Abstract
Curcumin is a safe, non‐toxic, readily available and naturally occurring compound, an active constituent of Curcuma longa (turmeric). Curcumin could potentially treat diseases, but faces poor physicochemical and pharmacological characteristics. To overcome these limitations, we developed a stable, water‐soluble formulation of curcumin called cyclodextrin‐complexed curcumin (CDC). We have previously shown that direct delivery of CDC to the lung following lipopolysaccharides exposure reduces acute lung injury (ALI) and effectively reduces lung injury, inflammation and mortality in mice following Klebsiella pneumoniae. Recently, we found that administration of CDC led to a significant reduction in angiotensin‐converting enzyme 2 and signal transducer and activator of transcription 3 expression in gene and protein levels following pneumonia, indicating its potential in treating coronavirus disease 2019 (COVID‐19). In this review, we consider the clinical features of ALI and acute respiratory distress syndrome (ARDS) and the role of curcumin in modulating the pathogenesis of bacterial/viral‐induced ARDS and COVID‐19.
Keywords: ALI, ARDS, COVID‐19, curcumin, NF‐κB, pneumonia
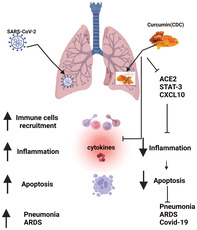
The schematics represent the potential mechanisms by which CDC effectively protects against ARDS/COVID‐19. Antiviral curcumin against SARS‐CoV‐2 mediated by distracting the ACE2, which prevents the entry of the virus into the cells. CDC induces antiviral responses by positively repressing the expression of ACE2, Nrf2, STAT‐3 and C–X–C motif chemokine 10 (CXCL10). CDC mediates immunomodulatory responses by inhibiting inflammation, cytokines, apoptosis and oxidative stress, therefore mitigating the progression to KP/ARDS following SARS‐CoV‐2 infection. CDC, cyclodextrin‐complexed curcumin; ARDS, acute respiratory distress syndrome; coronavirus disease 2019, COVID‐19; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; ACE2, angiotensin‐converting enzyme 2; Nrf2, nuclear factor erythroid 2 related factor 2; STAT‐3, signal transducer and activator of transcription 3; KP, Klebsiella pneumoniae
1. INTRODUCTION
Curcumin is a yellow compound produced by plants of the Curcuma longa (turmeric) species. 1 It is the principal curcuminoid of Curcuma longa, a member of the ginger family, Zingiberaceae. 2 , 3 It is sold as an herbal supplement, cosmetic ingredient, food flavouring and colouring. It has various medicinal preparations common in Ayurveda and Chinese medicine. The medicinal properties of curcumin have been known for thousands of years; however, the exact mechanism(s) of action and bioactive components, many of which have yet to be determined, have only recently been investigated. 2 , 3 , 4 Curcuminoids have been approved by the United States Food and Drug Administration (FDA) 2 and good tolerability and safety profiles have been shown by clinical trials. 3 , 5 According to the National Center for Complementary and Integrative Health, turmeric is generally safe, but consuming it in high doses or for extended periods may upset your stomach. Gupta et al 2 reported the safety of curcumin at doses as high as 12 g/day over 3 months in humans.
Curcumin, a polyphenol, has been shown to target multiple signalling molecules while also demonstrating activity at the cellular level, which is most likely implicated in its numerous health benefits. 2 , 3 , 6 It has been shown to aid in metabolic syndromes, 7 pain 8 and inflammatory conditions. 9 , 10 The diverse effects of curcumin result from its action on a wide range of cellular targets, including transcription factors, inflammatory cytokines, growth factors, apoptotic proteins and more. 11 , 12 With so many varied biological targets, curcumin elicits numerous pleiotropic effects, and this renders it therapeutically advantageous because many pathological disease states involve more than one signalling pathway, receptor protein/enzyme, or gene. 13
Curcumin has received worldwide attention for its multiple health benefits, which appear to act primarily through its antioxidant and anti‐inflammatory mechanisms. 10 These benefits are best achieved when curcumin is combined with agents such as piperine, which increases its bioavailability significantly. 3 It may also help manage exercise‐induced inflammation and muscle soreness and, as a result, enhance recovery and subsequent performance in inactive people. Because of its unique molecular chemical structure and functional groups, curcumin may bind with and either inhibit or activate a variety of endogenous biomolecules, including enzymes, receptors, signalling molecules, metals, transcription factors and even specific proteins located in cell membranes. 13 , 14
Curcumin represents one of the most diverse therapeutic agents yet isolated from natural sources. 2 , 10 , 11 For example, curcumin can act as a potent immunomodulatory agent that can modulate the activation of T cells, B cells, macrophages, neutrophils, natural killer (NK) cells and dendritic cells. 8 , 15 Curcumin can also downregulate the expression of various proinflammatory cytokines, including tumour necrosis factor (TNF), interleukin (IL)‐1, IL‐2, IL‐6, IL‐8, IL‐12 15 , 16 and chemokines most likely through inactivation of the transcription factor nuclear factor‐κB (NF‐κB). 17 Curcumin has also been shown to activate host macrophages and NK cells and modulate lymphocyte‐mediated functions. 18
Notably, most curcumin studies in humans have been in populations with existing health problems. Studies on healthy people can be challenging because of less immediate and measurable benefits if biomarkers are standard at baseline. Therefore, following subjects over time may provide the best insight into any potential health benefits for healthy people. However, the therapeutic application of curcumin is limited by its extremely low solubility in aqueous buffer, instability in body fluids and rapid metabolism. Nano‐delivery systems have shown excellent potential to improve the solubility, biocompatibility and therapeutic effect of curcumin. 19 Specifically, nanoparticles, including but not limited to liposomes, micelles, nanogels and niosomes, improve the bioavailability and therapeutic effects of curcumin such as in treating pulmonary tuberculosis using nanomicelles containing curcumin. 20 , 21 Moreover, nano‐curcumin, a powerful immunomodulatory agent, may help downregulate Th17 cell responses, lessen inflammation and accelerate patients' rehabilitation with coronavirus disease 2019 (COVID‐19). 22
Cyclodextrins (CDs) are cyclic oligosaccharides with a structure of a hollow truncated cone. Because of their hydrophilic outer surface and lipophilic cavity, CDs can solubilize hydrophobic drugs. To address the solubility concern, a novel curcumin formulation (cyclodextrin‐complexed curcumin (CDC)) was developed by complexing the compound with hydroxypropyl‐γ‐cyclodextrin (CD) 17 , 23 Specifically, curcumin was prepared at a concentration of 15 g/L. The solution was agitated, and after the complete dissolution of curcumin, the pH was adjusted to 6.0 with a mixture of hydrochloric acid and citric acid. The solution was sterile filtered, filled aseptically into sterile vials, capped and sealed. The recovered CDC solution contained 12 g/L curcumin and 93 g/L cyclodextrins in 20 mM sodium citrate and 100 mM NaCl solution. 23 This dramatically enhances water solubility and stability, facilitating direct pulmonary delivery. 24 Fluorescence microscopic examination revealed an association of curcumin with cells throughout the lung. 17 In vitro studies demonstrated that CDC increased curcumin's association with and transport across cultured human airway epithelial cells (Calu‐3) monolayers compared with uncomplexed curcumin solubilized using dimethyl sulfoxide or ethanol. 17 The pharmacokinetics of both curcumin and its principal metabolite (tetrahydrocurcumin) were assessed, leading to the discovery that tetrahydrocurcumin disappeared rapidly (undetectable after 30 min). 17 , 24 Saidi et al 23 reported that with improved bioavailability and stability, CDC has homogenous distribution in the hepatic tissue and was rapidly used by liver cells during preservation, indicating efficient uptake.
