Abstract
Doxorubicin (DOX) is a chemotherapeutic drug for a variety of malignancies, while its application is restricted by the cardiovascular toxic effects characterized by oxidative stress. Ferroptosis is a novel iron-dependent regulated cell death driven by lipid peroxidation. Our study aimed to investigate the role of Elabela (ELA) in DOX-induced oxidative stress and ferroptosis. In cultured rat aortic adventitial fibroblasts (AFs), stimulation with DOX dramatically induced cytotoxicity with reduced cell viability and migration ability, and enhanced lactate dehydrogenase (LDH) activity. Importantly, ELA and ferrostatin-1 (Fer-1) mitigated DOX-mediated augmentation of reactive oxygen species (ROS) in rat aortic AFs, accompanied by upregulated levels of Nrf2, SLC7A11, GPX4, and GSH. In addition, ELA reversed DOX-induced dysregulation of apoptosis- and inflammation-related factors including Bax, Bcl2, interleukin (IL)-1β, IL6, IL-10, and CXCL1. Intriguingly, knockdown of Krüppel-like factor 15 (KLF15) by siRNA abolished ELA-mediated alleviation of ROS production and inflammatory responses. More importanly, KLF15 siRNA impeded the beneficial roles of ELA in DOX-pretreated rat aortic AFs by suppressing the Nrf2/SLC7A11/GPX4 signaling. In conclusion, ELA prevents DOX-triggered promotion of cytotoxicity, and exerts anti-oxidative and anti-ferroptotic effects in rat aortic AFs via activation of the KLF15/GPX4 signaling, indicating a promising therapeutic value of ELA in antagonizing DOX-mediated cardiovascular abnormality and disorders.
Keywords: Doxorubicin, Elabela, Oxidative stress, Ferroptosis, Aortic adventitial fibroblast
Introduction
Doxorubicin (DOX) is highly prescribed as a chemotherapeutic antineoplastic drug with tremendous therapeutic efficacy and a wide antitumor spectrum, containing carcinomas, sarcomas, and hematological cancers (Carvalho et al. 2009). The major mechanisms of DOX-mediated inhibition of tumor refer to the generation of free radicals that cause damage to cellular proteins, and the direct inhibition of DNA replication to cause cell death by activating apoptosis, necroptosis, and recently identified ferroptosis (Burridge et al. 2016; Matt and Hofmann 2016; Rivankar 2014; Xue et al. 2020). Despite DOX displays extensive application value for clinical treatment of cancer, the unintended cytotoxicity targeting healthy tissues leads to side effects, such as acute nausea and vomiting, stomatitis, gastrointestinal disturbances, neurologic disturbances, bone marrow aplasia, and cardiotoxicity (Carvalho et al. 2009). Especially, DOX is highly correlated with increased incidence of dose-dependent toxic events in the cardiovascular system, including hypotension, arrhythmias, and congestive heart failure ultimately (Steinberg et al. 1987; Swain et al. 2003; Wallace et al. 2020).
The cardiotoxic effects of DOX are mainly attributed to its ability to generate reactive oxygen species (ROS) which directly causes membrane injury and lipid peroxidation (Burridge et al. 2016). What is more, ROS is a mediator to increase intracellular iron levels, and the DOX-iron free radical complexes also actively result in lipid peroxidation and ferroptosis (Park and Chung 2019; Renu et al. 2018). Ferroptosis, which is an iron-dependent oxidative form of cell death with increased lipid peroxidation and insufficient capacity for lipid peroxide clearance, participates in various cardiovascular diseases (Yu et al. 2021). Nuclear factor erythroid 2-related factor 2 (Nrf2)/SLC7A11 (also known as xCT)/glutathione peroxidase 4 (GPX4) are most known ferroptosis suppressing signaling, and simultaneously, they are established lipid peroxidation defending systems within cells (Dodson et al. 2019; Doll et al. 2019; Gao et al. 2022). The cysteine/glutathione (GSH)/GPX4 axis is recognized as the mainstay to repress ferroptosis (Seibt et al. 2019), and the activity of GPX4 relies on the activation of cystine-glutamate antiporter SLC7A11 (Chen et al. 2021). Nrf2 augments antioxidant capacity and suppresses the accumulation of lipid peroxidation by regulating SLC7A11 (Dong et al. 2021). These ferroptosis-suppressing signaling pathways collaborate to counteract ferroptotic cell injury, and the dysregulation of which leads to oxidative stress and ferroptosis. Additionally, abnormality of apoptotic factors and inflammatory mediators are found in DOX-triggered cardiotoxicity, such as Bax, Bcl2, interleukin (IL)-6, IL-1β, IL-18, and TNF-α (Alhazzani et al. 2021; Qi et al. 2020).
It is well recognized that the cytotoxicity of DOX is multidirectional and exists at the cellular level via diverse mechanisms (Speth et al. 1988). Targets of DOX-induced cardiotoxicity and vascular toxicity contain cardiomyocytes, vascular endothelial cells, smooth muscle cells and cardiac fibroblasts (Bosman et al. 2021; Chen et al. 2019; Mišúth et al. 2021; Octavia et al. 2012; Pan et al. 2021; Sonowal et al. 2018; Zhang et al. 2019; Zhou et al. 2022). However, one area left behind is the role of adventitial fibroblasts (AFs), a key cell type exerting intricate effects in the cardiovascular system in response to disease-related injury (Kuwabara and Tallquist 2017). We hypothesized that understanding their role in DOX-induced cytotoxicity is vital for prevention and therapy of DOX-mediated cardiovascular toxicity.
Krüppel-like factor 15 (KLF15) has emerged as a critical regulator of oxidative stress and inflammation in myocardial infarction, heart failure, and other cardiovascular diseases (He et al. 2021; Li et al. 2021; Liu et al. 2018; Lu et al. 2019; Wang et al. 2021; Zhao et al. 2019). However, the role of KLF15 in DOX-triggered cardiovascular dysfunction remains largely unclear. ELA (Elabela/Apela/Toddler) polypeptide, the second endogenous ligand for G protein-coupled receptor apelin peptide jejunum (APJ), is extensively studied in cardiovascular diseases and oncocardiology diseases (Zhou and Wu 2022). ELA exhibits a restricted distribution in various tissues. Among the adult brain, stomach, lung, adipose tissues, kidney, and pancreas, ELA is expressed only in the adult kidney (Wang et al. 2015). Notably, ELA exhibits tumor suppressor function in renal cell carcinoma by repressing tumor cell growth, migration, and survival (Soulet et al. 2020), indicating the antitumorigenic property of ELA. Furthermore, ELA is crucial for multiple biological processes via modulation of vasodilation, angiogenesis, fluid homeostasis along with cellular proliferation, differentiation, and survival. The distinct roles of ELA in attenuation of mitochondrial dysfunction, remodeling, and inflammatory injury have been explicitly implicated in the cardiovascular system (Chen et al. 2022; Ma et al. 2021; Song et al. 2021, 2020). Considering that ELA functions valuably in the cardiovascular system, this study was intended to investigate the regulatory roles and underlying mechanisms of ELA in DOX-mediated cellular injury and dysfunction in rat aortic AFs.
