Abstract
Several strategies have been put in place by organisms to adapt to their environment. One of these strategies is the production of stress proteins such as sHSPs, which have been widely described over the last 30 years for their role as molecular chaperones. Some sHSPs have, in addition, the particularity to exert a lipochaperone role by interacting with membrane lipids to maintain an optimal membrane fluidity. However, the mechanisms involved in this sHSP-lipid interaction remain poorly understood and described rather sporadically in the literature. This review gathers the information concerning the structure and function of these proteins available in the literature in order to highlight the mechanism involved in this interaction. In addition, analysis of primary sequence data of sHSPs available in database shows that sHSPs can interact with lipids via certain amino acid residues present on some β sheets of these proteins. These residues could have a key role in the structure and/or oligomerization dynamics of sHPSs, which is certainly essential for interaction with membrane lipids and consequently for maintaining optimal cell membrane fluidity.
Keywords: Small heat shock protein, Lipochaperone activity, Stress response, Protein structure
Introduction
A weak modification of the internal cell environment in response to external stress conditions (e.g., heat, nutritional, oxidative, alcohol stresses) is enough to lead to protein misfolding and a subsequent loss of biological function (Booth 1985; Berger et al. 1996; Lim et al. 2000). To maintain protein homeostasis, several stress mechanisms are involved in cells, leading to the production of a group of stress proteins called heat shock proteins (HSP). These proteins act as molecular chaperones which bind and sequester denatured proteins (Ellis et al. 1989; Ellis et al. 1990; Hartl 1996; Beissinger and Buchner 1998; Tyedmers et al. 2010). Some of them are able to refold the denatured protein in an ATP-dependent manner. HSP are universal stress proteins found in almost all organisms including eukaryotes (plants, metazoan, yeast), bacteria, archaebacteria, and viruses (Wirth 2003; Ponomarenko et al. 2013; Carra et al. 2017). Besides being present in all kingdoms (Narberhaus 2002; Horwitz 2003; Sun and MacRae 2005; Haslbeck et al. 2005), these proteins have a high degree of homology to each other and are found ubiquitously in cells. They can be grouped into six main families according to their molecular mass, HSP 110/100, 90, 70, 60, 47, and less than 35 kDa (Wirth 2003; Bukau and Horwich 1998; Chang et al. 2007; Tang et al. 2007; Marcion et al. 2014). However, each family has high specificity concerning notably its mechanisms of action, intracellular localization, substrate specificity, and ATP requirement.
The stress proteins under 35 kDa, called small heat shock protein (sHSP), act mainly as ATP-independent chaperones (Baneyx and Mujacic 2004). For some of them, a lipochaperone activity, i.e., cell membrane protection activity, is described from the outset of stress conditions. However, this lipochaperone activity is still poorly described and understood (Mogk et al. 2019). As this activity is found in very different model organisms that diverged quite early in the evolution of sHSP, it is quite possible that this role is more common than currently described. Although highly heterogeneous in their primary sequence, the secondary structures and activities are well-conserved among sHSPs (Jong et al. 1998; Poulain et al. 2010). Thus, the question is to predict the potential molecular lipochaperone role of a sHSP on the basis of its primary sequence. Indeed, bioinformatics work on the primary sequences of 50 sHSPs has shown that certain protein motifs are better conserved in sHSPs known for their role as molecular lipochaperones.
sHSP, a key stress fighter
sHSP through the ages
Since the discovery of the first sHSP by Craig and Ingola in 1982 (Ingolia and Craig 1982), the number of identified sHSPs has exploded. These proteins are present in every kingdom of life in which they act primarily as molecular chaperons to control and protect the quality of the protein network (Obuchowski and Liberek 2020). The number of sHSP per organism may vary; however, higher organisms (plants, human, animals …) generally have a large number of sHSP (Haslbeck et al. 2005). For example, there are nineteen sHSPs in the plant Arabidopsis thaliana (Siddique et al. 2008), one in the lactic acid bacterium Oenococcus oeni (Guzzo et al 1997). Although sHSPs are widespread, small genomes such as Helicobacter pylori 26,695 (1.66 Mb), Mycoplasma pneumoniae M129 (0.81 Mb), or Lactococcus lactis subsp. lactis II1403 (2.37 Mb) are devoid of this type of protein (Narberhaus 2002; Han et al. 2008).
sHSPs derive from a common ancestor of the vertebrate lens protein (Mörner 1894; Ingolia and Craig 1982) and now form a superfamily of small heat stress proteins of about 20 kDa (Horwitz et al. 1993; Jakob et al. 1993; de Jong et al. 1993), characterized by the presence of a central domain named the α-crystallin domain (Boelens 2014; Carra et al. 2017; Caspers et al. 1995; Jong et al. 1998). This domain has been under significant evolutionary pressure, and certain amino acids are still conserved among sHSPs (De Maio et al. 2019). The analysis of the sHSPs evolutionary tree by Vogel and colleagues showed that terminal domain specialization occurred during evolution later, several times and in parallel with and independently of the α-crystallin domain (Vogel et al. 2004; Kriehuber et al. 2010). This is reflected in rather high sequence heterogeneity between sHSPs (Mogk et al. 2019) that may be linked to the acquirement of multiple activities (De Jong et al. 1993; Carra et al. 2017; Obuchowski and Liberek 2020; Waters et al. 1996; Kappe et al. 2002).
The first trace of sHSPs in the history is probably from cyanobacteria such as Synechococcus sp. and Prochlorococcus sp. that lived on Earth two billion years ago (Fu et al. 2006). Indeed, sHSPs, which share some structural characteristics of the bacterial class A sHSP (corresponding to proteins with characteristics similar to those of Escherichia coli IbpA and IbpB) (Münchbach et al. 1999; Fu et al. 2006) have been identified in cyanophages (Fu et al. 2006) able to infect them (Schopf 2006; Maaroufi and Tanguay 2013). Animal sHSPs therefore evolved from bacterial class A sHSP via the lateral transfer gene either by the endosymbiotic mitochondria or by bacterial pathogen infections. Using the same mechanism of evolution, plant sHSPs might have been evolved from plant bacterial sHSPs of class B, like those found in Rhizobia (Fu et al. 2006). Because sHSPs are the least conserved family of chaperone proteins, the hypothesis of very early evolutionary divergence is reasonable (Kappe et al. 2002). However, according to the literature, 2 hypotheses have been put forward to explain the evolution of sHSPs: one proposes divergence from a common ancestor and the other convergence from several ancestral genes (Fu et al. 2006).
Structure of sHSPs
Contrary to sequence homology, the structure of sHSPs is well conserved in all organisms (Poulain et al. 2010; Klevit 2020). The majority of sHSPs adopt an IgG-type sandwich structure and are organized in large spherical oligomeric assemblies ranging from 10 to 18 nm in diameter (Haslbeck et al. 2005; Augusteyn 2004), even if others may adopt smaller forms (monomers or dimers) (Weeks et al. 2014; Boelens 2020). Each protein monomer has a low molecular weight (12–35 kDa) (Ingolia and Craig 1982; Kim et al. 1998; van Montfort et al. 2001; Kriehuber et al. 2010) and is mainly composed of β strands (60 to 70 percent) and few or no α-helixes (Augusteyn 2004). All sHSPs are composed of three domains, the two less conserved termini domains playing an important role in sHSP oligomerization and activities (Carra et al. 2017) and the well-preserved α-crystallin domain (ACD) (van Montfort et al. 2001; Kriehuber et al. 2010; Junprung et al. 2021; Klevit 2020; Mainz et al. 2015; Carra et al. 2017).
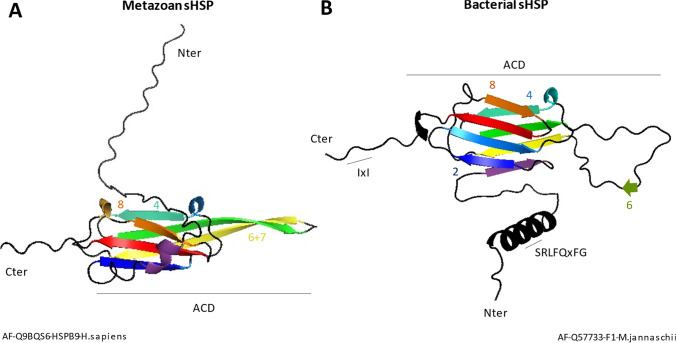
The α-crystallin is composed of around 90 amino acid residues among which the well-conserved signature motif GVLTL (between the β8 and β9 sheets) (Lentze et al. 2004 and Lentze and Narberhaus 2004; Augusteyn 2004; Sun and MacRae 2005 Bozaykut et al. 2014; MacRae 2000; Sun et al. 2002). The structure of the ACD can be split into two types of monomer (Fig. 1): the “bacterial” (Kim et al. 1998) and the “metazoan” structures (Delbecq and Klevit 2013). In both the ACD (involved in the oligomerization process), these are composed of seven or eight anti-parallel β strands that form a β-sandwich (Tikhomirova et al. 2017): one with β4, β5, and β6/7 strands and the second with β2, β3, β8, and β9 strands (Kim et al. 1998; Haslbeck et al. 2005; Kriehuber et al. 2010; Poulain et al. 2010; Basha et al. 2012).
