Dear Editor,
From the beginning of mass COVID‐19 vaccination campaign, several people sought dermatological attention due to cutaneous adverse manifestations following the three‐dose immunization regimen, as recently described. 1 , 2 SARS‐CoV‐2 vaccine‐related cutaneous adverse reactions encompass a protean spectrum of clinical courses, with primary cutaneous lymphoproliferative disorders (PCLDs) following SARS‐CoV‐2 vaccination only being partially explored. 3
In this regard, we report a retrospective review of patients attending seven Italian tertiary referral centres between January 2021 and July 2022. Information was collected through hospital operating systems. Inclusion criteria were (1) the presence of complete charts, (2) a biopsy‐proven PCLDs (3) onset, relapse or regression within 30 days after COVID‐19 vaccine administration. A total of 14 PCLDs were gathered: 6 classified as PCLDs relapse [2 Sézary syndrome (SS), 1 cutaneous lymphoid hyperplasia (CLH), 1 erythrodermic mycosis fungoides, 1 lymphomatoid papulosis (LyP), 1 primary cutaneous CD4+ small/medium T‐cell lymphoproliferative disorder (PCSMLPD)] and 8 diagnosed as new‐onset PCLDs [2 LyP, 2 CD4+ PCSMLPD, 1 atypical pityriasis lichenoides et varioliformis acuta (PLEVA), 2 CLH and 1 SS] (Figure 1a–h). Demographics, vaccine type, clinical features and onset time of cutaneous reactions are summarized in Table 1. More than half (64.2%) were males, and their median age was 61 years [interquartile range (IQR): 58–61]. The median onset time was 15 days (IQR: 9–15). No recrudescence either following the booster dose or thereafter was identified (mean follow‐up time: 13 months, IQR: 3–15).
FIGURE 1.
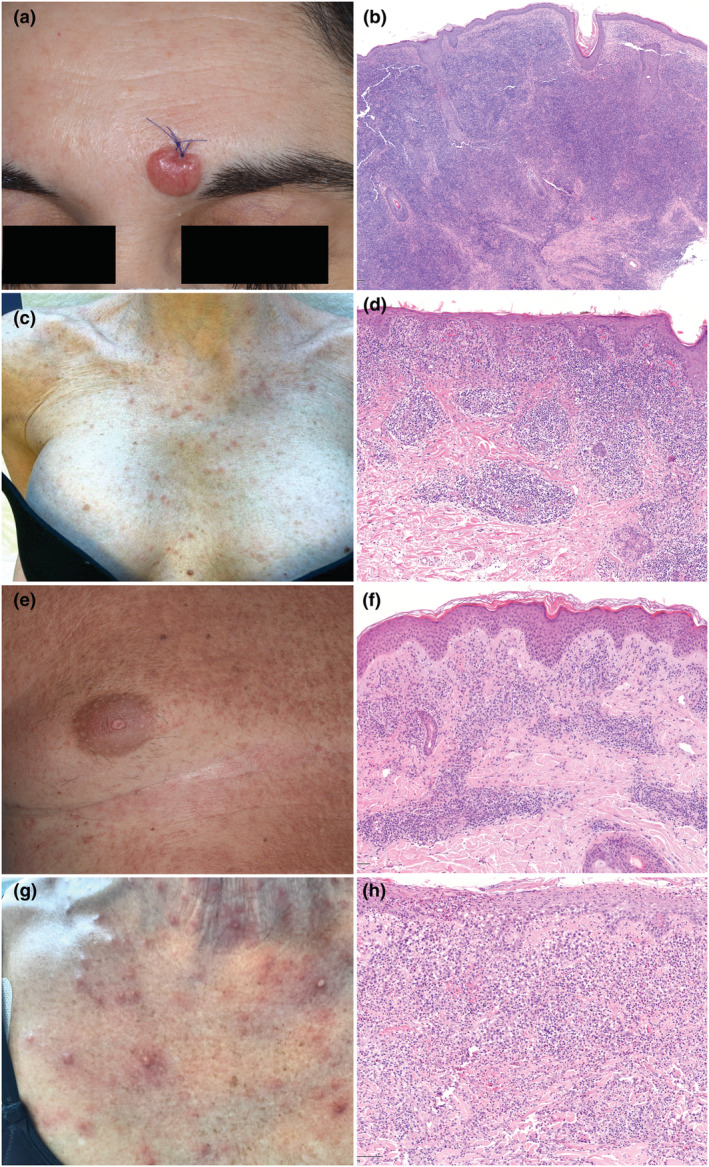
(a) A single erythematous nodule on the forehead. (b) A diffuse dense infiltrate in the dermis, sparing the epidermis and composed of small‐ to medium‐sized hyperchromatic lymphocytes. Atypical lymphocytes, arranged in clusters, are CD4+, PD1+ and exceptionally GATA3+ (data not shown) consistent with primary cutaneous CD4+ small/medium T‐cell lymphoproliferative disorder [haematoxylin and eosin (H&E), 10×]. (c) an erythematous maculopapular eruption on the chest. (d) Histopathological findings diagnostic for atypic pytiriasis lichenoides and varioliformis acuta: A dense lichenoid and perivascular infiltrate in the upper dermis composed of small‐medium sized, hyperchromatic lymphocytes mixed with some blasts, few plasma cells, mast cells and red blood cells. Lymphocytes are positive for CD3, CD4, CD7 (H&E 20×), Ki‐67 20% (data not shown). (e) A diffuse and confluent erythematous papular eruption on the trunk. (f) Histology revealing lymphomatoid papulosis