Abstract
Introduction
COVID‐19 has a wide spectrum of clinical severity and there is evidence that SARS‐Cov2 affects several organs and systems. Among the organs affected since the beginning of the pandemic, the relationship between SARS‐CoV‐2 infection and thyroid involvement has been demonstrated. Novel and highly effective messenger RNA and DNA‐based vaccines have been rapidly developed to decrease SARS‐CoV‐2 morbidity and mortality. Early after mass vaccinations, cases of thyroid dysfunction mainly including episodes of subacute thyroiditis, began to be reported like adverse effects. The objective of this study is to determine the impact of the pandemic, both due to SARS‐CoV2 infections and vaccinations, on the incidence of Graves' disease (GD).
Methods
Cross‐sectional, observational study comparing incidence of GD in adult population (over 18 years) before (2017–2019) and after (2020–2021) Covid‐19 pandemic. Only patients with new cases of GD, no relapsed diseases, were included. SARS‐CoV‐2 diagnosis was based on nucleic acid amplification tests on nasopharyngeal swabs or measurement of class M and class G antibodies to SARS‐CoV‐2 by highly specific assays. Data on incidence and vaccination related to SARS‐CoV‐2 infection were obtained from the public records from Castilla y León autonomous regional government.
Results
A total of 180 subjects were diagnosed and treated for GD during the study period. We observed a notable increase in expected GD cases in 2021 compared to 2017–19. The number of GD cases was higher in the second (Q2) quarter. Among 2021 GD cases, 42/66 patients (63.6%) had been vaccinated in the 90 days before symptom onset, but none of them in the first quarter of the year. A total of 97.7% were women with a mean age of 48.9 (SD 15.6) years. On average they were diagnosed 19.9 (SD 17.6) days after receiving the vaccine. A total of 7/42 (16.67%) had another previously diagnosed autoimmune disease and 11/42 (26.19%) were smokers.
Discussion
Our results show a notable increase in the incidence of GD during the year 2021, specially in women with a history of smoking. Hyper activation of the immune system induced by SARS‐CoV2 and by the recently released SARS‐COV‐2 vaccines has been highlighted in recent months. To assess whether this observed increase in the incidence of GD is sustained in the coming years or has simply been a precipitous trigger for individuals who were already predisposed to develop the disease, future studies will be needed.
Keywords: covid‐19, Graves' disease, hyperthyroidism, SARS‐CoV2, thyroid, vaccines
1. INTRODUCTION
The novel coronavirus disease COVID‐19, caused by SARS‐CoV‐2, was declared a pandemic by the World Health Organization (WHO) in March 2020. Since then, as of 7:33 pm CET, 18 March 2022, there have been 464,809,377 confirmed cases of COVID‐19 globally, including 6,062,536 deaths, reported to the WHO, with a total of 10,925,055,390 vaccine doses having been administered. 1
SARS‐CoV‐2 has a wide spectrum of clinical severity and evidence suggests that COVID‐19 can affect several organs and systems. Angiotensin‐converting enzyme 2 (ACE2) is involved in SARS‐CoV‐2 internalization into human cells, playing an important role in the pathogenesis of COVID‐19. ACE2 is expressed on thyroid follicular and parafollicular cells, allowing the entry of SARS‐CoV‐2 to the thyroid gland. 2 Because of this biological plausibility, there has been interest in trying to determine whether a history of thyroid dysfunction might be associated with a worse clinical course of COVID‐19 or an increased risk of SARS‐CoV‐2 infection. Several studies on this subject have apparently concluded that neither a history of hyperthyroidism nor a history of hypothyroidism is associated with worse clinical outcomes. 3 What does seem to be confirmed is that the direct entry of SARS‐CoV‐2 into thyroid cells 2 explains the association between a history of SARS‐CoV‐2 infection and the development of thyroid diseases, such as subacute thyroiditis. 4 It is also known that viral infections are involved in the pathogenesis of immune‐mediated thyroid diseases 5 which would explain the association described between a history of SARS‐CoV‐2 infection and new‐onset Graves' disease (GD) cases. 6
The severity of the SARS‐CoV‐2 pandemic has urged the rapid development of vaccines to decrease the associated morbidity and mortality. To date, in the Spanish healthcare system, the vaccines administered have been BNT162b2 from Pfizer‐BioNTech and mRNA1273 from Moderna Biotech, both using messenger RNA (mRNA) from viruses that are capable of generating immunity in the host cell, and recombinant human adenovirus vaccines such as ChAdOx1‐CoV‐2 from AstraZeneca and Ad26‐CoV‐2 from Johnson and Johnson. Early after mass vaccinations, cases of thyroid dysfunction began to be reported as adverse effects, mainly as episodes of subacute thyroiditis which have been described with both the use of mRNA vaccines 7 , 8 and the use of the adenovirus vaccine. 9 Somewhat later, there were also published cases of recurrence or new diagnoses of GD in clear relation to the recent history of vaccination; both mRNA vaccines and adenovirus vaccines have been implicated as the possible trigger mechanism. 10 , 11
The objective of this study is to determine the impact that the situation derived from the pandemic, both due to SARS‐CoV‐2 infections and vaccinations against this agent, has had on the incidence of GD.
