The management of COVID‐19 infection in cardiac transplant patients requires a careful balance between antiviral and immunosuppressive therapies to avoid allograft rejection. Routine surveillance endomyocardial biopsy (EMB) is the standard of care during the 6–12 months post‐transplant. 1 Non‐invasive monitoring methods using gene expression profiling (AlloMap) and donor‐derived cell‐free DNA (AlloSure) can also help rule out acute cellular rejection of grade 2R or higher. 1
A 34‐year‐old female with a history of end‐stage peripartum cardiomyopathy status post orthotopic heart transplant presented for routine surveillance EMB 7 months post‐transplant. Her calculated panel reactive antibodies were 0%. Her post‐transplant course was complicated by early episodes of acute cellular rejection due to subtherapeutic tacrolimus levels. Just prior to routine EMB, her AlloMap and AlloSure had significantly increased on therapeutic tacrolimus levels (Figure 1). She was found to be positive for COVID‐19 on pre‐procedural screening. Symptoms were mild fatigue and headache. EMB showed grade 2R acute cellular rejection (Figure 2), along with negative staining of perimyocyctic capillaries for C4d and C3d. No donor‐specific antibodies were present. The echocardiogram was unchanged with a normal left ventricular ejection fraction. SARS‐CoV‐2 levels were measured by PCR of RNA isolated from the biopsy specimen and were undetectable. Inflammatory marker levels, including white blood cell count, D‐dimer, ferritin, and lactate dehydrogenase, were mildly elevated. The patient was treated with two courses of high‐dose pulse steroids along with adjustments to her immunosuppressive regimen. Prednisone was increased from 15 to 40 mg daily and tacrolimus was increased from a goal trough of 8–10 to 12–15. Mycophenolate mofetil was maintained at a high dose throughout. Her transplant rejection abated and her COVID‐19 infection did not worsen. The patient remained positive on PCR testing for COVID‐19 over the next 6 months.
FIGURE 1.
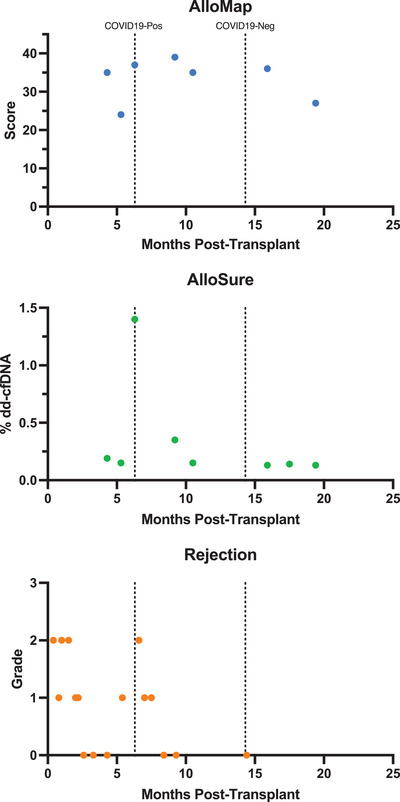
AlloMap, Allosure, and rejection levels of our patient over time. In patients ≥6 months post‐transplant, an Allomap score >34 is associated with a higher probability of acute rejection. However, the Allomap score must also be interpreted in relation to previous scores as a significant increase would also be considered a higher risk of rejection. For Allosure, >0.15% dd‐cfDNA is associated with a higher probability of acute rejection.
FIGURE 2.
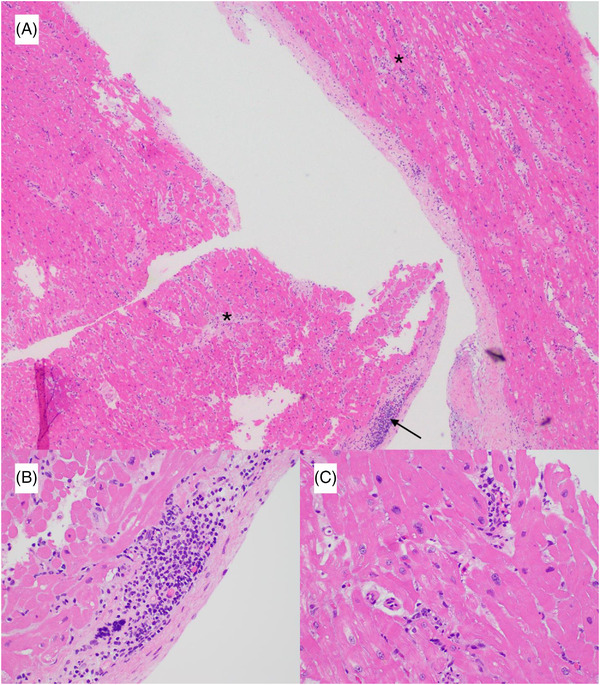
Hematoxylin and eosin stain at (A) 4× magnification of myocardial tissue showing >2 foci of lymphocytic infiltrate associated with myocyte damage (asterisk) and endocardial inflammatory infiltrates (arrow) (B) 40× magnification showing endocardial inflammatory infiltrates (C) 40× magnification showing myocyte injury.
