Abstract
Aim
To analyze the clinical patterns of new‐onset inflammatory arthritis after COVID‐19 vaccination among patients without pre‐existing rheumatic or autoimmune diseases.
Method
Case reports and series of new‐onset inflammatory arthritis after COVID‐19 vaccination were collected before April 2022. Clinical characteristics including diagnosis, age, gender, vaccine types, time interval between events, joint involvement (poly‐ or oligo−/monoarthritis), and laboratory data reflecting inflammatory status were sorted and P values between these parameters are calculated with independent sample Student's t test or 2 × 2 Fisher's exact test.
Results
Among 39 cases with new‐onset post‐vaccination arthritis including 25 females and 13 males (1 unknown), the most common diagnosis is adult‐onset Still's disease (AoSD, 10 cases), and the most common vaccine types are BNT162b2 (16 cases) and AZD‐1222 (or ChAdOx1‐nCoV19, 15 cases). Sub‐analysis reveals that post‐vaccination polyarthritis is more common among females (P = .016, by 2 × 2 Fisher's exact test, compared with male patients) and older patients (P = .006, by Student's t test). The C‐reactive protein level is significantly higher in cases with post‐vaccination inflammatory polyarthritis than oligoarthritis (P = .029), as well as in cases with AoSD than other causes of post‐vaccination arthritis (P = .004). However, serum level of erythrocyte sedimentation rate in patients with post‐vaccination AoSD are independent of other clinical variables in the analysis.
Conclusion
New‐onset post‐vaccination polyarthritis are more common in females and older patients. Although COVID‐19 vaccines may lead to inflammatory arthritis, the benefits of vaccination substantially outweigh the potential risks of such serious adverse effects due to their rarity.
Keywords: Coronavirus disease 19 (COVID‐19), inflammatory arthritis, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), vaccine
1. BACKGROUND
The pandemic of Coronavirus disease 2019 (COVID‐19) has overwhelmed the globe since late 2019, and the newly diagnosed cases have surpassed 500 million, causing more than 6 million deaths. 1 Recently, several effective, emergency use authorization (EUA)‐approved vaccines against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), have been developed and utilized to prevent infection, severe disease requiring hospitalization, and death. 2 However, few but serious side effects after vaccination may cause multiple bothersome issues, such as new‐onset of autoimmune‐related disorders and flare‐up of existing rheumatic inflammatory diseases. 3 It is noteworthy for clinicians to understand these conditions because of potential misdiagnosis, although they are relatively rare to be seen.
The safety of COVID‐19 vaccination for patients with rheumatic and musculoskeletal diseases (RMDs) has been widely evaluated in multiple studies. A large observational study from the European Alliance of Associations for Rheumatology (EULAR) Coronavirus Vaccine (COVAX) physician‐reported registry revealed that the rate of diseases flare‐up after COVID‐19 vaccination among patients with RMDs was not significantly higher than that of the unvaccinated patients. 4 The guidance statement published by American College of Rheumatology (ACR) task force consensus recommends that COVID‐19 vaccination should be administered in patients with RMDs even in a non‐life‐threatening or a high‐activity disease status, and they should be “prioritized for vaccination before the non‐prioritized general population” due to their greater risk of SARS‐CoV‐2 infection, hospitalization, and poorer prognosis of COVID‐19 compared with individuals without RMDs. 5
Nevertheless, some case reports and series have demonstrated the new‐onset of arthritis after vaccination among patients with neither RMDs nor autoimmune diseases. In the case series by Ursini and his collegues (2022), 66 patients comprising 43 females and 23 males had experienced transient inflammatory musculoskeletal manifestations after COVID‐19 vaccination. Analysis revealed that 18, 21, and 27 cases are expressed as polymyalgia rheumatica (PMR), mono/oligoarthritis and polyarthritis respectively; further, most of them had received BNT162b2 (39 cases) and AZD‐1222 (also known as ChAdOx1‐nCoV19, 23 cases) vaccines 11–13 days before. 6
Due to the limited number of cases, the underlying mechanisms and clinical characteristics of the condition are not well understood to date. In this article, we summarize the recent studies and reports of new‐onset inflammatory arthritis after COVID‐19 vaccination in patients without pre‐existing autoimmune or rheumatic diseases and analyze the clinical patterns.
2. METHODS
We searched for case reports, case series, observation studies, and systematic reviews of new‐onset arthritis after COVID‐19 vaccination via Medline (PubMed), Embase, and Web of Science. Because of the initiation of COVID‐19 outbreak and vaccines development in the late 2019 and the mid‐2020 respectively, we set the publication date between the range from January 2020 to March 2022. Keywords including “arthritis”, “arthralgia”, “COVID‐19 vaccine”, and “SARS‐CoV‐2 vaccine” were adopted with Boolean algebra and MeSH terms. To emphasize the “new‐onset” arthritis contributed from “COVID‐19 vaccines”, studies about arthritis after SARS‐CoV‐2 infection, and arthritis reactivation in patients with underlying or history of arthritis‐associated and other autoimmune diseases, were excluded for analysis. Other causes of arthritis, such as septic arthritis or shoulder bursitis after vaccination, would be discussed as well but they would not be included in the pooled analysis. All components of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) checklist have been adhered to and the results are illustrated with PRISMA algorithm shown in Figure 1.
FIGURE 1.

PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) algorithm of study selection
We used the critical appraisal checklists established by Joanna Briggs Institute (JBI) Manual for Evidence Synthesis 7 with multiple questions for appraisal of the selected case reports or series, and the results are demonstrated in Tables 1 and 2. The authors could have different viewpoints upon evaluation and appraisal of papers and on such occasions, the stricter one was selected. Most of the studies met the requirement with complete demonstration of clinical information.
TABLE 1.
Appraisal of case reports included in the analysis
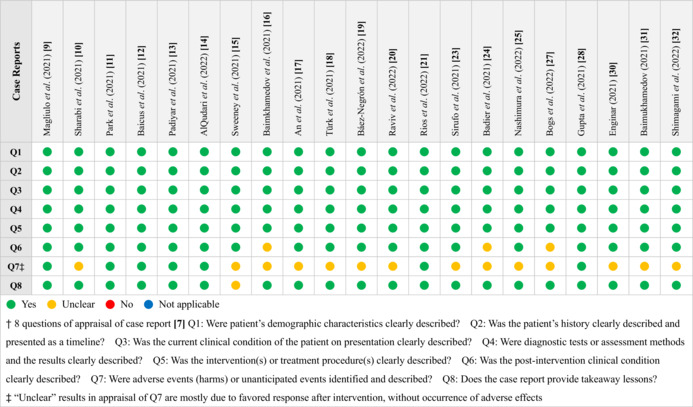
|
TABLE 2.