2. METHODS
2.1. Study design and population
This was a retrospective observational study. From January 2017 to December 2021, 180 subjects were diagnosed and treated for GD at the University Hospital of León. The referred hospital covers an area of the province of León (Spain) that has more than 300,000 inhabitants 12 and for which it is the only hospital of reference. The diagnosis of GD was based on both clinical and biochemical diagnoses, considering the elevation of free T4 with respect to laboratory reference values and TSH receptor antibodies value above the reference cut‐off point (>2.7 IU/ml). Patients with a relapse of previously diagnosed disease were excluded.
Patients from the period January 2017 to December 2019 are referred to as pre‐SARS‐CoV‐2. The SARS‐CoV‐2 diagnosis was based on nucleic acid amplification tests performed on nasopharyngeal swabs or the measurement of class M and class G antibodies to SARS‐CoV‐2 by highly specific assays. Data on incidence and vaccination related to SARS‐CoV‐2 infection were obtained from the public records from Castilla y León regional autonomous government 13 ; those referring to our healthcare area are shown in Figures 1 and 2.
Figure 1.
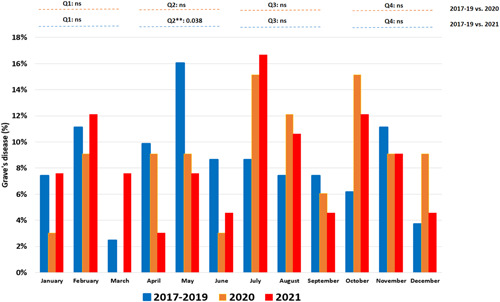
Percentage of cases of Graves' disease by month in 2017–2019, 2020 and 2021. Q1: January–March; Q2: April–June; Q3: July–August; Q4: October–December. NS, Not significant. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 2.
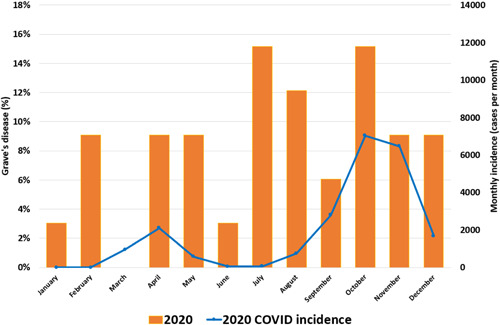
COVID monthly incidence in 2020 and percentages of Grave's disease by month [Color figure can be viewed at wileyonlinelibrary.com]
Data publication was approved by the local institutional review committee, registration number: 21170. In the years 2020 and 2021, the relationship between a history of SARS‐CoV‐2 infection or vaccination against SARS‐CoV‐2 and the onset of GD was considered probable if there was a history of SARS‐CoV‐2 infection or vaccination against SARS‐CoV‐2 within the previous 90 days. For the SARS‐CoV‐2 vaccination events, only the first vaccination dose was considered.
Previous autoimmune disease was considered if the patient has an established diagnosis of rheumatoid arthritis, multiple sclerosis, Hashimoto's thyroiditis, type 1 diabetes mellitus, psoriasis, celiac disease, pernicious anemia, myasthenia gravis, Addison's disease, or inflammatory bowel disease.
A positive family history of thyroid disease was defined if the patient had first‐line relatives with thyroid disease of immune origin: Hashimoto's thyroiditis or GD.
