Abstract
Increasing age appears to influence several morphologic changes in major tendons. However, the effects of aging on the cross‐sectional area (CSA) of different ankle tendons are much less understood. Furthermore, potential differences in specific tendon regions along the length of the tendons have not been investigated in detail. Sixty healthy adult participants categorized by age as young (n = 20; mean ± SD age = 22.5 ± 4.5 years), middle‐age (n = 20; age = 40.6 ± 8. 0 years), or old (n = 20; age = 69.9 ± 9.1 years), from both sexes, were included. The tendon CSA of tibialis anterior (TA), tibialis posterior (TP), fibularis (FT), and Achilles (AT) was measured from T1‐weighted 1.5 T MR images in incremental intervals of 10% along its length (from proximal insertion) and compared between different age groups and sexes. The mean CSA of the AT was greater in the middle‐age group than both young and old participants (p < 0.01) and large effect sizes were observed for these differences (Cohen's d > 1). Furthermore, there was a significant difference in CSA in all three groups along the length of the different tendons. Region‐specific differences between groups were observed in the distal portion (90% and 100% of the length), in which the FT presented greater CSA comparing middle‐age to young and old (p < 0.05). In conclusion, (1) great magnitude of morpho‐structural differences was discovered in the AT; (2) there are region‐specific differences in the CSA of ankle tendons within the three groups and between them; and (3) there were no differences in tendon CSA between sexes.
Keywords: Achilles tendon, aging, elderly, plantar flexors, tendon size
The main findings of this study are: (1) great magnitude of morpho‐structural differences was discovered in the AT; (2) there are region‐specific differences in the CSA of ankle tendons within the three groups and between them; and (3) there were no differences in tendon CSA between sexes.
1. INTRODUCTION
Several age‐associated changes in tendon properties have been described in animal and human models. Studies have shown that biochemical, cellular, and pathological changes can cause progressive tendon deterioration and contribute to age‐related debilities in physical function and clinical burden (de Cássia et al., 2017; Marqueti et al., 2018; Stenroth et al., 2015). A change in structural, mechanical, and material properties is probably linked to the decline of function and impairments observed in older individuals, which may increase the risk of injuries. Regarding structural and mechanical aspects, it is hard to separate the effects of aging per se and those effects of age‐related decline in levels of physical activity (Lindemann et al., 2020; Magnusson et al., 2007; Magnusson & Kjaer, 2019; McCrum et al., 2018; Svensson et al., 2016).
There are accurate and valid methods to measure tendon CSA (Hayes et al., 2019; Pons et al., 2018), which appear to be responsive to interventions, such as exercises, immobilization, and also aging (Arampatzis et al., 2007, 2010; Hansen et al., 2003; Magnusson et al., 2003; Mansur, de Noronha, et al., 2021; Svensson et al., 2016). What is known from these studies is that exercise increases tendon CSA (Arampatzis et al., 2007, 2010), and that immobilization leads to a rapid decline in the mechanical properties of the tendon (Psatha et al., 2012). No attempt has been made to fully investigate age‐induced differences and differences between sexes in tendon CSA of other ankle tendons such as tibialis anterior (TA), tibialis posterior (TP), and fibularis (FT). Among the likely reasons for this critical gap in the literature are that the Achilles tendon (AT) is the most common ankle tendon injured, especially in athletes, and the technical difficulty of measuring the other tendons due to their anatomical characteristics. The AT has a straight course, while the others have a curved course around the ankle. Furthermore, the anatomy of these tendons is less familiar to clinicians (De Maeseneer et al., 2018).
Interestingly, aging appears to result in reductions in the AT stiffness and its elastic modulus, whereas the data on changes in the CSA are conflicting. Some studies reported a CSA increase in older participants, while others showed a decline (Magnusson et al., 2003; Onambele et al., 2006; Pang & Ying, 2006; Stenroth et al., 2015). The discrepancy observed between these studies may be attributed to the use of ultrasonography to measure the AT CSA, which has been shown to have insufficient reliability (Bohm et al., 2016; Ekizos et al., 2013). Sample size and the limited number of transversal scans (typically from three regions) and the significant heterogeneity of participants among studies (Magnusson et al., 2003; Onambele et al., 2006; Stenroth et al., 2015) are additional possible confounding factors.
Most of the studies assessed the AT CSA at a specific tendon length (usually where the CSA is assumed to be the narrowest), which varied between three and four centimeters proximal to the insertion of the AT at the calcaneus (Csapo et al., 2014; Magnusson et al., 2003; Stenroth et al., 2015). The remaining studies used an average of a few measures of CSA (usually three) of different tendon lengths (Arampatzis et al., 2007, 2010; Magnusson et al., 2003). However, since AT has a variation in shape from proximal to distal as it approaches the calcaneal insertion site, a simple reference point is likely to be insufficient to generate measures that truly represent the condition of the tendon (Benjamin et al., 2008).
Previous studies have shown the presence of regional changes in the AT CSA induced by training in young adults (Arampatzis et al., 2007; Kongsgaard et al., 2005; Magnusson et al., 2003; Nuri et al., 2017). Non‐uniform stress concentration occurs along the tendon's length, especially at entheses (i.e., region of insertion of the tendon), where there is a high‐stress concentration (Benjamin et al., 2008; Magnusson et al., 2003). The different stress patterns may provoke diverse cellular reactions, and thus the tendon's vascularity, shape, and size can influence the tendons' morphology (Barkhausen et al., 2003). Factors like aging and gender may cause different changes in CSA in specific regions for each tendon of the ankle because they have distinct functions and morphology. A recent review (McCrum et al., 2018) analyzing age‐related differences in human leg muscle‐tendon units highlighted the lack of studies comparing specific regions of different tendons and between ages.
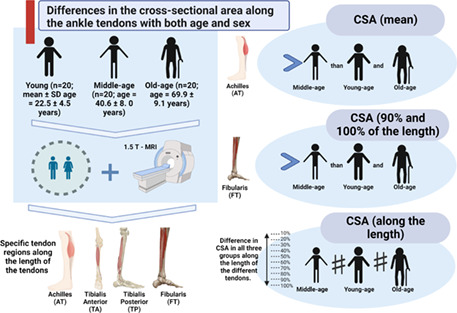
The present study investigates the age‐related changes and differences between sexes of the CSA for the main ankle tendons (TA, TP, FT, and AT), measured by MRI. We compared young, middle‐age, and old adults of both sexes; also, we evaluated the tendons in detail, comparing potential regional differences in CSA along the tendons, and comparing these regions between the three groups.
2. MATERIALS AND METHODS
2.1. Study design
This is a cross‐sectional observational study conducted to determine the effects of age on the CSA of the main ankle tendons (TA, TP, FT, and AT), by comparing young, middle‐age, and old adults of both sexes. In addition, we assessed possible differences in the CSA of specific regions at increments of 10% along the length of the tendons and the age‐related modifications in the CSA of these regions comparing between the three groups. Data were collected according to the Declaration of Helsinki and approved by an institutional Ethics Committee of Instituto de Pesquisa e Ensino HOME (IPE HOME) (Protocol n° 15816519.7.0000.0023) between November 2020 and May 2021. Informed consent was given by all participants before data collection.
2.2. Participants
Sixty men and women were divided into three groups according to their age as follows: young (age varying from 18 to 30 years), middle‐age (31 to 59 years), and old (≥60 years; Onambele et al., 2006; Stenroth et al., 2015; Thompson et al., 2013, 2014). We included healthy individuals that performed or not recreational physical activities. We excluded participants with history of previous ankle surgery or other orthopedic or neurological abnormalities of the lower limb; neurological, muscular, metabolic, or cardiovascular diseases or conditions that could affect muscle strength, such regular strength training or competitive sports.
