Abstract
Aim
The study examined the associated adverse events following SARS‐CoV‐2 vaccination among healthcare workers during the first dose of the vaccine in the Northern Region of Ghana.
Design
The study was a cross‐sectional survey involving 463 healthcare workers.
Method
The data were collected using a structured questionnaire. The data were analysed descriptively, and binary logistics was performed using SPSS version 25.
Results
The mean age was 33.4 ± 9.7 years, the majority (43.6%) being ≤30 years and males (57.2%). The self‐reported prevalence of SARS‐CoV‐2 vaccine adverse events was 75.5%. Common systemic adverse events comprised headache (47.5%), dizziness (18.4%) and local adverse events included generalized body pains (44.0%) and abscess around the injection sites (11.2%). The study found a high prevalence of self‐reported SARS‐CoV‐2 vaccine adverse events involving both systemic and local adverse events. Our study gives useful information that can be used for public health‐targeted interventions to boost public confidence in SARS‐CoV‐2 vaccines.
Keywords: adverse events, first dose, healthcare workers, SARS‐CoV‐2, vaccine
1. INTRODUCTION
The SARS‐CoV‐2 pandemic has become a major global health challenge since its outbreak in 2019 (Nzaji et al., 2020; Tran et al., 2021). It has affected several sectors of life including health, social structure, trade and national economies (Amponsah & Frimpong, 2020). Existing evidence shows that the SARS‐CoV‐2 pandemic has resulted in 1.9 million mortalities and about 86.4 million cases globally (Shekhar et al., 2021). The impact of the pandemic differs in terms of prevalence and mortality across different countries, the economic and social burden appears similar across the globe (Asante & Mills, 2020; Danquah & Schotte, 2020; Lee et al., 2020; Lone & Ahmad, 2020; Makoni, 2020; Peng et al., 2020; Ren et al., 2020; Zhao et al., 2020). Indeed, social life came to a standstill as individuals, and communities could not celebrate achievements and anniversaries, and many could not go on holidays. The human suffering, economic and social challenges resulted in the implementation of SARS‐CoV‐2 preventive strategies such as social distancing, proper hand hygiene practices, use of face masks, self‐isolation and quarantine (Liu et al., 2020; Nzaji et al., 2020; Schmidt, 2020).
Vaccination is an effective way of halting the spread of SARS‐CoV‐2 and reducing associated complications (Fu et al., 2020; Nzaji et al., 2020; Shekhar et al., 2021). However, the acceptance of vaccines has been a global challenge in some settings (Adeniyi et al., 2021; Dzieciolowska et al., 2021; Elhadi et al., 2021). Adverse events following immunizations (AEFIs) have compounded the acceptability of the SARS‐CoV‐2 vaccine as individuals and groups who have rejected previous vaccines indicated fear of the side effects (Datta et al., 2017; Yenyi, 2019). SARS‐CoV‐2 AEFIs range from moderate symptoms such as swelling and pain at the site of injection, localized skin redness, raised body temperature, drowsiness, vomiting and irritability (Datta et al., 2017; Joshi et al., 2018) to serious life‐threatening conditions such as seizures, hypotonic hyporesponsive episodes (HHE), prolonged crying and thrombocytopenia and could result in hospitalization (Datta et al., 2017; Joshi et al., 2018). As at the conduct of this study, five vaccines, namely Pfizer, Oxford/AstraZeneca, Moderna, Janssen and Sputnik V (Agyekum et al., 2021; Jones & Roy, 2021), have been approved for use globally (Agyekum et al., 2021). These vaccines have reportedly gone through a series of clinical trials and considered safe (Dal‐Ré et al., 2021). The immunization of individuals against the virus with the existing vaccines is currently ongoing across the globe with variable acceptability reports. As at the time of this study, 742,349 persons have been vaccinated with AstraZeneca/Oxford vaccine in Ghana (Ghana Health Service, 2021). However, there were social media reports suggesting that some individuals might not accept the vaccine, and this could prevent Ghana from achieving the herds' immunity that the vaccinations aim to achieve. Post‐vaccine surveillance to identify AEFIs and educating populations about them has been recommended as a strategy for boosting public confidence in vaccinations (Joshi et al., 2018). The current study was aimed at examining the associated adverse events flowing SARS‐CoV‐2 vaccination among healthcare workers during the first dose of the vaccine in the Northern Region of Ghana.
2. MATERIALS AND METHODS
2.1. Study design and setting
The study was a cross‐sectional survey conducted between April–June 2021 in sixteen (16) districts in the Northern Region, Ghana. Data were collected prospectively across the 16 districts of the Region. The 16 districts were considered because the vaccination exercise took place in all the districts.
2.2. Study population and sample size
The study involved all healthcare workers who took the first dose of the SARS‐CoV‐2 Vaccine in the Northern Region. The definition of healthcare workers in this study included all individuals who work in the healthcare sector in the Northern Region, Ghana. They also involved persons who were directly or indirectly involved in the management of SARS‐CoV‐2 patients and have been vaccinated against the infection.
At a population size of 742,349, thus those who received the first dose of the SAR‐CoV‐2 Vaccine (Ghana Health Service, 2021), the minimum sample size required for this study at a 95% confidence interval and 5% margin of error was estimated to be 384 (Sample Size Calculator by Raosoft, Inc. 2021).
2.3. Inclusion/Exclusion criteria
The study considered all healthcare workers from the 16 districts of the Northern Region who took the first dose of the SARS‐CoV‐2 vaccine. The person must also consent to participate in the study.