Material and methods
Isolation and culture of primary rat AFs
The experimental protocol of the study was approved by the Animal Research Ethics Committee of Beijing Chaoyang Hospital affiliated to Capital Medical University (approval number:2017-K-132). Primary rat aortic AFs were separated from the ascending aortas of 5- to 6-week-old male Sprague Dawley rats (Charles River, China, License number: SCXK (Beijing) 2016–0006) in accordance with the previous study (Song et al. 2020; Xu et al. 2016). Briefly, adventitia was cut into 1 mm3 section after the removal of the intimal and medial layers. Then the tissue sections were incubated with Dulbecco’s modified Eagle’s medium (DMEM) containing 20% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin in a humidified environment with 5% CO2 at 37 °C. Cells from passages 3–6 were used for subsequent experiments and were made quiescent by incubation in serum-free DMEM for 24 h before each experiment. The cultured rat AFs were stained and identified with vimentin (a marker of fibroblasts) and α-smooth muscle actin (a marker of SMCs) using immunofluorescence analysis.
Cell transfection and pre-treatments
AFs were transfected with 50-nM small interfering RNA (siRNA) against KLF15, and corresponding siRNA negative control (siNC) (GenePharma, Shanghai, China) using Lipofectamine 3000 (Invitrogen, CA, USA) for 48 h according to the manufacturer’s instructions. After 8 h transfection, the medium was replaced with fresh high-glucose DMEM. Silence efficiency was measured by Western blotting analysis. The KLF15 siRNA sequence was: sense 5′-GAACCUGUCCUCAAAGUUUTT-3′, antisense 5′-AAACUUUGAGGACAGGUUCTT-3′. AFs were stimulated with 1 μM ELA (MedChemExpress, NJ, USA), 5 μM ferrostatin-1 (Fer-1; Selleck Chemicals, TN, USA) dissolved in dimethyl sulfoxide (DMSO), DMSO (Beyotime, Shanghai, China) and 10 mM N-acetyl-L-cysteine (NAC; Sigma-Aldrich, MO, USA) for 1 h, respectively, followed by 24 h exposure to 10 μM DOX (MedChemExpress, USA).
Wound-healing assay and dihydroethidium (DHE) staining
Wound-healing assay was performed to determine the migration ability of AFs as previously described (Song et al. 2021). Vertical wounds were made with a 200 µl pipette tip when AFs achieved 80–90% confluence, and images were captured 0 h and 24 h after wounding with a light microscope. The scratch area was calculated using Image J software to examine the migration of AFs. Dihydroethidium (DHE) fluorescence staining was performed to observe the production of ROS. The treated AFs were incubated with DHE (Beyotime, Shanghai, China) in the dark at a dilution of 5 μM for 30 min at 37 °C. Fluorescent images were captured with the Olympus IX51 microscope (Olympus, Tokyo), and the intensity of fluorescence was calculated by Image J software.
Cell viability and lactate dehydrogenase activity
Cell viability was determined using the Cell Counting Kit-8 (CCK-8) (Beyotime, Shanghai, China) according to the manufacturer’s protocol. After treatment with Dox at different concentrations (0–10 μM) for 24 h, 10 μl CCK-8 solution was added into per well in 96-well plates for 3 h at 37 °C. Then the absorbance values were measured at 450 nm by a SpectraMax190 plate reader (Molecular Devices, CA, USA). The DOX-induced injury was quantified by the Lactate Dehydrogenase (LDH) Activity assay (KeyGEN, Jiangsu, China) as previously described (Liu et al. 2022). ELA, Fer-1, and DMSO were utilized for pretreatment 1 h before DOX incubation in rat aortic AFs, respectively. SiNC and siKLF15 were employed to transfect AFs for 48 h followed by stimulation of DOX with ELA, and the absorbance at 450 nm was analyzed by multifunctional microplate reader.
Lipid peroxidation analysis
The intracellular levels of MDA were quantified in rat aortic AFs using a Lipid Peroxidation MDA Assay Kit (Beyotime, Shanghai, China) according to the manufacturer’s protocol. In brief, the cells were harvested, ultrasonicated, and centrifuged at 12,000 g for 10 min at 4 °C. Then the supernatants were collected to react with thiobarbituric acid (TBA) to measure MDA levels at 532 nm and simultaneously used for measurement of protein concentration with Enhanced BCA Protein Assay Kit (Beyotime, Shanghai, China). The MDA levels were presented as μmol/mg protein.
ROS measurement
Mitochondrial and intracellular ROS were assessed for rat aortic AFs by the Mitochondrial ROS detection kit (BestBio, Shanghai, China) and the ROS Assay Kit (Beyotime, Shanghai, China), respectively, in accordance with manufacturing instructions. In brief, cells were collected and washed twice in phosphate-buffered saline (PBS) followed by incubation with sensitive fluorescent probe MitoROS or 5 μM 2,7-dichlorofuresc in diacetate (DCFH-DA) for 20 min at 37 °C. Then rat aortic AFs were washed three times by PBS before suspension in 1 ml PBS for ROS analysis. ROS levels were analyzed by Flow cytometer (BD Biosciences, NY, USA), and quantified as the mean fluorescence intensity using FlowJo V10 software.
Monobromobimane staining
Monobromobimane (MBB) staining was performed to measure the levels of GSH in rat aortic AFs. The AFs were incubated with MBB (MedChemExpress, USA) at a dilution of 50 μM for 30 min at 37 °C. Fluorescent images were captured with an Olympus IX51 microscope (Olympus, Tokyo), and the intensity of fluorescence was calculated by Image J software.
Western blotting analysis
Western blotting analysis was used to evaluate the protein expression in rat AFs as described previously (Xu et al. 2016). Briefly, the cells were lysed in radio immunoprecipitation assay (RIPA) lysis buffer (Beyotime, Shanghai, China) with protease inhibitors (Beyotime, Shanghai, China). Supernatants of extracts were collected after centrifugation at 4 °C, 12,000 g for 15 min. Then, protein concentrations were determined with the BCA Protein Assay Kit (Beyotime, Shanghai, China). Equal amounts of proteins were separated by SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, MA, USA), and then blocked with 5% nonfat milk for 1 h. The membranes were individually incubated with the following primary antibodies against KLF15 (1:500, Santa Cruz, CA), Nrf2 (1:1000, Gene Tex, CA), GPX4 (1:1000, Proteintech, IL, USA), xCT (1:1000, Abcam, USA), GAPDH (1:1000, Cell Signaling Technology, MA, USA), Bax (1:5000, Proteintech, USA), and Bcl2 (1:1000, Proteintech, USA) at 4 °C overnight. The proteins were then incubated with HRP-conjugated secondary antibodies (1:3000, Solarbio, Beijing, China) at room temperature for 1 h and visualized by ChemiDoc XRS + (Bio-Rad, USA). The intensity of blots was calculated by Image J software. In this work, GAPDH was used as a reference for normalizing the standard.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
The qRT-PCR was performed to evaluate the gene expression in rat AFs as described previously (Xu et al. 2016). Briefly, total RNAs were isolated using TRIzol reagent (Invitrogen, CA, USA), and reversely transcribed to cDNAs in accordance with the manufacturer’s protocols (Takara, Japan). The mRNA levels of KLF15, Nrf2, SLC7A11, GPX4, interleukin (IL)-1β, IL-6, IL-10, and CXCL1 were analyzed on the Roche LightCycler 480 System using SYBR Premix Ex Taq Kit (Takara, Japan), and the specific primer sequences used were listed in Table 1. The 2−ΔΔCt method was used to calculate the mRNA levels in rat aortic AFs.
