Abstract
Introduction
Diabetes mellitus worsens the prognosis of SARS‐CoV‐2 infection, and vaccination has been the major tool for reducing the risk of hospitalisation, and mortality.
The primary aim of this study was to evaluate the response to the SARS‐CoV‐2 vaccine in subjects with diabetes and controls. Differences between type 1 (T1D) and type 2 (T2D) diabetes and clinical determinants of vaccination response were also evaluated.
Methods
128 subjects with diabetes (60 with T1D and 62 with T2D) and 202 subjects acting as controls who completed a full vaccination cycle with two doses of mRNA vaccine were enroled. People with previous SARS‐CoV‐2 infection were excluded. Antibodies (Ab) directed against the spike protein of the SARS‐CoV‐2 were evaluated at one and 6 months after vaccination.
Results
In the whole cohort, the Ab level was higher among women than in men (p = 0.011) and negatively correlated with age (rho = −0.155, p = 0.005). Subjects with diabetes showed decreased levels of Ab after one month compared to controls (1217[747–1887]BAU/mL vs. 1477[942–2556]BAU/mL, p = 0.002), even after correction for age and gender (p = 0.002). No difference was found between subjects with T1D and T2D. After 6 months, antibody levels significantly decreased in people with and without diabetes, with no differences between groups, although some subjects were lost at follow‐up. In subjects with diabetes, only a significant correlation was found between Ab level and renal function (rho 0.190, p = 0.042).
Conclusions
Both T1D and T2D are associated with a reduced early response to vaccination. The serum concentration of Ab significantly reduced over time in both groups, highlighting the relevance of vaccination boosters independently of the presence of diabetes.
Keywords: antibodies, covid‐19, diabetes, SARS‐CoV‐2, vaccine
1. INTRODUCTION
After its breakdown in late 2019, Severe Acute Respiratory Syndrome Coronavirus‐2 (SARS‐CoV‐2) infection leading to Covid‐19 has been a major burden for healthcare systems and society worldwide.
Covid‐19 has proven to be a heterogeneous disorder that can cause an extremely severe disease leading to exitus if not supported by intensive care management. Diabetes is one of the most important negative prognostic factors in patients with Covid‐19. 1 , 2 Patients with diabetes have an increased risk of negative outcomes such as the need for invasive ventilation, intensive care unit admission, or death. 3 Several factors have been proposed to explain the increased risk of negative outcomes observed among subjects with diabetes, such as the presence of a pro‐inflammatory milieu and obesity 1 or the role of the so‐called “cytokine storm”. 4 Further, contrasting data have been gathered on the capability of subjects with diabetes to develop an appropriate immune response after SARS‐CoV‐2 infection. 5 , 6
The development and administration of large‐scale SARS‐CoV2 vaccines have been a turning point in the management of Covid‐19. 7 , 8 Nevertheless, impaired response to vaccination in subjects with diabetes has been reported 9 and poor glycaemic control seems to be a detrimental risk factor in this regard. 10
Therefore, the aim of this study was to assess differences in response to SARS‐CoV‐2 vaccination, in terms of SARS‐CoV‐2 anti‐spike antibodies (Ab), in the short (1 month) and medium (6 months) terms after the vaccination with two doses of mRNA vaccine between patients with and without diabetes. As secondary aims, we evaluated the differences in SARS‐CoV‐2 Ab levels between people with type 1 (T1D) and type 2 diabetes (T2D) and searched for determinants that could affect SARS‐CoV‐2 Ab levels in patients with diabetes.
2. MATERIALS AND METHODS
2.1. Study design and population
This longitudinal observational study was a collaboration between the Diabetes Unit at Umberto I Policlinico General Hospital, Sapienza University in Rome and the Laboratory Unit of Fondazione Campus Bio‐Medico University Hospital in Rome. Subjects with diabetes were enroled in the outpatient clinics of the Diabetes Units between April and June 2021, after the vaccination of fragile patients promoted by the Health Ministry in Italy. Healthy subjects were enroled amongst health care professionals in both centres between January and February 2021. All subjects underwent vaccination with BNY162b2 (Pfizer/BioNTech) mRNA vaccine, the only available mRNA vaccine at the time of enrolment of subjects in both Hospitals, for both subjects with and without diabetes.