type A: Perivascular infiltrate in the superficial dermis of medium/large‐sized pleomorphic lymphocytes mixed with eosinophils and blast cells (around 30% of the infiltrate). Atypical lymphocytes were CD3+, CD4+, CD7+, CD30+ (blasts included) and GATA3+ (data not shown) (H&E 20×). (g) A diffuse vesicular and papulo‐pustular eruption on the chest. (h) A dense perivascular and interstitial infiltrate in the superficial dermis of small‐sized and large, blast‐like, pleomorphic lymphocytes mixed with numerous neutrophils, eosinophils and histiocytes. Large cells were CD3+, CD4+, CD7+, CD30+, GATA3+ (data not shown) consistent with lymphomatoid papulosis type A (H&E 20×).
TABLE 1.
Demographics, vaccine type, clinicopathologic features and onset time of cutaneous adverse reactions in the study population
| Age (years) and sex (M/F) | History of previous PCLDs | Vaccine type (1st and 2nd dose) | Vaccine type (3rd and 4th dose) | Onset (days) | Clinical features | TCR | Diagnosis | Therapy | Outcome | Follow‐up time (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 47 F | CLH diagnosed in 2012. CR following TCS and ICS. Relapsed twice. In complete remission from 2 years | BNT162b2 | mRNA‐1273 a | 10 | Erythematous plaques on right deltoid and temporal region following the 2nd BNT162b2 dose | NP | CLH relapse | TCS, ICS | CR | 17 |
| 67 M | Lyp diagnosed in 2019, relapsed in 2020 | BNT162b2 | mRNA‐1273 a | 15 | Violaceous necrotic papules on upper and lower limbs following the 2nd BNT162b2 dose | γ + | Lyp type A relapse | MTX | CR | 16 |
| 49 M | CD4+ PCSM‐LPD diagnosed 2 years before in complete remission | BNT162b2 | BNT162b2 a | 15 | Erythematous plaque on the neck, no other symptoms were observed following the 2nd BNT162b2 dose | – | CD4+ PCSM‐LPD | None | SR | 14 |
| 58 M | Complete remission SS under tislelizumab | BNT162b2 | BNT162b2 | 4 | Erythroderma, itch and fever following the 2nd BNT162b2 dose | γ + | SS relapse | TCS, OCS, MOGA | CR | 2 |
| 61 M | Early‐stage mycosis fungoides diagnosed in 2018, well‐managed with the only use of TCS | BNT162b2 | BNT162b2 a | 14 | Erythroderma and itch following the 3rd BNT162b2 dose | γ + | Erythrodermic MF | OCS, PUVA | CR | 5 |
| 61 M | Well‐managed SS under mogamulizumab | BNT162b2 | BNT162b2 a | 15 | Sub‐erythroderma following the 3nd BNT162b2 dose | γ + | SS relapse | ECP | PR | 9 |
| 55 M | New onset | BNT162b2 | BNT162b2 a | 30 | Erythematous plaque on the back following the 3rd BNT162b2 dose | – | CLH | SE | CR | 2 |
| 55 M | New onset | BNT162b2 | NP | 7 | Erythematous plaque on right deltoid region following the 1st BNT162b2 dose | NP | CLH | TCS | CR | 19 |
