Abstract
Current knowledge related to employment of plasma exchange for treatment in COVID‐19 is predominantly based on casuistic evidence in the form of single‐center case‐series. Published data encompass reports from a total of approximately 225 patients. Based on published reports, therapeutic plasma exchange has generally been employed as rescue or adjunctive therapy in patients with critical illness, with proposed benefit attributed possible effects on mitigation and amelioration of cytokine storm syndrome. Treatment effects remain uncertain, with the impact of plasma exchange on critical illness in COVID inconclusive with regard to both efficacy and safety.
Keywords: COVID‐19, cytokine storm, plasma exchange
Abbreviations
- ASFA
American Society for Apheresis
- COVID‐19
Corona virus disease 2019
- IL
Interleukin
- RRT
Renal replacement therapy
- SARS‐CoV‐2
Severe acute respiratory syndrome due to coronavirus 2
- SOFA
Sequential organ function assessment
1. INTRODUCTION
Following the emergence of a novel betacoronavirus in Wuhan, China in November–December 2019 (COVID‐19), dissemination of infection has led to a global pandemic, and despite drastic containment measures, the spread of infection, termed severe acute respiratory syndrome due to coronavirus 2 (SARS‐CoV‐2), is ongoing. Currently the number of reported patients with COVID‐19 and associated deaths are, as of August 2021, >200 000 000 and > 4 250 000, respectively.
1.1. SARS‐CoV‐2
A number of pathogenic human coronaviruses have been classified throughout the last century. Following the identification of human coronavirus 229E (HCoV‐229E) (classified in the genus Alphacoronavirus) and HCoV‐OC43 (Betacoronavirus lineage 2a member) in the 1960s, SARS‐CoV‐1 (Betacoronavirus lineage 2b member) in 2003, HCoV‐NL63 (Alphacoronavirus lineage 1b member) in 2004, HCoV‐HKU1 (Betacoronavirus lineage 2a member) in 2005, and Middle East respiratory syndrome coronavirus in 2012 (Betacoronavirus lineage 2c), the current coronavirus pandemic represents the seventh human coronavirus known associated with respiratory infection [1]. Phylogenetic analyses currently indicate that although differences exist, SARS‐CoV‐2 is closely related to SARS‐CoV‐1; specifically, sequencing has identified a 79.5% shared genomic identity including a common cellular entry pathway mediated by the angiotensin converting enzyme II receptor [1].
SARS‐CoV‐2 causes respiratory tract infection. The severity of symptoms varies from mild to severe pneumonia and from mild to severe acute respiratory distress syndrome. In early observations from China in patients receiving no evidence‐based treatment, >40% of COVID‐19 patients admitted to hospital with pneumonia developed acute respiratory distress syndrome with subsequent mortality >50% [2]. Currently, evidence‐based therapeutic options available for severe COVID‐19 remain limited [3], and current care is predominantly supportive based on supplementary oxygen, mechanical ventilation, renal replacement therapy, extracorporeal membrane oxygenation in addition to nonspecific intensive care. Nonetheless, patients continue to receive divergent antiviral drugs and immunomodulatory agents, albeit few interventions remain adequately tested within the context of a randomized clinical trial, and benefits remain limited, particularly for patients with severe disease affliction.
1.2. Severe COVID‐19
Viral infection with SARS‐CoV‐2 is mediated via a viral Spike protein with affinity for the membrane angiotensin‐2 receptor [1]. Infected cells permit viral replication with consequent immune‐mediated systemic response. Among adults, SARS‐CoV‐2 primarily induces respiratory illness, that is, endotheliitis [4], albeit illness may afflict a number of other organs. A minority of patients progress to severe COVID‐19 characterized by acute respiratory distress syndrome with accompanying multi‐organ failure leading to requirement of intensive care support. Plausibly, viral pulmonary infection leads to an acquired immune response characterized by anti‐CoV‐2 antibody formation with subsequent induction of immune complexes as supported by the appearance of viral antibodies within 1–2 weeks following disease presentation [5]. Antibody sequencing of plasma from convalescent patients reveal potent antibodies directed against viral surface spike protein receptor binding domains; however, clinical implications with regard to the relationship of viral antigen and specific antibody type remain uncertain [5]. Nonetheless, a subset of vulnerable patients plausibly develop an antibody response leading to induction of soluble circulating immune complexes and local complement activating immune complexes in the vascular‐endothelial and alveolar‐epithelial layer with subsequent microvascular coagulation disturbance, alveolar edema and hemorrhage, and invasion of pulmonary space with white blood cells. Unsurprisingly, severe COVID‐19 has been shown to be associated with elevations in ferritin, erythrocyte sedimentation rate, C‐reactive protein, lactate dehydrogenase, tumor necrosis factors, interleukins, and other cytokines, with a particular predilection for elevation of D‐dimer and thrombocytopenia in patients with critical illness [6].