Subjects with the following conditions were excluded: previous SARS‐CoV‐2 infection, presence of any type of immunodeficiency, haematologic malignancies, cancers undergoing active treatment, long‐term corticosteroid therapy (more than 3‐month time), use of vaccines other than BNY162b2 (Pfizer/BioNTech), or administration of only one dose of mRNA vaccine. For the control group, subjects with diabetes or a previous history of gestational diabetes were excluded.
2.2. Study procedures
After 1 month (±7 days) from the second dose of vaccine against SARS‐CoV‐2, a blood sample was collected from each participant. An additional sample was collected after 6 months (±14 days) from the date of the second dose of vaccine against SARS‐CoV‐2. Subjects with diabetes who received a booster (third) dose of vaccine before the 6‐month period were excluded from the analysis at the 6‐month time, but they were included for the analysis at 1 month. Overall, 128 subjects with diabetes (60 with T1D and 68 with T2D) were enroled and 202 subjects without, after six months we were able to follow up 77 subjects with diabetes (31 with T1D and 46 with T2D) and 119 subjects without diabetes.
Anti‐spike Ab was evaluated on previously collected sera by using the Abbott SARS‐CoV‐2 IgG II Quant kit on ARCHITECT i2000SR immunoassay analyser (Abbott). It quantifies IgG immunoglobulins directed against the receptor‐binding domain of the S1 subunit of the Spike protein of SARS‐CoV‐2 by chemiluminescent microparticle immune assay. The test sensitivity (PPA, positive per cent agreement) and confidence interval (CI) 95% were 98.81% (93.56, 99.94) for samples collected after at least 15 days from the polymerase chain reaction detection of SARS‐CoV‐2, while specificity (NPA, negative percent agreement) and (CI) 95% of the test were 99.55% (99.15, 99.76) (Abbott). The results of the assay were expressed in binding Ab units per mL (BAU/mL) according to the World Health Organization standards.
For subjects with diabetes, demographic information and biometric parameters (weight, height, and body mass index (BMI), type of diabetes, duration of diabetes, biochemical parameters (fasting blood glucose and HbA1c) and information on comorbidities and/or complications of diabetes at the time of enrolment were also collected, as shown in supplementary table 1.
Subjects with diabetes were further divided into sub‐groups taking into account hypoglycaemic therapy (insulin, metformin, SGLT2i and GLP1‐RA), presence of microvascular complications (presence of at least one among diabetic nephropathy, neuropathy, or retinopathy) presence of macrovascular complications (presence of at least one among coronary artery disease, peripheral arterial disease or stroke), presence of obesity (based on a cut‐off of BMI >30 kg/m2), hypertension, or other autoimmune diseases, and finally smoking habit.
Side effects following vaccination were also collected: fever, myalgia, headache, pain at the injection site, and fatigue.
2.3. Statistic and ethics
At the time of study design, without any published data on Ab response in patients with diabetes, a priori sample size evaluation was performed using the software G*power version 3.1.9.2. In order to achieve reliable results on the difference in Ab after one month of vaccination, we decided to use a medium effect size of 0.5 (r), then setting α = 0.05 and β = 0.10 (power of 90%), the sample size required to identify a significant difference between subjects with and without diabetes was 86 patients per group. Considering a drop‐out of 10%, a total of 95 subjects per group had to be enroled. Considering the intention to extend the study to 6 months, the secondary aims of the study, and the high variability of the disease that could hamper the follow‐up evaluation, we decided to increase the number of subjects enroled to all people who agreed to participate. Descriptive statistics are presented for categorical variables as numbers with proportions, and for continuous variables as appropriate measures of central tendency and dispersion. Normality was tested with the Shapiro‐Wilk test. T‐test and ANOVA and Mann‐Whitney and Kruskall‐Wallis were used to analyse differences between groups for parametric and nonparametric continuous variables, respectively. Differences between basal and follow‐up levels of anti‐spike Ab in the different groups of patients were tested using the paired T‐test for continuous variables with parametric distribution, while the Wilcoxon matched test was used for non‐parametric variables. Categorical variables were compared with a χ2 or Fisher's exact test as appropriate.