Table 1.
Primer sequences for reverse transcription PCR analysis
| Gene | Primer sequences (5′ → 3′) |
|---|---|
| KLF15 |
F: TGTCCTCAAAGTTTGTGCGAATTGC R: GTTCTGCTGCTGGGTTCTTGGG |
| Nrf2 |
F: GCCTTCCTCTGCTGCCATTAGTC R: TGCCTTCAGTGTGCTTCTGGTTG |
| SLC7A11 |
F: CCATCATCATCGGCACCGTCATC R: TACTCCACAGGCAGACCAGAACAC |
| GPX4 |
F: CCAGCAACAGCCACGAGTTCC R: CACACGCAACCCCTGTACTTATCC |
| CXCL1 |
F: GCAGACAGTGGCAGGGATTCAC R: TGAGTGTGGCTATGACTTCGGTTTG |
| IL-10 |
F: TTGAACCACCCGGCATCTAC R: CCAAGGAGTTGCTCCCGTTA |
| IL-1β |
F: CAGCCAACGAATCCCAGACC R: ACAGATAGGGTCACAGCCAG |
| IL-6 |
F: CCACTGCCTTCCCTACTTC R: TCTGGCTTTGTCTTTCTTGTTA |
| GAPDH |
F: AGTGCCAGCCTCGTCTCATA R: TGAACTTGCCGTGGGTAGAG |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; KLF15, Kruppel-like factor 15; Nrf2, nuclear factor erythroid 2-related factor 2; SLC7A11, the cystine/glutamate antiporter; GPX4, glutathione peroxidase 4; CXCL1, chemokine C-X-C motif ligand-1; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-10, interleukin-10
Immunofluorescence studies and string analysis
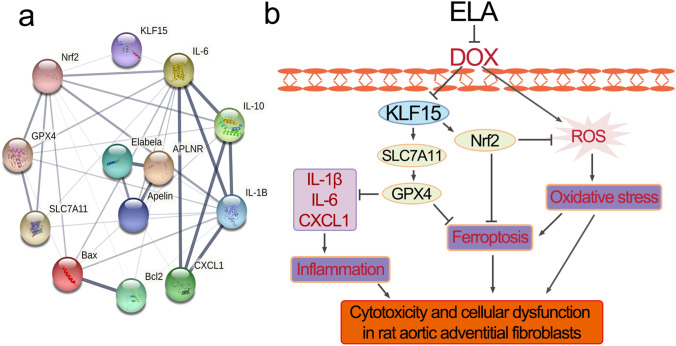
KLF15 localization in rat aortic AFs was detected by immunofluorescence staining as previously described (Song et al. 2020). Briefly, the treated cells were fixed with 4% paraformaldehyde for 15 min and permeabilized in 0.1% Triton X-100 for 20 min. They were blocked with 0.5% goat serum for 30 min and incubated with primary antibodies against KLF15 (Santa Cruz, CA) overnight at 4 °C and secondary antibodies for 1 h. The nuclei were probed by DAPI (Invitrogen, CA, USA). Finally, the cells were visualized by a fluorescence microscope (Olympus IX51, Japan). The protein–protein interaction (PPI) networks among Elabela, KLF15, Nrf2, SLC7A11, and GPX4 were obtained from the String database (https://string-db.org/).
Statistical analysis
GraphPad Prism version 9.0.2 and SPSS Statistics Version 25.0 software were used for statistical analysis. The data were presented as mean ± standard deviation (SD). Student’s t test was used for comparison between two groups, and one-way analysis of variance (ANOVA) with Bonferroni test was performed for comparison of multiple groups. P < 0.05 was considered statistically significant.
Results
Stimulation of DOX inhibits cell viability in rat aortic AFs
Staining of vimentin and α-SMA was performed to ensure the purity of rat aortic AFs (Fig. 1a). A wide variety of DOX dose ranging from 1 to 20 uM was used to calculate the cytotoxicity in cardiomyocytes (Jain and Rani 2018; Hou et al. 2021; Qi et al. 2020; Sirangelo et al. 2020; Zhang et al. 2020; Zhao et al. 2018). To explore dose-dependent impacts of DOX, rat AFs were incubated with DOX at concentrations of 0 μM, 2 μM, 5 μM, and 10 μM for 24 h, respectively. As shown in Fig. 1b, DOX treatment remarkably reduced the viability of AFs in a dose-dependent manner, especially at concentration of 10 μM. Then 10 μM was applied for further studies.
Fig. 1.
DOX causes dose-dependent cytotoxicity in rat aortic AFs. a Immunofluorescence staining of vimentin (green) and α-SMA (red) in rat aortic AFs. b The dose-dependent effects of DOX on the cell viability of rat aortic AFs (n = 6). AFs, adventitial fibroblasts; α-SMA, α-smooth muscle actin; DOX, doxorubicin. ** P < 0.01, *** P < 0.001 compared with control group
Administration of ELA suppresses DOX-induced promotion of oxidative stress in rat aortic AFs
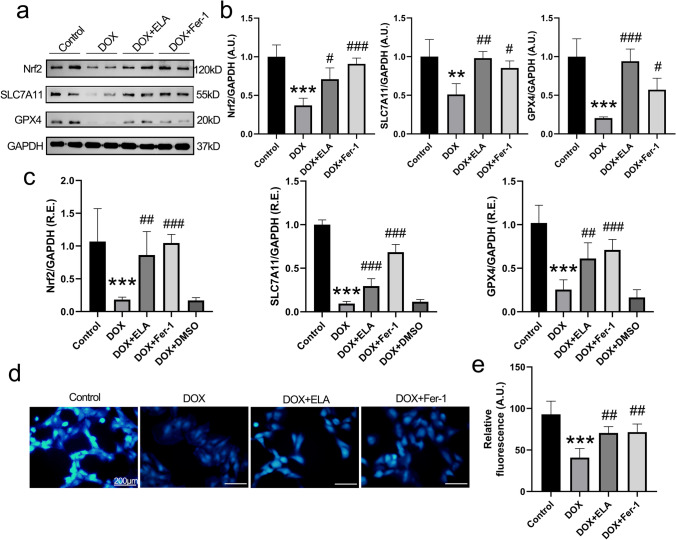
Oxidative stress contributes to DOX-mediated cardiovascular toxicity (Rawat et al. 2021). There was increased production of intracellular ROS (Fig. 2a–d) and mitochondrial ROS (Fig. 2e, f) in rat aortic AFs incubated by DOX, accompanied by increased MDA content (Fig. 2g). Importantly, the DOX-induced augmentated ROS levels were remarkably decreased by ELA treatment (Fig. 2) and antioxidant NAC (Fig. 2a, b and g), respectively. Excessive free radicals significantly promoted lipid peroxidation and ferroptosis (Octavia et al. 2012; Park and Chung 2019; Renu et al. 2018; Songbo et al. 2019), we, therefore, intended to discover whether ferroptosis inhibitor had effects on DOX-induced oxidative damage. Nrf2, SLC7A11, GPX4, and GSH were identified factors for anti-oxidative stress and anti-ferroptosis. Notably, Fer-1 alleviated DOX-induced enhanced oxidative stress in rat aortic AFs (Fig. 2), with enhanced Nrf2, SLC7A11, GPX4, and GSH levels (Fig. 3), thereby indicating that ferroptosis inhibition could maintain the cellular antioxidant capacity in DOX-treated rat AFs, and ferroptosis suppressing signaling may play a regulatory role.
