Abstract
Aspergillus fumigatus is an important pathogen of immunocompromised hosts, causing pneumonia and invasive disseminated disease with high mortality. To be able to analyze the expression of putative virulence-associated genes of A. fumigatus, the use of the enhanced green fluorescent protein (EGFP) as a reporter was established. Two 5′ sequences, containing the putative promoters of the pyrG gene, encoding orotidine-5′-phosphate decarboxylase, and the pksP gene, encoding a polyketide synthase involved in both pigment biosynthesis and virulence of A. fumigatus, were fused with the egfp gene. The PpksP-egfp construct was integrated via homologous recombination into the genomic pksP locus. EGFP production was analyzed by fluorescence spectrometry, Western blot analysis, and fluorescence microscopy. Differential gene expression in A. fumigatus was observed. Fluorescence derived from the PYRG-EGFP fusion protein was detected during all developmental stages of the fungus, i.e., during germination, during vegetative growth, in conidiophores, and weakly in conidia. In addition, it was also detected in germinating conidia when isolated from the lungs of immunocompromised mice. By contrast, PKSP-EGFP-derived fluorescence was not found in hyphae or stalks of conidiophores but was found in phialides and conidia in vitro when the fungus was grown under standard conditions, indicating a developmentally controlled expression of the gene. Interestingly, pksP-egfp expression was also detected in hyphae of germinating conidia isolated from the lungs of immunocompromised mice. This finding indicates that the pksP gene can also be expressed in hyphae under certain conditions and, furthermore, that the pksP gene might also contribute to invasive growth of the fungus.
Aspergillus fumigatus is a saprophytic fungus normally associated with decaying organic matter. It plays an essential role in recycling carbon and nitrogen sources and is ubiquitously distributed (7, 18). In immunocompromised patients, A. fumigatus can cause life-threatening diseases, such as pneumonia and invasive aspergillosis. The entry route of A. fumigatus is the inhalation of airborne conidia by humans and their deposition in the respiratory tract. Conidia are normally eliminated by innate immune defense mechanisms. In immunocompromised patients, however, conidia can germinate, outgrow, and invade the underlying tissue, leading to disseminated disease and making A. fumigatus the most important airborne fungal pathogen (reviewed in references 11, 15, and 21).
Previously, we and others isolated a mutant of A. fumigatus which lacked the ability to form the grey-green pigment characteristic of wild-type conidia (12, 14, 27, 28). Conidia of this mutant are white. Cloning of the gene defective in the mutant led to the identification of a gene designated pksP (alb1), for polyketide synthase involved in pigment biosynthesis (14, 28). Conidia of a pksP mutant strain showed reduced virulence in a mouse infection model and an altered surface structure compared with wild-type conidia. Furthermore, wild-type conidia were 10- to 20-fold more resistant against reactive oxygen species (ROS) than pksP mutant conidia and were able to scavenge ROS, presumably thereby detoxifying ROS (12, 13).
The pksP gene was found to be part of a cluster (29) involved in the biosynthesis of 1,8-dihydroxynaphthalene–melanin, which is present in conidia (5). It was shown that pksP was developmentally regulated; i.e., pksP mRNA was detectable only during sporulation of the fungus and not in vegetatively growing hyphae in vitro under standard conditions (29). This transcriptional pattern could be expected from the function of the PKSP protein, which is involved in the biosynthesis of the grey-green conidial pigment. To be able to monitor gene expression of virulence-determining genes like pksP in vivo, as reported here, the green fluorescent protein (GFP) was used in A. fumigatus. Previously, the GFP from the jellyfish Aequorea victoria has been used as a reporter protein (6) in a variety of heterologous systems, including fungal species like Aspergillus nidulans, Ustilago maydis, Candida albicans, Cryptococcus neoformans, Phanerochaete chrysosporium, and Absidia glauca (6, 8, 16, 17, 24, 25, 26). Although quantification of promoter strength is less accurate than that with other reporter genes, there are several advantages that make GFP an ideal tool for certain applications: (i) when GFP is expressed, it functions in the absence of any cofactors, with only blue or UV light and oxygen being required to induce green fluorescence, and (ii) the presence of GFP can be measured in living organisms at the level of single cells, making it a valuable tool in particular for the analysis of the interaction between a pathogen and its host (8, 25).
Here, we analyzed the expression derived from two different 5′ sequences, those of pksP and the pyrG gene, encoding orotidine-5′-phosphate decarboxylase (31), in vitro and in a mouse infection model, demonstrating that enhanced GFP (EGFP) fusions can be used to monitor gene expression in vitro and in vivo. Differential expression directed from the promoters was found. Interestingly, significant PpksP-egfp expression was detected in vivo in outgrowing hyphae isolated from the lungs of infected immunocompromised mice.
MATERIALS AND METHODS
Fungal and bacterial strains.
A. fumigatus strain ATCC 46645 is a wild-type isolate (12). The A. fumigatus strain AfPKSPEGFP4 (hygromycin B resistant, with white conidia) was derived from the wild-type ATCC 46645. The strain carries a PpksP-egfp gene fusion present on plasmid pUCGH-pksPI which was integrated in single copy at the chromosomal pksP gene locus (this study). The A. fumigatus strain AfPYRGEGFP1 (hygromycin B resistant) was also derived from the wild-type ATCC 46645. It contains two copies of plasmid pUCGH-pyrG (PpyrG-egfp) ectopically integrated into the genome (this study). Vectors and plasmids were propagated in Escherichia coli DH5α [F− F80d/lacZM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17 (rK− mK+) supE44 λ− thi1 gyrA96 relA1) (Bethesda Research Laboratories, Gaithersburg, Md.).