2.4. Data collection process
An electronically designed questionnaire was employed to collect data. The questionnaire was developed using protocols described elsewhere (Twene & Yawson, 2018; Ulendorf et al., 2018). The questionnaire was designed into three subthemes, including: (1) Sociodemographic characteristics, (2) Adverse events following the SARS‐CoV‐2 vaccine and (3) Previous history of vaccine adverse events and existing medical conditions.
The contact information of health workers who took the first dose of the SARS‐CoV‐2 vaccine was retrieved from the SARS‐CoV‐2 vaccination team. Eligible participants were contacted through a phone call to ascertain their willingness to partake in the research. After the consent, first, a link was sent to the health workers who have WhatsApp numbers to respond to items on the questionnaire. To ensure that such individuals do not attempt the questionnaire more than once, the electronic questionnaire was designed such that the same device (mobile phone or computer) cannot submit the questionnaire twice. Second, health workers without WhatsApp numbers were contacted directly via phone call to attempt the items on the questionnaire with the help of researchers. Third, persons who were to attempt the questionnaire via email equally had the questionnaire sent to their mail.
To validate the data collection tool, it was subjected to a critical review by comparing it with previously reported studies on adverse events following immunizations (Smith et al., 2017; Twene & Yawson, 2018; Ulendorf et al., 2018).
2.5. Data analysis plan
The raw data collected were extracted from the Google form online platform onto Microsoft Excel File (version 2016), cleaned and later exported to SPSS (version 25) for further data management and analysis. First, the data were descriptively analysed and presented in frequencies, percentages, charts and tables. Second, a binary logistic regression was performed to test the level of associations and predict factors of SARS‐CoV‐2 vaccine AEFIs. A statistical level of p < .05 was considered significant.
2.6. Ethical consideration
Administrative permissions to conduct the study was sought and obtained from the Northern Regional and Districts Health Directorates. The study equally observed critically, the Helsinki Declaration on Ethical Principles for Medical Research Involving Human Subjects, specifically, declaration 1‐32 (Kong, 2013). The risk level of this study to the participants was low and could not cause any physical harm. However, we considered that individuals who truly experienced AEFIs may be distressed recounting their experiences. We, therefore, indicated that participants could opt out if they considered revisiting their experiences as distressful. Generally, we ensured that participants' autonomy was upheld by allowing them to personally decide to participate or not in the study after using the participant information to explain the aim, content, process, potential outcome and impact of the study. Consent was obtained verbally or by signing an electronically consent form. Participants had the liberty to withdraw from the study at any point in time of the study.
To protect the privacy of participants and maintain the confidentiality of their information, we did not solicit participants' identifiable information including email addresses, names or initials among others. The data were coded and not shared with any third party. Only the research team had access to the data generated in this study.
3. RESULTS
3.1. Sociodemographic characteristics
The study consisted of 463 healthcare workers. The majority of participants were ≤30 years (43.6%) with a mean age of 33.4 years (SD ± 9.7). Most of the study participants were males (57.2%), married (54.9%), had diploma (30.9%) and frontline workers (26.1) as shown in Table 1.
TABLE 1.
Sociodemographic characteristics
| Variable | Categories | Frequency | Percentage |
|---|---|---|---|
| Age (years) | Mean (SD) 33.4 (9.7) | ||
| ≤30 | 202 | 43.6 | |
| 31–40 | 185 | 40.0 | |
| 41–50 | 40 | 8.6 | |
| 51–60 | 26 | 5.6 | |
| 61≥ | 10 | 2.2 | |
| Gender | Female | 198 | 42.8 |
| Male | 265 | 57.2 | |
| Highest level of education | |||
| None | 59 | 12.7 | |
| Certificate | 108 | 23.3 | |
| Diploma | 143 | 30.9 | |
| Degree | 106 | 22.9 | |
| Masters | 42 | 9.1 | |
| PhD | 5 | 1.1 | |
| Marital status | |||
| Single | 200 | 43.2 | |
| Married | 254 | 54.9 | |
| Co‐habiting | 3 | 0.6 | |
| Divorce | 6 | 1.3 | |
| Type of health worker | |||
| None frontline health staff | 342 | 73.9 | |
| Frontline health staff | 121 | 26.1 | |
In this study, the majority (75.0%) of the participants had adverse reactions after taking the SARS‐CoV‐2 vaccine as shown in Figure 1.
FIGURE 1.
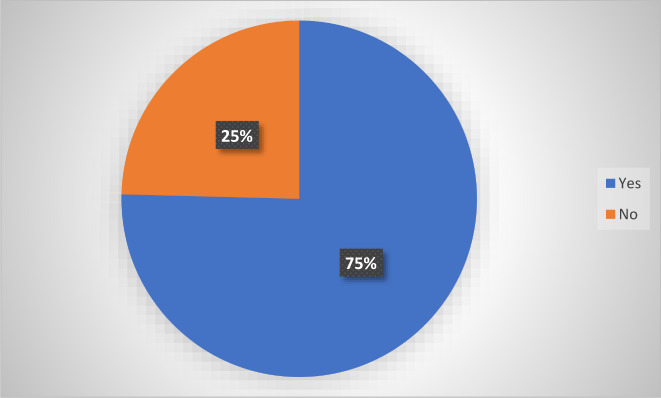
Prevalence of self‐reported adverse events following immunization SARS‐CoV‐2 vaccine
The common systemic adverse events comprised headache (47.5%), dizziness (18.4%), fever (temperature ≥ 38°C) (16.2%) and loss of appetite (11.2%) as shown in Figure 2. The common local adverse events reported included generalized body pains (44.0%), abscesses around the injection sites (11.2%) and rashes on the skin (5.2%) as shown in Figure 3.
