Abstract
Introduction
The current study evaluated the use of platelet‐rich plasma (PRP), an autologous blood product with supraphysiologic concentrations of growth factors, in the treatment of prolonged coronavirus disease 2019 (COVID‐19)–related smell loss.
Methods
This multi‐institutional, randomized controlled trial recruited patients with COVID‐19 who had objectively measured smell loss (University of Pennsylvania Smell Identification Test [UPSIT] ≤ 33) between 6 and 12 months. Patients were randomized to three intranasal injections of either PRP or sterile saline into their olfactory clefts. The primary outcome measure was change in Sniffin’ Sticks score (threshold, discrimination, and identification [TDI]) from baseline. The secondary end point measures included responder rate (achievement of a clinically significant improvement, ≥5.5 points TDI), change in individual TDI olfaction scores, and change in subjective olfaction via a visual analog scale.
Results
A total of 35 patients were recruited and 26 completed the study. PRP treatment resulted in a 3.67‐point (95% CI: 0.05–7.29, p = 0.047) greater improvement in olfaction compared with the placebo group at 3 months and a higher response rate (57.1% vs 8.3%, odds ratio 12.5 [95% exact bootstrap confidence interval, 2.2–116.7]). There was a greater improvement in smell discrimination following PRP treatment compared with placebo but no difference in smell identification or threshold. There was no difference in subjective scores between PRP and placebo. No adverse effects were reported.
Conclusion
Olfactory function following COVID‐19 can improve spontaneously after 6 months and can improve to a greater extent with PRP injection. These data build on the promise of PRP to be a safe potential treatment option for patients with COVID‐19–related smell loss, and larger‐powered studies will help further assess its efficacy.
Keywords: anosmia, COVID‐19, long COVID, olfaction, persistent olfactory dysfunction, platelet‐rich plasma, post COVID syndrome, PRP, smell loss, therapeutics
1. INTRODUCTION
Persistent postviral olfactory dysfunction (OD) caused by coronavirus disease 2019 (COVID‐19) is a prominent global health concern that continues to rise. 1 While many patients achieve spontaneous recovery from their OD, 2 persistent smell and associated taste loss are common symptoms of post‐COVID syndrome with significant impacts on quality of life. 3 , 4 , 5
Potential therapies for postviral and COVID‐19–related OD remain limited with low efficacy and a paucity of evidence‐based support. While a Cochrane review last updated in December 2020 found no definitive treatments for persistent COVID‐19 OD, 6 there are multiple ongoing clinical trials and a few recently published studies. The strongest evidence for the treatment of COVID‐19 OD recommends olfactory training. 7 , 8 , 9 , 10 , 11 Other proposed therapies based on prepandemic evidence involve the use of topical intranasal medications 12 , 13 , 14 and oral anti‐inflammatory/neuroprotective agents. 15 , 16 , 17 , 18 However, the efficacy remains moderate at best among all current proposed therapeutics.
This study evaluated the use of platelet‐rich plasma (PRP), an autologous blood product with supraphysiologic concentrations of growth factors, in the treatment of prolonged COVID‐19–related smell loss. PRP is widely used in other clinical fields and has demonstrated promise in peripheral nerve regeneration through stimulation of vascular and axonal regeneration via growth factors and by regulation of inflammatory response in the microenvironment. 19 This study builds from prior pilot studies published by our group and others that demonstrated the safety of PRP in its use for OD. 20 , 21 , 22 In a murine model of anosmia, topical intranasal PRP resulted in improved olfactory function and restoration of an intact olfactory epithelium. 23 Prior single‐arm clinical trials utilizing PRP for OD demonstrated no adverse outcomes and a potential improvement in olfactory function. 20 , 21 Notably, Steffens et al recently demonstrated the potential efficacy of a single intranasal injection of PRP for the treatment of COVID‐19–related OD compared with olfactory training. Although that study had a limited follow‐up period and lacked randomization or a blinded placebo arm, its results build on our group's pilot data that suggest PRP may play a role in the treatment of postviral OD. The aim of this randomized controlled clinical trial was to evaluate the efficacy and safety of intranasal PRP in a cohort of patients with COVID‐19–related persistent OD despite mainstay treatments including olfactory training.
2. METHODS
This study was a randomized, single‐blinded, placebo‐controlled trial comparing the use of PRP with sterile saline intranasal injection in participants with persistent COVID‐19–induced OD. The study was approved by Stanford University (IRB#55353) and the University of California San Diego (UCSD; IRB#210296) institutional review board committees and registered on Clinicaltrials.gov (NCT04406584).