2.2. Laboratory examinations
Thyroid hormones and TSH were tested using immunoenzymatic assays on the Roche Diagnostic Cobas e801 autoanalyzer. Reference ranges were 0.93–1.7 ng/dl for free T4 (FT4), 0.27–0.42 ng/dl for free triiodothyronine (FT3), and 0.27–4.2 mIU/L for TSH. TSH‐receptor antibodies (TRAbs) were tested by enzyme‐linked immunosorbent assay (ElisaRSR TRAb third generation, Cardiff, UK) (positive cut‐off >2.7 IU/ml).
2.3. Statistical analysis
Statistical data analysis was performed using the statistical software R version 4.1.2. Continuous variables are presented using their mean and standard deviation, while categorical variables are described with the number of individuals in each category and the corresponding percentage. The Shapiro–Wilk test was used to assess the normality of data distribution of continuous variables. Statistical tests used to compare groups included the Student t‐test for normally distributed variables and the Mann–Whitney U–test for variables with skewed distribution. The Chi‐squared test or Fisher exact test were used to compare counts and frequencies between groups for categorical variables, as appropriate. The Kruskal–Wallis test or one‐way analysis of variance with post hoc correction were also applied, depending on the distribution of variables.
3. RESULTS
3.1. Study population
In total, 180 patients were included: 87.8% were female, with a mean age of 48.11 (SD 16.51) years mean age and 8.3% had a previous diagnosis of another autoimmune disease.
3.2. Year distribution of GD incidence
Table 1 shows a similar number of GD patients per year throughout the period from 2017 to 2020, while a large increase is observed in 2021 with the total number of cases double that of 2020 (p < .001) and 2.44 times higher than the average incidence of cases in the 2017–2019 period (p < .001).
Table 1.
Graves' disease total number of cases by year in the period 2017–2021
| Year | Total cases | Population | Incidence |
|---|---|---|---|
| 2017 | 27 | 309,886 | 8.7 × 10− 4 |
| 2018 | 25 | 308,531 | 8.1 × 10−4 |
| 2019 | 29 | 304,534 | 9.5 × 10−4 |
| 2020 | 33 | 303,102 | 10.88 × 10‐4 |
| 2021 | 66 | 300,788 | 21.94 × 10−4 |
3.3. Seasonal distribution of GD incidence
3.3.1. (a) 2020 vs. 2017–19
We observed a similar number of GD patients per year throughout the period from 2017 to 2020 (Table 1). By seasonal distribution of GD cases, no differences were observed in the distribution by quarter period (Figure 1). The 2 waves of SARS‐CoV‐2 of 2020 incidence observed in our department was superimposable to that which happened across the rest of the country (Figure 2). From our point of view, the number of SARS‐CoV‐2 cases from the first wave is probably underestimated because of healthcare disruption during the early pandemic.
3.3.2. (b) 2021 vs. 2017–19
We observed a notable increase in expected GD cases in 2021 compared to 2017–19. The seasonal percent distribution of GD cases was different in the second quarter (Q2: April to June). During Q2 of the years 2017–2019, cases were more common compared with 2021 (28/81 vs. 10/66) (p = .038); no significant differences were found in the rest of the 3‐month periods (Figure 1). The 3 waves of SARS‐CoV‐2 of 2021 incidence observed in our department was superimposable to what happened in the rest of the country (Figure 3), the valley of the case curve during the year 2021 coincides with a period between SARS‐CoV‐2 waves. The vaccination curve of the population of the health area expressed as a percentage has also been superimposed in Figure 4.
Figure 3.
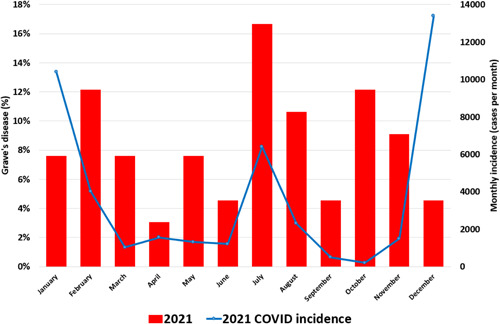
2021 COVID incidence and percentages of Grave's disease by month [Color figure can be viewed at wileyonlinelibrary.com]
Figure 4.