2.3. MRI acquisition
All participants underwent magnetic resonance imaging (MRI) of the ankle. The device used was the Philips Ingenia 1.5 T scanner, and the scanning time lasted approximately 20 min per participant. Participants laid supine with the hip and knee extended and the ankle fixed in a relaxed position, including axial, coronal, and sagittal sequences with fat saturation (SPIR; Bohm et al., 2014, 2016). Specific parameters of the axial T1‐weighted sequence: Acquisition time of 03:12 min, TR of 2285, TE of 10, matrix size 200 × 240, field of view 120 × 151 mm, pixel size 0.6 × 0.6 mm, section thickness of 4 mm with interspace thickness of 0.4 mm. The coil used was Head, centered just anterior to the tibiotalar joint with a serial reception of eight channels. Imaging was obtained continuously from the middle third of the leg to the lowest aspect of the calcaneus (Bohm et al., 2014, 2016).
2.4. Segmentation technique
The TA, TP, FT, and AT had the CSA manually measured in the axial T1‐weighted sequence using the software OsiriX® (Pixmeo SARL, version 2.5.1; Arampatzis et al., 2007, 2010; Bohm et al., 2014, 2016). The entire tendon area visible in each image was manually outlined, excluding the peritendinous sheath, through the tracing technique (Mansur, Estanislau, et al., 2021). All three raters (H.M., B.A.S.A, and G.E.) were physicians and blinded to patient identity and group.
The tendons were segmented and analyzed in increments of 10% along the length of the tendons through specific regions of interest (ROI). The sagittal images served to identify the locations of two landmarks to standardize the levels of the axial images. For the TA the landmarks were the most distal aspect of the muscle and at the level of the tibiotalar joint (Numkarunarunrote et al., 2007; Varghese & Bianchi, 2013); for TP the landmarks were the most distal portion of the muscle and the most distal portion of the medial malleolus (Lockard et al., 2019; Numkarunarunrote et al., 2007); for FT the landmarks were the most distal aspect of the fibularis longus muscle and the tip of the lateral malleolus, to include the peroneal tunnel (Davda et al., 2017; Numkarunarunrote et al., 2007); and for AT landmarks were the most distal portion of the soleus muscle and the most proximal portion of the calcaneal tuberosity (Arampatzis et al., 2007, 2010). We defined ROIs for the tendons because some ran down along the ankle and followed a curved course around the malleolus, resulting in an artefactual signal (“the magic angle effect”). Therefore, it is difficult to acquire images perpendicular to the tendon on MRI, making the tendon borders ill‐defined (Brushøj et al., 2006; Davda et al., 2017; Lockard et al., 2019).
We employed the intra‐class correlation coefficient (ICC) to define the reliability of the CSA measurements of each tendon analyzed. Two independent raters, both physicians (HM and BASA) measured the CSA of each tendon once and they were blinded to the other rater's measurements. The overall ICC reliability was in the range of 0.91–0.98 among the different tendon CSA.
2.5. Normalization of data
To compare the structural tendon properties between groups, CSA was normalized to body mass to the power of 3/4 (m3/4). The power of 3/4 was chosen because allometric parameters that relate surfaces (e.g., tendon CSA) to body mass are closer to 3/4 than to the 2/3 predicted by geometric similarity (Kongsgaard et al., 2005; Markovic & Jaric, 2004).
2.6. Statistical analysis
The quantitative outcomes were expressed as mean and standard deviation (±SD) or frequency distribution. Normality was consistently checked using the Shapiro–Wilk test. We used multiple one‐way analysis of variance (ANOVA) and Bonferroni post hoc comparisons to examine if differences existed along tendon length within each group and between each group, for each tendon (TA, TP, FT, and AT) or its segment analyzed. Mann–Whitney tests were used to determine if differences existed between women and men. An alpha level of 0.05 was considered significant. Effect size (Cohen's d) was calculated for variables with statistical significant differences and interpreted as trivial (0 < d < 0.2), small (0.2 ≤ d < 0.5), medium (0.5 ≤ d < 0.8), and large (d ≥ 0.8; Cohen, 1992). All statistical analyses were performed using IBM SPSS Statistics software version 24.0 for Windows (SPSS Inc.).
3. RESULTS
Our sample included 60 participants (mean age of 44.4 ± 19.9 years; mean body weight 77.9 ± 15.4 kg), with 20 individuals for each group, young (22.5 ± 4.5), middle‐age (40.6 ± 8.00), and old (69.9 ± 9.1), with the same numbers of men and women in each group. The baseline characteristics and tendon CSA for each group are described in Table 1.
TABLE 1.
Comparison of the mean cross‐sectional area (CSA) of the ankle tendons between different groups (young, middle‐age, and old)
| Variable | Sample (n = 60) | Young (n = 20) | Middle‐age (n = 20) | Old (n = 20) | p‐value | Middle‐age X Young (ES) | Middle‐age X Old (ES) |
|---|---|---|---|---|---|---|---|
| Side (R: L) | 30: 30 | 9: 11 | 8: 12 | 13: 7 | — | — | — |
| Age | 44.4 ± 19.9 | 22.5 ± 4.5 | 40.6 ± 8.00 | 69.9 ± 9.1 | <0.01 | — | — |
| Gender (M: F) | 30: 30 | 10: 10 | 10: 10 | 10: 10 | — | — | — |
| Weight | 77.9 ± 15.4 | 72.4 ± 16.5 | 80.6 ± 17.8 | 74.7 ± 9.8 | 0.32 | — | — |
| TA_CSA_normalized | 0.64 ± 0.15 | 0.65 ± 0.13 | 0.67 ± 0.09 | 0.61 ± 0.18 | 0.29 | 0.18 | 0.42 |
| TP_CSA_normalized | 0.85 ± 0.23 | 0.91 ± 0.22 | 0.87 ± 0.16 | 0.80 ± 0.28 | 0.28 | 0.21 | 0.31 |
| FT_CSA_normalized | 0.99 ± 0.25 | 0.93 ± 0.18 | 1.06 ± 0.21 | 0.93 ± 0.28 | 0.13 | 0.66 | 0.53 |
| AT_CSA_normalized | 2.59 ± 0.52 | 2.51 ± 0.58 | 3.42 ± 0.58 | 2.61 ± 0.51 | <0.01 a | 1.57 b | 1.48 b |
p‐value <0.01 as shown as bold.
Abbreviations: AT, Achilles; ES, effect size; FT, fibularis; mm2/Kg3/4, normalization; TA, tibialis anterior; TP, tibialis posterior.
There were significant differences of the AT CSA between middle‐age and young and between middle‐age and old groups (ANOVA one‐way and Bonferroni post hoc comparison; p < 0.01).
The effect size for both comparisons was considered large according to Cohen's d values.
The mean CSA of AT was significantly greater in the middle‐age group when compared with both the young group (p < 0.01) and the old group (p < 0.01), with a large effect for both comparisons (Cohen's d = 1.57 and 1.48, respectively) (Tables 1 and 2). Figures 1, 2, 3, 4, show the axial and sagittal identification MRI for each tendon and the differences within tendon CSA for each tendon at every 10% of length.
TABLE 2.