2.1. Participant selection
Participants were recruited from patients seen in the rhinology clinics at Stanford and UCSD between June 2021 and May 2022 who had polymerase chain reaction–confirmed diagnosis of severe acute respiratory syndrome coronavirus 2 (COVID‐19) between April 2020 and October 2021, and objective OD duration of >6 months but <12 months as depicted by the study flow diagram in Figure 1 according to CONSORT (Consolidated Standards of Reporting of Observational Studies) guidelines. Six months’ duration was used as a cutoff to ensure that the majority of patients known to spontaneously improve after COVID‐19–induced smell loss would not confound improvement from the intervention. 24 One year duration was used as a cutoff as we know the duration of loss often predicts recovery prognosis 25 and potentially how well any intervention may benefit our patients with smell loss and we did not want to miss a significant finding based on extended duration. Inclusion criteria comprised adult patients >18 years of age with confirmed OD who had a quantitative score of ≤33 points on the University of Pennsylvania Smell Identification Test (UPSIT) prior to study randomization. Participants must have previously trialed both olfactory training 8 and topical budesonide nasal irrigations 12 for at least 3 months and have a normal endoscopic examination of the nasal cavity and olfactory cleft. Exclusion criteria included a history of inflammatory sinonasal disease or evidence of rhinitis or sinusitis on endoscopy, prior sinonasal or anterior skull base surgery, self‐reported OD prior to COVID‐19 infection, neurodegenerative disease, history of bleeding disorders, or the use of blood thinner medications.
FIGURE 1.
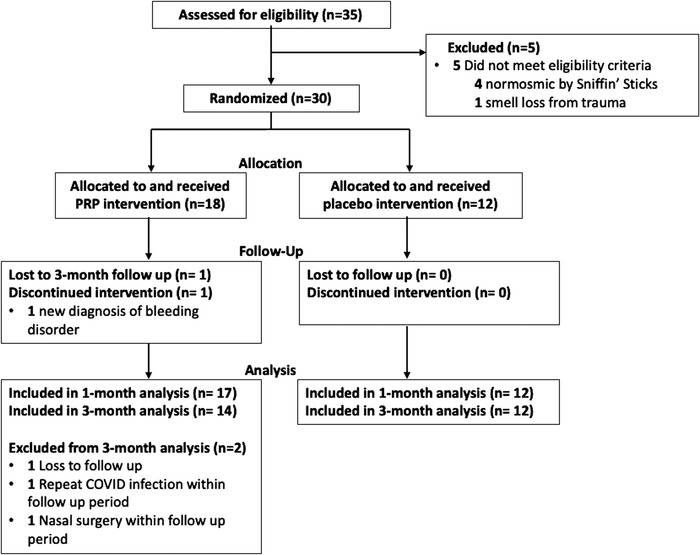
CONSORT (Consolidated Standards of Reporting of Observational Studies) flow diagram of the study recruitment and analysis. COVID, coronavirus disease 2019; PRP, platelet‐rich plasma.
2.2. Outcome measures
The outcome instrument was Sniffin’ Sticks, 26 a validated olfactory psychophysical test to determine odor threshold, discrimination, and identification (TDI), with each component score ranging from 0 to 16 for a total possible score of 48. Primary outcome measure was change in TDI score from baseline. Secondary end point measures included responder rate at 3 months, where a responder was defined as a clinically significant improvement on Sniffin’ Sticks TDI score (≥5.5 points). Additional secondary end points were the change in individual TDI component scores from baseline, and subjective olfaction via a 0‐ to 10‐point visual analog scale (VAS, 0 = no smell, 10 = perfect smell).
2.3. Study design
Patients underwent 1:1 randomization to either PRP or placebo (sterile saline) treatment via a random number generator. All recruited participants were initially screened for OD using UPSIT score ≤33 and then underwent baseline olfactory psychophysical testing using Sniffin’ Sticks. Repeat Sniffin’ Sticks testing was performed at the 4‐week (1‐month) and 3‐month follow‐up visits. Subjective smell function was queried at each time point.
Prior to treatment, participants were topically anesthetized with pledget application of 4% lidocaine and 0.1% phenylephrine. Patients received 1 mL of either PRP or sterile saline injected submucosally into bilateral olfactory clefts under endoscopic visualization. Treatments were given 2 weeks apart at three different time points (week 0, week 2, and week 4). All participants were blinded to the treatment received, underwent phlebotomy, and wore a blindfold during injections.
