Abstract
Gamma interferon (IFN-γ) and T1-phenotype immune responses are important components of host defense against a variety of intracellular pathogens, including Legionella pneumophila. The benefit of intrapulmonary adenovirus-mediated IFN-γ gene therapy was investigated in a nonlethal murine model of experimental L. pneumophila pneumonia. Intratracheal (i.t.) administration of 106 CFU of L. pneumophila induced the expression of T1 phenotype cytokines, such as IFN-γ and interleukin-12 (IL-12). Natural killer cells were identified as the major cellular source of IFN-γ. To determine if enhanced expression of IFN-γ in the lung could promote pulmonary clearance of L. pneumophila, we i.t. administered 5 × 108 PFU of a recombinant adenovirus vector containing the murine IFN-γ cDNA (AdmIFN-γ) concomitant with L. pneumophila. We observed a 10-fold decrease in lung bacterial CFU at day 2 in the AdmIFN-γ-treated group compared to controls (P < 0.01). Alveolar macrophages isolated from AdmIFN-γ-treated animals displayed enhanced killing of intracellular L. pneumophila organisms ex vivo. Similar improvements in bacterial clearance were observed with i.t. recombinant IFN-γ treatment. The transient transgenic expression of IL-12, a known inducer of IFN-γ and promoter of T1-type immune responses, resulted in more modest improvement in bacterial clearance (sixfold reduction; P < 0.05). These results demonstrate that, even in immunocompetent hosts, exogenous administration or transient transgenic expression of IFN-γ, and to a lesser extent IL-12, may be of potential therapeutic benefit in the treatment of patients with Legionella pneumonia.
Bacterial pneumonia is a leading cause of morbidity and mortality in the United States. With the emergence of multidrug-resistant organisms, treatment of patients with this disease has been difficult, particularly in immunocompromised and elderly patients. Therefore, the magnitude of host innate and adaptive immune responses is a critical determinant of the clinical outcome of patients with bacterial pneumonia. Interferon gamma (IFN-γ) is now recognized as an important cytokine in both innate and cell-mediated immune responses against a variety of microbial pathogens. The beneficial effects of IFN-γ on phagocytes include the induction of nitric oxide synthase expression and the generation of reactive oxygen intermediates (8). In addition, IFN-γ promotes T1-phenotype immune responses, which leads to enhanced cell-mediated killing of intracellular pathogens. As a result, IFN-γ has been used as an adjunctive treatment for several intracellular infections, including disseminated Mycobacterium avium complex, Leishmania major, and Toxoplasma gondii (23, 28).
Legionella pneumophila is an intracellular gram-negative organism that is a common cause of severe community-acquired and nosocomial pneumonia (45). Importantly, pulmonary infection due to this organism can result in substantial morbidity and mortality in both immunocompetent and immunocompromised individuals. Previous studies have demonstrated that endogenous IFN-γ is induced in response to Legionella infection and is believed to play an important role in the successful eradication of this organism (11, 46). Legionella infects and replicates within alveolar macrophages in permissive hosts, but its growth is dependent upon iron. Recombinant IFN-γ has been shown to activate monocytes and resident macrophages to inhibit growth of and even promote killing of L. pneumophila, in part by inducing nitric oxide and limiting the availability of intracellular iron in macrophages (13, 15–17). In vivo, recombinant IFN-γ has been demonstrated to be of benefit in neutropenic mice and corticosteroid-treated rats infected with L. pneumophila (42, 47). However, the benefit of IFN-γ in immunocompetent hosts with L. pneumophila pneumonia has not previously been demonstrated. Furthermore, rats are relatively resistant hosts for Legionella (51), making the biologic significance of these observations in immunocompetent animals unclear.
Therefore, we sought to test the hypothesis that either exogenous administration or enhanced endogenous expression of IFN-γ would be of therapeutic benefit in immunocompetent, permissive hosts using an A/J mouse model of experimental legionellosis (11). Unlike other mouse strains, A/J mouse macrophages are highly permissive for the growth of Legionella organisms, much like human macrophages. We used both recombinant IFN-γ (rmIFN-γ) and a recombinant adenovirus that results in enhanced endogenous expression of murine IFN-γ (AdmIFN-γ) to determine if either early administration or transient transgenic expression of IFN-γ would promote pulmonary bacterial clearance. We found that both treatment modalities resulted in enhanced bacterial clearance. Furthermore, our studies demonstrated that AdmIFN-γ, like rmIFN-γ, could activate macrophages to kill Legionella ex vivo; that the effects of AdmIFN-γ were independent of cell recruitment and proinflammatory cytokine induction; and that intrapulmonary rather than systemic IFN-γ expression was required for beneficial effects to be observed. In addition, because interleukin-12 (IL-12) is a key mediator of T1-type immune responses and IFN-γ production, we also investigated whether transient transgenic pulmonary expression of IL-12 would promote clearance of L. pneumophila, using a recombinant adenovirus that contains the murine IL-12 p35 and p40 cDNAs (AdIL-12). We found that intratracheally (i.t.) administered AdIL-12 also enhances bacterial clearance, but the results with AdIL-12 were modest compared to the effects of AdmIFN-γ or rmIFN-γ.
MATERIALS AND METHODS
Mice.
Female specific-pathogen-free 6- to 8-week old A/J mice were purchased from Jackson Laboratory (Bar Harbor, Maine) and housed under specific-pathogen-free conditions within the animal care facility at the University of Michigan until the day of sacrifice.
Preparation of L. pneumophila.
For animal experiments, we used a clinical isolate of L. pneumophila suzuki (serogroup 1), which was a gift of Kazuhiro Tateda (47). Bacteria were grown over 3 to 4 days on buffered charcoal-yeast extract agar supplemented with l-cysteine and ferric nitrate. A single colony was transferred to 3 ml of N-(2-acetamido)-2-aminoethanesulfonic acid (Sigma, St. Louis, Mo.) buffered yeast extract broth and incubated overnight at 37°C with constant shaking (18). A bacterial suspension was then transferred to fresh buffered yeast extract broth using serial fivefold dilutions and then again was incubated overnight under the same conditions. After confirmation of bacterial motility by microscopic observation, the concentration of bacteria was determined by measuring the amount of absorbance at 600 nm. According to a standard of absorbancies based on known CFU, the bacterial suspension was diluted to the desired concentration in saline and subsequently confirmed by plating the suspension. Animals were then anesthetized with a ketamine-xylazine mixture intraperitoneally (i.p.). The trachea was exposed, and 30 μl of inoculum was administered via a sterile 26-gauge needle. The skin incision was closed via surgical staples (11, 51).