The study was performed in accordance with the Declaration of Helsinki, and the study procedures were approved by the institutions' ethics committees (ref. 6382, prot 0539/2021).
3. RESULTS
Overall, 128 subjects with diabetes (male 56.3%) and 202 controls (male 40.1%) were enroled in the study. Amongst people with diabetes, 60 subjects had T1D (47%) and 68 subjects had T2D (53%). Controls were younger compared to people with diabetes (respectively—median [IR]: 37 [29–44] years vs. 54 [36–61] years).
In the whole population, women showed increased levels of Ab after one month of complete vaccination when compared with men (respectively 1495 [939–2457] BAU/mL vs. 1255 [856–2064] BAU/mL, p = 0.011). Moreover, a significant negative correlation was found between age and Ab levels (rho = −0.155, p = 0.005) in all subjects.
When comparing people with diabetes and controls, the former showed decreased levels of Ab after one month of complete vaccination (1217 [747–1887] BAU/mL vs. 1477 [942–2556] BAU/mL, p = 0.002), even after correction for age and gender (p = 0.002), Figure 1. No significant difference was found between subjects with T1D and T2D (1254 [861–1887] BAU/mL vs. 1156 [656–1944] BAU/mL, respectively, p = 0.569), Figure 2.
FIGURE 1.
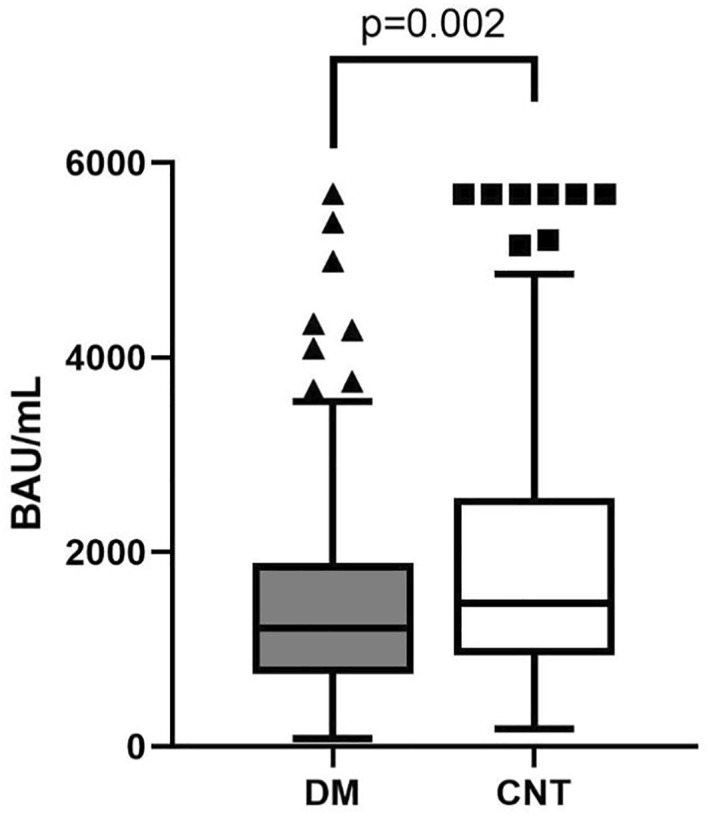
Differences in anti‐spike Ab between subjects with diabetes (DM) and without diabetes (CNT)
FIGURE 2.