2.4. PRP preparation and injection
PRP isolation and injections were performed as depicted (Figure S1) and previously described in our pilot study. 20 Emcyte GS30‐PURE II PRP kits (EmCyte Corporation) were utilized and PRP isolation was performed per GS30‐PURE II Protocol A. Of note, PRP kits were donated by the EmCyte Corporation, but the study design, completion, and data analysis were conducted solely by the authors. In brief, 25 mL of whole blood was obtained through a peripheral blood draw and added to a prefilled syringe with 5 mL of sodium citrate anticoagulant. The sample was centrifuged for 1 min at 4200 rpm, and the platelet plasma suspension was aspirated and recentrifuged for 5 min at 4200 rpm. The subsequent supernatant containing platelet‐poor plasma was discarded, leaving 2.5 mL of PRP that was resuspended and drawn up into two separate 1‐mL sterile syringes and injected submucosally at two sites within the olfactory cleft along the superior septum, posterior to the head of the middle turbinate. Participants in the placebo study arm received 1 mL of sterile saline injections bilaterally in the same locations.
To confirm the proper isolation of PRP, whole blood and PRP samples from select participants (n = 9) were processed for complete blood cell count analysis. Compared with their respective whole blood, PRP samples resulted in an average 5.9‐fold increase in platelet concentration (Figure S2) with low granulocyte and red blood cell counts.
2.5. Statistical analysis
We performed a power analysis based on data from our pilot study in which hyposmic participants with a mean baseline olfaction score (Sniffin’ Sticks) of 22.4 points and a standard deviation of 4.6 points improved by 5.85 points following PRP therapy. 20 Thus, we determined that a sample of 20 participants (10 control, 10 experimental) would provide this trial with 80% power to detect a similar effect size at 26% improvement at 3 months, at a two‐sided α level of 0.05.
A Shapiro–Wilk test was used to confirm the Gaussian distribution of TDI scores, TDI component scores, and subjective olfaction scores. To compare patients’ baseline demographic and clinical characteristics between the two study arms, a Fisher exact test was used for discreet variables and a t test for continuous variables. Linear mixed regression models were used to determine the effect of PRP and placebo interventions on olfaction scores over the 1‐ and 3‐month trial period, because such models avoid listwise deletion of an entire study participant and thus yield unbiased estimates when missing data occurred at a particular time point. The first degree of autocorrelation covariance structure was chosen for all of the models as it yields the best Bayesian Information Criterion model fitting score. An interaction term between the study arm and study month was included in the model to compare the differences in change of olfactory scores. The model also controlled for baseline olfactory scores.
At 1‐ and 3‐month time points, we calculated the responder rate, or the percentage that achieved a minimally clinically important difference in TDI score, previously determined as an improvement of ≥5.5 points. Because of our small sample size, we opted to use the median unbiased estimate of the probabilities of minimally clinically important difference to estimate the odds ratios at month 1 and month 3 and calculated 95% confidence intervals based on “exact” bootstrap distribution. 27
SAS software version 9.4 (SAS Institute Inc) was used to perform statistical analyses. A p value < 0.05 (2‐sided) was considered significant.
3. RESULTS
This multi‐institutional single‐blinded randomized controlled trial assessed 35 patients for eligibility; 29 of which completed the trial through month‐1(n = 17 intervention, n = 12 placebo), and 26 of which completed the month‐3 trial (n = 14 intervention, n = 12 placebo, Figure 1). Five subjects did not meet eligibility criteria (four tested normosmic by Sniffin’ Sticks despite testing hyposmic on UPSIT screening and one had a history of smell loss due to prior trauma). Of the 30 patients who were randomized, one in the PRP arm failed to complete intervention (disqualified with new diagnosis of a bleeding disorder/severe thrombocytopenia). Three additional subjects in the PRP arm completed the 1‐month follow‐up but were excluded from the 3‐month analysis due to loss to follow‐up, recurrent COVID‐19 infection, and nasal surgery within the follow‐up period. Baseline characteristics and clinical demographics for the participants were similar between the two study arms, as reported in Table 1. The mean age of recruited subjects was 44.1 years (SD 14.0 years) and 50% were female. There were no differences in the average duration of OD (placebo 8.6 months vs. PRP 8.9 months, p = 0.725). Baseline olfactory scores between placebo and PRP arms were similar as measured by UPSIT (25.2 vs 22.4, p = 0.283) and Sniffin’ Sticks (26.0 vs 24.3, p = 0.413). As part of the inclusion criteria, all subjects had OD for at least 6 months following their COVID‐19 infection and had previously trialed olfactory training and high volume topical nasal steroid rinses without resolution of their OD.
TABLE 1.