Fig. 1.
Predicted structure of model A metazoan sHSP (alphafold2 HSPB9 of H. sapiens) and B bacterial sHSP (alphafold2 sHSP of M. jannaschii). The numbers correspond to the sheets allowing interactions between the sHSP. The amino acid sequences SRLFQxFG and IXI correspond to highly conserved sequences in the N- and C-terminal domain in bacteria
The N-ter domain is variable in length and amino acid composition (Chen et al. 2010; Lelj-Garolla and Mauk 2011), with 90 residues on average from 40 in bacteria to 100 in animals (Kriehuber et al. 2010). Although they have low sequence identity, some motifs such as SRLFQxFG are highly conserved (Tikhomirova et al. 2017; Shatov et al. 2018). A hydrophobic groove formed by the association of the β2 and β7 sheets of the ACD of each monomer creates an open space into which the N-ter domain can engage to stabilize the dimer. This conformation is then said to be open because it facilitates the interaction of the sHSP with its substrate (Sudnitsyna et al. 2012; Tikhomirova et al. 2017, Boelens 2020).
The short variable C-ter domain (approximately 20 amino acids) contains the highly conserved I/L-x-I/L motif which acts as an “anchor” at the ACD and thus participates in oligomer formation (Saji et al. 2008; Poulain et al. 2010; Sudnitsyna et al. 2012; Delbecq and Klevit 2013; Hilton and Benesch 2012 and 2013).
Oligomerization of sHsp
As soon as they are produced, monomers are organized into dimers which are therefore the first level of the structural order (Delbecq and Klevit 2013). Common oligomerization mechanisms have been described between the sHPSs of different organisms according to the ACD organization, bacterial or metazoan. Either, there is an interaction between the β6 on one monomer with the β2 of the second monomer, which results in the so-called bacterial dimer (Fig. 2A) or the interaction between the β6 and β7 strands of two different monomers to produce the “metazoan”-type dimer (van Montfort et al. 2001; Sun and MacRae 2005; Delbecq and Klevit 2013; Hilton et al. 2013). Then sHSP dimers can interact to form a higher oligomeric structure. For most of sHSPs, the palindromic I/V-x-I/V motif in the C-ter domain on the first dimer interacts with the hydrophobic groove formed by β4 and β8 of the adjacent dimer ACD (Jong et al. 1993; van Montfort et al. 2001; Sun and MacRae 2005; Basha et al. 2012; Santhanagopalan et al. 2018; Mainz et al. 2015; Tikhomirova et al. 2017; Mogk et al. 2019) (Fig. 2B). The dimer assemblies will then interact with each other to form disks via the interaction between the still free C-ter domain and the β9 strand located at the end of the ACD domain, thus forming large oligomers (van Montfort et al. 2001) (Fig. 2C). However, for many mammalian, sHSP formation of large oligomers may be different, depending on N-terminal domain (Delbecq et al. 2015).
Fig. 2.
Oligomerization process of sHSP using the example of M. marinum sHSP (pdb 5ZUL) (Bhandari et al. 2019). A Dimerization: interaction between the β6 strand (green) of a monomer with the β2 strand of the adjacent monomer (blue). B Oligomerization: interaction between the C-ter domain of a dimer (red) with the hydrophobic groove formed by the β4 (turquoise) and β8 (orange) strands of an adjacent dimer. C Large oligomer formation: interaction between the free C-ter domain (pink) of the oligomer with the β9 (red) strand on another oligomer
These sHSPs are linked to the quality control of cellular proteins via the considerable dynamic dissociation and reassociation of oligomers around their substrates. The speed of these associations could be one of the main factors influencing chaperon activity (Fu et al. 2006 and 2012; Tikhomirova et al. 2017).
sHSPs, actors of stress response with multiple activities
The proper folding of proteins is essential for life (Klaips et al. 2017), but unfortunately stress conditions dramatically increase their level of unfolding (Tyedmers et al. 2010). Indeed, the modification of environmental conditions (i.e., acidification) may modify the physicochemical properties of amino acids, changing their interaction and consequently the final organization of the proteins. As the biological function of a protein is strongly linked to its tertiary structure, this denaturation will induce the loss of its function affecting the whole cellular and replicative metabolism (Berger et al. 1996; Lim et al. 2000). To survive, organisms have succeeded in adapting to their environment by developing various strategies for maintaining proteins. One of these strategies is the production of molecular chaperones such as sHSPs (Jakob et al. 1993; Boyle and Takemoto 1994; Singh et al. 1995; Wang and Spector 1995; Chang et al. 1996; Guzzo et al. 1997). Since the beginning of the 1990s, several studies have suggested the molecular chaperone roles played by eukaryotic and bacterial sHSPs. They constitute the first line of stress defense of the protein quality control system (Bukau et al. 2006; Chen et al. 2010; Haslbeck and Vierling 2015; Haslbeck et al. 2016; Mogk et al., 2019; Obuchowski and Liberek 2020). sHSPs confer resistance to several kinds of stresses such as heat, cold, salinity, ethanol, acid, and chemicals (Carra et al. 2017; Waters and Vierling 2020; Ma et al. 2021; Tian et al. 2012). sHSPs sequester denatured proteins in an ATP-independent manner (Jakob et al. 1993; Rodriguez Ospina et al. 2022) and thus could be considered more as “sequestrases” than “holdases” (Haslbeck and Vierling 2015; Haslbeck et al. 2016; Mogk et al. 2019; Reinle et al. 2022). To refold the misfolded protein, oligomeric sHSPs must collaborate with the Hsp70/100 complex (Veinger et al. 1998). The chaperone Hsp70 binds to the denatured sHSP-protein assemblies, allowing the recruitment of Hsp100 to extract and refold these proteins properly (Żwirowski et al. 2017). The same mechanisms have been observed both on eukaryotes such as Saccharomyces cerevisiae (Glover and Lindquist 1998; Cashikar et al. 2005), on bacteria like E. coli (Mogk et al. 2003), and cyanobacteria such as Synechocystis (Giese et al. 2005).
In addition to their role of molecular chaperone, the literature suggests a growing number of roles fulfilled by sHSPs. They have been described in some microorganisms as having an impact on virulence (Obuchowski and Liberek 2020), cyst and spore formation (Henriques et al. 1997; Cocotl-Yañez et al. 2014; Jee et al. 2018), dormancy (Yuan et al. 1997, Cunningham and Spreadbury 1998), cell cycle regulation (Kim et al. 2011), immune system modulation (Longo et al. 2020), and biofilm formation (Yeh et al. 1997; Zara et al. 2002; Diaz et al. 2006; Kuczynska-Wisnik et al. 2010; Pidot et al. 2010). Moreover, another role has been attributed to certain sHSPs corresponding to the capacity of maintaining cell membrane integrity. This new activity has given rise to the concept of sHSPs as lipochaperones (Maitre et al. 2014) and will be described more precisely in the next chapter.
Zoom on sHSP lipochaperone activity
The maintenance of the optimal state of fluidity of the cellular membrane is crucial to withstand stressful conditions. Organisms cannot synthetize the lipid bilayer de novo but can maintain a continuous process of lipid production and modification (Obuchowski and Liberek 2020). However, these two processes take time and are not sufficient at the beginning of stress to limit changes in the fluidity and/or structure of the membrane microdomains. These changes may act as a sensor perturbation, leading to the expression of sHSP that can exert their chaperon and eventually lipochaperone roles (Glatz et al. 2015, Vigh et al. 2007; Maitre et al. 2012). This second activity seems to be temporally favored, suggesting the importance to prioritize the regulation of membrane fluidity as demonstrated for Lo18 from O. oeni (Maitre et al. 2014). Currently, little is known about this lipochaperone activity; however, the few sHSPs described for it are present in model organisms representative of all kingdoms of life. Although they are known to interact with lipids (Table 1) (Coucheney et al. 2005; Balogi et al. 2005; Kim et al. 2011), the mechanism of interaction remains poorly understood. Among the knowledge, a distinction can be made between sHSPs described as interacting with the membrane and those that have a real role in maintaining membrane fluidity. Immunolabeling techniques allow to observe the interaction between sHSP 17.8 of Arabidopsis thaliana with the chloroplast membrane (Kim et al. 2011), human HSPB1 and HPB5 with the mitochondrial membrane system and lipid rafts (De Maio and Hightower 2020), HSPA from Synechococcus sp. with the thylakoid membrane (Nitta et al. 2005; Obuchowski and Liberek 2020), and HSP16.3 from Mycobacterium tuberculosis with positively charged lipid bilayers of M. tuberculosis (Sun and MacRae 2005; Zhang et al. 2005). Wilhelmy’s method showed that the interaction between the two sHSPs of Schizosaccharomyces pombe, HSP15.8 and HSP16, and different isolated lipids extracted from its membrane was sHsp-dose-dependent (Glatz et al. 2015).