Media, growth conditions, and preparation of conidial suspensions
For the cultivation of A. fumigatus strains, the previously described Aspergillus minimal medium (AMM) with 1% (wt/vol) glucose as the carbon source was used (12). Conidial suspensions were obtained from either AMM agar plates (12) or malt agar (2% [wt/vol] agar and 2% [wt/vol] malt in water) (Ristomalt-D; Materne-Fribourg, France) for mouse infections. If required, hygromycin B (Boehringer, Mannheim, Germany) was added to AMM and malt agar to give final concentrations of 100 and 150 μg ml−1, respectively. Fungal and bacterial strains were grown at 37°C. For preparation of spore suspensions, the method described previously was used without addition of antibiotics (12). E. coli strains were grown on Luria-Bertani agar plates or in Luria-Bertani medium at 37°C. If required, ampicillin was added to give a final concentration of 50 μg ml−1.
Standard DNA techniques.
Standard techniques for the manipulation of DNA were carried out as described by Sambrook et al. (22). Plasmid DNA used for transformation of A. fumigatus was prepared using anion-exchange columns from Macherey-Nagel (Düren, Germany) according to the manufacturer's instructions. Southern blot analysis was performed as previously described (4). DNA sequencing was carried out according to the method of Sanger et al. (23) using fluorescent dyes on a PE Biosystems (Weiterstadt, Germany) ABI 310 automated sequencer.
Plasmids and generation of recombinant plasmids.
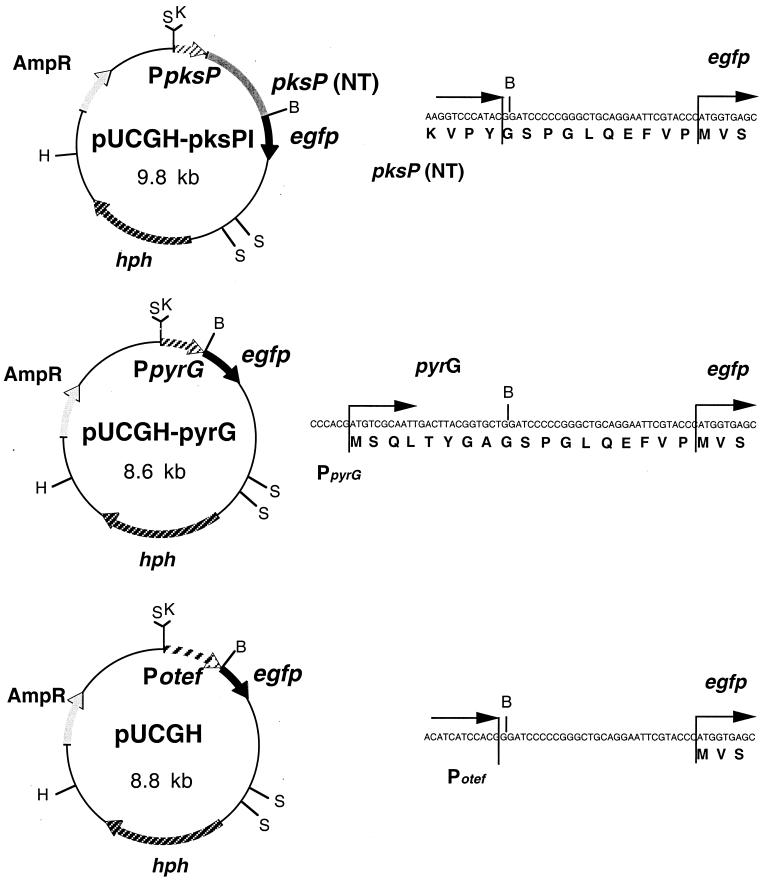
Two A. fumigatus 5′ sequences containing the putative promoters from the pyrG and pksP (alb1) genes, encoding orotidine-5′-phosphate decarboxylase (31) and a polyketide synthase involved in pigment biosynthesis (14, 28), respectively, were used. Plasmid p123 (kindly provided by C. Aichinger and R. Kahmann) served as the source for a gene encoding GFP. It contains the egfp gene (Clontech, Palo Alto, Calif.). The egfp cassette, including the otef promoter (25), the egfp gene, and a nos terminator, was excised from p123 by HincII-KpnI digestion and cloned into the pUC18 plasmid (32), which was also digested with HincII-KpnI to give pUCG. Following this, the hygromycin B resistance gene was cloned into plasmid pUCG. The hygromycin B resistance cassette is present on plasmid pANsCos1 (19). It encodes the hygromycin B phosphotransferase gene (hph) of E. coli under the control of the strong A. nidulans glyceraldehyde-3-phosphate dehydrogenase gene (gpd) promoter (20). Expression of hph leads to hygromycin B resistance of A. fumigatus transformants carrying this plasmid (see, e.g., references 10, 14, and 27). Transcription of hph is terminated by the A. nidulans trpC terminator, which follows the hph gene at its 3′ end (19). The hygromycin B resistance cassette was excised from plasmid pANsCos1 by BglII-HindIII digestion and ligated with BglII-HindIII-digested pUCG to create plasmid pUCGH. The restriction map of plasmid pUCGH is shown in Fig. 1.
FIG. 1.
Construction of expression plasmids and the corresponding nucleotide and amino acid sequences across the junctions. Abbreviations: AmpR, ampicillin resistance gene; B, BamHI; egfp, EGFP-encoding gene; H, HindIII; hph, hygromycin B resistance gene; K, KpnI; pksP (NT), region encoding the N-terminal part of PKSP; Potef, artificial promoter; PpksP, 5′ sequence of pksP; PpyrG, 5′ sequence of pyrG; S, SacI.