The significance of observed pro‐inflammatory disturbance in severe COVID‐19 remains debated. Overall, pro‐inflammatory cytokines and multiple chemokines including tumor necrosis factor alpha, interleukin 1β and ‐6, granulocyte‐colony stimulating factor, are all substantially elevated in COVID‐19 patients [6]. Arguably, loss of cytokine equilibrium following antibody‐mediated immune activation in the lungs leads to a systemic disturbance with implications for cytokine‐ and coagulation‐mediated end‐organ damage as supported by the lack of definitive evidence of SARS‐CoV‐2 in histopathologic studies on patients with severe acute respiratory distress syndrome [7]. A pronounced release of vasoactive mediators, that is, a cytokine release syndrome or storm has been proposed in patients with severe COVID‐19 [6], with particular focus on the implications of elevated interleukin‐6 levels due to associated negative effects on vasculature tone and membrane permeability, and possible contributory hyperviscosity leading to disturbances in coagulation and thrombosis [6]. Although uncertain, that is, the suggested mechanism remains unverified, the benefit observed with corticosteroids for treatment of severe COVID‐19 imparts support to a role of hyperinflammation and immunomodulation in the pathogenesis of COVID‐19.
1.3. Plasma exchange and mitigation of hypercytokinemia
Therapeutic plasma exchange entails removal of whole blood from patients, separation into components and subsequent removal and replacement of plasma. The procedure enables reduction of plasma components, that is, circulating antibodies, proteins, and inflammatory mediators. Therapeutic plasma exchange has long played a successful role in the depletion of injurious immunoglobulins and immune complexes across a diverse spectrum of diseases [8]. As such, plasma exchange continues to be proposed as a potential rescue therapy for attenuation of hyperinflammatory states including cytokine release syndrome. Historically, therapeutic plasma exchange has been evoked for treatment of divergent states of critical illness including disseminated intravascular coagulation, septic shock, and recently for rescue therapy in patients with acute respiratory distress syndrome due to pH1N1 influenza [9]. The rationale of plasma exchange is an overall reduction in circulating pro‐ and anti‐inflammatory cytokines and circulating mediators in hopes of mitigating complications associated with hyperinflammation. Accordingly, plasma exchange has been shown to reduce cytokine and chemokine levels, albeit clinical implications remain uncertain [10, 11, 12]. However, the efficacy of plasma exchange for amending hypercytokinemia remains debated, with results overall inconclusive [8]. Nonetheless, proposed possible benefit of plasma exchange in cytokine release syndrome includes clearance of antifibrinolytic mediators and fibrin degradation products, amelioration of injurious levels of free radicals and reduction in viscous components leading to mitigation of hyperviscosity; overall leading to normalization of pro‐ and anti‐inflammatory equilibrium with stabilization of endothelial membranes and disturbances in the coagulation pathway [8].
2. METHODS
The presented review was performed completed in accordance with the PRISMA protocol.
2.1. Information sources
A systematic search was conducted employing PubMed to search for published articles pertaining to use of plasma exchange for treatment in patients with COVID‐19 with no limits in timeframe or language. Last update was on August 20, 2021. The search strategy included the following terms: “COVID‐19,” “SARS‐CoV‐2,” “Plasma exchange,” and “Plasmapheresis” with supplementary manual searches in the reference list of the included studies.
2.2. Eligibility criteria
All titles and abstracts of retrieved articles were evaluated for eligibility. Relevant articles were read to determine final eligibility. Eligible studies fulfilled the following preset criteria: (1) Original data related to employment of plasmapheresis in COVID‐19; (2) ≥3 cases minimum; and (3) Outcomes reported. Titles and abstracts were reviewed with secondary evaluation of relevant full texts of eligible articles before final inclusion.
