Abstract
The long‐term protective efficacy of neutralizing antibodies (Nabs) against Omicron subvariants after inactivated booster vaccines remains elusive. During the follow‐up study, 54 healthy volunteers aged 20–31 years received inactivated CoronaVac booster vaccinations and were monitored for 221 days. The dynamic efficacy and durability of Nab against Omicron subvariants BA.1, BA.2, BA.2.12.2, and BA4/5 were assessed using a pseudotyped virus neutralization assay at up to nine time points post immunization. The antibody response against Omicron subvariants was substantially weaker than D614G, with BA.4/5 being the least responsive. The geometric mean titer (GMT) of Nab against Omicron subvariants BA.1, BA.2, BA.2.12.1, and BA.4/5 was 2.2‐, 1.7‐, 1.8‐, and 2.2‐fold lower than that against D614G (p s < 0.0001). The gap in Nab response between Omicron subvariants was pronounced during the 2 weeks–2 months following booster vaccination (p s < 0.05). Seven months post booster, the antibody potency against D614G was maintained at 100% (50% for Nab titers ≥ 100 50% inhibitory dilution [EC50]), whereas at 77.3% for BA.1, 90.9% for BA.2, 86.4% for BA.2.12.1, and 86.4% for BA.4/5 (almost 20% for Nab titers ≥ 100 EC50). Despite the inevitable immune escape, Omicron subvariants maintained sustained and measurable antibody potency post‐booster vaccination during long‐term monitoring, which could help optimize immunization strategies.
Keywords: booster vaccination, COVID‐19, neutralizing antibody, Omicron subvariants, SARS‐CoV‐2
Abbreviations
- COVID‐19
coronavirus disease 2019
- GMT
geometric mean titer
- IQR
interquartile range
- Nab
neutralizing antibody
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- VSV
vesicular stomatitis virus
1. INTRODUCTION
Since the Omicron jumped as a new variant of concern, its spike protein carrying more than 30 critical mutations has stirred up a new wave of infections worldwide and also raised widespread concerns about immune protection. 1 , 2 Successive new variants, including BA.1, BA.2, BA.2.12.1, and BA.4/5, are taking over as dominant strains, especially the additional L452R + F486V mutation of BA4/5, which seems to be the culprit of aggravated immune escapes. 3 , 4 , 5 CoronaVac is currently the most extensively administered coronavirus disease 2019 (COVID‐19) vaccine in China, with a global supply of over 2.8 billion doses. However, the immune escape profile after a booster vaccine against Omicron subvariants, especially the long‐term protective efficacy of neutralizing antibodies (Nabs), remains elusive and yet to be revealed.
Previous research has provided evidence regarding the potency of Nab responses against Omicron subvariants in healthy or infected individuals in the context of messenger RNA (mRNA) vaccine booster vaccination. 3 , 6 , 7 , 8 , 9 , 10 In general, these results were mainly derived from a cross‐sectional perspective. What exactly is the protective effect of inactivated vaccination booster‐induced antibodies against newly variants (particularly BA.4/5) and how long does it maintain a highly potent antiviral neutralizing activity? These stirring questions are fraught with uncertainty. Herein, during a 7‐month follow‐up, we assessed the efficacy and variability of Nabs against the Omicron subvariants BA.1, BA.2, BA.2.12.1, and BA.4/5 following the inactivated vaccine booster immunization (CoronaVac, Sinovac).
2. METHODS
2.1. Study population
In this research, 54 healthy adult volunteers were recruited from colleges (21 males and 33 females). Their ages ranged from 20 to 31 years, with a median (interquartile range, IQR) of 24 (22, 26) years. All participants fulfilled the following criteria at enrollment: (1) completed pre‐stage vaccinations and the booster dose of CoronaVac‐inactivated vaccines as scheduled; (2) no history of exposure or infection with COVID‐19; (3) no underlying diseases or surgical history; (4) no obesity, and without adverse lifestyle habits including smoking or alcoholism. Supporting Information: Table 1 contains detailed demographic information on participants, including age and gender statistics.
