Antibody responses to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) can be detected 10–15 days after symptom onset in most cases. Patients with mental disorders have a higher risk of developing coronavirus disease 2019 (COVID‐19) because of higher rates of metabolic syndrome, poor self‐care, and crowded psychiatric units. 1 However, the longitudinal kinetics of antibodies for post‐onset symptoms in individuals with psychiatric disorders remains poorly understood. In this study, we recruited 26 individuals hospitalized at the Tokyo Metropolitan Matsuzawa Hospital due to both mental disorders and SARS‐CoV‐2 infection diagnosed by reverse transcription‐quantitative polymerase chain reaction. This study was approved by the Ethics Committees of the Tokyo Metropolitan Matsuzawa Hospital and Tokyo Metropolitan Institute of Medical Science. Written informed consent was obtained from all patients. The longitudinal dynamics of immunoglobulin G (IgG) and immunoglobulin M (IgM) in response to SARS‐CoV‐2 infection were assessed using sequential serum samples. IgG and IgM levels were measured using an iFlash 3000 chemiluminescence immunoassay (CLIA) analyzer (Shenzhen YHLO Biotech Co., Ltd., Shenzhen, China) with an iFLash SARS‐CoV‐2 IgG and IgM kit (Shenzhen YHLO Biotech), 2 respectively. The severity of COVID‐19 was classified according to the criteria reported by Seow et al. 3 with minor modifications by Matsuzawa Hospital as follows: severity 0: asymptomatic or no requirement of supplemental oxygen; severity 1: requirement of supplemental oxygen by nasal cannula (gas flow 1–4 L/min) for at least 12 h; severity 2: requirement of supplemental oxygen by nasal cannula (gas flow 5–7 L/min) for at least 12 h; severity 3: requirement of supplemental oxygen by reservoir face mask (gas flow 6–7 L/min) for at least 12 h; severity 4: requirement of intubation and mechanical ventilation or supplemental oxygen by reservoir face mask (gas flow >8 L/min) for at least 12 h; severity 5: extracorporeal membrane oxygenation requirement. The participants' clinical information, including comorbidities and pharmacotherapy for COVID‐19, is summarized in Table S1. Information regarding pharmacotherapy for psychiatric disorders is provided in Table S2. A slight decrease in IgG and IgM levels was observed after 28 days (Figure S1). Differences in IgG and IgM responses were apparent when the patients were stratified according to disease severity. Patients with severity scores of 0 and 4 were classified into the non‐severe and severe groups, respectively. Similarly, the mild group included patients with a severity score of 1 or 2. Although statistical significance was not achieved, the magnitude of the IgG response at the peak was higher in the severe group than in the other groups. However, the peak IgM level in the severe group was not higher (Figure S2). The time to reach the highest IgM levels was significantly earlier in the severe group (11.8 ± 6.2 days, P = 0.02) and in the mild group (17.7 ± 10.3 days, P = 0.03) than in the non‐severe group (28.0 ± 10.2 days) (Fig. 1). Similarly, the time to the highest IgG levels tended to be shorter in the severe group than in the non‐severe group (Fig. 1). Seow et al. found that the peak IgM levels were significantly higher in the severe group, whereas the peak IgG levels were not when compared to those of the non‐severe group in the general population. 3 They also found no significant differences in the time to reach peak neutralization between the groups. 3 In contrast, the present study revealed that the time to the highest IgM levels was significantly earlier and that the magnitude of the IgG response was higher in severe cases than in less severe cases. These results were consistent after removing the patients with diabetes or those on steroid treatment. A possible factor that explains the discrepancy between our results and those of previous studies is that our participants were all diagnosed with psychiatric illnesses and most of them had taken long‐term antipsychotic medication. Gordon et al. reported that haloperidol has potential anti‐infectious effects using affinity‐purification mass spectrometry, and it showed antiviral activity through the sigma receptor. 4 Immune dysregulation is involved in the etiopathology of the schizophrenia subgroup. Thus, there could be a possibility of distortion of immunity due to the pathophysiology of psychiatric disorders or antipsychotic medicines. A limitation of this study is that the cases were small groups composed of Japanese patients. A larger cohort with other racial backgrounds is required to generalize the findings of this study. In summary, the longitudinal antibody response after acute SARS‐CoV‐2 infection in patients with psychiatric disorders showed different response patterns compared with previously reported responses in healthy subjects.
Fig. 1.