Table 1.
sHSPs described for their lipochaperone activity in model organisms
| Organism | sHSP | References | |
|---|---|---|---|
| Human | Homo sapiens | HSPB5 | De Maio et al. (2019) |
| HSPB1 | |||
| Plant | Arabidopsis thaliana | HSP17.8 | Balogi et al. (2008), Kim et al. (2011) |
| Daucus carota | HSP17.7 | Ahn and Zimmerman (2006), Balogi et al. (2008) | |
| Yeats | Schizosaccharomyces pombe | Hsp15.8 | Glatz et al. (2015) |
| Hsp16 | |||
| Bacteria | Lactiplantibacillus plantarum | Hsp18.55 | Capozzi et al. (2011) |
| Oenococcus oeni | Lo18 | Coucheney et al. (2005), Maitre et al. (2012, 2014), Weidmann et al. (2010, 2017) | |
| Synechocystis sp. | Hsp17 | Balogi et al. (2008), Tsvetkova et al. (2002), De Maio and Hightower (2020) | |
| Thermosynechococcus vulcanus | HspA | Roy et al. (1999) | |
| Synechococcus sp. | HspA | Nitta et al. (2005), Obuchowski and Liberek (2020) | |
| Mycobacterium tuberculosis | HSP16.3 | Lini et al. (2008); Sun and MacRae (2005); Zhang et al. (2005) |
Lipochaperone activity was observed in vivo and in vitro by fluorescence anisotropy measurements (reflecting the fluidity state of lipid bilayers) on Lo18 of O. oeni (Coucheney et al 2005; Maitre et al. 2012 and 2014; Weidmann et al. 2010 and 2017) and sHSP 18.55 from Lactiplantibacillus plantarum interacting with the charged membrane after ethanol stress and severe heat stress (> 48 °C) (Capozzi et al. 2011). Differential scanning calorimetry showed that sHSP17 from Synechocystis sp. can increase the physical order of the membrane to stabilize lipid membrane (Tsvetkova et al. 2002; De Maio and Hightower 2020) in response to heat stress (Balogi et al. 2008; Sun and MacRae 2005). Finally, electrolyte leakage analysis was performed to measure cell membrane stability at elevated temperature on sHSP17.7 of Daucus carota (Ahn and Zimmerman 2006).
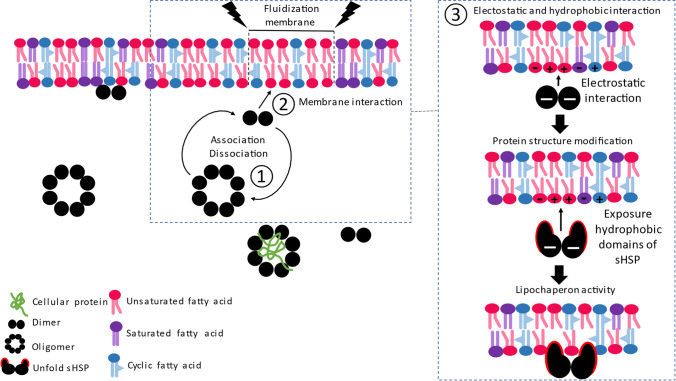
Although the interaction mechanism is certainly lipid/sHSP dependent, other characteristics are also fundamental (Fig. 3):
Fig. 3.
Mechanism of interaction described between sHSP and the membrane. (1) Dissociation of the sHSP oligomer near the membrane allows lipid-protein interaction. (2) Interaction with the polar head of phospholipids according to their structure and state of fluidization (Tsvetkova et al. 2002; Maitre et al. 2012). (3) Electrostatic force, occurring during the disassembly process, can attract the protein to the membrane and enhance hydrophobic force. This electrostatic force may expose hydrophobic domains of sHSP leading to protein structure modification (Zhang et al. 2005) which can then bind to the membrane (Chen et al. 2003; Tsvetkova et al. 2002)
Association dissociation
The dissociation of the sHSP oligomer is a prerequisite for lipid binding. The dynamics of oligomerization (association-dissociation) seems to be very important for all the sHSPs described above. Indeed, the loss of this equilibrium between dissociation and association leads to the inability of sHSPs to interact with the membrane (Chen et al. 2003; Balogi et al. 2008; Maitre et al. 2014; Zeng et al. 2015). Balogi and colleagues suggest that the dissociation of the oligomer into the dimeric structural unit near the membrane will allow their uptake by the membrane (Balogi et al. 2008), certainly due to the exposure of certain protein domains initially hidden in the oligomer (Zeng et al. 2015). It is interesting to note that the dissociation of oligomers appears to occur, in vitro, in a temperature range of 40 to 50 °C. For example, this dissociation happens at 43 °C for HSP26 from S. cerevisiae and 40 °C for sHSP 16.9 from wheat (Chen et al. 2003), temperature range similar to that of membrane fluidization.
Specificity of sHsp-membrane interaction
The reversible interaction between sHSPs and membrane lipids is very specific and depends, on the one hand, on the sHSP, and on the other hand on the fluidity state of the membrane due to the degree of unsaturation of the fatty acids and the nature of the polar heads (Balogi et al. 2008; Csoboz et al. 2022). These interactions prevent the formation of hyperfluidic states by regulating the lipid polymorphism of the membrane during phospholipid disorganization (Glatz et al. 2015; Tsvetkova et al. 2002; Coucheney et al. 2005). Phospholipid composition seems to be a key determinant of sHSP-membrane interaction. Indeed, the lipid bilayer is not just a hydrophobic wall, but a highly dynamic structure with a natural movement of phospholipids, making the membrane a very fluid environment (De Maio et al. 2019). For example, Lo18 from O. oeni seems to interact better with a mix of saturated, unsaturated, and cyclic fatty acid than a highly rigid membrane with a high content of saturated fatty acids (Maitre et al. 2012 and 2014). HSP15.8 from S. pombe has a higher affinity for monolayers (prepared in the Langmuir balance system) but interacts in an equivalent manner for all the major phospholipid groups comprising polar heads such as inositol, ethanolamine, and glycerol. In contrast, the second sHSP of S. pombe, HSP16, is more specific since it has a greater affinity for glycerol but less so for inositol (Glatz et al. 2015). Moreover, infrared spectroscopy performed on HSP16.3 of M. tuberculosis indicates that interaction is mediated via the head group of phospholipids and the hydrophobic part of the protein (Chen et al. 2003), as was also demonstrated for Lo18 of O. oeni by measuring the anisotropy fluorescence of liposomes (Maitre et al. 2012).
Electrostatic and hydrophobic interactions
Electrostatic force is an important element in these interactions which may occur during the disassembly process. All the sHSPs described display a negative charge in neutral buffer solution and mainly interact with positively charged membranes (Capozzi et al. 2011, Glatz et al. 2015, Sun and MacRae 2005). However, there are also hydrophobic interactions between sHSPs and the lipid bilayer that should not be neglected. Indeed, from the protein side, electrostatic interactions generate a slight conformational change in sHSP, exposing hydrophobic zones (Chen et al. 2003; Tsvetkova et al. 2002). Then, the flexibility and dynamism of M. tuberculosis HSP16.3 increases in the presence of Mycobacterium membrane lipids (Zhang et al. 2005). From the membrane side, fluidization creates a hydrophobic docking surface on the cell that allows the binding of sHSP, as described for the human HSPB1 (Csoboz et al. 2022).
Analysis of primary structure: the beginning of understanding
Although the primary sequence of sHSPs is poorly conserved, the protein structure (Poulain et al. 2010) and the primordial activities of these proteins are rather homogeneous (Jong et al. 1998). This suggests that certain motifs remain highly conserved between sHSPs (Jong et al. 1998), such as those of the α-crystallin domain (Morimoto and Santoro 1998).