2.2. Plasma sample collection
Booster immunization of all subjects was completed between October 2021 and January 2022, with follow‐up lasting until 221 days postbooster vaccination. After the CoronaVac booster, volunteers were monitored routinely and serum samples were collected to determine Nab levels at up to 9 time points for a single participant (Supporting Information: Table 1). Blood samples were collected and plasma was separated for subsequent assays by qualified personnel. Separated plasma samples were stored at −80°C until analysis. Supporting Information: Table 1 outlines the sample collection times for each participant. Supporting Information: Table 2 shows the time points covered by each major sample collection period, as well as the corresponding Nab levels against D614G and Omicron subvariants during these periods.
2.3. Cell lines and culture
All cell lines employed in the experiments were cultured in complete Dulbecco's modified Eagle's medium (DMEM medium [high glucose; Hyclone, cat. no. SH30243.01] supplemented with 10% fetal bovine serum [Pansera ES; PAN‐Biotech GmbH, cat. no. ST30‐2602], 1% penicillin–streptomycin, and 2% HEPES [Gibco, cat. no. 15630080]) and cultured in an incubator containing 5% CO2 at 37°C. HEK293T cells (American Type Culture Collection, cat. no. CRL‐3216; RRID: CVCL_0063) were used for the production of pseudotyped viral particles, whereas Huh‐7 cells (JCRB, cat. no. JCRB0403; RRID: CVCL_0336) served as target cells in titration and neutralization assays of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pseudotyped viruses.
2.4. Plasmid construction
The expression plasmid system for SARS‐CoV‐2 pseudotyped viruses is based on the vesicular stomatitis virus (VSV) pseudotyped virus production system and consists of two parts: pcDNA3.1.VSVG and pcDNA3.1.S2. pcDNA3.1.VSVG recombinant plasmid (GenBank: MT613045), constructed by inserting the codon‐optimized VSVG gene (Genbank: M27165) in pcDNA3.1, was used to amplify the VSV viral vector (G*ΔG‐VSV) (Kerafast: EH1020‐PM). 11 The pcDNA3.1.S2 recombinant plasmid (GenBank: MT613044) was constructed by inserting the S gene expressing the spike protein (C‐terminal truncation of 18 amino acid residues) of the SARS‐CoV‐2 D614G variant or Omicron subspecies, respectively, into the pcDNA3.1 plasmid. The virulent strain reference and plasmid construction were consistent with the previous studies. 5 Plasmids were extracted using endotoxin‐free reagents and assessed for plasmid quality by applying a spectrophotometer.
2.5. Pseudotype virus production and neutralization antibody assay
To quantify Nab titers, the firefly luciferase reporter gene‐based SARS‐CoV‐2 pseudotyped virus was performed as described previously. 12 Briefly, SARS‐CoV‐2 pseudotyped viruses were prepared by transfecting 293T cells with the SARS‐CoV‐2 S plasmid (providing membrane proteins) and G*δG‐VSV (providing the VSV genome). Culture supernatants containing pseudovirus particles were collected 24 h and 48 h after infection and transfection. For purification, the collected pseudotyped viruses were centrifuged at 1000g for 10 min and then passed through a 0.45 μm filter. The mixture was aliquoted and stored at −80°C until use.
A validated pseudotyped virus neutralization antibody assay was performed to determine the immune response against distinct variants. 5 , 11 , 12 For the neutralization assay, the serum samples were first thermally inactivated at 56°C for 30 min. Subsequently, samples were diluted from an initial dilution of 1:30, followed by five serial threefold dilutions in a 96‐well plate. After mixed with 650 TCID50/well (1.3 × 104 TCID50/ml, 50 μl) of pseudotyped viruses and incubated for 60 min at 37°C with 5% CO2, 2 × 104 Huh‐7 cells were added to each well and incubated for 24 h. The Multimode Microplate Reader (PerkinElmer, model no. HH34000000) was used to detect luciferase expression with relative light unit (RLU) values. Virus neutralization titers were calculated using Reed–Muench methodology. The 50% inhibitory dilution (EC50) was defined as the serum dilution at which the RLUs were reduced by 50% compared with the virus control wells (virus + cells) after subtraction of the background RLUs in the control groups with cells only. Results are based on three replicate wells with the %CV of the replicate wells set at ≤30%. For serum samples, EC50 < 30 is considered a negative result.