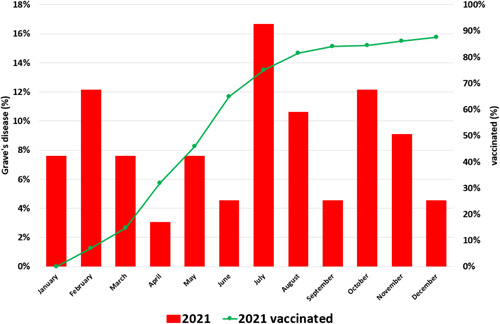
Percentage of cases of Grave's disease by month in 2021. COVID monthly incidence in 2021 and total % of population over 18 years vaccinated with complete vaccination regimen (2 doses for BNT162b2, 2 doses for mRNA1273, 2 doses ChAdOx1‐CoV‐2, and 1 dose for Ad26‐CoV‐2) in 2021 [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Clinical and laboratory features
No patient had a history of SARS‐CoV‐2 infection in 2017–2019. In 2020, 5/33 (15.2%) patients had a history of SARS‐CoV‐2 infection demonstrated by PCR (polymerase chain reaction) within 90 days before symptom onset, while 2/66 (3.0%) patients had a history of PCR‐demonstrated SARS‐CoV‐2 infection in the 90 days before symptom onset in 2021 (Table 2). Notable findings include a significant increase in initial FT4 numbers in both 2020 and 2021 compared to prepandemic years and a higher proportion of females in 2021 compared to 2020.
Table 2.
Clinical and demographic characteristics of the population with Graves' disease by period
| Years 2017–2019 | Year 2020 | Year 2021 | Overall | Years 2017–2019 vs. 2020 | Years 2017–2019 vs. 2021 | Year 2020 vs. 2021 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| (N = 81) | (N = 33) | (N = 66) | p‐value | p‐value | p‐value | p‐value | ||||
| Sex | ||||||||||
| Female (%) | 70 | 86.4% | 26 | 78.8% | 62 | 93.9% | .074 | .311 | .174 | .0387 |
| Male (%) | 11 | 13.6% | 7 | 21.2% | 4 | 6.1% | ||||
| Mean age (SD) | 46.3 | (17.5) | 51.5 | (13.5) | 48.6 | (16.5) | .234 | .081 | .368 | .453 |
| FT4 (SD) ng/dl | 2.43 | (1.39) | 3.32 | (1.89) | 3.24 | (1.57) | <.001 | .010 | <.001 | .777 |
| FT3 (SD) ng/dl | 0.65 | (0.39) | 1.11 | (0.77) | 1.55 | (2.56) | .216 | .370 | .089 | .927 |
| TSH (SD) mIU/L | 0.011 | (0.022) | 0.005 | (0.01) | 0.006 | (0.08) | .020 | .583 | .006 | .033 |
| TRAbs (SD) IU/ml | 21.44 | (34.08) | 15.91 | (16.78) | 13.99 | (14.90) | .286 | .360 | .126 | .746 |
| IS supplementation | 5 | 10.4% | 1 | 7.1% | 4 | 9.5% | .935 | .186 | .803 | .776 |
| No IS supplementation | 43 | 13 | 38 | |||||||
| Family history of TD | 10 | 27.8% | 7 | 38.9% | 14 | 31.8% | .710 | .407 | .593 | .695 |
| No family history of TD | 26 | 11 | 30 | |||||||
| Autoimmune disease | 5 | 6.2% | 2 | 6.1% | 8 | 12.1% | .428 | .999 | .249 | .488 |
| No autoimmune disease | 76 | 31 | 58 | |||||||
| Smoker | 10 | 12.3% | 13 | 39.4% | 14 | 21.2% | .005 | .001 | .148 | .056 |
| No smoker | 71 | 20 | 52 | |||||||
| SARS‐CoV‐2 infection | NA | 5 | 15.6% | 2 | 3.0% | NA | NA | .030 | ||
| No SARS‐CoV‐2 infection | NA | 21 | 64 | |||||||
Abbreviations: IS, iodine salt; TD, thyroid disease.
3.5. Distribution of GD incidence by vaccine
In 2021, 44/66 patients (66.7%) had a history of vaccination in the 90 days before symptom onset, although none of these were in the first 3 months of the year. Of these 44 patients, 23 developed GD after receiving the first dose of SARS‐CoV‐2 vaccine, compared to 21 who did so after the second dose. None had a recorded history of SARS‐CoV‐2 infection. Overall, 43/44 (97.7%) were women with a mean age of 48.9 (SD 15.6) years. On average, they were diagnosed 19.9 (SD 17.6) days after receiving the vaccine. Also, 7/44 (15.9%) had another previously diagnosed autoimmune disease and 11/44 (25%) were smokers.