Participant demographics
| Mean (SD) |
Placebo n = 12 |
PRP n = 18 |
p value |
|---|---|---|---|
| Age, years | 43.4 (16.3) | 44.6 (12.7) | 0.832 |
| Male gender, n (%) | 6 (50.0) | 9 (50.0) | 1.000 |
| Duration of olfactory loss, mo | 8.6 (2.4) | 8.9 (2.2) | 0.725 |
| Parosmia, n (%) | 5 (41.7) | 13 (72.2) | 0.101 |
| Subjective smell, 0‐10 | 3.8 (2.0) | 3.9 (1.4) | 0.876 |
| Baseline UPSIT score | 25.2 (6.9) | 22.4 (6.7) | 0.282 |
| Baseline Sniffin’ Sticks (TDI) score | 26.0 (4.4) | 24.3 (6.4) | 0.413 |
| Baseline T score | 5.0 (2.7) | 5.0 (3.9) | 0.975 |
| Baseline D score | 10.7 (1.7) | 9.5 (2.6) | 0.186 |
| Baseline I score | 10.4 (2.8) | 9.8 (2.6) | 0.533 |
| Race or ethnicity, n (%) | 0.446 | ||
| Hispanic | 2 (16.7) | 6 (33.3) | |
| White, non‐Hispanic | 9 (75.0) | 9 (50.0) | |
| Black, non‐Hispanic | 0 (0.0) | 1 (5.6) | |
| Two or more races | 1 (8.3) | 1 (5.6) | |
| Asian or Pacific Islander | 0 (0.0) | 1 (5.6) | |
| American Indian or Alaskan Native | 0 (0.0) | 0 (0.0) | |
| Medical history, n (%) | |||
| Diabetes | 0 (0.0) | 1 (5.6) | 0.424 |
| Hypertension | 1 (8.3) | 1 (5.6) | 0.775 |
| Asthma | 2 (16.7) | 2 (11.1) | 0.674 |
| Allergies | 1 (8.3) | 1 (5.6) | 0.775 |
| Post‐COVID symptoms, n (%) | |||
| Shortness of breath | 0 (0.0) | 0 (0.0) | – |
| Fatigue | 1 (8.3) | 2 (11.1) | 0.812 |
| Headache | 0 (0.0) | 0 (0.0) | – |
| Palpitations | 0 (0.0) | 1 (5.6) | 0.424 |
| Brain fog | 0 (0.0) | 2 (11.1) | 0.247 |
Abbreviations: COVID, coronavirus disease 2019; PRP, platelet‐rich plasma; SD, standard deviation; TDI, threshold, discrimination, identification; UPSIT, University of Pennsylvania Smell Identification Test.
Using a linear mixed model that adjusted for baseline score, estimated mean improvement in objective (TDI) and subjective (VAS) olfactory function are summarized for both placebo and PRP arms at 1‐month and 3‐months post‐intervention in comparison with baseline (Table 2). The PRP arm had a statistically significant improvement above baseline Δ4.31 TDI points, 95% CI: 1.69–6.93 at 1‐month post‐intervention (p = 0.002) and Δ6.25 points, 95% CI: 3.85–8.65 at 3‐months (p < 0.0001). The placebo arm had no statistically significant improvement above baseline (Δ1.17, −1.99–4.32 and Δ2.58, −0.13–5.29) at 1‐ and 3‐months, respectively. Examining individual components of olfaction (Table 2): threshold (T), discrimination (D), and identification (I) all improved post‐PRP treatment compared to baseline with the greatest improvement noted in smell discrimination at 3‐months post‐treatment (ΔD: 2.82, 1.76–3.87, p < 0.0001). In contrast, placebo intervention resulted in an improvement in smell threshold at 3‐months (ΔT: 1.75, 0.41–3.09, p = 0.011) but no changes in the other components of olfaction.
TABLE 2.