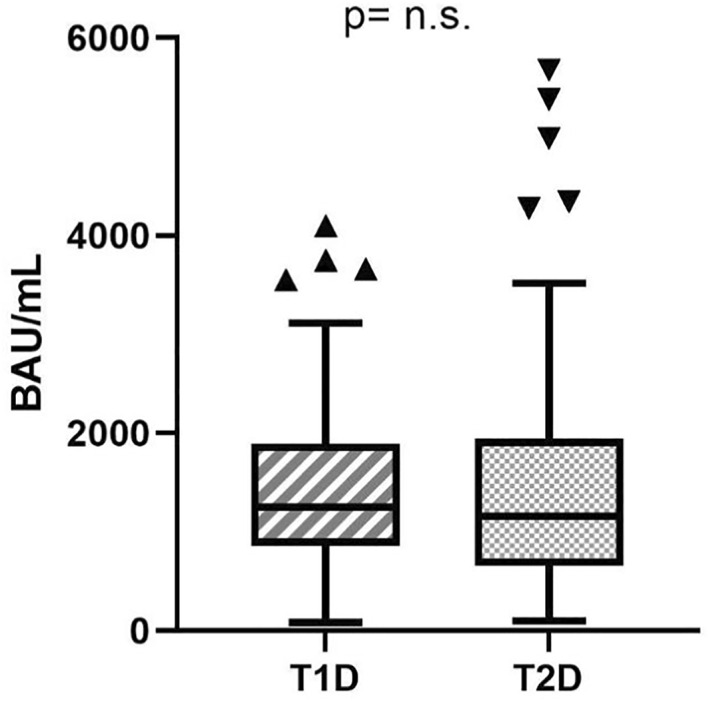
Differences in anti‐spike Ab between subjects with T1D and T2D
After 6 months a significant drop in Ab levels was observed in both people with diabetes and controls. Specifically, among people with diabetes Ab levels decreased from 1217 [747–1887] BAU/mL at month 1–154 [96–264] BAU/mL at month 6 (p < 0.001), and in subjects without diabetes from 1477 [942–2556] BAU/mL at month 1–185 [130–303] BAU/mL at month 6 (p < 0.001), Figure 3. The median absolute reduction in Ab levels over time did not differ between people with diabetes and controls (delta Ab: 1228 [673–1894] BAU/mL vs. 1208 [812–2123] BAU/mL, p = 0.252), Figure 4. However, since people with diabetes had a lower Ab response after 1 month, this resulted in significantly lower Ab levels after six months also (154 [96–264] BAU/mL vs. 185 [130–303] BAU/mL, p = 0.025). People with T1D and T2D did not differ in terms of Ab reduction (respectively 1128 [786–1985] BAU/mL vs. 1180 [558–1762] BAU/mL, p = 0.486).
FIGURE 3.
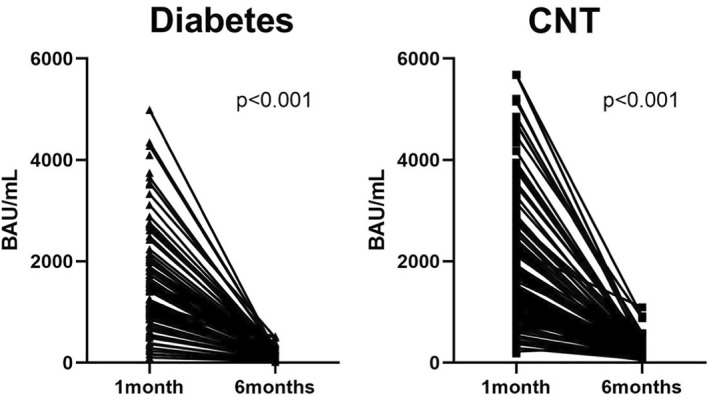
Differences in 6‐month levels of anti‐spike Ab in subjects with and without diabetes (CNT)
FIGURE 4.