FIGURE 2.
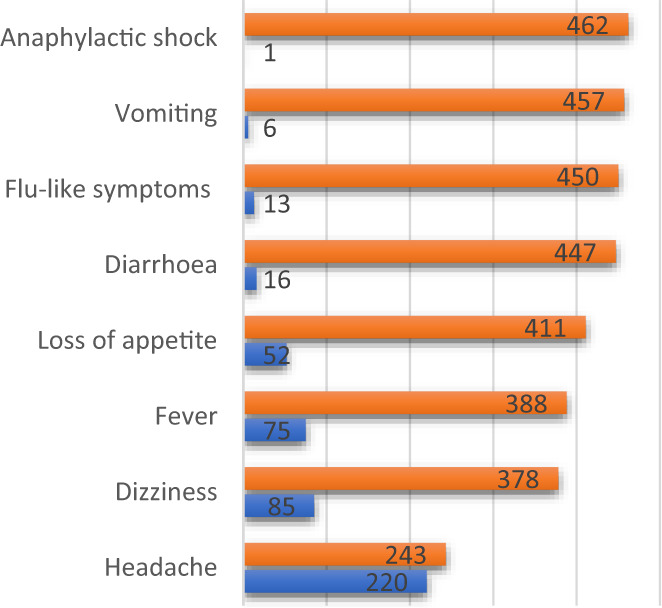
Systemic side effects
FIGURE 3.
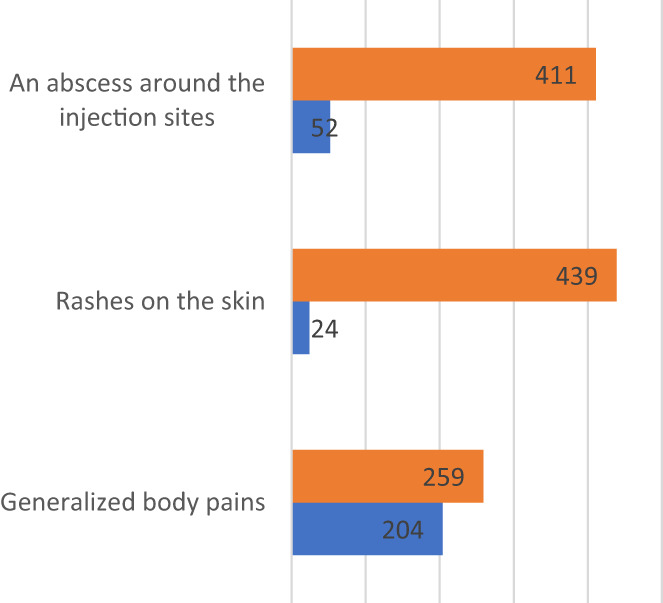
Local side effects
3.2. Adverse events following SARS‐CoV‐2 vaccination reporting practices
The majority (87.1%) of participants recorded these adverse effects or reactions within 24 hr. Out of the 75.4% of the participants who reported adverse events following immunization, only 30.1% reported these observed side effects to a health facility. With regard to counselling on how to manage adverse events, more than half (61.6%) were counselled. With the form of counselling given, 75.1% were told to report to the facility, 41.4% were counselled on home management of side effects and 4.6% were told to have enough rest as shown in Table 2.
TABLE 2.
Adverse events following SARS‐CoV‐2 vaccination reporting practices
| Variable | Categories | Frequency | Percentage |
|---|---|---|---|
| How long did it take you to see the side effect? | |||
| Within 24 hr | 304 | 87.1 | |
| After 24 hr | 45 | 12.9 | |
| Did you report the observed side effects or reactions to a health facility? | |||
| Yes | 104 | 30.1 | |
| No | 242 | 69.9 | |
| Were you advised on how to manage any observed side effects or reactions? | |||
| Yes | 178 | 61.6 | |
| No | 285 | 38.4 | |
| What form of counsel were you given? | |||
| Report any side effects to the health facility | 214 | 75.1 | |
| Manage side effects at home with iced cubes | 118 | 41.4 | |
| Take enough rest | 13 | 4.6 | |
3.3. History of vaccine‐related side effects and existing diseases
The majority (80.6%) of the participants have been immunized in the past with only 21.4% experiencing some side effects or adverse reactions. Most (61.6%) participants took the yellow fever vaccine, 21.0% took CSM, 41.1% took the hepatitis vaccine and 19.9% took tetanus. The side effect experienced by most participants in the past included: dizziness (100.0%), generalized body weakness (45.0%), headache (40.0%), fever (15.0%) and injection abscess (16.3%).
It was observed that 15.3% of the participants had a preexisting health disease such as hypertension (39.4%), ulcer (22.5%), diabetes (8.5%) and sickle cell disease (2.8%). Only 8.6% were on medication before taking the vaccine. About the second vaccination, 6.5% of the participants were not willing to take the next vaccination. The reasons for refusal of the next vaccination include: vaccines triggering other sicknesses (33.3%), severe discomfort (33.3%), vaccine not safe (20.0%), insomnia (10.0%) and diarrhoea (3.3%) as shown in Table 3.
TABLE 3.