Previous studies have identified specific motifs containing key amino acids for the structure and/or chaperone activity of sHSP (van Montfort et al. 2001; Mao and Chang 2001; Lentze et al. 2003; Weidmann et al. 2010). Lentze and colleagues (2003) have developed a library of HspH proteins from B. japonicum modified by site-directed mutagenesis on highly conserved amino acids identified by multiple alignment of 12 sHSPs from different organisms (plants, animals, bacteria). The majority of the modified proteins were altered in their quaternary structure (total loss or alteration) and/or their chaperone activity. The two modified proteins G74E and F94A/D (mutated at glycine and phenylalanine respectively at position 74 and 94) are able to form only dimeric structures, resulting in a loss of chaperone activity. The same findings were reported for the wheat protein Hsp16.9 modified for the same residues (G83 and F106) (van Montfort et al. 2001). A reduction in oligomer size is observed for the modified HspH proteins L100A/A109S/L116A leading to a reduction or abolition of chaperone activity. The same mutation in Hsp16.3 of M. tuberculosis (L122) or in Lo18 of O. oeni (A123S) results in the formation of unstable or small complexes with low chaperone activity, respectively (Mao and Chang 2001; Weidmann et al. 2010). Finally, the loss of chaperone activity for the modified HspH protein G114A is observed despite the maintenance of quaternary structures suggesting an altered substrate interaction site.
All these works show that some residues, highly conserved on the sHSP primary sequence, are essential for establishing chaperone activity. In line with these studies, it seems very relevant to use a similar method to identify conserved motifs among sHSPs having the additional lipochaperone activity.
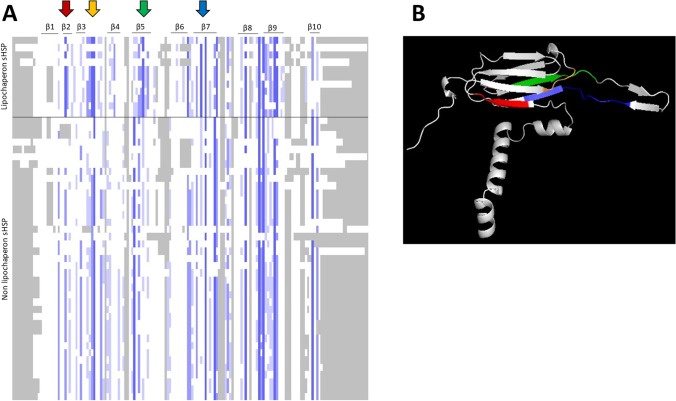
Twelve sequences of sHSPs (from human, plants, yeast, and bacteria) described as having lipochaperone activity (Table 1) were compared with 38 protein sequences of the sHSPs available in the literature, also representative of the different kingdoms (Fig. 4). However, in this second group, no lipochaperone activity is described, which does not exclude that some proteins may have it without being identified. Sequence alignment performed by ClustalW algorithms using Jalview 2.11.2.5 software between these two groups (described or not described for lipochaperone activity) shows the presence of four highly conserved amino acid motifs for the “lipochaperone activity” group (Fig. 4): “DKKET” on the β2, “ELPG” upstream of the β4 strand, the “LTISGKRE” on the β5, and “RSERSYGSFR” on the β7. Among these motifs, some amino acids are particularly conserved such as respectively glutamic acid (E), leucine (L), glycine (G), and phenylalanine (F) for each motif that are present in 77.3% of the cases for the sHSP of the lipochaperone group versus 50% for the other sHSPs (p value 0.042).
Fig. 4.
A sHSP primary sequence alignment by ClustalW algorithms using Jalview 2.11.2.5 software. Comparison of the alignments of 12 primary sHSP sequences described for their lipochaperone activity named “lipochaperone sHSP” with 38 primary sHSP sequences not described for this role named “Non lipochaperone sHSP.” Highly conserved (dark blue), conserved (blue), non-conserved (white), and unaligned (gray) residues are shown. Four domains, present on the β2 sheet (red arrow), before the β4 sheet (yellow arrow), β5 sheet (green arrow), and β7 sheet (blue arrow) respectively are more conserved on the lipochaperone sHSP. B Location of the four particularly conserved domains on a 3D predicted representation of an O. oeni sHSP Lo18 monomer
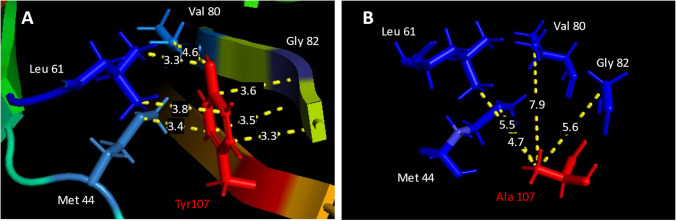
Almost no studies have investigated the primary structure for lipochaperone activity. Only the work on the Lo18 protein of O. oeni shows the importance of the second aromatic residue present on the last motif, “RSERSYGSFR” on β7 (Weidmann et al. 2010 and 2017; Maitre et al. 2012). Anisotropy measurements performed on the modified Lo18 protein Y107A (aromatic tyrosine replaced by alanine) showed its inability to prevent fluidization upon heat shock (Weidmann et al. 2010). Using a predicted model of Lo18 (obtained by trRosetta and confirmed by Alphafold 2), the aromatic ring of this tyrosine could act as a tenon between the two planes of the α-crystallin domain of Lo18, by interacting with adjacent amino acids present on different β sheets (Fig. 5A). The loss of the aromatic ring (transforming tyrosine into alanine) will change these interaction distances (Fig. 5A and B). In addition, it is interesting to note that three of them are present on highly conserved amino acid motifs described above (yellow and blue arrow, Fig. 4).
Fig. 5.
Amino acid interaction distances in O. oeni sHSPf Lo18. A Interaction between the aromatic ring of tyrosine at position 107 (red) and its adjacent amino acids (blue) within an average radius of 3.6 Å or B interaction of the alanine at position 107 from the tyrosine mutation (red) with adjacent amino acids (blue) within an average radius of 5.925 Å. The loss of the aromatic ring alters the interaction distances with other amino acids
Nevertheless, other sHSPs, not yet studied for lipochaperone activity, have shown that some amino acids of these motifs are important for their oligomeric structure. This is the case, for example, for HSP 16.5 from M. jannaschii modified for the second arginine present on the β7 sheet or for sHsP 16.9 from wheat described above (Jong et al. 1998; van Montfort et al. 2001).
It seems possible that these highly conserved amino acid motifs may play a role in the structure of sHSP, possibly allowing them to interact with the membrane. These results highlight the importance to study the lipochaperone activity in all sHSPs having these different motifs. Priority could be given to bacterial sHSPs, as they are more sensitive to point mutations, thanks to their structural rigidity (Studer et al. 2002; Lentze et al. 2003). They could be excellent candidates for in vitro and in vivo analysis of these four amino acid motifs.
Conclusion
To conclude, it appears that sHSPs can interact with lipids via certain amino acid residues present on the β2, inter β3/4, B5, and β7 strands. These residues could have an important role (i) in the structure and dynamics of oligomerization and (ii) for the interaction and maintenance of optimal cell membrane fluidity. To better understand the overall mechanisms allowing this sHSPs—cell membranes interaction, new experiments should focus on this activity and the highly conserved amino acid motifs described above.
sHSP deficiency may be involved in several human pathologies such as neurodegenerative diseases or familial exudative vitreoretinopathy. Indeed, this last pathology may be caused by the inability of human α-crystallin to interact with the retinal cell membrane suggesting a lipochaperone activity (Bakthisaran et al. 2015; Toth et al. 2015). Finally, the ability of sHSPs to protect membranes could be beneficial for probiotics or ferment production, especially for lactic acid bacteria. Indeed, this would allow them to resist production processes such as freeze-drying, which is known to induce a deleterious modification of the membrane fluidity of microorganisms (Arena et al. 2019). This has already been described for the Hsp18.55 of L. plantarum which induces cryotolerance by acting as an antifreeze protein (Arena et al. 2019).
It appears that understanding the lipochaperone activity of sHSPs could become an important area of research in the next decades.
Acknowledgements
We would like to thank Accent Europe company for their reading of the English text.