Appraisal of case series included in the analysis
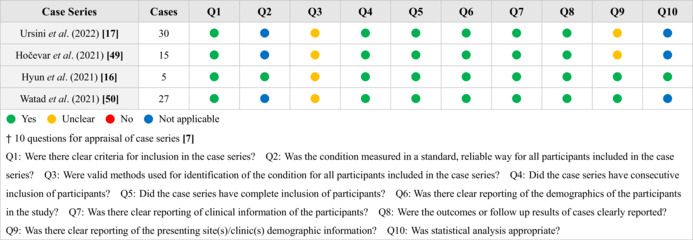
|
The P values of continuous variables including age, time interval between vaccination and arthritis onset, and laboratory data representing inflammatory status (white blood cell [WBC] count, serum erythrocyte sedimentation rate [ESR] and C‐reactive protein [CRP]) are calculated with double‐tailed independent sample Student's t test, while the discrete variables such as gender (female vs male), age (separated by 2 groups of <50 vs ≥50 years old), time interval (≤7 vs >7 days), vaccine type (messenger RNA [mRNA]‐based [BNT162b2 and mRNA‐1273] vs adenovirus‐based [AZD‐1222 and Sputnik‐V] vaccines), dosage (1st vs 2nd dose), and classification of arthritis (polyarthritis [defined as 4 or more joints involvement] 8 vs oligo‐/monoarthritis) are calculated by double‐tailed 2 × 2 Fisher's exact test. The P value threshold of significance is .05 in both tests.
3. RESULTS
The basic profiles and clinical characteristics of RMD‐naïve patients with new‐onset post‐vaccination arthritis are shown in Table 3 (more details are shown in Tables S1 and S2) and Table 4. In total, 39 cases (25 females, 13 males, and 1 case with unknown gender) within case reports and series are enrolled in the analysis, and the average age is 48.6 ± 20.1 years old. Among patients with given diagnosis of inflammatory arthritis, adult‐onset Still's disease (AoSD) accounts for the most common diagnosis (10 cases). 9 , 10 , 11 , 12 , 13 , 14 , 15 Other diagnoses include polyarthralgia and myalgia syndrome (PaMS, 5 cases), 16 undifferentiated connective tissue disease (UCTD, 4 cases), 17 immunoglobulin A (IgA) vasculitis (IgAV, or Henoch‐Schönlein purpura [HSP], 4 cases), 18 , 19 , 20 reactive arthritis (ReA, 3 cases), 21 , 22 systemic lupus erythematosus (SLE, 3 cases), 23 , 24 , 25 rheumatoid arthritis (RA, 1 cases), 26 and so on. Polyarthritis, defined as 4 or more joints involvment, 8 accounts for 23 cases, and the remaining 10 patients are classified as mono‐ or oligoarthritis (the remaining 6 cases are not clearly classified). The vaccines leading to arthritis are 16 cases with BNT162b2, 15 cases with AZD‐1222, 4 cases with CoronaVac, 2 cases with Sputnik‐V, and 2 cases with mRNA‐1273. The mean duration time between vaccine injection and the disease onset is 11.4 ± 16.7 days (adjusted, 7.4 ± 5.5 days), and the longest time interval is 3 months in a 35‐year‐old female who developed AoSD and macrophage activation syndrome (MAS) complicating multiple organ failure after AZD‐1222 vaccination. 13 Most patients with inflammatory arthritis had blood leukocytosis (mean WBC: 12.8 ± 7.8 × 109 per liter) with neutrophil predominance, elevated ESR (mean: 66.7 ± 25.3 mm/h, reference range: <20 mm/h) and CRP (mean: 124.2 ± 95.8 mg/L, reference range: <5 mg/L) levels (see Table 4). Two patients had received arthrocentesis from inflamed joints with effusion, and the synovial fluid analysis both revealed leukocytosis with polymorphonuclear leukocyte predominance, indicating exudative formation. 10 , 21 Autoimmune profiles which are clinically significant were generally negative in patients with post‐vaccination arthritis, with exception of 3 cases with SLE, 1 case with RA, and 1 case with anti‐Jo‐1 syndrome. 27 Whole‐body technetium‐99m methylene (Tc‐99 m) diphosphonate bone scans were performed in 4 patients with PaMS after AZD‐1222 injection, revealing with symmetrically increased uptake of radioactive tracer in multiple joints. 16
TABLE 3.