Fig. 2.
ELA mitigates DOX-induced oxidative stress in rat aortic AFs. a, b Representative dihydroethidium staining images (a) and quantification (b) to assess oxidative stress levels (n = 5). c, d Intracellular ROS production measured by flow cytometry (c), with quantification (d) (n = 5). e, f Mitochondrial ROS production measured by flow cytometry (e), with quantification (f) (n = 5). g Lipid oxidation levels revealed by MDA in rat aortic AFs (n = 6). DOX, doxorubicin; Fer-1, ferrostatin-1; MDA malondialdehyde; A.U. arbitrary units. *** P < 0.001 compared with control group; # P < 0.05, ### P < 0.001 compared with DOX group
Fig. 3.
Treatment with ELA and Fer-1 attenuates DOX-induced ferroptosis in rat aortic AFs. a, b Representative Western blot images (a) with quantification (b) of the proteins involved in ferroptotic signaling (including Nrf2, SLC7A11 and GPX4) in rat aortic AFs (n = 4). GAPDH was used as endogenous control. c Relative mRNA levels of ferroptosis-related factor Nrf2, SLC7A11 and GPX4 in rat aortic AFs by qRT-PCR analysis (n = 6). d, e The condition of intracellular GSH levels assessed by MBB staining (d), with quantification (e) (n = 5). AFs, adventitial fibroblasts; DOX, doxorubicin; Fer-1, ferrostatin-1; Nrf2, nuclear factor erythroid 2-related factor 2; SLC7A11, the cystine/glutamate antiporter; GPX4, glutathione peroxidase 4; A.U., arbitrary units; R.E., relative expression; MBB, monobromobimane. ** P < 0.01, *** P < 0.001 compared with control group; # P < 0.05, ## P < 0.01, ### P < 0.001 compared with DOX group
ELA mitigates DOX-induced augmentation of oxidative injury and ferroptosis in rat aortic AFs via activation of the Nrf2/SLC7A11/GPX4 signaling
Since ELA and Fer-1 displayed beneficial effects on DOX-triggered generation of ROS, we hypothesized that ELA functioned as antioxidant by modulating ferroptosis-related signaling. The levels of Nrf2, SLC7A11, GPX4, and intracellular GSH were measured in rat aortic AFs by Western blotting (Fig. 3a, b), qRT-PCR analysis (Fig. 3c), and MBB staining (Fig. 3d, e), respectively. DOX treatment resulted in marked decreases in Nrf2, SLC7A11, GPX4, and GSH levels in rat aortic AFs (Fig. 3). Notably, DOX-triggered decreases of Nrf2, SLC7A11, GPX4, and GSH levels were strikingly reversed by ELA in rat aortic AFs (Fig. 3), associated with improved oxidative and ferroptotic damage (Fig. 2). These findings suggest that ELA can protect against DOX-mediated oxidative stress and ferroptosis through normalizing the levels of Nrf2, SLC7A11, GPX4, and GSH.
ELA treatment ameliorates DOX-mediated abnormality of LDH release, apoptosis-and inflammation-related factors in rat aortic AFs
In accordance with the oxidative injury, we found that the LDH release (Fig. 4a), apoptotic factors (Fig. 4b, c), inflammatory mediators (Fig. 4d), and cellular migration ability (Fig. 4e, f) were dysregulated under DOX stimulation. Importantly, treatment of ELA alleviated DOX-induced enhanced levels of LDH release (Fig. 4a), pro-apoptotic factor Bax (Fig. 4b, c) and pro-inflammatory factor IL-1β, IL-6, and CXCL1 (Fig. 4d) in rat aortic AFs, accompanied by increased levels of anti-apoptotic factor Bcl2 (Fig. 4b, c), anti-inflammatory factor IL-10 (Fig. 4d) and cellular migration ability (Fig. 4e, f). These results reveal the protective role of ELA in DOX-induced cellular injury and dysfunction.
Fig. 4.
ELA prevents dysregulation of LDH release, apoptosis- and inflammation-related factors, and migration ability in rat aortic AFs subjected to DOX. a Protective effects of ELA in DOX-induced AFs injury revealed by LDH activity (n = 6). b, c Representative Western blot images (b) with quantification (c) of the proteins involved in apoptotic signaling (including Bax and Bcl2) (n = 4). GAPDH was used as an endogenous control. d Relative mRNA levels of IL-1β, IL-6, IL-10, and CXCL1 in rat aortic AFs (n = 6). e, f Representative wound healing images (e) of rat AFs at 0 h and 24 h, with quantification (f) (n = 5). LDH, lactate dehydrogenase; DOX, doxorubicin; Fer-1, ferrostatin-1; A.U., arbitrary units; IL, interleukin; CXCL1, chemokine C-X-C motif ligand-1; R.E., relative expression. *** P < 0.001 compared with control group; # P < 0.05, ## P < 0.01, ### P < 0.001 compared with DOX group
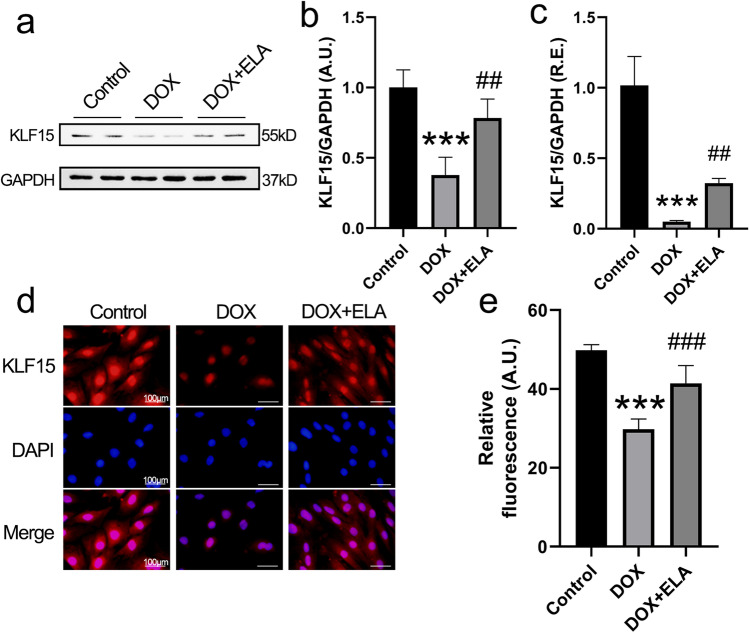
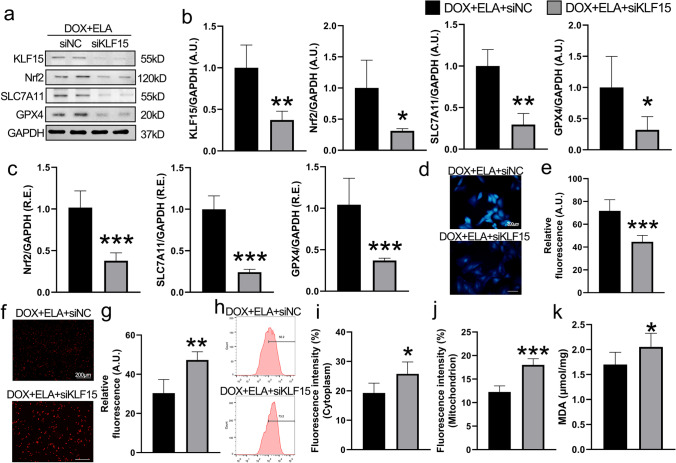
KLF15 is responsible for ELA-mediated anti-oxidative and anti-ferroptotic effects in DOX-treated rat aortic AFs
KLF15 plays an essential role in regulating cellular proliferation, migration, and death (Gao et al. 2017; Zhao et al. 2019). We next evaluated the effects of ELA on KLF15 signaling in rat AFs. Both protein and mRNA levels of KLF15 were significantly decreased in rat aortic AFs stimulated by DOX (Fig. 5a–c,). Immunofluorescence staining revealed that the expression of KLF15 was decreased in rat aortic AFs after DOX incubation (Fig. 5d, e). Intriguingly, as shown in Fig. 5, ELA treatment blunted the DOX-mediated decrease of KLF15 expression. To explore the involvement of KLF15 in DOX-triggered oxidative stress and ferroptosis, KLF15 siRNA was utilized to knock down the expression of KLF15 in rat aortic AFs. The efficacy of KLF15 knockdown was determined by Western Blotting analysis (Fig. 6a). Notably, ELA-mediated upregulation of Nrf2, SLC7A11, and GPX4 expressions were significantly reversed after knockdown of KLF15 by siRNA in the DOX-treated rat aortic AFs both at protein level (Fig. 6a, b) and mRNA level (Fig. 6c), with decreased GSH levels (Fig. 6d, e). Importantly, the inhibited Nrf2/SLC7A11/GPX4 signaling was associated with increased intracellular (Fig. 6f–i) and mitochondrial ROS production (Fig. 6j) and promoted MDA content (Fig. 6k) in rat aortic AFs pretreated with KLF15 siRNA. Taken together, these results suggest that ELA-mediated alleviation of oxidative stress and ferroptosis relies on KLF15-dependent Nrf2/SLC7A11/GPX4 signaling.