Comparison between groups (young, middle‐age, and old) for the CSA of ankle tendons
| TENDON | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | TA | TP | FT | AT | ||||||||
| Young | Middle‐age | Old | Young | Middle‐age | Old | Young | Middle‐age | Old | Young | Middle‐age | Old | |
| 1 | 0.84 | 0.69 | 0.95 | 1.29 | 0.93 | 1.26 | 1.41 | 0.93 | 1.42 | 2.95 | 2.48 | 3.45 |
| 2 | 0.69 | 0.78 | 0.60 | 0.83 | 0.83 | 0.57 | 0.83 | 1.30 | 0.85 | 2.33 | 3.51 | 2.42 |
| 3 | 0.67 | 0.66 | 0.70 | 0.88 | 0.87 | 0.66 | 0.92 | 0.89 | 1.36 | 2.23 | 2.68 | 2.99 |
| 4 | 0.70 | 0.69 | 0.47 | 0.81 | 1.04 | 0.91 | 0.91 | 1.11 | 1.26 | 2.70 | 2.91 | 2.98 |
| 5 | 0.58 | 0.56 | 0.59 | 0.84 | 0.76 | 0.67 | 1.00 | 1.11 | 0.98 | 2.48 | 2.61 | 2.70 |
| 6 | 0.62 | 0.80 | 0.61 | 1.07 | 0.74 | 0.62 | 0.85 | 1.26 | 0.83 | 2.51 | 3.37 | 2.40 |
| 7 | 0.49 | 0.74 | 0.62 | 0.97 | 0.93 | 0.99 | 0.84 | 1.20 | 0.83 | 2.31 | 3.55 | 2.83 |
| 8 | 0.76 | 0.65 | 0.63 | 0.93 | 0.71 | 0.94 | 0.84 | 1.13 | 0.92 | 2.34 | 2.71 | 2.53 |
| 9 | 0.55 | 0.53 | 0.82 | 0.82 | 0.87 | 1.06 | 0.87 | 1.14 | 1.29 | 1.80 | 3.60 | 2.98 |
| 10 | 0.38 | 0.53 | 0.73 | 0.53 | 0.92 | 0.77 | 0.75 | 0.56 | 0.92 | 1.69 | 3.17 | 2.66 |
| 11 | 0.45 | 0.53 | 0.61 | 0.64 | 0.64 | 0.93 | 0.69 | 0.92 | 1.01 | 1.96 | 3.12 | 1.98 |
| 12 | 0.71 | 0.80 | 0.50 | 1.07 | 0.83 | 1.27 | 1.29 | 1.12 | 0.76 | 2.45 | 3.50 | 1.86 |
| 13 | 0.83 | 0.66 | 0.73 | 1.06 | 0.91 | 0.92 | 1.20 | 0.96 | 1.16 | 2.63 | 3.47 | 2.84 |
| 14 | 0.80 | 0.57 | 0.88 | 0.88 | 1.32 | 1.09 | 0.95 | 1.02 | 1.04 | 2.67 | 3.21 | 2.95 |
| 15 | 0.75 | 0.74 | 0.76 | 1.32 | 1.13 | 0.96 | 1.03 | 1.11 | 0.90 | 3.62 | 4.00 | 3.33 |
| 16 | 0.55 | 0.66 | 0.56 | 0.74 | 0.73 | 0.70 | 0.76 | 0.80 | 1.04 | 3.64 | 3.95 | 2.78 |
| 17 | 0.65 | 0.80 | 0.37 | 0.80 | 0.76 | 0.42 | 0.84 | 1.48 | 0.40 | 2.16 | 3.91 | 1.65 |
| 18 | 0.50 | 0.79 | 0.37 | 0.71 | 0.76 | 0.33 | 0.78 | 1.13 | 0.61 | 2.30 | 3.75 | 1.98 |
| 19 | 0.75 | 0.60 | 0.20 | 1.32 | 0.79 | 0.31 | 1.03 | 0.80 | 0.47 | 3.62 | 4.17 | 3.09 |
| 20 | 0.76 | 0.76 | 0.43 | 0.68 | 0.90 | 0.55 | 0.87 | 1.25 | 0.57 | 1.75 | 4.73 | 1.87 |
| Mean | 0.65 | 0.67 | 0.61 | 0.91 | 0.87 | 0.80 | 0.93 | 1.06 | 0.93 | 2.51 * | 3.42 * | 2.61 * |
Note: There were significant differences in the mean AT CSA between middle‐age and young and between middle‐age and old groups (ANOVA one‐way test and Bonferroni post hoc comparison; p‐value <0.01).
Abbreviations: AT, Achilles; ES, effect size; FT, fibularis; mm2/Kg3/4, normalization; TA, tibialis anterior; TP, tibialis posterior.
FIGURE 1.
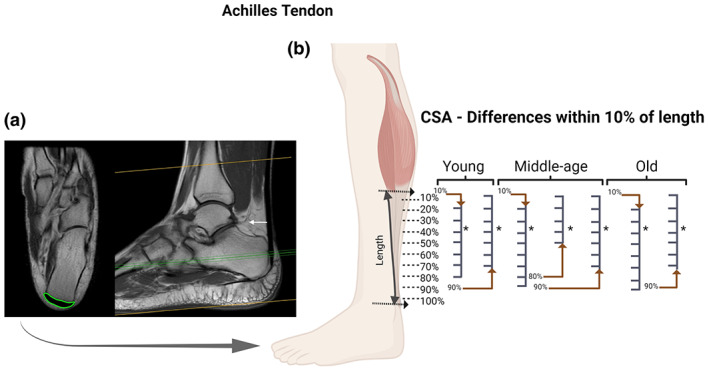
Achilles tendon (AT): Axial and coronal T1 MRI images (cross‐sectional area [CSA] measurement in “green” and white arrow pointing to the location of the proximal landmark) (a); and statistical differences identified between specific regions of the AT with CSA measurements at every 10% of tendon length in each age group (b). For the AT we observed region‐specific differences in the CSA along the length of the tendon within the three groups. BioRender web‐based software was used to create the figure (license number MT23TQ8O9Z)
FIGURE 2.
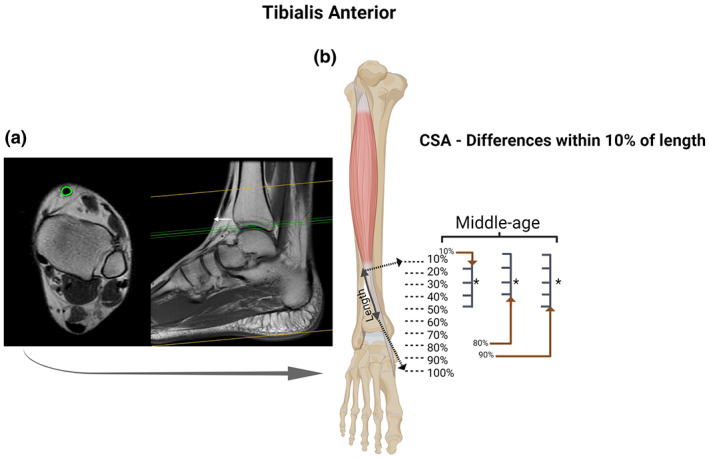
Tibialis anterior (TA) tendon: Axial and coronal T1 MRI images (cross‐sectional area [CSA] measurement in “green” and white arrow pointing to the location of the proximal landmark) (a); and statistical differences identified between specific regions of the TA with CSA measurements at every 10% of tendon length in each age group (b). For the TA we observed region‐specific differences in the CSA along the length of the tendon only in the middle‐age group. BioRender web‐based software was used to create the figure (license number HB23TQ9TX0)
FIGURE 3.