A PpksP-egfp gene fusion was constructed by PCR amplification of the sequence encoding the N-terminal region of PKSP (amino acids 1 to 436), including 547 bp of the 5′ sequence. The PCR was carried out using oligonucleotides KLNBaB (5′-CGTTGATCCAGGATCCGTATGGG-3′) and KLupsNF (5′-GATTTCTGCCATGGACTTGGG-3′). This led to the insertion of a BamHI site, 1,400 bp 3′ of the ATG start codon, in the DNA fragment amplified. This BamHI site and an existing KpnI site 547 bp upstream of the ATG start codon were used to clone the PCR fragment into the BamHI-KpnI-digested vector pUCGH. The resulting vector, carrying a PpksP-egfp fusion and the hygromycin B resistance cassette, was designated pUCGH-pksPI (Fig. 1).
For construction of the PpyrG-egfp fusion, the 5′ sequences of A. fumigatus pyrG (656 bp), including the sequence encoding the first 11 amino acids of the pyrG-encoded protein, was amplified by PCR, using oligonucleotides KLFpyrGBamHI (5′-GCTTGCTGGATCCAGCACCG-3′) and KLFpyrGKpnI (5′-CACCTGGTACCAGCAATTGGC-3′). The oligonucleotides created KpnI and BamHI sites at the 5′ and 3′ ends, respectively. The PCR fragment was then digested with KpnI and BamHI and ligated into pUCGH, which had also been digested with KpnI and BamHI, to give plasmid pUCGH-pyrG (Fig. 1).
Transformation of A. fumigatus.
Transformation of A. fumigatus was performed as described previously (14).
Fluorescence and light microscopy.
The microscopic analyses shown in Fig. 3 were performed using an Axioplan microscope (Carl Zeiss, Jena, Germany). Phase-contrast optics were used for light microscopy. For fluorescence microscopy the Zeiss filter set for fluorescein isothiocyanate fluorescence was employed (BP450-490 excitation filter, FT 493 beamsplitter, and BP 505-530 emission filter [Zeiss filter set 13]). Photographs were taken on Kodak Ektachrome 320T slide film. For digitalization, slides were scanned on a Nikon Coolscan III, using Silverfast software. In addition, the microscopic analyses shown in Fig. 5 were performed with a Leica TCS 4D confocal microscope system, using an argon-krypton laser for excitation at 488 nm. Fluorescence and phase-contrast images were observed with a 63× amplification planapo lens. The digital images were processed with Adobe (Seattle, Wash.) Photoshop 4.0 software.
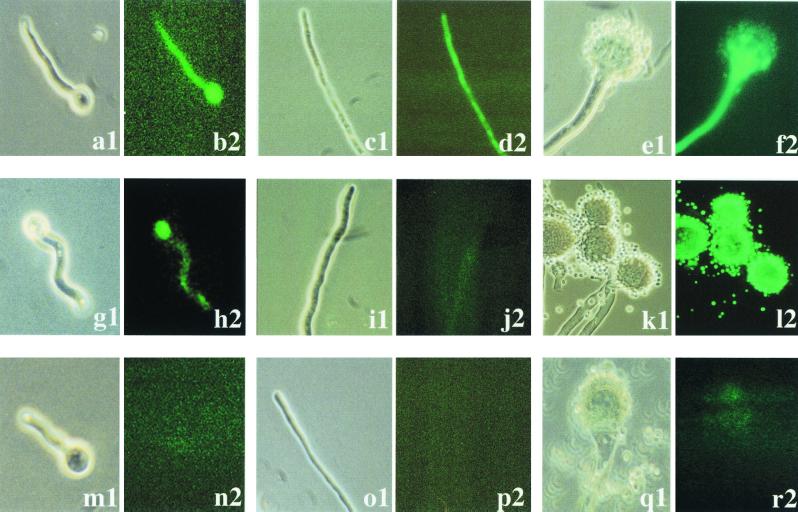
FIG. 3.
EGFP-derived fluorescence during different stages of A. fumigatus development. Germinating conidia (left two columns), hyphae (middle two columns), and conidiophore formation (right two columns) of the PpyrG-egfp-containing strain AfPYRGEGFP1 (a to f) and the PpksP-egfp-containing strain AfPKSPEGFP4 (g to l) are shown. As a control, the same stages of the untransformed wild-type strain are shown (m to r). Samples were analyzed by light microscopy (panels 1) or fluorescence microscopy (panels 2).
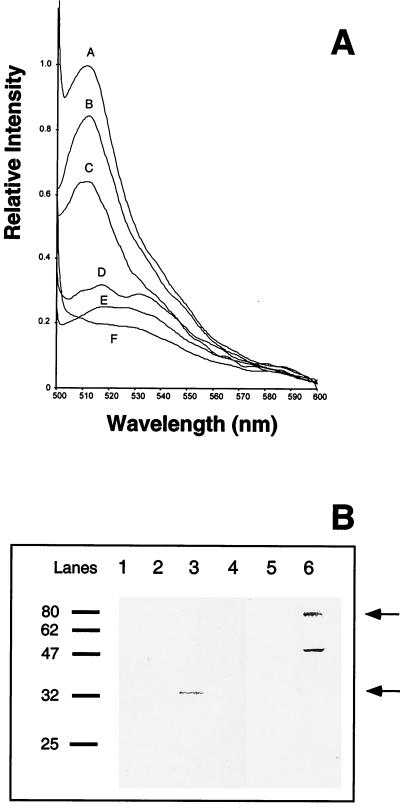
FIG. 5.