2. EFFECTS OF CURCUMIN ON NF‐κB
NF‐κB represents a family of transcription factors that regulate an extensive array of genes involved in different immune and inflammatory responses. 25 Accumulating evidence associates the transcription factor NF‐κB as a positive mediator of cell growth, but the molecular mechanism(s) involved in this process remains largely unknown. In addition, NF‐κB is critical in regulating the survival, activation and differentiation of innate immune cells and inflammatory T cells. 26 The activation of NF‐κB involves two major signalling pathways, the canonical and the non‐canonical. Both are important for regulating immune and inflammatory responses despite their differences in the signalling mechanism. 5 Canonical NF‐κB regulates CD4+ T‐cell differentiation by regulating cytokine production in innate immune cells and through T‐cell intrinsic mechanisms. The non‐canonical NF‐κB pathway is dispensable for naive T‐cell activation. 26
The activation of specific transcription factors, notably NF‐κB, and the consequent production of proinflammatory cytokines and other molecules comprise prominent features of inflammation. We have reported that reduced inflammation is reflected in the downregulation of NF‐κB activity. 17 In our experiments, the nuclear protein was isolated from the lungs of mice treated with lipopolysaccharide (LPS), and NF‐κB activity was determined in the presence and absence of CDC. The increased activity after LPS treatment was significantly reduced by CDC administration directly to the lungs as measured through protein and transcript levels. 17 We reported that the administration of CDC following LPS attenuated inflammation and injury and reduced activation of NF‐κBp65. 17 We also recently found that CDC administration significantly reduced the expression of NF‐κBp65 at both the transcript and protein levels following Klebsiella pneumoniae (KP). 24 Beyond our results regarding mice treated with LPS, CDC has been found to have numerous additional effects. The downstream impacts of NF‐κB are pleiotropic, regulating >200 genes involved in various cellular processes, including inflammation. 27 Previous studies show that curcumin reduces lung inflammation induced by influenza infection by inhibiting the NF‐κB signalling pathway. 28 Crucially, curcumin's downregulation of the NF‐κB pathway and anti‐inflammatory effect is dependent on the presence of peroxisome proliferator‐activated receptor γ. 26 , 29 Finally, further discussed below, NF‐κB and curcumin have important implications in the inflammasome activation cascade.
3. EFFECT OF CURCUMIN ON INFLAMMASOMES
Inflammasomes are cytosolic multiprotein complexes responsible for innate immunity, eventually resulting in cell death called pyroptosis. 26 Among the various inflammasomes, NOD‐like receptor pyrin domain‐containing 3 (NLRP3) is the most well‐characterized, activated in conditions of tissue damage, metabolic stress, reactive oxygen species (ROS) overload, inflammation and infection. 26 , 30 , 31 Puri et al 32 reported that NLRP3 inflammasome activation by mitochondrial ROS plays a critical role in the pathogenesis of exaggerated inflammation.
The NLRP3 complex consists of a sensor protein, an apoptosis‐associated speck‐like protein, a caspase recruitment domain (ASC) and a protease caspase 1. 33 , 34 Two different mechanisms can trigger NLRP3 activation. The first mechanism involves inflammatory bacterial products like LPS, which activate the NF‐κB pathway to induce NLRP3 and, consequently, pro–IL‐1β synthesis. 32 Second, some stimuli, such as nigericin, aluminium crystal and monosodium urate crystal (MSU), can activate the NLRP3 inflammasome and caspase 1 processing. 35 Curcumin can effectively suppress NLRP3 inflammasome activation and IL‐1β secretion by regulating NF‐κB signalling. In particular, the remarkable ability of curcumin to suppress inflammation is specific to the NLRP3 inflammasome. 35 , 36
The NLRP3 inflammasome has been shown to play a critical role in the pathogenesis of viral diseases. NF‐κB mediates NLRP3 activation in sterile and microbially induced inflammation. 37 Specifically, NF‐κB primes the NLRP3‐inflammasome for activation by inducing pro‐IL‐1β and NLRP3 expression. 26 , 31 Curcumin inhibits the NLRP3 inflammasome and reduces phosphorylation of NF‐κB subunits (p65 and p50), inhibiting the degradation of nuclear factor of kappa light polypeptide gene enhancer in B‐cells inhibitor α (IκBα) and ultimately inhibiting inflammation. 37 In our previous finding, KP led to upregulation of NLRP‐3 inflammasome activity, likely through NF‐κB.
Additionally, recent studies emphasise the critical role of the NLRP3 inflammasome in the immunopathogenesis of severe COVID‐19, especially in patients with increased risk (e.g. diabetes and obesity). Activation of the inflammasome is likely to form a severe ‘cytokine storm,’ which causes acute respiratory distress syndrome (ARDS) and, ultimately, death. This result positions curcumin to potentially have a role in treating COVID‐19. Curcumin's effects on the inflammasome have also been characterized in various other conditions. Li et al 35 reported that curcumin could effectively ameliorate MSU crystal‐induced gouty arthritis through NLRP3 inflammasome mediation via inhibiting NF‐κB signalling in vitro and in vivo.
4. EFFECT OF CURCUMIN ON OTHER CRITICAL PATHWAYS
Curcumin's effects on molecular pathways extend beyond just NF‐κB and the NLRP3 inflammasome, including its impact on signal transducer and activator of transcription 3 (STAT‐3), hypoxia‐inducible factor 1‐α (HIF‐1α) and nuclear factor erythroid 2 related factor 2 (Nrf2) pathways. Briefly, signal transducers and activators of transcription (STAT) are molecular pathways involved in various biological processes such as cell proliferation and apoptosis. 38 Curcumin can affect the STAT signalling pathway in the induction of its therapeutic impacts. Curcumin can enhance anti‐inflammatory cytokines and improve inflammatory disorders such as colitis by targeting STAT signalling pathways. 2 , 11 A recent study shows that STAT‐3 can induce inflammatory responses during coronavirus infections. 39
Hypoxia‐inducible factor‐1 (HIF‐1) is a transcription factor that consists of two subunits, HIF‐1α and HIF‐1β. 40 , 41 Under hypoxic conditions, HIF‐1α is an adaptive system that regulates the transcription of multiple genes associated with growth, angiogenesis, proliferation, glucose transport, metabolism, pH regulation and cell death. 42 , 43 However, aberrant HIF‐1α activation contributes to the pathophysiology of several human diseases, such as cancer, ischemic cardiovascular disorders and pulmonary and kidney diseases. 44 A study showed that curcumin significantly decreases hypoxia‐induced HIF‐1α protein levels in hepatocellular carcinoma cells (HepG2). Moreover, curcumin suppressed the transcriptional activity of HIF‐1 under hypoxia, leading to a decrease in the expression of vascular endothelial growth factor (VEGF), a major HIF‐1 target angiogenic factor. Curcumin also blocked hypoxia‐stimulated angiogenesis in vitro and down‐regulated HIF‐1α and VEGF expression in vascular endothelial cells. 45
Nrf2 is an essential transcription factor that maintains the cell's redox balance state and reduces inflammation in varying adverse stresses. 46 Curcumin can target the Nrf2 signalling pathway to protect the cells against oxidative damage. 33 Accumulating data demonstrates that curcumin applies four distinct methods to stimulate the Nrf2 signalling pathway, including inhibition of kelch‐like ECH‐associated protein 1 (Keap1), affecting the upstream mediators of Nrf2, influencing the expression of Nrf2 and target genes and improving the nuclear translocation of Nrf2. 47
5. EFFECT OF CURCUMIN ON TREATING COVID‐19
COVID‐19 is a rapidly spread disease, leading to high mortality rates. COVID‐19, which created a public health emergency of global concern, is caused by a coronavirus belonging to the Coronaviridae family 48 , 49 and has been identified in several mammalian hosts, especially in humans and bats. The clinical manifestation of COVID‐19 ranges from asymptomatic upper respiratory tract infection to critical illness and pneumonia associated with ARDS. 50 COVID–19 causes severe complications, including pneumonia, ARDS and multi‐organ failure. The main risk factors associated with greater severity and mortality caused by COVID‐19 includes hypertension, diabetes mellitus, cardiovascular disease (CVD), advanced age and obesity. 51 , 52 , 53 Procoagulant and pro‐thrombotic events are recurrent in patients with COVID‐19 and can cause significant damage. The virus attaches to the human host target cell receptor, the angiotensin‐converting enzyme 2 (ACE2), to cause infection.