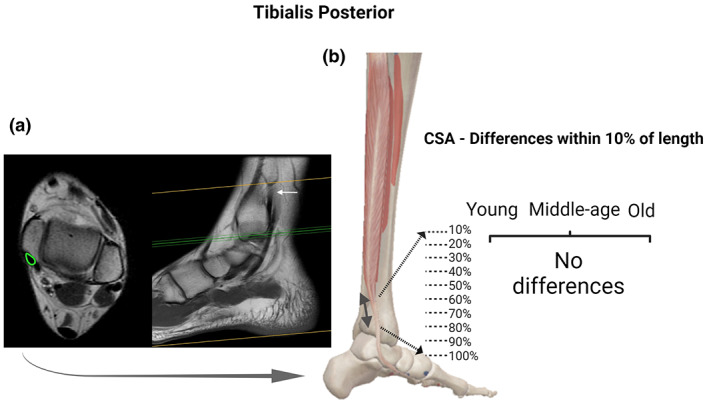
Tibialis posterior (TP) tendon: Axial and coronal T1 MRI images (cross‐sectional area [CSA] measurement in “green” and white arrow pointing to the location of the proximal landmark) (a); and statistical differences identified between specific regions of the TP with CSA measurements at every 10% of tendon length in each age group (b). For the TP we observed no region‐specific differences in the CSA along the length of the tendon. BioRender web‐based software was used to create the figure (license number QX23TQ8E6J)
FIGURE 4.
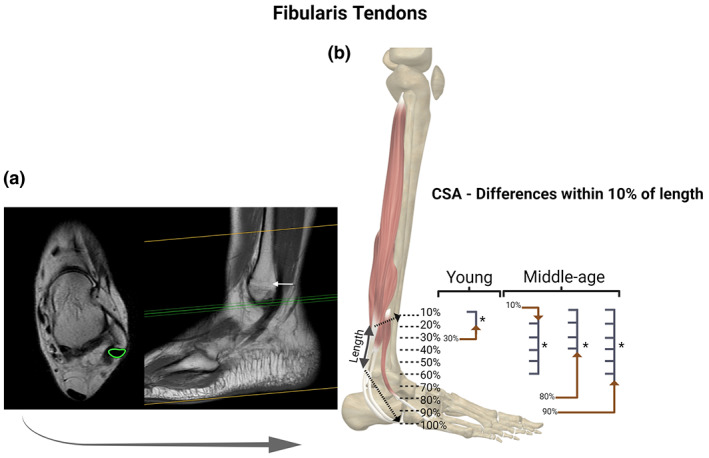
Fibularis tendon (FT): Axial and coronal T1 MRI images (cross‐sectional area [CSA] measurement in “green” and white arrow pointing to the location of the proximal landmark) (a); and statistical differences identified between specific regions of the FT with CSA measurements at every 10% of tendon length in each age group (b). For the FT we observed region‐specific differences in the CSA along the length of the tendon within young and middle‐age groups. BioRender web‐based software was used to create the figure (license number OS23TQ8JMQ)
In the three groups (young, middle‐age, and old) there were specific regions along the tendons length (TA, FT, and AT) with statistically different CSA. We observed differences within FT and AT for the young group (Figures 1b and 4b); AT, TA, and FT for the middle‐age group (Figures 1b, 2b and 4b, respectively); and in AT for the old group (Figure 1). Along the entire tendon length, the only differences observed between groups were in the FT (Figure 5). Middle‐age participants presented greater CSA in the portion of 90% and 100% of the FT length compared to young and old groups (p < 0.05; Figure 5d). Cohen's d values were 0.76 and 0.87 in the portion of 90% and 0.76 and 0.94 of 100%, comparing FT of middle‐age versus young and old, respectively.
FIGURE 5.
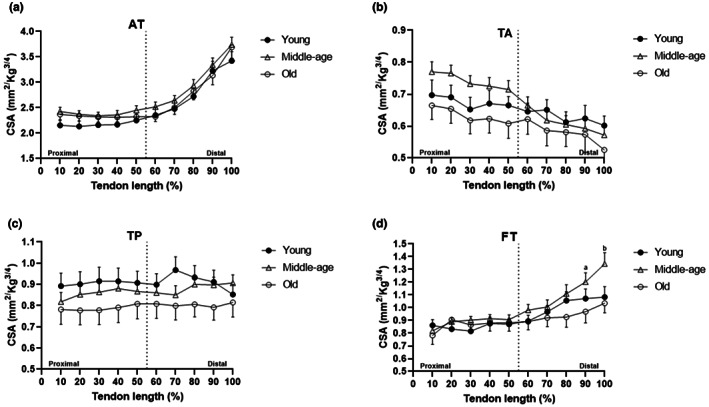
Statistical differences identified between specific regions with cross‐sectional areas (CSA) measurements at every 10% of tendon length between age groups for AT, TA, TP, and FT. for AT (a), TA (b), and TP (c) there were no differences between groups; for FT (d) there were statistically significant differences in the distal portion (90% [a] and 100% [b] of the length) between middle‐age and young and between middle‐age and old groups. BioRender web‐based software was used to create the figure (license number PF23TQ8ZY3)
There were the same number of men (n = 30; mean age of 45.7 ± 20.9 years; mean body weight 85.4 ± 12.6 kg) and women (n = 30; mean age of 43.1 ± 19.3 years; mean body weight 70.3 ± 14.3 kg) in our sample. The only difference observed between sexes was the weight (p < 0.01), with no difference in any tendon CSA, observed between women and men participants. However, when analyzing, per group, the non‐normalized data presented differences between sex for all tendons in the young group, TA and AT in the middle‐age group, and AT in the old group, with large effects observed for AT differences (Table 3).
TABLE 3.
Comparison of the mean cross‐sectional area (CSA) of the ankle tendons between men and women for young, middle‐age, and old groups, for normalized and non‐normalized data
| Group | Variable | Men | Women | Effect size (p‐value) | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Young (n = 20) | TA‐CSA_normalized | 0.68 | 0.12 | 0.63 | 0.16 | 0.53 (0.54) |
| TP‐CSA normalized | 0.92 | 0.22 | 0.88 | 0.24 | 0.17 (0.65) | |
| FT‐CSA normalized | 0.97 | 0.21 | 0.92 | 0.19 | 0.25 (0.82) | |
| AT‐CSA normalized | 2.60 | 0.65 | 2.34 | 0.57 | 0.43 (0.36) | |
| TA‐CSA non‐normalized | 18.88 | 3.89 | 14.41 | 3.02 | 1.28 b (0.019 a ) | |
| TP‐CSA non‐normalized | 25.08 | 3.26 | 19.69 | 3.14 | 1.68 b (<0.01 a ) | |
| FT‐CSA non‐normalized | 26.60 | 4.56 | 20.98 | 2.36 | 1.55 b (<0.01 a ) | |
| AT‐CSA non‐normalized | 71.04 | 13.20 | 53.14 | 8.86 | 1.59 b (<0.01 a ) | |
| Middle‐age (n = 20) | TA‐CSA normalized | 0.67 | 0.09 | 0.66 | 0.10 | 0.11 (0.89) |
| TP‐CSA normalized | 0.84 | 0.10 | 0.90 | 0.20 | 0.38 (0.59) | |
| FT‐CSA normalized | 1.09 | 0.24 | 1.09 | 0.26 | 0 (0.82) | |
| AT‐CSA normalized | 2.78 | 0.40 | 2.64 | 0.43 | 0.34 (0.45) | |
| TA‐CSA non‐normalized | 19.54 | 2.61 | 16.28 | 1.99 | 1.40 b (0.013 a ) | |
| TP‐CSA non‐normalized | 24.64 | 2.70 | 22.24 | 4.76 | 0.62 (0.13) | |
| FT‐CSA non‐normalized | 32.03 | 6.24 | 26.99 | 7.20 | 0.75 (0.17) | |
| AT‐CSA non‐normalized | 81.58 | 11.26 | 64.87 | 10.36 | 1.54 b (<0.01 a ) | |
| Old (n = 20) | TA‐CSA normalized | 0.64 | 0.29 | 0.58 | 0.06 | 0.29 (0.49) |
| TP‐CSA normalized | 0.78 | 0.34 | 0.79 | 0.22 | 0.03 (0.70) | |
| FT‐CSA normalized | 0.94 | 0.35 | 0.97 | 0.24 | 0.09 (0.89) | |
| AT‐CSA normalized | 2.68 | 0.64 | 2.49 | 0.42 | 0.35 (0.23) | |
| TA‐CSA non‐normalized | 16.71 | 7.07 | 14.11 | 2.18 | 0.49 (0.19) | |
| TP‐CSA non‐normalized | 20.52 | 8.51 | 19.24 | 5.65 | 0.18 (0.49) | |
| FT‐CSA non‐normalized | 24.61 | 8.52 | 23.83 | 6.73 | 0.10 (0.70) | |
| AT‐CSA non‐normalized | 71.06 | 16.84 | 60.26 | 8.83 | 0.80 b (0.07 a ) | |
p‐values are in parentheses as shown as bold.