Reagents.
rmIFN-γ was purchased from R&D systems (Minneapolis, Minn.). Polyclonal anti-murine IFN-γ, IL-12, tumor necrosis factor (TNF), gamma interferon-inducible protein 10 (IP-10) and MIG antibodies used in the enzyme-linked immunosorbent assay (ELISA) were obtained from R&D systems.
Adenovirus vectors.
AdmIFN-γ has been described previously (34). Briefly, this recombinant adenovirus 5, pACCMV.PLA, has murine IFN-γ cDNA inserted into the E1 region. Transfection of this adenovirus results in expression of biologically active murine IFN-γ. For experiments using AdmIFN-γ, we used Ademvplpa-loxP as the control adenovirus (AdCtl), which is an empty vector with an adenovirus 5 backbone and cytomegalovirus promoter (Vector Core, University of Michigan) (44). We also utilized an adenovirus vector which contains the cDNA for both the p35 and p40 subunits of murine interleukin-12 (AdIL-12) in the E1 and E3 regions, respectively. This adenovirus has previously been shown to express a biologically active form of IL-12 in vivo (10).
Lung harvesting.
At designated time points, mice were sacrificed by CO2 asphyxia. Prior to lung removal, the pulmonary vasculature was perfused via the right ventricle with 1 ml of phosphate-buffered saline (PBS) containing 5 mM EDTA. Whole lungs were then harvested for assessment of bacterial number and cytokine protein expression. After removal, whole lungs were homogenized in 1.0 ml of PBS with protease inhibitor (Boehringer Mannheim, Indianapolis, Ind.) using a tissue homogenizer (Biospec Products, Inc.) under a vented hood. Portions of homogenates (10 μl) were inoculated on buffered charcoal-yeast extract agar after serial 1:10 dilutions with PBS or 0.9 N saline (NS) to determine the number of CFU. The remaining homogenates were incubated on ice for 30 min and then centrifuged at 1,400 × g for 10 min. Supernatants were collected, passed through a 0.45-μm-pore-size filter (Gelman Sciences, Ann Arbor, Mich.), and then stored at −20°C for assessment of cytokine levels.
BAL and cytospins.
Mice were sacrificed 1, 2, and 4 days after inoculation with bacteria for the performance of bronchoalveolar lavage (BAL). The trachea was exposed and intubated using a 1.7-mm-outer-diameter polyethylene catheter. BAL was performed by instilling PBS containing 5 mM EDTA in 1-ml aliquots. The total volume of lavage was 10 ml per mouse to obtain cells or 1 ml for cytokine analysis. Cytocentrifugation slides (Cytospin 2; Shandon Inc., Pittsburgh, Pa.) were subsequently prepared from BAL cells and stained with Diff-Quik (Dade Behring, Newark, Del.) for cell differential (50).
Total lung leukocyte preparation.
Lungs were removed from euthanatized animals and leukocytes were prepared as previously described (35). Briefly, lungs were minced with scissors to a fine slurry in 15 ml of digestion buffer (RPMI–5% fetal calf serum–collagenase [1 mg/ml; Boehringer Mannheim]) plus DNase (30 μg/ml; Sigma). Lung slurries were enzymatically digested for 30 min at 37°C. Any undigested fragments were further dispersed by drawing the solution up and down through the bore of a 10-ml syringe. The total lung cell suspension was pelleted, resuspended, and spun through a 20% Percoll gradiant to enrich for leukocytes for flow analysis. Cell counts and viability were determined using Trypan blue exclusion counting on a hemacytometer.
Intracellular cytokine staining.
Cells from infected and uninfected control mice were isolated from lung digests as previously described. Intracytoplasmic cytokine staining was performed using the Cytofix/Cytoperm Plus kit and the manufacturer's protocol (BD PharMingen, San Diego, Calif.). Cells were stained for surface expression of CD4, CD8, or DX5 (pan-NK cell marker) using fluorescein isothiocyanate-labeled antibodies (BD PharMingen). Cells were then fixed with formaldehyde and permeabilized with sodium azide and saponin for 20 min on ice. After washing, cells were stained for intracytoplasmic IFN-γ expression with purified rat anti-murine IFN-γ antibodies (BD PharMingen) diluted in Perm/Wash solution for 30 min. Cells were analyzed on a FACSCalibur cytometer (Becton Dickinson) using Cellquest software (Becton Dickinson).
Murine cytokine ELISAs.
Murine cytokines were quantitated using a modification of a double ligand method as previously described (44). Standards were 0.5 log dilutions of murine recombinant cytokine from 1 pg/ml to 100 ng/ml. This ELISA method consistently detected murine IFN-γ, IL-12, TNF, IP-10, and MIG concentrations above 50 pg/ml. The ELISA did not cross-react with other cytokines, such as IL-1, IL-2, or IL-6. In addition, the ELISA did not cross-react with other members of the murine chemokine family, including murine KC, MIP-2, JE/MCP-1, MIP-1α, or RANTES.
Alveolar macrophage microbicidal assay.
Alveolar macrophages were isolated from BAL as described above. Briefly, the BAL fluid was spun down at 580 × g for 10 min, the supernatant was discarded, and the cell pellet was resuspended in RPMI-Dulbecco modified Eagle medium with 5% fetal bovine serum with antibiotics. The cell count was determined using a hemacytometer, and the cells were then diluted to a final concentration of 106 cells/ml. Trypan blue staining revealed the cells to be >95% viable. The cells were cultured in 24-well tissue culture plates (Costar, Cambridge, Mass.). After 2 h of incubation at 37°C in 5% CO2, the wells were washed of nonadherent cells, and a suspension of L. pneumophila was added at a multiplicity of infection (MOI) of 0.2 to 0.3 (i.e., 2 to 3 organism per 10 cells), as higher MOIs lead to significant early cytotoxicity (24, 29, 36). The cells were incubated with L. pneumophila for 2 more hours at 37°C, and then the wells were washed again to remove extracellular Legionella organisms. The cultured cells in the wells were lysed with cold distilled water 0, 2, and 3 days later to determine serial intracellular L. pneumophila CFU.
Statistical analysis.
Statistical significance was determined using one-way analysis of variance with the Bonferroni post-test for three or more groups or the Mann-Whitney test for two groups. To determine the main cellular source of IFN-γ in the lungs in animals with intrapulmonary Legionella infection, a chi-square test was performed. Calculations were performed using Prism 3.0 for Windows 95 and NT (GraphPad Software).
RESULTS
T1 cytokine expression and sources of IFN-γ after i.t. L. pneumophila administration.