2.3. Data extraction
Data from articles were extracted and recorded in a tabulated fashion.
2.4. Quality assessment
Quality of each publication was evaluated with assessment of study design, number of patients, intervention, and outcomes.
3. RESULTS
From a pool totaling 257 records identified, a total of 20 articles met the eligibility criteria. In total 228 cases published related to employment of plasmapheresis for treatment of COVID‐19. A flow chart depicting data identification and inclusion is provided in Figure 1. The majority of published data was presented as single center case series (n = 15) [10, 11, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25]. Two records were multicenter cases series [26, 27], one record was a retrospective propensity‐matched cohort study [28], one record was a single‐center pilot study [12], and one record was a single‐center open‐label randomized clinical trial [29]. All patients were attributed critical illness, with respiratory failure specifically denoted in the majority of records (n = 14) [10, 11, 12, 13, 14, 17, 18, 19, 22, 23, 24, 25, 27, 29]. Other supplementary inclusion criteria included cytokine release syndrome (n = 1) [28], hyperviscosity (n = 1) [20], neutralizing antibodies against type I interferons (n = 1) [16], severe autoimmune meningoencephalitis (n = 1) [15], and nonspecific severe COVID‐19 (n = 2) [21, 26].
FIGURE 1.
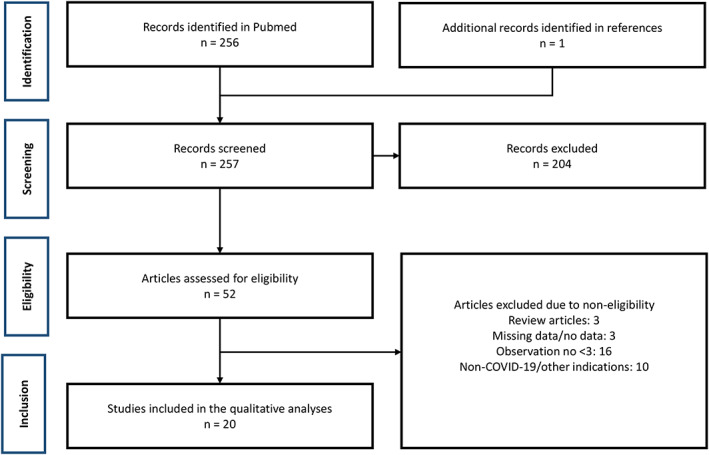
Flow chart depicting data inclusion
Three records related data from patients in Iran and the United states, respectively [10, 11, 13, 14, 20, 25]; two records related data from China, Saudi Arabia, and Turkey, respectively [12, 15, 21, 23, 26, 29]; with the remaining records relating data from France, Germany, Japan, Oman, Pakistan, Spain, the United Arab Emirates, and the United Kingdom [16, 17, 18, 19, 22, 24, 27, 28].
Plasmapheresis was performed in 1–9 sessions, plasma volumes ranged from 1.0 to 1.5, and replacement fluids varied between 0.9% saline, fresh frozen plasma, convalescent plasma, and Octaplas LG.
Laboratory effects pertaining to inflammatory markers was reported in 14 studies [10, 11, 14, 17, 18, 20, 21, 22, 23, 24, 25, 26, 27, 29], with reported decreases in levels of C‐reactive protein, Interleukin (IL)‐6, IL‐8, IL‐10, Tumor necrosis factor‐α, D‐dimer, Ferritin, neutrofile‐to‐lymphocyte ratio, Pro‐calcitonin, fibrinogen, and von Willebrand Factor. One study reported decreased hyperviscosity [20], and one study reported increased pro‐thrombin time and lymphocyte count [21]. Of note, decreased C‐reactive protein, D‐dimer and IL‐6 levels were also observed in the randomized clinical trial among patients in the control arm [29]. An overview of all studies reporting effects of treatment on inflammatory markers is provided in Table 1.
TABLE 1.