Abbreviations: AT, Achilles; ES, effect size; FT, fibularis; mm2/Kg3/4, normalization; SD, Standard deviation; TA, tibialis anterior; TP, tibialis posterior.
There were significant differences for non‐normalized CSA tendon between genders in young and middle‐age groups (ANOVA one‐way and Bonferroni post hoc comparison).
The effect size for comparisons was considered large according to Cohen's d values.
4. DISCUSSION
The main findings of the present study were the lack of differences in some of the ankle tendons (TA, TP, and FT) with aging; however, great morpho‐structural differences were discovered in the AT, as indicated by a greater CSA measured through MRI in the middle‐age group compared with both young and old individuals. In addition, region‐specific differences in the CSA along the length of all tendons were observed in all three groups. Furthermore, regional differences in the CSA between groups were present only in the distal portion (90% and 100%) of the FT, which was larger in the middle‐age than the young and old groups. Lastly, the differences in the CSA of the ankle tendons between men and women were observed just for non‐normalized data, for all groups.
Alterations in the structure, mechanical, and material properties of the tendons have been reported (Geremia et al., 2018; Lindemann et al., 2020; McCrum et al., 2018; Svensson et al., 2016). Since the tendon is a mechanosensitive and adaptive tissue, depending on its exposure to mechanical loading its properties can alter (McCrum et al., 2018; Svensson et al., 2016). For example, a consistent reduction in tendon stiffness (defined as force‐elongation relationship of the tendon) with age has been shown in the literature (McCrum et al., 2018). In addition, there is a relatively consistent reduction in Young's modulus (i.e., the slope of the stress–strain relationship, where stress is tendon force relative to CSA and strain is tendon elongation in relation to resting length) with age. However, there is no consensus in the literature regarding the effects of aging on tendon CSA, which is directly linked to the other properties (McCrum et al., 2018). Animal data propose that aging is associated with an increase in tendon CSA, however, the human model reported conflicting results (Birch et al., 1999; Magnusson et al., 2003; Onambele et al., 2006; Pang & Ying, 2006; Svensson et al., 2016). A previous study (Onambele et al., 2006) has suggested progressive reductions in the AT in middle‐age and old when compared to young individuals. Meanwhile, other authors (Couppé et al., 2014; Magnusson et al., 2003; Stenroth et al., 2015; Svensson et al., 2016; Tweedell et al., 2016) have reported an increase in tendon CSA in older individuals.
Some important points in the methodologies could explain the conflicting results among studies. One point is that the CSA measurement has mostly been performed with an ultrasound (US), a method with questionable accuracy (Bohm et al., 2016; Ekizos et al., 2013); a second point is the different landmarks to determine the tendon CSA; and a third point is heterogeneity in participants when comparing studies (McCrum et al., 2018). For example, Onambele et al. (Onambele et al., 2006) measured AT CSA of 70 participants using the US. The participants were men and women divided into three groups (24 young [24 ± 1 year], 10 middle‐aged [46 ± 1 year], and 36 older [68 ± 1 year]), and the average of three CSA measures was calculated. Stenroth et al. (2015) assessed the AT CSA of 100 participants (33 young [24 ± 2 years] and 67 old [75 ± 3 years]) also using US, and Magnusson et al. (2003) included 19 women (9 young and 10 elderly) in their study and determined the AT CSA by a single measure from MRI. To our knowledge, this is the first study measuring CSA of the four main tendons of the ankle (TA, TP, FT, and AT), including 60 participants equally divided into three groups, categorized by age as young, middle‐age, and old, with the same numbers of men and women. We used 10 CSA measures to calculate the average for each tendon and observed a significantly greater CSA only in the AT in the middle‐age group when compared to young and old groups. Great morpho‐structural differences were ratified by large effect sizes (Cohen's d values of 1.57 and 1.48 for the difference between young vs. middle‐age and middle‐age vs. old groups, respectively). These findings reinforce the importance of calculating p‐values accompanied by the effect size for observational and interventional studies (Fritz et al., 2012; Harrington et al., 2019).
The results observed in our study differ from previous studies and could be explained by several adaptive mechanisms. The reduced mechanical function of the tendon associated with lower voluntary neural activation of muscles occurs in older individuals compared to middle‐age individuals, inducing a decrease in tendon CSA (Tweedell et al., 2016; Wu et al., 2016). Moreover, in middle‐age individuals, the increase in the AT CSA may follow an intensification in the collagen formation and remodeling, counteracting the reduction in tendon material properties (stiffness and Young's modulus; Stenroth et al., 2015) as a tendon mechanical safety factor (Magnusson et al., 2003). Interestingly, a previous study (Thompson et al., 2013) showed results similar to ours with age‐related changes in the neuromuscular function of the ankle plantar flexors. The middle‐age group (43 ± 2 years) presented enhanced rates of muscle activation, with greater force compared to young (age = 22 ± 2 years) and old (69 ± 5 years) individuals (Thompson et al., 2013). Svensson et al (Thompson et al., 2013), investigated the mechanical properties of the human patellar tendon (PT) from the young (26 ± 4 years) and elderly (66 ± 1 years) men and women, and the results showed that mechanical properties were not substantially altered through age or sex. In contrast, the physiology and level of physical activity are entirely different in the elderly (Arampatzis et al., 2007; Stenroth et al., 2015). The reduction in activity leads to a lower biosynthesis of connective tissue components, such as diminished collagen formation and remodeling (Kjaer et al., 2005), indicating that the magnitude of tendon loading is a key factor in the adaptive responses. The present results suggest that age mainly affects the most loaded ankle tendon, namely the Achilles tendon. Future studies should explore the effect of age and the underlying cause of these changes in AT in greater detail.