Basic data of cases with post‐vaccination inflammatory arthritis
| Case and reference | Age, y | Gender | Vaccine type | Dosage | Interval, d | Classification of arthritis | WBC, ×109/L | ESR, mm/h | CRP, g/dL | Final diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. [9] | 45 | F | mRNA‐1273 | 2nd | 5 | Polyarthritis | 22.1 | 85 | 277 | AoSD |
| 2. [10] | 43 | M | BNT162b2 | 2nd | 10 | Oligoarthritis | 12.5 | N/A | 93.2 | AoSD |
| 3. [11] | 56 | F | BNT162b2 | 2nd | 7 | Polyarthritis | 40 | N/A | 300 | AoSD |
| 4. [11] | 36 | F | BNT162b2 | 1st | 10 | Polyarthritis | 12.2 | 56 | 162.8 | AoSD |
| 5. [12] | 22 | M | BNT162b2 | 1st | 13 | Oligoarthritis | N/A | N/A | 250 | AoSD |
| 6. [13] | 20 | F | AZD‐1222 | 1st | 10 | Oligoarthritis | 15.6 | 51 | 71 | AoSD |
| 7. [13] | 47 | F | AZD‐1222 | 1st | 21 | Polyarthritis | 12.1 | 86 | 169 | AoSD |
| 8. [13] | 35 | F | AZD‐1222 | 1st | 90 | Polyarthritis | 11.7 | 48 | 227 | AoSD |
| 9. [14] | 29 | M | AZD‐1222 | 1st | 2 | Oligoarthritis | 26.2 | 120 | >160 d | AoSD |
| 10. [15] | 53 | M | AZD‐1222 | 1st | 70 | Polyarthritis | N/A | 85 | 237 | AoSD |
| 11. [26] | 38 | F | Sputnik‐V | 1st | 20 | Polyarthritis | N/A | 39 | 10 | RA |
| 12. [21] | 23 | F | CoranoVac | 1st c | 3 c | Monoarthritis | N/A | 32 | 15 | ReA |
| 13. [22] | 72 | F | CoranoVac | 1st | 21 | Polyarthritis | 13.2 | 75 | 237 | ReA |
| 14. [22] | 79 | F | CoranoVac | 2nd | N/A | Polyarthritis | 11.9 | 77 | 215 | ReA |
| 15. [23] | 27 | F | mRNA‐1273 | 2nd | 14 | Polyarthritis | 7.6 | 88 | N/A | SLE |
| 16. [24] | 24 | M | BNT162b2 | 1st | 2 | Polyarthritis | N/A | N/A | N/A | SLE |
| 17. [25] | 42 | F | BNT162b2 | 1st | 14 | Polyarthritis | 5.3 | 55 | 91 | SLE, APS |
| 18. [17] | 61 | F | BNT162b2 | N/A | 3 | Polyarthritis | N/A | N/A | N/A | UCTD |
| 19. [17] | 50 | M | AZD‐1222 | N/A | 3 | N/A (unclear) | N/A | N/A | N/A | UCTD |
| 20. [17] | 45 | M | BNT162b2 | N/A | 5 | N/A (unclear) | N/A | N/A | N/A | UCTD |
| 21. [17] | 32 | F | BNT162b2 | N/A | 5 | N/A (unclear) | N/A | N/A | N/A | UCTD |
| 22. [18] | 76 | F | AZD‐1222 | 1st | 7 | Oligoarthritis | 7.6 | 36 | 40.9 | HSP |
| 23. [19] | 72 | M | AZD‐1222 | 1st | 15 | Polyarthritis | N/A | N/A | 55 | IgAV |
| 24. [20] | 30 | M | BNT162b2 | 2nd | 5 | Monoarthritis | 11.2 | N/A | 11.8 | IgAV |
| 25. [20] | 22 | M | BNT162b2 | 1st | 6 | Monoarthritis | 10.0 | N/A | 2.7 | IgAV |
| 26. [49] | 40 s b | U | AZD‐1222 | N/A | 8 | N/A (unclear) | N/A | N/A | N/A | ICV |
| 27. [46] | 15 | M | BNT162b2 | 2nd | N/A | Monoarthritis | 10.9 | N/A | 53.4 | suspected Behçet disease |
| 28. [27] | 46 | F | AZD‐1222 | 2nd | 7 | N/A (unclear) | 14.6 | 48 | 76 | Anti‐Jo‐1 syndrome |
| 29. [16] a | 68 | F | AZD‐1222 | 1st | 3 | Polyarthritis | 9.8 | >120 d | 135 | PaMS |
| 30. [16] a | 67 | F | AZD‐1222 | 1st | 4 | Polyarthritis | 2.6 | 32 | 13.1 | PaMS |
| 31. [16] a | 67 | F | AZD‐1222 | 1st | 4 | Polyarthritis | 5.8 | 76 | 41.3 | PaMS |
| 32. [16] a | 25 | F | AZD‐1222 | 1st | 3 | Polyarthritis | 7.1 | 64 | 18.5 | PaMS |
| 33. [16] a | 70 | F | AZD‐1222 | 1st | 7 | Polyarthritis | 11.8 | 60 | 293 | PaMS |
| 34. [47] | 74 | F | CoronaVac | 1st | 2 | Polyarthritis | N/A | 84 | 202 | ASIA |
| 35. [28] | 58 | M | Sputnik‐V | 2nd | 5 | Monoarthritis | N/A | 18 | 14 | N/A |
| 36. [48] | 90 s b | F | BNT162b2 | 2nd | 1 | Polyarthritis | N/A | 73 | 167 | N/A |
| 37. [48] | 70 s b | M | BNT162b2 | 1st | N/A | Polyarthritis | N/A | 69 | 37 | N/A |
| 38. [50] | 42 | F | BNT162b2 | 1st | 4 | Polyarthritis | N/A | N/A | N/A | N/A |
| 39. [50] | 70 | M | BNT162b2 | 1st | 3 | Polyarthritis | N/A | 90 | 175 | PMR |
Abbreviations: AoSD, adult‐onset Still's disease; APS, antiphospholipid syndrome; ASIA, autoimmune/inflammatory syndrome induced by adjuvants; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; F, female; HSP, Henoch‐Schönlein purpura; ICV, immune complex vasculitis; IgAV, immunoglobulin A vasculitis; M, male; N/A, not available; PaMS, polyarthralgia and myalgia syndrome; PMR, polymyalgia rheumatica; RA, rheumatoid arthritis; ReA, reactive arthritis; SLE, systemic lupus erythematosus; U, unknown; UTCD, undifferentiated connective tissue disease; WBC, white blood cell.
Cases No. 29 ~ 33 are adapted from the table in the article by Hyun et al (2021). 35
Cases with unclear age (No. 26, 36, and 37) are analyzed by median number of the decade, ie, case No. 26 (40s) is analyzed as 45 years old, No. 36 (90s) as 95 years old, and No. 37 (70s) as 75 years old.
Case No. 12 had arthritis after both dosage of vaccinations, and the situation of 1st dose is included in the analysis.
Cases with unclear laboratory data exceeding particular range (No. 9 and 29) are analyzed by the upper limit of the laboratory data informed in the article, ie, the CRP level of case No. 9 (> 160) is analyzed by 160, and the ESR level of No. 29 (> 120) by 120.
TABLE 4.