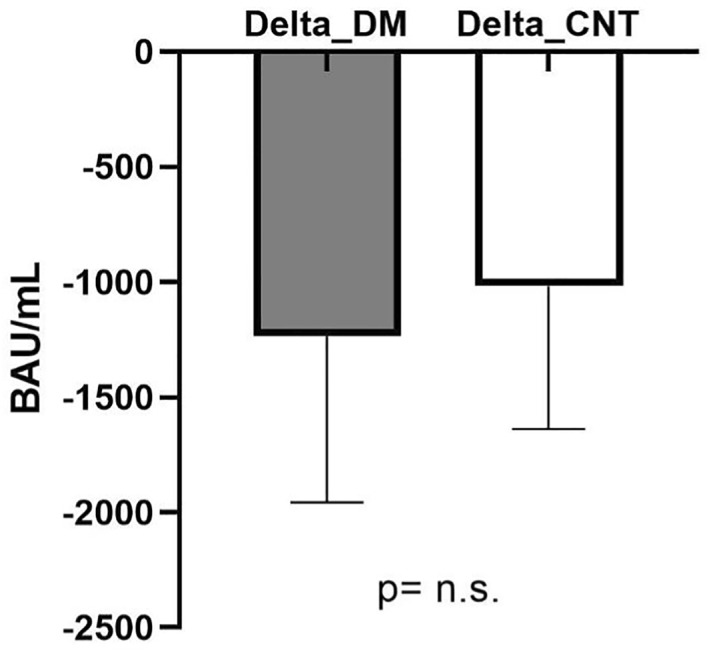
Comparison between delta anti‐spike Ab (1 month value—6 month value) between subjects with diabetes (DM) and without diabetes (CNT)
Among people with diabetes, Ab levels were at 1 month not related to HbA1c, fasting blood glucose, or body weight (data not shown). A significant correlation was found between Ab levels at 1 month and renal function in subjects with diabetes. In particular, Ab levels were higher among subjects with increased eGFR (rho 0.190, p = 0.042).
Further, no differences in Ab level at 1 month were observed dividing subjects with diabetes hypoglycaemic therapy based on the presence or absence of micro‐ or macro‐vascular complications, presence of obesity, hypertension, other autoimmune diseases, and smoking habit (data not shown).
Finally, patients who reported at least one sign or symptom after the second dose of the vaccine showed an increased level of Ab at one month compared to subjects who did not report any side effects (1404 [855–2000] BAU/mL vs. [1023 [458–1498] BAU/mL. p = 0.037).
At 6 months, no subject has been diagnosed with SARS‐CoV‐2 infection, both in subjects with diabetes and in controls, proving the efficacy of the vaccine against SARS‐CoV‐2 infection. Only two subjects with diabetes were excluded by the analysis at 6 months due to higher Ab levels compared to 1 month time, even if they did not report any documented infection.
4. DISCUSSION
This longitudinal study showed an impaired response to SARS‐CoV‐2 vaccination, in terms of anti‐spike Ab serum levels, in people with diabetes compared with those without, with no differences between T1D and T2D. We also confirmed that Ab levels significantly decreased 6‐months after the second vaccination dose. This reduction was similar among people with diabetes and controls; people with diabetes also maintained lower Ab levels 6 months after vaccination.
We also confirmed the role of gender and age in the development of anti‐spike Ab, in particular the increased levels of Ab in female subjects, according to previous studies. 11 , 12 , 13 The findings in the whole cohort of subjects enroled also confirmed that age is a relevant determinant of humoural response to SARS‐CoV‐2 vaccination. 14 , 15 The role played by age in vaccination response is well known both for antibody response and in terms of antibody persistence over time. 16
This study was one of the few that collected data on both subjects with T1D and T2D, adding novel findings on this topic for diabetologists. In particular, we were able to show that an impaired response to vaccination was present for both T1D and T2D subjects without major differences between them, even if there was a significant difference in age between the two groups of subjects. To our knowledge, comparisons between T1D and T2D were analysed only in the COVAC‐DM study. After the first dose of vaccine, the authors did not find any difference between T1D and T2D subjects; instead, they found a significant difference after 3 weeks from the second dose of vaccine, but the difference was not significant after correction for age and gender. 15 Further, this was one of the first studies that demonstrated the same reduction of Ab between subjects with T1D and T2D at the 6‐month period, even if some people were lost at follow‐up.