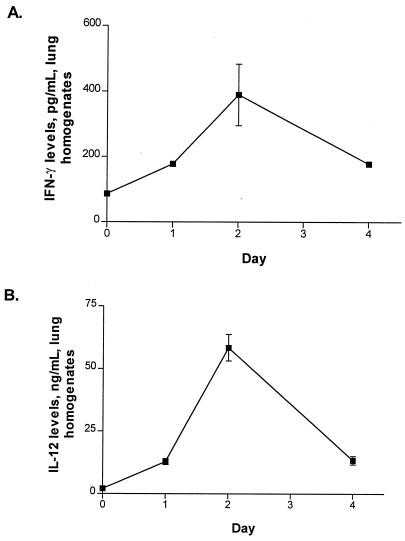
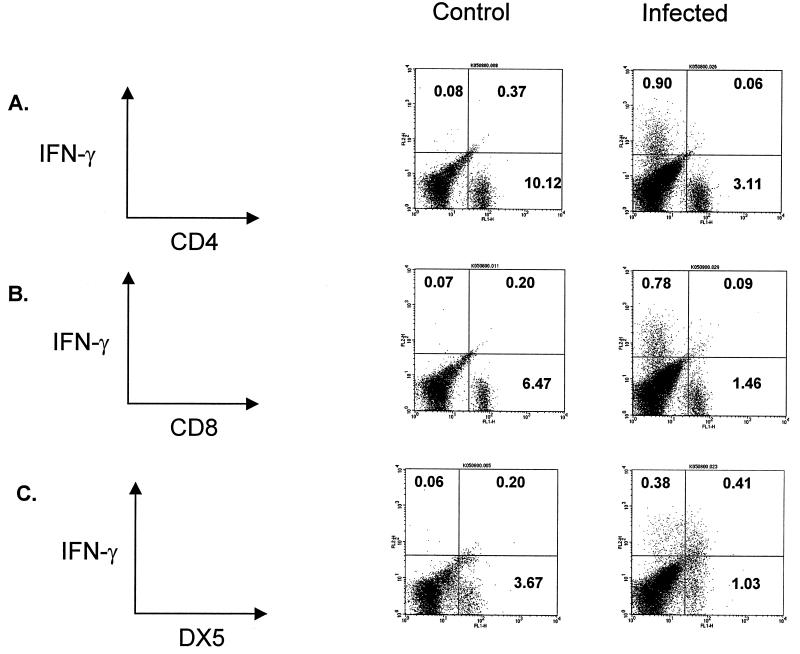
Initial experiments were performed to determine the time course and cellular source of T1-phenotype cytokines in mice with Legionella pneumonia. As shown in Fig. 1, the i.t. administration of L. pneumophila (106 CFU) resulted in the expression of both IL-12 and IFN-γ in the lung. The time course of T1-type cytokine expression correlated with the time course of bacterial CFU growth in the lung, with peak cytokine levels, and with bacterial CFU in lung homogenates occurring at day 2 (Fig. 1; also see Fig. 4). Using intracellular cytokine staining and flow cytometric analysis gated for lymphocytes, we observed that DX5+ cells were the predominant source of endogenous IFN-γ in our model (Fig. 2) by day 2 post-Legionella challenge (time of maximal IFN-γ production; P < 0.0001). However, other cells also contributed to IFN-γ production, including a smaller population of DX5− CD4− CD8− lung cells.
FIG. 1.
Cytokine production in lung during Legionella infection. Mice were sacrificed on days 0, 1, 2, and 4 following i.t. challenge with L. pneumophila (1.5 × 106 CFU/mouse). IFN-γ (A) and IL-12 (B) levels were measured in lung homogenates by ELISA. The experiment was performed three times (n = 5 animals per time point). Error bars, standard deviation.
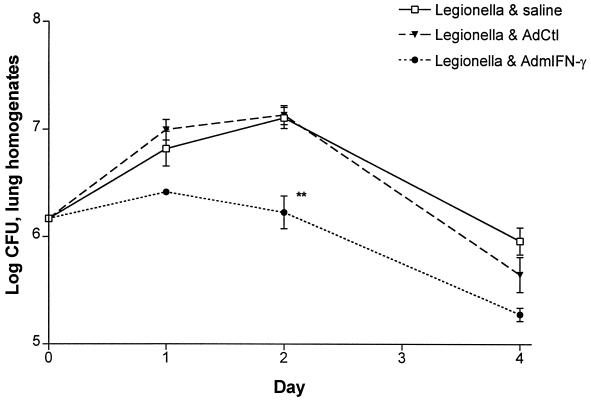
FIG. 4.
CFU in lung following i.t. challenge with L. pneumophila in AdmIFN-γ-treated animals. Mice received i.t. coadministrations of 1.5 × 106 CFU of L. pneumophila and AdmIFN-γ (5 × 108 PFU), saline, or AdCtl (5 × 108 PFU) and then were sacrificed on days 1, 2, and 4 following challenge for determination of L. pneumophila CFU in lung homogenates. The experiment was performed twice (n = 5 animals per group). ∗∗, P < 0.01; error bars, standard deviation.
FIG. 2.
Intracellular cytokine staining of IFN-γ in lung digest cells. On day 2 after i.t. challenge with L. pneumophila (i.e., when IFN-γ levels were at their peak), mice were sacrificed to obtain cells for lung digest. The cells were stained for surface expression of CD4 (A), CD8 (B), or DX5 (C) and then costained for intracytoplasmic IFN-γ. After staining, cells underwent analysis by flow cytometry with gating for lymphocytes by size and complexity characteristics (41). (C) The majority of cells staining positive for intracellular IFN-γ were DX5+, although a number of IFN-γ-positive cells were DX5−, CD4−, and CD8−. The experiment was performed twice (n = 2 animals per group).
Time- and dose-dependent production of IFN-γ in A/J mice after i.t. AdmIFN-γ administration.