Analysis of clinical characteristics of post‐vaccination arthritis in subgroups
| Subgroups | Cases count and gender | Average age, y | Mean time interval, d | Poly‐ vs oligo‐ or monoarthritis | Mean WBC, ×109/L | Mean ESR, mm/h | Mean CRP, mg/L |
|---|---|---|---|---|---|---|---|
| All | 39 (25F, 13M, 1U) | 48.6 ± 20.1 | 11.4 ± 16.7 (adj.) 7.4 ± 5.5 a | 23 vs 10 | 12.8 ± 7.8 | 66.7 ± 25.3 | 124.2 ± 95.8 |
| Diagnosis | |||||||
| AoSD | 10 (6F, 4M) | 38.6 ± 11.7 | (adj.) 9.8 ± 5.7 a | 6 vs 4 | 19.1 ± 9.4 | 75.9 ± 23.9 | 194.7 ± 72.3 |
| P value* | P = .071 | P = .181 a | P = .444 | P = .003 * | P = .278 | P = .004 * | |
| ReA | 3 (3F, 0M) | 58.0 ± 24.9 | 12.0 ± 9.0 | 2 vs 1 | N/A b | N/A b | N/A b |
| SLE | 3 (2F, 1M) | 31.0 ± 7.9 | 10.0 ± 5.7 | 3 vs 0 | N/A b | N/A b | N/A b |
| UCTD | 4 (3F, 1M) | 47.0 ± 10.4 | 4.0 ± 1.0 | 5 vs 0 | N/A b | N/A b | N/A b |
| IgAV or HSP | 5 (1F, 3M, 1U) | 41.3 ± 21.9 | 8.7 ± 4.5 | 1 vs 3 | N/A b | N/A b | 23.2 ± 22.8 |
| PaMS | 5 (5F, 0M) | 59.4 ± 17.2 | 4.2 ± 1.5 | 5 vs 0 | 7.4 ± 3.2 | 70.4 ± 28.7 | 100.2 ± 105.9 |
| Gender | |||||||
| Female | 25 | 51.5 ± 19.9 | (adj.) 7.8 ± 6.0 a | 18 vs 3 | 12.4 ± 8.2 | 64.3 ± 22.2 | 138.1 ± 97.6 |
| Male | 13 | 43.3 ± 20.3 | (adj.) 6.4 ± 4.4 a | 5 vs 7 | 14.2 ± 6.1 | 76.4 ± 33.5 | 99.0 ± 87.0 |
| Age | |||||||
| <50 y | 22 (14F, 7M, 1U) | 33.3 ± 9.9 | (adj.) 8.4 ± 5.5 a | 10 vs 8 | 12.8 ± 5.4 | 64.3 ± 24.3 | 105.5 ± 88.3 |
| ≥50 y | 17 (11F, 6M) | 68.4 ± 10.5 | (adj.) 6.1 ± 5.3 a | 13 vs 2 | 12.8 ± 10.8 | 68.8 ± 26.1 | 144.2 ± 99.5 |
| Vaccine type c | |||||||
| mRNA‐based | 18 (10F, 8M) | 43.4 ± 20.5 | 6.7 ± 4.1 | 10 vs 5 | 14.6 ± 10.0 | 73.7 ± 13.5 | 135.1 ± 98.6 |
| Adenoviral based | 17 (11F, 5M, 1U) | 50.9 ± 17.0 | (adj.) 7.9 ± 5.9 a | 10 vs 6 | 11.4 ± 6.0 | 63.7 ± 30.0 | 104.1 ± 90.0 |
| Dosage | |||||||
| 1st dose | 19 (11F, 8M) | 45.9 ± 20.5 | (adj.) 9.6 ± 6.8 a | 13 vs 6 | 12.7 ± 5.6 | 66.1 ± 24.2 | 126.0 ± 86.8 |
| 2nd dose | 10 (6F, 4M) | 49.4 ± 22.8 | 6.8 ± 3.6 | 5 vs 4 | 16.4 ± 9.8 | 64.8 ± 24.6 | 134.2 ± 103.4 |
| Time interval | |||||||
| ≤7 d | 23 (16F, 7M) | 51.1 ± 20.5 | 4.2 ± 1.8 | 12 vs 6 | 14.1 ± 10.5 | 67.0 ± 31.8 | 114.3 ± 107.3 |
| >7 d | 13 (8F, 4M, 1U) | 42.5 ± 16.2 | 24.3 ± 25.4 | 9 vs 3 | 11.3 ± 3.3 | 64.8 ± 18.7 | 145.7 ± 85.4 |
| Classification of arthritis | |||||||
| Polyarthritis | 23 (18F, 5M) | 55.6 ± 19.4 | (adj.) 8.4 ± 6.7 a | 23 vs 0 | 12.4 ± 8.9 | 71.7 ± 20.1 | 153.1 ± 95.2 |
| Oligo‐ or monoarthritis | 10 (3F, 7M) | 33.8 ± 18.5 | 6.8 ± 3.4 | 0 vs 10 | 13.4 ± 5.7 | 51.4 ± 35.9 | 71.2 ± 75.0 |
Abbreviations: adj., adjusted; AoSD, adult‐onset Still's disease; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; HSP, Henoch‐Schönlein purpura; IgAV, immunoglobulin A vasculitis; PaMS, polyarthralgia and myalgia syndrome; ReA, reactive arthritis; SLE, systemic lupus erythematosus; U, unknown; UTCD, undifferentiated connective tissue disease; WBC, white blood cell.
Time intervals of case No. 8 (90 d) and 10 (70 d) are excluded for adjustment because of outliers.
“N/A” of laboratory parameters (WBC, CRP, and ESR) resulted from insufficient or insignificant data in the report.
The mRNA‐based vaccines include BNT162b2 and mRNA‐1273, and the adenoviral‐based vaccines include AZD‐1222 and Sputnik‐V.
P‐value of AoSD vs other causes of post‐vaccination arthritis, calculated by Fisher's exact and Student's t test.
Sub‐analysis of clinical patterns and inflammation‐related laboratory data of different vaccines and diseases are shown in Table 5. Among cases with post‐vaccination inflammatory arthritis regardless of diagnosis, female patients have more joint involvement than male patients (P = .016, calculated by Fisher's exact test). The possibility of post‐vaccination polyarthritis increases by age (P = .006, by Student's t test); however, there is no significant difference when the cases are analyzed in 2 groups of age (<50 and ≥50 years, P = .07, by Fisher's exact test). The serum CRP level of all‐cause post‐vaccination inflammatory polyarthritis is significantly higher than oligo‐/monoarthritis (P = .022, by Student's t test); however, no similar results of serum ESR level (P = .125) and WBC count (P = .788) are noted. Apart from the above, no significant differences between the remaining parameters are seen. The gender, age, time interval between vaccination and disease onset, vaccine type, and dosage are independent of the inflammatory status of new‐onset post‐vaccination arthritis.
TABLE 5.