Author contribution
Conceptualization, TB and SW; methodology, TB and SW; formal analysis, TB and SW; investigation, TB and SW; resources, TB and SW; writing—original draft preparation, TB; writing—review and editing, TB and SW; visualization, TB and SW; supervision, SW; project administration, SW; funding acquisition, SW. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conseil Régional de Bourgogne, the Université de Bourgogne grant number 2021Y-09559, and the ministère de l’Enseignement supérieur de la Recherche, grant number MESR 2020–04.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tiffany Bellanger, Email: tiffany.bellanger@u-bourgogne.fr.
Stéphanie Weidmann, Email: stephanie.desroche@u-bourgogne.fr.
References
- Ahn YJ, Zimmerman JL. Introduction of the carrot HSP17.7 into potato (Solanum tuberosum L.) enhances cellular membrane stability and tuberization in vitro. Plant, Cell and Environment. 2006;29(1):95–104. doi: 10.1111/j.1365-3040.2005.01403.x. [DOI] [PubMed] [Google Scholar]
- Arena MP, Capozzi V, Longo A, Russo P, Weidmann S, Rieu A, Guzzo J, Spano G, Fiocco D (2019) The phenotypic analysis of Lactobacillus plantarum sHSP mutants reveals a potential role for HSP1 in cryotolerance. Front Microbiol, 10. 10.3389/fmicb.2019.00838 [DOI] [PMC free article] [PubMed]
- Augusteyn RC. α-crystallin: a review of its structure and function. Clin Exp Optom. 2004;87(6):356–366. doi: 10.1111/j.1444-0938.2004.tb03095.x. [DOI] [PubMed] [Google Scholar]
- Bakthisaran R, Tangirala R, Rao C. Small heat shock proteins: role in cellular functions and pathology. Biochimica et biophysica acta. 2015;1854(4):291–319. doi: 10.1016/j.bbapap.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Balogi Z, Török Z, Balogh G, Jósvay K, Shigapova N, Vierling E, Vígh L, Horváth I. “Heat shock lipid” in cyanobacteria during heat/light-acclimation. Arch Biochem Biophys. 2005;436(2):346–354. doi: 10.1016/j.abb.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Balogi Z, Cheregi O, Giese KC, Juhász K, Vierling E, Vass I, Vígh L, Horváth I. A mutant small heat shock protein with increased thylakoid association provides an elevated resistance against UV-B damage in synechocystis 6803. J Biol Chem. 2008;283(34):22983–22991. doi: 10.1074/jbc.m710400200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baneyx F, Mujacic M. Recombinant protein folding and misfolding in Escherichia coli. Nat Biotechnol. 2004;22(11):1399–1408. doi: 10.1038/nbt1029. [DOI] [PubMed] [Google Scholar]
- Basha E, O’Neill H, Vierling E. Small heat shock proteins and α-crystallins: dynamic proteins with flexible functions. Trends Biochem Sci. 2012;37(3):106–117. doi: 10.1016/j.tibs.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissinger M, Buchner J. How chaperones fold proteins. Biol Chem. 1998;379(3):245–259. [PubMed] [Google Scholar]
- Berger F, Morellet N, Menu F, Potier P. Cold shock and cold acclimation proteins in the psychrotrophic bacterium Arthrobacter globiformis SI55. J Bacteriol. 1996;178(11):2999–3007. doi: 10.1128/jb.178.11.2999-3007.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari S, Biswas S, Chaudhary A, Dutta S, Suguna K. Dodecameric structure of a small heat shock protein from Mycobacterium marinum M. Proteins: structure, function, and bioinformatics. 2019;87(5):365–379. doi: 10.1002/prot.25657. [DOI] [PubMed] [Google Scholar]
- Boelens WC. Cell biological roles of αB-crystallin. Prog Biophys Mol Biol. 2014;115(1):3–10. doi: 10.1016/j.pbiomolbio.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Boelens WC. Structural aspects of the human small heat shock proteins related to their functional activities. Cell Stress Chaperones. 2020;25(4):581–591. doi: 10.1007/s12192-020-01093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth IR. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985;49(4):359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle D, Takemoto L. Immunolocalization of the C-terminal and N-terminal regions of alpha-A and alpha-B crystallins. Curr Eye Res. 1994;13(7):497–504. doi: 10.3109/02713689408999881. [DOI] [PubMed] [Google Scholar]
- Bozaykut P, Ozer NK, Karademir B. Regulation of protein turnover by heat shock proteins. Free Radical Biol Med. 2014;77:195–209. doi: 10.1016/j.freeradbiomed.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92(3):351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125(3):443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Capozzi V, Fiocco D, Weidmann S, Guzzo J, Spano G. Increasing membrane protection in Lactobacillus plantarum cells overproducing small heat shock proteins. Annals of Microbiology. 2011;62(2):517–522. doi: 10.1007/s13213-011-0285-7. [DOI] [Google Scholar]
- Carra S, Alberti S, Arrigo PA, Benesch JL, Benjamin IJ, Boelens W, Bartelt-Kirbach B, Brundel BJJM, Buchner J, Bukau B, Carver JA, Ecroyd H, Emanuelsson C, Finet S, Golenhofen N, Goloubinoff P, Gusev N, Haslbeck M, Hightower LE, Tanguay RM. The growing world of small heat shock proteins: from structure to functions. Cell Stress Chaperones. 2017;22(4):601–611. doi: 10.1007/s12192-017-0787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashikar AG, Duennwald M, Lindquist SL. A chaperone pathway in protein disaggregation. J Biol Chem. 2005;280(25):23869–23875. doi: 10.1074/jbc.m502854200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers GJ, Leunissen JAM, de Jong WW. The expanding small heat-shock protein family, and structure predictions of the conserved “α-crystallin domain”. J Mol Evol. 1995;40(3):238–248. doi: 10.1007/bf00163229. [DOI] [PubMed] [Google Scholar]
- Chang Z, Primm TP, Jakana J, Lee IH, Serysheva I, Chiu W, Gilbert HF, Quiocho FA. Mycobacterium tuberculosis 16-kDa Antigen (Hsp16.3) functions as an oligomeric structure in vitro to suppress thermal aggregation. J Biol Chem. 1996;271(12):7218–7223. doi: 10.1074/jbc.271.12.7218. [DOI] [PubMed] [Google Scholar]
- Chang HC, Tang YC, Hayer-Hartl M, Hartl FU. SnapShot: molecular chaperones, part I. Cell. 2007;128(1):212.e1–212.e2. doi: 10.1016/j.cell.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lu YJ, Wang HW, Quan S, Chang Z, Sui SF. Two-dimensional crystallization of a small heat shock protein HSP16.3 on lipid layer. Biochem Biophys Res Commun. 2003;310(2):360–366. doi: 10.1016/j.bbrc.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Chen J, Feige MJ, Franzmann TM, Bepperling A, Buchner J. Regions outside the α-crystallin domain of the small heat shock protein hsp26 are required for its dimerization. J Mol Biol. 2010;398(1):122–131. doi: 10.1016/j.jmb.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Cocotl-Yañez M, Moreno S, Encarnación S, López-Pliego L, Castañeda M, Espín G. A small heat-shock protein (Hsp20) regulated by RpoS is essential for cyst desiccation resistance in Azotobacter vinelandii. Microbiology. 2014;160(3):479–487. doi: 10.1099/mic.0.073353-0. [DOI] [PubMed] [Google Scholar]
- Coucheney F, Gal L, Beney L, Lherminier J, Gervais P, Guzzo J. A small HSP, Lo18, interacts with the cell membrane and modulates lipid physical state under heat shock conditions in a lactic acid bacterium. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2005;1720(1–2):92–98. doi: 10.1016/j.bbamem.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Csoboz B, Gombos I, Kóta Z, Dukic B, Klement É, Varga-Zsíros V, Lipinszki Z, Páli T, Vígh L, Török Z. The small heat shock protein, HSPB1, interacts with and modulates the physical structure of membranes. Int J Mol Sci. 2022;23(13):7317. doi: 10.3390/ijms23137317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AF, Spreadbury CL. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton α-crystallin homolog. J Bacteriol. 1998;180(4):801–808. doi: 10.1128/jb.180.4.801-808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong WW, Leunissen JA, Voorter CE. Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol. 1993 doi: 10.1093/oxfordjournals.molbev.a039992. [DOI] [PubMed] [Google Scholar]