Quantification of EGFP-derived fluorescence. (A) The fluorescence intensities of protein extracts of different A. fumigatus strains were measured by fluorescence spectrometry. Equal amounts of protein (500 μg) were measured. The fluorescence value of conidia of strain AfPKSPEGFP4 was set equal to 1, as a reference. Traces: A, conidia of strain AfPKSPEGFP4; B, mycelia of strain AfPYRGEGFP1; C, conidia of strain AfPYRGEGFP1; D, mycelia of strain AfPKSPEGFP4; E, conidia of wild-type strain ATCC 46645; F, mycelia of wild-type strain ATCC 46645. (B) Western blot analysis. The EGFP protein was detected using a polyclonal antiserum (see Materials and Methods). Sixty micrograms of total protein extract of each strain analyzed was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The full-length products of EGFP protein fusions are indicated by arrows. Molecular masses (in kilodaltons) of marker proteins are given on the left. Lanes: 1, mycelia of wild-type strain ATCC 46645; 2, conidia of wild-type strain ATCC 46645; 3, mycelia of strain AfPYRGEGFP1; 4, conidia of strain AfPYRGEGFP1; 5, mycelia of strain AfPKSPEGFP4; 6, conidia of strain AfPKSPEGFP4.
Western blot analysis.
A. fumigatus cultures were cultivated overnight in AMM at 37°C with shaking at 180 rpm. Protein extracts of mycelia were obtained as previously described for A. nidulans (4). For analysis of conidial proteins, conidia were isolated from mycelia grown for 3 days on AMM agar plates at 37°C using 10 ml of H2O. The conidial suspension was centrifuged at 12,000 × g at 4°C. Proteins from the pellet were extracted by using a pestle and mortar with liquid nitrogen, as previously described for mycelia of A. nidulans (4). Western blot analysis was performed as described by Brakhage and Van den Brulle (4) except that each slot of the protein gel was loaded with 60 μg of protein. A GFP antiserum was obtained from Invitrogen (Groningen, The Netherlands). Since the specificity of antiserum against EGFP was low, the antibodies were first saturated overnight with crude extracts of non-EGFP-containing A. fumigatus wild-type strain ATCC 46645. Precipitates were centrifuged for 5 min at 4°C, and the supernatant was used for the immune reaction.
Fluorimetric determination of fluorescence intensity.
Protein extracts of A. fumigatus were obtained as described above for Western blot analysis. The intensity of fluorescence of the protein extract was determined using an LS 50B luminescence spectrometer (Perkin-Elmer, Norwalk, Conn.) with an excitation wavelength of 490 nm. The fluorescence value of conidia of strain AfPKSPEGFP4 was set at 1 as a reference. All other values were determined as relative values.
Determination of protein concentrations.
Protein concentrations were determined according to the method of Bradford (3).
Analysis of expression of egfp-containing gene fusions in mice.
Six- to 8-week-old OF1 male mice (Swiss outbred, 32 to 34 g; Ilta Credo, St. Germain sur l'Abreole, France) were immunosuppressed with 25 mg of cortisone acetate, which was injected intraperitoneally twice (day −3 and day 0). Mice were anesthetized by intramuscular injection of 100 μl of ketamine (100 mg per ml) (Mérial, Lyon, France)-xylazine (2 mg per ml) (Bayer AG, Leverkusen, Germany) solution. Twenty-five microliters of a solution of A. fumigatus conidia in phosphate-buffered saline–Tween 20 (4 × 107 conidia per ml) was inoculated intranasally. Bronchoalveolar lavage was performed 24 h after inoculation. For this purpose, mice were sacrificed by cervical dislocation. The tracheae were exposed after incision of the cervical area. Germinating conidia and mycelia were isolated together with alveolar macrophages. This was done by washing the lungs 10 times with 1 ml of ice-cold Ca2+- and Mg2+-free phosphate-buffered saline through an 18-gauge plastic catheter inserted into the trachea. The extracted cells (germinating conidia, hyphae, and alveolar macrophages) were separated from the lavage fluid by centrifugation at 400 × g for 8 min at 4°C. The pellet was resuspended in Ca2+-containing phosphate-buffered saline, and germlings and hyphae were directly examined with a microscope. The animal experiments were repeated twice with identical results.
RESULTS
Construction of A. fumigatus strains carrying fusions of different 5′ sequences with the EGFP-encoding gene.
To establish whether GFP can be used to monitor gene expression in A. fumigatus, EGFP was used. Two different 5′ sequences containing the putative promoters were analyzed, i.e., the 5′ sequences of the A. fumigatus pyrG gene, encoding orotidine-5′-phosphate decarboxylase (31), and of the A. fumigatus pksP gene (14). The 5′ sequences, including the ATG start codons and codons encoding some N-terminal amino acids of the respective proteins, were fused in frame with the egfp gene (Fig. 1). The construction of translational fusions was checked by DNA sequence analysis across the junctions.
The A. fumigatus wild-type strain ATCC 46645 was transformed with plasmids pUCGH-pksPI (PpksP-egfp) and pUCGH-pyrG (PpyrG-egfp). Hygromycin B-resistant transformants exhibiting EGFP fluorescence were checked by Southern blot analysis. For the PpyrG-egfp gene fusion, transformant strain AfPYRGEGFP1 containing the gene fusion ectopically integrated in double copy into the genome was used for further studies (not shown), because the strain exhibited easily detectable fluorescence. For the PpksP-egfp plasmid, several transformants, designated AfPKSPEGFP2 to -5, were analyzed. Southern blot analysis of chromosomal DNAs of the transformant strains revealed that one of the transformants, AfPKSPEGFP4, carried the gene fusion integrated in single copy at the chromosomal pksP gene locus (Fig. 2B, lanes 4). Consequently, the expression of the PpksP-egfp gene fusion was directed by the endogenous promoter. Because of this mode of integration into the genome (Fig. 2), the endogenous pksP gene was controlled only by the 547-bp fragment immediately upstream of the pksP gene, which was present in front of the PpksP-egfp on plasmid pUCGH-pksPI. Interestingly, these 547 bp were apparently not sufficient for expression of the pksP gene, because transformant AfPKSPEGFP4 produced only white conidia.