Sub‐analysis of P value between different variables
|
Gender F vs M |
.723 | 1.000 | .625 | 1.000 | 1.000 | 1.000 | .190 | .965 | .077 | .764 | ||
| Age a | Act. | .251 | N/A | .333 | .672 | .119 | .023 * | N/A | N/A | N/A | ||
| Vs. | 1.000 | – | .375 | 1.000 | 1.000 | .467 | – | – | .132 | |||
| Interval a | Act. | .517 | N/A | .249 | .667 | .394 | .657 | N/A | N/A | N/A | ||
| Vs. | 1.000 | .224 | .076 | 1.000 | .464 | 1.000 | .017 * | .141 | .117 | |||
|
Vaccines b mRNA vs adenovirus‐based |
.497 | .263 | .176 | .512 | 1.000 | .167 | 1.000 | .498 | .762 | .420 | ||
|
Dosage 1st vs 2nd |
1.000 | .688 | 1.000 | .306 | .388 | .229 | 1.000 | .228 | – | .468 | ||
|
Classification polyarthritis vs oligo‐/monoarthritis |
.016 * | .006 * | .070 | .510 | 1.000 | 1.000 | .677 | .853 | .581 | .080 | ||
| WBC | .678 | N/A | .990 | N/A | .502 | .398 | .376 | .788 | N/A | N/A | ||
| ESR | .359 | N/A | .672 | N/A | .838 | .405 | .918 | .125 | N/A | N/A | ||
| CRP | .293 | N/A | .277 | N/A | .767 | .420 | .840 | .029 * | N/A | N/A | ||
|
F vs M Gender |
Act. | Vs. | Act. | Vs. |
mRNA vs adenovirus‐based Vaccines c |
1st vs 2nd Dosage |
polyarthritis vs oligo‐/monoarthritis Classification |
WBC | ESR | CRP | ||
| Age a | Interval a | |||||||||||
Note: The P values between variables of gender (female or male), age, time interval (between vaccination and disease onset), vaccine type (mRNA or adenoviral‐based), dosage (1st or 2nd dose), classification of arthritis (poly‐ or oligoarthritis), and laboratory data reflecting inflammatory status (WBC, ESR, and CRP) are listed in the chart. Each P value represents the relationship of the 2 parameters corresponding in the left side and bottom of the table. The left‐lower sections are P values of all included cases (39 patients), and the right‐upper part are P values of those with post‐vaccination AoSD (10 patients). The P values related to “interval” had been adjustive calculated after exclusion of 2 cases (mentioned in the previous table) with 70 and 90 d between the vaccination and onset of arthritis. The P values in the table with underline are calculated with 2 × 2 Fisher's exact test, and the rest are calculated with double‐tailed Student's t test.
Abbreviations: CRP, C‐reactive protein; ESR erythrocyte sedimentation rate; F, female; M, male; mRNA, messenger RNA‐based vaccines; N/A not available; oligo, oligoarthritis; poly, polyarthritis; WBC white blood cell.
The “Act.” of age and time interval are analyzed with actual number of the data (continuous variables), and the “Vs.” are analyzed with subgroup (ie, age are separated by <50 and ≥50 years old, and time interval are separated by ≤7 and >7 d).
The mRNA‐based vaccines include BNT162b2 and mRNA‐1273, and adenoviral‐based vaccines include AZD‐1222 and Sputnik‐V.
Significant P values (<.05).
Among 10 patients (6 females and 4 males, average age 38.6 ± 12.4 years) with post‐vaccination AoSD, 5 cases were following by AZD‐1222, 4 cases by BNT162, and 1 case by mRNA‐1273. Manifestation of polyarthritis (6 cases) 9 , 11 , 13 , 15 is more common than oligo‐ or monoarthritis (4 cases) 10 , 12 , 13 , 14 in patients with post‐vaccination AoSD, and the adjusted mean time interval between events is 9.8 ± 5.7 days (Table 4). Most patients have characteristic features of AoSD including spiking fever, Still's rash, lymphadenopathy, and pleurisy with effusion, and 2 cases had complications of hyperinflammatory syndromes 12 , 13 (Table S1). Elevations of WBC count, ESR, CRP, and ferritin levels are seen in most patients with post‐vaccination AoSD, and their blood WBC count (mean: 19.1 ± 9.4 × 109/L) and serum CRP (mean: 194.7 ± 72.3 mg/L) levels are significantly higher than other causes of post‐vaccination inflammatory arthritis (P = .003 and .004, respectively). However, the serum ESR level (mean, 75.9 ± 23.9 mm/h; P = .278) does not show similar results as CRP and WBC (Table 4). Further, older patients with post‐vaccination AoSD have more joint involvements than younger patients (P = .023) (Table 5).
The major treatments of patients with post‐vaccination inflammatory arthritis include systemic or intra‐articular administration 21 , 28 of glucocorticoids and pain control with non‐steroidal anti‐inflammatory drugs (NSAIDs). Some patients with more severe or systemic features were given conventional or biological immunomodulators based on the diagnosis such as methotrexate, 13 , 17 , 26 , 27 hydroxychloroquine, 23 , 24 , 25 mycophenolate mofetil, 23 , 27 intravenous immunoglobulin (IVIg), 13 tocilizumab (an interleukin [IL]‐6 antagonist), 11 , 13 or anakinra (an IL‐1 antagonist). 12 , 17
Other than inflammatory arthritis, septic arthritis and shoulder injury related to vaccine administration (SIRVA) manifest arthralgia after vaccination as well. Two cases of Streptococcus gordonii septic arthritis in the glenohumeral joints ipsilateral to injection site after COVID‐19 vaccination were reported, and the patients were treated with surgical intervention and antibiotics. 29 , 30 6 cases of SIRVA with subacromial‐subdeltoid bursitis after COVID‐19 vaccination were reported and all the patients experienced severe shoulder pain and limited range of motion (ROM). 31 , 32 , 33 , 34 , 35 Meanwhile, a case with intramuscular hematoma after vaccination resulting from iatrogenically erroneous injection site was also reported. 31 Another case with pre‐existing asymptomatic supraspinatus calcific tendinopathy suffered from shoulder pain and ROM limitation 3 hours after AZD‐1222 vaccination. 36 Although these etiologies of arthritis are also clinically important, they are neither listed in Table 3 nor included in analysis because they are beyond the scope of the review.