It is well‐accepted that tendons can remodel their mechanical and morphological properties in response to mechanical loading (Magnusson et al., 2003; McCrum et al., 2018). In addition, in an innovative study, Magnusson and Kjaer (2003) showed a significant difference in AT CSA along its length, with the most distal portion up to 85% greater than the proximal portion, suggesting variability in the structural properties of the tendon. Confirming these findings, a previous study (Arampatzis et al., 2007) demonstrated region‐specific hypertrophy of the AT, with an increase in the CSA at 60 and 70% of its length after 14 weeks of high‐strain‐magnitude exercise. However, to our knowledge, no previous study has measured the potential‐specific region differences along the length of the four main ankle tendons, and between groups of different ages (McCrum et al., 2018). Here, we observed that besides AT, the TA, TP, and FT also presented significant differences in the CSA of specific regions along their lengths in young, middle‐age, and old individuals. Moreover, the only region‐specific differences observed between groups were a greater CSA in the distal portion (90% and 100% of the length) of the FT in the middle‐age group compared to young and old groups. Therefore, the regional differences in tendon metabolism or non‐uniform elongations of the tendons along their length may result in different region‐specific adaptations (Magnusson et al., 2003; Magnusson & Kjaer, 2003). Furthermore, our findings strongly suggest that these tendon capacities remain with aging, with region‐specific differences in the CSA along the length of all tendons observed similarly in all three groups of age (McCrum et al., 2018; Svensson et al., 2016).
Few studies have investigated the effect of gender on tendon properties, most of them analyzing patellar and AT properties (Kongsgaard et al., 2007; Magnusson et al., 2007; Sarver et al., 2017). In the present study, there were no differences in CSA of ankle tendons between men and women for normalized data. Previous studies reported similar results for different lower limb tendons. Burgess et al. (Burgess et al., 2009) reported similarities between genders in the patellar properties, including CSA, tendon stiffness, and Young's modulus, in elderly participants. Furthermore, Morrison et al. (Morrison et al., 2015) showed no differences in the AT stiffness or maximum isometric force between genders. A recent study (Sarver et al., 2017) with mice showed that male and female tendons have very similar mechanical properties and biochemical composition, without AT CSA differences. Interestingly, (Magnusson et al., 2007) highlighted the different adaptability of patellar tendon between genders showing a significant increase in tendon CSA only for trained men, while trained and untrained women had similar tendon sizes. However, comparing both untrained men and women, similar patellar tendon CSA was observed (Magnusson et al., 2007). Curiously, we observed that non‐normalized data presented differences between genders for some tendons in the young, middle‐age, and old groups. A comparable finding was observed in a recent study (Abián et al., 2021) examining patellar tendon morphology. When absolute measurements were analyzed, greater strength was recorded for men; however, when strength was normalized by lower limb muscle mass the results showed no difference between men and women. Therefore, it suggests the importance of adequate data normalization for the correct assessment of tendon properties.
There were several limitations within the present study. First, we could not measure the length, nor the entire tendons CSA from the point where a curved course around the ankle would be present for some of the tendons, resulting in an artefactual signal called “the magic angle effect,” making it difficult to correctly measure its borders (Davda et al., 2017; Lockard et al., 2019; Varghese & Bianchi, 2013). Second, the FT (i.e., brevis and longus) appear black in the MRI and run in close connection, creating further difficulties in defining the borders for each tendon. Therefore, we calculated the CSA of both tendons as one tendon (FT; Lockard et al., 2019; Mansur, Estanislau, et al., 2021). Third, we did not precisely evaluate the level of activity or the exercise regimes of each participant; however, we excluded individuals who were engaged in regular strength training or competitive sports since it had already been shown the capacity of the tendon to adapt to exercises (Arampatzis et al., 2007; Magnusson et al., 2003; McCrum et al., 2018; Svensson et al., 2016).
To our knowledge, this was the first study that collectively analyzed the effect of aging on CSA of the four main ankle tendons, and in detail investigated the potential region‐specific differences of tendon CSA by comparing between different age groups, and the differences between men and women. Besides expanding the current concepts of the literature, our findings can be used as normative values for clinical studies once all participants were healthy individuals. However, further studies are necessary for the complete understanding of the differences observed between the CSA of young, middle‐age, and old groups.
5. CONCLUSIONS
This study assessed the age‐related changes and differences between gender of the main tendon's ankle CSA for the first time using MRI by comparing young, middle‐age, and old adults of both sexes. The major results suggest that: (1) great magnitude of morpho‐structural differences were discovered in the AT, as demonstrated by large effects sizes; (2) there were region‐specific differences in the CSA of the ankle tendons within the three groups and between them; and (3) no differences between men and women were observed for any tendon CSA.
AUTHOR CONTRIBUTION
HM conceived and designed the research, performed the experiments, analyzed the data, interpreted results of experiments, drafted manuscript, edited and revised manuscript, and approved final version of manuscript. JLQD, MN, MK, and PM edited and revised manuscript and approved final version of manuscript. BASA performed experiments and approved final version of manuscript. RCM prepared figures, edited and revised manuscript, and approved final version of manuscript.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
Data S1
ACKNOWLEDGEMENTS
This study was financed in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior ‐ Brasil (CAPES) ‐ Finance Code 001, Fundação de Apoio a Pesquisa do Distrito Federal (FAPDF) (grant number 00193.00000773/2021‐72, 00193.00000859/2021‐3; 00193.00001222/2021‐26), and the National Council for Scientific and Technological Development (CNPq; process numbers 309435/2020‐0 and 310269/2021). The authors are also grateful for the financial support provided by the Decanato de Pós‐Graduação (grant DPG/DPI N. 02/2022) and PPGEF 11 n.11/2022.
Mansur, H. , Durigan, J.L.Q. , de Noronha, M. , Kjaer, M. , Magnusson, S.P. & de Araújo, B.A.S. et al. (2023) Differences in the cross‐sectional area along the ankle tendons with both age and sex. Journal of Anatomy, 242, 213–223. Available from: 10.1111/joa.13774
This paper was carried out at Santa Helena Hospital, Brasilia – DF, Brazil.
DATA AVAILABILITY STATEMENT
It is available.