Inflammatory outcomes of COVID‐19 patients treated with plasma exchange
| Author | Published | Design | Population | n | Treatment (number of sessions, plasma volume, replacement fluid) | Outcomes |
|---|---|---|---|---|---|---|
| Gluck et al. | December 2020 | Single center, case series | Requirement of supplemental oxygen or mechanical ventilation | 10 | Five sessions, 1.0 plasma volumes, 5% Human albumin or FFP | Decreased C‐reactive protein, IL‐6, IL‐8, IL‐10 and TNF‐α |
| Luo et al. | May 2020 | Single center, case series | Severe COVID‐19 | 3 | NA, NA, NA | Decrease in C‐reactive protein and IL‐6 and increase in Prothrombin time and Lymphocyte count |
| Fernandez et al. | December 2020 | Single center, case series | Critical illness with pneumonia | 4 | Two to six sessions, 1.2 plasma volumes, 5% Human albumin | Decreased levels TNF‐α, IL‐6, G‐CSF, Ferritin and D‐dimer |
| Zhang et al. | May 2020 | Single center, case series | Acute respiratory distress | 3 | One session, 3000 ml volume, FFP | Reduced neutrofile‐to‐lymphocyte ratio |
| Faqihi et al. | April 2021 | Single‐center open‐label, randomized clinical trial | Mechanical ventilation and multi‐organ failure | PLEX: 43 Control: 44 | One to five sessions, 1.0–1.5 plasma volumes, FFP or Octaplas LG | PLEX: Decreased Ferritin, C‐reactive protein, D‐dimer, and IL‐6 Controls: Decreased C‐reactive protein, D‐dimer and IL‐6 |
| Gucyetmez et al. | August 2020 | Multi‐center, case series | Critical illness, ICU admission | 18 | Three sessions, NA, NA | Decreased D‐dimer, Ferritin, IL‐6, C‐reactive protein and Pro‐calcitonin |
| Jaiswal et al. | January 2021 | Multi‐center, case series | Respiratory failure and acute respiratory distress or shock | 14 | One session, 30–40 ml/kg plasma volume, convalescent plasma | Decreased C‐reactive protein |
| Truong et al. | December 2020 | Single‐center, case series | Critical illness and hyperviscosity | 6 | Two to three sessions, NA, NA | Decreased plasma viscosity, fibrinogen, D‐dimer, and C‐reactive protein |
| Arulkumaran et al. | November 2020 | Single‐center, case series | Respiratory failure | 5 | Five sessions, 3000 ml plasma volume, Octaplas LG | Reduced levels of vWF, Ferritin, D‐dimer, and Fibrinogen |
| Roshandel et al. | April 2021 | Single center, case series | Acute respiratory distress and mechanical ventilation | 5 | Two sessions, 1500–2000 ml, 0.9% saline with 5% Human albumin and FFP | Reduced levels of C‐reactive protein and IL‐6 |
| Hashemian et al. | December 2020 | Single‐center, case series | Acute respiratory distress | 15 | NA, 40 ml/kg plasma volume, 5% Human albumin and 0.9% saline or FFP | Decreased TNF‐α, IL‐6, Ferritin, C‐reactive protein |
| Keith et al. | September 2020 | Single‐center, case series | Sepsis, acute respiratory distress and multi‐organ failure | 8 | Two to seven sessions, 1.0 plasma volume, FFP | Decrease in C‐reactive protein and Ferritin |
| Khamis et al. | June 2020 | Single‐center, case‐series | Acute respiratory distress and multi‐organ failure | 11 | Five sessions, hematocrit‐adjusted plasma volume, FFP | Decreased IL‐6, C‐reactive protein, D‐dimer and Ferritin |
| Morath et al. | August 2020 | Single center, case series | Respiratory failure | 5 | One to two sessions, median 3.4 L plasma volume, FFP | Reduced levels of C‐reactive protein, IL‐6, Ferritin, and D‐dimer |
Abbreviations: FFP, Fresh frozen plasma; G‐CSF, Granulocyte colony‐stimulating factor; ICU, Intensive care unit; IL, Interleukin; NA, Not available; PLEX, Plasma exchange; TNF, Tumor necrosis factor; vWF, Von Willebrand Factor.