- de Jong WW, Caspers GJ, Leunissen JAM. Genealogy of the α-crystallin—small heat-shock protein superfamily. Int J Biol Macromol. 1998;22(3–4):151–162. doi: 10.1016/s0141-8130(98)00013-0. [DOI] [PubMed] [Google Scholar]
- De Maio A, Hightower LE. Heat shock proteins and the biogenesis of cellular membranes. Cell Stress Chaperones. 2020;26(1):15–18. doi: 10.1007/s12192-020-01173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maio A, Cauvi DM, Capone R, Bello I, Egberts WV, Arispe N, Boelens W. The small heat shock proteins, HSPB1 and HSPB5, interact differently with lipid membranes. Cell Stress Chaperones. 2019;24(5):947–956. doi: 10.1007/s12192-019-01021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbecq SP, Klevit RE. One size does not fit all: the oligomeric states of αB crystallin. FEBS Lett. 2013;587(8):1073–1080. doi: 10.1016/j.febslet.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbecq SP, Rosenbaum JC, Klevit RE. A mechanism of subunit recruitment in human small heat shock protein oligomers. Biochemistry. 2015;54(28):4276–4284. doi: 10.1021/acs.biochem.5b00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz D, Döbeli H, Yeboah-Manu D, Mensah-Quainoo E, Friedlein A, Soder N, Rondini S, Bodmer T, Pluschke G. Use of the immunodominant 18-kilodalton small heat shock protein as a serological marker for exposure to Mycobacterium ulcerans. Clin Vaccine Immunol. 2006;13(12):1314–1321. doi: 10.1128/cvi.00254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ. The molecular chaperone concept. Seminars in cell biology. 1990;1(1):1–9. [PubMed] [Google Scholar]
- Ellis RJ, van der Vies SM, Hemmingsen SM. The molecular chaperone concept. Biochemical Society symposium. 1989;55:145–153. [PubMed] [Google Scholar]
- Fu MS, De Sordi L, Mühlschlegel FA (2012) Functional characterization of the small heat shock protein Hsp12p from Candida albicans. PLoS ONE 7(8):e42894. 10.1371/journal.pone.0042894 [DOI] [PMC free article] [PubMed]
- Fu X, Jiao W, Chang Z. Phylogenetic and biochemical studies reveal a potential evolutionary origin of small heat shock proteins of animals from bacterial class A. J Mol Evol. 2006;62(3):257–266. doi: 10.1007/s00239-005-0076-5. [DOI] [PubMed] [Google Scholar]
- Giese KC, Basha E, Catague BY, Vierling E. Evidence for an essential function of the N terminus of a small heat shock protein in vivo, independent of in vitro chaperone activity. Proc Natl Acad Sci. 2005;102(52):18896–18901. doi: 10.1073/pnas.0506169103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz A, Pilbat AM, Németh GL, Vince-Kontár K, Jósvay K, Hunya Á, Udvardy A, Gombos I, Péter M, Balogh G, Horváth I, Vígh L, Török Z. Involvement of small heat shock proteins, trehalose, and lipids in the thermal stress management in Schizosaccharomyces pombe. Cell Stress Chaperones. 2015;21(2):327–338. doi: 10.1007/s12192-015-0662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40. Cell. 1998;94(1):73–82. doi: 10.1016/s0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- Guzzo J, Delmas F, Pierre F, Jobin M, Samyn B, Van Beeumen J, Cavin J, Diviès C. A small heat shock protein from Leuconostoc oenos induced by multiple stresses and during stationary growth phase. Lett Appl Microbiol. 1997;24(5):393–396. doi: 10.1046/j.1472-765x.1997.00042.x. [DOI] [PubMed] [Google Scholar]
- Han MJ, Yun H, Lee SY. Microbial small heat shock proteins and their use in biotechnology. Biotechnol Adv. 2008;26(6):591–609. doi: 10.1016/j.biotechadv.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381(6583):571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Miess A, Stromer T, Walter S, Buchner J. Disassembling protein aggregates in the yeast cytosol. J Biol Chem. 2005;280(25):23861–23868. doi: 10.1074/jbc.m502697200. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Peschek J, Buchner J, Weinkauf S (2016) Structure and function of α-crystallins: traversing from in vitro to in vivo. Biochim Biophys Acta Gen Subj 1860(1):149–166. 10.1016/j.bbagen.2015.06.008 [DOI] [PubMed]
- Haslbeck M, Vierling E. A first line of stress defense: small heat shock proteins and their function in protein homeostasis. J Mol Biol. 2015;427(7):1537–1548. doi: 10.1016/j.jmb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques AO, Beall BW, Moran CP. CotM of Bacillus subtilis, a member of the alpha-crystallin family of stress proteins, is induced during development and participates in spore outer coat formation. J Bacteriol. 1997;179(6):1887–1897. doi: 10.1128/jb.179.6.1887-1897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton GR, Benesch JLP. Two decades of studying non-covalent biomolecular assemblies by means of electrospray ionization mass spectrometry. J R Soc Interface. 2012;9(70):801–816. doi: 10.1098/rsif.2011.0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton GR, Hochberg GKA, Laganowsky A, McGinnigle SI, Baldwin AJ, Benesch JLP. C-terminal interactions mediate the quaternary dynamics of αB-crystallin. Phil Trans R Society b: Biol Sci. 2013;368(1617):20110405. doi: 10.1098/rstb.2011.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J (1993) Proctor lecture. The function of alpha-crystallin. Investigative ophthalmology & visual science 34(1):10–22 [PubMed]
- Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76(2):145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- Ingolia TD, Craig EA. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci. 1982;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268(3):1517–1520. doi: 10.1016/s0021-9258(18)53882-5. [DOI] [PubMed] [Google Scholar]
- Jee B, Singh Y, Yadav R, Lang F. small Heat Shock Protein16.3 of Mycobacterium tuberculosis: after two decades of functional characterization. Cell Physiol Biochem. 2018;49(1):368–380. doi: 10.1159/000492887. [DOI] [PubMed] [Google Scholar]
- Junprung W, Supungul P, Tassanakajon A. Structure, gene expression, and putative functions of crustacean heat shock proteins in innate immunity. Dev Comp Immunol. 2021;115:103875. doi: 10.1016/j.dci.2020.103875. [DOI] [PubMed] [Google Scholar]
- Kappé G, Leunissen, JAM, de Jong WW (2002).Evolution and diversity of prokaryotic small heat shock proteins. In Small Stress Proteins (pp. 1–17). Springer Berlin Heidelberg. 10.1007/978-3-642-56348-5_1 [DOI] [PubMed]
- Kim R, Kim KK, Yokota H, Kim SH. Small heat shock protein of Methanococcus jannaschii, a hyperthermophile. Proc Natl Acad Sci. 1998;95(16):9129–9133. doi: 10.1073/pnas.95.16.9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Xu ZY, Na YJ, Yoo YJ, Lee J, Sohn EJ, Hwang I. Small heat shock protein hsp17.8 functions as an AKR2A cofactor in the targeting of chloroplast outer membrane proteins in arabidopsis. Plant Physiology. 2011;157(1):132–146. doi: 10.1104/pp.111.178681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaips CL, Jayaraj GG, Hartl FU. Pathways of cellular proteostasis in aging and disease. J Cell Biol. 2017;217(1):51–63. doi: 10.1083/jcb.201709072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevit RE. Peeking from behind the veil of enigma: emerging insights on small heat shock protein structure and function. Cell Stress Chaperones. 2020;25(4):573–580. doi: 10.1007/s12192-020-01092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriehuber T, Rattei T, Weinmaier T, Bepperling A, Haslbeck M, Buchner J. Independent evolution of the core domain and its flanking sequences in small heat shock proteins. FASEB J. 2010;24(10):3633–3642. doi: 10.1096/fj.10-156992. [DOI] [PubMed] [Google Scholar]
- Kuczyńska-Wiśnik D, Matuszewska E, Laskowska E. Escherichia coli heat-shock proteins IbpA and IbpB affect biofilm formation by influencing the level of extracellular indole. Microbiology. 2010;156(1):148–157. doi: 10.1099/mic.0.032334-0. [DOI] [PubMed] [Google Scholar]