In total, 25/44 (56.8%) received the Pfizer vaccine (BNT162b2), 5 (11.4%) the Moderna vaccine (mRNA1273), 7 (15.9%) the AstraZeneca vaccine (ChAdOx1‐COV‐2), and 7 (15.9%) the Janssen vaccine (Ad26‐CoV‐2). Significant differences were observed with an increase in the GD cases recorded after vaccination with the Janssen vaccine and that expected according to the percentage of administered doses (2.1%, p < .001). 74.0% of the population was vaccinated with Pfizer's vaccine, while the percentage of observed cases of GD decreased to 59.52%, p = .033.
4. DISCUSSION
GD is an organ‐specific autoimmune disorder, associated with the presence of circulating TSH‐R autoantibodies, and is the most common cause of hyperthyroidism in developed countries. Although GD can affect anyone, it is more common between 30 and 60 years of age, and about 5–6 times higher in females than in males. 14 Previous studies carried out in other developed countries indicate a variable incidence of 10–50 cases per 100,000 inhabitants/year. 15 This variability responds to the strong genetic component in the likelihood of developing GD, which is estimated at 79%, 16 but many other risk factors have been associated with autoimmune thyroid disorders involving T‐cell dysfunction, among which smoking stands out with an odds ratio of 1.9, 17 or iodine intake. 18 Unfortunately, we have a lack of knowledge on the incidence of GD in Spain and only have data about the estimated prevalence of 0.8% 19 of the population being diagnosed with hyperthyroidism and 0.14% receiving antithyroid treatment. 20
During the COVID‐19 pandemic thyroid gland alteration/dysfunction has been emerged as a possible endocrine complication. 21 Compared with pre‐pandemic, the post‐SARS‐CoV‐2 era patients presented some differences from a clinical and epidemiological point of view, with a more severe thyrotoxicosis, an increase in the levels of FT4 and an even greater gender gap than traditionally described, of up 10 times higher in females; in fact, only one man presented GD with the possibility that it was triggered by a history of vaccination. There is also a higher incidence of smoking in the cases observed in 2020; one possibility is that a history of SARS‐CoV‐2 infection may have triggered a higher number of cases in predisposed subjects, just as a history of smoking is known to be associated with a more severe course of COVID‐19, 22 but another consideration is that smoking may be a predisposing factor for developing GD after SARS‐CoV2 infection.
For obvious reasons, no patient in this study had a history of SARS‐CoV‐2 infection in 2017–2019. It should also be considered that the number of SARS‐CoV‐2 cases from the first wave in 2020 is probably underestimated because of the disruption to healthcare during the early pandemic. In 2020, 5/33 (15.2%) patients had a history of SARS‐CoV‐2 infection demonstrated by PCR within 90 days before symptom onset, while only 2/66 (3.0%) patients had a history of PCR‐demonstrated SARS‐CoV‐2 infection in the 90 days before symptom onset in 2021. Although the real incidence of SARS‐CoV‐2 infection in 2020 was probably underestimated, the seroprevalence IgG‐SARS‐CoV‐2 data in 2020 was 9.7% in León province 23 ; this difference contrasts with the fact that the recorded incidence of SARS‐CoV‐2 infection during 2021 was much higher than that recorded during 2020 (Figure 1, Figure 2) and, while there was only a small increase in GD cases during 2020, the incidence of GD more than doubled during 2021, which suggests that large‐scale vaccination has had an impact of its own. It should be considered that the doubled diagnosis of GD in 2021 might reflect a delayed diagnosis of cases in 2020 due to healthcare disruption which occurred during the first year of the pandemic in addition to vaccination.