History of vaccine‐related side effects and existing diseases
| Variable | Categories | Frequency | Percentage |
|---|---|---|---|
| Have you been immunized before? | |||
| Yes | 373 | 80.6 | |
| No | 90 | 19.4 | |
| Ever experience the side effect | |||
| Yes | 80 | 21.4 | |
| No | 293 | 78.6 | |
| What were the side effects? | |||
| Generalized body pains | 36 | 45.0 | |
| Rashes on the skin | 2 | 2.5 | |
| Headache | 32 | 40.0 | |
| Fever | 12 | 15.0 | |
| An abscess around the injection sites | 13 | 16.3 | |
| Anaphylactic shock | 1 | 1.3 | |
| Vomiting | 1 | 1.3 | |
| Diarrhoea | 2 | 2.5 | |
| Flu‐like symptoms | 3 | 3.8 | |
| Dizziness | 80 | 100.0 | |
| Loss of appetite | 7 | 8.8 | |
| What type of vaccines did you experience the side effects or reactions? | |||
| Yellow fever | 229 | 61.6 | |
| CSM | 78 | 21.0 | |
| Hepatitis | 153 | 41.1 | |
| Tetanus | 74 | 19.9 | |
| Did you have any preexisting disease? | |||
| Yes | 71 | 15.3 | |
| No | 392 | 84.7 | |
| What was the preexisting disease? | |||
| Sickle cell | 2 | 2.8 | |
| Hypertension | 28 | 39.4 | |
| Diabetes | 6 | 8.5 | |
| Ulcer | 16 | 22.5 | |
| Others | 19 | 26.8 | |
| Were you on any medication before taking the vaccine? | |||
| Yes | 40 | 8.6 | |
| No | 423 | 91.4 | |
| With your experience with the first vaccination, are you willing to take the next vaccination | |||
| Yes | 433 | 93.5 | |
| No | 30 | 6.5 | |
| Why will you not take the next vaccination? | |||
| Trigger other sicknesses | 10 | 33.3 | |
| Vaccine not safe | 6 | 20 | |
| Severe discomfort | 10 | 33.3 | |
| Insomnia | 3 | 10.0 | |
| Severe Diarrhoea | 1 | 3.3 | |
Note: Flu‐like symptoms: running nose, cough and chills.
3.4. Factors influencing COVID‐19 vaccine AEFIs
Study participants who were ≤40 years were 53% less likely to experience an adverse reaction after the SARS‐CoV‐2 vaccine as compared with those more than 40 years (aOR; 0.47, 95% CI [0.24–0.92], p = .027). Males were 45% less likely as compared with females to experience adverse reactions after the SARS‐CoV‐2 vaccine (aOR; 0.55, 95% CI [0.32–0.92], p = .03). Study participants who were on medications for other existing health conditions were 1.6 times more likely to experience an adverse reaction after the SARS‐CoV‐2 vaccine compared with those who were not on any medication before the vaccination (aOR; 1.6, 95% CI [0.50–5.04], p = .43). Those without any existing disease were 59% less likely to experience adverse reactions after the SARS‐CoV‐2 vaccine compared with those with preexisting disease (aOR; 0.41, 95% CI [0.15–5.04], p = .049).
Participants with no history of side effects of immunization were 36% less likely to experience an adverse reaction after the SARS‐CoV‐2 vaccine compared with those with side effects following immunization (aOR; 0.64, 95% CI [0.34–1.23], p = .18) as shown in Table 4.
TABLE 4.
Factors influencing SARS‐CoV‐2 vaccine AEFIs
| Variables | Categories | aOR | 95% CI | p‐Value |
|---|---|---|---|---|
| Age | Over 40 years | Ref | ||
| 40 years and below | 0.47 | 0.24–0.92 | .027 | |
| Gender | Females | Ref | ||
| Males | 0.55 | 0.32–0.94 | .03 | |
| Were you on medication for other existing health conditions? | ||||
| Yes | Ref | |||
| No | 1.60 | 0.50–5.04 | .43 | |
| Did you have any preexisting diseases? | ||||
| Yes | Ref | |||
| No | 0.41 | 0.15–5.04 | .049 | |
| Ever experienced the side effects of immunization? | ||||
| Yes | Ref | |||
| No | 0.64 | 0.34–1.23 | .18 | |
Note: Significance of bold values are p < .05.
4. DISCUSSION
The need to enumerate the adverse events associated with the SARS‐CoV‐2 vaccination is essential in this era of continuous increase in infections, mutation of original strains and depletion of immunity of vaccinated individuals (Kaya & Pirincci, 2021; Nilsson et al., 2021; Sampath et al., 2021). We, therefore, examined the associated adverse events following SARS‐CoV‐2 vaccination among healthcare workers during the first dose of the vaccine in the Northern Region of Ghana and found a high (75.5%) prevalence of self‐reported SARS‐CoV‐2 vaccine adverse events. Adverse events following the administration of medication for either preventive purposes or curative purposes have been associated with reduced compliance with the full regimen of the medication (Adeniyi et al., 2021; Chen et al., 2021; Yenyi, 2019). It is therefore important that vaccine manufacturers and immunologists continue to improve the quality, especially the SARS‐CoV‐2 vaccines to reduce the current high rates of adverse events associated with the vaccine. Our study participants reported both local and systemic SARS‐CoV‐2 vaccine adverse events as reported in another study (Menni et al., 2021). Although media (CDC, 2021) and other scientific studies (Azimi et al., 2021; Jeon et al., 2021; Kaya & Pirincci, 2021) have reported fatal adverse events following the SARS‐CoV‐2 vaccination, the participants in this study reported simple to moderate adverse local and systemic events following their vaccination. SARS‐CoV‐2 vaccine adverse events have been characterized as mild and moderate if they do not cause life‐threatening and permanent disability including hospitalization and death (Hu et al., 2021; No et al., 2013). The reports of this study participants include common systemic adverse events comprising headache, dizziness, fever (temperature ≥ 38°C) and loss of appetite, and local adverse events reported included generalized body pains, abscesses around the injection sites and rashes on the skin. Like other studies, SARS‐CoV‐2 vaccine‐associated systemic adverse events are reported to include fatigue, headache, malaise, arthralgia, chills, fever, nausea/vomiting and diarrhoea, and local symptoms such as tenderness, pain, redness, swelling (Jeon et al., 2021; Menni et al., 2021). These symptoms though not considered as severe by the participants could cause discomfort and alter daily routines such as absence from work due to some participants' inability to carry out their routine activities with the symptoms. Important in the findings of this study is the indication by most participants reported adverse events to health facilities, with non‐hospitalization. Indeed some previous studies (Jeon et al., 2021; Joshi et al., 2018; Tozzi et al., 2013) reported that most individuals who experienced mild or moderate vaccine adverse events had their symptoms resolved without direct physical interventions. Like our study, other studies reported that participants who did not receive any physical therapy for their symptoms received counselling on home management such as having enough bed rest (Jeon et al., 2021).
