Abstract
Background
Published data on coronavirus disease 2019 (COVID‐19) convalescent plasma (CCP) use in children and obstetric patients are limited. We describe a single‐center experience of hospitalized patients who received CCP for acute COVID‐19.
Methods
A retrospective review of children 0–18‐years‐old and pregnant patients hospitalized with laboratory‐confirmed acute COVID‐19 who received CCP from March 1, 2020 to March 1, 2021 was performed. Clinical and laboratory data were collected to assess the safety of CCP administration. Antibodies to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) were measured in the CCP products and in patients before transfusion and at various time points post‐transfusion. Correlation between the administered SARS‐CoV‐2 administered versus the SARS‐CoV‐2 anti‐spike immunoglobulin response in patient serum was assessed.
Results
Twenty‐two children and ten obstetric patients were eligible. Twelve pediatric and eight obstetric patients had moderate disease and ten pediatric and two obstetric patients had severe disease. Five pediatric patients died. Eighteen of 37 (48.6%) CCP titers that were measured met US Food and Drug Administration (FDA) criteria for high immunoglobulin G (IgG) antibody titer. There were no complications with transfusion. High‐titer CCP showed a positive correlation with rise in patient total immunoglobulin levels only in obstetric patients but not in pediatric patients. Among pediatric patients, the median serum antibody level increased over time after transfusion.
Conclusions
Coronavirus 2019 convalescent plasma was administered safely to our patients. Our study suggested that CCP did not interfere with endogenous antibody production. The antibody titer of CCP correlated with post‐transfusion response only in obstetric patients. Randomized trials in pediatric and obstetric patients are needed to further understand how to dose CCP and evaluate efficacy.
Keywords: child, COVID‐19, COVID‐19 serotherapy, pregnancy, SARS‐CoV‐2
INTRODUCTION
Historically, convalescent plasma has been utilized during outbreaks of emergent infectious diseases to provide passive antibody‐based immunity to vulnerable individuals and reduce the risk of infection (prophylaxis) or lessen the impact of disease (therapeutic use). 1 , 2 , 3
There are limited therapeutic options for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. Multiple treatments, including convalescent plasma, monoclonal antibodies, antivirals, and anti‐inflammatory agents are being investigated. In the USA, the use of COVID‐19 convalescent plasma (CCP) obtained from donors who recovered from acute COVID‐19 was initially available only through Investigational New Drug (INDs) application for compassionate use in children and an extended access research protocol for adults, including pregnant persons, but since August 2020, it has been approved under Emergency Use Authorization (EUA) by the US Food and Drug Administration (FDA). In February 2021, the FDA modified the EUA to limit CCP use to only high‐titer products, defined as high neutralizing antibody titer measured by the c‐PASS Neutralization Antibody assay (Genscript) or immunoglobulin G (IgG) using the VITROS 5600 II enzyme‐linked immunoassay (OrthoClinical Diagnostics, Inc.). 4 , 5
Houston has seen more than 955,000 people with confirmed SARS‐CoV‐2 infection and 8200 deaths since the beginning of pandemic as of July, 2022. 6 At Texas Children's Hospital and its affiliated maternity Pavillion for Women in Houston, over 14,000 patients tested positive for SARS‐CoV‐2 and 1982 were admitted as of May 14, 2021. Convalescent plasma was used as per FDA guidance for the treatment of hospitalized COVID‐19 patients during the period of March 1, 2020 to March 1, 2021.
The safety, optimal dosing, timing, and frequency of transfusions and the efficacy of CCP in pediatric and obstetric patients hospitalized with COVID‐19 are yet to be established. We report on our center's experience with the use of CCP under EUA for pediatric and obstetric patients hospitalized with moderate to severe COVID‐19 during the first year of the pandemic. In addition to assessing the safety of CCP administration, we sought to understand better how to utilize CCP in these populations for whom limited data are available. We evaluated the association between CCP dose (antibody titers and volume) and post‐transfusion antibody titers over time in transfusion recipients.
MATERIALS AND METHODS
Study design
A retrospective review was conducted of acute COVID‐19 patients who were treated with CCP at the Texas Children's Hospital and Pavilion for Women from March 1, 2020 to March 1, 2021. The protocol was approved by the Baylor College of Medicine Institutional Review Board (IRB) protocol H‐47500.
Study objectives
The primary objective of this study was to describe the patient characteristics and safety of CCP administration in hospitalized pediatric and obstetric patients treated under EUA for moderate to severe COVID‐19. Secondary objectives included the assessment of SARS‐CoV‐2 antibody levels of transfused CCP and serologic responses in treated patients when data were available. We also sought to determine the correlation of post‐transfusion antibodies with CCP unit titer and administered volume of the transfusion. Exploratory objectives included the assessment of a clinical response and mortality from COVID‐19 after CCP administration.