Fig. 5.
ELA reverses DOX-mediated downregulated expression of KLF15 in rat aortic AFs. a, c The expression of KLF15 in rat aortic AFs in the absence and presence of DOX and ELA by Western blotting (a and b) (n = 4) and qRT-PCR analysis (c) (n = 6), respectively. GAPDH was used as an endogenous control. d, e Images of immunofluorescence staining (d) and quantification (e) of KLF15 in rat aortic AFs in response to DOX and ELA (n = 5). KLF15, Krüppel-like factor 15. *** P < 0.001 compared with control group; ## P < 0.01, ### P < 0.001 compared with DOX group
Fig. 6.
KLF15 knockdown blocks ELA-meidated benificial roles in the ferroptosis and ROS generation in DOX-pretreated rat aortic AFs. a, b Representative Western blots (a) with quantification (b) of KLF15, Nrf2, SLC7A11, and GPX4 in rat aortic AFs (n = 4). GAPDH was used as an endogenous control. c Relative mRNA levels of Nrf2, SLC7A11, and GPX4 (n = 6). d, e The levels of intracellular GSH by MBB staining (d) with quantification (e) (n = 5). f, g) Images of representative dihydroethidium staining (f) and quantification (g) to determine oxidative stress levels (n = 5). h, i Intracellular ROS production measured by flow cytometry (h), with quantification (i) (n = 5). j Quantification of mitochondrial ROS measured by flow cytometry (n = 5). k Lipid oxidation levels revealed by MDA in rat aortic AFs (n = 6). siNC, small interfering RNA negative control; siRNA, small interfering RNA. * P < 0.05, ** P < 0.01, *** P < 0.001 compared with DOX + ELA + siNC group
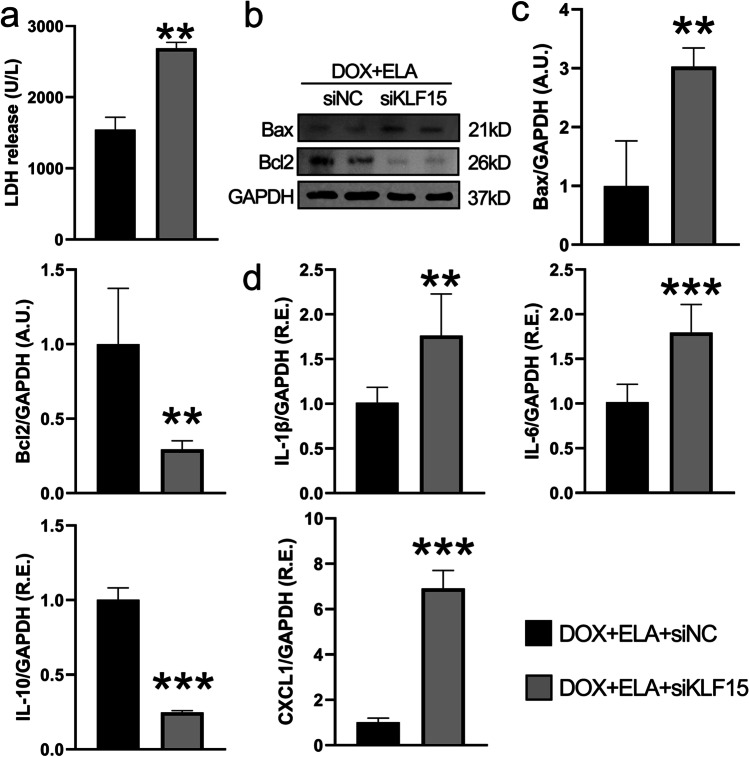
Knockdown of KLF15 disturbs ELA-mediated beneficial roles in apoptosis- and inflammation-related factors in DOX-pretreated rat aortic AFs
As mentioned above, ELA also conferred protection against DOX-induced cytotoxicity by normalizing the levels of LDH, Bax, Bcl2, IL-1β, IL-6, IL-10, and CXCL1. Then we demonstrated the levels of LDH, apoptosis- and inflammation-related factors when cells were treated with KLF15 siRNA. Furthermore, knockdown of KLF15 resulted in marked increases in LDH (Fig. 7a), Bax (Fig. 7b and 7c), IL-1β, IL-6, and CXCL1 (Fig. 7d) levels, and decreases in Bcl2 (Fig. 7b, c) and IL-10 (Fig. 7d) expression in rat aortic AFs. We also constructed the PPI network using String to predict the functional protein associating network among Elabela, KLF15, Nrf2, SLC7A11, and GPX4 (Fig. 8a). Above all, these data suggest that ELA inhibits DOX-induced promotion of oxidative stress and ferroptosis through KLF15-dependent Nrf2/SLC7A11/GPX4 signaling (Fig. 8b).
Fig. 7.