2.6. Statistical analysis
Data were plotted as points with connecting lines or shown as percentage bars. Differences in Nab titers against SARS‐CoV‐2 variants were expressed as geometric mean titers (GMTs) with 95% confidence intervals. Statistical significance was determined using the Friedman nonparametric repeated‐measures analysis of variance test with Bonferroni's multiple testing correction or the Wilcoxon matched‐pairs signed‐rank test. Continuous variables were displayed as medians and IQR. Two‐tailed p < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistic version 26.0 (IBM Corp.). Visualization was performed using GraphPad Prism 8.0 (GraphPad Software, Inc.).
3. RESULTS
3.1. Long‐term Nab variations against Omicron subvariants after the CoronaVac booster
By monitoring the serum Nab levels of 54 volunteers who received CoronaVac booster vaccinations for 7 months, antibody response profiles against different Omicron subvariants were plotted, which reflected the long‐term vaccine efficacy. In general, serum Nab titers against all variants rose consistently after 4 days post booster, peaked at about 2–3 weeks, and declined significantly after 2 months. The antibody response against Omicron subvariants was substantially weaker than D614G throughout the follow‐up, with BA.4/5 and BA.1 the worst (Figure 1 and Supporting Information: Table 2). From 4 days following booster immunization, nearly all volunteers maintained high or consistently detectable levels of Nabs against D614G for 7 months. Some respondents, in contrast, consistently failed to generate detectable antibodies against Omicron subvariants (Figure 1). Based on the total of 302 observation sites, the median (IQR) Nab titer for D614G was 184.0 (87.5, 346.8), whereas for Omicron subvariants, the titers were 96.6 (53.7, 179.8) for BA.1, 113.2 (62.8, 193.6) for BA.2, and 107.7 (57.5, 185.8) for BA.2.12.1, and 96.5 (55.4, 160.5) for BA.4/5, respectively (Supporting Information: Table 2).
Figure 1.
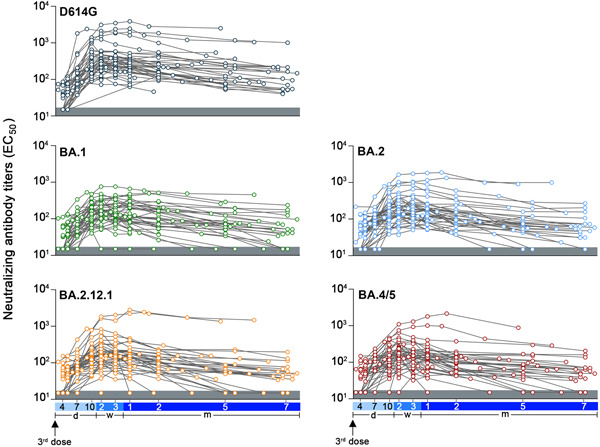
Profile of Nab titers in plasma against D614G and Omicron subvariants after booster vaccination of healthy volunteers. Nab titers were expressed as 50% inhibitory dilution [EC50] values based on pseudotype virus neutralization assays. Samples with EC50 values below 30 were considered negative and assigned a value of 15, as displayed in the gray shaded area. The blue horizontal axis corresponds to time point markers following the booster immunization.
3.2. Nab response against Omicron subvariants was impaired than that against D614G
To visually observe the distinction in antibody response among Omicron variants and the previous variant over the follow‐up period, we assessed and compared the GMT of Nab against the variants at different time periods (Figure 2). Ten days after a booster vaccination, significantly higher titers of Nabs against D614G were observed in sera than in all Omicron subvariants, which tendency persisted until the end of follow‐up (p s < 0.0001). During the titer peak at 3 weeks, the GMT of Nab against Omicron subvariants BA.1, BA.2, BA.2.12.1, and BA.4/5 was 2.2‐, 1.7‐, 1.8‐, and 2.2‐fold lower than those against D614G (p s < 0.0001). Following booster vaccination, the gap in Nab response between Omicron subvariants was pronounced during the 2 weeks–2 months (p s < 0.05) (Figure 2 and Supporting Information: Table 3). During the follow‐up period, the highest Nab titers against Omicron subvariant were observed in BA.2, whereas the lowest were seen against BA.4/5, which could cause immune escape, triggering a severe impairment of vaccine effectiveness (Supporting Information: Tables 2 and 3). These results above supported that the Omicron subvariants, particularly BA.4/5, appear to elicit a weaker and less effective antibody response than D614G, even after a booster vaccination.