An understanding of the age and gender‐related risk of the SARS‐CoV‐2 vaccine is essential in customizing health education strategies and physical interventions that are both receptive and compatible with the individuals' dynamics. We, therefore, investigated participants' risk for SARS‐CoV‐2 vaccine adverse events and found that individuals ≤40 years were 47% less likely to experience an adverse reaction after injection with the SARS‐CoV‐2 vaccine as compared with those more than 40 years. Similarly, though significant males were less likely to have adverse events post‐SARS‐CoV‐2 vaccination. The age‐related risk of SARS‐CoV‐2 vaccine adverse events appears to be more common in older individuals, as this has equally been reported in a study (Menni et al., 2021). In the latter, it is reported that individuals aged ≤55 years were nearly two times more likely to sustain at least a systemic SARS‐CoV‐2 vaccine adverse event compared with individuals aged 55 > years (Menni et al., 2021). Contrary to our study’s finding that males were 55% less likely to react to the SARS‐CoV‐2 vaccine as compared with females, Menni et al. (2021) found that females had increased odds of experiencing SARS‐CoV‐2 vaccine adverse events compared with males. Although it is not out of place to obtain these contradictory findings in different studies, it might be very useful to review the factors that contributed to these differences to ensure that literature that is reported for public use is free of uncertainties that could mislead practitioners when they seek to apply evidence to practice.
The current study’s findings show that few of the participants were on medication before the SARS‐CoV‐2 vaccination, however, were 1.6 times more likely to experience an adverse reaction after the SARS‐CoV‐2 vaccination compared with those who were not on any medication before the vaccination (aOR; 1.6, 95% CI [0.50–5.04], p = .430). Considering that global health agencies aim for herd immunity to limit the continuous mutation of the virus and fluctuating infection rates with fatal outcomes, the need to take action to promote the uptake of the SARS‐CoV‐2 vaccine is critical. It is therefore important for the SARS‐CoV‐2 vaccine task force to establish the use of medications among individuals that may have additive or potentiated effectiveness to the SARS‐CoV‐2 vaccine, as our findings suggest that individuals with no known disease were 41% less likely to experience adverse reactions after the SARS‐CoV‐2 vaccine compared with persons with preexisting disease (aOR; 0.41, 95% CI [0.15–5.04], p = .049). Besides, individuals with no history of side effects of immunization were 64% less likely to experience an adverse reaction after the SARS‐CoV‐2 vaccine compared with those with side effects following immunization (aOR; 0.64, 95% CI [0.34–1.23], p = .180). These findings may be particularly important in our context (Ghana), for the SARS‐CoV‐2 vaccination exercise currently ongoing. It will be important to target persons with preexisting disease conditions for the SARS‐CoV‐2 vaccine under close monitoring, due to their risk of dying as a result of SARS‐CoV‐2 infection. To boost public interest and confidence in the SARS‐CoV‐2 vaccine, persons without existing disease conditions and a history of previous immunization adverse events could also be targeted, as they have less tendency to report SARS‐CoV‐2 vaccine adverse events.
Another important finding in our study is the indication of a greater proportion (93.5%) of study participants' willingness to take the second dose of the SARS‐CoV‐2 vaccine, regardless of the reported SARS‐CoV‐2 vaccine adverse events. This might explain the appreciation of the importance of the SARS‐CoV‐2 vaccine among study participants. Their willingness to take the second dose of the SARS‐CoV‐2 vaccine may also be influenced by their background (healthcare workers). Suggestively, if persons are made to appreciate the level of risk and the importance of the SARS‐CoV‐2 vaccine may influence their willingness to accept the vaccine. A call for all health practitioners to increase vaccine education among the public. Among the few participants who indicated an unwillingness to take the second dose of the vaccine, attributed reasons comprised the tendency of the vaccine to trigger other sicknesses, severe discomfort, vaccine not being safe, insomnia and diarrhoea. These findings are consistent with previous studies reports on SARS‐CoV‐2 vaccine hesitancy among the public (Agyekum et al., 2021; Butler et al., 2015; Dal‐Ré et al., 2021; Dubé et al., 2014; Fu et al., 2020; Shekhar et al., 2021; Tran et al., 2021). Addressing the assigned reasons by participants through public health education on vaccine safety and expected mild to moderate adverse events combined with its management may be a step towards increasing vaccine uptake and expelling predetermined misconceptions.
5. STUDY STRENGTHS AND LIMITATIONS
A strength of our study comprised the fact that the SARS‐CoV‐2 vaccine adverse events of the participants were captured in real time prospectively, more likely to be devoid of recall bias. A limitation of the study is the use of self‐reported data, subjective to the participants' judgement. Additionally, our study participants were a part of the population (healthcare workers) and may not be representative of the population, therefore generalizability may be a challenge. Comparable with other studies elsewhere reporting similar systemic and local SARS‐CoV‐2 vaccine adverse events, our study can be relied on for public health interventional purposes.