The pandemic years have witnessed a boom in the production and export of the humble underground stem called turmeric, along with a renewal of interest among the scientific community in the spice's therapeutic qualities, especially against COVID‐19. This coincides with the findings of existing studies. 39 , 40 , 41 We recently found that the CDC administration after KP significantly attenuated ACE2 and STAT‐3 expression protein and gene levels (unpublished data). Ultimately, because of CDC's promising effects on relevant markers, curcumin could be used as supportive therapy to treat ARDS and COVID‐19 disease to save lives.
Beyond the findings of our laboratory, several other studies have evaluated curcumin's potential to treat COVID through varying mechanisms. Modelling studies have shown that curcumin inhibits the virus‐receptor interaction in two ways, through the spike protein and the ACE2 receptor. 22 , 54 , 55 ACE2 is an enzyme located in various body parts, including alveolar epithelial cells of the lung, intestinal absorptive cells or enterocytes of the small intestine, venous endothelial cells of the kidney, endothelial cells of the heart and renal tubular epithelial cells. 56 , 57 ACE2 is also present in the lower respiratory tract of humans. 55 , 57 Therefore, potential drug therapies could target and block the cell surfacereceptors from binding coronavirus and activating specific cell signalling pathways, which help in viral replication. Hamed et al reported that nano‐curcumin might be an innovative therapeutic agent for COVID‐19 patients by regulating the inflammatory response. 22 , 58
Thennakoon et al recently reported that nanocellulose/polyvinyl alcohol/curcumin (CNC/PVA/curcumin) nanoparticles with enhanced drug loading properties were developed by the dispersion of nanocellulose in curcumin/polyvinyl alcohol aqueous medium. 59 The enhancement of curcumin's solubility will significantly improve drug loading in nanocellulose, and this curcumin presents a promising nano‐based approach for treating COVID‐19. 60 Silymarin, extracted from milk thistle, has a protective effect during lung injury because of its ability to decrease the production of nitric oxide and the infiltration of inflammatory cells. 61 Additionally, silymarin has recently been considered a potent inhibitor for ACE2, preventing its host‐cell entry. 62 Nemany et al recently found silymarin/curcumin‐loaded albumin nanoparticles coated with chitosan and used in a muco‐inhalable delivery system had anti‐inflammatory and anti‐COVID‐19 effects. 63 , 64
Studies on viral infections have shown overactive inflammasome and therefore, destructive and systemic inflammation in patients. 65 The NLRP3 inflammasome has been shown to play a critical role in the pathogenesis of viral diseases. 34 , 55 , 65 The proliferation of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in a wide range of cells can be combined with numerous observations of direct and indirect inflammasome activation by other coronaviruses.
6. EFFECT OF CURCUMIN IN LONG COVID‐19
Despite ongoing vaccination efforts, there is an urgent demand for safe and effective treatments to help reduce the debilitating effects of SARS‐CoV‐2 disease. The pathophysiology of COVID‐19 is highly heterogeneous, and the way COVID‐19 modulates the different systems in the host remains unknown. This complex and multifactorial response requires a comprehensive therapeutic approach. Curcumin has beneficial effects on the progression of inflammatory diseases because of its numerous action mechanisms: antiviral, anti‐inflammatory, anticoagulant, antiplatelet and cytoprotective. These features make it a promising therapeutic in the long adjuvant treatment of COVID‐19. 34 , 66 , 67
Pawar et al 54 reported that the administration of oral curcumin with piperine as symptomatic adjuvant therapy in COVID‐19 treatment could substantially reduce morbidity and mortality and ease the logistical and supply related burdens on the healthcare system. Curcumin could be a safe and natural therapeutic option to prevent post‐COVID thromboembolic events. 54 Vahedian‐Azimi et al 68 conducted a meta‐analysis in June 2021 to find studies assessing the effects of curcumin‐related compounds in mild to severe COVID‐19 patients. Six studies showed that curcumin supplementation led to a significant decrease in typical symptoms, duration of hospitalisation and deaths. Curcumin administration leads to a substantial reduction in proinflammatory cytokines, such as IL‐1β and IL‐6, with a significant increase in anti‐inflammatory cytokines, including IL‐10, IL‐35 and transforming growth factor alpha (TGF‐α). These findings suggested that curcumin exerts beneficial effects by partially restoring the proinflammatory/anti‐inflammatory balance during COVID‐19 infection. 68
Askari et al 69 reported that 46 outpatients with COVID‐19 disease were randomly allocated to receive two capsules of curcumin‐piperine for 14 days. There was a significant improvement in dry cough, sputum cough, ague, sore throat, weakness, muscular pain, headache and dyspnoea in curcumin‐piperine groups. 69 Kumar et al 70 reported that using curcumin, Piper Nigrum Piperine and catechin could cure and prevent COVID‐19 outbreaks and infection. Curcumin and piperine interact and form a π–π intermolecular complex, which enhances curcumin's bioavailability. Additionally, the molecules curcumin and catechin bind directly to the receptor binding domain of spike protein and ACE‐2 receptors of the host cell, inhibiting the virus entry in the host cell. 70
Nag et al 71 recently reported curcumin as a potential therapeutic candidate against the SARS‐CoV‐2 omicron variant among seven phytochemicals studied. It is reported to cause impaired heart function, lung injuries and increased C‐reactive protein levels in severely ill patients. 71 Kushwaha et al 72 reported that a nano curcumin‐based formulation (NCF) with improved bioavailability showed several holistic therapeutic effects, including myocardial protection, oedema prevention, anti‐inflammatory and antioxidant properties, and metabolic and mitochondrial homeostasis maintenance under hypoxic conditions. 72
Saber‐Moghaddam et al 73 reported that an open‐label, non‐randomised clinical trial evaluated the efficacy of nano curcumin oral formulation in 41 hospitalised patients with mild–moderate COVID‐19. Most symptoms, including fever and chills, tachypnea, myalgia and cough, resolved significantly faster in the curcumin group. 73 Thimmulappa et al 66 evaluated phytochemical curcumin for the treatment of COVID‐19 in a randomised clinical trial. They found that curcumin showed broad‐spectrum antiviral activity against enveloped viruses, and curcumin may suppress SARS‐CoV‐2 infection by directly modifying the spike protein and ACE2. Additionally, curcumin exerts immunomodulatory activity by blocking NF‐κB, inflammasome, high mobility group box 1 (HMGB1) and IL‐6‐driven inflammatory responses. 66
Nrf2 is a central transcription factor that regulates the antioxidant defence system and is considered a modifier for several inflammatory diseases. It has previously been reported that curcumin is a promising Nrf2 agonist, and the administration of curcumin activates the Nrf2 pathway in the lungs of mice. 66 , 74 Sabre‐Moghaddam et al 73 suggest that curcumin's anti‐inflammatory properties and inhibition of p21‐activated kinases (PAK1), activator protein 1 (AP1) and NF‐κB could be a potentially beneficial treatment for COVID‐19‐related ARDS. Therefore, curcumin may also exert antiviral activity against SARS‐COV‐2 by activating the Nrf2 pathway. 73
The death of severely ill COVID‐19 patients is associated with respiratory failure or multi‐organ failure caused by ARDS and septic shock.63 ARDS and sepsis pathogenesis involves an early hyperactivated inflammatory response characterized by a cytokine storm. 19 Because of anti‐inflammatory and anti‐inflammasome properties, without any side effects, curcumin can potentially play a role in treating pneumonia and COVID‐19 infection along with other drug regimens. The present data reveal that oral curcumin therapy in COVID‐19 treatment could dramatically reduce morbidity and mortality (See graphical abstract).