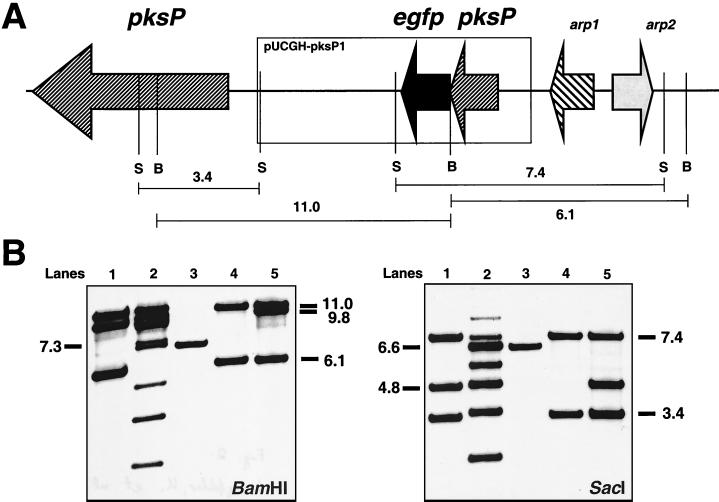
FIG. 2.
Identification of an A. fumigatus transformant carrying the PpksP-egfp fusion integrated in single copy at the pksP gene locus. (A) Partial restriction map of the genomic region containing pksP and schematic representation of the integration of a single copy of PpksP-egfp. The arrows indicate the orientations of the genes. B and S, BamHI and SacI restriction sites, respectively. Sizes of DNA fragments to which the probe hybridizes and which are important for the molecular characterization of transformants are indicated. arp1 and arp2 are located in the vicinity of pksP and encode scytalone dehydratase and 1,3,6,8-tetrahydroxynaphthalene reductase, respectively. The products of both genes are also involved in 1,8-tetrahydroxynaphthalene–melanin biosynthesis (27, 28). (B) Southern blot analyses. Chromosomal DNAs of the wild-type strain and transformant strains were cut by either BamHI or SacI, as indicated. The DNA was hybridized with a DNA probe of 2.0 kb generated by PCR amplification using oligonucleotides KLNBaB and KLupsNF and chromosomal DNA of the wild-type strain ATCC 46645 as the template. Transformant AfPKSPEGFP4 (lanes 4) showed the expected hybridization pattern. The band characteristic of the wild type (lanes 3) had disappeared and two new bands had appeared, indicating that the gene fusion was integrated in single copy at the chromosomal pksP gene locus. The transformants in lanes 1 and 5 carry the gene fusion integrated in double copy at the chromosomal pksP gene locus. Numbers on the right and left indicate approximate sizes of hybridizing bands in kilobase pairs. Lanes: 1, transformant AfPKSPEGFP2; 2, transformant AfPKSPEGFP3; 3, wild-type strain ATCC 46645; 4, transformant AfPKSPEGFP4; 5, transformant AfPKSPEGFP5.
Differential expression of gene fusions in vitro.
The expression of the egfp gene fusions was assessed in AMM at 37°C. The results are shown in Fig. 3. The PpyrG-egfp-containing transformant AfPYRGEGFP1 expressed intense fluorescence activity (Fig. 3a to f). Considerable fluorescence was observed during germination of conidia, during mycelial growth, and also in conidiophores (Fig. 3a to f). Conidia showed only weak fluorescence (Fig. 3f). By contrast, for PpksP-egfp expression a different pattern was observed. Under standard conditions, i.e., growth of mycelia for 24 h in AMM at 37°C, there was no fluorescence detectable in hyphae of transformant strain AfPKSPEGFP4 (Fig. 3i and j). Fluorescence of germinating conidia was very weak and hardly above the background observed in hyphae (Fig. 3g to j). It probably represented only background fluorescence due to the dilution of the PKSPEGFP protein fusion from conidia to the outgrowing hyphae. However, when mycelia were allowed to sporulate on AMM agar plates, strong fluorescence was detected exclusively in phialides and conidia (Fig. 3k and l). Even in the stalk of the conidiophore no fluorescence was visible, indicating a strictly spatial expression of the pksP gene in certain cell types (Fig. 4). This observation confirmed previous results showing that the pksP transcript was detectable only during sporulation of the fungus (28). Interestingly, PpksP-egfp expression was even confined to two cell types, i.e., phialides and conidia. The analysis of the untransformed wild-type strain as a negative control showed that almost no background fluorescence could be detected (Fig. 3m to r).
FIG. 4.
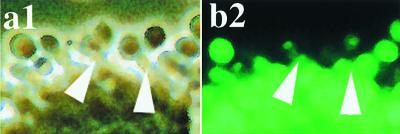
EGFP-derived fluorescence in phialides and conidia of strain AfPKSPEGFP4. The photographs show enlargements of Fig. 3k and l. Some phialides are indicated by arrowheads. The sample was analyzed by light microscopy (a1) or fluorescence microscopy (b2).