Patient selection
Pediatric patients aged <18 years and pregnant patients were included if they were hospitalized with COVID‐19 confirmed by SARS‐CoV‐2 detection via molecular methods – reverse‐transcriptase polymerase chain reaction (RT‐PCR) or transcription mediated amplification (TMA) testing – from a nasopharyngeal swab either at our facility or outside institutions and met FDA criteria and our institutional criteria for CCP administration (Appendix S1, COVID‐19 convalescent plasma institutional criteria for use). 5 Due to its limited availability in Houston early in the pandemic, CCP was used in hospitalized patients with moderate to severe disease and patients who had underlying comorbidities, including pregnancy, which increased their risk for severe COVID‐19 as per guidance from the Centers for Disease Control and Prevention (CDC). 4 , 7 Our institutional criteria for CCP administration included all of the following criteria: (1) Confirmed SARS‐CoV‐2 infection by positive SARS‐CoV‐2 molecular assay, (2) SARS‐CoV‐2 antibody negative prior to CCP administration, (3) no history of severe reactions to transfusion of blood products, and (4) not diagnosed with multisystem inflammatory syndrome in children (MIS‐C), AND at least one of the following: having a high‐risk condition for severe COVID‐19 OR having moderate or severe COVID‐19 (Appendix S1). The criterion for testing negative for SARS‐CoV‐2 antibody to be eligible for CCP was implemented during the national shortage of CCP and was not a strict requirement, depending on availability of CCP and the individual assessment of the patient's underlying comorbidities and clinical presentation.
COVID‐19 convalescent plasma administration
Pediatric and obstetric patients received the entire CCP unit whenever feasible. Among pediatric patients weighing less than 20 kg, and those with concerns for volume overload, CCP was administered divided in 5–10 ml/kg transfusions every 12 h until depletion of the unit (~200 ml). The number of units administered to each patient was determined by the primary clinical team in consultation with Transfusion Medicine and Infectious Diseases.
Antibody titers were not always available from the local blood center prior to CCP administration until February 2021 and, when measured, the units were labeled as “high” or “low titer” per FDA guidance, without quantification. As per EUA guidance, a sample of all CCP units administered was stored and subsequently tested for SARS‐CoV‐2 antibodies.
Data collection
We extracted demographic, clinical, radiographic, and laboratory data from patient electronic medical records to standardized data collection forms using REDCap electronic data capture tools hosted at Texas Children's Hospital. 8 We assessed it for any adverse events based on the Centers for Disease Control and Prevention, Hemovigilance Module Surveillance Protocol. 9 The number of days after CCP for fever resolution, removal of all respiratory support and recovery of lymphocytopenia, and the results of PCR testing (positive or negative) after the CCP administration were also recorded.
Blood samples were obtained from all patients prior to and after CCP administration per CCP administration protocol and when requested by the primary physicians as part of routine clinical care to measure antibodies to SARS‐CoV‐2.
Antibody titers
Antibody titers of CCP were measured using blood segments attached to the CCP units when available retrospectively. They were tested for quantitative SARS‐CoV‐2 IgG antibodies against the SARS‐CoV‐2 spike protein using the VITROS 5600 II enzyme‐linked immunoassay (OrthoClinical Diagnostics, Inc.) and neutralizing antibody titers assayed by serial dilution using the c‐PASS Neutralization Antibody assay (GenScript). All assays used had received FDA emergency use authorization. The CCP unit was categorized as “high titer” if the signal‐to‐cutoff (S/C) value, arbitrary unit (AU)/ml, was IgG 12 AU/ml or greater (during our study period) or if the neutralization titer was >1:80 based on FDA definitions. 5
Patient serum samples were tested for quantitative total SARS‐CoV‐2 immunoglobulins – Ig = Immunoglobin G (IgG), immunoglobulin M (IgM) and immunoglobulin A (IgA) – against the SARS‐CoV‐2 spike protein using the VITROS 5600 II enzyme‐linked immunoassay (OrthoClinical Diagnostics, Inc.).
Only patients who had documented CCP unit transfusion and as 1 day pre‐ and post‐transfusion total anti‐spike Ig quantification, obtained between 24 to 48 h after CCP transfusion, were included for evaluation of antibody response to CCP. The CCP dose administered was then calculated in terms of CCP volume per weight (ml/kg), total IgG transfused per kg (calculated by total IgG x unit volume/patient weight), and total IgG transfused (calculated by total IgG x unit volume). Serologic response in the patients was measured as change in total Ig levels between pre‐ and post‐transfusion states (ΔIg).
The correlation between the ΔIg and the total neutralizing CCP Ig administered was analyzed using linear regression using Microsoft Excel. Strong, moderate, mild, weak, and no correlation were defined as R 2 of 0.8–1.0, 0.6–0.8, 0.4–0.6, 0.2–0.4, and 0.0–0.2, respectively.
RESULTS
Patient demographics and clinical characteristics
In the first year of the pandemic, 32 patients with polymerase chain reaction (PCR)‐confirmed COVID‐19 received CCP under FDA authorization. The first administration occurred in June 2020, and the last in February 2021. Twenty‐two pediatric (age 3 weeks to 17 years) and 10 obstetric patients (age 20–36 years) met inclusion criteria for treatment during hospitalization. Demographics, and clinical and radiographic presentation are shown in Table 1. All but one pediatric patient had at least one underlying medical condition and there were often multiple conditions in the same patient (Table S1, Clinical details of the pediatric patients). Similarly, seven of the 10 obstetric patients had an underlying condition but were not as complex as the pediatric patients. The most common race and ethnicity was non‐Hispanic White. The most common presenting symptoms were fever, cough, and shortness of breath. The predominant diagnoses associated with COVID‐19 were acute respiratory failure and COVID‐19 pneumonia. Among pediatric patients, 12/22 (54.5%) had moderate disease and 10/22 (45.5%) had severe disease based on our institutional criteria (Appendix S1). Most obstetric patients were moderately ill (8/10, 80%) and two patients (20%) had severe disease. The majority of patients had lymphocytopenia and elevated inflammatory markers. Eleven of 22 (50%) pediatric patients had one or more coinfections during hospitalization, which included two (9.1%) patients with Pseudomonas aeruginosa (1 bacteremia and pneumonia/tracheitis, and one with pneumonia/tracheitis), two (9.1%) Escherichia coli (one enterocutaneous fistula abscess and one bacteremia), one (4.5%) Streptomyces sp. bacteremia, two (9.1%) Candida tropicalis urinary tract infection, one (4.5%) Candida albicans urinary tract infection, one (4.5%) clinically suspected progressive disseminated fungal infection, three (13.6%) Cytomegalovirus viremia and viruria, one (4.5%) rhinovirus infection (nasopharyngeal specimen), and one (4.5%) parainfluenza infection (nasopharyngeal specimen). None of the obstetric patients had coinfections.