Figure 2.
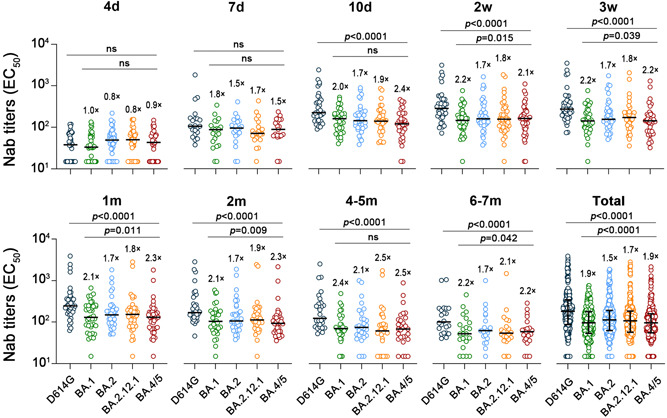
Comparison of geometric mean titer (GMT) of Nab against D614G and Omicron subvariants over various periods. The GMT fold differences between the D614G and Omicron subvariants were calculated. Statistical significance was determined using a two‐tailed Friedman nonparametric repeated‐measures analysis of variance (ANOVA) test with Bonferroni's multiple testing correction. GMT, geometric mean titer; Nab, neutralizing antibody.
3.3. Detectable Nab titers against Omicron subvariants were maintained 7 months after booster vaccination
Fully detectable Nabs were available earliest against D614G (7d) and the last against BA4/5 (3w) (Figure 3). Two weeks after the booster vaccination, Nabs' potency against D614G was 100%, whereas potency against Omicron subvariants was 97.5% for BA.1, 100% for BA.2, 97.5% for BA.2.12.1, and 95% for BA.4/5, respectively. Meanwhile, Nab titers above 100 against D614G were as high as 95%, whereas this percentage for Omicron subvariants was 75% (BA.1), 82.5% (BA.2), 77.5% (BA.2.12.1), and 72.5% (BA.4/5). However, 7 months post booster, the antibody potency against D614G was maintained at 100% (50% above 100), dropping to 77.3% for BA.1, 90.9% for BA.2, 86.4% for BA.2.12.1%, and 86.4% for BA.4/5. Notably, among all Omicron subvariants, volunteers with antibody titers above 100 accounted for only slightly over 20% and high titers of Nab (EC50 ≥ 500) were almost absent (Figure 3). In addition, to further observe the variations in the titers of Nabs against SARS‐CoV‐2 variants after the booster immunization, Nab titer decay rates were visualized. We identified similar patterns of antibody decay over the long term, despite the wide variation in titers between strains (Figure 4).
Figure 3.
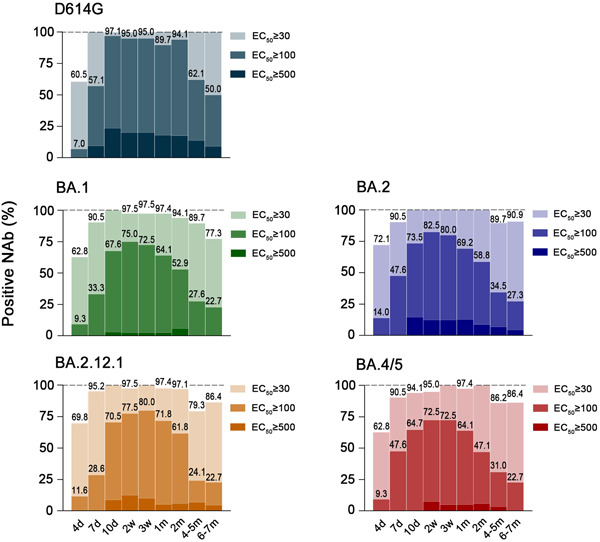
Patterns of Nab potency against D614G and Omicron subvariants during 7 months after booster vaccination. The 50% inhibitory dilution (EC50) representing Nab titers was subdivided into ≥30, ≥100, and ≥500 levels. The graph showed the proportional bars of antibody levels for different gradients, denoted by shades of color from light to dark. Values of 100% were not indicated directly and were marked by dashed lines.