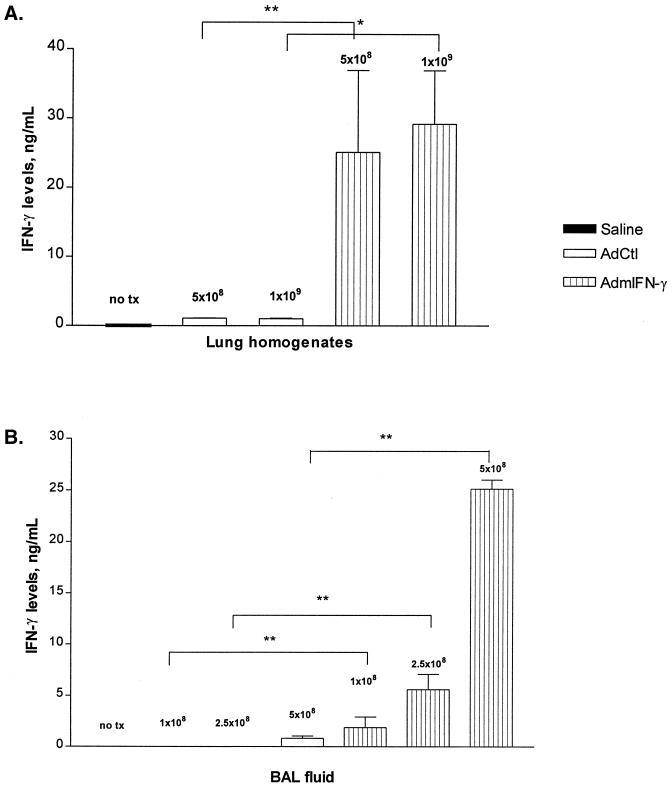
Previous studies have demonstrated an important role for IFN-γ in the clearance of intracellular bacteria, including L. pneumophila, from the lung. To determine if enhanced pulmonary IFN-γ expression could augment Legionella clearance, we utilized an intrapulmonary adenovirus-mediated gene therapy approach. In initial characterization studies, we observed substantial expression of IFN-γ in a time-dependent fashion after i.t. administration of AdmIFN-γ (109 PFU). Peak levels occurred at day 1, with sustained elevation until at least day 7 post-AdmIFN-γ administration (data not shown). Levels of IFN-γ returned to baseline by day 14. In contrast, mice treated with AdCtl did not have significantly elevated lung levels of IFN-γ at any time point. We then administered increasing doses of AdmIFN-γ, which resulted in a significant dose-dependent induction of IFN-γ (Fig. 3). A dose of 5 × 108 PFU was used for all subsequent experiments, as at doses of 109 PFU and above, systemic toxicity was observed (lethargy and ruffled fur). Thus, these studies indicate that the i.t. administration of AdIFN-γ results in a significant induction in the expression of IFN-γ within the lung that is both time and dose dependent.
FIG. 3.
Dose-dependent IFN-γ expression after i.t. AdmIFN-γ administration. Increasing doses of AdmIFN-γ or AdCtl were administered (doses in PFU indicated by numbers above bars). Two days later, mice were sacrificed for determination of either lung homogenate (A) or BAL (B) cytokine levels. The experiment was performed twice (n = 5 animals per group). Statistical significance: ∗, P < 0.05; ∗∗, P < 0.01. Error bars, standard deviation. no tx, saline.
Effect of transient transgenic IFN-γ expression or intrapulmonary rmIFN-γ administration on bacterial clearance in A/J mice with Legionella pneumonia.
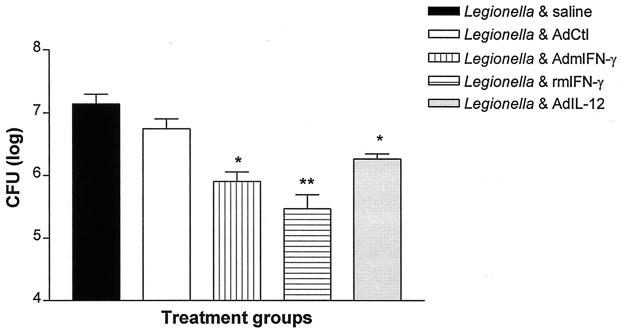
To determine if transient transgenic expression of IFN-γ could enhance lung bacterial clearance, mice received coadministration of L. pneumophila (106 CFU) and AdmIFN-γ, saline, or Adctl. As shown in Fig. 4, i.t. treatment with AdmIFN-γ (5 × 108 PFU) enhanced bacterial clearance compared to that observed in infected animals treated with either saline or AdCtl (reduction of [10 ± 1.2]-fold [mean ± standard deviation] CFU in lung homogenate on day 2 from all experiments; P < 0.01). Intratracheal rmIFN-γ (100 ng) treatment was also associated with a marked reduction in CFU in lung at day 2 compared to that observed in control animals (35-fold reduction; P < 0.001) (Fig. 5). Since IL-12 is known to be a major inducer of IFN-γ and a key promoter of a T1-type host response, we performed additional studies to assess the effects of transgenic expression of IL-12 in our model. The i.t. administration of a recombinant adenovirus containing IL-12 p35 and p40 cDNAs (5 × 108 PFU) resulted in a mean sixfold reduction in CFU in lung compared to that in control animals (P < 0.05) (Fig. 5). These results indicate that transient transgenic expression of IL-12 also has beneficial effects on bacterial clearance in Legionella pneumonia, albeit to a lesser extent than that observed with IFN-γ.
FIG. 5.
CFU in lung homogenate in animals with Legionella pneumonia treated with AdmIFN-γ, rmIFN-γ, or AdIL-12. Animals received i.t. coadministrations of L. pneumophila (106 CFU) and saline, AdCtl, AdmIFN-γ, rmIFN-γ (100 ng), or AdIL-12 (5 × 108-PFU dose for all adenovirus vectors). Two days later, mice were sacrificed for determination of CFU lung homogenate. The experiment was performed twice (n = 5 animals per group). Statistical significance: ∗, P < 0.05; ∗∗, P < 0.001 (compared to control). Error bars, standard deviations.
Effect of i.t. versus i.p. administration of AdmIFN-γ on lung bacterial clearance in mice with Legionella pneumonia.
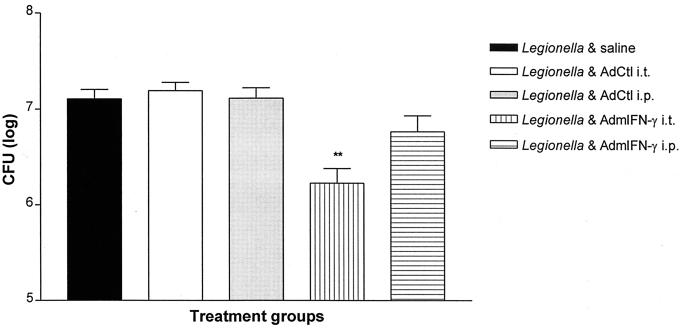
To determine if enhanced bacterial clearance in response to transient transgenic expression of IFN-γ required compartmentalized therapy, animals were administered AdmIFN-γ either i.t. or i.p. concomitant with i.t. L. pneumophila administration. The number L. pneumophila CFU in lung was determined 2 days later. As shown in Fig. 6, i.p. administration of AdmIFN-γ (5 × 108 PFU) led to only a small and statistically insignificant reduction in lung bacterial CFU (twofold reduction; P > 0.05). In contrast, i.t. administration of AdmIFN-γ resulted in a significant improvement in bacterial clearance similar to that observed in previous experiments.
FIG. 6.
Effect of systemic versus localized administration of AdmIFN-γ vector on bacterial clearance. Animals were co-administered L. pneumophila (1.5 × 106 CFU) i.t., and i.t. AdmIFN-γ, i.p. AdmIFN-γ, i.t. AdCtl, i.p. AdCtl (all adenovirus doses 5 × 108 PFU), or saline. On day 2 post-challenge, mice were sacrificed for lung CFU determination. ∗∗, P < 0.01 compared to control. The experiment was performed once (n = 5 animals per group). Error bars, standard deviation.