A total of 14 studies reported on mortality outcomes [11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 26, 27, 28, 29], with mean mortality 20.2% with a range from 9.1% to 50%. An overview of studies reporting mortality outcomes is provided in Table 2. Notably, 35‐day mortality was nonsignificantly reduced in the intervention arm of the randomized clinical trial (20.9% vs. 34.1%, p = 0.582) [29], with post hoc analyses demonstrating an overall significant decrease in Sequential Organ Function Assessment (SOFA) score in patients receiving plasma exchange (p < 0.05); benefit was however limited to day 7 (p = 0.001) and day 14 (p = 0.03), with no significant difference observed at day 35 (SOFA score 1 [IQR 0.5–1.5] and 0.5 [IQR 0.0–1.0], p = 0.07). Comparably, mortality benefit was demonstrated in the retrospective propensity‐matched cohort study (9.9% vs. 38.5%, p < 0.01) based on propensity score and covariate matching with subsequent evaluation of outcomes using Kaplan–Meier and Cox proportional hazards modeling. Notably, only 63.4% of cases were included in the matched model with no women present in the final cohort [28].
TABLE 2.
Mortality outcomes in COVID‐19 patients treated with plasma exchange
| Author | Published | Design | Population | n | Treatment | Outcomes |
|---|---|---|---|---|---|---|
| Adeli et al. | May 2020 | Single‐center, case series | Respiratory distress or mechanical ventilation | 8 | Three to five sessions, 2000 ml plasma volume, 500 ml 5% Human albumin, 4 units FFP, and 0.9% saline | In‐hospital mortality 12.5% |
| Keith et al. | September 2020 | Single‐center, case series | Sepsis, acute respiratory distress, and multi‐organ failure | 8 | Two to seven sessions, 1.0 plasma volume, FFP | In‐hospital‐mortality 25% |
| Hashemian et al. | December 2020 | Single‐center, case series | Acute respiratory distress | 15 | NA (every second day), 40 ml/kg plasma volume, 5% Human albumin and 0.9% saline or FFP | In‐hospital mortality 40% |
| Dogan et al. | May 2020 | Single center, case series | COVID‐19 related autoimmune meningoencephalitis | 6 | Six to nine sessions, NA, 5% Human albumin | In‐hospital mortality 16.7% |
| Gucyetmez et al. | August 2020 | Multi‐center, case series | Critical illness, ICU admission | 18 | Three sessions, NA, NA | In‐hospital mortality 16.7% |
| Faqihi et al. | December 2020 | Single‐center, pilot study | Mechanical ventilation and multi‐organ failure | 10 | Five to seven sessions, 1.0–1.5 plasma volumes, 5% Human albumin or FFP | 28‐day mortality 10% |
| de Prost et al. | January 2021 | Single‐center, case series | Critical illness and neutralizing antibodies against type I interferons | 4 | Three to four sessions, NA, NA | In‐hospital mortality 50% |
| Khamis et al. | June 2020 | Single‐center, case series | Acute respiratory distress and multi‐organ failure | 11 | Five sessions, hematocrit‐adjusted plasma volume, FFP | Mortality 9.1% |
| Morath et al. | August 2020 | Single center, case series | Respiratory failure | 5 | One to two sessions, median 3.4 L plasma volume, FFP | Mortality 40% |
| Matsushita et al. | December 2020 | Single‐center, case series | Respiratory distress or mechanical ventilation | 5 | Three to seven sessions, 2500–3000 ml plasma volumes, FFP | 30‐day mortality 40% |
| Faqihi et al. | April 2021 | Single‐center open‐label, randomized clinical trial | Mechanical ventilation and multi‐organ failure | PLEX: 43 Controls: 44 | One to five sessions, 1.0–1.5 plasma volumes, FFP or Octaplas LG | 35‐day mortality: PLEX 20.9% Controls 11.4%, p = 0.572 |
| Truong et al. | December 2020 | Single‐center, case series | Critical illness and hyperviscosity | 6 | Two to three sessions, NA, NA | In‐hospital mortality 33% |
| Jaiswal et al. | January 2021 | Multi‐center, case series | Respiratory failure with acute respiratory distress or shock | 14 | One session, 30–40 ml/kg plasma volume, convalescent plasma | 28‐day mortality 28.6% |
| Kamran et al. | January 2021 | Retrospective propensity‐score matched cohort | Cytokine release syndrome (male only) | PLEX: 45 Controls: 45 | NA (once daily), 1.5 plasma volumes, FFP and 0.9% saline 2:1 | All‐cause mortality: PLEX 9.9% Matched controls 38.5%, p < 0.001 |
| 198 | Mortality: 20.2% |
Abbreviations: FFP, Fresh frozen plasma; ICU, Intensive care unit; NA, Not available; PLEX, Plasma exchange.