7. CURCUMIN AND THE ACE2 RECEPTOR
ACE2, a carboxypeptidase that degrades angiotensin II into angiotensin, has been identified as a potent receptor for SARS‐CoV‐2. ACE inhibition has assumed a central role in reducing cardiovascular and renal events. However, with the advent of COVID‐19, attention has been turned to ACE2 as a possible target to reduce virus binding to different human cells. 75 SARS‐CoV‐2 uses ACE2 for host cellular entry. This is mediated via spike proteins on SARS‐CoV‐2, especially the spike glycoprotein receptor binding domain. ACE2 is present in the lung, heart, kidney, venous endothelial cells and arterial smooth muscle cells. Furthermore, ACE2 is abundantly present in humans in the epithelia of the lung alveolar epithelial cells and small intestine, which might provide possible routes of entry for SARS‐CoV. 76
Gheware et al 77 found negligible expression of ACE2 in non‐COVID‐19 lungs irrespective of gender and a uniform increase in ACE2 expression in all severe COVID‐19 lungs in their study. It seems unlikely that baseline pulmonary ACE2 expression levels contribute to the risk of developing COVID‐19. 77 Shanmugarajan et al 78 reported that ACE2 is mediated via proteins of SARS‐CoV‐2, especially the spike glycoprotein receptor binding domain. Accordingly, their study of virus replication and binding to the host system led to probing curcuminoids' efficiency toward essential surface drug target proteins using the computational biology paradigm approach. Fourteen natural curcuminoids were studied for their possibility of inhibiting SARS‐CoV‐2. 78
Pal et al 79 reported that SARS‐CoV‐2 are enveloped viruses containing non‐segmented positive‐sense, single‐stranded RNA. 79 Recent data showed that the spike protein (S protein) of SARS‐CoV‐2 binds to ACE2 with a higher affinity than SARS‐CoV. For this reason, it spreads rapidly in human populations. 80 Recent studies show curcumin maintains binding efficiency to the receptor‐binding domain (RBD) of the viral spike COVID‐19 protein and human ACE2, triggering the blockage of the ACE2 receptor and resulting in inhibition of the viral attachment with the host cell. 78 , 81 In addition, Zhang et al,79 in 2020, reported that curcumin could potentially increase soluble ACE2 protein, which may competitively attach with COVID‐19 to neutralise the virus and rescue cellular ACE2 activity, which negatively controls the renin‐angiotensin system (RAS) to protect the lung from injury. 80 , 82
Gheware et al 77 reported that a relatively large cohort of patients with fatal COVID‐19 demonstrated high pulmonary expression of ACE2 protein in their post‐mortem lung tissues compared to negligible expression in control lung tissues, highlighting the critical role of ACE2 protein in the pathogenesis of SARS‐CoV‐2 infection. 77 The level of ACE2 expression in the lung correlates with an increased risk of severe infection and complications in COVID‐19. Ultimately, the broad, multifaceted and beneficial effect that curcumin has on the progression and severity of COVID‐19 makes it a promising therapeutic.
8. EFFECT OF CURCUMIN IN TREATING ACUTE LUNG INJURY/ARDS
Acute lung injury (ALI) and ARDS are characterized by rapid‐onset respiratory failure following various direct and indirect insults to the parenchyma or vasculature of the lungs. 83 ALI remains a significant cause of morbidity and mortality in critically ill patients. 84 ALI is characteristic of the wholesale destruction of the lung endothelial barrier, which results in protein‐rich lung oedema, the influx of proinflammatory leukocytes and intractable hypoxemia, contributing to high mortality. 85 Despite an improved understanding of the pathogenesis of ALI, supportive care with a lung‐protective strategy of mechanical ventilation remains the only treatment with a proven survival advantage. 86 , 87 Most ALI patients die or are weaned from supportive therapy within 1–2 weeks, although up to 10% of patients require prolonged treatment (30 days or more). Ultimately, there is an urgent need to develop therapies to halt the progression of this devastating syndrome. Most people who get ARDS are already at the hospital for trauma or illness. Pathologically, ARDS is characterized by diffuse alveolar damage, alveolar‐capillary leakage and protein‐rich pulmonary oedema leading to the clinical manifestation of poor lung compliance, severe hypoxemia and bilateral infiltrates. Most clinical trials in ALI have targeted mechanically ventilated patients. Past pharmacological agents may have failed to demonstrate efficacy due partly to the resultant delay in initiation of therapy until several days after the onset of lung injury. 88
ALI and its excessive inflammatory responses in the lung, known as ‘cytokine storms’, result in pulmonary oedema, atelectasis and fatal ARDS. 28 ARDS, the most severe form of ALI, is a clinical manifestation of the response of the lung to pulmonary insults brought on by infectious, non‐infectious and other damaging events. It affects up to 200 000 patients annually in the United States, with a mortality rate approaching 50%, with inflammation and tissue fibrosis being the leading cause of morbidity and mortality. 89 Factors predisposing patients to ARDS include sepsis, aspiration and pneumonia. 28 , 90 , 91
LPS is a bacterial bio‐active component found in gram‐negative bacteria and involved in many pathological conditions because of its role in activating the inflammatory cascade. 17 The mechanism of LPS causing ALI depends on its proinflammatory activities. 17 LPS is proposed to recruit and promote monocyte infiltration and aggregation, promote provocative cytokine synthesis and secretion and induce apoptosis in alveolar epithelial cells. 48 Previous studies from our lab demonstrated that targeted delivery of CDC to lung cells following exposure to LPS reduces the severity of ALI in mice. 17 In another study, the novel curcumin analogue c26 was found to have remarkable protective effects on rats' LPS‐induced ALI. These effects may be related to its ability to suppress the production of inflammatory cytokines through the extracellular signal‐regulated kinases (ERK) pathway. Compound c26, with improved chemical stability and bioactivity, may have the potential to be further developed into an anti‐inflammatory candidate for the prevention and treatment of ALI. 92
Importantly, curcumin has been found to have broad anti‐inflammatory activities both in vitro and in vivo. Additionally, other studies indicate that pre‐treatment with curcumin showed beneficial effects on ALI induced by oleic acid, 93 sepsis 94 , 95 and aspiration. 96 Finally, curcumin is an effective inhibitor of bleomycin (BLM)‐induced inflammation, apoptosis and migration of basal alveolar epithelial cells. 97 Ultimately, the full potential benefits of curcumin and its mechanisms of action have just begun to be elucidated to treat pulmonary diseases, including fibrosis and ALI/ARDS 24 , 28 (Figure 1).
FIGURE 1.