Effect of AdmIFN-γ on intrapulmonary cytokine levels.
Since overexpression of IFN-γ had impressive effects on bacterial clearance, we investigated the possibility that AdmIFN-γ may enhance bacterial clearance by inducing the expression of other cytokines that contribute to antibacterial host defense. As expected, IFN-γ levels in lung homogenates from AdmIFN-γ treated animals were significantly higher than in those from infected animals treated with AdCtl or saline (mean fourfold increase on day 2; P < 0.01) (data not shown) at all time points studied, although these animals had a lower bacterial burden in the lungs. However, no appreciable differences in levels of other cytokines studied—including TNF alpha, IL-12, or the ELR-CXC chemokines (IP-10 and MIG, which are known to be induced by IFN-γ) (20)—were noted in the AdmIFN-γ-treated animals compared to controls (data not shown). Based upon these results, the induction of other cytokines does not appear to contribute to the beneficial effects of AdmIFN-γ.
Effect of AdmIFN-γ on lung leukocyte influx in mice with Legionella pneumonia.
To determine the effects of intrapulmonary transgenic expression of IFN-γ on the development of lung inflammation, we administered L. pneumophila concurrently with AdmIFN-γ or AdCtl and then performed BAL at various time points postchallenge. As shown in Table 1, we observed that challenge with L. pneumophila resulted in a substantial increase in leukocytes in the lungs over the baseline, particularly neutrophils. On days 1 and 2 post-infectious challenge, when IFN-γ expression was at its peak in AdmIFN-γ-treated animals, AdmIFN-γ-treated animals did not display significant differences in the total number of neutrophils or mononuclear cells in lung airspace compared to control animals. By day 4, the AdmIFN-γ-treated animals had a trend towards fewer total leukocytes and neutrophils in lavage fluid compared to controls, consistent with their lower lung bacterial counts, but these differences were not statistically significant (data not shown). Thus, the beneficial effect of AdmIFN-γ treatment was not associated with significant differences in cell recruitment to the airspace during Legionella infection.
TABLE 1.
Cell counts and differentials in lavage fluid after Legionella challenge in AdmIFN-γ-treated animalsa
| Treatment of Legionella | Mean ± SEM
|
||
|---|---|---|---|
| Total no. of cells | No. of PMN | No. of mononuclear cells | |
| Untreated | (1.40 ± 0.24) × 105 | 425 ± 425 | (1.39 ± 0.23) × 105 |
| Day 1 | |||
| 0.9 NS | (3.01 ± 0.42) × 105 | (2.66 ± 0.38) × 105 | (0.35 ± 0.04) × 105 |
| AdCtl | (3.01 ± 0.74) × 105 | (2.68 ± 0.68) × 105 | (0.34 ± 0.07) × 105 |
| AdmIFN-γ | (2.85 ± 0.47) × 105 | (2.35 ± 0.44) × 105 | (0.50 ± 0.22) × 105 |
| Day 2 | |||
| 0.9 NS | (1.20 ± 0.19) × 106 | (1.08 ± 0.17) × 106 | (1.21 ± 0.24) × 105 |
| AdCtl | (7.78 ± 0.96) × 105 | (7.20 ± 0.89) × 105 | (5.77 ± 0.80) × 104 |
| AdmIFN-γ | (8.98 ± 0.25) × 105 | (8.05 ± 2.28) × 105 | (9.27 ± 2.72) × 104 |
Mice were challenged with 2 × 106 CFU of L. pneumophila concomitantly with AdmIFN-γ (5 × 108 PFU), AdCtl (5 × 108 PFU), or saline. On day 0, 1, and 2 after challenge, animals were sacrificed for BAL. (n = 5 animals per group). The experiment was performed once.
Effect of AdmIFN-γ administration on alveolar macrophage bactericidal activity ex vivo.
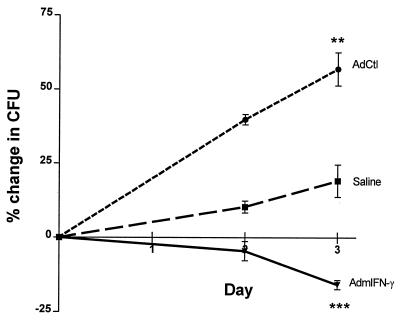
Given that the transgenic expression of IFN-γ resulted in no significant changes in leukocyte recruitment to the airspace, we next investigated whether AdmIFN-γ treatment resulted in enhanced ability of alveolar macrophages to kill intracellular L. pneumophila ex vivo. Animals were administered i.t. AdmIFN-γ, AdCtl, or saline, and then BAL was performed on day 2 to recover alveolar macrophages for ex vivo studies. We observed that alveolar macrophages from uninfected animals treated with AdmIFN-γ in vivo inhibited the intracellular growth of L. pneumophila organisms (15% reduction in CFU between day 0 and day 3; P<0.001), whereas intracellular growth continued in alveolar macrophages isolated from either saline- or AdCtl-treated animals (Fig. 7). Furthermore, we observed that alveolar macrophages from AdCtl-treated animals were more permissive to intracellular Legionella growth than alveolar macrophages from saline-treated animals (P < 0.001), suggesting that the adenovirus vector itself might be detrimental to macrophage microbicidal responses.
FIG. 7.
Effect of AdmIFN-γ treatment on intracellular growth of Legionella in macrophages ex vivo. Mice were treated i.t. with AdmIFN-γ (5 × 108 PFU), AdCtl (5 × 108 PFU), or saline. Two days later, alveolar macrophages were recovered from lavage fluid and cultured with L. pneumophila organisms at an MOI of 0.2 to 0.3. Cells were washed and lysed on day 0 following a 2 of coincubation of Legionella and macrophages to determine the level of initial intracellular or cell-associated Legionella organisms. Two and three days later, macrophages were washed and lysed to determine serial CFU counts. Results are expressed as the net percent change in CFU on days 2 and 3 compared to initial (i.e., day 0) CFU. The experiment was performed twice. Statistical significance: ∗∗, P < 0.01 (compared to alveolar macrophages from saline and AdCtl-treated animals); ∗∗∗, P < 0.001 (compared to alveolar macrophages from saline-treated animals). Error bars, standard deviations.
DISCUSSION
L. pneumophila is a major cause of severe bacterial pneumonia in both immunocompromised and immunocompetent hosts. Therefore, modulating the immune response to this and other pathogens continues to be an attractive therapeutic strategy. For our studies, we have used an A/J mouse model, as macrophages from this mouse strain, like human macrophages, are permissive for Legionella growth. In our preliminary studies with this model and our Legionella strain, we observed that intrapulmonary Legionella infection leads to a reproducible upregulation of IFN-γ and IL-12 production in the lung which correlates temporally with bacterial burden. This is consistent with earlier reports of T1-phenotype cytokine induction following Legionella infection in animal models and human patients (11, 12, 14, 46, 47).