Change of olfactory score from baseline to 1‐month and 3‐month post intervention visit
| Month 1 vs Baseline | Month 3 vs Baseline | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Arm | Change | Lower CI | Upper CI | p value | Change | Lower CI | Upper CI | p value | |
| Total TDI | |||||||||
| Placebo | 1.17 | –1.99 | 4.32 | 0.464 | 2.58 | –0.13 | 5.29 | 0.061 | |
| Treatment | 4.31 | 1.69 | 6.93 | 0.002 | 6.25 | 3.85 | 8.65 | <0.0001 | |
| Difference | 3.15 | –0.96 | 7.25 | 0.131 | 3.67 | 0.05 | 7.29 | 0.047 | |
| T score | |||||||||
| Placebo | 0.42 | –1.07 | 1.91 | 0.579 | 1.75 | 0.41 | 3.09 | 0.011 | |
| Treatment | 2.04 | 0.79 | 3.30 | 0.002 | 1.82 | 0.64 | 3.00 | 0.003 | |
| Difference | 1.63 | –0.32 | 3.57 | 0.100 | 0.07 | –1.71 | 1.85 | 0.935 | |
| D score | |||||||||
| Placebo | 0.92 | –0.36 | 2.19 | 0.157 | 0.42 | –0.78 | 1.62 | 0.488 | |
| Treatment | 1.14 | 0.06 | 2.21 | 0.038 | 2.82 | 1.76 | 3.87 | <0.0001 | |
| Difference | 0.22 | –1.45 | 1.89 | 0.793 | 2.40 | 0.80 | 4.00 | 0.004 | |
| I score | |||||||||
| Placebo | –0.17 | –1.72 | 1.39 | 0.831 | 0.42 | –0.99 | 1.83 | 0.555 | |
| Treatment | 1.54 | 0.23 | 2.85 | 0.022 | 1.53 | 0.29 | 2.78 | 0.017 | |
| Difference | 1.71 | –0.32 | 3.74 | 0.098 | 1.12 | –0.76 | 3.00 | 0.239 | |
| VAS | |||||||||
| Placebo | 1.20 | 0.05 | 2.35 | 0.040 | 1.25 | 0.27 | 2.23 | 0.014 | |
| Treatment | 1.50 | 0.51 | 2.49 | 0.004 | 2.13 | 1.33 | 2.93 | <0.0001 | |
| Difference | 0.30 | –1.22 | 1.82 | 0.694 | 0.88 | –0.38 | 2.15 | 0.167 | |
Abbreviations: CI, 95% confidence interval; TDI, threshold, discrimination, identification (Sniffin’ Sticks); VAS, visual analog scale.
Bolded value p < 0.05.
When assessing subjective changes in smell function, both the placebo and the PRP arms demonstrated a significant improvement in VAS at 1‐ and 3‐months compared to baseline (Table 2). VAS scores improved Δ1.2, 0.05–2.35, p = 0.040 (1‐month) and Δ1.25, 0.27–2.23, p = 0.014 (3‐month) in the placebo arm and Δ1.5, 0.51–2.49, p = 0.004 (1‐month) and Δ2.13, 1.33–2.93, p < 0.0001 (3‐month) in the PRP arm.
PRP treatment resulted in a 3.67‐point greater improvement in olfaction (TDI score) compared to the placebo group at 3 months (95% CI, 0.05–7.29, p = 0.047) in a mixed linear model adjusted for baseline olfactory score (Table 2, Figure 2). There was also a 2.40‐point greater improvement in discrimination scores in the PRP versus placebo group at 3‐months (95% CI, 0.80–4.00, p = 0.004). There was no statistical difference in the improvement of overall olfaction score or individual component scores between PRP and placebo at 1‐month posttreatment. The change in olfaction threshold and identification were also similar in both study arms at 3 months. No significant difference was found in the change of subjective olfaction scores (VAS) at either month 1 or month 3 between placebo and intervention (Table 2, Figure 2).
FIGURE 2.
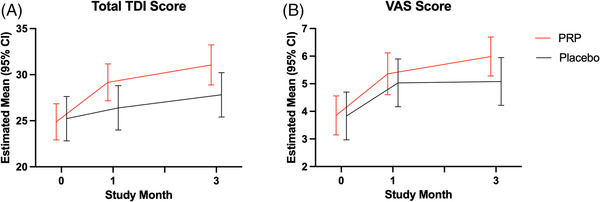
Measured psychophysical (threshold, discrimination, identification [TDI]) and subjective (visual analog scale [VAS]) olfaction scores at baseline and 1 month and 3 months after treatment, from linear mixed models adjusted for baseline score. Error bars represent 95% confidence intervals (CIs).
In evaluating responder rate, at 1‐month post‐intervention, 3 of 12 (25.0%) subjects in the placebo arm had clinically significant improvement in olfactory function compared to 7 of 17 (41.2%) patients in the PRP arm (OR, 2.0 [95% exact bootstrap CI, 0.4–17.0]). By completion of the trial (3‐month post‐intervention), the responder rate was 8.3% in the placebo arm (1 of 12) compared to 57.1% (8 of 14) of subjects in the PRP arm (OR, 12.5 [95% exact bootstrap CI, 2.2–116.7]).