| 80 F | New onset | BNT162b2 | BNT162b2 | 15 | Erythroderma, itch and blood skin compatible with mSWAT 100 following the 3rd BNT162b2 dose | γ + | SS | TCS, OCS | CR | 1 |
| 60 M | New onset | BNT162b2 | NP | 30 | Erythematous papular lesions diffuse at trunk and arms following the 2nd BNT162b2 dose | γ + | Lyp type A | CS iv + Trimeton iv | CR | 14 |
| 52 F | New onset | BNT162b2 | NP | 3 | Erythematous nodule (diameter: 15 mm) on the forehead following the 1st BNT162b2 dose | – | CD4+ PCSM‐LPD | RT | CR | 15 |
| 62 F | New onset | ChAdOx1 nCoV‐19, BNT162b2 | NP | 7 | Erythematous maculo‐papular and vesicular lesions on the trunk and upper limbs following the 1st ChAdOx1 nCoV‐19 dose | – | Atypical PLEVA | OCS | CR | 16 |
| 61 F | New onset | BNT162b2 | BNT162b2 | 10 | Erythematous‐vesicular pustular lesions on the chest following the 1st BNT162b2dose. Occurrence of similar elements at trunk, face and limbs | γ + | Lyp type A | none | SR | 13 |
| 45 M | New onset | BNT162b2 | BNT162b2 a | 20 | Single nodule on the left cheek (diameter: 8 mm) following the 3rd BNT162b2 dose | γ + | CD4+ PCSM‐LPD | SE | CR | 3 |
Abbreviations: CD4+ PCSM‐LPD, primary cutaneous CD4+ small/medium T‐cell lymphoproliferative disorder; CLH, cutaneous lymphoid hyperplasia; CR, complete response; ECP, extracorporeal photopheresis; F, female; ICS, intralesional corticosteroids; Lyp, lymphomatoid papulosis; M, male; MOGA, mogamulizumab; mSWAT, Modified Severity‐Weighted Assessment Tool; MTX, methotrexate; NBUVB, narrowband ultraviolet B; NP, not performed; OCS, oral corticosteroids; PLEVA, pityriasis lichenoides et varioliformis acuta; PUVA, psoralen plus ultraviolet‐A radiation; RT, radiotherapy; SE, surgical excision; SR, spontaneous resolution; SS, Sézary syndrome; TCR, T‐cell receptor (γ/β) gene rearrangement assay; TCS, topical corticosteroids.
4th dose not performed.
The outlined manifestations appear quite heterogeneous, generally of short duration, with a tendency to self‐resolve and easily treatable with standard therapies, when needed. One of the patients with LyP exceptionally reported the same skin manifestations immediately after HBV vaccine administration several decades before. This latter and a second patient with new‐onset Lyp type A showed a non‐diagnostic diffuse cutaneous eruption with marked cellular atypia and numerous GATA3+ blasts. Similarly, a case of new‐onset PLEVA had atypical histological findings with marked nuclear pleomorphisms and blasts, falling within the PCLD spectrum. 4 To our knowledge, this is the first multicentre study displaying the largest number of patients and unreported vaccine‐related clinical presentations to date (e.g. SS following the third BNT162b2 dose).