6. CONCLUSION
Our study found a high prevalence of self‐reported SARS‐CoV‐2 vaccine adverse events involving both systemic and local adverse events. Regardless of the SARS‐CoV‐2 vaccine adverse events, a greater number of participants indicated willingness for the second dose of the vaccine. Our study gives useful information that can be used for public health‐targeted interventions to boost public confidence in SARS‐CoV‐2 vaccines.
AUTHORS CONTRIBUTIONS
Authors EKD, MNA and IAW conceived the study. Author EKD designed the study protocol in collaboration with IAW, YNA and TA. Author EKD wrote the first draft in collaboration with RNN, YNA, MWK and ABB. All authors (EKD, MNA, IAW, RNN, YNA, MKW, ABB, TA and TMM) contributed significantly to the Data collection. Authors MNA and IAW performed the data analysis. Authors MNA, MKW, TA and TMM performed the critical review. The final manuscript was read and approved by all authors.
FUNDING INFORMATION
No funding was received for this study.
CONFLICT OF INTEREST
The authors declared no competing interest.
ETHICS STATEMENT
An informed consent was sought from participants prior to recruitment onto the study. The confidentiality and privacy of participants were respected. An administrative permission to conduct the study was sought and obtained from the Northern Regional and Districts Health Directorates. The study equally observed critically, the Helsinki Declaration on Ethical Principles for Medical Research Involving Human Subjects, specifically, declaration 1 to 32 (Kong, 2013).
ACKNOWLEDGEMENT
Appreciation goes to the Northern Regional Health Directorate (Ghana Health Service) and participants for participating in the study.
Dzantor, E. K. , Asumah, M. N. , Inusah, A.‐W. , Nukpezah, N. R. , Agyeman, Y. N. , Kukeba, M. W. , Braimah, B. A. , Adjeso, T. , & Tahiru, M. M. (2023). Adverse events reported after first dose of SARS‐CoV‐2 vaccine in the Northern Region of Ghana. Nursing Open, 10, 1785–1793. 10.1002/nop2.1438
DATA AVAILABILITY STATEMENT
Data will be made available upon request from the corresponding author (via edzantor21pg@sph.uhas.edu.gh/edemdzantor2011@gmail.com).
REFERENCES
- Adeniyi, O. V. , Stead, D. , Singata‐Madliki, M. , Batting, J. , Wright, M. , Jelliman, E. , Abrahams, S. , & Parrish, A. (2021). Acceptance of COVID‐19 vaccine among the Healthcare Workers in the Eastern Cape, South Africa: A cross sectional study. Vaccine, 9(6), 666. 10.3390/vaccines9060666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agyekum, M. W. , Afrifa‐Anane, G. F. , Kyei‐Arthur, F. , & Addo, B. (2021). Acceptability of COVID‐19 vaccination among health care workers in Ghana. Advances in Public Health, 2021, 1–8. 10.1155/2021/9998176 [DOI] [Google Scholar]
- Amponsah, R. , & Frimpong, I. A. (2020). Ghana in the Face of COVID‐19 : Economic Impact of Coronavirus (2019‐NCOV) Outbreak on Ghana. March , 1404–1411. 10.4236/ojbm.2020.84089 [DOI]
- Asante, L. A. , & Mills, R. O. (2020). Exploring the socio‐economic impact of COVID‐19 pandemic in marketplaces in Urban Ghana. Africa Spectrum, 55, 170–181. 10.1177/0002039720943612 [DOI] [Google Scholar]
- Azimi, M. , Dehzad, W. M. , Atiq, M. A. , Bahain, B. , & Asady, A. (2021). Adverse effects of the COVID‐19 vaccine reported by lecturers and staff of Kabul University of Medical Sciences, Kabul, Afghanistan. Infection and Drug Resistance, 14, 4077–4083. 10.2147/IDR.S332354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, R. , MacDonald, N. E. , Eskola, J. , Liang, X. , Chaudhuri, M. , Dube, E. , Gellin, B. , Goldstein, S. , Larson, H. , Manzo, M. L. , Reingold, A. , Tshering, K. , Zhou, Y. , Duclos, P. , Guirguis, S. , Hickler, B. , & Schuster, M. (2015). Diagnosing the determinants of vaccine hesitancy in specific subgroups: The Guide to Tailoring Immunization Programmes (TIP). Vaccine, 33(34), 4176–4179. 10.1016/j.vaccine.2015.04.038 [DOI] [PubMed] [Google Scholar]
- CDC . (2021). (Vaccine Adverse Event Reporting System (VAERS) . Available from: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vaers/index.html [last accessed 04 June 2021].
- Chen, M. , Yuan, Y. , Zhou, Y. , Deng, Z. , Zhao, J. , Feng, F. , Zou, H. , & Sun, C. (2021). Safety of SARS‐CoV‐2 vaccines: A systematic review and meta‐analysis of randomized controlled trials. Infectious Diseases of Poverty, 10(1), 1–12. 10.1186/s40249-021-00878-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal‐Ré, R. , Stephens, R. , & Sreeharan, N. (2021). Let me choose my COVID‐19 vaccine. European Journal of Internal Medicine, 87(2021), 104–105. 10.1016/j.ejim.2021.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danquah, M. , & Schotte, S. (2020). COVID‐19 and the socioeconomic impact in Africa: The case of Ghana. WIDER Background Note 2020/5, June.