Overall, treatment‐related adverse reactions were sporadically reported (n = 4). Importantly, no treatment‐related allergic reactions, fever, coagulopathy, cardiac and/or renal failure were observed in patients randomized to plasma exchange, and incidence of hospital‐acquired infections was comparable between groups (p = 0.85) [29].
4. DISCUSSION
As shown, our current understanding of the potential benefit and harm of plasma exchange in patients with critical illness due to COVID‐19 is based on a total of 20 publications encompassing 228 cases. Overall, evidence as to the efficacy of plasma exchange remains inadequate, with the vast majority of published data deriving from small case‐based series. As such, the effects of plasma exchange have only been evaluated in one randomized clinical trial with passing benefit on organ dysfunction but no significant benefit on mortality [29]. Overall, although plasma exchange seems well tolerated, evidence remains wholly inconclusive with regard to efficacy and safety.
4.1. Plasma exchange and COVID‐19
Therapeutic plasma exchange has previously been employed for adjunctive or rescue therapy in a variety of infectious diseases, with therapy aimed at potential mitigation and amelioration of disease complications rather than treatment of the infectious disease itself. The American Society for Apheresis (ASFA) guidelines suggests (grade 2b, weak recommendation) possible use of therapeutic plasma exchange in patients with sepsis and multi‐organ failure [8]. In accordance with the guideline, employment of plasma exchange remains an option in patients with critical illness due to COVID‐19. Proposed benefit of treatment is based on a perceived mitigation of cytokine release syndrome through direct removal of toxic substances and viral particles with concomitant stabilization of endothelial membrane function and coagulation.
A number of studies have proposed a close association of respiratory failure with cytokine levels in severe COVID‐19 [6, 30]. Specifically, blood levels of a number of various cytokines including granulocyte colony‐stimulating factor, tumor necrosis factor‐α, and interleukin 6 have been implicated in outcomes related to COVID‐19 [6], leading to a focus on treatments permitting possible mitigation of the host immune response. Plasma exchange has repeatedly been shown to decrease circulating levels of divergent cytokines in COVID‐19. As such, treatment is associated with decreases in both acute phase reactants such as C‐reactive protein, ferritin, and pro‐calcitonin [10, 11, 14, 17, 21, 22, 25, 27, 29], and reduction in cytokine and chemokine levels including IL‐6, IL‐8, IL‐10, Tumor necrosis factor‐α, and granulocyte colony‐stimulating factor [10, 17, 18, 21, 22, 25, 26]. The implications on clinical outcomes, however, remain uncertain, particularly given the uncertainties regarding the impact of nonspecific removal of both circulating pro‐ and anti‐inflammatory cytokines.
The impact of treatment on clinical outcomes, specifically mortality, remains indeterminate. Overall mortality was approximately 20%, with the majority of reported mortality rates representing in‐hospital mortality. Rates did however range from approximately 10% to 50%. Comparisons of mortality rates between patients treated with plasmapheresis and nontreated patients were only provided in two studies [28, 29]. Crude mortality rate of patients with acute respiratory distress due to COVID‐19 is currently estimated to be 28.0% (IQR 13.9%–53.6%) based on pooled data [2]. Considering the predominance of non‐randomized data, the divergent mortality rates reported plausibly reflect deviating selection biases rather than genuine treatment‐associated benefit or harm. Notably, marked benefit was reported in a retrospective propensity score matched study [28]. Interpretations of proposed study benefit are however uncertain due to possible nonexchangeability (only approximately 60% of cases were matched in the cohort), nonadjustment for residual confounding secondary to indication bias, issues related to sparseness of data, and the nonemployment of conditional methods for evaluating outcomes. As such, the current best estimate of possible treatment effect stems from a single‐center open‐label randomized trial prematurely terminated due to perceived mitigation of the SARS‐CoV‐2 outbreak in Saudi Arabia [29]. In total 87 patients with critical illness due to COVID‐19 were randomized to either standard care with therapeutic plasma exchange (maximum five consecutive daily sessions, early termination in patients with clinical improvement) or standard care alone om a 1:1 ratio. Although plasma exchange is reported to be associate with lower 35‐day mortality (20% vs. 34%, p = 0.09), reported survival is discrepant, with Kaplan–Meier survival curves, Cox proportional hazards ratios, and reported survival rates in Table 3 of the published manuscript indicating a 35‐day survival from intensive care admission of 88.6% (39 of 44 patients) and 79.1% (34 of 43 patients) in the control and intervention arm, that is, 35‐day mortality rates of 11.4% and 20.9% (p = 0.582), respectively [29].