Studies were performed to identify the cellular source of IFN-γ in our model. Previously, it was reported that in vitro stimulation of splenocyte cultures by Legionella antigens led to IFN-γ production by natural killer phenotype cells (7). Intact Legionella organisms can also stimulate human peripheral blood CD4+ T lymphocytes to produce IFN-γ in vitro (32). Using intracellular cytoplasmic staining of lung digest cells isolated from Legionella-infected animals, we have determined that the predominant source of early IFN-γ in the lung are cells positive for DX5, which is a pan-NK cell marker. Although DX5 may also be expressed on CD3+ NK-T cells, this population probably comprises a minority of the DX5+ IFN-γ-producing cells in the lung early on, as shown previously following respiratory syncytial virus infection (30). Thus, the majority of the cells producing intrapulmonary IFN-γ following Legionella infection in our model are NK cells. However, a sizeable population of IFN-γ producing cells are CD4−, CD8−, and DX5−. A likely candidate for this cell population is γδ-T cells, which have previously been linked to early localized production of IFN-γ following i.t. Klebsiella infection and i.p. Listeria infection (21, 35). Studies are ongoing to identify these additional cellular sources of IFN-γ within the lung.
IFN-γ plays an important role in the clearance of many intracellular pathogens, including T. gondii, L. monocytogenes, and Chlamydia trachomatis (4, 8, 39, 52). Endogenous IFN-γ clearly plays a significant role in clearance of L. pneumophila since IFN-γ knockout mice are more susceptible than wild-type mice, and neutralization of IFN-γ by a monoclonal antibody increases bacterial burden in mice challenged intravenously with L. pneumophila (22, 27). We therefore investigated whether modulation of the immune system by transient transgenic IFN-γ expression was beneficial to immunocompetent, permissive hosts with Legionella pneumonia. Our results indicate that either exogenous administration or enhanced transgenic expression of IFN-γ augments bacterial clearance in immunocompetent hosts in vivo. IFN-γ-treated animals had significantly lower CFU counts in lung compared to those in infected animals treated with saline or AdCtl. We demonstrated that adenovirus-mediated IFN-γ expression has beneficial effects similar to that seen with recombinant protein. Thus, we have shown that even immunocompetent hosts may benefit from increased intrapulmonary IFN-γ expression or administration.
AdmIFN-γ likely has multiple effects on the innate immune response. Since we did not observe enhanced leukocyte influx into the pulmonary airspaces in Legionella-infected animals treated with AdmIFN-γ, our results suggest that the actions of AdmIFN-γ are independent of an augmented recruitment response. Rather, the IFN-γ transgene is likely exerting important activating effects on the resident leukocytes in the lungs. This argument is supported by previously published findings that recombinant IFN-γ activates alveolar macrophages and other monocytes to limit the growth of intracellular Legionella organisms in vitro (3, 15, 33, 37, 43). Likewise, our observations indicated that macrophages isolated from AdmIFN-γ-treated mice inhibited the growth of Legionella. Thus, AdmIFN-γ appears to activate alveolar macrophages in vivo in a fashion similar to that shown with rmIFN-γ treatment in vitro. However, it is also quite possible that IFN-γ has important activating effects on neutrophils recruited to the lung, which has been demonstrated in vitro against Legionella and fungal pathogens (5, 6, 40).
The importance of proximal T1-phenotype cytokines, such as IL-12 and IL-18, in Legionella and other intracellular infections has previously been demonstrated (12, 14, 19, 48, 49). These cytokines are key modulators of intrapulmonary production of IFN-γ in Legionella pneumonia and underscore the significance of a T1-type host response towards promoting bacterial clearance. The transient transgenic expression of IL-12 resulted in some improvement in Legionella clearance. However, the fact that this effect was less than that observed with AdmIFN-γ suggests that IL-12 overexpression by itself is not sufficient to maximize IFN-γ responses. Thus, as a potential therapy, IL-12 expression or administration may be a less-attractive alternative than IFN-γ administration. It is possible that other molecules are required (such as IL-18) to synergistically enhance IFN-γ expression, as has been demonstrated in vitro (25, 38, 53). However, the neutralization of both IL-12 and IL-18 simultaneously had only modest effects on IFN-γ levels over neutralization of IL-12 alone, suggesting that IL-12 is still the key mediator in terms of IFN-γ production (12). IL-12 and IL-18 may also have activating effects on the innate immune response that are not mediated by IFN-γ, such as promoting NK cell-mediated cytotoxicity (2, 31, 49). Whether the synergistic activities of exogenous IL-12 and IL-18 are beneficial in Legionella pneumonia—through either IFN-γ-dependent or -independent effects—is the focus of ongoing studies.
Our studies also illustrate several key points to consider when using a gene therapy approach. First, our results demonstrate the importance of compartmentalized administration of gene therapy vectors. Specifically, i.p. administered AdmIFN-γ had limited effects on pulmonary clearance of Legionella. Importantly, we did not observe enhanced protein expression in the lung after i.p. vector administration relative to the expression observed in untreated animals. In contrast, i.t. treatment clearly augmented pulmonary IFN-γ production and enhanced bacterial clearance. Second, the adenovirus vector itself may have potentially detrimental effects on the immune system, particularly on macrophage function. To this end, it has been shown that AdCtl treatment can impair pulmonary clearance of Klebsiella, especially when given at higher doses (≥5 × 108 PFU) (26). Although we did not observe similar effects on Legionella clearance in vivo, we did find that macrophages recovered from AdCtl-treated animals were more permissive for the growth of Legionella ex vivo than were macrophages from untreated animals. A possible explanation is that the vector itself depresses macrophage activation. Previously, it was observed that alveolar macrophages infected with type 1 and type 8 adenoviruses displayed reduced expression of Fc and complement receptors and a decreased ability to kill ingested Candida albicans (1). Thus, the adverse effects of the adenovirus vector may be partially counteracting the potential benefits of transgenic overexpression of the target molecule, underscoring the need for less-immunoreactive gene therapy vectors.
Finally, the kinetics of cytokine expression and activity must be addressed. IFN-γ appears to play its most important role early in the time course of Legionella infection. The half-life of the recombinant protein administered in vivo is very short (up to 7 h, depending on the route of administration) (9), but this early burst of activity is sufficient to enhance clearance out to 2 days. This may have important implications for the design of potential therapy for acute infections as opposed to chronic infections.
ACKNOWLEDGMENTS
We thank Jay Kolls for providing us with the recombinant adenovirus IFN-γ vector, Thomas A. Moore for many informative discussions and his assistance in flow cytometric analysis, and Pamela M. Lincoln and Holly L. Evanoff for their assistance in ELISA.
