Dear Editor,
Previous studies suggested that nonpharmaceutical interventions during the COVID‐19 pandemic have also affected the spread of other pathogens. 1 , 2 Here, we analyzed the transmission patterns of 22 infectious diseases in England in the context of the COVID‐19 prevention measures, using data derived from the UK Health Security Agency, the UK Office for National Statistics, and the Royal College of General Practitioners Research and Surveillance Centre (Suppporting Information: Methods and Tables 1 and 2).
Reported cases for all investigated infectious diseases dipped in response to the first lockdown except from methicillin‐resistant Staphylococcus aureus (MRSA), Lyme disease, and hepatitis E (Figure 1 and Supporting Information: Figures 1–22). MRSA infections are usually diagnosed in healthcare settings, 3 and some studies reported an increase of MRSA cases during COVID‐19. 3 Hepatitis E is predominantly transmitted by contaminated food in England, in particular from farmed pigs. 4 Therefore, these finding do not seem to be surprising.
Figure 1.
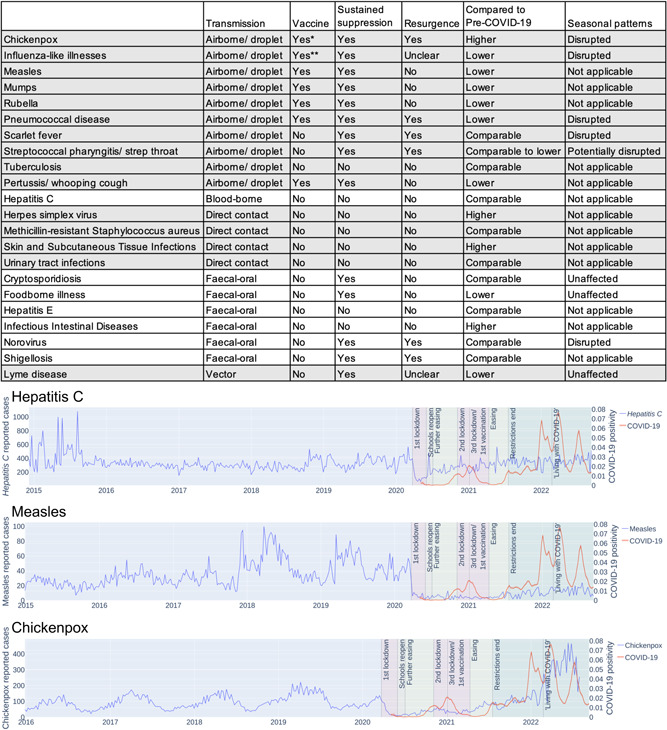
Impact of COVID‐19 prevention measures on the circulation of other infectious diseases. Overview table providing a qualitative description of the impact of the COVID‐19 measures on the investigated pathogens in England and curves illustrating the impact of the COVID‐19 measures on hepatitis C, measles, and chickenpox. A detailed information is presented in Supporting Information: Figures 1–22.
Lyme disease was not reduced during the initial lockdown but a decrease has been reported later in the pandemic (Figure 1 and Supporting Information: Figure 22), which is probably attributed to underreporting. 5 Generally, the drop in documented cases during the first lockdown is difficult to interpret, as it might be the consequence of underreporting. 5 , 6
Thirteen diseases displayed a sustained reduction when prevention measures were in place (Figure 1 and Supporting Information: Figures 1–22), including 9 of the 10 diseases that spread via the air and 4 of the 6 diseases characterized by fecal–oral transmission (Figure 1 and Supporting Information: Figures 1–10 and 16–21).
The prevention measure‐associated associated reduction of airborne pathogens confirms other findings. 2 , 7 The only exception was tuberculosis (Figure 1 and Supporting Information: Figure 9). Most tuberculosis infections are asymptomatic and go undiagnosed. 8 , 9 Delayed diagnoses due to limited access to tuberculosis services during the pandemic may have caused a rise of severe cases, including COVID‐19/tuberculosis co‐infections. 9 , 10 Hence, the pandemic measures may not have reduced severe tuberculosis cases, which are typically diagnosed.
Moreover, our findings agree with others showing that hygiene measures and physical distancing reduce the transmission of enteric diseases that are transmitted via the fecal–oral route. 1 , 6 , 7 Exceptions may indicate pathogens that are predominantly spread by food contaminations without significant further human‐to‐human transmission. 4 Also in agreement with previous findings, 2 the pandemic‐related prevention measures disrupted the seasonal transmission patterns of different infectious diseases (Figure 1 and Supporting Information: Figures 1, 2, 6, 7 and 20).
There are concerns that the disruption of routine vaccinations may affect population immunity resulting in larger outbreaks of vaccine‐preventable diseases. 2 However, our findings indicate a sustainable suppression of vaccine‐preventable diseases also beyond the lifting of restrictions (Figure 1). This included measles, mumps, rubella, pertussis, and pneumococcal disease (Supporting Information: Figures 3–6 and 10). Although our data also indicate a sustained reduction of influenza‐like illnesses, other data suggest that influenza cases should be expected to rebound. 11 This may reflect the relatively low influenza vaccination rates and influenza vaccine efficacy compared to other vaccine‐preventable diseases. 10
By contrast, non‐vaccine preventable respiratory infections including chickenpox (not part of routine vaccinations in the UK), scarlet fever, and streptococcal pharyngitis displayed an immediate resurgence after the removal of prevention measures (Supporting Information: Figures 1, 7 and 8), suggesting that similar transmission peaks have been prevented by the vaccine‐mediated immunity for the diseases with high vaccine coverage in the UK.