In order to quantify fluorescence and to allow a comparison of the promoter constructs, the presence of EGFP was quantified using two different methods. As shown in Fig. 5A, protein extracts isolated from the different A. fumigatus strains were analyzed by fluorescence spectrometry. The protein extract of the untransformed wild-type strain of A. fumigatus only showed marginal fluorescence activity at 509 nm irrespective of whether the protein extract was isolated from hyphae (Fig. 5A, trace F) or conidia (Fig. 5A, trace E). The same was found with protein derived from hyphae of strain AfPKSPEGFP4 (Fig. 5A, trace D). By contrast, strong fluorescence was measured in protein extracts derived from conidia of strain AfPKSPEGFP4 (Fig. 5A, trace A) and mycelia from strain AfPYRGEGFP1 (Fig. 5A, trace B). Weaker fluorescence was observed when protein extract of conidia of strain AfPYRGEGFP1 was analyzed (Fig. 5A, trace C). Taken together, these data showed that the cellular amount of EGFP protein determined by fluorescence spectrometry correlated well with the fluorescence detected microscopically. These findings were independently supported by Western blotting, using an antiserum against EGFP (Fig. 5B). Since the PKSP-EGFP and PYRG-EGFP fusion proteins have different molecular masses, of 75.7 and 28.8 kDa, respectively, due to the different number of amino acids fused to the EGFP, they migrated differently in the gel. In the untransformed wild-type strain, no EGFP was detected (Fig. 5B, lanes 1 and 2). In hyphae of strain AfPYRGEGFP1, a band with the expected size of 33 kDa was detected; it could not be detected in conidia. This is most likely due to the low specificity of the antiserum used for Western blot analysis, resulting in a lower sensitivity compared with the fluorescence spectrometry. It further confirms, however, that there is only a small amount of EGFP in conidia of this strain. A similar observation was made by Valdez-Taubas et al. (31) for the general purine transporter protein UAPC of A. nidulans, which could not be detected by conventional Western blot analysis although a high-titer antiserum was used. In contrast, it was possible to identify the cellular location of the protein using a GFP fusion protein. In conidia of transformant strain AfPKSPEGFP4, the expected EGFP fusion with a molecular mass of 80 kDa was observed (Fig. 5B, lane 6). There was also visible a faster-migrating band which seems to correspond to a proteolytically cleaved PKSP-EGFP fusion protein. In hyphae of strain AfPKSPEGFP4 grown under standard conditions, no EGFP protein was detectable by Western blot analysis (lane 5). As expected, no EGFP protein was found in hyphae or conidia of the untransformed wild-type strain (lanes 1 and 2).
GFP as a reporter for the study of fungus-host interaction in vivo.
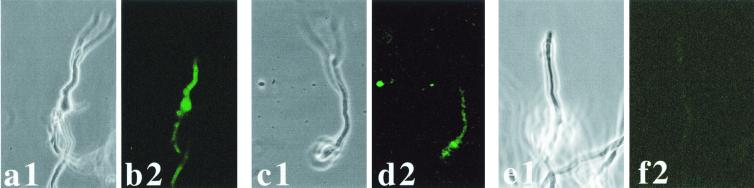
To investigate whether EGFP can be used as a reporter for gene expression in vivo and also whether PpksP-egfp expression showed the same expression pattern in vitro and in vivo, immunocompromised mice were infected with the different A. fumigatus strains. At 24 h after inoculation, germlings were isolated from the lungs by broncheoalveolar lavage and analyzed for egfp expression. The results are shown in Fig. 6. Unexpectedly, in contrast to the in vitro results, the PpksP-egfp gene fusion was expressed in germlings isolated from the lungs of immunocompromised mice (Fig. 6c and d). The fluorescence was clearly above the background fluorescence observed with the untransformed wild-type strain (Fig. 6e and f). The fluorescence in germlings isolated from mice was also stronger than the weak background fluorescence that could be detected in germinating conidia in vitro (Fig. 3h). This finding indicates that the pksP gene not only is expressed during conidiation but also can be expressed in hyphae. As a control, the expression of the PpyrG-egfp gene in germlings isolated from the lungs of immunocompromised mice was analyzed. As shown in Fig. 6a and b, the PpyrG-egfp gene was expressed in vivo in hyphae.
FIG. 6.
EGFP-derived fluorescence of A. fumigatus reisolated from the lungs of immunocompromised mice 24 h after inoculation. Germlings were isolated by bronchoalveolar lavage. Germlings of the PpyrG-egfp-containing strain AfPYRGEGFP1 (a and b), the PpksP-egfp-containing strain AfPKSPEGFP4 (c and d), and, as a negative control, the untransformed wild-type strain (e and f) are shown. Samples were analyzed by light microscopy (panels 1) or fluorescence microscopy (panels 2).
DISCUSSION
Here, we demonstrate that the egfp gene can be used as reporter gene in A. fumigatus to measure gene expression both in vitro and in vivo. The PpksP-egfp construct was integrated in single copy at the chromosomal pksP locus. It therefore allows the precise measurement of gene regulation and makes artifacts, which could be due to the integration of gene fusions at unknown genetic loci, unlikely. For the PpyrG-egfp-carrying plasmid, no attempt was made to isolate single-copy transformants because the expression of this gene fusion served only as a control to check whether EGFP is functional in case there was no PKSP-EGFP-dependent fluorescence in A. fumigatus. However, in the A. fumigatus strain that was analyzed, which contained two ectopically integrated copies of the PpyrG-egfp gene, the expression pattern of the pyrG gene fusion reflected that expected for the pyrG gene 5′ sequence; i.e., the PpyrG-egfp fusion was constitutively expressed. Fluorescence was visible in germlings, hyphae, and conidiophores and also in germlings derived from the lungs of immunocompromised mice. This can be expected from a gene which is required for the biosynthesis of an essential metabolic compound such as uracil. It further supports the theory that pyrG is an essential gene for the fungus at almost all developmental stages and is also required for invasive growth in a murine model of invasive aspergillosis (9). Therefore, this gene fusion might also be of use to observe the infectious process in the mouse infection model. By contrast, under the conditions applied in vitro, the PpksP-egfp gene fusion was expressed only during sporulation. These data agree well with the result of Tsai et al. (28), who detected pksP mRNA only in sporulating fungi in vitro but not in hyphae, and with the function of the PKSP protein, which is involved in the biosynthesis of the grey-green conidial pigment.