This work was supported in part by NIH grants IIL 57243 and P50HL60289 (T.J.S.) and 5 T32 HL07749-08 (J.C.D.).
REFERENCES
- 1.Adair B M, McNulty M S, Foster J C. Effects of two adenoviruses (type 1 and type 8) on functional properties of bovine alveolar macrophages in vitro. Am J Vet Res. 1992;53:1010–1014. [PubMed] [Google Scholar]
- 2.Akira S. The role of IL-18 in innate immunity. Curr Opin Immunol. 2000;12:59–63. doi: 10.1016/s0952-7915(99)00051-5. [DOI] [PubMed] [Google Scholar]
- 3.Bhardwaj N, Nash T W, Horwitz M A. Interferon-gamma-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1986;137:2662–2669. [PubMed] [Google Scholar]
- 4.Black C M, Catterall J R, Remington J S. In vivo and in vitro activation of alveolar macrophages by recombinant interferon-gamma. J Immunol. 1987;138:491–495. [PubMed] [Google Scholar]
- 5.Blanchard D K, Djeu J Y, Klein T W, Friedman H, Stewart W E. Protective effects of tumor necrosis factor in experimental Legionella pneumophila infections of mice via activation of PMN function. J Leukoc Biol. 1988;43:429–435. doi: 10.1002/jlb.43.5.429. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard D K, Friedman H, Klein T W, Djeu J Y. Induction of interferon-gamma and tumor necrosis factor by Legionella pneumophila: augmentation of human neutrophil bactericidal activity. J Leukoc Biol. 1989;45:538–545. doi: 10.1002/jlb.45.6.538. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard D K, Friedman H, Stewart W E, Klein T W, Djeu J Y. Role of gamma interferon in induction of natural killer activity by Legionella pneumophila in vitro and in an experimental murine infection model. Infect Immun. 1988;56:1187–1193. doi: 10.1128/iai.56.5.1187-1193.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 9.Bolinger A M, Taeubel M A. Recombinant interferon gamma for treatment of chronic granulomatous disease and other disorders. Clin Pharm. 1992;11:834–850. [PubMed] [Google Scholar]
- 10.Bramson J, Hitt M, Gallichan W S, Rosenthal K L, Gauldie J, Graham F L. Construction of a double recombinant adenovirus vector expressing a heterodimeric cytokine: in vitro and in vivo production of biologically active interleukin-12. Hum Gene Ther. 1996;7:333–342. doi: 10.1089/hum.1996.7.3-333. [DOI] [PubMed] [Google Scholar]
- 11.Brieland J, Freeman P, Kunkel R, Chrisp C, Hurley M, Fantone J, Engleberg C. Replicative Legionella pneumophila lung infection in intratracheally inoculated A/J mice. A murine model of human Legionnaires' disease. Am J Pathol. 1994;145:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- 12.Brieland J K, Jackson C, Hurst S, Loebenberg D, Muchamuel T, Debets R, Kastelein R, Churakova T, Abrams J, Hare R, O'Garra A. Immunomodulatory role of endogenous interleukin-18 in gamma interferon-mediated resolution of replicative Legionella pneumophila lung infection. Infect Immun. 2000;68:6567–6573. doi: 10.1128/iai.68.12.6567-6573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brieland J K, Remick D G, Freeman P T, Hurley M C, Fantone J C, Engleberg N C. In vivo regulation of replicative Legionella pneumophila lung infection by endogenous tumor necrosis factor alpha and nitric oxide. Infect Immun. 1995;63:3253–3258. doi: 10.1128/iai.63.9.3253-3258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brieland J K, Remick D G, LeGendre M L, Engleberg N C, Fantone J C. In vivo regulation of replicative Legionella pneumophila lung infection by endogenous interleukin-12. Infect Immun. 1998;66:65–69. doi: 10.1128/iai.66.1.65-69.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrd T F, Horwitz M A. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J Clin Investig. 1989;83:1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrd T F, Horwitz M A. Lactoferrin inhibits or promotes Legionella pneumophila intracellular multiplication in nonactivated and interferon gamma-activated human monocytes depending upon its degree of iron saturation. Iron-lactoferrin and nonphysiologic iron chelates reverse monocyte activation against Legionella pneumophila. J Clin Investig. 1991;88:1103–1112. doi: 10.1172/JCI115409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byrd T F, Horwitz M A. Regulation of transferrin receptor expression and ferritin content in human mononuclear phagocytes. Coordinate upregulation by iron transferrin and downregulation by interferon gamma. J Clin Investig. 1993;91:969–976. doi: 10.1172/JCI116318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrne B, Swanson M S. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai G, Kastelein R, Hunter C A. Interleukin-18 (IL-18) enhances innate IL-12-mediated resistance to Toxoplasma gondii. Infect Immun. 2000;68:6932–6938. doi: 10.1128/iai.68.12.6932-6938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farber J M. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- 21.Ferrick D A, Schrenzel M D, Mulvania T, Hsieh B, Ferlin W G, Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 22.Fujio H, Yoshida S, Miyamoto H, Mitsuyama M, Mizuguchi Y. Investigation of the role of macrophages and endogenous interferon-gamma in natural resistance of mice against Legionella pneumophila infection. FEMS Microbiol Immunol. 1992;4:183–191. doi: 10.1111/j.1574-6968.1992.tb04993.x. [DOI] [PubMed] [Google Scholar]
- 23.Gallin J I, Farber J M, Holland S M, Nutman T B. Interferon-gamma in the management of infectious disease. Ann Intern Med. 1995;123:216–224. doi: 10.7326/0003-4819-123-3-199508010-00009. [DOI] [PubMed] [Google Scholar]
- 24.Gao L Y, Abu Kwaik Y. Apoptosis in macrophages and alveolar epithelial cells during early stages of infection by Legionella pneumophila and its role in cytopathogenicity. Infect Immun. 1999;67:862–870. doi: 10.1128/iai.67.2.862-870.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia V E, Uyemura K, Sieling P A, Ochoa M T, Morita C T, Okamura H, Kurimoto M, Rea T H, Modlin R L. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J Immunol. 1999;162:6114–6121. [PubMed] [Google Scholar]
- 26.Greenberger M J, Kunkel S L, Strieter R M, Lukacs N W, Bramson J, Gauldie J, Graham F L, Hitt M, Danforth J M, Standiford T J. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J Immunol. 1996;157:3006–3012. [PubMed] [Google Scholar]