Figure 4.
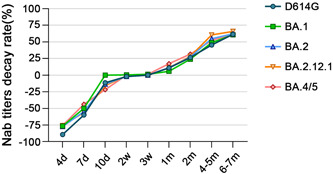
Decay rates of Nab titer against D614G and Omicron subvariants over time (compared to the peak Nab geometric mean titer [GMT] of its own). Although Nab titers varied greatly among severe acute respiratory syndrome coronavirus 2 (SARS‐Cov‐2) variants over time, the long‐term trend and pattern of Nab decay were comparable. The decay rate for a period was calculated as (the peak GMT of the strain – the GMT of that period)/the peak GMT × 100%. Nab, neutralizing antibody; GMT, geometric mean titer.
4. DISCUSSION
With the subsequent emergence and turnover of Omicron subvariants, concerns are mounting about whether the newest subvariant, BA4/5, could pose a severe threat to the immune protective efficacy of booster vaccinations. Through the postvaccination monitoring of the CoronaVac‐inactivated booster, we revealed immune escape profiles and immune protection efficacy among Omicron subvariants.
To our knowledge, this is the longest follow‐up report on antibody responses against Omicron subvariants after receiving inactivated vaccine boosters. Evidence from this long‐term follow‐up suggests that Nab immune responses against the Omicron subvariants are notably reduced compared with the previously widespread variant (D614G) post boosters, which has been supported by previous studies. 3 , 5 , 8 , 10 , 13 , 14 We also found that the gap in Nab titers against Omicron subvariants is most pronounced at the peak of antibody production after booster vaccination. This gap, however, gradually decreased over time, and by 7 months of follow‐up, the protective efficacy was almost equivalent for all Omicron subvariants. Fortunately, despite the inevitable immunological escape and antibody decay, most booster recipients retained a respectable detection rate of Nab titers post 7 months, albeit a limited percentage of high titer levels. This evidence indicates that the antibody protective response against the Omicron subvariants triggered by inactivated booster vaccinations persisted during prolonged monitoring. Additional antibody attenuation against the BA4/5 subvariant can indeed be observed, which causes inefficacy of antibody production during the peak. However, the booster could still play an indispensable role in long‐term immune protection.
In a follow‐up study of mRNA booster vaccination, Nab titers for BA.1, BA.2.12.1, and BA.4/5 were 4.6‐, 7.0‐, and 13.4‐fold lower than D614G, respectively. 8 Consistently, we also observed the reduction trend, but with a relatively lower fold, which may be due to the heterogeneity of different vaccines. Due to the presence of intact virus particles, inactivated vaccines may contain a wider range of neutralizing epitopes, which could be helpful in response to newly emerging mutant strains. 15,16 In addition, assay methods and quantitative criteria variations could also contribute to the variability of follow‐up results.
5. CONCLUSIONS
Collectively, the immune response of Nab against Omicron subvariants showed an inevitable decrease after the booster in contrast with the previous variant. Nevertheless, antibody‐associated immune escape is relatively limited in BA.4/5 compared with other subspecies. Of interest is that Omicron subvariants maintained sustained and measurable antibody potency during the long‐term monitoring, which instils confidence in the development of new vaccines and the optimization of vaccine strategies.