Because of the mode of integration of the PpksP-egfp fusion into the genome, its expression was directed by the endogenous promoter, whereas the endogenous pksP gene was controlled only by 547 bp upstream of the pksP gene which was present in front of PpksP-egfp on plasmid pUCGH-pksPI. These 547 bp were apparently not sufficient for expression of the pksP gene, because transformant AfPKSPEGFP4 produced only white conidia. The same was found for other transformants containing the PpksP-egfp gene fusion integrated at the chromosomal pksP gene locus. All of these strains produced white conidia and showed the same developmentally dependent pattern of expression of PpksP-egfp in vitro as strain AfPKSPEGFP4 (not shown). Two putative binding sites for the transcriptional activator ABAA are located upstream of position −547. Since ABAA is required for developmental expression of many genes in A. nidulans (2; reviewed in reference 1), it appears very likely that these sites are required for expression of pksP. Furthermore, theoretically it was conceivable that pksP expression was subject to feedback inhibition by products of the conidial pigment biosynthesis pathway. This is lacking in strain AfPKSPEGFP4, which could lead to an artificial expression pattern for the PpksP-egfp fusion. However, since the PpksP-egfp gene fusion showed the identical developmentally dependent regulation as the pksP gene examined by Northern blot analysis in the wild-type strain (28), the lack of pigment biosynthesis in strain AfPKSPEGFP4 apparently has no effect on the expression pattern of the PpksP-egfp fusion.
Interestingly, PpksP-egfp expression was detected in germlings isolated from the lungs of immunocompromised mice. This result was unexpected and suggests that under certain stress conditions, pksP can even be expressed in hyphae. Therefore, pksP might also be of importance for the invasive growth of the fungus, e.g., by producing polyketide derivatives which have a toxic potential on residual immune effector cells. There is also a highly conserved stress response element present in the pksP 5′ sequence. It remains to be shown, however, whether pksP expression in germlings and hyphae is mediated via this element. We and others have shown that PKSP is an important factor in A. fumigatus virulence (12, 14, 28, 29). However, although the pigment is able to scavenge ROS, it seems unlikely that this is the major PKSP-dependent virulence determinant (13). This is also supported by preliminary results suggesting that the biosynthesis steps immediately succeeding those catalyzed by PKSP appear to have little impact on virulence (29). Taken together, these findings suggest that PKSP might be involved in additional processes that are important for infection. Expression in hyphae during invasive growth would lend support to this theory.
ACKNOWLEDGMENTS
We thank Christian Aichinger and Regine Kahmann for the gift of plasmid p123 encoding egfp.
This work was supported by the Deutsche Forschungsgemeinschaft (grant Br-1130/5-3 to A.A.B.).
REFERENCES
- 1.Adams T H, Wieser J K, Yu J H. Asexual sporulation in Aspergillus nidulans. Microbiol Mol Biol Rev. 1998;62:35–54. doi: 10.1128/mmbr.62.1.35-54.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrianopoulos A, Timberlake W E. The Aspergillus nidulans abaA gene encodes a transcriptional activator that acts as a genetic switch to control development. Mol Cell Biol. 1994;14:2505–2515. doi: 10.1128/mcb.14.4.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–252. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Brakhage A A, Van den Brulle J. Use of reporter genes to identify recessive trans-acting mutations specifically involved in the regulation of Aspergillus nidulans penicillin biosynthesis genes. J Bacteriol. 1995;177:2781–2788. doi: 10.1128/jb.177.10.2781-2788.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brakhage A A, Langfelder K, Wanner G, Schmidt A, Jahn B. Pigment biosynthesis and virulence. In: Brakhage A A, Jahn B, Schmidt A, editors. Aspergillus fumigatus: biology, clinical aspects and molecular approaches to pathogenicity. Contributions to microbiology. Vol. 2. Basel, Switzerland: Karger Medical and Scientific Publishers; 1999. pp. 205–215. [DOI] [PubMed] [Google Scholar]
- 6.Chalfie M C, Tu Y, Euskirchen G, Ward W W, Stuhl K. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 7.Debeaupuis J-P, Sarfat J, Chazale V, Latgé J-P. Genetic diversity among clinical and environmental isolates of Aspergillus fumigatus. Infect Immun. 1997;65:3080–3085. doi: 10.1128/iai.65.8.3080-3085.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.del Poeta M, Toffaletti D L, Rude T H, Sparks S D, Heitman J, Perfect J R. Cryptococcus neoformans differential gene expression detected in vitro and in vivo with green fluorescent protein. Infect Immun. 1999;67:1812–1820. doi: 10.1128/iai.67.4.1812-1820.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.d'Enfert C, Diaquin M, Delit A, Wuscher N, Debeaupuis J-P, Huerre M, Latgé J-P. Attenuated virulence of uridine-uracil auxotrophs of Aspergillus fumigatus. Infect Immun. 1996;64:4401–4405. doi: 10.1128/iai.64.10.4401-4405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.d'Enfert C, Weidner G, Mol P C, Brakhage A A. Transformation systems of Aspergillus fumigatus. In: Brakhage A A, Jahn B, Schmidt A, editors. Aspergillus fumigatus: biology, clinical aspects and molecular approaches to pathogenicity. Contributions to microbiology. Vol. 2. Basel, Switzerland: Karger Medical and Scientific Publishers; 1999. pp. 149–166. [DOI] [PubMed] [Google Scholar]