- 27.Heath L, Chrisp C, Huffnagle G, LeGendre M, Osawa Y, Hurley M, Engleberg C, Fantone J, Brieland J. Effector mechanisms responsible for gamma interferon-mediated host resistance to Legionella pneumophila lung infection: the role of endogenous nitric oxide differs in susceptible and resistant murine hosts. Infect Immun. 1996;64:5151–5160. doi: 10.1128/iai.64.12.5151-5160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holland S M, Eisenstein E M, Kuhns D B, Turner M L, Fleisher T A, Strober W, Gallin J I. Treatment of refractory disseminated nontuberculous mycobacterial infection with interferon gamma. A preliminary report. N Engl J Med. 1994;330:1348–1355. doi: 10.1056/NEJM199405123301904. [DOI] [PubMed] [Google Scholar]
- 29.Husmann L K, Johnson W. Cytotoxicity of extracellular Legionella pneumophila. Infect Immun. 1994;62:2111–2114. doi: 10.1128/iai.62.5.2111-2114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussell T, Openshaw P J. Intracellular IFN-gamma expression in natural killer cells precedes lung CD8+ T cell recruitment during respiratory syncytial virus infection. J Gen Virol. 1998;79:2593–2601. doi: 10.1099/0022-1317-79-11-2593. [DOI] [PubMed] [Google Scholar]
- 31.Hyodo Y, Matsui K, Hayashi N, Tsutsui H, Kashiwamura S, Yamauchi H, Hiroishi K, Takeda K, Tagawa Y, Iwakura Y, Kayagaki N, Kurimoto M, Okamura H, Hada T, Yagita H, Akira S, Nakanishi K, Higashino K. IL-18 up-regulates perforin-mediated NK activity without increasing perforin messenger RNA expression by binding to constitutively expressed IL-18 receptor. J Immunol. 1999;162:1662–1668. [PubMed] [Google Scholar]
- 32.Kitsukawa K, Nakamoto A, Koito H, Matsuda Y, Saito A, Yamamoto H. Interferon-gamma (IFN-gamma) production by human T lymphocytes upon Legionella pneumophila stimulation in vitro. Clin Exp Immunol. 1995;99:76–81. doi: 10.1111/j.1365-2249.1995.tb03475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein T W, Yamamoto Y, Brown H K, Friedman H. Interferon-gamma induced resistance to Legionella pneumophila in susceptible A/J mouse macrophages. J Leukoc Biol. 1991;49:98–103. doi: 10.1002/jlb.49.1.98. [DOI] [PubMed] [Google Scholar]
- 34.Lei D, Lancaster J R, Joshi M S, Nelson S, Stoltz D, Bagby G J, Odom G, Shellito J E, Kolls J K. Activation of alveolar macrophages and lung host defenses using transfer of the interferon-gamma gene. Am J Physiol. 1997;272:L852–L859. doi: 10.1152/ajplung.1997.272.5.L852. [DOI] [PubMed] [Google Scholar]
- 35.Moore T A, Moore B B, Newstead M W, Standiford T J. Gamma delta-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J Immunol. 2000;165:2643–2650. doi: 10.4049/jimmunol.165.5.2643. [DOI] [PubMed] [Google Scholar]
- 36.Muller A, Hacker J, Brand B C. Evidence for apoptosis of human macrophage-like HL-60 cells by Legionella pneumophila infection. Infect Immun. 1996;64:4900–4906. doi: 10.1128/iai.64.12.4900-4906.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nash T W, Libby D M, Horwitz M A. IFN-gamma-activated human alveolar macrophages inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1988;140:3978–3981. [PubMed] [Google Scholar]
- 38.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–264. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 39.Rank R G, Ramsey K H, Pack E A, Williams D M. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect Immun. 1992;60:4427–4429. doi: 10.1128/iai.60.10.4427-4429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roilides E, Uhlig K, Venzon D, Pizzo P A, Walsh T J. Enhancement of oxidative response and damage caused by human neutrophils to Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect Immun. 1993;61:1185–1193. doi: 10.1128/iai.61.4.1185-1193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shapiro H. Practical flow cytometry. 3rd ed. New York, N.Y: Wiley-Liss; 1995. [Google Scholar]
- 42.Skerrett S J, Martin T R. Intratracheal interferon-gamma augments pulmonary defenses in experimental legionellosis. Am J Respir Crit Care Med. 1994;149:50–58. doi: 10.1164/ajrccm.149.1.8111597. [DOI] [PubMed] [Google Scholar]
- 43.Skerrett S J, Martin T R. Recombinant murine interferon-gamma reversibly activates rat alveolar macrophages to kill Legionella pneumophila. J Infect Dis. 1992;166:1354–1361. doi: 10.1093/infdis/166.6.1354. [DOI] [PubMed] [Google Scholar]
- 44.Standiford T J, Wilkowski J M, Sisson T H, Hattori N, Mehrad B, Bucknell K A, Moore T A. Intrapulmonary tumor necrosis factor gene therapy increases bacterial clearance and survival in murine gram-negative pneumonia. Hum Gene Ther. 1999;10:899–909. doi: 10.1089/10430349950018300. [DOI] [PubMed] [Google Scholar]
- 45.Stout J E, Yu V L. Legionellosis. N Engl J Med. 1997;337:682–687. doi: 10.1056/NEJM199709043371006. [DOI] [PubMed] [Google Scholar]
- 46.Tateda K, Matsumoto T, Ishii Y, Furuya N, Ohno A, Miyazaki S, Yamaguchi K. Serum cytokines in patients with Legionella pneumonia: relative predominance of Th1-type cytokines. Clin Diagn Lab Immunol. 1998;5:401–403. doi: 10.1128/cdli.5.3.401-403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tateda K, Moore T A, Deng J C, Newstead M W, Zeng X, Matsukawa A, Swanson M S, Yamaguchi K, Standiford T J. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J Immunol. 2001;166:3355–3361. doi: 10.4049/jimmunol.166.5.3355. [DOI] [PubMed] [Google Scholar]
- 48.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- 49.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 50.Watson P. A slide centrifuge: an apparatus for concentrating cells in suspension onto a microscope slide. J Lab Clin Med. 1966;68:494–501. [PubMed] [Google Scholar]
- 51.Winn W C, Davis G S, Gump D W, Craighead J E, Beaty H N. Legionnaires' pneumonia after intratracheal inoculation of guinea pigs and rats. Lab Investig. 1982;47:568–578. [PubMed] [Google Scholar]
- 52.Yang J, Kawamura I, Mitsuyama M. Requirement of the initial production of gamma interferon in the generation of protective immunity of mice against Listeria monocytogenes. Infect Immun. 1997;65:72–77. doi: 10.1128/iai.65.1.72-77.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang T, Kawakami K, Qureshi M H, Okamura H, Kurimoto M, Saito A. Interleukin-12 (IL-12) and IL-18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect Immun. 1997;65:3594–3599. doi: 10.1128/iai.65.9.3594-3599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]