- Lelj-Garolla B, Mauk AG. Roles of the N- and C-terminal sequences in Hsp27 self-association and chaperone activity. Protein Sci. 2011;21(1):122–133. doi: 10.1002/pro.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentze N, Narberhaus F. Detection of oligomerisation and substrate recognition sites of small heat shock proteins by peptide arrays. Biochem Biophys Res Commun. 2004;325(2):401–407. doi: 10.1016/j.bbrc.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Lentze N, Studer S, Narberhaus F. Structural and functional defects caused by point mutations in the α-crystallin domain of a bacterial α-heat shock protein. J Mol Biol. 2003;328(4):927–937. doi: 10.1016/s0022-2836(03)00356-5. [DOI] [PubMed] [Google Scholar]
- Lentze N, Aquilina JA, Lindbauer M, Robinson CV, Narberhaus F. Temperature and concentration-controlled dynamics of rhizobial small heat shock proteins. Eur J Biochem. 2004;271(12):2494–2503. doi: 10.1111/j.1432-1033.2004.04180.x. [DOI] [PubMed] [Google Scholar]
- Lim J, Thomas T, Cavicchioli R. Low temperature regulated DEAD-box RNA helicase from the Antarctic archaeon, Methanococcoides burtonii. J Mol Biol. 2000;297(3):553–567. doi: 10.1006/jmbi.2000.3585. [DOI] [PubMed] [Google Scholar]
- Lini N, Rehna EA, Shiburaj S, Maheshwari JJ, Shankernarayan NP, Dharmalingam K. Functional characterization of a small heat shock protein from Mycobacterium leprae. BMC Microbiol. 2008;8:208. doi: 10.1186/1471-2180-8-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo A, Russo P, Capozzi V, Spano G, Fiocco D. Knock out of sHSP genes determines some modifications in the probiotic attitude of Lactiplantibacillus plantarum. Biotech Lett. 2020;43(3):645–654. doi: 10.1007/s10529-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma P, Li J, Qi L, Dong X. The archaeal small heat shock protein hsp17.6 protects proteins from oxidative inactivation. Int J Mol Sci. 2021;22(5):2591. doi: 10.3390/ijms22052591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaroufi H, Tanguay RM. Analysis and phylogeny of small heat shock proteins from marine viruses and their cyanobacteria host. PLoS ONE. 2013;8(11):e81207. doi: 10.1371/journal.pone.0081207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae TH. Structure and function of small heat shock/α-crystallin proteins: established concepts and emerging ideas. Cell Mol Life Sci. 2000;57(6):899–913. doi: 10.1007/pl00000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainz A, Peschek J, Stavropoulou M, Back KC, Bardiaux B, Asami S, Prade E, Peters C, Weinkauf S, Buchner J, Reif B. The chaperone αB-crystallin uses different interfaces to capture an amorphous and an amyloid client. Nat Struct Mol Biol. 2015;22(11):898–905. doi: 10.1038/nsmb.3108. [DOI] [PubMed] [Google Scholar]
- Maitre M, Weidmann S, Rieu A, Fenel D, Schoehn G, Ebel C, Coves J, Guzzo J. The oligomer plasticity of the small heat-shock protein Lo18 from Oenococcus oeni influences its role in both membrane stabilization and protein protection. Biochemical Journal. 2012;444(1):97–104. doi: 10.1042/bj20120066. [DOI] [PubMed] [Google Scholar]
- Maitre M, Weidmann S, Dubois-Brissonnet F, David V, Covès J, Guzzo J. Adaptation of the wine bacterium Oenococcus oeni to ethanol stress: role of the small heat shock protein lo18 in membrane integrity. Appl Environ Microbiol. 2014;80(10):2973–2980. doi: 10.1128/aem.04178-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Q, Chang Z. Site-Directed mutation on the only universally conserved residue leu122 of small heat shock protein hsp16.3. Biochem Biophys Res Commun. 2001;289(5):1257–1261. doi: 10.1006/bbrc.2001.6062. [DOI] [PubMed] [Google Scholar]
- Marcion G, Seigneuric R, Chavanne E, Artur Y, Briand L, Hadi T, Gobbo J, Garrido C, Neiers F. C-terminal amino acids are essential for human heat shock protein 70 dimerization. Cell Stress Chaperones. 2014;20(1):61–72. doi: 10.1007/s12192-014-0526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogk A, Deuerling E, Vorderwülbecke S, Vierling E, Bukau B. Small heat shock proteins, ClpB and the DnaK system form a functional triade in reversing protein aggregation. Molecular microbiology. 2003;50(2):585–595. doi: 10.1046/j.1365-2958.2003.03710.x. [DOI] [PubMed] [Google Scholar]
- Mogk A, Ruger-Herreros C, Bukau B. Cellular functions and mechanisms of action of small heat shock proteins. Annu Rev Microbiol. 2019;73(1):89–110. doi: 10.1146/annurev-micro-020518-115515. [DOI] [PubMed] [Google Scholar]
- Morimoto R, Santoro M. Stress–inducible responses and heat shock proteins: New pharmacologic targets forcytoprotection. Nat Biotechnol. 1998;16:833–838. doi: 10.1038/nbt0998-833. [DOI] [PubMed] [Google Scholar]
- Mörner CT. (1894) Untersuchen der Proteinsubstanzen in licht-brechenden Medien des Auges. Hoppe-Seyler’s Z Physiol Chem. 1894;18:61–106. [Google Scholar]
- Münchbach M, Nocker A, Narberhaus F. Multiple small heat shock proteins in rhizobia. Journal of bacteriology. 1999;181(1):83–90. doi: 10.1128/JB.181.1.83-90.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narberhaus F. α-crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol Mol Biol Rev. 2002;66(1):64–93. doi: 10.1128/mmbr.66.1.64-93.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta K, Suzuki N, Honma D, Kaneko Y, Nakamoto H. Ultrastructural stability under high temperature or intensive light stress conferred by a small heat shock protein in cyanobacteria. FEBS Lett. 2005;579(5):1235–1242. doi: 10.1016/j.febslet.2004.12.095. [DOI] [PubMed] [Google Scholar]
- Obuchowski I, Liberek K. Small but mighty: a functional look at bacterial sHSPs. Cell Stress Chaperones. 2020;25(4):593–600. doi: 10.1007/s12192-020-01094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidot SJ, Porter JL, Tobias NJ, Anderson J, Catmull D, Seemann T, Kidd S, Davies JK, Reynolds E, Dashper S, Stinear TP. Regulation of the 18 kDa heat shock protein in Mycobacterium ulcerans: an alpha-crystallin orthologue that promotes biofilm formation. Mol Microbiol. 2010;78(5):1216–1231. doi: 10.1111/j.1365-2958.2010.07401.x. [DOI] [PubMed] [Google Scholar]
- Ponomarenko M, Stepanenko I, Kolchanov N (2013) Heat shock proteins in Brenner’s encyclopedia of genetics (pp. 402–405). Elsevier. 10.1016/b978-0-12-374984-0.00685-9
- Poulain P, Gelly JC, Flatters D. Detection and architecture of small heat shock protein monomers. PLoS ONE. 2010;5(4):e9990. doi: 10.1371/journal.pone.0009990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinle K, Mogk A, Bukau B. The diverse functions of small heat shock proteins in the proteostasis network. J Mol Biol. 2022;434(1):167157. doi: 10.1016/j.jmb.2021.167157. [DOI] [PubMed] [Google Scholar]
- Rodriguez Ospina S, Blazier D, Criado-Marrero M, Gould L, Gebru N, Beaulieu-Abdelahad D, Wang X, Remily-Wood E, Chaput D, Stevens S, Uversky V, Bickford P, Dickey C, Blair L. Small heat shock protein 22 improves cognition and learning in the tauopathic brain. Int J Mol Sci. 2022;23(2):851. doi: 10.3390/ijms23020851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SK, Hiyama T, Nakamoto H. Purification and characterization of the 16-kDa heat-shock-responsive protein from the thermophilic cyanobacterium Synechococcus vulcanus, which is an alpha-crystallin-related, small heat shock protein. Eur J Biochem. 1999;262(2):406–416. doi: 10.1046/j.1432-1327.1999.00380.x. [DOI] [PubMed] [Google Scholar]
- Saji H, Iizuka R, Yoshida T, Abe T, Kidokoro S, Ishii N, Yohda M. Role of the IXI/V motif in oligomer assembly and function of StHsp14.0, a small heat shock protein from the acidothermophilic archaeon, Sulfolobus tokodaii strain 7. Proteins: Structure, Function, and Bioinformatics. 2008;71(2):771–782. doi: 10.1002/prot.21762. [DOI] [PubMed] [Google Scholar]