None of the participants reported long‐standing adverse effects related to the injections. Short‐term side effects were related to the injection itself and consisted of nasal congestion and pressure that lasted up to 24 hours, experienced by both PRP and placebo arms. One participant in the placebo arm reported photophobia lasting for a few hours post‐injection that self‐resolved. Follow up endoscopic visualization showed no gross effects to the olfactory cleft mucosa at 3 months post‐treatment.
4. DISCUSSION
In this single‐blinded, randomized controlled study, PRP treatment resulted in a greater improvement in overall olfaction scores compared with placebo with a 12.5 times greater likelihood in achieving a treatment response at 3 months. Submucosal injections of PRP into the olfactory cleft were well tolerated without significant adverse effects and did not worsen smell function, as previously noted in our pilot study and other studies utilizing intranasal PRP. 20 , 21 , 22 , 28 , 29 These data suggest that PRP has the potential as a safe treatment option for patients with COVID‐19 smell loss.
However, there was no statistical difference in overall subjective improvement between the PRP and placebo arms. Both arms of the study demonstrated significant improvement at 1 month and 3 months after treatment. The lack of difference may be attributable to an underpowered study sample that did not account for the magnitude of spontaneous recovery or placebo effect. Furthermore, the greatest improvement with PRP therapy was seen in smell discrimination. Subjective olfactory improvement is likely variable with each individual placing a different weighted importance on smell intensity, discrimination, and identification. However, it has also been shown that subjective improvement lags objective recovery in COVID‐19–related OD. 30 Thus, it is possible that subjective improvement may be more notable with a longer follow‐up period.
In their study, Steffens et al reported olfaction outcomes using a cohort of patients who underwent a single intranasal injection of PRP with a 1‐month follow‐up 22 and found that PRP treatment resulted in higher TDI scores compared with control. Our two studies differ in that ours was a randomized, blinded study that involved a placebo injection, had a longer follow‐up period of 3 months, and included only patients who had failed olfactory training. Both studies had similar levels of improvement in TDI scores following PRP treatment but with different follow‐up periods (Δ6.25 points at 3 months vs Δ6.7 points at 1 month, respectively). In our study, the control group had greater olfactory improvement (Δ3.0 points at 3 months vs Δ0.5 points at 1 month). This difference in olfactory improvement between the two studies’ control groups likely reflects the placebo effect of receiving a sham procedural intervention and the differences in spontaneous resolution with a longer follow‐up period.
Although not a named outcome of this study, we did make a note of those with coinciding parosmia as many COVID‐19 patients with smell loss also experience smell distortion. We did not notice any change in parosmia following PRP treatment. Additionally, the presence of parosmia did not affect objective olfaction recovery based on adjusted linear mixed models (data not shown). While our analysis controlled for baseline olfactory scores, we also noted that the duration of OD did not affect smell recovery. The study recruited patients over the course of a year (2021–2022) with a least 6 months of OD, and while there have been multiple variants of COVID‐19 during this period, the randomization between PRP and placebo was well balanced over the entire duration of enrollment.
Limitations of this study include the small sample size. PRP treatments resulted in significantly improved olfactory function compared with placebo with a higher responder rate, but the wide confidence intervals in our model highlight the variability of response and small sample size and thus the high odds ratio should be interpreted with caution. Two participants in the placebo arm were responders at month 1 (Δ6.0 TDI points) but were no longer responders at month 3 (Δ4.0 points at month 3). This difference is likely within the anticipated retesting margin of error. Future larger studies will allow for a better understanding of the effect size between PRP and placebo. In performing a power analysis based on our pilot study, we estimated that the ability to detect a type I error with 80% power (α= 0.05), would require 20 patients (10 control, 10 experimental). However, this analysis did not account for olfaction improvement in the placebo arm, which is likely attributable to spontaneous recovery, a placebo effect of obtaining an intervention, or the effects of other ongoing, pretrial treatments. The effect sizes from this clinical trial will help guide sample size calculations for future studies.
Other limitations include the lack of prior data to inform the optimal dosage or concentration of our PRP injections, which may have an impact on olfaction recovery. Given our past experience, we injected 1 mL of PRP into the olfactory cleft bilaterally at two different sites (each 0.5 mL) along the superoposterior septum, a region previously shown to have high concentrations of olfactory nerve fibers. 31 Steffens et al utilized our protocol and injection volume in their recent PRP study. 22 In this study, our PRP preparation technique resulted in an average 5.9‐fold increase in platelet concentration compared with whole blood (Figure S2). This yield is in keeping with prior clinical studies for PRP preparation, 32 although further studies are required to determine the optimal PRP therapy protocols for OD. Similarly, a better understanding of the mechanism of action in the use of PRP for postviral olfactory loss is warranted and would benefit from preclinical studies.