TABLE 1.
Demographics, and clinical and radiographic presentation
| n (%) a | ||
|---|---|---|
| Pediatric, N = 22 | Obstetrics, N = 10 | |
| Age, median (range) | 12.9 years (3 weeks −17 years) | 31.2 years (20–36 years) |
| Female sex | 13 (59.1) | 10 (100) |
| Race/ethnicity | ||
| Hispanic White | 9 (40.9) | 10 (100) |
| NH Black | 1 (4.5) | 0 (0) |
| NH White | 11 (50) | 0 (0) |
| Asian | 1 (4.5) | 10 (100) |
| Weight, median [kg (range)] | 33.4 (5.6–170.6) | 79.4 (71–121.1) |
| BMI, median (range) | 23.9 (10.9–53.9) b | 31.4 (27–52.1) |
| Exposure known | 9 (40.9) c | 6 (60) d |
| Exposure unknown but family with similar symptoms | 2 (9.1) e | 1 (10) f |
| Underlying illness | 21 (95.5) g | 7 (70) h |
| Symptoms | ||
| Median duration of symptoms until admission [days (range)] | 2 (0–8) | 8 (2–13) |
| Fever | 20 (90.9) | 10 (100) |
| Cough | 17 (77.3) | 10 (100) |
| Shortness of breath/respiratory distress | 16 (72.7) | 10 (100) |
| Nausea/vomiting | 7 (31.8) | 2 (20) |
| Nasal congestion/rhinorrhea | 10 (45.5) | 4 (40) |
| Chest pain/discomfort/tightness | 5 (22.7) | 4 (40) |
| Abdominal pain | 3 (13.6) | 1 (10) |
| Loss of taste/smell | 0 | 4 (40) |
| Musculoskeletal pain | 3 (13.6) | 3 (30) |
| Loss of appetite/inability to eat | 2 (9.1) | 2 (20) |
| Seizures | 3 (13.6) | 0 |
| Headache | 1 (4.5) | 2 (20) |
| Blood group | ||
| O | 15 (68.2) | 7 (70) |
| A | 5 (22.7) | 3 (30) |
| B | 1 (4.5) | 0 |
| AB | 1 (4.5) | 0 |
| Laboratory data (median) | ||
| WBC [1000/μl (range)] | 7.49 (0.59–17.08) | 8.36 (5.52–14.70) |
| Peak WBC [1000/mcl (range)] | 13.76 (2.0–29.36) | 14.56 (7.38–21.77) |
| Lowest WBC [1000/mcl (range)] | 4.2 (0–9.91) | 6.04 (4.84–12.28) |
| Absolute lymphocyte count [1000/mcl (range)] | 0.935 (0.18–5.91) | 0.855 (0.43–1.21) |
| Lowest Absolute lymphocyte count [1000/mcl (range)] | 0.48 (0–1.83) | 0.625 (0.43–0.82) |
| Hemoglobin [g/dl (range)] | 11.55 (8.7–15.9) | 11.45 (10.0–13.1) |
| Lowest Hb [g/dl (range)] | 7.75 (5.2–12.9) | 9.55 (6.0–12.7) |
| Platelet [1000/mcl (range)] | 228 (20–548) | 218 (113–294) |
| Lowest Platelet [1000/mcl (range)] | 99 (5–294) | 200 (107–279) |
| Peak AST [U/L (range)] | 173 (41–1452) | 109 (38–509) |
| Peak ALT [U/L (range)] | 144 (6–305) | 116 (26–843) |
| Peak Creatinine [mg/dl (range)] | 0.50 (0.18–2.95) | 0.45 (0.35–0.65) |
| C reactive protein [mg/dl (range)] i | 6.85 (0.9–26.3) | 15.5 (1.5–29.0) |
| Peak C reactive protein [mg/dl (range)] | 15.4 (1.5–38.1) | 17.3 (4.5–32.7) |
| Peak procalcitonin [ng/dl (range)] j | 0.86 (0.05–194.05) | 0.195 (0.04–0.86) |
| Peak ferritin [ng/ml, ref 10–60 (range)] k | 432.5 (46–19,900) | 55 (18–314) |
| Peak fibrinogen [mg/dl, ref 220–440, (range)] l | 520.5 (270–927) | 672 (483–836) |
| Peak IL‐6 [pg/ml, ref ≤2.0, (range)] m | 31.3 (5.1–474.1) | 179 (179) |
| D‐dimer [μg/ml, ref ≤0.40, (range)] | 0.79 (0.27–11.77) | 1.01 (0.73–2.19) |
| Peak D‐dimer [μg/ml, ref ≤0.40, (range)] | 1.69 (0.41–20.0) | 1.33 (0.73–2.22) |
| Peak B type natriuretic peptide [pg/ml, ref < 100, (range)] n | 235 (10.0–4622.9) | 15.9 (6.0–71.4) |
| Chest radiograph finding upon presentation | ||
| Atelectasis/opacities | 16 (72.7) | 8 (80) |
| Infiltrate/consolidation | 8 (36.4) | 1 (10) |
| Bronchopneumonia/pneumonia | 9 (40.9) | 6 (60) |
| leural effusion | 4 (18.2) | 2 (20) |
| Echocardiogram | Total N = 17 | Total N = 4 |
| Normal | 7/17 (41.2) | 4/4 (100) |
| Depressed ventricular function | 3/17 (17.6) | ‐ |
| Valve regurgitation o | 3/17 (17.6) | ‐ |
| Dilation of coronary artery | 4/17 (23.5) | ‐ |
| Flattening of the interventricular in systole | 2/17 (11.8) | ‐ |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; IL‐6, interleukin 6; NH, non‐Hispanic; WBC, White blood cell.