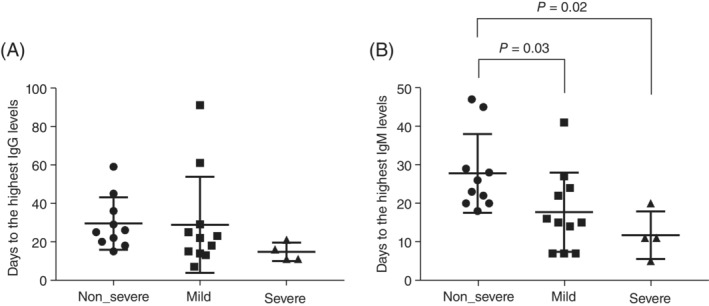
Time to reach the highest antibody levels. The days to the highest levels of IgG (A) and IgM (B) against SARS‐CoV‐2 in each severity group are shown as dot blots. Error bars present mean and standard deviation (SD). P‐values are calculated by one‐way analysis of variance and subsequent Holm‐Sidak's multiple comparisons test.
Disclosure statement
The authors declare no conflict of interest.
Funding Information
This study was financially supported by the Tokyo Metropolitan Government of Japan. We appreciate the donation of the iFlash 3000 device from the Murakami Foundation.
Author contributions
MM had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: EI, FY, MM, MK, and MS. Substantial contributions to acquisition, analysis, or interpretation of data: EI, FY, MM, KM, KS, EH, MO, and MK. Drafting of the manuscript: MM. Critical revision of the manuscript for important intellectual content: FY, MM, and MK. Statistical analysis: MM. Obtained funding: MK. Administrative, technical, or material support: FY, and MK. Substantial supervision: SS, MK, and MS.
Supporting information
Table S1.Demographics of the participants
Table S2.Pharmacotherapy for psychiatric disorders
Figure S1. Antibody response against SARS‐CoV‐2. Levels of IgG (A) and IgM (B) against SARS‐CoV‐2 in all patients at different time points after symptom onset are shown. For two patients, the days after first routine clinical laboratory testing were used instead of post‐onset of symptoms due to lack of clinical records for symptom onset. Data are presented as mean ± SD.
Figure S2. Sequential comparison of SARS‐CoV‐2 antibodies between non‐severe, mild, and severe groups. The levels of IgG (A) and IgM (B) against SARS‐CoV‐2 at different time points after symptom onset are shown. Non‐severe, mild, and severe cases correspond to group 0, groups 1–2, and group 4, respectively. The number of samples for each non‐severe, mild, and severe group are 2, 7, and 3 for 0–7 days, 8, 9, and 4 for 8–14 days, 8, 7, and 3 for 15–21 days, 6, 7, and 3 for 22–28 days, and 6, 3, and 2 for over 28 days, respectively. Data are presented as mean ± SD.
Acknowledgments
We are grateful to all the staff of the Clinical Laboratory in Tokyo Metropolitan Matsuzawa Hospital for assisting with the sampling of serum from patients, and Ms. Eri Horiuchi for assisting with the operation of the iFlash 3000 chemiluminescence immunoassay analyzer. This study was supported by the Tokyo Metropolitan Government of Japan.
References
- 1. Druss BG. Addressing the COVID‐19 pandemic in populations with serious mental illness. JAMA Psychiat. 2020; 77: 891–892. [DOI] [PubMed] [Google Scholar]
- 2. Kaneko Y, Sugiyama A, Tanaka T et al. The serological diversity of serum IgG/IgA/IgM against the SARS‐CoV‐2 nucleoprotein, spike, and receptor‐binding domain and neutralizing antibodies in patients with COVID‐19 in Japan. Health Sci Rep 2022; 5: e572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seow J, Graham C, Merrick B et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS‐CoV‐2 infection in humans. Nat. Microbiol. 2020; 5: 1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gordon DE, Jang GM, Bouhaddou M et al. A SARS‐CoV‐2 protein interaction map reveals targets for drug repurposing. Nature 2020; 583: 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.Demographics of the participants
Table S2.Pharmacotherapy for psychiatric disorders
Figure S1. Antibody response against SARS‐CoV‐2. Levels of IgG (A) and IgM (B) against SARS‐CoV‐2 in all patients at different time points after symptom onset are shown. For two patients, the days after first routine clinical laboratory testing were used instead of post‐onset of symptoms due to lack of clinical records for symptom onset. Data are presented as mean ± SD.
Figure S2. Sequential comparison of SARS‐CoV‐2 antibodies between non‐severe, mild, and severe groups. The levels of IgG (A) and IgM (B) against SARS‐CoV‐2 at different time points after symptom onset are shown. Non‐severe, mild, and severe cases correspond to group 0, groups 1–2, and group 4, respectively. The number of samples for each non‐severe, mild, and severe group are 2, 7, and 3 for 0–7 days, 8, 9, and 4 for 8–14 days, 8, 7, and 3 for 15–21 days, 6, 7, and 3 for 22–28 days, and 6, 3, and 2 for over 28 days, respectively. Data are presented as mean ± SD.