- Santhanagopalan I, Degiacomi MT, Shepherd DA, Hochberg GKA, Benesch JLP, Vierling E. It takes a dimer to tango: oligomeric small heat shock proteins dissociate to capture substrate. J Biol Chem. 2018;293(51):19511–19521. doi: 10.1074/jbc.ra118.005421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopf JW. Fossil evidence of Archaean life. Phil Trans R Soc b: Biol Sci. 2006;361(1470):869–885. doi: 10.1098/rstb.2006.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatov V, Weeks S, Strelkov S, Gusev N. The role of the arginine in the conserved n-terminal domain RLFDQxFG motif of human small heat shock proteins hspb1, hspb4, hspb5, hspb6, and hspb8. Int J Mol Sci. 2018;19(7):2112. doi: 10.3390/ijms19072112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique M, Gernhard S, von Koskull-Döring P, Vierling E, Scharf KD. The plant sHSP superfamily: five new members in Arabidopsis thaliana with unexpected properties. Cell Stress Chaperones. 2008;13(2):183–197. doi: 10.1007/s12192-008-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Groth-Vasselli B, Kumosinski TF, Farnsworth PN. α-Crystallin quaternary structure: molecular basis for its chaperone activity. FEBS Lett. 1995;372(2–3):283–287. doi: 10.1016/0014-5793(95)00980-n. [DOI] [PubMed] [Google Scholar]
- Studer S, Obrist M, Lentze N, Narberhaus F. A critical motif for oligomerization and chaperone activity of bacterial α-heat shock proteins. Eur J Biochem. 2002;269(14):3578–3586. doi: 10.1046/j.1432-1033.2002.03049.x. [DOI] [PubMed] [Google Scholar]
- Sudnitsyna MV, Mymrikov ES, Seit-Nebi A, Gusev N. The role of intrinsically disordered regions in the structure and functioning of small heat shock proteins. Curr Protein Pept Sci. 2012;13(1):76–85. doi: 10.2174/138920312799277875. [DOI] [PubMed] [Google Scholar]
- Sun Y, MacRae TH. Small heat shock proteins: molecular structure and chaperone function. Cell Mol Life Sci. 2005;62(21):2460–2476. doi: 10.1007/s00018-005-5190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Van Montagu M, Verbruggen N. Small heat shock proteins and stress tolerance in plants. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 2002;1577(1):1–9. doi: 10.1016/s0167-4781(02)00417-7. [DOI] [PubMed] [Google Scholar]
- Tang YC, Chang HC, Hayer-Hartl M, Hartl FU. SnapShot: molecular chaperones, part II. Cell. 2007;128(2):412.e1–412.e2. doi: 10.1016/j.cell.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Tian H, Tan J, Zhang L, Gu X, Xu W, Guo X, Luo Y. Increase of stress resistance in Lactococcus lactis via a novel food-grade vector expressing a shsp gene from Streptococcus thermophilus. Brazilian journal of microbiology: [publication of the Brazilian Society for Microbiology] 2012;43(3):1157–1164. doi: 10.1590/S1517-838220120003000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhomirova TS, Selivanova OM, Galzitskaya OV. α-Crystallins are small heat shock proteins: functional and structural properties. Biochem Mosc. 2017;82(2):106–121. doi: 10.1134/s0006297917020031. [DOI] [PubMed] [Google Scholar]
- Tóth ME, Sántha M, Penke B, Vígh L (2015). How to stabilize both the proteins and the membranes: diverse effects of shsps in neuroprotection. in heat shock proteins (pp. 527–562). Springer International Publishing. 10.1007/978-3-319-16077-1_23
- Tsvetkova NM, Horváth I, Török Z, Wolkers WF, Balogi Z, Shigapova N, Crowe LM, Tablin F, Vierling E, Crowe JH, Vígh L. Small heat-shock proteins regulate membrane lipid polymorphism. Proc Natl Acad Sci. 2002;99(21):13504–13509. doi: 10.1073/pnas.192468399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyedmers J, Mogk A, Bukau B. Cellular strategies for controlling protein aggregation. Nat Rev Mol Cell Biol. 2010;11(11):777–788. doi: 10.1038/nrm2993. [DOI] [PubMed] [Google Scholar]
- van Montfort RLM, Basha E, Friedrich KL, Slingsby C, Vierling E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat Struct Biol. 2001;8(12):1025–1030. doi: 10.1038/nsb722. [DOI] [PubMed] [Google Scholar]
- Veinger L, Diamant S, Buchner J, Goloubinoff P. The small heat-shock protein IBPb from escherichia coli stabilizes stress-denatured proteins for subsequent refolding by a multichaperone network. J Biol Chem. 1998;273(18):11032–11037. doi: 10.1074/jbc.273.18.11032. [DOI] [PubMed] [Google Scholar]
- Vigh L, Horváth I, Maresca B, Harwood JL. Can the stress protein response be controlled by ‘membrane-lipid therapy’? Trends Biochem Sci. 2007;32(8):357–363. doi: 10.1016/j.tibs.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Vogel C, Bashton M, Kerrison ND, Chothia C, Teichmann SA. Structure, function and evolution of multidomain proteins. Curr Opin Struct Biol. 2004;14(2):208–216. doi: 10.1016/j.sbi.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Wang K, Spector A. Alpha-crystallin can act as a chaperone under conditions of oxidative stress. Invest Ophthalmol vis Sci. 1995;36(2):311–321. [PubMed] [Google Scholar]
- Waters ER, Vierling E. Plant small heat shock proteins – evolutionary and functional diversity. New Phytol. 2020;227(1):24–37. doi: 10.1111/nph.16536. [DOI] [PubMed] [Google Scholar]
- Waters ER, Lee GJ, Vierling E. Evolution, structure and function of the small heat shock proteins in plants. J Exp Bot. 1996;47(3):325–338. doi: 10.1093/jxb/47.3.325. [DOI] [Google Scholar]
- Weeks SD, Baranova EV, Heirbaut M, Beelen S, Shkumatov AV, Gusev NB, Strelkov SV. Molecular structure and dynamics of the dimeric human small heat shock protein HSPB6. J Struct Biol. 2014;185(3):342–354. doi: 10.1016/j.jsb.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Weidmann S, Rieu A, Rega M, Coucheney F, Guzzo J. Distinct amino acids of the Oenococcus oeni small heat shock protein Lo18 are essential for damaged protein protection and membrane stabilization. FEMS Microbiol Lett, No-No. 2010 doi: 10.1111/j.1574-6968.2010.01999.x. [DOI] [PubMed] [Google Scholar]
- Weidmann S, Maitre M, Laurent J, Coucheney F, Rieu A, Guzzo J. Production of the small heat shock protein Lo18 from Oenococcus oeni in Lactococcus lactis improves its stress tolerance. Int J Food Microbiol. 2017;247:18–23. doi: 10.1016/j.ijfoodmicro.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Wirth (2003) Les protéines de choc thermique (heat shock proteins-Hsps). II. Hsp70 : biomarqueur et acteur du stress cellulaire. . Facmv.Ulg.Ac.Be; meertens, marina. (n.d.). Annales02/2003. http://www.facmv.ulg.ac.be/amv/articles/2003_147_2_05.pdf
- Yeh CH, Chang PFL, Yeh KW, Lin WC, Chen YM, Lin CY. Expression of a gene encoding a 16.9-kDa heat-shock protein, Oshsp16.9, in Escherichia coli enhances thermotolerance. Proc Natl Acad Sci. 1997;94(20):10967–10972. doi: 10.1073/pnas.94.20.10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan CX, Czarnecka-Verner E, Gurley WB. Expression of human heat shock transcription factors 1 and 2 in HeLa cells and yeast. Cell Stress Chaperones. 1997;2(4):263. doi: 10.1379/1466-1268(1997)002<0263:eohhst>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zara S, Antonio Farris G, Budroni M, Bakalinsky AT. HSP12 is essential for biofilm formation by a Sardinian wine strain of S. cerevisiae. Yeast. 2002;19(3):269–276. doi: 10.1002/yea.831. [DOI] [PubMed] [Google Scholar]
- Zeng L, Tan J, Lu T, Lei Q, Chen C, Hu Z. Small heat shock proteins and the endoplasmic reticulum: potential attractive therapeutic targets? Curr Mol Med. 2015;15(1):38–46. doi: 10.2174/1566524015666150114111745. [DOI] [PubMed] [Google Scholar]
- Zhang H, Fu X, Jiao W, Zhang X, Liu C, Chang Z. The association of small heat shock protein Hsp16.3 with the plasma membrane of Mycobacterium tuberculosis: dissociation of oligomers is a prerequisite. Biochem Biophys Res Commun. 2005;330(4):1055–1061. doi: 10.1016/j.bbrc.2005.03.092. [DOI] [PubMed] [Google Scholar]
- Żwirowski S, Kłosowska A, Obuchowski I, Nillegoda NB, Piróg A, Ziętkiewicz S, Bukau B, Mogk A, Liberek K. Hsp70 displaces small heat shock proteins from aggregates to initiate protein refolding. EMBO J. 2017;36(6):783–796. doi: 10.15252/embj.201593378. [DOI] [PMC free article] [PubMed] [Google Scholar]