Knockdown of KLF15 blocks the protective effects of ELA on DOX-induced dysregulation of levels of LDH, apoptosis- and inflammation-related factors in rat aortic AFs. a Effects of ELA in DOX-induced AFs injury revealed by LDH activity (n = 6). b, c Representative Western blots (b) with quantification (c) to determine protein levels of Bax and Bcl2 in rat aortic AFs (n = 4). GAPDH was used as an endogenous control. d Relative mRNA levels of inflammatory factors including IL-1β, IL-6, IL-10, and CXCL1 in rat aortic AFs (n = 6). ** P < 0.01, *** P < 0.001 compared with DOX + ELA + siNC group
Fig. 8.
a Protein–protein interaction network elucidating interactions between Elabela, KLF15, pro-inflammatory cytokines, ferroptosis- and apoptosis-related factors. b Overview of the protective role of ELA in DOX-mediated oxidative stress and ferroptosis in rat aortic adventitial fibroblasts. ELA negatively regulates DOX-induced oxidative stress, ferroptosis and inflammatory responses in rat adventitial fibroblasts by modulating the KLF15/Nrf2/SLC7A11/GPX4 signaling
Discussion
Doxorubicin is an effectively applicated antitumor agent and frequently leads to severe cardiovascular toxic events in a dose-dependent manner (Rawat et al. 2021). A wide variety of DOX doses ranging from 1 to 20 uM is utilized to calculate the cytotoxicity in cardiomyocytes (Jain and Rani 2018; Hou et al. 2021; Qi et al. 2020; Sirangelo et al. 2020; Zhang et al. 2020; Zhao et al. 2018). Mice usually receive a singly or cumulatively total dose of 15–25 mg/kg (Catanzaro et al. 2019; Hou et al. 2021; Zhang et al. 2020; Zheng et al. 2020). We demonstrated that 10 uM of DOX remarkably causes damage to cell viability in rat aortic AFs, and ELA is meaningful to promote cellular viability when the concentration of DOX is 5 and 10 µM. We previously evaluated the protective effects of ELA in cardiovascular disorders and found that ELA maintains the homeostasis of rat aortic AFs by inhibiting oxidative stress, autophagy, inflammation, and apoptosis (Song et al. 2021, 2020). In this study, cultured rat aortic AFs were pretreated with ELA followed by DOX incubation. As expected, ELA treatment markedly rescues DOX-induced oxidative stress and ferroptosis, accompanied by normalized levels of LDH, IL-1β, IL-6, IL-10, CXCL1, Bax, and Bcl2 in rat aortic AFs. Nrf2, SLC7A11, GPX4, and GSH are classical ferroptosis-suppressing signaling pathways, which are also known for anti-oxidative injury. Abnormality in this system causes oxidative and ferroptotic cell injury. Intriguingly, we found that DOX-mediated decreased levels of Nrf2, SLC7A11, GPX4, and GSH are reversely increased by ELA treatment. We next intended to discover how Elabela regulates these signaling pathways. The transcription factor KLF15 regulates cellular activity and cell death via modulating diverse transcriptional pathways and sustained cardiovascular homeostasis under physiological and pathological conditions (Zhao et al. 2019). Then, knockdown KLF15 by siRNA was used in rat aortic AFs to determine its role. Importantly, KLF15 deficiency aggravates DOX-induced decrease in Nrf2/SLC7A11/GSH/GPX4 levels, which antagonizes the protective function of ELA. In accordance with the inhibited anti-ferroptotic signaling, oxidative stress is significantly exacerbated by knockdown of KLF15 in rat aortic AFs.
Collectively, our study identified that ELA ameliorates DOX-induced augmentation of oxidative stress and ferroptosis by activating the KLF15/GPX4 signaling pathway. ELA could be regarded as a therapeutic candidate for DOX-induced cardiovascular toxicity. The next step in vivo study would be performed and the dose of ELA would be determined based on its efficacy to antagonize DOX-mediated cardiovascular oxidative injury, ferroptosis, and dysfunction.
Conclusions
In summary, our findings demonstrated for the first time that ELA treatment protects against DOX-triggered enhancement of oxidative stress and ferroptosis in rat aortic adventitial fibroblasts. ELA exerts anti-ferropoptic, anti-apoptotic, anti-oxidant and anti-inflammatory roles in rat aortic adventitial fibroblasts via activation of the KLF15/Nrf2 and SLC7A11/GPX4 signaling pathways. This study provides novel insight into the protective mechanisms of the anti-cytotoxic effects of ELA. Targeting ELA and KLF15/GPX4 signaling may be a prospective therapy to overcome the intractable nodes in DOX-mediated cardiovascular abnormality and disorders.
Acknowledgements
This study was supported by the General Program and the National Major Research Plan Training Program of the National Natural Science Foundation of China (No. 92168117; 81770253; 91849111), Beijing Natural Science Foundation (7222068), Clinical Research Incubation Program of Beijing Chaoyang Hospital Affiliated to Capital Medical University (CYFH202209) and Reform and Development Program of Beijing Institute of Respiratory Medicine (ysrh2022002). We appreciate all graduate students and the doctors who participated in this study.
Author contribution
Mi-Wen Zhang and Jiu-Chang Zhong: writing—original draft, methodology, supervision, writing—review and editing, read and approved the final manuscript. Xue-Ting Li, Zhen-Zhou Zhang, and Ying Liu: collected and recorded the samples, methodology, read and approved the final manuscript. Jia-Wei Song and Xin-Ming Liu: collected and recorded the samples, read and approved the final manuscript. Yihang Chen, Ning Wang, and Ying Guo: formal analysis, read and approved the final manuscript. Li-Rong Liang and Jiu-Chang Zhong: Writing—review and editing, supervision, methodology, read and approved the final manuscript.
Funding
This study was supported by the General Program and the National Major Research Plan Training Program of the National Natural Science Foundation of China (No. 92168117; 81770253; 91849111), Beijing Natural Science Foundation (7222068), Clinical Research Incubation Program of Beijing Chaoyang Hospital Affiliated to Capital Medical University (CYFH202209) and Reform and Development Program of Beijing Institute of Respiratory Medicine (ysrh2022002).
Data availability
The data that support the findings of this study are available on reasonable request from the corresponding author.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Li-Rong Liang, Email: llrcruie@163.com.
Jiu-Chang Zhong, Email: jczhong@sina.com.