REFERENCES
- Abián, P. , Martínez, F. , Jiménez, F. & Abián‐Vicén, J. (2021) Morphology of the patellar tendon and the contractility response of the quadriceps: symmetry and gender analysis. International Journal of Environmental Research and Public Health, 18, 5309. 10.3390/ijerph18105309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arampatzis, A. , Karamanidis, K. & Albracht, K. (2007) Adaptational responses of the human Achilles tendon by modulation of the applied cyclic strain magnitude. The Journal of Experimental Biology, 210(August [Pt 15]), 2743–2753. 10.1242/jeb.003814 [DOI] [PubMed] [Google Scholar]
- Arampatzis, A. , Peper, A. , Bierbaum, S. & Albracht, K. (2010) Plasticity of human Achilles tendon mechanical and morphological properties in response to cyclic strain. Journal of Biomechanics, 43(December 16), 3073–3079. 10.1016/j.jbio-mech.2010.08.014 [DOI] [PubMed] [Google Scholar]
- Barkhausen, T. , van Griensven, M. , Zeichen, J. & Bosch, U. (2003) Modulation of cell functions of human tendon fibroblasts by different repetitive cyclic mechanical stress patterns. Experimental and Toxicologic Pathology, 55(2–3), 153–158. 10.1078/0940-2993-00302 [DOI] [PubMed] [Google Scholar]
- Benjamin, M. , Kaiser, E. & Milz, S. (2008) Structure‐function relationships in tendons: a review. Journal of Anatomy, 212(3), 211–228. 10.1111/j.1469-7580.2008.00864.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch, H.L. , McLaughlin, L. , Smith, R.K. & Goodship, A.E. (1999) Treadmill exercise‐induced tendon hypertrophy: assessment of tendons with different mechanical functions. Equine Veterinary Journal. Supplement, 30, 222–226. 10.1111/j.2042-3306.1999.tb05222.x [DOI] [PubMed] [Google Scholar]
- Bohm, S. , Mersmann, F. , Schroll, A. , Mäkitalo, N. & Arampatzis, A. (2016) Insufficient accuracy of the ultrasound‐based determination of Achilles tendon cross‐sectional area. Journal of Biomechanics, 49, 2932–2937. 10.1016/j.jbiomech.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Bohm, S. , Mersmann, F. , Tettke, M. , Kraft, M. & Arampatzis, A. (2014) Human Achilles tendon plasticity in response to cyclic strain: effect of rate and duration. The Journal of Experimental Biology, 217(November Pt 22), 4010–4017. 10.1242/jeb.112268 [DOI] [PubMed] [Google Scholar]
- Brushøj, C. , Henriksen, B.M. , Albrecht‐Beste, E. , Hölmich, P. , Larsen, K. & Bachmann, N.M. (2006) Reproducibility of ultrasound and magnetic resonance imaging measurements of tendon size. Acta Radiologica, 47(9), 954–959. 10.1080/02841850600854936 [DOI] [PubMed] [Google Scholar]
- Burgess, K.E. , Pearson, S.J. , Breen, L. & Onambélé, G.N. (2009) Tendon structural and mechanical properties do not differ between genders in a healthy community‐dwelling elderly population. Journal of Orthopaedic Research, 27(6), 820–825. 10.1002/jor.20811 [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1992) A power primer. Psychological Bulletin, 112(1), 155–159. 10.1037//0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- Couppé, C. , Svensson, R.B. , Grosset, J.F. , Kovanen, V. , Nielsen, R.H. , Olsen, M.R. et al. (2014) Life‐long endurance running is associated with reduced glycation and mechanical stress in connective tissue. Age (Dordrecht, Netherlands), 36(4), 9665. 10.1007/s11357-014-9665-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csapo, R. , Malis, V. , Hodgson, J. & Sinha, S. (2014) Age‐related greater Achilles tendon compliance is not associated with larger plantar flexor muscle fascicle strains in senior women. Journal of Applied Physiology, 116, 961–969. 10.1152/japplphysiol.01337.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davda, K. , Malhotra, K. , O'Donnell, P. , Singh, D. & Cullen, N. (2017) Peroneal tendon disorders. EFORT Open Reviews, 2(6), 281–292. 10.1302/2058-5241.2.160047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cássia, M.R. , Almeida, J.A. , Nakagaki, W.R. , Guzzoni, V. , Boghi, F. , Renner, A. et al. (2017) Resistance training minimizes the biomechanical effects of aging in three different rat tendons. Journal of Biomechanics, 28(53), 29–35. 10.1016/j.jbiomech.2016.12.029 [DOI] [PubMed] [Google Scholar]
- De Maeseneer, M. , Madani, H. , Lenchik, L. , De Mey, J. , Provyn, S. & Shahabpour, M. (2018) Ultrasound of the distal insertions of the ankle and foot tendons with anatomical correlation: a review. Canadian Association of Radiologists Journal, 69(3), 282–292. 10.1016/j.carj.2017.11.005 [DOI] [PubMed] [Google Scholar]
- Ekizos, A. , Papatzika, F. , Charcharis, G. , Bohm, S. , Mersmann, F. & Arampatzis, A. (2013) Ultrasound does not provide reliable results for the measurement of the patellar tendon cross sectional area. Journal of Electromyography and Kinesiology, 23, 1278–1282. 10.1016/j.jelekin.2013.08.004 [DOI] [PubMed] [Google Scholar]
- Fritz, C.O. , Morris, P.E. & Richler, J.J. (2012) Effect size estimates: current use, calculations, and interpretation. Journal of Experimental Psychology: General, 141(1), 2–18. 10.1037/a0024338 Epub 2011 Aug 8. Erratum in: J Exp Psychol Gen. 2012 Feb;141(1):30. [DOI] [PubMed] [Google Scholar]
- Geremia, J.M. , Baroni, B.M. , Bobbert, M.F. , Bini, R.R. , Lanferdini, F.J. & Vaz, M.A. (2018) Effects of high loading by eccentric triceps surae training on Achilles tendon properties in humans. European Journal of Applied Physiology, 118(8), 1725–1736. 10.1007/s00421-018-3904-1 [DOI] [PubMed] [Google Scholar]
- Hansen, P. , Aagaard, P. , Kjaer, M. , Larsson, B. & Magnusson, S.P. (2003) Effect of habitual running on human Achilles tendon load‐deformation properties and cross‐sectional area. Journal of Applied Physiology (1985), 95(6), 2375–2380. 10.1152/japplphysiol.00503.2003 [DOI] [PubMed] [Google Scholar]
- Harrington, D. , D'Agostino, R.B., Sr. , Gatsonis, C. , Hogan, J.W. , Hunter, D.J. , Normand, S.T. et al. (2019) New guidelines for statistical reporting in the journal. The New England Journal of Medicine, 381(3), 285–286. 10.1056/NEJMe1906559 [DOI] [PubMed] [Google Scholar]
- Hayes, A. , Easton, K. , Devanaboyina, P.T. , Wu, J.P. , Kirk, T.B. & Lloyd, D. (2019) A review of methods to measure tendon dimensions. Journal of Orthopaedic Surgery and Research, 14(1), 18. 10.1186/s13018-018-1056-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer, M. , Langberg, H. , Miller, B.F. , Boushel, R. , Crameri, R. , Koskinen, S. et al. (2005) Metabolic activity and collagen turnover in human tendon in response to physical activity. Journal of Musculoskeletal & Neuronal Interactions, 5(1), 41–52. [PubMed] [Google Scholar]
- Kongsgaard, M. , Aagaard, P. , Kjaer, M. & Magnusson, S.P. (2005) Structural Achilles tendon properties in athletes subjected to different exercise modes and in Achilles tendon rupture patients. Journal of Applied Physiology (1985), 99(5), 1965–1971. 10.1152/japplphysiol.00384.2005 [DOI] [PubMed] [Google Scholar]
- Kongsgaard, M. , Reitelseder, S. , Pedersen, T.G. , Holm, L. , Aagaard, P. , Kjaer, M. et al. (2007) Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiologica (Oxford, England), 191(2), 111–121. 10.1111/j.1748-1716.2007.01714.x [DOI] [PubMed] [Google Scholar]