- Datta, A. , Baidya, S. , Das, S. , Mog, C. , & Datta, S. (2017). Assessment of mother’s knowledge and practices regarding adverse events following immunization of their children in a rural area of Tripura. National Journal of Community Medicine, 8(4), 159–163. [Google Scholar]
- Dubé, E. , Gagnon, D. , Nickels, E. , Jeram, S. , & Schuster, M. (2014). Mapping vaccine hesitancy‐Country‐specific characteristics of a global phenomenon. Vaccine, 32(49), 6649–6654. 10.1016/j.vaccine.2014.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzieciolowska, S. , Hamel, D. , Gadio, S. , Dionne, M. , Gagnon, D. , Robitaille, L. , Cook, E. , Caron, I. , Talib, A. , Parkes, L. , Dubé, È. , & Longtin, Y. (2021). Covid‐19 vaccine acceptance, hesitancy, and refusal among Canadian healthcare workers: A multicenter survey. American Journal of Infection Control, 49(2021), 1152–1157. 10.1016/j.ajic.2021.04.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhadi, M. , Alsoufi, A. , Alhadi, A. , Hmeida, A. , Alshareea, E. , Dokali, M. , Abodabos, S. , Alsadiq, O. , Abdelkabir, M. , Ashini, A. , Shaban, A. , Mohammed, S. , Alghudban, N. , Bureziza, E. , Najah, Q. , Abdulrahman, K. , Mshareb, N. , Derwish, K. , Shnfier, N. , … Msherghi, A. (2021). Knowledge, attitude, and acceptance of healthcare workers and the public regarding the COVID‐19 vaccine: A cross‐sectional study. BMC Public Health, 21(1), 1–21. 10.1186/s12889-021-10987-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, C. , Wei, Z. , Pei, S. , Li, S. , Sun, X. , & Liu, P. (2020). Acceptance and preference for COVID‐19 vaccination in health‐care workers (HCWs). MedRxiv, 2962(548), 1–35. 10.1101/2020.04.09.20060103 [DOI] [Google Scholar]
- Ghana Health Service . (2021). COVID‐19 Vaccine Update . https://www.ghs.gov.gh/covid19/ [last accessed 19 July 2021].
- Hu, R. , Peng, S. , Liu, Y. , Tang, F. , Wang, Z. , Zhang, L. , Gao, J. , & Guo, H. (2021). The characteristics and trend of adverse events following immunization reported by information system in Jiangsu province, China, 2015–2018. BMC Public Health, 21(1), 1–8. 10.1186/s12889-021-11387-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon, M. , Kim, J. , Oh, C. E. , & Lee, J. Y. (2021). Adverse events following immunization associated with coronavirus disease 2019 vaccination reported in the mobile vaccine adverse events reporting system. Journal of Korean Medical Science, 36(17), 1–8. 10.3346/jkms.2021.36.e114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, I. , & Roy, P. (2021). Sputnik V COVID‐19 vaccine candidate appears safe and effective. The Lancet, 397(10275), 642–643. 10.1016/S0140-6736(21)00191-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi, J. , Das, M. K. , Polpakara, D. , Aneja, S. , Agarwal, M. , & Arora, N. K. (2018). Vaccine safety and surveillance for Adverse Events Following Immunization (AEFI) in India. Indian Journal of Pediatrics, 85(2), 139–148. [DOI] [PubMed] [Google Scholar]
- Kaya, F. , & Pirincci, E. (2021). Determining the frequency of serious adverse reactions of inactive SARS‐COV‐2 vaccine. Work, 69(3), 735–739. 10.3233/WOR-210473 [DOI] [PubMed] [Google Scholar]
- Kong, H. W. S. (2013). ETHICAL PRINCIPLES FOR Scientific Requirements and Research Protocols. Wma, October 29, 1975–32. https://www.wma.net/policies‐post/wma‐declaration‐of‐helsinki‐ethical‐principles‐for‐medical‐research‐involving‐human‐subjects/
- Lee, H. , Jun Moon, S. , Ossak Ndombi, G. , Kim, K.‐N. , Berhe, H. , & Woo Nam, E. (2020). COVID‐19 perception, knowledge, and preventive practice: Comparison between South Korea, Ethiopia, and Democratic Republic of Congo. African Journal of Reproductive Health, 24(2), 66–85. 10.29063/ajrh2020/v24i2s.11 [DOI] [PubMed] [Google Scholar]
- Liu, M. , Cheng, S. Z. , Xu, K. W. , Yang, Y. , Zhu, Q. T. , Zhang, H. , Yang, D. Y. , Cheng, S. Y. , Xiao, H. , Wang, J. W. , Yao, H. R. , Cong, Y. T. , Zhou, Y. Q. , Peng, S. , Kuang, M. , Hou, F. F. , Cheng, K. K. , & Xiao, H. P. (2020). Use of personal protective equipment against coronavirus disease 2019 by healthcare professionals in Wuhan, China: Cross sectional study. The BMJ, 369, 6–11. 10.1136/bmj.m2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lone, S. A. , & Ahmad, A. (2020). COVID‐19 pandemic – An African perspective. Emerging Microbes and Infections, 9(1), 1300–1308. 10.1080/22221751.2020.1775132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makoni, M. (2020). COVID‐19 in Africa: Half a year later. The Lancet Infectious Diseases, 20(10), 1127. 10.1016/s1473-3099(20)30708-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menni, C. , Klaser, K. , May, A. , Polidori, L. , Capdevila, J. , Louca, P. , Sudre, C. H. , Nguyen, L. H. , Drew, D. A. , Merino, J. , Hu, C. , Selvachandran, S. , Antonelli, M. , Murray, B. , Canas, L. S. , Molteni, E. , Graham, M. S. , Modat, M. , Joshi, A. D. , … Spector, T. D. (2021). Vaccine side‐effects and SARS‐CoV‐2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. The Lancet Infectious Diseases, 21(7), 939–949. 10.1016/S1473-3099(21)00224-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson, L. , Csuth, Á. , Storsaeter, J. , Garvey, L. H. , & Jenmalm, M. C. (2021). Vaccine allergy: Evidence to consider for COVID‐19 vaccines. Current Opinion in Allergy and Clinical Immunology, 21(4), 401–409. 10.1097/ACI.0000000000000762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- No, D. , Date, F. A. , & No, I. V. (2013). Food and Drugs Authority Guidelines for Surveillance of Adverse Events Following. February, 1–59.