4. DISCUSSION
Compared with the case series by Ursini and his collegues (2022), 6 the most common vaccine types contributing to inflammatory arthritis are similar to our study (ie, BNT162b2 and AZD‐1222). Females account for more cases than males in both studies (60% in this review and 65% in their case series); however, in their case series, polyarthritis cases had lower age distribution compared to that of oligoarthritis (polyarthritis 54 ± 16 vs oligoarthritis 64 ± 15 years old), and such finding was quite different from ours (polyarthritis 55.6 ± 19.4 vs oligo‐/monoarthritis 33.8 ± 18.5 years old) (Table 3). The time intervals between vaccination and disease onset are longer in their case series (11 ~ 13 days) than in this review (adjusted, 7.4 ± 5.5 days). Our study reveals a higher proportion of polyarthritis within all cases with post‐vaccination inflammatory arthritis (70%) than their case series (41%); further, more females presented with polyarthritis in our series (P = .016) than that of their case series (P = .196, by Fisher's exact test). Although the mean ESR and CRP levels in their case series are both lower than those in our study, these laboratory parameters are generally higher in patients with post‐vaccination polyarthritis than oligoarthritis (case series: mean ESR 51 ± 34 vs 36 ± 25 mm/h; mean CRP 21.3 vs 19.0 mg/L).
Although arthritis has been reported after the administration of COVID‐19 vaccines, the relationship is still not established to date. Investigations of hypothetical mechanisms and clinical manifestations of autoimmune phenomena after COVID‐19 vaccination have been reported. Adjuvants or the vaccine itself may trigger an overactive immune reaction, autoimmune consequences, or even inflammation in susceptible individuals. 3 Molecular mimicry, leading to cross‐reaction of immune response between pathogens' antigen in the vaccines and the tissue or organic molecular structures in vivo, can otherwise activate overwhelming systemic or local inflammation. 37 Arthritis, as a common manifestation of autoimmune or inflammatory diseases, is expected to be involved in the adverse effects after vaccination despite very low incidence in the phase 3 trials of these widely used vaccines.
Adult‐onset Still's disease (AoSD) is an idiopathic systemic autoinflammatory disease presenting with prolonged spiking fever, arthritis, and characteristic salmon‐colored Still's rash, and other manifestations such as sore throat, hepatosplenomegaly, lymphadenopathy, and serositis. 38 It is estimated that up to 15% of patients with AoSD develop life‐threatening macrophage activation syndrome (MAS), and the administration of potent immunosuppressants such as pulse steroid therapy, methotrexate, anakinra, and tocilizumab are suggested. 39 Significant elevation of serum CRP level is shown in the analysis (Tables 3 and 4), which is consistent with clinical acknowledgement of hyperinflammatory features of AoSD. The exact connection between vaccination and AoSD remains unclear, although there have been published case reports concerning the development of AoSD following influenza vaccination. 40 , 41 SARS‐CoV‐2 infection‐related AoSD has been confirmed in several studies, and the core pathogenesis of hyperinflammatory status involves the proinflammatory cytokines (e.g., IL‐1, IL‐6, interferon‐gamma [IFN‐γ]) which is cross‐reacting in the development of AoSD. 9 , 11 , 13 , 38 Similar to other autoimmune diseases, molecular mimicry by vaccine antigens and precipitates related to adjuvants can be possible mechanisms. 37
Shoulder injury related to vaccine administration (SIRVA) is a special complication of vaccination which is hypothetically due to local immune response and inflammatory consequences triggered by pre‐existing antibodies. 42 Inappropriate injection sites, which are much closer to the acromial side of the deltoid muscle than regular standard procedures, may provoke the inflammatory reaction in the bursae around the shoulder girdle. 42 , 43 Subacromial‐subdeltoid bursitis is a common consequence of SIRVA and it usually occurs within 48 hours after vaccine injection with serious symptoms of shoulder pain and limited ROM. 43 Patients complicated with septic arthritis 29 , 30 and SIRVA 31 , 32 , 33 , 34 , 35 are considered the direct effects from vaccine injections, and the time interval between vaccination and symptoms onset can be varied and similar to those who had inflammatory arthritis, which may lead to misdiagnosis. Therefore, careful history‐taking, physical examinations, and differential diagnostic methods such as cultures (blood, synovial fluid, and/or tissue sample yielded by surgical intervention), autoimmune profiles, arthrocentesis for synovial fluid analysis consisting of microscopic crystals detection, joint ultrasonography, or articular magnetic resonance imaging (MRI) may be needed to avoid misdiagnosis and improper treatments.
4.1. Limitations
There are several limitations in this review. First, the number of cases in the analysis is relatively too small to consolidate the findings, and the heterogeneity of clinical information in the case reports or series increases the complexity of the analysis. Therefore, population‐based retrospective studies with the application of healthcare databases can be conducted to clarify the results. Second, the illness status of AoSD is generally more severe than other causes of arthritis, causing a potential selection bias that post‐vaccination AoSD owns more publication opportunities than other less severe or indolent conditions. Third, the incidence of the arthritis caused by different types of COVID‐19 vaccine may have bias due to the uneven distribution of global vaccination. Also, since there has been limited access for children and adolescents to COVID‐19 vaccination recently, the reports of post‐vaccination inflammatory arthritis in those under 18 years old are still very insufficient. Fourth, insufficient descriptions of past medical history, which may potentially contribute to arthritis afterwards, were noted in some reports and may leading to more confounding factors. As Baimukhamedov and his collegues (2021) discussed in their article, there was a possibility that the patient had undiscovered indolent rheumatoid arthritis (RA) before COVID‐19 vaccination, which might provoke flare‐up of the “silent” disease. 26
Finally, some non‐specific diagnostic terms such as reactive arthritis (ReA), autoimmune/inflammatory syndrome induced by adjuvants (ASIA), and polyarthralgia and myagia syndrome (PaMS) may lead to misunderstanding due to their characteristics of “exclusiveness”. Previously known as Reiter's syndrome, ReA is originally defined as the asymmetric seronegative spondyloarthritis with preceding gastrointestinal or genitourinary infection from specific pathogens (e.g., Campylobactor, Shigella, Salmonella, Chlamydia, and Yersinia species), 44 instead of the vaccines or their adjuvants. Although SARS‐CoV‐2 and other causative agents are possible triggering factors of ReA 45 and there are several substances that are molecularly structurally similar to SARS‐CoV‐2 in the vaccines, some physicians may take advantage of the concept of “reactive” and expand the clinical usage without any proven connection between them. Further, the concepts of adjuvant‐induced arthritis and methods for clinical diagnosis require more research because one is unable to confirm that a patient with post‐vaccination arthritis is directly contributed to the adjuvant. Hence, a novel diagnostic term such as “vaccine‐induced idiopathic arthritis” may be needed for this type of arthritis to avoid confusion unless more published evidence becomes available.