Unless otherwise specified.
Obtained in 16/22 patients.
Exposure: multiple members (mother, sibling and non‐relative) 1, mother 2, grandfather 1, aunt 2, non‐relative 2, and undocumented 1.
Husband 3, Mother 1, a family member from Mexico 1, and workplace (Patient MA and exposed to a patient) 1.
Father 2.
Husband and stepson.
Main diagnosis are as follows: 5 (22.7%) with obesity, 3 (13.6%) with primary immunodeficiency, 3 (13.6%) with malignancy receiving treatment during the study period of which one had bone marrow transplant, 2 (9.1%) with prematurity with global development delay, 2 (9.1%) with Down syndrome with congenital heart disease, and neurologic disability, 1 (4.5%) with remote history of treated brain tumor with panhypopituitarism, 1 (4.5%) with mitochondrial disease with history of heart transplant, 1 (4.5%) rheumatic disease, 1 (4.5%) extensive vascular anomaly, 1 (4.5%) with autoimmune hepatitis and 1 (4.5%) neurological disease. Many pediatric patients had more than one risk factors for severe COVID‐19 disease according to CDC including eight with chronic lung disease including asthma, seven with obesity (defined as body mass index [BMI] ≥30), seven with immunosuppression use, four with liver disease, four with hypertension (HTN), four with a neurological disorder, three with a primary immune deficiency, three with prematurity, three with malignancy (additionally one patient with a history of brain tumor), two that were overweight (BMI 25 to <30) with Down syndrome, two with congenital heart disease, two with type 2 diabetes mellitus, one with a heart transplant, one with autologous bone marrow transplant, one with a vascular anomaly, one with chronic kidney disease, one with a history of cerebrovascular disease and one with metabolic disorder.
Seven had obesity of which one had type 2 diabetes and hypertension and one had asthma. Three others were overweight.
C‐reactive protein level obtained in all pediatric patients and 9/10 obstetric patients.
Procalcitonin level obtained in 20/22 pediatric patients and 10/10 obstetric patients.
Ferritin obtained in 20/22 pediatric patients and 10/10 obstetric patients.
Fibrinogen obtained in all pediatric patients and 9/10 obstetric patients.
Interleukin‐6 obtained in 8/22 pediatric patients and 1/10 obstetric patients.
B type natriuretic peptide obtained in 17/22 pediatric patients and 8/10 obstetric patients.
Trivial valve regurgitation not included.
COVID‐19 treatment and clinical care
Treatment and supportive care for all patients is shown in Table 2. In addition to CCP, almost all patients received remdesivir, dexamethasone, and antibiotics. One pediatric patient did not receive remdesivir due to elevated transaminases and a second due to lack of parental consent. One obstetric patient did not receive remdesivir due to lack of availability of the drug. Most received dexamethasone and the two patients who did not received other steroids.
TABLE 2.
Treatment, supportive care and outcomes
| n (%) a | ||
|---|---|---|
| Pediatric, N = 22 | Obstetric, N = 10 | |
| Treatments b | ||
| Remdesivir | 20 (90.9) | 9 (90) |
| Azithromycin | 10 (45.5) | 10 (100) |
| Antibiotics | 22 (100) | 10 (100) |
| Vasopressors | 8 (36.4) | 1 (10) |
| Heparin or enoxaparin | 18 (81.8) | 10 (100) |
| Anakinra | 5 (22.7) | 0 (0) |
| Dexamethasone | 20 (90.9) | 10 (100) |
| Betamethasone | 0 | 7 (70) |
| Respiratory support | ||
| Invasive mechanical ventilation | 10 (45.5) | 2 (20) |
| BiPAP/CPAP | 16 (72.7) | 0 (0) |
| High flow nasal cannula | 15 (68.2) | 7 (70) |
| Low flow oxygen | 11 (50) | 7 (70) |
| VV‐ECMO | 1 (4.5) | 1 (10) |
| VA‐ECMO | 2 (9.1) | 0 (0) |
| Duration of hospitalization days [median, (range)] | 18 (5–117) c | 8 (5–14) |
| Duration of ICU admission days [median, (range)] | 15.5 (5–42) | 4.5 (2–14) d |
| Discharged | 17 (77.3) | 10 (100) |
| Deceased | 5 (22.7) | 0 |
| New diagnosis/problem e | ||
| Pneumonia | 19 (86.4) | 10 (100) |
| Respiratory failure | 22 (100) | 10 (100) |
| Lymphocytopenia | 21 (95.5) | 10 (100) |
| Thrombocytopenia | 13 (59.1) | 1 (10) |
| ARDS | 3 (13.6) | 1 (10) |
| Cardiogenic shock | 2 (9.1) | 0 |
| Acute kidney injury | 7 (31.8) | 0 |
| Increased transaminases | 11 (50) | 5 (50) |
| Myocarditis | 1 (4.5) | 0 |
| Dilation of coronary artery | 4 (18.2) | 0 |
Note: Lymphocytopenia defined as absolute lymphocyte count <1000 for adults and <1500 for children 18 and under, thrombocytopenia defined as platelet count less than 150,000/mcl, ARDS defined per Berlin definition for clinical diagnosis, AKI defined as increase in creatinine of 0.3 mg/dl within 48 h and increased transaminases defined as three times upper limit of normal for AST and ALT.