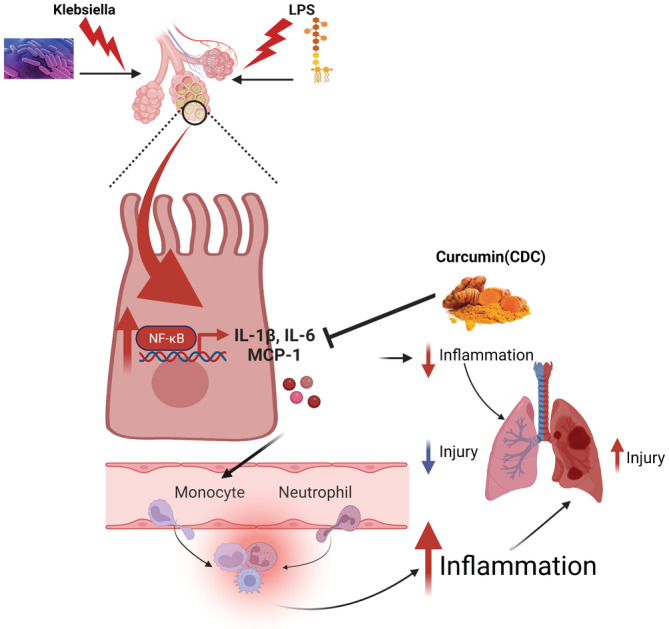
The proposed mechanism model responsible for curcumin's protective effects in mice following LPS/KP. Cyclodextrin‐complexed curcumin (CDC) inhibits the production of proinflammatory cytokines by targeting the NF‐κB pathway. CDC targets NF‐κB signalling by inhibiting the activation of IL‐1β, IL‐6 and MCP1. KP, Klebsiella pneumoniae; CDC,; NF‐κB, nuclear factor‐κB; IL, interleukin; MCP1, monocyte chemoattractant protein‐1
9. EFFECT OF CURCUMIN ON TREATING BACTERIAL PNEUMONIA
Pneumonia is a significant cause of morbidity and mortality worldwide and causes a substantial burden on healthcare systems. 98 The main types of pneumonia are bacterial, viral and mycoplasma pneumonia. The bacteria and viruses that most commonly cause pneumonia in the community differ from those in healthcare settings, some of which are discussed here. 95 , 96 A common cause of bacterial pneumonia is Streptococcus pneumoniae (SP). 99 Ventilator‐associated pneumonia (VAP) is when someone gets pneumonia after being on a ventilator, a machine that supports breathing. 100 , 101 KP is a gram‐negative pathogen with a large accessory genome of plasmids and chromosomal loci. 102 , 103 The gram‐positive bacterium, methicillin‐resistant Staphylococcus aureus (MRSA), is resistant to β‐lactam antibiotics because of the lowered β‐lactam affinity to penicillin‐binding proteins (PBP) and PBP2a. 104 , 105 Pseudomonas aeruginosa is an important pathogen frequently associated with healthcare‐associated infections, particularly in critically ill or immunocompromised patients. 99
Plant compounds such as curcumin may serve as a source of novel antibiotics. 106 One study found synergistic effects of curcumin and common antibiotics against 60 strains of gram‐positive and gram‐negative biofilm‐producing bacteria, including common pneumonia‐causing pathogens introduced earlier. 107 In vitro disc diffusion assays have demonstrated that extracts from plants in the Curcuma genus can inhibit the growth of several pneumonia‐causing bacteria, such as K. pneumoniae. 2 , 92 Promisingly, curcumin has effectively inhibited the development of several species of multidrug‐resistant bacteria. 3 , 103 , 104 Additionally, Gunes et al 108 found in vitro antibacterial activity of curcumin against methicillin‐sensitive Staphylococcus aureus (MSSA), methicillin‐resistant Staphylococcus aureus (MRSA), Enterococcus faecalis, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli (E.coli) and KP using the microdilution broth susceptibility test method. 108 One study found that MRSA and MSSA have demonstrated in vitro sensitivities to curcumin. 108 This finding could suggest that curcumin acts on cellular targets that bypass the resistance mechanisms of MRSA. 99 On another note, Alikiaii reported that curcumin lessens pneumonia severity by reducing neutrophil infiltration and ameliorating the exaggerated immune response in preclinical pneumonia models. 109
Our studies demonstrate that CDC administration improves cell survival and reduces injury, inflammation and mortality in a murine model of lethal gram‐negative (KP) pneumonia. CDC, therefore, has promising anti‐inflammatory potential in pneumonia and likely other inflammatory lung diseases, demonstrating the importance of optimising the physicochemical properties of active natural products to optimise their clinical application. 24
10. OTHER THERAPEUTIC EFFECTS OF CURCUMIN
It has been found that cyclodextrin‐complexed curcumin (CDC) has a greater cellular uptake and a longer half‐life in cells than free curcumin, indicating that CDC has superior attributes to free curcumin for cellular uptake. In addition, the improvement of curcumin permeability across mammalian cells and animal tissue was observed in CD‐encapsulated curcumin and was approximately 1.8‐fold compared with the free curcumin. Therefore, these studies suggest that CDC has improved in vitro and in vivo bioavailability and chemotherapeutic efficacy compared to curcumin alone.
Multiple studies have demonstrated the safety and efficacy of curcumin in numerous animals, including rodents, monkeys, horses, rabbits and cats. They have provided a solid basis for evaluating its safety and effectiveness in humans. More than 65 human clinical trials of curcumin, which included >1000 patients, have been completed, and as many as 35 clinical trials are underway. Curcumin is a free radical scavenger and hydrogen donor and exhibits pro‐oxidant and antioxidant activity. It binds metals, particularly iron and copper, and can function as an iron chelator. Curcumin is remarkably non‐toxic and exhibits limited bioavailability. Curcumin shows excellent promise as a therapeutic agent. It is currently in human clinical trials for various conditions, including multiple myeloma, pancreatic cancer, myelodysplastic syndromes, colon cancer, psoriasis and Alzheimer's disease. Curcumin protects against hepatic disorders, chronic arsenic exposure and alcohol intoxication. Dose‐escalating studies have indicated the safety of curcumin at doses as high as 12 g/day over 3 months. Various formulations of curcumin, including nanoparticles, liposomal encapsulation, emulsions, capsules, tablets and powder, have been examined. Additionally, curcumin treatment is suitable for mental health conditions and reduces antidepressant and anxiolytic effects.
Curcumin, in inflamed organs, reduces the expression levels of NLRP3, IL‐1β, IL‐18 and caspase 1, inhibiting the inflammasome. In LPS‐stimulated mouse macrophages, curcumin inhibits the activity of the NLRP3 inflammasome. Curcumin activated Nrf2 and inhibited NF‐κB. In the lungs, curcumin effectively prevented the increase of neurogenic locus notch homologue protein 1 (Notch1). Curcumin has a regulatory effect on several molecules in the intracellular signal transduction pathways involved in inflammation, including extracellular signal‐regulated kinase 1 (ERK1), STAT‐3 and AMP‐activated protein kinase (AMPK). Curcumin down‐regulates the expression of inflammatory enzymes, such as cyclooxygenase‐2 (COX2) and inducible nitric oxide synthase (iNOS) and inhibits proinflammatory enzymes and chemokines. Studies have shown that curcumin inhibits severe inflammation and cytokine storm in COVID‐19 infection, which may help prevent ARDS, ALI and multiple organ dysfunction syndromes such as in the lungs, liver, kidneys, brain and eventually death. Oral curcumin supplementation may inhibit COVID‐19‐caused inflammation alongside other drug regimens by affecting these pathways and molecules and applying anti‐inflammatory, antioxidant and anti‐apoptotic properties without specific side effects. Ultimately, curcumin shows massive potential as a therapeutic agent in different contexts.
AUTHOR CONTRIBUTION
None of the authors has a financial relationship with a commercial company.
ACKNOWLEDGEMENT
This work was supported by the National Institutes of Health (HL102013 and GM111305).