- 11.Denning D W. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–805. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 12.Jahn B, Koch A, Schmidt A, Wanner G, Gehringer H, Bhakdi S, Brakhage A A. Isolation and characterization of an Aspergillus fumigatus mutant strain with pigmentless conidia and reduced virulence. Infect Immun. 1997;65:5110–5117. doi: 10.1128/iai.65.12.5110-5117.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jahn B, Boukhallouk F, Lotz J, Langfelder K, Wanner G, Brakhage A A. Interaction of human phagocytes with pigmentless Aspergillus conidia. Infect Immun. 2000;68:3736–3739. doi: 10.1128/iai.68.6.3736-3739.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langfelder K, Jahn B, Gehringer H, Bhakdi S, Brakhage A A. Identification of polyketide synthase gene (pksP) of Aspergillus fumigatus involved in conidial pigment biosynthesis and virulence. Med Microbiol Immunol. 1998;187:79–89. doi: 10.1007/s004300050077. [DOI] [PubMed] [Google Scholar]
- 15.Latgé J-P. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma B, Mayfield M B, Gold M H. The green fluorescent protein gene functions as a reporter of gene expression in Phanerochaete chrysosporium. Appl Environ Microbiol. 2001;67:948–955. doi: 10.1128/AEM.67.2.948-955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morschhäuser J, Michel S, Hacker J. Expression of a chromosomally integrated, single copy GFP gene in Candida albicans, and its use as a reporter of gene regulation. Mol Gen Genet. 1998;257:412–420. doi: 10.1007/s004380050665. [DOI] [PubMed] [Google Scholar]
- 18.Mullins J, Harvey R, Seaton A. Sources and incidence of airborne Aspergillus fumigatus (Fres.) Clin Allergy. 1976;6:209–217. doi: 10.1111/j.1365-2222.1976.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 19.Osiewacz H D. A versatile shuttle cosmid vector for the efficient construction of genomic libraries and for the cloning of fungal genes. Curr Genet. 1994;26:87–90. doi: 10.1007/BF00326309. [DOI] [PubMed] [Google Scholar]
- 20.Punt P J, Oliver R P, Dingemanse M A, Powels P H, van den Hondel C A M J J. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene. 1987;56:117–124. doi: 10.1016/0378-1119(87)90164-8. [DOI] [PubMed] [Google Scholar]
- 21.Rüchel R, Reichard U. Pathogenesis and clinical presentation of aspergillosis. In: Brakhage A A, Jahn B, Schmidt A, editors. Aspergillus fumigatus: biology, clinical aspects and molecular approaches to pathogenicity. Contributions to microbiology. Vol. 2. Basel, Switzerland: Karger Medical and Scientific Publishers; 1999. pp. 21–43. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 23.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schilde C, Wöstemeyer J, Burmester A. Green fluorescent protein as a reporter for gene expression in the mucoralean fungus Absidia glauca. Arch Microbiol. 2001;175:1–7. doi: 10.1007/s002030000228. [DOI] [PubMed] [Google Scholar]
- 25.Spellig T, Bottin A, Kahmann R. Green fluorescent protein (GFP) as a new vital marker in the phytopathogenic fungus Ustilago maydis. Mol Gen Genet. 1996;252:503–509. doi: 10.1007/BF02172396. [DOI] [PubMed] [Google Scholar]
- 26.Suelmann R, Sievers N, Fischer R. Nuclear traffic in fungal hyphae: in vivo study of nuclear migration and positioning in Aspergillus nidulans. Mol Microbiol. 1997;25:757–769. doi: 10.1046/j.1365-2958.1997.5131873.x. [DOI] [PubMed] [Google Scholar]
- 27.Tsai H-F, Washburn R G, Chang Y C, Kwon-Chung K J. Aspergillus fumigatus arp1 modulates conidial pigmentation and complement deposition. Mol Microbiol. 1997;26:175–183. doi: 10.1046/j.1365-2958.1997.5681921.x. [DOI] [PubMed] [Google Scholar]
- 28.Tsai H-F, Yun C C, Washburn R G, Wheeler M H, Kwon-Chung K J. The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J Bacteriol. 1998;180:3031–3038. doi: 10.1128/jb.180.12.3031-3038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai H-F, Wheeler M H, Chang Y C, Kwon-Chung K J. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J Bacteriol. 1999;181:6469–6477. doi: 10.1128/jb.181.20.6469-6477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valdez-Taubas J, Diallinas G, Scazzocchio C, Rosa A L. Protein expression and subcellular localization of the general purine transporter UapC from Aspergillus nidulans. Fungal Genet Biol. 2000;30:105–113. doi: 10.1006/fgbi.2000.1197. [DOI] [PubMed] [Google Scholar]
- 31.Weidner G, d'Enfert C, Koch A, Mol P, Brakhage A A. Development of a homologous transformation system for the human pathogenic fungus Aspergillus fumigatus based on the pyrG gene encoding orotidine monophosphate decarboxylase. Curr Genet. 1998;33:378–385. doi: 10.1007/s002940050350. [DOI] [PubMed] [Google Scholar]
- 32.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]