Abbreviations: ARDS, acute respiratory distress syndrome; BiPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure; VA‐ECMO, veno‐arterial extracorporeal membrane oxygenation; VV‐ECMO, veno‐venous extracorporeal membrane oxygenation.
Unless otherwise specified.
For pediatrics, additional treatments used include: hydroxychloroquine for systemic lupus erythematosus in one patient, intravenous immune globulin (IVIG) in two patients, canakinumab in one patient and bivalirudin in two patients.
The patient admitted for 117 days had prolonged hospitalization due to problems related to worsening complications of his underlying chronic granulomatous disease, which exacerbated upon recovery from acute COVID‐19.
Excludes patients who were not admitted to the ICU.
New diagnosis or problem during admission.
All pediatric patients were admitted to the intensive care unit (ICU), and 10/22 (45.5%) received mechanical ventilation for a median of 12 (range 4–38) days. Seventeen patients were discharged home after resolution of illness and their median length of stay for hospitalization was 18 (range 5–117) days. Five (22.7%) pediatric patients died after being hospitalized for median 37 (range 8–42) days. All five deceased patients had underlying illnesses including systemic lupus erythematosus, neuroblastoma, Rett syndrome, extensive vascular anomaly, and autoimmune hepatitis. These patients had complications such as E. coli bacteremia, severe pulmonary fibrosis and acute respiratory distress syndrome (ARDS), mucous plugging, and pneumothorax (two cases).
Eight of 10 (80%) obstetric patients were admitted to the ICU and two (20%) required mechanical ventilation for a median period of 18.5 days (range 5–32 days). One patient on extracorporeal membrane oxygenation (ECMO) was transferred to another institution. Three (30%) patients required premature deliveries via cesarean section during their hospitalization at gestational ages 32 weeks 2 days, 29 weeks 2 days, and 28 weeks 3 days, due to abnormal fetal heart rate and worsening maternal respiratory status in two patients and another due to suspected placental abruption. All newborns delivered had negative nasopharyngeal molecular testing for SARS‐CoV‐2 and had no complications other than prematurity needing supportive care and were discharged home. All obstetric patients were discharged after median 8 days of hospitalization (range 5–14).
COVID‐19 convalescent plasma administration
The details of the CCP administration and antibody levels, patient serology data, and outcome measures are shown in Table 3. None of the patients had adverse events associated with CCP administration including allergic reaction, transfusion‐associated circulatory overload, and transfusion‐related acute lung injury. Of the 36 CCP units administered to pediatric patients, antibody levels were available in 25 patients (69.4%), of which 11/25 (30.6%) were high‐titer products according to the FDA criteria using IgG level. For obstetric patients, of the 13 CCP units given, antibody levels were available in 12 patients (92.3%), of which 7/12 (58.3%) were high‐titer products using IgG level. Titers were not measured for 11 (30.6%) units given to pediatric patients and one (7.7%) obstetric patient due to unavailability of segments. Post‐transfusion plasma serology was available for 18/22 pediatric patients obtained at a median of 3 days (range 0–129) post‐transfusion with a median level of 119 AU/ml (range 2–1220). The median total anti‐spike immunoglobulin level was higher during the 8–12 days post and >21 days post transfusion compared with the period of 0–7 days post transfusion. In obstetric patients, post‐CCP total anti‐spike immunoglobulin level was available for five patients, and obtained at a median of 1 day (range 0–2) post transfusion. The median for total immunoglobulin level was 45 AU/ml (range 9.5–100).
TABLE 3.
