Abstract
Abbreviations
- COV‐SPOT
COVID‐19 Safe Prescribing of Oncological Treatments
- COVID‐19
Coronavirus disease 2019
- LFD
lateral flow device
- NHS
National Health Service
- PCR
polymerase chain reaction
- REDCap
Research Electronic Data Capture
- SACT
systemic anticancer therapy
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- UKCCP
United Kingdom Coronavirus Cancer Project
- VOC
variants of concern
Dear editor,
Over half a billion people worldwide have been infected by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2), the virus responsible for the COVID‐19 pandemic. Research has indicated that risk factors for poor outcomes following SARS‐CoV‐2 infection include increased age, comorbidities and being immunocompromised. 1 , 2 Patients with cancer are more susceptible to infection due to their underlying malignancy and the use of immunosuppressive treatments, and have been identified as a high‐risk group for adverse outcomes following COVID‐19 infection. 2
Vaccines against SARS‐CoV‐2 are effective in reducing symptomatic disease, hospital admissions and death. 3 Global vaccination strategies have prioritised cancer patients, given their high‐risk for severe disease and associated mortality. Studies consistently indicate that available vaccines are safe and effective in reducing severe COVID‐19 risk in these patients. 3 However, concerns have been raised over vaccination efficacy following the emergence of immunity‐escape SARS‐CoV‐2 variants of concern (VOC) such as Omicron (B·1·1·529) and Delta (B·1·617·2). While protection against symptomatic infection is reduced with these VOC, vaccines remain effective at reducing the risk of severe infection or hospital admission. Moreover, cancer patients receiving a booster vaccine dose following primary immunisation display strong neutralising capacity against VOC. 4 Nonetheless, the high frequency of COVID‐19 breakthrough infections in immunocompromised patients, including those with cancer on active treatment, highlights the need for further protection in these cohorts. 5 It is therefore paramount that healthcare systems deliver the highest level of evidence‐based safe prescribing of oncological treatments during the ongoing pandemic. Lessons learnt during the COVID‐19 pandemic can also be applied to any future pandemic events.
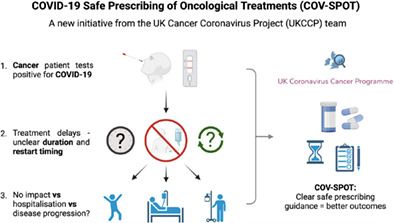
Due to the high level of circulating COVID‐19 infections, many cancer patients commencing, or currently receiving systemic anticancer therapy (SACT) or radiotherapy, have tested positive for COVID‐19. These patients may be symptomatic or asymptomatic, with COVID‐19 diagnosed on routine testing before treatment delivery. Studies demonstrate persistent COVID‐19 polymerase chain reaction (PCR) positivity up to 105 days after recovery, including in asymptomatic individuals with negative lateral flow device (LFD) tests after recovering from infection. 6 There is no strong evidence to guide treatment decisions for these patients. Current national guidance supports delaying SACT in patients testing positive for COVID‐19 and to treat only when significant symptoms have resolved. 7 Treatment delays may risk disease progression and subsequent negative cancer outcomes. Conversely, initiating cancer therapy too early could lead to rebound viraemia and COVID‐19 with adverse sequelae. Currently, there are variations in guidance across NHS trusts which have led to nonevidenced‐based practice.
Given the lack of evidence to support decision‐making in patients testing positive for COVID‐19, potential areas of clinical uncertainty include: (i) the appropriateness of prompt initiation of cancer treatment in high‐risk individuals with rapidly progressive cancers (germ cell tumours, lymphomas, small cell lung cancer, etc), (ii) treating asymptomatic, vaccinated individuals, (iii) treating symptomatic but clinically stable patients, (iv) treating individuals who are PCR‐positive but LFD‐negative and (v) safe timing to restart treatment following positive tests.
The United Kingdom Coronavirus Cancer Programme (UKCCP) was established in March 2020 as a national, clinician‐led reporting network for COVID‐19 cases in cancer patients. 8 It is one of the longest‐running pandemic response programmes, linking 86 cancer centres across the United Kingdom. It provides meaningful real‐time evidence to the oncology community to inform clinical decision‐making around issues involving COVID‐19 and cancer patients. 9 The programme was the first to demonstrate that chemotherapy can be safely delivered during the pandemic in UK cancer centres. 10 Further information can be found at www.ukcovidcancerprogramme.org.