5. CONCLUSION
This review reveals that post‐vaccination polyarthritis among patients without history of autoimmune or rheumatic diseases are significantly more common in females and older patients. The inflammatory status is more pronounced in adult‐onset Still's diseases (compared with all causes of arthritis) and polyarthritis (compared with oligo‐ or monoarthritis). New‐onset inflammatory arthritis after COVID‐19 vaccination can be troublesome and potentially lethal despite its low incidence. In approach to patients with post‐vaccination arthralgia, clinicians should carefully differentiate side effects including SIRVA from inflammatory arthritis to avoid mistreatment. Insufficient cases and incomplete data resulting in significant bias in the analysis are the major limitations of this review; therefore, a healthcare database‐based study should be conducted in the future to reinforce the conclusions. Lastly, although there were such serious adverse effects or other autoimmune manifestations discovered after COVID‐19 vaccinations, the benefits of complete vaccinations still substantially out weigh the potential risks because of their extremely rare incidence.
CONFLICT OF INTEREST
The named authors have no conflict of interest, finance or otherwise.
Supporting information
Table S1‐S2
ACKNOWLEDGEMENTS
We sincerely thank Dr. Wen‐Chan Tsai, MD, PhD, for providing numerous useful recommendations, advice, editing, and feedback for the study. This article could have been done without his help. Further, we are very grateful to Mr. Yen‐Po Chen, who is majoring in information engineering, for establishing a web program (Numerical Python, NumPy) which was helpful in sorting and arranging the database.
Chen C‐C, Chen C‐J. New‐onset inflammatory arthritis after COVID‐19 vaccination: A systematic review. Int J Rheum Dis. 2022;00:1‐11. doi: 10.1111/1756-185X.14482
REFERENCES
- 1. COVID‐19 Map, Johns Hopkins Coronavirus Resource Center 2022, Accessed 1st April 2022. https://coronavirus.jhu.edu/map.html
- 2. COVID‐19 Vaccines, United States Food and Drug Administration (U.S. FDA) 2022, Accessed 1st April https://www.fda.gov/emergency‐preparedness‐and‐response/coronavirus‐disease‐2019‐covid‐19/covid‐19‐vaccines
- 3. Chen Y, Xu ZW, Wang P, et al. New‐onset autoimmune phenomena post‐COVID‐19 vaccination. Immunology. 2022;165(4):386‐401. [DOI] [PubMed] [Google Scholar]
- 4. Machado PM, Lawson‐Tovey S, Strangfeld A, et al. Safety of vaccination against SARS‐CoV‐2 in people with rheumatic and musculoskeletal diseases: results from the EULAR Coronavirus Vaccine (COVAX) physician‐reported registry. Ann Rheum Dis. 2021;81:695‐709. [DOI] [PubMed] [Google Scholar]
- 5. Curtis JR, Johnson SR, Anthony DD, et al. American College of Rheumatology (ACR) guidance for COVID‐19 vaccination in patients with rheumatic and musculoskeletal diseases (RMD): version 3. Arthritis Rheumatol. 2021;73(10):e60‐e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ursini F, Ruscitti P, Raimondo V, et al. Spectrum of short‐term inflammatory musculoskeletal manifestations after COVID‐19 vaccine administration: a report of 66 cases. Ann Rheum Dis. 2022;81(3):440‐441. [DOI] [PubMed] [Google Scholar]
- 7. Moola S, Munn Z, Tufanaru C, et al. Chapter 7: systematic reviews of etiology and risk. In: Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. Joanna Briggs Institute (JBI); 2020. https://synthesismanual.jbi.global [Google Scholar]
- 8. Cush JJ. Approach to articular and musculoskeletal disorders. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison's Principles of Internal Medicine. Vol 20 e; McGraw Hill; 2018:2615. [Google Scholar]
- 9. Magliulo D, Narayan S, Ue F, Boulougoura A, Badlissi F. Adult‐onset Still's disease after mRNA COVID‐19 vaccine. Lancet Rheumatol. 2021;3(10):e680‐e682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharabi A, Shiber S, Molad Y. Adult‐onset Still's disease following mRNA COVID‐19 vaccination. Clin Immunol. 2021;233:108878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park SY, Lee KH. Adult‐onset Still's disease after BNT162b2 mRNA COVID‐19 vaccine. J Korean Med Sci. 2021;36(50):e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baicus C, Delcea C, Pinte L, Dan GA. Hyper‐inflammation after COVID‐19 mARN vaccination: at the crossroads of multisystem inflammatory disease and adult‐onset Still's disease. Does terminology matter? Rom J Intern Med. 2022;60(1):3‐5. [DOI] [PubMed] [Google Scholar]
- 13. Padiyar S, Kamath N, Mathew J, et al. New‐onset adult‐onset Still's disease‐like syndrome after ChAdOx1 nCoV‐19 vaccination‐a case series with review of literature. Clin Rheumatol. 2022;18:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. AlQudari EA, Alabdan L, Alkhathami AA, Alotaibi MD, Alhamzi HA. Adult‐onset Still's disease after the ChAdOx1 nCoV‐19 vaccine. Cureus. 2022;14(1):e21279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sweeney A, Tracey G, Garnham K. Adult‐onset Still disease post‐adenovirus vector COVID‐19 vaccine. Intern Med J. 2021;51(12):2144‐2145. [DOI] [PubMed] [Google Scholar]
- 16. Hyun H, Song JY, Seong H, et al. Polyarthralgia and myalgia syndrome after ChAdOx1 nCOV‐19 vaccination. J Korean Med Sci. 2021;36(34):e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ursini F, Ruscitti P, Raimondo V, et al. Systemic syndromes of rheumatological interest with onset after COVID‐19 vaccine administration: a report of 30 cases. Clin Rheumatol. 2022;41(7):2261‐2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sirufo MM, Raggiunti M, Magnanimi LM, Ginaldi L, De Martinis M. Henoch‐Schönlein purpura following the first dose of COVID‐19 viral vector vaccine: a case report. Vaccines (Basel). 2021;9(10):1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Badier L, Toledano A, Porel T, et al. IgA vasculitis in adult patient following vaccination by ChadOx1 nCoV‐19. Autoimmun Rev. 2021;20(11):102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nishimura N, Shiomichi Y, Takeuchi S, Akamine S, Yoneda R, Yoshizawa S. IgA vasculitis following COVID‐19 vaccination. Mod Rheumatol Case Rep. 2022;rxac014. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. An QJ, Qin DA, Pei JX. Reactive arthritis after COVID‐19 vaccination. Hum Vaccin Immunother. 2021;17(9):2954‐2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Türk SM, Öztürk Z, Karataş D, Gönüllü E. Inactivated COVID‐19 vaccine can induce reactive polyarthritis in older patients: report of two cases. Georgian Med News. 2021;319:100‐102. [PubMed] [Google Scholar]