- Nzaji, M. K. , Ngombe, L. K. , Mwamba, G. N. , Ndala, D. B. B. , Miema, J. M. , Lungoyo, C. L. , Mwimba, B. L. , Bene, A. C. M. , & Musenga, E. M. (2020). Acceptability of vaccination against COVID‐19 Among Healthcare Workers in The Democratic Republic of the Congo. Pragmatic and Observational Research, 11, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Y. , Pei, C. , Zheng, Y. , Wang, J. , Zhang, K. , Zheng, Z. , & Zhu, P. (2020). Knowledge, attitude and practice associated with COVID‐19 among university students: A cross‐sectional survey in China . 10.21203/rs.3.rs-21185/v1 [DOI] [PMC free article] [PubMed]
- Raosoft . (2021). Sample size calculator by Raosoft, Inc . http://www.raosoft.com/samplesize.html.
- Ren, H. , Zhao, L. , Zhang, A. , Song, L. , Liao, Y. , Lu, W. , & Cui, C. (2020). Early forecasting of the potential risk zones of COVID‐19 in China’s megacities. Science of the Total Environment, 729, 138995. 10.1016/j.scitotenv.2020.138995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath, V. , Rabinowitz, G. , Shah, M. , Jain, S. , Diamant, Z. , Jesenak, M. , Rabin, R. , Vieths, S. , Agache, I. , Akdis, M. , Barber, D. , Breiteneder, H. , Chinthrajah, S. , Chivato, T. , Collins, W. , Eiwegger, T. , Fast, K. , Fokkens, W. , O’Hehir, R. E. , … Nadeau, K. C. (2021). Vaccines and allergic reactions: The past, the current COVID‐19 pandemic, and future perspectives. Allergy: European Journal of Allergy and Clinical Immunology, 76(6), 1640–1660. 10.1111/all.14840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, C. W. (2020). Lack of handwashing access: A widespread deficiency in the age of COVID‐19. Environmental Health Perspectives, 128(6), 1–2. 10.1289/EHP7493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar, R. , Sheikh, A. B. , Upadhyay, S. , Singh, M. , Kottewar, S. , Mir, H. , Barrett, E. , & Pal, S. (2021). COVID‐19 vaccine acceptance among health care workers in the United States. Vaccine, 9(2), 1–18. 10.3390/vaccines9020119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, L. E. , Amlôt, R. , Weinman, J. , Yiend, J. , & Rubin, G. J. (2017). A systematic review of factors affecting vaccine uptake in young children. Vaccine, 35(45), 6059–6069. 10.1016/j.vaccine.2017.09.046 [DOI] [PubMed] [Google Scholar]
- Tozzi, A. E. , Asturias, E. J. , Ram, M. , Halsey, N. A. , Law, B. , & Zuber, P. L. F. (2013). Assessment of causality of individual adverse events following immunization (AEFI): A WHO tool for global use. Vaccine, 31(44), 5041–5046. 10.1016/j.vaccine.2013.08.087 [DOI] [PubMed] [Google Scholar]
- Tran, V. D. , Pak, T. V. , Gribkova, E. I. , Galkina, G. A. , Loskutova, E. E. , Dorofeeva, V. V. , Dewey, R. S. , Nguyen, K. T. , & Pham, D. T. (2021). Determinants of COVID‐19 vaccine acceptance in a high infection‐rate country: A cross‐sectional study in Russia. Pharmacy Practice, 19(1), 2276. 10.18549/pharmpract.2021.1.2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twene, P. , & Yawson, A. (2018). Adverse Events Following Immunization (AEFI) reporting in a Rural District in Ghana. Postgraduate Medical Journal of Ghana, 7(2), 1–10. [Google Scholar]
- Ulendorf, S. , Valentiner‐branth, P. , & Mølbak, K. (2018). Examining determinants for reporting suspected adverse events following HPV vaccination in Denmark. Vaccine, 1–5, 6158–6162. 10.1016/j.vaccine.2018.08.061 [DOI] [PubMed] [Google Scholar]
- Yenyi, S. E. (2019). Influence of low rate of reporting of adverse events following immunization on immunization dropout. Public Health Education and Promotion Commons. https://scholarworks.waldenu.edu/dissertations [last accessed 1 June 2021]. [Google Scholar]
- Zhao, Z. , Li, X. , Liu, F. , Zhu, G. , Ma, C. , & Wang, L. (2020). Prediction of the COVID‐19 spread in African countries and implications for prevention and control: A case study in South Africa, Egypt, Algeria, Nigeria, Senegal and Kenya. Science of the Total Environment, 729, 138959. 10.1016/j.scitotenv.2020.138959 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon request from the corresponding author (via edzantor21pg@sph.uhas.edu.gh/edemdzantor2011@gmail.com).