- Lindemann, I. , Coombes, B.K. , Tucker, K. , Hug, F. & Dick, T.J.M. (2020) Age‐related differences in gastrocnemii muscles and Achilles tendon mechanical properties in vivo. Journal of Biomechanics, 9(112), 110067. 10.1016/j.jbiomech.2020.110067 [DOI] [PubMed] [Google Scholar]
- Lockard, C.A. , Chang, A. , Clanton, T.O. & Ho, C.P. (2019) T2* mapping and subregion analysis of the tibialis posterior tendon using 3 tesla magnetic resonance imaging. The British Journal of Radiology, 92(1104), 20190221. 10.1259/bjr.20190221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson, S.P. , Beyer, N. , Abrahamsen, H. , Aagaard, P. , Neergaard, K. & Kjaer, M. (2003) Increased cross‐sectional area and reduced tensile stress of the Achilles tendon in elderly compared with young women. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 58(2), 123–127. 10.1093/gerona/58.2.b123 [DOI] [PubMed] [Google Scholar]
- Magnusson, S.P. , Hansen, M. , Langberg, H. , Miller, B. , Haraldsson, B. , Westh, E.K. et al. (2007) The adaptability of tendon to loading differs in men and women. International Journal of Experimental Pathology, 88(4), 237–240. 10.1111/j.1365-2613.2007.00551.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson, S.P. & Kjaer, M. (2003) Region‐specific differences in Achilles tendon cross‐sectional area in runners and non‐runners. European Journal of Applied Physiology, 90(5–6), 549–553. 10.1007/s00421-003-0865-8 [DOI] [PubMed] [Google Scholar]
- Magnusson, S.P. & Kjaer, M. (2019) The impact of loading, unloading, ageing and injury on the human tendon. The Journal of Physiology, 597(5), 1283–1298. 10.1113/JP275450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur, H. , de Noronha, M. , Marqueti, R.C. & Durigan, J.L.Q. (2021) Acute lateral ankle sprain alters muscle and tendon properties: case series. Foot and Ankle Surgery, S1268‐7731(21), 00098‐9. 10.1016/j.fas.2021.05.008 [DOI] [PubMed] [Google Scholar]
- Mansur, H. , Estanislau, G. , Noronha, M. , Marqueti, R.C. , Fachin‐Martins, E. & Durigan, J.L.Q. (2021) Intra‐ and inter‐rater reliability for the measurement of the cross‐sectional area of ankle tendons assessed by magnetic resonance imaging. Acta Radiologica, 11, 2841851211003284. 10.1177/02841851211003284 [DOI] [PubMed] [Google Scholar]
- Markovic, G. & Jaric, S. (2004) Movement performance and body size: the relationship for different groups of tests. European Journal of Applied Physiology, 92(1–2), 139–149. 10.1007/s00421-004-1076-7 [DOI] [PubMed] [Google Scholar]
- Marqueti, R.C. , Durigan, J.L.Q. , Oliveira, A.J.S. , Mekaro, M.S. , Guzzoni, V. , Aro, A.A. et al. (2018) Effects of aging and resistance training in rat tendon remodeling. The FASEB Journal, 32(1), 353–368. 10.1096/fj.201700543R [DOI] [PubMed] [Google Scholar]
- McCrum, C. , Leow, P. , Epro, G. , König, M. , Meijer, K. & Karamanidis, K. (2018) Alterations in leg extensor muscle‐tendon unit biomechanical properties with ageing and mechanical loading. Frontiers in Physiology, 28(9), 150. 10.3389/fphys.2018.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison, S.M. , Dick, T.J.M. & Wakeling, J.M. (2015) Structural and mechanical properties of the human Achilles tendon: sex and strength effects. Journal of Biomechanics, 48(12), 3530–3533. 10.1016/j.jbiomech.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numkarunarunrote, N. , Malik, A. , Aguiar, R.O. , Trudell, D.J. & Resnick, D. (2007) Retinacula of the foot and ankle: MRI with anatomic correlation in cadavers. AJR. American Journal of Roentgenology, 188(4), W348–W354. 10.2214/AJR.05.1066 [DOI] [PubMed] [Google Scholar]
- Nuri, L. , Obst, S.J. , Newsham‐West, R. & Barrett, R.S. (2017) Regional three‐dimensional deformation of human Achilles tendon during conditioning. Scandinavian Journal of Medicine & Science in Sports, 27(11), 1263–1272. 10.1111/sms.12742 [DOI] [PubMed] [Google Scholar]
- Onambele, G.L. , Narici, M.V. & Maganaris, C.N. (2006) Calf muscle‐tendon properties and postural balance in old age. Journal of Applied Physiology (1985), 100(6), 2048–2056. 10.1152/japplphysiol.01442.2005 [DOI] [PubMed] [Google Scholar]
- Pang, B.S. & Ying, M. (2006) Sonographic measurement of achilles tendons in asymptomatic subjects: variation with age, body height, and dominance of ankle. Journal of Ultrasound in Medicine, 25(10), 1291–1296. 10.7863/jum.2006.25.10.1291 [DOI] [PubMed] [Google Scholar]
- Pons, C. , Borotikar, B. , Garetier, M. , Burdin, V. , Ben Salem, D. , Lempereur, M. et al. (2018) Quantifying skeletal muscle volume and shape in humans using MRI: a systematic review of validity and reliability. PLoS One, 13(11), e0207847. 10.1371/journal.pone.0207847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psatha, M. , Wu, Z. , Gammie, F.M. , Ratkevicius, A. , Wackerhage, H. , Lee, J.H. et al. (2012) A longitudinal MRI study of muscle atrophy during lower leg immobilization following ankle fracture. Journal of Magnetic Resonance Imaging, 35(3), 686–695. 10.1002/jmri.22864 [DOI] [PubMed] [Google Scholar]
- Sarver, D.C. , Kharaz, Y.A. , Sugg, K.B. , Gumucio, J.P. , Comerford, E. & Mendias, C.L. (2017) Sex differences in tendon structure and function. Journal of Orthopaedic Research, 35(10), 2117–2126. 10.1002/jor.23516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenroth, L. , Sillanpää, E. , Mcphee, J.S. , Narici, M.V. , Gapeyeva, H. , Pääsuke, M. et al. (2015) Plantarflexor muscle‐tendon properties are associated with mobility in healthy older adults. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 70, 996–1002. 10.1093/gerona/glv011 [DOI] [PubMed] [Google Scholar]
- Svensson, R.B. , Heinemeier, K.M. , Couppé, C. , Kjaer, M. & Magnusson, S.P. (2016) Effect of aging and exercise on the tendon. J Appl Physiol (1985), 121(6), 1237–1246. 10.1152/japplphysiol.00328.2016 [DOI] [PubMed] [Google Scholar]
- Thompson, B.J. , Ryan, E.D. , Herda, T.J. , Costa, P.B. , Herda, A.A. & Cramer, J.T. (2014) Age‐related changes in the rate of muscle activation and rapid force characteristics. Age (Dordrecht, Netherlands), 36(2), 839–849. 10.1007/s11357-013-9605-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, B.J. , Ryan, E.D. , Sobolewski, E.J. , Conchola, E.C. & Cramer, J.T. (2013) Age related differences in maximal and rapid torque characteristics of the leg extensors and flexors in young, middle‐aged and old men. Experimental Gerontology, 48(2), 277–282. 10.1016/j.exger.2012.10.009 [DOI] [PubMed] [Google Scholar]
- Tweedell, A.J. , Ryan, E.D. , Scharville, M.J. , Rosenberg, J.G. , Sobolewski, E.J. & Kleinberg, C.R. (2016) The influence of ultrasound measurement techniques on the age‐related differences in Achilles tendon size. Experimental Gerontology, 76, 68–71. 10.1016/j.exger.2016.01.015 [DOI] [PubMed] [Google Scholar]
- Varghese, A. & Bianchi, S. (2013) Ultrasound of tibialis anterior muscle and tendon: anatomy, technique of examination, normal and pathologic appearance. Journal of Ultrasound, 17(2), 113–123. 10.1007/s40477-013-0060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, R. , Delahunt, E. , Ditroilo, M. , Lowery, M. & De Vito, G. (2016) Effects of age and sex on neuromuscular‐mechanical determinants of muscle strength. Age (Dordrecht, Netherlands), 38(3), 57. 10.1007/s11357-016-9921-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data Availability Statement
It is available.