Suresh MV, Francis S, Aktay S, Kralovich G, Raghavendran K. Therapeutic potential of curcumin in ARDS and COVID‐19. Clin Exp Pharmacol Physiol. 2023;1‐10. doi: 10.1111/1440-1681.13744
Sairah Francis and Sinan Aktay contributed equally to this article.
Funding information National Institutes of Health, Grant/Award Number: HL102013
DATA AVAILABILITY STATEMENT
All data collected and analysed within this study are available from the corresponding author on request.
REFERENCES
- 1. Tonnesen HH, Masson M, Loftsson T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int J Pharm. 2002;244(1–2):127‐135. [DOI] [PubMed] [Google Scholar]
- 2. Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15(1):195‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hewlings SJ, Kalman DS. Curcumin: a review of its effects on human health. Foods. 2017;6(10):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharifi‐Rad J, Rayess YE, Rizk AA, et al. Turmeric and its major compound curcumin on health: bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front Pharmacol. 2020;11:01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lao CD, Ruffin MT, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti‐inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41(1):40‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Panahi Y, Hosseini MS, Khalili N, et al. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: a post‐hoc analysis of a randomized controlled trial. Biomed Pharmacother. 2016;82:578‐582. [DOI] [PubMed] [Google Scholar]
- 8. Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging. 2014;9:451‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giblin J, Podesta R, White J. Dimensional stability of impression materials immersed in an iodophor disinfectant. Int J Prosthodont. 1990;3(1):72‐77. [PubMed] [Google Scholar]
- 10. Basnet P, Skalko‐Basnet N. Curcumin: an anti‐inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16(6):4567‐4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fossey SL, Bear MD, Lin J, et al. The novel curcumin analog FLLL32 decreases STAT3 DNA binding activity and expression, and induces apoptosis in osteosarcoma cell lines. BMC Cancer. 2011;11:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353‐356. [DOI] [PubMed] [Google Scholar]
- 13. Hatamipour M, Johnston TP, Sahebkar A. One molecule, many targets and numerous effects: the pleiotropy of curcumin lies in its chemical structure. Curr Pharm Des. 2018;24(19):2129‐2136. [DOI] [PubMed] [Google Scholar]
- 14. Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. J Tradit Complement Med. 2017;7(2):205‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jagetia GC, Aggarwal BB. ‘Spicing up’ of the immune system by curcumin. J Clin Immunol. 2007;27(1):19‐35. [DOI] [PubMed] [Google Scholar]
- 16. Cho JW, Lee KS, Kim CW. Curcumin attenuates the expression of IL‐1beta, IL‐6, and TNF‐alpha as well as cyclin E in TNF‐alpha‐treated HaCaT cells; NF‐kappaB and MAPKs as potential upstream targets. Int J Mol Med. 2007;19(3):469‐474. [PubMed] [Google Scholar]
- 17. Suresh MV, Wagner MC, Rosania GR, et al. Pulmonary administration of a water‐soluble curcumin complex reduces severity of acute lung injury. Am J Respir Cell Mol Biol. 2012;47(3):280‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sa G, Das T. Anti cancer effects of curcumin: cycle of life and death. Cell Div. 2008;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghalandarlaki N, Alizadeh AM, Ashkani‐Esfahani S. Nanotechnology‐applied curcumin for different diseases therapy. Biomed Res Int. 2014;2014:394264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galdoporpora JM, Martinena C, Bernabeu E, et al. Inhalable Mannosylated rifampicin‐curcumin Co‐loaded Nanomicelles with enhanced In vitro antimicrobial efficacy for an optimized pulmonary tuberculosis therapy. Pharmaceutics. 2010;299(4):G833‐G843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tahmasebi S, El‐Esawi MA, Mahmoud ZH, et al. Immunomodulatory effects of nanocurcumin on Th17 cell responses in mild and severe COVID‐19 patients. J Cell Physiol. 2021;236(7):5325‐5338. [DOI] [PubMed] [Google Scholar]
- 23. Saidi SA, Meurisse N, Jochmans I, et al. Hepatocellular uptake of cyclodextrin‐complexed curcumin during liver preservation: a feasibility study. Biopharm Drug Dispos. 2018;39(1):18‐29. [DOI] [PubMed] [Google Scholar]
- 24. Zhang B, Swamy S, Balijepalli S, et al. Direct pulmonary delivery of solubilized curcumin reduces severity of lethal pneumonia. FASEB J. 2019;33(12):13294‐13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL‐1beta‐mediated up‐regulation of HIF‐1alpha via an NFkappaB/COX‐2 pathway identifies HIF‐1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17(14):2115‐2117. [DOI] [PubMed] [Google Scholar]
- 26. Liu T, Zhang L, Joo D, Sun SC. NF‐kappaB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu AR, Ramakrishnan P. Regulation of nuclear factor‐kappaB function by O‐GlcNAcylation in inflammation and cancer. Front Cell Dev Biol. 2021;9:751761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu Z, Ying Y. The inhibitory effect of curcumin on virus‐induced cytokine storm and its potential use in the associated severe pneumonia. Front Cell Dev Biol. 2020;8:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jimenez‐Flores LM, Lopez‐Briones S, Macias‐Cervantes MH, Ramirez‐Emiliano J, Perez‐Vazquez V. A PPARγ, NF‐κB and AMPK‐dependent mechanism may be involved in the beneficial effects of curcumin in the diabetic db/db mice liver. Molecules. 2014;19(6):8289‐8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang H, He H, Chen Y, et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med. 2017;214(11):3219‐3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sahoo M, Ceballos‐Olvera I, del Barrio L, Re F. Role of the inflammasome, IL‐1beta, and IL‐18 in bacterial infections. ScientificWorldJournal. 2011;11:2037‐2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puri G, Naura AS. Implication of mitochondrial ROS‐NLRP3 inflammasome axis during two‐hit mediated acute lung injury in mice. Free Radic Res. 2022;56:1‐15. [DOI] [PubMed] [Google Scholar]
- 33. Ahmed SM, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. 2017;1863(2):585‐597. [DOI] [PubMed] [Google Scholar]
- 34. Saeedi‐Boroujeni A, Mahmoudian‐Sani MR, Bahadoram M, Alghasi A. COVID‐19: a case for inhibiting NLRP3 inflammasome, suppression of inflammation with curcumin? Basic Clin Pharmacol Toxicol. 2021;128(1):37‐45. [DOI] [PubMed] [Google Scholar]
- 35. Li X, Xu DQ, Sun DY, Zhang T, He X, Xiao DM. Curcumin ameliorates monosodium urate‐induced gouty arthritis through nod‐like receptor 3 inflammasome mediation via inhibiting nuclear factor‐kappa B signaling. J Cell Biochem. 2019;120(4):6718‐6728. [DOI] [PubMed] [Google Scholar]
- 36. Zhao C, Zhao W. NLRP3 inflammasome‐a key player in antiviral responses. Front Immunol. 2020;11:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen B, Li H, Ou G, Ren L, Yang X, Zeng M. Curcumin attenuates MSU crystal‐induced inflammation by inhibiting the degradation of IkappaBalpha and blocking mitochondrial damage. Arthritis Res Ther. 2019;21(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mitchell TJ, John S. Signal transducer and activator of transcription (STAT) signalling and T‐cell lymphomas. Immunology. 2005;114(3):301‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jafarzadeh A, Nemati M, Jafarzadeh S. Contribution of STAT3 to the pathogenesis of COVID‐19. Microb Pathog. 2021;154:104836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Feinman R, Deitch EA, Watkins AC, et al. HIF‐1 mediates pathogenic inflammatory responses to intestinal ischemia‐reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2010;299(4):G833‐G843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Krick S, Eul BG, Hanze J, et al. Role of hypoxia‐inducible factor‐1alpha in hypoxia‐induced apoptosis of primary alveolar epithelial type II cells. Am J Respir Cell Mol Biol. 2005;32(5):395‐403. [DOI] [PubMed] [Google Scholar]