Convalescent plasma data, serological data and outcome measures
| Pediatrics, N = 22 | Obstetrics, N = 10 | |
|---|---|---|
| Timing of 1st dose since admission [days (range)] | 2 (0–9) | 1 (0–4) |
| Median number of transfusion episodes (range) | 1 (1–5) | 1 (1–2) |
| Total volume of CCP administered | ||
| ml (range) | 221 (33–1050) | 218 (194–435) |
| ml/kg (range) | 6.7 (1.4–33.1) | 2.9 (1.9–4.9) |
| Volume per transfusion episodes | ||
| ml (range) | 200 (25–250) | 210 (172–225) |
| ml/kg (range) | 4.3 (0.6–10.2) | 2.6 (1.4–3.0) |
| CCP titer in each unit a | ||
| Total immunoglobulins [AU/ml, (range)] | 249.0 (44.0–1090.0) | 480.0 (3.9–960.0) |
| IgG [AU/ml, (range)] | 9.55 (ND–21.2) | 14.0 (ND–21.2) |
| Neutralizing antibodies | >1:80 (ND to >1:640) | >1:80 (ND to >1:320) |
| CCP titer category b | ||
| High based on IgG [n (%)] | 11/25 (44) | 7/12 (58.3) |
| Low based on IgG [n (%)] | 14/25 (56) | 5/12 (41.7) |
| High based on neutralizing antibodies [n (%)] | 14/25 (56) | 7/12 (58.3) |
| Low based on neutralizing antibodies [n (%)] | 11/25 (44) | 5/12 (41.7) |
| Information unavailable [n (%)] | 11/36 (30.6) | 1/13 (7.7) |
| Patient serology (total anti‐spike Ig) | ||
| Pre‐transfusion c | ||
| Number of negative patients [n, (%)] | 16/21 (76.2%) | 3/5 (60%) |
| Number of positive patients [n, (%)] | 5/21 (23.8%) | 2/5 (40%) |
| Median for positive patients [AU/ml (range)] | 22 (2.5–99) | 4.5 (1.51–7.57) |
| Post‐transfusion serology median [AU/ml (range)] | 119.0 (2–1220) | 45.0 (9.5–100) |
| Post‐transfusion serology [total Ig AU/ml (range)] d | ||
| Tested 0–7 days after CCP transfusion e | 80.6 (2–1070) | 45.0 (9.5–100) |
| Tested 8–21 days after CCP transfusion f | 180.0 (12–661) | ‐ |
| Tested >21 days after CCP transfusion g | 210.0 (4.1–1220) | ‐ |
| Outcome measures | ||
| Duration from CCP administration to fever resolution [days (range)] | 2 (0–30) | 0 (0 to 15) |
| Duration of CCP administration to off respiratory support [days (range)] | 7 (3–43) | 5 (1–7) |
| PCR | ||
| Remained positive n (%) | 5/11 (45.5) | 0/3 (0) |
| Turned negative n (%) | 6/11 (54.5) | 3/3 (100) |
| Days to turn negative [days (range)] | 30.5 (5–50) | 24 (18–98) |
| Not tested | 11 | 7 |
| Recovery from lymphopenia | 13/21 patients | 9/10 patients |
| Number of days (range) | 5 (0–30) | 3 (1 to 12) |
Abbreviations: CCP, COVID‐19 convalescent plasma; Ig, immunoglobulin; ND, not detected.
CCP titer was available in 28/36 units (77.8%) units given to pediatric patients and 11/13 (84.6%) units given to obstetric patients.
The CCP unit was categorized as “high titer” if the signal‐to‐cutoff (S/C) value, arbitrary unit (AU)/ml, was IgG 12 AU/ml or greater (during our study period) or if the neutralization titer was >1:80 based on FDA definition.
Obtained in 21 pediatric patients and 5 obstetric patients.
Obtained in 18 pediatric and 5 obstetric patients.
Obtained in 17 pediatric and 5 obstetric patients.
Obtained in five pediatric patients.
Obtained in six pediatric patients.
Nine pediatric and four obstetric patients had one day pre‐ and 24–48 h post‐transfusion total anti‐spike Ig quantification measured and were included to evaluate for serologic response after CCP transfusion (Table 4). Figure 1 shows the correlation between the ΔIg and total CCP anti‐spike IgG dose for those who received high‐titer products. Pediatric patients receiving high‐titer CCP had higher mean ΔIg than those receiving low‐titer plasma (81.0 vs. 38.0 AU/ml); however, there was no correlation between CCP dose (ml/kg), AU/ml in CCP, IgG transfused per kg or total Ig transfused on the ΔIg (R 2 = 0.00, 0.02, 0.05, and 0.04, respectively). The low‐titer CCP demonstrated no correlation with any of the parameters evaluated. Furthermore, low‐titer plasma resulted in smaller increments in ΔIg (range = 20.0–64.0 AU/ml). All four obstetric patients received high‐titer CCP and moderate to strong correlations were found among total IgG dose (AU), total IgG dose per unit weight (AU/kg), and CCP dose (AU/kg) on ΔIg (R 2 = 0.987, 0.983, 0.756, respectively).
TABLE 4.
Coronavirus 2019 convalescent plasma administration details and serological response data for selected patients
| Pediatric | Obstetric | |
|---|---|---|
| N = 9 | N = 4 | |
| Median age, (range) | 14 (9–17) | 30 (26–33) |
| Number of patients that received high‐titer CCP [n (%)] | 5 (55.6) | 4 (100) |
| Number of patients that received low‐titer CCP [n (%)] | 4 (44.4) | ‐ |
| Median volume [ml/kg (range)] | 4.8 (1.4–12.2) | 2.5 (1.8–3.0) |
| Median IgG dose per unit [AU/ml (range)] | 2976.0 (100.3–6707.7) | 1192.6 (423.7–2834.7) |
| Median ΔIg [AU/ml (range)] | 64.0 (20.0–122.0) | 41.2 (9.5–98.5) |
| Median ΔIg recipients of high‐titer plasma [AU/ml (range)] | 81.0 (28.0–122.0) | 41.2 (9.5–98.5) |
| Median ΔIg recipients of low‐titer plasma [AU/ml (range)] | 38.0 (20.0–64.0) | ‐ |
Note: Δ total anti‐spike protein immunoglobulin (Δ Ig): Differences between the total anti‐spike immunoglobulin level obtained pre‐CCP administration and 24–48 h post‐transfusion.
Abbreviations: CCP, COVID‐19 convalescent plasma; Δ Ig, delta immunoglobulin.
FIGURE 1.