This case describes concurrent COVID‐19 infection and presumed acute cellular rejection detected via AlloMap and AlloSure with confirmation by EMB. However, the diagnosis of rejection, in this case, is complicated by concurrent COVID‐19 infection as SARS‐CoV‐2 has been implicated as an etiology of myocarditis. While her AlloMap and AlloSure were elevated, these are not specific for rejection and instead broadly indicate increased immune system activity and allograft injury, respectively, both of which can be seen in acute myocarditis. Furthermore, acute cellular rejection and myocarditis are difficult to distinguish on pathology due to overlapping criteria. The Dallas criteria for myocarditis requires the presence of inflammatory cellular infiltrates with or without myocyte necrosis. 2 Similarly, acute cellular rejection is defined by the presence of inflammatory cellular interstitial and/or perivascular infiltrate with at least one focus of myocyte damage. 3
Mechanistically, SARS‐CoV‐2 may enter myocardial cells via ACE2 receptors leading to direct viral damage. 4 SARS‐CoV‐2 can trigger the systemic release of proinflammatory cytokines from which immune‐mediated myocardial damage can occur. 4 In heart transplant patients, we hypothesize whether SARS‐CoV‐2 may stimulate the immune system (despite immunosuppressive medications) and cause an autoimmune attack on the transplanted organ. In our case, SARS‐CoV‐2 levels were undetectable by PCR on four different biopsy occasions despite persistent positivity on nasopharyngeal testing, making direct viral damage less likely. Our patient had mild COVID‐19 symptoms and did not have significantly elevated inflammatory markers; thus, a cytokine release syndrome causing acute myocarditis is less likely. Given our patient's history of rejection, a favorable response to increased immunosuppression, and the lack of SARS‐CoV‐2 detected in myocardial tissue, we believe acute cellular rejection to be the diagnosis. While our case supports the possibility that SARS‐CoV‐2 triggers an elevated immune response that in transplant recipients may induce allograft rejection, we cannot exclude the possibility that her COVID‐19 infection and acute cellular rejection were two independent processes.
Interestingly, our patient's AlloMap remained elevated in association with her persistent COVID‐19 infection, while her AlloSure and EMB results reflected successful treatment of her rejection. This suggests that persistent COVID‐19 infection may lead to a persistently heightened immune response that does translate into persistent subclinical myocardial injury.
Management should be personalized based on the individual's risk of rejection and the severity of COVID‐19 infection. Considering our patient had a history of rejection and mild COVID‐19 symptoms, she was managed with high‐dose pulse steroids as well as increases to her oral prednisone and tacrolimus trough goals in her daily immunosuppressive regimen. While antimetabolites are typically discontinued or reduced in the setting of active infection, her mycophenolate mofetil dose remained unchanged.
In conclusion, we describe a case of acute cellular rejection of a transplanted heart associated with mild COVID‐19 infection. In heart transplant recipients with mild COVID‐19 infection, treatment for acute cellular rejection may not worsen COVID‐19 infection.
AUTHOR CONTRIBUTION
Crystal Lihong Yan made substantial contributions to the acquisition and interpretation of data and the drafting and critical revision of the manuscript. Gaurav Gupta made substantial contributions to the interpretation of data and drafting of the manuscript. Luiz Paulo Guido and Philip Ruiz made substantial contributions to the acquisition and analysis of data and review of the manuscript. Eugene Joseph Bauerlein III made substantial contributions to the concept and review of the manuscript. Nina Thakkar Rivera made substantial contributions to the concept, design, and critical revision of the manuscript. All authors approve of the submitted and final versions.
CONFLICTS OF INTEREST
The authors have no conflicts of interest related to this work.
ACKNOWLEDGMENTS
The authors have no acknowledgments. This work received no funding.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Costanzo MR, Dipchand A, Starling R, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29(8):914‐956. [DOI] [PubMed] [Google Scholar]
- 2. Cooper LT Jr. Myocarditis. N Engl J Med. 2009;360(15):1526‐1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24(11):1710‐1720. [DOI] [PubMed] [Google Scholar]
- 4. Tajbakhsh A, Gheibi Hayat SM, Taghizadeh H, et al. COVID‐19 and cardiac injury: clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev Anti Infect Ther. 2021;19(3):345‐357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