- 42. Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia‐inducible transcription factor depends primarily upon redox‐sensitive stabilization of its alpha subunit. J Biol Chem. 1996;271(50):32253‐32259. [DOI] [PubMed] [Google Scholar]
- 43. Jiang H, Huang Y, Xu H, Hu R, Li QF. Inhibition of hypoxia inducible factor‐1alpha ameliorates lung injury induced by trauma and hemorrhagic shock in rats. Acta Pharmacol Sin. 2012;33(5):635‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bahrami A, Atkin SL, Majeed M, Sahebkar A. Effects of curcumin on hypoxia‐inducible factor as a new therapeutic target. Pharmacol Res. 2018;137:159‐169. [DOI] [PubMed] [Google Scholar]
- 45. Bae MK, Kim SH, Jeong JW, et al. Curcumin inhibits hypoxia‐induced angiogenesis via down‐regulation of HIF‐1. Oncol Rep. 2006;15(6):1557‐1562. [PubMed] [Google Scholar]
- 46. Davidson BA, Vethanayagam RR, Grimm MJ, et al. NADPH oxidase and Nrf2 regulate gastric aspiration‐induced inflammation and acute lung injury. J Immunol. 2013;190(4):1714‐1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ashrafizadeh M, Ahmadi Z, Mohammadinejad R, Farkhondeh T, Samarghandian S. Curcumin activates the Nrf2 pathway and induces cellular protection against oxidative injury. Curr Mol Med. 2020;20(2):116‐133. [DOI] [PubMed] [Google Scholar]
- 48. Godeau D, Petit A, Richard I, Roquelaure Y, Descatha A. Return‐to‐work, disabilities and occupational health in the age of COVID‐19. Scand J Work Environ Health. 2021;47(5):408‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guha S, Chakraborty A. Coronavirus management and control: nutrition and alternative medicines. Nutr Health. 2022;28(4):635‐645. [DOI] [PubMed] [Google Scholar]
- 50. Wen W, Su W, Tang H, et al. Immune cell profiling of COVID‐19 patients in the recovery stage by single‐cell sequencing. Cell Discov. 2020;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome Coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020;28(7):1195‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu J, Sheng L, Ma Y, et al. The analysis of risk factors of impacting mortality rate in severe multiple trauma patients with posttraumatic acute respiratory distress syndrome. Am J Emerg Med. 2008;26(4):419‐424. [DOI] [PubMed] [Google Scholar]
- 53. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. Jama. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 54. Pawar KS, Mastud RN, Pawar SK, et al. Oral curcumin with Piperine as adjuvant therapy for the treatment of COVID‐19: a randomized clinical trial. Front Pharmacol. 2021;12:669362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rattis BAC, Ramos SG, Celes MRN. Curcumin as a potential treatment for COVID‐19. Front Pharmacol. 2021;12:675287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Simmons G, Bertram S, Glowacka I, et al. Different host cell proteases activate the SARS‐coronavirus spike‐protein for cell‐cell and virus‐cell fusion. Virology. 2011;413(2):265‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ni W, Yang X, Yang D, et al. Role of angiotensin‐converting enzyme 2 (ACE2) in COVID‐19. Crit Care. 2020;24(1):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Valizadeh H, Abdolmohammadi‐Vahid S, Danshina S, et al. Nano‐curcumin therapy, a promising method in modulating inflammatory cytokines in COVID‐19 patients. Int Immunopharmacol. 2020;89(Pt B):107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gunathilake TMSU, Ching YC, Uyama H, Hai ND, Chuah CH. Enhanced curcumin loaded nanocellulose: a possible inhalable nanotherapeutic to treat COVID‐19. Cellulose (Lond). 2022;29(3):1821‐1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gunathilake T, Ching YC, Uyama H, Hai ND, Chuah CH. Enhanced curcumin loaded nanocellulose: a possible inhalable nanotherapeutic to treat COVID‐19. Cellulose (Lond). 2022;29(3):1821‐1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nieuwenhuizen L, de Groot PG, Grutters JC, Biesma DH. A review of pulmonary coagulopathy in acute lung injury, acute respiratory distress syndrome and pneumonia. Eur J Haematol. 2009;82(6):413‐425. [DOI] [PubMed] [Google Scholar]
- 62. Aguilar‐Lemarroy A, Lopez‐Uribe A, Sanchez‐Corona J, Jave‐Suarez LF. Severe acute respiratory syndrome coronavirus 2 ORF3a induces the expression of ACE2 in oral and pulmonary epithelial cells and the food supplement Vita Deyun((R)) diminishes this effect. Exp Ther Med. 2021;21(5):485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hanafy NAN, El‐Kemary MA. Silymarin/curcumin loaded albumin nanoparticles coated by chitosan as muco‐inhalable delivery system observing anti‐inflammatory and anti COVID‐19 characterizations in oleic acid triggered lung injury and in vitro COVID‐19 experiment. Int J Biol Macromol. 2022;198:101‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hanafy NAN, El‐Kemary MA. Silymarin/curcumin loaded albumin nanoparticles coated by chitosan as muco‐inhalable delivery system observing anti‐inflammatory and anti COVID‐19 characterizations in oleic acid triggered lung injury and in vitro COVID‐19 experiment. Int J Biol Macromol. 2022;198:101‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Marin‐Palma D, Tabares‐Guevara JH, Zapata‐Cardona MI, et al. Curcumin inhibits In vitro SARS‐CoV‐2 infection In Vero E6 cells through multiple antiviral mechanisms. Molecules. 2021;26(22):690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Thimmulappa RK, Mudnakudu‐Nagaraju KK, Shivamallu C, et al. Antiviral and immunomodulatory activity of curcumin: a case for prophylactic therapy for COVID‐19. Heliyon. 2021;7(2):e06350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Babaei F, Nassiri‐Asl M, Hosseinzadeh H. Curcumin (a constituent of turmeric): new treatment option against COVID‐19. Food Sci Nutr. 2020;8(10):5215‐5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vahedian‐Azimi A, Abbasifard M, Rahimi‐Bashar F, et al. Effectiveness of curcumin on outcomes of hospitalized COVID‐19 patients: a systematic review of clinical trials. Nutrients. 2022;14(2):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Askari G, Sahebkar A, Soleimani D, et al. The efficacy of curcumin‐piperine co‐supplementation on clinical symptoms, duration, severity, and inflammatory factors in COVID‐19 outpatients: a randomized double‐blind, placebo‐controlled trial. Trials. 2022;23(1):472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kumar G, Kumar D, Singh NP. Therapeutic approach against 2019‐nCoV by inhibition of ACE‐2 receptor. Drug Res (Stuttg). 2021;71(4):213‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nag A, Banerjee R, Paul S, Kundu R. Curcumin inhibits spike protein of new SARS‐CoV‐2 variant of concern (VOC) omicron, an in silico study. Comput Biol Med. 2022;146:105552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kushwaha AD, Mishra KP, Singh M, Ganju L, Saraswat D. Nanocurcumin formulation: a possible therapeutic agent for post COVID inflammatory syndrome. Immunopharmacol Immunotoxicol. 2022;44(2):141‐146. [DOI] [PubMed] [Google Scholar]