The relationship between PCLDs and SARS‐CoV‐2 vaccination appears multifaceted and the exact pathogenic mechanisms, if any, are not fully understood. Recurrent primary cutaneous anaplastic large‐cell lymphoma, as well as primary cutaneous follicle centre cell lymphoma, spontaneously regressed after the first dose COVID‐19 vaccination, has been reported. 5 , 6 These findings might reflect, in a genetically predisposed individual, an immune system overstimulation secondary to SARS‐CoV‐2 vaccination, potentially enhancing anti‐tumor response. Interestingly, regression has not been detected in our series and its role should be further elucidated. Conversely, in a recent case series, low‐grade cutaneous lymphoid reactions after COVID‐19 vaccination have been observed. 7 Recurrence of pre‐existing complete remission PCLDs has also been described following COVID‐19 vaccine, with several case reported so far. 3 , 8 , 9 The overproduction and exhaustion of CD4+ and CD8+ lymphocytes, expressing CD30 after being triggered by the vaccine, might be held responsible for the disease recurrence. Although recurrences have been observed even in the current multicentre experience, these entities are known for displaying a waxing and waning course of the disease, which could account for the aforementioned observation.
Owing to the retrospective nature of the present study and to the small sample, definite conclusions regarding the causal link between COVID‐19 vaccine and the observed event cannot be drawn. However, the temporal relation of our findings points at the potential effects of COVID‐19 vaccine on PCLDs and calls for further studies. Compelling COVID‐19 vaccines safety profiles, not only perceived from the dermatology perspectives, remain ascertained and reassuring in the ongoing second booster era.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
ETHICS STATEMENT
The patients in this manuscript have given written informed consent to the publication of their case details.
Gianluca Avallone and Carlo Alberto Maronese authors contributed equally to this article and share first authorship.
Emilio Berti and Silvia Alberti‐Violetti authors contributed equally to this article and share senior authorship.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Avallone G, Cavallo F, Astrua C, Caldarola G, Conforti C, de Simone C, et al. Cutaneous adverse reactions following SARS‐CoV‐2 vaccine booster dose: a real‐life multicentre experience. J Eur Acad Dermatol Venereol. 2022;36(11):e876–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brumfiel CM, Patel MH, DiCaudo DJ, Rosenthal AC, Pittelkow MR, Mangold AR. Recurrence of primary cutaneous CD30‐positive lymphoproliferative disorder following COVID‐19 vaccination. Leuk Lymphoma. 2021;62(10):2554–5. [DOI] [PubMed] [Google Scholar]
- 4. Borra T, Custrin A, Saggini A, Fink‐Puches R, Cota C, Vermi W, et al. Pityriasis Lichenoides, atypical Pityriasis Lichenoides, and related conditions: a study of 66 cases. Am J Surg Pathol. 2018;42(8):1101–12. [DOI] [PubMed] [Google Scholar]
- 5. Gambichler T, Boms S, Hessam S, Tischoff I, Tannapfel A, Lüttringhaus T, et al. Primary cutaneous anaplastic large‐cell lymphoma with marked spontaneous regression of organ manifestation after SARS‐CoV‐2 vaccination. Br J Dermatol. 2021;185(6):1259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aouali S, Benkaraache M, Almheirat Y, Zizi N, Dikhaye S. Complete remission of primary cutaneous follicle Centre cell lymphoma associated with COVID‐19 vaccine. J Eur Acad Dermatol Venereol. 2022;36(9):e676–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hooper MJ, Veon FL, LeWitt TM, Chung C, Choi J, Zhou XA, et al. Cutaneous T‐cell‐rich lymphoid infiltrates after SARS‐CoV‐2 vaccination. JAMA Dermatol. 2022;158(9):1073–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Panou E, Nikolaou V, Marinos L, Kallambou S, Sidiropoulou P, Gerochristou M, et al. Recurrence of cutaneous T‐cell lymphoma post viral vector COVID‐19 vaccination. J Eur Acad Dermatol Venereol. 2022;36(2):e91–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koumaki D, Marinos L, Nikolaou V, Papadakis M, Zografaki K, Lagoudaki E, et al. Lymphomatoid papulosis (LyP) after AZD1222 and BNT162b2 COVID‐19 vaccines. Int J Dermatol. 2022;61(7):900–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