As such, current results remain inconclusive as to the possible benefit of therapeutic plasma exchange. Both due to paucity of emphatic data supporting benefit, but also due possible negative effects related to nonselective removal of beneficiary plasma constituents including viral antibodies. The issue remains uncertain, although preliminary trials using convalescent plasma as replacement fluid have been reported, and as randomized clinical trial is ongoing (Clinical trials id NCT04634422).
5. CONCLUSIONS
Existing knowledge related to use of plasma exchange in COVID‐19 is chiefly based on published case‐/series‐based evidence from approximately 225 patients. Plasma exchange remains predominantly employed as rescue or adjunctive therapy in patients with critical illness, with proposed benefit due to mitigation and amelioration of cytokine storm syndrome. The impact of plasma exchange on critical illness in COVID‐19 has however only been evaluated in one randomized clinical trial, and evidence remains wholly inconclusive with regard to efficacy and safety: albeit treatment is published materials seems well tolerated and safe given the condition of treated patients.
CONFLICT OF INTEREST
No conflicts of interest to declare.
ETHICS STATEMENT
Systematic reviews do not require pre‐existent ethical approval in Danish law.
Carlson N, Szpirt WM. Use of therapeutic plasma exchange in COVID‐19: A systematic review of current evidence. Ther Apher Dial. 2022;26(S1):3–11. 10.1111/1744-9987.13779
Funding information The systematic review was completed without support of any external funding, that is, no funding to declare.
REFERENCES
- 1. Zhou P, Yang X‐L, Wang X‐G, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hasan SS, Capstick T, Ahmed R, Kow CS, Mazhar F, Merchant HA, et al. Mortality in COVID‐19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta‐analysis. Expert Rev Respir Med. 2020;14(11):1149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. RECOVERY Collaborative Group , Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med. 2021;384(8):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383(2):120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee Y‐L, Liao C‐H, Liu P‐Y, Cheng C‐Y, Chung M‐Y, Liu C‐E, et al. Dynamics of anti‐SARS‐Cov‐2 IgM and IgG antibodies among COVID‐19 patients. J Infect. 2020;81(2):e55–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moore JB, June CH. Cytokine release syndrome in severe COVID‐19. Science. 2020;368(6490):473–4. [DOI] [PubMed] [Google Scholar]
- 7. Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y, et al. Kidney biopsy findings in patients with COVID‐19. J Am Soc Nephrol. 2020;31(9):1959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Padmanabhan A, Connelly‐Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, et al. Guidelines on the use of therapeutic apheresis in clinical practice ‐ evidence‐based approach from the writing Committee of the American Society for apheresis: the eighth special issue. J Clin Apher. 2019;34(3):171–354. [DOI] [PubMed] [Google Scholar]
- 9. Patel P, Nandwani V, Vanchiere J, Conrad SA, Scott LK. Use of therapeutic plasma exchange as a rescue therapy in 2009 pH1N1 influenza A‐an associated respiratory failure and hemodynamic shock. Pediatr Crit Care Med. 2011;12(2):e87–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gluck WL, Callahan SP, Brevetta RA, Stenbit AE, Smith WM, Martin JC, et al. Efficacy of therapeutic plasma exchange in the treatment of penn class 3 and 4 cytokine release syndrome complicating COVID‐19. Respir Med. 2020;175:106188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hashemian SM, Shafigh N, Afzal G, Jamaati H, Tabarsi P, Marjani M, et al. Plasmapheresis reduces cytokine and immune cell levels in COVID‐19 patients with acute respiratory distress syndrome (ARDS). Pulmonology. 2020;S2531‐0437(20):30254–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Faqihi F, Alharthy A, Alodat M, Kutsogiannis DJ, Brindley PG, Karakitsos D. Therapeutic plasma exchange in adult critically ill patients with life‐threatening SARS‐CoV‐2 disease: a pilot study. J Crit Care. 2020;60:328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adeli SH, Asghari A, Tabarraii R, Shajari R, Afshari S, Kalhor N, et al. Therapeutic plasma exchange as a rescue therapy in patients with coronavirus disease 2019: a case series. Pol Arch Intern Med. 2020;130(5):455–8. [DOI] [PubMed] [Google Scholar]