- 73. Saber‐Moghaddam N, Salari S, Hejazi S, et al. Oral nano‐curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease‐19 patients: an open label nonrandomized clinical trial. Phytother Res. 2021;35:2616‐2623. [DOI] [PubMed] [Google Scholar]
- 74. Muchtaridi M, Amirah SR, Harmonis JA, Ikram EHK. Role of nuclear factor erythroid 2 (Nrf2) in the recovery of long COVID‐19 using natural antioxidants: a systematic review. Antioxidants (Basel). 2022;11(8):1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Junior AG, Tolouei SEL, Dos Reis Livero FA, Gasparotto F, Boeing T, de Souza P. Natural agents modulating ACE‐2: a review of compounds with potential against SARS‐CoV‐2 infections. Curr Pharm des. 2021;27(13):1588‐1596. [DOI] [PubMed] [Google Scholar]
- 76. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gheware A, Ray A, Rana D, et al. ACE2 protein expression in lung tissues of severe COVID‐19 infection. Sci Rep. 2022;12(1):4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shanmugarajan D, Prabitha P, Kumar BP, Suresh B. Curcumin to inhibit binding of spike glycoprotein to ACE2 receptors: computational modelling, simulations, and ADMET studies to explore curcuminoids against novel SARS‐CoV‐2 targets. RSC Adv. 2020;10(52):31385‐31399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pal M, Berhanu G, Desalegn C, Kandi V. Severe acute respiratory syndrome Coronavirus‐2 (SARS‐CoV‐2): an update. Cureus. 2020;12(3):e7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dhar S, Bhattacharjee P. Promising role of curcumin against viral diseases emphasizing COVID‐19 management: a review on the mechanistic insights with reference to host‐pathogen interaction and immunomodulation. J Funct Foods. 2021;82:104503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Raghavendran K, Napolitano LM. Definition of ALI/ARDS. Crit Care Clin. 2011;27(3):429‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Albertine KH, Soulier MF, Wang Z, et al. Fas and fas ligand are up‐regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol. 2002;161(5):1783‐1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Huang YD, Fang Y, Ma L, et al. Kindlin‐2 mediates lipopolysaccharide‐induced acute lung injury partially via Pyroptosis in mice. Inflammation. 2022;45:1199‐1208. [DOI] [PubMed] [Google Scholar]
- 86. Adams AB, Cakar N, Marini JJ. Static and dynamic pressure‐volume curves reflect different aspects of respiratory system mechanics in experimental acute respiratory distress syndrome. Respir Care. 2001;46(7):686‐693. [PubMed] [Google Scholar]
- 87. ARDS_Network . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome Network. N Engl J Med. 2000;342:1301‐1308. [DOI] [PubMed] [Google Scholar]
- 88. Levitt JE, Matthay MA. Clinical review: early treatment of acute lung injury‐‐paradigm shift toward prevention and treatment prior to respiratory failure. Crit Care. 2012;16(3):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Uhal BD, Joshi I, Hughes WF, Ramos C, Pardo A, Selman M. Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung. Am J Physiol. 1998;275(6 Pt 1):L1192‐L1199. [DOI] [PubMed] [Google Scholar]
- 90. Arroliga AC, Ghamra ZW, Perez Trepichio A, et al. Incidence of ARDS in an adult population of Northeast Ohio. Chest. 2002;121:1972‐1976. [DOI] [PubMed] [Google Scholar]
- 91. Bernard GR, Luce JM, Sprung CL, et al. High‐dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987;317(25):1565‐1570. [DOI] [PubMed] [Google Scholar]
- 92. Zhang Y, Liang D, Dong L, et al. Anti‐inflammatory effects of novel curcumin analogs in experimental acute lung injury. Respir Res. 2015;16:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhu RF, Zhou M, He JL, Ding FY, Yu SQ, Xu GL. Protective effect of curcumin on oleic‐induced acute lung injury in rats. Zhongguo Zhong Yao Za Zhi. 2008;33(17):2141‐2145. [PubMed] [Google Scholar]
- 94. Nahra R, Dellinger RP. Targeting the lipopolysaccharides: still a matter of debate? Curr Opin Anaesthesiol. 2008;21(2):98‐104. [DOI] [PubMed] [Google Scholar]
- 95. Benjamim CF, Hogaboam CM, Kunkel SL. The chronic consequences of severe sepsis. J Leukoc Biol. 2004;75(3):408‐412. [DOI] [PubMed] [Google Scholar]
- 96. Guzel A, Kanter M, Aksu B, et al. Preventive effects of curcumin on different aspiration material‐induced lung injury in rats. Pediatr Surg Int. 2009;25(1):83‐92. [DOI] [PubMed] [Google Scholar]
- 97. Gouda MM, Prabhu A, Bhandary YP. Curcumin alleviates IL‐17A‐mediated p53‐PAI‐1 expression in bleomycin‐induced alveolar basal epithelial cells. J Cell Biochem. 2018;119(2):2222‐2230. [DOI] [PubMed] [Google Scholar]
- 98. Adnet F, Baud F. Relation between Glasgow coma scale and aspiration pneumonia. Lancet. 1996;348(9020):123‐124. [DOI] [PubMed] [Google Scholar]
- 99. Agrawal A, Suresh MV, Singh SK, Ferguson DA Jr. The protective function of human C‐reactive protein in mouse models of Streptococcus pneumoniae infection. Endocr Metab Immune Disord Drug Targets. 2008;8(4):231‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bauer TT, Ferrer R, Angrill J, Schultze‐Werninghaus G, Torres A. Ventilator‐associated pneumonia: incidence, risk factors, and microbiology. Semin Respir Infect. 2000;15:272‐279. [DOI] [PubMed] [Google Scholar]
- 101. Boonsarngsuk V, Thungtitigul P, Suwatanapongched T. Chronic Klebsiella pneumonia: a rare manifestation of Klebsiella pneumonia. J Thorac Dis. 2015;7(9):1661‐1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Balamayooran G, Batra S, Theivanthiran B, Cai S, Pacher P, Jeyaseelan S. Intrapulmonary G‐CSF rescues neutrophil recruitment to the lung and neutrophil release to blood in gram‐negative bacterial infection in MCP‐1−/− mice. J Immunol. 2012;189(12):5849‐5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Negi N, Prakash P, Gupta ML, Mohapatra TM. Possible role of curcumin as an efflux pump inhibitor in multi drug resistant clinical isolates of Pseudomonas aeruginosa. J Clin Diagn Res. 2014;8(10):DC04‐DC07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mun SH, Kim SB, Kong R, et al. Curcumin reverse methicillin resistance in Staphylococcus aureus. Molecules. 2014;19(11):18283‐18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Okwu MU, Olley M, Akpoka AO, Izevbuwa OE. Methicillin‐resistant Staphylococcus aureus (MRSA) and anti‐MRSA activities of extracts of some medicinal plants: a brief review. AIMS Microbiol. 2019;5(2):117‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Khameneh B, Iranshahy M, Soheili V, Fazly Bazzaz BS. Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob Resist Infect Control. 2019;8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kali A, Bhuvaneshwar D, Charles PM, Seetha KS. Antibacterial synergy of curcumin with antibiotics against biofilm producing clinical bacterial isolates. J Basic Clin Pharm. 2016;7(3):93‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gunes H, Gulen D, Mutlu R, Gumus A, Tas T, Topkaya AE. Antibacterial effects of curcumin: an in vitro minimum inhibitory concentration study. Toxicol Ind Health. 2016;32(2):246‐250. [DOI] [PubMed] [Google Scholar]
- 109. B Alikiaii, M Bagherniya, G Askari, T Sathyapalan, A Sahebkar. Evaluation of the effect of curcumin on pneumonia: A systematic review of preclinical studies. Phytother Res. 2021;35(4):1939‐1952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data collected and analysed within this study are available from the corresponding author on request.