AUTHOR CONTRIBUTIONS
Tao Shen, Qinghua Zou, and Weijin Huang conceptualized and designed the whole study. Xinjie Li, Yue Yin, and Qianqian Cui contributed to sample collection, formal analysis, methodology, investigation, and validation. Yue Yin visualized the data. Xinjie Li contributed to statistical analyses and wrote the original draft. Xinjie Li, Tao Shen, and Qinghua Zou reviewed and edited the manuscript. All authors reviewed and approved the manuscript for publication.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the Peking University Biomedical Ethics Board (PUIRB) (ethics approval number: IRB00001052‐22030). All volunteers have signed an informed consent form. The study was conducted in compliance with all International Council for Harmonization Good Clinical Practice guidelines and the ethical principles of the Declaration of Helsinki.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
We are grateful to all volunteers who participated in this follow‐up study. The study was supported by the Beijing Municipal Natural Science Foundation (M21021).
Li X, Yin Y, Cui Q, Huang W, Zou Q, Shen T. Long‐term variations and potency of neutralizing antibodies against Omicron subvariants after CoronaVac‐inactivated booster: a 7‐month follow‐up study. J Med Virol. 2022;95:e28279. 10.1002/jmv.28279
Xinjie Li, Yue Yin, and Qianqian Cui contributed equally to this study.
Contributor Information
Qinghua Zou, Email: zouqinghua@hsc.pku.edu.cn.
Tao Shen, Email: taoshen@hsc.pku.edu.cn.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article (and its Supporting Information files).
REFERENCES
- 1. Gobeil SMC, Henderson R, Stalls V, et al. Structural diversity of the SARS‐CoV‐2 Omicron spike. Mol Cell. 2022;82(11):2050‐2068.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS‐CoV‐2 Omicron to antibody neutralization. Nature. 2022;602(7898):671‐675. [DOI] [PubMed] [Google Scholar]
- 3. Tuekprakhon A, Nutalai R, Dijokaite‐Guraliuc A, et al. Antibody escape of SARS‐CoV‐2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185(14):2422‐2433.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tegally H, Moir M, Everatt J, et al. Emergence of SARS‐CoV‐2 Omicron lineages BA.4 and BA.5 in South Africa. Nat Med. 2022;28:1785‐1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao Y, Yisimayi A, Jian F, et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608:593‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang L, Kainulainen MH, Jiang N, et al. Differential neutralization and inhibition of SARS‐CoV‐2 variants by antibodies elicited by COVID‐19 mRNA vaccines. Nat Commun. 2022;13(1):4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bellusci L, Grubbs G, Zahra FT, et al. Antibody affinity and cross‐variant neutralization of SARS‐CoV‐2 Omicron BA.1, BA.2 and BA.3 following third mRNA vaccination. Nat Commun. 2022;13(1):4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qu P, Faraone J, Evans JP, et al. Neutralization of the SARS‐CoV‐2 Omicron BA.4/5 and BA.2.12.1 subvariants. N Engl J Med. 2022;386(26):2526‐2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arora P, Kempf A, Nehlmeier I, et al. Augmented neutralisation resistance of emerging omicron subvariants BA.2.12.1, BA.4, and BA.5. Lancet Infect Dis. 2022;22(8):1117‐1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hachmann NP, Miller J, Collier AY, et al. Neutralization escape by SARS‐CoV‐2 Omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med. 2022;387(1):86‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nie J, Li Q, Wu J, et al. Establishment and validation of a pseudovirus neutralization assay for SARS‐CoV‐2. Emerg Microbes Infect. 2020;9(1):680‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nie J, Li Q, Wu J, et al. Quantification of SARS‐CoV‐2 neutralizing antibody by a pseudotyped virus‐based assay. Nat Protoc. 2020;15(11):3699‐3715. [DOI] [PubMed] [Google Scholar]
- 13. Wang Q, Guo Y, Iketani S, et al. Antibody evasion by SARS‐CoV‐2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608:603‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quandt J, Muik A, Salisch N, et al. Omicron BA.1 breakthrough infection drives cross‐variant neutralization and memory B cell formation against conserved epitopes. Sci Immunol. 2022;7:eabq2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li M, Wang H, Tian L, et al. COVID‐19 vaccine development: milestones, lessons and prospects. Signal Transduct Target Ther. 2022;7(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dai L, Gao GF. Viral targets for vaccines against COVID‐19. Nat Rev Immunol. 2021;21(2):73‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supporting Information files).