References
- Alhazzani K, Alotaibi MR, Alotaibi FN, Aljerian K, As Sobeai HM, Alhoshani AR, Alanazi AZ, Alanazi WA, Alswayyed M. Protective effect of valsartan against doxorubicin-induced cardiotoxicity: histopathology and metabolomics in vivo study. J Biochem Mol Toxicol. 2021;35:e22842. doi: 10.1002/jbt.22842. [DOI] [PubMed] [Google Scholar]
- Bosman M, Krüger DN, Favere K, Wesley CD, Neutel CHG, Van Asbroeck B, Diebels OR, Faes B, Schenk TJ, Martinet W, De Meyer GRY, Van Craenenbroeck EM, Guns PJDF (2021) Doxorubicin impairs smooth muscle cell contraction: novel insights in vascular toxicity. Int J Mol Sci 22. 10.3390/ijms222312812 [DOI] [PMC free article] [PubMed]
- Burridge PW, Li YF, Matsa E, Wu H, Ong SG, Sharma A, Holmström A, Chang AC, Coronado MJ, Ebert AD, Knowles JW, Telli ML, Witteles RM, Blau HM, Bernstein D, Altman RB, Wu JC. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med. 2016;22:547–556. doi: 10.1038/nm.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, Moreira PI. Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem. 2009;16:3267–3285. doi: 10.2174/092986709788803312. [DOI] [PubMed] [Google Scholar]
- Catanzaro MP, Weiner A, Kaminaris A, Li C, Cai F, Zhao F, Kobayashi S, Kobayashi T, Huang Y, Sesaki H, Liang Q. Doxorubicin-induced cardiomyocyte death is mediated by unchecked mitochondrial fission and mitophagy. FASEB J. 2019;33:11096–11108. doi: 10.1096/fj.201802663R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Hong HJ, Hao WR, Cheng TH, Liu JC, Sung LC. Nicorandil prevents doxorubicin-induced human umbilical vein endothelial cell apoptosis. Eur J Pharmacol. 2019;859:172542. doi: 10.1016/j.ejphar.2019.172542. [DOI] [PubMed] [Google Scholar]
- Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17:2054–2081. doi: 10.1080/15548627.2020.1810918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Yu W, Zhong C, Hong Q, Huang G, Que D, Wang Y, Yang Y, Rui B, Zhuang Z, Liang M, Ye Z, Yan X, Lv J, Zhang R, Yan J, Yang P. Elabela ameliorates doxorubicin-induced cardiotoxicity by promoting autophagic flux through TFEB pathway. Pharmacol Res. 2022;178:106186. doi: 10.1016/j.phrs.2022.106186. [DOI] [PubMed] [Google Scholar]
- Dodson M, Castro Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius E, Scheel CH, Mourão A, Buday K, Sato M, Wanninger J, Vignane T, Mohana V, Rehberg M, Flatley A, Schepers A, Kurz A, White D, Sauer M, Sattler M, Tate EW, Schmitz W, Schulze A, O'Donnell V, Proneth B, Popowicz GM, Pratt DA, Angeli JPF, Conrad M. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- Dong H, Xia Y, Jin S, Xue C, Wang Y, Hu R, Jiang H. Nrf2 attenuates ferroptosis-mediated IIR-ALI by modulating TERT and SLC7A11. Cell Death Dis. 2021;12:1027. doi: 10.1038/s41419-021-04307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Guo Y, Liu X, Shang D, Du Y. KLF15 protects against isoproterenol-induced cardiac hypertrophy via regulation of cell death and inhibition of Akt/mTOR signaling. Biochem Biophys Res Commun. 2017;487:22–27. doi: 10.1016/j.bbrc.2017.03.087. [DOI] [PubMed] [Google Scholar]
- Gao M, Fan K, Chen Y, Zhang G, Chen J, Zhang Y. Understanding the mechanistic regulation of ferroptosis in cancer: the gene matters. J Genet Genomics. 2022;S1673–8527(22):00160–166. doi: 10.1016/j.jgg.2022.06.002. [DOI] [PubMed] [Google Scholar]
- He S, Lu Y, Guo Y, Li S, Lu X, Shao S, Zhou H, Wang R, Wang J, Gao P, Li X. Krüppel-like factor 15 modulates CXCL1/CXCR2 signaling-mediated inflammatory response contributing to angiotensin II-induced cardiac remodeling. Front Cell Dev Biol. 2021;9:644954. doi: 10.3389/fcell.2021.644954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou K, Shen J, Yan J, Zhai C, Zhang J, Pan JA, Zhang Y, Jiang Y, Wang Y, Lin RZ, Cong H, Gao S, Zong WX. Loss of TRIM21 alleviates cardiotoxicity by suppressing ferroptosis induced by the chemotherapeutic agent doxorubicin. EBioMedicine. 2021;69:103456. doi: 10.1016/j.ebiom.2021.103456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Rani V. Mode of treatment governs curcumin response on doxorubicin-induced toxicity in cardiomyoblasts. Mol Cell Biochem. 2018;442:81–96. doi: 10.1007/s11010-017-3195-6. [DOI] [PubMed] [Google Scholar]
- Kuwabara JT, Tallquist MD. Tracking adventitial fibroblast contribution to disease: a review of current methods to identify resident fibroblasts. Arterioscler Thromb Vasc Biol. 2017;37:1598–1607. doi: 10.1161/ATVBAHA.117.308199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xu W, Zhang L. KLF15 regulates oxidative stress response in cardiomyocytes through NAD. Metabolites. 2021;11:620. doi: 10.3390/metabo11090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Xu L, Yu X, Li W, Sun X, Xiao S, Guo M, Wang H. Protective effect of KLF15 on vascular endothelial dysfunction induced by TNF-α. Mol Med Rep. 2018;18:1987–1994. doi: 10.3892/mmr.2018.9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yang Y, Song J, Li D, Liu X, Li C, Ma Z, Zhong J, Wang L. Knockdown of forkhead box protein P1 alleviates hypoxia reoxygenation injury in H9c2 cells through regulating Pik3ip1/Akt/eNOS and ROS/mPTP pathway. Bioengineered. 2022;13:1320–1334. doi: 10.1080/21655979.2021.2016046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YY, Li XD, Zhou HD, Shao S, He S, Hong MN, Liu JC, Xu YL, Wu YJ, Zhu DL, Wang JG, Gao PJ. Transactivation domain of Krüppel-like factor 15 negatively regulates angiotensin II-induced adventitial inflammation and fibrosis. FASEB J. 2019;33:6254–6268. doi: 10.1096/fj.201801809R. [DOI] [PubMed] [Google Scholar]
- Ma Z, Song JJ, Martin S, Yang XC, Zhong JC. The Elabela-APJ axis: a promising therapeutic target for heart failure. Heart Fail Rev. 2021;26:1249–1258. doi: 10.1007/s10741-020-09957-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt S, Hofmann TG. The DNA damage-induced cell death response: a roadmap to kill cancer cells. Cell Mol Life Sci. 2016;73:2829–2850. doi: 10.1007/s00018-016-2130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mišúth S, Uhrinová M, Klimas J, Vavrincová Yaghi D, Vavrinec P. Vildagliptin improves vascular smooth muscle relaxation and decreases cellular senescence in the aorta of doxorubicin-treated rats. Vascul Pharmacol. 2021;138:106855. doi: 10.1016/j.vph.2021.106855. [DOI] [PubMed] [Google Scholar]
- Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Pan JA, Zhang H, Lin H, Gao L, Zhang HL, Zhang JF, Wang CQ, Gu J. Irisin ameliorates doxorubicin-induced cardiac perivascular fibrosis through inhibiting endothelial-to-mesenchymal transition by regulating ROS accumulation and autophagy disorder in endothelial cells. Redox Biol. 2021;46:102120. doi: 10.1016/j.redox.2021.102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Chung SW. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019;10:822. doi: 10.1038/s41419-019-2064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Boliang W, Xiaoxi T, Guoqiang F, Jianbo X, Gang W. Cardamonin protects against doxorubicin-induced cardiotoxicity in mice by restraining oxidative stress and inflammation associated with Nrf2 signaling. Biomed Pharmacother. 2020;122:109547. doi: 10.1016/j.biopha.2019.109547. [DOI] [PubMed] [Google Scholar]