The possible interrelationship of vaccines developed for SARS‐COV‐2 and the activation of the autoimmune system has been highlighted in recent months. 24 The most widely accepted theory is the “adjuvant‐induced autoimmune/inflammatory syndrome” (AISIS); for example, aluminum salts are the adjuvant in the Ad26‐CoV‐2 from Johnson and Johnson vaccine, Pfizer‐BioNTech's BNT162b2 vaccine contains lipid nanoparticles to facilitate the transport of mRNA into the host cell and two of them contain polyethylene glycol (PEG) extracts that can trigger an exaggerated immune response. In addition to AISIS (also known as ASIA syndrome), other possible explanations for GD being triggered by SARS‐CoV‐2 vaccinations include molecular mimicry of the spike protein with endogenous thyroid antigens, or a toxic activity of the spike protein due to the interaction with ACE‐II, which is highly expressed in the thyroid gland, triggering inflammation and then autoimmunity. Adjuvants are generally well tolerated, but they can cause an autoimmune response in genetically predisposed subjects. This can occur by a variety of mechanisms, including altered host immunity, polyclonal activation of B cells, effects on cell‐mediated immunity, virus‐induced antibodies and accelerated molecular mimicry. 14
Although some differences have been observed with an apparently higher relative risk in adenovirus vaccines compared to mRNA vaccines, this could be explained by the fact that adenovirus vaccines were used in the vaccination strategy of young populations that are more susceptible to immunological problems. It should also be taken into consideration that the vaccination strategy in our population was changeable due to the limited availability of new doses and some subjects ended up combining different types of vaccines in the first and second doses, although this was not the case for any of the individuals in this study, but it did occur in the general population.
Above all, we must emphasize that the data from this study do not allow us to assess the impact of SARS‐CoV2 infection or vaccination or other possible confounding factors involved in the development of new‐onset GD. With the aforementioned limitations, it seems that the results obtained in this work should invite the healthcare community to be especially vigilant about the possible impact of mass vaccination campaigns and/or a history of SARS‐CoV‐2 infection on the development of this and other autoimmune diseases. In our opinion, it is necessary to perform larger studies that will allow us to deepen our knowledge of the specific risk attributable to each type of vaccine, revaccination or history of infection, and to complement this with data that have been observed in other geographic areas. In addition, long‐term studies should be considered to assess differences in the clinical course and therapeutic needs depending on the triggering factor. Finally, it is worth analyzing in the coming years whether the increase observed corresponds to patients with a predisposition to develop the disease, where the precipitating factor has brought the process forward a few years, or whether the increase in incidence will be maintained in the long‐term.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Barajas Galindo DE, Ramos Bachiller B, González Roza L, et al. Increased incidence of Graves' disease during the SARS‐CoV2 pandemic. Clin Endocrinol. 2022;1‐8. 10.1111/cen.14860
REFERENCES
- 1. WHO Coronavirus (COVID‐19) Dashboard [Internet] . Covid19. who.int. 2022. [cited 21 March 2022]. Available from: https://covid19.who.int/
- 2. Rotondi M, Coperchini F, Ricci G, et al. Detection of SARS‐COV‐2 receptor ACE‐2 mRNA in thyroid cells: a clue for COVID‐19‐related subacute thyroiditis. J Endocrinol Invest. 2021;44(5):1085‐1090. 10.1007/s40618-020-01436-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brix TH, Hegedüs L. Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) infection and thyroid disease. An update. Curr Opin Endocrinol Diabetes Obes. 2021;8(5):525‐532. 10.1097/MED.0000000000000654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De la Higuera López‐Frías M, Perdomo CM, Galofré JC. Tiroiditis subaguda tras infección por COVID‐19. Rev Clin Esp. 2021;221(6):370‐372. 10.1016/j.rce.2021.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hammerstad S, Villanueva R, Tomer Y. Infection and autoimmune thyroid diseases. Infect Autoimmun. 2015;Chapter 54:891‐918. ISBN 9780444632692. 10.1016/B978-0-444-63269-2.00048-9 [DOI] [Google Scholar]