- 14. Keith PD, Scott LK, Weaver KE, Day M, Choe C, Perkins L, et al. Treatment of critically ill coronavirus disease 2019 patients with adjunct therapeutic plasma exchange: a single‐center retrospective case series. Crit Care Explor. 2020;2(9):e0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dogan L, Kaya D, Sarikaya T, Zengin R, Dincer A, Akinci IO, et al. Plasmapheresis treatment in COVID‐19‐related autoimmune meningoencephalitis: case series. Brain Behav Immun. 2020;87:155–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Prost N, Bastard P, Arrestier R, Fourati S, Mahévas M, Burrel S, et al. Plasma exchange to rescue patients with autoantibodies against type I interferons and life‐threatening COVID‐19 pneumonia. J Clin Immunol. 2021;41(3):536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khamis F, Al‐Zakwani I, Al Hashmi S, Al Dowaiki S, Al Bahrani M, Pandak N, et al. Therapeutic plasma exchange in adults with severe COVID‐19 infection. Int J Infect Dis. 2020;99:214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morath C, Weigand MA, Zeier M, Speer C, Tiwari‐Heckler S, Merle U. Plasma exchange in critically ill COVID‐19 patients. Crit Care. 2020;24(1):481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsushita Y, Kusaoi M, Hiki M, Murayama G, Abe Y, Nozawa K, et al. Combination therapy with plasma exchange and glucocorticoid may be effective for severe COVID‐19 infection: a retrospective observational study. Ther Apher Dial. 2021;25(4):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Truong AD, Auld SC, Barker NA, Friend S, Wynn AT, Cobb J, et al. Therapeutic plasma exchange for COVID‐19‐associated hyperviscosity. Transfusion. 2021;61(4):1029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luo S, Yang L, Wang C, Liu C, Li D. Clinical observation of 6 severe COVID‐19 patients treated with plasma exchange or tocilizumab. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(2):227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fernandez J, Gratacos‐Ginès J, Olivas P, Costa M, Nieto S, Mateo D, et al. Plasma exchange: an effective rescue therapy in critically ill patients with coronavirus disease 2019 infection. Crit Care Med. 2020;48(12):e1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang L, Zhai H, Ma S, Chen J, Gao Y. Efficacy of therapeutic plasma exchange in severe COVID‐19 patients. Br J Haematol. 2020;190(4):e181–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arulkumaran N, Thomas M, Brealey D, Alwan F, Singh D, Lunn M, et al. Plasma exchange for COVID‐19 thrombo‐inflammatory disease. EJHaem. 2020. 10.1002/jha2.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roshandel E, Sankanian G, Salimi M, Jalili A, Salari S, Sadeghi A, et al. Plasma exchange followed by convalescent plasma transfusion in COVID‐19 patients. Transfus Apher Sci. 2021;60:103141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gucyetmez B, Atalan HK, Sertdemir I, Cakir U, Telci L, COVID‐19 Study Group . Therapeutic plasma exchange in patients with COVID‐19 pneumonia in intensive care unit: a retrospective study. Crit Care. 2020;24(1):492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jaiswal V, Nasa P, Raouf M, Gupta M, Dewedar H, Mohammad H, et al. Therapeutic plasma exchange followed by convalescent plasma transfusion in critical COVID‐19‐an exploratory study. Int J Infect Dis. 2021;102:332–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kamran SM, Mirza Z‐E‐H, Naseem A, Liaqat J, Fazal I, Alamgir W, et al. Therapeutic plasma exchange for coronavirus disease‐2019 triggered cytokine release syndrome; a retrospective propensity matched control study. PLoS One. 2021;16(1):e0244853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Faqihi F, Alharthy A, Abdulaziz S, Balhamar A, Alomari A, AlAseri Z, et al. Therapeutic plasma exchange in patients with life‐threatening COVID‐19: a randomised controlled clinical trial. Int J Antimicrob Agents. 2021. May;57(5):106334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jose RJ, Manuel A. COVID‐19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8(6):e46–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