- Rawat PS, Jaiswal A, Khurana A, Bhatti JS, Navik U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed Pharmacother. 2021;139:111708. doi: 10.1016/j.biopha.2021.111708. [DOI] [PubMed] [Google Scholar]
- Renu K, Abilash VG, Tirupathi Pichiah PB, Arunachalam S (2018) Molecular mechanism of doxorubicin-induced cardiomyopathy - an update. Eur J Pharmacol 818:241–253. 10.1016/j.ejphar.2017.10.043 [DOI] [PubMed]
- Rivankar S. An overview of doxorubicin formulations in cancer therapy. J Cancer Res Ther. 2014;10:853–858. doi: 10.4103/0973-1482.139267. [DOI] [PubMed] [Google Scholar]
- Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med. 2019;133:144–152. doi: 10.1016/j.freeradbiomed.2018.09.014. [DOI] [PubMed] [Google Scholar]
- Sirangelo I, Sapio L, Ragone A, Naviglio S, Iannuzzi C, Barone D, Giordano A, Borriello M. Vanillin prevents doxorubicin-induced apoptosis and oxidative stress in rat H9c2 cardiomyocytes. Nutrients. 2020;12:2317. doi: 10.3390/nu12082317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Yang M, Liu Y, Song JW, Wang J, Chi HJ, Liu XY, Zuo K, Yang XC, Zhong JC. MicroRNA-122 aggravates angiotensin II-mediated apoptosis and autophagy imbalance in rat aortic adventitial fibroblasts via the modulation of SIRT6-elabela-ACE2 signaling. Eur J Pharmacol. 2020;883:173374. doi: 10.1016/j.ejphar.2020.173374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Yang M, Liu Y, Song JW, Liu XY, Miao R, Zhang ZZ, Liu Y, Fan YF, Zhang Q, Dong Y, Yang XC, Zhong JC. Elabela prevents angiotensin II-induced apoptosis and inflammation in rat aortic adventitial fibroblasts via the activation of FGF21-ACE2 signaling. J Mol Histol. 2021;52:905–918. doi: 10.1007/s10735-021-10011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songbo M, Lang H, Xinyong C, Bin X, Ping Z, Liang S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol Lett. 2019;307:41–48. doi: 10.1016/j.toxlet.2019.02.013. [DOI] [PubMed] [Google Scholar]
- Sonowal H, Pal P, Shukla K, Saxena A, Srivastava SK, Ramana KV. Aldose reductase inhibitor, fidarestat prevents doxorubicin-induced endothelial cell death and dysfunction. Biochem Pharmacol. 2018;150:181–190. doi: 10.1016/j.bcp.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulet F, Bodineau C, Hooks KB, Descarpentrie J, Alves I, Dubreuil M, Mouchard A, Eugenie M, Hoepffner JL, López JJ, Rosado JA, Soubeyran I, Tomé M, Durán RV, Nikolski M, Villoutreix BO, Evrard S, Siegfried G, Khatib AM. ELA/APELA precursor cleaved by furin displays tumor suppressor function in renal cell carcinoma through mTORC1 activation. JCI Insight. 2020;5:e129070. doi: 10.1172/jci.insight.129070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth PA, van Hoesel QG, Haanen C. Clinical pharmacokinetics of doxorubicin. Clin Pharmacokinet. 1988;15:15–31. doi: 10.2165/00003088-198815010-00002. [DOI] [PubMed] [Google Scholar]
- Steinberg JS, Cohen AJ, Wasserman AG, Cohen P, Ross AM. Acute arrhythmogenicity of doxorubicin administration. Cancer. 1987;60:1213–1218. doi: 10.1002/1097-0142(19870915)60:6<1213::aid-cncr2820600609>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- Wallace KB, Sardão VA, Oliveira PJ. Mitochondrial determinants of doxorubicin-induced cardiomyopathy. Circ Res. 2020;126:926–941. doi: 10.1161/CIRCRESAHA.119.314681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yu D, Wang M, Wang Q, Kouznetsova J, Yang R, Qian K, Wu W, Shuldiner A, Sztalryd C, Zou M, Zheng W, Gong DW. Elabela-apelin receptor signaling pathway is functional in mammalian systems. Sci Rep. 2015;5:8170. doi: 10.1038/srep08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Xu H, Kong J, Liu D, Qin W, Bai W. Krüppel-like factor 15 reduces ischemia-induced apoptosis involving regulation of p38/MAPK signaling. Hum Gene Ther. 2021;32:1471–1480. doi: 10.1089/hum.2021.075. [DOI] [PubMed] [Google Scholar]
- Xu R, Zhang ZZ, Chen LJ, Yu HM, Guo SJ, Xu YL, Oudit GY, Zhang Y, Chang Q, Song B, Chen DR, Zhu DL, Zhong JC. Ascending aortic adventitial remodeling and fibrosis are ameliorated with Apelin-13 in rats after TAC via suppression of the miRNA-122 and LGR4-β-catenin signaling. Peptides. 2016;86:85–94. doi: 10.1016/j.peptides.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Xue CC, Li MH, Zhao Y, Zhou J, Hu Y, Cai KY, Zhao Y, Yu SH, Luo Z. Tumor microenvironment-activatable Fe-doxorubicin preloaded amorphous CaCO3 nanoformulation triggers ferroptosis in target tumor cells. Sci Adv. 2020;6:eaax1346. doi: 10.1126/sciadv.aax1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Yan Y, Niu F, Wang Y, Chen X, Su G, Liu Y, Zhao X, Qian L, Liu P, Xiong Y. Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021;7:193. doi: 10.1038/s41420-021-00579-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhu JX, Ma ZG, Wu HM, Xu SC, Song P, Kong CY, Yuan YP, Deng W, Tang QZ. Rosmarinic acid alleviates cardiomyocyte apoptosis via cardiac fibroblast in doxorubicin-induced cardiotoxicity. Int J Biol Sci. 2019;15:556–567. doi: 10.7150/ijbs.29907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Hu C, Kong CY, Song P, Wu HM, Xu SC, Yuan YP, Deng W, Ma ZG, Tang QZ. FNDC5 alleviates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via activating AKT. Cell Death Differ. 2020;27:540–555. doi: 10.1038/s41418-019-0372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Tao X, Qi Y, Xu L, Yin L, Peng J. Protective effect of dioscin against doxorubicin-induced cardiotoxicity via adjusting microRNA-140-5p-mediated myocardial oxidative stress. Redox Biol. 2018;16:189–198. doi: 10.1016/j.redox.2018.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Song W, Wang L, Rane MJ, Han F, Cai L. Multiple roles of KLF15 in the heart: underlying mechanisms and therapeutic implications. J Mol Cell Cardiol. 2019;129:193–196. doi: 10.1016/j.yjmcc.2019.01.024. [DOI] [PubMed] [Google Scholar]
- Zheng X, Zhong T, Ma Y, Wan X, Qin A, Yao B, Zou H, Song Y, Yin D. Bnip3 mediates doxorubicin-induced cardiomyocyte pyroptosis via caspase-3/GSDME. Life Sci. 2020;242:117186. doi: 10.1016/j.lfs.2019.117186. [DOI] [PubMed] [Google Scholar]
- Zhou M, Wu Y. Effects and signaling pathways of Elabela in the cardiovascular system. Peptides. 2022;147:170674. doi: 10.1016/j.peptides.2021.170674. [DOI] [PubMed] [Google Scholar]
- Zhou L, Han Y, Yang Q, Xin B, Chi M, Huo Y, Guo C, Sun X. Scutellarin attenuates doxorubicin-induced oxidative stress, DNA damage, mitochondrial dysfunction, apoptosis and autophagy in H9c2 cells, cardiac fibroblasts and HUVECs. Toxicol in Vitro. 2022;82:105366. doi: 10.1016/j.tiv.2022.105366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author.