5. CONCLUSION
In this randomized controlled trial, treatment with intranasal PRP resulted in a greater improvement in measured olfactory function compared with placebo for COVID‐19–related OD. Yet there was no subjective olfactory improvement over placebo. Given the paucity of definitive therapeutic options for postviral OD, PRP therapy may be a promising addition to existing therapies such as olfactory training and steroid irrigations. However, it would be important to counsel potential patients that subjective improvement following PRP therapy can vary by individual. Larger studies are required to better determine optimal candidacy, further assess efficacy, and standardize protocols.
Supporting information
Supporting Information
ACKNOWLEDGMENT
PRP isolation kits were donated by EmCyte Corporation. No relevant financial disclosures for all authors. ZMP: consultant/advisory board for Medtronic, Ethicon J&J, Regeneron/Sanofi, Optinose, Mediflix, Consumer Medical, Dianosic, Olfera Therapeutics. CHY receives support from the ARS/AAO‐HNS New Investigator Award and from the grant K08DC019956 from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders.
Yan CH, Jang SS, Lin H‐FC, et al. Use of platelet‐rich plasma for COVID‐19–related olfactory loss: a randomized controlled trial. Int Forum Allergy Rhinol. 2022;1‐9. 10.1002/alr.23116
Contributor Information
Carol H. Yan, Email: c1yan@health.ucsd.edu.
Zara M. Patel, Email: zmpatel@stanford.edu.
REFERENCES
- 1. Tan BKJ, Han R, Zhao JJ, et al. Prognosis and persistence of smell and taste dysfunction in patients with covid‐19: meta‐analysis with parametric cure modelling of recovery curves. BMJ. 2022;378:e069503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel ZM, Holbrook EH, Turner JH, et al. International consensus statement on allergy and rhinology: olfaction. Int Forum Allergy Rhinol. 2022;12(4):327‐680. [DOI] [PubMed] [Google Scholar]
- 3. Said M, Luong T, Jang SS, Davis ME, DeConde AS, Yan CH. Clinical factors associated with lower health scores in COVID‐19–related persistent olfactory dysfunction. Int Forum Allergy Rhinol. 2022;12(10):1242‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burges Watson DL, Campbell M, Hopkins C, Smith B, Kelly C, Deary V. Altered smell and taste: anosmia, parosmia and the impact of long Covid‐19. PLoS One. 2021;16(9):e0256998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vaira LA, Gessa C, Deiana G, et al. The effects of persistent olfactory and gustatory dysfunctions on quality of life in long‐COVID‐19 patients. Life. 2022;12(2):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Webster KE, O'Byrne L, MacKeith S, Philpott C, Hopkins C, Burton MJ. Interventions for the prevention of persistent post‐COVID‐19 olfactory dysfunction. Cochrane Database Syst Rev. 2022;9(9):CD013877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel ZM, Wise SK, DelGaudio JM. Randomized controlled trial demonstrating cost‐effective method of olfactory training in clinical practice: essential oils at uncontrolled concentration. Laryngoscope Investig Otolaryngol. 2017;2(2):53‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Damm M, Pikart LK, Reimann H, et al. Olfactory training is helpful in postinfectious olfactory loss: a randomized, controlled, multicenter study. Laryngoscope. 2014;124(4):826‐831. [DOI] [PubMed] [Google Scholar]
- 9. Lechner M, Liu J, Counsell N, et al. The COVANOS trial – insight into post‐COVID olfactory dysfunction and the role of smell training. Rhin. 2022;60(3):188‐199. [DOI] [PubMed] [Google Scholar]
- 10. Yaylacı A, Azak E, Önal A, Aktürk DR, Karadenizli A. Effects of classical olfactory training in patients with COVID‐19‐related persistent loss of smell. Eur Arch Otorhinolaryngol. 2022;Jul 29:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pires Íde AT, Steffens ST, Mocelin AG, et al. Intensive olfactory training in post‐COVID‐19 patients: a multicenter randomized clinical trial. Am J Rhinol Allergy. 2022:36(6):780‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nguyen TP, Patel ZM. Budesonide irrigation with olfactory training improves outcomes compared with olfactory training alone in patients with olfactory loss. Int Forum Allergy Rhinol. 2018;8(9):977‐981. [DOI] [PubMed] [Google Scholar]