- 6. Mateu‐Salat M, Urgell E, Chico A. SARS‐COV‐2 as a trigger for autoimmune disease: report of two cases of Graves' disease after COVID‐19. J Endocrinol Invest. 2020;43(10):1527‐1528. 10.1007/s40618-020-01366-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bornemann C, Woyk K, Bouter C. Case report: two cases of subacute thyroiditis following SARS‐CoV‐2 vaccination. Front Med. 2021;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan F, Brassill MJ. Subacute thyroiditis post‐Pfizer‐BioNTech mRNA vaccination for COVID‐19. Endocrinol Diabetes Metab Case Rep. 2021;2021:21‐142. 10.1530/EDM-21-0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Franquemont S, Galvez J. Subacute thyroiditis after mRNA vaccine for COVID‐19. J Endocr Soc. 2021;5(Suppl_1):A956‐A957. [Google Scholar]
- 10. Lui DTW, Lee KK, Lee CH, Lee ACH, Hung IFN, Tan KCB. Development of Graves' disease after SARS‐CoV‐2 mRNA vaccination: a case report and literature review. Front Public Health. 2021;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vera‐Lastra O, Ordinola Navarro A, Cruz Domiguez MP, Medina G, Sánchez Valadez TI, Jara LJ. Two cases of Graves' disease following SARS‐CoV‐2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants. Thyroid. 2021;31(9):1436‐1439. [DOI] [PubMed] [Google Scholar]
- 12.[Internet]. 2022. [cited 28 June 2022]. Available from: https://www.sanidad.gob.es/en/organizacion/sns/informeAnualSNS/docs/CCAA2005/castillayLeon.pdf
- 13.Situación epidemiológica del coronavirus en Castilla y León — Análisis de datos abiertos JCyL [Internet]. Analisis.datosabiertos.jcyl.es. 2022. [cited 28 March 2022]. Available from: https://analisis.datosabiertos.jcyl.es/pages/coronavirus/
- 14. Abraham‐Nordling M, Byström K, Törring O, et al. Incidence of hyperthyroidism in Sweden. Eur J Endocrinol. 2011;165(6):899‐905. [DOI] [PubMed] [Google Scholar]
- 15. Smith TJ, Hegedüs L. Graves' disease. N Engl J Med. 2016;375(16):1552‐1565. [DOI] [PubMed] [Google Scholar]
- 16. Brix TH, Hegedüs L. Twins as a tool for evaluating the influence of genetic susceptibility in thyroid autoimmunity. Ann Endocrinol (Paris). 2011;72(2):103‐107. [DOI] [PubMed] [Google Scholar]
- 17. Prummel MF. Smoking and risk of Graves' disease. JAMA: The Journal of the American Medical Association. 1993;269(4):479‐482. 10.1001/jama.1993.03500040045034 [DOI] [PubMed] [Google Scholar]
- 18. Laurberg P, Cerqueira C, Ovesen L, et al. Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab. 2010;24(1):13‐27. ISSN 1521‐690X 10.1016/j.beem.2009.08.013 [DOI] [PubMed] [Google Scholar]
- 19. Valdés S, Maldonado‐Araque C, Lago‐Sampedro A, et al. Population‐based national prevalence of thyroid dysfunction in Spain and associated factors: Di@bet.es Study. Thyroid. 2017;27(2):156‐166. [DOI] [PubMed] [Google Scholar]
- 20. Torrejón S, Vila L, Soldevila B, Martín M, Puig‐Domingo M. Estimation of the prevalence of thyroid dysfunction in catalonia through two different registries: pharmaceutical dispensing and diagnostic registration. Endocrinol Diabetes Metab. 2020;4(1):e00167. 10.1002/edm2.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruggeri RM, Campennì A, Deandreis D, et al. SARS‐COV‐2‐related immune‐inflammatory thyroid disorders: facts and perspectives. Expert Rev Clin Immunol. 2021;17(7):737‐759. 10.1080/1744666X.2021.1932467 Epub 2021 Jun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simons D, Shahab L, Brown J, Perski O. The association of smoking status with SARS‐CoV‐2 infection, hospitalization and mortality from COVID‐19: a living rapid evidence review with Bayesian meta‐analyses (version 7). Addiction. 2021;116(6):1319‐1368. 10.1111/add.15276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.[Internet]. Lamoncloa.gob.es . 2022. [cited 28 June 2022]. Available from: https://www.lamoncloa.gob.es/serviciosdeprensa/notasprensa/sanidad14/Documents/2020/151220_informe_definitivo_cuarta_ronda_enecovid.pdf
- 24. Ruggeri RM, Giovanellla L, Campennì A SARS‐CoV‐2 vaccine may trigger thyroid autoimmunity: real‐life experience and review of the literature. J Endocrinol Invest 2022;45:2283‐2289. 10.1007/s40618-022-01863-x [DOI] [PMC free article] [PubMed] [Google Scholar]