- 23. Báez‐Negrón L, Vila LM. New‐onset systemic lupus erythematosus after mRNA SARS‐CoV‐2 vaccination. Case Rep Rheumatol. 2022;2022:6436839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raviv Y, Betesh‐Abay B, Valdman‐Grinshpoun Y, Boehm‐Cohen L, Kassirer M, Sagy I. First presentation of systemic lupus erythematosus in a 24‐year‐old male following mRNA COVID‐19 vaccine. Case Rep Rheumatol. 2022;2022:9698138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Molina Rios S, Rojas Martinez R, Estevez Ramirez GM, Medina YF. Systemic lupus erythematosus and antiphospholipid syndrome after COVID‐19 vaccination. A case report. Mod Rheumatol Case Rep. 2022;rxac018. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baimukhamedov C, Makhmudov S, Aliya Botabekova A. Seropositive rheumatoid arthritis after vaccination against SARS‐CoV‐2 infection. Int J Rheum Dis. 2021;24(11):1440‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gupta K, Sharma GS, Kumar A. COVID‐19 vaccination‐associated anti‐Jo‐1 syndrome. Reumatologia. 2021;59(6):420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baimukhamedov C. Arthritis of the left elbow joint after vaccination against SARS‐CoV‐2 infection. Int J Rheum Dis. 2021;24(9):1218‐1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Massel DH, Haziza S, Rivera S, Mohile N, Subhawong TK, Hernandez VH. Septic arthritis of the shoulder after SARS‐CoV‐2 Pfizer vaccination: a case report. JBJS Case Connect. 2021;11(3):e21.00090. [DOI] [PubMed] [Google Scholar]
- 30. Flowers RC, Rodriguez BR, Corbitt K. Streptococcus gordonii septic arthritis of the glenohumeral joint following deltoid intramuscular vaccination. BMJ Case Rep. 2021;14(5):e243066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chow JC, Koles SL, Bois AJ. Shoulder injury related to SARS‐CoV‐2 vaccine administration. CMAJ. 2022;194(2):E46‐E49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Honarmand AR, Mackey J, Hayeri R. Shoulder injury related to vaccine administration (SIRVA) following mRNA COVID‐19 vaccination: report of 2 cases of subacromial‐subdeltoid bursitis. Radiol Case Rep. 2021;16(12):3631‐3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rodrigues TC, Hidalgo PF, Skaf AY, Serfaty A. Subacromial‐subdeltoid bursitis following COVID‐19 vaccination: a case of shoulder injury related to vaccine administration (SIRVA). Skeletal Radiol. 2021;50(11):2293‐2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boonsri P, Chuaychoosakoon C. Combined subacromial‐subdeltoid bursitis and supraspinatus tear following a COVID‐19 vaccination: a case report. Ann Med Surg (Lond). 2021;69:102819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chuaychoosakoon C, Parinyakhup W, Tanutit P, Maliwankul K, Klabklay P. Shoulder injury related to Sinovac COVID‐19 vaccine: a case report. Ann Med Surg (Lond). 2021;68:102622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klabklay P, Boonsri P, Kanyakool P, Chuaychoosakoon C. A COVID‐19 vaccination precipitating symptomatic calcific tendinitis: a case report. Ann Med Surg (Lond). 2022;74:103347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Segal Y, Shoenfeld Y. Vaccine‐induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15:586‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Giacomelli R, Ruscitti P, Shoenfeld Y. A comprehensive review on adult onset Still's disease. J Autoimmun. 2018;93:24‐36. [DOI] [PubMed] [Google Scholar]
- 39. Gerfaud‐Valentin M, Jamilloux Y, Jean I, Sève P. Adult‐onset Still's disease. Autoimmun Rev. 2014;13(7):708‐722. [DOI] [PubMed] [Google Scholar]
- 40. Yoshioka K, Fujimoto S, Oba H, Minami M, Aoki T. Onset of adult‐onset Still's disease following influenza vaccination. Mod Rheumatol. 2011;21(4):432‐435. [DOI] [PubMed] [Google Scholar]
- 41. Yoo WH. Adult‐onset Still's disease following influenza vaccination. Joint Bone Spine. 2010;77:373‐374. [DOI] [PubMed] [Google Scholar]
- 42. Wiesel BB, Keeling LE. Shoulder injury related to vaccine administration. J Am Acad Orthop Surg. 2021;29(17):732‐739. [DOI] [PubMed] [Google Scholar]
- 43. Wood CT, Ilyas AM. Shoulder injury related to vaccine administration: diagnosis and management. J Hand Surg Glob Online. 2022;4(2):111‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Selmi C, Gershwin ME. Diagnosis and classification of reactive arthritis. Autoimmun Rev. 2014;13(4–5):546‐549. [DOI] [PubMed] [Google Scholar]
- 45. Zeidler H, Hudson AP. Reactive arthritis update: spotlight on new and rare infectious agents implicated as pathogens. Curr Rheumotol Rep. 2021;23(7):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bogs T, Saleh N, Yavuz ST, Fazeli W, Ganschow R, Schreiner F. Aseptic meningitis, mucocutaneous lesions and arthritis after COVID‐19 vaccination in a 15‐year‐old boy. Vaccines (Basel). 2022;10(2):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Enginar AU. Arthritis following COVID‐19 vaccination: report of two cases. Int Immunopharmacol. 2021;101:108256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shimagami H, Yamaguchi Y, Kato Y, Kumanogoh A. Marked increase of interferon‐β after BNT162b2 mRNA vaccination: a case of polyarthritis with pleurisy. BMJ Case Rep. 2022;15(3):e246533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hočevar A, Simonović Z, Rotar Ž, Tomšič M. Vasculitis as temporally associated with COVID‐19 infection or vaccination: a single‐center experience. J Rheumatol. 2022;49(2):232‐233. [DOI] [PubMed] [Google Scholar]
- 50. Watad A, De Marco G, Mahajna H, et al. Immune‐mediated disease flares or new‐onset disease in 27 subjects following mRNA/DNA SARS‐CoV‐2 vaccination. Vaccines (Basel). 2021;9(5):435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2