- 13. Gupta S, Lee JJ, Perrin A, et al. Efficacy and safety of saline nasal irrigation plus theophylline for treatment of COVID‐19‐related olfactory dysfunction: the SCENT2 Phase 2 randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2022;148(9):830‐837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kasiri H, Rouhani N, Salehifar E, Ghazaeian M, Fallah S. Mometasone furoate nasal spray in the treatment of patients with COVID‐19 olfactory dysfunction: a randomized, double blind clinical trial. Int Immunopharmacol. 2021;98:107871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yan CH, Rathor A, Krook K, et al. Effect of omega‐3 supplementation in patients with smell dysfunction following endoscopic sellar and parasellar tumor resection: a multicenter prospective randomized controlled trial. Neurosurgery. 2020;87(2):E91‐E98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaira LA, Hopkins C, Petrocelli M, et al. Efficacy of corticosteroid therapy in the treatment of long‐ lasting olfactory disorders in COVID‐19 patients. Rhin. 2021;59(1):21‐25. [DOI] [PubMed] [Google Scholar]
- 17. Di Stadio A, D'Ascanio L, Vaira LA, et al. Ultramicronized palmitoylethanolamide and luteolin supplement combined with olfactory training to treat post‐COVID‐19 olfactory impairment: a multi‐center double‐blinded randomized placebo‐controlled clinical trial. CN. 2022:20(10):2001‐2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lerner D, Garvey K, Arrighi‐Allisan A, et al. Letter to the editor: study summary ‐ randomized control trial of omega‐3 fatty acid supplementation for the treatment of COVID‐19 related olfactory dysfunction. Trials. 2020;21(1):942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang S, Liu X, Wang Y. Evaluation of platelet‐rich plasma therapy for peripheral nerve regeneration: a critical review of literature. Front Bioeng Biotechnol. 2022;10:808248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yan CH, Mundy DC, Patel ZM. The use of platelet‐rich plasma in treatment of olfactory dysfunction: a pilot study. Laryngoscope Investig Otolary. 2020;5(2):187‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mavrogeni P, Kanakopoulos A, Maihoub S, Krasznai M, Szirmai A. Anosmia treatment by platelet rich plasma injection. Int Tinnitus J. 2017;20(2):102‐105. [DOI] [PubMed] [Google Scholar]
- 22. Steffens Y, Le Bon SD, Lechien J, et al. Effectiveness and safety of PRP on persistent olfactory dysfunction related to COVID‐19. Eur Arch Otorhinolaryngol. 2022:279(12):5951‐5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yasak AG, Yigit O, Araz Server E, Durna Dastan S, Gul M. The effectiveness of platelet‐rich plasma in an anosmia‐induced mice model. Laryngoscope. 2018;128(5):E157‐E162. [DOI] [PubMed] [Google Scholar]
- 24. Khan AM, Lee J, Rammaha T, et al. Natural trajectory of recovery of COVID‐19 associated olfactory loss. Am J Otolaryngol. 2022;43(5):103572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. London B, Nabet B, Fisher AR, White B, Sammel MD, Doty RL. Predictors of prognosis in patients with olfactory disturbance. Ann Neurol. 2008;63(2):159‐166. [DOI] [PubMed] [Google Scholar]
- 26. Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odour identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22(1):39‐52. [DOI] [PubMed] [Google Scholar]
- 27. Parzen M, Lipsitz S, Ibrahim J, Klar N. An estimate of the odds ratio that always exists. J Comput Graph Statist. 2002;11(2):420‐436. [Google Scholar]
- 28. Tutar B, Ekincioglu E, Karaketir S, et al. The impact of platelet‐rich fibrin (PRF) on olfactory function and pain after septoplasty operations. Eur Arch Otorhinolaryngol. 2020;277(4):1115‐1120. [DOI] [PubMed] [Google Scholar]
- 29. Goljanian Tabrizi A, Asadi M, Mohammadi M, Abedi Yekta A, Sohrabi M. Efficacy of platelet‐rich plasma as an adjuvant therapy to endoscopic sinus surgery in anosmia patients with sinonasal polyposis: a randomized controlled clinical trial. Med J Islam Republ Iran. 2021;35:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prajapati DP, Shahrvini B, Said M, Srinivas S, DeConde AS, Yan CH. Assessment of patient recognition of coronavirus disease 2019 (COVID‐19)‐associated olfactory loss and recovery: a longitudinal study. Int Forum Allergy Rhinol. 2021;11(11):1529‐1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holbrook EH, Rebeiz L, Schwob JE. Office‐based olfactory mucosa biopsies. Int Forum Allergy Rhinol. 2016;6(6):646‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amable PR, Carias RBV, Teixeira MVT, et al. Platelet‐rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4(3):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