Due to the clinical uncertainty around safe prescribing following COVID‐19 infection, the UKCCP has launched the COVID‐19 Safe Prescribing of Oncological Treatment (COV‐SPOT) initiative to provide definitive evidence to guide clinical decisions for COVID‐19‐positive patients undergoing anticancer treatment.
This prospective cohort study aims to:
Describe outcomes for cancer patients who test positive for COVID‐19 during treatment, with a focus on aforementioned areas of clinical uncertainty.
Describe COVID‐19 outcomes following cancer treatment restart, including worsening of symptoms, hospitalisation and mortality.
Determine whether certain types of treatment are safer to restart than others, such as immunotherapy, radiotherapy and targeted treatments, which are less immunosuppressive than chemotherapy.
The COV‐SPOT project will be delivered through a UK‐wide multicentre service evaluation. Data collection, analysis and dissemination will be performed through a central research electronic data capture (REDCap) 10 platform, hosted by The Medical Sciences Division, University of Oxford. Sites wishing to partake should submit an expression of interest form at https://ukcovidcancerprogramme.org/cov-spot.
Our goal is to provide definitive, high‐quality data to support clinical decision‐making in cancer patients with COVID‐19 and limit the impact of the ongoing pandemic on cancer treatment.
AUTHOR CONTRIBUTIONS
Conceptualisation and visualisation: Lennard Lee, Catherine Harper‐Wynne. Writing—original draft: Nathan Appanna. Writing—editing: Nathan Appanna, Grisma Patel, Lennard Lee. Writing—review: All authors. Supervision and project administration: Lennard Lee, Catherine Harper‐Wynne. All authors were involved in decision to submit this work. The work reported in the article has been performed by the authors, unless clearly specified in the text.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
ACKNOWLEDGEMENTS
The authors would like to thank all patients, oncologists, physicians and healthcare staff working tirelessly on the frontlines of the COVID‐19 pandemic.
Nathan Appanna and Grisma Patel contributed equally to this work.
Catherine Harper‐Wynne and Lennard Y. W. Lee shared joint senior authorship.
REFERENCES
- 1. Pinato DJ, Patel M, Scotti L, et al. Time‐dependent COVID‐19 mortality in patients with cancer: an updated analysis of the OnCovid registry. JAMA Oncol. 2022;8:114‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee LYW, Cazier JB, Starkey T, et al. COVID‐19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Monin L, Laing AG, Muñoz‐Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID‐19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naranbhai V, St Denis KJ, Lam EC, et al. Neutralization breadth of SARS‐CoV‐2 viral variants following primary series and booster SARS‐CoV‐2 vaccines in patients with cancer. Cancer Cell. 2022;40:103‐108.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fendler A, de Vries EGE, GeurtsvanKessel CH, et al. COVID‐19 vaccines in patients with cancer: immunogenicity, efficacy and safety. Nat Rev Clin Oncol. 2022;19:385‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Plebani M. Persistent viral RNA shedding in COVID‐19: caution, not fear. EBioMedicine. 2021;64:103234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. NICE . Systemic Anticancer Treatments. COVID‐19 Rapid Guideline: Delivery of Systemic Anticancer Treatments . Guidance. NICE. https://www.nice.org.uk/guidance/ng161/chapter/3‐Systemic‐anticancer‐treatments. Accessed November 3, 2022.
- 8. Anil I, Arnold R, Benkwitz‐Beford S, et al. The UK coronavirus cancer monitoring project: protecting patients with cancer in the era of COVID‐19. Lancet Oncol. 2020;21:622‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Booth S, Curley HM, Varnai C, et al. Key findings from the UKCCMP cohort of 877 patients with haematological malignancy and COVID‐19: disease control as an important factor relative to recent chemotherapy or anti‐CD20 therapy. Br J Haematol. 2022;196:892‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee LYW, Cazier JB, Angelis V, et al. COVID‐19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919‐1926. [DOI] [PMC free article] [PubMed] [Google Scholar]