Concerns have been raised that a lack of exposure to common pathogens may result in decreased immunity enabling larger and more deleterious outbreaks. 2 However, only four infectious diseases (chickenpox, herpes simplex virus, skin and subcutaneous tissue infections, infectious intestinal diseases) have since the removal of all restrictions in England on July 19, 2021 resulted in higher spread levels than pre‐COVID‐19 (Figure 1). It remains to be investigated whether these increases may be related to COVID‐19.
In conclusion, our analysis shows that the COVID‐19 prevention measures reduced the spread of pathogens that are transmitted via the air and the fecal–oral route. Despite concerns that a lack of exposure to common pathogens may affect population immunity and result in large outbreaks by various pathogens post‐COVID‐19, only 4 of the 22 investigated diseases and disease groups displayed higher post‐ than prepandemic levels without an obvious causative relationship. This included chickenpox for which an effective vaccine is available but not used in the UK. Notably, the COVID‐19 prevention measures resulted in the sustained suppression of vaccine‐preventable infectious diseases also after the removal of restrictions, while non‐vaccine preventable diseases displayed a rapid rebound, supporting the importance of effective vaccination programmes. More research investigating how disease burden can be reduced by tolerable non‐pharmaceutical interventions is warranted.
AUTHOR CONTRIBUTIONS
Lauren J. Hayes and Martin Michaelis developed the project idea. Lauren J. Hayes and Hannah Uri performed the research. Mark N. Wass and Martin Michaelis supervised the research. All authors analyzed data. Martin Michaelis wrote the initial manuscript draft. All authors contributed to the finalization of the manuscript. All authors approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
This work was supported by the BBSRC via the SoCoBio DTP and the Frankfurter Stiftung für krebskranke Kinder.
Lauren J. Hayes and Hannah Uri contributed equally.
Contributor Information
Mark N. Wass, Email: M.N.Wass@kent.ac.uk.
Martin Michaelis, Email: M.Michaelis@kent.ac.uk.
DATA AVAILABILITY STATEMENT
All data are provided in the manuscript and its supplements.
REFERENCES
- 1. Eldesouki RE, Uhteg K, Mostafa HH. The circulation of non‐SARS‐CoV‐2 respiratory viruses and coinfections with SARS‐CoV‐2 during the surge of the Omicron variant. J Clin Virol. 2022;153:105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oh KB, Doherty TM, Vetter V, Bonanni P. Lifting non‐pharmaceutical interventions following the COVID‐19 pandemic—the quiet before the storm? Expert Rev Vaccines. 2022;21:1541‐1553. 10.1080/14760584.2022.2117693 [DOI] [PubMed] [Google Scholar]
- 3. Segala FV, Bavaro DF, Di Gennaro F, et al. Impact of SARS‐CoV‐2 epidemic on antimicrobial resistance: a literature review. Viruses. 2021;13:2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oeser C, Vaughan A, Said B, et al. Epidemiology of hepatitis E in England and Wales: a 10‐year retrospective surveillance study, 2008‐2017. J Infect Dis. 2019;220:802‐810. [DOI] [PubMed] [Google Scholar]
- 5. McCormick DW, Kugeler KJ, Marx GE, et al. Effects of COVID‐19 pandemic on reported Lyme disease, United States, 2020. Emerging Infect Dis. 2021;27:2715‐2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim S, Kim J, Choi BY, Park B. Trends in gastrointestinal infections before and during non‐pharmaceutical interventions in Korea in comparison with the United States. Epidemiol Health. 2022;44:e2022011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nielsen RT, Dalby T, Emborg HD, et al. COVID‐19 preventive measures coincided with a marked decline in other infectious diseases in Denmark, spring 2020. Epidemiol Infect. 2022;150:e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boom WH, Schaible UE, Achkar JM. The knowns and unknowns of latent Mycobacterium tuberculosis infection. J Clin Invest. 2021;131:e136222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trajman A, Felker I, Alves LC, et al. The COVID‐19 and TB syndemic: the way forward. Int J Tuberc Lung Dis. 2022;26:710‐719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Q, Guo S, Wei X, et al. Global prevalence, treatment and outcome of tuberculosis and COVID‐19 coinfection: a systematic review and meta‐analysis (from November 2019 to March 2021). BMJ Open. 2022;12:e059396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pérez‐López A, Al Mana H, Iqbal M, Suleiman M, Hasan MR, Tang P. Resurgence of influenza A infections in children after the relaxation of COVID‐19‐related social distancing measures and normalization of international travel in Qatar. J Travel Med. 2022:taac107. 10.1093/jtm/taac107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Uyeki TM, Hui DS, Zambon M, Wentworth DE, Monto AS. Influenza. Lancet. 2022;400:693‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
All data are provided in the manuscript and its supplements.


