinical theories, regardless of their precedence, so that the balance of evidence-based practice can be maintained with high-quality scientific evidence.
The primary limitation of this study is the use of bone-to-bone distance measures to investigate a potential mechanism of soft tissue injury. For example, the glenoid-to-footprint minimum distance fell below the estimated labral thickness (suggesting glenoid-to-footprint contact) at an average humerothoracic elevation angle of 114.6°±1.7°. However, based on visual inspections of the models and the presumed course of the supraspinatus muscle-tendon unit from the insertional footprint to the supraspinatus fossa, it does not appear that the glenoid begins to redirect the tendon until after 130° humerothoracic elevation, thereby placing it in a position to be entrapped or impinged (Figure 4). Therefore, it is likely that the bone-to-bone glenoid-to-footprint minimum distance measure is an oversimplification of a complex phenomenon. Even so, the results of this study remain consistent with previous investigations that glenoid-to-tendon contact is common8; 11; 13; 15 and may begin to occur by 90–130° humerothoracic elevation.11; 13; 15
This study has additional limitations to consider when interpreting the results. First, an estimated labral thickness was used to determine the prevalence of contact, given the labrum was not well-visualized on the CT. Although labral morphology is variable between individuals, 31 the results of the sensitivity analysis suggests that the estimated (i.e., assumed) labral thickness did not substantially impact the results of the study. Second, only unloaded, scapular plane abduction was investigated. It is possible that other motions or conditions may influence glenoid-to-footprint proximities and may be more representative of functional conditions common to daily activities. Third, only asymptomatic individuals aged 50–60 were enrolled as part of a larger investigation and it remains unclear how glenoid-to-footprint proximities may change across the lifespan or in the presence of symptoms. Fourth, the distribution of participant gender was biased towards women. However, exploratory analysis did not reveal any significant differences between men and women in any outcome measure, which suggests the unbalanced gender distribution did not likely affect the results of the study. Finally, the questionnaire of involvement in overhead athletics is subject to recall bias. However, no validated measure currently exists to estimate lifetime exposure to overhead athletics.
In conclusion, glenoid-to-footprint contact appears to be very common during active, unloaded scapular plane elevation. The location of contact occurred within the region of the rotator cuff footprint where degenerative rotator cuff tears are thought to originate. This information may be helpful to understand the role of internal impingement in the etiology of degenerative rotator cuff tears.
Acknowledgements
The authors would like to thank the study volunteers. This work was supported by the National Institutes of Health (grant number K99AR075876). The NIH did not play a role in the design, conduct, or reporting of this study. The authors do not have any conflicts of interest.
References
- 1.Lawrence RL, Moutzouros V, Bey MJ. 2019. Asymptomatic Rotator Cuff Tears. JBJS Rev 7:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tempelhof S, Rupp S, Seil R. 1999. Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg 8:296–299. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto A, Takagishi K, Osawa T, et al. 2010. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg 19:116–120. [DOI] [PubMed] [Google Scholar]
- 4.Modi CS, Smith CD, Drew SJ. 2012. Partial-thickness articular surface rotator cuff tears in patients over the age of 35: Etiology and intra-articular associations. Int J Shoulder Surg 6:15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaeffeler C, Mueller D, Kirchhoff C, et al. 2011. Tears at the rotator cuff footprint: prevalence and imaging characteristics in 305 MR arthrograms of the shoulder. Eur Radiol 21:1477–1484. [DOI] [PubMed] [Google Scholar]
- 6.Waldt S, Bruegel M, Mueller D, et al. 2007. Rotator cuff tears: assessment with MR arthrography in 275 patients with arthroscopic correlation. Eur Radiol 17:491–498. [DOI] [PubMed] [Google Scholar]
- 7.Budoff JE, Nirschl RP, Ilahi OA, et al. 2003. Internal impingement in the etiology of rotator cuff tendinosis revisited. Arthroscopy 19:810–814. [DOI] [PubMed] [Google Scholar]
- 8.Walch G, Boileau P, Noel E, et al. 1992. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: An arthroscopic study. J Shoulder Elbow Surg 1:238–245. [DOI] [PubMed] [Google Scholar]
- 9.Jobe CM. 1996. Superior glenoid impingement. Current concepts. Clin Orthop Relat Res:98–107. [PubMed] [Google Scholar]
- 10.Perry J 1983. Anatomy and biomechanics of the shoulder in throwing, swimming, gymnastics, and tennis. Clin Sports Med 2:247–270. [PubMed] [Google Scholar]
- 11.Saini G, Lawrence RL, Staker JL, et al. 2021. Supraspinatus-to-Glenoid Contact Occurs During Standardized Overhead Reaching Motion. Orthop J Sports Med 9:23259671211036908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coats-Thomas MS, Massimini DF, Warner JJP, et al. 2018. In Vivo Evaluation of Subacromial and Internal Impingement Risk in Asymptomatic Individuals. Am J Phys Med Rehabil 97:659–665. [DOI] [PubMed] [Google Scholar]
- 13.Kim TK, McFarland EG. 2004. Internal impingement of the shoulder in flexion. Clin Orthop Relat Res:112–119. [DOI] [PubMed] [Google Scholar]