An impaired immunological response in subjects with T1D was also confirmed by the recent data gathered by D’Addio F. and Sabiu G. et al., showing a compromised cellular cytotoxic immune response after administration of mRNA vaccine for SARS‐CoV‐2 in T1D subjects compared to healthy controls 17 ; however, data were collected after the second dose of vaccine with a short follow‐up. Nevertheless, it is relevant to highlight that the vaccination in subjects with T1D was safe in terms of local and systemic side effects 17 and in terms of glucose control in the short time after vaccine administration. 18
Previous studies suggested that cellular and humoural responses to vaccination could be influenced by glycaemic control. 10 In particular, in a study conducted on healthy workers in Japan, diabetes was associated with a reduced level of anti‐spike Ab, and HbA1c > 6.5% further impaired the immune response. 19 Additionally, in the CAVEAT study, subjects with type 2 diabetes with an HbA1c >7% showed a reduced response to the vaccine 21 days after the second vaccination. 20 In our study, we did not find any correlation between glycaemic control, in terms of both fasting blood glucose and HbA1c, with Ab levels; the same neutral findings were also observed in the COVAC‐DM study, 15 leaving an open question about the relationship between glycaemic control and response to the vaccine. Moreover, observing the median HbA1c of subjects enroled, the good glycaemic control at baseline for both T1D (7.6 [7.0–8.6]%) and T2D (6.9 [6.3–7.6]%), could also explain the neutral findings, limiting the detrimental effect of hyperglycaemia. Further studies should investigate the relationship between glucose levels and the long‐term vaccination response to understand how blood glucose impacts vaccine response and thereby understand how relevant blood glucose management could be in terms of vaccination response to maximise the protective effect of vaccination in people with diabetes.
Interestingly, a positive correlation was found between eGFR and anti‐spike Ab in subjects with diabetes; this evidence is supported by the same findings of the COVAC‐DM study. 15 Indeed, a reduction in kidney function could be related to the reduced efficacy of the SARS‐CoV‐2 vaccine, 21 , 22 but the same was also observed for other vaccination. 23 Considering that the presence of chronic kidney disease is also associated with hospitalisation for Covid‐19 in subjects with DM2, 24 further studies are needed to evaluate the efficacy of SARS‐CoV‐2 vaccination in people with impaired kidney function. Moreover, we did not find any impact of features related to diabetes management, in particular hypoglycaemic therapy or of the presence of micro‐ and macro‐vascular complications, with the vaccine response in subjects with diabetes. This suggests no relevant impact of chronic management of diabetes on the ability of the immune system to develop Ab after vaccination. Although this was one of the secondary aims of the study, no conclusive interpretation could be drawn in the absence of proper studies designed with this specific aim.
Further, in line with evidence from the COVAC‐DM study, 15 increased levels of anti‐spike Ab were reported in subjects who reported side effects of vaccination, suggesting that a more robust immune activation, clinically expressed in terms of side effects, is associated with a greater response in terms of anti‐spike Ab.
Finally, a contrasting conclusion could be drawn about the relationship between anti‐spike Ab and weight. Our study, similar to a large study conducted on healthy subjects, 12 did not show any relationship between weight or BMI and anti‐spike Ab level, in contrast to Watanabe M. et al. 25
Strengths of this study include the relatively large cohort of subjects with and without diabetes, the comparison between different forms of diabetes (T1D and T2D) and the longitudinal study design with a 6‐month follow‐up, which allowed us to observe and compare for the first time the trajectory of anti‐spike Ab levels in people with and without diabetes. However, on the other side, the number of people lost at follow‐up was one of the major limitations of the study, which could hamper the interpretation of the results obtained at 6 months. Further prospective studies, with specific sample size evaluation, should be designed to evaluate the trajectory of anti‐spike Ab levels over time and over booster of vaccine to evaluate the differences between subjects with and without diabetes over time.
The study should however be interpreted in the light of some limitations, in addition to the previously stated, another limitation was the lack of anthropometric and biochemical data for the group of people without diabetes.