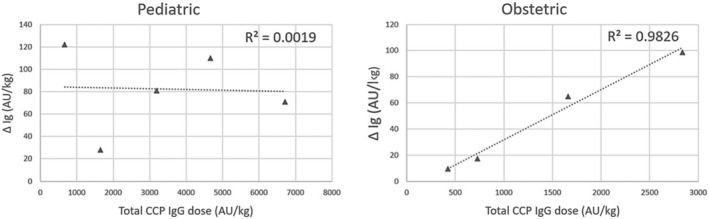
Correlation between the total COVID‐19 convalescent plasma anti‐spike IgG dose (AU/kg) for high‐titer products and the changes in the delta total anti‐spike immunoglobulin pre‐and post‐transfusion (AU/kg). Abbreviations: CCP, COVID‐19 convalescent plasma; Ig, immunoglobulin; Δ Ig, delta immunoglobulin. Δ total anti‐spike protein immunoglobulin (Δ Ig): Differences between the total anti‐spike immunoglobulin level obtained pre‐CCP administration and 24–48 h post‐transfusion. Total CCP IgG dose: amount of IgG (AU/kg) administered per CCP transfusion.
DISCUSSION
Effective therapeutic modalities for acute COVID‐19, other than supportive care, are needed. Our observation suggests that CCP can safely be administered to moderately and severely ill hospitalized pediatric and obstetric patients with acute COVID‐19 but the data on its efficacy is limited. To the best of our knowledge, this is the first case series of both pediatric and obstetric patients with acute COVID‐19 who received CCP with an institutional protocol in place for administration where titers of the CCP product were also available.
For hospitalized adults, no difference in clinical outcome among hospitalized patients was shown in a limited number of randomized control trials (RCTs) of CCP. 10 , 11 There are studies showing the safety of the convalescent plasma use in adults. 12 A systematic review and meta‐analysis of 10 RCTs concluded that treatment with convalescent plasma compared with placebo or standard of care was not significantly associated with a decrease in all‐cause mortality or with other improved clinical outcomes. 13 However, early administration of high‐titer convalescent plasma against SARS‐CoV‐2 to mildly ill older adults was reported to be effective to reduce progression of COVID‐19. 14 Limited case series in pediatric patients with severe pulmonary disease demonstrated potential benefits without significant side effects. 15 , 16 , 17 , 18 The use of CCP among critically ill obstetric patients is limited to case reports. 19
Our patients were hospitalized with various comorbidities including pregnancy. Pediatric patients were sicker, and we were limiting the use of CCP during the first wave of pandemic in Houston to our sick patients with comorbidities. We were able to provide and share our experiences on the number of doses, volume, and the timing of administration where data are limited. Data on titer levels were also available.
In general, CCP is considered a safe option for treatment with a risk profile similar to other blood products. All products were appropriately matched for blood type and all patients were screened for history of severe allergic reactions. We did not want to waste any of this precious resource but our primary concern for pediatric patients was the risk of volume overload. For this reason, smaller patients (<20 kg) received aliquots from the same unit approximately 12 h apart. Our pediatric patients tolerated both the volume and the repeated doses. There were two pediatric patients who developed fever within a few hours of CCP administration, which prompted transfusion reaction investigations. These were concluded by the transfusion medicine specialists to be of low suspicion for a transfusion reaction in accordance with the Centers for Disease Control and Prevention, NHSN Biovigilance Component: Hemovigilance Module Surveillance Protocol, 9 as both patients had been intermittently febrile prior to and following transfusion, most likely as a result of COVID‐19. All of our pregnant women tolerated the CCP well with no documented adverse events. There were three patients who delivered premature babies and all newborns did well without complications.
Our institution was able to measure antibody levels for 36 units transfused to pediatric patients and 13 units transfused to obstetric patients using methods authorized by the FDA under emergency use. Many of our patients had post‐transfusion serology testing performed, which showed an increase in antibody levels even after more than 21 days. This suggests mounting of a post‐transfusion de novo response and that CCP did not diminish the serological response. It is also supported by another case series of pediatric patients. 17
Notably, the serologic response data from the small subset of patients examined appears to suggest that for high‐titer CCP there is a positive association between CCP dose, total Ig delivered, and the magnitude of change in patient serum total Ig, especially in obstetric patients. These same data also suggest that, at least among pediatric patients, high‐titer CCP is appreciably better at increasing immunoglobulin levels post‐transfusion than the low‐titer CCP. These results support the FDA EUA recommendation to administer high‐titer products only. This was implemented towards the end of our study period. There is, however, significant ambiguity about the usefulness of serologic response measurements in guiding clinical treatment among pediatric patients. The seroresponse was more notable among obstetric patients showing a dose–response in the delta total Ig. The exact reason why there was no dose–response correlation in the pediatric patients is unclear. The pediatric patients had more complex clinical manifestations of underlying illnesses and severe diseases, which may have led to variable consumption or vascular permeability of the antibodies affecting the antibody response. Furthermore, the immune repertoire in pediatric patients in hospital is varied and could explain this variability.
Our study has important limitations given its retrospective design, and as such, not all data points were available. This was a single‐center study, and the sample size was small with a short follow up. Our study was also limited to our hospitalized patients with moderate to severe acute COVID‐19. In addition to CCP, most of our patients were receiving multiple interventions including remdesivir and dexamethasone, and we had no control group, making it challenging to evaluate the efficacy of CCP.