- 14.Neer CS 2nd. 1972. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am 54:41–50. [PubMed] [Google Scholar]
- 15.McFarland EG, Hsu CY, Neira C, et al. 1999. Internal impingement of the shoulder: a clinical and arthroscopic analysis. J Shoulder Elbow Surg 8:458–460. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan LD, McMahon PJ, Towers J, et al. 2004. Internal impingement: findings on magnetic resonance imaging and arthroscopic evaluation. Arthroscopy 20:701–704. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence RL, Zauel R, Bey MJ. 2021. Measuring 3D In-vivo Shoulder Kinematics using Biplanar Videoradiography. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson JA. 2018. Shoulder ultrasound. Fundamentals of Musculoskeletal Ultrasound, 3rd ed. Philadelphia, PA: Elsevier; pp. 55–126. [Google Scholar]
- 19.Lee MH, Sheehan SE, Orwin JF, et al. 2016. Comprehensive Shoulder US Examination: A Standardized Approach with Multimodality Correlation for Common Shoulder Disease. Radiographics 36:1606–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laucis NC, Rosen KA, Thodge A, et al. 2021. Sonographic evaluation of the association between calcific tendinopathy and rotator cuff tear: a case-controlled comparison. Clin Rheumatol 40:2897–2905. [DOI] [PubMed] [Google Scholar]
- 21.Minagawa H, Yamamoto N, Abe H, et al. 2013. Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: From mass-screening in one village. J Orthop 10:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gill TK, Shanahan EM, Allison D, et al. 2014. Prevalence of abnormalities on shoulder MRI in symptomatic and asymptomatic older adults. Int J Rheum Dis 17:863–871. [DOI] [PubMed] [Google Scholar]
- 23.Wu G, van der Helm FC, Veeger HE, et al. 2005. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion--Part II: shoulder, elbow, wrist and hand. J Biomech 38:981–992. [DOI] [PubMed] [Google Scholar]
- 24.Peltz CD, Zauel R, Ramo N, et al. 2015. Differences in glenohumeral joint morphology between patients with anterior shoulder instability and healthy, uninjured volunteers. J Shoulder Elbow Surg 24:1014–1020. [DOI] [PubMed] [Google Scholar]
- 25.Bey MJ, Zauel R, Brock SK, et al. 2006. Validation of a new model-based tracking technique for measuring three-dimensional, in vivo glenohumeral joint kinematics. J Biomech Eng 128:604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phadke V, Braman JP, LaPrade RF, et al. 2011. Comparison of glenohumeral motion using different rotation sequences. J Biomech 44:700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peltz CD, Divine G, Drake A, et al. 2015. Associations between in-vivo glenohumeral joint motion and morphology. J Biomech 48:3252–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mochizuki T, Sugaya H, Uomizu M, et al. 2008. Humeral insertion of the supraspinatus and infraspinatus. New anatomical findings regarding the footprint of the rotator cuff. J Bone Joint Surg Am 90:962–969. [DOI] [PubMed] [Google Scholar]
- 29.Jeong JY, Min SK, Park KM, et al. 2018. Location of Rotator Cuff Tear Initiation: A Magnetic Resonance Imaging Study of 191 Shoulders. Am J Sports Med 46:649–655. [DOI] [PubMed] [Google Scholar]
- 30.Kim HM, Dahiya N, Teefey SA, et al. 2010. Location and initiation of degenerative rotator cuff tears: an analysis of three hundred and sixty shoulders. J Bone Joint Surg Am 92:1088–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zanetti M, Carstensen T, Weishaupt D, et al. 2001. MR arthrographic variability of the arthroscopically normal glenoid labrum: qualitative and quantitative assessment. Eur Radiol 11:559–566. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence RL, Braman JP, Ludewig PM. 2019. The impact of decreased scapulothoracic upward rotation on subacromial proximities. J Orthop Sports Phys Ther 49:180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davidson PA, Elattrache NS, Jobe CM, et al. 1995. Rotator cuff and posterior-superior glenoid labrum injury associated with increased glenohumeral motion: a new site of impingement. J Shoulder Elbow Surg 4:384–390. [DOI] [PubMed] [Google Scholar]
- 34.Lawrence RL, Braman JP, Staker JL, et al. 2014. Comparison of 3-dimensional shoulder complex kinematics in individuals with and without shoulder pain, part 2: glenohumeral joint. J Orthop Sports Phys Ther 44:646–655, B641–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ludewig PM, Cook TM. 2002. Translations of the humerus in persons with shoulder impingement symptoms. J Orthop Sports Phys Ther 32:248–259. [DOI] [PubMed] [Google Scholar]
- 36.Hallstrom E, Karrholm J. 2009. Shoulder rhythm in patients with impingement and in controls: dynamic RSA during active and passive abduction. Acta Orthop 80:456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seitz AL, McClure PW, Finucane S, et al. 2011. Mechanisms of rotator cuff tendinopathy: intrinsic, extrinsic, or both? Clin Biomech (Bristol, Avon) 26:1–12. [DOI] [PubMed] [Google Scholar]
- 38.Lawrence RL, Ludewig PM, Ward SR. 2021. An Integrated Approach to Musculoskeletal Performance, Disease, and Recovery. Phys Ther 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawrence RL, Schlangen DM, Schneider KA, et al. 2017. Effect of glenohumeral elevation on subacromial supraspinatus compression risk during simulated reaching. J Orthop Res 35:2329–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawrence RL, Sessions WC, Jensen MC, et al. 2018. The effect of glenohumeral plane of elevation on supraspinatus subacromial proximity. J Biomech 79:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neer CS 2nd. 1983. Impingement lesions. Clin Orthop Relat Res:70–77. [PubMed] [Google Scholar]