In conclusion, our study showed a reduced response to vaccination in people with diabetes after 1 month and a significant reduction over time of anti‐spike Ab, without difference between people with T1D and T2D. These data could be carefully considered to plan future vaccination campaigns in order to protect people with diabetes from the development of the severe form of Covid‐19 disease. Additional studies are also needed to better clarify the role of glycaemic control in response to vaccination.
AUTHOR CONTRIBUTIONS
Luca D’Onofrio and Marta Fogolari designed the study and wrote the manuscript, and Luca D’Onofrio conducted data collection and analysis. Antonio Siena, Riccardo De Fata, Lucia Coraggio, Carmen Mignogna, and Chiara Moretti data collection. Marta Fogolari, Rocco Amendolara, and Flavio Davini conducted laboratory analysis. Ernesto Maddaloni data analysis and writing manuscript. Raffaella Buzzetti and Silvia Angeletti revised the manuscript for critical content. Raffaella Buzzetti designed and wrote the manuscript. All authors approved the final version of the work to be published.
CONFLICT OF INTEREST
All authors declare no conflicts of interest related to this manuscript.
ETHICS STATEMENT
The study was performed in accordance with the Declaration of Helsinki, and the study procedures were approved by the institutions' ethics committees (ref. 6382, prot 0539/2021).
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/dmrr.3601.
Supporting information
Table S1
ACKNOWLEDGEMENT
This study was funded by Sapienza University of Rome (“Progetti per Avvio alla Ricerca – Tipo 1”: protocol number: AR12117A63C69596).
D’Onofrio L, Fogolari M, Amendolara R, et al. Reduced early response to SARS‐CoV2 vaccination in people with type 1 and type 2 diabetes, a 6 months follow‐up study: The CoVaDiab study I. Diabetes Metab Res Rev. 2022;e3601. 10.1002/dmrr.3601
Luca D’Onofrio and Marta Fogolari the authors should be considered joint first author
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID‐19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782‐7. Published online July 17. 10.1016/S2213-8587(20)30238-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahrooz A, Muscogiuri G, Buzzetti R, Maddaloni E. The complex combination of COVID‐19 and diabetes: pleiotropic changes in glucose metabolism. Endocrine. 2021;72(2):317‐325. 10.1007/s12020-021-02729-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maddaloni E, D’Onofrio L, Alessandri F, et al. Cardiometabolic multimorbidity is associated with a worse Covid‐19 prognosis than individual cardiometabolic risk factors: a multicentre retrospective study (CoViDiab II). Cardiovasc Diabetol. 2020;19(1):164. 10.1186/s12933-020-01140-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maddaloni E, Buzzetti R. Covid‐19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020;36(7):e33213321. Published online. 10.1002/dmrr.3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pal R, Sachdeva N, Mukherjee S, et al. Impaired anti‐SARS‐CoV‐2 antibody response in non‐severe COVID‐19 patients with diabetes mellitus: a preliminary report. Diabetes Metab Syndr. 2020;15(1):193‐196. 10.1016/j.dsx.2020.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lampasona V, Secchi M, Scavini M, et al. Antibody response to multiple antigens of SARS‐CoV‐2 in patients with diabetes: an observational cohort study. Diabetologia. 2020;63(12):2548‐2558. 10.1007/s00125-020-05284-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Praet JT, Vandecasteele S, De Roo A, Vynck M, De Vriese AS, Reynders M. Dynamics of the cellular and humoral immune response after BNT162b2 mRNA Covid‐19 vaccination in Covid‐19 naive nursing home residents. J Infect Dis. 2021. Published online. 10.1093/infdis/jiab458 [DOI] [PubMed] [Google Scholar]