With the ongoing pandemic of COVID‐19, CCP may offer a relatively cheap and accessible option for treatment in low‐ to middle‐income countries where access to more advanced treatment may be limited. With the emergence of new SARS‐CoV‐2 variants, CCP may offer protection from the circulating virus, especially in immunocompromised patients. 20 Given the safety we observed and as supported by other studies, CCP may offer treatment in pediatric and immunocompromised patients.
In summary, CCP was safely administered to our 32 hospitalized pediatric and obstetric patients with COVID‐19. Further randomized studies are needed to address efficacy in pediatric and obstetric patients.
AUTHOR CONTRIBUTIONS
Saki Ikeda conceptualized and designed the study, drafted the manuscript, and reviewed and revised the manuscript. Flor M. Munoz conceptualized and designed the study, and reviewed and revised the manuscript. Eduardo Benzi, Sridevi Devaraj, Lisa A. Hensch, and Shiu‐Ki Rocky Hui, provided and analyzed certain laboratory data and conceptualized the study. Jun Teruya participated in the writing and critical review of the manuscript. Manisha Gandhi, and Karin A. Fox provided expertise in obstetric data and critically reviewed the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1
Table S1
ACKNOWLEDGMENTS
We would like to thank the Gulf Coast Regional Blood Center for providing CCP to our patients. Partial funding was provided by the National Institute of Health (NIH) 1R61HD105593 to Dr. Devaraj.
Ikeda S, Benzi E, Hensch LA, Devaraj S, Hui S‐K, Gandhi M, et al. Convalescent plasma in hospitalized pediatric and obstetric coronavirus disease 2019 (COVID‐19) patients. Pediatr Int. 2022;64:e15407. 10.1111/ped.15407
Contributor Information
Saki Ikeda, Email: sakikeda@it.ncgm.go.jp.
Flor M. Munoz, Email: florm@bcm.edu.
REFERENCES
- 1. van Griensven J, Edwards T, de Lamballerie X, Semple MG, Gallian P, Baize S, et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374(1):33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta‐analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599–609. [DOI] [PubMed] [Google Scholar]
- 3. Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta‐analysis. J Infect Dis. 2015;211(1):80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. US Food & Drug Administration . COVID‐19 Convalescent Plasma EUA Clinical Memorandum. [cited 2022 Jul 6]. Available from: https://www.fda.gov/media/141480/download
- 5. US Food & Drug Administration . Convalescent Plasma EUA Letter of Authorization; 2021. [cited 2022 Jul 6]. Available from: https://www.fda.gov/media/141477/download
- 6. Harris County/City of Houston COVID‐19 Data Hub. [cited 2022 Jul 6]. Available from: https://covid‐harriscounty.hub.arcgis.com/pages/cumulative‐data
- 7. Centers for Disease Control and Prevention . People with certain medical conditions. [cited 2022 Jul 6]. Available from: https://www.cdc.gov/coronavirus/2019‐ncov/need‐extra‐precautions/people‐with‐medical‐conditions.html
- 8. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)‐a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Center for Disease Control—Division of Healthcare Quality Promotion . National Healthcare Safety Network biovigilance component hemovigilance module surveillance Protocol. 2018; 1–30. March 2021; [cited 2022 Jul 6]. Available from: https://www.cdc.gov/nhsn/pdfs/biovigilance/bv‐hv‐protocol‐current.pdf
- 10. Li L, Li L, Zhang W, Hu Y, Tong X, Zheng S, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19: a randomized clinical trial. J Am Med Assoc. 2020;324(5):460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, et al. A randomized trial of convalescent plasma in Covid‐19 severe pneumonia. N Engl J Med. 2021;384(7):619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, Chen J, et al. Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol. 2020;190(11):2290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Janiaud P, Axfors C, Schmitt AM, Gloy V, Ebrahimi F, Hepprich M, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID‐19: a systematic review and meta‐analysis. J Am Med Assoc. 2021;325(12):1185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early high‐titer plasma therapy to prevent severe Covid‐19 in older adults. N Engl J Med. 2021;384(7):610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diorio C, Anderson EM, McNerney KO, Goodwin EC, Chase JC, Bolton MJ, et al. Convalescent plasma for pediatric patients with SARS‐CoV‐2‐associated acute respiratory distress syndrome. Pediatr Blood Cancer. 2020;67(11):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schwartz SP, Thompson P, Smith M, Lercher DM, Rimland CA, Bartelt L, et al. Convalescent plasma therapy in four critically ill pediatric patients with coronavirus disease 2019: a case series. Crit Care Explor. 2020;2(10):e0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arrieta A, Galvis AE, Morphew T, Ehwerhemuepha L, Osborne S, Enriquez C, et al. Safety and antibody kinetics of COVID‐19 convalescent plasma for the treatment of moderate to severe cases of SARS‐COV‐2 infection in pediatric patients. Pediatr Infect Dis J. 2021;40(7):606–11. [DOI] [PubMed] [Google Scholar]
- 18. Gordon O, Brosnan MK, Yoon S, Jung D, Littlefield K, Ganesan A, et al. Pharmacokinetics of high‐titer anti–SARS‐CoV‐2 human convalescent plasma in high‐risk children. JCI Insight. 2022;7(2):e151518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Franchini M, Prefumo F, Grisolia G, Bergamini V, Glingani C, Pisello M, et al. Convalescent plasma for pregnant women with covid‐19: a systematic literature review. Viruses. 2021;13(7):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thompson MA, Henderson JP, Shah PK, Rubinstein SM, Joyner MJ, Choueiri TK, et al. Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID‐19. JAMA Oncol. 2021;7(8):1167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Table S1