- 10. Pieralice S, D’Onofrio L, Pozzilli P, Buzzetti R. Third dose of COVID‐19 vaccine in diabetes: relevance of good metabolic control to improve its efficacy. Diabetes Metab Res Rev. 2022;38(5):e3533. Published online April 25. 10.1002/dmrr.3533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lo Sasso B, Giglio RV, Vidali M, et al. Evaluation of anti‐SARS‐Cov‐2 S‐RBD IgG antibodies after COVID‐19 mRNA BNT162b2 vaccine. Diagnostics. 2021;11(7):1135. 10.3390/diagnostics11071135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lustig Y, Sapir E, Regev‐Yochay G, et al. BNT162b2 COVID‐19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single‐centre, longitudinal cohort study in health‐care workers. Lancet Respir Med. 2021;9(9):999‐1009. 10.1016/S2213-2600(21)00220-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nomura Y, Sawahata M, Nakamura Y, et al. Age and smoking predict antibody titres at 3 Months after the second dose of the BNT162b2 COVID‐19 vaccine. Vaccines. 2021;9(9):1042. 10.3390/vaccines9091042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao M, Slotkin R, Sheth AH, et al. Serum neutralizing antibody titers 12 months after COVID‐19 mRNA vaccination: correlation to clinical variables in an adult, US‐population. Clin Infect Dis. 2022. Published online May 26. 10.1093/cid/ciac416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sourij C, Tripolt NJ, Aziz F, et al. Humoral immune response to COVID‐19 vaccination in diabetes is age‐dependent but independent of type of diabetes and glycaemic control: the prospective COVAC‐DM cohort study. Diabetes Obes Metab. 2022;24(5):849‐858. 10.1111/dom.14643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32(2). 10.1128/CMR.00084-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. D’Addio F, Sabiu G, Usuelli V, et al. Immunogenicity and safety of SARS‐CoV‐2 mRNA vaccines in a cohort of patients with type 1 diabetes. Diabetes. 2022;71(8):1800‐1806. Published online May 12. 10.2337/db22-0053 [DOI] [PubMed] [Google Scholar]
- 18. D’Onofrio L, Coraggio L, Zurru A, et al. Short‐term safety profile of Sars‐Cov2 vaccination on glucose control: continuous glucose monitoring data in people with autoimmune diabetes. Diabetes Res Clin Pract. 2021;179:109022. 10.1016/j.diabres.2021.109022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitsunaga T, Ohtaki Y, Seki Y, et al. The evaluation of factors affecting antibody response after administration of the BNT162b2 vaccine: a prospective study in Japan. PeerJ. 2021;9:e12316. 10.7717/peerj.12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marfella R, D’Onofrio N, Sardu C, et al. Does poor glycaemic control affect the immunogenicity of the COVID‐19 vaccination in patients with type 2 diabetes: the CAVEAT study. Diabetes Obes Metab. 2022;24(1):160‐165. 10.1111/dom.14547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Swai J, Gui M, Long M, Wei Z, Hu Z, Liu S. Humoral and cellular immune response to severe acute respiratory syndrome coronavirus‐2 vaccination in haemodialysis and kidney transplant patients. Nephrology. 2022;27(1):7‐24. 10.1111/nep.13974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao M, Slotkin R, Sheth AH, et al. Clinical variables correlate with serum neutralizing antibody titers after COVID‐19 mRNA vaccination in an adult, US‐based population. Medrxiv Prepr Serv Heal Sci. 2022. Published online May 4. 10.1101/2022.04.03.22273355 [DOI] [Google Scholar]
- 23. Ma BM, Yap DYH, Yip TPS, Hung IFN, Tang SCW, Chan TM. Vaccination in patients with chronic kidney disease‐Review of current recommendations and recent advances. Nephrology. 2021;26(1):5‐11. 10.1111/nep.13741 [DOI] [PubMed] [Google Scholar]
- 24. Maddaloni E, D’Onofrio L, Alessandri F, et al. Clinical features of patients with type 2 diabetes with and without Covid‐19: a case control study (CoViDiab I). Diabetes Res Clin Pract. 2020;169:108454. 10.1016/j.diabres.2020.108454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Watanabe M, Balena A, Tuccinardi D, et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID‐19 mRNA vaccine. Diabetes Metab Res Rev. 2021;38(1). Published online May 6. 10.1002/dmrr.3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


